Abstract
Background/Aim: With diabetes, skeletal muscle mitochondrial quality (fusion, fission & mitophagy) and muscle mass are compromised. Geranylgeraniol (GGOH) can prevent mitochondrial damage, inflammation, and improve muscle health; however, the effect of GGOH on a diabetic model is not known. This study aimed to determine the effect of GGOH on mitochondrial quality and muscle mass in diabetic rats.
Materials and Methods: Sprague-Dawley rats were divided into three groups: regular diet (CON; n=7), high-fat-diet with 35 mg/kg body weight of streptozotocin (STZ) (HFD; n=7), and HFD/STZ with 800 mg/kg of GGOH (GG; n=7) for a total of 8 weeks. At the end of the study, soleus and gastrocnemius muscles were collected and analyzed for gene and protein expression of OPA1, MFN2, DRP1, p-DRP, LC3AB, PINK1, Parkin, SOD2, NF-ĸB, IL-6, TNF-α, and IL-1β. Additionally, the cross-sectional area (CSA) of soleus muscles was analyzed.
Results: In soleus, HFD group had significantly higher IL-1β and lower LC3A, MFN2, DRP1, and SOD2 mRNA expression compared to CON group. The GG group had higher PINK1 mRNA expression than the HFD group. Additionally, the GG group had lower LC3B and DRP1 protein than the HFD group and lower LC3A and MFN2 protein than the HFD and CON groups. Lastly, HFD and GG groups had a smaller CSA than CON group, whereas GG had a greater CSA than HFD.
Conclusion: GGOH supplementation could prevent mitochondrial fragmentation and potentially decrease the demand for mitochondrial fusion. Additionally, autophagosome degradation occurred at a greater rate than formation, indicating increased clearance of damaged organelles. Improved mitochondrial quality could potentially rescue muscle CSA in diabetic rats with GGOH supplementation.
Keywords: Autophagosomes, inflammation, fusion, fission, mitochondria, muscle mass, type 2 diabetes
Type 2 Diabetes (T2D) is characterized by hyperglycemia and insulin resistance (1) and its incidence is approximately 8.8% (415 million people) worldwide. Hyperglycemia can increase reactive oxygen species (ROS) and reduce mitochondrial biogenesis, resulting in inflammation, tissue damage, and mitochondrial dysfunction (2). In addition, the mitochondrial dysfunction reduces β-oxidation and ATP production and can also further increase ROS, leading to insulin resistance and diabetes (2). Dysfunctional mitochondria and hyperglycemia play a major role in this vicious cycle that causes inflammation, insulin resistance, and diabetes. Therefore, to prevent mitochondrial dysfunction observed in T2D, mitochondrial quality must be well regulated and maintained through mitochondrial fusion, fission, and mitophagy (3).
Mitochondria are regularly reorganized through mitochondrial fusion [outer mitochondrial membrane (OMM): MFN1/2 and inner mitochondrial membrane (IMM): OPA1] and fission (DRP1) (4). The dysfunctional mitochondria are removed via mitophagy (PINK1, Parkin, LC3A, & LC3B) to maintain proper function (4). These processes have been demonstrated to maintain muscle mass (5,6), muscle force production (6,7) and myofibril contractility (7), and prevent mitochondrial dysfunction (7,8) in the skeletal muscles of mice. However, the balance between fusion and fission must be maintained to prevent the accumulation of dysfunctional mitochondria (9). Therefore, mitochondrial fragmentation can occur through increased fission, decreased fusion, or a combination of both (9). For example, over-expression of MFN2 in liver cells generated mitochondrial clusters composed of small damaged mitochondria (9), while DRP1 knockdown mice had reduced clearance of dysfunctional mitochondria and caused muscle atrophy in skeletal muscle (5). This suggests that the dynamics between fusion and fission are critical in preventing increases in damaged mitochondria.
Inflammation observed in T2D is associated with increased mitochondrial fragmentation caused by the upregulation of DRP1 or downregulation of MFN2 (10). Increased mitochondrial fragmentation and reduced fusion lead to increased ROS production in myoblasts treated with high concentrations of glucose (11) and in MFN2 knockout model (12). Studies have demonstrated a reduction in fusion (MFN2 and OPA1) (13-15) and mitophagy protein expression (PINK1 and LC3B) in T2D patients (16,17) and high-fat diet-fed mice (15). In contrast, fission protein expression (DRP1) is upregulated in obese high-fat diet-fed mice (15). Additionally, evidence has suggested that the observed muscular atrophy (18), impaired metabolism (4), and fiber-type transition (4) in T2D could potentially stem from the mitochondria (4). This leads to skeletal muscle dysfunction characterized by reduced muscle strength/power, poor functional capacity (19,20), and exercise intolerance (4). These results demonstrate that improving mitochondrial health could mitigate the skeletal muscle dysfunction observed in T2D.
Most T2D patients are prescribed statins depending on their age and risk factors (21), and statins have been shown to reduce the synthesis of ubiquinone (CoQ10) and geranylgeraniol (GGOH) (22). Compared to healthy individuals, T2D individuals had lower levels of CoQ10 (23-25), which could ultimately lead to mitochondrial dysfunction. GGOH supplementation with statins has been shown to prevent statin toxicity by promoting CoQ10 synthesis in monocytic cells (26). These results have suggested that GGOH could mitigate statin-mediated mitochondrial dysfunction. Additionally, GGOH supplementation to Wistar rats has been shown to exert anti-inflammatory effects by inhibiting nuclear factor-ĸB (NF-ĸB), which caused a reduction in inflammatory cytokines (IL-6, IL-1β, and TNF-α) in the plasma and the liver (27). Similarly, when incubating neuronal cells with GGOH, there was a reduction in inflammatory markers, and preservation of the integrity of the mitochondria (28). Notably, supplementing GGOH in a denervated rat model has prevented reduction in gastrocnemius cross-sectional area (CSA) (29). These results suggest that GGOH could be a viable supplementation for a diabetic model as GGOH mitigates inflammation, preserves mitochondrial health and shape, and protects against reduction in muscle size. Improving mitochondrial quality is essential to enhance metabolic regulation in diabetic populations; however, to our knowledge, no research has examined the effect of GGOH supplementation on mitochondrial quality (mitochondrial fusion, fission, and mitophagy) and muscle cross-sectional area (CSA) in the skeletal muscle of T2D rats. Therefore, this study aimed to determine the effect of GGOH on mitochondrial quality and muscle CSA in rats with diabetes.
Materials and Methods
Animals and treatments. Twenty-one Sprague-Dawley rats were randomly assigned to three groups: regular diet (CON; n=7), high-fat diet (HFD; n=7), and geranylgeraniol+high-fat diet (GG; n=7). CON was given an AIN-93G diet (10% calories from fat) throughout the eight weeks of the study. After two weeks of feeding, the CON group was given a vehicle citrate buffer in a dose volume of 1 ml/kg. The HFD group was fed with a high-fat diet (45% calories came from fat, Research Diets), while the GG group was given a high-fat diet with 800 mg/kg GGOH (American River Nutrition, LLC., Hadley, MA, USA) for eight weeks. After two weeks of feeding, HFD and GG were given a streptozotocin (STZ) dose of 35 mg/kg body weight at 0.1 mmol/L citrate buffer dissolved in citrate buffer at a pH of 4.4 (30) to induce diabetes. Fasting blood sugar was collected 42-72 h after STZ injection, and rats were considered diabetic if fasting blood sugar was above 200 mg/dl. Additionally, non-fasting blood sugar (NFBS) was measured one week after STZ injection from 8-10 am. The rats in HFD and GG groups were confirmed to have diabetes based on their fasting glucose levels (data not shown). The rats were kept in individual cages with the temperature set at 21±2˚C with a 12 h light-dark cycle. They were fed their respective diets twice a week and had free access to food and water. All conditions and handling of the animals were approved by the Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee. All experiments were performed by the relevant guidelines and regulations.
Sample collection. At the end of the study, blood was collected from rats fasted for 4 h and then anesthetized with isoflurane. Blood samples were centrifuged and kept at −80˚C for further analysis. In addition, the right soleus muscles were harvested and placed in optimal cutting temperature molds and flash-frozen in liquid nitrogen for CSA analysis. In contrast, the left soleus muscle and gastrocnemius were flash-frozen in liquid nitrogen for gene and protein expression analysis. Muscle samples were kept at −80˚C for further analysis.
Muscle tissue homogenization and RNA isolation. Muscle samples were weighed, stripped from surrounding structures, and placed in a homogenization safe tube. Homogenizing buffer and glass beads were added and homogenized using the preset setting for rat muscle in the FastPrep-24 5G (MP Biomedicals, Solon, OH, USA). The tube was then incubated at room temperature for 10 min. The homogenized sample was centrifuged at 10,640×g for 5 min at 4˚C, and the supernatant was transferred to a new tube and aliquoted for protein concentration and western blot analysis. Using the manufacturer’s protocol, the soleus muscle RNA was extracted using RNeasy Fibrous Tissue Mini Kit (Qiagen, Germantown, MD, USA). The supernatant was then mixed with ethanol and added to the RNeasy Mini Column, and centrifuged. Next, DNase stock solution was added to the RNeasy membrane and incubated at 20-30oC for 15 min. Following incubation, Buffer RW1 was added to the RNeasy column, centrifuged for 15 s, and the flow-through was discarded. RNeasy column was then placed in a 2 ml tube, centrifuged for 1 min, and finally, put into a new 1.5 ml tube with RNase-free water, and the RNA was eluted.
Muscle gene expression. Muscle samples were analyzed for muscle gene expression of NF-ĸB, IL-1β, IL-6, TNF-α, SOD2, MFN2, DRP1, PINK1, Parkin, LC3A, and LC3B in the CON, HFD, and GG groups. Real-time PCR was performed using # 2 ABI PRISM70500 Sequence Detection System (Applied Biosystems, Waltham, MA, USA) with iTaq SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and was normalized to β-actin. Relative fold change in transcript abundance was determined using the 2-ΔΔCt method.
CSA analysis. Muscle samples were sectioned at 10 μm using a Shandon Cryotome (Thermo-Fisher, Waltham, MA, USA). Soleus muscles from CON, HFD, and GG groups were placed on the same microscope slide in duplicates and immediately fixed using 4% paraformaldehyde (PFA) for ten min. Once fixed, the slides were washed in phosphate-buffered saline with Tween-20 (PBST) 3 times for 5 min (3´5). After washing, the slides were placed in hematoxylin for 10 min and then washed in PBST 3X5. Slides were then dipped in Eosin for 5 min. A mounting medium was added, and slides were mounted and stored in the darkened box at room temperature. Slides were then visualized using a Zeiss Axiovert 200m Inverted Fluorescent Motorized Microscope (Ziess, Dublin, CA, USA). Images were taken, and muscle CSA was analyzed using Image J (National Institute for Health, Bethesda, MD, USA). One hundred muscle fiber areas were measured for each sample.
Western blot analysis. The supernatant was isolated and analyzed for protein concentration using Pierce™ BCA Protein Assay Kit and was stored a -80˚C. Supernatant from each sample was combined with Tris-buffered saline (TBS), 2X Laemmli buffer, and dithiothreitol. The supernatant was sonicated and heated at 95˚C. Then, 50 μg of protein were loaded into 20% polyacrylamide gel (4-20% Mini-PROTEAN TGX gel, Bio-Rad) and separated at 120V for 45 min at room temperature. All samples from CON, HFD, and GG groups were loaded on the same gel in duplicates. Samples were then electrophoretically transferred to a Polyvinylidene fluoride membrane at 70V at 4˚C for 2.5 h for immunoblotting. Membranes were washed with TBST (TBS with Tween-20) 3X5 and dried using methanol for 1 min. The ladder was marked with a WesternBright ChemiPen (Advansta Inc., San Jose, CA, USA) and then rewetted and washed with TBST 3X5. Next, 5% nonfat dry milk with TBST was used to block the membranes for 1 h, and after that, the membrane was incubated with primary antibodies against OPA1 (1:1,000; Cell Signaling, Danvers, MA, USA), MFN2 (1:1,000; Cell Signaling), PINK1 (1:1,000; Novus, Centennial, CO, USA), Parkin (1:1,000; Cell Signaling), LC3AB (1:1,000; Cell Signaling), IL-1β (1:1,000; Novus), IL-6 (1:1,000; Santa Cruz, Dallas, TX, USA), SOD2 (1:1,500; Novus), and GAPDH (1:4,000; Cell Signaling) with 3% nonfat dry milk with TBST at 4˚C for 16 h. DRP1 (1:500; Cell Signaling) and p-DRP1 (1:500; Cell Signaling) were blocked with 5% bovine serum albumin (BSA) in TBST for both primary and secondary antibody incubation. After overnight incubation the membranes blocked with mouse and rabbit monoclonal primary antibodies were incubated with secondary anti-mouse IgG (1:1,000; Cell Signaling) and anti-rabbit IgG (1:1,000; Cell Signaling), respectively, for 1 h at room temperature with 5% milk in TBST. Chemiluminescent substrate (WesternBright Sirius HRP substrate Advanta, Menlo Park, CA, USA) and the C-Digit imaging system (Li-Cor, Lincoln, NE, USA) were used to visualize the stained protein bands. Image Studio Digits Ver 4.0 (Li-Cor) was used for band densitometry. Membranes were stripped using 5X Western reprobe for 60 min and reblotted with antibodies. Total protein concentrations were normalized to GAPDH and expressed in arbitrary units.
Statistical analysis. SPSS (IBM version 26; IBM Corp, Armonk, NY, USA) was used for all statistical analyses. Log10 transformation was used when the assumption of normality was violated. Gene expression, protein expression, and soleus muscle CSA were analyzed using a one-way analysis of variance. Bonferroni post hoc tests were used for pairwise comparisons. The statistical significance was set at p≤0.05. Data are reported as mean±SE.
Results
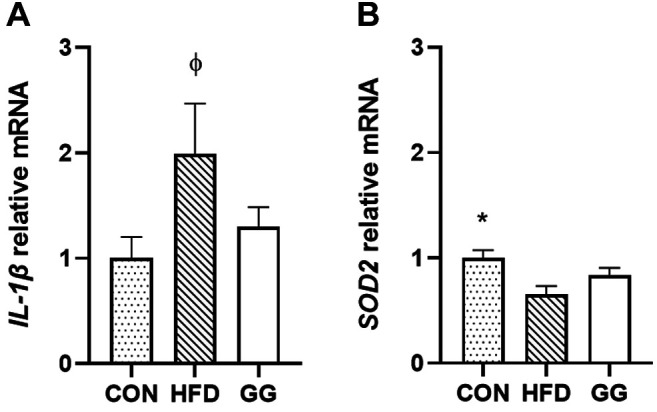
Pro-inflammatory cytokines. The expression of IL-1β gene in the soleus muscle was significantly higher in the HFD group (1.99±0.48) (p<0.05) than that in the CON group (1.00±0.20; p=0.033; Figure 1A) while no significant (p>0.05) differences were observed between GG and CON groups and GG and HFD groups. No significant differences were observed for NF-ĸB, IL-6, and TNF-α among groups. No significant differences were observed in protein expression of IL-1β, IL-6, and TNF-α in the soleus muscle among all groups.
Figure 1. Gene expression analyses for (A) IL-1β and (B) SOD2. A significant condition effect was observed for IL-1β and SOD2. Values are mean±SE. *p<0.05 vs. high-fat-diet (HFD). φp<0.05 vs. regular diet (CON).
The expression IL-1β gene in the gastrocnemius muscle was significantly higher in the HFD group (2.72±0.64; p=0.016) than in the CON group (1.00±0.19), while no differences were observed between GG and HFD groups, and CON and GG groups. No significant differences were observed for NF-ĸB, IL-6, and TNF-α gene expression among groups. Furthermore, no significant differences were observed regarding the expression of IL-1β, IL-6, and TNF-α proteins in the gastrocnemius muscle among the groups.
Antioxidant marker. The expression of SOD2 gene in the soleus muscle was significantly lower in the HFD group (0.65±0.08) than in the CON group (1.00±0.07; p=0.007; Figure 1B), while no differences were observed between CON and GG groups and GG and HFD groups. No significant differences were observed in soleus SOD2 protein expression among groups. Furthermore, no significant differences were observed regarding SOD2 gene and protein expression in the gastrocnemius muscle among the groups.
Mitochondrial quality.
Mitochondrial fusion. MFN2 gene expression in the soleus muscle was significantly lower in the HFD group (0.74±0.10) than in the CON group (1.00±0.06; p=0.041; Figure 2A), while no differences were observed between GG and CON groups and GG and HFD groups.
Figure 2. Gene expression analyses for (A) MFN2, (B) DRP1, and (C) PINK1. A significant condition effect was observed for MFN2, DRP1, and PINK1. Values are mean±SE. *p<0.05 vs. high-fat-diet (HFD).
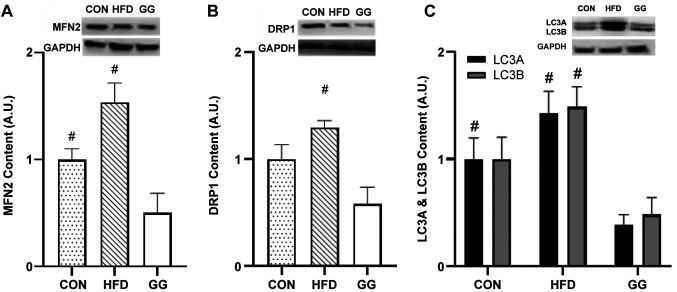
MFN2 protein expression in the soleus muscle was significantly lower in the GG group (0.43±0.17) than in the CON (1.00±0.10; p=0.007) and HFD (1.54±0.18; p=0.010; Figure 3A) groups, while no differences were observed between CON and HFD groups. No significant differences were observed in OPA1 protein expression in the soleus muscle among groups. For the gastrocnemius gene and protein expression, no significant differences were observed for MFN2 and OPA1 among the groups.
Figure 3. Protein expression analyses for (A) MFN2, (B) DRP1, (C) LC3A & LC3B. Protein expression analysis [regular diet (CON): n=7; highfat-diet (HFD): n=7; GG: n=7] data were normalized to GAPDH. A significant condition effect was observed for MFN2, DRP1, LC3A, and LC3B. The western blots display an example of protein expression for MFN2, DRP1, LC3A & LC3B, and the corresponding GAPDH in CON, HFD, and GG groups of rats. Values are mean±SE. #p<0.05 vs. GG.
Mitochondrial fission. DRP1 gene expression in the soleus muscle was significantly lower in the HFD group (0.61±0.09) than in the CON group (1.00±0.04; p=0.002; Figure 2B), while no differences were observed between GG and CON groups and GG and HFD groups. DRP1 protein expression in the soleus muscle was significantly lower in the GG group (0.58±0.16) than that in the HFD group (1.30±0.06; p=0.019; Figure 3B), while no differences were observed between the GG and CON groups and HFD and CON groups. Additionally, no significant differences were observed in soleus p-DRP protein expression and the ratio of p-DRP/DRP-1 among the groups.
For the gastrocnemius gene and protein expression, no significant differences were observed for DRP1, p-DRP1, and p-DRP1/DRP-1 among the groups.
Mitophagy. PINK1 gene expression in the soleus muscle was significantly higher in the GG group (1.30±0.13) than in the HFD group (0.81±0.16; p=0.034; Figure 2C), while no differences were observed between GG and CON groups and CON and HFD groups. No significant differences were observed among the groups for Parkin expression. The LC3A protein expression in the soleus muscle was significantly lower in the GG group (0.38±0.10) than in the CON (1.00±0.20; p=0.028; Figure 2C) and HFD (1.42±0.21; p=0.010; Figure 3C) groups, while no differences were observed between CON and HFD groups. The LC3B protein expression of in the soleus muscle was significantly lower in the GG group (0.48±0.17) than in the HFD group (1.49±0.19; p=0.012; Figure 3D), while no differences were observed between the HFD and CON groups and GG and CON groups. No significant differences were observed in soleus protein expression for PINK1, Parkin, and the ratio of LC3B/A among the groups.
The PINK1 gene expression in the gastrocnemius muscle was significantly greater in the HFD (2.07±0.21; p=0.012) and GG (2.73±0.81; p=0.006) groups than in the CON group (1.00±0.10) while no differences were observed between HFD and GG groups. No significant differences were observed for Parkin gene expression in the gastrocnemius muscle among the groups. No significant differences were observed for PINK1, Parkin, LC3A, and LC3B protein expression, and the ratio of LC3B/A in the gastrocnemius muscle among the groups.
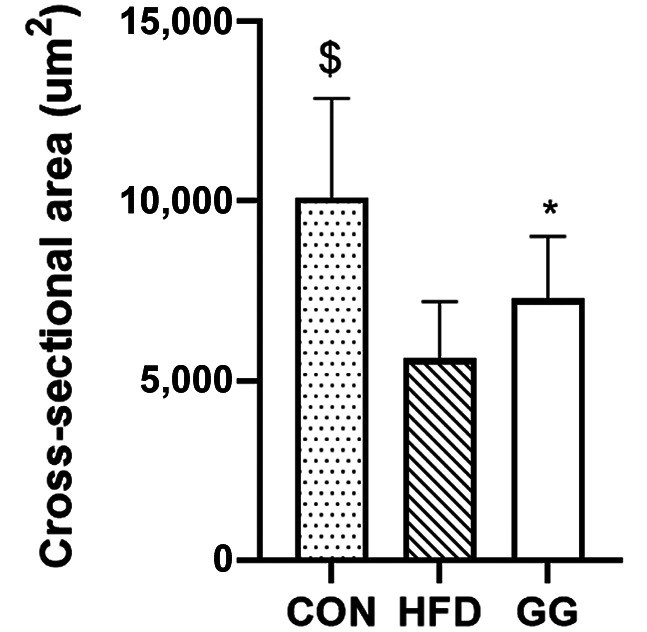
Muscle CSA. The GG group (7,284.69±70.91 μm2) had significantly greater CSA than the HFD group (5,615.59±59.97 μm2; p=0.001), while the CON group (10,092.88±104.67 μm2) had significantly greater CSA than the GG (7,284.69±70.91 μm2) and HFD (5,615.59±59.97 μm2; p=0.001; Figure 4) groups.
Figure 4. Cross-sectional area (CSA) analyses for soleus muscle of rats. For CSA analysis [regular diet (CON): n=7; high-fat-diet (HFD): n=7; GG: n=7), 100 muscle fibers from each rat (n=700 muscle fibers) were analyzed. A significant condition effect was observed. Values are mean±SE. $p<0.05 vs. GG and HFD groups. *p<0.05 vs. HFD group.
Discussion
The major finding of this study is that, in the soleus muscle, diabetic rats (HFD) had increased levels of the pro-inflammatory cytokine (IL-1β), decreased oxidative capacity (SOD2), fusion (MFN2), and fission (DRP1) transcriptional activity when compared to non-diabetic rats (CON). However, supplementation of their diet with GGOH (GG) resulted in increased mitophagy (PINK1) transcriptional activity, decreased levels of fusion (MFN2), fission (DRP1), and mitophagy (LC3A, LC3B) proteins compared to those in the HFD group. Concomitantly, the GG group had a greater soleus CSA than the HFD group; however, CSA in the GG group was still smaller than that in the CON group. These findings suggest that GGOH supplementation may have an integral role in preserving muscle mass which could, at least partly, be due to the attenuation of inflammation and favorable mitochondrial dynamics.
Diabetes is characterized by insulin resistance and hyperglycemia, which are implicated with chronic inflammation (31) and oxidative stress (32). In the current study, diabetic rats (HFD) had higher expression of IL-1β (in gastrocnemius and soleus) and lower mitochondria-specific SOD2 genes in the soleus muscle than control rats (CON) with no difference in IL-6 and TNF-α. These suggested that 8-week high fat diet with STZ was sufficient to promote inflammatory response and impair mitochondrial oxidative capacity in the soleus muscle. In accordance with our results, a greater IL-1β gene expression was observed in myoblasts from T2D individuals (33) and the soleus muscle from a T2D (34) and insulin-resistance (35) mouse models with no differences in IL-6 and TNF-α (33). For SOD2, despite the different measurement sites [i.e., spinal cord (36)], our result is consistent with that in mice fed a high-fat diet (36). However, SOD2 protein content was decreased in the gastrocnemius muscle of type 1 diabetic (T1D) rats (37). The discordance result between Pottecher et al. (2018) and ours could be due to the muscle (gastrocnemius vs. soleus), model (T1D vs. T2D), and STZ dose (65 mg/kg vs. 35 mg/kg) (37). The transcription factor Nuclear Factor-Kappa B (NF-ĸB) modulates gene expression of many cellular processes, e.g., inflammation and oxidative stress (38). It has been well elucidated that NF-ĸB regulates the transcriptional activity for IL-1β, IL-6, and TNF-α. In addition, NF-ĸB-induced p53 has been shown to suppress SOD2 transcription activity (39). It is important to note that although one-way ANOVA results revealed no difference in NF-ĸB among groups since IL-1β was different between HFD and CON groups, a secondary analysis using an independent t-test was conducted. Results showed that NF-ĸB was higher in the HFD group than in the CON group, which could partly explain the greater IL-1β and lower SOD2 in the HFD group than in the CON group. Previously, Carlsen et al. (2009) had shown that mice fed with a high-fat diet (similar nutritional contents compared to the current high-fat diet) had a higher whole body NF-ĸB activity than mice fed with a low-fat diet (40). In addition, compared to healthy adults, insulin-resistant adults had higher NF-ĸB activity in the skeletal muscle (41). Together, these results might, at least partly, suggest that the NF-ĸB signaling cascade could mediate high IL-1β and low SOD2 in the HFD group.
Emerging evidence has demonstrated that mitochondrial fusion (MFN2 and OPA1) and fission (DRP1) are dysregulated in T2D model (3) and could contribute to muscle atrophy (6,8,42,43). Regarding fusion and fission, the diabetic rats had lower mitochondrial fusion (lower MFN2) and fission (lower DRP1) transcriptional capacity than the control rats. Interestingly, when a high-fat diet was supplemented with GGOH (GG), no changes were observed for transcriptional capacity; however, MFN2 and total DRP1 protein levels were lower in the HFD group and the CON group, with no change in DRP1 activational state (no difference in the ratio between p-DRP1Ser637 and total DRP1). Our results for MFN2 and OPA1 were consistent with those of previous studies. Previous results have demonstrated that MFN2 gene or protein expression was reduced in skeletal muscle of obese mice fed a high-fat diet (15), newly diagnosed diabetic subjects (44), and type 2 obese and lean diabetic individuals (13). Additionally, no differences were observed in OPA1 protein expression in obese mice fed a high-fat diet than in mice fed a normal diet (15). Despite the similar role of MFN2 and OPA1 in mitochondrial fusion, it has been shown that MFN is essential for fusion as no OMM fusion was observed in MFN-null cells, while in OPA1-null cells partial fusion was observed (45). Although it is not uncommon, our findings regarding MFN2 protein expression were not consistent with the gene data. The discordance between gene and protein expression could be due to the complicated post-translational and variation in in vivo protein half-life (46).
In contrast, our gene and protein results for DRP1 are inconsistent with those of others showing an increase in total DRP1 protein in diabetic individuals and rodent models (15,47,48). Nevertheless, these studies (15,48) did not assess the DRP1 activational state, which is a better indication of mitochondrial fission than total DRP1. Interestingly, when mice over-expressed DRP1, they experienced a severe reduction in muscle mass in both the soleus and gastrocnemius muscles, suggesting that DRP1 could be critical in maintaining skeletal muscle CSA (43). The decrease in fusion indicated a reduction in mitochondrial dynamics and has been implicated in muscle mass loss (6,8,42,43). The decreased fusion and fission were previously observed during cellular aging (49). Rather than considering it as a negative physiological change, Figge et al. suggested that when mitochondria were damaged and lost their function due to oxidative stress, the rate of fusion and fission could be reduced as an adaptative measure to prolong cellular function (50). Thus, the greater CSA in the GG group than in the HFD group might, at least partly, be explained by the mitochondria’s adaptative response to a high-fat diet.
Fragmented mitochondria must be removed via PINK1/Parkin-mediated mitophagy (4). Otherwise, increased accumulation of fragmented mitochondria could increase ROS and further add to the inflammatory state commonly observed in diabetic models (10). In contrast to previous studies that observed a decrease in PINK1 and Parkin compared to control (17,44,51), our results showed that PINK1 and Parkin were not different between the HFD and CON groups. Interestingly, with GGOH supplementation (GG), the transcript abundance for PINK1 was higher than that in the HFD group and no changes were observed in PINK1 protein and Parkin gene and protein expression. The discordance in the PINK1 gene and protein could potentially be due to the rate of PINK1 protein degradation when the mitochondrial membrane potential is maintained (52). PINK1 phosphorylation is essential for the recruitment and activation of Parkin (53); hence, the absence of changes in PINK1 protein did not allow for activation and recruitment of the Parkin protein. Additionally, LC3A/B can also selectively remove damaged mitochondria. The LC3A protein undergoes lipidation and forms LC3B protein (54), and an increase in LC3B is indicative of autophagosome formation (55). Our results showed no difference between CON and HFD groups, whereas the GG group had lower LC3A and LC3B protein expression than the HFD and CON groups. Previously, it has been demonstrated that skeletal muscle LC3B was lower, and LC3A trended to be lower in T2D patients (16). With GGOH, the reduction in LC3A and LC3B could suggest an overall greater rate of LC3A lipidation (forming LC3B) and LC3B degradation. Therefore, these changes could improve the clearance of damaged mitochondria in the GG group. The inhibition of overall clearance of damaged mitochondria can reduce muscle mass and is therefore essential for preventing atrophy (56).
In this study, GGOH appeared to mitigate the decrease in soleus muscle CSA observed in HFD; but it was still smaller than that in the CON group. The potential mechanism of effects of GGOH on the anti-inflammatory properties could be through improving the mitochondrial quality (reduced total DRP1, LC3A, and LC3B protein expression). Studies have revealed that over-expression of DRP1 and inhibition of damaged mitochondria clearance can reduce skeletal muscle size (43,56). This could suggest that GGOH may have a protective role in preserving soleus muscle CSA in diabetic rats, at least partly, due to the decrease in mitochondrial fission (DRP1) and improved autophagy (LC3A and LC3B), which could prevent excessive mitochondrial fragmentation leading to mitophagy to clear out the damaged mitochondria.
The lack of differences in fission and fusion markers between HFD and CON groups was inconsistent with previous studies (15,57,58) and could be related to the model and duration of feeding (individuals with T2D, human podocytes, and mice administered HFD for 40 weeks). The advantage of using the STZ/HFD model was that its progression to diabetes is similar to that observed in humans with T2D (59); however, Zucker diabetic fatty rat and db/db mouse could be a better model for studying the pathogenesis of diabetes (59). In addition, the HFD/STZ model can result in sustained hyperglycemia and diabetic symptoms (59); however, the mitochondrial changes might require a longer duration to manifest pathogenic symptoms.
In conclusion, GGOH supplementation in diabetic rats mitigated the CSA reduction, possibly through decreased mitochondrial fragmentation and a greater rate of autophagosome degradation. Additionally, GGOH supplementation also prevented a significant increase in the levels of the pro-inflammatory cytokine IL-1β and prevented a decrease in the levels of the antioxidant marker SOD2, which may have also helped preserve muscle CSA in diabetic rats. Thus, changes in mitochondrial quality and reduced inflammation could potentially attenuate the reduction of muscle CSA in diabetic rats with GGOH supplementation. Further research is necessary to investigate the effects of GGOH supplementation on human skeletal muscle regarding mitochondrial quality and muscle size. GGOH is inexpensive and could be used in various clinical and orthopedic conditions susceptible to muscle mass reduction.
Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by the Authors. Additionally, the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Authors’ Contributions
H.Y.L and C.L.S. conceived and designed the research. C.L.S. & R.W. conducted data collection. C.L.S., R.W., H.Y.L., C.A., N.C.J collected samples. N.C.J., H.Y.L., and C.A. performed sample and data analysis. N.C.J. and H.Y.L. wrote the manuscript. N.C.J., C.L.S., R.W., H.Y.L., and C.A. reviewed the draft. All Authors read and approved the manuscript.
Acknowledgements
This project was supported by the Texas Tech University startup funds.
References
- 1.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength. World J Diabetes. 2014;5(4):444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017;11:637–645. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimaki S, Kuwabara T. Diabetes-Induced Dysfunction of Mitochondria and Stem Cells in Skeletal Muscle and the Nervous System. Int J Mol Sci. 2017;18(10):2147. doi: 10.3390/ijms18102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulac M, Leduc-Gaudet JP, Reynaud O, Ayoub MB, Guérin A, Finkelchtein M, Hussain SN, Gouspillou G. Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation. J Physiol. 2020;598(17):3691–3710. doi: 10.1113/JP279802. [DOI] [PubMed] [Google Scholar]
- 6.Tezze C, Romanello V, Desbats MA, Fadini GP, Albiero M, Favaro G, Ciciliot S, Soriano ME, Morbidoni V, Cerqua C, Loefler S, Kern H, Franceschi C, Salvioli S, Conte M, Blaauw B, Zampieri S, Salviati L, Scorrano L, Sandri M. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 2017;25(6):1374–1389.e6. doi: 10.1016/j.cmet.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouspillou G, Godin R, Piquereau J, Picard M, Mofarrahi M, Mathew J, Purves-Smith FM, Sgarioto N, Hepple RT, Burelle Y, Hussain SNA. Protective role of Parkin in skeletal muscle contractile and mitochondrial function. J Physiol. 2018;596(13):2565–2579. doi: 10.1113/JP275604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141(2):280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang P, Yu T, Yoon Y. Mitochondrial clustering induced by overexpression of the mitochondrial fusion protein Mfn2 causes mitochondrial dysfunction and cell death. Eur J Cell Biol. 2007;86(6):289–302. doi: 10.1016/j.ejcb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Williams M, Caino MC. Mitochondrial dynamics in type 2 diabetes and cancer. Front Endocrinol (Lausanne) 2018;9:211. doi: 10.3389/fendo.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U.S.A. 2006;103(8):2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebastián D, Hernández-Alvarez MI, Segalés J, Sorianello E, Muñoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Orešič M, Pich S, Burcelin R, Palacín M, Zorzano A. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U.S.A. 2012;109(14):5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, Laville M, Guillet C, Boirie Y, Wallberg-Henriksson H, Manco M, Calvani M, Castagneto M, Palacín M, Mingrone G, Zierath JR, Vidal H, Zorzano A. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54(9):2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 14.Joseph AM, Joanisse DR, Baillot RG, Hood DA. Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp Diabetes Res. 2012;2012:642038. doi: 10.1155/2012/642038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu R, Jin P, Yu L, Wang Y, Han L, Shi T, Li X. Impaired mitochondrial dynamics and bioenergetics in diabetic skeletal muscle. PLoS One. 2014;9(3):e92810. doi: 10.1371/journal.pone.0092810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Møller AB, Kampmann U, Hedegaard J, Thorsen K, Nordentoft I, Vendelbo MH, Møller N, Jessen N. Altered gene expression and repressed markers of autophagy in skeletal muscle of insulin resistant patients with type 2 diabetes. Sci Rep. 2017;7:43775. doi: 10.1038/srep43775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheele C, Nielsen AR, Walden TB, Sewell DA, Fischer CP, Brogan RJ, Petrovic N, Larsson O, Tesch PA, Wennmalm K, Hutchinson DS, Cannon B, Wahlestedt C, Pedersen BK, Timmons JA. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration. FASEB J. 2007;21(13):3653–3665. doi: 10.1096/fj.07-8520com. [DOI] [PubMed] [Google Scholar]
- 18.Perry BD, Caldow MK, Brennan-Speranza TC, Sbaraglia M, Jerums G, Garnham A, Wong C, Levinger P, Asrar Ul Haq M, Hare DL, Price SR, Levinger I. Muscle atrophy in patients with Type 2 Diabetes Mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc Immunol Rev. 2016;22:94–109. [PMC free article] [PubMed] [Google Scholar]
- 19.Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, van Loon LJ. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14(8):585–592. doi: 10.1016/j.jamda.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, Zuliani G, Ferrucci L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35(8):1672–1679. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamari Y, Bitzur R, Cohen H, Shaish A, Harats D. Should all diabetic patients be treated with a statin. Diabetes Care. 2009;32 Suppl 2:S378–S383. doi: 10.2337/dc09-S344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teclebrhan H, Olsson J, Swiezewska E, Dallner G. Biosynthesis of the side chain of ubiquinone:trans-prenyltransferase in rat liver microsomes. J Biol Chem. 1993;268(31):23081–23086. [PubMed] [Google Scholar]
- 23.Ates O, Bilen H, Keles S, Alp HH, Keleş MS, Yıldırım K, Ondaş O, Pınar LC, Civelekler M, Baykal O. Plasma coenzyme Q10 levels in type 2 diabetic patients with retinopathy. Int J Ophthalmol. 2013;6(5):675–679. doi: 10.3980/j.issn.2222-3959.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-ghoroury EA, Raslan HM, Badawy EA, El-Saaid GS, Agybi MH, Siam I, Salem SI. Malondialdehyde and coenzyme Q10 in platelets and serum in type 2 diabetes mellitus: correlation with glycemic control. Blood Coagul Fibrinolysis. 2009;20(4):248–251. doi: 10.1097/mbc.0b013e3283254549. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa G, Yamamoto Y, Zhi JG, Tanino Y, Yamasaki M, Yano M, Nakajima T, Fukui M, Yoshikawa T, Nakamura N. Daily profile of plasma %CoQ10 level, a biomarker of oxidative stress, in patients with diabetes manifesting postprandial hyperglycaemia. Acta Diabetol. 2005;42(4):179–181. doi: 10.1007/s00592-005-0199-6. [DOI] [PubMed] [Google Scholar]
- 26.Campia I, Lussiana C, Pescarmona G, Ghigo D, Bosia A, Riganti C. Geranylgeraniol prevents the cytotoxic effects of mevastatin in THP-1 cells, without decreasing the beneficial effects on cholesterol synthesis. Br J Pharmacol. 2009;158(7):1777–1786. doi: 10.1111/j.1476-5381.2009.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giriwono PE, Shirakawa H, Ohsaki Y, Hata S, Kuriyama H, Sato S, Goto T, Komai M. Dietary supplementation with geranylgeraniol suppresses lipopolysaccharide-induced inflammation via inhibition of nuclear factor-ĸB activation in rats. Eur J Nutr. 2013;52(3):1191–1199. doi: 10.1007/s00394-012-0429-y. [DOI] [PubMed] [Google Scholar]
- 28.Marcuzzi A, Piscianz E, Zweyer M, Bortul R, Loganes C, Girardelli M, Baj G, Monasta L, Celeghini C. Geranylgeraniol and neurological impairment: Involvement of apoptosis and mitochondrial morphology. Int J Mol Sci. 2016;17(3):365. doi: 10.3390/ijms17030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyawaki A, Rojasawasthien T, Hitomi S, Aoki Y, Urata M, Inoue A, Matsubara T, Morikawa K, Habu M, Tominaga K, Kokabu S. Oral administration of geranylgeraniol rescues denervation-induced muscle atrophy via suppression of Atrogin-1. In Vivo. 2020;34(5):2345–2351. doi: 10.21873/invivo.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahlawat A, Sharma S. A new promising simultaneous approach for attenuating type II diabetes mellitus induced neuropathic pain in rats: iNOS inhibition and neuroregeneration. Eur J Pharmacol. 2018;818:419–428. doi: 10.1016/j.ejphar.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 32.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura S, Yonekura S, Shimosato T, Takaya T. Myogenetic oligodeoxynucleotide (myoDN) recovers the differentiation of skeletal muscle myoblasts deteriorated by diabetes mellitus. Front Physiol. 2021;12:679152. doi: 10.3389/fphys.2021.679152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molanouri Shamsi M, Hassan ZH, Gharakhanlou R, Quinn LS, Azadmanesh K, Baghersad L, Isanejad A, Mahdavi M. Expression of interleukin-15 and inflammatory cytokines in skeletal muscles of STZ-induced diabetic rats: effect of resistance exercise training. Endocrine. 2014;46(1):60–69. doi: 10.1007/s12020-013-0038-4. [DOI] [PubMed] [Google Scholar]
- 35.Américo-Da-Silva L, Aguilera J, Quinteros-Waltemath O, Sánchez-Aguilera P, Russell J, Cadagan C, Meneses-Valdés R, Sánchez G, Estrada M, Jorquera G, Barrientos G, Llanos P. Activation of the NLRP3 inflammasome increases the IL-1β Level and decreases GLUT4 translocation in skeletal muscle during insulin resistance. Int J Mol Sci. 2021;22(19):10212. doi: 10.3390/ijms221910212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langley MR, Yoon H, Kim HN, Choi CI, Simon W, Kleppe L, Lanza IR, LeBrasseur NK, Matveyenko A, Scarisbrick IA. High fat diet consumption results in mitochondrial dysfunction, oxidative stress, and oligodendrocyte loss in the central nervous system. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3):165630. doi: 10.1016/j.bbadis.2019.165630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pottecher J, Adamopoulos C, Lejay A, Bouitbir J, Charles AL, Meyer A, Singer M, Wolff V, Diemunsch P, Laverny G, Metzger D, Geny B. Diabetes worsens skeletal muscle mitochondrial function, oxidative stress, and apoptosis after lower-limb ischemia-reperfusion: Implication of the RISK and SAFE pathways. Front Physiol. 2018;9:579. doi: 10.3389/fphys.2018.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lingappan K. NF-ĸB in oxidative stress. Curr Opin Toxicol. 2018;7:81–86. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drane P, Bravard A, Bouvard V, May E. Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene. 2001;20(4):430–439. doi: 10.1038/sj.onc.1204101. [DOI] [PubMed] [Google Scholar]
- 40.Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, Blomhoff R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4(3):215–222. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tantiwong P, Shanmugasundaram K, Monroy A, Ghosh S, Li M, DeFronzo RA, Cersosimo E, Sriwijitkamol A, Mohan S, Musi N. NF-ĸB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am J Physiol Endocrinol Metab. 2010;299(5):E794–E801. doi: 10.1152/ajpendo.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sebastián D, Sorianello E, Segalés J, Irazoki A, Ruiz-Bonilla V, Sala D, Planet E, Berenguer-Llergo A, Muñoz JP, Sánchez-Feutrie M, Plana N, Hernández-Álvarez MI, Serrano AL, Palacín M, Zorzano A. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016;35(15):1677–1693. doi: 10.15252/embj.201593084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Touvier T, De Palma C, Rigamonti E, Scagliola A, Incerti E, Mazelin L, Thomas JL, D’Antonio M, Politi L, Schaeffer L, Clementi E, Brunelli S. Muscle-specific Drp1 overexpression impairs skeletal muscle growth via translational attenuation. Cell Death Dis. 2015;6:e1663. doi: 10.1038/cddis.2014.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhansali S, Bhansali A, Walia R, Saikia UN, Dhawan V. Alterations in mitochondrial oxidative stress and mitophagy in subjects with prediabetes and type 2 diabetes mellitus. Front Endocrinol (Lausanne) 2017;8:347. doi: 10.3389/fendo.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525–3532. doi: 10.1091/mbc.e09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4(9):117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S, Wang Y, Gan X, Fang D, Zhong C, Wu L, Hu G, Sosunov AA, McKhann GM, Yu H, Yan SS. Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes. 2015;64(5):1728–1742. doi: 10.2337/db14-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D, Sesaki H, Roy S. Reduced levels of Drp1 protect against development of retinal vascular lesions in diabetic retinopathy. Cells. 2021;10(6):1379. doi: 10.3390/cells10061379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jendrach M, Pohl S, Vöth M, Kowald A, Hammerstein P, Bereiter-Hahn J. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev. 2005;126(6-7):813–821. doi: 10.1016/j.mad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Figge MT, Reichert AS, Meyer-Hermann M, Osiewacz HD. Deceleration of fusion-fission cycles improves mitochondrial quality control during aging. PLoS Comput Biol. 2012;8(6):e1002576. doi: 10.1371/journal.pcbi.1002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czajka A, Ajaz S, Gnudi L, Parsade CK, Jones P, Reid F, Malik AN. altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBioMedicine. 2015;2(6):499–512. doi: 10.1016/j.ebiom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191(5):933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhuang N, Li L, Chen S, Wang T. PINK1-dependent phosphorylation of PINK1 and Parkin is essential for mitochondrial quality control. Cell Death Dis. 2016;7(12):e2501. doi: 10.1038/cddis.2016.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27(6):421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 55.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, Ahn HJ, Ait-Mohamed O, Ait-Si-Ali S, Akematsu T, Akira S, Al-Younes HM, Al-Zeer MA, Albert ML, Albin RL, Alegre-Abarrategui J, Aleo MF, Alirezaei M, Almasan A, Almonte-Becerril M, Amano A, Amaravadi R, Amarnath S, Amer AO, Andrieu-Abadie N, Anantharam V, Ann DK, Anoopkumar-Dukie S, Aoki H, Apostolova N, Arancia G, Aris JP, Asanuma K, Asare NY, Ashida H, Askanas V, Askew DS, Auberger P, Baba M, Backues SK, Baehrecke EH, Bahr BA, Bai XY, Bailly Y, Baiocchi R, Baldini G, Balduini W, Ballabio A, Bamber BA, Bampton ET, Bánhegyi G, Bartholomew CR, Bassham DC, Bast RC Jr, Batoko H, Bay BH, Beau I, Béchet DM, Begley TJ, Behl C, Behrends C, Bekri S, Bellaire B, Bendall LJ, Benetti L, Berliocchi L, Bernardi H, Bernassola F, Besteiro S, Bhatia-Kissova I, Bi X, Biard-Piechaczyk M, Blum JS, Boise LH, Bonaldo P, Boone DL, Bornhauser BC, Bortoluci KR, Bossis I, Bost F, Bourquin JP, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady NR, Brancolini C, Brech A, Brenman JE, Brennand A, Bresnick EH, Brest P, Bridges D, Bristol ML, Brookes PS, Brown EJ, Brumell JH, Brunetti-Pierri N, Brunk UT, Bulman DE, Bultman SJ, Bultynck G, Burbulla LF, Bursch W, Butchar JP, Buzgariu W, Bydlowski SP, Cadwell K, Cahová M, Cai D, Cai J, Cai Q, Calabretta B, Calvo-Garrido J, Camougrand N, Campanella M, Campos-Salinas J, Candi E, Cao L, Caplan AB, Carding SR, Cardoso SM, Carew JS, Carlin CR, Carmignac V, Carneiro LA, Carra S, Caruso RA, Casari G, Casas C, Castino R, Cebollero E, Cecconi F, Celli J, Chaachouay H, Chae HJ, Chai CY, Chan DC, Chan EY, Chang RC, Che CM, Chen CC, Chen GC, Chen GQ, Chen M, Chen Q, Chen SS, Chen W, Chen X, Chen X, Chen X, Chen YG, Chen Y, Chen Y, Chen YJ, Chen Z, Cheng A, Cheng CH, Cheng Y, Cheong H, Cheong JH, Cherry S, Chess-Williams R, Cheung ZH, Chevet E, Chiang HL, Chiarelli R, Chiba T, Chin LS, Chiou SH, Chisari FV, Cho CH, Cho DH, Choi AM, Choi D, Choi KS, Choi ME, Chouaib S, Choubey D, Choubey V, Chu CT, Chuang TH, Chueh SH, Chun T, Chwae YJ, Chye ML, Ciarcia R, Ciriolo MR, Clague MJ, Clark RS, Clarke PG, Clarke R, Codogno P, Coller HA, Colombo MI, Comincini S, Condello M, Condorelli F, Cookson MR, Coombs GH, Coppens I, Corbalan R, Cossart P, Costelli P, Costes S, Coto-Montes A, Couve E, Coxon FP, Cregg JM, Crespo JL, Cronjé MJ, Cuervo AM, Cullen JJ, Czaja MJ, D’Amelio M, Darfeuille-Michaud A, Davids LM, Davies FE, De Felici M, de Groot JF, de Haan CA, De Martino L, De Milito A, De Tata V, Debnath J, Degterev A, Dehay B, Delbridge LM, Demarchi F, Deng YZ, Dengjel J, Dent P, Denton D, Deretic V, Desai SD, Devenish RJ, Di Gioacchino M, Di Paolo G, Di Pietro C, Díaz-Araya G, Díaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dikic I, Dinesh-Kumar SP, Ding WX, Distelhorst CW, Diwan A, Djavaheri-Mergny M, Dokudovskaya S, Dong Z, Dorsey FC, Dosenko V, Dowling JJ, Doxsey S, Dreux M, Drew ME, Duan Q, Duchosal MA, Duff K, Dugail I, Durbeej M, Duszenko M, Edelstein CL, Edinger AL, Egea G, Eichinger L, Eissa NT, Ekmekcioglu S, El-Deiry WS, Elazar Z, Elgendy M, Ellerby LM, Eng KE, Engelbrecht AM, Engelender S, Erenpreisa J, Escalante R, Esclatine A, Eskelinen EL, Espert L, Espina V, Fan H, Fan J, Fan QW, Fan Z, Fang S, Fang Y, Fanto M, Fanzani A, Farkas T, Farré JC, Faure M, Fechheimer M, Feng CG, Feng J, Feng Q, Feng Y, Fésüs L, Feuer R, Figueiredo-Pereira ME, Fimia GM, Fingar DC, Finkbeiner S, Finkel T, Finley KD, Fiorito F, Fisher EA, Fisher PB, Flajolet M, Florez-McClure ML, Florio S, Fon EA, Fornai F, Fortunato F, Fotedar R, Fowler DH, Fox HS, Franco R, Frankel LB, Fransen M, Fuentes JM, Fueyo J, Fujii J, Fujisaki K, Fujita E, Fukuda M, Furukawa RH, Gaestel M, Gailly P, Gajewska M, Galliot B, Galy V, Ganesh S, Ganetzky B, Ganley IG, Gao FB, Gao GF, Gao J, Garcia L, Garcia-Manero G, Garcia-Marcos M, Garmyn M, Gartel AL, Gatti E, Gautel M, Gawriluk TR, Gegg ME, Geng J, Germain M, Gestwicki JE, Gewirtz DA, Ghavami S, Ghosh P, Giammarioli AM, Giatromanolaki AN, Gibson SB, Gilkerson RW, Ginger ML, Ginsberg HN, Golab J, Goligorsky MS, Golstein P, Gomez-Manzano C, Goncu E, Gongora C, Gonzalez CD, Gonzalez R, González-Estévez C, González-Polo RA, Gonzalez-Rey E, Gorbunov NV, Gorski S, Goruppi S, Gottlieb RA, Gozuacik D, Granato GE, Grant GD, Green KN, Gregorc A, Gros F, Grose C, Grunt TW, Gual P, Guan JL, Guan KL, Guichard SM, Gukovskaya AS, Gukovsky I, Gunst J, Gustafsson AB, Halayko AJ, Hale AN, Halonen SK, Hamasaki M, Han F, Han T, Hancock MK, Hansen M, Harada H, Harada M, Hardt SE, Harper JW, Harris AL, Harris J, Harris SD, Hashimoto M, Haspel JA, Hayashi S, Hazelhurst LA, He C, He YW, Hébert MJ, Heidenreich KA, Helfrich MH, Helgason GV, Henske EP, Herman B, Herman PK, Hetz C, Hilfiker S, Hill JA, Hocking LJ, Hofman P, Hofmann TG, Höhfeld J, Holyoake TL, Hong MH, Hood DA, Hotamisligil GS, Houwerzijl EJ, Høyer-Hansen M, Hu B, Hu CA, Hu HM, Hua Y, Huang C, Huang J, Huang S, Huang WP, Huber TB, Huh WK, Hung TH, Hupp TR, Hur GM, Hurley JB, Hussain SN, Hussey PJ, Hwang JJ, Hwang S, Ichihara A, Ilkhanizadeh S, Inoki K, Into T, Iovane V, Iovanna JL, Ip NY, Isaka Y, Ishida H, Isidoro C, Isobe K, Iwasaki A, Izquierdo M, Izumi Y, Jaakkola PM, Jäättelä M, Jackson GR, Jackson WT, Janji B, Jendrach M, Jeon JH, Jeung EB, Jiang H, Jiang H, Jiang JX, Jiang M, Jiang Q, Jiang X, Jiang X, Jiménez A, Jin M, Jin S, Joe CO, Johansen T, Johnson DE, Johnson GV, Jones NL, Joseph B, Joseph SK, Joubert AM, Juhász G, Juillerat-Jeanneret L, Jung CH, Jung YK, Kaarniranta K, Kaasik A, Kabuta T, Kadowaki M, Kagedal K, Kamada Y, Kaminskyy VO, Kampinga HH, Kanamori H, Kang C, Kang KB, Kang KI, Kang R, Kang YA, Kanki T, Kanneganti TD, Kanno H, Kanthasamy AG, Kanthasamy A, Karantza V, Kaushal GP, Kaushik S, Kawazoe Y, Ke PY, Kehrl JH, Kelekar A, Kerkhoff C, Kessel DH, Khalil H, Kiel JA, Kiger AA, Kihara A, Kim DR, Kim DH, Kim DH, Kim EK, Kim HR, Kim JS, Kim JH, Kim JC, Kim JK, Kim PK, Kim SW, Kim YS, Kim Y, Kimchi A, Kimmelman AC, King JS, Kinsella TJ, Kirkin V, Kirshenbaum LA, Kitamoto K, Kitazato K, Klein L, Klimecki WT, Klucken J, Knecht E, Ko BC, Koch JC, Koga H, Koh JY, Koh YH, Koike M, Komatsu M, Kominami E, Kong HJ, Kong WJ, Korolchuk VI, Kotake Y, Koukourakis MI, Kouri Flores JB, Kovács AL, Kraft C, Krainc D, Krämer H, Kretz-Remy C, Krichevsky AM, Kroemer G, Krüger R, Krut O, Ktistakis NT, Kuan CY, Kucharczyk R, Kumar A, Kumar R, Kumar S, Kundu M, Kung HJ, Kurz T, Kwon HJ, La Spada AR, Lafont F, Lamark T, Landry J, Lane JD, Lapaquette P, Laporte JF, László L, Lavandero S, Lavoie JN, Layfield R, Lazo PA, Le W, Le Cam L, Ledbetter DJ, Lee AJ, Lee BW, Lee GM, Lee J, Lee JH, Lee M, Lee MS, Lee SH, Leeuwenburgh C, Legembre P, Legouis R, Lehmann M, Lei HY, Lei QY, Leib DA, Leiro J, Lemasters JJ, Lemoine A, Lesniak MS, Lev D, Levenson VV, Levine B, Levy E, Li F, Li JL, Li L, Li S, Li W, Li XJ, Li YB, Li YP, Liang C, Liang Q, Liao YF, Liberski PP, Lieberman A, Lim HJ, Lim KL, Lim K, Lin CF, Lin FC, Lin J, Lin JD, Lin K, Lin WW, Lin WC, Lin YL, Linden R, Lingor P, Lippincott-Schwartz J, Lisanti MP, Liton PB, Liu B, Liu CF, Liu K, Liu L, Liu QA, Liu W, Liu YC, Liu Y, Lockshin RA, Lok CN, Lonial S, Loos B, Lopez-Berestein G, López-Otín C, Lossi L, Lotze MT, Lőw P, Lu B, Lu B, Lu B, Lu Z, Luciano F, Lukacs NW, Lund AH, Lynch-Day MA, Ma Y, Macian F, MacKeigan JP, Macleod KF, Madeo F, Maiuri L, Maiuri MC, Malagoli D, Malicdan MC, Malorni W, Man N, Mandelkow EM, Manon S, Manov I, Mao K, Mao X, Mao Z, Marambaud P, Marazziti D, Marcel YL, Marchbank K, Marchetti P, Marciniak SJ, Marcondes M, Mardi M, Marfe G, Mariño G, Markaki M, Marten MR, Martin SJ, Martinand-Mari C, Martinet W, Martinez-Vicente M, Masini M, Matarrese P, Matsuo S, Matteoni R, Mayer A, Mazure NM, McConkey DJ, McConnell MJ, McDermott C, McDonald C, McInerney GM, McKenna SL, McLaughlin B, McLean PJ, McMaster CR, McQuibban GA, Meijer AJ, Meisler MH, Meléndez A, Melia TJ, Melino G, Mena MA, Menendez JA, Menna-Barreto RF, Menon MB, Menzies FM, Mercer CA, Merighi A, Merry DE, Meschini S, Meyer CG, Meyer TF, Miao CY, Miao JY, Michels PA, Michiels C, Mijaljica D, Milojkovic A, Minucci S, Miracco C, Miranti CK, Mitroulis I, Miyazawa K, Mizushima N, Mograbi B, Mohseni S, Molero X, Mollereau B, Mollinedo F, Momoi T, Monastyrska I, Monick MM, Monteiro MJ, Moore MN, Mora R, Moreau K, Moreira PI, Moriyasu Y, Moscat J, Mostowy S, Mottram JC, Motyl T, Moussa CE, Müller S, Muller S, Münger K, Münz C, Murphy LO, Murphy ME, Musarò A, Mysorekar I, Nagata E, Nagata K, Nahimana A, Nair U, Nakagawa T, Nakahira K, Nakano H, Nakatogawa H, Nanjundan M, Naqvi NI, Narendra DP, Narita M, Navarro M, Nawrocki ST, Nazarko TY, Nemchenko A, Netea MG, Neufeld TP, Ney PA, Nezis IP, Nguyen HP, Nie D, Nishino I, Nislow C, Nixon RA, Noda T, Noegel AA, Nogalska A, Noguchi S, Notterpek L, Novak I, Nozaki T, Nukina N, Nürnberger T, Nyfeler B, Obara K, Oberley TD, Oddo S, Ogawa M, Ohashi T, Okamoto K, Oleinick NL, Oliver FJ, Olsen LJ, Olsson S, Opota O, Osborne TF, Ostrander GK, Otsu K, Ou JH, Ouimet M, Overholtzer M, Ozpolat B, Paganetti P, Pagnini U, Pallet N, Palmer GE, Palumbo C, Pan T, Panaretakis T, Pandey UB, Papackova Z, Papassideri I, Paris I, Park J, Park OK, Parys JB, Parzych KR, Patschan S, Patterson C, Pattingre S, Pawelek JM, Peng J, Perlmutter DH, Perrotta I, Perry G, Pervaiz S, Peter M, Peters GJ, Petersen M, Petrovski G, Phang JM, Piacentini M, Pierre P, Pierrefite-Carle V, Pierron G, Pinkas-Kramarski R, Piras A, Piri N, Platanias LC, Pöggeler S, Poirot M, Poletti A, Poüs C, Pozuelo-Rubio M, Prætorius-Ibba M, Prasad A, Prescott M, Priault M, Produit-Zengaffinen N, Progulske-Fox A, Proikas-Cezanne T, Przedborski S, Przyklenk K, Puertollano R, Puyal J, Qian SB, Qin L, Qin ZH, Quaggin SE, Raben N, Rabinowich H, Rabkin SW, Rahman I, Rami A, Ramm G, Randall G, Randow F, Rao VA, Rathmell JC, Ravikumar B, Ray SK, Reed BH, Reed JC, Reggiori F, Régnier-Vigouroux A, Reichert AS, Reiners JJ Jr, Reiter RJ, Ren J, Revuelta JL, Rhodes CJ, Ritis K, Rizzo E, Robbins J, Roberge M, Roca H, Roccheri MC, Rocchi S, Rodemann HP, Rodríguez de Córdoba S, Rohrer B, Roninson IB, Rosen K, Rost-Roszkowska MM, Rouis M, Rouschop KM, Rovetta F, Rubin BP, Rubinsztein DC, Ruckdeschel K, Rucker EB 3rd, Rudich A, Rudolf E, Ruiz-Opazo N, Russo R, Rusten TE, Ryan KM, Ryter SW, Sabatini DM, Sadoshima J, Saha T, Saitoh T, Sakagami H, Sakai Y, Salekdeh GH, Salomoni P, Salvaterra PM, Salvesen G, Salvioli R, Sanchez AM, Sánchez-Alcázar JA, Sánchez-Prieto R, Sandri M, Sankar U, Sansanwal P, Santambrogio L, Saran S, Sarkar S, Sarwal M, Sasakawa C, Sasnauskiene A, Sass M, Sato K, Sato M, Schapira AH, Scharl M, Schätzl HM, Scheper W, Schiaffino S, Schneider C, Schneider ME, Schneider-Stock R, Schoenlein PV, Schorderet DF, Schüller C, Schwartz GK, Scorrano L, Sealy L, Seglen PO, Segura-Aguilar J, Seiliez I, Seleverstov O, Sell C, Seo JB, Separovic D, Setaluri V, Setoguchi T, Settembre C, Shacka JJ, Shanmugam M, Shapiro IM, Shaulian E, Shaw RJ, Shelhamer JH, Shen HM, Shen WC, Sheng ZH, Shi Y, Shibuya K, Shidoji Y, Shieh JJ, Shih CM, Shimada Y, Shimizu S, Shintani T, Shirihai OS, Shore GC, Sibirny AA, Sidhu SB, Sikorska B, Silva-Zacarin EC, Simmons A, Simon AK, Simon HU, Simone C, Simonsen A, Sinclair DA, Singh R, Sinha D, Sinicrope FA, Sirko A, Siu PM, Sivridis E, Skop V, Skulachev VP, Slack RS, Smaili SS, Smith DR, Soengas MS, Soldati T, Song X, Sood AK, Soong TW, Sotgia F, Spector SA, Spies CD, Springer W, Srinivasula SM, Stefanis L, Steffan JS, Stendel R, Stenmark H, Stephanou A, Stern ST, Sternberg C, Stork B, Strålfors P, Subauste CS, Sui X, Sulzer D, Sun J, Sun SY, Sun ZJ, Sung JJ, Suzuki K, Suzuki T, Swanson MS, Swanton C, Sweeney ST, Sy LK, Szabadkai G, Tabas I, Taegtmeyer H, Tafani M, Takács-Vellai K, Takano Y, Takegawa K, Takemura G, Takeshita F, Talbot NJ, Tan KS, Tanaka K, Tanaka K, Tang D, Tang D, Tanida I, Tannous BA, Tavernarakis N, Taylor GS, Taylor GA, Taylor JP, Terada LS, Terman A, Tettamanti G, Thevissen K, Thompson CB, Thorburn A, Thumm M, Tian F, Tian Y, Tocchini-Valentini G, Tolkovsky AM, Tomino Y, Tönges L, Tooze SA, Tournier C, Tower J, Towns R, Trajkovic V, Travassos LH, Tsai TF, Tschan MP, Tsubata T, Tsung A, Turk B, Turner LS, Tyagi SC, Uchiyama Y, Ueno T, Umekawa M, Umemiya-Shirafuji R, Unni VK, Vaccaro MI, Valente EM, Van den Berghe G, van der Klei IJ, van Doorn W, van Dyk LF, van Egmond M, van Grunsven LA, Vandenabeele P, Vandenberghe WP, Vanhorebeek I, Vaquero EC, Velasco G, Vellai T, Vicencio JM, Vierstra RD, Vila M, Vindis C, Viola G, Viscomi MT, Voitsekhovskaja OV, von Haefen C, Votruba M, Wada K, Wade-Martins R, Walker CL, Walsh CM, Walter J, Wan XB, Wang A, Wang C, Wang D, Wang F, Wang F, Wang G, Wang H, Wang HG, Wang HD, Wang J, Wang K, Wang M, Wang RC, Wang X, Wang X, Wang YJ, Wang Y, Wang Z, Wang ZC, Wang Z, Wansink DG, Ward DM, Watada H, Waters SL, Webster P, Wei L, Weihl CC, Weiss WA, Welford SM, Wen LP, Whitehouse CA, Whitton JL, Whitworth AJ, Wileman T, Wiley JW, Wilkinson S, Willbold D, Williams RL, Williamson PR, Wouters BG, Wu C, Wu DC, Wu WK, Wyttenbach A, Xavier RJ, Xi Z, Xia P, Xiao G, Xie Z, Xie Z, Xu DZ, Xu J, Xu L, Xu X, Yamamoto A, Yamamoto A, Yamashina S, Yamashita M, Yan X, Yanagida M, Yang DS, Yang E, Yang JM, Yang SY, Yang W, Yang WY, Yang Z, Yao MC, Yao TP, Yeganeh B, Yen WL, Yin JJ, Yin XM, Yoo OJ, Yoon G, Yoon SY, Yorimitsu T, Yoshikawa Y, Yoshimori T, Yoshimoto K, You HJ, Youle RJ, Younes A, Yu L, Yu L, Yu SW, Yu WH, Yuan ZM, Yue Z, Yun CH, Yuzaki M, Zabirnyk O, Silva-Zacarin E, Zacks D, Zacksenhaus E, Zaffaroni N, Zakeri Z, Zeh HJ 3rd, Zeitlin SO, Zhang H, Zhang HL, Zhang J, Zhang JP, Zhang L, Zhang L, Zhang MY, Zhang XD, Zhao M, Zhao YF, Zhao Y, Zhao ZJ, Zheng X, Zhivotovsky B, Zhong Q, Zhou CZ, Zhu C, Zhu WG, Zhu XF, Zhu X, Zhu Y, Zoladek T, Zong WX, Zorzano A, Zschocke J, Zuckerbraun B. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10(6):507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Audzeyenka I, Rachubik P, Typiak M, Kulesza T, Topolewska A, Rogacka D, Angielski S, Saleem MA, Piwkowska A. Hyperglycemia alters mitochondrial respiration efficiency and mitophagy in human podocytes. Exp Cell Res. 2021;407(1):112758. doi: 10.1016/j.yexcr.2021.112758. [DOI] [PubMed] [Google Scholar]
- 58.Bach D, Naon D, Pich S, Soriano F, Vega N, Rieusset J, Laville M, Guillet C, Boirie Y, Wallberg-henriksson H, Manco M, Calvani M, Castagneto M, PalaciÌn M, Mingrone G, Zierath J, Vidal H, Zorzano A. Expression of Mfn2, the Charcot-Marie-Tooth Neuropathy Type 2A Gene, in Human Skeletal Muscle. Diabetes. 2022;54(9):2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 59.Gheibi S, Kashfi K, Ghasemi A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed Pharmacother. 2017;95:605–613. doi: 10.1016/j.biopha.2017.08.098. [DOI] [PubMed] [Google Scholar]