Abstract
The ecological environment of quarry mining area is fragile, and the vegetation restoration cycle is long and difficult, so scientific and appropriate artificial vegetation is of great significance to ecological restoration. The purpose of this study was to evaluate the herbaceous and woody vegetation restoration, including Medicago sativa (Me), artificial miscellaneous grass (Mg), Rhus typhina (Rh), fruit orchard (Or) and Pinus tabulaeformis (Pi), to investigate the soil physicochemical properties and the structure of the microbial communities, and to reveal the correlation between them. The results addressed that Medicago sativa and artificial miscellaneous grass had significant effect on soil remediation, which were conducive to scientific and efficient ecological restoration, and could promote ecological restoration in the damaged ecosystems. While, the modes of Rh and Pi were not suitable for ecological restoration in this study area because they had strong allelopathy. Another arborous restoration mode of Or showed a better improvement effect (including soil nutrients, soil microbial diversity, etc.) than that of Rh and Pi. The findings also indicated that the herbaceous vegetation restoration modes of Me and Mg significantly increased the relative abundance of Proteobacteria, Acidobacteria, Actinobacteria bacteria, Ascomycota and Mortierllomycota fungi, and reduced the relative abundance of Firmicutes bacteria and Basidiomycota fungi. This study also revealed that the trend of bacterial localization in the fruit orchard, artificial miscellaneous grass and Medicago sativa was more obvious. Among many soil abiotic factors, the contents of organic matter, available nitrogen and pH were the most important factors affecting soil microbial community.
Keywords: Quarry mining, Vegetation restoration, Soil microorganisms, Yanshan mountains
Introduction
Quarry mining area is an extremely degraded ecosystem. Due to the stripping of the original topsoil and vegetation, the soil erosion is intensified and the ecosystem function is reduced. Vegetation restoration is the only strategy to control soil erosion and restore ecosystem function (An, Huang & Zheng, 2009), and soil quality also determines the nature of vegetation succession and the success of ecological restoration (Putten et al., 2013). Soil is the carrier of many ecological processes in the ecosystem, such as nutrient cycling, water balance and litter decomposition (Yang et al., 2014). The main obstacle to the vegetation restoration of mine wasteland is the soil factor with special and adverse properties (Wang et al., 2011). The soil structure and nutrient status play a key role in the growth of plants, directly affect the composition and physiological vitality of plant communities, determine the structure, function and productivity level of the ecosystem. And more importantly, soil is one of the key indicators to measure the restoration and maintenance of ecological functions of degraded ecosystems (Yu et al., 2007). Therefore, it is necessary to evaluate the soil quality under different vegetation restoration modes (Song et al., 2021). The monitoring of soil microorganisms provides an important basis for ecosystem restoration in mining areas to evaluate the realization of objectives (Harris, 2003; Kiehl et al., 2010; Eaton et al., 2015).
Theoretically speaking, natural restoration is the most scientific and sustainable natural integration process closest to the original ecosystem (Chazdon, 2008; Herath et al., 2009; Chiquoine, Abella & Bowker, 2016). However, in northern China, due to lack of water resources, poor soil conditions and fragile ecological environment, natural restoration usually takes a long time, and people may face long-term ecological risks (Hobbs, Higgs & Harris, 2009; Gastauer et al., 2019). Therefore, proper artificial vegetation restoration is of great significance. So far, many ecological disturbance areas, especially extreme disturbance areas such as mining, can be quickly restored by artificial restoration according to “human will”, but the restoration cost is high. The restoration of “rapid greening”, which simply pursues visual beauty, is favored by more and more people (especially decision-makers) (Ren, 2006), while the restoration of ecosystem structure and function is neglected. Therefore, the disadvantages of artificial restoration in integrating natural ecological processes and characteristics have also emerged (Lu et al., 2021).
As early as the beginning of the 20th century, developed countries began to carry out land restoration in mining areas, including classified planting, ecological landscape reconstruction of abandoned mines, environmental monitoring and supervision (Hu, 2019). In China, vegetation restoration and ecological environment management in mining areas started later. The research on vegetation restoration in mining areas focuses on the technology of vegetation restoration and reconstruction (Zhao et al., 2018), the selection of vegetation species (Wei, Zhen & Du, 2022), and the impact of vegetation restoration on other biological and non biological factors in the ecosystem (Liu, 2022), while there are few studies on the sustainability of the vegetation restoration mode and the integration with the local ecosystem (Wang et al., 2020). Under the background of sustainable development, the establishment of an efficient and sustainable artificial vegetation restoration system in mining areas has gradually attracted people’s attention (Bai et al., 2020; Yi et al., 2020). The purpose of this study was to clarify the improvement and sustainability of soil abiotic elements (nutrients) and the integration of biological elements (bacteria and fungi) with native habitat by Medicago sativa, artificial miscellaneous grass, Rhus typhina, Pinus tabulaeformis, etc., which are widely used in vegetation restoration in northern China (Mou, Zhang & Liu, 2021; Song et al., 2021; Yin, Li & Du, 2021; Irene et al., 2022).
The Yanshan Mountains are not only a green barrier and water source of the capital Beijing China, but also a key area for ecological construction around Beijing. At the same time, Yanshan Mountains are also important mineral base in China. The eastern mining area in Sanhe city of Hebei Province is rich in dolomite, which lies at the southern foot of the Yanshan Mountains. It was once used as the supply base of construction raw materials from 1970s to 2015. An abandoned quarry with an area of 22 km2 had been formed because of decades of over exploitation. In our study, comparing to unrestored area and unmined area, we examined and compared the effects of herbaceous (including Medicago sativa, miscellaneous grass) and arboreal (including Rhus Typhina, orchard, Pinus tabuliformis) vegetation on soil characteristics and soil microbial community diversity and composition. This study also attempted to explore the following scientific issues: (1) Compared with unrestored plot, did vegetation restoration continuously improve soil nutrient status? Were there differences in soil nutrients under different vegetation restoration modes? (2) Compared with the original habitat plot, was there a trend of localization of soil microbial communities under different vegetation restoration modes? (3) If there were differences in soil microbial communities, which soil abiotic factors were related to them? The results were helpful to understand the response of reclaimed soil to vegetation restoration and provided reference for the selection of artificial vegetation.
Materials and Methods
Study area
Field experiments were carried out in the dolomite quarry (40°00′23″ N–40°02′34″ N, 117°05′13″ E–117°11′10″ E) in the east of the Yanshan piedmont plain. It has a typical warm temperate continental climate. The average annual temperature is 11.1 °C, the average annual precipitation is 617.4 mm, and the annual evaporation is 1,681.9 mm. The soil type in the study area is mainly calcareous saline-alkali brown soil, loose soil, poor erosion and erosion resistance, serious soil erosion, low content of organic matter. The original topsoil and vegetation of the quarry have been completely stripped, mostly new gravel conglomerate soil and sandy soil, lack of nutrients and water.
Sample plot setting and experimental designing
In 2010, a large-scale artificial vegetation restoration measure was carried out in the quarry mining area by using native or non-native plant species, which mainly consisted of leguminous alfalfa (Medicago sativa) and miscellaneous grass (mainly Gramineae), and woody plants included Rhus Typhina, orchard (Malus spp., Pyrus spp., Prunus spp., Crataegus pinnatifida, etc.), Pinus Tabuliformis, etc. These species are more adaptable to the local fragile ecological environment and have a high survival rate, so they are often selected as pioneer species to improve the soil conditions (Table 1). In July 2020, seven sample plots of Medicago sativa (Me), miscellaneous grass (Mg), Rhus Typhina (Rh), orchard (Or), Pinus tabuliformis (Pi), unrestored plot (CK1) and unmined plot (CK2) were collected.
Table 1. The information of sample plots.
| Sample plots | Experimental design and vegetation overview |
|---|---|
| CK1 | CK1 was an unrestored plot. The site was a quarry platform formed in 2020, which had been covered with soil and leveled, but no artificial vegetation restoration had been carried out. Local annual herbaceous vegetation such as Setaria viridis, Chenopodium album, Chloris virgata and Lepidium apetalum were distributed. CK1 was taken as the starting point of vegetation restoration effect evaluation. |
| CK2 | CK2 was an unmined plot. This sample plot was the natural habitat with less human disturbance. Natural vegetation included Vitex negundo var. heterophylla, Elaeagnus angustifolia, Themeda triandra, Artemisia stechmanniana, Bothriochloa ischaemum, etc. This sample plot was the evaluation standard of vegetation soil restoration effect. |
| Rh | Rh was a Rhus Typhina plot. This sample area was a pure Rhus Typhina forest, and there were few herbs under the forest. It had been restored for 10 years. |
| Mg | Mg was a miscellaneous grass plot. This sample plot was mainly gramineous plants such as Eleusine indica, Zoysia japonica and Buchloe dactyloides, mixed with cosmos bipinnata and Lespedeza formosa. It had been restored for 10 years. |
| Me | Me was a Medicago sativa plot. This sample plot had been artificially restored with herb (Medicago sativa) for 10 years. Medicago sativa declined, and local annual herbs such as Setaria viridis, Salsola collina and Chenopodium album appeared. It had been restored for 10 years. |
| Or | Or was an orchard plot. This sample plot was a variety of fruit trees, and there were a large number of natural weeds under the tree, including Cymodon doctylon, Digitaria sanguinalis, Eleusine indica, Imperata cyindrica, Amaranthus lividus, Amaranthus retroflexus, Descurainia sophia, Humulus scandens etc. It had been restored for 10 years. |
| Pi | Pi was a Pinus Tabuliformis plot. This sample plot was pure Pinus tabulaeformis forest, and there were few herbs under the forest. It had been restored for 10 years. |
Note:
Among, CK1 was an unrestored plot. CK2 was an unmined plot. Rh was a Rhus Typhina plot. Mg was a miscellaneous grass plot. Me was a Medicago sativa plot. Or was an orchard plot. Pi was a Pinus Tabuliformis plot.
Methods of soil investigation and measurement
The field work was carried out in August 2021, seven quadrats (10 m × 10 m) were set randomly according to the S-shape in the above four sample plots. On the diagonal of each quadrat, litters on the soil surface were removed, and 20 cm soil columns were randomly drilled with a 5 cm diameter soil drill. The above operation was repeated seven times for each sample plot. Finally, the seven soil samples of each plot were mixed adequately, and were sealed with a sterile plastic bag refrigerating in an ice box, and were quickly taken back to the laboratory. The soil samples were divided into two parts, one part (sieved through 2 mm mesh) was used to measure soil nutrients after natural air-drying in the room, and the other part (frozen at −20 °C) was used to measure soil microorganisms.
In this study, soil organic matter, nitrogen, phosphorus, potassium and pH were used as the indicators to characterize soil quality under different restoration modes. The corresponding methods are as follows, total nitrogen (TN): elemental analyzer (Euro Vector EA3000); total phosphorus (TP): spectrophotometer (UV-9000S) after digestion with H2SO4-HClO4.; total potassium (TK): sodium hydroxide melting method; pH: pH meter (Mettler Toledo pH (FE20)); organic matter (OM): potassium dichromate volumetric-external heating method; available nitrogen (AN): alkaline hydrolysis diffusion method; available phosphorus (AP): hydrochloric acid and sulfuric acid solution extraction method; available potassium (AK): ammonium acetate extraction-flame spectrophotometry. Seven repetitions were done per sample.
Analysis of soil microbial community
Total DNA was extracted by CTAB assay and later measured by 1% agarose gel electrophoresis. V3+V4-b segment of bacterial 16S rRNA gene was amplified using 338F (5′-ACTCCTACGGAGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTC TAAT-3′) as primers (Xu et al., 2016). The ITS1-f segment of fungal 18S rRNA was sequenced using ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and 2043R (5′-GCTGCGTTCTTCATCGATGC-3′) as primers (Caban et al., 2018; Nottingham et al., 2018). The amplification conditions were as follows: initial deformation at 98 °C for 1 min, followed by 30 cycles at 98 °C for 10 s, 50 °C for 30 s, 72 °C for 60 s, and a final extension of 72 °C for 5 min (Applied Biosystems, Foster City, CA, USA), all purified PCR products were mixed in equimolar amounts by Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). After DNA was extracted from the soil samples, they were sent to Biomarker Technologies Co, LTD, Beijing, China for high throughput sequencing of Illumina (Illumina, San Diego, CA, USA). Seven repetitions were done per sample.
Statistical analyses
In this study, excel and R-3.6.3 Vegan Picante and other software were used for statistical analysis of all data. The Alpha (α) diversities of soil fungal and bacterial community were analyzed by QIIME2 (https://qiime2.org/). In order to analyze Beta (β) diversity of soil fungal and bacterial communities, firstly, the OTUs (Operational Taxonomic Units) table was standardized by Hellinger, Non-metric multidimensional scaling (NMDS) analysis of the phylogenetic of OTUs based on Bray-Curtis distance (Bray-Curtis Dissimilarity, dBCD) was calculated by using the Vegan package of R-3.6.3. Redundancy analysis was carried out using CANOCO for Windows, Version 4.5. VPA (variance partitioning canonical correspondence analysis). The samples were analyzed by using ANOSIM (Analysis of Similarities) and PerMANOVA (permutation-based multivariate ANOVA) variance analysis, so as to analyze whether there are significant differences among sites, and further analyze the differences among groups by PCoA (Principal Co-ordinates Analysis) (Edgar, 2013; Kõljalg et al., 2013).
Results
Soil physicochemical properties
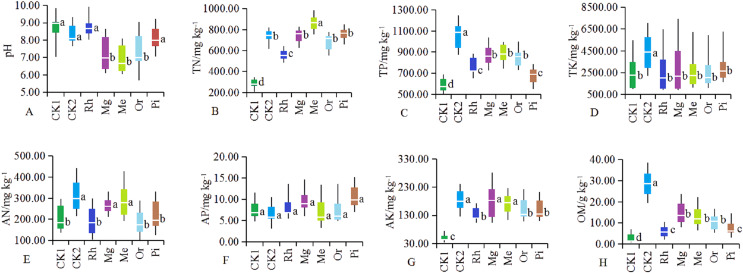
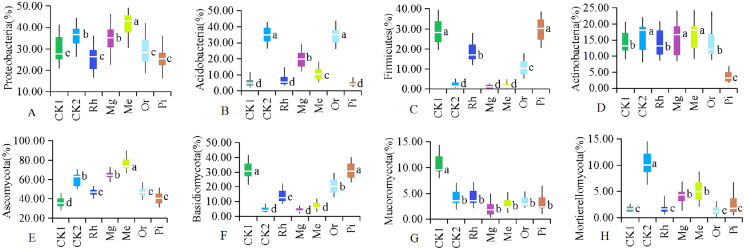
Soil parameters such as pH, TN, TP, TK, AN, AP, AK and OM were significantly different in the different plots (Fig. 1). After vegetation restoration, the pH values of CK1 and CK2 were 8.89 and 8.22, while those of Me, Mg and Or were 6.59, 6.83 and 7.08, respectively (Fig. 1A). Obviously, herbaceous plots Me, Mg and woody plot Or could significantly improve alkaline soil, while the arboreal plots of Rh and Pi had no obvious effect on alkaline soil.
Figure 1. (A–H) Soil physicochemical properties in different sample plots.
The different letters indicated significant differences between treatments according to ANOVA and Tukey’s test (n = 7, P < 0.05). The white line segments in each box indicated the average of the data group. The tops and bottoms of boxes represent the 75th and 25th percentiles, respectively. The upper and lower vertical bars extend to the maximum and minimum values, respectively.
The concentrations of TN (Fig. 1B), TP (Fig. 1C), AK (Fig. 1G) and OM (Fig. 1H) in restored plots (Rh, Or, Pi, Mg and Me) showed significantly higher values compared with CK1 (P < 0.05), while TK (Fig. 1D) and AP (Fig. 1F) no significant differences were observed among restored plots (P > 0.05). In addition to the arboreal plots such as Rh, Or and Pi, herbaceous vegetation restoration also significantly increased the concentration of AN (Fig. 1E), with the most significant increase in the plots of Me and Mg. In general, the effect of Mg and Me on the improvement of soil nutrients were more prominent, followed by Or.
Soil microbial properties
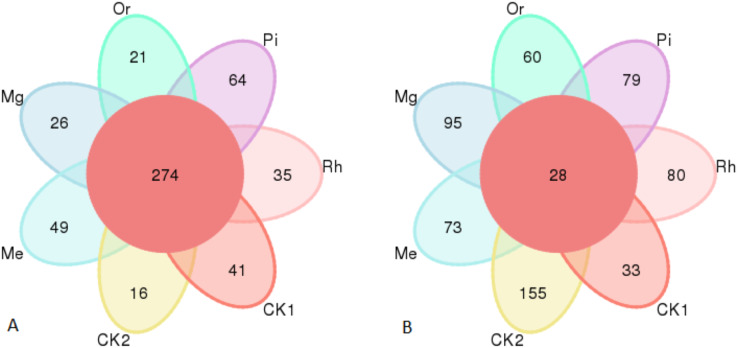
Gene sequence analysis of bacteria and fungi in 49 samples provided a total of 460,556 and 538,732 effective sequences clustered into 2,381 OTUs and 1,288 OTUs at the similarity level of 97.0%, respectively, belonged to 32 phyla, 662 genera and 724 species for bacteria, 10 phyla, 298 genera and 326 species for fungi. The numbers of identified bacterial OTUs in the plots of CK1, CK2, Me, Mg, Or, Pi and Rh were 1,231, 1,684, 1,119, 1,633, 1,632, 1,222 and 878, respectively. Among them, there were 274 core OTUs in the seven groups, and soils of CK1, CK2, Me, Mg, Or, Pi and Rh had 41, 16, 49, 26, 21, 64 and 35 unique OTUs, respectively (Fig. 2A). Then, the fungal OTUs Venn diagram showed that there were 28 core OTUs in the seven groups, and the unique OTUs in the above plots were 33, 155, 73, 95, 60, 79 and 80, respectively (Fig. 2B).
Figure 2. Venn diagram of soil bacterial OTUs (A) and fungal OTUs (B).
The OTUs not unique to a single sample or common to all samples were not shown in the diagram.
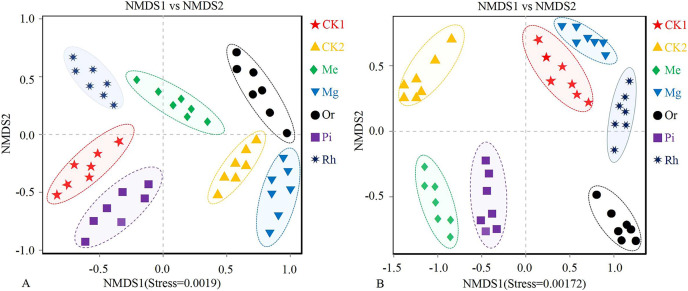
NMDS analysis showed that no overlap was observed among different plots, which illustrated that soil bacterial (Fig. 3A) communities (stress = 0.0019) and fungal (Fig. 3B) communities (stress = 0.00172) in plots were clearly different, especially the bacterial (Fig. 3A) composition between CK1 and restored plots (Mg and Or), and the fungal (Fig. 3B) composition between CK1 and all plots except Mg.
Figure 3. NMDS plots of the soil bacterial (A) and fungal (B) OTUs.
Non-metric multidimensional scaling (NMDS) of the soil bacterial (A) and fungal (B) community composition (Bray–Curtis distance). Ellipses indicate 95% confidence intervals around centroids for each site.
Furthermore, the cluster dendrogram (Fig. S1) based on Unweighted Pair-group Method with Arithmetic Mean (UPGMA) showed that the soil bacterial communities in CK1 were far from that in Mg and Or, which were close to that of the CK2, Mg and Or (Fig. S1A). It showed that vegetation restoration could change the soil bacterial community to varying degrees. The results demonstrated that among various restoration modes, the localization trend of soil bacteria in the Or and Mg were more obvious. The variation law inconsistent with that of bacteria was found in the soil fungal community. Seven samples were divided into four clusters (Fig. 3B) by cluster analysis (each cluster was almost located in different quadrants). The larger cluster group were from the plots of CK1, Mg and Rh plots (Fig. S1B), indicating that the modes of Me, Or and Pi could significantly change the soil fungal community compared with Mg and Rh. The sample grouping of CK2 plot was clearly separated from other plots. Obviously, at this stage, the soil fungal communities in each restoration mode had not shown the trend of localization.
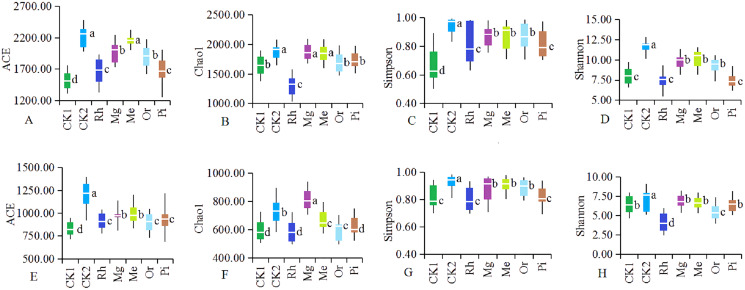
As the number of sequences per sample increased, the Rarefaction Curve of OTUs (Fig. S2) reached the plateau. Re-vegetation could significantly increase soil bacterial community diversity as shown in Fig. 4. The unrestored plot CK1 exhibited the lowest values of ACE index (Fig. 4A1) and Simpson index (Fig. 4A3). The highest values of bacterial ACE index and Simpson index were observed in the herbaceous plot Me, followed by the herbaceous plot Mg and the woody plot Or respectively. Woody plot Rh exhibited the lowest values of Chao1 index (Fig. 4A2) and Shannon index (Fig. 4A4). While Me owned the highest values of bacterial Chao1 index and Shannon index with 1,873.28 and 10.84. With regard to soil fungal community diversity (Figs. 4B1– 4B4), showing a change law almost consistent with that of bacteria: the fungal diversity indexes of the natural habitat plot CK2, the herbaceous plots Me and Mg were higher, followed by the woody plots Or and Pi, and the unrestored plot CK1 and the woody plot Rh were the lowest. Thus, in this study area, herbaceous vegetation restoration modes of Me and Mg better improved the diversity of soil microorganism, while woody modes of Or was better, but Rh was the worst.
Figure 4. Diversity of the soil bacteria (A–D) and fungi (E–H).
Alpha diversity of the soil bacteria (A–D) and fungi (E–H). The white horizontal bars inside each box indicates the mean value. The tops and bottoms of boxes represent the 75th and 25th percentiles, respectively. The upper and lower vertical bars extend to the maximum and minimum values, respectively. The numbers of replicated samples in this figure are n = 7. Letters are used to distinguish whether there are significant differences between groups. Different letters indicate that there are display differences between groups (P < 0.05).
In studied plots, the OTUs could be divided into different categories, and four dominant bacterial phyla with the average relative abundances >12% were obtained, including Proteobacteria (Fig. 5A1), Acidobacteria (Fig. 5A2), Firmicutes (Fig. 5A3) and Actinobacteria (Fig. 5A4), accounting for 67.84–76.49% of the total relative abundances. Among them, Proteobacteria and Acidobacteria had absolute advantages, accounting for 31.49% and 15.99% of the average relative abundance, respectively.
Figure 5. The relative abundances of dominant soil bacterial (A–D) and fungal (E–H).
Communities at the phylum level. Letters are used to distinguish whether there are significant differences between groups. Different letters indicate that there are display differences between groups (P < 0.05, ANOVA).
It could be seen from Fig. 5A1 that the relative abundances of Proteobacteria in the plots of Mg, Me and CK2 were significantly higher than that of CK1, and that of plot Me were significantly highest; The relative abundance of Proteobacteria were no significant difference between Mg and CK2; There was no significant difference in the relative abundance of Proteobacteria among Rh, Or, Pi and CK1. The relative abundances of Acidobacteria (Fig. 5A2) in plots CK2 and Or were significantly higher than that of all other plots, and which in Me and Mg were significantly higher than that of CK1, Rh and Pi. Obviously, compared with plot CK1, plots Or, Me and Mg significantly increased the abundance of Acidobacteria. In the studied area, the bacteria of Firmicutes (Fig. 5A3) were mainly distributed in plot CK1 and the arbor forest plots such as Rh, or and Pi. In summary, the relative abundances of Actinobacteria (Fig. 5A4) in herbaceous plots Me, Mg and natural habitat plot CK2 were significantly higher than that of unrestored plot CK1 and arbor forest plots such as Rh, Or and Pi.
Soil fungal phyla with the average relative abundances >3% were Ascomycota (Fig. 5B1), Basidiomycota (Fig. 5B2), Mucoromycota (Fig. 5B3) and Mortierellomycota (Fig. 5B4), accounting for 63.25–90.68% of the total relative abundance. Among them, Ascomycota and Basidiomycota were absolutely dominant, accounting for 53.19% and 17.45% of the average relative abundance, respectively.
In the herbaceous plots Me and Mg, the relative abundances of Ascomycota (Fig. 5B1) were significantly higher than that of other restored plots and the unrestored plot CK1; The relative abundances of Ascomycota in the arboreal plots of Rh, Or and Pi were lower and had no significant difference, but they were significantly higher than that of CK1 and significantly lower than CK2 in the original habitat plots. The relative abundance of Basidiomycota (Fig. 5B2) showed the opposite change law to that of Ascomycota, that was, the relative abundances of Basidiomycota in arboreal plots were significantly higher than that in herbaceous plots and CK2. Vegetation restoration significantly reduced the abundance of Mucoromycota. The relative abundance of Mucoromycota (Fig. 5B3) in plot CK1 was significantly higher than that in all other plots, while there was no significant difference in the relative abundance of Mucoromycota among other sample plots (including CK2). The relative abundance of Mortierellomycota (Fig. 5B4) showed the same variation law as that of Ascomycota, which were significantly higher in herbaceous plots Me and Mg than that in unrestored plot CK1 and arboreal Rh, Or and Pi.
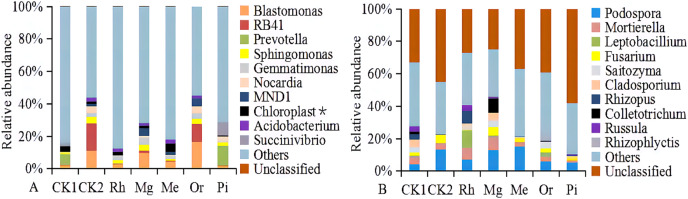
At the genus level, the bacterial groups with the average relative abundances greater than 1.0% were Blastomonas, RB41, Prevotella, Sphingomonas, Gemmatimonas, Nocardia, MND1, uncultured_bacterium_o_Chloroplast, Acidobacterium and Succinivibrio, accounting for 12.21% (Rh)–45.27% (Or) in different plots (Fig. 6A). In addition, the fungal groups with the relative abundances greater than 1.0% were Podospora, Mortierella, Leptobacillium, Fusarium, Saitozyma, Cladosporium, Rhizopus, Colletotrichum, Russula and Rhizophlyctis, accounting for 11.06% (Pi)–47.18% (Mg) in different plots (Fig. 6B). The heatmap (Fig. S3) indicated that the soil bacterial community structure of seven plots could be divided into five relatively concentrated clusters, including CK2-Or-Mg, CK1, Rh, Pi and Me, the results showed that the soil bacterial community could be changed by vegetation restoration (Fig. S3A). Among them, soil bacterial community composition from Mg and Or existed much more similar in comparison to CK2, demonstrating that the plots Mg and Or could better improve the soil bacterial community structure compared to CK2 (Fig. S3A). While, soil fungal community structure were clustered into five groups, including Rh-Mg-CK1, Me, Or, Pi and CK2 (Fig. S3B). The similarities of soil fungal community composition of Mg, me, Rh, Or and Pi were very low compared with the natural habitat plot CK2. While, a certain similarity was reflected between Mg and the unrecovered plot CK1, demonstrating that all recovered plots could not better improve the soil fungal community structure compared to CK2 (Fig. S3B).
Figure 6. The relative abundances of soil bacterial (A) and fungal communities (B).
With the relative abundance >1% at the genus level.
Correlation between soil abiotic and biotic characteristics
The correlations between soil characteristics and microbial community diversity were shown in Table 2. The concentrations of soil TN, TP, AN, AK and Om had a strong positive effect on soil bacterial and fungal diversity. The soil microbial diversity indexes of Simpson, Chao1 and ACE increased with the decrease of Soil pH.
Table 2. Spearman’s rank correlations between soil characteristics and soil microbial communities.
| Microbial community | Diversity index | Soil characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | TN | TP | TK | AN | AP | AK | OM | ||
| Bacteria | Simpson | −0.53* | 0.54* | 0.51* | 0.31 | 0.38 | 0.39 | 0.69** | 0.37 |
| Chao1 | −0.68** | 0.67** | 0.43 | 0.53* | 0.58* | 0.22 | 0.49 | 0.67** | |
| ACE | −0.58** | 0.67** | 0.41 | 0.26 | 0.65** | 0.12 | 0.51* | 0.65** | |
| Shannon | −0.41 | 0.76** | 0.60* | 0.21 | 0.54* | 0.36 | 0.59** | 0.63** | |
| Fungi | Simpson | −0.51* | 0.21 | 0.31 | 0.18 | 0.32 | 0.21 | 0.23 | 0.52* |
| Chao1 | −0.77** | 0.77** | 0.59* | 0.33 | 0.59* | 0.33 | 0.65** | 0.69** | |
| ACE | −0.63** | 0.68** | 0.56* | 0.36 | 0.66** | 0.41 | 0.71** | 0.66** | |
| Shannon | −0.29 | 0.47 | 0.27 | 0.28 | 0.55* | 0.39 | 0.43 | 0.55* | |
Notes:
n = 7. Significance are demonstrated as: P < 0.05 (*) (two tailed), P < 0.01 (**) (two tailed).
Soil TN, TP, AN, AK and OM concentrations strongly positively influenced soil bacterial and fungal community diversity. However, the diversity indexes of soil microorganisms, such as Simpson, Chao 1 and Ace, increased with the decrease of soil pH.
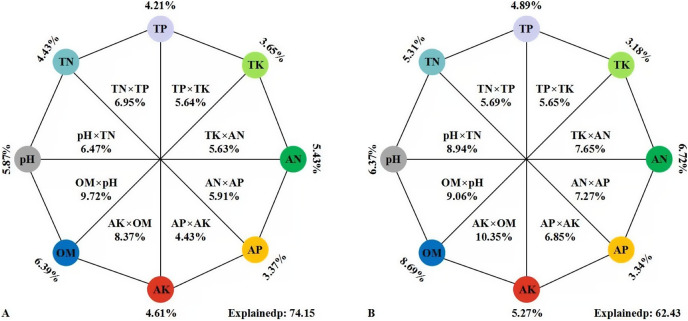
A variety of soil environmental factors act on soil microbial communities. In this study, VPA (Fig. 7) was conducted to quantify the relative contribution of different environmental factors to changes in microbial community composition. These eight environmental variables accounted for 74.15% of the changes in soil bacterial community. The alone relative contribution of soil properties to soil bacterial community structure showed a downward trend: OM (6.39%), pH (5.87%), AN (5.43%), AK (4.61%), TN (4.43%), TP (4.21%), TK (3.65%) and AP (3.37%) of the total variation, respectively (Fig. 7A). These variables explained 62.43% of the variation of soil fungi. The effect of soil properties on soil fungal community structure decreased in turn of OM (8.69%) > AN (6.72%) > pH (6.37%) > TN (5.31%) > AK (5.27%) > TP (4.89%) > AP (3.34%) > TK (3.18%) (Fig. 7B). The results showed that soil OM was the most important factor, which was highest as a single factor for explaining the variation, followed by pH and AN, while soil TK and AP were the least important factors.
Figure 7. VPA of the effects of soil environment factors on soil bacterial (A) and fungal (B) communities.
The percentages in the figures were the variation of the bacterial/fungal community structure explained by the eight sets of environmental factors. The external percentages were the single interpretation rate of environmental factors, the internal percentages were the common interpretation rate of two environmental factors, and the relative abundances of OTUs were used as input in the analysis.
Discussion
Soil physicochemical properties under different modes
Soil quality is a significant component of ecosystem restoration, and its physical, chemical and biological properties sustain plant regeneration and establishment (Yu et al., 2020). After mining activities, soil had been destroyed severely with a higher pH, a lower soil nutrients (Fig. 1). The results in this study showed that vegetation restoration could generally improve the soil quality (Fig. 1). One common reason was that in the process of vegetation restoration, the gradual accumulation of surface litter increases the humus in the soil, so as to effectively improve the soil nutrient status (Li, Liu & Zhou, 2015; Li et al., 2018). And there were significant differences in the effects of different restoration modes on different soil nutrients.
Medicago sativa is a widely distributed artificial vegetation type in northern China, with strong alkali resistance, especially in accelerating vegetation restoration (Franchini et al., 2005), preventing soil erosion (Wu et al., 2013a), repairing degraded soil and so on (He et al., 2017). A large number of rhizobia are formed in alfalfa roots, which have the function of nitrogen fixation. Mg was a miscellaneous grass plot, which was mainly gramineous plants such as Eleusine indica, Zoysia japonica and Buchloe dactyloides, mixed with cosmos bipinnata and Lespedeza formosa (Table 1). In the plots Me and Mg, a large number of roots and litter rotted into the soil, which significantly increased the content of soil nutrients (Figs. 1B, 1C, 1E, 1G and 1H) and improved the alkaline soil (Fig. 1A). The research of Guo et al. (2009) shows that the soil pH value in the restored area is significantly lower than that in the unrestored area, which may be caused by the decay of plant residues and roots (Mukhopadhyay, Maiti & Masto, 2013).
In the ecological restoration, vegetation and soil are coordinated restoration. Restoration of plant diversity in nutrient-poor soil leads to an increase in soil fertility (George & David, 2019). Moreover, the modes of Me and Mg also help to improve the biodiversity of plant communities. In the process of vegetation restoration in northern China, the decline and localization of alfalfa (Li & Shao, 2005; Chen & Li, 2015) and artificial miscellaneous grass (Niu & Jiang, 2004; Pan et al., 2020) are inevitable trends. At present, there were a large number of native dominant herbs such as Bothriochloa ischaemum, Themeda triandra, Melilotus albus in the plots Me and Mg (Table 1). There was no doubt that Me and Mg greatly improved the nutritional status of the soil, and then promoted the emergence (settlement) of native plants, which in turn promoted the localization of soil microorganisms (Fig. 3A) (Faivre et al., 2017; He et al., 2020).
In the studied area, the modes of Rhus typhina and Pinus tabulaeformis had been used on a large scale. Rhus typhina has a wide ecological range and strong ability of reproduction and stress resistance, which plays an important role in vegetation restoration in arid and mining areas. Due to the characteristics of Rhus typhina, some scholars believe that it has the characteristics of invasive species and may develop into invasive species (Liu, Yu & Zhou, 2002; Wang et al., 2012), which poses an ecological threat to native species.
Although Rhus typhina can rapidly increase vegetation coverage and biomass in the short term. However, in the long run, with the rapid propagation of Rhus typhina, the herbs under the forest decrease sharply, forming a single excellent community of Rhus typhina, but unable to form a stable local plant community (You et al., 2013). This study showed that in the plot Rh, because the Rhus typhina had the characteristics of super root tiller reproductive ability and stress resistance, the native plants were difficult to grow (Table 1).
Pinus tabulaeformis is an important greening tree species in northern China, but in the process of its long-term management, it is often found that there are problems such as productivity decline, soil fertility decline and renewal obstacles (Cao et al., 2015). Pinus tabulaeformis has a strong autotoxic effect, which is caused by the leaching of its surface stems and leaves through rain and fog droplets or the generation of odor substances (Li, Wang & Yao, 2010). The Autotoxicity of Pinus tabulaeformis not only limits the growth and regeneration of its own seedlings, but also hinders the entry of other native species (Wang et al., 2007; Pan et al., 2009).
In the studied area, as an attempt of ecological restoration of mining wasteland, many fruit orchards had been planted because of their economic value. Compared with the modes of Rh and Pi, the allelopathy of Or mode is not obvious (Wu et al., 2013b). Therefore, there were also a large number of naturally occurring weeds (Table 1) in the plot Or, and previous studies (Hao et al., 2003; Li, Zhao & Zhang, 2004) have shown that planting grass in orchard can increase the content of soil OM (Fig. 1H), TN (Fig. 1B) and TP (Fig. 1C), which was conducive to the absorption of nutrient elements by fruits. The soil pH in this study area was generally high and belonged to weakly alkaline soil. Alkaline soil can cause physiological disturbance and element deficiency in fruit trees (Guo et al., 2015).
Moreover, according to this study, it should also be pointed out that some soil nutrients were not related to the restoration modes, that was, compared with the non restored plot CK1, there were no significant difference of the soil TK (Fig. 1D) and AP (Fig. 1F) between the restoration modes. The concentration of soil AP in the study area was generally low, all below 10 mg . kg−1, and the consumption of soil AP was very small (Guo et al., 2009). Another study (Qian et al., 2014) shows that the weak mobility and low utilization of AP in alkaline soils (Fig. 1F) resulted in an overall low accumulation of TP (relative to CK2) (Fig. 1C). The TK level in all soils was significantly lower than that of natural habitat soils, which may reflect that each restoration mode had no obvious effect on the accumulation of total potassium to a certain extent (Liu et al., 2004). This may be related to the low concentration of potassium in saline alkali soil in the study area, which mainly exists in the form of mineral potassium and non exchangeable potassium, and the low concentration of exchangeable potassium and water-soluble potassium.
To sum up, compared with woody modes (Rh, Pi and Or), herbaceous modes (Me, Mg) reduced topsoil erosion, increased litter, and improved soil environment and plant community, creating a good environment for the growth of more native plants and soil microorganisms.
Soil microorganisms under different modes
Soil microbial community diversity indexes changed with different vegetation restoration modes and exhibited higher in some restored plots compared with the unrestored plot CK1 (Fig. 4). On the whole, the herbaceous modes were better than the woody modes. Previous studies have shown that the establishment of rich plant communities promotes the development of a variety of microorganisms. Soil microbial community composition changed with different restored plants. The NMDS (Fig. 3) showed that the composition of bacterial and fungal communities were different between all plots, indicating the existence of different soil microbial ecosystems, similar results obtained in previous studies (Kim et al., 2018).
At the phylum level, the bacterial populations of Proteobacteria (Fig. 5A1), Acidobacteria (Fig. 5A2), Firmicutes (Fig. 5A3) and Actinobacteria (Fig. 5A4) were dominant, indicating that these microbial communities had high adaptability and played a vital role in these ecosystems.
Proteobacteria, which are the main dominant community in alkaline soil and widely exist in mining area (Li, Liu & Zhou, 2015), are widely distributed in different environments and have strong adaptability (Liu, Huang & Zeng, 2016). Proteobacteria are often the dominant taxa in some black soil and semi humid soil, the larger the proportion of Proteobacteria in the soil, to some extent, it represents the more fertile the soil (Liu et al., 2014). This study showed that compared with CK1, the relative abundance of Proteobacteria (Fig. 5A1) increased in other sample plots, which showed the same trend as the change law of bacterial community composition in the natural succession of grassland vegetation on the Loess Plateau (Zhang et al., 2016). This study also confirmed that Medicago sativa (Me) and artificial miscellaneous grass (Mg) could significantly improve the content of Proteobacteria. However, woody species such as Rhus typhina (Rh), orchard (Or) and Pinus tabulaeformis (Pi) did not show obvious effect. The similar findings in zinc smelting slag (Luo et al., 2015), as well as copper mine tailing in Jiangxi Provience demonstrated that the predominant phylum was Proteobacteria (Sun et al., 2018).
Acidobacteria are also very common in the natural environment and can degrade macromolecular polymers such as plant cellulose (Lv, Zhao & Zhang, 2020). The abundance of Acidobacteria in some forest soil bacteria is more than 65% (Lee, Ka & Cho, 2008). This study showed that Acidobacteria (Fig. 5A2) mainly existed in the plot Or, secondly, followed by the plots Mg and Me, which were related to a large number of soil OM (Fig. 1H) and soil nutrients (Fig. 1B, 1C, 1E and 1G) in the plots Or, Mg and Me. Additionally, previous report in restored mine soils also established that phytoremediation of leguminous and gramineous herbs could significantly increase the relative abundance of Acidobacteria (Wei et al., 2018).
In arid and oligotrophic soils, the relative abundance of Firmicutes is high (Vanessa et al., 2013). The cell wall of Firmicutes is thick, which can produce spores and resist dehydration, so it can better adapt to the harsh environment in the mining area (Ji et al., 2014). In the plots CK1, Rh, Or and Pi, there were almost no local herbaceous vegetation cover, and the soil moisture content was low, so the relative abundance of Firmicutes (Fig. 5A3) was high. In the plots Me and Mg, the vegetation cover increased significantly and the soil water content increased, so the relative abundance of Firmicutes showed a downward trend. The relative abundance of Firmicutes in the plot CK2 was the lowest, which was related to soil water content under different restoration modes (Yu et al., 2020).
Actinomycetes play an active role in the material cycle, promote the formation of soil aggregate structure and improve the soil (Johnson et al., 2007). As a marker phylum of soil nutrients, Actinomycetes have higher abundance in the weak acidity, arid and organic matter rich soils, and exhibits significant resilience to thrive in hostile environments (Stevenson & Hallsworth, 2014). Just like Proteobacteria and Acidobacteria, the relative abundance of Actinomycetes (Fig. 5A4) in the plots Me and Mg, where soils were rich in organic matter and soil nutrients, were significantly higher (relative to the unrestored plot CK1 and the arboreal plots Rh, Pi and Or), which was similar with the research in the gold mine tails (Sibanda et al., 2019).
Ascomycota and Basidiomycota are the two most widely distributed and abundant fungal groups, which are related to their metabolic characteristics and strong viability in a variety of habitats (Al-Sadi et al., 2017). In the studied area, the relative abundances of Ascomycota (Fig. 5B1) and Basidiomycota (Fig. 5B2) were high, which were similar to the results of Yang et al. (2017) in returning farmland to grassland in the semi-arid area of the Loess Plateau. Most Ascomycota and Basidiomycota are saprophytic bacteria. In this study, the pH values of soil in various plots were 6.9–8.9 (Fig. 1A), and neutral and alkaline soil is most suitable for the growth of saprophytic fungi, which may be one reason why Ascomycota and Basidiomycota are dominant bacteria (Zhang et al., 2018a, 2018b).
Ascomycota are susceptible to plant species and plant residues, and their function is to decompose lignified debris (De Boer et al., 2005). In this study, the soil nutrients, vegetation coverage and vegetation residues in alfalfa plot Me and artificial miscellaneous grass plot Mg increased significantly, so that Ascomycota can make better use of degradable vegetation residues and promote the rapid growth and reproduction of Ascomycota (Ma et al., 2013). Mortierellomycota is a marker group of rich soil nutrients. The relative abundance of Mortierellomycota (Fig. 5B4) in various plots presented the same change law as Ascomycota (Fig. 5B1), which is closely related to soil nutrients (Yuan et al., 2020).
More than 98% of terrestrial fungi in nature belong to Ascomycota and Basidiomycota, and the species diversity of the former is significantly more than that of the latter. The number of species of Ascomycetes is more than twice that of Basidiomycota (Hibbett et al., 2007). The relative abundance of Basidiomycota (Fig. 5B2) showed the opposite change law to that of Ascomycota (Fig. 5B1), which is related to the increase of fungal dominance of Ascomycota (Tian et al., 2017).
The spore germination and mycelial growth of Mucoromycota are fast (Lü et al., 2019). Therefore, dominant fungi are first formed in the early stage of vegetation restoration. However, Mucoromycota fungi are more sensitive to the accumulation of their own metabolic by-products, especially the accumulation of CO2 in the environment, which makes them stop growing, produce dormant structure and enter dormant state (Fröhlich-Nowoisky et al., 2009; Amend et al., 2010). In this study, the CK1 was a sample plot without artificial restoration, and only local annual herbaceous vegetation such as Setaria viridis, Chenopodium album, Chloris virgata and Lepidium apetalum were distributed. The relative abundance of Mucoromycota (Fig. 5B3) in sample plot CK1 was the highest. With the increase of recovery years, the relative abundance of Mucoromycota in all other plots decreased with the consumption of available nutrients and the accumulation of CO2 in soil, and which were no significant difference among various plots.
From the analysis of NMDS (Fig. 3), we could also obtain the following information: Compared with the original habitat (CK2), the recovery progress of soil bacteria and fungi was different in different restoration modes. The localization trend of soil bacteria in herbaceous plots was obvious, that was, the bacterial composition of Mg and Me was similar to that of CK2. However, in woody plots, only soil bacteria in Or showed an obvious localization trend. The similarity of fungal components between each model and CK2 was very low. Obviously, the influence of herbaceous vegetation on bacteria was more obvious at present, which was related to factors such as soil cover (saline-alkaline soil), recovery time (10 years, relatively short) and natural environment (more rainfall). The pH values of soils in the study area ranged from 7.5 to 8.5. Some studies have shown that the conditions of saline-alkali land are not conducive to the growth of fungi, and the bacterial diversity of microorganisms in saline-alkali land often has absolute advantages (Zhang & Feng, 2008; Zhou et al., 2006). There was more rainfall in the study area, and plants provided more carbon sources for soil bacterial communities. Thus, bacteria are resilient (de Franciska et al., 2018), so in this study, the recovery process was faster for bacteria, whereas fungi were less competitive (Harris, 2003). Zhang et al. (2021) shows that even after nearly 30 years, the soil quality after reconstruction was still difficult to return to the same level as that of ordinary agricultural land. This study also fully showed that soil nutrient status could be effectively regulated by artificial methods, but the localization of soil microorganisms was long and uncertain (Singh, Pandey & Singh, 2011). Therefore, from the perspective of soil microbial recovery, ecological restoration in the study area was still at an early stage (Bai et al., 2018; Zhang et al., 2021).
Factors impacting on soil microbial communities
In each vegetation restoration mode, the significant abiotic changes were the accumulation of TN, TP, AN, AK and OM, which strongly affect the diversity of soil bacterial and fungal community (Table 2). These results were similar to previous findings (Bradley, Drijber & Knops, 2006; Griffiths et al., 2011).
In this study, the indexes of Simpson, Chao1 and ACE of bacteria and fungi were significantly negatively correlated with soil pH, which was inconsistent with the positive correlation between soil fungal richness and pH of non-alkaline soil reported in previous research (Liu et al., 2018), which was obviously related to saline-alkaline soil in the study area (Zhang & Feng, 2008). Our results were consistent with those of previous studies, suggesting that soil pH was a major factor controlling bacterial and fungal community structure (Yang et al., 2018; Rousk et al., 2010; Li et al., 2021).
In addition, other investigations have shown that TN, TP and AN in soil have effect on the composition of bacterial communities (Cho, Kim & Lee, 2016; Dawud et al., 2016; Zeng, Dong & An, 2016). Together, these findings support the idea that soil microorganisms are closely related to soil nutrients induced by different vegetation (Siles & Margesin, 2016). Our results revealed the effects of artificial vegetation and soil nutrients on soil microbial diversity and composition, and provided guidance for the artificial regulation of soil microbial diversity and composition in the restoration of abandoned mining areas.
Conclusions
This study mainly analyzed soil nutrients and microorganisms under different vegetation restoration modes from two aspects: the improvement of physical and chemical properties and the localization process of soil ecosystem based on soil microbial community analysis. First, we had found that the herbaceous vegetation of Medicago sativa and artificial miscellaneous grass were more suitable for rapid and efficient soil restoration in Yanshan Mountains of Hebei Province, China. After more than 10 years of continuous cultivation, most of the soil physical and chemical properties had been improved. And the arboreal modes of Rhus typhina and Pinus tabuliformis had no significant effect on soil improvement. Moreover, the herbaceous vegetation restoration modes significantly increased the relative abundance of Proteobacteria, Acidobacteria, Actinobacteria, Ascomycota and Mortierllomycota. This study also revealed that the trend of bacterial localization in the fruit orchard, artificial miscellaneous grass and Medicago sativa soil was more obvious. Among many soil abiotic factors, OM, AN and pH were the most important factors affecting soil microbial community.
Supplemental Information
Rarefaction curves about the OTUs reached the plateau with the increase of the number of sequences per sample, stressing that the 35,000 and 20,000 sequences for each sample were sufficient to characterize soil bacterial and fungal communities in the studied soils.
The heatmap based on bray distance indicated that the soil bacterial community structure of seven plots could be divided into five relatively concentrated clusters.
The original data of bacterial OTUs quantity, involved seven samples (seven replicates for each sample) and a total of 49 original data.
The original data of fungal OTU quantity, involved seven samples (seven replicates for each sample) and a total of 49 original data.
The relative abundance of major bacteria in soil microorganisms, involved seven samples (seven replicates for each sample) and a total of 49 original data.
The relative abundance of major fungi in soil microorganisms, involved seven samples (seven replicates for each sample) and a total of 49 original data.
The soil physical and chemical properties, involved seven samples (seven replicates for each sample) and a total of 49 original data.
Acknowledgments
We thank Hong Yao for its help with meteorological data collection.
Funding Statement
This study was supported financially by the Natural Science Foundation of China (No. 32260006), the science and technology project of Inner Mongolia Autonomous Region (No. 2019GG002), the Natural Science Foundation of Hebei Province (No. C2020408015), the Inner Mongolia Natural Science Foundation (No. 2020MS03001), the Science and Technology Project of Ordos (No. 2022YY008), and the Open Research Foundation of Yinshanbeilu Grassland Eco-hydrology National Observation and Research Station, China Institute of Water Resources and Hydropower Research (No. YSS2022012). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Jing Wang, Email: jingwangabc@163.com.
Yongjun Fan, Email: fanyj1975@163.com.
Additional Information and Declarations
Competing Interests
Hui Liu is an employee of Langfang Zetong Forestry Engineering Design Co., Ltd. Hebei Province, China.
Author Contributions
Jianjun Ma conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Chenyao Li performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Liu Hui performed the experiments, prepared figures and/or tables, and approved the final draft.
Jing Wang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Yongjun Fan conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The Illumina sequencing data is available at NCBI: PRJNA857463 (fungi), and PRJNA857576 (bacteria).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Al-Sadi et al. (2017).Al-Sadi AM, Al-Khatri B, Nasehi A, Al-Shihi M, Maharachchikumbura S. High fungal diversity and dominance by ascomycota in dam reservoir soils of arid climates. International Journal of Agriculture & Biology. 2017;19(4):682–688. doi: 10.17957/IJAB/15.0328. [DOI] [Google Scholar]
- Amend et al. (2010).Amend AS, Seifert KA, Samson R, Bruns TD. Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(31):13748–13753. doi: 10.1073/pnas.1000454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Huang & Zheng (2009).An SS, Huang YM, Zheng FL. Evaluation of soil microbial indices along a revegetation chronosequence in grassland soils on the Loess Plateau, Northwest China. Applied Soil Ecology. 2009;41(3):286–292. doi: 10.1016/j.apsoil.2008.12.001. [DOI] [Google Scholar]
- Bai et al. (2020).Bai ZK, Shi XY, Zhou W, Wang JM, Zhao ZQ, Cao YG. How does artificiality support and guide the natural restoration of ecosystems. China Land Science. 2020;34(9):1–9. doi: 10.11994/zgtdkx.20200918.123606. [DOI] [Google Scholar]
- Bai et al. (2018).Bai ZK, Zhou W, Wang JM, Zhao ZQ, Cao YG, Zhou Y. Rethink on ecosystem restoration and rehabilitation of mining areas. China Land Science. 2018;32(11):1–9. doi: 10.11994/zgtdkx.20181107.162318. [DOI] [Google Scholar]
- Bradley, Drijber & Knops (2006).Bradley K, Drijber RA, Knops J. Increased N availability in grassland soils modifies their microbial communities and decreases the abundance of arbuscular mycorrhizal fungi. Soil Biology & Biochemistry. 2006;38(7):1583–1595. doi: 10.1016/j.soilbio.2005.11.011. [DOI] [Google Scholar]
- Caban et al. (2018).Caban JR, Kuppusamy S, Kim JH, Yoon YF, Kim SY, Lee YB. Green manure amendment enhances microbial activity and diversity in antibiotic-contaminated soil. Applied Soil Ecology. 2018;129:72–76. doi: 10.1016/j.apsoil.2018.04.013. [DOI] [Google Scholar]
- Cao et al. (2015).Cao GQ, Lin SZ, Wang AP, Peng YR. Bioassay and identification of allelochemicals in pinus massoniana root. Chinese Journal of Applied & Environmental biology. 2015;11(6):686–689. doi: 10.3321/j.issn:1006-687X.2005.06.006. [DOI] [Google Scholar]
- Chazdon (2008).Chazdon RL. Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science. 2008;320:1458–1460. doi: 10.1126/science.1155365. [DOI] [PubMed] [Google Scholar]
- Chen & Li (2015).Chen ZY, Li JY. Effects of growth years on the yield of Medicago sativa L. and soil nutrient. Journal of Shandong Agricultural University (Natural Science Edition) 2015;46(2):214–220. doi: 10.3969/j.issn.1000-2324.2015.02.010. [DOI] [Google Scholar]
- Chiquoine, Abella & Bowker (2016).Chiquoine LP, Abella SR, Bowker MA. Rapidly restoring biological soil crusts and ecosystem functions in a severely disturbed desert ecosystem: a publication of the Ecological Society of America. Ecological Applications. 2016;26(4):1260–1272. doi: 10.1002/15-0973. [DOI] [PubMed] [Google Scholar]
- Cho, Kim & Lee (2016).Cho SJ, Kim MH, Lee YO. Effect of pH on soil bacterial diversity. Journal of Ecology and Environment. 2016;40(1):75–83. doi: 10.1186/s41610-016-0004-1. [DOI] [Google Scholar]
- Dawud et al. (2016).Dawud SM, Raulund-Rasmussen K, Domisch T, Finér L, Jaroszewicz B, Vesterdal L. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N Ratio, and pH? Ecosystems. 2016;19(4):645–660. doi: 10.1007/s10021-016-9958-1. [DOI] [Google Scholar]
- De Boer et al. (2005).De Boer W, Folman LB, Summerbell RC, Lynne B. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiology Reviews. 2005;29(4):795–811. doi: 10.1016/j.femsre.2004.11.005. [DOI] [PubMed] [Google Scholar]
- de Franciska et al. (2018).de Franciska VT, Griffiths RI, Bailey M, Craig H, Girlanda M, Gweon HS, Hallin S, Kaisermann A, Keith AM, Kretzschmar M, Lemanceau P, Lumini E, Mason KE, Oliver A, Ostle N, Prosser JI, Thion C, Thomson B, Bardgett RD. Soil bacterial networks are less stable under drought than fungal networks. Nature Communications. 2018;9(1):3033. doi: 10.1038/s41467-018-05516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton et al. (2015).Eaton WD, Shokralla S, McGee KM, Hajibabaei M. Using metagenomics to show the efficacy of forest restoration in the New Jersey Pine Barrens. Genome. 2015;60(10):825–836. doi: 10.1139/gen-2015-0199. [DOI] [PubMed] [Google Scholar]
- Edgar (2013).Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods. 2013;10(10):996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Faivre et al. (2017).Faivre N, Fritz M, Freitas T, de Boissezon B, Vandewoestijne S. Nature-based solutions in the EU: innovating with nature to address social, economic and environmental challenges. Environmental Research. 2017;159(3):509–518. doi: 10.1016/j.envres.2017.08.032. [DOI] [PubMed] [Google Scholar]
- Franchini et al. (2005).Franchini JC, Crispino CC, Souza RA, Torres E, Hungria M. Microbiological parameters as indicators of soil quality under various soil management and crop rotation systems in southern Brazil. Soil and Tillage Research. 2005;92(1–2):18–29. doi: 10.1016/j.still.2005.12.010. [DOI] [Google Scholar]
- Fröhlich-Nowoisky et al. (2009).Fröhlich-Nowoisky J, Pickersgill DA, Després VR, Pöschl U. High diversity of fungi in air particulate matter. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12814–12819. doi: 10.1073/pnas.0811003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastauer et al. (2019).Gastauer M, Souza Filho PWM, Ramos SJ, Caldeira CF, Silva JR, Siqueira JO, Furtini Neto AE. Mine land rehabilitation in Brazil: goals and techniques in the context of legal requirements. Ambio. 2019;48(1):74–88. doi: 10.1007/s13280-018-1053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George & David (2019).George NF, David T. Plant biodiversity and the regeneration of soil fertility. Proceedings of the National Academy of Sciences of the United States of America. 2019;118(49):e2111321118. doi: 10.1073/pnas.2111321118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths et al. (2011).Griffiths RI, Thomso BC, James P, Bell T, Bailey M, Whiteley AS. The bacterial biogeography of British soils. Environmental Microbiology. 2011;13(6):1642–1654. doi: 10.1111/j.1462-2920.2011.02480.x. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2015).Guo H, Du YF, Wang HT, Wang ZK, Fang KK, Zhang XX, Mao PJ, Li HK. Analysis of soil and leaf nutrients in apple orchards in the loess plateau—a case study of Huangling County, Shaanxi Province. Soils. 2015;47(4):682–689. doi: 10.13758/j.cnki.tr.2015.04.010. [DOI] [Google Scholar]
- Guo et al. (2009).Guo YJ, Ni Y, Han JG, Han L. Effects of steppe cultivation and alfalfa plantation on the availability of soil phosphorus. Journal of Soil and Water Conservation. 2009;23(1):88–92. doi: 10.13870/j.cnki.stbcxb.2009.01.044. [DOI] [Google Scholar]
- Hao et al. (2003).Hao SY, Lu HD, Niu JL, Xie XH, Li DK. Effects of herbage-mulching to the apple yield and soil water and other soil physical properties in the Loess Plateau. Soil and Fertilizer Sciences in China. 2003;1:25–27. doi: 10.11838/sfsc.20030108. [DOI] [Google Scholar]
- Harris (2003).Harris JA. Measurements of the soil microbial community for estimating the success of restoration. European Journal of Soil Science. 2003;54(4):801–808. doi: 10.1046/j.1351-0754.2003.0559.x. [DOI] [Google Scholar]
- He et al. (2020).He JS, Bu HY, Hu XW, Feng YH, Li SL, Zhu JX, Liu GH, Wang YR, Nan ZB. Close-to-nature restoration of degraded alpine grasslands: theoretical basis and technical approach. Chinese Science Bulletin. 2020;65(34):3898–3908. doi: 10.1360/TB-2020-0405. [DOI] [Google Scholar]
- He et al. (2017).He H, Peng Q, Wang X, Fan CB, Pang JY, Lambers H. Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant and Soil. 2017;146(1/2):565–584. doi: 10.1007/s11104-017-3242-9. [DOI] [Google Scholar]
- Herath et al. (2009).Herath DN, Lamont BB, Enright NJ, Miller BP. Comparison of post-mine rehabilitated and natural shrubland communities in south-western Australia. Restoration Ecology. 2009;17(5):577–585. doi: 10.1111/j.1526-100X.2008.00464.x. [DOI] [Google Scholar]
- Hibbett et al. (2007).Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai Y-C, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson K-H, Lichtwardt R, Longcore J, Miądlikowska J, Miller A, Moncalvo J-M, Mozley-Standridge S, Oberwinkler F, Parmasto E, Vérie Reeb, Rogers JD, Roux C, Ryvarden L, Sampaio Jé P, Schüßler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao Y-J, Zhang N. A higher-level phylogenetic classification of the fungi. Mycological Research. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Hobbs, Higgs & Harris (2009).Hobbs RJ, Higgs E, Harris JA. Novel ecosystems: implications for conservation and restoration. Trends in Ecology & Evolution. 2009;24(11):599–605. doi: 10.1016/j.tree.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Hu (2019).Hu ZQ. The 30 years’ land reclamation and ecological restoration in China: review, rethinking and prospect. Coal Science and Technology. 2019;47(1):25–35. doi: 10.13199/j.cnki.cst.2019.01.004. [DOI] [Google Scholar]
- Irene et al. (2022).Irene MU, Robert JK, Kristen SV, Keith WG. Immediate and long-term effects of invasive plant species on soil characteristics. Soil Ecology Letters. 2022;4(3):276–288. doi: 10.1007/s42832-021-0104-4. [DOI] [Google Scholar]
- Ji et al. (2014).Ji XL, Yang LL, Li B, Lin LB, Zhang Q, Wei YL. Diversity of culturable bacteria inside the ore from Beiya gold deposit: a preliminary study. Chinese Journal of Microecology. 2014;26(2):157–162. doi: 10.13381/j.cnki.cjm.201402008. [DOI] [Google Scholar]
- Johnson et al. (2007).Johnson SS, Hebsgaard MB, Christensen TR, Mastepanov M, Nielsen R, Munch K, Brand T, Gilbert MTP, Zuber MT, Bunce M, Rønn R, Gilichinsky D, Froese D, Willerslev E. Ancient bacteria show evidence of DNA repair. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(36):14401–14405. doi: 10.1073/pnas.0706787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl et al. (2010).Kiehl K, Kirmer A, Donath TW, Rasran L, Hölzel N. Species introduction in restoration projects-evaluation of different techniques for the establishment of semi-natural grasslands in Central and Northwestern Europe. Basic and Applied Ecology. 2010;11(4):285–299. doi: 10.1016/j.baae.2009.12.004. [DOI] [Google Scholar]
- Kim et al. (2018).Kim S, Zang HD, Mortimer P, Shi LL, Li YJ. Tree species and recovery time drives soil restoration after mining: a chronosequence study. Land Degradation & Development. 2018;29(6):1738–1747. doi: 10.1002/ldr.2951. [DOI] [Google Scholar]
- Kõljalg et al. (2013).Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl Börn D, Lücking R, Martín Mía P, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson K-H. Towards a unified paradigm for sequence-based identification of fungi. Molecular Ecology. 2013;22(21):5271–5277. doi: 10.1111/mec.12481. [DOI] [PubMed] [Google Scholar]
- Lee, Ka & Cho (2008).Lee SH, Ka JO, Cho JC. Members of the phylum acidobacteria are dominant and metabolically active in rhizsphere soil. FEMS Microbiology Letters. 2008;285(2):263–269. doi: 10.1111/j.1574-6968.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- Li et al. (2018).Li DJ, Li W, Shan J, Song TQ, Wang KL. Responses of soil nutrients and microbial communities to three restoration strategies in a karst area, southwest China. Journal of Environmental Management. 2018;207:456–464. doi: 10.1016/j.jenvman.2017.11.067. [DOI] [PubMed] [Google Scholar]
- Li, Liu & Zhou (2015).Li JJ, Liu F, Zhou XM. Effects of different reclaimed scenarios on soil microbe and enzyme activities in mining areas. Environmental Science. 2015;36(5):1836–1841. doi: 10.13227/j.hjkx.2015.05.044. [DOI] [PubMed] [Google Scholar]
- Li & Shao (2005).Li YY, Shao MA. Degradation process and plant diversity of alfalfa grassland in north Loess Plateau of China. Chinese Journal of Applied Ecology. 2005;16(12):2321–2327. doi: 10.3321/j.issn:1001-9332.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Li, Wang & Yao (2010).Li DW, Wang DM, Yao WX. Autotoxicity of Pinus tabulaeformis and its ecology significance. Scientia Silvae Sinicae. 2010;46(11):174–178. doi: 10.11707/j.1001-7488.20101128. [DOI] [Google Scholar]
- Li et al. (2021).Li X, Zhang Y, Song S, Zhou Y, Zhang J. Bacterial diversity patterns differ in different patch types of mixed forests in the upstream area of the Yangtze River Basin. Applied Soil Ecology. 2021;161:103868. doi: 10.1016/j.apsoil.2020.103868. [DOI] [Google Scholar]
- Li, Zhao & Zhang (2004).Li HK, Zhao ZY, Zhang GJ. Effects of planting different herbage on soil fertility of apple orchard in Weibei areas. Journal of Northwest Forestry University. 2004;9(2):31–34. doi: 10.3969/j.issn.1001-7461.2004.02.009. [DOI] [Google Scholar]
- Liu (2022).Liu T. A research on ecological restoration strategy of industrial and mining wasteland under the background of land space planning. Territory & Natural Resources Study. 2022;4:24–26. doi: 10.16202/j.cnki.tnrs.2022.04.001. [DOI] [Google Scholar]
- Liu, Huang & Zeng (2016).Liu Y, Huang YM, Zeng QC. Soil bacterial communities under different vegetation types in the Loess Plateau. Environmental Science. 2016;10(10):3928–3938. doi: 10.13227/j.hjkx.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu D, Liu G, Chen L, Wang J, Zhang L. Soil pH determines fungal diversity along an elevation gradient in Southwestern China. Science China-Life Sciences. 2018;61(6):718–726. doi: 10.1007/s11427-017-9200-1. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2014).Liu JJ, Sui YY, Yu ZH, Shi Y, Chu HY, Jin J, Liu XB, Wang GH. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biology and Biochemistry. 2014;70:113–122. doi: 10.1016/j.soilbio.2013.12.014. [DOI] [Google Scholar]
- Liu, Yu & Zhou (2002).Liu QR, Yu M, Zhou YL. A preliminary study on the invasive plants in Beijing. Journal of Beijing Normal University (Natural Science) 2002;38(3):399–404. doi: 10.3321/j.issn:0476-0301.2002.03.021. [DOI] [Google Scholar]
- Liu et al. (2004).Liu SA, Zhao FB, Zhao HF, Liu ZM, Hao JH. Investigate and countermeasures of apple orchard soil nutrients state in Luochuan. Shaanxi Journal of Agricultural Science. 2004;4:48–50. doi: 10.3969/j.issn.0488-5368.2004.04.022. [DOI] [Google Scholar]
- Lü et al. (2019).Lü ML, Liu Z, Song Z, Wang YN, Liu XY. Diversity and distribution of culturable Mucoromycota fungi in the Greater Khinggan Mountains, China. Biodiversity Science. 2019;27(8):821–832. doi: 10.17520/biods.2019058. [DOI] [Google Scholar]
- Lu et al. (2021).Lu Q, Ge GT, Sa DW, Wang ZJ, Hou ML, Jia YS. Effects of salt stress levels on nutritional quality and microorganisms of alfalfa-influenced soil. PeerJ. 2021;9(2):e11729. doi: 10.7717/peerj.11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo et al. (2015).Luo ZZ, Li LL, Niu YN, Cai LQ, Xie JH. Soil dryness characteristics of alfalfa cropland and optimal growth years of alfalfa on the Loess Plateau of central Gansu, China. Chinese Journal of Applied Ecology. 2015;26(10):3059–3065. doi: 10.13287/j.1001-9332.20150921.010. [DOI] [PubMed] [Google Scholar]
- Lv, Zhao & Zhang (2020).Lv YH, Zhao Y, Zhang YP. Research on the distribution characteristics of soil bacteria communities under the influence of two main sand-fixing plants in Minqin desert area of Gansu province. Ecology and Environmental Sciences. 2020;29(4):717–724. doi: 10.16258/j.cnki.1674-5906.2020.04.010. [DOI] [Google Scholar]
- Ma et al. (2013).Ma AZ, Zhuang XL, Wu JM, Cui MM, Lv D, Liu CZ. Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. PLOS ONE. 2013;8(6):e66146. doi: 10.1371/journal.pone.0066146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou, Zhang & Liu (2021).Mou HX, Zhang WW, Liu BR. Soil fungi community diversity in Medicago sativa field of different planting years at Yellow River diversion Irrigation area. Research of Soil and Water Conservation. 2021;28(4):91–96+104. doi: 10.13869/j.cnki.rswc.2021.04.013. [DOI] [Google Scholar]
- Mukhopadhyay, Maiti & Masto (2013).Mukhopadhyay S, Maiti SK, Masto RE. Use of reclaimed mine soil index (RMSI) for screening of tree species for reclamation of coal mine degraded land. Ecological Engineering. 2013;57(2):133–142. doi: 10.1016/j.ecoleng.2013.04.017. [DOI] [Google Scholar]
- Niu & Jiang (2004).Niu SL, Jiang GM. Function of artificial grassland in restoration of degraded natural grassland and its research advance. Chinese Journal of Applied Ecology. 2004;9:1662–1666. doi: 10.3321/j.issn:1001-9332.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Nottingham et al. (2018).Nottingham AT, Fierer N, Turner BL, Whitaker J, Ostle NJ, McNamara NP, Bardgett RD, Leff JW, Salinas Norma SM, Kruuk L, Meir P. Microbes follow Humboldt: temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology. 2018;99(11):2455–2466. doi: 10.1002/bes2.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. (2020).Pan P, Wang CT, Hu L, Liu SL, Li J. The synergetic responses of plant community and soil to the restorative succession of cultivated grassland. Ecology and Environment Sciences. 2020;29(12):2355–2364. doi: 10.16258/j.cnki.1674-5906.2020.12.006. [DOI] [Google Scholar]
- Pan et al. (2009).Pan CD, Wang Q, Ruan X, Li ZH. Biological activity and quantification of potential autotoxins from the leaves of picea schrenkiana. Chinese Journal of Plant Ecology. 2009;33(1):186–196. doi: 10.3773/j.issn.1005-264x.2009.01.021. [DOI] [Google Scholar]
- Putten et al. (2013).Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos J, Kulmatiski A, Schweitzer JA, Suding KN, Voorde TFJ, Wardle DA. Plant-soil feedbacks: the past, the present and future challenges. Journal Ecology. 2013;101(2):265–276. doi: 10.1111/1365-2745.12054. [DOI] [Google Scholar]
- Qian et al. (2014).Qian X, Gu J, Sun W, Li YD, Fu QX, Wang XJ, Gao H. Changes in the soil nutrient levels, enzyme activities, microbial community function, and structure during apple orchard maturation. Applied Soil Ecology. 2014;77:18–25. doi: 10.1016/j.apsoil.2014.01.003. [DOI] [Google Scholar]
- Ren (2006).Ren ME. Sediment discharge of the Yellow River, China: past, present and future—a synthesis. Advances in Earth Science. 2006;21(6):551–563. doi: 10.1007/s13131-015-0619-6. [DOI] [Google Scholar]
- Rousk et al. (2010).Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N. Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME Journal. 2010;4(10):1340–1351. doi: 10.1038/ismej.2010.58. [DOI] [PubMed] [Google Scholar]
- Sibanda et al. (2019).Sibanda T, Selvarajan R, Msagati T, Venkatachalam S, Meddows-Taylord S. Defunct gold mine tailings are natural reservoir for unique bacterial communities revealed by high-throughput sequencing analysis. Science of the Total Environment. 2019;650:2199–2209. doi: 10.1016/j.scitotenv.2018.09.380. [DOI] [PubMed] [Google Scholar]
- Siles & Margesin (2016).Siles JA, Margesin R. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient in alpine forest soils: what are the driving factors? Microbial Ecology. 2016;72(1):207–220. doi: 10.1007/s00248-016-0748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, Pandey & Singh (2011).Singh JS, Pandey VC, Singh DP. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agriculture. Ecosystems and Environment. 2011;140(3–4):339–353. doi: 10.1016/j.agee.2011.01.017. [DOI] [Google Scholar]
- Song et al. (2021).Song X, Fang C, Yuan ZQ, Li FM. Long-term growth of alfalfa increased soil organic matter accumulation and nutrient mineralization in a semi-arid environment. Frontiers in Environmental Science. 2021;9:649346. doi: 10.3389/fenvs.2021.649346. [DOI] [Google Scholar]
- Stevenson & Hallsworth (2014).Stevenson A, Hallsworth JE. Water and temperature relations of soil Actinobacteria. Environmental Microbiology Reports. 2014;6(6):744–755. doi: 10.1111/1758-2229.12199. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2018).Sun XY, Zhou YL, Tan YJ, Wu ZX, Lu P, Zhang GH, Yu FX. Restoration with pioneer plants changes soil properties and remodels the diversity and structure of bacterial communities in rhizosphere and bulk soil of copper mine tailings in Jiangxi Province, China. Environmental Science and Pollution Research. 2018;25(22):22106–22119. doi: 10.1007/s11356-018-2244-3. [DOI] [PubMed] [Google Scholar]
- Tian et al. (2017).Tian JQ, Qiao YC, Wu B, Chen H, Li W, Jiang N, Zhang XL, Liu XZ. Ecological succession pattern of fungal community in soil along a retreating glacier. Frontiers in Microbiology. 2017;8:1028. doi: 10.3389/fmicb.2017.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanessa et al. (2013).Vanessa NK, Gouvêa TR, Duarte LM, Andreote FD, Mendes R, Soares de Melo I. Water regime influences bulk soil and rhizosphere of cereus jamacaru bacterial communities in the Brazilian Caatinga biome. PLOS ONE. 2013;8(9):e73606. doi: 10.1371/journal.pone.0073606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2011).Wang Y, Ouyang ZY, Zheng H, Wang X, Chen F, Zeng J. Carbon metabolism of soil microbial communities of restored forests in Southern China. Journal of Soils & Sediments. 2011;11(5):789–799. doi: 10.1007/s11368-011-0352-5. [DOI] [Google Scholar]
- Wang et al. (2020).Wang F, Pan XB, Gerlein-Safdi C, Cao XM, Wang S, Gu LH, Wang DF, Lu Q. Vegetation restoration in Northern China: a contrasted picture. Land Degradation & Development. 2020;31(6):669–676. doi: 10.1002/ldr.3314. [DOI] [Google Scholar]
- Wang et al. (2007).Wang Q, Ruan X, Li Z, Pan CD. Autotoxicity of plants and research of coniferous forest autotoxicity. Scientia Silvae Sinicae. 2007;43(6):134–142. doi: 10.3321/j.issn:1001-7488.2007.06.024. [DOI] [Google Scholar]
- Wang et al. (2012).Wang SM, Zhang N, Yu LQ, Zhao RH, Hao P, Li JW, Jiang YS, Sha HF, Liu Y, Zhang ZX. Distribution pattern and their influcing factors of invasive alien plants in Beijing. Acta Ecologica Sinica. 2012;32(15):4618–4629. doi: 10.5846/stxb201201120064. [DOI] [Google Scholar]
- Wei et al. (2018).Wei H, Peng CH, Yang B, Song HX, Li Q, Jiang L, Wei G, Wang KF, Wang H, Liu SR, Liu XJ, Chen DX. Contrasting soil bacterial community, diversity and function in two forests in China. Frontiers in Microbiology. 2018;9:1693. doi: 10.3389/fmicb.2018.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Zhen & Du (2022).Wei YJ, Zhen L, Du BZ. The evolution of desertification control and restoration technology in typical ecologically vulnerable regions. Journal of Resources and Ecology. 2022;13(5):775–785. doi: 10.5814/j.issn.1674-764x.2022.05.003. [DOI] [Google Scholar]
- Wu et al. (2013a).Wu N, Wu XY, Wang DQ, Zhang YS, Zhang YQ, Zhao K. Allelopathy experiments of vegetative organs for nanguo pear on seeds of crops. Protection Forest Science and Technology. 2013a;9:18–20. doi: 10.13601/j.issn.1005-5215.2013.09.031. [DOI] [Google Scholar]
- Wu et al. (2013b).Wu XD, Zhang XJ, Xie YZ, Xu K, Yang J. Vertical distribution characters of soil organic carbon and soil enzyme activity in alfalfa field with different growing years. Acta Prataculturae Sinica. 2013b;22(1):245–251. doi: 10.11686/cyxb20130129. [DOI] [Google Scholar]
- Xu et al. (2016).Xu N, Tan GC, Wang HY, Gai XP. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. European Journal of Soil Biology. 2016;74:1–8. doi: 10.1016/j.ejsobi.2016.02.004. [DOI] [Google Scholar]
- Yang et al. (2017).Yang Y, Dou YX, Huang YM, An S. Links between soil fungal diversity and plant and soil properties on the Loess Plateau. Frontiers in Microbiology. 2017;8:2198–2210. doi: 10.3389/fmicb.2017.02198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2018).Yang C, Zhang FG, Liu N, Hu J, Zhang YJ. Changes in soil bacterial communities in response to the fairy ring fungus Agaricus gennadii in the temperate steppes of China. Pedobiologia. 2018;69:34–40. doi: 10.1016/j.pedobi.2018.05.002. [DOI] [Google Scholar]
- Yang et al. (2014).Yang N, Zou DS, Yang MY, Lin ZG, Song GT, Chen ZY, Zhao LF. Changes of soil properties in re-vegetation stages on sloping-land with purple soils in Hengyang of Hunan Province, South-central China. Acta Ecologica Sinica. 2014;34(10):2693–2701. doi: 10.5846/stxb201301030012. [DOI] [Google Scholar]
- Yi et al. (2020).Yi X, Bai CQ, Liang LW, Zhao ZC, Song WX, Zhang Y. The evolution and frontier development of land ecological restoration research. Journal of Natural Resources. 2020;35(1):37–52. doi: 10.31497/zrzyxb.20200105. [DOI] [Google Scholar]
- Yin, Li & Du (2021).Yin Y, Li Q, Du H. Near-natural transformation of Pinus tabuliformis better improve soil nutrients and soil microbial community. PeerJ. 2021;9(120):e12098. doi: 10.7717/peerj.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You et al. (2013).You HZ, Ge C, Wang C, Jun BI. Comparison of community structure and species diversity throughout the succession process between two restoration types in iron mine dump in east of Hebei. Ecology and Environmental Sciences. 2013;22(09):1482–1487. doi: 10.16258/j.cnki.1674-5906.2013.09.008. [DOI] [Google Scholar]
- Yu et al. (2007).Yu HL, Gu W, Jiang Y, Liu YB. Characteristics of artificial plantation communities and soil properties along highway slopes of semi-arid regions. Chinese Journal of Eco-agriculture. 2007;15(6):22–25. [Google Scholar]
- Yu et al. (2020).Yu FM, Yao YW, Xie DY, Wang XR, Li Y. Study on the soil microbial community structure associated with six land use in Siding mining area. China Environmental Science. 2020;40(5):2262–2269. doi: 10.19674/j.cnki.issn1000-6923.2020.0259. [DOI] [Google Scholar]
- Yuan et al. (2020).Yuan J, Wen T, Zhang H, Zhao M, Shen Q. Predicting disease occurrence with high accuracy based on soil macroecological patterns of Fusarium wilt. The ISME Journal. 2020;14(12):2936–2950. doi: 10.1038/s41396-020-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Dong & An (2016).Zeng Q, Dong Y, An S. Bacterial community responses to soils along a latitudinal and vegetation gradient on the Loess Plateau, China. PLOS ONE. 2016;11(4):e0152894. doi: 10.1371/journal.pone.0152894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2021).Zhang ZJ, Cao YG, Wang SF, Guo CY, Wang X, Lu N, Zhou W, Bai ZK. Characteristics and differences of surface soil microbial population and enzyme activities in opencast mining area of Pingshuo. Acta Ecologica Sinica. 2021;41(1):110–123. doi: 10.5846/stxb202002240326. [DOI] [Google Scholar]
- Zhang & Feng (2008).Zhang W, Feng YJ. Distribution of soil microorganism and their relations with soil factors of saline-alkaline grasslands in Songnen Plain. Grassland and Turf. 2008;3:7–11. doi: 10.13817/j.cnki.cyycp.2008.03.013. [DOI] [Google Scholar]
- Zhang et al. (2018a).Zhang SM, Huang YM, Ni YX, Zhong QQ. Effects of artificial forest and grass on soil fungal community at southern Ningxia mountain. China Environmental Science. 2018a;38(4):1449–1458. doi: 10.19674/j.cnki.issn1000-6923.2018.0175. [DOI] [Google Scholar]
- Zhang et al. (2018b).Zhang C, Liu GB, Song ZL, Wang JL, Guo L. Interactions of soil bacteria and fungi with plants during long-term grazing exclusion in semiarid grasslands. Soil Biology & Biochemistry. 2018b;124:47–58. doi: 10.1016/j.soilbio.2018.05.026. [DOI] [Google Scholar]
- Zhang et al. (2016).Zhang C, Liu G, Xue S, Wang G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biology and Biochemistry. 2016;97(22):40–49. doi: 10.1016/j.soilbio.2016.02.013. [DOI] [Google Scholar]
- Zhao et al. (2018).Zhao JJ, Lu MX, Gu HH, Yuan XT, Li FP. Study on the ecological restoration of mining area based on the change of vegetation coverage. Mining Research and Development. 2018;38(10):115–118. doi: 10.13827/j.cnki.kyyk.2018.10.025. [DOI] [Google Scholar]
- Zhou et al. (2006).Zhou Z, Sun OJ, Huang J, Gao XH. Land use affects the relationship between species diversity and productivity at the local scale in a semi-arid steppe ecosystem. Functional Ecology. 2006;20(5):753–762. doi: 10.1111/j.1365-2435.2006.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rarefaction curves about the OTUs reached the plateau with the increase of the number of sequences per sample, stressing that the 35,000 and 20,000 sequences for each sample were sufficient to characterize soil bacterial and fungal communities in the studied soils.
The heatmap based on bray distance indicated that the soil bacterial community structure of seven plots could be divided into five relatively concentrated clusters.
The original data of bacterial OTUs quantity, involved seven samples (seven replicates for each sample) and a total of 49 original data.
The original data of fungal OTU quantity, involved seven samples (seven replicates for each sample) and a total of 49 original data.
The relative abundance of major bacteria in soil microorganisms, involved seven samples (seven replicates for each sample) and a total of 49 original data.
The relative abundance of major fungi in soil microorganisms, involved seven samples (seven replicates for each sample) and a total of 49 original data.
The soil physical and chemical properties, involved seven samples (seven replicates for each sample) and a total of 49 original data.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.