Abstract
Our previous efforts have led to the development of two potent NNRTIs K-5a2 and 25a, exhibiting effective anti-HIV-1 potency and resistance profiles compared with etravirine. However, both inhibitors suffered from potent hERG inhibition and short half-life. In this article, with K-5a2 and etravirine as leads, series of novel fluorine-substituted diarylpyrimidine derivatives were designed via molecular hybridization and bioisosterism strategies. The results indicated 24b was the most active inhibitor, exhibiting broad-spectrum activity (EC50 = 3.60 - 21.5 nM) against resistant strains, significantly lower cytotoxicity (CC50 = 155 μM) and reduced hERG inhibition (IC50 > 30 μM). Crystallographic studies confirmed the binding of 24b and the role of the fluorine atom, as well as optimal contacts of a nitrile group with the main-chain carbonyl group of H235. Furthermore, 24b showed longer half-life and favorable safety properties. All the results demonstrated that 24b has significant promise in circumventing drug resistance as an anti-HIV-1 candidate.
Graphical Abstract

INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT), an RNA-dependent DNA polymerase that uses single-stranded RNA templates to synthesize double-stranded viral DNA, is a validated target for AIDS therapy.1 In addition to being a central target for anti-HIV drugs, HIV-1 RT has the advantage of providing well-characterized biochemical mechanisms and abundant structural information.2 Eight nucleoside/nucleotide RT inhibitors (NRTIs/NtRTIs) and six non-nucleoside RT inhibitors (NNRTIs) have been approved by the U.S. Food and Drug Administration (FDA) so far.3 In particular, NNRTIs are widely used in the combination antiretroviral therapy (also called cART) because of their potent antiviral activity and high selectivity. Among them, nevirapine (NVP), delavirdine (DLV), and efavirenz (EFV) are first-generation NNRTIs, but they show a significant loss of activity against single-point mutations with their clinical application, such as Y181C and K103N.4 Etravirine (1, ETR), rilpivirine (2, RPV), and doravirine (DOR) are second-generation NNRTIs. Although they exhibit promising activity against the Y181C and K103N mutations, their clinical application has been complicated by emergence of drug-resistance mutations (such as double mutant strain F227L+V106A) and adverse effects (such as hypersensitivity reactions).5, 6 Therefore, the development of next-generation NNRTIs with greater potency, improved drug-resistance profiles, and less toxicity is still required.7, 8
Our previous efforts have led to the development of five novel and potent fused-pyrimidine-bearing NNRTIs, including thiophene[3,2-d]pyrimidines 3 (K-5a2) and 4 (25a), dihydrofuro[3,4-d]pyrimidine 5, thiazolo[5,4-d]pyrimidine 6, and thiazolo[4,5-d]pyrimidine 7.9-13 Although the five compounds demonstrated greater anti-HIV-1 potency and improved resistance profiles compared with that of ETR, most of them still have some shortcomings as ideal drug candidates. More specifically, 3 displayed reduced activity against the particularly challenging double-mutant HIV-1 strain RES056 (K103N+Y181C) (EC50 = 30.6 nM) compared with ETR (EC50 = 17.0 nM), and compounds 4, 5, and 6 exhibited increased cytotoxicity (CC50 = 2.30 μM, 25.1 μM and 23.2 μM, respectively) compared with 3 (CC50 > 227 μM). Moreover, they all suffered from potent human ether-à-go-go related gene (hERG) inhibition with IC50 values ranging from 0.13-0.83 μM. Binding hERG channels (Kv11.1) can be the concurrent cause of a severe tachyarrhythmia, which is regarded as a very serious drug side effect.14 In Phase IIb trials of RPV, HIV-negative patients receiving 75 mg and 300 mg doses were found to have a dose-dependent prolonged QT interval, caused by a dose-dependent blockade of hERG channels by RPV (IC50 = 0.50 μM).15 Therefore, the hERG binding liability of these novel NNRTIs has greatly challenged the discovery of next-generation anti-HIV-1 drugs with improved safety profiles.
It has been reported that the hERG-encoded channel is predominantly blocked by lipophilic amines, which are included in many noncardiovascular drugs in common clinical application for prolonging the QT interval.16 Besides, a hydrogen-bond acceptor may also contribute to high-affinity binding to hERG.17 When comparing the structures of compounds 3-7, they all feature a flexible piperidine-linked benzenesulfonamide or benzamide motif, which contains a tertiary amine atom and hydrogen-bond acceptor. Flexibility and a basic aliphatic amine in the center of this motif have been suggested as prominent characteristics in the hERG pharmacophore,18, 19 thus the piperidine-bearing motif may be responsible for the potent hERG inhibitory activities, which we took as the starting point in the current study. According to the modification strategy to avoid hERG blockage, some subtle peripheral structural modifications to the piperidine-linked benzenesulfonamide structure could be favorable, but that portion of the structure plays a crucial role in inhibiting wild-type (WT) and mutant HIV-1 strains based on the existing structure-activity relationships and co-crystal structures.20 Changes in this region could decrease potency at the hERG channel, but could also decrease the potency against the intended target.
In this work, series of novel fluorine-substituted diarylpyrimidines derivatives (9a-e and 11a-e) were designed via molecular hybridization and bioisosterism strategies, with K-5a2 and ETR as lead compounds. The target compounds abandoned the hERG-perturbing piperidine-linked benzenesulfonamide scaffold and incorporated the central core of 3 and right wing of ETR. Simultaneously, a fluorine atom and trifluoromethyl group were introduced to the right wing, with the aim of developing novel hydrogen bonding and compensate for the loss of the beneficial hydrogen bonding seen in the piperidine-linked benzenesulfonamide structure.21 Furthermore, based on the preliminary activity results, bioisosterism and scaffold-hopping strategies were employed to design the novel diarylpyrimidine derivatives 12-23 and explore the influence of the central core on the activity. Eventually, the left wing of the compounds was modified (24a and 24b) with the aim of improving the compounds’ resistance profiles.
CHEMISTRY
The synthetic protocols for the newly designed compounds 9a-e and 11a-e are outlined in Scheme 1. The previously prepared compounds 8 and 10 were selected as starting materials,10 which were reacted with 4-amino-3-fluorobenzonitrile, 4-amino-2-fluorobenzonitrile, 4-amino-2,3-difluorobenzonitrile, 4-amino-2-(trifluoromethyl)benzonitrile and 4-amino-3-(trifluoromethyl)benzonitrile in the presence of (±)-2,2'-bis(diphenylphosphino)-1,1'-binaphthalene (BINAP) and PdCl2(PPh3)2 to yield the target compounds 9a-e and 11a-e via Buchwald-Hartwig reaction. In an analogous way, compounds 12, 13, 15-23 and 24a-e were prepared (Scheme 2, 4). In Scheme 3, treatment 26 with benzene sulfonyl chloride to afford 27, which was treated with 4-amino-2-fluorobenzonitrile to give the key intermediate 28. Remove the benzenesulfonyl protecting group to yield the target compound 14. In Scheme 4, 2,4-dichlorothieno[2,3-d]pyrimidine (30) was treated with 3,5-dimethyl-4-hydroxybenzaldehyde gave 31. Subsequent reaction with diethyl cyanomethylphosphonate via Wittig–Horner reaction afforded intermediate 32, which was converted to target compounds 25a-e via Buchwald-Hartwig reaction.
Scheme 1.
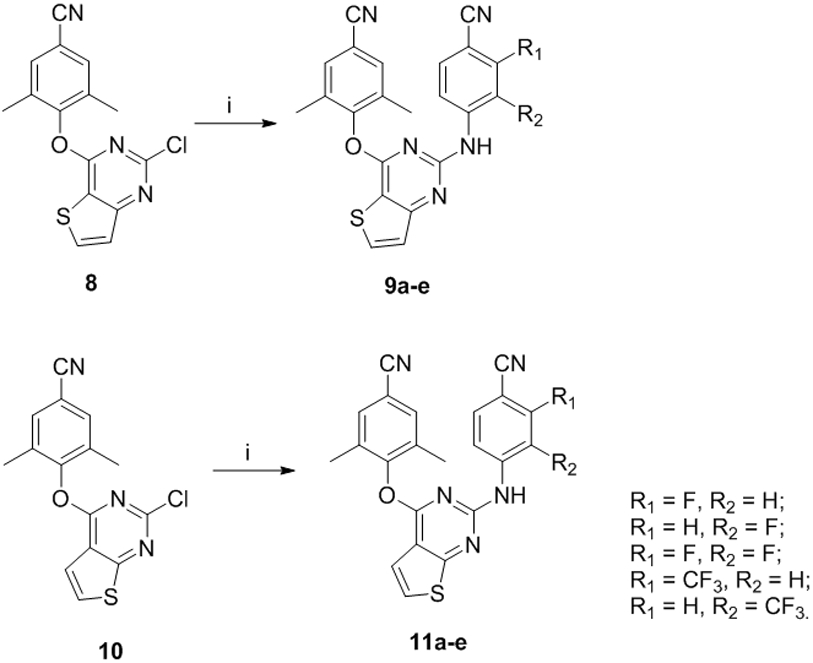
Synthesis of 9a-e and 11a-e a
a Reagents and conditions: (i) BINAP, PdCl2(PPh3)2, Cs2CO3, 120°C, 12h, 46-71%.
Scheme 2.

Synthesis of 12-13 and 15-23 a
a Reagents and conditions: (i) BINAP, PdCl2(PPh3)2, Cs2CO3, 120°C, 12h, 37-72%.
Scheme 4.

Synthesis of 24a-e and 25a-e a
a Reagents and conditions: (i) BINAP, PdCl2(PPh3)2, Cs2CO3, 120°C, 12h, 43-61%; (ii) 3,5-dimethyl-4-hydroxybenzaldehyde, DMF, K2CO3, r.t., 1.5 h, 87%; (iii) t-BuOK, THF, (EtO)2P(O)CH2CN, 0°C to r.t., 6 h, 75%.
Scheme 3.

Synthesis of 14 a
a Reagents and Conditions: (i) NaOH, CH3CN, 3h, 93%; (ii) BINAP, PdCl2(PPh3)2, Cs2CO3, 120°C, 12h, 64%; (iii) NaOH, MeOH, 85°C, 8h, 53%.
HIV-1 RT Crystallization and Structure Determination
An engineered HIV-1 RT construct, RT52A, here referred to as wild-type (WT) RT, was expressed and purified as described previously.22, 23 Prior to crystallization, RT52A (20 mg mL−1) was incubated with 24b at a 1:1.5 protein:drug molar ratio at room temperature for 30 min. Co-crystals of RT with 24b were produced in hanging drops at 4°C with a 1:1 ratio of protein solution and well solution consisting of 10%(v/v) PEG 8000, 4%(v/v) PEG 400, 100 mM MES pH 6.3, 10 mM spermine, 15 mM MgSO4, 100 mM ammonium sulfate, and 5 mM tris(2-carboxyethyl)phosphine together with an experimentally optimized concentration of microseeds from previously generated and crushed RT/rilpivirine crystals (pre-seeding). The crystals were cryo-protected by dipping them into the above solution with 25% ethylene glycol and plunge-frozen in liquid N2. X-ray data were collected from two of the plunge-frozen crystals at the APS 23-ID-B beamline. The crystallographic software packages HKL2000,24 Phenix,25 and COOT26 were used for data processing, structure refinement, and model building, respectively. The structure was solved by molecular replacement using the 1.51 Å resolution structure of the HIV-1 RT/rilpivirine complex (PDB ID: 4G1Q) as the template. The diffraction data and refinement statistics are summarized in Table S1.
RESULTS AND DISCUSSION
The target compounds 9a-e, 11a-e, 12-23, 24a-e and 25a-e were evaluated against WT HIV-1 (IIIB) and the NNRTI-resistant strain K103N+Y181C (RES056) in the MT4 cell line using the MTT method. EFV and ETR were selected as control drugs. The values of EC50 (anti-HIV potency), CC50 (cytotoxicity), and SI (selectivity index, CC50/EC50 ratio) of the synthesized compounds are summarized in Tables 2-4.
Table 2.
Activity against HIV-1 IIIB and RES056 and cytotoxicity of 9a-e and 11a-e

| |||||||
|---|---|---|---|---|---|---|---|
| Compd | R1 | R2 | EC50 (nM)a |
CC50 (μM)b | SIc |
||
| IIIB | RES056 | IIIB | RES056 | ||||
| 9a | H | F | 30.4±18.4 | >300800 | >300 | >9881 | NAd |
| 9b | F | H | 6.74±0.71 | 50.1±4.93 | >300 | >44643 | >5995 |
| 9c | F | F | 226±51.4 | >288400 | >288 | >1273 | NAd |
| 9d | CF3 | H | 29.2±11.6 | >255600 | 255±11.1 | 8749 | <1 |
| 9e | H | CF3 | 896±851.5 | >237300 | 237±26.4 | 265 | <1 |
| 11a | H | F | 24.0±9.22 | >300800 | >300 | >12531 | NAd |
| 11b | F | H | 10.1±3.28 | 119±17.1 | >300 | >29586 | >2518 |
| 11c | F | F | 40.3±25.8 | >288300 | >288 | >7143 | NAd |
| 11d | CF3 | H | 44.9±11.3 | >268500 | >268 | >5974 | NAd |
| 11e | H | CF3 | 1284±136 | >268500 | >268 | >209 | NAd |
| EFV | - | - | 2.31±0.23 | 186±22.4 | >6.35 | >2667 | >34 |
| ETR | - | - | 2.55±0.37 | 37.9±1.60 | >4.58 | >1818 | >121 |
EC50: concentration of compound required to achieve 50% protection of MT-4 cell cultures against HIV-1-induced cytopathic effect, as determined by the MTT method.
CC50: concentration required to reduce the viability of mock-infected cell cultures by 50%, as determined by the MTT method.
SI: selectivity index, the ratio of CC50/EC50.
NA: not available
Table 4.
Activity against HIV-1 IIIB and RES056 and cytotoxicity of 24a-e and 25a-e

| |||||||
|---|---|---|---|---|---|---|---|
| Compd | R1 | R2 | EC50 (nM)a |
CC50 (μM)b | SIc |
||
| IIIB | RES056 | IIIB | RES056 | ||||
| 24a | H | F | 6.34±1.32 | 561±91.1 | 216±22.4 | 34138 | 386 |
| 24b | F | H | 8.60±4.80 | 21.5±1.60 | 155±0.461 | 18041 | 7216 |
| 24c | F | F | 8.92±1.72 | 1094±351 | 102±37.3 | 11505 | 94 |
| 24d | CF3 | H | 146±38.9 | >238600 | 238±3.13 | 1630 | <1 |
| 24e | H | CF3 | 24.5±14.5 | 48.4±0.80 | 198±23.2 | 8089 | 4104 |
| 25a | H | F | 7.13±3.10 | 473±17.4 | 264±1.64 | 37090 | 559 |
| 25b | F | H | 7.64±1.06 | 33.4±0.80 | 262±11.9 | 34385 | 7868 |
| 25c | F | F | 18.6±8.5 | 2351±380 | 240±31.0 | 12873 | 102 |
| 25d | CF3 | H | 35.3±12.7 | 140±9.90 | 193±8.46 | 5480 | 1375 |
| 25e | H | CF3 | 166±66.3 | >254300 | >254 | >1525 | <1 |
| EFV | - | - | 2.31±0.23 | 186±22.4 | >6.35 | >2667 | >34 |
| ETR | - | - | 2.55±0.37 | 37.9±1.60 | >4.58 | >1818 | >121 |
EC50: concentration of compound required to achieve 50% protection of MT-4 cell cultures against HIV-1-induced cytopathic effect, as determined by the MTT method.
CC50: concentration required to reduce the viability of mock-infected cell cultures by 50%, as determined by the MTT method.
SI: selectivity index, the ratio of CC50/EC50.
As depicted in Table 2, the newly synthesized compounds exhibited modest to high potency against the HIV-1 IIIB strain with EC50 values ranging from 6.74 to 1284 nM. Based on the structural features and preliminary activity data, the structure-activity relationships (SAR) are summarized, indicating that the types of substituents R1 and R2 in the right wing significantly influenced anti-HIV activity. Among all the derivatives, only compounds 9b and 11b (R1 = F, R2 = H) exhibited comparable activity (EC50 = 6.74 and 10.1 nM, respectively) to the reference drugs EFV (EC50 = 2.31 nM) and ETR (EC50 = 2.55 nM). Changing the position of the R1 and R2 substituents (9a and 11a, R1 = H, R2 = F, EC50 = 30.4 and 24.0 nM) or replacing both positions by fluorine atoms (9c and 11c, R1 = F, R2 = F, EC50 = 226 and 40.3 nM) decreased the compounds activity. Replacement of the F atom in the R1 position of 9b and 11b with CF3 group resulted in reduced potency (9d and 11d, EC50 = 29.2 and 44.9 nM, respectively). Especially, introducing CF3 group at the R2 position sharply decreased the activity (9e and 11e, EC50 = 896 and 1248 nM, respectively). For the double mutant HIV-1 strain RES056, 9b and 11b proved to be the only two effective inhibitors with EC50 values of 50.1 and 119 nM, being superior to that of EFV (EC50 = 186 nM) and inferior to that of ETR (EC50 = 37.9 nM). Notably, 9b was almost 2-fold more potent than 11b against both HIV-1 IIIB and RES056, confirming that again the central core have a marked impact on the activity. In addition, all compounds displayed lower cytotoxicity. The two most potent inhibitors 9b and 11b exhibited no cytotoxicity at the concentration of 300 μM and showed a higher selectivity index (SI) value against IIIB and RES056 (9b: SI > 44643 and >5995; 11b: SI >44643 and >5995, respectively).
Based on the preliminarily established SAR, the designed compounds retained the privileged right wing (R1 = F, R2 = H), and we turned our attention to the central core. Bioisosterism and scaffold-hopping strategies were employed to design the novel diarylpyrimidine derivatives 12-23 with the aim of improving the drug resistance profiles. As depicted in Table 3, compound 14 featuring the 5H-pyrrolo[3,2-d]pyrimidine central core exhibited the most active potency against IIIB and RES056 with EC50 values of 2.89 and 29.4 nM, being equipotent to that of ETR (EC50 = 3.13 and 32.2 nM). However, 14 showed higher cytotoxicity (CC50 = 5.98 μM), which led to a lower selectivity index (SI = 2069 and 203, respectively). Replacement of the central core of 14 with thiazolo[4,5-d]pyrimidine (12) and thiazolo[5,4-d]pyrimidine (13) yielded potent inhibitors against the HIV-1 IIIB with EC50 values of 3.00 and 3.72 nM, but both inhibitors showed decreased potency in the case of RES056 (EC50 = 131 and 162 nM, respectively). Compound 15 with the quinazoline central core displayed acceptable activity against HIV-1 IIIB and RES056 (EC50 = 8.07 and 148 nM), introducing fluorine at the C5 position of the quinazoline core led to a decreased potency (16, EC50 = 12.3 and 182 nM). Removing the pyrrole core of 14 yielded a single-digit nanomolar inhibitor toward the HIV-1 IIIB (17, EC50 = 6.33 nM), while its activity against RES056 sharply decreased (EC50 = 388 nM). Elaboration with a chlorine atom at the C6 position of 17 afforded 18 with reduced activity to HIV-1 IIIB and RES056 (EC50 = 48.9 and 898 nM).
Table 3.
Activity against HIV-1 IIIB and RES056 and cytotoxicity of compounds 12-23

| ||||||
|---|---|---|---|---|---|---|
| Compd | Central Core | EC50 (nM)a |
CC50 (μM)b | SIc |
||
| IIIB | RES056 | IIIB | RES056 | |||
| 12 |

|
3.00±0.693 | 131±22.1 | 125±18.6 | 41735 | 953 |
| 13 |

|
3.72±0.416 | 162±12.0 | 176±16.4 | 47387 | 1087 |
| 14 |

|
2.89±0.630 | 29.4±1.98 | 5.98±0.704 | 2069 | 203 |
| 15 |

|
8.07±3.04 | 148±36.1 | 197±3.68 | 24396 | 1334 |
| 16 |

|
12.3±2.31 | 182±37.8 | 171±35.2 | 13901 | 943 |
| 17 |

|
6.33±1.72 | 388±58.2 | >348 | >54945 | >897 |
| 18 |

|
48.9±12.7 | 898±247 | 245±6.81 | 5015 | 273 |
| 19 |
|
6.31±1.57 | 107±46.7 | 226±6.84 | 35805 | 2106 |
| 20 |

|
20.7±7.74 | 611±349 | >300 | >14487 | >490 |
| 21 |
|
2.99±0.539 | 274±143 | >312 | >104167 | >1137 |
| 22 |
|
37.7±13.8 | 951±280 | >313 | >8310 | >329 |
| 23 |

|
11.3±1.78 | 180±10.1 | 173±10.6 | 15389 | 958 |
| EFV | - | 2.49±0.547 | 260±47.6 | >6.34 | >2540 | >24 |
| ETR | - | 3.13±0.902 | 32.2±12.2 | >4.59 | >1468 | >143 |
EC50: concentration of compound required to achieve 50% protection of MT-4 cell cultures against HIV-1-induced cytopathic effect, as determined by the MTT method.
CC50: concentration required to reduce the viability of mock-infected cell cultures by 50%, as determined by the MTT method.
SI: selectivity index, the ratio of CC50/EC50.
Furthermore, some alicyclic cores were also introduced in the central scaffold, such as 6,7-dihydrothieno[3,2-d]pyrimidine (19), 5,7-dihydrothieno[3,4-d]pyrimidine (20), 5,7-dihydrofuro[3,4-d]pyrimidine (21) and 6,7-dihydro-5H-cyclopenta[d]pyrimidine (22) and 5,6,7,8-tetrahydroquinazoline (23). Among these compounds, 21 turned out to be the most potent inhibitor against HIV-1 IIIB with an EC50 value of 2.99 nM, being comparable to that of ETR and EFV. However, all compounds showed reduced antiviral activity (EC50 = 107-951 nM) against RES056 compared to ETR (EC50 = 32.2 nM).
Further SAR demonstrated that the compounds’ activity is strongly dependent on their central core, especially for the mutant strain RES056. Although compound 14 featuring the 5H-pyrrolo[3,2-d]pyrimidine central core exhibited more active potency against RES065 than 9b featuring the thieno[3,2-d]pyrimidine core, it displayed greatly increased cytotoxicity compared with other compounds. Thus, with 9b as a lead, we focused our attention to its left wing, hoping to further improve the activity against mutant strain RES056. Ten other compounds were designed and synthesized with the thiophene[3,2-d]pyrimidine and thiophene[2,3-d]pyrimidine central scaffolds. As depicted in Table 4, the results were consistent with the established SAR. 24b and 25b (R1 = F, R2 = H) proved to be the most potent inhibitors. Especially against RES056, both compounds exhibited more potent activity (EC50 = 21.5 and 33.4 nM) than ETR (EC50 = 37.9 nM). More importantly, they showed lower cytotoxicity (CC50 = 155 and 262 μM). In addition, other compounds exhibited reduced activity against the RES056 compared with ETR.
Based on the preliminary activity results, the promising compounds 24b and 25b were further evaluated for their activity against HIV-1 viral isolates carrying a variety of NNRTI-resistance mutations, including L100I, K103N, Y181C, Y188L, E138K, and F227L+V106A. As depicted in Table 5, 24b demonstrated single-digit nanomolar potency against all mutant isolates, with EC50 values of <3.62 nM (L100I), <3.62 nM (K103N), 8.49 nM (Y181C), 8.15 nM (Y188L), 9.40 nM (E138K) and 9.28 nM (F227L+V106A), being equipotent to or superior to ETR in the same cellular assay. In addition, 25b was also a highly potent inhibitor against L100I (EC50 = 5.55 nM) and K103N (EC50 = 7.36 nM); however, it exhibited reduced inhibition of Y181C (EC50 = 26.3 nM), Y188L (EC50 = 35.7 nM), E138K (EC50 = 29.7 nM), and F227L+V106A (EC50 = 32.8 nM) mutant viruses compared to ETR.
Table 5.
Activity of 24b and 25b against mutant HIV-1 strains
| Compds | EC50 (nM)a |
|||||
|---|---|---|---|---|---|---|
| L100I | K103N | Y181C | Y188L | E138K | F227L+V106A | |
| 24b | <3.62 | <3.62 | 8.49±0.48 | 8.15±0.64 | 9.40±2.72 | 9.28±1.60 |
| 25b | 5.55±1.44 | 7.36±1.12 | 26.3±7.21 | 35.7±1.28 | 29.7±0.48 | 32.8±0.00 |
| ETR | 6.08±1.55 | 3.33±0.69 | 14.5±8.22 | 20.4±8.64 | 9.76±6.90 | 19.7±7.30 |
EC50: concentration of compound required to achieve 50% protection of MT-4 cell cultures against HIV-1-induced cytopathic effect, as determined by the MTT method.
To validate the binding target of these derivatives, 24b and 25b were selected to test their inhibitory activity against recombinant WT HIV-1 RT enzyme. As displayed in Table 6, the results demonstrated that 24b and 25b showed effective inhibitory activity against WT HIV-1 RT with IC50 values of 36.2 and 56.6 nM, being superior to that of NVP (IC50 = 181 nM) and inferior to that of ETR (IC50 = 11.0 nM). The inhibitory activity against RT contribute to the conclusion that 24b and 25b are classical NNRTIs.
Table 6.
Inhibitory Activity against WT HIV-1 RT
IC50: inhibitory concentration of test compounds required to inhibit biotin deoxyuridine triphosphate (biotin-dUTP) incorporation into WT HIV-1 RT by 50%.
See reference 9.
Crystal Structure of HIV-1 RT in Complex with 24b
To gather further understanding of the previously explained SAR, we have determined the crystal structure of HIV-1 wild-type (WT) RT in complex with 24b at 2.24 Å resolution (Table S1 and Figure S1). Overall, the structure is very similar to previously reported RT-NNRTI structures20, 23 (Figure 3A). The pyrimidine moiety in the central ring and the right wing anilino linker show hydrogen-bonding interactions with the main chain of K101, conserved in most diarylpyrimidine (DAPY) NNRTIs. The thiophene moiety in the central ring of 24b forms a water-mediated hydrogen bond network with the main-chain atoms of E138 and of I180 (Figure 3B). The water molecule bridging 24b with E138 is conserved in the RT complexes with 3, 4, and RPV. The hydrophobic interactions of the left wing of 24b with the tunnel (Y181, Y188, F227, W229, and L234 side chains) are reminiscent of those observed with RPV and 4 (Figure 3B). The right wing, bearing the fluorine (F) substituent in R1, also shows similar interactions as in other RT/DAPY structures. A critical observation in this structure is the precise positioning of the fluorine, oriented towards the side chain of F227 (F-Cε2 F227 distance: 3.3 Å). Potentially, the fluorobenzonitrile ring could rotate 180º, but this may be unfavorable because of electrostatic repulsion with the main-chain carbonyl oxygen atoms of H235 and P236 (Figure 3C). A bulky substituent like -CF3 in R1 would clash with F227, forcing its repositioning, explaining the 18-fold drop in EC50 (24d, EC50 = 146 nM). R2 substituents oriented toward the left wing would likely cause intramolecular steric hindrance. Thus, they preferentially orient towards residue P236. As they would clash with main-chain atoms of K101, K102, and K103, the energetic cost of rearranging these residues may explain their worse EC50 values, such as 25a, 25c and 25e. An interesting feature in the RT complex with 24b is that there is a favorable association of the nitrile substituent of the right wing with the carbonyl group of H235, in which the complementary electrostatics of the two groups are neatly aligned (C of the nitrile with H235 O: 3.0 Å; N of the nitrile with H235 C: 3.0 Å). In conclusion, addition of the F substituent in R1 results in 24b with remarkable potency and broad activity against drug-resistant HIV-1 strains.
Figure 3.

Crystal structure of HIV-1 RT in complex with 24b (PDB ID: 6UL5). (A) 24b binding in the NNIBP. (B) 24b hydrogen bonding-interactions with RT and water molecules. (C) Details of the interactions of 24b (with a single F substituent at R1, Table 4) with RT, and potential clashes (red dashed lines) of the superposed 24c (with two F substituents at R1 and R2, Table 4) in two alternative orientations. Color legend: i) RT p66 subdomains: fingers in blue, palm in dark red, thumb in green, and connection in yellow; ii) RT p51 subunit in silver.
In Vitro Metabolic Stability of 24b and 25b in the Human Liver Microsomal Assay.
With the aim of evaluating the effect of the stability of 24b and 25b in liver in vitro, the human liver microsomal assay was performed. The lead compounds K-5a2 and 25a were selected as controls. In addition, propafenone was also taken as the control compound in view of its fast clearance rate in human liver microsomes. As shown in Table 7, 24b exhibited significant liver microsomal stability (T1/2 = 80.1 min). Although 25b displayed reduced stability (T1/2 = 32.7 min) compared to 24b, it still has greater stability than K-5a2 and 25a (T1/2 = 2.4 and 1.6 min).
Table 7.
Human Microsomal Stability of 24b and 25b.
| Compound | Human Liver Microsome |
||||
|---|---|---|---|---|---|
| R2 | T1/2 (min) | CLint(mic) (μL/min/mg) |
CLint(liver) (mL/min/kg) |
Remaining (T=60min) |
|
| 24b | 0.9412 | 80.1 | 17.3 | 15.6 | 55.8% |
| 25b | 0.9322 | 32.7 | 42.4 | 38.2 | 23.8% |
| K-5a2 | 0.9973 | 2.4 | 577.3 | 519.6 | 0.8% |
| 25a | 0.992 | 1.6 | 847.3 | 762.5 | 0.2% |
| Propafenone | 0.9846 | 5.2 | 265.9 | 239.3 | 0.1% |
R2 is the correlation coefficient of the linear regression for the determination of kinetic constant
CLint(mic) = 0.693/half-life/mg microsome protein per mL
CLint (liver) = CLint (mic)*mg microsomal protein/g liver weight*g liver weight/kg body weight
Liver weight: 20 g/kg for human.
In Vitro Effects of 24b on CYP Enzymatic Inhibitory Activity.
The approved drug ETR is an inhibitor of CYP2C9 and CYP2C19 and an inducer of CYP3A4, which can account for many drug–drug interactions.27 So, with the aim of evaluating the compounds’ side effects in liver in vitro, the selected compound 24b was tested for its CYP enzyme inhibitory activity. As shown in Table 8, 24b exhibited no significant inhibition of CYP1A2 (IC50 > 50 μM), CYP2C19 (IC50 = 5.85 μM), CYP2D6 (IC50 > 50 μM), and CYP3A4M (IC50 > 50 μM). However, it exhibited higher inhibitory activity against CYP2C9 (IC50 = 1.99 μM) and may be an inhibitor of CYP2C9, as is ETR.
Table 8.
Effects of 24b on Inhibition of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4M
| CYP Isozyme | Standard Inhibitor | IC50 (μM) | Compd | IC50 (μM) |
|---|---|---|---|---|
| 1A2 | α-Naphthoflavone | 0.216 | 24b | >50 |
| 2C9 | Sulfaphenazole | 0.609 | 24b | 1.99 |
| 2C19 | (+)-N-3-benzylnirvanol | 0.227 | 24b | 5.85 |
| 2D6 | Quinidine | 0.134 | 24b | >50 |
| 3A4M | Ketoconazole | 0.0375 | 24b | >50 |
Assessment of hERG Activity
Moreover, 24b was evaluated for inhibition of hERG potassium channel activity in a manual patch-clamp electrophysiology assay.28 As depicted in Table 9, 24b exhibited a lower inhibition rate (22.22%) at a concentration of 30 μM, which means 24b has an IC50 value above 30 μM, and displayed a significantly improved hERG profile over the leads K-5a2 and 25a (IC50 = 0.13 and 0.18 μM, respectively).
Table 9.
Inhibition of hERG potassium channel activity by 24b in HEK293 cells.
| cell | hERG inhibitory effect (%) |
||||
|---|---|---|---|---|---|
| 0.3 μM | 1 μM | 3 μM | 10 μM | 30 μM | |
| cell 1 | 4.12 | 8.01 | 13.35 | 18.48 | 21.59 |
| cell 2 | 1.75 | NA | NA | NA | NA |
| cell 3 | NA | 9.21 | 11.90 | 18.96 | 22.85 |
| Mean (%) | 2.93 | 8.61 | 12.63 | 18.72 | 22.22 |
| SD | 1.68 | 0.84 | 1.03 | 0.33 | 0.89 |
In Vivo Pharmacokinetics Study.
The highly desirable potency of 24b against mutant HIV-1 strains along with its reduced hERG inhibition warranted further study of its pharmacokinetics in the Wistar rat and Kunming mice PK model (Table 10). Initially, 24b was characterized by a low clearance (0.23 L/h/kg in rat and 0.42 L/h/kg in mice), low volume of distribution (0.29 L/kg in rat and 0.38 L/kg in mice), and a longer terminal half-life (T1/2) after po administration (8.82 h in rat and 6.38 h in mice), while the peak serum concentrations were quickly observed at 0.50 h and 1.00 h, respectively. However, the maximum concentration (Cmax) after po administration exhibited great differences between species (9.72 mg/L in rat and 0.56 mg/L h in mice). In addition, the terminal half-life also showed obvious differences between the two species after iv administration, which was 0.89 h in rat and 4.67 h in mice. The oral bioavailability doesn't make much of a difference between species of 24b, being 6.31% in rat and 5.38% in mice. The lower oral bioavailability may be caused by its poor solubility and need further improvement for a drug candidate.
Table 10.
Pharmacokinetic Profile of 24ba
| Subject | Species | T1/2 | Tmax | Cmax | AUC0-t | AUC0−∞ | CL | Vdss | F |
|---|---|---|---|---|---|---|---|---|---|
| (h) | (h) | (mg/L) | (h*mg/L) | (h*mg/L) | (L/h/kg) | (L/kg) | (%) | ||
| 24b (iv)b | Rats | 0.89±0.02 | 0.033 | 269±17.3 | 88.1±1.66 | 88.4±1.94 | 0.23 | 0.29±0.04 | - |
| 24b (po)c | 8.82±0.38 | 0.50±0.00 | 9.72±1.80 | 25.8±4.97 | 27.9±3.23 | - | 6.31 | ||
| 24b (iv)b | Mice | 4.67±1.01 | 0.033 | 186±1.52 | 4.68±0.32 | 4.72±0.30 | 0.42 | 0.38±0.07 | - |
| 24b (po)c | 6.38±2.38 | 1.00±0.00 | 0.56±0.08 | 1.24±0.26 | 1.27±0.26 | - | 5.38 |
PK parameters (mean ± SD, n = 3)
Dosed intravenously at 2 mg/kg
Dosed orally at 10 mg/kg.
Safety Assessment.
Assessment of Acute Toxicity.
Next, an acute toxicity study of 24b in Kunming mice was carried out (Figure 4). After giving the animals single oral doses of 2000 mg/kg, no mortality or poisoning symptoms were found during the following 7 days. In addition, no abnormal behaviors or significant changes of body weight were observed compared to the control group during the experiments.
Figure 4.
The relative body weight changes of mice in different groups (control and 24b).
Conclusion
We use rationally conceived molecular hybridization and bioisosterism strategies to generate novel high-efficiency and low-toxicity NNRTIs. This led to the identification of novel promising diarylpyrimidine inhibitors with fluorine-substituted aminobenzonitrile in the right wing instead of the piperidine-linked benzenesulfonamide motif in the lead K-5a2. We report the synthesis, and biological and crystallographic evaluation of these novel analogs. The antiviral activity results show that compound 24b displays excellent broad-spectrum anti-HIV-1 potency against WT virus and a variety of NNRTI-resistant mutations, with EC50 values ranging from 3.6 nM to 21.5 nM. The structural analysis shows that several important features of 24b, including the substituent placement and pocket location of a fluorine atom, as well as optimized interaction of the nitrile of the right wing aryl group with the main-chain carbonyl group of pocket residue H235, contributes to its potency and broad-spectrum activity against NNRTI-resistance mutations. Importantly, 24b demonstrated a significantly improved hERG profile (IC50 > 30 μM). Pharmacokinetic parameters for 24b were determined in the rat and mice model, showing a lower clearance and longer half-life. Furthermore, 24b showed no acute toxicity at a dose of 2000 mg/kg in Kunming mice. In total, all the results demonstrated that 24b has great potential to be developed as an anti-HIV-1 drug candidate with its improved resistance profiles and low hERG inhibitory activity.
EXPERIMENTAL SECTION
Chemistry
All melting points were determined on a micro melting point apparatus (RY-1G, Tianjin TianGuang Optical Instruments). 1H-NMR and 13C-NMR spectra were recorded in DMSO-d6 on a Bruker AV-400 spectrometer with tetramethylsilane (TMS) as the internal standard. Chemical shifts are reported in δ values (ppm) from TMS and coupling constants are given in hertz; signals are abbreviated as s (singlet), d (doublet), t (triplet), and m (multiplet). The mass spectra were measured in AG1313A Standard LC Autosampler (Agilent). All reactions were routinely monitored by thin layer chromatography (TLC) on Silica Gel GF254 for TLC (Merck), and spots were visualized with iodine vapor or by irradiation with UV light (λ = 254 and 356 nm). Flash column chromatography was performed on columns packed with Silica Gel (200-300 mesh, Qingdao Haiyang Chemical Company). Solvents were purified and dried by standard methods. Compounds purity was analyzed on a Shimadzu SPD-20A/20AV HPLC system with a Inertsil ODS-SP, 5 μm C18 column (150 mm × 4.6 mm). HPLC conditions: methanol/water 80:20; flow rate 1.0 mL/min; UV detection from 210 to 400 nm; temperature, ambient; injection volume, 20 μL. Purity of all final compounds was >95%.
General procedure for the preparation of final compounds 9a-e and 11a-e
In a Schlenk-type flask, starting materials fluoro- or trifluoromethyl-substituted 4-aminobenzonitrile (1.2 mmol) and 8 (or 10, 0.1 mol) were dissolved in dry dioxane (10 mL), and then PdCl2(PPh3)2 (0.07 g, 0.01 mmol), BINAP (0.06 g, 0.01 mmol) and Cs2CO3 (0.97 g, 3.0 mmol) were added. The flask was evacuated and backfilled with nitrogen. The mixture was stirred at 120°C for 12 hours. Then solvent was evaporated under reduce pressure and the obtained residue was dissolved in 20 mL ethyl acetate. The organic phase was washed with saturated sodium chloride (3 × 5 mL), then dried over anhydrous Na2SO4, filtered, and purified by flash column chromatography to give the target compounds 9a-e and 11a-e.
4-((2-((4-cyano-2-fluorophenyl)amino)thieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (9a)
Recrystallized from EA/PE as a white solid, 46% yield, mp: 282-283°C.1H NMR (400 MHz, DMSO-d6) δ 9.52 (s, 1H, NH), 8.42 (d, J = 5.4 Hz, 1H, C6-thienopyrimidine-H), 7.88 (t, J = 8.4 Hz, 1H, C6-Ph’-H), 7.79 (s, 2H, C3,C5-Ph”-H), 7.75 (dd, J = 11.2, 1.9 Hz, 1H, C3-Ph’-H), 7.46 (d, J = 5.4 Hz, 1H, C7-thienopyrimidine-H), 7.38 (dd, J = 8.5, 1.9 Hz, 1H, C5-Ph’-H), 2.14 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 165.2, 162.7, 157.2, 153.7, 153.3 (JCF = 199 Hz), 138.5, 133.6 (JCF = 10 Hz), 133.2, 133.1, 129.0 (JCF = 3 Hz), 123.9, 122.5, 119.5 (JCF = 23 Hz), 118.9, 118.6 (JCF = 2 Hz), 109.4, 109.0, 104.4 (JCF = 9 Hz), 16.2. ESI-MS: m/z 416.5 [M + 1]+, 433.5 [M + NH4]+, 438.4 [M + Na]+. C22H14FN5OS (415.09). HPLC purity: 96.88%.
4-((2-((4-cyano-3-fluorophenyl)amino)thieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (9b)
Recrystallized from EA/PE as a white solid, 59% yield, mp: 297-299°C. 1H NMR (400 MHz, DMSO-d6) δ 10.41 (s, 1H, NH), 8.46 (d, J = 5.3 Hz, 1H, C6-thienopyrimidine-H), 7.82 (s, 2H, C3,C5-Ph”-H), 7.66 (d, J = 8.3 Hz, 1H, C5-Ph’-H), 7.63 – 7.57 (m, 1H, C2-Ph’-H), 7.53 (d, J = 5.3 Hz, 1H, C7-thienopyrimidine-H), 7.33 (dd, J = 8.8, 2.0 Hz, 1H, C6-Ph’-H), 2.15 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 165.0, 164.8, 162.7, 162.3, 156.9, 153.3 (JCF = 168 Hz), 147.6 (JCF = 12 Hz), 138.8, 133.8, 133.3, 133.1, 124.0, 118.9, 115.2 (JCF = 2.5 Hz), 109.7, 109.0, 104.5 (JCF = 2.6 Hz), 16.1. ESI-MS: m/z 416.5 [M + 1]+, 433.6 [M + NH4]+, 438.4 [M + Na]+. C22H14FN5OS (415.09). HPLC purity: 97.43%.
4-((4-(4-cyano-2,6-dimethylphenoxy)thieno[3,2-d]pyrimidin-2-yl)amino)-2,3-difluorobenzonitrile (9c)
Recrystallized from EA/PE as a white solid, 51% yield, mp: 290-292°C. 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H, NH), 8.44 (d, J = 5.4 Hz, 1H, C6-thienopyrimidine-H), 7.77 (s, 2H, C3,C5-Ph”-H), 7.68 (ddd, J = 8.9, 7.0, 1.7 Hz, 1H, C5-Ph’-H), 7.48 (d, J = 5.4 Hz, 1H, C7-thienopyrimidine-H), 7.44 (ddd, J = 8.8, 6.8, 1.9 Hz, 1H, C6-Ph’-H), 2.13 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 165.1, 162.7, 156.9, 153.2 (JCF = 182 Hz), 140.1, 138.7, 133.2, 133.1, 128.0 (JCF = 1 Hz), 123.9, 118.9, 117.7, 114.0, 109.5 (JCF = 6 Hz), 16.1. ESI-MS: m/z 434.5 [M + 1]+, 456.4 [M + Na]+. C22H13F2N5OS (433.08). HPLC purity: 95.98%.
4-((2-((4-cyano-3-(trifluoromethyl)phenyl)amino)thieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (9d)
Recrystallized from EA/PE as a white solid, 41% yield, mp: 289-291°C. 1H NMR (400 MHz, DMSO-d6) δ 10.42 (s, 1H, NH), 8.46 (d, J = 5.3 Hz, 1H, C6-thienopyrimidine-H), 8.19 (s, 1H, C2-Ph’-H), 8.01 (d, J = 8.7 Hz, 1H, C5-Ph’-H), 7.88 (d, J = 8.7 Hz, 1H, C6-Ph’-H), 7.79 (s, 2H, C3,C5-Ph”-H), 7.53 (d, J = 5.4 Hz, 1H, C7-thienopyrimidine-H), 2.16 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 164.8, 162.7, 156.9, 153.1, 145.6, 138.8, 136.3, 133.2, 133.1, 124.0, 120.9, 118.9, 116.7, 115.9 (JCF = 2 Hz), 109.6 (JCF = 3 Hz), 98.9 (JCF = 2 Hz), 16.2. ESI-MS: m/z 466.4 [M + 1]+, 483.4 [M + NH4]+. C23H14F3N5OS (465.09). HPLC purity: 98.54%.
4-((2-((4-cyano-2-(trifluoromethyl)phenyl)amino)thieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (9e)
Recrystallized from EA/PE as a white solid, 53% yield, mp: 240-243°C. 1H NMR (400 MHz, DMSO-d6) δ 8.86 (s, 1H, NH), 8.39 (d, J = 5.3 Hz, 1H, C6-thienopyrimidine-H), 8.18 (d, J = 1.9 Hz, 1H, C3-Ph’-H), 7.90 (dd, J = 8.6, 1.9 Hz, 1H, C5-Ph’-H), 7.81 (d, J = 8.6 Hz, 1H, C6-Ph’-H), 7.73 (s, 2H, C3,C5-Ph”-H), 7.40 (d, J = 5.4 Hz, 1H, C7-thienopyrimidine-H), 2.11 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 165.3, 162.7, 158.3, 153.1, 142.2, 138.5, 136.5, 133.1, 133.0, 131.5 (JCF = 5 Hz), 127.9, 123.8, 123.0 (JCF = 30 Hz), 118.8, 118.1, 109.3, 108.8, 107.1, 16.1. ESI-MS: m/z 466.4 [M + 1]+, 483.5 [M + NH4]+. C23H14F3N5OS (465.09). HPLC purity: 98.43%.
4-((2-((4-cyano-2-fluorophenyl)amino)thieno[2,3-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (11a)
Recrystallized from EA/PE as a white solid, 62% yield, mp: 251-254°C. 1H NMR (400 MHz, DMSO-d6) δ 9.70 (s, 1H, NH), 7.78 (s, 2H, C3,C5-Ph”-H), 7.77 – 7.72 (m, 2H, C4,C6-Ph’-H), 7.62 (d, J = 6.0 Hz, 1H, C7-thienopyrimidine-H), 7.56 (d, J = 5.9 Hz, 1H, C6-thienopyrimidine-H), 7.34 (dd, J = 8.5, 1.8 Hz, 1H, C5-Ph’-H), 2.13 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.7, 162.3, 155.9, 153.6 (JCF = 187 Hz), 151.4, 133.1, 128.9 (JCF = 3 Hz), 123.2, 122.6, 119.6 (JCF = 24 Hz), 118.9, 118.7, 118.5 (JCF = 2 Hz), 112.5, 109.2, 104.7 (JCF = 9 Hz), 16.2. ESI-MS: m/z 416.5 [M + 1]+, 433.6 [M + NH4]+, 438.4 [M + Na]+. C22H14FN5OS (415.09). HPLC purity: 99.21%.
4-((2-((4-cyano-3-fluorophenyl)amino)thieno[2,3-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (11b)
Recrystallized from EA/PE as a white solid, 48% yield, mp: 272-274°C. 1H NMR (400 MHz, DMSO-d6) δ 10.54 (s, 1H, NH), 7.82 (s, 2H, C3,C5-Ph”-H), 7.66 (d, J = 6.0 Hz, 1H, C7-thienopyrimidine-H), 7.63 (d, J = 8.2 Hz, 1H, C5-Ph’-H), 7.59 (d, J = 5.9 Hz, 1H, C6-thienopyrimidine-H), 7.45 (d, J = 13.4 Hz, 1H, C2-Ph’-H), 7.26 (d, J = 8.0 Hz, 1H, C6-Ph’-H), 2.15 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.6, 162.3, 155.5, 153.5 (JCF = 176 Hz), 147.2 (JCF = 12 Hz), 133.8, 133.2, 133.0, 123.6, 119.0, 118.7, 115.2(JCF = 9 Hz), 112.5, 109.6, 104.5 (JCF = 26 Hz), 91.2 (JCF = 15 Hz), 16.2. ESI-MS: m/z 416.5 [M + 1]+, 433.6 [M + NH4]+. C22H14FN5OS (415.09). HPLC purity: 97.98%.
4-((4-(4-cyano-2,6-dimethylphenoxy)thieno[2,3-d]pyrimidin-2-yl)amino)-2,3-difluorobenzonitrile (11c)
Recrystallized from EA/PE as a white solid, 71% yield, mp: 260-262°C. 1H NMR (400 MHz, DMSO-d6) δ 10.06 (s, 1H, NH), 7.76 (s, 2H, C3,C5-Ph”-H), 7.65 (d, J = 5.9 Hz, 1H, C7-thienopyrimidine-H), 7.58 (d, J = 5.9 Hz, 1H, C6-thienopyrimidine-H), 7.55 (d, J = 1.8 Hz, 1H, C5-Ph’-H), 7.40 (ddd, J = 8.8, 6.7, 1.8 Hz, 1H, C6-Ph’-H), 2.12 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.6, 162.3, 155.6, 153.5 (JCF = 165 Hz), 135.4, 133.1, 128.0 (JCF = 4 Hz), 123.6, 118.9 (JCF = 24 Hz), 118.7, 117.8, 114.0 (JCF = 3 Hz), 112.8, 109.3, 16.2. ESI-MS: m/z 434.4 [M + 1]+, 451.5 [M + NH4]+. C22H13F2N5OS (433.08). HPLC purity: 96.38%.
4-((2-((4-cyano-3-(trifluoromethyl)phenyl)amino)thieno[2,3-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (11d)
Recrystallized from EA/PE as a white solid, 50% yield, mp: 296-298°C. 1H NMR (400 MHz, DMSO-d6) δ 10.53 (s, 1H, NH), 8.08 (s, 1H, C2-Ph’-H), 7.93 (d, J = 9.0 Hz, 1H, C5-Ph’-H), 7.86 (d, J = 8.7 Hz, 1H, C6-Ph’-H), 7.79 (s, 2H, C3,C5-Ph”-H), 7.67 (d, J = 5.9 Hz, 1H, C7-thienopyrimidine-H), 7.59 (d, J = 5.9 Hz, 1H, C6-thienopyrimidine-H), 2.15 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.3, 162.3, 155.5, 153.4, 145.2, 136.3, 133.2, 133.0, 132.0 (JCF = 32 Hz), 124.2, 123.7, 121.5, 121.0, 118.9 (JCF = 15 Hz), 116.6, 116.0 (JCF = 5 Hz), 113.0, 109.4, 99.2, 16.2. ESI-MS: m/z 466.3 [M + 1]+, 483.4 [M + NH4]+. C23H14F3N5OS (465.09). HPLC purity: 95.49%.
4-((2-((4-cyano-2-(trifluoromethyl)phenyl)amino)thieno[2,3-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (11e)
Recrystallized from EA/PE as a white solid, 63% yield, mp: 244-246°C. 1H NMR (400 MHz, DMSO-d6) δ 9.13 (s, 1H, NH), 8.18 (d, J = 1.9 Hz, 1H, C3-Ph’-H), 7.89 (dd, J = 8.6, 1.9 Hz, 1H, C5-Ph’-H), 7.72 (d, J = 8.5, 1H, C6-Ph’-H), 7.70 (s, 2H, C3,C5-Ph”-H), 7.57 (d, J = 5.9 Hz, 1H, C7-thienopyrimidine-H), 7.52 (d, J = 6.0 Hz, 1H, C6-thienopyrimidine-H), 2.09 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.8, 162.2, 157.1, 153.4, 141.9, 136.5, 133.2, 133.0, 132.9, 132.0, 131.5 (JCF = 5 Hz), 128.8, 124.0 (JCF = 30 Hz), 122.8, 118.8 (JCF = 24 Hz), 118.0, 112.3, 109.0, 107.6, 16.1. ESI-MS: m/z 466.4 [M + 1]+, 483.4 [M + NH4]+, 488.4 [M + Na]+. C23H14F3N5OS (465.09). HPLC purity: 97.98%.
General procedure for the preparation of final compounds 12-13 and 15-23
The synthetic procedures for target compounds 12-13 and 15-23 were similar to that of 9a, only with the difference that the starting materials were selected from our previously reported intermediates.
4-((5-((4-cyano-3-fluorophenyl)amino)thiazolo[4,5-d]pyrimidin-7-yl)oxy)-3,5-dimethylbenzonitrile (12)
Recrystallized from EA/PE as a white solid, 44% yield, mp: 323-324℃. 1H NMR (400 MHz, DMSO-d6) δ 10.67 (s, 1H, NH), 9.29 (t, J = 1.4 Hz, 1H), 7.83 (s, 2H, C3,C5-Ph”-H), 7.72 – 7.59 (m, 1H), 7.43 (d, J = 13.1 Hz, 1H), 7.27 (d, J = 8.8 Hz, 1H), 2.17 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 168.3, 164.8, 160.5, 155.6, 153.5 (JCF = 202 Hz), 152.8, 146.9 (JCF = 12 Hz), 133.9, 133.3, 133.0, 127.0, 118.9, 115.2 (JCF = 17 Hz), 115.1, 109.7, 104.7 (JCF = 26 Hz), 91.6 (JCF = 16 Hz), 16.2. ESI-MS: m/z 417.2 [M + H]+, 439.09 [M + Na]+. C21H13FN6OS (416.09). HPLC purity: 99.04%.
4-((5-((4-cyano-3-fluorophenyl)amino)thiazolo[5,4-d]pyrimidin-7-yl)oxy)-3,5-dimethylbenzonitrile (13)
Recrystallized from EA/PE as a white solid, 51% yield, mp: 319-321°C. 1H NMR (400 MHz, DMSO-d6) δ 10.67 (s, 1H, NH), 9.29 (s, 1H), 7.83 (s, 2H, C3,C5-Ph”-H), 7.65 (t, J = 8.2 Hz, 1H), 7.42 (d, J = 13.3 Hz, 1H), 7.27 (d, J = 8.7 Hz, 1H), 2.17 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 168.3, 160.4, 155.5, 153.5 (JCF = 187 Hz), 152.8, 146.9 (JCF = 12 Hz), 133.9, 133.3, 133.0, 127.0, 119.0, 115.2 (JCF = 17 Hz), 109.7, 104.6 (JCF = 26 Hz), 91.6 (JCF = 16 Hz), 16.2. ESI-MS: m/z 417.3 [M + H]+, 439.2 [M + Na]+. C21H13FN6OS (416.09). HPLC purity: 98.83%.
4-((2-((4-cyano-3-fluorophenyl)amino)-5H-pyrrolo[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (14)
As shown in Scheme 3, the starting material 26 (0.60 g, 2.0 mmol) and benzenesulfonyl chloride (0.38 g, 2.2 mmol) were dissolved in CH3CN (15 mL), and NaOH (50% aq., 0.32 mL, 4.0 mmol) was added. The reaction mixture stirred at room temperature for 3 h. Then the reaction mixture diluted with H2O and ethyl acetate and separated. The organic phase was washed with saturated sodium chloride (20 mL), dried over anhydrous Na2SO4, filtered, and purified by flash column chromatography. The product was recrystallized from ethyl acetate to provide the white solid intermediate 27 with 93% yield, mp: 263-265°C. HRMS m/z C21H15ClN4O3S: calcd 438.0553, found 439.0623 [M + H]+, 440.0646 [M + 2]+.
Intermediate 27 (0.44 g, 1.0 mmol) and 4-amino-2-fluorobenzonitrile (0.15 g, 1.1 mmol) were dissolved in dry dioxane (10 mL), and then PdCl2(PPh3)2 (0.07 g, 0.01 mmol), BINAP (0.06 g, 0.01 mmol) and Cs2CO3 (0.97 g, 3.0 mmol) were added. The reaction was carried out under the protection of nitrogen. The resulting mixture was stirred at 120°C for 12 hours. The solvent was evaporated under reduce pressure and the residue was dissolved in 20 mL ethyl acetate. The organic phase was washed with saturated sodium chloride (3 × 5 mL), dried over anhydrous Na2SO4, filtered, and then purified by flash column chromatography to give the compound 28 with 64% yield, mp: 276-277°C. HRMS m/z C28H19FN6O3S: calcd 538.1223, found 539.1433 [M + H]+.
A solution of NaOH (3M, 1.0 mL, 3.0 mmol) was added to a stirring solution of 28 (0.54g, 1.0 mmol) in 20 mL of methanol and heated to reflux at 85 °C until for 8 hours. Then solvent was removed and the residue was extracted with ether (3 × 10 mL), washed with saturated sodium chloride (10 mL), dried over anhydrous Na2SO4, and then purified by flash column chromatography to provide the target compound 14.
Recrystallized from EA/PE as a white solid, 53% yield, mp: 280-282℃. 1H NMR (400 MHz, DMSO-d6) δ 12.27 (s, 1H), 10.00 (s, 1H), 7.81-7.80 (m, 3H), 7.67-7.53 (m, 2H), 7.29 (dd, J = 8.7, 1.9 Hz, 1H), 6.51 (t, J = 2.3 Hz, 1H), 2.18 (s, 7H). 13C NMR (100 MHz, DMSO-d6) δ 165.0 (JCF = 249 Hz), 162.5, 153.8, 153.6, 153.4 (JCF = 181 Hz), 152.5, 148.5 (JCF = 13 Hz), 133.5, 133.2, 133.2, 119.0, 115.5, 114.2, 110.1, 109.4, 103.3 (JCF = 26 Hz), 101.3, 89.4 (JCF = 16 Hz), 16.3. ESI-MS: m/z 399.4 [M + H]+, 421.4 [M + Na]+. C22H15FN6O (398.13). HPLC purity: 98.54%.
4-((2-((4-cyano-3-fluorophenyl)amino)quinazolin-4-yl)oxy)-3,5-dimethyl benzonitrile (15)
Recrystallized from EA/PE as a white solid, 39% yield, mp: 300-301°C. 1H NMR (400 MHz, DMSO-d6) δ 10.37 (s, 1H, NH), 8.36 – 8.28 (m, 1H), 7.95 (ddd, J = 8.5, 6.9, 1.6 Hz, 1H), 7.84 – 7.76 (m, 4H), 7.73 – 7.63 (m, 1H), 7.59 – 7.52 (m, 1H), 7.47 (d, J = 8.7 Hz, 1H), 2.16 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 166.0, 155.2, 153.7, 153.1 (JCF = 190 Hz), 147.5 (JCF = 12 Hz), 135.9, 133.9, 133.2, 133.0, 126.1, 125.4, 124.1, 119.0, 115.3, 115.2, 111.6, 109.5, 104.9 (JCF = 26 Hz), 91.2 (JCF = 15 Hz), 16.2. ESI-MS: m/z 410.4 [M + H]+, 432.2 [M + Na]+. C24H16FN5O (409.13). HPLC purity: 95.76%.
4-((2-((4-cyano-3-fluorophenyl)amino)-5-fluoroquinazolin-4-yl)oxy)-3,5-dimethylbenzonitrile (16)
Recrystallized from EA/PE as a yellow solid, 47% yield, mp: 272-274°C. 1H NMR (400 MHz, DMSO-d6) δ 10.36 (s, 1H, NH), 8.43 – 8.39 (m, 1H), 7.80 – 7.75 (m, 3H), 7.73 – 7.60 (m, 2H), 7.55 – 7.51 (m, 1H), 7.47 – 7.45 (m, 1H), 2.12 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 168.3, 162.3, 161.7, 153.6 (JCF = 200 Hz), 146.6 (JCF = 12 Hz), 133.8, 132.6, 129.1, 119.0, 115.7, 110.5, 109.3, 105.3 (JCF = 32 Hz), 100.1, 91.3 (JCF = 22 Hz), 39.6, 39.4, 16.2. ESI-MS: m/z 428.3 [M + H]+, 450.2 [M + Na]+. C24H15F2N5O (427.12). HPLC purity: 96.76%.
4-((2-((4-cyano-3-fluorophenyl)amino)pyrimidin-4-yl)oxy)-3,5-dimethyl benzonitrile (17)
Recrystallized from EA/PE as a yellow solid, 72% yield, mp: 263-264°C. 1H NMR (400 MHz, DMSO-d6) δ 10.48 (s, 1H), 8.56 (d, J = 5.6 Hz, 1H), 7.78 (s, 2H), 7.63 (t, J = 8.2 Hz, 1H), 7.52 – 7.36 (m, 1H), 7.25 (d, J = 8.8 Hz, 1H), 6.82 (d, J = 5.6 Hz, 1H), 2.13 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 168.3, 162.3, 161.3, 159.2, 153.6 (JCF = 204 Hz), 147.1 (JCF = 12 Hz), 133.8, 133.1, 133.0, 129.4, 119.0, 115.2, 115.1, 109.4, 104.6 (JCF = 26 Hz), 100.0, 91.3 (JCF = 15 Hz), 39.6, 39.4, 16.2. ESI-MS: m/z 360.3 [M + H]+, 382.4 [M + Na]+. C20H14FN5O (359.12). HPLC purity: 95.88%.
4-((6-chloro-2-((4-cyano-3-fluorophenyl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (18)
Recrystallized from EA/PE as a yellow solid, 62% yield, mp: 245-247°C. 1H NMR (400 MHz, DMSO-d6) δ 10.46 (s, 1H), 8.52 (d, J = 5.6 Hz, 1H), 7.78 (s, 2H), 7.53 – 7.45 (m, 1H), 7.12 (s, 1H), 6.81 (d, J = 5.6 Hz, 1H), 2.13 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 168.4, 162.3, 162.0, 159.6, 153.2 (JCF = 187 Hz), 146.5 (JCF = 14 Hz), 133.8, 133.4, 130.5, 119.2, 116.1, 110.7, 105.2 (JCF = 22 Hz), 100.2, 91.3 (JCF = 15 Hz), 39.6, 39.3, 16.2. ESI-MS: m/z 394.2 [M + H]+, 416,5 [M + Na]+. C20H14FN5O (393.08). HPLC purity: 99.35%.
4-((2-((4-cyano-3-fluorophenyl)amino)-6,7-dihydrothieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (19)
Recrystallized from EA/PE as a white solid, 40% yield, mp: 285-287°C. 1H NMR (400 MHz, DMSO-d6) δ 10.46 (s, 1H, NH), 7.78 (s, 2H), 7.58 (t, J = 8.2 Hz, 1H), 7.27 (d, J = 13.4 Hz, 1H), 7.13 (d, J = 8.7 Hz, 1H), 3.51 (t, J = 8.1 Hz, 2H), 3.40 – 3.32 (m, 2H), 2.11 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 174.6, 164.8 (JCF = 329 Hz), 156.6, 153.7 (JCF = 176 Hz), 147.2 (JCF = 12 Hz), 133.7, 133.1, 132.9, 119.0, 115.2, 114.8, 109.5, 108.9, 104.0 (JCF = 26 Hz), 90.8 (JCF = 16 Hz), 36.6, 29.5, 16.1. ESI-MS: m/z 418.3 [M + H]+, 440.2 [M + Na]+. C22H16FN5OS (417.11). HPLC purity: 97.90%.
4-((2-((4-cyano-3-fluorophenyl)amino)-5,7-dihydrothieno[3,4-d]pyrimidin-4-yl) oxy)-3,5-dimethylbenzonitrile (20)
Recrystallized from EA/PE as a yellow solid, 37% yield, mp: 178-180°C. 1H NMR (400 MHz, DMSO-d6) δ 10.30 (s, 1H, NH), 7.72 (s, 2H), 7.54-7.53 (m, 1H), 7.26 (d, J = 12.6Hz, 1H), 7.02 (d, J = 8.0 Hz, 1H), 3.51-3.50 (m, 2H), 3.40-3.37 (m, 2H), 2.12 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 172.6, 164.8 (JCF = 330 Hz), 161.5, 156.6, 153.7 (JCF = 206 Hz), 146.2 (JCF = 13 Hz), 133.7, 133.1, 132.9, 115.6, 114.8, 109.5, 108.9, 104.2 (JCF = 26 Hz), 90.8 (JCF = 16 Hz), 36.6, 29.5, 16.1. ESI-MS: m/z 418.2 [M + H]+, 440.4 [M + Na]+. C22H16FN5OS (417.11). HPLC purity: 96.87%.
4-((2-((4-cyano-3-fluorophenyl)amino)-5,7-dihydrofuro[3,4-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (21)
Recrystallized from EA/PE as a yellow solid, 48% yield, mp: 291-293°C. 1H NMR (400 MHz, DMSO-d6) δ 10.58 (s, 1H, NH), 7.79 (s, 2H, C3,C5-Ph”-H), 7.62 (t, J = 8.3 Hz, 1H), 7.34 (d, J = 13.3 Hz, 1H), 7.19 (d, J = 8.7 Hz, 1H), 5.16 (d, J = 2.7 Hz, 2H), 4.97 (d, J = 2.3 Hz, 2H), 2.13 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 174.5, 162.4 (JCF = 279 Hz), 153.4, 147.1 (JCF = 12 Hz), 133.8, 133.2, 133.0, 119.0, 115.1, 109.5, 107.1, 91.3, 72.3, 69.4, 16.1. ESI-MS: m/z 402.4 [M + H]+, 424.2 [M + Na]+. C22H16FN5O2 (401.13). HPLC purity: 98.42%.
4-((2-((4-cyano-3-fluorophenyl)amino)-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (22)
Recrystallized from EA/PE as a white solid, 51% yield, mp: 212-214°C. 1H NMR (400 MHz, DMSO-d6) δ 7.61 (t, J = 8.1 Hz, 1H), 7.54 (s, 4H), 7.17 (dd, J = 12.0, 2.1 Hz, 1H), 6.75 (dd, J = 8.6, 2.1 Hz, 1H), 2.92 (dt, J = 28.9, 7.6 Hz, 9H), 1.96 (s, 12H). 13C NMR (100 MHz, DMSO-d6) δ 168.3, 161.7 (JCF = 243 Hz), 153.4, 150.3, 146.5 (JCF = 22 Hz), 134.3, 133.7, 133.1, 119.0, 116.3, 109.1, 106.5, 91.1, 27.0, 24.6, 23.4, 16.2. ESI-MS: m/z 400.2 [M + H]+, 422.4 [M + Na]+. C23H18FN5O (399.15). HPLC purity: 97.29%.
4-((2-((4-cyano-3-fluorophenyl)amino)-5,6,7,8-tetrahydroquinazolin-4-yl)oxy)-3,5-dimethylbenzonitrile (23)
Recrystallized from EA/PE as a brown solid, 48% yield, mp: 234-236°C. 1H NMR (400 MHz, DMSO-d6) δ 7.60 (t, J = 8.2 Hz, 1H), 7.54 (s, 4H), 7.21 (dd, J = 12.3, 2.1 Hz, 1H), 6.72 (dd, J = 8.6, 2.1 Hz, 1H), 2.69 (d, J = 24.7 Hz, 9H), 1.86 (s, 9H). 13C NMR (100 MHz, DMSO-d6) δ 172.1, 162.1 (JCF = 254 Hz), 156.7, 153.4, 146.8 (JCF = 18 Hz), 140.6, 133.8, 132.6, 119.2, 116.5, 109.5, 106.8, 91.7, 25.1, 23.9, 22.7, 16.3. ESI-MS: m/z 414.2 [M + H]+, 436.3 [M + Na]+. C24H20FN5O (413.17). HPLC purity: 98.16%.
General procedure for the preparation of final compounds 24a-e
The synthetic procedures for target compounds 24a-e were similar to that of 9a, with the difference that the starting material was 29 (Scheme 4).
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[3,2-d]pyrimidin-2-yl)amino)-3-fluorobenzonitrile (24a)
Recrystallized from EA/PE as a white solid, 60% yield, mp: 278-280°C. 1H NMR (400 MHz, DMSO-d6) δ 9.50 (s, 1H, NH), 8.40 (d, J = 5.4 Hz, 1H, C6-thienopyrimidine-H), 7.89 (t, J = 8.4 Hz, 1H, C6-Ph’-H), 7.73 (dd, J = 11.2, 1.9 Hz, 1H, C3-Ph’-H), 7.67 (d, J = 16.6 Hz, 1H, ArCH=), 7.55 (s, 2H, C3,C5-Ph”-H), 7.45 (d, J = 5.4 Hz, 1H, C7-thienopyrimidine-H), 7.30 (dd, J = 8.4, 1.8 Hz, 1H, C5-Ph’-H), 6.49 (d, J = 16.7 Hz, 1H, =CHCN), 2.11 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 165.0, 163.1, 157.3, 151.6 (JCF = 186 Hz), 150.3, 138.4, 133.7 (JCF = 10 Hz), 132.2, 131.8, 129.0 (JCF = 2 Hz), 128.7, 123.8, 122.4, 119.5 (JCF = 22 Hz), 118.6 (JCF = 2 Hz), 109.0, 104.2 (JCF = 9 Hz), 97.2, 16.4. ESI-MS: m/z 442.5 [M + 1]+, 459.6 [M + NH4]+. C24H16FN5OS (441.11). HPLC purity: 99.05%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[3,2-d]pyrimidin-2-yl)amino)-2-fluorobenzonitrile (24b)
Recrystallized from EA/PE as a white solid, 43% yield, mp: 290-292°C. 1H NMR (400 MHz, DMSO-d6) δ 10.38 (s, 1H, NH), 8.44 (d, J = 5.3 Hz, 1H, C6-thienopyrimidine-H), 7.71 (d, J = 14.6 Hz, 1H, ArCH=), 7.64 (d, J = 7.9 Hz, 1H, C5-Ph’-H), 7.60 (d, J = 8.3 Hz, 1H, C4-Ph’-H), 7.58 (s, 2H, C3,C5-Ph”-H), 7.53 (d, J = 5.4 Hz, 1H, C7-thienopyrimidine-H), 7.41 – 7.37 (m, 1H, C2-Ph’-H), 6.49 (d, J = 16.7 Hz, 1H, =CHCN), 2.13 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 164.8, 163.1, 157.0, 151.6 (JCF = 176 Hz), 150.4, 147.7 (JCF = 12 Hz), 138.5, 133.8, 132.4, 131.7, 128.8, 123.9, 119.3, 115.3 (JCF = 32 Hz), 109.2, 104.6 (JCF = 25 Hz), 97.1, 90.8 (JCF = 15 Hz), 16.4. ESI-MS: m/z 422.6 [M + 1]+, 459.6 [M + NH4]+. C24H16FN5OS (441.11). HPLC purity: 97.94%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[3,2-d]pyrimidin-2-yl)amino)-2,3-difluorobenzonitrile (24c)
Recrystallized from EA/PE as a white solid, 44% yield, mp: 283-285°C. 1H NMR (400 MHz, DMSO-d6) δ 9.88 (s, 1H, NH), 8.42 (d, J = 5.3 Hz, 1H, C6-thienopyrimidine-H), 7.71 (d, J = 7.0 Hz, 1H, C5-Ph’-H), 7.66 (d, J = 16.6 Hz, 1H, ArCH=), 7.54 (s, 2H, C3,C5-Ph”-H), 7.47 (d, J = 5.3 Hz, 1H, C7-thienopyrimidine-H), 7.35 (ddd, J = 8.9, 6.8, 1.8 Hz, 1H, C6-Ph’-H), 6.48 (d, J = 16.7 Hz, 1H, =CHCN), 2.11 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 164.9, 163.1, 157.0, 151.6 (JCF = 202 Hz), 150.3, 138.5, 135.9, 132.2, 131.8, 128.7, 128.0 (JCF = 3 Hz), 123.9, 119.3, 117.6, 114.1 (JCF = 3 Hz), 109.4, 97.2, 93.8 (JCF = 12 Hz), 16.4. ESI-MS: m/z 460.5 [M + 1]+, 477.4 [M + NH4]+. C24H15F2N5OS (459.10). HPLC purity: 98.43%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[3,2-d]pyrimidin-2-yl)amino)-2-(trifluoromethyl)benzonitrile (24d)
Recrystallized from EA/PE as a white solid, 59% yield, mp: 250-253°C. 1H NMR (400 MHz, DMSO-d6) δ 8.81 (s, 1H, NH), 8.37 (d, J = 5.4 Hz, 1H, C6-thienopyrimidine-H), 8.16 (s, 1H, C2-Ph’-H), 7.83 (s, 2H, C5,C6-Ph’-H), 7.63 (d, J = 16.7 Hz, 1H, ArCH=), 7.50 (s, 2H, C3,C5-Ph”-H), 7.39 (d, J = 5.4 Hz, 1H, C7-thienopyrimidine-H), 6.47 (d, J = 16.7 Hz, 1H, =CHCN), 2.09 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 165.1, 163.1, 158.3, 151.5, 150.2, 142.2, 138.3, 136.5, 132.1, 131.7, 131.5 (JCF = 6 Hz), 128.6, 127.7, 123.8, 123.0 (JCF = 30 Hz), 119.3, 118.1, 108.9, 106.8, 97.2, 16.3. ESI-MS: m/z 492.4 [M + 1]+, 509.4 [M + NH4]+. C25H16F3N5OS (491.10). HPLC purity: 97.67%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[3,2-d]pyrimidin-2-yl)amino)-3-(trifluoromethyl)benzonitrile (24e)
Recrystallized from EA/PE as a white solid, 51% yield, mp: 274-276°C. 1H NMR (400 MHz, DMSO-d6) δ 10.41 (s, 1H, NH), 8.44 (d, J = 5.3 Hz, 1H, C6-thienopyrimidine-H), 8.23 (d, J = 2.1 Hz, 1H, C3-Ph’-H), 8.07 (d, J = 8.8 Hz, 1H, C5-Ph’-H), 7.83 (d, J = 8.7 Hz, 1H, C6-Ph’-H), 7.67 (d, J = 16.7 Hz, 1H, ArCH=), 7.56 (s, 2H, C3,C5-Ph”-H), 7.52 (d, J = 5.3 Hz, 1H, C7-thienopyrimidine-H), 6.49 (d, J = 16.7 Hz, 1H, =CHCN), 2.13 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 164.6, 163.1, 157.0, 151.5, 150.3, 145.6, 138.6, 136.3, 132.3 (JCF = 25 Hz), 131.7, 128.8, 124.3 (JCF = 37 Hz), 120.8, 119.3, 116.7, 116.1 (JCF = 6 Hz), 109.6, 98.8, 97.1, 16.4. ESI-MS: m/z 492.4 [M + 1]+, 509.4 [M + NH4]+. C25H16F3N5OS (491.10). HPLC purity: 99.26%.
(E)-3-(4-((2-chlorothieno[2,3-d]pyrimidin-4-yl)oxy)-3,5-dimethylphenyl) acrylonitrile (32)
A mixture of 4-hydroxy-3,5-dimethylbenzaldehyde (0.88 g, 5.85 mmol) and K2CO3 (1.35 g, 9.75 mmol) in 15 mL of DMF was stirred at room temperature for 15 min, and then 2,4-dichlorothiopheno[2,3-d]pyrimidine (30, 1.0 g, 4.88 mmol) was added to the mixed solution. The mixture was stirred for another 1.5 h and then poured into ice water (30 mL) and left to stand for 20 min. The obtained precipitated was filtrated and washed with cold water, recrystallized from DMF-H2O to provide 31as a white solid in 87% yield, mp: 263-265°C. ESI-MS: m/z 319.4 (M + 1). C15H11ClN2O2S (318.02).
A mixture of (EtO)2P(O)CH2CN (0.67 g, 3.76 mmol) and t-BuOK (0.71 g, 6.28 mmol) in THF (10 mL) was stirred for 1 h at 0 °C, and then a solution of 31 (1.0 g, 3.14 mmol) in THF (5 mL) and DCM (5 mL) was slowly added to it over 1 h. The mixture was stirred for another 4 hours at room temperature (monitored by TLC) and then poured into ice water (20 mL). The precipitate was collected and washed with water to give 32 as a white solid in 75% yield, mp: 235-237°C. ESI-MS: m/z 342.4 (M + 1). C17H12ClN3OS (341.04). HPLC purity: 97.43%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[2,3-d]pyrimidin-2-yl)amino)-3-fluorobenzonitrile (25a)
Recrystallized from EA/PE as a white solid, 46% yield, mp: 261-262°C. 1H NMR (400 MHz, DMSO-d6) δ 9.67 (s, 1H, NH), 7.76 (d, J = 8.4 Hz, 1H, C6-Ph’-H), 7.73 (d, J = 1.5 Hz, 1H, C3-Ph’-H), 7.71-7.69 (m, 1H, C7-thienopyrimidine-H), 7.64 (d, J = 16.7 Hz, 1H, ArCH=), 7.58 (d, J = 5.9 Hz, 1H, C6-thienopyrimidine-H), 7.55 (s, 2H, C3,C5-Ph”-H), 7.24 (dd, J = 8.6, 1.8 Hz, 1H, C5-Ph’-H), 6.49 (d, J = 16.7 Hz, 1H, =CHCN), 2.11 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.6, 162.7, 155.9, 153.7, 151.3 (JCF = 206 Hz), 150.3, 133.3 (JCF = 11 Hz), 132.0, 131.7, 128.9 (JCF = 2 Hz), 128.7, 123.0, 122.5, 119.5 (JCF = 23 Hz), 118.7, 118.5 (JCF = 2 Hz), 112.6, 104.5 (JCF = 10 Hz), 97.0, 14.5. ESI-MS: m/z 442.6 [M + 1]+, 459.6 [M + NH4]+. C24H16FN5OS (441.11). HPLC purity: 95.90%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[2,3-d]pyrimidin-2-yl)amino)-2-fluorobenzonitrile (25b)
Recrystallized from EA/PE as a white solid, 53% yield, mp: 265-267°C. 1H NMR (400 MHz, DMSO-d6) δ 10.49 (s, 1H, NH), 7.82-7.81 (m, 1H, C7-thienopyrimidine-H), 7.71 (d, J = 11.1 Hz, 1H, ArCH=), 7.66-7.65 (m, 1H, C2-Ph’-H), 7.64-7.63 (m, 1H, C6-Ph’-H), 7.60-7.58 (m, 1H, C6-thienopyrimidine-H), 7.58 (s, 2H, C3,C5-Ph”-H), 7.31 (d, J = 8.9 Hz, 1H, C5-Ph’-H), 6.48 (d, J = 16.7 Hz, 1H, =CHCN), 2.12 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.4, 162.7, 155.6, 151.8 (JCF = 196 Hz), 150.4, 147.3 (JCF = 12 Hz), 133.8, 133.2 (JCF = 20 Hz), 132.3, 131.6, 128.8, 123.4, 118.8, 115.2 (JCF = 17 Hz), 112.7, 104.6 (JCF = 26 Hz), 97.0, 91.1, 16.2. ESI-MS: m/z 442.6 [M + 1]+, 459.6 [M + NH4]+. C24H16FN5OS (441.11). HPLC purity: 96.81%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[2,3-d]pyrimidin-2-yl)amino)-2,3-difluorobenzonitrile (25c)
Recrystallized from EA/PE as a white solid, 61% yield, mp: 259-261°C. 1H NMR (400 MHz, DMSO-d6) δ 10.04 (s, 1H, NH), 7.84-7.83 (m, 1H, C7-thienopyrimidine-H), 7.67 (d, J = 14.0 Hz, 1H), 7.64 (d, J = 3.2 Hz, 1H), 7.58 (d, J = 6.0 Hz, 1H, C6-thienopyrimidine-H), 7.54 (s, 2H, C3,C5-Ph”-H), 7.28 (ddd, J = 8.8, 6.8, 1.8 Hz, 1H), 6.48 (d, J = 16.7 Hz, 1H, =CHCN), 2.10 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.5, 162.7, 155.6, 151.9 (JCF = 184 Hz), 150.3, 132.1, 131.6, 128.6, 127.9 (JCF = 4 Hz), 123.4, 119.3, 118.7, 117.7, 114.0 (JCF = 3 Hz), 112.9, 97.0, 94.1 (JCF = 12 Hz), 16.4. ESI-MS: m/z 460.5 [M + 1]+, 477.4 [M + NH4]+. C24H15F2N5OS (459.10). HPLC purity: 96.89%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[2,3-d]pyrimidin-2-yl)amino)-2-(trifluoromethyl)benzonitrile (25d)
Recrystallized from EA/PE as a white solid, 51% yield, mp: 210-212°C. 1H NMR (400 MHz, DMSO-d6) δ 9.08 (s, 1H, NH), 8.10 (s, 1H, C2-Ph’-H), 7.91 (dd, J = 8.6 Hz, 1H, C5-Ph’-H), 7.82 (d, J = 8.5, 1H, C6-Ph’-H), 7.79 (s, 2H, C3,C5-Ph”-H), 7.65 (d, J = 6.0 Hz, 1H, C7-thienopyrimidine-H), 7.61 (d, J = 16.4 Hz, 1H, ArCH=), 7.54 (d, J = 6.0 Hz, 1H, C6-thienopyrimidine-H), 6.44 (d, J = 16.7 Hz, 1H, =CHCN), 2.09 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.3, 162.7, 157.2, 151.8, 149.5, 141.3, 136.5, 133.2 (JCF = 7 Hz), 131.9, 131.5, 131.3 (JCF = 4 Hz), 128.5, 122.6, 120.3, 118.7, 118.1, 112.5 (JCF = 12 Hz), 108.2, 97.0, 16.2. ESI-MS: m/z 492.5 [M + 1]+, 509.5 [M + NH4]+. C25H16F3N5OS (491.10). HPLC purity: 99.05%.
(E)-4-((4-(4-(2-cyanovinyl)-2,6-dimethylphenoxy)thieno[2,3-d]pyrimidin-2-yl)amino)-3-(trifluoromethyl)benzonitrile (25e)
Recrystallized from EA/PE as a white solid, 54% yield, mp: 208-210°C. 1H NMR (400 MHz, DMSO-d6) δ 9.07 (s, 1H, NH), 7.79 (d, J = 2.0 Hz, 1H, C3-Ph’-H), 7.71 (d, J = 9.3 Hz, 2H, C5,C6-Ph’-H), 7.61 (d, J = 16.7 Hz, 1H, ArCH=), 7.55 (d, J = 5.5 Hz, 1H, C7-thienopyrimidine-H), 7.52 (d, J = 6.0 Hz, 1H, C6-thienopyrimidine-H), 7.47 (s, 2H, C3,C5-Ph”-H), 6.45 (d, J = 16.7 Hz, 1H, =CHCN), 2.08 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 171.6, 162.7, 157.1, 151.8, 150.2, 141.9, 136.5, 133.0 (JCF = 6 Hz), 131.9, 131.5, 131.4 (JCF = 5 Hz), 128.5, 122.6, 119.3, 118.7, 118.1, 112.5 (JCF = 12 Hz), 107.4, 97.0, 16.1. ESI-MS: m/z 492.4 [M + 1]+, 509.5 [M + NH4]+. C25H16F3N5OS (491.10). HPLC purity: 97.56%.
Supplementary Material
Figure 1.

Chemical structures of U.S. FDA-approved second-generation NNRTI drugs (1 and 2) and our previously reported potent HIV-1 NNRTIs (3-7).
Figure 2.
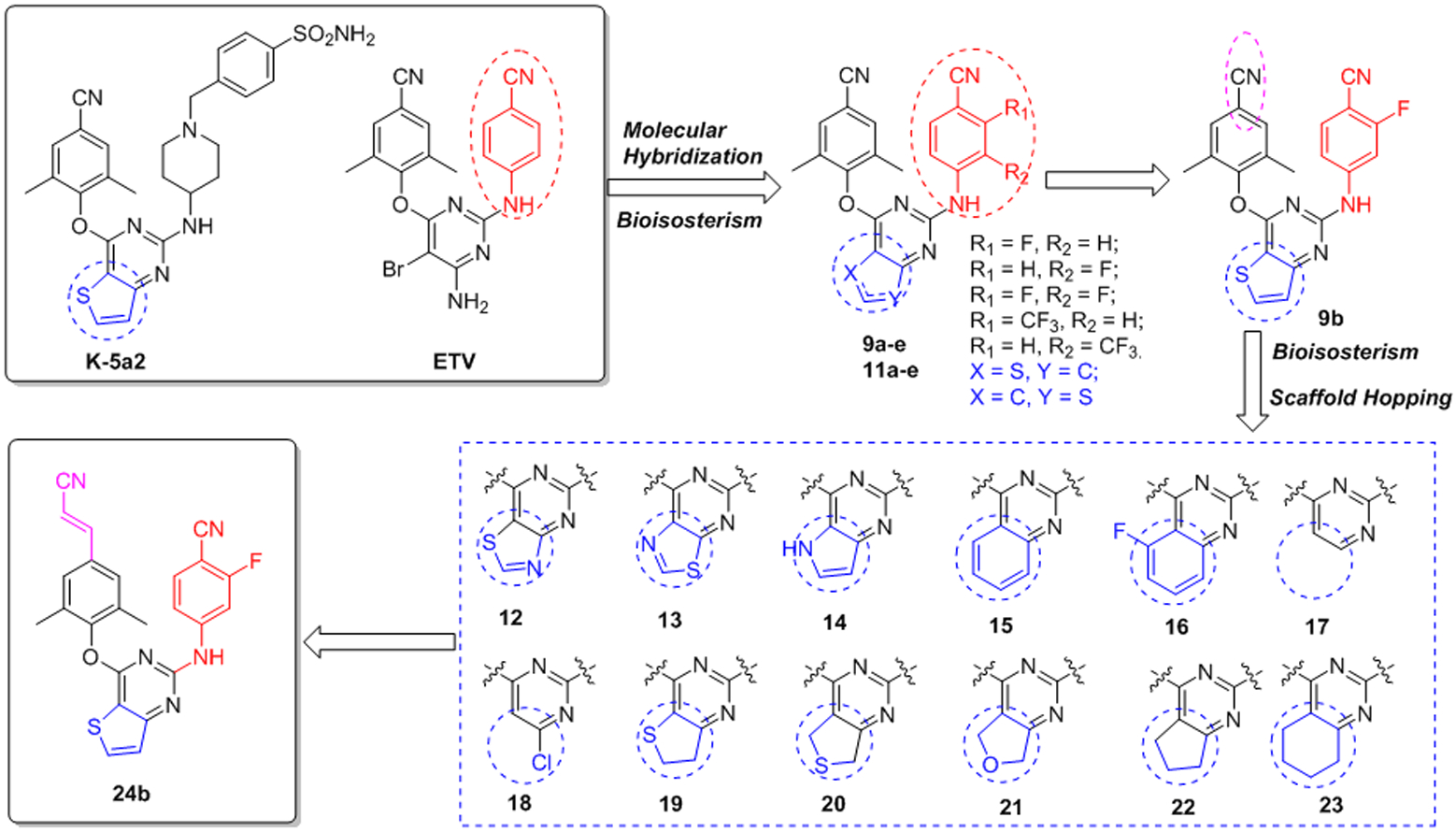
Design and optimization of the fluorine-substituted diarylpyrimidine derivatives.
Table 1.
| Compounds | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| EC50 (nM) | WT | 4.1±0.1 | 1.0±0.3 | 1.4±0.4 | 1.2±0.2 | 1.6±0.4 | 2.2±0.9 | 1.6±0.2 |
| K103N | 2.4±0.6 | 1.3±0.36 | 2.9 | 0.95±0.07 | 0.9±0.1 | 3.9±1.9 | 6.5±1.5 | |
| E138K | 14±2.2 | 5.7±0.11 | 2.9 | 4.7±0.16 | 7.0±2.5 | 1.9±0.1 | 7.9±0.1 | |
| RES056 | 17±1.8 | 11±1.9 | 31±12 | 5.5±0.81 | 41±9.9 | 26±1.5 | 18±2.3 | |
| F227L+V106A | 29±7.7 | 82±21 | 4.2±1.2 | 2.7±1.7 | 19±2.2 | 19±4.5 | 16±4.6 | |
| CC50 (μM) | > 4.6 | 4.0±1.2 | 2.3±0.4 | 2.3±0.4 | > 250 | 25±0.75 | 23±1.7 | |
| hERG IC50 (μM) | - | 0.50 | 0.13 | 0.18 | 0.83 | 0.18 | 0.12 | |
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from the National Natural Science Foundation of China (NSFC Nos. 81573347, 81973181, 81903453), the Key Project of NSFC for International Cooperation (No. 81420108027), Shandong Provincial Natural Science Foundation (ZR2019BH011), Natural Science Foundation of Jiangsu Province (BK2019041035), China Postdoctoral Science Foundation (2019T120596, 2018M640641), Young Scholars Program of Shandong University (YSPSDU No. 2016WLJH32), Shandong Provincial Key research and development project (Nos. 2017CXGC1401, 2019JZZY021011), the Taishan Scholar Program at Shandong Province, KU Leuven (GOA 10/014), and NIH MERIT Award R37 AI027690 (to E.A.). We thank Anthony Hoang for production of the RT52A protein; and the APS 23-ID-B beamline facility for data collection. The technical assistance of Mr. Kris Uyttersprot and Mrs. Kristien Erven, for the HIV experiments is gratefully acknowledged.
ABBREVIATIONS USED
- AIDS
acquired immune deficiency syndrome
- CC50
50% cytotoxicity concentration
- Cmax
maximum concentration
- DAPY
diarylpyrimidine
- DLV
delavirdine
- DOR
doravirine
- EFV
efavirenz
- ETV
etravirine
- EC50
the effective concentration causing 50% inhibition of viral cytopathogenicity
- cART
combination antiretroviral therapy
- HIV
human immunodeficiency virus
- hERG
the human ether-à-go-go related gene
- NNIBP
NNRTI-binding pocket
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- NVP
nevirapine
- RF
fold-resistance
- RPV
rilpivirine
- RT
reverse transcriptase
- SAR
structure-activity relationship
- SI
selectivity index
- WT
wild-type
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.9b01769
Molecular formula strings and some data (CSV)
In vitro assay of anti-HIV activities in MT-4 cells, recombinant HIV-1 reverse transcriptase (RT) inhibitory assays, cytochrome P450 inhibition assay, pharmacokinetic methods, acute toxicity experiment, and assay procedures for hERG activity (PDF).
The authors declare that all experimental work complied with the institutional guidelines on animal studies (care and use of laboratory animals).
The authors declare no competing financial interest.
Reference
- 1.Bec G; Meyer B; Gerard MA; Steger J; Fauster K; Wolff P; Burnouf D; Micura R; Dumas P; Ennifar E Thermodynamics of HIV-1 reverse transcriptase in action elucidates the mechanism of action of non-nucleoside inhibitors. J. Am. Chem. Soc 2013, 135, 9743–9752. [DOI] [PubMed] [Google Scholar]
- 2.Zhan P; Chen X; Li D; Fang Z; De Clercq E; Liu X HIV-1 NNRTIs: structural diversity, pharmacophore similarity, and implications for drug design. Med. Res. Rev 2013, 33 Suppl 1, E1–72. [DOI] [PubMed] [Google Scholar]
- 3.Namasivayam V; Vanangamudi M; Kramer VG; Kurup S; Zhan P; Liu X; Kongsted J; Byrareddy SN The journey of HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) from lab to clinic. J. Med. Chem 2019, 62, 4851–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehman DA; Wamalwa DC; McCoy CO; Matsen FA; Langat A; Chohan BH; Benki-Nugent S; Custers-Allen R; Bushman FD; John-Stewart GC; Overbaugh J Low-frequency nevirapine resistance at multiple sites may predict treatment failure in infants on nevirapine-based treatment. J. Acquired Immune Defic. Syndr 2012, 60, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyrer C; Pozniak A HIV drug resistance - an emerging threat to epidemic control. N. Engl. J. Med 2017, 377, 1605–1607. [DOI] [PubMed] [Google Scholar]
- 6.Wensing AM; Calvez V; Gunthard HF; Johnson VA; Paredes R; Pillay D; Shafer RW; Richman DD 2017 update of the drug resistance mutations in HIV-1. Top. HIV Med 2017, 24, 132–133. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan P; Pannecouque C; De Clercq E; Liu X Anti-HIV drug discovery and development: current innovations and future trends. J. Med. Chem 2016, 59, 2849–2878. [DOI] [PubMed] [Google Scholar]
- 8.Kang D; Feng D; Ginex T; Zou J; Wei F; Zhao T; Huang B; Sun Y; Desta S; De Clercq E; Pannecouque C; Zhan P; Liu X Exploring the hydrophobic channel of NNIBP leads to the discovery of novel piperidine-substituted thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 NNRTIs. Acta Pharm. Sinica B 2019, 10.1016/j.apsb.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang D; Fang Z; Li Z; Huang B; Zhang H; Lu X; Xu H; Zhou Z; Ding X; Daelemans D; De Clercq E; Pannecouque C; Zhan P; Liu X Design, synthesis, and evaluation of thiophene[3,2-d]pyrimidine derivatives as HIV-1 non-nucleoside reverse transcriptase inhibitors with significantly improved drug resistance profiles. J. Med. Chem 2016, 59, 7991–8007. [DOI] [PubMed] [Google Scholar]
- 10.Kang D; Fang Z; Huang B; Lu X; Zhang H; Xu H; Huo Z; Zhou Z; Yu Z; Meng Q; Wu G; Ding X; Tian Y; Daelemans D; De Clercq E; Pannecouque C; Zhan P; Liu X Structure-based optimization of thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 non-nucleoside reverse transcriptase inhibitors with improved potency against resistance-associated variants. J. Med. Chem 2017, 60, 4424–4443. [DOI] [PubMed] [Google Scholar]
- 11.Kang D; Zhang H; Wang Z; Zhao T; Ginex T; Luque FJ; Yang Y; Wu G; Feng D; Wei F; Zhang J; De Clercq E; Pannecouque C; Chen CH; Lee KH; Murugan N; Steitz T; Zhan P; Liu X Identification of dihydrofuro[3,4-d]pyrimidine derivatives as novel HIV-1 non-nucleoside reverse transcriptase inhibitors with promising antiviral activities and desirable physicochemical properties. J. Med. Chem 2019, 62, 1484–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang D; Zhao T; Wang Z; Feng D; Zhang H; Huang B; Wu G; Wei F; Zhou Z; Jing L; Zuo X; Tian Y; Poongavanam V; Kongsted J; De Clercq E; Pannecouque C; Zhan P; Liu X Discovery of piperidine-substituted thiazolo[5,4-d]pyrimidine derivatives as potent and orally bioavailable HIV-1 non-nucleoside reverse transcriptase inhibitors. Commu. Chem 2019, 2, 74. [Google Scholar]
- 13.Jiang X; Yu J; Zhou Z; Kongsted J; Song Y; Pannecouque C; De Clercq E; Kang D; Poongavanam V; Liu X; Zhan P Molecular design opportunities presented by solvent-exposed regions of target proteins. Med. Res. Rev 2019, 39, 2194–2238. [DOI] [PubMed] [Google Scholar]
- 14.Sanguinetti MC; Tristani-Firouzi M HERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [DOI] [PubMed] [Google Scholar]
- 15.Kang D; Huo Z; Wu G; Xu J; Zhan P; Liu X Novel fused pyrimidine and isoquinoline derivatives as potent HIV-1 NNRTIs: a patent evaluation of WO2016105532A1, WO2016105534A1 and WO2016105564A1. Expert Opin. Ther. Pat 2017, 27, 383–391. [DOI] [PubMed] [Google Scholar]
- 16.Shamovsky I; Connolly S; David L; Ivanova S; Norden B; Springthorpe B; Urbahns K Overcoming undesirable HERG potency of chemokine receptor antagonists using baseline lipophilicity relationships. J. Med. Chem 2008, 51, 1162–1178. [DOI] [PubMed] [Google Scholar]
- 17.Mitcheson JS; Chen J; Lin M; Culberson C; Sanguinetti MC A structural basis for drug-induced long QT syndrome. P. Natl. Acad. Sci. USA 2000, 97, 12329–12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho JF; Louvel J; Doornbos ML; Klaasse E; Yu Z; Brussee J; AP IJ Strategies to reduce HERG K+ channel blockade. Exploring heteroaromaticity and rigidity in novel pyridine analogues of dofetilide. J. Med. Chem 2013, 56, 2828–2840. [DOI] [PubMed] [Google Scholar]
- 19.Mitcheson JS; Chen J; Lin M; Culberson C; Sanguinetti MC A structural basis for drug-induced long QT syndrome. P. Natl. Acad. Sci. USA 2000, 97, 12329–12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y; Kang D; Nguyen L; Smithline Z; Pannecouque C; Zhan P; Liu X; Steitz TA Structural basis for potent and broad inhibition of HIV-1 RT by thiophene[3,2-d]pyrimidine non-nucleoside inhibitors. Elife 2018, 7, pii: e36340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meanwell NA Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem 2018, 61, 5822–5880. [DOI] [PubMed] [Google Scholar]
- 22.Bauman JD; Das K; Ho WC; Baweja M; Himmel DM; Clark AD Jr.; Oren DA; Boyer PL; Hughes SH; Shatkin AJ; Arnold E Crystal engineering of HIV-1 reverse transcriptase for structure-based drug design. Nucleic Acids Res. 2008, 36, 5083–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das K; Bauman JD; Clark AD Jr.; Frenkel YV; Lewi PJ; Shatkin AJ; Hughes SH; Arnold E High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. P. Natl. Acad. Sci. USA 2008, 105, 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otwinowski Z; Minor W Processing of X-ray diffraction data collected in oscillation mode. Method. Enzymol 1997, 276, 307–326. [DOI] [PubMed] [Google Scholar]
- 25.Adams PD; Afonine PV; Bunkoczi G; Chen VB; Davis IW; Echols N; Headd JJ; Hung LW; Kapral GJ; Grosse-Kunstleve RW; McCoy AJ; Moriarty NW; Oeffner R; Read RJ; Richardson DC; Richardson JS; Terwilliger TC; Zwart PH PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr 2010, 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emsley P; Cowtan K Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr 2004, 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- 27.Perez VE; Sanchez-Parra C; Serrano Villar S Etravirine drug interactions. Enferm. Infecc. y Micr. Cl 2009, 27 Suppl 2, 27–31. [DOI] [PubMed] [Google Scholar]
- 28.Vandenberg JI; Perry MD; Perrin MJ; Mann SA; Ke Y; Hill AP HhERG K(+) channels: structure, function, and clinical significance. Physiol. Rev 2012, 92, 1393–1478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


