Abstract
Although immunotherapy research to date has focused largely on T cells, there is mounting evidence that tumour-infiltrating B cells and plasma cells (collectively referred to as tumour-infiltrating B lymphocytes (TIL-Bs)) have a crucial, synergistic role in tumour control. In many cancers, TIL-Bs have demonstrated strong predictive and prognostic significance in the context of both standard treatments and immune checkpoint blockade, offering the prospect of new therapeutic opportunities that leverage their unique immunological properties. Drawing insights from autoimmunity, we review the molecular phenotypes, architectural contexts, antigen specificities, effector mechanisms and regulatory pathways relevant to TIL-Bs in human cancer. Although the field is young, the emerging picture is that TIL-Bs promote antitumour immunity through their unique mode of antigen presentation to T cells; their role in assembling and perpetuating immunologically ‘hot’ tumour microenvironments involving T cells, myeloid cells and natural killer cells; and their potential to combat immune editing and tumour heterogeneity through the easing of self-tolerance mechanisms. We end by discussing the most promising approaches to enhance TIL-B responses in concert with other immune cell subsets to extend the reach, potency and durability of cancer immunotherapy.
Although the critical role of T cells in antitumour immunity has become indisputable in the era of immune checkpoint blockade and adoptive T cell therapy, these same advances have revealed the many limitations and vulnerabilities of the T cell response, prompting an urgent need to understand and harness orthogonal immunological mechanisms. A flood of new evidence implicates tumour-infiltrating B cells and plasma cells (PCs) (collectively referred to as tumour-infiltrating B lymphocytes (TIL-Bs)) as powerful, multifaceted players in antitumour responses. TIL-Bs are rarely found on their own but, rather, associate intimately with T cells, myeloid cells and other immune cells in the most immunologically ‘hot’ tumours, often substantially exceeding the levels of B-lineage cells in healthy non-lymphoid tissues (FIG. 1; see Supplementary Table 1). Indeed, the fact that exhausted or dysfunctional CD8+ and CD4+ TILs frequently express the B cell-recruiting C–X–C motif chemokine ligand 13 (CXCL13) suggests they are programmed to solicit B cell help in the face of tumour persistence1–3. Such interactions can culminate in the formation of tertiary lymphoid structures (TLSs), lymph node-like structures that arise de novo in tumour stroma and appear actively engaged in priming and sustaining adaptive immune responses. Similar to T cells, TIL-Bs are associated with a positive prognostic value in most cancers (FIG. 1; see Supplementary Table 2). Moreover, they can strongly enhance the prognostic impact of CD4+ and CD8+ TILs, an effect that appears particularly robust in tumours harbouring TLSs4. Also similar to T cells, TIL-Bs comprise various phenotypes, including both effector and regulatory B cell (Breg cell) subsets.
Fig. 1 |. B cells in health and malignancy.
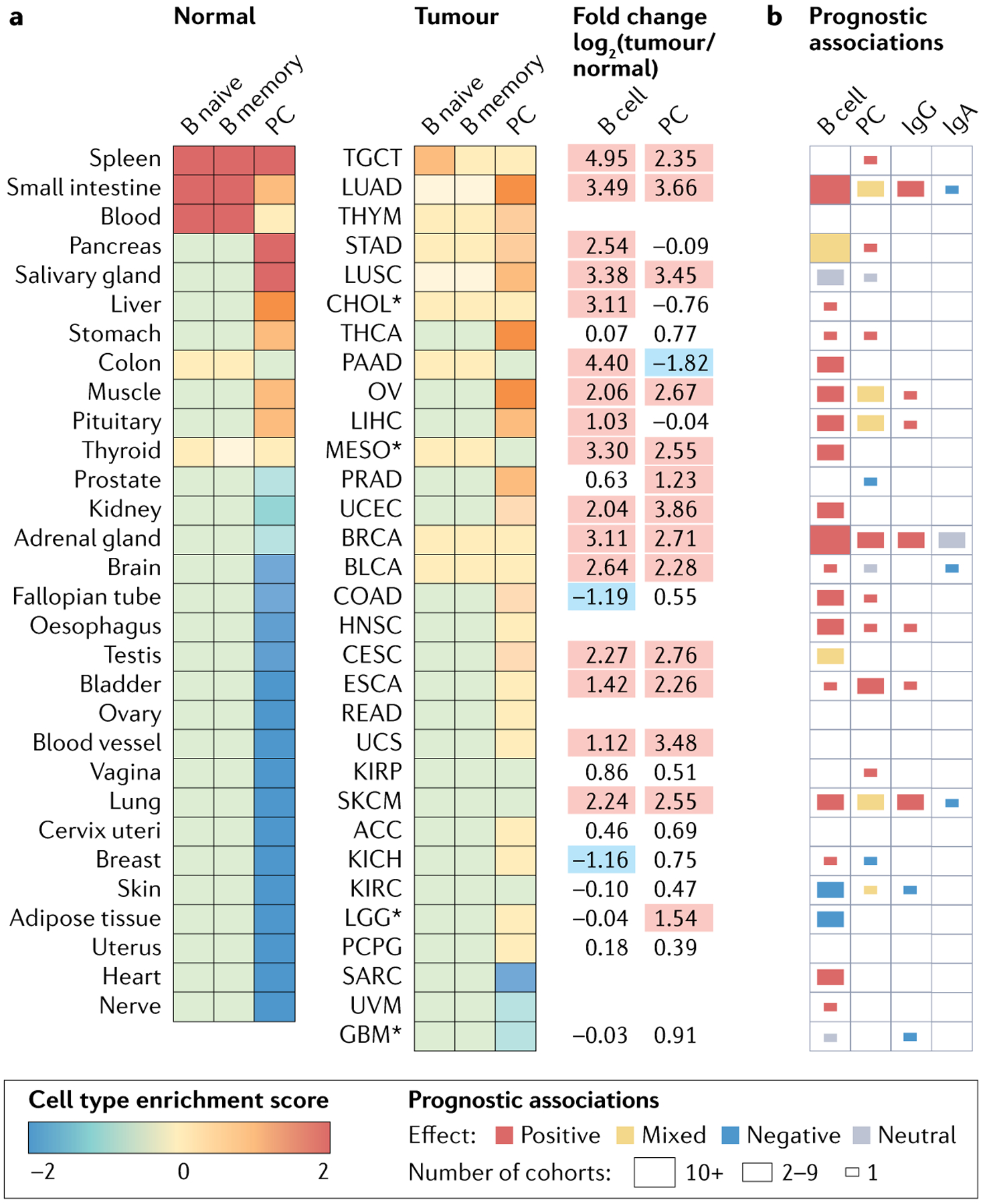
a | Heat maps comparing abundance of B cells and plasma cells (PCs) in normal and tumour tissues. Data from Genotype-Tissue Expression consortium (GTEx v. 8, n = 16,704 samples) and The Cancer Genome Atlas (TCGA) consortium (harmonized dataset, n = 9,922 primary solid tumours) were analysed using xCell199 to generate cell type enrichment scores for naive B cells (B naive), memory B cells (B memory) and PCs. Scores averaged across samples of the same origin, scaled across columns and ranked by decreasing order. Asterisk indicates an identical normal tissue comparator was not available, so a closely related tissue was used instead. Log-fold change for B cell (average of naive B cells and memory B cells) and PC scores between tumours and their normal counterparts are reported (right). Red, tumour tissue > normal tissue; no colour, tumour tissue ~ normal tissue; blue, tumour tissue < normal tissue. b | Heat map summarizing prognostic associations for intratumoural B cells, PCs, IgG and IgA based on an update of previous literature searches93,200. Tumour sites are deemed positive, neutral, or negative based on the majority of cohorts. Supplementary Tables 1 and 2 provide supporting data and references. ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; ESCA, oesophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadeno-carcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma and squamous cell carcinoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumours; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma.
Exhausted or dysfunctional CD8+ and CD4+ TILs.
(Exhausted or dysfunctional CD8+ and CD4+ tumour-infiltrating lymphocytes). Adysfunctional T cell state triggered by chronic antigen exposure and characterized by poor effector functions (for example, cytokine production), sustained expression of inhibitory receptors (for example, PD-1, cytotoxic T lymphocyte-associated protein 4 (CTLA4)) and widespread transcriptomic changes. Exhausted T cells can be placed on a dysfunction continuum (pre-dysfunctional, early dysfunctional and late dysfunctional) based on their clonality (intermediate to high) and their expression of C–X–C motif chemokine ligand 13 (CXCL13) and inhibitory receptors (intermediate to high).
TIL-Bs exhibit the hallmarks of antigen recognition, including somatic hypermutation, class switch recombination, clonal expansion and differentiation into IgG or IgA-producing PCs. Whereas tumour-reactive CD4+ and CD8+ TILs often recognize mutated proteins (neoantigens)5, the target antigens of TIL-Bs identified to date are predominantly self-proteins6, suggesting that tumours can disrupt B cell tolerance mechanisms. Indeed, the orthogonal tolerance mechanisms used by B cells and T cells raise fascinating immunological issues and compelling therapeutic opportunities in the setting of cancer. Adding to this theme of complementarity, TIL-Bs have a plethora of effector mechanisms that enable them to enhance not only adaptive immune responses but also innate mechanisms involving complement, myeloid cells and natural killer cells, as well as the myriad possibilities afforded by antibodies.
Although B cells and PCs remain poorly understood in the cancer immunotherapy field, they have received intensive study in autoimmunity, where they use multi-pronged effector mechanisms to mediate potent, persistent and often unstoppable destruction of target tissues, sometimes with remarkable selectivity. Drawing insights from the autoimmunity field, this Review summarizes our current understanding of TIL-Bs in human cancers and highlights the knowledge gaps that require urgent attention. After considering the role of TIL-Bs across the major facets of cancer immunity, we discuss strategies to harness their cell-based and antibody-based effector mechanisms to enable a new generation of cancer immunotherapies.
B cell development and tolerance
B cells arise and mature in bone marrow, where they undergo V(D)J recombination, resulting in cell surface expression of B cell receptors (BCRs) of the IgM isotype (FIG. 2a). Up to 75% of immature B cells are self-reactive7 and undergo either clonal deletion or receptor editing, wherein a second immunoglobulin light chain is generated, providing a second chance to reduce self-reactivity (FIG. 2b). Nonetheless, a considerable proportion of self-reactive B cells enter the periphery and comprise about 20% of mature naive circulating B cells in healthy individuals8. This reservoir of autoreactive B cells is thought to be critical to avoid large ‘gaps’ in the B cell repertoire that could otherwise serve as havens for pathogens expressing antigens that resemble host proteins9. Indeed, self-reactive B cells can participate in responses to infectious agents through the process of clonal redemption10,11 (FIG. 2b). Peripheral B cell tolerance is maintained by several mechanisms, chief ones being the suppressive effects of CD4+ regulatory T (Treg) cells counterbalanced with the stimulatory effects of CD4+ T follicular helper cells (TFH cells) and other T cell subsets. Indeed, either a decrease in Treg cells or an increase in TFH cells is sufficient to generate autoreactive B cells8,12. Thus, whereas both B cells and T cells are subject to central and peripheral tolerance, the precise mechanisms and underlying antigenic repertoires are different, resulting in complementary, interdependent definitions of self that carry important implications for antitumour immunity. As discussed later, there are numerous examples of TIL-Bs recognizing self-antigens (TABLE 1; see Supplementary Table 3), suggesting that many TIL-B responses do indeed involve disruption of peripheral tolerance mechanisms (FIG. 2b) as seen in other inflammatory contexts such as acute and chronic infection13,14.
Fig. 2 |. B cell differentiation and tolerance.
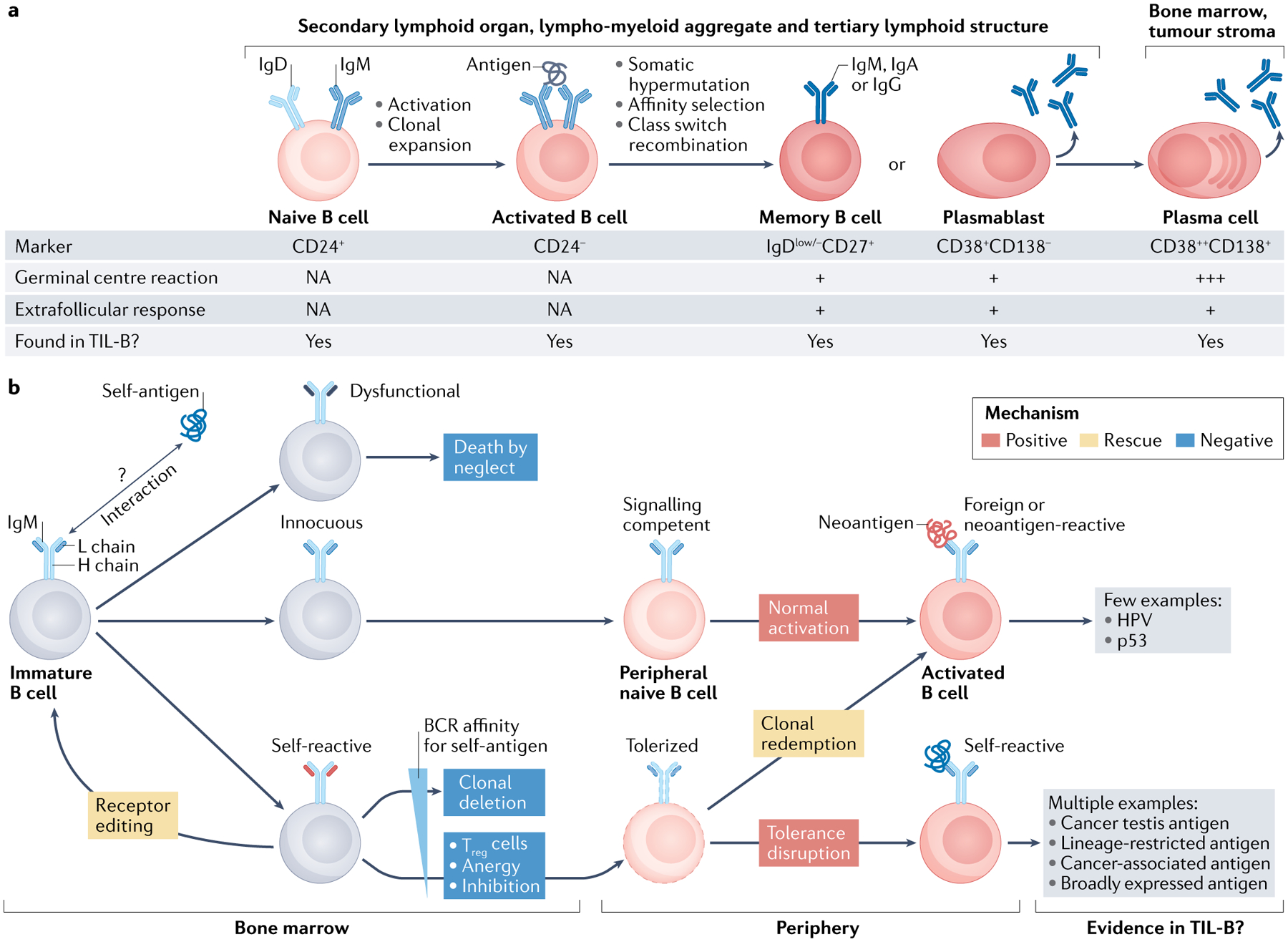
a | Stages of B cell activation, clonal expansion, somatic hypermutation, affinity selection, class switch recombination and differentiation to plasma cells (PCs). These events can involve germinal centre reactions or extrafollicular responses, with the former generally being the better source of long-lived PCs. All of these B cell subsets are found in human tumours, with the most commonly reported subsets being activated B cells, memory B cells and PCs. b | Central tolerance takes place in bone marrow and eliminates immature self-reactive B cells via clonal deletion; receptor editing of immunoglobulin light chains (L chains) offers one chance at rescue. Peripheral tolerance maintains weakly and moderately self-reactive B cells in a hypo-responsive or inactive state through several mechanisms. B cell responses to foreign antigens (or potentially neoantigens in cancer) can occur through conventional activation or clonal redemption. Disruption of peripheral tolerance during inflammation, autoimmunity or cancer can license self-reactive B cells and PCs to participate in immune responses. With the exception of human papillomavirus (HPV) antigens and tumour protein p53 (p53) neoantigens (although most p53-specific autoantibodies appear to recognize non-mutated epitopes), studies to date indicate that TIL-Bs generally recognize self-antigens with varying degrees of tumour-specific expression. BCR, B cell receptor; H chain, heavy chain; NA, not applicable; TIL-B, tumour-infiltrating B lymphocyte; Treg cell, regulatory T cell.
Table 1 |.
Antigens recognized by TIL-Bs
| Antigen name | Antigen location (known or predicted) | Cancer of origin | Isotype | Refs |
|---|---|---|---|---|
| Cancer testis and stem cell antigens | ||||
| CTAG1B | Intracellular | Lung (NSCLC), breast | IgG > IgA (lung), IgG < IgA (breast) | 105,194 |
| CTAG2 | Nucleoplasm, nuclear bodies, vesicles | Lung (NSCLC) | IgG > IgA | 194 |
| MAGEA3 | Intracellular | Lung (NSCLC) | IgG | 194 |
| NXF2 | Intracellular | Lung (NSCLC) | IgG | 194 |
| SPAG8 | Intracellular | Breast | IgA, IgG | 105 |
| SOX2 | Nucleoplasm | Lung (NSCLC) | IgG | 194 |
| Viral antigens | ||||
| E2 | Nucleus | Head and neck (HPV + HNSCC) | IgG | 53 |
| E6 | Nucleus | Head and neck (HPV + HNSCC) | IgG | 53 |
| E7 | Nucleus | Head and neck (HPV + HNSCC) | IgG | 53 |
| Lineage-restricted antigens | ||||
| BDNF | Secreted | Ovary (HGSC) | IgA | 138 |
| MLANA | Membrane | Lung (NSCLC) | IgG | 194 |
| Commonly mutated antigens | ||||
| BRCA2 | Nucleoplasm, cytosol | Breast | IgA, IgG | 105 |
| TP53 | Nucleoplasm | Lung (adenocarcinoma, LCC, NSCLC, SCC) | IgG | 194–196 |
| Antigens commonly dysregulated in cancer | ||||
| ACAT2 | Nucleoplasm, cytosol | Lung (LCC) | IgG | 195 |
| COPS4 | Nuclear speckles | Breast | IgA, IgG | 105 |
| GAPDH | Nuclear membrane, vesicles, plasma membrane, cytosol | Lung (LCC) | IgG | 195 |
| MUC1 | Plasma membrane | Breast | NE | 197 |
| RHOC | Intracellular | Lung (NSCLC) | IgG | 48 |
| TSPAN7 | Plasma membrane | Ovary (HGSC) | IgA | 138 |
| Post-translationally modified antigens | ||||
| ACTB | Cytosol, apoptotic blebs | Breast (MBC) | IgG | 88 |
| Hspa4 | Nucleoplasm, cytosol, plasma membrane | Breast (4T1) | IgG | 149 |
| Self-antigens with no reported aberrancies | ||||
| GAS8 | Plasma membrane, cytosol | Lung (LCC) | IgG | 195 |
| MAPK9 | Intracellular | Lung (LCC) | IgG | 195 |
| PHF3 | Nucleoplasm | Lung (LCC) | IgG | 195 |
| RPL3 | Nucleoli, cytosol | Lung (NSCLC) | IgG | 48 |
| Lipid antigens | ||||
| Ganglioside D3 | Plasma membrane | Breast (MBC) | NE | 198 |
| Nucleic acid antigens | ||||
| Mitochondrial DNA | Mitochondria | Lung (LCC) | IgG | 195 |
Known antigens locations are reported in roman font, predicted locations in italics. Data were extracted from the Human Protein Atlas. Cancer subtype indicated in parentheses where available. 4T1, murine BALB/c mammary gland tissue-derived breast cancer cell line; BDNF, brain-derived neurotrophic factor; BRCA2, breast cancer type 2 susceptibility protein; HGSC, high-grade serous ovarian cancer; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; LCC, large cell carcinoma; MBC, medullary breast carcinoma; MUC1, mucin 1; NE, not evaluated; NSCLC, non-small-cell lung carcinoma; SCC, squamous cell carcinoma; TIL-B, tumour-infiltrating B lymphocyte.
Clonal redemption.
A process by which moderately self-reactive anergic B cells are recruited to the germinal centre, where somatic hypermutation gives them the opportunity to mutate away from self-reactivity.
The emerging range of TIL-B phenotypes
The advent of high-dimensional flow cytometry and single-cell RNA sequencing (scRNA-seq) has advanced our understanding of B cell maturation from nine canonical stages (FIG. 2a) and seven core markers15 to a plethora of molecular phenotypes that integrate developmental, functional and clonal information. Although the interpretation of such data is evolving, there is evidence for at least 10 B cell subsets in healthy human blood16, 12–13 in lymphoid tissues17,18 and 14 in peripheral organs19. Tissue-resident memory B cells have been reported in skin, gut and lung epithelium20,21 and, similar to tissue-resident memory T cells22, are characterized by expression of CD45RB and CD69 (REFS21,23) and provide a first line of defence against reinfection.
In the autoimmunity field, there is strong interest in so-called double-negative B cells that lack expression of IgD and CD27. In particular, the double-negative 2 (DN2) subset of double-negative B cells, which exhibit a T-box transcription factor 21 (TBX21, or T-bet)+CD11c+ C–X–C motif chemokine receptor 5 (CXCR5)−CD21− phenotype, produce autoreactive antibodies, and correlate with more aggressive forms of systemic lupus erythematous and other conditions24,25. Also of keen interest in autoimmunity are memory PCs that reside in inflamed tissues with support from cytokines such as IL-6, IL-12, tumour necrosis factor (TNF) and type 1 interferon and can induce chronic immunopathology in mouse models26. Finally, Breg cells have been described to have a role in autoimmunity, transplantation and infection. Breg cells are more difficult to define than Treg cells, as they lack a lineage marker analogous to the Treg cell marker forkhead box protein P3 (FOXP3) and they can arise from any B cell developmental stage27 under the influence of BCR engagement28, Toll-like receptor (TLR)–CD40 co-stimulation29,30 or cytokine exposure31–36. Breg cells are mainly defined by their effector molecules, chief among these being IL-10 as well as IL-35, tumour growth factor-β (TGFβ) and granzyme B (GZMB)36,37. Breg cells exert inhibitory effects against CD4+ and CD8+ T cells, dendritic cells and monocytes, while supporting Treg cells and invariant natural killer T cells36.
CD40.
A molecule central to B cell activation; upon binding to its ligand expressed by activated T cells, CD40 promotes B cell survival and proliferation, germinal centre formation and development of memory B cells and long-lived plasma cells (PCs).
Early studies in human cancer revealed that TIL-B phenotypes include naive, activated and memory B cells, germinal centre B cells, and PCs and their intermediates38. Some studies have described atypical CD27− memory B cells among TIL-Bs39–42, which could correspond to the double-negative B cells described above. Moreover, an exhausted TIL-B phenotype (CD69+CD27−CD21−) has been described in association with Treg cells in patients with lung cancer43. Recent scRNA-seq studies of tumour tissues from patients report anywhere from 2 to 13 TIL-B phenotypes spanning the entire continuum from naive B cells to PCs42,44–57. In general, the TIL-B phenotypes described so far align with canonical B cell subsets identified in healthy donors (FIG. 2a), although TIL-Bs appear to exhibit greater diversity within some of these subsets44,47. By scRNA-seq, the most heterogeneous TIL-B subsets are memory B cells, which generally cluster according to their class switch and CD27 status44, and PCs, which cluster according to immunoglobulin isotype46,51. Germinal centre B cells (presumably derived from TLSs) may form clusters corresponding to light, transitional and dark zone phenotypes47.
Breg cells have been described in human tumours and, similar to what has been observed in autoimmunity, can arise from memory B cells58, plasmablasts59 and plasmocytes60,61. Notably, Breg cells have been reported in only two scRNA-seq studies so far44,54. This could, in part, reflect a bioinformatic issue, as IL10, GZMB and PDCD1-expressing TIL-Bs were identified in breast cancer but did not form a distinct phenotypic cluster44. It is essential to define more robust markers of Breg cells in human cancer so that their properties and therapeutic vulnerabilities can be deciphered.
Various factors can influence the phenotype of TIL-Bs, including disease stage and tumour type (FIG. 1). For example, a transition from naive to PC-like B cells was observed in more advanced lung cancers48, which underscores the need to account for stage as a confounding factor in prognostic studies. TIL-B phenotypes can differ between spontaneous and transplanted models of the same cancer62, an important consideration for murine studies. The antigenic landscape is another important factor. For example, TLSs and germinal centre B cells are enriched in HPV+ versus HPV− head and neck cancers47,55, and an increased tumour mutation burden enhanced both B cell and PC infiltrates in mouse breast cancer models63. The importance of cell–cell interactions was illustrated by a study using a cancer mouse model in which tumour-associated neutrophils induced the recruitment and differentiation of B cells to PCs via cytokines such as TNF and TNF ligand superfamily member 13B (TNFSF13B, or BAFF)64. Finally, Breg cell differentiation has been linked to interaction with semi-mature dendritic cells in the tumour microenvironment (TME) of hepatocellular carcinoma65, TLR2 stimulation by tumour-derived autophagosomes in breast, lung and ovarian adenocarcinomas66, tumour-derived leukotriene B4 in breast cancer67 and IL-21 in the case of GZMB+ Breg cells in breast, cervical, ovarian and colorectal carcinomas68.
The increasing understanding of TIL-B phenotypes provided by scRNA-seq is beginning to translate into prognostic studies that go beyond simple markers such as CD2042,44,47,48,51,52,54. For example, a scRNA-seq study of tumour tissue from patients with nasopharyngeal carcinoma led to the identification of double-negative B cells and PCs that carried negative and positive associations with survival, respectively42. In contrast, a colorectal cancer scRNA-seq study identified an IgA+IGLC2+ PC subset that correlated with poor prognosis in The Cancer Genome Atlas (TCGA) cohort51 and may correspond to a previously described immunosuppressive IgA+ cell subset60,61. Additional studies involving other tumour types are required to elucidate the contributions of newly discovered TIL-B phenotypes to antitumour immunity and clinical outcomes.
TIL-B neighbourhoods
Similar to tumour-infiltrating T cells, TIL-Bs are found in at least three different structural zones of the TME: highly organized TLSs, which resemble lymph nodes; less organized stromal infiltrates involving TIL-Bs, T cells and macrophages, which we refer to here as lympho-myeloid aggregates (LMAs); and intra-epithelial infiltrates, where TIL-Bs, T cells and macrophages come into direct contact with tumour cells (FIG. 3). We will discuss what is known about TIL-Bs in each of these compartments, emphasizing the major gaps and controversies in our understanding.
Fig. 3 |. TIL-B neighbourhoods.
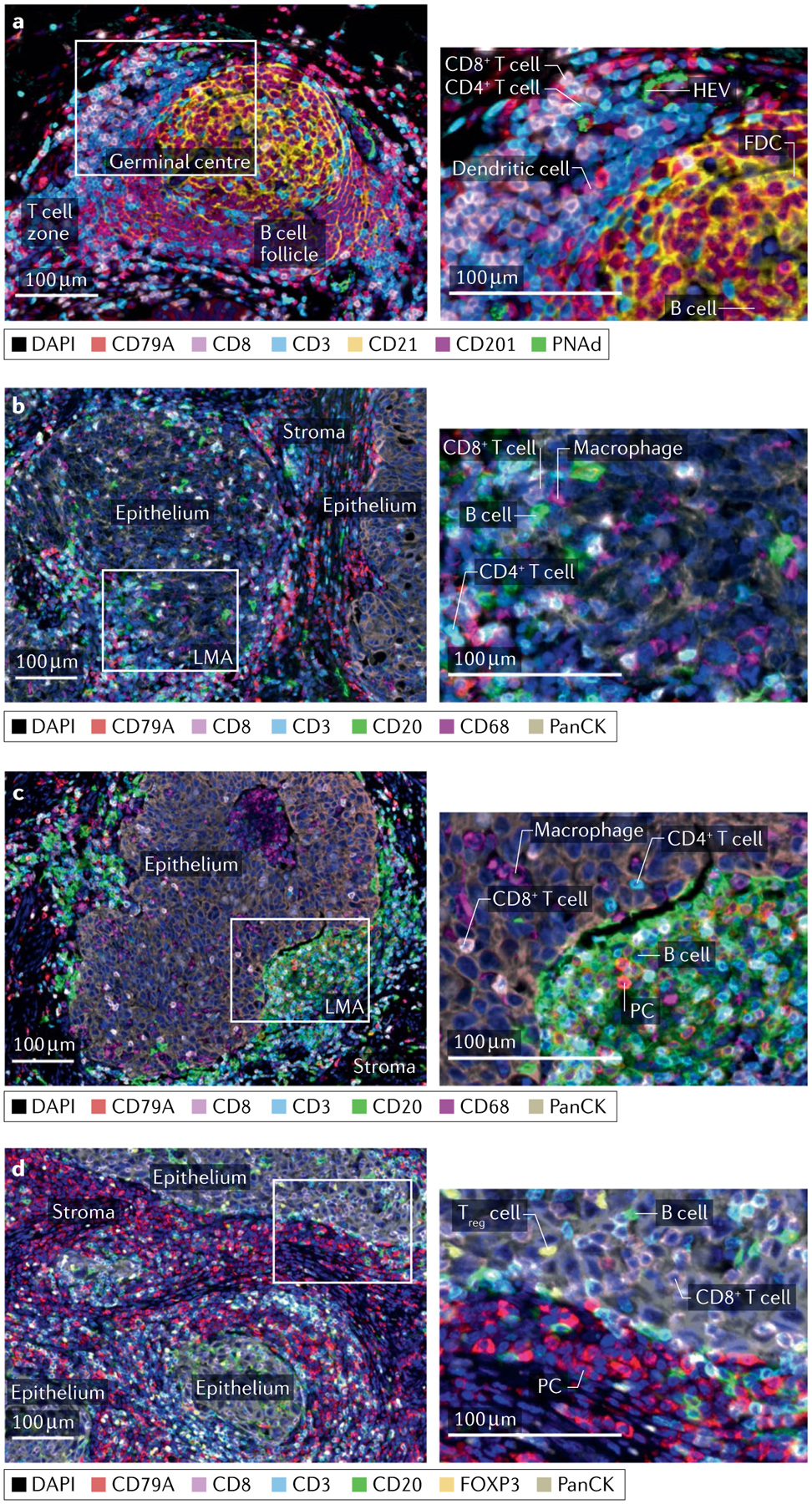
a–d | Multiplex immunofluorescence staining of untreated human high-grade serous ovarian cancer (parts a–c) and medullary breast cancer tissues (part d) showing examples of a secondary follicular tertiary lymphoid structure (part a), astromal lympho-myeloid aggregate (LMA) with infiltration of a neighbouring epithelial region by CD4 and CD8 T cells and B cells (part b), a dense infiltrate of B cells restricted to the stromal compartment despite infiltration of adjacent epithelium by T cells and macrophages (part c) and a dense stromal infiltrate of plasma cells (PCs) accompanied by intra-epithelial B cells (part d). Left column: low-magnification views with relevant structural zones labelled. Right column: high-magnification views of boxed regions from left column showing examples of CD8 T cell (CD8+CD3+), presumptive CD4 T cell (CD8−CD3+), dendritic cell (CD201+), follicular dendritic cell (FDC; CD21+), high endothelial venule (HEV; PNAd+), B cell (CD20+), PC (CD20−CD79A+), macrophage (CD68+) and regulatory T cells (Treg cells; CD3+FOXP3+). Markers and associated colours specified below each image. Epithelium, tumour epithelium; FOXP3, forkhead box protein P3; PanCK, pan cytokeratin; PNAd, peripheral node addressin; stroma, tumour stroma; TIL-B, tumour-infiltrating B lymphocyte. Image courtesy of Katy Milne, Tashi Rastogi and Karanvir Singh, BC Cancer.
Tertiary lymphoid structures
TLSs are lymph node-like structures that arise de novo in the stroma of hot tumours in response to antigens and inflammatory stimuli4,69. Although the term TLS is used by some to describe any lymphoid aggregate, we recommend a stricter definition that, at a minimum, includes clearly demarcated B cell follicles containing CD21+ follicular dendritic cells (FDCs) and adjacent T cell zones containing conventional dendritic cells and high endothelial venules (HEVs) (FIG. 3a). In some TLSs, B cell follicles contain germinal centres defined by features (and markers) such as activated FDCs (CD21, CD23), B cells undergoing proliferation and somatic hypermutation (Ki67, B cell lymphoma 6 protein (BCL6), activation-induced cytidine deaminase (AICDA)), TFH cells (CD4, BCL6, inducible T cell co-stimulatory (ICOS), PD-1), plasmablasts and PCs4. In a recent breast cancer study, the presence of germinal centres was associated with a favourable balance of TFH cells to follicular Treg cells in TLSs70. Analogous to lymph nodes, TLSs without versus with germinal centres have been referred to as primary follicular TLSs and secondary follicular TLSs, respectively71.
Despite many similarities, TLSs show structural differences from lymph nodes that may have significant implications for TIL-B responses. TLSs lack a capsule, which likely results in greater exposure to immunoregulatory factors and apoptotic and/or necrotic debris from the TME72. The non-capsular structure and proximal location of TLSs may facilitate the uptake and presentation of antigens that are at low abundance or highly context-dependent (for example, post-translationally modified antigens). These features may also afford better access to tissue-specific self-antigens by B cells and other TLS-associated antigen-presenting cells (APCs), thereby facilitating the maintenance of self-tolerance, as discussed below. Similarly, TLSs lack subcapsular sinus macrophages, which play an integral role in antigen presentation to B cells in lymph nodes. It is unclear whether tumour-associated TLSs contain the well-structured conduits that transport soluble antigens in lymph nodes73 or the stromal cell networks that support B cell and T cell interactions, including CXCL12-producing reticular cells in germinal centres and fibroblastic reticular cells in T cell zones74. Indeed, tumour-associated germinal centres typically lack well-defined dark and light zones, which in normal lymph nodes are integral to the process of BCR affinity maturation. Thus, it seems reasonable to speculate that, compared with lymph nodes, TLSs may generate TIL-B responses with distinct antigen specificities, as discussed next.
In addition to their well-known capacity to generate high-affinity antibodies against foreign antigens, germinal centres play an important role in mitigating the dual risks that somatic hypermutation and affinity maturation pose to self-tolerance: new BCR mutations can inadvertently confer autoreactivity, and B cells that are autoreactive at baseline can become activated and undergo somatic hypermutation during the process of clonal redemption10,11.To maintain tolerance, lymph nodes and germinal centres in healthy tissues have elaborate mechanisms to detect, inhibit, edit and delete autoreactive B cells (FIG. 2b). Critical to reducing the presence and/or activity of autoreactive B cells are tightly regulated interactions between B cells, TFH cells and follicular Treg cells, with disruptions in any one being sufficient to promote autoantibody production75. Of particular relevance to patients with cancer, the PD-1 and cytotoxic T lymphocyte-associated protein 4 (CTLA4) pathways are also integral to this process75. We know very little about how effectively these sophisticated functions are performed by tumour-associated TLSs, where the difference between self and non-self can be as subtle as a single amino acid change, a post-translational modification or, simply, overexpression. On the positive side, TLSs could be a place where self-tolerance mechanisms are sufficiently relaxed to allow the production of autoreactive antibodies that counteract the effects of immune editing of neoantigens and other tumour-specific antigens (FIG. 4). On the negative side, TLSs could give rise to autoreactive B cells and PCs that underlie the autoimmune-mediated toxicities associated with immune checkpoint blockade and, in rare instances, paraneoplastic syndromes76. Indeed, an association between TLSs and paraneoplastic syndromes has been reported in ovarian cancer77. These risks need to be considered when developing immunotherapies aimed at enhancing TLSs.
Fig. 4 |. Model for intramolecular epitope spreading from neo-epitopes to self-epitopes in cancer.

Top panel: a–h | Antigen released by tumour cells (part a) is taken up by professional antigen presenting cells (APCs) (part b) which then prime neo-epitope-specific CD4 T follicular helper (TFH) cells and/or T peripheral helper (TPH) cells as well as CD8 T cells. TFH cells and/or TPH cells prime a B cell response against a self-epitope on the same antigen (part c), triggering clonal expansion and plasma cell (PC) differentiation (part d). PC-derived autoantibodies form immune complexes with antigen, which facilitates antigen uptake and presentation by APCs (part e). Additional self-reactive CD4 and CD8 T cells undergo priming, expansion and differentiation (part f). TFH cells and/or TPH cells prime and amplify self-reactive B cells (part g), leading to further epitope spreading (part h). Bottom panel: neo-epitope-specific CD8 T cell (cell 1) eliminates neoantigen-expressing tumour cells. Without epitope spreading, this can result in outgrowth of variant tumour cells that no longer express the neoantigen. With epitope spreading, CD4 and CD8 T cells (cell 2, 3 and 4) can additionally target self-epitopes which may drive responses less susceptible to immune editing. Moreover, orthogonal antibody-mediated effector mechanisms can be engaged, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), resulting in better tumour control. BCR, B cell receptor; MHC, major histocompatibility complex; TCR, T cell receptor.
Somatic hypermutation.
A process by which antigen-activated B cells acquire somatic mutations in their B cell receptor (BCR) sequence, thereby leading to receptor diversification.
Affinity maturation.
A process by which the diverse repertoire of B cell receptors (BCRs) generated by somatic hypermutation is screened to select clones with the highest affinity for their cognate antigen.
Lympho-myeloid aggregates
As mentioned, we propose the generic term ‘lympho-myeloid aggregates’ to describe the various non-follicular aggregates of TIL-Bs, T cells and myeloid cells found in tumours, anticipating that future studies will reveal LMA subtypes with distinct functions (FIG. 3b,c). LMAs are invariably found in TLS-positive tumours, yet they have an even broader distribution that includes virtually all immunologically hot tumours78. The following are examples of known or anticipated LMA subclasses that warrant consideration.
Early, immature, stalled or contracting TLSs.
Undoubtedly, some of the LMAs found in tumours correspond to the precursors of bona fide TLSs. Furthermore, as TLSs are transient, antigen-dependent structures73, they presumably undergo a contraction phase if their cognate antigen(s) disappears from the TME. Although mouse models involving experimentally induced TLSs have provided useful insights into these stages73, the field is in need of spontaneous, orthotopic tumour models that engender natural TLS responses, as seen in human cancers.
Extrafollicular response zones.
B cell activation, differentiation and affinity maturation can also occur in so-called extrafollicular responses that take place outside germinal centres in secondary lymphoid organs or inflamed peripheral tissues72,79. In place of TFH cells, extrafollicular responses in inflamed tissues involve a CD4+ T cell subset known as T peripheral helper cells (TPH)80. Both TFH cells and TPH cells are PD-1high and express CXCL13 and IL-21; however, TPH cells are generally negative for BCL6 and CXCR5 (REF.81). Notably, these definitions are fluid, as our understanding of non-canonical TFH-like cells continues to grow82. In autoimmunity, extrafollicular responses have been shown to involve activated B cells, plasmablasts and double-negative B cells alongside TPH cells12,83,84. Although extrafollicular responses have not been formally described in cancer, TIL-Bs were found to associate with CXCL13-producing CD4+ T cells with a PD-1highT-bethighBCL6lowCXCR5− phenotype in putative early TLSs in breast cancer85, and similar findings were recently reported in nasopharyngeal carcinoma86.
Secondary lymphoid organs.
Peripheral organs, such as the spleen and lymph nodes, that provide the optimal environment to generate and quench adaptive immune responses.
Antitumour effector zones.
Although antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) are widely viewed as important effector mechanisms for TIL-Bs, the architectural setting for these activities remains poorly defined in cancer. In human ovarian cancer, large stromal LMAs have been described in which TIL-Bs, T cells and macrophages congregate and intermix. These are sites of intense PD-L1 expression by macrophages and neighbouring tumour cells, suggesting high T cell activity and associated interferon-γ (IFNγ) production. Given the strong, favourable prognostic significance of these PD-L1+ zones87, they are compelling candidates for sites of ADCC and/or ADCP fuelled by TIL-B-derived antibodies.
Antibody-dependent cellular cytotoxicity.
(ADCC). A process by which Fc receptor-expressing effector cells, such as natural killer cells and macrophages, recognize antibody-coated cells and orchestrate their destruction through the release of cytotoxic granules.
Antibody-dependent cellular phagocytosis.
(ADCP). A process by which Fc receptor-expressing phagocytes, typically macrophages, recognize antibody-coated cells and destroy them by engulfment.
Plasma cell-rich zones.
In very hot tumours, PCs can dominate the stromal compartment, replacing cancer-associated fibroblasts and other stromal components (FIG. 3d). This is well illustrated in patients with medullary breast cancer, where dense stromal PCs have been shown to produce tumour-reactive antibodies and are associated with an exceptionally favourable prognosis88.
Intra-epithelial B cells
Finally, B cells expressing markers such as CD19 and CD20 can be found directly infiltrating tumour epithelium alongside T cells and macrophages (FIG. 3b), a pattern that is prognostically favourable in most cancers (FIG. 1; see Supplementary Table 2). Notably, intra-epithelial B cells are found in only a subset of hot tumours39,89, suggesting that they have unique entry cues or other requirements relative to T cells. The phenotype, clonality, antigen specificity and function of intra-epithelial B cells is unknown but may soon be revealed by methods such as spatial transcriptomics.
Initiation and progression of TIL-B neighbourhoods
From the foregoing, one can construct a working model of how different TIL-B neighbourhoods may arise and interrelate. The presence of T cells and myeloid cells in the TME appears to be a precondition for any form of TIL-Bs, as the latter are rarely seen without the former. Indeed, dysfunctional or exhausted T cells are known to produce CXCL13 (REFS1–3), which along with other factors may initiate the recruitment of activated B cells and PCs primed in secondary lymphoid organs. This would result in simple LMAs that may evolve to become sites of extrafollicular responses, further amplifying TIL-Bs and T cell responses. Progression to primary follicular TLS formation likely requires permissive stroma and a favourable cytokine milieu, whereas secondary follicular TLS formation may additionally require factors such as high antigen load (including neoantigens, cancer testis antigens and viral antigens) and a conducive somatic mutation profile69,90. Productive secondary follicular TLSs could ‘supercharge’ the TIL-B response by generating long-lived, inflammatory PCs that colonize tumour stroma and release affinity matured antibodies against tumour-associated, tissue-specific and/or self-antigens. A robust, local supply of antibody, in turn, would promote ADCC, ADCP and other effector mechanisms (discussed later), resulting in LMAs corresponding to antitumour effector zones. In line with this model, a recent study provides evidence that TLSs do promote in situ B cell maturation in the TME91. Applying spatial transcriptomics and immunohistochemistry to clear cell renal cell carcinoma, it was shown that, compared with TLS− tumours, TLS+ tumours present with a wider diversity of B cell phenotypes, increased somatic hypermutation and clonal expansion, pronounced infiltration of tumour stroma by plasma cells, and enhanced coating of tumour cells by IgG. BOX 1 discusses the prognostic facets of this model.
Box 1 |. Which lymphoid structures are the strongest drivers of prognosis?
Tertiary lymphoid structures (TLSs) have received considerable attention lately due to their generally favourable association with patient survival and response to both conventional and immune-based therapies4. However, TLSs, lympho-myeloid aggregates (LMAs) and intra-epithelial B cells are often found together, underscoring the importance of discerning their independent effects. In a pancreatic cancer study, B cells were only prognostically favourable when organized as TLSs201. Moreover, secondary follicular TLSs were found to have a stronger prognostic effect than primary follicular TLSs or LMAs in lung cancer, melanoma and pancreatic cancer153,202,203. Accordingly, in another melanoma study, favourable prognosis was associated with TLSs containing higher fractions of B cells undergoing somatic hypermutation and decreased fractions of CD21+CD20+ B cells204. Similarly, across several cancer types, secondary follicular TLSs were a better predictor of clinical response to PD-1–PD-L1 blockade than less mature TLSs, PD-L1 expression or CD8+ tumour-infiltrating cell (TIL) density205. A pre-eminent role for secondary follicular TLSs suggests that affinity matured, long-lived PCs may be the most important prognostic and predictive driver. Notably, however, in the autoimmunity and transplantation fields, the importance of secondary follicular TLSs remains unresolved206. In lupus nephritis, LMA-like infiltrates involving B cells and PCs are common and correlate with poor kidney outcome, whereas clearly organized ectopic germinal centres are only present in a minority (6%) of patients72. Furthermore, a tolerogenic role for TLSs in transplantation is suggested by their presence in long-surviving heart and kidney allografts, as well as the presence of Treg cells in many TLSs73. On reflection, one might question why TLSs alone should be a reliable prognostic indicator in any setting given that TLSs are sites where immune responses are initiated, not completed. One might predict that LMAs corresponding to ‘effector zones’, once better defined and objectively measured, will ultimately represent the most robust prognostic and predictive indicators.
Targets of TIL-B-derived antibodies
The landscape of antigens recognized by serum antibodies in patients with cancer has been interrogated extensively over the past 25 years using methods such as SEREX, phage display and protein arrays92–94, providing a foundation for understanding TIL-B-associated antibody responses. Serum antibodies have been shown to recognize tumour-associated proteins such as tumour protein p53 (REF.95) and cancer testis antigen 1B (CTAG1B; also known as NY-ESO-1)96,97; polypeptides arising from non-canonical reading frames98, frameshift mutations99 and endogenous retroviruses100; and post-translational modifications such as aberrantly glycosylated mucin 1 (MUC1)101. Despite examples such as these, the majority of serum antibody targets identified to date are non-mutated self-proteins, which can be nuclear, cytoplasmic, transmembrane or secreted6. Moreover, despite concerted efforts to identify serological antigens unique to specific cancers102, most antigens discovered to date have broad or ubiquitous expression patterns, suggesting that they arise from disrupted peripheral B cell tolerance mechanisms rather than conventional adaptive immune responses. p53 is informative in this regard, as anti-p53 antibodies have been found to primarily recognize wild type rather than mutant epitopes103, again suggesting the involvement of self-reactive B cells.
Relatively few antigens recognized by TIL-B-derived antibodies have been identified to date, as experimentally this requires either expanding TIL-Bs from fresh tumours and purifying the antibodies they secrete or molecular cloning of antibodies from TIL-Bs and producing recombinant versions for use in antigen discovery. To date, only 70 TIL-B target antigens have been identified, mainly in patient tissue samples from breast, lung and ovarian tumours (see TABLE 1 and Supplementary Table 3 for antigens, cloning methods and references). These antigens span a broad range of cellular compartments (that is, nuclear, cytoplasmic, extracellular) and molecular composition (for example, nucleic acids, lipids, proteins), although protein antigens have received the most study to date. Many correspond to known T cell antigens, including cancer testis antigens (NY-ESO-1, CT45, CT47), differentiation antigens (melanoma-associated antigen family members and MLANA), overexpressed antigens (MUC1) and mutated antigens (p53, breast cancer type 2 susceptibility protein (BRCA2)). Some are implicated in tumorigenesis (for example, p53, BRCA2, brain-derived neurotrophic factor (BDNF)), whereas others are broadly or ubiquitously expressed, such as heat shock and ribosomal proteins. In HPV-induced head and neck cancer, TIL-B-derived antibodies have been shown to recognize viral antigens (TABLE 1). To date, no studies have reported (or been designed to detect) neoantigen-specific TIL-B-derived antibodies. Thus, the view so far is that TIL-B responses predominantly involve self-antigens, which may reflect a methodological bias arising from the predominant use of wild-type protein libraries and arrays in TIL-B antigen identification efforts so far, a lower propensity for neoantigens to elicit antibody responses, and/or a higher propensity for cancers to disrupt peripheral tolerance mechanisms relevant to B cells (FIG. 2b). With regard to this last point, TIL-B responses to the self-protein matrix metalloproteinase 14 (MMP14) were recently shown to involve both germline-encoded and somatically hypermutated BCRs104.
Notably, even though serum and TIL-B antibody responses involve similar classes of antigen, they appear uncoupled. In ovarian cancer, autoantibodies to p53 and NY-ESO-1 showed no association with CD20+ TIL-Bs39 and persisted even after extensive tumour debulking, ruling out tumour-resident B cells and PCs as their main source. Similarly, in breast cancer, TIL-B-derived and serum-derived autoantibody responses were uncoupled for the majority of antigens105. These observations suggest that serum antibodies and TIL-B responses can arise and persist independently. As such, it is conceivable that serological responses, hosted primarily in tumour-draining lymph nodes and bone marrow rather than the TME, may represent the major source of antitumour antibodies.
Effector mechanisms used by TIL-Bs
Arguably, TIL-Bs have the most diverse set of effector mechanisms of all immune cells in the TME, enabling them to play central roles in both innate and adaptive responses (FIG. 5). We will review cell-based mechanisms first, followed by those mediated by antibodies.
Fig. 5 |. TIL-B effector mechanisms.
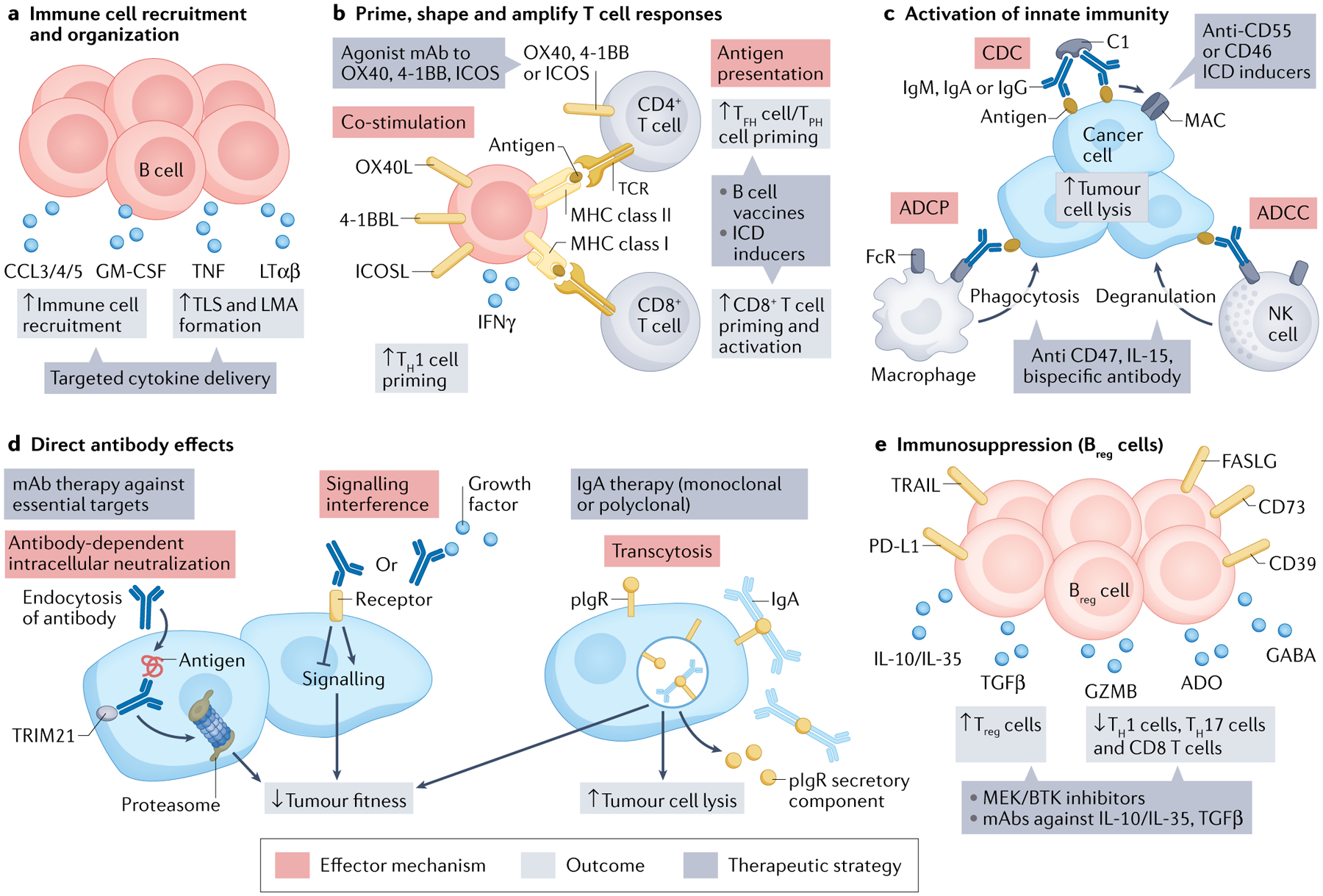
a–e | Five major categories of effector mechanism. ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; ADO, adenosine; 4–1BBL, 4–1BB ligand; CCL, C–C motif chemokine ligand; CDC, complement-dependent cytotoxicity; FASLG, FAS ligand; GABA, γ-aminobutyric acid; GZMB, granzyme B; ICD, immunogenic cell death; ICOS, inducible T cell co-stimulatory; ICOSL, ICOS ligand; IFNγ, interferon-γ; LMA, lympho-myeloid aggregate; LTαβ, lymphotoxin-αβ; mAb, monoclonal antibody; MAC, membrane attack complex; MHC, major histocompatibility complex; NK cell, natural killer cell; OX40L, OX40 ligand; PD-L1, programmed cell death 1 ligand 1; pIgR, polymeric immunoglobulin receptor; TCR, T cell receptor; TFH cell, T follicular helper cell; TGFβ, tumour growth factor-β; TH1 cell, T helper 1 cell; TIL-B, tumour-infiltrating B lymphocyte; TLS, tertiary lymphoid structure; TNF, tumour necrosis factor; TPH cell, T peripheral helper cell; TRAIL (or TNFSF10), TNF superfamily member 10; Treg cell, regulatory T cell; TRIM21, tripartite motif containing 21.
Cytokine production and lymphoid organization
Normal B cells produce various immunostimulatory cytokines, including IL-6, IFNγ, TNF, C–C motif chemokine ligand 3 (CCL3), IL-2 and colony-stimulating factor 2 (CSF2; also known as GM-CSF)106. Breast cancer-derived TIL-Bs were found to express elevated levels of IFNG, TNFA and IL4 transcripts, as well as elevated transcript levels of the immunosuppressive cytokines IL-10 and TGFβ107. In metastatic ovarian tumours, TIL-Bs secreted GM-CSF, IFNγ, IL-12p40, CXCL10 and IL-7, which can stimulate macrophages, dendritic cells, T cells and natural killer cells41. By scRNA-seq, plasmablast-like TIL-Bs from patients with ovarian cancer and melanoma expressed higher levels of transcripts encoding IFNγ and chemokines that attract T cells, macrophages and natural killer cells (for example, CCL3, CCL4 and CCL5) compared with other TIL-B subsets, and indeed were associated with higher T cell infiltration50,57. Accordingly, anti-CD20-antibody-mediated B cell depletion in patients with melanoma led to reduced T cell and macrophage tumour infiltrates57. B cells can also contribute to TLS formation and maintenance through secretion of lymphotoxin α1β2106, a role that proved essential for reducing tumour growth in a mouse melanoma model108. Conversely, B cell-derived lymphotoxin promoted the outgrowth of androgen-independent prostate tumours in mice109, underscoring the context dependence of lymphotoxin signalling.
Antigen presentation and spreading
Along with dendritic cells and macrophages, B cells serve as professional APCs that can process and present major histocompatibility complex (MHC) class I and II epitopes to CD8+ and CD4+ T cells, allowing them to induce, shape and amplify T cell responses110 (FIG. 5b). Whereas dendritic cells and macrophages use phagocytosis, pinocytosis and Fc receptor-mediated endocytosis to sample antigens from their milieu, B cells use their surface immunoglobulin molecules to take up cognate antigen, even using physical extraction via repetitive pulling and, if necessary, lysosome-mediated enzymatic digestion to do so111,112. Consequently, antigen-specific B cells are more efficient than dendritic cells and macrophages at presenting low-abundance antigens to T cells113. Moreover, B cells are the only professional APCs capable of clonal expansion, enabling them to skew the immune response towards specific antigens in a manner unavailable to other APCs. Classic studies demonstrated that autoreactive B cells are sufficient to prime autoreactive CD4+ T cells114. Similarly, the APC function of B cells is critical in mouse models of autoimmune diseases115–118, human coeliac disease119 and multiple sclerosis120. TIL-Bs from human tumours have a phenotype consistent with an APC role, including expression of MHC class I and II43 and co-stimulatory molecules such as CD80, CD86 and ICOS ligand (ICOSL)39,54,121 (FIG. 5b). However, direct evidence that TIL-Bs present tumour antigens acquired in vivo has been provided by only one study to date, where CD69+CD21+CD27+ TIL-Bs isolated directly from human lung cancer samples were shown to stimulate autologous CD4+ TILs in vitro43. A recent murine study using model antigens showed evidence of antigen presentation by tumour-specific B cells, leading to enhanced TFH cell and CD8+ T cell responses against tumours122. Thus, further studies are warranted to elucidate this important aspect of TIL-B biology.
An APC role for TIL-Bs has fundamental implications for epitope spreading, a process in which an initial immune response to epitope A spreads to epitopes B, C and so on (FIG. 4). Epitope spreading is well documented in autoimmunity and is considered a desirable outcome of cancer immunotherapy, owing to its potential to mitigate the challenge of tumour heterogeneity. Indeed, epitope spreading from mutant to wild type antigens may underlie successful anti-PD-1 antibody therapy in melanoma123. B cells have an integral role in epitope spreading through their ability to acquire cognate antigen via surface immunoglobulin, and process and present associated MHC class II epitopes to CD4+ T cells. In turn, CD4+ T cells can provide help to B cells specific for other epitopes on the same antigen (intramolecular spreading) or on physically associated antigens (intermolecular spreading). In cancer, such a mechanism could enable an initial CD4+ T cell response to a mutated neoantigen to trigger B cell and T cell responses to wild type flanking epitopes, which could mitigate the effects of neoantigen loss through immune editing. Evidence supporting such a mechanism was obtained in the setting of scleroderma (an autoimmune connective tissue disease) with coincident malignancy, where patients were found to harbour both CD4+ T cells specific for a mutated autoantigen and serum autoantibodies to the wild type autoantigen124. Similar findings were reported for the Yo autoantigen in ovarian cancer77. Such a process may explain the favourable prognostic impact of certain autoimmune conditions in cancer125 and, in extreme cases, the development of the runaway autoimmune responses seen in paraneoplastic syndromes76.
Regulatory B cells
The most commonly reported Breg cell effector mechanism in human cancer is secretion of IL-10 (REF.126) (FIG. 5e). Although IL-10-expressing Breg cells are rare or absent in scRNA-seq datasets, IL-10+ Breg cells have been detected by immunohistochemistry or flow cytometry in various human cancers127–129 and after anti-PD-1 antibody treatment in patients with hepatocellular carcinoma130. Most studies report a positive correlation between Breg cells and Treg cells, suggesting interaction between these subsets. IL-35 is another key regulatory cytokine produced by Breg cells, as shown in a mouse pancreatic cancer model and further supported by bioinformatic analysis performed on human tumour data from TCGA131. GZMB+ Breg cells have been detected in multiple human cancers and can inhibit T cell proliferation in vitro68. Paradoxically, however, GZMB expression is also associated with ‘killer B cells’, which can have a cytolytic effect against tumour cells in vitro132, similar to Fas ligand (FASLG)+ and TNF superfamily member 10 (TNFSF10; also known as TRAIL)+ B cells133,134. Finally, other potential Breg cell effector molecules include adenosine (ADO)135, TGFβ128 and γ-aminobutyric acid (GABA)136, although more studies are needed in human cancer.
Antibody-dependent cell-mediated phagocytosis
ADCP is mediated by myeloid cells upon Fc receptor-mediated recognition of antibody–antigen complexes, leading to phagocytosis of the target cell. ADCP has garnered increased attention lately due to the arrival of therapeutic antibodies that block the CD47-signal regulatory protein-α (SIRPα) ‘don’t eat me’ pathway137. Although very few studies have investigated the induction of ADCP by TIL-B-derived antibodies (FIG. 5c), Biswas et al. recently described a monoclonal IgA against tetraspanin 7, which in association with CD351+ myeloid cells mediated an antitumour effect in an ovarian cancer mouse model138.
Antibody-dependent cell-mediated cytotoxicity
ADCC is mediated by natural killer cells and myeloid cells upon Fc receptor-mediated recognition of antibody–antigen complexes, leading to target cell lysis. Although natural killer cells and myeloid cells are abundant in multiple cancer types139 and therapeutic antibodies such as rituximab can induce ADCC140, there is no direct evidence to date of ADCC induction by TIL-B-derived antibodies (FIG. 5c). Nonetheless, in a bioinformatic analysis of TCGA data, B cell signatures were positively associated with expression of GZMB and Fc fragment of IgG receptor IIIa (FCGR3A), suggesting natural killer cell-mediated ADCC141. Moreover, around 20% of tumours showed increased expression of genes encoding metalloproteinases that cleave the natural killer cell-stimulating ligands MHC class I polypeptide-related sequence A and B141, consistent with a previously described mechanism of natural killer cell evasion142.
Complement-based mechanisms
The complement cascade can be triggered by the classical, lectin or alternative pathways, culminating in the formation of the membrane attack complex (MAC) and target cell lysis143. In general, complement-dependent cytotoxicity (CDC) is a difficult mechanism to profile in tumour tissues, as it involves more than 30 protein components that are regulated primarily through post-translational mechanisms. Nonetheless, the finding that most cancer types express high levels of complement inhibitory receptors, such as CD46, CD55 and CD59, strongly implicates CDC as important in human cancer143 (FIG. 5c). Indeed, neoadjuvant chemotherapy in patients with breast cancer induced an ICOSL+ B cell population via the complement–complement C3d receptor 2 axis, which correlated with an increased T effector cell to Treg cell ratio and tumour clearance54. This effect was antagonized by tumour cells expressing the complement inhibitory receptor CD55. By contrast, in clear cell renal cell carcinoma, classical pathway activation (as evidenced by co-localization of C1q and IgG on tumour cells) was associated with CD163+ M2 macrophages, T cell exhaustion and poor prognosis144, in accord with the immunosuppressive properties of C1q (REF.145). Similarly, IgM-dependent activation of the classical pathway was reported in human lung cancer, and was shown to inhibit T cell responses in a corresponding mouse model146.
Whereas ADCP, ADCC and CDC are generally directed towards cell surface antigens, a significant proportion of TIL-B-derived antibodies recognize intracellular antigens (TABLE 1; see Supplementary Table 3), raising the question of how such responses might contribute to antitumour immunity. The following effector mechanisms, although understudied in cancer so far, represent potential means by which TIL-Bs could impact both extracellular and intracellular processes in cancer cells, representing a new avenue that warrants further study.
Antibody-dependent intracellular neutralization
Antibody-dependent intracellular neutralization is a process by which antibodies induce the proteasomal degradation of intracellular proteins147,148. Although antibody-dependent intracellular neutralization has been studied primarily in the context of viral infection, TIL-B-derived IgG from lung cancer was shown to promote degradation of the tumour protein Ras homologue family member C by a mechanism resembling antibody-dependent intracellular neutralization48 (FIG. 5d).
Antibody-mediated signalling interference
Upon binding to their cognate antigen, antibodies can modulate signalling pathways that influence tumour growth or behaviour (FIG. 5d). For example, in a mouse model, IgG against glycosylated heat shock protein A4 induced CXCR4 expression on tumour cells which facilitated lymph node metastasis149. Moreover, TIL-Bs from a patient with ovarian cancer were found to produce antibody that antagonized the tumour-promoting effects of BDNF138.
Transcytosis
The above-mentioned study also introduced IgA transcytosis as a new TIL-B-mediated antitumour mechanism138 (FIG. 5d). Mucosal epithelial cells express the polymeric immunoglobulin receptor (pIgR) on their surface, which binds to PC-derived IgAs and is internalized. This internalized complex is then transported from the basolateral to the luminal space. Remarkably, in vitro exposure of pIgR+ ovarian tumour lines to IgA (either tumour antigen-specific or nonspecific) inhibited oncogenic signalling and promoted T cell-mediated killing by a pIgR-dependent mechanism. Similarly, IgA infusions slowed the growth of pIgR+ human ovarian tumours engrafted in mice138, suggesting a provocative new therapeutic concept with potential relevance to many human cancers.
Therapeutic manipulation of TIL-Bs
Few cancer therapies have been designed to intentionally induce TIL-B responses; however, our evolving understanding of the myriad cellular and antibody-based mechanisms used by TIL-Bs offers many exciting opportunities (FIG. 5). Below, we provide an overview of recent insights and advances.
Chemotherapy and targeted agents
Baseline TIL-B density is positively associated with response to chemotherapy in humans and mice54,63. Furthermore, chemotherapy can further increase TIL-B density41,150 and expression of co-stimulatory molecules54,151, as well as induce TLSs152. Notably, the use of corticosteroids during chemotherapy can be detrimental to germinal centre formation in TLSs153. Compared with cytotoxic chemotherapy, targeted agents may interact differently with TIL-Bs, as a negative association between TIL-Bs and response to BRAF and MEK inhibitors was seen in patients with melanoma154. STING agonists can promote the formation of immature TLSs in a mouse melanoma model and may prove useful in combination with agents that induce TLS maturation155. Finally, a triple regimen involving low-dose chemotherapy, oncolytic virus and dual checkpoint blockade led to enhanced TIL-Bs and increased tumour regression in a breast cancer mouse model156. Building on these early studies, further work is warranted to identify optimal strategies for using chemotherapeutic and targeted agents to amplify TIL-B responses.
Immune checkpoint blockade
The presence of PD-1+ and PD-L1+ TIL-Bs has been reported in multiple human cancers130,157–159, providing an initial suggestion that TIL-Bs may be directly influenced by PD-1 and/or PD-L1 targeting immune checkpoint blockade; analogous data for CTLA4 expression have not yet been reported. Substantial evidence is accumulating for a strong, positive effect of anti-CTLA4 and anti-PD-1 antibody blockade on TIL-Bs and TLSs, reflecting the aforementioned roles played by these pathways in regulating interactions between B cells, TFH cells and follicular Treg cells. Baseline TIL-B densities predict response to PD-1 and CTLA4 blockade in patients with multiple cancers89,160–162 and, indeed, are augmented by immune checkpoint blockade160,161,163. In patients with non-small-cell lung cancer treated with neoadjuvant anti-PD-1 antibody, regressing tumours were associated with dense tumour immune infiltrates, including the presence of PCs and TLSs, whereas unstructured lymphoid aggregates in tumours showed no association with response164. In patients with urothelial cancer, the presence of stromal B cells and immature TLSs in tumours was associated with a lack of response to combined anti-CTLA4 and anti-PD-1 antibody treatment, yet mature TLSs were seen in responding tumours after treatment165, suggesting a substantial, favourable transformation of the TIL-B response.
Preclinical models provide direct mechanistic evidence for a favourable influence of immune checkpoint blockade on TIL-Bs and TLSs. B cells were required for response to CTLA4 and PD-1 blockade in a mouse breast cancer model63, and addition of an anti-PD-L1 antibody to radiation therapy converted a Breg cell response to an effector B cell response associated with improved tumour control in a squamous cell carcinoma model166. Similarly, in a mouse melanoma model, immune checkpoint blockade led to quantitative and qualitative improvements in TLSs and increased efficacy in a melanoma model108. Finally, in a lung cancer model, anti-PD-1 antibody treatment increased the number of TLSs and the production of IgG by TLSs, along with improved tumour control167. Thus, immune checkpoint blockade provides a powerful means to enhance both T cell and B cell responses in cancer.
Agonistic anti-CD40 antibodies
Agonistic anti-CD40 antibodies are under clinical evaluation for several cancers168 and are expected to strongly influence TIL-B responses, given the central role of CD40 in B cell biology169. Indeed, in a mesothelioma model, anti-CD40 antibody treatment enhanced TIL-B density and B cell-dependent tumour control170. In mouse models and patients with cancer, anti-CD40 antibody treatment induced a sharp decrease in the levels of circulating B cells, while increasing expression of co-stimulatory molecules, possibly reflecting activation-induced extravasation from blood171,172. In a glioma model, anti-CD40 antibody treatment induced TLSs and other lymphoid aggregates, but these were associated with hypofunctional T cells, suppressive CD11b+ B cells and impaired response to immune checkpoint blockade173. Thus, further work is required to determine how best to modulate the CD40 pathway in favour of TIL-B responses.
Cytokine therapy
Cytokines such as IL-2, IL-15 and IL-21 not only support the differentiation and proliferation of T cells and natural killer cells but also promote B cell development and isotype switching174. Indeed, IL-21 was required for curative responses to immune checkpoint therapy in breast cancer models63. Although IL-2 is commonly used to stimulate cytolytic T cells, it inhibits TFH cell differentiation and promotes Treg cell responses, and hence may be detrimental to TIL-B responses175. Other cytokines that could potentially be deployed to promote TIL-B responses and TLS formation include BAFF (a B cell maturation and survival factor) and CXCL13 (a B cell chemoattractant), both of which are generally prognostically favourable in human cancer176,177. Notably, however, CXCL13 can also mediate pro-tumour effects177. Finally, a drug targeting TNFSF14 to tumour vessels through linkage to a vascular targeting peptide induced TLS formation and normalized tumour vasculature, leading to enhanced T cell infiltration and therapeutic synergy with a tumour vaccine178. Thus, cytokines offer several promising avenues for promoting TIL-B responses, although as with all cytokine therapies, the challenge is to develop strategies that specifically enhance antitumour immunity while avoiding unwanted immunological effects.
Vaccines
Various cancer vaccines have been shown to induce TLS formation in patients, including a heterologous DNA viral vaccine against HPV antigens in cervical intra-epithelial neoplasia179 and an allogeneic tumour cell-based vaccine in pancreatic cancer180. Furthermore, antigen-loaded, CD40-activated B cells are being investigated as cell-based vaccines and proved equivalent to dendritic cells in models of melanoma and lymphoma181; this approach was further enhanced by co-transfer of tumour antigen-specific PCs182. Recently, TNFSF9+ (also known as 4–1BB ligand, 4–1BBL) B cells stimulated with CD40 agonist and IFNγ showed impressive efficacy in combination with radiation, CD8+ T cells and PD-L1 blockade in a glioblastoma model when compared with the same combination minus stimulated B cells183. There are no reports so far about B cell or antibody responses to personalized neoantigen-targeting vaccines184, but these may be expected based on known mechanisms of antigen presentation and epitope spreading (FIG. 4).
Treg cell and Breg cell depletion
Given the essential role of Treg cells in preventing systemic autoantibody responses8,12, Treg cell depletion is a compelling approach to enhance TIL-B responses. Indeed, in a lung cancer model, Treg cell depletion led to expansion of TLSs that supported improved T cell proliferation and dendritic cell function, leading to tumour destruction185. Although Breg cell depletion is challenging given the lack of unique markers, Breg cell differentiation and function can be impacted by certain chemotherapeutic agents54,166,186 and inhibitors of STAT3 (REF.187), MEK188 and BTK189. Furthermore, Treg cell and Breg cell activity can potentially be inhibited by antagonism of IL-10, IL-35, TGFβ and other suppressive factors.
Antibody-related therapies
Finally, there are several approaches to leverage the antibody-mediated effects of TIL-Bs. Transformative methods such as scRNA-seq enable high-throughput cloning of TIL-B-derived antibodies, so the challenge now is to identify which antibodies and cognate antigens are best suited for safe and effective therapeutic targeting, as recently reviewed93. An important consideration is that some antibodies can have pro-tumour effects149 and carry the risk of autoimmune sequelae76. That said, high-affinity, autoreactive antibodies do not necessarily induce unacceptable immunopathology, as demonstrated by the use of autoreactive, broadly neutralizing antibodies against HIV-1 (REF.9). Once antibodies with appropriate specificities are identified, they can be further engineered or used in combination therapies to enhance desired antitumour properties190. Of particular relevance to TIL-B effector mechanisms, Heemsherk et al. recently described a bispecific antibody that induced tumour regression in vivo by triggering neutrophil-mediated cytotoxicity, ADCC and ADCP191. Other promising approaches include CD47 blockade to promote ADCP192 or blockade of CD55 or CD46 to promote complement-mediated killing mechanisms193.
Conclusion
There is now abundant evidence that TIL-Bs and the antibodies they produce are prominent features of the immune response to human cancer. TIL-Bs are found alongside T cells, natural killer cells, myeloid cells and others in the TMEs of the ‘hottest’ tumours and show hallmarks of active antigen recognition and diverse effector functions. They carry strong prognostic and predictive significance in many indications and treatment scenarios, including CTLA4-directed and PD-1-directed immune checkpoint blockade. Moreover, their numbers and functional attributes can be modulated by standard treatments, immune checkpoint blockade, therapeutic vaccines and various other experimental approaches. Beginning with pioneering studies two decades ago, the target antigens of TIL-Bs are being elucidated, so far revealing a preponderance of self-proteins with varying degrees of tumour-specific features. In this and other ways, TIL-Bs share many features with the pathogenic B cells and PCs that mediate autoimmune responses. If TIL-B specificities are indeed skewed towards self-reactivity, one can envision this providing an effective means for the immune system to contend with intratumoural heterogeneity through the recognition of non-mutated antigens with more ‘truncal’ expression patterns within the tumour phylogeny.
Having garnered intense interest in the past few years, the TIL-B field faces exciting new challenges. Most, if not all, TIL-B effector functions depend on the nature of their cognate antigens; for example, ADCC requires surface antigens, whereas signal interference requires antigens that drive tumour cell fitness. Therefore, to fully understand the mechanisms of action of TIL-Bs, we require a broader, unbiased view of their antigenic landscape, which will require the use of multiple, orthogonal discovery methods. scRNA-seq is enabling the first mapping of TIL-B clonotypes to phenotypes, ushering in an era when recombinant antibodies from TIL-B phenotypes of interest can be readily produced and used to probe antigen specificities and effector mechanisms on an unprecedented scale. In parallel, we need to decipher and more precisely describe the various cellular neighbourhoods in which TIL-Bs are found, moving beyond TLSs to include the other fascinating structures revealed by multiplex immunohistological methods. To this end, spatial transcriptomics with matched BCR and TCR sequencing data promise to be transformative. Finally, we need to leverage insights from the above endeavours to design a new generation of immunotherapies purpose-built to leverage TIL-B effector mechanisms in concert with T cells and other immune cells. Again, the autoimmunity field provides rich examples of powerful, multipronged, tissue-restricted and unrelenting immune responses against many of the same peripheral tissues that challenge us as malignancies. To develop this next generation of immunotherapies, new preclinical models are urgently needed that recapitulate the complex cellular ecosystems in which TIL-Bs operate. Investment in the above objectives will pay dividends in the form of new target antigens, therapeutic antibodies and integrated immunotherapies that can contend with the extensive heterogeneity and unremitting evolution of metastatic cancers.
Supplementary Material
Acknowledgements
The authors thank K. Singh, T. Rastogi and K. Milne for technical assistance with multicolour immunofluorescence staining and image processing for Fig. 3. C.M.L. is supported by postdoctoral fellowships from Canadian Institutes of Health Research (CIHR) (Banting postdoctoral fellowships programme, 429161) and Michael Smith Foundation for Health Research (MSFHR) (RT-2020-0630). A.C.B. is supported by a Doctoral Award from CIHR (Frederick Banting and Charles Best Canada Graduate Scholarship, FBD — 177882). M.G. and D.P.H. were supported by funds from the National Institutes of Health (NIH P30 CA014195), 2021 Metavivor Early Career Investigator Award, and the San Diego Padres Pedal the Cause C3 Collaborative Translational Cancer Pilot Project Award.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Cancer thanks Claudia Jakubzick, who co-reviewed with Kavita Rawat, Shiv Pillai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41568-022-00466-1.
References
- 1.Thommen DS et al. A transcriptionally and functionally distinct PD-1+CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med 24, 994–1004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Workel HH et al. A transcriptionally distinct CXCL13+CD103+CD8+ T-cell population is associated with B-cell recruitment and neoantigen load in human cancer. Cancer Immunol. Res 7, 784–796 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Li H et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sautès-Fridman C, Petitprez F, Calderaro J & Fridman WH Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Jhunjhunwala S, Hammer C & Delamarre L Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Reuschenbach M, von Knebel Doeberitz M & Wentzensen N A systematic review of humoral immune responses against tumor antigens. Cancer Immunol. Immunother 58, 1535–1544 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardemann H et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Sng J et al. AIRE expression controls the peripheral selection of autoreactive B cells. Sci. Immunol 4, eaav6778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that defects in T cell central tolerance can markedly affect the B cell compartment, leading to autoantibody production.
- 9.Finney J, Watanabe A, Kelsoe G & Kuraoka M Minding the gap: the impact of B-cell tolerance on the microbial antibody repertoire. Immunol. Rev 292, 24–36 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnett DL, Reed JH, Christ D & Goodnow CC Clonal redemption and clonal anergy as mechanisms to balance B cell tolerance and immunity. Immunol. Rev 292, 61–75 (2019). [DOI] [PubMed] [Google Scholar]; This Review describes the concept of clonal redemption, in which anergic B cells can mutate away from self-reactivity during somatic hypermutation to produce antibodies against foreign antigens.
- 11.Brink R & Phan TG Self-reactive B cells in the germinal center reaction. Annu. Rev. Immunol 36, 339–360 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Meffre E & O’Connor KC Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunol. Rev 292, 90–101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang EY et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Qiu CC, Caricchio R & Gallucci S Triggers of autoimmunity: the role of bacterial infections in the extracellular exposure of lupus nuclear autoantigens. Front. Immunol 10, 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanz I et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front. Immunol 10, 1–17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This Review describes the core markers required to reproducibly distinguish the different phenotypic subsets of B cells.
- 16.Stewart A et al. Single-cell transcriptomic analyses define distinct peripheral B cell subsets and discrete development pathways. Front. Immunol 12, 602539 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass DR et al. An integrated multi-omic single-cell atlas of human B cell identity. Immunity 53, 217–232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work presents an atlas of peripheral B cell subsets isolated from normal lymphoid tissues from human participants.
- 18.Holmes AB et al. Single-cell analysis of germinal-center B cells informs on lymphoma cell of origin and outcome. J. Exp. Med 217, e20200483 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He S et al. Single-cell transcriptome profiling an adult human cell atlas of 15 major organs. Genome Biol. 21, 294 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allie SR & Randall TD Resident memory B cells. Viral Immunol 33, 282–293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisel NM, Weisel FJ, Farber DL & Borghesi LA Comprehensive analyses of B-cell compartments across the human body reveal novel subsets and a gut-resident memory phenotype. Blood 136, 2774–2785 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenkel JM & Masopust D Tissue-resident memory T cells. Immunity 41, 886–897 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allie SR et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol 20, 97–108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenks SA et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 49, 725–739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies DN2 B cells as predominant producers of autoantibodies in lupus.
- 25.Li Y, Li Z & Hu F Double-negative (DN) B cells: an under-recognized effector memory B cell subset in autoimmunity. Clin. Exp. Immunol 205, 119–127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang HD et al. Pathogenic memory plasma cells in autoimmunity. Curr. Opin. Immunol 61, 86–91 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oleinika K, Mauri C & Salama AD Effector and regulatory B cells in immune-mediated kidney disease. Nat. Rev. Nephrol 15, 11–26 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto M et al. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 34, 703–714 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Miles K et al. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc. Natl Acad. Sci. USA 109, 887–892 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshizaki A et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491, 264–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosser EC et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat. Med 20, 1334–1339 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Menon M, Blair PA, Isenberg DA & Mauri C A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 44, 683–697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saulep-Easton D et al. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia 30, 163–172 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fehres CM et al. APRIL induces a novel subset of IgA+ regulatory B cells that suppress inflammation via expression of IL-10 and PD-L1. Front. Immunol 10, 1368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang RX et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med 20, 633–641 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosser EC & Mauri C Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Kaltenmeier C et al. CD4+ T cell–derived IL-21 and deprivation of CD40 signaling favor the in vivo development of granzyme B-expressing regulatory B cells in HIV patients. J. Immunol 194, 3768–3777 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Fridman WH et al. B cells and cancer: to B or not to B? J. Exp. Med 218, e20200851 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen JS et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27− memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res 18, 3281–3292 (2012). [DOI] [PubMed] [Google Scholar]; This study is the first to describe the co-localization and combined prognostic effects of tumour-infiltrating CD20+ B cells and CD8+ T cells in human cancer, highlighting the importance of T cell–B cell interactions in effective antitumour immunity.
- 40.Centuori SM et al. Double-negative (CD27−IgD−) B cells are expanded in NSCLC and inversely correlate with affinity-matured B cell populations. J. Transl. Med 16, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montfort A et al. A strong B-cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin. Cancer Res 23, 250–262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong L et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat. Commun 12, 1540 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruno TC et al. Antigen-presenting intratumoral B cells affect CD4+ TIL phenotypes in non–small cell lung cancer patients. Cancer Immunol. Res 5, 898–907 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work is the first report of exhausted B cells and their association with Treg cells in human cancer.
- 44.Hu Q et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat. Commun 12, 2186 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides a comprehensive atlas of TIL-Bs in patients with breast cancer, and reports B cell signatures (identified by single-cell sequencing) that significantly associate with improved survival.
- 45.Azizi E et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian J et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res. 30, 745–762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruffin AT et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun 12, 3349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J et al. Single-cell transcriptome and antigen-immunoglobin analysis reveals the diversity of B cells in non-small cell lung cancer. Genome Biol. 21, 152 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei Y et al. Single-cell analyses reveal suppressive tumor microenvironment of human colorectal cancer. Clin. Transl. Med 11, e422 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olalekan S, Xie B, Back R, Eckart H & Basu A Characterizing the tumor microenvironment of metastatic ovarian cancer by single-cell transcriptomics. Cell Rep. 35, 109165 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Wang W et al. Multiregion single-cell sequencing reveals the transcriptional landscape of the immune microenvironment of colorectal cancer. Clin. Transl. Med 11, e253 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, Liu Z, Wu D, Chen L & Xie L Single-cell RNA-seq analysis reveals microenvironmental infiltration of plasma cells and hepatocytic prognostic markers in HCC with cirrhosis. Front. Oncol 10, 596318 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wieland A et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that in patients with HPV+ tumours, TIL-Bs produce HPV-specific IgG antibodies, providing strong evidence that TIL-B can recognize tumour-specific antigens.
- 54.Lu Y et al. Complement signals determine opposite effects of B cells in chemotherapy-induced immunity. Cell 180, 1081–1097 (2020). [DOI] [PubMed] [Google Scholar]; This study shows that neoadjuvant chemotherapy of breast cancer induces ICOSL+ B cells via complement signalling, which in turn boosts T cell-mediated antitumour responses.
- 55.Cillo AR et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity 52, 183–199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lambrechts D et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med 24, 1277–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Griss J et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun 10, 4186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakami Y et al. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci. Rep 9, 13083 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai C et al. Interleukin 10-expressing B cells inhibit tumor-infiltrating T cell function and correlate with T cell Tim-3 expression in renal cell carcinoma. Tumor Biol. 37, 8209–8218 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Shalapour S et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows that chronic liver inflammation is accompanied by IgA+PD-L1+IL-10+ plasmocytes that suppress CD8+ T cells and accelerate the development of hepatocellular carcinoma.
- 61.Shalapour S et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spear S et al. Discrepancies in the tumor microenvironment of spontaneous and orthotopic murine models of pancreatic cancer uncover a new immunostimulatory phenotype for B cells. Front. Immunol 10, 542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hollern DP et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell 179, 1191–1206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that in mouse breast cancer models, responses to immune checkpoint inhibitors depend on B cell activation by TFH cells, highlighting that B cell/T cell signatures could be used as a biomarker to predict such responses.
- 64.Shaul ME et al. Tumor-associated neutrophils drive B-cell recruitment and their differentiation to plasma cells. Cancer Immunol. Res 9, 811–824 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Ouyang FZ et al. Dendritic cell-elicited B-cell activation fosters immune privilege via IL-10 signals in hepatocellular carcinoma. Nat. Commun 7, 13453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou M et al. Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88−NF-κB signal pathway. Oncoimmunology 5, e1180485 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wejksza K et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor α. J. Immunol 190, 2575–2584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindner S et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 73, 2468–2479 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Domblides C et al. Tumor-associated tertiary lymphoid structures: from basic and clinical knowledge to therapeutic manipulation. Front. Immunol 12, 698604 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noël G et al. Functional TH1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J. Clin. Invest 131, e139905 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that T follicular regulatory cells (CD25+CXCR5+GARP+FOXP3+) can inhibit TFH cells in TLSs, thereby dampening TLS-driven humoral responses.
- 71.Kinker GS et al. B cell orchestration of anti-tumor immune responses: a matter of cell localization and communication. Front. Cell Dev. Biol 9, 678127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jenks SA, Cashman KS, Woodruff MC, Lee FEH & Sanz I Extrafollicular responses in humans and SLE. Immunol. Rev 288, 136–148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This Review describes the three main B cell activation pathways and further highlights how the extrafollicular pathway might help to produce pathogenic autoantibodies in lupus.
- 73.Ruddle NH Basics of inducible lymphoid organs. Curr. Top. Microbiol. Immunol 426, 1–19 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Kranich J & Krautler NJ How follicular dendritic cells shape the B-cell antigenome. Front. Immunol 7, 225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cyster JG & Allen CDC B cell responses: cell interaction dynamics and decisions. Cell 177, 524–540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Graus F & Dalmau J Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol 16, 535–548 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Small M et al. Genetic alterations and tumor immune attack in Yo paraneoplastic cerebellar degeneration. Acta Neuropathol. 135, 569–579 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Kroeger DR, Milne K & Nelson BH Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin. Cancer Res 22, 3005–3015 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Elsner RA & Shlomchik MJ Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 53, 1136–1150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rao DA et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the synovium of rheumatoid arthritis contains expanded TPH cells that, similar to TFH cells, express a wide array of factors to support B cell activation and function.
- 81.Yoshitomi H & Ueno H Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell. Mol. Immunol 18, 523–527 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eisenbarth SC et al. CD4+ T cells that help B cells — a proposal for uniform nomenclature. Trends Immunol. 42, 658–669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun 9, 1758 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arazi A et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol 20, 902–914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.GuTrantien C et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2, e91487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the importance of tumour-infiltrating CXCL13-producing TFH cells in promoting memory B cell differentiation and humoral antitumour immunity in human breast cancer.
- 86.Li JP et al. PD-1+CXCR5−CD4+Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J. Immunother. Cancer 9, e002101 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Webb JR, Milne K, Kroeger DR & Nelson BH PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol 141, 293–302 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Hansen MH, Nielsen H & Ditzel HJ The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc. Natl Acad. Sci. USA 98, 12659–12664 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabrita R et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Lin Z et al. Pan-cancer analysis of genomic properties and clinical outcome associated with tumor tertiary lymphoid structure. Sci. Rep 10, 21530 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maxime MF et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541 (2022). [DOI] [PubMed] [Google Scholar]
- 92.Zaenker P & Ziman MR Serologic autoantibodies as diagnostic cancer biomarkers — a review. Cancer Epidemiol. Biomark. Prev 22, 2161–2181 (2013). [DOI] [PubMed] [Google Scholar]
- 93.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV & Chudakov DM B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol 20, 294–307 (2020). [DOI] [PubMed] [Google Scholar]
- 94.Macdonald IK, Parsy-Kowalska CB & Chapman CJ Autoantibodies: opportunities for early cancer detection. Trends Cancer 3, 198–213 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Shimada H, Ochiai T & Nomura F Titration of serum p53 antibodies in 1085 patients with various types of malignant tumors a multiinstitutional analysis by the Japan p53 Antibody Research Group. Cancer 97, 682–689 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Oshima Y et al. NY-ESO-1 autoantibody as a tumor-specific biomarker for esophageal cancer: screening in 1969 patients with various cancers. J. Gastroenterol 51, 30–34 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Raza A et al. Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy. J. Transl. Med 18, 140 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fischer E et al. Cryptic epitopes induce high-titer humoral immune response in patients with cancer. J. Immunol 185, 3095–3102 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Ishikawa T et al. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 63, 5564–5572 (2003). [PubMed] [Google Scholar]
- 100.Reis BS et al. Prostate cancer progression correlates with increased humoral immune response to a human endogenous retrovirus GAG protein. Clin. Cancer Res 19, 6112–6125 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Blixt O et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 13, R25 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang X et al. Autoantibody signatures in prostate cancer. N. Engl. J. Med 353, 1224–1235 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Soussi T p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 60, 1777–1788 (2000). [PubMed] [Google Scholar]
- 104.Mazor RD Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell 10.1016/j.cell.2022.02.012 (2022). [DOI] [PubMed] [Google Scholar]
- 105.Garaud S et al. Antigen specificity and clinical significance of IgG and IgA autoantibodies produced in situ by tumor-infiltrating B cells in breast cancer. Front. Immunol 9, 2660 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen P & Fillatreau S Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol 15, 441–451 (2015). [DOI] [PubMed] [Google Scholar]
- 107.Garaud S et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight 4, e129641 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rodriguez AB et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep. 36, 109422 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows that in a mouse melanoma model, TLS development is organized by cancer-associated fibroblasts via the TNF receptor pathway, and their presence correlates with the response to immunotherapy.
- 109.Ammirante M, Luo JL, Grivennikov S, Nedospasov S & Karin M B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hua Z & Hou B The role of B cell antigen presentation in the initiation of CD4+ T cell response. Immunol. Rev 296, 24–35 (2020). [DOI] [PubMed] [Google Scholar]
- 111.Lanzavecchia A Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu. Rev. Immunol 8, 773–793 (1990). [DOI] [PubMed] [Google Scholar]
- 112.Tolar P Cytoskeletal control of B cell responses to antigens. Nat. Rev. Immunol 17, 621–634 (2017). [DOI] [PubMed] [Google Scholar]
- 113.Rivera A, Chen CC, Ron N, Dougherty JP & Ron Y Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int. Immunol 13, 1583–1593 (2001). [DOI] [PubMed] [Google Scholar]
- 114.Lin RH, Mamula MJ, Hardin JA & Janeway CA Induction of autoreactive B cells allows priming of autoreactive T cells. J. Exp. Med 173, 1433–1439 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith MJ, Simmons KM & Cambier JC B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat. Rev. Nephrol 13, 712–720 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giles JR, Kashgarian M, Koni PA & Shlomchik MJ B cell-specific MHC class II deletion reveals multiple nonredundant roles for B cell antigen presentation in murine lupus. J. Immunol 195, 2571–2579 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Molnarfi N et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J. Exp. Med 210, 2921–2937 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.O’Neill SK et al. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J. Immunol 179, 5109–5116 (2007). [DOI] [PubMed] [Google Scholar]
- 119.Iversen R et al. Efficient T cell–B cell collaboration guides autoantibody epitope bias and onset of celiac disease. Proc. Natl Acad. Sci. USA 116, 15134–15139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jelcic I et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 175, 85–100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wennhold K et al. CD86+ antigen-presenting B cells are increased in cancer, localize in tertiary lymphoid structures, and induce specific T-cell responses. Cancer Immunol. Res 9, 1098–1108 (2021). [DOI] [PubMed] [Google Scholar]
- 122.Cui C et al. Neoantigen driven B cell and CD4+ T follicular helper cell collaboration promotes robust antitumor CD8+ T cell responses. Cell 184, 1–18 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lo JA et al. Epitope spreading toward wild type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci. Transl. Med 13, eabd8636 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joseph CG et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 343, 152–157 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that in patients with both scleroderma and cancer, somatic mutations in the POLR3A gene are associated with autoantibodies against non-mutated regions of the corresponding protein (RPC1), suggesting that immune responses to tumour-associated neoantigens may trigger humoral responses against self-epitopes via antigen spreading.
- 125.Zitvogel L, Perreault C, Finn OJ & Kroemer G Beneficial autoimmunity improves cancer prognosis. Nat. Rev. Clin. Oncol 18, 591–602 (2021). [DOI] [PubMed] [Google Scholar]
- 126.Michaud D, Steward CR, Mirlekar B & Pylayeva-Gupta Y Regulatory B cells in cancer. Immunol. Rev 299, 74–92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou X, Su Y-X, Lao X-M, Liang Y-J & Liao G-Q CD19+IL-10+ regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4+ T cells to CD4+Foxp3+ regulatory T cells. Oral. Oncol 53, 27–35 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Wang WW et al. CD19+CD24hiCD38hi Bregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget 6, 33486–33499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ishigami E et al. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer 26, 180–189 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Xiao X et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 6, 546–559 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Mirlekar B et al. B cell-derived IL35 drives STAT3-dependent CD8+ T-cell exclusion in pancreatic cancer. Cancer Immunol. Res 8, 292–308 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hagn M et al. Human B cells differentiate into granzyme B-secreting cytotoxic B lymphocytes upon incomplete T-cell help. Immunol. Cell Biol 90, 457–467 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Lundy SK Killer B lymphocytes: the evidence and the potential. Inflamm. Res 58, 345–357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tao H et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur. J. Immunol 45, 999–1009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jeske SS et al. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol. Immunother 69, 1205–1216 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang B et al. B cell-derived GABA elicits IL-10+ macrophages to limit antitumour immunity. Nature 599, 471–476 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Logtenberg MEW, Scheeren FA & Schumacher TN The CD47–SIRPα immune checkpoint. Immunity 52, 742–752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Biswas S et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature 591, 464–470 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Charoentong P et al. Pan-cancer immunogenomic analyses reveal genotype–immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 18, 248–262 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Ferris RL, Jaffee EM & Ferrone S Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J. Clin. Oncol 28, 4390–4399 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu X et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat. Genet 51, 560–567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper presents a bioinformatic survey of B cell responses across cancers using data from TCGA.
- 142.Doubrovina ES et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J. Immunol 171, 6891–6899 (2003). [DOI] [PubMed] [Google Scholar]
- 143.Roumenina LT, Daugan MV, Petitprez F, Sautès-Fridman C & Fridman WH Context-dependent roles of complement in cancer. Nat. Rev. Cancer 19, 698–715 (2019). [DOI] [PubMed] [Google Scholar]
- 144.Roumenina LT et al. Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol. Res 7, 1091–1105 (2019). [DOI] [PubMed] [Google Scholar]
- 145.Kouser L et al. Emerging and novel functions of complement protein C1q. Front. Immunol 6, 317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kwak JW et al. Complement activation via a C3a receptor pathway alters CD4+ T lymphocytes and mediates lung cancer progression. Cancer Res. 78, 143–156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Foss S et al. TRIM21 — from intracellular immunity to therapy. Front. Immunol 10, 2049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rhodes DA & Isenberg DA TRIM21 and the function of antibodies inside cells. Trends Immunol. 38, 916–926 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Gu Y et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat. Med 25, 312–322 (2019). [DOI] [PubMed] [Google Scholar]; This work shows that in a mouse breast cancer model, anti-HSPA4 IgG produced by TIL-Bs activates the CXCR4/SDF1a pathway in tumour cells, thereby promoting lymph node metastasis.
- 150.Lo CS et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin. Cancer Res 23, 925–934 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Zirakzadeh AA et al. Doxorubicin enhances the capacity of B cells to activate T cells in urothelial urinary bladder cancer. Clin. Immunol 176, 63–70 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Morcrette G et al. APC germline hepatoblastomas demonstrate cisplatin-induced intratumor tertiary lymphoid structures. Oncoimmunology 8, e1584547 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Silina K et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 78, 1308–1320 (2018). [DOI] [PubMed] [Google Scholar]
- 154.Brase JC et al. Role of tumor-infiltrating B cells in clinical outcome of patients with melanoma treated with dabrafenib plus trametinib. Clin. Cancer Res 27, 4500–4510 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chelvanambi M, Fecek RJ, Taylor JL & Storkus WJ STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother. Cancer 9, e001906 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vito A et al. Immune checkpoint blockade in triple negative breast cancer influenced by B cells through myeloid-derived suppressor cells. Commun. Biol 4, 859 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang X et al. PD-1-expressing B cells suppress CD4+ and CD8+ T cells via PD-1/PD-L1-dependent pathway. Mol. Immunol 109, 20–26 (2019). [DOI] [PubMed] [Google Scholar]
- 158.Das R et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest 128, 715–720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guan H et al. PD-L1 mediated the differentiation of tumor-infiltrating CD19+ B lymphocytes and T cells in invasive breast cancer. Oncoimmunology 5, e1075112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Petitprez F et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020). [DOI] [PubMed] [Google Scholar]
- 161.Helmink BA et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Budczies J et al. A gene expression signature associated with B cells predicts benefit from immune checkpoint blockade in lung adenocarcinoma. Oncoimmunology 10, 1860586 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Amaria RN et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med 24, 1649–1654 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cottrell TR et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann. Oncol 29, 1853–1860 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.van Dijk N et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat. Med 26, 1839–1844 (2020). [DOI] [PubMed] [Google Scholar]
- 166.Kim SS et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin. Cancer Res 26, 3345–3359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sánchez-Alonso S et al. A new role for circulating T follicular helper cells in humoral response to anti-PD-1 therapy. J. Immunother. Cancer 8, e001187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vonderheide RH CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med 71, 47–58 (2020). [DOI] [PubMed] [Google Scholar]
- 169.Elgueta R et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev 229, 152–172 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jackaman C, Cornwall S, Graham PT & Nelson DJ CD40-activated B cells contribute to mesothelioma tumor regression. Immunol. Cell Biol 89, 255–267 (2011). [DOI] [PubMed] [Google Scholar]
- 171.Irenaeus SMM et al. First-in-human study with intratumoral administration of a CD40 agonistic antibody, ADC-1013, in advanced solid malignancies. Int. J. Cancer 145, 1189–1199 (2019). [DOI] [PubMed] [Google Scholar]
- 172.Vonderheide RH et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol 25, 876–883 (2007). [DOI] [PubMed] [Google Scholar]
- 173.van Hooren L et al. Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma. Nat. Commun 12, 4127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leonard WJ, Lin JX & O’Shea JJ The γc family of cytokines: basic biology to therapeutic ramifications. Immunity 50, 832–850 (2019). [DOI] [PubMed] [Google Scholar]
- 175.Papillion A & Ballesteros-Tato A The potential of harnessing IL-2-mediated immunosuppression to prevent pathogenic B cell responses. Front. Immunol 12, 667342 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yarchoan M et al. Effects of B cell-activating factor on tumor immunity. JCI Insight 5, e136417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rubio AJ, Porter T & Zhong X Duality of B cell–CXCL13 axis in tumor immunology. Front. Immunol 11, 521110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johansson-Percival A et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat. Immunol 18, 1207–1217 (2017). [DOI] [PubMed] [Google Scholar]
- 179.Maldonado L et al. Vaccination: intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci. Transl. Med 6, 221ra13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lutz ER et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res 2, 616–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wennhold K et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget 8, 27740–27753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wennhold K et al. Using antigen-specific B cells to combine antibody and T cell–based cancer immunotherapy. Cancer Immunol. Res 5, 730–743 (2017). [DOI] [PubMed] [Google Scholar]
- 183.Lee-Chang C et al. Activation of 4–1BBL+ B cells with CD40 agonism and IFNγ elicits potent immunity against glioblastoma. J. Exp. Med 218, e20200913 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Blass E & Ott PA Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol 18, 215–229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Joshi NS et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ziebart A et al. The influence of chemotherapy on adenosine-producing B cells in patients with head and neck squamous cell carcinoma. Oncotarget 9, 5834–5847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee-Chang C et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J. Immunol 191, 4141–4151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yarchoan M et al. MEK inhibition suppresses B regulatory cells and augments anti-tumor immunity. PLoS ONE 14, e0224600 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Das S & Bar-Sagi D BTK signaling drives CD1dhiCD5+ regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene 38, 3316–3324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lu RM et al. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci 27, 1–30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Heemskerk N et al. Augmented antibody-based anticancer therapeutics boost neutrophil cytotoxicity. J. Clin. Invest 131, e134680 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang W et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front. Immunol 11, 18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Geller A & Yan J The role of membrane bound complement regulatory proteins in tumor development and cancer immunotherapy. Front. Immunol 10, 1074 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Germain C et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med 189, 832–844 (2014). [DOI] [PubMed] [Google Scholar]; This work shows that in lung cancer, B cells display features of an ongoing humoral response, including recognition of tumour-associated antigens, and predict patient outcome when present with mature dendritic cells.
- 195.Yasuda M et al. Antigens recognized by IgG derived from tumor-infiltrating B lymphocytes in human lung cancer. Anticancer. Res 26, 3607–3611 (2006). [PubMed] [Google Scholar]
- 196.Yasuda M et al. Tumor-infiltrating B lymphocytes as a potential source of identifying tumor antigen in human lung cancer. Cancer Res. 62, 1751–1756 (2002). [PubMed] [Google Scholar]
- 197.Pavoni E et al. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 7, 1–17 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kotlan B et al. Novel ganglioside antigen identified by B cells in human medullary breast carcinomas: the proof of principle concerning the tumor-infiltrating B lymphocytes. J. Immunol 175, 2278–2285 (2005). [DOI] [PubMed] [Google Scholar]
- 199.Aran D, Hu Z & Butte AJ xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wouters MCA & Nelson BH Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res 24, 6125–6135 (2018). [DOI] [PubMed] [Google Scholar]
- 201.Castino GF et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology 5, e1085147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Posch F et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology 7, e1378844 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gunderson AJ et al. Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer. Oncoimmunology 10, e1900635 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lynch KT et al. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J. Immunother. Cancer 9, e002273 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vanhersecke L et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2, 794–802 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This large study involving three independent cohorts shows that the presence of mature TLSs is associated with response to checkpoint blockade of the PD-1 pathway.
- 206.Gago da Graça C, van Baarsen LGM & Mebius RE Tertiary lymphoid structures: diversity in their development, composition, and role. J. Immunol 206, 273–281 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


