Abstract
Cell-mediated immunity is critical for the host defense to Cryptococcus neoformans, as demonstrated by numerous animal studies and the prevalence of the infection in AIDS patients. Previous studies have established that the polysaccharide capsule contributes to the virulence of C. neoformans by suppressing T-lymphocyte proliferation, which reflects the clonal expansion of T lymphocytes that is a hallmark of cell-mediated immunity. The present studies were performed to identify the major mechanism by which polysaccharide impairs lymphocyte proliferation, since capsular polysaccharide has the potential to affect the development of T-lymphocyte responses by stimulating production of interleukin-10 (IL-10), inhibiting phagocytosis, and inducing shedding of cell surface receptors. We demonstrate that polysaccharide inhibits lymphocyte proliferation predominantly by blocking uptake of C. neoformans, which is crucial for subsequent lymphocyte proliferation. In addition, we show that polysaccharide did not suppress lymphocyte proliferation via an IL-10-dependent mechanism, nor did it affect critical surface receptor interactions on the T cell or antigen-presenting cell. Having established that polysaccharide impairs phagocytosis, we performed studies to determine whether opsonization with human serum or with anticapsular antibody could reverse this effect. Impaired uptake and lymphocyte proliferation that were induced by polysaccharide can be enhanced through opsonization with monoclonal antibodies or human serum, suggesting that antipolysaccharide antibodies might enhance the host defense by restoring uptake of the organism and subsequent presentation to T lymphocytes. These studies support the therapeutic potential of stimulating cell-mediated immunity to C. neoformans with anticapsular antibody.
Cryptococcus neoformans is one of the leading fatal mycoses in AIDS (4, 13, 18). Although cell-mediated immunity is of paramount importance in the host defense to C. neoformans, the dominant mechanism by which the major virulence factor, the polysaccharide capsule, might influence the development of cell-mediated immunity and how this mechanism might be overcome have not been determined.
We and others have established that capsular polysaccharide (CPS) suppresses T-lymphocyte responses to both live and killed C. neoformans (5, 30, 33, 43). We have previously shown that strains of C. neoformans with a large capsule are less able to stimulate proliferation of human lymphocytes than minimally encapsulated strains and that addition of purified CPS inhibits lymphocyte proliferation (33). CPS also impairs alveolar macrophage-dependent T-cell responses to C. neoformans (43). Since the clonal proliferation of T cells is a hallmark of the cell-mediated immune response, we considered the possibility that CPS-mediated suppression of lymphocyte proliferation may be an important mechanism of virulence.
There are a number of mechanisms by which CPS could suppress the development of the T-lymphocyte response. CPS can induce immunosuppressive cytokines such as interleukin-10 (IL-10) (45), which suppresses lymphocyte proliferation by a number of mechanisms (8, 12, 35, 40). CPS also causes shedding of some cell surface receptors by an unknown mechanism (14). Since antigen presentation is critically dependent on the expression of costimulatory surface receptors, it is possible that CPS could interfere with a critical receptor-ligand interaction between the antigen-presenting cell and the T cell. CPS also inhibits uptake of the organism by phagocytic cells (27, 32), which could inhibit the antigen available for processing and presentation to the T cell. Thus, there is the potential for CPS to suppress the antigen available for presentation by inhibiting the uptake of C. neoformans by the antigen-presenting cell, by inhibiting cell-cell interactions necessary for costimulatory signals, or by stimulating production of immunosuppressive IL-10.
By contrast to the role of cell-mediated immunity, the role of humoral immunity has provided an arcanum in our understanding of cryptococcal host defense. While administration of antibodies to CPS is protective (37, 39), deficiencies of humoral immunity do not predispose the host to cryptococcal infections. This suggests that natural humoral mechanisms are unimportant in the host defense to C. neoformans but that administration of antibody, or vaccination with the development of antibodies, can augment mechanisms of host defense. If CPS inhibits antigen presentation by inhibiting uptake of C. neoformans, there is the potential to overcome this effect by opsonizing the organism with anticapsular antibody. In a murine model, specific anticryptococcal antibodies that opsonize C. neoformans can augment cellular uptake (15, 36, 38), and antibodies to glucoronxylmannan conjugated to tetanus toxoid promote phagocytosis of C. neoformans in the absence of complement (51) and enhance survival via a CD4-dependent mechanism (50). Thus, it is possible that specific anticapsular antibody might enhance uptake and ultimately presentation to T cells, resulting in activation, proliferation, and development of cell-mediated immune responses that would provide an explanation for the therapeutic efficacy of anticapsular antibodies.
To determine whether CPS suppresses lymphocyte proliferation by production of IL-10, lymphocytes were stimulated with CPS-treated C. neoformans in the presence or absence of neutralizing antibody to IL-10. To determine if CPS was affecting interactions between antigen-presenting cells and T cells, CPS was added to the peripheral blood mononuclear cells (PBMC) and the excess was removed before stimulation with C. neoformans. To determine whether the antiphagocytic properties of CPS contributed to a reduction in lymphocyte proliferation, phagocytosis was correlated with [3H]thymidine ([3H]TdR) incorporation. Finally, the ability of complement or anticapsular antibody to ameliorate the effect on lymphocyte proliferation was tested with pooled human sera and anticapsular monoclonal antibodies (MAb).
MATERIALS AND METHODS
Isolation of PBMC and selection of lymphocyte populations.
Human peripheral blood was obtained from healthy adults by venipuncture. The blood was anticoagulated with 10 U of heparin (Organon Teknika-Cappel, Scarborough, Ontario, Canada) per ml. PBMC were purified by centrifugation (800 × g for 20 min) on a Ficoll-Hypaque density gradient (Lymphoprep; Labquip, Woodbridge, Ontario, Canada). PBMC were washed three times in Hanks balanced salt solution (Gibco, Burlington, Ontario, Canada), counted, and suspended in medium containing RPMI 1640 (Gibco); 5% heat-inactivated pooled human AB serum (lot 7M1809; BioWhittaker, Walkersville, Md.); and 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 0.2 μg of amphotericin B/ml, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids (all from Gibco).
Preparation of C. neoformans and CPS.
C. neoformans 67 (ATCC 52817; acapsular mutant) (21), 68 (ATCC 24064; lightly encapsulated, serotype A) (47), 3501 (ATCC 34873; lightly encapsulated, serotype D), 613 (ATCC 36556; lightly encapsulated, serotype D) (24), T145 (ATCC 62070; moderately encapsulated, serotype A) (41), and 6 (ATCC 62066; heavily encapsulated, serotype A) (41) were obtained from the American Type Culture Collection (Rockville, Md.). The organisms were maintained as previously described (34) on Sabouraud slants (Difco, Detroit, Mich.) and passaged to fresh slants monthly. The organisms were killed as previously described (33) by autoclaving at 121°C for 15 min and were stored at 4°C. CPS was obtained from strain 68, serotype A (ATCC 24064), as previously described (22). All reagents were prepared in endotoxin-free water (Baxter, Mississauga, Ontario, Canada), and glassware was baked prior to use.
Polysaccharide coating and staining of C. neoformans.
Acapsular C. neoformans (strain 67) was incubated in purified polysaccharide for 1 h at 37°C. Unbound polysaccharide was removed by washing in phosphate-buffered saline (PBS). The polysaccharide-coated C. neoformans was then used in proliferation and phagocytosis studies. Mucicarmine (Sigma, St. Louis, Mo.) staining and microscopic examination were used to determine whether CPS had bound to the surface of C. neoformans.
Treatment of C. neoformans with antibody or sera.
For some experiments, heat-killed C. neoformans was incubated for 1 h at 37°C with undiluted non-heat-inactivated human AB serum (lots 5M1937 and 7M1809; BioWhittaker), heat-inactivated (56°C for 60 min) serum, or an anticapsular MAb (MAb 471) that was purified as previously described (16, 42). This MAb is a murine immunoglobulin G1 antibody that binds to serotype A and D polysaccharide. The organisms were then washed three times in PBS and used in proliferation or phagocytosis assays.
Lymphocyte proliferation in response to C. neoformans.
To determine whether C. neoformans stimulated lymphocyte proliferation, PBMC (2 × 105 cells/well) were cultured in round-bottom wells of 96-well tissue culture plates (Corning Glass Works, Corning, N.Y.). Whole C. neoformans cells (2 × 105/well) were used to stimulate the lymphocytes. Cultures were incubated for 7 days at 37°C with 5% CO2. Sixteen hours before the end of incubation, 1 μCi of [3H]TdR (ICN, Montreal, Quebec, Canada) was added. Cells were harvested on glass filters, and counts per minute were determined in a liquid scintillation counter. [3H]TdR incorporation into cultures containing C. neoformans alone was routinely less than 300 cpm. As a control, PBMC were stimulated with 10 μg of concanavalin A (Sigma) per ml or 10−2 Leaf units of tetanus toxoid (Connaught Laboratories, Mississauga, Ontario, Canada). In some experiments, lidocaine (10 to 10,000 μM; Baxter) was added to the culture wells. In other experiments, cells were incubated in the presence of 100 to 1,000 ng of anti-IL-10 (Pharmingen) or isotype-matched control antibody (Sigma) per ml.
ELISA for IL-10.
The concentration of IL-10 in culture supernatants was determined by an enzyme-linked immunosorbent assay (ELISA). The capture antibody was monoclonal anti-IL-10 (1 μg/ml) (18551 D; Pharmingen, San Diego, Calif.) or JES3-19F (American Type Culture Collection). The secondary antibody was a biotinylated anti-IL-10 MAb (1.5 μg/ml) (18562 D; Pharmingen), followed by avidin-peroxidase (Sigma). The ELISA was developed by adding 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma; A-1888) in 0.1 M citric acid buffer with 1 μl of 30% hydrogen peroxide per ml. The ELISA was read spectrophotometrically at 405 nm. All results were the means from duplicate samples, and the standard curve was generated by using IL-10 from the Biological Response Modifiers Program, National Institutes of Health, Bethesda, Md.
Phagocytosis of C. neoformans.
PBMC were cultured in 24-well plates containing plastic 13-mm-diameter coverslips (Nunc, Naperville, Illinois) at 37°C in RPMI medium. After 1 h, the nonadherent cells were removed by washing, and C. neoformans (106 organisms/well) was added to the wells. At various times, medium and unbound Cryptococcus were removed by washing with PBS. Coverslips were removed, fixed in methanol, stained with Giemsa stain (ICN), and then examined by light microscopy for the number of cells that had bound or ingested C. neoformans (3). Studies determined that Giemsa staining was as reliable as fluorescein isothiocyanate labeling of C. neoformans and quenching of extracellular fluorescence with trypan blue.
Statistics.
Data are given as the mean ± standard error of the mean (SEM) for the indicated number of experiments. Each experiment was performed with different donors on different days. [3H]TdR incorporation is expressed as the mean counts per minute ± SEM for quadruplicate wells. To analyze the data statistically, one-way analysis of variance was performed when allowed by the F test (Statview 512+; Brainpower Inc., Calabasas, Calif.). For experiments in which phagocytosis and lymphocyte proliferation were determined, Wilcoxon-Mann-Whitney statistics were used. In experiments comparing human serum to anti-CPS MAb, Friedman two-way analysis of variance by ranks was performed. For these tests, a P value of <0.05 was considered significant.
RESULTS
Inhibition of lymphocyte proliferation by CPS is independent of IL-10.
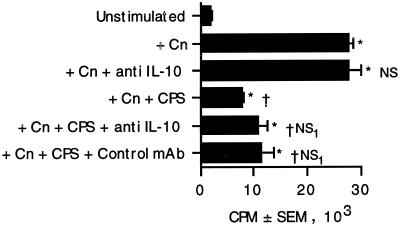
Recombinant IL-10 can abrogate lymphocyte proliferation in response to C. neoformans (35). Since CPS stimulates production of IL-10 (8, 12), it was possible that CPS could suppress lymphocyte proliferation via production of IL-10. To determine whether the CPS induced sufficient IL-10 to influence lymphocyte proliferation, PBMC were stimulated with C. neoformans in the presence or absence of purified polysaccharide and anti-IL-10 or isotype-matched control antibody. The anti-IL-10 MAb tended to augment lymphocyte proliferation but did not restore it to statistically significant levels (Fig. 1). To ensure that the CPS was capable of stimulating IL-10 production, ELISA for IL-10 was performed on CPS-stimulated supernatants. Modest concentrations of IL-10 were detected (128 ± 40 pg/ml; n = 4), which were greater than the concentration found in prior studies (45). The anti-IL-10 antibody was active, since it enhanced C. neoformans-stimulated tumor necrosis factor alpha (TNF-α) release. (The concentrations of TNF-α were 4,742 pg/ml in supernatants of PBMC that were stimulated with C. neoformans plus polysaccharide plus control immunoglobulin G and 9,057 pg/ml in supernatants of PBMC stimulated with C. neoformans plus polysaccharide plus anti-IL-10.) This data suggests that IL-10 is not the primary mechanism responsible for the CPS-induced lymphocyte suppression.
FIG. 1.
Blocking of IL-10 does not restore lymphocyte proliferation in response to acapsular C. neoformans 67 cultured in the presence of polysaccharide. PBMC and C. neoformans (Cn) were cultured in the presence or absence of capsular polysaccharide (10 μg/ml) and anti-IL-10 or control antibody (1 μg/ml). Lymphocyte proliferation was assessed 7 days later by [3H]TdR incorporation. ∗, P < 0.05 by analysis of variance. NS, not significantly different compared to stimulated PBMC. †, P < 0.05 compared to PBMC plus C. neoformans. NS1, not significantly different from PBMC plus C. neoformans plus CPS. The experiment was repeated three times with similar results.
CPS suppresses lymphocyte proliferation by binding to C. neoformans rather than affecting interactions between antigen-presenting cells and T cells.
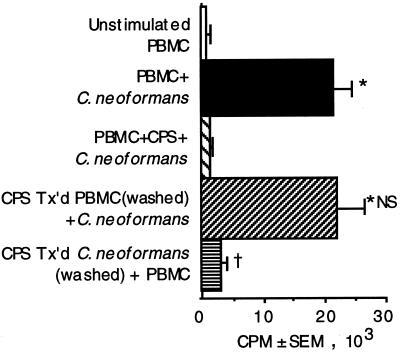
CPS causes shedding of some cell surface receptors (14). Since receptor-ligand interactions are important in antigen presentation, the possibility that CPS interferes with a critical costimulatory signal was considered. PBMC were incubated with purified CPS, and the excess was removed by washing. The CPS-treated PBMC were stimulated with C. neoformans. Preincubation of PBMC with CPS had no effect on lymphocyte proliferation (Fig. 2). In parallel experiments, acapsular C. neoformans that had previously been incubated with purified polysaccharide and washed to remove the excess CPS was used to stimulate PBMC. Preincubation of C. neoformans with purified polysaccharide abrogated lymphocyte proliferation (Fig. 2). Mucicarmine staining confirmed that CPS was binding to C. neoformans (data not shown). Thus, the polysaccharide does not affect lymphocyte proliferation by affecting the interaction between antigen-presenting cells and T cells but rather exerts its effect by binding to the organism.
FIG. 2.
Lymphocyte proliferation in response to acapsular
C. neoformans 67 was abrogated when the organism was
preincubated in purified polysaccharide. PBMC alone (□) were compared
to PBMC that were stimulated with C. neoformans (■); PBMC
that were stimulated with C. neoformans in the presence of
10 μg of CPS per ml
( ); PBMC
that were pretreated for 1 h at 37°C (Tx’d) with CPS, washed,
and stimulated with C. neoformans (
); or C.
neoformans that was pretreated for 1 h at 37°C with CPS,
washed, and used to stimulate PBMC (▤). After 7 days, lymphocyte
proliferation was determined by [3H]TdR incorporation.
∗, P < 0.05 compared to unstimulated PBMC. NS, not
significantly different compared to C. neoformans-stimulated
PBMC. †, P < 0.05 compared to stimulated PBMC. The
experiment was repeated four times with similar results.
); PBMC
that were pretreated for 1 h at 37°C (Tx’d) with CPS, washed,
and stimulated with C. neoformans (
); or C.
neoformans that was pretreated for 1 h at 37°C with CPS,
washed, and used to stimulate PBMC (▤). After 7 days, lymphocyte
proliferation was determined by [3H]TdR incorporation.
∗, P < 0.05 compared to unstimulated PBMC. NS, not
significantly different compared to C. neoformans-stimulated
PBMC. †, P < 0.05 compared to stimulated PBMC. The
experiment was repeated four times with similar results.
Blocking of phagocytosis inhibits lymphocyte proliferation in response to C. neoformans.
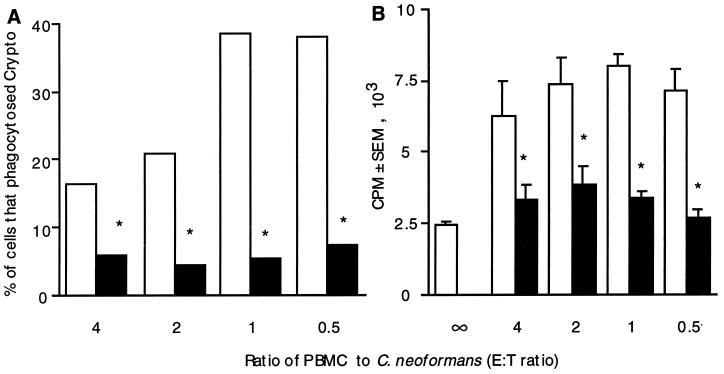
One of the major effects of the polysaccharide capsule is to inhibit phagocytosis (25). To determine whether impaired phagocytosis might explain the reduced lymphocyte proliferation, the uptake of untreated acapsular C. neoformans was compared to that of CPS-coated C. neoformans and correlated with lymphocyte proliferation. Preincubation of C. neoformans with purified polysaccharide reduced the number of cells that had taken up by C. neoformans by 60 to 70% across a broad range of numbers of organisms (Fig. 3). The number of organisms that had been internalized was proportional to the number that had bound to cells (data not shown), and the number of cells that had internalized C. neoformans correlated with a reduction in lymphocyte proliferation (Fig. 3).
FIG. 3.
Preincubation of C. neoformans 67 in purified CPS reduced phagocytosis and decreased lymphocyte proliferation. (A) PBMC (2 × 105/well) were put into culture with various numbers of untreated, acapsular C. neoformans cells (□) or acapsular C. neoformans cells that had been pretreated with purified CPS (■). Eighteen hours later, coverslips were examined for the percentage of cells that had phagocytosed C. neoformans (Crypto). (B) In parallel, the proliferative responses of PBMC (2 × 105/well) stimulated with various numbers of untreated C. neoformans cells (□) and with C. neoformans that had been treated with purified CPS (■) were compared. After 7 days, lymphocyte proliferation was assessed by [3H]TdR incorporation. The experiment was repeated twice with similar results. ∗, P < 0.01 compared to untreated organisms. E:T, effector/target.
To examine the correlation between the uptake of organisms and lymphocyte proliferation, larger numbers of CPS-coated organisms were added to the culture in the hope of increasing the number of internalized organisms to the level attained with acapsular C. neoformans. However, this approach failed to produce an increase in the number of internalized CPS-coated organisms, and there was no increase in lymphocyte proliferation (data not shown). Therefore, another approach was used to correlate the uptake of C. neoformans with lymphocyte proliferation.
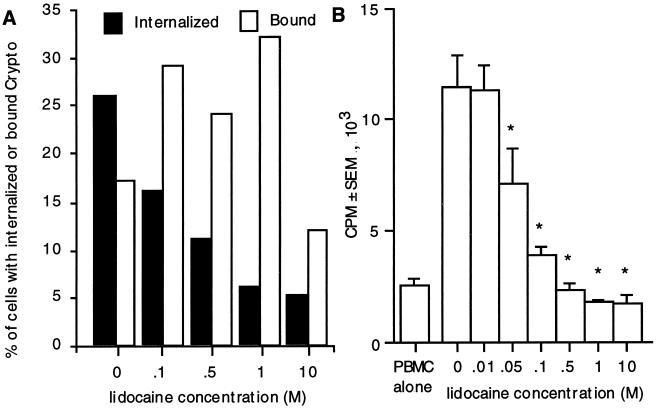
To confirm that phagocytosis correlates with the magnitude of lymphocyte proliferation in response to C. neoformans, acapsular organisms were cultured in the presence of lidocaine. Lidocaine inhibits phagocytosis (6), and the reduction in phagocytosed organisms was correlated with lymphocyte proliferation. There was a dose-dependent inhibition in phagocytosis with between 0.1 and 10 M lidocaine (Fig. 4A) but no consistent change in the number of cells that bound C. neoformans. There was a dose-dependent reduction in lymphocyte proliferation with between 0.05 and 10 M lidocaine (Fig. 4B) that correlated with a reduction in the percentage of cells that had phagocytosed C. neoformans but not with the percentage of cells that had bound the organism. To ensure that lidocaine did not inhibit lymphocyte proliferation by another mechanism, the responses to a mitogen (concanavalin A) and a superantigen (staphylococcal enterotoxin B), which do not require phagocytosis, were tested. Lidocaine did not affect proliferation in response to these stimuli (data not shown). These studies demonstrate that T-cell proliferation is dependent on uptake of the organism.
FIG. 4.
Inhibition of phagocytosis abrogates uptake and lymphocyte proliferation of acapsular C. neoformans 67. PBMC were stimulated with C. neoformans in the presence of various concentrations of lidocaine. Coverslips were examined for the percentage of cells that had bound or internalized C. neoformans (Crypto) (A), and [3H]TdR incorporation was assessed (B). ∗, P < 0.01 compared to 0 M lidocaine by analysis of variance. The experiment was repeated three times with similar results.
Opsonization of polysaccharide-treated C. neoformans overcomes suppression of lymphocyte proliferation.
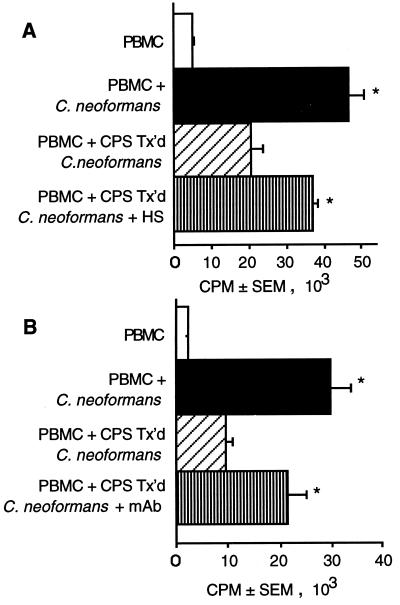
To determine if opsonization with human serum could neutralize the inhibition of lymphocyte proliferation in response to acapsular C. neoformans that had been coated with CPS, treated C. neoformans was incubated for 1 h in non-heat-inactivated human serum and then used to stimulate lymphocytes to proliferate. Human serum augmented lymphocyte proliferation in response to polysaccharide-coated C. neoformans (Fig. 5A). This correlated with an increase in the percentage of cells that had taken up CPS-treated C. neoformans, which went from 7.0% ± 3.5% of the cells in the absence of serum to 26.9% ± 7.5% of the cells in the presence of serum (n = 4 experiments).
FIG. 5.
Treatment with non-heat-inactivated human serum or
anticapsular MAb augments lymphocyte proliferation in response to
CPS-coated acapsular C. neoformans 67. PBMC (□) were
stimulated with untreated C. neoformans (■) or C.
neoformans that had been preincubated with 10 μg of purified CPS
per ml
( ).
CPS-treated organisms were incubated in human serum (HS) (A) or with 10
μg of MAb to polysaccharide per ml (B) (▥). In panel A the
percentage of cells that had taken up CPS-treated C.
neoformans increased from 8% of cells without serum to 29% of
cells with serum opsonization. In panel B the percentage of cells that
had taken up CPS-treated C. neoformans increased from 10%
of cells in the absence of antibody to 45% of cells in the presence of
antibody. ∗, P < 0.05 compared to PBMC plus C.
neoformans. †, P < 0.05 compared to PBMC plus
CPS-treated C. neoformans. The experiment was repeated three
times with similar results.
).
CPS-treated organisms were incubated in human serum (HS) (A) or with 10
μg of MAb to polysaccharide per ml (B) (▥). In panel A the
percentage of cells that had taken up CPS-treated C.
neoformans increased from 8% of cells without serum to 29% of
cells with serum opsonization. In panel B the percentage of cells that
had taken up CPS-treated C. neoformans increased from 10%
of cells in the absence of antibody to 45% of cells in the presence of
antibody. ∗, P < 0.05 compared to PBMC plus C.
neoformans. †, P < 0.05 compared to PBMC plus
CPS-treated C. neoformans. The experiment was repeated three
times with similar results.
To determine if MAb to CPS could also overcome suppression of lymphocyte proliferation, C. neoformans that had been coated with CPS was incubated with anticapsular antibody and then used to stimulate lymphocytes to proliferate. Anti-CPS MAb augmented lymphocyte proliferation in response to polysaccharide-treated C. neoformans (Fig. 5B). This correlated with an increase in the percentage of cells that had taken up CPS-treated C. neoformans, which went from 11.3% ± 2.3% of the cells in the absence of antibody to 34.2% ± 4.7% of the cells in the presence of antibody (n = 3 experiments).
Opsonization of encapsulated C. neoformans enhances lymphocyte proliferation.
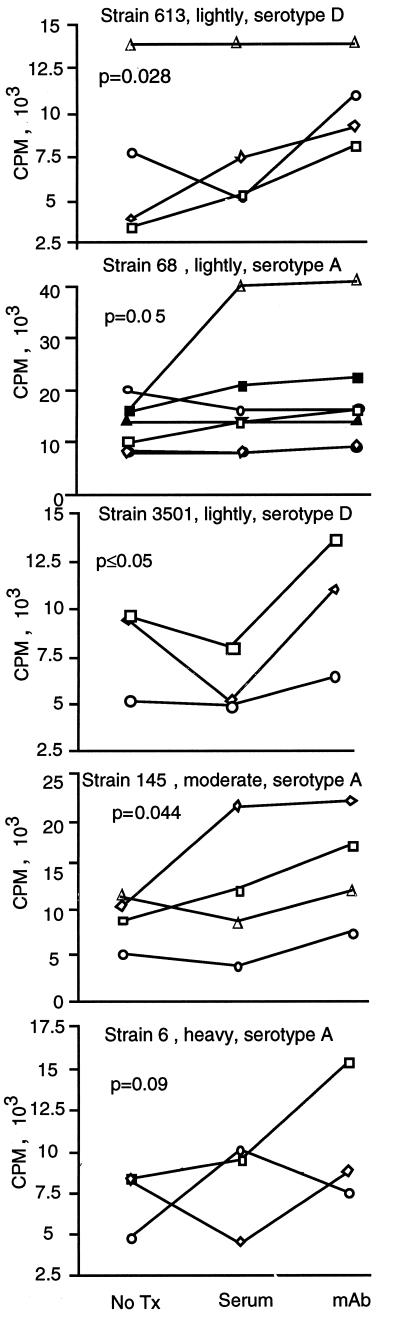
Having established the effects of an opsonic antibody on acapsular organisms that had been coated with capsular polysaccharide, we performed experiments to compare the abilities of anti-CPS MAb and human serum to augment lymphocyte proliferation in response to four different encapsulated strains of C. neoformans. Preincubation in normal human serum or with anticryptococcal antibody increased lymphocyte proliferative responses to encapsulated strains of C. neoformans regardless of the serotype (Fig. 6), which correlated with increased association of C. neoformans with adherent PBMC (data not shown). For all but the most highly encapsulated strains tested, lymphocyte proliferation was greater when organisms were treated with anticapsular antibody than when they were treated with human serum. Thus, treatment with MAb to CPS was an effective way to augment lymphocyte proliferation.
FIG. 6.
Anticapsular antibody is more effective than human serum in augmenting T-cell responses. Various encapsulated strains of C. neoformans (613, 68, 3501, T145, and 6) were not pretreated (No Tx) or pretreated by being incubated in normal human serum or with 10 μg of anti-CPS antibody per ml for 1 h at 37°C. The organisms were washed and put into culture at 2 × 105/well with PBMC (2 × 105/well) in medium containing heat-inactivated serum. Seven days later, lymphocyte proliferation was assessed by [3H]TdR incorporation. Each symbol represents an individual experiment. Two-way analysis of variance was performed by the Friedman test, and multiple comparisons determined that significant differences were found between the group that was not pretreated and the group that was treated with MAb.
DISCUSSION
We have made three observations: (i) CPS did not inhibit lymphocyte proliferation by an IL-10-dependent mechanism or by directly affecting antigen-presenting or accessory cells; (ii) CPS suppressed phagocytosis, and this correlated with lymphocyte proliferation; and (iii) opsonization with an anticapsular MAb increased lymphocyte proliferation and phagocytosis and was more effective than opsonization with human serum.
One of the most important virulence factors of C. neoformans is its polysaccharide capsule. CPS has a number of important effects on the immune response. It inhibits phagocytosis (25) and induces the release of immunosuppressive cytokines such as IL-10 (45). CPS can also inhibit production of TNF-α and IL-1β (44), which are important in the host defense to C. neoformans (1, 2, 20). Further, CPS can affect leukocyte infiltration in inflammatory responses by causing shedding of L-selectin and TNF receptors (14). We and others have previously shown that there is an inverse correlation between the size of the capsule and lymphocyte proliferation (5, 33). Further, the addition of exogenous capsule inhibits lymphocyte proliferation in response to an acapsular strain (33). In this study, we found that the major mechanism by which CPS suppressed lymphocyte proliferation was by inhibiting uptake of the organisms that was necessary for presentation to lymphocytes, rather than by inducing IL-10 or by directly suppressing the function of antigen-presenting cells or T cells.
A previous study suggested that CPS-induced IL-10 production was an important mechanism in the CPS-mediated suppression of lymphocyte proliferation (40). However, three pieces of evidence indicate that CPS-induced IL-10 production was not responsible for suppression of lymphocyte proliferation in our studies. First, preincubation of responding cells with CPS failed to affect subsequent lymphocyte proliferation. If incubation of PBMC with CPS had stimulated the production of inhibitory concentrations of IL-10, it should have affected lymphocyte proliferation. We found no suppression when PBMC were incubated with CPS, suggesting that the effect was not due to IL-10. Second, previous studies have demonstrated that an encapsulated strain of C. neoformans does not suppress the response to an acapsular strain (33). If the encapsulated strain had been inducing IL-10 production that suppressed lymphocyte proliferation, the IL-10 produced in response to the encapsulated strain should have suppressed the proliferation in response to the acapsular strain. The fact that the encapsulated strain did not suppress lymphocyte proliferation in response to the acapsular strain suggests that IL-10 was not responsible for the effect. Finally, an anti-IL-10 antibody did not restore lymphocyte proliferation, suggesting that IL-10 is not the primary reason that CPS reduces proliferative responses to C. neoformans.
The reason for the discrepancy between our studies and the previous studies is not apparent; however, the previous studies evaluated the contribution of IL-10 in response to two nonisogenic strains of C. neoformans (40), while in our studies, the same strain was used to compare the responses with and without CPS. Thus, it may be that the effect is related to phenotypic differences of the strains. We considered the possibility that the amount of polysaccharide in our cultures overwhelmed the ability of anti-IL-10 to neutralize the cytokine. This seems unlikely, since low levels of IL-10 are secreted in response to CPS, and anti-IL-10 antibody was able to enhance CPS-induced TNF-α release. Our data does agree with the finding by Retini et al. (40) that CPS impairs phagocytosis and extends this observation to demonstrate that this is an important mechanism responsible for reduced lymphocyte proliferation.
We found that CPS affected lymphocyte proliferation by binding to C. neoformans. Binding of free polysaccharide to cryptococcal organisms has been well described (22). Incubation of nonencapsulated Cryptococcus with purified cryptococcal polysaccharide renders nonencapsulated cells resistant to phagocytosis (23, 25, 26). We found that CPS bound to the organisms, which limited uptake and resulted in a significant reduction in lymphocyte proliferation.
Knowing that diminished lymphocyte proliferation in the presence of CPS was due to decreased uptake of the organism, we were interested in determining whether opsonizing the organism might increase uptake and restore lymphocyte proliferation. Initially, we used human serum as a source of opsonins. Human serum with active complement can opsonize C. neoformans, while heat-inactivated serum does not (7). Cryptococcus is opsonized by C3 fragments, which bind to the capsule and opsonize cryptococci, increasing uptake (28, 29, 31). Highly encapsulated strains are more potent activators of complement than acapsular strains (48). We found that preincubation in complement-sufficient human serum enhanced uptake of polysaccharide-coated organisms and that this was associated with improved lymphocyte proliferation.
The role of natural antibody in the host defense to C. neoformans is controversial. Patients who are predisposed to cryptococcal infections have defects in cell-mediated immunity. By contrast, patients with isolated defects in antibody production do not have a meaningful increase in the incidence of cryptococcal infections. This has led to the assumption that T-cell-mediated immunity is important, while humoral immunity is not. However, there are numerous studies demonstrating that specific anticryptococcal antibody enhances granuloma formation (17) and is protective in murine models (9, 15, 19, 50). Since T cells are clearly important in cryptococcal host defense, we considered the possibility that protective antibody might somehow influence T-cell responses and enhance the host defense by this mechanism. This is supported by recent studies demonstrating that T cells can cooperate with administered antibody to induce protective responses and increase survival in a murine model (50) and by other studies where anticryptococcal antibody augmented lymphocyte proliferation (46). We were interested in determining whether an anticapsular MAb was more effective at restoring lymphocyte proliferation than human serum, reasoning that following vaccination (passive or active therapy with specific anti-CPS antibody), both would exert their effect in vivo.
Anticryptococcal antibodies can have protective, nonprotective, or disease-enhancing effects on the host defense (36, 38, 49), suggesting that they have multiple effects on the immune response. For our studies, we selected antibodies that had been demonstrated to be protective in a murine system. We found that lymphocyte proliferation in the presence of a specific anticapsular antibody was significantly better than that in the presence of human serum for all but the most highly encapsulated strains of C. neoformans.
Recently, the presence of anticapsular antibody has been found to increase production of IL-1β, TNF-α, and IL-2 (46). Our studies indicate that one mechanism by which anticapsular antibody enhances cell-mediated immunity is by promoting the uptake of organisms by the antigen-presenting cells, which facilitates presentation to T cells. Additionally, increased uptake of the organisms could enhance activation of antigen-presenting cells, which would in turn enhance production of IL-1β and TNF-α (46). The increased presentation of antigen that resulted from enhanced antigen uptake might also enhance IL-2 production (46) and hence lymphocyte proliferation. Thus, the effects of anti-CPS antibody on cytokine levels can be explained by increased antigen being presented to T cells as well as increased stimulation and production of favorable cytokines.
Our studies support the rationale for vaccine therapy for cryptococcosis. A vaccine has been prepared by conjugating glucoronxylmannan to tetanus toxoid (11). This vaccine elicits murine and human antibody responses (10, 11, 39). Antibodies to glucoronxylmannan conjugated to tetanus toxoid promote phagocytosis of C. neoformans in the absence of complement (51), and the vaccine is protective in a murine model (10). Our data suggests that one mechanism by which these antibodies may be effective is by enhancing the uptake of organisms by antigen-presenting cells and thus enhancing presentation to T cells, augmenting cell-mediated immunity.
In summary, we have shown that exogenous polysaccharide can inhibit cell-mediated immune responses by binding to organisms and reducing their uptake by antigen-presenting cells. Further, opsonization by normal human serum and by anticapsular antibody can augment the responses to C. neoformans. Our studies support the therapeutic potential of stimulating cell-mediated immunity with anticapsular antibody.
ACKNOWLEDGMENTS
We thank Jason Spurrell and Peter Warren for technical assistance.
This work is supported by a grants from the Medical Research Council and The Canadian Foundation for AIDS Research and by Public Health Service grant AI14209. R.M.S. was supported by a National Health Research and Development Program Studentship. C.H.M. is a Scholar of the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Aguirre K, Havell E A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformansin the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi E, Barluzzi R, Mazzolla R, Pitzurra L, Puliti M, Saleppico S, Bistoni F. Biomolecular events involved in anticryptococcal resistance in the brain. Infect Immun. 1995;63:1218–1222. doi: 10.1128/iai.63.4.1218-1222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaka W, Scharringa J, Verheul A F, Verhoef J, Van Strijp A G, Hoepelman I M. Quantitative analysis of phagocytosis and killing of Cryptococcus neoformansby human peripheral blood mononuclear cells by flow cytometry. Clin Lab Immunol. 1995;2:753–759. doi: 10.1128/cdli.2.6.753-759.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coker R J. Cryptococcal infection in AIDS. Int J Sex Transm Dis AIDS. 1992;3:168–172. doi: 10.1177/095646249200300303. [DOI] [PubMed] [Google Scholar]
- 5.Collins H L, Bancroft G J. Encapsulation of Cryptococcus neoformansimpairs antigen-specific T-cell responses. Infect Immun. 1991;59:3883–3888. doi: 10.1128/iai.59.11.3883-3888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das K C, Misra H P. Impairment of RAW 264.7 macrophage function by antiarrhythmic drugs. Mol Cell Biochem. 1994;132:151–162. doi: 10.1007/BF00926924. [DOI] [PubMed] [Google Scholar]
- 7.Davies S R, Clifford D P, Hoidal J R, Repine J E. Opsonic requirements for the uptake of Cryptococcus neoformansby human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1982;145:870–874. doi: 10.1093/infdis/145.6.870. [DOI] [PubMed] [Google Scholar]
- 8.Del Prete G, De Carli M, Almerigogna F, Giudizi M G, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–360. [PubMed] [Google Scholar]
- 9.Deshaw M, Pirofski L A. Antibodies to the Cryptococcus neoformanscapsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clin Exp Immunol. 1995;99:425–432. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devi S J. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformansin a murine model. Vaccine. 1996;14:841–844. doi: 10.1016/0264-410x(95)00256-z. [DOI] [PubMed] [Google Scholar]
- 11.Devi S G N, Schneerson R, Egan W, Ulrich T J, Bryla D, Robbins J B, Bennett J E. Cryptococcus neoformansserotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991;59:3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocyte: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dismukes W E. Cryptococcal meningitis in patients with AIDS. J Infect Dis. 1988;157:624–628. doi: 10.1093/infdis/157.4.624. [DOI] [PubMed] [Google Scholar]
- 14.Dong Z M, Murphy J W. Cryptococcal polysaccharides induce L-selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J Clin Invest. 1996;97:689–698. doi: 10.1172/JCI118466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dromer J, Perronne C, Barge J, Vilde J L, Yeni P. Role of IgG and the complement component C5 in the initial course of experimental cryptococcosis. Clin Exp Immunol. 1989;78:412–417. [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert T F, Kozel T R. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformanscapsular polysaccharide. Infect Immun. 1987;55:1895–1899. doi: 10.1128/iai.55.8.1895-1899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 18.Grant I H, Armstrong D. Fungal infections in AIDS. Cryptococcosis. Infect Dis Clin N Am. 1988;2:457–464. [PubMed] [Google Scholar]
- 19.Houpt D C, Pfrommer G S, Young B J, Larson T A, Kozel T R. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformansglucuronoxylomannan. Infect Immun. 1994;62:2857–2864. doi: 10.1128/iai.62.7.2857-2864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huffnagle G B, Toews G B, Burdick M D, Boyd M B, McAllister K S, McDonald R A, Kunkel S L, Strieter R M. Afferent phase production of TNF-α is required for development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 21.Jacobson E S, Ayers D J, Harrell A C, Nicholas C C. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J Bacteriol. 1982;150:1292–1296. doi: 10.1128/jb.150.3.1292-1296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozel T R, Hermerath C A. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect Immun. 1984;43:879–886. doi: 10.1128/iai.43.3.879-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozel T R. Nonencapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977;16:99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozel T R, Cazin J. Nonencapsulated variant of Cryptococcus neoformans. I. Virulence studies of characterization of soluble polysaccharide. Infect Immun. 1971;3:287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozel T R, Mastroianni R P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976;14:62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozel T R, McGaw T G. Opsonization of Cryptococcus neoformansby human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect Immun. 1979;25:255–261. doi: 10.1128/iai.25.1.255-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozel T R, Pfrommer G S T, Guerlain A S, Highison B A, Highison G J. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun. 1988;56:2794–2800. doi: 10.1128/iai.56.11.2794-2800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitz S M, DiBenedetto D J. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988;56:2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitz S M, Farrell T P. Growth inhibition of Cryptococcus neoformansby cultured human monocytes: role of the capsule opsonins, the culture surface, and cytokines. Infect Immun. 1990;58:1201–1209. doi: 10.1128/iai.58.5.1201-1209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levitz S M, Farrell T P, Maziarz R T. Killing of Cryptococcus neoformans by human peripheral blood mononuclear cells stimulated in culture. J Infect Dis. 1991;163:1108–1113. doi: 10.1093/infdis/163.5.1108. [DOI] [PubMed] [Google Scholar]
- 31.Levitz S M, Tabuni A. Binding of Cryptococcus neoformansby human cultured macrophages. Requirements for multiple complement receptors and actin. J Clin Invest. 1991;87:528–535. doi: 10.1172/JCI115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell T G, Friedman L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect Immun. 1972;5:491–498. doi: 10.1128/iai.5.4.491-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mody C H, Syme R M. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61:464–469. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mody C H, Toews G B, Lipscomb M F. Cyclosporin A inhibits the growth of Cryptococcus neoformansin a murine model. Infect Immun. 1988;56:7–12. doi: 10.1128/iai.56.1.7-12.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monari C, Retini C, Palazzetti B, Bistoni F, Vecchiarelli A. Regulatory role of exogenous IL-10 in the development of immune response versus Cryptococcus neoformans. Clin Exp Immunol. 1997;109:242–247. doi: 10.1046/j.1365-2249.1997.4021303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee J, Nussbaum G, Scharff M D, Casadevall A. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformansoriginating from one B cell. J Exp Med. 1995;181:405–409. doi: 10.1084/jem.181.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee J, Zuckier L S, Scharff M D, Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformansglucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994;38:580–587. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee S, Lee S C, Casadevall A. Antibodies to Cryptococcus neoformansglucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1994;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirofski L, Lui R, DeShaw M, Kressel A B, Zhong Z. Analysis of human monoclonal antibodies elicited by vaccination with a Cryptococcus neoformansglucuronoxylomannan capsular polysaccharide vaccine. Infect Immun. 1995;63:3005–3014. doi: 10.1128/iai.63.8.3005-3014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel T R. Encapsulation of Cryptococcus neoformanswith glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun. 1998;66:664–669. doi: 10.1128/iai.66.2.664-669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Small J M, Mitchell T G, Wheat R W. Strain variation in composition and molecular size of the capsular polysaccharide of Cryptococcus neoformansserotype A. Infect Immun. 1986;54:735–741. doi: 10.1128/iai.54.3.735-741.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiropulu C, Eppard R A, Otteson E, Kozel T R. Antigenic variation within serotypes of Cryptococcus neoformansdetected by monoclonal antibodies specific for the capsular polysaccharide. Infect Immun. 1989;57:3240–3242. doi: 10.1128/iai.57.10.3240-3242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformansregulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217–223. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformansinduces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vecchiarelli A, Retini C, Monari C, Casadevall A. Specific antibody to Cryptococcus neoformansalters human leukocyte cytokine synthesis and promotes T-cell proliferation. Infect Immun. 1998;66:1244–1247. doi: 10.1128/iai.66.3.1244-1247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson D E, Bennett J E, Bailey J W. Serologic grouping of Cryptococcus neoformans. Proc Soc Exp Biol Med. 1968;127:820–823. doi: 10.3181/00379727-127-32812. [DOI] [PubMed] [Google Scholar]
- 48.Young B J, Kozel T R. Effects of strain variation, serotype, and structural modification on kinetics for activation and binding of C3 to Cryptococcus neoformans. Infect Immun. 1993;61:2966–2972. doi: 10.1128/iai.61.7.2966-2972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan R, Casadevall A, Spira G, Scharff M D. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformansinto a protective antibody. J Immunol. 1995;154:1810–1816. [PubMed] [Google Scholar]
- 50.Yuan R, Casadevall A, Scharff M D. T cells cooperate with passive antibody to modify Cryptococcus neoformansinfection in mice. Proc Natl Acad Sci USA. 1997;94:2483–2488. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong Z, Pirofski L. Opsonization of Cryptococcus neoformansby human anticryptococcal glucuronoxylomannan antibodies. Infect Immun. 1996;64:3446–3450. doi: 10.1128/iai.64.9.3446-3450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]