Abstract
Transcription factors (TFs) play important roles in regulating multiple biological processes by binding to promoter regions and regulating the global gene transcription levels. Pseudomonas syringae is a Gram-negative phytopathogenic bacterium harbouring 301 putative TFs in its genome, approximately 50 of which are responsible for virulence-related gene and pathway regulation. Over the past decades, RNA sequencing, chromatin immunoprecipitation sequencing, high-throughput systematic evolution of ligands by exponential enrichment, and other technologies have been applied to identify the functions of master regulators and their interactions in virulence-related pathways. This review summarises the recent advances in the regulatory networks of TFs involved in the type III secretion system (T3SS) and non-T3SS virulence-associated pathways, including motility, biofilm formation, quorum sensing, nucleotide-based secondary messengers, phytotoxins, siderophore production, and oxidative stress. Moreover, this review discusses the future perspectives in terms of TF-mediated pathogenesis mechanisms and provides novel insights that will help combat P. syringae infections based on the regulatory networks of TFs.
Keywords: Pseudomonas syringae, Transcription factors, Virulence, Regulatory networks
1. Introduction
Pseudomonas syringae is a model phytopathogenic bacterium and can infect many plants, including major crops. To establish infection and incite disease in plants, P. syringae first enters the host plant, overcomes its immune system, and then causes disease [1]. P. syringae uses flagella- and type IV pili-mediated motility and performs active taxis for entering the host cells [2], [3]. Once P. syringae cells enter the apoplasts, they secrete a group of hypersensitive response and pathogenicity (Hrp) type III secretion system (T3SS) effectors and phytotoxins to suppress host resistance and enhance disease development [4], [5], [6]. Additionally, P. syringae can exert its virulence via biofilm formation [7], siderophore production [8], and c-di-GMP-mediated signaling [9]. To successfully infect plants and cause disease, bacteria require many regulators, including nucleotide-based secondary messengers and transcription factors (TFs). Herein, we summarise the integrative virulence-related TFs and complex regulatory networks, providing insights into the regulatory mechanisms of P. syringae pathogenesis.
TFs bind genomic DNA and play crucial roles in regulating numerous biological pathways [10]. Several important virulence-related TFs have been previously identified and characterised in P. syringae [11], [12], [13], [14], [15]. For example, RhpRS, a two-component system (TCS), has been identified as a master regulator of T3SS genes by directly regulating hrpRS and other virulence-related genes by sensing environmental and polyphenol signals in plants [16], [17], [18]. T3SS genes are regulated by at least 12 additional regulators, including sigma factors (HrpL, RpoN, and AlgU) [16], [19], [20], [21] and other regulators (AefR, CorR, HrpA, Lon, HrpV, GacA, CvsR, PsrA, and RhpPC) [22], [23], [24], [25], [26], [27], [28], [29], [30]. In recent years, owing to the development of research technologies, including RNA sequencing (RNA-Seq), chromatin immunoprecipitation sequencing (ChIP-Seq) [14], [31], [32], [33], and high-throughput systematic evolution of ligands by exponential enrichment (HT-SELEX) [34], new regulators have been identified and predicted in P. syringae virulence-related pathways. We map the genome-wide regulons of 16 key virulence-related transcriptional regulators and designated their crosstalk as an intricate network named ‘PSRnet’. We find five additional T3SS regulators (EnvZ-OmpR, CbrAB2, PhoPQ, PilRS, and MgrA) in this network [31]. In another study, we have identified binding motifs for 100 of the 301 putative annotated P. syringae TFs using HT-SELEX [34]. These 100 TFs have 118 different position weight matrix motifs: 106 dimer-binding motifs, 8 monomer-binding motifs, and 4 motifs with trimer-binding specificity. The target genes of 25 of the 100 TFs are related to virulence-associated pathways, including the T3SS, bacterial motility, siderophore production, and surface attachment.
A deeper understanding of virulence-related regulatory crosstalk can provide significant insights into the mechanisms underlying the pathogenesis of P. syringae, allowing researchers to develop novel approaches to tackle the infection and the associated economic losses. This review presents a global overview of virulence-related regulators, especially TFs, and maps five regulatory networks of the T3SS and non-T3SS pathways of P. syringae, namely motility, biofilm formation, and nucleotide-based secondary messenger function; quorum sensing (QS); phytotoxins; oxidative stress resistance; and siderophore production.
2. Twenty-two TFs control T3SS in different environments in P. syringae
The T3SS has a complicated structure that allows bacterial pathogens to inject effector proteins directly into the host cell cytoplasm, thereby causing infection and diseases [20]. Numerous environmental signals and factors can regulate the T3SS, which is induced in planta or in nutrient-poor, low osmotic, and acidic conditions [e.g., in minimal medium (MM)] but inhibited under nutrient-rich, high pH, and high osmotic conditions [e.g., King’s B medium (KB)] in vitro [35], [36], [37]. Because the pathogenicity of P. syringae mainly relies on the T3SS [38], in the present review, we focus on summarising the critical and complicated regulatory networks related to the T3SS under different conditions (Fig. 1, Fig. 2).
Fig. 1.
The T3SS regulatory network in MM (inducingconditions) in P. syringae. T3SS is primarily activated by the HrpRSL pathway, which is under the control of the top TCS RhpRS under inducing conditions (MM or in planta). More than 20 regulators collectively control this regulatory network (CorRS, CvsRS, GacAS, RhpRS, PilRS, HrpRS, HrpL, HrpA, HrpT, AefR, PsrA, RhpP, RpoN, HrpV, HrpG, HrpF, HrpJ, Lon, and PSPPH_3618). In MM, HrpRS binds the hrpL promoter region to induce its expression, and HrpL directly activates the hrp box and the transcription of T3SS-related genes. GacA indirectly inhibits hrpRS transcription, whereas CvsR, HrpA, and AlgU directly induce the transcription of hrpRS. HrpT, PilR, and HrpL repress hrpL expression, whereas AefR and PsrA activate its expression in MM. PilR also suppresses the expression of T3SS effectors, including hrpW1, hopX1, hopG1, and hopF3. Lon inhibits the expression of hopR1, hrpB, and hopAJ1. PSPPH_3618 positively regulates the transcription of avrB2 and rhpP. RhpP directly degrades the HrpL protein. In planta, RhpS senses polyphenols through the functions of the Pro40 residue and inhibits T3SS by reducing the phosphate activity.
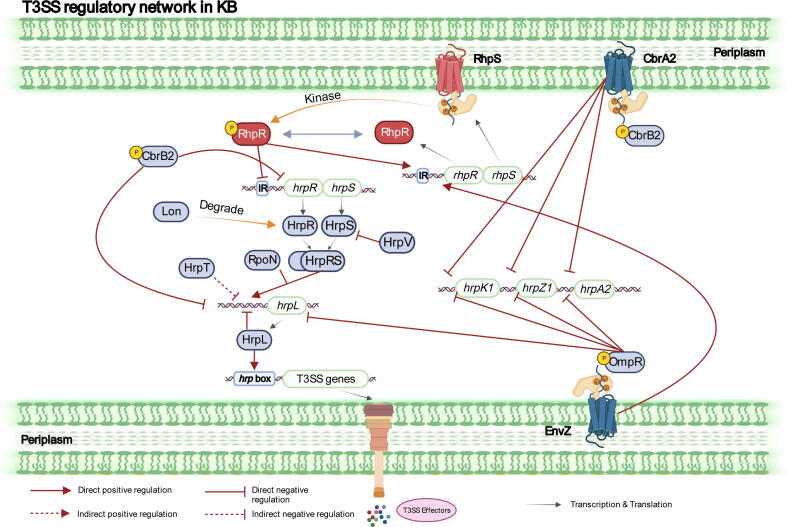
Fig. 2.
The T3SS regulatory network in KB (inhibitory conditions) in P. syringae. T3SS is inhibited in nutrient-rich media such as KB. RhpS is a histidine kinase. P-RhpR represses the transcription of hrpRS by binding the inverted repeats in the upstream regions. Other regulators, including CbrB2, EnvZ/OmpR, HrpV, and HrpT, also control this pathway under inhibitory conditions. CbrB2 and OmpR suppress the expression of hrpL, hrpK1, hrpZ1, and hrpA2. CbrB2 directly restrains hrpRS transcription. Lon acts as a negative regulator of T3SS by degrading HrpRS.
In P. syringae, T3SS activation largely depends on the HrpRSL (hrpRS and hrpL) pathway in planta or in MM. hrpR and hrpS are adjacently located and share the same promoter that is present in front of hrpR. HrpRS forms a hetero-hexamer and induces hrpL transcription under the assistance of σ54 (RpoN), which encodes an alternative RNA polymerase sigma factor [13]. HrpL binds the hrp box (GGAACC-N15/16-CCACNNA), which is found in the promoter regions of most T3SS effector genes. The induction of hrpRS operon transcription is mainly mediated by regulators such as AlgU, HrpA, and several TCSs (RhpRS, CvsRS, and GacAS) [14], [17], [33], [39], [40]. For example, AlgU controls the transcription of hrpL, hrpRS, and other T3SS effector genes [33]. Moreover, HrpA can induce the expression of hrpRS, hrpL, and hrcC [39].
RhpRS is the best-studied master TCS that regulates T3SS genes and bacterial virulence. Our previous studies have shown that an rhpS mutant remarkably reduces disease severity [14], [17], [40], [41]. rhpRS is located in the same operon as the hrpRS operon. RhpS is a histidine kinase (HK) and functions as an autokinase upon itself and as a phosphatase on its cognate response regulator (RR) RhpR [14]. These components help P. syringae sense and respond to external environmental stress by harmonising T3SS gene expression. Phosphorylated RhpR (P-RhpR) has a high binding affinity to the inverted repeats at the 5′ end of the upstream hrpRS and directly suppresses hrpRS transcription. Our recent studies have identified three polyphenols (tannic acid; 1,2,3,4,6-pentagalloylglucose; and epigallocatechin gallate) that suppress both the kinase and phosphatase activities of RhpS in vitro and induce the expression of rhpRS operon by directly targeting RhpS. Three above-mentioned polyphenols can be sensed by Pro40 of RhpS and repress its phosphatase activity, thereby inhibiting virulence through phosphorelay crosstalk with three other non-cognate HKs (PSPPH_3550, 5115, and 3736) [18]. In T3SS-induced conditions, RhpS performs phosphatase activity on P-RhpR and thus reverses the inhibition of hrpRS operon, leading to induction of the downstream hrpRSL pathway. The non-cognate HKs phosphorylate RhpR, suppressing T3SS and virulence in P. syringae. CvsRS is a metal ion-sensing TCS, and its expression is induced by Ca2+, whose concentration is higher in the apoplast during bacterial infection [42]. CvsR can bind to the promoter of the hrpRS operon and activate T3SS-related genes expression in MM [26]. On the other hand, GacAS modulates hrpL transcription and contributes to hrpRS operon induction, which induces T3SS [39], [43]. On the other hand, GacAS is considered a negative regulator of the T3SS in P. syringae and is not even required for its pathogenesis in Arabidopsis leaf tissue [29]. We propose that GacAS have different functions on T3SS in different subspecies [31]. On the leaf surface, GacAS is activated, leading to the repression of T3SS and induction of flagellar-dependent motility. When bacteria enter the leaf apoplast, the GacAS system is deactivated, leading to de-suppression of T3SS and virulence. Once bacteria reach a high density, GacAS might be re-activated to repress T3SS [44].
Similar to the ExsADCE system in P. aeruginosa, an HrpSVG downbeat feedback regulatory system tunes the T3SS in P. syringae [45]. In P. aeruginosa, ExsA is a master transcription activator of T3SS. ExsD is an anti-activator that inhibits T3SS by disrupting the self-association of ExsA and its binding to DNA. ExsC is an anti-anti activator that forms a complex with ExsD to relieve the inhibitory effects of ExsD on ExsA [46]. In P. syringae, the crosstalk of HrpS/V/G in the regulation of T3SS is characteristically equivalent to ExsA/D/C of P. aeruginosa [47]. HrpV directly binds HrpS via the AAA+ domain to destroy the HrpRS heterodimer, resulting in the negative regulation of the T3SS [48]. Meanwhile, HrpG can remove the anti-activator HrpV from HrpS [49], and HrpJ allows the de-repression of the T3SS by binding the HrpG/HrpV heterodimeric chaperone [50]. Moreover, HrpF plays two roles in the regulation of T3SS assembly; it either functions as a negative regulator by possibly participating in HrpSVG circuit regulation via interactions with HrpG or stabilises HrpA in the cytoplasm [47].
hrpL is also co-regulated by other regulators, including AefR, PsrA, HrpT, and TCS CorRS. CorR can bind to the promoter region of hrpL and directly induce its early transcription, thereby impacting the expression of hrp regulon [22]. PsrA plays indirect and positive regulatory roles in hrpL transcription [27]. AefR indirectly upregulates the transcription of hrpL and thus contributes to pathogenicity in bean plants [51], [52], whereas HrpT suppresses hrpL expression in an environment-dependent manner [53]. Moreover, Lon and RhpP proteases play essential roles in T3SS regulation by targeting HrpR and HrpL, respectively [54]. The levels of various T3SS proteins, including HrcV, HrpW1, and AvrB2, are higher in KB in the lon mutant compared with the wild-type [55]. RhpP reduces the HrpL protein levels in P. syringae [28].
RhpRS is a master regulator of T3SS in P. syringae. In inhibited conditions, RhpS acts as a kinase that transfers phosphoryl on RhpR (P-RhpR), which represses the transcription of the hrpRS operon and inhibits T3SS. In contrast, in T3SS-inducing environments such as in planta or MM, RhpS mainly functions as phosphatase and removes the phosphoryl from RhpR, which relieves its suppression of hrpRS on T3SS.
In recent studies, we use ChIP-Seq and RNA-Seq to identify novel T3SS regulators, including TCSs and TFs. The expression of T3SS genes, including hrpR, hrpL, hrpA2, hrpZ1, and hrpK1, is significantly upregulated in ΔompR and ΔenvZ, indicating that TCS EnvZ-OmpR represses the T3SS in P. syringae in KB. The other two TCSs, CbrAB2 and PilRS, exert similar adverse regulatory effects on T3SS effector gene expression in KB in P. syringae [31]. Additionally, HT-SELEX identifies a novel master regulator, PSPPH_3618, which is involved in the T3SS regulatory network. PSPPH_3618 can directly bind the promoters of avrB2 and rhpPC, downregulate their expression levels, and further reduce the pathogenicity of P. syringae [34]. We conclude that two regulatory networks act on the T3SS under either inducing or inhibiting conditions, contributing to a better understanding of the essential virulence pathways (Fig. 1, Fig. 2).
3. P. syringae employs 14 TFs to control motility
Motility is essential for a bacterial pathogen to spread on the plant surface and colonise host plants. Several types of motility occur in P. syringae: flagella-dependent liquid swimming, surface swimming, swarming motility, and type IV pili (T4P)-dependent twitching motility. Motility is an important virulence factor in P. syringae because the mutation of flagella-related genes may cause P. syringae to lose its motility, remarkably decreasing its virulence [56], [57]. The type of motility via which a pathogen causes infection mainly depends on environmental factors, including wetness and viscosity. Both flagella- and T4P-mediated surface motilities are essential for the translocation of P. syringae in leaf apoplasts.
The motility regulatory network of P. syringae is centred around the transcriptional activator FleQ [58] (Fig. 3). It directly induces the expression of fleSR and two essential flagellar basal body rod protein operons – flgBCDE and flgFGHIJKL – as well as many other flagella-related genes, including fliH, fliE, fliG, and fliJ [58], [59]. Moreover, the transcriptional levels of flhA, flhF, fleN, and fliA are lower in pathogens lacking FleQ than in wild-type pathogens, which provides basal concentrations of these proteins [59]. The regulators OmpR, GacA, and CvsR can bind to the promoter region of fleQ and positively regulate its transcription [31]. CbrB2 negatively controls fleQ by binding to its upstream region. ChIP-Seq results reveal that MgrA and PhoP simultaneously bind to the promoter of fleQ [31]. GacA regulates swarming [43], whereas CvsR contributes to swimming and swarming motility [26].
Fig. 3.
The motility regulatory network of P. syringae. Motility in P. syringae majorly occurs via flagella-dependent liquid, surface swimming, and T4P-mediated swarming, allowing the bacterial cells to enter apoplasts. Motility is mainly regulated by the master regulator FleQ. It directly induces the flagellar-related expression of genes (including flgBCDE, flgFGHJKKL, fliH, fliE, fliJ, fliG, fleSR, fleN, fliA, and flhAF). FleQ is induced by CvsR, OmpR, and GacA; but repressed by PilR, CbrB2, RphR, and AlgU. P-RhpR regulates motility by directly inhibiting the transcription of fimA and flhA. AlgU activates the expression of amrZ and pilR and induces the expression of fleQas, which directly enhances flagellar motility. PilR regulates motility by inducing pilBCD and inhibiting algU and mucAB. FleR and PSPPH_2720 regulate the transcription of fliH, fliE, fliJ, and fliG.
AlgU, an extracytoplasmic function sigma factor, is a global virulence regulator that plays essential roles in regulating the motility of P. syringae. Previous studies present the AlgU-dependent regulatory binding landscape and confirms that it downregulates many flagella-related and chemotaxis motility genes [33], [60], [61]. However, AlgU induces antisense transcript expression of the fleQ gene (fleQas) in P. syringae, enhancing its swimming motility and positively controlling its flagellar activity [60]. In addition, P-RhpR can bind to the promoter region of flhA, encoding a flagellar export-related protein, thereby affecting swimming motility [40]. Motility plate culture experiments verify that P-RhpR negatively regulates the swimming motility of P. syringae. fimA, which encodes a type-I fimbrial subunit and is related to twitching motility, is also controlled by P-RhpR. Twitching phenotype tests demonstrate that P-RhpR positively regulates twitching motility by directly binding the promoter region of fimA [40]. Thus, an essential regulatory network is involved in the motility of P. syringae (Fig. 3).
4. Twelve TFs modulate biofilm formation in P. syringae
When P. syringae enters the apoplasts of host plants, they need to establish colonies and form biofilms by secreting highly viscous compounds such as extracellular polysaccharides (EPSs), extracellular DNA, proteins, and lipids, which protects them from host immune resistance and attack by antibiotics [62], [63]. P. syringae generally produces two types of EPS, namely alginate and levan; of the two, alginate is more common [64]. Alginate is a co-polymer composed of O-acetylated β-1,4-linked d-mannuronic acid and its C-5 epimer, l-guluronic acid. Alginate contributes to the rigidity and flexibility of biofilms as it provides a robust and sticky skeleton, as well as interlocking chains that hold bacterial cells together. Alginate biosynthesis is controlled by 12 regulators – AlgU, AlgR, RpoN, HrpL, AmrZ, MgrA, TCSs CvsRS, GacAS, RhpRS, PilRS, OmpR/EnvZ, and PhoPQ; these are summarised as a regulatory network in Fig. 4.
Fig. 4.
Biofilm formation regulatory networks in P. syringae. The biofilm matrix of P. syringae comprises EPS, extracellular DNA, protein, and lipid. EPS primarily consists of alginates produced by alg-related genes, including algD, alg8, alg44, algKEGXL, algIJF, and algA, which are mainly regulated by the sigma factor AlgU. Regulators including MgrA, AmrZ, AlgR, GacA, RpoN, and HrpL also induce alginate synthesis genes (algU, algD, algC, argB, and algR3). Regulators including PilR, OmpR, CvsR, RhpR, and PhoP negatively regulate alginate synthesis, thereby inhibiting the expression of algU and algD in P. syringae. GacA positively regulates EPS production by inducing salA expression.
AlgU not only suppresses flagella-dependent motility but also plays essential roles in regulating alginate production by activating the expression of genes such as algD and alg8, which are essential to this function [31]. AlgU also controls the expression of additional regulators (e.g., AmrZ) that positively participate in this pathway-regulatory crosstalk [65]. Moreover, GacS and SalA activate the modest expression of alginate production-related genes in planta and iron-enriched conditions while contributing significantly to their regulation in vitro in an iron-limited manner [21], [23]. However, CvsR inversely controls biofilm formation by indirectly suppressing the algU transcription levels, thereby repressing alginate production in a Ca2+-dependent manner [26]. Additionally, OmpR plays a key role in the negative regulation of alginate synthesis by directly binding the promoter region of algD and algU, whereas HrpL induces algR3 expression to positively regulate alginate production [31]. AlgR, an alginate biosynthesis regulatory protein, regulates the expression of algC and argB under the assistance of sigma factor RpoN, which is required for bacterial colonisation and biofilm formation [66]. These regulators reveal the intricate regulatory mechanisms underlying biofilm formation in P. syringae.
5. Nucleotide-based secondary messengers play significant roles in many virulence-related pathways
In prokaryotes, nucleotide-based secondary messengers, including cyclic adenosine monophosphate (cAMP), guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (collectively known as (p)ppGpp), and cyclic di-guanosine monophosphate (c-di-GMP), have emerged as essential signal molecules that play important roles in regulating multiple survival- and virulence-related biological functions in bacterial pathogens [67]. A summary of the architecture of these secondary messengers is presented in Fig. 5, with the related regulators and genes indicated.
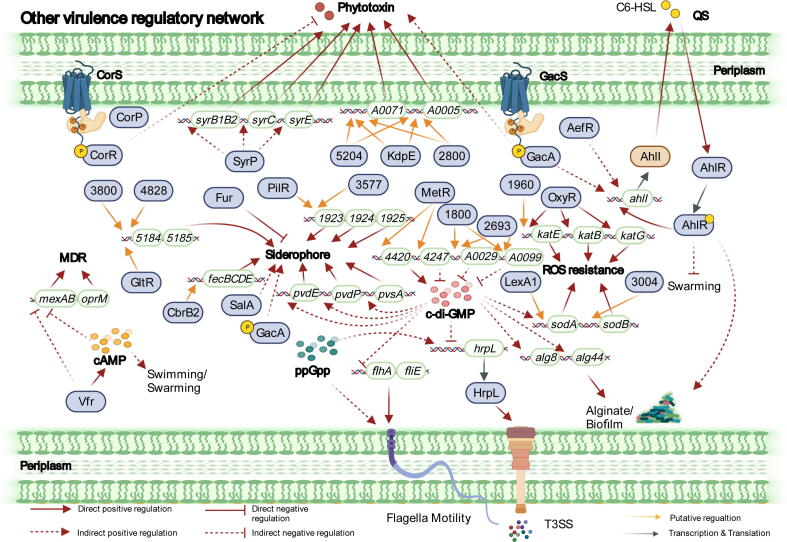
Fig. 5.
Other virulence-related regulatory networks in P. syringae. Other virulence-related pathways, including nucleotide-based secondary messengers (cAMP, (p)ppGpp, and c-di-GMP), QS, phytotoxins, siderophore production, and ROS resistance, play roles in the pathogenesis of P. syringae. Vfr induces cAMP production and suppresses the expression of multidrug resistance genes such as mexAB and oprM. c-di-GMP is regulated by MetR, PSPPH_1800, and PSPPH_2693. c-di-GMP regulates the T3SS, siderophore production, biofilm formation, and ROS resistance by affecting the expression of hrpL, pvdE, pvdP, pvsA, alg8, alg44, sodA, and sodB. AhlR controls the QS system in P. syringae by binding to the promoter region of ahlI with the assistance of GacA and AefR. Six regulators, including CorRS, CorP, SyrP, KdpE, PSPPH_5204, and PSPPH_2800, regulate phytotoxin production via the function of the genes syrB1B2, syrC, syrE, PSPPH_A0071, and PSPPH_A0005. Siderophore production-related genes involved in PSPPH_5184/5185, PSPPH_1923/1924/1925, and fecBCDE are controlled by nine regulators (GltR, CbrB2, Fur, GasAS, SalA, PilR, PSPPH_3800, PSPPH_4828, and PSPPH_3577). The TFs OxyR, PSPPH_1960, LexA1, and PSPPH_3004 regulate ROS resistance by targeting katE, katB, katG, sodA, and sodB.
cAMP, catalysed by adenylyl cyclases, is a cyclic nucleotide that regulates essential processes in bacteria [68]. In P. syringae, cAMP production is reduced in the vfr mutant and abolished in the cyaA mutant. Vfr is a cAMP-regulatory protein, and cyaA encodes an adenylate cyclase [69]. Both mutants show reduced virulence in their hosts, decreased surface motilities, and higher sensitivity to antibiotic compounds through the activation of the expression of multidrug efflux pump genes (mexAB and oprM), which are located in the same operon in P. syringae [69].
(p)ppGpp is a signalling compound that responds to limited nutrient conditions, including amino acid-, fatty acid-, carbon-, and iron-starved conditions [68]. In P. syringae, the intracellular concentration of (p)ppGpp is controlled by two homologous proteins, RelA and SpoT. RelA is a monofunctional synthase that uses GTP and ATP to produce (p)ppGpp. SpoT is a bifunctional hydrolase–synthase that generates and hydrolyses (p)ppGpp [68]. (p)ppGpp participates in multiple bacterial physiological pathways, such as metabolic processes including DNA replication, transcription, and translation, and virulence-associated pathways by inducing virulence factors. In P. syringae, SpoT is an essential virulence effector that affects its motility as well as pyoverdine production and induces T3SS gene expression by activating the transcription of hrpRS and hrpL [70] (Fig. 5).
As one of the best-studied bacterial secondary messengers, c-di-GMP is produced by diguanylate cyclases and eliminated by phosphodiesterases, which are highly conserved in many bacterial pathogens [71]. Studies have revealed that c-di-GMP can modulate several virulence-related pathways in P. syringae, including the T3SS, biofilm formation, reactive oxygen species (ROS) resistance, and siderophore production. High levels of c-di-GMP repress the transcription of hrpR and hrpL, projecting adverse regulatory effects on the T3SS [72]. In addition, increased c-di-GMP levels reduce the swarming and swimming motilities of P. syringae by regulating the expression of flagellar synthesis-related genes. In contrast, c-di-GMP positively regulates biofilm formation by upregulating the expressions of alg8 and alg44, which are required for EPS biosynthesis. Additionally, c-di-GMP can increase siderophore production by inducing the expression of pvdE, pvdP, and pvsA, which encode pyoverdine transporter and peptide synthases [72]. Moreover, a higher level of c-di-GMP allows P. syringae to exert higher tolerance to oxidative stress by upregulating sodA expression [72] (Fig. 5).
6. Three TFs control the QS system in P. syringae
QS involves the production, release, and group-wide detection of extracellular signalling molecules among bacterial communities. Once the QS molecules are secreted by the bacteria and accumulated to a threshold level, the bacteria change their global gene expression traits to exert their virulence and adapt to a high-level cell population density situation [73]. In P. syringae, 3-oxo-hexanoyl-homoserine lactone (C6-AHL) is the primary QS molecule synthesised by an AHL synthase, which is a product of ahlI. C6-AHL interacts with its receptor AhlR, which forms a stable complex to activate the expression of ahlI, further accumulating the concentration of QS molecules [52] (Fig. 5). In addition, aefR mutants cannot produce AHL, thereby reducing the motility and virulence and increasing tolerance to antibiotics by activating mexEF/oprN gene expression [3], [74]. TCS GacAS is also required for AHL production in P. syringae [43], [75]. Of note, AefR and GacAS regulate the QS pathway independently in P. syringae [52], as shown in Fig. 5.
7. Three TFs control phytotoxin secretion in P. syringae
Bacterial pathogens produce phytotoxins to injure host cells and cause diseases. The disease symptoms can be generated with the treatment of phytotoxins without bacterial infection. Pathogens that lose the ability to secret phytotoxins reduce or completely lose their virulence [1]. Phytotoxins, such as coronatine and syringomycin, are produced during infection with P. syringae and produce disease symptoms [76]. P. syringae can produce coronatine to re-open the stomata at biological concentrations during the night; moreover, the pathogen uses syringomycin to target the plasma membranes of host plants to form ion channels and cause cytolysis [77], [78], [79]. Coronatine yields are affected by temperature changes, and its synthesis is induced at 18℃ but significantly repressed at 28℃ [80], [81]. A modified TCS with one HK (CorS) and two RRs (CorR and CorP) serves as a regulatory system that can modulate coronatine production [82] (Fig. 5). The environmental sensor CorS senses temperature changes and allows response regulators to function as positive activators of the coronatine synthesis process by binding to the specific target sequences. In addition, the regulation of syringomycin synthesis is complicated because of nutritional factors and plant signal molecules. SyrB1, SyrB2, SyrC, and SyrE are responsible for catalysing syringomycin production [83]. SyrD, which functions as a transporter protein, allows P. syringae to export this toxin across the cytoplasmic membrane once cyclized [84]. syrP, which encodes a regulatory protein, plays a significant role in controlling syringomycin production and virulence in a phosphorylated manner [85]. However, although the GacAS system is likely at the top of the regulatory hierarchy network, the intermediary regulators remain unknown [86]. This regulatory pathway is presented in Fig. 5.
8. Siderophore production is controlled by 3 TFs in P. syringae
Because the concentration of Fe3+ is relatively low in the environment, iron is required for the growth and complete virulence of many pathogenic bacteria [87]. Mutants lacking siderophore-associated genes exhibit reduced EPS production but increased antibiotic tolerance, and express dysregulated QS and multidrug efflux [8]. In P. syringae, siderophore production is regulated by three TFs (Fur, GacAS, SalA) (Fig. 5). Fur, which encodes a ferric uptake regulation protein, is a master negative regulator of iron homeostasis and is found to control swarming motility and tabtoxin and AHL production [88]. Fur acts as an iron-responsive repressor by binding to its target gene elements, known as Fur box [89]. In iron-limited or iron-rich conditions, Fur slightly and strongly represses siderophore production [88]. In addition, GacAS and SalA play roles in the modulation of siderophore-related genes. In a previous study, genes related to pyoverdine production are downregulated in both ΔsalA and ΔgacS strains under low-Fe conditions, suggesting that SalA and GacS function as important activators of pyoverdine production responding to iron limitation [21].
9. OxyR participates in the regulation of ROS resistance in P. syringae
Plants are always surrounded by many microorganisms, including pathogens such as P. syringae. These pathogens have developed two layers of defence system to prevent themselves from infection. The first line is pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) that recognizes the conserved molecules from pathogens. The second line is effector-triggered immunity (ETI) under the control of individual plant resistance genes [90]. These two layers of defence system can induce similar plant defence responses, including the oxidative burst (superoxide and hydrogen peroxide). PTI induces a weaker and/or short ROS burst, while ETI induces a stronger one. The ROS leads to physical barriers or direct toxicity against the invading pathogens. Therefore, pathogenic bacteria like P. syringae must detoxify the apoplastic ROS and overcome ROS-mediated defence signalling in host plants. OxyR is an oxidative regulatory protein required for the complete virulence and protection of P. syringae from exogenous/endogenous ROS toxicity (Fig. 5). OxyR positively regulates the expression of thioredoxins and catalase genes, including katB, katE, and katG, in response to the effects of H2O2 or during infection in host plants [91]. Overall, we present siderophore production and the ROS resistance regulatory networks in Fig. 5.
10. Similarities, distinctions, and crosstalk between networks
Many TFs play different roles in multiple regulatory systems, while some TFs are specific to certain regulatory networks. For example, HrpRSL and HrpSVG pathways are specific to the T3SS regulatory network, while four TCSs (RhpRS, GacAS, PilRS, and CbrAB2) are participating in T3SS, motility, and biofilm formation systems. Biofilm formation largely relies on the sigma factor AlgU and regulates motility by downregulating flagella-related genes. c-di-GMP plays an essential role in mediating the switch between motility and biofilm formation. Besides, GacAS is a flexible and plastic TCS that can regulate the QS, phytotoxin, and siderophore production system. In addition, AhlR and AefR are required for the QS system, and the latter can also induce T3SS while inhibiting swarming motility indirectly. Furthermore, OxyR and SyrP are specific to the ROS system and phytotoxin secretion, respectively.
c-di-GMP is essential for the crosstalk between motility and biofilm formation. During the initial attachment period, P. syringae needs flagellar motility to swim before entering apoplastic spaces and attaching to the leaf surface. Subsequently, the flagellar and pilus motility allows P. syringae to form an early attachment stage. The motility is then suppressed, while the EPS production-related genes are upregulated by the increased c-di-GMP level to permit EPS biosynthesis and promote biofilm formation. The decrease in flagellar motility might contribute to stabilizing the complicated structure of the biofilm. Taken together, the proper crosstalk between motility and biofilm formation plays an important role in contributing to P. syringae virulence and survival.
11. HT-SELEX predicts 13 novel TFs involved in virulence-related regulatory networks
Although virulence-related TFs in P. syringae have been studied for decades using many methods, the direct and specific binding patterns of many TFs remain elusive. To this end, we have used HT-SELEX to globally profile the DNA interaction specificities of annotated TFs in P. syringae. The ligand libraries are first produced by PCR with 40-bp randomized oligo as a template. Purified TFs and selection ligands are incubated together, followed by equilibrating with resin beads. The bound DNA is subject to PCR. The PCR products from selection ligands are used for the next HT-SELEX cycle, which is repeated four times before next-generation sequencing. Autoseed [92] is used to identify initial seeds for generating position weight matrix (PWM) models. We successfully reveal 14 previously uncharacterised TFs that control virulence-associated pathways, including the T3SS, motility, c-di-GMP, siderophore production, phytotoxin secretion, and ROS resistance [34]. We determine the predicted virulence-related regulators within the above-mentioned regulatory networks.
HT-SELEX identifies two regulators involved in flagella-dependent motility: FleR and PSPPH_2720. These regulators harbour similar DNA binding motifs, suggesting that they co-regulate similar motility-related genes, including fliH, fliE, fliJ, and fliG. Moreover, we predict five TFs (MgrA, GacA, PSPPH_0442, PSPPH_3297, and PSPPH_3294) as regulators of T4P pathways. MgrA can bind the promoters of algU, mucA, and mucB, which are also controlled by another TF, PilR [34]. In terms of c-di-GMP, we use HT-SELEX to identify three potential TFs (MetR, PSPPH_1800, and PSPPH_2693) that might play roles in regulating this pathway by binding to the promoter region of PSPPH_4247, PSPPH_4420, PSPPH_A0029, and PSPPH_A0099, which encodes a known phosphodiesterase and putative diguanylate cyclases [34]. In addition, we show that KdpE, PSPPH_2800, and PSPPH_5204 might regulate the production of phytotoxins by binding genes related to PSPPH_A0071 and PSPPH_A0005. In the siderophore pathway, we predict six TFs, including PilR, CbrB2, GltR, PSPPH_4828, PSPPH_3800, and PSPPH_3577, that might regulate this pathway. PilR and PSPPH_3577 are predicted to regulate the operon of PSPPH_1923, 1924, and 1925, which encode pyoverdine sidechain peptide synthetase I, II, and III, respectively. GltR, TF PSPPH_4828, and PSPPH_3800 might play roles in regulating PSPPH_5184 and PSPPH_5185, which are related to the iron ABC transporter protein. Additionally, CbrB2 is predicted to bind to the promoter region of the operon fecBCDE, suggesting that CbrB2 controls iron transport in P. syringae [34]. Our HT-SELEX analysis also has revealed that three regulators (LexA1, PSPPH_1960, and PSPPH_3004) might participate in the ROS resistance pathway by regulating the expression of katE, sodA, and sodB, respectively [34]. We review these predicted TFs in their respective regulatory networks.
12. Conclusions and perspectives
Bacterial pathogens primarily rely on the plasticity of transcription levels to exert their virulence in different growth periods. During the infection, P. syringae first utilizes flagellar motility to adhere to the plant's cell surface and expand attachment. Subsequently, the attachment signals may change the P. syringae genes expression pattern (including T3SS) to secrete effectors injuring plant cells and EPS to form biofilm preventing the access of antimicrobial compounds. Meanwhile, P. syringae employs phytotoxins, ROS resistance system, and T3 effectors to suppress the plant defence. QS senses the threshold-level molecules and induces biofilm maturation. Finally, the bacterial cells at the surface layer of biofilm detach from the matrix and disperse to cause a new infection cycle [1].
In the past decades, several virulence-related regulatory mechanisms have been studied and illustrated in P. syringae. This review summarises these regulatory networks, including T3SS and non-T3SS pathways, to profile a global visualisation of crosstalk among master regulators in P. syringae (Table 1). The ChIP-Seq, RNA-Seq, and HT-SELEX approaches help us identify the binding sites, differentially expressed genes, and DNA-binding specificities of TFs, which demonstrate a vast regulatory landscape.
Table 1.
Overview of known transcription factors in Pseudomonas syringae.
| Genes | Produced proteins/secondary messengers | Roles |
|---|---|---|
| hrpRS | HrpR and HrpS | HrpR and HrpS together activate T3SS by inducing hrpL transcription [13]. |
| hrpL | HrpL | HrpL regulates most T3SS genes and alginate production [31]. |
| algU | AlgU | AlgU controls the transcription of T3SS effector genes [33]. AlgU negatively regulates flagella-dependent motility [33], [60], [61] and positively regulates alginate production [31]. |
| hrpA | HrpA | HrpA induces the expression of hrpRS, hrpL, and hrcC[39] |
| rhpRS | RhpR and RhpS | P-RhpR negatively regulates the hrpRS by directly binding to its promoter region. P-RhpR also negatively regulates swimming, c-di-GMP, and biofilm formation by targeting flhA, PSPPH2590, and algD. It positively regulates twitching motility by targeting fimA[14], [17], [40], [41]. |
| cvsRS | CvsR and CvsS | CvsRS upregulates T3SS induction [26]. CvsR positively regulates swimming and swarming [26]. CvsR represses alginate production in a Ca2+-dependent manner [26]. |
| gacAS | GacA and GacS | GacAS is a negative regulator of T3SS [29]. GacA regulates swarming [43] and activates the modest expression of alginate-related genes [21]. GacAS is also required for the QS system [43], [75], and positively regulates siderophore-related genes expression [21]. |
| hrpV | HrpV | HrpV negatively regulates T3SS [48]. |
| hrpG | HrpG | HrpG can remove the anti-activator HrpV from HrpS [49]. |
| hrpJ | HrpJ | HrpJ derepresses the T3SS genes by directly binding with the HrpG/HrpV heterodimeric chaperone [50]. |
| hrpF | HrpF | HrpF functions as a negative regulator of T3SS and stabilises HrpA in the cytoplasm [47]. |
| corRS | CorR and CorS | CorR induces the expression of hrpL[22]. CorS activates the coronatine synthesis [82]. |
| psrA | PsrA | PsrA indirectly and positively regulates hrpL expression [27]. |
| aefR | AefR | AefR indirectly upregulates the transcription of hrpL[30], [52]. AefR positively regulates motility, virulence, and antibiotic tolerance [3], [74]. |
| hrpT | HrpT | HrpT suppresses hrpL expression [53]. |
| lon | Lon | Lon negatively regulates T3SS by degrading HrpR and T3 effectors in KB. Lon also regulates motility by binding to the promoter of gacA[24], [55]. |
| ompR/envZ | OmpR and EnvZ | EnvZ-OmpR represses the T3SS in KB [31]. OmpR positively and directly regulates the expression of fleQ and negatively regulates alginate synthesis [31]. |
| cbrAB2 | CbrA2 and CbrB2 | CbrAB2 represses the T3SS in KB [31]. CbrB2 negatively controls fleQ[31] and regulates siderophore pathway and iron transport [34]. |
| pilRS | PilR and PilS | PilRS represses the T3SS in KB [31] and regulates motility-related genes [34]. PilR modulates the siderophore pathway [34]. |
| PSPPH_3618 | PSPPH_3618 | PSPPH_3618 downregulates the expression of avrB2 and rhpRC[34]. |
| fleQ | FleQ | FleQ is a transcriptional activator of motility [58]. |
| mgrA | MgrA | MgrA binds to the promoter of fleQ[31] and regulates the expression of motility-related genes [34]. |
| phoP | PhoP | PhoP binds to the promoter of fleQ[31]. |
| salA | SalA | SalA activates the modest expression of alginate production-related genes [23] and positively regulates siderophore-related genes expression [21]. |
| algR | AlgR | AlgR is an alginate biosynthesis regulatory protein that regulates the expression of algC and argB[66]. |
| vfr | Vfr | Vfr positively regulates cAMP production [69]. |
| ahlR | AhlR | AhlR participates in QS regulation, activating the expression of ahlI[52]. |
| corP | CorP | CorP modulates coronatine production [82]. |
| syrP | SyrP | SyrP plays a significant role in controlling syringomycin production and virulence in a phosphorylation-dependent manner [85]. |
| fur | Fur | Fur is a master negative regulator of iron homeostasis. Fur controls swarming motility, tabtoxin, and AHL production [88]. Fur also represses siderophore production [88]. |
| oxyR | OxyR | OxyR positively regulates ROS resistance [91]. |
| fleR | FleR | FleR positively regulates motility-related genes [34]. |
| PSPPH_2720 | PSPPH_2720 | PSPPH_2720 positively regulates motility-related genes [34]. |
| PSPPH_0442 | PSPPH_0442 | PSPPH_0442 regulates T4P pathway [34]. |
| PSPPH_3297 | PSPPH_3297 | PSPPH_3297 regulates T4P pathway [34]. |
| PSPPH_3294 | PSPPH_3294 | PSPPH_3294 regulates T4P pathway [34]. |
| metR | MetR | MetR regulates the expression of c-di-GMP-related genes [34]. |
| PSPPH_1800 | PSPPH_1800 | PSPPH_1800 regulates the expression of c-di-GMP-related genes [34]. |
| PSPPH_2693 | PSPPH_2693 | PSPPH_2693 regulates the expression of c-di-GMP-related genes [34]. |
| kdpE | KdpE | KdpE regulates the production of phytotoxins [34]. |
| PSPPH_2800 | PSPPH_2800 | PSPPH_2800 regulates the production of phytotoxins[34] |
| PSPPH_5204 | PSPPH_5204 | PSPPH_5204 regulates the production of phytotoxins [34]. |
| gltR | GltR | GltR modulates the siderophore pathway [34]. |
| PSPPH_4828 | PSPPH_4828 | PSPPH_4828 modulates siderophore pathway [34]. |
| PSPPH_3800 | PSPPH_3800 | PSPPH_3800 modulates siderophore pathway [34]. |
| PSPPH_3577 | PSPPH_3577 | PSPPH_3577 modulates siderophore pathway [34]. |
| lexA1 | LexA1 | LexA1participates in ROS resistance pathway [34]. |
| PSPPH_1960 | PSPPH_1960 | PSPPH_1960 participates in ROS resistance pathway [34]. |
| PSPPH_3004 | PSPPH_3004 | PSPPH_3004 participates in ROS resistance pathway [34]. |
Many of the abovementioned virulent pathways are shared in other Pseudomonas species, but the associated TFs are largely different. Take the T3SS for example, different from the T3SS regulatory network (22 TFs and Hrp-T3SS) in P. syringae reviewed in this article, two key pathways (Gac/Rsm-T3SS and Vfr-ExsA-T3SS) regulate T3SS in P. aeruginosa [93]. GacAS is the vital switch for the upstream activation of T3SS. HptB is an activator of RetS, which can inhibit GacS [94]. Besides, GacA can induce rsmY and rsmZ, while PtrR and SuhB repress GacA. In the Vfr-ExsA-T3SS pathway, Vfr is influenced by the level of cAMP, and the transcription activator ExsA directly induces the T3SS-related genes expression in P. aeruginosa. ExsA is also modulated by nine other regulators (VqsM, Fis, RcoA1, PsrA, HigB, PtrA, ExsD, MvaT/U, and HigA) [93]. However, a self-feedback regulatory pathway is shared by both species, including ExsADCE in P. aeruginosa and HrpSVG in P. syringae.
The diseases caused by P. syringae have caused massive economic losses. It is essential to find measures to combat this bacterial pathogen. The most common treatment currently applied to combat P. syringae infection is copper-based bactericides. However, it is decreasingly popular because pathogens develop copper resistance, and the copper accumulation has caused environmental damage [95]. Targeting TFs can treat human diseases such as cancer or inflammation [96], [97]. For example, phthalimide-based drugs can treat multiple myeloma cells by targeting TFs IKZF1 and IKZF2, both as the cereblon substrates. The most clinically approved antibiotics negatively affect bacterial viability in bacterial pathogens by targeting cell wall growth, DNA replication, transcription, and translation. In this situation, bacteria that can mutate to become antibiotic resistance may have a selective growth advantage [98]. An advantageous method to combat bacterial infection and prevent drug resistance is targeting virulence instead of viability because inhibiting virulence reduces the selective pressure and results in fewer antibiotic-resistance strains [99]. In addition, therapies targeting TFs would not harm the normal microbiota of hosts. Therefore, several drugs have been designed to target virulence-related TFs to inhibit bacterial infection. For example, structure-based small-molecule can inhibit several TFs (such as MarA, SoxS, and Rob), which regulate virulence in pathogenic Escherichia coli [100]. Besides, virstatin and benzimidazole compounds can limit infection by targeting vital virulence TFs ToxT and LcrF in Vibrio cholerae and Yersinia pseudotuberculosis, respectively [101], [102]. Given these studies, we propose that targeting virulence-associated TFs in P. syringae is a potential method to combat its infection.
We propose the following approaches to inhibit P. syringae virulence: 1) The drugs or compounds that directly inhibit T3SS effectors or switch off upstream master regulators such as RhpRS. 2) The measures to alleviate bacterial motility, such as swimming, swarming, and surface attachment, by targeting the master motility regulators (e.g., FleQ and AlgU). We further predict that drugs targeting virulence-related TFs in P. syringae may act synergistically with conventional antibiotics to control diseases caused by P. syringae.
Taken together, this review demonstrates that eight virulence-related regulatory networks are key to successful P. syringae infection. Given the crucial roles of the transcriptional regulatory networks (TRN) of P. syringae , an exploration of a more comprehensive TRN with all TFs in the genome would help us to further understand its pathogenicity and metabolism. We believe that our findings will contribute to the development of environmentally friendly and sustainable strategies to relieve the infections caused by P. syringae .
CRediT authorship contribution statement
Jiadai Huang: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. Chunyan Yao: Investigation, Writing – review & editing. Yue Sun: Investigation, Writing – review & editing. Quanjiang Ji: Conceptualization. Xin Deng: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by General Research Fund of Hong Kong (11102720, 21103018, 11101619, and 11103221 to XD), National Natural Science Foundation of China (31870116 and 32172358 to XD), and Shenzhen Science and Technology Fund (JCYJ20210324134000002).
References
- 1.Ichinose Y., Taguchi F., Mukaihara T. Pathogenicity and virulence factors of Pseudomonas syringae. J Gen Plant Pathol. 2013;79(5):285–296. [Google Scholar]
- 2.Taguchi F., Ichinose Y. Role of type IV Pili in virulence of Pseudomonas syringae pv. tabaci 6605: correlation of motility, multidrug resistance, and HR-inducing activity on a nonhost plant. Mol Plant Microbe In. 2011;24(9):1001–1011. doi: 10.1094/MPMI-02-11-0026. [DOI] [PubMed] [Google Scholar]
- 3.Kanda E., Tatsuta T., Suzuki T., Taguchi F., Naito K., Inagaki Y., et al. Two flagellar stators and their roles in motility and virulence in Pseudomonas syringae pv. tabaci 6605. Mol Genet Genomics. 2011;285(2):163–174. doi: 10.1007/s00438-010-0594-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5(4):148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 5.Nomura K., Melotto M., He S.Y. Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Curr Opin Plant Biol. 2005;8(4):361–368. doi: 10.1016/j.pbi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Lindgren P.B., Peet R.C., Panopoulos N.J. Gene-cluster of Pseudomonas-Syringae Pv Phaseolicola controls pathogenicity of bean-plants and hypersensitivity on nonhost plants. J Bacteriol. 1986;168(2):512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laue H., Schenk A., Li H., Lambertsen L., Neu T.R., Molin S., et al. Contribution of alginate and levan production to biofilm formation by Pseudomonas syringae. Microbiol-Sgm. 2006;152:2909–2918. doi: 10.1099/mic.0.28875-0. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi F., Suzuki T., Inagaki Y., Toyoda K., Shiraishi T., Ichinose Y. The siderophore pyoverdine of Pseudomonas syringae pv. tabaci 6605 Is an intrinsic virulence factor in host tobacco infection. J Bacteriol. 2010;192(1):117–126. doi: 10.1128/JB.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin K.H., Lee Y.C., Tu Z.L., Chen C.H., Tseng Y.H., Yang J.M., et al. The cAMP receptor-like protein CLP Is a novel c-di-GMP receptor linking cell-cell signaling to virulence gene expression in Xanthomonas campestris. J Mol Biol. 2010;396(3):646–662. doi: 10.1016/j.jmb.2009.11.076. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Jin Q.L., Thilmony R., Zwiesler-Vollick J., He S.Y. Type III protein secretion in Pseudomonas syringae. Microbes Infect. 2003;5(4):301–310. doi: 10.1016/s1286-4579(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 12.Wang J.R., Shao X.L., Zhang Y.C., Zhu Y.N., Yang P., Yuan J., Wang T.T., Yin C.Y., Wang W., Chen S., Liang H.H., Deng X. HrpS Is a Global Regulator on Type III Secretion System (T3SS) and Non-T3SS Genes in Pseudomonas savastanoi pv. phaseolicola. Mol Plant Microbe In. 2018;31(12):1232–1243. doi: 10.1094/MPMI-02-18-0035-R. [DOI] [PubMed] [Google Scholar]
- 13.Hendrickson E.L., Guevera P., Ausubel F.M. The alternative sigma factor RpoN is required for hrp activity in Pseudomonas syringae pv. maculicola and acts at the level of hrpL transcription. J Bacteriol. 2000;182(12):3508–3516. doi: 10.1128/jb.182.12.3508-3516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng X., Liang H.H., Chen K., He C., Lan L.F., Tang X.Y. Molecular mechanisms of two-component system RhpRS regulating type III secretion system in Pseudomonas syringae. Nucleic Acids Res. 2014;42(18):11472–11486. doi: 10.1093/nar/gku865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X.Y., Xiao Y.M., Zhou J.M. Regulation of the type III secretion system in phytopathogenic bacteria. Mol Plant Microbe In. 2006;19(11):1159–1166. doi: 10.1094/MPMI-19-1159. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Y., Heu S., Yi J., Lu Y., Hutcheson S.W. Identification of a putative alternate sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J Bacteriol. 1994;176(4):1025–1036. doi: 10.1128/jb.176.4.1025-1036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng X., Lan L.F., Xiao Y.M., Kennelly M., Zhou J.M., Tang X.Y. Pseudomonas syringae two-component response regulator RhpR regulates promoters carrying an inverted repeat element. Mol Plant Microbe In. 2010;23(7):927–939. doi: 10.1094/MPMI-23-7-0927. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y.P., Ding Y.Q., Shao X.L., Yao C.Y., Li J.W., Liu J.G., et al. Pseudomonas syringae senses polyphenols via phosphorelay crosstalk to inhibit virulence. Embo Rep. 2021;22(12) doi: 10.15252/embr.202152805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutcheson S.W., Bretz J., Sussan T., Jin S., Pak K. Enhancer-binding proteins HrpR and HrpS interact to regulate hrp-encoded type III protein secretion in Pseudomonas syringae strains. J Bacteriol. 2001;183(19):5589–5598. doi: 10.1128/JB.183.19.5589-5598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindeberg M., Cartinhour S., Myers C.R., Schechter L.M., Schneider D.J., Collmer A. Closing the circle on the discovery of genes encoding Hrp regulon members and type III secretion system effectors in the genomes of three model Pseudomonas syringae strains. Mol Plant Microbe Interact. 2006;19(11):1151–1158. doi: 10.1094/MPMI-19-1151. [DOI] [PubMed] [Google Scholar]
- 21.Yu X., Lund S.P., Greenwald J.W., Records A.H., Scott R.A., Nettleton D., et al. Transcriptional analysis of the global regulatory networks active in Pseudomonas syringae during leaf colonization. mBio. 2014;5(5):e01683–e01714. doi: 10.1128/mBio.01683-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sreedharan A., Penaloza-Vazquez A., Kunkel B.N., Bender C.L. CorR regulates multiple components of virulence in Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact. 2006;19(7):768–779. doi: 10.1094/MPMI-19-0768. [DOI] [PubMed] [Google Scholar]
- 23.Willis D.K., Holmstadt J.J., Kinscherf T.G. Genetic evidence that loss of virulence associated with gacS or gacA mutations in Pseudomonas syringae B728a does not result from effects on alginate production. Appl Environ Microb. 2001;67(3):1400–1403. doi: 10.1128/AEM.67.3.1400-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou T.H., Yin C.Y., Zhang Y.C., Shi H., Wang J.R., Sun L.B., et al. Lon protease is involved in RhpRS-mediated regulation of type III secretion in Pseudomonas syringae. Mol Plant Microbe In. 2016;29(10):807–814. doi: 10.1094/MPMI-06-16-0114-R. [DOI] [PubMed] [Google Scholar]
- 25.Preston G., Deng W.L., Huang H.C., Collmer A. Negative regulation of hrp genes in Pseudomonas syringae by HrpV. J Bacteriol. 1998;180(17):4532–4537. doi: 10.1128/jb.180.17.4532-4537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fishman M.R., Zhang J., Bronstein P.A., Stodghill P., Filiatrault M.J. Ca2+-induced two-component system CvsSR regulates the type III secretion system and the extracytoplasmic function sigma factor AlgU in Pseudomonas syringae pv. tomato DC3000. J Bacteriol. 2018;200(5) doi: 10.1128/JB.00538-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee A., Cui Y., Hasegawa H., Chatterjee A.K. PsrA, the Pseudomonas sigma regulator, controls regulators of epiphytic fitness, quorum-sensing signals, and plant interactions in Pseudomonas syringae pv. tomato strain DC3000. Appl Environ Microb. 2007;73(11):3684–3694. doi: 10.1128/AEM.02445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li K., Zhu Y.N., Yan W., Deng X., Xiao Y.M., Song L.Y., et al. Two components of the rhpPC operon coordinately regulate the type III secretion system and bacterial fitness in Pseudomonas savastanoi pv. phaseolicola. Plos Pathog. 2019;15(4) doi: 10.1371/journal.ppat.1007673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Malley M.R., Chien C.F., Peck S.C., Lin N.C., Anderson J.C. A revised model for the role of GacS/GacA in regulating type III secretion by Pseudomonas syringae pv. tomato DC3000, Mol. Plant Pathol. 2020;21(1):139–144. doi: 10.1111/mpp.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng X., Xiao Y.M., Lan L.F., Zhou J.M., Tang X.Y. Pseudomonas syringae pv. phaseolicola mutants compromised for type III secretion system gene induction. Mol Plant Microbe In. 2009;22(8):964–976. doi: 10.1094/MPMI-22-8-0964. [DOI] [PubMed] [Google Scholar]
- 31.Shao X.L., Tan M.M., Xie Y.P., Yao C.Y., Wang T.T., Huang H., et al. Integrated regulatory network in Pseudomonas syringae reveals dynamics of virulence. Cell Rep. 2021;34(13) doi: 10.1016/j.celrep.2021.108920. [DOI] [PubMed] [Google Scholar]
- 32.Butcher B.G., Bronstein P.A., Myers C.R., Stodghill P.V., Bolton J.J., Markel E.J., et al. Characterization of the fur regulon in Pseudomonas syringae pv. tomato DC3000. J Bacteriol. 2011;193(18):4598–4611. doi: 10.1128/JB.00340-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markel E., Stodghill P., Bao Z.M., Myers C.R., Swingle B. AlgU controls expression of virulence genes in Pseudomonas syringae pv. tomato DC3000. J Bacteriol. 2016;198(17):2330–2344. doi: 10.1128/JB.00276-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan L.G., Wang T.T., Hua C.F., Sun W.J., Li X.Y., Grunwald L., et al. A compendium of DNA-binding specificities of transcription factors in Pseudomonas syringae. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-18744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huynh T.V., Dahlbeck D., Staskawicz B.J. Bacterial-blight of soybean - Regulation of a pathogen gene determining host cultivar specificity. Science. 1989;245(4924):1374–1377. doi: 10.1126/science.2781284. [DOI] [PubMed] [Google Scholar]
- 36.Rahme L.G., Mindrinos M.N., Panopoulos N.J. Plant and environmental sensory signals control the expression of hrp genes in Pseudomonas-Syringae-Pv Phaseolicola. J Bacteriol. 1992;174(11):3499–3507. doi: 10.1128/jb.174.11.3499-3507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Dijk K., Fouts D.E., Rehm A.H., Hill A.R., Collmer A., Alfano J.R. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J Bacteriol. 1999;181(16):4790–4797. doi: 10.1128/jb.181.16.4790-4797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He S.Y. Type III protein secretion systems in plant and animal pathogenic bacteria. Annu Rev Phytopathol. 1998;36:363–392. doi: 10.1146/annurev.phyto.36.1.363. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz-Martin I., Thwaites R., Macho A.P., Mansfield J.W., Beuzon C.R. Positive regulation of the Hrp type III secretion system in Pseudomonas syringae pv. phaseolicola. Mol Plant Microbe Interact. 2010;23(5):665–681. doi: 10.1094/MPMI-23-5-0665. [DOI] [PubMed] [Google Scholar]
- 40.Xie Y.P., Shao X.L., Zhang Y.C., Liu J.G., Wang T.T., Zhang W.T., et al. Pseudomonas savastanoi two-component system RhpRS switches between virulence and metabolism by tuning phosphorylation state and sensing nutritional conditions. Mbio. 2019;10(2) doi: 10.1128/mBio.02838-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao Y.M., Lan L.F., Yin C.T., Deng X., Baker D., Zhou J.M., et al. Two-component sensor rhpS promotes induction of Pseudomonas syringae type III secretion system by repressing negative regulator RhpR. Mol Plant Microbe In. 2007;20(3):223–234. doi: 10.1094/MPMI-20-3-0223. [DOI] [PubMed] [Google Scholar]
- 42.Stael S., Wurzinger B., Mair A., Mehlmer N., Vothknecht U.C., Teige M. Plant organellar calcium signalling: an emerging field. J Exp Bot. 2012;63(4):1525–1542. doi: 10.1093/jxb/err394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee A., Cui Y.Y., Yang H.L., Collmer A., Alfano J.R., Chatterjee A.K. GacA, the response regulator of a two-component system, acts as a master regulator in Pseudomonas syringae pv. tomato DC3000 by controlling regulatory RNA, transcriptional activators, and alternate sigma factors. Mol Plant Microbe In. 2003;16(12):1106–1117. doi: 10.1094/MPMI.2003.16.12.1106. [DOI] [PubMed] [Google Scholar]
- 44.O'Malley M.R., Anderson J.C. Regulation of the Pseudomonas syringae type III secretion system by host environment signals. Microorganisms. 2021;9(6) doi: 10.3390/microorganisms9061227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buttner D. Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiol Mol Biol R. 2012;76(2):262–310. doi: 10.1128/MMBR.05017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaz M.R., King J.M., Yahr T.L. Intrinsic and extrinsic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Front Microbiol. 2011;2 doi: 10.3389/fmicb.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y.C., Lin Y.C., Wei C.F., Deng W.L., Huang H.C. The pathogenicity factor HrpF interacts with HrpA and HrpG to modulate type III secretion system (T3SS) function and t3ss expression in Pseudomonas syringae pv. averrhoi. Mol Plant Pathol. 2016;17(7):1080–1094. doi: 10.1111/mpp.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jovanovic M., James E.H., Burrows P.C., Rego F.G.M., Buck M., Schumacher J. Regulation of the co-evolved HrpR and HrpS AAA plus proteins required for Pseudomonas syringae pathogenicity. Nat Commun. 2011;2 doi: 10.1038/ncomms1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei C.F., Deng W.L., Huang H.C. A chaperone-like HrpG protein acts as a suppressor of HrpV in regulation of the Pseudomonas syringae pv. syringae type III secretion system. Mol Microbiol. 2005;57(2):520–536. doi: 10.1111/j.1365-2958.2005.04704.x. [DOI] [PubMed] [Google Scholar]
- 50.Charova S.N., Gazi A.D., Mylonas E., Pozidis C., Sabarit B., Anagnostou D., et al. Migration of type III secretion system transcriptional regulators links gene expression to secretion. Mbio. 2018;9(4) doi: 10.1128/mBio.01096-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng X., Xiao Y.M., Lan L.F., Zhou J.M., Tang X.Y. Pseudomonas syringae pv. phaseolicola mutants compromised for type III secretion system gene induction, molecular plant microbe. Interactions. 2009;22(8):964–976. doi: 10.1094/MPMI-22-8-0964. [DOI] [PubMed] [Google Scholar]
- 52.Quinones B., Pujol C.J., Lindow S.E. Regulation of AHL production and its contribution to epiphytic fitness in Pseudomonas syringae. Mol Plant Microbe In. 2004;17(5):521–531. doi: 10.1094/MPMI.2004.17.5.521. [DOI] [PubMed] [Google Scholar]
- 53.Ortiz-Martin I., Thwaites R., Mansfield J.W., Beuzon C.R. Negative regulation of the hrp type III secretion system in Pseudomonas syringae pv. phaseolicola. Mol Plant Microbe In. 2010;23(5):682–701. doi: 10.1094/MPMI-23-5-0682. [DOI] [PubMed] [Google Scholar]
- 54.Xie Y.P., Shao X.L., Deng X. Regulation of type III secretion system in Pseudomonas syringae. Environ Microbiol. 2019;21(12):4465–4477. doi: 10.1111/1462-2920.14779. [DOI] [PubMed] [Google Scholar]
- 55.Hua C.F., Wang T.T., Shao X.L., Xie Y.P., Huang H., Liu J.G., et al. Pseudomonas syringae dual-function protein Lon switches between virulence and metabolism by acting as both DNA-binding transcriptional regulator and protease in different environments. Environ Microbiol. 2020;22(7):2968–2988. doi: 10.1111/1462-2920.15067. [DOI] [PubMed] [Google Scholar]
- 56.Haefele D.M., Lindow S.E. Flagellar motility confers epiphytic fitness advantages upon Pseudomonas-Syringae. Appl Environ Microb. 1987;53(10):2528–2533. doi: 10.1128/aem.53.10.2528-2533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hattermann D.R., Ries S.M. Motility of Pseudomonas-Syringae Pv Glycinea and its role in infection. Phytopathology. 1989;79(3):284–289. [Google Scholar]
- 58.Dasgupta N., Wolfgang M.C., Goodman A.L., Arora S.K., Jyot J., Lory S., et al. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol Microbiol. 2003;50(3):809–824. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 59.Nogales J., Vargas P., Farias G.A., Olmedilla A., Juan S.J., Gallegos M.T. FleQ coordinates flagellum-dependent and -independent motilities in Pseudomonas syringae pv. tomato DC3000. Appl Environ Microb. 2015;81(21):7533–7545. doi: 10.1128/AEM.01798-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markel E., Dalenberg H., Monteil C.L., Vinatzer B.A., Swingle B. An AlgU-regulated antisense transcript encoded within the Pseudomonas syringae fleQ gene has a positive effect on motility. J Bacteriol. 2018;200(7) doi: 10.1128/JB.00576-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao Z.M., Wei H.L., Ma X., Swingle B. Pseudomonas syringae AlgU downregulates flagellin gene expression, helping evade plant immunity. J Bacteriol. 2020;202(4) doi: 10.1128/JB.00418-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitchurch C.B., Tolker-Nielsen T., Ragas P.C., Mattick J.S. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295(5559):1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 63.Sutherland I.W. Biofilm exopolysaccharides: a strong and sticky framework. Microbiol-Uk. 2001;147:3–9. doi: 10.1099/00221287-147-1-3. [DOI] [PubMed] [Google Scholar]
- 64.Lecourieux D., Raneva R., Pugin A. Calcium in plant defence-signalling pathways. New Phytol. 2006;171(2):249–269. doi: 10.1111/j.1469-8137.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 65.Prada-Ramirez H.A., Perez-Mendoza D., Felipe A., Martinez-Granero F., Rivilla R., Sanjuan J., et al. AmrZ regulates cellulose production in Pseudomonas syringae pv. tomato DC3000. Mol Microbiol. 2016;99(5):960–977. doi: 10.1111/mmi.13278. [DOI] [PubMed] [Google Scholar]
- 66.Penaloza-Vazquez A., Fakhr M.K., Bailey A.M., Bender C.L. AlgR functions in algC expression and virulence in Pseudomonas syringae pv. syringae. Microbiol-Sgm. 2004;150:2727–2737. doi: 10.1099/mic.0.27199-0. [DOI] [PubMed] [Google Scholar]
- 67.Pesavento C., Hengge R. Bacterial nucleotide-based second messengers. Curr Opin Microbiol. 2009;12(2):170–176. doi: 10.1016/j.mib.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Kalia D., Merey G., Nakayama S., Zheng Y., Zhou J., Luo Y.L., et al. Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p)ppGpp signaling in bacteria and implications in pathogenesis. Chem Soc Rev. 2013;42(1):305–341. doi: 10.1039/c2cs35206k. [DOI] [PubMed] [Google Scholar]
- 69.Taguchi F., Ichinose Y. Virulence factor regulator (Vfr) controls virulence-associated phenotypes in Pseudomonas syringae pv. tabaci 6605 by a quorum sensing-independent mechanism. Mol Plant Pathol. 2013;14(3):279–292. doi: 10.1111/mpp.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chatnaparat T., Li Z., Korban S.S., Zhao Y.F. The bacterial alarmone (p)ppGpp is required for virulence and controls cell size and survival of Pseudomonas syringae on plants. Environ Microbiol. 2015;17(11):4253–4270. doi: 10.1111/1462-2920.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romling U., Galperin M.Y., Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol R. 2013;77(1):1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang T.T., Cai Z., Shao X.L., Zhang W.T., Xie Y.P., Zhang Y.C., et al. Pleiotropic effects of c-di-GMP content in Pseudomonas syringae. Appl Environ Microb. 2019;85(10) doi: 10.1128/AEM.00152-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von Bodman S.B., Bauer W.D., Coplin D.L. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003;41:455–482. doi: 10.1146/annurev.phyto.41.052002.095652. [DOI] [PubMed] [Google Scholar]
- 74.Kawakita Y., Taguchi F., Inagaki Y., Toyoda K., Shiraishi T., Ichinose Y. Characterization of each aefR and mexT mutant in Pseudomonas syringae pv. tabaci 6605. Mol Genet Genomics. 2012;287(6):473–484. doi: 10.1007/s00438-012-0693-9. [DOI] [PubMed] [Google Scholar]
- 75.Marutani M., Taguchi F., Ogawa Y., Hossain M.M., Inagaki Y., Toyoda K., et al. Gac two-component system in Pseudomonas syringae pv. tabaci is required for virulence but not for hypersensitive reaction. Mol Genet Genomics. 2008;279(4):313–322. doi: 10.1007/s00438-007-0309-y. [DOI] [PubMed] [Google Scholar]
- 76.Bender C.L., Alarcon-Chaidez F., Gross D.C. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol R. 1999;63(2):266–292. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panchal S., Roy D., Chitrakar R., Price L., Breitbach Z.S., Armstrong D.W., et al. Coronatine facilitates Pseudomonas syringae infection of Arabidopsis leaves at night. Front Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hutchison M.L., Gross D.C. Lipopeptide phytotoxins produced by Pseudomonas syringae pv syringae: comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Mol Plant Microbe In. 1997;10(3):347–354. doi: 10.1094/MPMI.1997.10.3.347. [DOI] [PubMed] [Google Scholar]
- 79.Hutchison M.L., Tester M.A., Gross D.C. Role of biosurfactant and Ion channel-forming activities of syringomycin in transmembrane Ion flux - A model for the mechanism of action in the plant-pathogen interaction. Mol Plant Microbe In. 1995;8(4):610–620. doi: 10.1094/mpmi-8-0610. [DOI] [PubMed] [Google Scholar]
- 80.Wang L., Bender C.L., Ullrich M.S. The transcriptional activator CorR is involved in biosynthesis of the phytotoxin coronatine and binds to the cmaABT promoter region in a temperature-dependent manner. Mol Gen Genet. 1999;262(2):250–260. doi: 10.1007/s004380051081. [DOI] [PubMed] [Google Scholar]
- 81.Palmer D.A., Bender C.L. Effects of environmental and nutritional factors on production of the polyketide phytotoxin coronatine by Pseudomonas-Syringae Pv Glycinea. Appl Environ Microb. 1993;59(5):1619–1626. doi: 10.1128/aem.59.5.1619-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ullrich M., Peñaloza-Vázquez A., Bailey A.-M., Bender C.L. A modified two-component regulatory system is involved in temperature-dependent biosynthesis of the Pseudomonas syringae phytotoxin coronatine. J Bacteriol. 1995;177(21):6160–6169. doi: 10.1128/jb.177.21.6160-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guenzi E., Galli G., Grgurina I., Gross D.C., Grandi G. Characterization of the syringomycin synthetase gene cluster - A link between prokaryotic and eukaryotic peptide synthetases. J Biol Chem. 1998;273(49):32857–32863. doi: 10.1074/jbc.273.49.32857. [DOI] [PubMed] [Google Scholar]
- 84.Quigley N.B., Mo Y.Y., Gross D.C. Syrd is required for syringomycin production by Pseudomonas-Syringae pathovar Syringae and is related to a family of Atp-binding secretion proteins. Mol Microbiol. 1993;9(4):787–801. doi: 10.1111/j.1365-2958.1993.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang J.H., Quigley N.B., Gross D.C. Analysis of the syrP gene, which regulates syringomycin synthesis by Pseudomonas syringae pv syringae. Appl Environ Microb. 1997;63(7):2771–2778. doi: 10.1128/aem.63.7.2771-2778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitten T., Kinscherf T.G., McEvoy J.L., Willis D.K. A newly identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol Microbiol. 1998;28(5):917–929. doi: 10.1046/j.1365-2958.1998.00842.x. [DOI] [PubMed] [Google Scholar]
- 87.Ravel J., Cornelis P. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol. 2003;11(5):195–200. doi: 10.1016/s0966-842x(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 88.Cha J.Y., Lee J.S., Oh J.I., Choi J.W., Baik H.S. Functional analysis of the role of Fur in the virulence of Pseudomonas syringae pv. tabaci 11528: fur controls expression of genes involved in quorum-sensing. Biochem Bioph Res Co. 2008;366(2):281–287. doi: 10.1016/j.bbrc.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 89.Pohl E., Haller J.C., Mijovilovich A., Meyer-Klaucke W., Garman E., Vasil M.L. Architecture of a protein central to iron homeostasis: crystal structure and spectroscopic analysis of the ferric uptake regulator. Mol Microbiol. 2003;47(4):903–915. doi: 10.1046/j.1365-2958.2003.03337.x. [DOI] [PubMed] [Google Scholar]
- 90.Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol. 2008;20(1):10–16. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Ishiga Y., Ichinose Y. Pseudomonas syringae pv. tomato OxyR Is required for virulence in tomato and arabidopsis. Mol Plant Microbe In. 2016;29(2):119–131. doi: 10.1094/MPMI-09-15-0204-R. [DOI] [PubMed] [Google Scholar]
- 92.Jolma A., Yan J., Whitington T., Toivonen J., Nitta K.R., Rastas P., et al. DNA-binding specificities of human transcription factors. Cell. 2013;152(1–2):327–339. doi: 10.1016/j.cell.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 93.Shao X., Yao C., Ding Y., Hu H., Qian G., He M., et al. The transcriptional regulators of virulence for Pseudomonas aeruginosa: therapeutic opportunity and preventive potential of its clinical infections. Genes Diseases. 2022 doi: 10.1016/j.gendis.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bordi C., Lamy M.C., Ventre I., Termine E., Hachani A., Fillet S., et al. Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis. Mol Microbiol. 2010;76(6):1427–1443. doi: 10.1111/j.1365-2958.2010.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rabiey M., Roy S.R., Holtappels D., Franceschetti L., Quilty B.J., Creeth R., et al. Phage biocontrol to combat Pseudomonas syringae pathogens causing disease in cherry. Microb Biotechnol. 2020;13(5):1428–1445. doi: 10.1111/1751-7915.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhagwat A.S., Vakoc C.R. Targeting transcription factors in cancer. Trends Cancer. 2015;1(1):53–65. doi: 10.1016/j.trecan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cabrini G., Bezzerri V., Mancini I., Nicolis E., Dechecchi M.C., Tamanini A., et al. Targeting transcription factor activity as a strategy to inhibit pro-inflammatory genes involved in cystic fibrosis: decoy oligonucleotides and low-molecular weight compounds. Curr Med Chem. 2010;17(35):4392–4404. doi: 10.2174/092986710793361243. [DOI] [PubMed] [Google Scholar]
- 98.Emanuele A.A., Adams N.E., Chen Y.-C., Maurelli A.T., Garcia G.A. Potential novel antibiotics from HTS targeting the virulence-regulating transcription factor, VirF, from Shigella flexneri. J Antibiot. 2014;67(5):379–386. doi: 10.1038/ja.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alksne L.E. Virulence as a target for antimicrobial chemotherapy. Expert Opin Inv Drug. 2002;11(8):1149–1159. doi: 10.1517/13543784.11.8.1149. [DOI] [PubMed] [Google Scholar]
- 100.Bowser T.E., Bartlett V.J., Grier M.C., Verma A.K., Warchol T., Levy S.B., et al. Novel anti-infection agents: small-molecule inhibitors of bacterial transcription factors. Bioorg Med Chem Lett. 2007;17(20):5652–5655. doi: 10.1016/j.bmcl.2007.07.072. [DOI] [PubMed] [Google Scholar]
- 101.Hung D.T., Shakhnovich E.A., Pierson E., Mekalanos J.J. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science. 2005;310(5748):670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 102.Kim O.K., Garrity-Ryan L.K., Bartlett V.J., Grier M.C., Verma A.K., Medjanis G., et al. N-hydroxybenzimidazole inhibitors of the transcription factor LcrF in Yersinia: novel antivirulence agents. J Med Chem. 2009;52(18):5626–5634. doi: 10.1021/jm9006577. [DOI] [PMC free article] [PubMed] [Google Scholar]