Summary
Background
Influenza virus infection is associated with incident ischemic heart disease (IHD) events. Here, we estimate the global, regional, and national IHD mortality burden attributable to influenza.
Methods
We used vital registration data from deaths in adults ≥50 years (13.2 million IHD deaths as underlying cause) to assess the relationship between influenza activity and IHD mortality in a non-linear meta-regression framework from 2010 to 2019. This derived relationship was then used to estimate the global influenza attributable IHD mortality. We estimated the population attributable fraction (PAF) of influenza for IHD deaths based on the relative risk associated with a given level of weekly influenza test positivity rate and multiplied PAFs by IHD mortality from the Global Burden of Disease study.
Findings
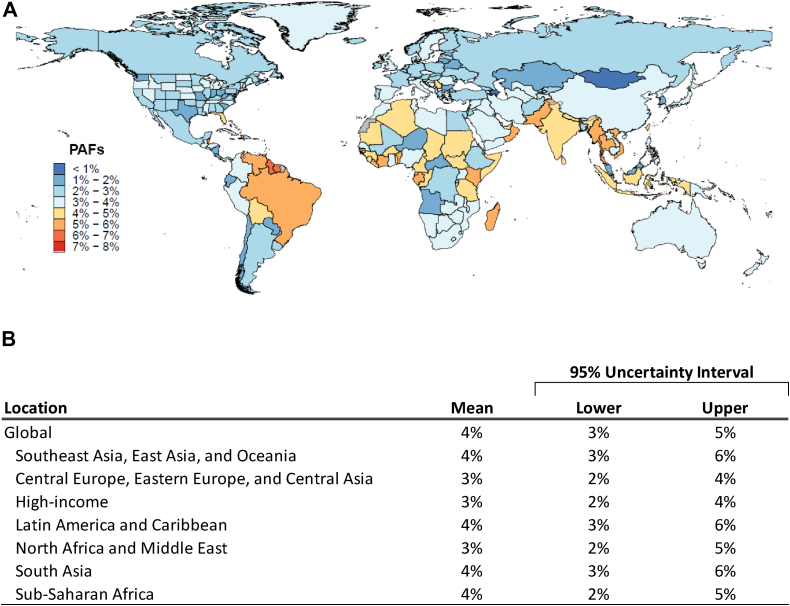
Influenza activity was associated with increased risk of IHD mortality across all countries analyzed. The mean PAF of influenza for IHD mortality was 3.9% (95% uncertainty interval [UI] 2.5–5.3%), ranging from <1% to 10%, depending on country and year. Globally, 299,858 IHD deaths (95% UI 191,216–406,809) in adults ≥50 years could be attributed to influenza, with the highest rates per 100,000 population in the Central Europe, Eastern Europe and Central Asia Region (32.3; 95% UI 20.6–43.8), and in the North Africa and Middle East Region (26.7; 95% UI 17–36.2).
Interpretation
Influenza may contribute substantially to the burden of IHD. Our results suggest that if there were no influenza, an average of 4% of IHD deaths globally would not occur.
Funding
Collaborative study funded by Sanofi Vaccines.
Keywords: Influenza, Ischemic heart disease, Mortality, Cardiovascular, Global disease burden
Research in context.
Evidence before this study
Associations between influenza epidemics and cardiovascular deaths, including from acute cardiac events, were first reported in the early 20th century following observations of synchronous influenza activity and excess mortality. Since then, an increasing body of literature has confirmed these associations using population- and individual-level studies of increasing complexity and quality. We conducted a search using PubMed and Embase databases on 20th June 2022 including terms for influenza and heart diseases and identified approximately 90 publications describing relationships between influenza and cardiocirculatory disease from specified geographies and timepoints using heterogeneous effect measures and metrics of limited comparability. None used cause-specific mortality data from multiple countries and continents to understand influenza-attributable ischemic heart disease mortality burden and the variation in its association due to differences in influenza virus circulation and underlying characteristics of populations at the global level.
Added value of this study
This study provides the first global estimates of influenza-attributable ischemic heart disease mortality, using a harmonized approach generating exposure–response relationships in countries of differing geographical location, hemisphere and socio-economic status. These relationships were extrapolated globally and converted to readily interpretable units for policymaking. We found that, globally, 3–5% of the total number of ischemic heart disease deaths could be attributed to influenza, corresponding to 200,000–400,000 ischemic heart disease deaths annually. Whilst global modeling approaches are imperfect, the study provides country-specific estimates allowing policymakers to prioritize influenza prevention in context of this additional public health impact, and provides a benchmark for replication using other exposures, outcomes, and methods.
Implications of all the available evidence
Influenza virus infection is an important trigger for cardiac events and may be responsible for 3–5% of ischemic heart disease deaths, annually. Ischemic heart disease is the leading cause of global mortality. If influenza exposure could be removed, a substantial number of ischemic heart disease deaths would be prevented. Policymakers should consider the benefit of influenza vaccination, especially in people at risk for cardiovascular events and complications. Additional research may be required to quantify the vaccine-preventable fraction of ischemic heart disease burden in different countries with varying influenza seasonality and prevalence of underlying risk factors for cardiovascular diseases.
Introduction
Ischemic heart disease (IHD) is the leading cause of death worldwide.1 Studies have shown a significant and clinically important association between influenza and major cardiovascular events,2, 3, 4, 5 highlighted by a study describing patients six times more likely to experience a heart attack the week after influenza virus infection than at any other point during the year before or after that infection.5 Experimental and observational reports found that influenza virus infection can cause direct cardiac changes, and the host's response to influenza virus infection can increase circulation of inflammatory mediators and activate immune cells that can induce damage throughout the body, not just locally within the respiratory tract.6 Nonetheless, the contribution of influenza virus infection to IHD mortality is not well quantified and the impact of influenza vaccination as part of routine care to prevent IHD morbidity and mortality remains uncertain.7, 8, 9
Historically, studies evaluating influenza-associated cardiovascular deaths have used time series approaches to estimate influenza-attributable events and have been limited to settings where vital statistics records are available,10, 11, 12, 13, 14 with no global estimates reported previously. In those time-series analyses, baseline mortality was usually estimated using multi-year averages, and influenza-attributable mortality defined as excess mortality above expected baselines during an influenza season. The variation in background rates in all-cause or cause-specific mortality time series may mask influenza signals, revealing only high-intensity peaks and potentially missing epidemics that span longer periods at lower influenza incidence (as can occur in tropical areas).15
Here, we estimate the global IHD mortality attributable to influenza from 2010 through 2019, before the emergence of the COVID-19 as a global pandemic. To extend previous excess mortality estimations we directly modeled a risk-response function between influenza activity and IHD deaths from vital registration data that was then applied to age and location-specific estimates of IHD mortality from the Global Burden of Disease (GBD) Study.16,17
Methods
Data sources and exposure modelling
A total of 13.2 million IHD diagnoses as underlying cause of deaths in adults ≥50 years were retrieved from vital registration systems from 10 countries (Brazil, Chile, China, Denmark, Guatemala, Italy, New Zealand, Mexico, South Africa, and United States), using International Classification of Diseases, 10th Revision (ICD-10) codes I20–I25. Additionally, we gathered weekly all-cause mortality data (119.5 million all-cause deaths) from Australia, Canada, France, Germany, Israel, Norway, Poland, Portugal, Spain and Sweden (Table S1 in the Supplementary Appendix – Section 1).
We used public health influenza surveillance data to quantify influenza activity, defined as the proportion of influenza positive samples tested weekly, from 2010 through 2019 and modelled location-specific weekly influenza exposure using RegMod,18 a Bayesian Regression model developed for the GBD project.16,17,19 The model was fit to influenza surveillance data obtained from the World Health Organization, the Brazilian Ministry of Health and the U.S. Centers for Disease Control and Prevention influenza surveillance systems (Table S2), and adjusted by environmental covariates including time (year), seasonality (week), temperature, dewpoint, and population density (Table S3 – Section I). More detailed information about the exposure-modelling approach with RegMod is included in the Supplementary Material Section II. Because >50% influenza positivity rate was observed very rarely and mostly in weeks with small number of samples tested for influenza, we excluded these weeks from the analysis (Fig. S1, Supplementary Appendix – Section II modelling influenza activity). Influenza activity varied by country, region and calendar year (Fig. S2).
We filled missing data points within each country-specific time series and estimated data for countries without influenza surveillance by imputing information from surrounding locations and covariates (Table S4 shows the list of countries with missing data on weekly influenza surveillance). The average observed versus modeled influenza activity as well as the out-of-sample prediction error by GBD super-region20 and year are presented in Table S5. To evaluate the predictive capacity of the model, we conducted an out-of-sample validation. We split the data set into training data (80%) to which we fit the model, and validation data (20%) which we used for validating the model. Table S6 in the Supplementary Material shows predicted values, measured values and root squared mean error (RSME) for the training data set by super region. The model performed best in high-income areas and worst in South Asia, North Africa and the Middle East.
Statistical analysis
We used generalized additive models with shape constraints (SCAMs) for individual locations to estimate the relationship between influenza activity and excess risk of IHD mortality in the ten countries with cause-specific mortality data. We calculated means of influenza activity across the same week of exposure and previous weeks. For instance, a lag of two weeks reflects the mean of the current week and 1 week prior. To avoid noise in the curves due to residual confounding or data quality issues, we constrained the curve to be monotonically increasing. The models considered weekly IHD deaths as outcome variables, and influenza activity, defined as the fraction of samples testing positive for influenza, as a predictor variable. Models were adjusted for trend, season, temperature, and dewpoint and explored temporal delay between exposure (influenza activity) and the occurrence of IHD deaths (Supplementary Appendix – Section III). For the US and Brazil, where subnational data were available, we incorporated a random effect by region.
The Meta-regression-Bayesian, Regularized, Trimmed (MR-BRT) tool21 was used to meta-regress the country-specific exposure-response curves into a single relationship between influenza activity and the relative risk (RR) of IHD death. Initially we included all-cause and IHD exposure-response curves and indicated the mortality category through a covariate. This was done to a) assess whether there is a systematic difference between both mortality curve types and b) to include the all-cause curves in the uncertainty range estimations. Since there was no difference in both curve types (meaning the covariate did not show to be significant – Fig. S3), we decided to only include IHD data in the final meta-regression and influenza attributable IHD burden estimations were exclusively based on the exposure-response curves that include only IHD-specific mortality data. The exposure-response curves from each country were normalized so that the lowest relative risk was zero when there was no positivity rate. The curves were then meta-regressed to fit a 3rd degree spline with a knot centered around 0.25. Though we included a number of covariates (population density, urbanicity, mean temperature, mean dewpoint temperature, air pollution [population-weighted mean PM2.5 and Ozone exposures], mean influenza positivity, temperature range, Healthcare Access and Quality Index [HAQI] and Socio-demographic index [SDI], Table S3) that could potentially explain the difference in country-specific exposure–response relationships, none were selected during the MR-BRT bias covariate selection process.22 (Supplementary Appendix – Section IIIa and IIIb for details).
We calculated the population attributable fraction (PAF) for each week and country, based on the RR associated solely on differences in influenza activity in a specific location and country. The PAF reflects the proportion of IHD deaths attributable to influenza. Equation 1 describes the calculation of weekly PAFs.
where l is the location, and a is the weekly influenza activity a given location. RRa is the relative risk associated with influenza activity a.
Weekly PAFs were then aggregated for each year from 2010 to 2019. To generate higher level PAFs (i.e., for super regions) we calculated population weighted averages.
We used estimates from GBD 2019 for IHD mortality16,17 and estimated the influenza-attributable IHD mortality for three age groups (50–64 years, 65–74 years and ≥75 years) as the product of the total IHD mortality and the corresponding PAF for each GBD location by year and age group. We provide a schematic of the analytical strategy used for the GBD IHD estimation in the Supplementary Material Section IIIc (Fig. S4).16,17
Our approach allowed for quantitative assessments of uncertainty that reflect data variance, model uncertainty, and stochastic effects. We propagated uncertainty from all components of the modelling chain using posterior simulation: for each quantity of interest, we pulled 1000 random draws from the posterior distribution of the estimate and performed all calculations at the draw level to estimate 95% uncertainty intervals (UI).
Ethics statement
Human subject review was not required for this study as only aggregate national data without personal identifiers were used in analyses. Data was aggregated by age group and week.
Role of funding source
The study was funded by Sanofi Vaccines. Three authors (S.S.C., J.N., C.M.) are employees of Sanofi and were involved in formulating the study question, study design, interpretation of the results. K.G.B., V.M.V.-T., L.E.W. and M.B. had access to the data and were responsible for data analysis with input from all the other co-authors. S.S.C. wrote the first draft of the manuscript. All authors critically reviewed the manuscript and contributed to the decision to publish its final version.
Results
Regression results
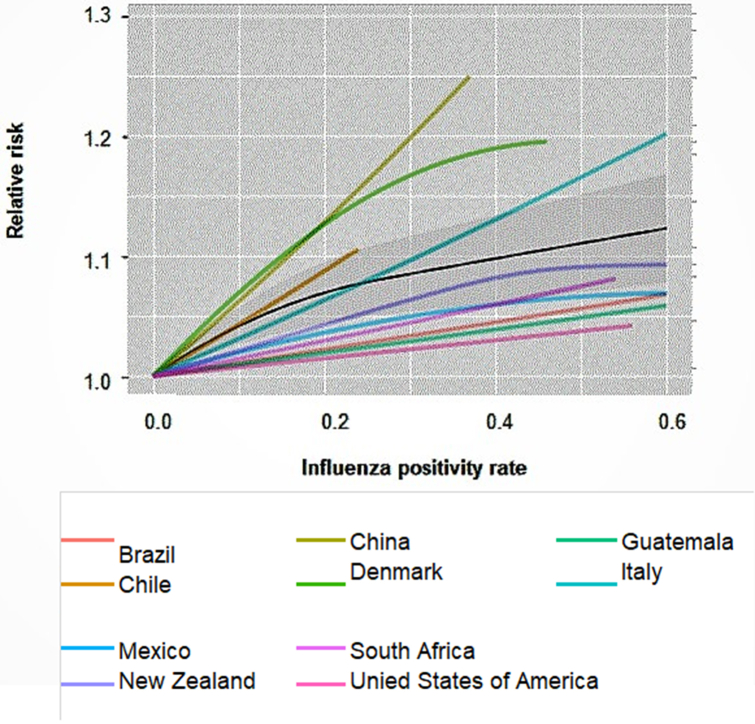
We found a significant increase in IHD mortality related to increased influenza activity and observed the main effect for the same week of exposure, with no evidence of temporal delays in effects or mortality displacement. Fig. 1 shows meta-regression conducted for no lag (same week of exposure or week 0). The curves display a steeper slope at lower exposure levels before leveling off at higher exposures. In Supplementary Material (Fig. S5) we show the average of the influenza activity in the week of exposure and n previous weeks (up to a lag of 6 weeks).
Fig. 1.
Meta-regression exposure-response curves between ischemic heart disease death and influenza activity for individual countries for no lag derived from country-specific SCAMs. Black solid line depicts meta-regressed curve with grey areas displaying 95% confidence intervals. The area under the curve (AuC), the area under the meta-regressed curve, is an indicator of the effect magnitude for each lag and an evaluation of the potential temporal delays that could either imply lagged effects or harvesting. For the exposure in the same week the AuC is 0.649.
Population attributable fraction and attributable mortality
The annual mean PAF, i.e., the percentage of IHD deaths that can be attributed to influenza exposure globally, was 3.9% (UI 2.5–5.3%), with slightly lower annual mean in Central Europe, Eastern Europe and Central Asia, high income and North Africa and Middle East Regions. Fig. 2 shows the annual mean PAF for each country considering all years (2010–2019). Country-specific mean PAF ranged from <1% to 8%, with some countries in Southeast Asia, Africa and South America showing ≥4% of IHD mortality attributed to influenza. Variation in country specific PAF estimates by year can be seen in Fig S6 (Panels A–J) and Table S7 in Supplementary Appendix section IV.
Fig. 2.
The influenza-attributable fraction (PAF) of ischemic heart disease mortality by country and super regions. Panel A shows a map with each country's annual mean percentage of ischemic heart disease death attributed to influenza (population attributable fraction) among adults aged ≥50 years, from 2010 to 2019. Panel B shows table with annual mean population attributable fraction (in percentage) of ischemic heart disease deaths globally and by super regions, with 95% uncertainty interval.
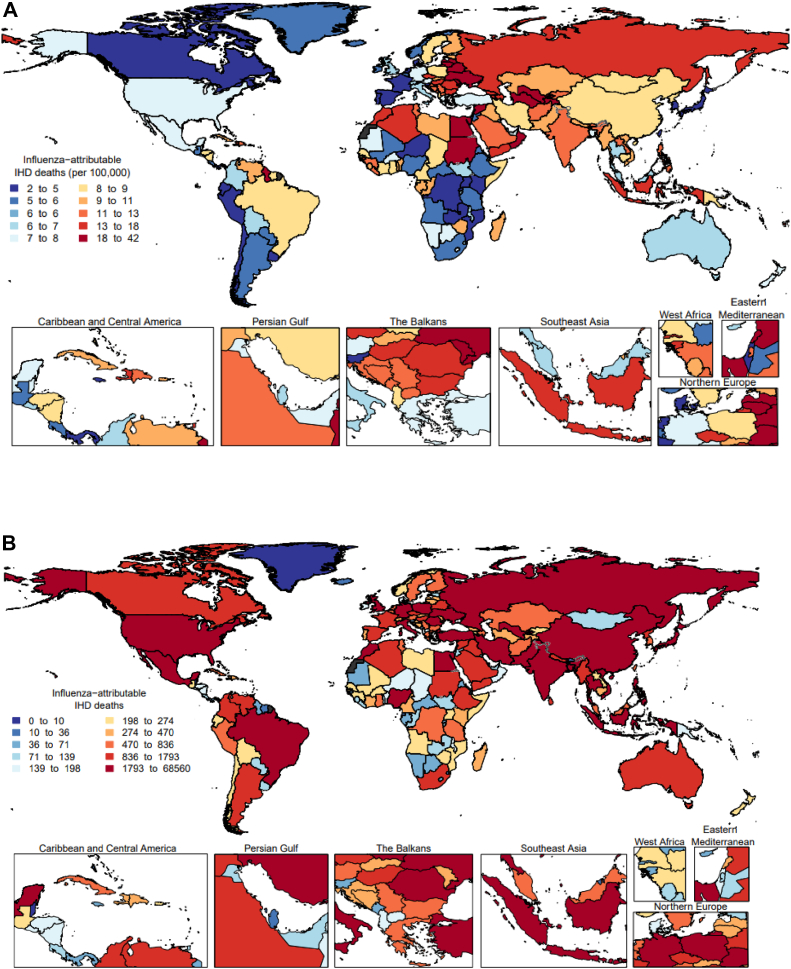
On average, from 2010 to 2019 approximately 299,858 IHD deaths (95% UI 191.216–406,809) or 18.4 deaths (95% UI 11.8, 25) per 100,000 adults ≥50 years could be attributed to influenza activity globally. The frequency of IHD deaths attributed to influenza activity increased by age, with 165,955 (95% CI 105,828–221,145) occurring in the population ≥75 years, 70,032 (95% CI 44,659–95,010) in those aged 65–74 years, and 63,870 (95% CI 40,729–86,653) in the age 50–64 years (Table 1). From 2010 to 2019, the mean annual influenza-attributable IHD mortality rate per 100,000 adults ≥50 years was the highest in Central Europe, Eastern Europe and Central Asia region (32.3 per 100,000 population; 95% UI 20.6–43.8), and in North Africa and Middle East region (26.7 per 100,000 population; 95% UI 17–36.2). Fig. 3 shows the mean rates (Panel A) and number of IHD deaths (Panel B) attributed to influenza activity by country.
Table 1.
Mean number and rates of influenza attributable ischemic heart disease deaths by age groups and super region, 2010–2019.
| Super region | Age (in years) | Mean number | 95% Uncertainty interval | Mean rates per 100,000 | 95% Uncertainty interval |
|---|---|---|---|---|---|
| Global | ≥50 | 2,99,858 | 1,91,216, 4,06,809 | 18.4 | 11.8, 25.0 |
| 50–64 | 63,871 | 40,729, 86,653 | 6.4 | 4.1, 8.6 | |
| 65–74 | 70,032 | 44,659, 95,010 | 18.7 | 11.9, 25.3 | |
| 75+ | 1,65,955 | 1,05,828, 2,25,147 | 67.6 | 43.1, 91.7 | |
| Southeast Asia, East Asia, and Oceania | ≥50 | 99,009 | 63,167, 1,34,270 | 18.2 | 11.6, 24.7 |
| 50–64 | 18,591 | 11,861, 25,212 | 5.3 | 3.4, 7.1 | |
| 65–74 | 22,491 | 14,349, 30,500 | 18.5 | 11.8, 25.2 | |
| 75+ | 57,928 | 36,957, 78,558 | 85.0 | 54.2, 115.3 | |
| Central Europe, Eastern Europe, and Central Asia | ≥50 | 42,483 | 27,091, 57,636 | 32.3 | 20.6, 43.8 |
| 50–64 | 7093 | 4523, 9623 | 9.0 | 5.8, 12.3 | |
| 65–74 | 8724 | 5563, 11,837 | 29.3 | 18.7, 39.8 | |
| 75+ | 26,666 | 17,005, 36,177 | 114.6 | 73.1, 155.4 | |
| High-income | ≥50 | 45,582 | 29,050, 61,869 | 11.7 | 7.4, 15.8 |
| 50–64 | 5105 | 3253, 6929 | 2.5 | 1.6, 3.4 | |
| 65–74 | 6888 | 4390, 9349 | 7.0 | 4.4, 9.5 | |
| 75+ | 33,589 | 21,407, 45,591 | 38.9 | 24.8, 52.9 | |
| Latin America and Caribbean | ≥50 | 17,751 | 11,319, 24,084 | 15.6 | 9.9, 21.1 |
| 50–64 | 3812 | 2430, 5171 | 5.3 | 3.4, 7.2 | |
| 65–74 | 4143 | 2642, 5620 | 16.2 | 10.4, 22.0 | |
| 75+ | 9797 | 6247, 13,292 | 58.2 | 37.1, 78.9 | |
| North Africa and Middle East | ≥50 | 22,564 | 14,382, 30,624 | 26.7 | 17.0, 36.2 |
| 50–64 | 6039 | 3849, 8197 | 10.6 | 6.7, 14.3 | |
| 65–74 | 5853 | 3731, 7944 | 33.7 | 21.5, 45.7 | |
| 75+ | 10,671 | 6802, 14,483 | 106.5 | 67.9, 144.5 | |
| South Asia | ≥50 | 61,093 | 38,952, 82,895 | 22.6 | 14.4, 30.6 |
| 50–64 | 20,200 | 12,879, 27,409 | 11.5 | 7.4, 15.7 | |
| 65–74 | 18,586 | 11,850, 25,219 | 29.1 | 18.5, 39.5 | |
| 75+ | 22,307 | 14,222, 30,267 | 71.4 | 45.5, 96.9 | |
| Sub-Saharan Africa | ≥50 | 11,375 | 7255, 15,432 | 12.7 | 8.1, 17.2 |
| 50–64 | 3032 | 1933, 4113 | 4.9 | 3.1, 6.7 | |
| 65–74 | 3347 | 2135, 4541 | 17.9 | 11.4, 24.3 | |
| 75+ | 4997 | 3187, 6778 | 53.4 | 34.1, 72.5 |
Fig. 3.
Mean annual ischemic heart disease mortality rate per 100,000 population (A) and mean annual number of deaths (B) attributable to influenza among adults ≥50 years, 2010–2019.
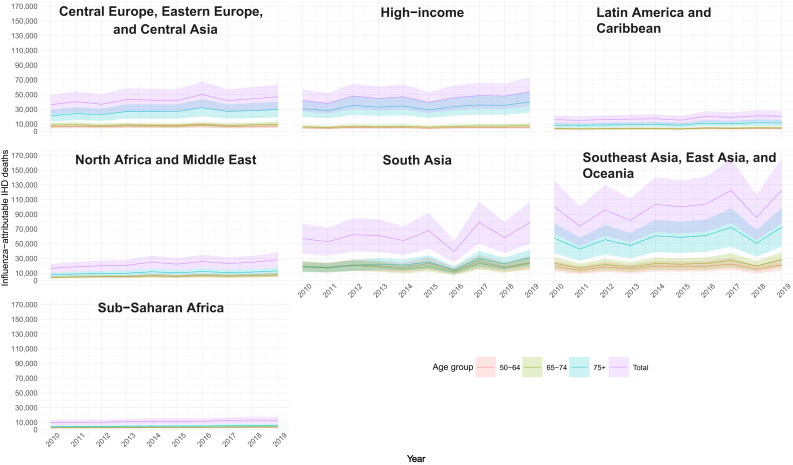
The lowest global number of attributable deaths was observed in 2011 with 248,900 (95% UI 158,672–337,819) deaths and the highest in 2019 with 363,800 (95% UI 232,105–432,262) deaths (Table S8). We observed a variation in influenza attributable IHD mortality rates by super region, with the highest rate in 2016 observed in Central Europe, Eastern Europe and Central Asia Region, while 2017 and 2018 were associated with higher mortality rates in South Asia. In Sub-Saharan Africa, influenza-attributable mortality was more equally distributed across years (Fig. 4; Table S8).
Fig. 4.
Influenza-attributable ischemic heart disease (IHD) deaths by age group, super regions, and year, from 2010 through 2019. Shaded area indicates 95% uncertainty interval (UI).
Discussion
Our analysis showed a consistent association between influenza activity and IHD deaths across different countries over a 9-year period. By applying this relationship to influenza activity measures globally, we estimated that an average of approximately 4% (95% UI 3–5%) of IHD deaths could be attributed to influenza activity, with some variations by year and country/region. The highest contribution of influenza to IHD deaths was seen in South Asia and parts of Africa and the Americas, with 5–7% of influenza attributable IHD deaths. The highest rates of influenza attributable IHD mortality were seen in North Africa and Eastern Europe countries. The global annual average of IHD deaths attributed to influenza was approximately 300,000 (200,000–400,000).
Globally, influenza is associated with 290,000–650,000 annual deaths from respiratory causes alone.23 The full range of influenza-associated mortality world-wide could be twice as high, as previous estimates did not account for non-respiratory deaths, such as those associated with cardiovascular disease (CVD).4,23,24 A study among adults ≥65 years in New York City, which looked at excess IHD deaths during influenza epidemics, demonstrated differences in the timing and magnitude of the association as dependent on influenza activity and CVD outcome, with estimated influenza-associated IHD mortality varying from 2.4% to 6.9%.10 Similarly, we found an annual average of 3% (95% UI 2–5%) in IHD deaths attributable to influenza in the United States (data in Table S5). Influenza-associated health effects can vary by season and depend on population immunity and other host factors, vaccine coverage and effectiveness, and virulence of the circulating influenza viruses.12,25,26 Nonetheless, improving influenza surveillance worldwide and supporting global civil registration and vital statistics,27 especially in low and middle income countries, could improve our attribution estimations, strengthening the evidence-base for clinical and health policy recommendations for the use of influenza vaccination.
A recent study estimated approximately 8 million global IHD deaths in 2019,16 with an age standardized death rate increasing in many locations across South, East and Southeastern Asia since the 90s. More than 80% of IHD deaths are estimated to occur in low- and lower-middle-income countries.28 In China, IHD mortality rates increased significantly (from 742 to 938 per 100,000 population, p = 0.002) during 2011–2018.29 International guidelines for CVD prevention focus on social determinants of health, particularly lifestyle changes (e.g., smoking cessation, diet, exercises) and medications to control elevated levels of cholesterol and blood pressure.30,31 More recently, the European Society of Cardiology32 and the American College of Cardiology33 have recommended influenza vaccination for prevention of heart failure and IHD. Nonetheless, despite the growing evidence of the role of influenza virus infection as a trigger for cardiovascular events, the use of influenza vaccination is suboptimal. A study assessing the uptake of influenza vaccination among patients with heart failure found coverage ranging from 2.6% in Asia and 53% in North America.34 Moreover, a survey done among the World Health Organization Member States found that only 6.4% of African Region countries, and 9.1% of South-East Asian Region countries reported having national influenza immunization programs for adults.35 Efforts to increase access to influenza vaccines worldwide could have an important impact in reducing IHD mortality, especially in areas where access to healthcare may be limited.
We observed marked PAF variation among countries. This variation in attribution could not be explained by differences related to population density, urbanicity, temperature, air pollution, influenza positivity, health care access or socio-demographics. Most importantly, we lacked information on other potential risk factors for IHD deaths such as diet, sedentarism, tobacco and alcohol consumption, or adherence to preventive measures for both IHD and influenza, which could partially explain some of the differences. Similarly, other potential disease modifiers at household level (e.g., in-house air-pollution) or individual level (e.g., frailty, adherence to treatments, impaired heart diseases) were not available. Moreover, we were unable to estimate the impact of influenza vaccination on IHD mortality in our study as influenza vaccine uptake data are not available for most countries. We did not find a strong time lag between influenza exposure and IHD mortality. Similar to our findings, a study in China, using a time series approach, found no apparent time lag for influenza virus activity and increased CVD, IHD and ischemic stroke mortality, with the strongest association being during or within the week of activity.29 Some other studies have reported an association between influenza and myocardial infarction,5,36 particularly in the first few days after infection and for up to 4 weeks.4,37 Our analysis was at the week-level which may have reduced the modelling sensitivity to identify (at least short) time lags. Another contributing factor could be the fact that most surveillance for influenza world-wide is done in outpatient settings where children are over-represented, and we know that children drive the influenza epidemic, with infection in older adults typically following.38
There are other limitations in our study that warrant discussion. Influenza ascertainment is limited by the lack of laboratory test confirmation, both in high-resource and low-resource settings.39,40 Moreover, national influenza surveillance systems, for most countries, have limited geographic representativeness and many countries do not have monitoring systems in place,41 limiting our assessment to certain countries where both influenza surveillance and vital statistics data sources were available. Death certificates may misclassify causes of death, and this may be different from country to country. Moreover, the quality of data from countries with limited resources was likely not as good as in some of the high-income countries since the former may rely on sample registration or verbal autopsy data instead of vital registry. This variation in diagnostic coding and cause of death classification compounded with limited (or absence) influenza surveillance data, may also explain some of the large variation observed in the association between influenza and IHD mortality. Moreover, because IHME GBD estimates are done using underlying cause of death, there may be events classified IHD as contributing causes that were not included. Another point to consider is that we used calendar year to investigate the association between influenza and IHD in the Northern Hemisphere countries where influenza circulation is seasonal, with peak activity varying from December through February and therefore spanning into two calendar years. This may have diluted the impact of influenza on IHD deaths in these countries. Future work should consider incorporating seasonal variations in the estimates, allowing influenza transmission in temperate countries to be assessed differently than tropical countries.
In conclusion, influenza may contribute substantially to the burden of IHD. Our study suggests that if there were no influenza, an average of 4% of IHD deaths globally would not occur. Estimates of influenza vaccination program impact on IHD mortality should be conducted to help understand the full public health value of influenza vaccination. Our study supports clinical practice guidelines recommending influenza vaccination as an important component IHD to preventive care.
Contributors
S.S.C.: conception, design, interpretation, writing. J.N.: conception, design, interpretation. K.G.B.: design, analysis, interpretation. D.M.: data access, analysis, interpretation. T.B.-S.: data access, analysis, interpretation. J.R.O.: interpretation; V.M.V.-T.: analysis; L.E.W.: analysis; G.R.: design, analysis, interpretation; C.M.: conception, design, interpretation; M.B.: design, analysis, interpretation. K.G.B., V.M.V.-T., L.E.W. and M.B. had direct access to and verified the data in the manuscript. All authors critically reviewed the manuscript and contributed to the decision to publish its final version.
Data sharing statement
To download the disease burden data on ischemic heart disease used in these analyses, please visit the Global Health Data Exchange website [link: https://ghdx.healthdata.org/]. Source of information for covariates and influenza surveillance data are available in the Supplementary Material (Tables S2 and S3).
Declaration of interests
J.R.O. reports that he has received fees from IHME for consulting on the submitted work; honoraria from Moderna, Pfizer, and Seqirus to serve on scientific advisory boards; fees for serving on a DSMB and as an independent safety monitor for Pharmaron; and his institution has received research funding from Bill & Melinda Gates Foundation, NIH, Pfizer, and the World Health Organization. J.N. worked for Sanofi during the study analysis and may own stocks. S.S.C. and C.M. work for Sanofi. T.B.-S. reports steering committee member of the Amgen financed GALACTIC-HF trial, the Boston Scientific “LUX-Dx TRENDS Evaluates Diagnostics Sensors in Heart Failure Patients Receiving Boston Scientific's Investigational ICM System” trial. Chief investigator and steering committee chair of the Sanofi Pasteur financed “NUDGE-FLU” trial, “DANFLU-1” trial and the Sanofi Pasteur financed “DANFLU-2” trial. Advisory Board: Sanofi Pasteur, Amgen and GSK. Speaker Honorarium: Novartis, Sanofi Pasteur and GSK. Research grants: GE Healthcare and Sanofi Pasteur. D.M. reported that his institution has received a grant from Sanofi Pasteur unrelated to this study. M.B. reported that his institution had a contract to Sanofi for the current study.
Acknowledgment
The authors would like to thank all the countries that shared cause of death data with the Institute for Health Metrics and Evaluation, allowing for this analysis. This study received funding from Sanofi Vaccines, Lyon, France.
Footnotes
Full professors: Tor Biering-Sørensen and Justin R. Ortiz.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101740.
Contributor Information
Sandra S. Chaves, Email: Sandra.Chaves@Sanofi.com.
Joshua Nealon, Email: jnealon@hku.hk.
Appendix A. Supplementary data
References
- 1.World Health Organization (WHO) Cardiovascular diseases (CVDs) https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 2.Clayton T.C., Thompson M., Meade T.W. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29(1):96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 3.Madjid M., Aboshady I., Awan I., Litovsky S., Casscells S.W. Influenza and cardiovascular disease: is there a causal relationship? Tex Heart Inst J. 2004;31(1):4–13. [PMC free article] [PubMed] [Google Scholar]
- 4.Warren-Gash C., Smeeth L., Hayward A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:601–610. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 5.Guan X., Yang W., Sun X., et al. Association of influenza virus infection and inflammatory cytokines with acute myocardial infarction. Inflamm Res. 2012;61:591–598. doi: 10.1007/s00011-012-0449-3. [DOI] [PubMed] [Google Scholar]
- 6.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(26):2540–2541. doi: 10.1056/NEJMc1805679. [DOI] [PubMed] [Google Scholar]
- 7.Froggatt H.M., Heaton N.S. Nonrespiratory sites of influenza-associated disease: mechanisms and experimental systems for continued study. FEBS J. 2022;289(14):4038–4060. doi: 10.1111/febs.16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singleton J.A., Wortley P., Lu P.J. Influenza vaccination of persons with cardiovascular disease in the United States. Tex Heart Inst J. 2004;31(1):22–27. [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control Seasonal influenza vaccination and antiviral use in EU/EEA member states. https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-vaccination-antiviral-use-eu-eea-member-states
- 10.Nguyen J.L., Yang W., Ito K., Matte T.D., Shaman J., Kinney P.L. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1(3):274–281. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Liu Y., Wu P., et al. Influenza-associated excess respiratory mortality in China, 2010-15: a population-based study. Lancet Public Health. 2019;4(9):e473–e481. doi: 10.1016/S2468-2667(19)30163-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen C.L., Chaves S.S., Demont C., Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999-2018. JAMA Netw Open. 2022;5(2) doi: 10.1001/jamanetworkopen.2022.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu P., Goldstein E., Ho L.M., et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998-2009. J Infect Dis. 2012;206(12):1862–1871. doi: 10.1093/infdis/jis628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen J., Mazick A., Glismann S., Mølbak K. Excess mortality related to seasonal influenza and extreme temperatures in Denmark, 1994-2010. BMC Infect Dis. 2011;11:350. doi: 10.1186/1471-2334-11-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamerius J., Nelson M.I., Zhou S.Z., Viboud C., Miller M.A., Alonso W.J. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect. 2011;119(4):439–445. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study [published correction appears in J Am Coll Cardiol. 2021 Apr 20;77(15):1958-1959] J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019 [published correction appears in Lancet. 2020 Nov 14;396(10262):1562] Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RegMod: general regression models. https://pypi.org/project/regmod/
- 19.Johnson S.C., Cunningham M., Dippenaar I.N., et al. Public health utility of cause of death data: applying empirical algorithms to improve data quality. BMC Med Inform Decis Mak. 2021;21(1):175. doi: 10.1186/s12911-021-01501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GBD regions and super regions. https://www.iapb.org:8443/learn/vision-atlas/about/definitions-and-regions/
- 21.Zheng P., Aravkin A.Y., Barber R., Sorensen R.J.D., Murray C.J.L. Trimmed constrained mixed effects models: formulations and algorithms. 2019. http://arxiv.org/abs/1909.10700
- 22.Burnett R.T., Pope C.A., 3rd, Ezzati M., et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure [published correction appears in Environ Health Perspect. 2014 Sep;122(9):A235] Environ Health Perspect. 2014;122(4):397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iuliano A.D., Roguski K.M., Chang H.H., et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study [published correction appears in Lancet. 2018 Jan 19] Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellers S.A., Hagan R.S., Hayden F.G., Fischer W.A., 2nd The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11(5):372–393. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosano A., Bella A., Gesualdo F., et al. Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14-2016/17 seasons) Int J Infect Dis. 2019;88:127–134. doi: 10.1016/j.ijid.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Petrova V.N., Russell C.A. The evolution of seasonal influenza viruses [published correction appears in Nat Rev Microbiol. 2017 Nov 07] Nat Rev Microbiol. 2018;16(1):47–60. doi: 10.1038/nrmicro.2017.118. [DOI] [PubMed] [Google Scholar]
- 27.Global civil registration and vital statistics scaling up investment plan 2015–2024. World Bank. https://www.worldbank.org/content/dam/Worldbank/document/HDN/Health/CRVS%20Scaling-up%20plan%20final%205-28-14web.pdf
- 28.Gupta R., Yusuf S. Challenges in management and prevention of ischemic heart disease in low socioeconomic status people in LLMICs. BMC Med. 2019;17(1):209. doi: 10.1186/s12916-019-1454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu R., Liu X., Yang P., et al. Influenza-associated cardiovascular mortality in older adults in Beijing, China: a population-based time-series study. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-042487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart J., Manmathan G., Wilkinson P. Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc Dis. 2017;6 doi: 10.1177/2048004016687211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta R., Wood D. Primary prevention of ischemic heart disease: populations, individuals and health professionals. Lancet. 2019;394:585–596. doi: 10.1016/S0140-6736(19)31893-8. [DOI] [PubMed] [Google Scholar]
- 32.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev Esp Cardiol. 2022;75(6):523. doi: 10.1016/j.rec.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 33.King S.C., Fiebelkorn A.P., Sperling L.S. Expert analysis: influenza vaccination: proven and effective cardiovascular disease prevention. Accessed at American College of Cardiology. https://www.acc.org/Error/NotFound?item=%2flatest-in-cardiology%2farticles%2f2020%2f11%2f02%2f14%2f42%2finfluenza-vaccination-proven-and-effective-cvd-prevention+on+june+1&user=extranet%5cAnonymous&site=acc
- 34.Vardeny O., Claggett B., Udell J.A., et al. Influenza vaccination in patients with chronic heart failure: the PARADIGM-HF trial. JACC Heart Fail. 2016;4:152–158. doi: 10.1016/j.jchf.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Williams S.R., Driscoll A.J., LeBuhn H.M., Chen W.H., Neuzil K.M., Ortiz J.R. National routine adult immunisation programmes among World Health Organization member states: an assessment of health systems to deploy COVID-19 vaccines. Euro Surveill. 2021;26(17) doi: 10.2807/1560-7917.ES.2021.26.17.2001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren-Gash C., Hayward A.C., Hemingway H., et al. Influenza infection and risk of acute myocardial infarction in England and Wales: a CALIBER self-controlled case series study. J Infect Dis. 2012;206(11):1652–1659. doi: 10.1093/infdis/jis597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madjid M., Miller C.C., Zarubaev V.V., et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J. 2007;28(10):1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worby C.J., Chaves S.S., Wallinga J., Lipsitch M., Finelli L., Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10–16. doi: 10.1016/j.epidem.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrozzino J.J., Smith C., Atkinson M.J. Rapid diagnostic testing for seasonal influenza: an evidence-based review and comparison with unaided clinical diagnosis. J Emerg Med. 2010;39:476–490.e1. doi: 10.1016/j.jemermed.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 40.Talbot H.K., Williams J.V., Zhu Y., Poehling K.A., Griffin M.R., Edwards K.M. Failure of routine diagnostic methods to detect influenza in hospitalized older adults. Infect Control Hosp Epidemiol. 2010;31(7):683–688. doi: 10.1086/653202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortiz J.R., Sotomayor V., Uez O.C., et al. Strategy to enhance influenza surveillance worldwide. Emerg Infect Dis. 2009;15(8):1271–1278. doi: 10.3201/eid1508.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.