Abstract
Low back pain (LBP) is a common disease that imposes a huge social and economic burden on people. Intervertebral disc (IVD) degeneration (IVDD) is often considered to be the leading cause of LBP and further aggravate and cause serious spinal problems. The established treatment strategy for IVDD consists of physiotherapy, pain medication by drug therapy, and, if necessary, surgery, but none of them can be treated from the etiology; that is, it cannot fundamentally reverse IVD and reconstruct the mechanical function of the spine. With the development of nanotechnology and regenerative medicine, nano-drug delivery systems (NDDSs) have improved treatment results because of their good biodegradability, biocompatibility, precise targeted specific drug delivery, prolonged drug release time, and enhanced drug efficacy, and various new NDDSs for drugs, proteins, cells, and genes have brought light and hope for the treatment of IVDD. This review summarizes the research progress of NDDSs in the treatment of IVDD and provides prospects for using NDDSs to address the challenges of IVDD. We hope that the ideas generated in this review will provide insight into the precise treatment of IVDD.
Keywords: Intervertebral disc degeneration, Nanotechnology, Drug delivery system, Nanocarriers, Nanoparticles
Graphical abstract
Highlights
-
•
IVDD is a chronic degenerative disease, which is the main cause of LBP, causing a heavy burden on individuals and society.
-
•
The efficacy of IVDD drugs is hampered by their rapid clearance and lack of targeting.
-
•
The choice of delivery routes and design of NDDSs may ameliorate the limited drug efficacy of IVDD.
-
•
Outlooks and immediate challenges regarding NDDSs-based IVDD therapies are discussed.
1. Introduction
Low back pain (LBP) is a common public health problem worldwide that leads to serious lifelong disability and a huge economic burden on society and patients [1,2]. According to relevant research, more than 80% of adults will suffer from LBP at some stage. About 10% of LBP patients will develop chronic disabilities [3,4]. With the aging of the population, the economic burden of LBP has increased significantly [5]. LBP has become one of the major diseases affecting public health and quality of life [6]. The etiology of LBP is multifaceted and includes genetic causes, lifestyle factors (such as occupational exposure, lack of exercise, drinking, and smoking), and aging [4,7,8]. Although the etiology of LBP is complex, intervertebral disc (IVD) degeneration (IVDD) is the most common etiology, representing clear morphological changes caused by aging or mechanical stress [9].
The IVD is a fibrocartilage tissue that connects adjacent vertebrae and is located between vertebrae to provide flexibility and maintain pressure. Continuous mechanical stimulation during development and aging inevitably causes dysfunction and degradation of the IVD [10]. Existing research proves that the progressive destruction of the extracellular matrix (ECM), changes in the IVD cell phenotype, loss of active IVD cells, increased cell aging and death, and excessive inflammatory reactions are considered the key developmental factors of IVDD [[11], [12], [13]]; which aggravate the disorder and destruction of normal IVD function [14]. Currently, most patients use rest or conservative treatment only to relieve pain, as well as a variety of drugs such as non-steroidal anti-inflammatory drugs (NSAIDs), analgesics, and other blockers. When these methods are ineffective, surgery is usually used to alleviate symptoms and improve the quality of life [15,16]. In brief, these interventions do not allow the recovery of IVD function, and there are some defects such as high invasiveness, high risk of recurrence, loss of mechanical properties, and adjacent IVD degeneration. Since non-surgical and surgical treatments are suboptimal, finding more effective strategies for IVDD treatment is important. Different treatment strategies are available, including biomaterial substitution, drug injection, gene therapy, and stem cell therapy [17]. Among these, drug therapy and gene therapy involve the challenge of identifying specific targets. Equally important, the half-life of bioactive substances for IVD treatment is short, which limits the duration of drug treatment activities, thereby reducing efficacy [18]. In this regard, nano-drug delivery systems (NDDSs) may play a crucial role as part of new treatment strategies because they can combine different types of drugs or therapeutic agents, have adjustable release and targeting capabilities, and may help to improve the therapeutic effects of drugs by concentrating and prolonging their presence in IVD tissues [19,20].
This review aims to briefly describe the pathogenesis of IVDD and the limitations of the current treatments. Most importantly, this review demonstrates the progress of NDDS therapy for IVDD. Finally, challenges and future directions in this field are presented to advance the development of NDDS in IVDD therapy.
2. IVD: histological, biochemical, and pathophysiology features
2.1. IVD structure and function
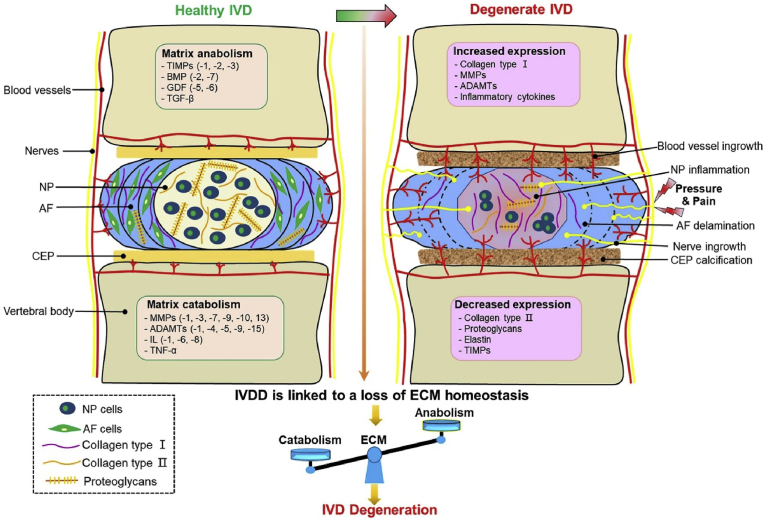
The IVD is a cartilaginous structure between the upper and lower vertebrae, which is firmly connected to the vertebrae and is an important structure of the spine. Due to its unique load distribution, sliding, and wear resistance, IVD is an important structure for human movement and shock absorption [4,21]. Each IVD is composed of three complete tissues: nucleus pulposus (NP), annulus fibrosus (AF), and upper and lower cartilage endplates (CEPs) (Fig. 1).
Fig. 1.
Schematic representation of healthy IVD and the main pathological and morphological changes in IVDD.
The NP is a hydrophilic high-pressure structure surrounded by fibrous rings. Its main function is to resist pressure from the spine. The NP is composed of water, inorganic salts, and NP cells (small chondrocyte-like cells) embedded in the ECM and spinal cord cell clusters [22]. ECM is composed of type II collagen (collagen-II) fibers, proteoglycans, and elastin. Elastin contains negatively charged side chains of proteoglycans, which contribute to the high hydration and permeability of NP and enable IVD to resist the burden of pressure and retain their morphology [23]. Proteoglycans and collagen-II are the main components of NP. Their function is to maintain moisture and make the IVD bear compression load, completed through the hydraulic distribution of the entire IVD [24]. Therefore, NP is considered a buffer that works as a shock absorber to avoid direct and severe mechanical friction and impact on cartilage and bone.
AF is composed of internal and external parts, and the main difference lies in their collagen composition. The inner fiber ring comprises several layers of fibrocartilage, mainly collagen-II and more proteoglycans. The outer fiber ring is a fibrous tissue composed of type I collagen (collagen-I); thus, its structure can be stretched and withstand pressure [25]. Close to the outer ring fibrosis, the content of collagen-I fibers increases, while the content of collagen-II fibers and proteoglycans decreases [7,26].The main function of AF is to suppress and maintain the osmotic pressure exerted by NP through its tensile strength, providing natural resistance to bending, torsion, and shear, especially in the flexion and extension or bending and torsion of the IVD. Unlike NP, the cells in AF are fibroblasts, and their slender cell shape contributes to the arrangement of ECM rich in collagen-I, which may provide the basic structure for the lamella in AF [27].
The CEP is a thin-layered cartilaginous structure covering the upper and lower ends of the IVD (approximately 0.6–1.1 mm thick each), which plays a key role in transporting liquids and solutes into and out of the IVD and provides the IVD nutrition [28]. The IVD is the largest known avascular tissue in the human body. Most of the nutritional supply of the IVD depends on the infiltration of CEPs. At the same time, the internal healing potential of the IVD is very limited, which is also why the IVD easily degenerates. In addition, owing to its cartilaginous structure, the endplate provides a flexible entity that can support a large load and distribute pressure in the IVD to the adjacent vertebrae [29,30].
2.2. IVDD pathophysiology
IVDD is a multifactorial degenerative disease caused by aging, injury, nutrition, biomechanics, genetics, and environmental factors [[31], [32], [33], [34], [35]]. In all cases, degenerated IVD is mainly manifested by an imbalance of anabolic and catabolic processes and a reduction in the number and activity of NP cells, which may lead to tissue weakness and cell morphological and functional changes [36,37]. Clinically, IVDD is a progressive chronic disease characterized by low back and leg pain and can even lead to serious disability [14,38]. In severe cases, IVDD can lead to disc herniation, radiculopathy, spondylolisthesis, spinal canal stenosis, and even degenerative scoliosis, the most direct cause of chronic disability [39,40]. These painful symptoms are due to molecular and cellular changes in tissues that damage the structure and mechanical properties of the IVD (Fig. 1).
2.2.1. Cellular and molecular changes
With aging, the physiological process of the IVD changes naturally, and its ability to withstand mechanical stress decreases. However, other factors accelerate the degradation of these pathophysiological processes and drive the abnormal response of IVD cells [41].
Some studies have emphasized the key role of disappearing large vacuolar notochordal cells and the interruption of information exchange between them and NP cells in IVDD, which is closely related to reduced NP cell survival [[42], [43], [44]]. With the decrease in notochord cells (the dominant cells that produce proteoglycans and maintain the consistency of NP gel) and the phenotypic changes of NP cells, there is a reduction in normal ECM and proteoglycan synthesis. The balance of collagen production is converted from collagen II to collagen I, accompanied by increased matrix-degrading enzyme production, which ultimately reduces the height and loading capacity of the IVD [45,46]. With the development of disease, NP cells abnormally synthesize and secrete matrix metalloproteinase (MMP)-1, MMP-3, and a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS), which inhibit the synthesis of proteoglycan and collagen-II, resulting in changes in ECM integrity [[47], [48], [49]]. In addition, NP cells secrete large amounts of interleukin (IL)-6, IL-8, and prostaglandin E2 (PGE2), which can then stimulate the production of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) [20]. Abnormal nerve ingrowth and vascularization occur in normal avascular and non-innervated IVD tissues. These new nerve fibers interact with inflammatory mediators in NP, causing discogenic low back pain [14]. Moreover, NP cells induce angiogenesis and chemokine synthesis, especially CCL2, 3, 4, 5, 7, and 13, CXCL10, and IL-8, which stimulate immune cell recruitment and produce IL-1β and tumor necrosis factor α (TNF-α) [14,50]. These two major pro-inflammatory cytokines jointly mediate catabolism and anti-anabolism in NP and play important roles in the progress of IVDD [10,51]. With metabolic dysregulation, advanced glycation end products (AGEs) accumulate in NP tissues and promote their degeneration, further increasing oxidative stress and secretion of major inflammatory cytokines [52,53]. In conclusion, inflammatory mediators, such as IL-1β and TNF-α, regulate catabolic reactions in IVD, leading to decreased proteoglycan and collagen-II content and increased collagen-I expression, causing NP to gradually lose its gelatinous and highly hydrating properties, becoming more fibrotic, less elastic, and cracked, resulting in morphological changes and height reduction of IVD. Thus, the ability of IVD to act as a hydraulic shock absorber is weakened.
Furthermore, chondrocyte hypertrophy, bone marrow space occlusion, sclerosis, calcification, and loss of permeability of CEPS change the balance of energy supply and demand and reduce the diffusion rate of nutrients and metabolites [[54], [55], [56]]. During immune cell recruitment, the nutrient supply decreases, and demand increases. This imbalance between demand and supply reduces the availability of nutrients to IVD cells, resulting in adverse effects on cell activity and vitality [57]. Moreover, with the calcification of the tiny pores within CEPs and changes in the mechanical mechanism, metabolite excretion is reduced, and the IVD microenvironment is acidified, which leads to decreased cell proliferation and viability, stimulates apoptosis and senescence of NP cells, and further aggravates the degenerative cascade [21,58]. Eventually, the ECM of AF also weakens with the irregular thinning of collagen fibers, and its concentric structure dissolves and may form fissures [59].
2.2.2. Structural and mechanical changes
IVD degeneration involves cellular, molecular, and inflammatory mechanisms resulting in significant structural, physical, and mechanical changes and LBP. The degradation of proteoglycan and the weakening of the water-binding capacity of NP lead to dehydration and the loss of its mechanical properties. As NP denaturation becomes more fibrotic, it begins to behave more non-hydrostatically. The fibers in AF gradually lose direction and degenerate, and the tensile force of AF increases [60]. Therefore, the AF can tear; in severe cases, NP (IVD herniation) prolapse can occur, usually accompanied by significant LBP and leg pain owing to compression of adjacent nerve roots [61,62]. The AF is also damaged internally, and subsequent nerve growth and secretion of inflammatory factors magnify the degenerative cascade and the patient's pain. Moreover, degenerative changes in the IVD are related to damage to nearby structures, such as ligaments, joints, and vertebral muscles, which lead to functional changes and injury susceptibility [63]; Due to overload, the osteoid joint must bear a higher load, which leads to arthritis of surrounding tissues and the formation of bone spurs; at the same time, there is also a reduction in the strength of the yellow ligament, leading to its hypertrophy and protrusion into the spinal canal, narrowing and compressing the nerve structure [40,46,64]. In conclusion, the associated hydrostatic pressure loss leads to an overall reduction in IVD height, compromising the overall mechanical integrity of the IVD and encouraging further degradation, thus initiating a vicious cycle.
3. Diagnosis and current treatments of IVDD
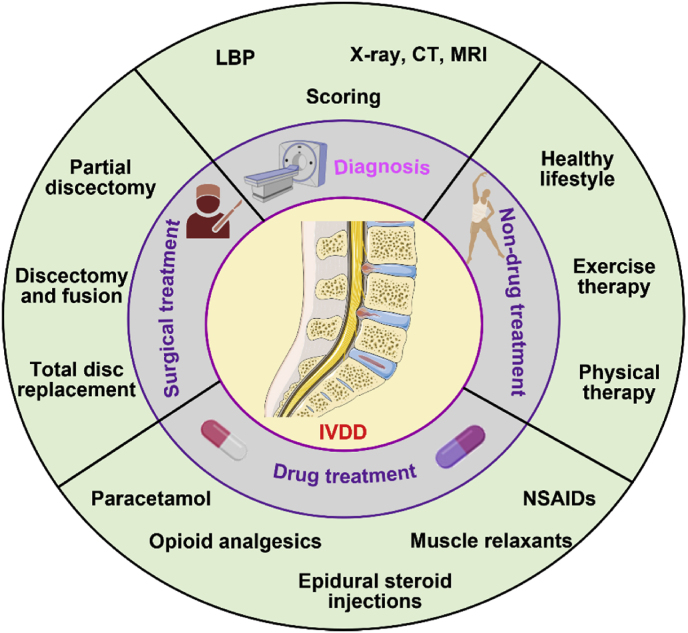
The diagnosis of IVDD is mainly based on the clinical symptoms of LBP. It is then confirmed by complementary imaging analysis (such as X-ray, CT scan, or magnetic resonance imaging (MRI)), and the severity of degeneration is evaluated by scoring [20,65,66]. Scoring is based on the high hydration state (Pfirrmann) of NP and IVD or the Modic change in CEPs [[67], [68], [69]]. Currently, there are two common methods of IVDD treatment: conservative treatment (including non-drug treatment and drug treatment) and invasive surgical treatment [70]. If degeneration is not severe, a healthy lifestyle, appropriate and regular exercise, weight loss, and physical therapy are key and simple non-drug approaches to treating IVDD. Conventional drugs include paracetamol, opioid analgesics, epidural steroid injections, muscle relaxants, and NSAIDs, all of which aim to achieve pain control, and improved function and quality of life. However, with their own risk of side effects, some people may develop addiction and dependence [71,72]. In the early stage of the disease, to reduce pain, treatment methods (such as painkillers, physiotherapy, and anti-inflammatory drugs) can be used to provide a platform for the body to adapt to improve the patient's physical health and quality of life. In advanced cases, steroids can be injected locally to treat nerve root symptoms [73]. Once conservative treatment fails, acute nerve interruption, cauda equina syndrome, spinal deformity (such as degenerative kyphosis), or spinal cord functional instability, surgical treatment is required. Surgical interventions include partial or complete discectomy, fusion, and total disc replacement (TDR) (Fig. 2). Spinal fusion is the fusing or connecting of two bones. It is currently considered the gold standard for IVDD treatment [74]. TDR, replacing degenerative intervertebral discs with artificial IVDs, is usually used only when there is a single segment of IVD disease and no disease of the small joints [72,75]. Although back pain may be reduced after spinal surgery, there is an increased risk of further degeneration at the surgery site or adjacent segments, and pain often recurs [76]. In conclusion, although these drugs and surgical treatment strategies positively impact patients' pain, their long-term efficacy is still moderate and unreliable. There is a high risk of vertebral fracture and adjacent segment degeneration [77,78]. Hence, based on the structural and pathophysiological characteristics of IVD, there is an urgent need for more ideal and valuable treatment methods designed to prevent, reduce, and even reverse the incidence of IVD degenerative cascade.
Fig. 2.
Diagnosis and current IVDD treatments.
4. Beneficial aspects of NDDSs for the treatment of IVDD
Currently, most drugs or bioactive molecules used to treat IVDD are administered systemically or via in situ IVD injection, but they all have the disadvantage of poor therapeutic effects. First, prolonged systemic overexposure and subsequent off-target side effects (e.g., enzyme inhibitors), resulting in very short bioavailability within the IVD, often also have side effects on healthy organs [18]; In addition, for IVD, the bioavailability in local tissues after systemic administration is limited owing to the presence of avascular, endplate, and synovial spaces [19]. For in situ IVD injection, the disadvantage is that the injected drugs are quickly cleared in the IVD, and the absorption of drugs by target cells is limited, which reduces the drug concentration and therapeutic effect at the action site. However, more frequent injections will lead to the risk of infection, inflammation, may puncture the NP, and even accelerate the IVDD process [20,79,80]. Furthermore, drugs directly injected into the IVD or epidural space will increase the risk of adverse reactions of the central nervous system with the dispersion or leakage of drugs [72,81]. Therefore, owing to the side effects and low drug availability of traditional drugs and delivery routes, NDDSs may potentially play a crucial role as part of the new IVDD treatment strategy because they can combine different types of drugs, provide local and continuous drug release, and achieve targeted drug delivery through passive or active targeting, reducing off-target toxicity [[82], [83], [84]].
Nanotechnology is at the forefront of medical diagnosis, imaging, and therapeutic drug delivery. Nanotechnology has the potential to alter the dimensions of drugs and materials, thereby facilitating control over their various properties. Simultaneously, owing to the innovative and impressive development of nanotechnology, many NDDSs have been developed and introduced into the field related to IVDD treatment. The field of NDDS in IVDD treatment is constantly evolving and expanding [[18], [19], [20]]. NDDSs have the following outstanding advantages, which have been widely confirmed to significantly improve and repair IVDD: (1) NDDSs are non-toxic, biodegradable, and have perfect biocompatibility with IVD, creating a favorable tissue microenvironment for promoting IVD regeneration; (2) some specific NDDSs can cross cellular barriers into the cytoplasmic space or activate specific transport mechanisms to improve drug retention; (3) continuous drug release prolongs the maintenance time of effective drug concentrations, reducing the frequency of drug administration and treatment cost, and improves compliance [[85], [86], [87]]; (4) natural materials may have additional benefits because their composition is similar to cartilage and other IVD-related matrices, providing a superior cellular microenvironment for the treatment of IVDD [88,89]. At present, nanoparticles, dendrimers, liposomes, micelles, and exosomes have been used as nanocarriers to construct nano-drugs; these nanocarriers contribute to IVD repair or healing, relieving symptoms (e.g., pain and inflammation) and improving function. Published studies have shown that nanodrugs may be safer, more reliable, and less risky than surgical treatment [17,90,91].
5. Major types of NDDS for IVDD
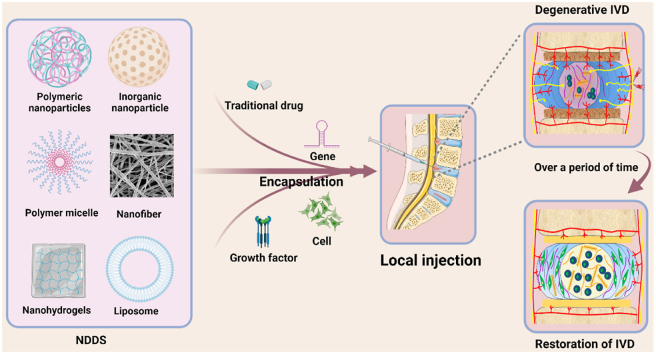
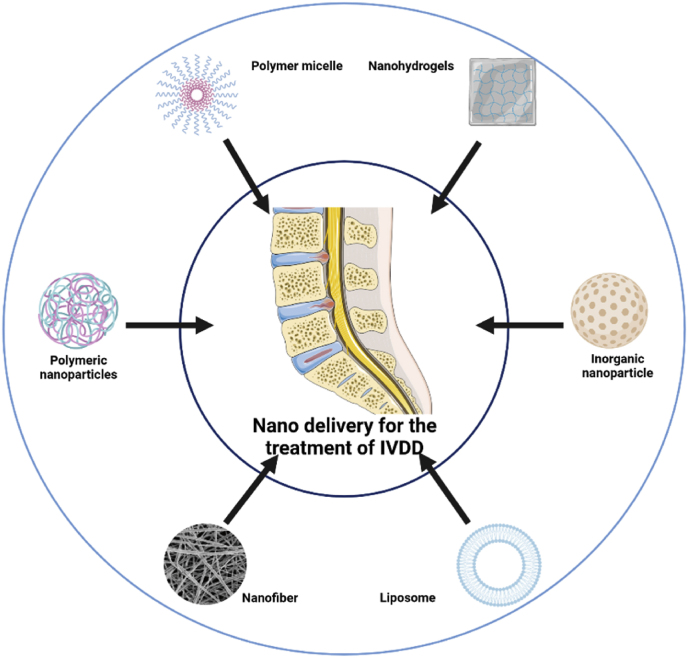
Numerous NDDSs carrying therapeutic agents have been used to promote IVD repair and regeneration. Several approaches have been adopted to apply biocompatible and safe drug delivery platforms to improve therapeutic outcomes. Herein, we summarized several promising nanocarriers for the management of IVDD, including liposomes, polymeric nanoparticles, inorganic nanoparticles, polymer micelles, nanofibers, nanohydrogels, and exosomes (Fig. 3), and look for new types of NDDS that are more valuable and effective for the treatment of IVDD in the future. The advantages and limitations of some common NDDSs used for the treatment of IVDD are summarized in Table 1. In addition, recent research of NDDSs for IVDD treatment are listed in Table 2.
Fig. 3.
Commonly used nano-drug delivery systems for IVDD treatment.
Table 1.
Main nano-drug delivery systems for IVDD.
| Category | Size | Removal manner | Scalability | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|---|
| Liposome | 50 nm −5 μm | Biodegradation | High |
|
|
[92,95] |
|
|
|||||
|
|
|||||
| PNPs | 1–1000 nm | Biodegradation | Medium |
|
|
[186,192] |
| ||||||
| ||||||
| Inorganic nanoparticles | 1–100 nm | Cell internalization or foreign body reaction | Medium |
|
|
[187,188] |
| ||||||
| ||||||
| Polymer micelles | Vared | Biodegradation | High |
|
|
[193] |
| ||||||
| ||||||
| Nanofibers | Varied | Cell internalization or foreign body reaction | Medium |
|
|
[194,195] |
| Nano-hydrogels | Mesh size of 5–100 nm | Biodegradation | High |
|
|
[196,197] |
| ||||||
| ||||||
|
Table 2.
Recent research of nano-drug delivery systems for IVDD treatment.
| Study | Year | Formulation | Therapeutic agents | Encapsulation efficiency | Particle size (nm) | Release rate | In vivo model tested | Timing of first administration after induction | Administration methods | Compared to free agents | Experiment duration | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Banala et al. [99] | 2019 | Liposome | siRNA | NA | NA | NA | Rabbits | 7 d | In situ injection (IS) | No | 8 w | Reduced the extent of apoptosis in the discs |
| Wang et al. [100] | 2020 | Liposome | oxymatrine | 73.4 ± 4.1% | 178.1 ± 2.9 | 67.2 ± 3.0% in 48 h | Mice | 2 h | IS | Yes | 4 w | Attenuated NP cell apoptosis, reduced the expression of MMP 3/9 and IL-6, and decreased degradation of collagen-II |
| Liang et al. [111] | 2013 | PNPs | ADSCs, dexamethasone and TGF-β3 | NA | 140 | NA | Rats | 2 w | IS | Yes | 24 w | Regenerated the degenerated disc |
| Lim et al. [112] | 2022 | PNPs | ABT263 | 57.%±13.4% | 494.3 ± 64.4 | 80.8% in 48 h | Rats | 2 w | IS | Yes | 6 w | Eliminated senescent cells, reduced pro-inflammatory cytokines and matrix proteases |
| Nguyen et al. [120] | 2019 | Carbon-based nanoparticles | Simvastatin | NA | 300–500 | NA | Rabbit (in ex vivo) | NA | IS | Yes | 2 w | NA |
| Zhou et al. [121] | 2022 | Carbon-based nanoparticles | NA | NA | ≈80 | NA | Rats | 2 w | IS | NA | 4 w | Alleviated oxidative stress and increased the activities of SOD1 |
| Zhu et al. [129] | 2022 | Magnetic nanoparticles (h-MnO2) | TGF-β3 | NA | ≈50 | ≈80% in 5 d | Rats | at the day of surgery | IS | Yes | 8 w | Prevented the degeneration and promoted self-regeneration |
| Feng et al. [132] | 2018 | Micelles | miRNA-29 | NA | ≈72 | ≈90% in 14 d | Rabbits | 2 w | IS | No | 4 w | Inhibited the fibrosis process, and reverse IVDD |
| Yu et al. [133] | 2021 | Micelles | KGN, APO | KGN: 3.7'%; APO: 38.8% | 49.8 | KGN: 90% in 4 h | Rats | at the day of surgery | IS | Yes | 16 w | Stimulated differentiation of human ADSCs and protected cells by suppressing oxidative stress |
| Chang et al. [135] | 2022 | Micelles | Runx1 | NA | 86.85 ± 17 | NA | Rats | at the day of surgery | IS | No | 4 w | Increased hydration content, disc space height and ECM production. |
| Feng et al. [142] | 2017 | Nanofibers | NR4A1 | NA | 100 | 80% in 2 months | Rats | 2 w | IS | No | 4 w | Repressed fibrosis and supported IVDS regeneration |
| Yu et al. [145] | 2022 | Nanofibers | Fucoidan | NA | 770 ± 0.240 | 40% in 10 h | Rats | at the day of surgery | In situ implantation | No | 4 w | Reduced the expression of COX-2 and deposited more ECM between the scaffold layers |
| Wang et al. [151] | 2021 | Nanohydrogels | MSCs | NA | 286 | NA | Rabbits | 4 w | IS | No | 16 w | Supported the differentiation of MSCs into NP-like cells and regenerated degenerated NPs |
| Chen et al. [153] | 2020 | Nanohydrogels | agomir-874 | NA | NA | 90% in 10 d | Rats | at the day of surgery | IS | Yes | 8 w | Modulated ECM balance thereby lowing the process of IVDD |
| Chang et al. [154] | 2022 | Nanohydrogels | circSTC2 | 95.60 ± 0.002% | 103.5 ± 1.06 | 18.32 ± 3.03% in 3 d and over 80% in 27 d | Rats | at the day of surgery | IS | No | 8 W | Promoted ECM synthesis and repaired NP tissue |
| Shen et al. [162] | 2021 | Nanohydrogels | Lactate oxidase | 93% | 100 | NA | Rabbits | 3 d | IS | Yes | 8 w | Downregulated the local lactate level in degenerative IVDs |
| Zheng et al. [165] | 2021 | Nanohydrogels | MR409 | NA | 150 | >80% in 3 d | Rats | 8 w | IS | Yes | 12 w | Attenuated IVDD by inhibiting autophagy |
| Liu et al. [167] | 2021 | Nanohydrogels | Aspirin | NA | 141.8 | (85.43 ± 2.30% | Rabbits | at the day of surgery | IS | Yes | 2 w | Inhibited the inflammatory response |
| Bai et al. [169] | 2020 | Nanohydrogels | Rapamycin | NA | NA | 80% in 3 d | Rats | 4 w | IS | Yes | 16 w | Reduced the level of ROS and regulated the polarization of macrophages |
5.1. Liposomes
Liposomes, nanosized spherically enclosed lipid bilayers, were the first nanoparticles to be transformed into clinical applications. Similar to cell membranes, liposomes are composed mainly of phospholipids and cholesterol. Liposomes are composed of a hydrophilic core and one or more hydrophobic spaces surrounded by lipid bilayers. This unique amphiphilic structure enables the encapsulation of both hydrophobic and hydrophilic compounds [92]. Moreover, the main advantages of nanoliposomes are their high biocompatibility, good flexibility in regulating biophysical and physicochemical properties, and established preparation methods, which can be customized according to their intended use [93,94]. They can deliver drugs directly into target cells or tissues, avoiding the use of high concentrations of free drugs and thereby reducing toxicity and adverse effects [95].
Owing to these advantages, liposomes are widely used as drug carriers [96]. For example, liposomal bupivacaine is often used clinically to relieve postoperative pain [97,98]. In 2011, Yang et al. employed a thin-film hydration method to encapsulate hydroxycamptothecin in liposomes and successfully transplanted them into the laminectomy area of rabbits [95].
Caspase 3 plays a central role in apoptosis, and ADAMTS5 plays a critical role in ECM degradation. Banala et al. injected liposomal small interfering RNA (siRNA) designed against caspase 3 and ADAMTS5 genes into a rabbit model of IVDD. They found that liposomal siRNA could significantly downregulate the expression levels of pro-apoptotic markers, indicating that direct delivery of siRNA to the IVD has the potential to achieve non-surgical treatment of IVDD [99]. Therefore, developing an induction system using this strategy to regulate transgene expression is possible. siRNA developed against the target produced gene downregulation and contributed to the production of amplified ECM components. In another study, Wang et al. encapsulated oxymatrine (OMT) in liposomes using a pH gradient and conducted a series of experiments. They found that compared with OMT in neutral saline, OMT-liposomes showed excellent stability and a significantly longer sustained release time and had more drug accumulation in the IVD. OMT-liposomes play an anti-IVDD role by attenuating the apoptosis of NP cells, decreasing the expression of matrix metalloproteinase-3/9 and IL-6, and reducing the degradation of collagen-II [100].
To date, minimal investigations have been conducted on the use of liposomes in the treatment of IVDD. More studies are needed to explain the effectiveness of liposome-mediated drug delivery for treating IVDD. In addition, there are several points to note: (1) The overall encapsulation volume of liposomes is low, which can be greatly improved by using reverse evaporation and freeze-thaw cycles [101,102]. (2) After entering the body, phospholipids are easily degraded, which can be addressed by adding different components to lipid bilayers or surface-modifying liposomes to achieve longer circulation times and controlled release [103,104]. (3) Low liposomal reproducibility is a major obstacle. (4) The current scale of liposome production is small, and there is an urgent need to develop large-scale methods suitable for clinical applications. (5) The pathological changes following IVDD are even more unfavorable for the absorption and release of drugs, making delivery of drugs to the IVD by liposomes more difficult.
5.2. Polymeric nanoparticles (PNPs)
PNPs are usually nanoscale homogeneous spherical structures composed of biocompatible and biodegradable polymers that encapsulate drugs inside or attached to the surface of the particles [105]. PNPs can be synthesized chemically, such as poly (lactic acid) (PLA), poly (l-lactide-co-glycolide) (PLGA), polycaprolactone, and polyethylene glycol (PEG), or obtained from natural polymers, such as alginate, chitosan, fibrin, gelatin, collagen, and albumin [106,107]. Synthetic polymers are preferred because of their inertness, low toxicity, and degradability [108]. PNPs are generally used as drug carriers and are biodegradable. Drugs can be released via desorption, diffusion, or nanoparticle erosion at the target tissue site. To reduce immune and intermolecular interactions between chemical groups on the surface of PNPs, they are usually coated with non-ionic active agents. Moreover, to target drugs to specific tissues or cells, PNPs are usually combined with ECM or cell-binding ligands [18].
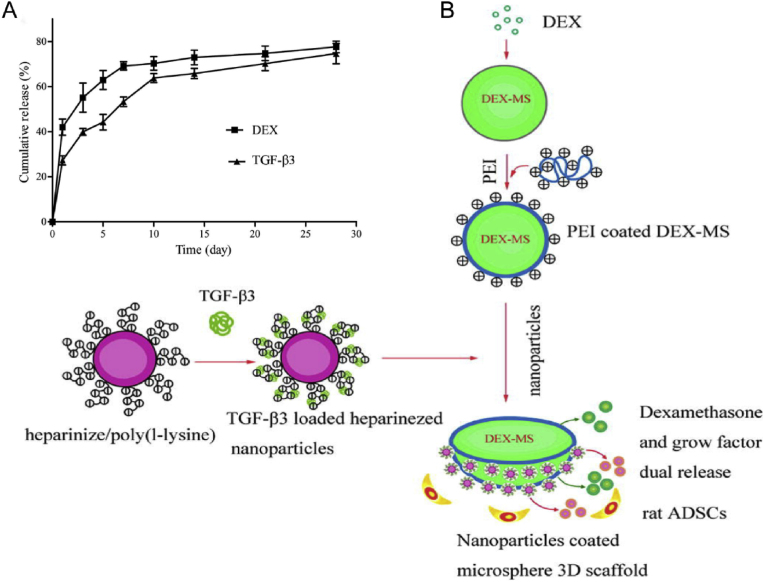
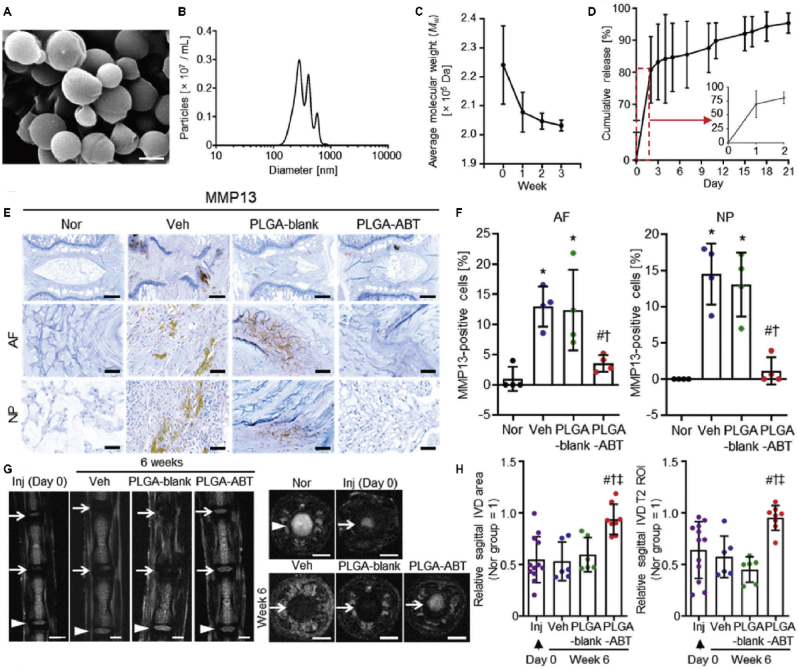
In 2012, Liang et al. constructed nanostructured 3D PLGA microspheres loaded with dexamethasone and growth factor nanoparticles, in which stem cells could generate more NP matrix than in the control group in vitro [109]. This system is non-cytotoxic, biocompatible, and compatible with bioactive factors, which provides a new opportunity for simple and effective stable IVD drug delivery [110]. Later, TGF-β3 and dexamethasone (DEX) were combined on PLGA microspheres loaded with adipose-derived stem cells (ADSCs) (Fig. 4), and found that 24 weeks after implanting the system into the IVD of rats, the PM (with a needle puncture, PLGA microspheres-only injection) and PMA (with a needle puncture, PLGA microspheres loaded with ADSC injection) groups regained disc height values of 63% and 76% and MRI signal intensities of 47% and 76%, respectively, compared to the NC group [111]. A major concern with using PLGA is that its degradation products (i.e., lactic and glycolic acids) decrease local pH, which may cause an inflammatory reaction. However, Lim et al. dispelled this concern [112]. They loaded ABT263 (a senolytic drug) into PLGA nanoparticles (PLGA-ABT) with an encapsulation efficiency of 57.3% ± 13.4% and then intradiscally administered PLGA-ABT into the needle punch injury-induced IVDD rat model. They found that PLGA-ABT released 80.8% of loaded ABT263 in vitro for the first two days and 14.6% of the encapsulated drug for the next 19 days. Importantly, inflammation or apoptosis at the injection site was not observed. They demonstrated that both senescent AF and NP cells from degenerative discs could be removed by a single intradiscal injection of PLGA-ABT 6 weeks post-treatment. This strategy also reduced the expression of pro-inflammatory cytokines (IL-6) and MMP-13 in the IVD, inhibited the progress of IVDD, and even restored the structure of the IVD, which demonstrates for the first time that local delivery of senolytic drugs can effectively treat senescence-related IVDD (Fig. 5) [112].
Fig. 4.
Schematic diagram and characterization of DEX-PLGA microspheres. (A) DEX and TGF-β3 release profiles from dual bead PLGA microspheres. (B) Schematic diagram of TGF-β3-loaded heparinized nanoparticles coated onto a DEX–PLGA microsphere. Reproduced with permission [111]. Copyright 2013, Acta Materialia Inc. Published by Elsevier Ltd.
Fig. 5.
PLGA-ABT injected intradiscally inhibits IVDD and restores intervertebral disc structure. (A) SEM image of PLGA-ABT. Scale bars, 500 nm. (B) The diameter range of PLGA-ABT. (C) Biodegradation of PLGA-nanoparticles as evaluated by the molecular weight change of PLGA nanoparticles over the incubation time in buffer at 37 °C. (D) The cumulative release of ABT263 from PLGA-ABT in vitro. (E–F) Immunostaining for MMP13 in AF and NP tissues of normal IVD (Nor) and injured IVD lesions treated with either vehicle (Veh), PLGA-blank, or PLGA-ABT for six weeks. Scale bars, 500 μm. (G–H) Representative coronal (left) and axial (right) MRI, sagittal area, and T2 ROI. Reproduced with permission [112]. Copyright 2021, Wiley-VCH GmbH.
In 2017, Antunes et al. combined poly(γ-glutamic acid) (γ-PGA) and chitosan (Ch) to form a nano complex, which was used to culture nucleotomized bovine IVDs, and found that γ-PGA/Ch could promote the production of sulfated glycosaminoglycan and synthesis of collagen-II in a neutral environment, indicating that it promotes the recovery of the IVD native matrix [113]. Coincidentally, Teixeira et al. used γ-PGA/Ch and diclofenac (Df) to form nanoparticles and evaluated the effect of injecting Df/γ-PGA/Ch into degenerated IVD. Df/γ-PGA/Ch can be internalized by IVD cells and downregulate the expression of pro-inflammatory factors (IL-6, IL-8, and PGE2). At the same time, it can also reduce ECM degradation by downregulating MMP1 and MMP3 and upregulating collagen-II and proteoglycan [114]. Thus, PNPs have great potential for application in IVDD treatment. We believe that with more discoveries and inventions of PNPs, more studies on the targeted therapy of IVDD based on PNPs will emerge.
5.3. Inorganic nanoparticles
Inorganic nanoparticles are derived from inorganic materials, including metal nanoparticles, carbon-based nanoparticles, silica nanoparticles, calcium nanoparticles, ceramic nanoparticles, and quantum dots [115]. Inorganic nanoparticles with various morphologies and particle sizes ranging from 1 to ∼100 nm. Inorganic nanoparticle surfaces are difficult to modify, and combine with drug molecules in different ways, such as electrostatic interactions, hydrophobic interactions, and covalent bonds of enzyme-sensitive groups, to achieve responsive release and enhance therapeutic effects.
5.3.1. Carbon-based nanoparticles
Carbon-based nanoparticles (NPs) are the most widely used inorganic nanoparticles for IVD drug delivery. Some carbon-based nanoparticles have their own biological activities. For example, fullerene (C60), composed of 60 carbon atoms, forms a hollow sphere approximately 1 nm in diameter. It is called a "free radical sponge," Its antioxidant effect is hundreds of times higher than other conventional antioxidants. However, their low water solubility and poor biocompatibility limit their application. Subsequently, researchers modified fullerene with hydrophilic groups (such as carboxyl, hydroxyl, and epoxy groups) to make it more biocompatible. Among the numerous fullerene derivatives reported, fullerol has been the most widely studied. Fullerol is a polyhydroxylated derivative of fullerene. In 2014, Jin et al. reported that fullerol nanoparticles could increase the water and proteoglycan content and inhibit heterotopic bone formation in degenerated IVDs, thus effectively preventing IVDD [116]. Later, they demonstrated that fullerol nanoparticles effectively alleviated the inflammatory cascade caused by IVDD [117]. Moreover, In 2018, Yeh et al. found that the combined effect of link protein N-terminal peptide (LN) and fullerol counteracted IL-1α-induced increases in pro-inflammatory mediators (IL-6 and cyclooxygenase-2) and matrix metalloproteinase (MMP-1, -2, -9, and -13) [118]. The limitation of this study is that the effects of LN and fullerol in vivo have not been tested. Xiao et al. reported a functionalized nano-fullerene conjugated with a peptide that specifically binds to formyl peptide receptor-1 (FPR-1) expressed on activated macrophages [119]. This research provides a new idea for targeted IVD delivery. It is possible to develop a drug that can specifically bind to a certain cell receptor following IVD and then combine it with carriers to achieve highly targeted IVD drug delivery.
In 2019, Nguyen et al. developed stable nanoscale phase-transition liquid perfluorocarbon droplets that could be activated multiple times by high-intensity focused ultrasound (HIFU). Ultrasound-generated pressure waves can transform droplets into bubbles, a process called acoustic droplet vaporization (ADV), and the phase-change bubbles can release drugs when the droplets undergo ADV. They encapsulated hydrophobic simvastatin in perfluorocarbon droplets and found that the droplets exhibited high stability, low cytotoxicity, and controllably triggered release under ex vivo conditions for at least 14 days [120]. This new system can be applied to IVDD in the future.
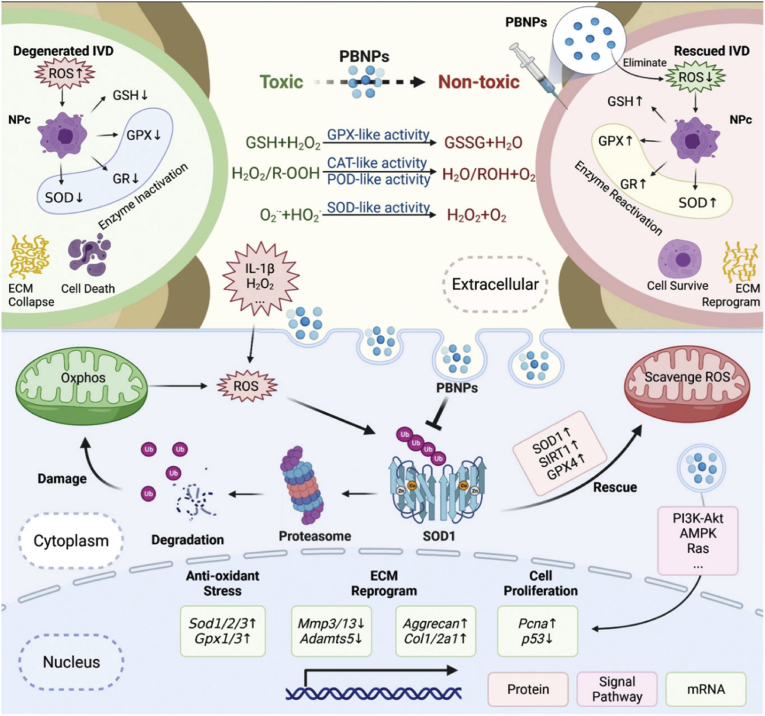
In 2022, Zhou et al. locally injected Prussian blue nanoparticles (PBNPs) into IVDD rat models and found that PBNPs could alleviate intracellular oxidative stress by inhibiting the ubiquitination of superoxide dismutase 1 (SOD1) and alleviating IVDD induced by reactive oxygen species (ROS) (Fig. 6). Moreover, PBNPs can be used as a potential antioxidation-protective discography contrast agent to avoid the anoxic environment in IVDs caused by conventional discography that can accelerate disc degeneration [121].
Fig. 6.
Molecular mechanism diagrams of PBNPs alleviate ROS in NP cells. The mechanism is divided into three levels. First, antioxidant enzyme‐like PBNPs can directly scavenge extracellular ROS; second, PBNPs can inhibit the ubiquitin‐proteasome degradation of intracellular SOD1, increase the number of mitochondria‐related SOD1 protein and enzyme activity, and eliminate intracellular ROS; third, PBNPs can activate Ras, p53, PI3K Akt, AMPK, and other pathways, directly affecting the transcription of antioxidant enzymes (SOD, GPX, and SIRT). Under these three key mechanisms, PBNPs can improve the antioxidant capacity inside and outside NPs, which further activates the viability of cells, improves the anabolic ability of cells, and reconstructs the ECM of the nucleus pulposus, which rescues ROS‐mediated IVDD in animal models. Reproduced with permission [121]. Copyright 2022, The Authors. Advanced Science published by Wiley-VCH GmbH.
5.3.2. Magnetic nanoparticles
In 1956, Gilchrist applied magnetic nanoparticles to biomedical research for the first time [122]. Magnetic NDDSs can be used to modify the surfaces of individual particles, hollow structures, and hybrid structures of magnetic nanoparticles. Magnetic nanoparticles can be coated with silica, aurum, or polymer into magnetic nanoparticles with a core-shell structure, which is more conducive to their surface modification and drug loading. Manganese dioxide (MnO2) nanoparticles are the main magnetic nanoparticles for IVD drug delivery.
MnO2 is a common mineral with several unique chemical and physical properties. Owing to their low toxicity, strong adsorption, and good biocompatibility, MnO2 nanoparticles can carry a variety of drugs and cytokines and then degrade into manganese ions under weakly acidic conditions to slowly release the encapsulated cargo and achieve a therapeutic effect. Zhang et al. proposed that direct injection of proteins encapsulated in hollow MnO2 (h-MnO2) microspheres can achieve slow drug release, relieve the oxidative stress reaction in organisms, provide an oxygen (O2) equivalent for cells, improve the low O2 concentration and low pH state in the microenvironment, and thus restore tissue strength and the process of cell metabolism [90]. At the same time, MnO2 particles can also regulate the production of cytokines at the level of regulatory genes and reduce the degree of inflammation. In this process, MnO2 gradually decomposes into Mn2+ and is discharged with excreted body fluids, thus restoring the internal environment to the optimal state [123]. MnO2 can change the living environment of anaerobic nucleus pulposus cells in terms of gene expression and achieve IVD repair. Most importantly, MnO2 microspheres can achieve a sustained release of encapsulated drugs and cooperate with drug mechanisms to resist the negative effects caused by various pro-inflammatory factors. Moreover, MnO2 and protein may act synergistically, which is important for degrading pro-inflammatory factors in the IVDD microenvironment [90].
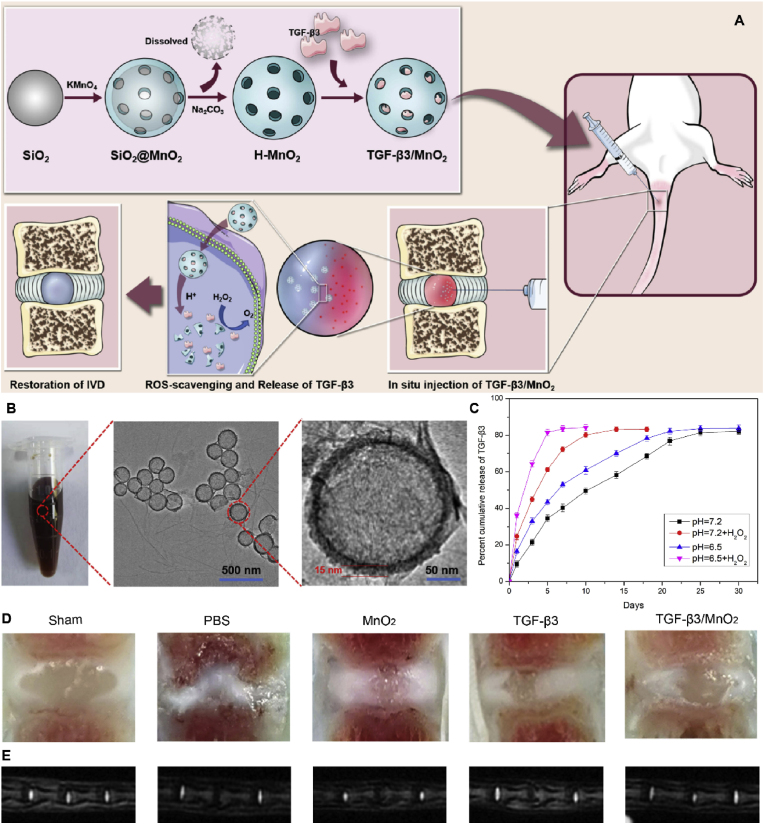
TGF-β3, an important member of the TGF-β superfamily, regulates biological processes such as cell proliferation, survival, and differentiation [124,125]. TGF-β3 upregulates the expression of genes related to cartilage formation, promotes cartilage repair, and accelerates cartilage differentiation [126]. Importantly, TGF-β3 stimulation promoted IVD cell survival and ECM deposition [127,128]. Therefore, targeting TGF-β3 could ameliorate the metabolic imbalance of the ECM in IVDD and possibly delay its progression. However, delivering TGF-β3 alone to diseased tissues creates a localized excess, and the protein is rapidly degraded or flushed by excreted body fluids. Methods for delivering sustained doses of TGF-β3 to target tissues can facilitate IVD repair. Zhu et al. loaded TGF-β3 onto h-MnO2 nanoparticles to form intelligent biodegradable NDDSs, which can dissociate at low pH or in the presence of hydrogen peroxide (H2O2) environments to release loaded TGF-β3 [129]. The release curve showed that TGF-β3 was continuously released from MnO2 in a time-dependent manner. Compared to the slow drug release observed at pH 7.4, the rate of TGF-β3 release was higher at pH 6.5. Furthermore, incubation with H2O2 at pH 6.5 further accelerated drug release by triggering the decomposition of MnO2 nanocarriers into Mn2+ ions. When injected into IVDD rats, TGF-β3/MnO2 effectively slowed Col-II degradation and decreased iNOS expression for up to eight weeks after treatment. This was not observed in the TGF-β3 and MnO2 groups (Fig. 7) [129]. However, further research is needed to improve the efficacy and extend the treatment time, as well as to confirm the long-term efficacy of this nanoplatform. In brief, targeting oxidative stress or acidity and designing multifunctionally responsive-DDS can improve the harsh microenvironment of intervertebral disc degeneration and may provide a new strategy for IVDD treatment.
Fig. 7.
Schematic diagram and characterization of MnO2 nanoparticles. (A) Schematic of MnO2 nanoparticles in IVDD treatment. (B) Digital picture and TEM images of MnO2 nanoparticles. (C) Nanoparticle decomposition and drug behaviors of TGF-β3/MnO2. In vitro release of TGF-β3 from MnO2 NPs at different pH values (7.4 and 6.5) in the absence or presence of 100 μM H2O2. (D–E) Evaluation of disc degeneration with T2-weighted MRI and gross appearance at 8 weeks. Representative images of disc gross appearance (D) and MRI signal intensity (E). Reproduced with permission [129]. Copyright 2022, Zhu et al., published and licensed by Dove Medical Press Limited.
5.4. Polymer micelles
Polymeric micelles are core-shell structures formed by the self-assembly of amphiphilic block copolymers during hydration, and contain a hydrophobic core with a hydrophilic shell facing the hydrophilic medium, allowing insoluble drugs to be wrapped in hydrophobic cores. Their size is usually determined by the ratio of hydrophilic to hydrophobic parts of amphiphilic molecules and can vary depending on the nature of the encapsulated drug. Polymer micelles were first proposed as drug delivery carriers by Yokoyama et al. [130] in 1992. In recent years, polymer micelles as a "nanoreactor" with unique properties, have played an increasingly important role in the in vivo delivery of insoluble drugs [131].
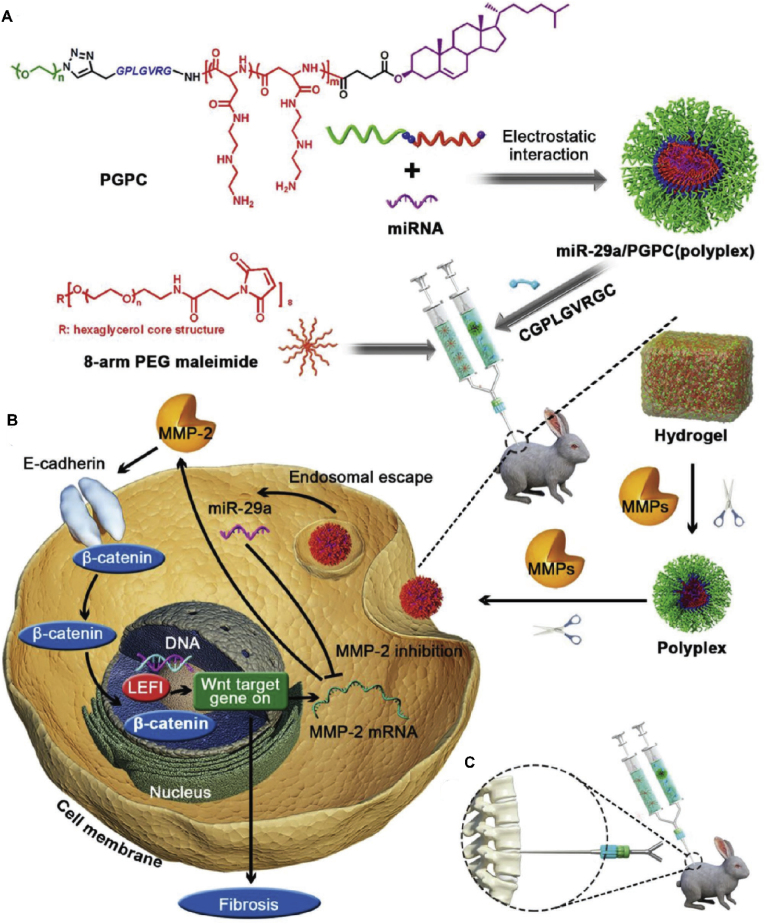
In 2018, Feng et al. used overexpressed MMPs after IVDD to develop a continuous and bioresponsive two-stage miRNA-29 delivery system, which consists of polyplex micelles as miRNA-29 carriers and injectable hydrogel capsules (Fig. 8). In the first stage, the increased MMP levels in degenerated IVD led to the MMP-responsive degradation of hydrogels, resulting in the continuous release of micelles. In the second stage, the released micelles separated the PEG shell under the action of MMPs. Then, enhanced cell uptake and endosomal escape could be achieved in NP cells. Inhibition of fibrosis by miRNA-29a was demonstrated to silence MMP-2 expression and block the β-catenin translocation pathway from the cytoplasm to the nucleus [132]. This strategy effectively delivers miRNA-29 to NP cells in IDD tissue, thus achieving continuous MMP inhibition and delaying the progression of IVDD. In addition, Yu et al. designed a novel amphiphilic copolymer, PEG-PAPO, which can be self-assembled into injectable esterase-reactive nano micelles that can load lipophilic kartogenin (KGN) and apolipoprotein (APO). When the micelles were co-injected with human ADSCs into IVDD rats, oxidative stress promoted esterase activity in human ADSCs, thus accelerating micelle degradation. The results proved the biosafety of the micelles and their ability to stimulate human ADSCs differentiation and inhibit oxidative stress. Therefore, these micelles may be used as a potential adjuvant for cell transplantation in IVDD treatment [133]. In another study, Lin et al. designed a highly secure, polyamine-based PEG-polyplex nano micelle nucleic acid delivery system, which carries Runx1 (a cartilage anabolic factor directly involved in KGN-mediated chondrogenesis) mRNA. The results showed that Runx1 mRNA delivered by nano micelles increased IVD height and prevented fibrosis at the degeneration site [134]. Later, they confirmed that this system also increased the water content of the punctured IVD by approximately 43% at 4 weeks after injection. In addition, the IVD space and ECM production were also significantly improved [135].
Fig. 8.
Sustained and bioresponsive two-stage delivery of therapeutic miRNA-29 via polyplex micelle-loaded injectable hydrogels to inhibit intervertebral disc fibrosis. (A) Schematic illustration for the formation of miRNA/PGPC polyplex micelles. (B) Encapsulation of miRNA/PGPC polyplexes in PEG hydrogels and molecular mechanism of MMP-2 silence in NP cells for fibrosis inhibition. (C) Injection sites in the IVDs of rabbits. Reproduced with permission [132]. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinhei.
5.5. Nanofibers
As an important biomaterial, nanofibers have the characteristics of a high surface-area-to-volume ratio, high porosity, and low density. Currently, most nanofibers are composed of biodegradable synthetic polymers and natural polymers. In the field of biomedicine, nanofibers are mainly used as a scaffold for tissue engineering. In recent years, drug delivery has become an important new application. Nanofibers have structural characteristics similar to the ECM, which can improve the interactions between cells and drugs.
As early as 2011, nanofibers were used as tissue-engineering scaffolds in vitro to support AF cell growth [136]. Nerurkar et al. used electrospinning to produce nano-poly (ε-caprolactone) scaffolds that mimic the layered structure of natural AF and have the same function as natural tissue. These scaffolds were combined with a cell seed hydrogel (as a substitute for NP) to form a disc-like angle-ply structure (DAPS) [137,138]. Martin et al. implanted DAPS into the caudal vertebrae of rats and found that the scaffold did not allow endogenous cell infiltration [139]. In 2014, Turner et al. found that different tensions in the scaffold matrix can cause differences in the function of AF cells in vitro. This shows that appropriate tension is required to produce correctly formed fibrous annulus tissue [140]. In 2015, Tao et al. demonstrated a scaffold with a nanofiber structure formed by the self-assembly of functional peptides under physiological conditions. The scaffold can promote the proliferation, migration, and secretion of the extracellular matrix (collagen-II, aggrecan, and Sox-9) of human degenerated NP cells in vitro, and is biocompatible in vivo. However, this study did not explore the effects of scaffolds on IVDD [141].
Moreover, Feng et al. developed a hyperbranched polymer (HP) with high plasmid DNA (pDNA) binding affinity and negligible cytotoxicity, which can be self-assembled into nano-polyplexes with a "double shell" structure, and can be encapsulated in PLGA nanospheres or loaded onto spongy nanofibrous microspheres (NF-SMS), thus forming an injectable two-stage gene delivery system for continuous delivery of high plasmid pDNA to IVD cells. This novel delivery system is biodegradable and biocompatible, can release polyplexes in a time-controlled manner, and can efficiently transfer anti-fibrosis genes (NR4A1) into IVD cells, significantly reverse fibrosis, and promote IVD regeneration in rat models [142]. In addition, Uysal et al. designed a collagen peptide-presenting nanofiber, assembled into nanofibers to form a scaffold. The scaffold provides a biocompatible environment for MSCs in vitro to differentiate into chondrocytes and maintain the morphology and function of MSCs in vivo. These scaffolds were injected into degenerated rabbit IVDs, which showed that the scaffolds could promote functional recovery and prevent IVDD progression [143]. Liu et al. fabricated a decellularized AF matrix (DAFM)/poly (ether carbonate urethane) urea (DAFM/PECUU)-composite electrospun scaffolds with high hydrophilicity and porosity using coaxial electrospinning technology. AF-derived stem cells proliferated well on the DAFM/PECUU scaffolds and showed increased expression of collagen I and II and aggrecan. When the scaffolds were transplanted into rabbits, inflammation around the DAFM/PECUU scaffolds was significantly lower than that around the PECUU scaffolds [144]. Therefore, nanofibrous scaffolds with special characteristics can repair AF and have good therapeutic potential for IVDD.
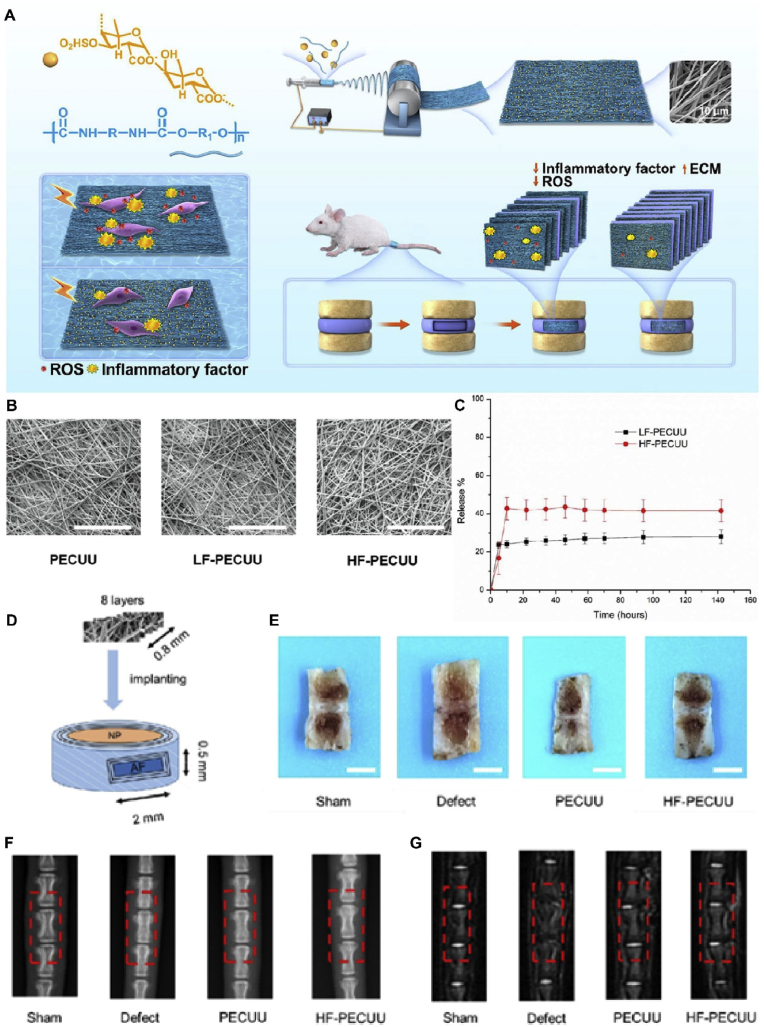
When nanofibrous scaffolds were integrated with other drugs, they achieved an impressive synergistic anti-IVDD effect. In 2022, Yu et al. loaded fucoidan, a natural bioactive polysaccharide with good anti-inflammatory and antioxidant properties, onto PECUU scaffolds using the electrospinning technique (Fig. 9). Compared with the pure PECUU scaffold, the fucoidan-loaded PECUU nanofibrous scaffold (F-PECUU) significantly decreased the gene and protein expression related to inflammation (IL-1, IL-6, and TNF) and oxidative stress in lipopolysaccharide (LPS)-induced annulus fibrosus cells (AFCs). F-PECUU decreased the expression of COX-2 in vivo and deposited more ECM between scaffold layers than pure PECUU. The disc height and nucleus pulposus hydration of repaired IVD reached 75% and 85% of those in the sham group. In addition, F-PECUU helped maintain an integrated tissue structure with a compression modulus similar to the sham group [145]. Taken together, the nanofibrous F-PECUU scaffold developed in this study may provide a new method for promoting AF repair in IVDD treatment by ameliorating the harsh degenerative microenvironment.
Fig. 9.
Schematic diagram and characterization of F-PECUU for IVDD treatment. (A) Schematic diagram of F-PECUU synthesis and in vivo experimental design. (B) The characterization of F-PECUU scaffolds by scanning electron microscopy (SEM). Scale bar, 100 μm; (C) The release curves of fucoidan. (D) The scheme of operation in vivo. (E) Preliminary observation of IVD specimens after 4 weeks implantation. Scale bars, 2 cm; (F) The images of X-ray and the %DHI of IVDs; (G) The images of MRI and the relative water content of IVDs. Reproduced with permission [145]. Copyright 2022, Acta Materialia Inc. Published by Elsevier Ltd.
5.6. Nanohydrogels
Nanohydrogels are hydrophilic three-dimensional polymer networks formed by chemical or physical cross-linking hydrogels and nanomaterials. Nanohydrogels have the unique properties of hydrogels and nanoparticles. Conventional hydrogels (such as microgels) usually involve intermolecular cross-linking, whereas, in nanohydrogels, intramolecular cross-linking is mainly determined [146]. Nanohydrogels have several advantages (Table 1): (1) They can swell in water and have a certain adhesion, and (2) the drug can be encapsulated in their internal three-dimensional network structures to protect it from destruction by the external environment, (3) high drug loading and sustained release, (4) small size and high permeability, (5) large specific surface area for modification, and (6) high biocompatibility and biodegradability. Nanohydrogels are being widely studied for IVD delivery because of their longer plasma half-life, better loading capacity, and excellent tissue absorption capacity. In IVDD treatment, nanohydrogels have a gel-like structure similar to that of the NP; they are often used as scaffolds to deliver cells, genes, bioactive factors, and drugs (Table 2).
5.6.1. Nanohydrogel-based cell therapy
As mentioned earlier, the biological activity and number of NP cells decreased significantly following IVDD, resulting in decreased secretion of ECM-related proteins. Therefore, increasing NP cell numbers may slow IVDD progression. Due to the poor self-regeneration of cells isolated from degenerated IVD, transplanting exogenous cells may be a promising strategy for IVDD treatment [147]. However, acidic, hypoxic, inflammatory, and nutrient-deficient environments in degenerated IVDs are unsuitable for cell survival [148]. Hydrogels are considered suitable carriers because of their similar rheological properties and good cytocompatibility with NP tissue. However, ordinary hydrogels are generally unsuitable for the early IVDD treatment because local administration also destroys AF, thus accelerating IVDD progression. Therefore, the current trend is to use injectable nanohydrogel carriers; that is, the nanohydrogels are in a liquid state before injection, and phase hardening occurs after reaching the degenerated IVD, which ensures minimum damage to AF and enables the carrier to adapt to irregular shapes in the IVD.
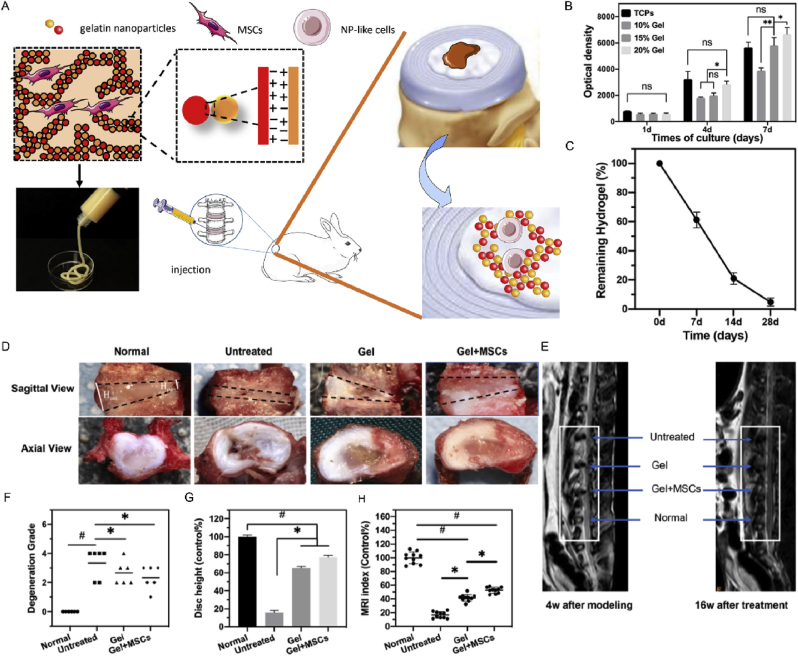
In 2016, Gan et al. prepared TGF-β3-loaded PLGA nanoparticles, seeded onto dextran/gelatin nanohydrogels to form a delivery system. This system exhibited good biocompatibility, stably released TGF-β3, and successfully induced MSCs into NP-like cells [149]. In 2019, Ligorio et al. designed a hydrogel using graphene oxide (GO) as a nanofiller. The mechanical properties of the hydrogels were similar to those of NP tissue. NP cells cultured on the hydrogel showed a high survival rate and metabolic activity, indicating that the system has great potential to transport NP cells [150]. However, neither of the above studies validated this effect in vivo. Later, Wang et al. used colloidal nanohydrogels as injectable scaffolds, which were tested for their injectability, biocompatibility, biodegradability, and ability to support the differentiation of MSCs into NP-like cells (Fig. 10). After they were delivered to rabbit IVDD models through minimally invasive surgery, they showed excellent anti-leakage performance, improved cell survival rates, and robustly regenerated degenerating NPs [151].
Fig. 10.
Injectable nanostructured colloidal gels resembling native NPs as MSC carriers for the repair of degenerated intervertebral discs. (A) The scheme shows the colloidal network formation as a matrix for 3D cell encapsulation and culture in the IVD degeneration rat model. (B) MSC proliferation on different concentrations of gelatin colloidal gels after 1, 4, and 7 days of culture were evaluated by a CCK-8 assay and compared to MSCs on tissue culture plates (TCPs). *P < 0.05; **P < 0.01. (C) After 7, 14, and 28 days of subcutaneous implantation, the residual gels were quantified. (D) Morphological differences of IVDs by macroscopic views showing the changes of IVD structure after 16 weeks of implantation. (E) Representative MRI images of discs 4 weeks after modeling and 16 weeks after treatment. (F) Degeneration grade of IVDs was morphologically evaluated, and (G) the disc height index was quantitatively assessed. (H) MRI signal intensity index of IVDs after 16 weeks of treatment. Reproduced with permission [151]. Copyright 2021, Published by Elsevier B.V.
The above studies have demonstrated the potential of cell-loaded nanohydrogels in IVDD; however, although injectable nanohydrogels can minimize the leakage of transplanted cells, they may lead to unwanted osteophyte formation once they leak or flow back into the structures around the IVDs. One possible solution is to use markers to label transplanted MSCs or other cells to achieve real-time observation with imaging equipment (such as 3T MRI) in vitro [152]. In addition, ensuring the directional differentiation of transplanted cells is a major problem. The current general method uses inducers or gene-editing techniques to induce differentiation. However, in a complex in vivo environment, directional differentiation is difficult to guarantee and may even cause unnecessary differentiation results. Future studies should focus on developing highly specific inducers and finding ways to achieve real-time monitoring of cell differentiation. Therefore, emerging studies have used nanohydrogels as delivery systems to enhance drug efficacy.
5.6.2. Nanohydrogel-based gene therapy
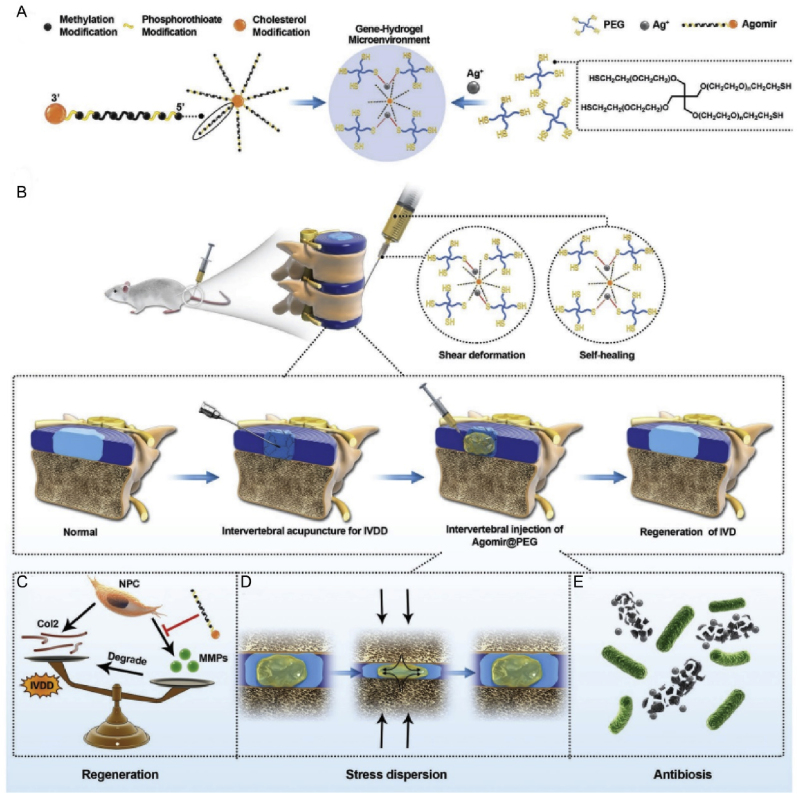
The continuous development of gene sequencing technology has provided a basis for gene therapy. Through differential gene expression analysis of degenerative NP tissue, genes related to IVDD and regeneration and repair have been continuously discovered. Currently, research on gene therapy is mostly focused on miRNAs. In 2020, Chen et al. prepared a PEG nanohydrogel with excellent agomir loading (agomir is a modified miRNA fragment that mimics the role of miRNA in regulating target gene expression) (Fig. 11). The nanohydrogel system has mechanical properties similar to those of a normal IVD and can match and compensate for the elasticity of the degenerated IVD. This system can be directly injected into the intervertebral space in a minimally invasive manner, thereby reducing the risk of leakage and rupture. The results showed that the system could deliver agomir-874 locally to IVDs, which regulates the catabolism of ECM and improves the microenvironment of nucleus pulposa tissue [153]. Besides miRNAs, more attention has been paid to the role of some circular RNA (circRNA) in IVDD in recent years. Chang et al. constructed an injectable nanohydrogel microsphere loaded with liposomes containing a circRNA (circSTC2) silencing gene. As a safe and controllable targeted gene delivery system, it can silence pro-IVDD genes in NP cells and regulate ECM metabolic homeostasis. In rat models, it promoted ECM synthesis and repair of NP tissue after 2 months [154].
Fig. 11.
Gene-hydrogel microenvironments for regeneration in IVDD. (A) The construction of the gene-hydrogel microenvironment. (B) The Agomir@PEG was injected into the intervertebral space to construct the gene-hydrogel microenvironment. (C–E) The multi-functions provided by the gene-hydrogel microenvironment match the regeneration of IVDD. Reproduced with permission [153]. Copyright 2019, The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
These studies demonstrate the surprising versatility of gene-loaded nanohydrogel systems in IVDD treatment. However, the delivery of genes to the IVD remains challenging. The poor performance of carriers may lead to gene inactivation, low transfection efficiency, and a short half-life. Therefore, developing a reliable delivery system is a primary prerequisite for IVDD gene therapy.
5.6.3. Bioactive factor-loaded nanohydrogels for IVDD treatment
The bioactive factors commonly used in the treatment of IVDD are growth and differentiation factor (GDF), TGF-β, osteogenic protein-1 (OP-1), and IGF. However, it has been proven that direct injection of bioactive factors into the IVD has a very short half-life. Continuous local administration is needed to maintain therapeutic effects, which can accelerate degeneration [155]. Additionally, improper injections may lead to heterotopic ossification. Therefore, there is an urgent need to develop a continuous drug delivery system (DDS) to reduce side effects and improve therapeutic effects. Ligorio et al. developed an injectable graphene oxide (GO)-self-assembled peptide FeFKFeFK (F: phenylalanine; K: lysine; E: glutamate) hybrid hydrogel as a potential delivery platform for cells and drugs in NP. GO in the hydrogel was used as a carrier to deliver TGF-β3, resulting in the high expression of aggrecan and collagen-II in NP cells [156]. This study highlights the potential of using GO as a nanocarrier for designing functional hydrogels.
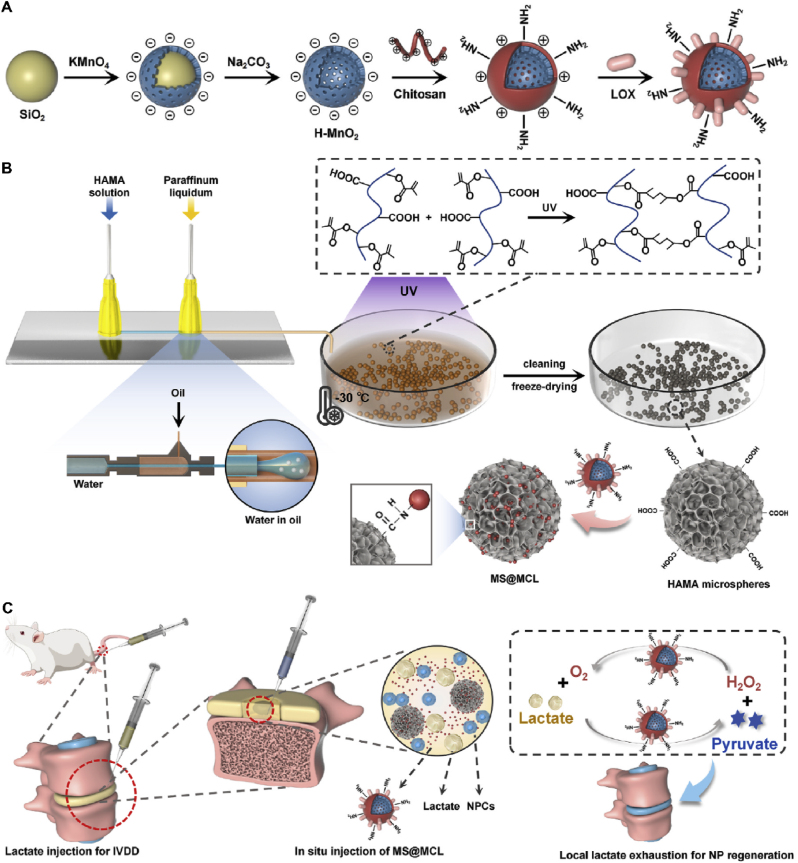
Anaerobic glycolysis is the main energy metabolism pathway in NP cells and is the largest avascular tissue in vivo [157]. During IVDD, especially due to the decreased diffusion of metabolites and nutrients through nearby capillaries, lactic acid gradually accumulates in local NPs, with a concentration of eight to ten times that of the surrounding plasma [158]. Lactate accumulation within the IVD downregulates matrix synthesis and promotes NP cell apoptosis, destroying tissue structure and discogenic pain [159]. Lactate oxidase enzyme (LOX), a member of the flavonoid enzyme family, is an α-hydroxyl acid oxidase that catalyzes the oxidation of lactic acid to pyruvate and H2O2 [160]. However, H2O2-induced oxidative stress has been shown to induce IVDD pathogenesis, which greatly limits the application of LOX in IVDD treatment [161]. In recent years, MnO2-based nanoplatforms have attracted increasing attention because they catalyze H+/H2O2 to generate O2 and Mn2+ [123], providing a large amount of O2 locally, promoting the oxidation catalysis of LOX, and alleviating the oxidative stress damage caused by H2O2. Shen et al. produced an injectable microsphere (MS@MCL) for local lactate exhaustion by grafting a MnO2-LOX composite nanozyme onto microfluidic hyaluronic acid methacrylate (HAMA) microspheres via chemical bonding (Fig. 12), which can effectively achieve O2-promoted lactate exhaustion through a cyclic reaction to alleviate the lactate-accumulative microenvironment and promote the regeneration of degenerative NP cells. After injection for 8 weeks, MS@MCL showed a long-term therapeutic reduction of intervertebral height narrowing and prevented ECM degradation and inflammatory damage [162].
Fig. 12.
Schematic illustrations of nanozyme-functionalized hydrogel microsphere and degenerative NP tissue regenerative process. (A) Preparation steps of the MnO2-Chitosan-LOX (MCL) nanozyme. (B) Microfluidic fabrication of injectable HAMA microspheres and grafting nanozymes onto microspheres via covalent bonding (MS@MCL). (C) In situ injection of MS@MCL into a rat caudal IDD model for local lactate exhaustion and NP regeneration. Reproduced with permission [162]. Copyright 2021, The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
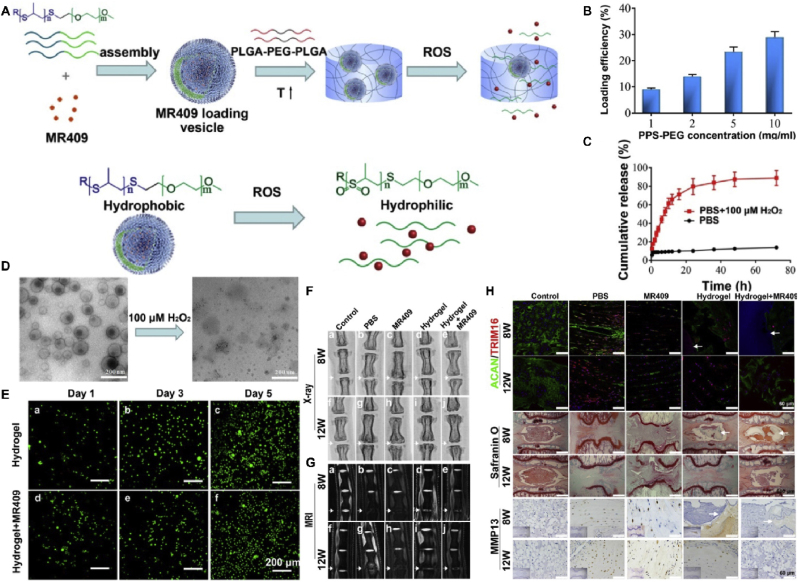
The synthetic growth hormone-releasing hormone, analog MR409, regulates immune cell infiltration and IL-1β synthesis and plays a significant anti-inflammatory role [163,164]. In addition, MR409 is an antioxidant that can downregulate ROS accumulation or block ROS signaling. As a polypeptide, MR409 is easily degraded in vivo, particularly in the harsh environment of intervertebral disc degeneration. Therefore, there may be a need for a specially designed carrier to protect against the controlled, targeted release of MR409 for therapeutic applications. Zheng et al. designed a thermosensitive ROS-responsive nanohydrogel loaded with MR409 to prevent puncture-induced IVDD in rats [165]. In their study, water-soluble MR409 was loaded into ROS-responsive vesicles composed of an mPEG20-b-PPS30 (PPS-PEG) amphiphilic polymer. These loaded vesicles were then embedded in a thermosensitive poly(lactic-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic-co-glycolic acid) copolymer (PLGA-PEGPLGA) hydrogel for protection and controlled release. According to the in vitro results, the injectable hydrogel containing ROS-responsive vesicles is a reliable delivery system for the controlled release of MR409. In vivo administration of MR409 suppressed secretory autophagy, thereby slowing mouse age-related disc degeneration. The intradiscal application of an ROS-responsive MR409-encapsulated hydrogel achieved locally controlled release and attenuated needle puncture-induced disc degeneration in rats by inhibiting the secretory autophagy pathway and associated IL-1β secretion [165] (Fig. 13).
Fig. 13.
ROS-responsive MR409-encapsulated thermosensitive hydrogel ameliorates IVDD in rats by inhibiting the secretory autophagy pathway. (A) Schematic illustration of the thermosensitive hydrogel loaded with ROS-responsive PPS-PEG vesicles for controlled release of MR409. (B) MR409-loading efficiencies of PPS-PEG vesicles. (C) Cumulative release of MR409 from PPS-PEG vesicles or hydrogel-containing vesicles in the presence of 100 μM H2O2. (D) Change in the morphology of MR409-loaded vesicles in the presence of external H2O2 (100 μM). (E) Images of live/dead cell staining of rat NP cells cultured with hydrogel confirming good biocompatibility. (F–G) Representative spine X-ray (F) and MR images (G) from the five experimental groups at 8 and 12 weeks. (H) Representative immunofluorescence staining of ACAN (green) and TRIM16 (red), Safranin O staining, and immunohistochemical staining of MMP13 in experimental discs at postoperative weeks 8 and 12. Arrows indicate residual hydrogel. Reproduced with permission [165]. Copyright 2021, The Authors.
5.6.4. Drug-loaded nanohydrogels for IVDD treatment
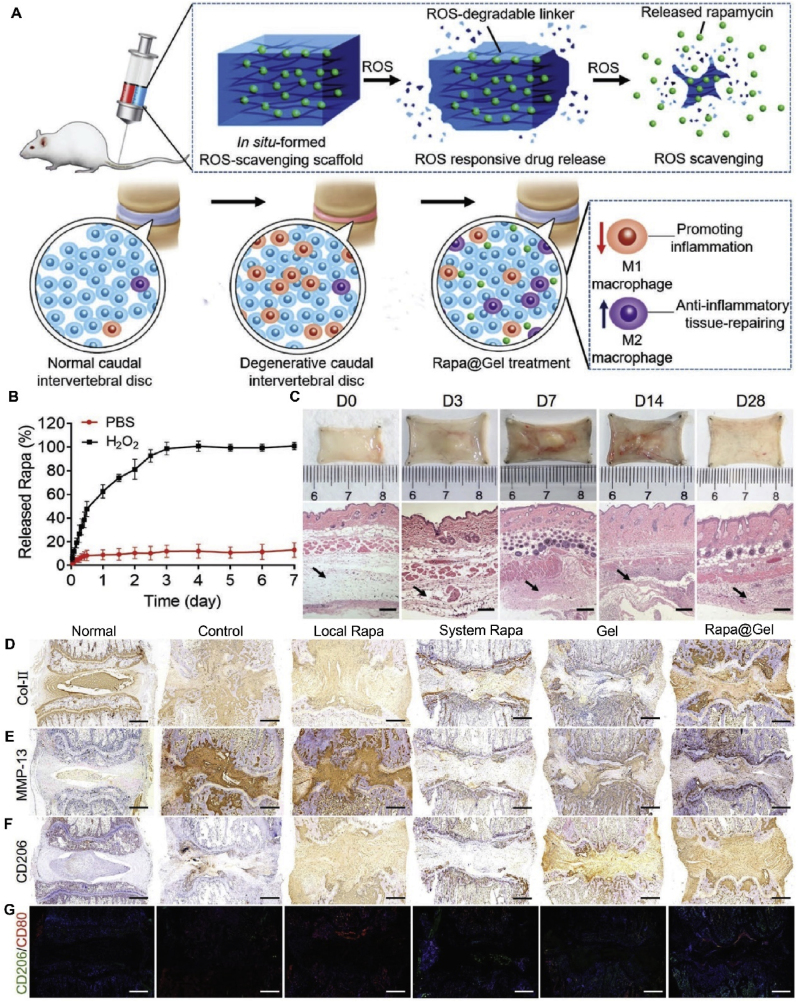
In addition to delivering a variety of bioactive molecules and genes, nanohydrogels also show great potential as a carrier for traditional drugs for treating IVDD. As mentioned earlier, the inflammatory microenvironment plays a vital role in IVDD pathogenesis. Many matrix metalloproteinases, nitric oxide, prostaglandins, IL-1, IL-6, and TNF-α are overexpressed in IVDD. Therefore, anti-inflammatory therapy is a promising therapeutic strategy. Traditional anti-inflammatory drugs can effectively relieve pain and other symptoms; at the same time, they can cause gastrointestinal ulcers and other side effects [166]. Furthermore, because the IVD is avascular, it is often difficult for drugs to reach the treatment site. Nanohydrogels have been widely used to deliver anti-inflammatory drugs because of their well-controlled release properties. Liu et al. used a blending method to composite aspirin (ASP)-loaded liposomes and photo-crosslinkable gelatin-methacryloyl (GelMA) into a hydrogel with properties similar to those of the ECM, which effectively inhibited the inflammatory response of local tissue after lumbar disc surgery and filled the local tissue defect. This also permitted a slow drug release [167]. Many studies have shown that multiple factors, such as macrophage M1/M2 phenotype transition, elevated ROS levels, and mitochondria-associated autophagy, contribute to chronic inflammation and progressive regression in IVDD [168]. Nanohydrogel-mediated drug delivery might improve this situation. Bai et al. designed an ROS-scavenging nanohydrogel scaffold loaded with rapamycin, which had good biocompatibility and could release rapamycin programmatically. In vivo studies have shown that it can reduce ROS levels and promote M2-type macrophage polarization, thus delaying the progression of IVDD (Fig. 14) [169]. This represents a new strategy to modulate the local inflammatory microenvironment to promote IVD regeneration.
Fig. 14.
Reactive oxygen species (ROS)-scavenging responsive hydrogel with rapamycin (Rapa) for treatment of IVDD. (A) Schematic of Rapa-loaded ROS-responsive hydrogel regulating IVD immune microenvironment and ameliorating tissue repair. (B) Cumulative release characterization of Rapa from hydrogels in PBS with or without H2O2 (1 × 10−3 m). (C) Gel formation and degradation were detected in healthy mice. Most gels were degraded at the injection site four weeks after injection, and H&E staining results showed biodegradability and biocompatibility. Scale bars, 200 μm. (D) Collagen-II immunohistochemistry of the experimental and control group. (E) Matrix metalloproteinase 13 (MMP13) immunohistochemistry. (F) CD206 immunohistochemistry. (G) The CD206/CD80 immunofluorescence assay. The blue color represents the DAPI staining of nuclei. Scale bars, 500 μm. Reproduced with permission [169]. Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
5.7. Exosomes
Exosomes are nanoscale extracellular vesicles secreted by cells, consistent with the cell membrane, and composed of phospholipid bilayers. Exosomes mainly affect the biological activities of receptor cells through their contents. In recent years, because of its low immunogenicity and good internal stability, exosome-based acellular therapy has been used to treat various diseases [170]. Recently, it has been used as a carrier for genes and drugs to treat various diseases [171]. The role of exosomes in the treatment of IVDD has also been widely studied [172]. Still, most current studies focus on the exosome itself, and few studies have used exosomes as drug carriers to deliver various drugs to the IVD. This may be due to the complexity of the internal components of exosomes; various small molecular substances in exosomes have different effects on IVDD, and beneficial and harmful genetic materials coexist. In addition, the role of exosomes in IVDD is closely related to that of their parent cells. Exosomes from different sources have different effects on IVDD, which can not only adversely affect IVD by promoting vascularization [173] but also protect IVD by promoting ECM synthesis and NP cell proliferation [174]. Because the IVD is a non-vascular structure, an exosome preparation injected intravenously poorly reaches the IVD, so most IVDD treatment studies based on exosomes utilize local injection. However, pure exosome injection does not seem advantageous compared with other delivery systems. Therefore, we speculated that exosomes alone might not be good drug carriers for IVDD, at least at this stage. One possible solution is to combine exosomes with other nanosystems to enhance their delivery efficiency. In 2021, Xing et al. combined ADSC-derived exosomes with a thermosensitive acellular ECM hydrogel (dECM@exo). The dECM@exo system provided in situ gelation to replenish ECM leakage and an environment for the growth of NP cells [175].
6. Conclusions and future perspectives
IVDD is a common degenerative joint disease and is the leading cause of lower back pain. It seriously affects patients’ quality of life and places a heavy financial burden on their families and society [176]. The current main treatment for IVDD is aimed at controlling clinical symptoms rather than treating the etiology of degeneration. It is accompanied by high invasiveness, high risk of recurrence, and degeneration of adjacent IVDs, and cannot prevent progression or reverse the degeneration of IVDD [[177], [178], [179]]. Over the past few decades, emerging therapies, including growth factors, platelet-rich plasma, and gene-editing techniques, have been tested in preclinical and clinical studies [[180], [181], [182], [183], [184]]. However, the reality is that the efficacy is not surprising. The main obstacles to IVDD repair include avascular architecture, inadequate endogenous cell repair, and the presence of an unfavorable microenvironment at the site of degeneration. Thus, overcoming these obstacles is an ideal approach for treating IVDD. However, this is difficult to achieve in clinical practice. Importantly, the anatomical peculiarities of IVDs make it difficult for oral or intravenous drugs to reach the degeneration site. The activity of endogenous enzymes in IVD degrades the injected therapeutic biomolecules, indicating that the bioavailability and persistence of injected biomolecules may be transient, suggesting that high doses and frequent repetitions may be required [81,185], which in turn may exacerbate degeneration and make it difficult to maintain drug activity within IVDs. Therefore, pharmacological treatment of IVDD has become a dilemma, and effective treatment has become a major clinical problem to be solved.
In recent years, the rapid development of NDDSs has brought dawn to the treatment of IVDD. Ideally, IVD-targeted DDS should be low or even non-toxic, minimally invasive, have good drug entrapment efficiency, enable controlled release, be delivered continuously, and be easy to perform clinically. Unfortunately, current technologies cannot meet all these requirements simultaneously. In turn, existing NDDSs should also be thoroughly evaluated in large-scale studies to determine their controlled release properties and repeatability. It is also challenging to ensure that the nanodrug exhibits high biological stability and activity without being degraded by enzymes in degenerated IVDs. Therefore, it can be argued that the problem of obtaining an effective drug delivery method for targeting IVD is currently unresolved, largely due to the complex structure of IVDs. Hence, a more efficient DDS is urgently needed to treat IVDD. In the past two decades, rapid developments in nanotechnology have provided new ideas for IVD-targeted drug delivery. Compared with larger biomaterials, nanoparticles exhibit unique structural, chemical, mechanical, magnetic, and electrical properties and well-controlled release characteristics. Using various types of nanoparticles, drugs can be precisely and controllably released into IVDs and even specific cells without disturbing the normal anatomical structure. We believe that NDDSs will undoubtedly be a major trend in the future of drug therapies for IVDD.
The advantage of liposomes is that they can be loaded with both hydrophilic and lipophilic drugs and customized as needed. However, liposomes are prone to degradation in vivo, and improving the stability of drugs in liposomes is a major challenge. Currently, the role of liposomes in IVDD is mainly in the laboratory stage, and more studies are needed to evaluate the drug-loading characteristics of liposomes. In addition, the drug entrapment efficiency of liposomes is relatively low, which may limit their use in IVDD treatment.
Similar to liposomes, some PNPs are degradable but have a higher encapsulation efficiency. Of the many PNPs available, we speculate that natural polymers, such as chitosan, may be good drug delivery carriers. Chitosan is a renewable, naturally occurring, cationic polysaccharide. As a drug delivery carrier, chitosan has the advantages of simple synthesis and characterization, good biocompatibility, excellent biodegradability, good bioadhesion, nonimmunogenicity, nontoxicity, and water solubility. Remarkable progress has been made as a drug delivery carrier in other diseases. Both in vitro and in vivo studies have confirmed that it has good drug encapsulation efficiency for effective drug release.
Moreover, the electrostatic interaction between the positively charged chitosan and negatively charged IVD cartilage matrix enabled targeted drug delivery to a certain extent. However, the synthetic process of PNPs is relatively cumbersome and unstable; therefore, developing a simple carrier to replace PNPs is also an idea. In 2018, Zhang et al. developed an injectable nanodelivery system based on albumin/heparin nanoparticles (BHNPs). The system is simple to prepare, has a high entrapment efficiency, is biocompatible, and is suitable for large-scale production. They utilized this system as the carrier for stromal cell-derived factor-1 α (SDF-1α). BHNPs gradually released SDF-1α in a first-order manner. Compared with SDF-1α and BHNPs alone, BHNPs/SDF can induce better regeneration of AF and NP cells [186].
Inorganic nanoparticles play a unique role in imaging and photothermal therapy due to their magnetism, radioactivity, or plasticity, and most of them have good biocompatibility and stability. However, there are still many challenges in clinical transformation, with the biggest issue being long-term in vivo toxicity. Although numerous studies have confirmed that inorganic nanomaterials have low acute toxicity, whether they can eventually be cleared by the body and cause long-term toxicity has not yet been verified [187,188]. Another concern was the low clearance rate. If these problems are solved, they can only be used in clinical research. Currently, some inorganic nanoparticles have been used in clinical trials, mostly for in vivo imaging and rarely for drug delivery. Therefore, the clinical transformation of inorganic nanoparticles as DDS is a key issue that needs to be addressed. The combination of inorganic and organic nanoparticles, or the development of new inorganic nanoparticles with lower toxicity may be an effective way to apply inorganic nanoparticles in the clinic.
As drug delivery carriers, polymer micelles have many good properties, such as increasing the solubility of hydrophobic drugs, improving drug efficacy, prolonging drug action time, and improving drug bioavailability. Through targeted modification, polymer micelles can also guide drugs to specific action sites and reduce their toxicity and side effects. In future research, improving efficacy, reducing the dosage, and reducing side effects will be key to improving clinical drug use. The polymer micelle shell functions as "navigation" while connecting water-soluble drugs. The core encapsulates hydrophobic drugs and achieves slow release, thus realizing the combination of two or more drugs, which may become another highlight of polymer micelles in the treatment of IVDD. However, most polymer micelles are still in the basic research stage of the laboratory, and their properties as drug carriers cannot fully meet the requirements of clinical drug delivery. Second, the toxicological properties and sustained release mechanism of polymer micelles and their transformation into clinical practice are also important challenges to be addressed.
Currently, there is little research on nanofibers as NDDSs for treating IVDD, and more studies are needed to demonstrate their safety and drug-release capabilities. More efficient nanofiber drug delivery materials should be developed to enhance the therapeutic effect. Multiaxial nanofibers may be the best solution to this challenge, as different drugs can be encapsulated in different layers, and their release can be controlled. Given the specificity of the anatomical structure of the IVD, a low-toxicity or even non-toxic, biodegradable nanofiber is welcome in the treatment of IVDD. Currently, 3D printing technology is booming in the field of orthopedics. We speculate that combining 3D printing technology with nanofibers is possible to achieve drug delivery and IVD replacement.
Nanohydrogels play an important role in the regeneration, repair, and replacement of NP. They provide an aqueous environment that rehydrates the degraded microenvironment, protects biological agents, and partially restores the mechanical properties of IVDs. The permeability of hydrogels facilitates the delivery of nutrients to targeted cells. Furthermore, as biomaterials for NP regeneration and repair, hydrogels not only have good mechanical properties to the intervertebral height and bear the body load but also positively affect the proliferation, differentiation, and signal transduction of IVD cells. Nanohydrogel has a good chimeric effect with the surrounding tissue and is non-toxic. Another significant advantage of nanohydrogels is that they can be combined with other nano-delivery systems, such as liposomes [135,167] and exosomes [175], to delay their degradation and enhance delivery efficiency.
Moreover, 3D biomimetic hydrogel scaffolds based on bioprinting have significantly progressed in treating spinal cord injuries. We believe that future hydrogels can also make great achievements in IVDD treatment, including the ability to withstand physiological pressure, support the biological activity of mesenchymal stem cells, and have a sufficiently high porosity to allow the diffusion of growth factors, O2, and nutrients, thereby effectively inducing the regeneration of cartilage and nucleus pulposus cells [189]. Therefore, developing nanohydrogels with biocompatibility and mechanical properties similar to those of normal NPs remains a future development. When the injectable nanohydrogel is punctured into the IVD tissue, it inevitably damages the tissue integrity of the AF and may cause IVDD. Moreover, the safe and accurate delivery of hydrogel biomaterials has become an open question. As mentioned earlier, IVD tissue has a complex microenvironment, such as hypoxia and acidic and different stress environments, and microenvironment-responsive hydrogels also have good prospects in the future.
Furthermore, although NDDSs have progressed greatly in treating IVDD, they remain in the cellular and animal experimental stages. They have a long way to go for clinical application. Most current NDDSs target only one aspect of the pathological process of IVDD, for example, by delivering anti-inflammatory cytokines, growth factors, or cells. Although these strategies have shown promising results, they are not yet sufficient to achieve complete recovery, mainly because the progression of IVDD is complex and multifactorial, with multiple processes disrupted, including inflammatory and catabolic cascades, progressive cellular loss, and decreased cellular anabolic activity [20]. The three aforementioned events do not occur sequentially but are intertwined and act in a vicious cycle that creates barriers to treatment. Therefore, Frapin et al. proposed the concept of a sequential DDS [20]: (1) the release of anti-inflammatory or anti-catabolic cytokines to target the inflammatory, pro-catabolic microenvironment; (2) an increase in cell density in a less hostile microenvironment by endogenous cell recruitment or exogenous cell injection; and (3) promotion of ECM synthesis through the release of anabolic factors. Therefore, combining this strategy with NDDSs may be a more advanced treatment strategy for IVDD. Furthermore, few current studies on the regeneration and repair of IVDD are related to pain relief, yet pain relief is a major clinical concern for patients. Thus, establishing animal models of discogenic pain and good evaluation criteria are important for future clinical applications.
In addition, research on NDDSs for the treatment of IVDD is still in the exploratory stage, with a small number of studies mostly based on rats and rabbits. However, there are anatomical differences between IVDs and humans, which may make them unsuitable animal models. We believe sheep may be a more suitable model for studying human IVDD than rats and rabbits, as sheep IVDs do not exhibit an abnormally high spontaneous healing response or the persistence of notochord cells with age [190]. Sheep have IVD mechanics and NP proteoglycan content similar to humans, which makes them ideal experimental animals for the study of IVDD treatment [191]. And, the experimental periods of the current studies are all relatively short, while the progression of IVDD is a chronic process; therefore, the long-term efficacy of NDDS needs to be evaluated. Furthermore, because the NP is more prone to degeneration, the regeneration of this tissue has received more attention. However, future treatments should focus on the synergistic recovery of NP and AF. In addition, the pathogenesis of IVDD is very complex, and treatment with only one factor or signaling pathway is often ineffective. Thus, NDDS with multitarget synergistic therapy may be the focus of future research.
In summary, ongoing research into the pathophysiology of IVDD, development of therapies aimed at halting disease progression or inducing a regenerative response, and optimization of drug delivery strategies has the potential to achieve effective clinical management of IVDD.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Natural Science Foundation of Gansu province (21JR7RA406) and Cuiying Technology Innovation Project of Lanzhou University Second Hospital (CY2020-MS20). Zhanjun Ma is supported by a co-founding from the UCLouvain-China Scholarship Council (CSC). Jingjing Yang is supported by a grant from the CSC.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.11.006.
Contributor Information
Zhanjun Ma, Email: zhanjun.ma@uclouvain.be.
Jingjing Yang, Email: jingjing.yang@uclouvain.be.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kague E., Turci F., Newman E., Yang Y., Brown K.R., Aglan M.S., Otaify G.A., Temtamy S.A., Ruiz-Perez V.L., Cross S., et al. 3D assessment of intervertebral disc degeneration in zebrafish identifies changes in bone density that prime disc disease. Bone Res. 2021;9(1):39. doi: 10.1038/s41413-021-00156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchbinder R., van Tulder M., Öberg B., Costa L.M., Woolf A., Schoene M., Croft P. Low back pain: a call for action. Lancet. 2018;391(10137):2384–2388. doi: 10.1016/s0140-6736(18)30488-4. [DOI] [PubMed] [Google Scholar]
- 3.Ma K., Chen S., Li Z., Deng X., Huang D., Xiong L., Shao Z. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis Cartilage. 2019;27(1):41–48. doi: 10.1016/j.joca.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Urban J.P., Roberts S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003;5(3):120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips C.J. Economic burden of chronic pain. Expert Rev. Pharmacoecon. Outcomes Res. 2006;6(5):591–601. doi: 10.1586/14737167.6.5.591. [DOI] [PubMed] [Google Scholar]
- 6.Wu A., Dong W., Liu S., Cheung J.P.Y., Kwan K.Y.H., Zeng X., Zhang K., Sun Z., Wang X., Cheung K.M.C., et al. The prevalence and years lived with disability caused by low back pain in China, 1990 to 2016: findings from the global burden of disease study 2016. Pain. 2019;160(1):237–245. doi: 10.1097/j.pain.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng Y., Egan B., Wang J. Genetic factors in intervertebral disc degeneration. Genes Dis. 2016;3(3):178–185. doi: 10.1016/j.gendis.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemanta D., Jiang X.X., Feng Z.Z., Chen Z.X., Cao Y.W. Etiology for degenerative disc disease. Chin. Med. Sci. J. 2016;31(3):185–191. doi: 10.1016/s1001-9294(16)30049-9. [DOI] [PubMed] [Google Scholar]
- 9.Millecamps M., Tajerian M., Naso L., Sage H.E., Stone L.S. Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing SPARC-null mice. Pain. 2012;153(6):1167–1179. doi: 10.1016/j.pain.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Che M., Xin J., Zheng Z., Li J., Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110660. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H.J., Liao H.Y., Bai D.Y., Wang Z.Q., Xie X.W. MAPK/ERK signaling pathway: a potential target for the treatment of intervertebral disc degeneration. Biomed. Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112170. [DOI] [PubMed] [Google Scholar]
- 12.Weber K.T., Jacobsen T.D., Maidhof R., Virojanapa J., Overby C., Bloom O., Quraishi S., Levine M., Chahine N.O. Developments in intervertebral disc disease research: pathophysiology, mechanobiology, and therapeutics. Curr. Rev. Musculoskelet. Med. 2015;8(1):18–31. doi: 10.1007/s12178-014-9253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadow T., Sowa G., Vo N., Kang J.D. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin. Orthop. Relat. Res. 2015;473(6):1903–1912. doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risbud M.V., Shapiro I.M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binch A.L.A., Fitzgerald J.C., Growney E.A., Barry F. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat. Rev. Rheumatol. 2021;17(3):158–175. doi: 10.1038/s41584-020-00568-w. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W., Sun T., Li Y., Yang M., Zhao Y., Liu J., Li Z. Application of stem cells in the repair of intervertebral disc degeneration. Stem Cell Res. Ther. 2022;13(1):70. doi: 10.1186/s13287-022-02745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clouet J., Fusellier M., Camus A., Le Visage C., Guicheux J. Intervertebral disc regeneration: from cell therapy to the development of novel bioinspired endogenous repair strategies. Adv. Drug Deliv. Rev. 2019;146:306–324. doi: 10.1016/j.addr.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Colella F., Garcia J.P., Sorbona M., Lolli A., Antunes B., D'Atri D., Fpy Barré, Oieni J., Vainieri M.L., Zerrillo L., et al. Drug delivery in intervertebral disc degeneration and osteoarthritis: selecting the optimal platform for the delivery of disease-modifying agents. J. Contr. Release. 2020;328:985–999. doi: 10.1016/j.jconrel.2020.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Blanquer S.B., Grijpma D.W., Poot A.A. Delivery systems for the treatment of degenerated intervertebral discs. Adv. Drug Deliv. Rev. 2015;84:172–187. doi: 10.1016/j.addr.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Frapin L., Clouet J., Delplace V., Fusellier M., Guicheux J., Le Visage C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv. Drug Deliv. Rev. 2019;149–150:49–71. doi: 10.1016/j.addr.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Dowdell J., Erwin M., Choma T., Vaccaro A., Iatridis J., Cho S.K. Intervertebral disk degeneration and repair. Neurosurgery. 2017;80(3s):S46–s54. doi: 10.1093/neuros/nyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohtori S., Inoue G., Miyagi M., Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15(6):1347–1355. doi: 10.1016/j.spinee.2013.07.490. [DOI] [PubMed] [Google Scholar]
- 23.Maroudas A., Stockwell R.A., Nachemson A., Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J. Anat. 1975;120(Pt 1):113–130. [PMC free article] [PubMed] [Google Scholar]
- 24.Chan W.C., Sze K.L., Samartzis D., Leung V.Y., Chan D. Structure and biology of the intervertebral disk in health and disease. Orthop. Clin. N. Am. 2011;42(4):447–464. doi: 10.1016/j.ocl.2011.07.012. vii. [DOI] [PubMed] [Google Scholar]
- 25.Bach F.C., Willems N., Penning L.C., Ito K., Meij B.P., Tryfonidou M.A. Potential regenerative treatment strategies for intervertebral disc degeneration in dogs. BMC Vet. Res. 2014;10:3. doi: 10.1186/1746-6148-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchand F., Ahmed A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15(5):402–410. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Oichi T., Taniguchi Y., Oshima Y., Tanaka S., Saito T. Pathomechanism of intervertebral disc degeneration. JOR Spine. 2020;3(1) doi: 10.1002/jsp2.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontana G., See E., Pandit A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv. Drug Deliv. Rev. 2015;84:146–158. doi: 10.1016/j.addr.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Lotz J.C., Fields A.J., Liebenberg E.C. The role of the vertebral end plate in low back pain. Global Spine J. 2013;3(3):153–164. doi: 10.1055/s-0033-1347298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boubriak O.A., Watson N., Sivan S.S., Stubbens N., Urban J.P. Factors regulating viable cell density in the intervertebral disc: blood supply in relation to disc height. J. Anat. 2013;222(3):341–348. doi: 10.1111/joa.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts S. Disc morphology in health and disease. Biochem. Soc. Trans. 2002;30(Pt 6):864–869. doi: 10.1042/bst0300864. [DOI] [PubMed] [Google Scholar]
- 32.Battié M.C., Videman T., Levalahti E., Gill K., Kaprio J. Heritability of low back pain and the role of disc degeneration. Pain. 2007;131(3):272–280. doi: 10.1016/j.pain.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Hadjipavlou A.G., Tzermiadianos M.N., Bogduk N., Zindrick M.R. The pathophysiology of disc degeneration: a critical review. J. Bone Joint Surg. Br. 2008;90(10):1261–1270. doi: 10.1302/0301-620x.90b10.20910. [DOI] [PubMed] [Google Scholar]
- 34.Elmasry S., Asfour S., de Rivero Vaccari J.P., Travascio F. Effects of tobacco smoking on the degeneration of the intervertebral disc: a finite element study. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kos N., Gradisnik L., Velnar T. A brief review of the degenerative intervertebral disc disease. Med. Arch. 2019;73(6):421–424. doi: 10.5455/medarh.2019.73.421-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freemont A.J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 37.Smith L.J., Nerurkar N.L., Choi K.S., Harfe B.D., Elliott D.M. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis. Model Mech. 2011;4(1):31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luoma K., Riihimäki H., Luukkonen R., Raininko R., Viikari-Juntura E., Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25(4):487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 39.Adams M.A., Roughley P.J. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Z., Xiang Q., Wang J., Zhang Y. The potential role of melatonin in retarding intervertebral disc ageing and degeneration: a systematic review. Ageing Res. Rev. 2021;70 doi: 10.1016/j.arr.2021.101394. [DOI] [PubMed] [Google Scholar]
- 41.Feng C., Liu H., Yang M., Zhang Y., Huang B., Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle. 2016;15(13):1674–1684. doi: 10.1080/15384101.2016.1152433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erwin W.M., Ashman K., O'Donnel P., Inman R.D. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54(12):3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- 43.Colombier P., Clouet J., Boyer C., Ruel M., Bonin G., Lesoeur J., Moreau A., Fellah B.H., Weiss P., Lescaudron L., et al. TGF-β1 and GDF5 act synergistically to drive the differentiation of human adipose stromal cells toward nucleus pulposus-like cells. Stem Cell. 2016;34(3):653–667. doi: 10.1002/stem.2249. [DOI] [PubMed] [Google Scholar]
- 44.Balagué F., Mannion A.F., Pellisé F., Cedraschi C. Non-specific low back pain. Lancet. 2012;379(9814):482–491. doi: 10.1016/s0140-6736(11)60610-7. [DOI] [PubMed] [Google Scholar]
- 45.Romaniyanto, Mahyudin F., Sigit Prakoeswa C.R., Notobroto H.B., Tinduh D., Ausrin R., Rantam F.A., Suroto H., Utomo D.N., Rhatomy S. An update of current therapeutic approach for Intervertebral Disc Degeneration: a review article. Ann. Med. Surg. (Lond) 2022;77 doi: 10.1016/j.amsu.2022.103619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirnaz S., Capadona C., Wong T., Goldberg J.L., Medary B., Sommer F., McGrath L.B., Jr., Härtl R. Fundamentals of intervertebral disc degeneration. World Neurosurg. 2022;157:264–273. doi: 10.1016/j.wneu.2021.09.066. [DOI] [PubMed] [Google Scholar]
- 47.Craddock R.J., Hodson N.W., Ozols M., Shearer T., Hoyland J.A., Sherratt M.J. Extracellular matrix fragmentation in young, healthy cartilaginous tissues. Eur. Cell. Mater. 2018;35:34–53. doi: 10.22203/eCM.v035a04. [DOI] [PubMed] [Google Scholar]
- 48.Bachmeier B.E., Nerlich A., Mittermaier N., Weiler C., Lumenta C., Wuertz K., Boos N. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur. Spine J. 2009;18(11):1573–1586. doi: 10.1007/s00586-009-1031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pockert A.J., Richardson S.M., Le Maitre C.L., Lyon M., Deakin J.A., Buttle D.J., Freemont A.J., Hoyland J.A. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60(2):482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi H., Suguro T., Okazima Y., Motegi M., Okada Y., Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21(2):218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 51.Vasiliadis E.S., Pneumaticos S.G., Evangelopoulos D.S., Papavassiliou A.G. Biologic treatment of mild and moderate intervertebral disc degeneration. Mol. Med. 2014;20(1):400–409. doi: 10.2119/molmed.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Y., Wang Y., Zhang Y., Geng W., Liu W., Gao Y., Li S., Wang K., Wu X., Kang L., et al. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J. Cell Mol. Med. 2017;21(7):1373–1387. doi: 10.1111/jcmm.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J., Nisar M., Huang C., Pan X., Lin D., Zheng G., Jin H., Chen D., Tian N., Huang Q., et al. Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Exp. Mol. Med. 2018;50(11):1–14. doi: 10.1038/s12276-018-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aigner T., Gresk-otter K.R., Fairbank J.C., von der Mark K., Urban J.P. Variation with age in the pattern of type X collagen expression in normal and scoliotic human intervertebral discs. Calcif. Tissue Int. 1998;63(3):263–268. doi: 10.1007/s002239900524. [DOI] [PubMed] [Google Scholar]
- 55.Paietta R.C., Burger E.L., Ferguson V.L. Mineralization and collagen orientation throughout aging at the vertebral endplate in the human lumbar spine. J. Struct. Biol. 2013;184(2):310–320. doi: 10.1016/j.jsb.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Grant M.P., Epure L.M., Bokhari R., Roughley P., Antoniou J., Mwale F. Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium-sensing receptor in the intervertebral disc. Eur. Cell. Mater. 2016;32:137–151. doi: 10.22203/ecm.v032a09. [DOI] [PubMed] [Google Scholar]
- 57.Huang Y.C., Urban J.P., Luk K.D. Intervertebral disc regeneration: do nutrients lead the way? Nat. Rev. Rheumatol. 2014;10(9):561–566. doi: 10.1038/nrrheum.2014.91. [DOI] [PubMed] [Google Scholar]
- 58.Kamali A., Ziadlou R., Lang G., Pfannkuche J., Cui S., Li Z., Richards R.G., Alini M., Grad S. Small molecule-based treatment approaches for intervertebral disc degeneration: current options and future directions. Theranostics. 2021;11(1):27–47. doi: 10.7150/thno.48987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Connell G.D., Guerin H.L., Elliott D.M. Theoretical and uniaxial experimental evaluation of human annulus fibrosus degeneration. J. Biomech. Eng. 2009;131(11) doi: 10.1115/1.3212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heuer F., Schmidt H., Wilke H.J. The relation between intervertebral disc bulging and annular fiber associated strains for simple and complex loading. J. Biomech. 2008;41(5):1086–1094. doi: 10.1016/j.jbiomech.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 61.Kepler C.K., Anderson D.G., Tannoury C., Ponnappan R.K. Intervertebral disk degeneration and emerging biologic treatments. J. Am. Acad. Orthop. Surg. 2011;19(9):543–553. doi: 10.5435/00124635-201109000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Adams M.A., Lama P., Zehra U., Dolan P. Why do some intervertebral discs degenerate, when others (in the same spine) do not? Clin. Anat. 2015;28(2):195–204. doi: 10.1002/ca.22404. [DOI] [PubMed] [Google Scholar]
- 63.Choi Y., Park M.H., Lee K. Tissue engineering strategies for intervertebral disc treatment using functional polymers. Polymers. 2019;11(5) doi: 10.3390/polym11050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao Z., Wu X., Song Y., Luo R., Yin H., Zhan S., Li S., Wang K., Zhang Y., Yang C. Angiopoietin-like protein 8 expression and association with extracellular matrix metabolism and inflammation during intervertebral disc degeneration. J. Cell Mol. Med. 2019;23(8):5737–5750. doi: 10.1111/jcmm.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kongsted A., Leboeuf-Yde C. The Nordic back pain subpopulation program--individual patterns of low back pain established by means of text messaging: a longitudinal pilot study. Chiropr. Osteopathy. 2009;17:11. doi: 10.1186/1746-1340-17-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maher C., Underwood M., Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736–747. doi: 10.1016/s0140-6736(16)30970-9. [DOI] [PubMed] [Google Scholar]
- 67.Modic M.T., Steinberg P.M., Ross J.S., Masaryk T.J., Carter J.R. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 68.Pfirrmann C.W., Metzdorf A., Zanetti M., Hodler J., Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 69.Anderson D.G., Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5(6 Suppl):260s–266s. doi: 10.1016/j.spinee.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 70.Foster N.E., Anema J.R., Cherkin D., Chou R., Cohen S.P., Gross D.P., Ferreira P.H., Fritz J.M., Koes B.W., Peul W., et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391(10137):2368–2383. doi: 10.1016/s0140-6736(18)30489-6. [DOI] [PubMed] [Google Scholar]
- 71.Kloppenburg M., Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage. 2020;28(3):242–248. doi: 10.1016/j.joca.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Krut Z., Pelled G., Gazit D., Gazit Z. Stem cells and exosomes: new therapies for intervertebral disc degeneration. Cells. 2021;10(9) doi: 10.3390/cells10092241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandez-Moure J., Moore C.A., Kim K., Karim A., Smith K., Barbosa Z., Van Eps J., Rameshwar P., Weiner B. Novel therapeutic strategies for degenerative disc disease: review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018;6 doi: 10.1177/2050312118761674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee Y.C., Zotti M.G., Osti O.L. Operative management of lumbar degenerative disc disease. Asian Spine J. 2016;10(4):801–819. doi: 10.4184/asj.2016.10.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fritzell P., Hägg O., Wessberg P., Nordwall A. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26(23):2521–2532. doi: 10.1097/00007632-200112010-00002. ; discussion 2532-2524. [DOI] [PubMed] [Google Scholar]
- 76.Han I., Ropper A.E., Konya D., Kabatas S., Toktas Z., Aljuboori Z., Zeng X., Chi J.H., Zafonte R., Teng Y.D. Biological approaches to treating intervertebral disk degeneration: devising stem cell therapies. Cell Transplant. 2015;24(11):2197–2208. doi: 10.3727/096368915x688650. [DOI] [PubMed] [Google Scholar]
- 77.van den Eerenbeemt K.D., Ostelo R.W., van Royen B.J., Peul W.C., van Tulder M.W. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur. Spine J. 2010;19(8):1262–1280. doi: 10.1007/s00586-010-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deyo R.A., Mirza S.K. Trends and variations in the use of spine surgery. Clin. Orthop. Relat. Res. 2006;443:139–146. doi: 10.1097/01.blo.0000198726.62514.75. [DOI] [PubMed] [Google Scholar]
- 79.Guterl C.C., See E.Y., Blanquer S.B., Pandit A., Ferguson S.J., Benneker L.M., Grijpma D.W., Sakai D., Eglin D., Alini M., et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur. Cell. Mater. 2013;25:1–21. doi: 10.22203/ecm.v025a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henry N., Clouet J., Le Bideau J., Le Visage C., Guicheux J. Innovative strategies for intervertebral disc regenerative medicine: from cell therapies to multiscale delivery systems. Biotechnol. Adv. 2018;36(1):281–294. doi: 10.1016/j.biotechadv.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Moriguchi Y., Alimi M., Khair T., Manolarakis G., Berlin C., Bonassar L.J., Härtl R. Biological treatment approaches for degenerative disk disease: a literature review of in vivo animal and clinical data. Global Spine J. 2016;6(5):497–518. doi: 10.1055/s-0036-1571955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parhi P., Mohanty C., Sahoo S.K. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov. Today. 2012;17(17–18):1044–1052. doi: 10.1016/j.drudis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 83.Rosenblum D., Joshi N., Tao W., Karp J.M., Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9(1):1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarannum M., Vivero-Escoto J.L. Nanoparticle-based therapeutic strategies targeting major clinical challenges in pancreatic cancer treatment. Adv. Drug Deliv. Rev. 2022;187 doi: 10.1016/j.addr.2022.114357. [DOI] [PubMed] [Google Scholar]
- 85.Sandhiya S., Dkhar S.A., Surendiran A. Emerging trends of nanomedicine--an overview. Fundam. Clin. Pharmacol. 2009;23(3):263–269. doi: 10.1111/j.1472-8206.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 86.Losi P., Briganti E., Magera A., Spiller D., Ristori C., Battolla B., Balderi M., Kull S., Balbarini A., Di Stefano R., et al. Tissue response to poly(ether)urethane-polydimethylsiloxane-fibrin composite scaffolds for controlled delivery of pro-angiogenic growth factors. Biomaterials. 2010;31(20):5336–5344. doi: 10.1016/j.biomaterials.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 87.Wang W., Lu K.J., Yu C.H., Huang Q.L., Du Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019;17(1):82. doi: 10.1186/s12951-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parchi P.D., Vittorio O., Andreani L., Battistini P., Piolanti N., Marchetti S., Poggetti A., Lisanti M. Nanoparticles for tendon healing and regeneration: literature review. Front. Aging Neurosci. 2016;8:202. doi: 10.3389/fnagi.2016.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohd Isa I.L., Mokhtar S.A., Abbah S.A., Fauzi M.B., Devitt A., Pandit A. Intervertebral disc degeneration: biomaterials and tissue engineering strategies toward precision medicine. Adv. Healthc Mater. 2022;11(13) doi: 10.1002/adhm.202102530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang W., Yang M., Sun T., Zhang J., Zhao Y., Li J., Li Z. Can manganese dioxide microspheres be used as intermediaries to alleviate intervertebral disc degeneration with strengthening drugs? Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.866290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmocker A., Khoushabi A., Frauchiger D.A., Gantenbein B., Schizas C., Moser C., Bourban P.-E., Pioletti D.P. A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement. Biomaterials. 2016;88:110–119. doi: 10.1016/j.biomaterials.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 92.Sarfraz M., Afzal A., Yang T., Gai Y., Raza S.M., Khan M.W., Cheng Y., Ma X., Xiang G. Development of dual drug loaded nanosized liposomal formulation by A reengineered ethanolic injection method and its pre-clinical pharmacokinetic studies. Pharmaceutics. 2018;10(3) doi: 10.3390/pharmaceutics10030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kang Y.J., Cutler E.G., Cho H. Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg. 2018;5(1):35. doi: 10.1186/s40580-018-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 95.Yang J., Ni B., Liu J., Zhu L., Zhou W. Application of liposome-encapsulated hydroxycamptothecin in the prevention of epidural scar formation in New Zealand white rabbits. Spine J. 2011;11(3):218–223. doi: 10.1016/j.spinee.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 96.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kolcun J.P.G., Brusko G.D., Basil G.W., Epstein R., Wang M.Y. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurg. Focus. 2019;46(4):E14. doi: 10.3171/2018.12.Focus18701. [DOI] [PubMed] [Google Scholar]
- 98.Roh M.S., Kucher O.A., Shick K.M., Knolhoff D.R., McGarvey J.S., Peterson S.C. Intramuscular liposomal bupivacaine decreases length of stay and opioid usage following lumbar spinal fusion. Clin. Spine Surg. 2020;33(8):E359–e363. doi: 10.1097/bsd.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 99.Banala R.R., Vemuri S.K., Dar G.H., Palanisamy V., Penkulinti M., Surekha M.V., Gurava Reddy A.V., Nalam M.R., Subbaiah G. Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD) Spine J. 2019;19(5):896–904. doi: 10.1016/j.spinee.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 100.Wang H., Ding Y., Zhang W., Wei K., Pei Y., Zou C., Zhang C., Ding J., Fang H., Tan S. Oxymatrine liposomes for intervertebral disc treatment: formulation, in vitro and vivo assessments. Drug Des. Dev. Ther. 2020;14:921–931. doi: 10.2147/dddt.S242493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu X., Costa A., Burgess D.J. Protein encapsulation in unilamellar liposomes: high encapsulation efficiency and a novel technique to assess lipid-protein interaction. Pharm. Res. (N. Y.) 2012;29(7):1919–1931. doi: 10.1007/s11095-012-0720-x. [DOI] [PubMed] [Google Scholar]
- 102.Ohsawa T., Miura H., Harada K. Improvement of encapsulation efficiency of water-soluble drugs in liposomes formed by the freeze-thawing method. Chem. Pharm. Bull. (Tokyo) 1985;33(9):3945–3952. doi: 10.1248/cpb.33.3945. [DOI] [PubMed] [Google Scholar]
- 103.Fonseca-Santos B., Gremião M.P., Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer's disease. Int. J. Nanomed. 2015;10:4981–5003. doi: 10.2147/ijn.S87148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alyautdin R., Khalin I., Nafeeza M.I., Haron M.H., Kuznetsov D. Nanoscale drug delivery systems and the blood-brain barrier. Int. J. Nanomed. 2014;9:795–811. doi: 10.2147/ijn.S52236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kothamasu P., Kanumur H., Ravur N., Maddu C., Parasuramrajam R., Thangavel S. Nanocapsules: the weapons for novel drug delivery systems. Bioimpacts. 2012;2(2):71–81. doi: 10.5681/bi.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Madamsetty V.S., Tavakol S., Moghassemi S., Dadashzadeh A., Schneible J.D., Fatemi I., Shirvani A., Zarrabi A., Azedi F., Dehshahri A., et al. Chitosan: a versatile bio-platform for breast cancer theranostics. J. Contr. Release. 2022;341:733–752. doi: 10.1016/j.jconrel.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 107.Modi D., Mohammad, Warsi M.H., Garg V., Bhatia M., Kesharwani P., Jain G.K. Formulation development, optimization, and in vitro assessment of thermoresponsive ophthalmic pluronic F127-chitosan tacrolimus gel. J. Biomater. Sci. Polym. Ed. 2021;32(13):1678–1702. doi: 10.1080/09205063.2021.1932359. [DOI] [PubMed] [Google Scholar]
- 108.Taliyan R., Kakoty V., Sarathlal K.C., Kharavtekar S.S., Karennanavar C.R., Choudhary Y.K., Singhvi G., Riadi Y., Dubey S.K., Kesharwani P. Nanocarrier mediated drug delivery as an impeccable therapeutic approach against Alzheimer's disease. J. Contr. Release. 2022;343:528–550. doi: 10.1016/j.jconrel.2022.01.044. [DOI] [PubMed] [Google Scholar]
- 109.Liang C.Z., Li H., Tao Y.Q., Zhou X.P., Yang Z.R., Xiao Y.X., Li F.C., Han B., Chen Q.X. Dual delivery for stem cell differentiation using dexamethasone and bFGF in/on polymeric microspheres as a cell carrier for nucleus pulposus regeneration. J. Mater. Sci. Mater. Med. 2012;23(4):1097–1107. doi: 10.1007/s10856-012-4563-0. [DOI] [PubMed] [Google Scholar]
- 110.Liang C., Li H., Li C., Yang Z., Zhou X., Tao Y., Xiao Y., Li F., Chen Q. Fabrication of a layered microstructured polymeric microspheres as a cell carrier for nucleus pulposus regeneration. J. Biomater. Sci. Polym. Ed. 2012;23(18):2287–2302. doi: 10.1163/156856211x614789. [DOI] [PubMed] [Google Scholar]
- 111.Liang C.Z., Li H., Tao Y.Q., Peng L.H., Gao J.Q., Wu J.J., Li F.C., Hua J.M., Chen Q.X. Dual release of dexamethasone and TGF-β3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater. 2013;9(12):9423–9433. doi: 10.1016/j.actbio.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 112.Lim S., An S.B., Jung M., Joshi H.P., Kumar H., Kim C., Song S.Y., Lee J.R., Kang M., Han I., et al. Local delivery of senolytic drug inhibits intervertebral disc degeneration and restores intervertebral disc structure. Adv. Healthc Mater. 2022;11(2) doi: 10.1002/adhm.202101483. [DOI] [PubMed] [Google Scholar]
- 113.Antunes J.C., Pereira C.L., Teixeira G.Q., Silva R.V., Caldeira J., Grad S., Gonçalves R.M., Barbosa M.A. Poly(γ-glutamic acid) and poly(γ-glutamic acid)-based nanocomplexes enhance type II collagen production in intervertebral disc. J. Mater. Sci. Mater. Med. 2017;28(1):6. doi: 10.1007/s10856-016-5787-1. [DOI] [PubMed] [Google Scholar]
- 114.Teixeira G.Q., Leite Pereira C., Castro F., Ferreira J.R., Gomez-Lazaro M., Aguiar P., Barbosa M.A., Neidlinger-Wilke C., Goncalves R.M. Anti-inflammatory Chitosan/Poly-γ-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc. Acta Biomater. 2016;42:168–179. doi: 10.1016/j.actbio.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 115.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang X., Jin L., Yao L., Shen F.H., Shimer A.L., Li X. Antioxidative nanofullerol prevents intervertebral disk degeneration. Int. J. Nanomed. 2014;9:2419–2430. doi: 10.2147/ijn.S60853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jin L., Ding M., Oklopcic A., Aghdasi B., Xiao L., Li Z., Jevtovic-Todorovic V., Li X. Nanoparticle fullerol alleviates radiculopathy via NLRP3 inflammasome and neuropeptides. Nanomedicine. 2017;13(6):2049–2059. doi: 10.1016/j.nano.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yeh C.H., Chen D., Aghdasi B., Xiao L., Ding M., Jin L., Li X. Link protein N-terminal peptide and fullerol promote matrix production and decrease degradation enzymes in rabbit annulus cells. Connect. Tissue Res. 2018;59(2):191–200. doi: 10.1080/03008207.2017.1330333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiao L., Huang R., Zhang Y., Li T., Dai J., Nannapuneni N., Chastanet T.R., Chen M., Shen F.H., Jin L., et al. A new formyl peptide receptor-1 antagonist conjugated fullerene nanoparticle for targeted treatment of degenerative disc diseases. ACS Appl. Mater. Interfaces. 2019;11(42):38405–38416. doi: 10.1021/acsami.9b11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nguyen K., Pan H.Y., Haworth K., Mahoney E., Mercado-Shekhar K.P., Lin C.Y., Zhang Z., Y C.P. Multiple-exposure drug release from stable nanodroplets by high-intensity focused ultrasound for a potential degenerative disc disease treatment. Ultrasound Med. Biol. 2019;45(1):160–169. doi: 10.1016/j.ultrasmedbio.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou T., Yang X., Chen Z., Yang Y., Wang X., Cao X., Chen C., Han C., Tian H., Qin A., et al. Prussian blue nanoparticles stabilize SOD1 from ubiquitination-proteasome degradation to rescue intervertebral disc degeneration. Adv. Sci. 2022;9(10) doi: 10.1002/advs.202105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gilchrist R.K., Medal R., Shorey W.D., Hanselman R.C., Parrott J.C., Taylor C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957;146(4):596–606. doi: 10.1097/00000658-195710000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ding B., Zheng P., Ma Pa, Lin J. Manganese oxide nanomaterials: synthesis, properties, and theranostic applications. Adv. Mater. 2020;32(10) doi: 10.1002/adma.201905823. [DOI] [PubMed] [Google Scholar]
- 124.Kimbrough-Allah M.N., Millena A.C., Khan S.A. Differential role of PTEN in transforming growth factor β (TGF-β) effects on proliferation and migration in prostate cancer cells. Prostate. 2018;78(5):377–389. doi: 10.1002/pros.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roger Y., Sydow S., Burmeister L., Menzel H., Hoffmann A. Sustained release of TGF-β from polysaccharide nanoparticles induces chondrogenic differentiation of human mesenchymal stromal cells. Colloids Surf. B Biointerfaces. 2020;189 doi: 10.1016/j.colsurfb.2020.110843. [DOI] [PubMed] [Google Scholar]
- 126.Kazemnejad S., Khanmohammadi M., Mobini S., Taghizadeh-Jahed M., Khanjani S., Arasteh S., Golshahi H., Torkaman G., Ravanbod R., Heidari-Vala H., et al. Comparative repair capacity of knee osteochondral defects using regenerated silk fiber scaffolds and fibrin glue with/without autologous chondrocytes during 36 weeks in rabbit model. Cell Tissue Res. 2016;364(3):559–572. doi: 10.1007/s00441-015-2355-9. [DOI] [PubMed] [Google Scholar]
- 127.Haberstroh K., Enz A., Zenclussen M.L., Hegewald A.A., Neumann K., Abbushi A., Thomé C., Sittinger M., Endres M., Kaps C. Human intervertebral disc-derived cells are recruited by human serum and form nucleus pulposus-like tissue upon stimulation with TGF-beta3 or hyaluronan in vitro. Tissue Cell. 2009;41(6):414–420. doi: 10.1016/j.tice.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 128.Risbud M.V., Di Martino A., Guttapalli A., Seghatoleslami R., Denaro V., Vaccaro A.R., Albert T.J., Shapiro I.M. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine. 2006;31(8):884–890. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 129.Zhu L., Yang Y., Yan Z., Zeng J., Weng F., Shi Y., Shen P., Liu L., Yang H. Controlled release of TGF-β3 for effective local endogenous repair in IDD using rat model. Int. J. Nanomed. 2022;17:2079–2096. doi: 10.2147/ijn.S358396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yokoyama M., Kwon G.S., Okano T., Sakurai Y., Seto T., Kataoka K. Preparation of micelle-forming polymer-drug conjugates. Bioconjugate Chem. 1992;3(4):295–301. doi: 10.1021/bc00016a007. [DOI] [PubMed] [Google Scholar]
- 131.Kwon G.S., Kataoka K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 2012;64:237–245. doi: 10.1016/j.addr.2012.09.016. [DOI] [Google Scholar]
- 132.Feng G., Zha Z., Huang Y., Li J., Wang Y., Ke W., Chen H., Liu L., Song Y., Ge Z. Sustained and bioresponsive two-stage delivery of therapeutic miRNA via polyplex micelle-loaded injectable hydrogels for inhibition of intervertebral disc fibrosis. Adv. Healthc Mater. 2018;7(21) doi: 10.1002/adhm.201800623. [DOI] [PubMed] [Google Scholar]
- 133.Yu C., Li D., Wang C., Xia K., Wang J., Zhou X., Ying L., Shu J., Huang X., Xu H., et al. Injectable kartogenin and apocynin loaded micelle enhances the alleviation of intervertebral disc degeneration by adipose-derived stem cell. Bioact. Mater. 2021;6(10):3568–3579. doi: 10.1016/j.bioactmat.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin C.Y., Crowley S.T., Uchida S., Komaki Y., Kataoka K., Itaka K. Treatment of intervertebral disk disease by the administration of mRNA encoding a cartilage-anabolic transcription factor. Mol. Ther. Nucleic Acids. 2019;16:162–171. doi: 10.1016/j.omtn.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang C.C., Tsou H.K., Chang H.H., Chan L.Y., Zhuo G.Y., Maeda T., Lin C.Y. Runx1 messenger RNA delivered by polyplex nanomicelles alleviate spinal disc hydration loss in a rat disc degeneration model. Int. J. Mol. Sci. 2022;23(1) doi: 10.3390/ijms23010565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koepsell L., Zhang L., Neufeld D., Fong H., Deng Y. Electrospun nanofibrous polycaprolactone scaffolds for tissue engineering of annulus fibrosus. Macromol. Biosci. 2011;11(3):391–399. doi: 10.1002/mabi.201000352. [DOI] [PubMed] [Google Scholar]
- 137.Nerurkar N.L., Sen S., Huang A.H., Elliott D.M., Mauck R.L. Engineered disc-like angle-ply structures for intervertebral disc replacement. Spine. 2010;35(8):867–873. doi: 10.1097/BRS.0b013e3181d74414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nerurkar N.L., Mauck R.L., Elliott D.M. Modeling interlamellar interactions in angle-ply biologic laminates for annulus fibrosus tissue engineering. Biomech. Model. Mechanobiol. 2011;10(6):973–984. doi: 10.1007/s10237-011-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martin J.T., Milby A.H., Chiaro J.A., Kim D.H., Hebela N.M., Smith L.J., Elliott D.M., Mauck R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014;10(6):2473–2481. doi: 10.1016/j.actbio.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Turner K.G., Ahmed N., Santerre J.P., Kandel R.A. Modulation of annulus fibrosus cell alignment and function on oriented nanofibrous polyurethane scaffolds under tension. Spine J. 2014;14(3):424–434. doi: 10.1016/j.spinee.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 141.Tao H., Wu Y., Li H., Wang C., Zhang Y., Li C., Wen T., Wang X., He Q., Wang D., et al. BMP7-Based functionalized self-assembling peptides for nucleus pulposus tissue engineering. ACS Appl. Mater. Interfaces. 2015;7(31):17076–17087. doi: 10.1021/acsami.5b03605. [DOI] [PubMed] [Google Scholar]
- 142.Feng G., Zhang Z., Dang M., Zhang X., Doleyres Y., Song Y., Chen D., Ma P.X. Injectable nanofibrous spongy microspheres for NR4A1 plasmid DNA transfection to reverse fibrotic degeneration and support disc regeneration. Biomaterials. 2017;131:86–97. doi: 10.1016/j.biomaterials.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Uysal O., Arslan E., Gulseren G., Kilinc M.C., Dogan I., Ozalp H., Caglar Y.S., Guler M.O., Tekinay A.B. Collagen peptide presenting nanofibrous scaffold for intervertebral disc regeneration. ACS Appl. Bio Mater. 2019;2(4):1686–1695. doi: 10.1021/acsabm.9b00062. [DOI] [PubMed] [Google Scholar]
- 144.Liu C., Xiao L., Zhang Y., Zhao Q., Xu H. Regeneration of annulus fibrosus tissue using a DAFM/PECUU-blended electrospun scaffold. J. Biomater. Sci. Polym. Ed. 2020;31(18):2347–2361. doi: 10.1080/09205063.2020.1812038. [DOI] [PubMed] [Google Scholar]
- 145.Yu Q., Han F., Yuan Z., Zhu Z., Liu C., Tu Z., Guo Q., Zhao R., Zhang W., Wang H., et al. Fucoidan-loaded nanofibrous scaffolds promote annulus fibrosus repair by ameliorating the inflammatory and oxidative microenvironments in degenerative intervertebral discs. Acta Biomater. 2022 doi: 10.1016/j.actbio.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 146.Dalwadi C., Patel G. Application of nanohydrogels in drug delivery systems: recent patents review. Recent Pat. Nanotechnol. 2015;9(1):17–25. doi: 10.2174/1872210509666150101151521. [DOI] [PubMed] [Google Scholar]
- 147.Oehme D., Goldschlager T., Ghosh P., Rosenfeld J.V., Jenkin G. Cell-based therapies used to treat lumbar degenerative disc disease: a systematic review of animal studies and human clinical trials. Stem Cell. Int. 2015;2015 doi: 10.1155/2015/946031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lyu F.J., Cheung K.M., Zheng Z., Wang H., Sakai D., Leung V.Y. IVD progenitor cells: a new horizon for understanding disc homeostasis and repair. Nat. Rev. Rheumatol. 2019;15(2):102–112. doi: 10.1038/s41584-018-0154-x. [DOI] [PubMed] [Google Scholar]
- 149.Gan Y., Li S., Li P., Xu Y., Wang L., Zhao C., Ouyang B., Tu B., Zhang C., Luo L., et al. A controlled release codelivery system of MSCs encapsulated in dextran/gelatin hydrogel with TGF-β3-loaded nanoparticles for nucleus pulposus regeneration. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/9042019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ligorio C., Zhou M., Wychowaniec J.K., Zhu X., Bartlam C., Miller A.F., Vijayaraghavan A., Hoyland J.A., Saiani A. Graphene oxide containing self-assembling peptide hybrid hydrogels as a potential 3D injectable cell delivery platform for intervertebral disc repair applications. Acta Biomater. 2019;92:92–103. doi: 10.1016/j.actbio.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y., Zhang Y., Chen K., Shao F., Wu Y., Guo C., Wu H., Zhang D., Li W., Kong Q., et al. Injectable nanostructured colloidal gels resembling native nucleus pulposus as carriers of mesenchymal stem cells for the repair of degenerated intervertebral discs. Mater. Sci. Eng. C Mater Biol. Appl. 2021;128 doi: 10.1016/j.msec.2021.112343. [DOI] [PubMed] [Google Scholar]
- 152.Barczewska M., Wojtkiewicz J., Habich A., Janowski M., Adamiak Z., Holak P., Matyjasik H., Bulte J.W., Maksymowicz W., Walczak P. MR monitoring of minimally invasive delivery of mesenchymal stem cells into the porcine intervertebral disc. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen W., Chen H., Zheng D., Zhang H., Deng L., Cui W., Zhang Y., Santos H.A., Shen H. Gene-hydrogel microenvironment regulates extracellular matrix metabolism balance in nucleus pulposus. Adv. Sci. 2020;7(1) doi: 10.1002/advs.201902099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chang H., Cai F., Zhang Y., Jiang M., Yang X., Qi J., Wang L., Deng L., Cui W., Liu X. Silencing gene-engineered injectable hydrogel microsphere for regulation of extracellular matrix metabolism balance. Small Methods. 2022;6(4) doi: 10.1002/smtd.202101201. [DOI] [PubMed] [Google Scholar]
- 155.Michalek A.J., Buckley M.R., Bonassar L.J., Cohen I., Iatridis J.C. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine J. 2010;10(12):1098–1105. doi: 10.1016/j.spinee.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ligorio C., O'Brien M., Hodson N.W., Mironov A., Iliut M., Miller A.F., Vijayaraghavan A., Hoyland J.A., Saiani A. TGF-β3-loaded graphene oxide - self-assembling peptide hybrid hydrogels as functional 3D scaffolds for the regeneration of the nucleus pulposus. Acta Biomater. 2021;127:116–130. doi: 10.1016/j.actbio.2021.03.077. [DOI] [PubMed] [Google Scholar]
- 157.Madhu V., Boneski P.K., Silagi E., Qiu Y., Kurland I., Guntur A.R., Shapiro I.M., Risbud M.V. Hypoxic regulation of mitochondrial metabolism and mitophagy in nucleus pulposus cells is dependent on HIF-1α-BNIP3 Axis. J. Bone Miner. Res. 2020;35(8):1504–1524. doi: 10.1002/jbmr.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Silagi E.S., Schoepflin Z.R., Seifert E.L., Merceron C., Schipani E., Shapiro I.M., Risbud M.V. Bicarbonate recycling by HIF-1-Dependent carbonic anhydrase isoforms 9 and 12 is critical in maintaining intracellular pH and viability of nucleus pulposus cells. J. Bone Miner. Res. 2018;33(2):338–355. doi: 10.1002/jbmr.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Keshari K.R., Lotz J.C., Link T.M., Hu S., Majumdar S., Kurhanewicz J. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine. 2008;33(3):312–317. doi: 10.1097/BRS.0b013e31816201c3. [DOI] [PubMed] [Google Scholar]
- 160.Alam F., RoyChoudhury S., Jalal A.H., Umasankar Y., Forouzanfar S., Akter N., Bhansali S., Pala N. Lactate biosensing: the emerging point-of-care and personal health monitoring. Biosens. Bioelectron. 2018;117:818–829. doi: 10.1016/j.bios.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 161.Feng C., Yang M., Lan M., Liu C., Zhang Y., Huang B., Liu H., Zhou Y. ROS: crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/5601593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shen J., Chen A., Cai Z., Chen Z., Cao R., Liu Z., Li Y., Hao J. Exhausted local lactate accumulation via injectable nanozyme-functionalized hydrogel microsphere for inflammation relief and tissue regeneration. Bioact. Mater. 2022;12:153–168. doi: 10.1016/j.bioactmat.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bagno L.L., Kanashiro-Takeuchi R.M., Suncion V.Y., Golpanian S., Karantalis V., Wolf A., Wang B., Premer C., Balkan W., Rodriguez J., et al. Growth hormone-releasing hormone agonists reduce myocardial infarct scar in swine with subacute ischemic cardiomyopathy. J. Am. Heart Assoc. 2015;4(4) doi: 10.1161/JAHA.114.001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Czikora I., Sridhar S., Gorshkov B., Alieva I.B., Kasa A., Gonzales J., Potapenko O., Umapathy N.S., Pillich H., Rick F.G., et al. Protective effect of Growth Hormone-Releasing Hormone agonist in bacterial toxin-induced pulmonary barrier dysfunction. Front. Physiol. 2014;5:259. doi: 10.3389/fphys.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng Q., Shen H., Tong Z., Cheng L., Xu Y., Feng Z., Liao S., Hu X., Pan Z., Mao Z., et al. A thermosensitive, reactive oxygen species-responsive, MR409-encapsulated hydrogel ameliorates disc degeneration in rats by inhibiting the secretory autophagy pathway. Theranostics. 2021;11(1):147–163. doi: 10.7150/thno.47723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pallay R.M., Seger W., Adler J.L., Ettlinger R.E., Quaidoo E.A., Lipetz R., O'Brien K., Mucciola L., Skalky C.S., Petruschke R.A., et al. Etoricoxib reduced pain and disability and improved quality of life in patients with chronic low back pain: a 3 month, randomized, controlled trial. Scand. J. Rheumatol. 2004;33(4):257–266. doi: 10.1080/03009740410005728. [DOI] [PubMed] [Google Scholar]
- 167.Liu Y., Du J., Peng P., Cheng R., Lin J., Xu C., Yang H., Cui W., Mao H., Li Y., et al. Regulation of the inflammatory cycle by a controllable release hydrogel for eliminating postoperative inflammation after discectomy. Bioact. Mater. 2021;6(1):146–157. doi: 10.1016/j.bioactmat.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 2011;121(3):985–997. doi: 10.1172/jci44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bai J., Zhang Y., Fan Q., Xu J., Shan H., Gao X., Ma Q., Sheng L., Zheng X., Cheng W., et al. Reactive oxygen species-scavenging scaffold with rapamycin for treatment of intervertebral disk degeneration. Adv. Healthc Mater. 2020;9(3) doi: 10.1002/adhm.201901186. [DOI] [PubMed] [Google Scholar]
- 170.Wang C., Li Z., Liu Y., Yuan L. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11(8):3996–4010. doi: 10.7150/thno.56035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/ijn.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bhujel B., Shin H.E., Choi D.J., Han I. Mesenchymal stem cell-derived exosomes and intervertebral disc regeneration: review. Int. J. Mol. Sci. 2022;23(13) doi: 10.3390/ijms23137306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun Z., Zhao H., Liu B., Gao Y., Tang W.H., Liu Z.H., Luo Z.J. AF cell derived exosomes regulate endothelial cell migration and inflammation: implications for vascularization in intervertebral disc degeneration. Life Sci. 2021;265 doi: 10.1016/j.lfs.2020.118778. [DOI] [PubMed] [Google Scholar]
- 174.Guo Z., Su W., Zhou R., Zhang G., Yang S., Wu X., Qiu C., Cong W., Shen N., Guo J., et al. Exosomal MATN3 of urine-derived stem cells ameliorates intervertebral disc degeneration by antisenescence effects and promotes NPC proliferation and ECM synthesis by activating TGF-β. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/5542241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xing H., Zhang Z., Mao Q., Wang C., Zhou Y., Zhou X., Ying L., Xu H., Hu S., Zhang N. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J. Nanobiotechnol. 2021;19(1):264. doi: 10.1186/s12951-021-00991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Johnson Z.I., Schoepflin Z.R., Choi H., Shapiro I.M., Risbud M.V. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur. Cell. Mater. 2015;30:104–116. doi: 10.22203/ecm.v030a08. ; discussion 116-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pennicooke B., Moriguchi Y., Hussain I., Bonssar L., Härtl R. Biological treatment approaches for degenerative disc disease: a review of clinical trials and future directions. Cureus. 2016;8(11):e892. doi: 10.7759/cureus.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Denozière G., Ku D.N. Biomechanical comparison between fusion of two vertebrae and implantation of an artificial intervertebral disc. J. Biomech. 2006;39(4):766–775. doi: 10.1016/j.jbiomech.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 179.Disch A.C., Schmoelz W., Matziolis G., Schneider S.V., Knop C., Putzier M. Higher risk of adjacent segment degeneration after floating fusions: long-term outcome after low lumbar spine fusions. J. Spinal Disord. Tech. 2008;21(2):79–85. doi: 10.1097/BSD.0b013e3180577259. [DOI] [PubMed] [Google Scholar]
- 180.Chang Y., Yang M., Ke S., Zhang Y., Xu G., Li Z. Effect of platelet-rich plasma on intervertebral disc degeneration in vivo and in vitro: a critical review. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/8893819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chujo T., An H.S., Akeda K., Miyamoto K., Muehleman C., Attawia M., Andersson G., Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31(25):2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 182.Willems N., Bach F.C., Plomp S.G., van Rijen M.H., Wolfswinkel J., Grinwis G.C., Bos C., Strijkers G.J., Dhert W.J., Meij B.P., et al. Intradiscal application of rhBMP-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res. Ther. 2015;17(1):137. doi: 10.1186/s13075-015-0625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matta A., Erwin W.M. Injectable biologics for the treatment of degenerative disc disease. Curr. Rev. Musculoskelet. Med. 2020;13(6):680–687. doi: 10.1007/s12178-020-09668-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Farhang N., Ginley-Hidinger M., Berrett K.C., Gertz J., Lawrence B., Bowles R.D. Lentiviral CRISPR epigenome editing of inflammatory receptors as a gene therapy strategy for disc degeneration. Hum. Gene Ther. 2019;30(9):1161–1175. doi: 10.1089/hum.2019.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Walsh A.J., Bradford D.S., Lotz J.C. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29(2):156–163. doi: 10.1097/01.Brs.0000107231.67854.9f. [DOI] [PubMed] [Google Scholar]
- 186.Zhang H., Yu S., Zhao X., Mao Z., Gao C. Stromal cell-derived factor-1α-encapsulated albumin/heparin nanoparticles for induced stem cell migration and intervertebral disc regeneration in vivo. Acta Biomater. 2018;72:217–227. doi: 10.1016/j.actbio.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 187.Arias L.S., Pessan J.P., Vieira A.P.M., Lima T.M.T., Delbem A.C.B., Monteiro D.R. Iron oxide nanoparticles for biomedical applications: a perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7(2) doi: 10.3390/antibiotics7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Manshian B.B., Jiménez J., Himmelreich U., Soenen S.J. Personalized medicine and follow-up of therapeutic delivery through exploitation of quantum dot toxicity. Biomaterials. 2017;127:1–12. doi: 10.1016/j.biomaterials.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 189.Koffler J., Zhu W., Qu X., Platoshyn O., Dulin J.N., Brock J., Graham L., Lu P., Sakamoto J., Marsala M., et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25(2):263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shu C.C., Smith M.M., Smith S.M., Dart A.J., Little C.B., Melrose J. A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells for intervertebral disc regeneration. Int. J. Mol. Sci. 2017;18(5) doi: 10.3390/ijms18051049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Beckstein J.C., Sen S., Schaer T.P., Vresilovic E.J., Elliott D.M. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine. 2008;33(6):E166–E173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 192.Kreuter J. Nanoparticles and nanocapsules--new dosage forms in the nanometer size range. Pharm. Acta Helv. 1978;53(2):33–39. [PubMed] [Google Scholar]
- 193.Aranda-Lara L., García B.E.O., Isaac-Olivé K., Ferro-Flores G., Meléndez-Alafort L., Morales-Avila E. Drug delivery systems-based dendrimers and polymer micelles for nuclear diagnosis and therapy. Macromol. Biosci. 2021;21(3) doi: 10.1002/mabi.202000362. [DOI] [PubMed] [Google Scholar]
- 194.Singh A., Rath G., Singh R., Goyal A.K. Nanofibers: an effective tool for controlled and sustained drug delivery. Curr. Drug Deliv. 2018;15(2):155–166. doi: 10.2174/1567201814666171002115230. [DOI] [PubMed] [Google Scholar]
- 195.Š Zupančič. Core-shell nanofibers as drug delivery systems. Acta Pharm. 2019;69(2):131–153. doi: 10.2478/acph-2019-0014. [DOI] [PubMed] [Google Scholar]
- 196.Kabanov A.V., Alakhov V.Y. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carrier Syst. 2002;19(1):1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 197.Vinogradov S.V. Nanogels in the race for drug delivery. Nanomedicine. 2010;5(2):165–168. doi: 10.2217/nnm.09.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.