Abstract
Atrial fibrillation is the most common cardiac arrhythmia with its prevalence expected to increase to 12.1 million people in the United States by 2030. Chronic underlying conditions that affect the heart and lungs predispose patients to develop atrial fibrillation. Obstructive sleep apnea is strongly associated with atrial fibrillation. Several pathophysiological mechanisms have been proposed to elucidate this relationship which includes electrophysiological substrate modification and the contribution of the autonomic nervous system. In this comprehensive review, we highlight important relationships and plausible causality between obstructive sleep apnea and atrial fibrillation which will improve our understanding in the evaluation, management, and prevention of atrial fibrillation. This is the most updated comprehensive review of the relationship between obstructive sleep apnea and atrial fibrillation.
Keywords: Cardiovascular, electrophysiology, heart, hypoxia, medicine, pulmonary
Impact Statement
This comprehensive review elucidates the close relationship between the presence of obstructive sleep apnea and atrial fibrillation incidence and recurrence. It reviews their common and dependent etiologies and modifiable variables that will impact the prevention and management strategies of atrial fibrillation.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia. It is expected to affect 12.1 million people in the United States by 2030.1,2 Chronic underlying conditions that affect the heart and lungs predispose patients to develop AF, particularly coronary artery disease, valvular disease, heart failure, pulmonary hypertension, and obstructive sleep apnea (OSA). AF is associated with increased incidence of thromboembolic events, cerebrovascular events, and hospitalizations.
OSA is a common respiratory illness that is strongly associated with AF3,4 with incidence of AF 88% higher in patients with OSA. 5 In a study of 2911 males, participants with greater severity of sleep-disordered breathing (SDB) had increased odds of AF (odds ratio [OR] = 2.15, 95% confidence interval [CI], 1.12–1.82), and the severity of hypoxia was associated with complex ventricular ectopy (P < 0.001). 6 Furthermore, the prevalence of AF in OSA patient is likely an underestimate, given that many patients with AF have undiagnosed OSA. Certain predictors such as plasma visfatin concentration are associated with the diagnosis of OSA in patients with AF. 7 In rat model, cumulative exposure to transient OSA-related conditions increases AF susceptibility. 8 There is a correlation between OSA and increased cardioembolic risk of a cerebrovascular accident in patients with AF,9,10 this is even greater when there are increased indices of hypoxia during sleep. 11
Several pathophysiological mechanisms have been proposed to elucidate the development of AF in OSA, which include hypoxia-induced arrhythmogenesis, structural remodeling, autonomic imbalance, and SDB and arrhythmogenesis.12,13 This is summarized in Figure 1. Some studies have proposed that the current treatment of OSA via reoxygenation promotes conduction abnormalities, resulting in AF vulnerability. This review of the link between OSA and AF will discuss the current proposed pathophysiological mechanisms associated with the occurrence of AF in OSA, prevalence of AF in OSA, role of ablative therapy in untreated OSA, and current treatment modalities for AF.
Figure 1.
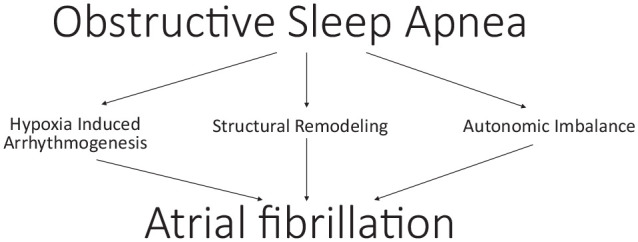
Pathophysiological mechanisms causing atrial fibrillation in patients with obstructive sleep apnea.
Hypoxia-induced arrhythmogenesis
Several mechanisms by which hypoxia induces a propensity toward arrhythmogenesis have been proposed. Studies in animals and humans have been performed and suggest that hypoxia induces expression of factors that contribute to structural remodeling and conduction abnormalities. For patients investigated for OSA, there is an association of nocturnal hypoxemia and pulse rate variability, which are independent predictors of incidental AF. 14 Hypoxic conditions created by OSA favor the upregulation of hypoxia-induced factor-1a (HIF-1a), which is the transcription factor of hypoxic-induced vascular endothelial growth factor (VEGF). HIF-1a/VEGF both share a potential role in the increased expression of matrix metalloproteinase-9 (MMP-9), particularly in patients with paroxysmal/persistent AF, thus suggesting a possible significant role in atrial structural remodeling.15 –17 However, an emerging theory has been proposed that the Warburg effect, a form of tumor cell metabolism under hypoxic conditions, plays a significant role in transcription of HIF-1a leading to increased expression of VEGF and upregulation of inflammatory cells, resulting in the progression of AF through fibrosis and development conduction abnormalities. 18 In other studies, hypoxia contributed to alterations in cardiac conduction via shortening action potentials, prolongation, and heterogeneity of refractory periods, early afterdepolarizations, and increased pulmonary vein (PV) firings. However, the correction of the hypoxia resulted in increased vulnerability to AF. 19 In a study by Lin et al., 20 hypoxia shortened the action potential duration and reduced PV firing, but reoxygenation induced PV burst firings. In another study, hydrogen peroxide caused increased PV burst firings and early afterdepolarizations, suggesting that oxidative stress favors PV arrhythmogenesis. 21 Connexin-43 is a major gap junction protein with expression reversibly reduced by hypoxia with increased correlation of AF in rats, and interestingly, cardiac sympathetic denervation which increases the connexin-43 expression in these hypoxic mouse models reduces AF occurrence. 22
Stretch-induced structural remodeling
Structural remodeling of the atria in OSA-causing AF has been previously investigated. Intrathoracic pressure shifts, because of repetitive apnea episodes, is a proposed mechanism of structural remodeling, leading to atrial dilation and remodeling near the PV. 23 Negative tracheal pressures, up to −100 mbar, were shown to shorten atrial effective refractory period (AERP) and enhance susceptibility to AF in anesthetized pigs. 24 Moreover, OSA is a risk factor for diastolic dysfunction, which also contributes to manifestations of AF by interfering with cardiac structure. In a study assessing how obesity promotes AF in rats, obstructive apnea-induced AF due to diastolic dysfunction in obese rats compared to lean rats, suggesting another arrhythmogenic substrate for AF. 25
Atrial fibrosis is the primary substrate for the development of AF.26,27 The long-term effects of repeated apnea phases demonstrated increased duration of AF in chronic OSA rats compared to control rats, inducibility in 82.4% (P < 0.05) of chronic AF rats, and significant conduction slowing. 23 This study was one of the initial studies to introduce the role connexin-43 may have in conduction abnormalities. Recurrent apneas promote atrial fibrosis via local and systemic inflammation (IL-6) and selective reduction in collagen degradation (MMP-2). 28 Furthermore, it has been proposed that the organization of interstitial collagen rather than the amount of collagen present seems to contribute to changes in conduction velocity. Krul et al in 2015 investigated conduction velocities in 35 left atrial (LA) appendages during pulmonary vein isolation (PVI) for AF and concluded that the thickness of the collagen fibrils was associated with increased longitudinal conduction velocities and activation times. 29 Other studies show these findings in the left atrium are not similar for the right atrium where no observable histological differences in human right atrial tissue was seen for individuals at high or low risk of OSA. 30 OSA causes fibrosis in the atrium which was seen by late gadolinium enhancement technique of cardiac magnetic resonance, and this was an independently associated predictor of AF in patients with OSA. 31
Autonomic imbalance
Autonomic changes have been implicated in the development of AF. Alterations in sympathetic tone during apnea episodes stimulate sympathetic activation via chemoreceptors and/or decreased parasympathetic tone that has been demonstrated through impaired vagal input, diminished baroreflex sensitivity, and impairment of the parasympathetic components of heart rate variability. 32
Severe intermittent hypoxemia, acidosis, and hypercapnia can result in sympathetic activation leading to heart rate and blood pressure elevation. 17 Conversely, hypoxemia in setting of apnea can also trigger a vagal response 33 and the negative intrathoracic pressure stimulates the parasympathetic nervous system. 34 The ANS imbalance may precipitate electrical changes in the atrium that predisposes to AF.19,35
A study reported that there was increased sympathetic activity, in the form of blood pressure elevation, in patients with OSA during wakefulness and during sleep; however, the use of CPAP improved these findings. 36
Since then, many studies have investigated the association between OSA and hypertension, which is a known risk factor for AF possibly through stretch-induced changes contributing to diastolic dysfunction.37 –39 The neural activity of the left stellate ganglion was enhanced in OSA compared to control dog model and this was thought to accelerate LA neural remodeling to increase AF. 40 Superior left ganglionic plexus ablation was shown to suppress AF in chronic OSA mouse model by inhibition of its sympathovagal hyperactivity. 41 Low-level vagus nerve stimulation has also been shown to suppress acute OSA and suppressing AF. 42
SDB events and arrhythmogenesis
SDB is defined by repeated episodes of apnea and hypopnea during slumber 43 and has been shown to increase the risk of AF incidence. 44 In addition, studies have revealed a temporal association between nocturnal AF episodes and individual apneic events.45,46 One mechanism of apnea-related arrhythmogenesis involves the wide fluctuations of intrathoracic pressures during airway obstruction. More specifically, inspiratory effort on the collapsed upper airway during sleep generates negative intrathoracic pressures resulting in wider atrial transmural pressure gradients and atrial wall distension.45,46 Orban et al simulated this phenomenon using the Mueller maneuver (inhalation with mouth and nostrils closed) in 20 healthy adults and demonstrated a decrease in LA volume during the maneuver with a subsequent compensatory increase above baseline following release. 47 Over time, continual obstructive events cause recurrent atrial wall stretching and can result in structural and functional atrial remodeling. 48 Furthermore, atrial dilatation has been shown to further promote electrophysiologic changes. This was demonstrated by Linz et al who applied negative tracheal pressure during tracheal occlusion in pigs and observed shortened atrial refractory periods with amplified AF inducibility. 24 Large, prospective studies have also shown increased risk of new-onset AF in patients with larger LA diameters.49,50
During an apneic episode, upper airway obstruction impairs gas exchange and leads to hypoxia, an independent risk factor for AF incidence. 44 Upon resolution of obstruction, reoxygenation ensues and may result in cardiac structural oxidative injury due to reactive oxygen species 51 which are associated with arrhythmogenesis. 52 In addition, Stevenson et al studied the electrophysiologic effects of episodic hypoxia and hypercapnia using sheep under autonomic blockade. Hypercapnia resulted in prolongation of the AERP and increased right atrial conduction times; the propensity for AF was notably decreased during hypercapnia likely by its effect on the refractory period. However, upon restoring eucapnia, an increase in AF susceptibility was observed as the AERP had recovered while atrial conduction time remained slow for 2 h following normalization of blood gas levels. 19
Overall, the pathophysiologic interplay between AF and SDB is best described by the changes in intrathoracic pressure, hypoxia, and hypercapnia thereby promoting atrial structural and electrical remodeling and thus arrhythmogenesis.
Prevalence of comorbid OSA and AF
Publications of the relationship between OSA and cardiac arrhythmias date to 1983. Guilleminault et al observed 400 patients with OSA under 24 h electrocardiogram (ECG) monitoring and demonstrated resolution of some arrhythmias following tracheostomy. 53 Since that time, studies have continually indicated a robust association between OSA and AF.54 –56 The Sleep Heart Health Study reported four times higher risk of AF in patients with severe OSA as compared to those without OSA (adjusted odds ratio: 4.02; 95% CI, 1.03–15.74). 45 In addition, a retrospective analysis of 3542 adults with no prior AF history demonstrated several strong predictors of AF incidence, including BMI, OSA, and OSA severity. 44 The probability of AF recurrence following ablation is threefold higher in those with OSA. 57 Another study revealed that the odds of OSA were doubled in those with AF even after risk factor adjustment. 58 Furthermore, in a study involving 150 patients with AF, 74% of patients demonstrated SDB with 42.7% having OSA. 59 In conclusion, while the mechanisms of association between OSA and AF are complex, multiple studies have demonstrated the increased prevalence of comorbid OSA and AF.
Relation of OSA and AF to congestive heart failure
Congestive heart failure (CHF) is associated with OSA and AF. They have similar risk factors, such as hypertension, advanced age, and obesity. OSA affects CHF by compromising filling and emptying of the left ventricle by intrathoracic pressure variation and by sympathetic stimulation. 60 The increased venous and extravascular fluid volume leads to SDB disorder which includes OSA. 61 Current recommendation suggests CPAP therapy improves CHF in patients with OSA diagnoses. 62
CHF plays a major role in the etiology and morbidity of AF. CHF over time could lead to atrial remodeling through direct atrial enlargement from increased ventricular volume and pressure and indirectly through substrates, such as the reactive oxygen species from CHF and SDB. 63 These atrial changes increase the probability of AF occurrence. CHF increases the risk of stroke in AF. AF can directly lead to worsening of CHF symptoms by eliminating the atrial contraction component of left ventricular filling and lead to short-term and long-term worsening of ventricular myocardial function due to tachycardia from rapid ventricular response. A holistic management of CHF, OSA, AF, and their modifiable risk factors is essential to optimize each individual condition. In addition to monitoring body fluid content for CHF management, and detection of cardiac arrhythmias, such as AF, some cardiac implantable electronic devices have algorithms to detect advanced OSA 64 which could aid in detection and management of sleep apnea.
The relationship of therapy of OSA and AF
Diagnosing and treating OSA is an important part of the workup prior to considering catheter-based PVI for the treatment of AF.65,66 One meta-analysis showed a 25% overall greater risk of AF recurrence with patients with OSA compared to patients without OSA. 65 Although a study showed no statistically significant reduction in the burden of AF with CPAP therapy, 67 most other studies showed a correlation with therapy for OSA associated with AF recurrence with an increase in AF recurrence after catheter ablation for untreated OSA 68 and a decrease in AF recurrence after PVI for patients treated with CPAP. 69 Additional studies showed a positive correlation with treatment of OSA with CPAP and a reduction in recurrence of AF, irrespective of patient undergoing PVI. 70
The Sauer Heart Rhythm study 71 looked more into issues with the actual procedure itself. Specifically, the effectiveness of PVI for the treatment of AF and the possibility of recurrence of PV conduction post procedure due to acute PV reconnection and the associated long-term clinical outcomes. The study showed acute return of PV conduction after a successful ablation occurred more in the older populations, patients with non-paroxysmal AF, hypertension, a large atrium, and OSA and less dependent on the power and duration of the energy delivered, or even the specific catheter tip used during the procedure (4 vs 8 mm catheter).
There is a reduced risk of AF recurrence after PVI for patients with OSA who use CPAP for at least greater than 4 h a night. 70 The study showed that patients with OSA have increased blood pressures, pulmonary artery pressures, right ventricular volumes, LA sizes, and left ventricular masses, all of which improved following CPAP treatment. The lower blood pressure, ventricular mass, and left atrium size following treatment decreased the risk of AF recurrence after PVI. 72 There is limited benefit for patients with OSA going through PVI for AF if not treated with CPAP. 73
Other risk factors for AF recurrence within a 12-month period include early recurrence of AF, defined as recurrent AF within 0- to 3-month blanking period, and an LA diameter of greater than 40 mm. 72 OSA continues to be identified as a risk factor for the persistent AF group undergoing cryoballoon ablation.
Cardiac arrhythmias can be decreased after three months of CPAP therapy for patients with untreated OSA, with the severity of OSA being correlated to the prevalence of cardiac arrhythmias. 74 Weight control, in addition to CPAP compliance and early AF intervention, reduces the risk of AF recurrence in OSA patients. 75 One of the risk factors for OSA is obesity and weight loss can temporarily reduce AF burden. 76 Ongoing weight loss can be associated with a significant reduction of AF prevalence and the actual maintenance of sinus rhythm. 76 OSA needs to be diagnosed and treated for patients with AF. History-taking with various standard OSA symptoms and demographic questionnaires (such as Epworth Sleepiness Scale, Berlin Questionnaire, Sleep Apnea Clinical Score, STOP-Bang, MOODS, and OSA50) is known to have accuracy in screening for OSA in those who would need more specific sleep study testing in the general population, but the accuracy of this is reduced in those with cardiac conditions, such as AF. These questionnaires will be a good starting point to identify patients with AF who have OSA but more objective test, such as the home sleep apnea testing, is encouraged for those with AF to rule out OSA.77 –80 Metoprolol has been shown to prevent AF in animal models with chronic OSA by inhibiting atrial structural, sympathetic nervous and metabolic remodeling81,82 studies to demonstrate this effect in humans will be valuable. OSA has been associated with increased incidence of extra-PV triggers of AF, and elimination of these triggers reduces arrhythmia recurrence. 83 A strong association exists between OSA and cardiovascular conditions including AF, 84 in which prompt diagnosis and management of OSA lead to better outcomes.
Conclusions
The link between OSA and AF is a combination of sympathetic and parasympathetic drivers and electrophysiologic substrate modification. This strong relationship includes common etiologies, increased prevalence of AF in patients with untreated OSA, and improved prognosis when OSA is treated independent of PVI therapy for AF. These factors should be strongly considered in patients with risk factors of OSA presenting with an initial episode or recurrence of AF. For patients who have an unacceptable burden of AF episodes despite repeat ablations and antiarrhythmic therapy, diagnosis and management of OSA should be optimized before further rhythm control or proceeding to other measures of control, such as invasive neuromodulation or atrioventricular node ablation with permanent pacemaker implantation.
Footnotes
Authors’ Contributions: All authors participated in the design, interpretation of the studies, analysis, conclusions, and writing various segments of the article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Ikechukwu Ifedili  https://orcid.org/0000-0002-8098-2280
https://orcid.org/0000-0002-8098-2280
Eva Ingram  https://orcid.org/0000-0003-3441-2348
https://orcid.org/0000-0003-3441-2348
References
- 1. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota,1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25 [DOI] [PubMed] [Google Scholar]
- 2. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142–7 [DOI] [PubMed] [Google Scholar]
- 3. Abbasi A, Gupta SS, Sabharwal N, Meghrajani V, Sharma S, Kamholz S, Kupfer Y. A comprehensive review of obstructive sleep apnea. Sleep Sci 2021;14:142–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linz D, Hendriks J. Central sleep apnea in atrial fibrillation: risk factor or marker of untreated underlying disease. Int J Cardiol Heart Vasc 2020;30:100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moula AI, Parrini I, Tetta C, Luca F, Parise G, Rao CM, Mauro E, Parise O, Matteucci F, Gulizia MM, La Meir M, Gelsomino S. Obstructive sleep apnea and atrial fibrillation. J Clin Med 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehra R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T, Ibrahim OA, Salem R, Redline S. Nocturnal Arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009;169:1147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Szymanska A, Platek AE, Sierdzinski J, Szymanski FM. Visfatin as a predictor of obstructive sleep apnea in atrial fibrillation patients. Sleep Breath 2020;24:1215–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linz B, Hohl M, Lang L, Wong DWL, Nickel AG, De La Torre C, Sticht C, Wirth K, Boor P, Maack C, Speer T, Jespersen T, Schotten U, Sanders P, Böhm M, Linz D. Repeated exposure to transient obstructive sleep apnea-related conditions causes an atrial fibrillation substrate in a chronic rat model. Heart Rhythm 2021;18:455–64 [DOI] [PubMed] [Google Scholar]
- 9. Lipford MC, Flemming KD, Calvin AD, Mandrekar J, Brown RD, Jr, Somers VK, Caples SM. Associations between cardioembolic stroke and obstructive sleep apnea. Sleep 2015;38:1699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yaranov DM, Smyrlis A, Usatii N, Butler A, Petrini J, Mendez J, Warshofsky M. Obstructive sleep apnea and cardioembolic risk assessment in patients with atrial fibrillation: CHADS2 OR CHADS2S? J Am Coll Cardiol 2014;63:A1435 [Google Scholar]
- 11. Pengo MF, Faini A, Grote L, Ludka O, Joppa P, Pataka A, Dogas Z, Mihaicuta S, Hein H, Anttalainen U, Ryan S, Lombardi C, Parati G, ESADA Working Group. Impact of sleep apnea on cardioembolic risk in patients with atrial fibrillation: data from the ESADA cohort. Stroke 2021;52:712–5 [DOI] [PubMed] [Google Scholar]
- 12. Goudis CA, Ketikoglou DG. Obstructive sleep and atrial fibrillation: pathophysiological mechanisms and therapeutic implications. Int J Cardiol 2017;230:293–300 [DOI] [PubMed] [Google Scholar]
- 13. Linz D, Linz B, Hohl M, Bohm M. Atrial arrhythmogenesis in obstructive sleep apnea: therapeutic implications. Sleep Med Rev 2016;26:87–94 [DOI] [PubMed] [Google Scholar]
- 14. Blanchard M, Gervès-Pinquié C, Feuilloy M, Le Vaillant M, Trzepizur W, Meslier N, Paris A, Pigeanne T, Racineux JL, Balusson F, Oger E, Girault JM, Gagnadoux F. Association of nocturnal hypoxemia and pulse rate variability with incident atrial fibrillation in patients investigated for obstructive sleep apnea. Ann Am Thorac Soc 2021;18:1043–51 [DOI] [PubMed] [Google Scholar]
- 15. Ogi H, Nakano Y, Niida S, Dote K, Hirai Y, Suenari K, Tonouchi Y, Oda N, Makita Y, Ueda S, Kajihara K, Imai K, Sueda T, Chayama K, Kihara Y. Is structural remodeling of fibrillated atria the consequence of tissue hypoxia. Circ J 2010;74:1815–21 [DOI] [PubMed] [Google Scholar]
- 16. Li M, Yang G, Xie B, Babu K, Huang C. Changes in matrix metalloproteinase-9 levels during progression of atrial fibrillation. J Int Med Res 2014;42:224–30 [DOI] [PubMed] [Google Scholar]
- 17. Hohl M, Linz B, Bohm M, Linz D. Obstructive sleep apnea and atrial arrhythmogenesis. Curr Cardiol Rev 2014;10:362–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Bai F, Liu N, Ouyang F, Liu Q. The Warburg effect: a new insight into atrial fibrillation. Clin Chim Acta 2019;499:4–12 [DOI] [PubMed] [Google Scholar]
- 19. Stevenson IH, Roberts-Thomson KC, Kistler PM, Edwards GA, Spence S, Sanders P, Kalman JM. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7:1263–70 [DOI] [PubMed] [Google Scholar]
- 20. Lin YK, Lai MS, Chen YC, Cheng CC, Huang JH, Chen SA, Chen YJ, Lin CI. Hypoxia and reoxygenation modulate the arrhythmogenic activity of the pulmonary vein and atrium. Clin Sci 2012;122:121–32 [DOI] [PubMed] [Google Scholar]
- 21. Lin YK, Lin FZ, Chen YC, Cheng CC, Lin CI, Chen YJ, Chen SA. Oxidative stress on pulmonary vein and left atrium arrhythmogenesis. Circ J 2010;74:1547–56 [DOI] [PubMed] [Google Scholar]
- 22. Yang X, Zhang L, Liu H, Shao Y, Zhang S. Cardiac sympathetic denervation suppresses atrial fibrillation and blood pressure in a chronic intermittent hypoxia rat model of obstructive sleep apnea. J Am Heart Assoc 2019;8:e010254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwasaki YK, Kato T, Xiong F, Shi YF, Naud P, Maguy A, Mizuno K, Tardif JC, Comtois P, Stanley N. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol 2014;64:2013–23 [DOI] [PubMed] [Google Scholar]
- 24. Linz D, Schotten U, Neuberger HR, Bohm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm 2011;8:1436–43 [DOI] [PubMed] [Google Scholar]
- 25. Iwasaki YK, Shi Y, Benito B, Gillis MA, Mizuno K, Tardif JC, Nattel S. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012;9:1409–16 [DOI] [PubMed] [Google Scholar]
- 26. Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 2008;51:802–9 [DOI] [PubMed] [Google Scholar]
- 27. Chimenti C, Russo MA, Carpi A, Frustaci A. Histological substrate of human atrial fibrillation. Biomed Pharmacother 2010;64:177–83 [DOI] [PubMed] [Google Scholar]
- 28. Ramos P, Rubies C, Torres M, Batlle M, Farre R, Brugada J, Montserrat JM, Almendros I, Mont L. Atrial fibrosis in a chronic murine model of obstructive sleep apnea: mechanisms and prevention by mesenchymal stem cells. Respir Res 2014; Apr2815:54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krul SP, Berger WR, Smit NW, van Amersfoorth SC, Driessen AH, van Boven WJ, Fiolet JW, van Ginneken AC, van der Wal AC, de Bakker JM, Coronel R, Groot J. Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation. Circ Arrhythm Electrophysiol 2015;8:288–95 [DOI] [PubMed] [Google Scholar]
- 30. van Oosten EM, Boag AH, Cunningham K, Veinot J, Hamilton A, Petsikas D, Payne D, Hopman WM, Redfearn DP, Song W, Lamothe S, Zhang S, Baranchuk A. The histology of human right atrial tissue in patients with high-risk Obstructive Sleep Apnea and underlying cardiovascular disease: a pilot study. Int J Cardiol Heart Vasc 2015;6:71–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Oliveira FG, Pinto I, Valdigem B, Senra T, Bertolami A. Evaluation of late atrial enhancement by cardiac magnetic resonance imaging in patients with obstructive sleep apnea. Sleep Med 2020;74:204–10 [DOI] [PubMed] [Google Scholar]
- 32. Baranchuk A, Simpson CS, Redfearn DP, Fitzpatrick M. It’s time to wake up! Sleep apnea and cardiac arrhythmias. Europace 2008;10:666–7 [DOI] [PubMed] [Google Scholar]
- 33. Naughton MT, Kee K. Sleep apnoea in heart failure: to treat or not to treat? Respirology 2017;22:217–29 [DOI] [PubMed] [Google Scholar]
- 34. Zhang L, Hou Y, Po SS. Obstructive sleep apnoea and atrial fibrillation. Arrhythm Electrophysiol Rev 2015;4:14–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu Z, Nie L, He B, Yu L, Salim M, Huang B, Cui B, He W, Wu W, Jiang H. Increase in vulnerability of atrial fibrillation in an acute intermittent hypoxia model: importance of autonomic imbalance. Auton Neurosci 2013;177:148–53 [DOI] [PubMed] [Google Scholar]
- 36. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao LQ, Liu SW. Atrial fibrillation in essential hypertension: an issue of concern. J Cardiovasc Med 2014;15:100–6 [DOI] [PubMed] [Google Scholar]
- 38. Torres G, Sanchez-de-la-Torre M, Barbe F. Relationship between OSA and hypertension. Chest 2015;148:824–32 [DOI] [PubMed] [Google Scholar]
- 39. de Abreu-Silva EO, Beltrami-Moreira M. Sleep apnea: an underestimated cause of resistant hypertension. Curr Hypertens Rev 2014;10:27. [DOI] [PubMed] [Google Scholar]
- 40. Xiaokereti J, Guo YK, Dong ZY, Ma M, Lu YM, Li YD, Zhou XH, Zhang L, Tang BP. Enhanced atrial internal-external neural remodeling facilitates atrial fibrillation in the chronic obstructive sleep apnea model. PLoS ONE 2021;16:e0247308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang L, Guo Y, Xiaokereti J, Cao G, Li H, Sun H, Li K, Zhou X, Tang B. Ganglionated plexi ablation suppresses chronic obstructive sleep apnea-related atrial fibrillation by inhibiting cardiac autonomic hyperactivation. Front Physiol 2021;12:640295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo Y, Xiaokereti J, Meng Q, Cao G, Sun H, Zhou X, Zhang L, Tang B. Low-level vagus nerve stimulation reverses obstructive sleep apnea-related atrial fibrillation by ameliorating sympathetic hyperactivity and atrial myocyte injury. Frontiers in Physiology 2021;11:620655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Rusell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008;118:1080–111 [DOI] [PubMed] [Google Scholar]
- 44. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007;49:565–71 [DOI] [PubMed] [Google Scholar]
- 45. Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S, Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006;173:910–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Monahan K, Storfer-Isser A, Mehra R, Shahar E, Mittleman M, Rottman J, Punjabi N, Sanders M, Wuan SF, Resnick H, Redline S. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009;54:1797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Orban M, Bruce CJ, Pressman GS, Leinveber P, Romero-Corral A, Korinek J, Konecny T, Vilarraga H, Kara T, Caples SM, Somers VK. Dynamic changes of left ventricular performance and left atrial volume induced by the Mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am J Cardiol 2008;102:1557–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim SM, Cho KI, Kwon JH, Lee HG, Kim TI. Impact of obstructive sleep apnea on left atrial functional and structural remodeling beyond obesity. J Cardiol 2012;60:475–83 [DOI] [PubMed] [Google Scholar]
- 49. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. Circulation 1994;89:724–30 [DOI] [PubMed] [Google Scholar]
- 50. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455–61 [DOI] [PubMed] [Google Scholar]
- 51. Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem 2004;385:217–21 [DOI] [PubMed] [Google Scholar]
- 52. Jeong EM, Liu M, Sturdy M, Gao G, Varghese ST, Sovari AA, Dudley SC., Jr. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol 2012;52:454–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia, and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 1983;52:490–4 [DOI] [PubMed] [Google Scholar]
- 54. Somers VK. Sleep: a new cardiovascular frontier. N Engl J Med 2005; 353:2070–3 [DOI] [PubMed] [Google Scholar]
- 55. Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman SM, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589–94 [DOI] [PubMed] [Google Scholar]
- 56. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–41 [DOI] [PubMed] [Google Scholar]
- 57. Jongnarangsin K, Chugh A, Good E, Mukerji S, Dey S, Crawford T, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Boonyapisit W, Pelosi F, Jr, Bogun F, Morady F, Oral H. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:668–72 [DOI] [PubMed] [Google Scholar]
- 58. Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110:364–7 [DOI] [PubMed] [Google Scholar]
- 59. Bitter T, Langer C, Vogt J, Lange M, Horstkotte D, Oldenburg O. Sleepdisordered breathing in patients with atrial fibrillation and normal systolic left ventricular function. Dtsch Arztebl Int 2009;106:164–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Levy P, Naughton MT, Tamisier R, Cowie MR, Bradley TD. Sleep apnoea and heart failure. Eur Respir J 2022;59:2101640. [DOI] [PubMed] [Google Scholar]
- 61. Pearse SG, Cowie MR. Sleep-disordered breathing in heart failure. Eur J Heart Fail 2016;18:353–61 [DOI] [PubMed] [Google Scholar]
- 62. Khattak HK, Hayat F, Pamboukian SV, Hahn HS, Schwartz BP, Stein PK. Obstructive sleep apnea in heart failure: review of prevalence, treatment with continuous positive airway pressure, and prognosis. Tex Heart Inst J 2018;45:151–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hegner P, Lebek S, Maier LS, Arzt M, Wagner S. The effect of gender and sex hormones on cardiovascular disease, heart failure, diabetes, and atrial fibrillation in sleep apnea. Front Physiol 2021;12:741896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boriani G, Diemberger I, Pisano ECL, Pieragnoli P, Locatelli A, Capucci A, Talarico A, zecchin M, rapacciuolo A, Piacenti M, Indolfi C, Arias MA, Checchinato C, La Rovere MT, Sinagra G, Emdin M, Ricci RP, D’Onofrio A. Association between implantable defibrillator-detected sleep apnea and atrial fibrillation: the DASAP-HF study. J Cardiovasc Electrophysiol 2022;33:1472–9 [DOI] [PubMed] [Google Scholar]
- 65. Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011;108:47–51 [DOI] [PubMed] [Google Scholar]
- 66. Linz B, Norup Hertel J, Hendriks J, Saljic A, Dobrev D, Baumert M, Jespersen T, Linz D. Sleep apnea and atrial fibrillation: challenges in clinical and translational research. Expert Rev Cardiovasc Ther 2022;20:101–9 [DOI] [PubMed] [Google Scholar]
- 67. Traaen GM, Aakerøy L, Hunt TE, Øverland B, Bendz C, Sande LØ, Aakhus S, Fagerland MW, Steinshamn S, Anfinsen OG, Massey RJ, Broch K, Ueland T, Akre H, Loennechen JP, Gullestad L. Effect of continuous positive airway pressure on arrhythmia in atrial fibrillation and sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2021;204:573–82 [DOI] [PubMed] [Google Scholar]
- 68. Li L, Wang ZW, Li J, Ge X, Guo LZ, Wang Y, Guo WH, Jiang CX, Ma CS. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace 2014;16:1309–14 [DOI] [PubMed] [Google Scholar]
- 69. Shukla A, Aizer A, Holmes D, Fowler S, Park DS, Bernstein S, Bernstein N, Chinitz L. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electrophysiol 2015;1:41–51 [DOI] [PubMed] [Google Scholar]
- 70. Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP, Michaud GF, van der Geest R, Blankstein R, Steigner M, John RM, Jerosch-Herold M, Malhotra A, Kwong RY. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013;2:e000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sauer WH, McKernan ML, Lin D, Gerstenfeld EP, Callans DJ, Marchlinski FE. Clinical predictors and outcomes associated with acute return of pulmonary vein conduction during pulmonary vein isolation for treatment of atrial fibrillation. Heart Rhythm 2006;3:1024–8 [DOI] [PubMed] [Google Scholar]
- 72. Bavishi AA, Kaplan RM, Peigh G, Diaz CL, Baman JR, Trivedi A, Wasserlauf J, Shen MJ, Sattayaprasert P, Chicos AB, Kim S, Verma N, Arora R, Lin A, Knight BP, Passman RS. Patient characteristics as predictors of recurrence of atrial fibrillation following cryoballoon ablation. Pacing Clin Electrophysiol 2019;42:694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, Zimetbaum PJ, Buxton AE, Josephson ME, Anter E. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62:300–5 [DOI] [PubMed] [Google Scholar]
- 74. Varga PC, Rosianu HS, Vesa ÅžC, Hancu BGD, Beyer R, Pop CM. The impact of continuous positive airway pressure on cardiac arrhythmias in patients with sleep apnea. J Res Med Sci 2020;25:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang Y, Ning Y, Wen W, Jia Y, Chen X, Huang M, Sara JD, Qin Y, Fang F, Zhang H, Du Y, Li L, Jiao X, Yang Y, Han X, Zhang M, Wei Y. CPAP is associated with decreased risk of AF recurrence in patients with OSA, especially those younger and slimmer: a meta-analysis. J Interv Card Electrophysiol 2020;58:369–79 [DOI] [PubMed] [Google Scholar]
- 76. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69 [DOI] [PubMed] [Google Scholar]
- 77. Desteghe L, Hendriks JML, McEvoy RD, Chai-Coetzer CL, Dendale P, Sanders P, Heidbuchel H, Linz D. The why, when, and how to test for obstructive sleep apnea in patients with atrial fibrillation. Clin Res Cardiol 2018;107:617–31 [DOI] [PubMed] [Google Scholar]
- 78. Delesie M, Knaepen L, Hendrickx B, Huygen L, Verbraecken J, Weytjens K, Dendale P, Heidbuchel H, Desteghe L. The value of screening questionnaires/scoring scales for obstructive sleep apnoea in patients with atrial fibrillation. Arch Cardiovasc Dis 2021;114:737–47 [DOI] [PubMed] [Google Scholar]
- 79. Desteghe L, Hendriks JML, Heidbuchel H, Potpara TS, Lee GA, Linz D. Obstructive sleep apnoea testing and management in atrial fibrillation patients: a joint survey by the European Heart Rhythm Association (EHRA) and the Association of Cardiovascular Nurses and Allied Professions (ACNAP). Europace 2021;23:1677–84 [DOI] [PubMed] [Google Scholar]
- 80. Starkey SY, Jonasson DR, Alexis S, Su S, Johal R, Sweeney P, Brasher PMA, Fleetham J, Ayas N, Orenstein T, Ahmed IH. Screening for obstructive sleep apnea in an atrial fibrillation population: what’s the best test. CJC Open 2021;3:442–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sun L, Yan S, Wang X, Zhao S, Li H, Wang Y, Lu S, Dong X, Zhao J, Yu S, Li M, Li Y. Metoprolol prevents chronic obstructive sleep apnea-induced atrial fibrillation by inhibiting structural, sympathetic nervous and metabolic remodeling of the atria. Sci Rep 2017;7:14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dai H, Yuan Y, Yin S, Zhang Y, Han Y, Sun L, Li T, Xu J, Sheng L, Gong Y, Li Y. Metoprolol inhibits profibrotic remodeling of epicardial adipose tissue in a canine model of chronic obstructive sleep apnea. J Am Heart Assoc 2019; Feb58:e011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Anter E, Di Biase L, Contreras-Valdes FM, Gianni C, Mohanty S, Tschabrunn CM, Viles-Gonzalez JF, Leshem E, Buxton AE, Kulbak G, Halaby RN, Zimetbaum PJ, Waks JW, Thomas RJ, Natale A, Josephson ME. Atrial substrate and triggers of paroxysmal atrial fibrillation in patients with obstructive sleep apnea. Circ Arrhythm Electrophysiol 2017;10:e005407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, Mehra R, Bozkurt B, Ndumele CE, Somers V. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021;144:e56–67 [DOI] [PubMed] [Google Scholar]


