Abstract
Objective:
Naltrexone is an effective treatment for heavy drinking among young adults, and laboratory-based studies have shown that naltrexone dampens the subjective response to alcohol and craving. However, few studies have tested naltrexone’s dynamic, within-person effects on subjective response and craving among young adults in natural drinking environments.
Methods:
Using daily diary data from a randomized, placebo-controlled study of naltrexone efficacy in young adults, we examined the between-person effects of treatment condition (i.e., naltrexone vs. placebo) and medication dosage (daily, targeted, daily+targeted) on the subjective response to alcohol and craving on drinking days. Multilevel mediation models predicted subjective response and craving from treatment condition (between-person) and medication dosage (within-person), accounting for drinking levels. All effects were disaggregated within- and between-person.
Results:
At the between-person level, naltrexone directly blunted intense subjective effects (i.e., “impaired”, “drunk”), and indirectly blunted subjective effects through reduced drinking. Naltrexone was not associated with craving. Between-person effects were not significant after alpha correction, but their effect sizes (bs=.14-.17) exceeded the smallest effect size of interest. At the within-person level, taking 2 (vs. 1) pills was associated with heavier drinking, and taking 1 (vs. 0) pills was associated with lighter drinking, and lighter drinking was associated with a lower subjective response and craving. Treatment condition did not moderate the within-person effects of dosing on outcomes.
Conclusions:
The findings suggest that the direct between-person effect of naltrexone was largest on intense subjective responses, blunting feeling “drunk” and “impaired”. Future research using momentary (rather than daily) assessments could confirm and extend these findings.
Keywords: Craving, Naltrexone, Subjective Response, Heavy Drinking
Introduction
Heavy drinking (4+ drinks for women, 5+ for men) is a serious public health problem, as it has myriad negative consequences, including risk of Alcohol Use Disorder (AUD; NIAAA, 2018; Perkins, 2002; Waddell et al., 2021; Waddell, 2021; Wechsler et al., 2004). In response to growing rates of heavy drinking (Grant et al., 2017), several studies have found that motivational and skills-based interventions reduce heavy drinking, particularly in young adults (Marlatt et al., 1998; Turrisi et al., 2009). However, these interventions have small effects on typical drinking quantity and are least effective for the heaviest drinkers (Carey et al., 2007; Huh et al., 2015). Naltrexone, an opioid antagonist, is thought to blunt the rewarding effects of alcohol and reduce craving (Ray et al., 2019), and may be efficacious in augmenting motivational/skills-based interventions. However, research on naltrexone for prevention/early intervention is limited, and little is known about its underlying dynamics in heavy drinking young adults. In addition, most studies investigating mechanisms of change have not employed ecological methods, nor have they differentiated between-person from within-person effects. Therefore, the current study tested the effects of naltrexone on within- and between-person changes in subjective response to alcohol and craving in a randomized, placebo-controlled trial of young adults utilizing daily diaries during the course of the intervention.
Prior studies have shown that naltrexone reduces drinking and relapse rates in clinical settings, with small to medium effect sizes (Anton et al., 2006; Maisel et al., 2013; O’Malley et al., 1992; Srisurapanont & Jarusuraisin, 2005; Volpicelli et al., 1992). However, naltrexone may also be useful in treating heavy-drinking young adults who are not interested in pursuing a goal of abstinence. Targeting this subgroup may reach more heavy drinkers, reducing their risk of developing an AUD or facilitating their recovery from AUD. Young adults often report low motivation to change or abstain from drinking (Dimeff et al., 1999), but may be more open to the relatively low-burden commitment of taking naltrexone (Epler et al., 2009). Several recent studies have shown that naltrexone reduces drinks per drinking day, BAC levels, and abstinent days in non-treatment seeking samples (Bold et al., 2017; Leeman et al., 2008; Miranda et al., 2014; O’Malley et al., 2015; Tidey et al., 2008), and several of these effects are maintained over time (DeMartini et al., 2016). Thus, promoting naltrexone treatment in young adults may reduce heavy drinking in a broader group of drinkers, and introduce little burden to their daily lives.
The efficacy of naltrexone in clinical and non-clinical samples has spurred research on mechanisms of change, primarily using laboratory-based studies. Several studies have shown that naltrexone reduces alcohol craving (Ray et al., 2019; Anton et al., 2004; Drobes et al., 2004; Hendershot et al., 2017; O’Malley et al., 2002), a proximal predictor of drinking behavior (Fazzino et al., 2014). In addition, several studies have shown that naltrexone blunts the subjective effects of alcohol (Ray et al., 2019; Drobes et al., 2004; McCaul et al., 2000; Ray et al., 2007; Setiawan et al., 2011), particularly those that are rewarding and highly arousing (Ray et al., 2019), which are also a proximal predictor of drinking behavior (King et al., 2011; 2014; Morean et al., 2013; Waddell et al., 2020). Naltrexone may simultaneously enhance the sedative effects of alcohol, though meta-analytic findings show this effect is only seen in light/novice drinkers (Ray et al., 2019).
Although prior studies have examined effects of naltrexone on subjective response, most use laboratory-based assessments, reducing generalizability to naturalistic drinking environments. Some studies have begun to explore naltrexone effects on craving at the daily level (e.g., Mann et al., 2018; Witkiewitz et al., 2019), however we are aware of only two studies that have tested the effects of naltrexone on subjective response using ecological methods. In a sample of adolescents, Miranda et al. (2014). found that naltrexone was associated with less stimulant and greater sedative subjective responses. This study also showed that naltrexone blunted craving at higher estimated breath alcohol concentrations (eBACs). Tidey et al. (2008) found that, in adult (Mage=29.01) heavy drinkers, naltrexone was associated with less urge to drink and with blunted stimulant alcohol effects (in women) using 3 weeks of daily EMA data. Given particularly high rates of heavy drinking and AUDs (Grant et al., 2017) in young adults (e.g., aged 18-25), it is important to replicate and extend the results of prior studies with adolescents and older adults in samples of heavy drinking young adults. In addition, measuring relations among naltrexone, subjective response, and craving over a longer period of time is important to capture effects across multiple drinking episodes during treatment.
Therefore, the current study used data from a randomized controlled trial (O’Malley et al., 2015) that tested the efficacy of daily + targeted naltrexone vs. placebo in reducing drinking in young adults. In this study, participants were asked to take one pill (25 mg of either naltrexone or placebo) each day of the study and another pill (25 mg of the same substance) in anticipation of drinking. Participants were asked to fill out daily diaries during the study to report on the past-day number of pills taken (0, 1, or 2), amount of alcohol consumed, subjective response when drinking, and alcohol craving. We tested the effects of naltrexone (vs. placebo) on alcohol craving and subjective response, accounting for levels of drinking during the 8-week treatment period. Controlling for the level of drinking is important because naltrexone could have direct effects on subjective response and craving or indirectly reduce alcohol response by reducing drinking. At the between-person level (averaged across drinking days), we hypothesized that naltrexone treatment (compared to placebo) would be associated with both dampened subjective response and craving on drinking days. We anticipated naltrexone to have the largest effect on rewarding/stimulant subjective response. Participants were asked how “buzzed”, “drunk”, and “impaired” they felt when drinking, and we hypothesized the strongest effects for the “buzzed” subjective response item. Although “buzzed” does not appear as an item on established measures of subjective response, some research suggests that “buzzed” maps onto the same factor as other positively-reinforcing/stimulating effects such as feeling sociable and talkative (Fleming et al., 2016). Thus, of the items measured, we believe “buzzed” best represents rewarding/positively reinforcing effects. Because past research suggests that items measuring impairment (e.g., “drunk”, “impaired”) load onto a sedative alcohol effects factor (Morean et al., 2013), and naltrexone’s effects on sedation are unclear in heavy drinking/clinical populations (Ray et al., 2019), we considered the examination of these effects exploratory. We also anticipated indirect effects of naltrexone on subjective response/craving through reduced drinking as individuals who drink less are likely to experience weaker overall effects; however, our main hypotheses concerned the direct effects of naltrexone on the subjective response and craving. Finally, we hypothesized that treatment condition (at the between-person level) would moderate the effect of medication dosage (at the within-person level) on within-day drinking. Because research has shown that naltrexone may be most efficacious on a targeted basis (i.e., when taken before a drinking episode; Kranzler et al., 2014), we hypothesized that the effect of medication dosage on day-to-day changes in subjective response would be stronger in the naltrexone condition (compared to placebo). This was based on the supposition that the blunting effect of naltrexone on subjective response would be more prominent on days when more medication was taken.
Materials and Methods
Participants
This study involved secondary analysis of data from an 8-week randomized controlled trial of naltrexone’s efficacy in reducing heavy drinking (O’Malley et al., 2015). The original study randomized 140 young adult drinkers (79% current AUD) to receive either naltrexone (25 mg daily+25 mg targeted) or placebo (placebo daily+placebo targeted) medication. A total of 115 (47% naltrexone) participants provided data between weeks 3-8 (see data analytic plan for details). Eligible participants were aged 18-25, endorsed 4+ past-month heavy drinking days, were fluent in English, and not pregnant/nursing. Participants were excluded if they endorsed serious psychiatric disorders or past-month DSM-IV drug dependence other than nicotine. The current sample (See Tables 1–3) had a mean age of 21.24 (SD = 2.16) and was predominately male (68%).
Table 1:
Descriptive Statistics Across Treatment Group
| Naltrexone | Placebo | |
|---|---|---|
| 1. Treatment Condition | 47% (N=54) | 53% (N=61) |
| 2. Family History | 41% Positive | 36% Positive |
| 3. Sex | 70% Male | 67% Male |
| 4. Medication Dosage | 73% Both Pills | 63% Both Pills |
| 5. Weekend (day) | 56% Weekends | 61% Weekends |
| 6. Drinking Quantity | 4.46 (2.93) | 5.74 (3.85) |
| 7. Buzzed SR | 3.94 (3.09) | 4.38 (3.22) |
| 8. Drunk SR | 2.32 (2.76) | 3.29 (3.26) |
| 9. Impaired SR | 1.78 (2.51) | 2.72 (3.07) |
| 10. Craving | 5.93 (1.54) | 5.72 (1.58) |
Note: All between-person descriptive statistics are aggregated at the person level (N=115), whereas all within-person descriptives are aggregated at the daily level (N=1543). SR = subjective response. Variables 4-10 were measured during the dialy diary protocol.
Table 3:
Descriptive Statistics and Bivariate Correlations
| % or Mean (SD) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Treatment | 47% Naltrexone | .10** | .01 | .19* | −.10 | −.28** | −.15 | −.30** | −.30** | −.02 |
| 2. Family History | 38% Positive | -- | .12 | .08 | −.13 | .09 | .07 | .11 | .17† | .10 |
| 3. Sex | 69% Male | -- | .07 | −.03 | −.23* | .09 | −.10 | .01 | .13 | |
| 4. Medication Dosage | 68% Both Pills | -- | .09** | .05* | .13** | .06* | .04 | .12** | ||
| 5. Weekend (day) | 58% Weekends | -- | .22** | .19** | .18** | .18** | .09** | |||
| 6. Drinks Per Day | 5.10 (3.48) | -- | .69** | .74** | .66** | .34** | ||||
| 7. Buzzed SR | 4.16 (3.16) | -- | .83** | .70** | .41** | |||||
| 8. Drunk SR | 2.8 (3.05) | -- | .87** | .37** | ||||||
| 9.. Impaired SR | 2.25 (2.84) | -- | .34** | |||||||
| 10. Craving | 5.83 (1.56) | -- |
Note: Treatment is coded as 1=Naltrexone, 0=Placebo; Family History is coded as 1=positive, 0=negative; Sex is coded as 1=female, 0=male; Medication Dosage is coded as 0=no pill, 1=targeted or daily, 2=targeted and daily; Weekend is coded as 1=weekend, 0=weekday; SR is measured on a scale from 0-10; Craving is measured on a scale from 1 to 9. All within-person variables were group averaged when correlating with other between-level variables.
p < .10
p < .05
p < .01
Procedures
Study procedures were approved by the Institutional Review Board at Yale University. Participants were recruited throughout the surrounding community via fliers and online advertisements, screened via phone, and then brought into the clinic for informed consent and in-person evaluation. Eligible participants were randomized to receive either naltrexone or placebo. Individuals were instructed to take a daily dose of the assigned study medication (25 mg active naltrexone or placebo), and at the second week, were instructed to also take a targeted dose (25 mg active or placebo) in anticipation of drinking or high-risk situations. Each morning, as part of a web-based daily diary, participants were asked to report their previous-day medication usage and drinking quantity, and to rate their alcohol craving and subjective response. Participants could receive up to $415 for attending all scheduled appointments and completing all daily assessments.
Measures
Demographics
Participant’s biological sex was assessed at baseline.
Family History.
Family history of AUD (0=no family history, 1=family history) was assessed via the Family History Assessment Module (Rice et al., 1995).
Daily Measures
All daily measures were assessed the next morning, during which participants rated variables from the past day/evening.
Alcohol Craving.
Craving was assessed via 3 items from the Alcohol Urge Questionnaire (Bohn et al., 1995). These items (i.e., “The idea of drinking was appealing”, “I felt like I could have really used a drink”, “I really didn’t feel like drinking [reverse coded]”) were averaged to create a composite craving measure (α = .69). Responses ranged from (1) Strongly Disagree to (9) Strongly Agree.
Subjective Response.
Subjective response was assessed via three items asking how “buzzed”, “drunk”, and “impaired” the participant felt the past evening on a scale of 0-10.
Drinking Quantity.
Participants were asked “how many standard drinks did you have yesterday? A standard drink was defined as a 12-ounce beer, a 5-ounce glass of wine, or 1 ½ ounces of liquor”.
Medication Dosage.
Participants were asked whether they took a daily and/or targeted dose of medication the past day. We created two contrast codes comparing no pills to 1 pill (none vs. daily), and 2 pills to 1 pill (daily + targeted vs. daily).
Data Analysis
All variable distributions were examined for outliers. Variables with values higher than 3 standard deviations (SDs) above the mean (1% of all cases) were replaced with the next highest value outside the distribution (Tabachnick & Fidell, 2001). We corrected for any further non-normality by using Robust Maximum Likelihood (MLR) estimation in Mplus Version 8.5. Due to the focus on within-person effects of daily/targeted naltrexone, and because participants were asked to start taking daily naltrexone in week one but were not instructed to begin taking targeted naltrexone until week two, we excluded data from the first two weeks of the study. This was done to give participants ample time to acclimate to the medication regimen. Further, due to the focus on subject response and in line with prior EMA naltrexone studies (e.g., Miranda et al., 2014), we restricted analyses to drinking days (non-drinking days were casewise deleted).
A series of multilevel mediation models were tested with study day (Level 1) nested within person (Level 2). For each multilevel mediation model, all paths were specified within one mediation model in Mplus Version 8.5. First, we tested whether treatment condition (naltrexone vs. placebo) had a direct effect on subjective response and craving (separately), controlling for the effect of drinking quantity. We believe that these analyses are important as they represent a between-person test of naltrexone’s effects on subjective response using ecologically valid, daily measures of subjective response in the individual’s life. In addition, accounting for drinking quantity at the day level allows for a test both of naltrexone’s direct effects on subjective response and its indirect effects that operate through reduced consumption. Although Miranda et al. (2014) accounted for eBAC in analyses, Tidey et al. (2008) did not.
Each subjective response item (buzzed, drunk, impaired) was entered into a separate model. Because treatment condition was a fixed level 2 effect (i.e., no variability within person), the effects of treatment condition on subjective response and craving were modeled as aggregate effects at the between-person level (Level 2). Drinking quantity was specified as a predictor of subjective response and craving at both the between- (average drinking quantity on drinking days) and within-person (drinks on a particular day) levels. Although it is theoretically appealing to model drinking quantity as the ultimate outcome at both levels, accounting for drinking quantity when predicting subjective response and craving is essential. Subjective response is contingent on the amount of alcohol consumed, and prior research also suggests that drinking may affect ratings of alcohol craving (e.g., Trela et al., 2018). Thus, treatment condition was specified as a predictor of subjective response, drinking quantity, and craving, and drinking quantity was specified as a predictor of subjective response and craving at the between-person level. At the within-person level, we modeled effects of medication dose on drinking quantity, subjective response, and craving, and the effects of drinking quantity on subjective response and craving. We included whether it was a weekday/weekend as a within-person covariate, as weekends are associated with heavier drinking (Kuntsche & Cooper, 2010); we also included day of treatment (with the 3-week mark coded as day 1) to identify changes over time in the effects of dose on the outcomes. Between-person covariates included family history of AUD, percent drinking days that medication was taken, and sex (only for drinking quantity). Family history of AUD and sex are strong correlates of heavier drinking, and we deemed percent drinking days that medication was taken an important covariate to account for variance due to participant dosing variability. We also covaried the number of drinking days available for each participant across the study period to account for non-compliance with daily diaries. Indirect effects were tested using the product of coefficients method (Tofighi & MacKinnon, 2011).
Next, we tested cross-level interactions via a multiple group multilevel mediation model to assess whether a higher daily medication dosage was associated with less subjective response and/or craving in the naltrexone condition (compared to placebo). This effectively tests whether the effect of medication dosage on subjective response and craving at the daily level depends on treatment condition (i.e., whether higher medication dosage predicts lower subjective response only for those in the naltrexone condition). All paths from previous models (except for treatment condition as a predictor variable) remained the same. Treatment group differences were tested by comparing scaled chi-square difference estimates from a model allowing paths from medication dose to subjective response (separate models) and craving to freely vary to a model constraining these paths to be equal. In the presence of a significant chi-square difference test, single degree of freedom testing was used to determine which paths were moderated by treatment condition.
All multilevel models used group/person-mean (Level 1) and grand-mean (Level 2) centering for all continuous predictors. For each within-person effect, we refer to deviation from a person’s mean score for each continuous predictor. All models included random intercepts for each mediator and outcome, which is the default in MPlus Version 8.51. Residual variances were specified at level 2, in line with recommendations from Preacher et al. (2010). Model fit was assessed using standard guidelines (Hu & Bentler, 1999), where adequate fit is reflected by CFI and TLI values close to 0.95, RMSEA values close to 0.06, and SRMR values close to 0.08.
For the current study, we specified b=.13 as the post hoc Smallest Effect Size of Interest (SESOI). In a review of naltrexone efficacy, Ray et al. (2019) found small effects of naltrexone on craving (hedges g=.20) and stimulant subjective response (hedges g=.23), which correspond to effect sizes of b=.13 and b=.15, respectively (Brydges, 2019). Thus, we used the lower of the two effect sizes (i.e., b=.13) as our SESOI. The current study was designed to detect a small-to-moderate between-person effect of naltrexone on reduced drinking; power calculations from the initial grant submission suggested that the current sample had 80% power to detect a between-person effect of b=.26.
Results
Descriptive Statistics
Daily drinking showed significant outliers (16 cases; 1% of total cases), and all values outside 3 SDs from the mean were winsorized39. Participants (N=115) recorded 1,543 drinking days, of which 775 were nested within the naltrexone and 768 within the placebo condition. On average, participants took no medication on 5.5% of drinking days recorded, 1 pill on 26.9% of days, and 2 pills on 67.6% of days. At the between-person level (e.g., averaged across all drinking days), naltrexone treatment was significantly correlated with all subjective response variables and drinking, such that participants receiving naltrexone reported lower average levels of subjective response and drank less on average across drinking days. This highlights the importance of accounting for direct effects of naltrexone on daily drinking when examining its effects on subjective response and craving. A higher average medication dosage (either naltrexone or placebo) was correlated with stronger subjective response, stronger craving, and more drinking averaged across drinking days. For a full list of descriptive statistics and correlations between primary study variables during the daily diary protocol, see Table 3. Intraclass correlations showed substantial within-person variability in drinking quantity (ICC = .36), subjective response (ICC = .32), and craving (ICC = .36), supporting the use of multilevel models. In addition, there was substantial within-person variability in daily medication dosage (ICC = .33). Descriptive statistics for all model outcomes (i.e., drinking quantity, craving, subjective effects) by treatment condition are shown in Table 1 and descriptive statistics by treatment condition and medication dosage are shown in Table 2.
Table 2:
Descriptive Statistics Across Treatment Group and Medication Dose
| Naltrexone | Placebo | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 Pills | 1 Pill | 2 Pills | 0 Pills | 1 Pill | 2 Pills | |
| Drinking Quantity | 3.97 (2.29) | 3.59 (2.56) | 4.8 (3.14) | 6.90 (5.40) | 5.50 (1.66) | 5.91 (3.67) |
| Craving | 5.5 (1.49) | 5.47 (1.81) | 6.11 (1.41) | 5.78 (1.32) | 5.51 (1.66) | 5.82 (1.56) |
| Buzzed SR | 2.81 (2.39) | 2.88 (2.95) | 4.34 (3.08) | 4.53 (3.27) | 3.79 (3.16) | 4.65 (3.22) |
| Drunk SR | 1.86 (2.26) | 1.69 (2.49) | 2.54 (2.83) | 3.47 (3.32) | 2.94 (3.23) | 3.44 (3.25) |
| Impaired SR | 1.47 (1.78) | 1.28 (2.27) | 1.96 (2.60) | 2.71 (2.80) | 2.64 (3.19) | 2.77 (3.05) |
Note. SR = subjective response.
Model fit was adequate for all models, as shown in Table 4. All standardized model parameters for craving are shown in Table 5 and standardized parameters for all subjective response models are shown in Table 6. Parameters reported in the text are unstandardized effects.
Table 4:
Fit Indices for All Models
| Chi Square Test | RMSEA | CFI | SRMR | |
|---|---|---|---|---|
| Craving Model | 4.39, p = .036 | .047 | .992 | .001 (W) .048 (B) |
| SR – Buzzed | .53, p = .466 | .00 | 1.00 | .000 (W) .016 (B) |
| SR – Drunk | .63, p = .427 | .00 | 1.00 | .000 (W) .014 (B) |
| SR – Impaired | 1.87, p = .172 | .024 | .999 | .000 (W) .027 (B) |
| SR - Intense | 37.94, p < .001 | .037 | .985 | .010 (W) .032 (B) |
Note: SR = subjective response.
Table 5:
Craving Model Standardized Parameters
| b | SE | p-value | 95% CI | |
|---|---|---|---|---|
| Within-Person | ||||
| Craving/SR | ||||
| Drink Quant | .45 | .03 | < .001 | (.40, .51) |
| Weekend | .01 | .03 | .71 | (−.04, .06) |
| Day of Study | −.01 | .03 | .98 | (−.06, .06) |
| 1 vs. 0 | .01 | .04 | .85 | (−.07, .08) |
| 2 vs. 1 | .06 | .04 | .15 | (−.02, .14) |
| Drink Quant | ||||
| Weekend | .18 | .03 | < .001 | (.13, .23) |
| Day of Study | −.07 | .02 | .004 | (−.11, −.02) |
| 1 vs. 0 | −.06 | .03 | .013 | (−.11, −.01) |
| 2 vs. 1 | .14 | .03 | < .001 | (.09, .20) |
| Between-Person | ||||
| Craving/SR | ||||
| Drink Quant | .20 | .10 | .051 | (−.001, .41) |
| Fam History | .08 | .09 | .41 | (−.11, .26) |
| Days Completed | −.10 | .11 | .37 | (−.31, .12) |
| Percent Med | −.01 | .08 | .95 | (−.16, .15) |
| Treatment | .05 | .10 | .65 | (−.15, .25) |
| Drink Quant | ||||
| Sex | −.25 | .08 | .001 | (−.40, −.11) |
| Family History | .13 | .09 | .13 | (−.04, .30) |
| Days Completed | −.01 | .08 | .91 | (−.16, .14) |
| Percent Med | −.13 | .09 | .15 | (−.30, .05) |
| Treatment | −.28 | .08 | < .001 | (−.43, −.12) |
Note. Treatment is coded such that 1=Naltrexone vs. 0=Placebo; Family History is coded as 1=positive, 0=negative; Sex is coded as 1=female, 0=male; Weekend is coded as 1=weekend, 0=weekday; Days Completed = Total Number of Diary Days Completed; Percent Med = percentage of days when medication was taken; Day of Study = day of treatment, with beginning of week two coded as 1.
Table 6:
Subjective Response Models Standardized Parameters
| “Buzzed” Model | “Drunk” Model | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| b | SE | p-value | 95% CI | b | SE | p-value | 95% CI | |
| Within-Person | ||||||||
| SR | ||||||||
| Drink Quant | .72 | .02 | < .001 | (.69, .76) | .74 | .02 | < .001 | (.65, .74) |
| Weekend | .03 | .02 | .12 | (−.01, .07) | .02 | .02 | .11 | (−.01 .08) |
| Day of Study | −.01 | .02 | .68 | (−.06, .04) | −.02 | .03 | .42 | (−.07, .03) |
| 1 vs. 0 | .01 | .02 | .55 | (−.03, .05) | .02 | .03 | .41 | (−.03, .07) |
| 2 vs. 1 | .08 | .03 | .004 | (.03, .13) | .03 | .03 | .91 | (−.06, .05) |
| Between-Person | ||||||||
| SR | ||||||||
| Drink Quant | .60 | .07 | < .001 | (.47, .73) | .69 | .08 | < .001 | (.38, .69) |
| Fam History | .03 | .09 | .75 | (−.14, .19) | .05 | .08 | .083 | (−.02, .29) |
| Days Completed | −.03 | .10 | .74 | (−.23, .17) | −.12 | .09 | .11 | (−.31, .03) |
| Percent Med | −.01 | .06 | .99 | (−.13, .12) | .03 | .09 | .86 | (−.16, .19) |
| Treatment | −.01 | .09 | .97 | (−.17, .16) | −.14 | .08 | .027 | (−.33, −.02) |
| “Impaired” Model | Intense SR Model | |||||||
|
| ||||||||
| b | SE | p-value | 95% CI | b | SE | p-value | 95% CI | |
|
| ||||||||
| Within-Person | ||||||||
| SR | ||||||||
| Drink Quant | .69 | .02 | < .001 | (.65, .74) | .62 | .03 | < .001 | (.56, .67) |
| Weekend | .04 | .02 | .11 | (−.01 .08) | .08 | .03 | .004 | (.03, .14) |
| Day of Study | −.02 | .03 | .42 | (−.07, .03) | −.01 | .03 | .82 | (−.05, .04) |
| 1 vs. 0 | .02 | .03 | .41 | (−.03, .07) | .02 | .03 | .41 | (−.03, .08) |
| 2 vs. 1 | −.01 | .03 | .91 | (−.06, .05) | −.04 | .05 | .41 | (−.14, .06) |
| Between-Person | ||||||||
| SR | ||||||||
| Drink Quant | .54 | .08 | < .001 | (.38, .69) | .62 | .07 | < .001 | (.50, .75) |
| Fam History | .14 | .08 | .083 | (−.02, .29) | .10 | .07 | .13 | (−.05, .24) |
| Days Completed | −.14 | .09 | .11 | (−.31, .03) | −.14 | .10 | .15 | (−.32, .05) |
| Percent Med | .02 | .09 | .86 | (−.16, .19) | .04 | .06 | .47 | (−.07, .16) |
| Treatment | −.18 | .08 | .027 | (−.33, −.02) | −.16 | .07 | .029 | (−.30, −.02) |
Note. Treatment is coded such that 1=Naltrexone and 0=Placebo; Family History is coded as 1=positive, 0=negative; Sex is coded as 1=female, 0=male; Weekend is coded as 1=weekend, 0=weekday; Days Completed = Total Number of Diary Days Completed; Percent Med = percentage of days when medication was taken; Day of Study = day of treatment, with beginning of week two coded as 1; Drinking quantity estimates at the between and within-person levels were identical to those in Table 5 and are not shown here.
Craving Model
At the between-person level, naltrexone (vs. placebo) was associated with lighter drinking (b = −1.27, SE = .39, p < .001, 95% CI = [−2.04, −.50]) but not with craving. Men drank more heavily (b = −1.25, SE = .40, p = .001, 95% CI = [−2.04, −.46]) and heavier drinking was non-significantly associated with higher craving (b = .09, SE = .04, p = .051, 95% CI = [.00, .17]). Naltrexone treatment was non-significantly associated with dampened craving indirectly through an effect on drinking quantity (see Table 7 for all indirect effects).
Table 7:
Indirect Effects from All Models
| Beta | SE | 95% CI | |
|---|---|---|---|
| Between-Person | |||
| Treatment – Drinking – Craving | −.11 | .07 | (−.24, .03) |
| Treatment – Drinking – Buzzed | −.64 | .21 | (−1.04, −.24) |
| Treatment – Drinking – Drunk | −.70 | .22 | (−1.13, −.27) |
| Treatment – Drinking – Impaired | −.54 | .19 | (−.91, −.17) |
| Treatment – Drinking – Intense SR | −.48 | .16 | (−.80, −.16) |
| Within-Person | |||
| 2 (vs. 1) Pills – Drinking – Craving | .17 | .04 | (.10, .25) |
| 1 (vs 0) Pills – Drinking – Craving | −.16 | .06 | (−.28, −.04) |
| 2 (vs 1) Pills – Drinking – Buzzed | .56 | .12 | (.34, .79) |
| 1 (vs 0) Pills – Drinking – Buzzed | −.51 | .21 | (−.92, −.11) |
| 2 (vs 1) Pills – Drinking – Drunk | .57 | .12 | (.34, .80) |
| 1 (vs 0) Pills – Drinking – Drunk | −.51 | .21 | (−.92, −.10) |
| 2 (vs 1) Pills – Drinking – Impaired | .48 | .10 | (.28, .68) |
| 1 (vs 0) Pills – Drinking – Impaired | −.43 | .18 | (−.79, −.08) |
| 2 (vs 1) Pills – Drinking – Intense SR | .24 | .05 | (.14, .35) |
| 1 (vs 0) Pills – Drinking – Intense SR | −.22 | .09 | (−.40, −.04) |
Note: All indirect effects were tested using the product of coefficients method. Treatment is coded as 1=Naltrexone, 0=Placebo. SR = subjective response.
At the within-person level, taking 1 (vs. 0) pill was associated with lower than average drinking quantity (b = −.75, SE = .31, p = .013, 95% CI = [−.1.36, −.14]), and taking 2 (vs. 1) pills was associated with higher than average drinking quantity (b = .82, SE = .17, p < .001, 95% CI = [.49, 1.15]). Weekend days were associated with heavier drinking (b = .96, SE = .15, p < .001, 95% CI = [.66, 1.26]), and days later in treatment were associated with lighter drinking (b = −.02, SE = .01, p = .004, 95% CI = [−.03, −.01]). Medication dose was not associated with craving, but heavier drinking was strongly associated with craving (b = .21, SE = .02, p < .001, 95% CI = [.17, .25]). There was also a significant indirect effect of medication dose on craving through drinking, such that taking 1 (vs. 0) pill was associated with weaker craving through lighter drinking, and taking 2 (vs. 1) pills was associated with stronger craving through heavier drinking (Figure 1).2
Figure 1: Craving Model.
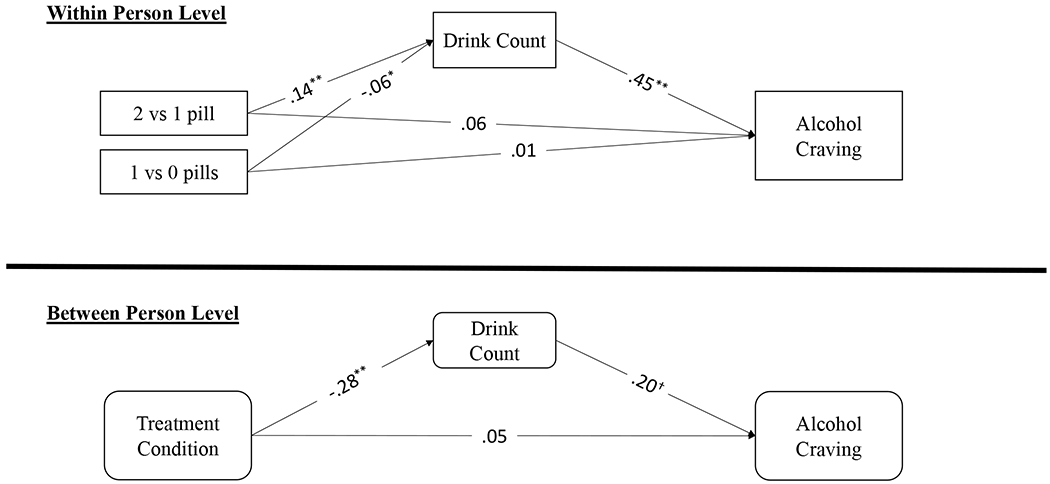
Note: All parameters are standardized estimates; Treatment is coded as 1=Naltrexone, 0=Placebo. At the within-person level, taking 1 (vs. 0) pills was associated with less drinking whereas taking 2 (vs. 1) pills was associated heavier drinking. There were significant indirect effects of medication dose on craving through drinking, such that taking 1 (vs. 0) pills was associated with weaker craving through lighter drinking and taking 2 (vs. 1) pills was associated with stronger craving through heavier drinking. ** P < .01, * P < .05, † P < .10
Subjective Response Models: Buzzed, Drunk, Impaired
At the between-person level, naltrexone (vs. placebo) treatment was not associated with feeling buzzed (b = −.01, SE = .33, p = .97, 95% CI = [−.68, .64]), was non-significantly associated with feeling less drunk (b = −.51, SE = .27, p = .055, 95% CI = [−1.03, .01]), and was significantly associated with feeling less impaired (b = −.64, SE = .29, p = .027, 95% CI = [−1.21, −.07]). Thus, naltrexone treatment was associated with a .64 unit decrease in levels of feeling impaired. Drinking quantity was associated with feeling more buzzed (b = .51, SE = .07, p < .001, 95% CI = [.36, .65]), more drunk (b = .55, SE = .05, p < .001, 95% CI = [.46, .65]), and more impaired (b = .43, SE = .07, p < .001, 95% CI = [.29, 56]). No covariate effects were significant. There were significant indirect effects of naltrexone on all subjective response items, such that naltrexone was associated with blunted subjective responses through lighter drinking.
At the within-person level, taking 2 (vs. 1) pills was associated with feeling more buzzed (b = .43, SE = .15, p = .004, 95% CI = [.14, .72]), but there were no significant relations between medication dosage and feeling drunk or impaired. Drinking quantity was associated with feeling more buzzed (b = .69, SE = .03, p < .001, 95% CI = [.62, .75]), more drunk (b = .69, SE = .03, p < .001, 95% CI = [.63, .74]), and more impaired (b = .58, SE = .03, p < .001, 95% CI = [.52, 64]). Neither weekend drinking nor the day of the study was associated with feeling buzzed, drunk, or impaired. There were significant indirect effects of medication dosage on all three subjective response items through drinking in the same direction as craving; taking 1 (vs. 0) pills was associated with weaker subjective response through lighter drinking, and taking 2 (vs. 1) pills was associated with stronger subjective response through heavier drinking (Figure 2)2.
Figure 2: Specific Subjective Response Item Models.
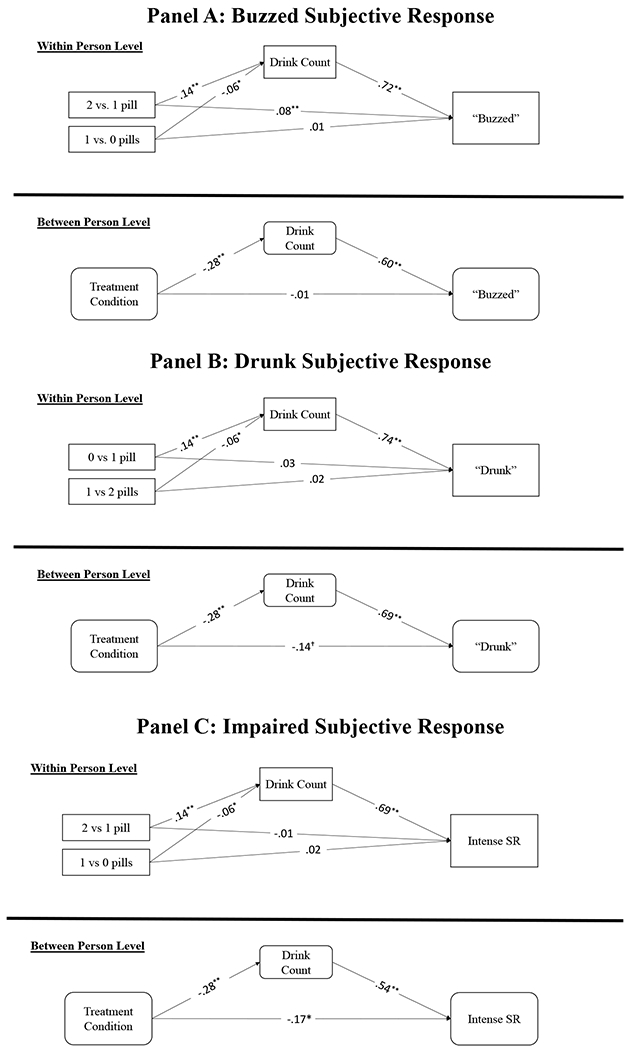
Note: All parameters are standardized estimates; Treatment is coded as 1=Naltrexone, 0=Placebo. At the between-person level, naltrexone treatment was directly associated with feeling less impaired, and there were significant indirect effects of naltrexone medication on all subjective response items, such that naltrexone was associated with blunted subjective response through lighter drinking. At the within-person level, taking 2 (vs. 1) pills was associated with feeling more buzzed, and there were significant indirect effects of medication dosage on all subjective response items through drinking, such that taking 1 (vs. 0) pills was associated with weaker subjective response through lighter drinking, and taking 2 (vs. 1) pills was associated with stronger subjective response through heavier drinking. ** P < .01, * P < .05, † P < .10
Post-Hoc Analysis: Drunk and Impaired
Based on the results of the prior analyses, we conducted a post-hoc test to determine whether treatment condition had an effect on a latent factor for feeling drunk and impaired, which represent higher-dose subjective response items. We estimated a latent factor partialling out the commonality between the two subjective response items. This was done by setting standardized factor weights of the two indicators to be equal. Both “impaired” and “drunk” loaded significantly onto the factor (β = .93, SE = .01, p < .001, 95% CI = [.92, .95]), which we refer to as “intense” subjective response from this point forward. This latent factor replaced the individual subjective response items in the post-hoc model.
At the between-person level, naltrexone treatment was associated with feeling lower levels of intense subjective response (b = −.45, SE = .21, p = .029, 95% CI = [−.30, −.02]) (Figure 3), and there was a significant indirect effect of naltrexone on intense subjective response through lighter drinking. Thus, naltrexone treatment was associated with a .45 unit decrease in levels of feeling intense subjective response. At the within-person level, heavier drinking (b = .30, SE = .02, p < .001, 95% CI = [.56, .67]) and weekend days (b = .21, SE = .07, p = .004, 95% CI = [.03, .14]) were associated with stronger intense subjective response. Neither contrast code of medication dose was associated with intense subjective response, though indirect effects mirrored those in previous models.3
Figure 3. Intense Subjective Response Model.
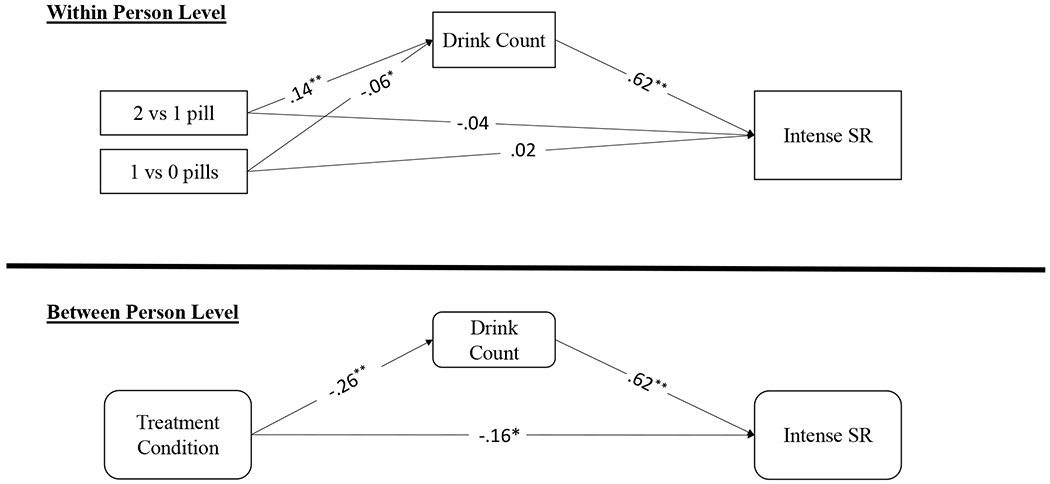
Note: All parameters are standardized estimates; Treatment is coded as 1=Naltrexone, 0=Placebo. At the between-person level, naltrexone treatment was directly associated with feeling less intense subjective response, and there were significant indirect effects of naltrexone medication on intense subjective response, such that naltrexone was associated with blunted intense subjective response through lighter drinking. At the within-person level, there were significant indirect effects of medication dosage on intense subjective response through drinking, such that taking 1 (vs. 0) pills was associated with weaker intense subjective response through lighter drinking and taking 2 (vs. 1) pills was associated with stronger intense subjective response through heavier drinking. ** P < .01, * P < .05, † P < .10
Alpha Adjustment
Because the current study tested 4 a priori hypotheses and 1 post hoc hypothesis (i.e., the effects of naltrexone on craving, “buzzed”, “drunk”, and “impaired”, and intense subjective response), we corrected for alpha inflation using the Benjamini-Hochberg false discovery rate correction procedure (Benjamini and Hochberg, 1995). Accounting for all 5 comparisons, previously significant effects of naltrexone on feeling “impaired” and intense subjective response were no longer significant. Naltrexone was not significantly associated with craving, feeling “buzzed”, or feeling “drunk”, both before and after correction. However, the size of the effects of naltrexone on feeling less “drunk” (b=.14), “impaired” (b=.18), and intense subjective response (b=.16) were all above the threshold for the post hoc determined SESOI (i.e., b=.13) and consistent with the small but meaningful effects of naltrexone in prior studies. Indirect blunting effects of naltrexone (vs. placebo) through lighter drinking remained statistically significant after alpha correction.
Medication Dosage
To determine whether treatment condition (naltrexone vs. placebo) moderated the effect of daily medication dosage on subjective response and craving, we estimated a multiple group multilevel mediation model. A model that constrained structural paths from medication dose to craving to be equal (X2 (4) = 5.35, p = .25) showed no significant decrement in model fit from a model that allowed these paths to freely vary (X2 (2) = 5.62, p = .06; ΔX2 (2) =1.26, p = .53). For subjective response, there were also no differences in relations between medication dose and feeling buzzed (X2 (2) = 3.10, p = .21; X2 (4) = 3.51, p = .48; ΔX2 (2) =.22, p = .90), drunk (X2 (2) = .59, p = .75; X2 (4) = 2.06, p = .73; ΔX2 (2) =1.54, p = .46), impaired (X2 (2) =1.64, p = .44; X2 (4) = 4.44, p = .35; ΔX2 (2) = 2.84, p = .24), or intense subjective response (X2 (31) = 59.62, p = .001; X2 (33) = 59.67, p = .003; ΔX2 (2) = 1.13, p = .57) by treatment condition. Thus, there was no indication that treatment condition moderated effects of daily dose on subjective response or craving.
Post-Hoc Power Analyses
We conducted several post hoc power analyses using GPower 3.1 to evaluate the size of between-person and within-person effects that future studies similar to the current study would be powered to detect. Power calculations assumed a moderate-to-high correlation among repeated measures (r=.50), specified two groups of interest (i.e., naltrexone and placebo), and 13 measurements per person (i.e., 1543 daily reports/115 participants). For tests of between-person naltrexone effects, future studies that have the same size (N=115) and number of measurements (1543 daily reports) would have 82.6% power to detect an effect size of b=.20, 58.4% power to detect an effect size of b=.15, and 46.9% power to detect an effect size of b=.13. For tests of within-person dosing effects, future studies that have the same size (N=115) and number of measurements (1543 daily reports) would have over 99% power to detect effects of b=.20, b=.15, and b=.13.
To detect between-person effect sizes of b=.13, b=.15, and b=20, sample sizes of N=254, N=190, and N=108, respectively, would be required to achieve 80% a-priori power.
Discussion
The current study tested the effects of naltrexone (vs. placebo) on two hallmark treatment mediators: subjective response and craving. This is one of the first studies to investigate naltrexone’s effects on both subjective response and craving at the daily level in young adults. We found that naltrexone blunted more intense subjective effects (“impaired”, “drunk”), but not feeling “buzzed” on drinking days. We also found that, at the daily level, regardless of treatment condition, there was an effect of taking 2 (vs. 1) medication pills on feeling more “buzzed”. Finally, we also found indirect effects of naltrexone through reduced drinking quantity at both levels. Between-person effects of naltrexone were not statistically significant after adjusting the alpha level, but all were above the SESOI. Thus, the current study interprets paths that were above the SESOI as “effects”, but we do not refer to statistical significance levels here forward.
At the between-person level, there was an indirect effect of naltrexone (vs. placebo) on blunted subjective effects through lighter drinking. Although the indirect effect of naltrexone on craving through drinking did not exceed the SESOI (standardized b = −.11, p = .051), it was in the expected direction, whereas the direct effect of naltrexone on craving was opposite in direction from predictions (standardized b = .05). At the within-person level, there was an effect of taking 1 (vs. 0) pills on weaker subjective response/craving through lighter drinking, and taking 2 (vs. 1) pills on stronger subjective response/craving through heavier drinking.
Study findings support a model in which there may be direct blunting effects of naltrexone treatment on subjective measures (particularly intense subjective ones), above and beyond the indirect effects of naltrexone on subjective response through lighter drinking. The lack of global naltrexone effects on feeling “buzzed” may be due to the fact that these effects are experienced at lower blood alcohol concentrations, and may be associated with lighter drinking patterns. Heavier drinkers may feel less “buzzed” when drinking (Piasecki et al., 2012) and “buzzed” may be present at lower levels of intoxication (e.g., Linden-Carmichael et al., 2021). In contrast, feeling “drunk” and “impaired” are subjective effects that may only be endorsed during heavier drinking episodes, showing mean-level differences in number of drinks needed to feel these effects compared to feeling “buzzed”. Therefore, one interpretation of the current findings is that naltrexone may blunt acute effects present at higher BAC levels, but may not blunt effects experienced early in a drinking episode. These findings also fit into the literature on trajectories of drinking, as Gueorguieva et al. (2007) found that naltrexone was associated with lower odds of being in the heaviest drinking category, and thus there may be a higher likelihood of naltrexone blunting effects such as “impaired” and “drunk”. Future research is needed to confirm these hypotheses.
It is also possible that feeling “buzzed” captures more expectancy effects than pharmacologic ones. Individuals may feel “buzzed” simply from being in the drinking environment, even before they have an appreciable BAC. Such a placebo response has been demonstrated in several lab-based alcohol administration paradigms (e.g., George et al., 2012). Relatedly, one study found a positive, rewarding atmosphere (e.g., a bar) produced equivalent rewarding alcohol effects in placebo and alcohol conditions (Corbin et al., 2015). In summary, findings suggest that naltrexone may reduce subjective effects that depend on alcohol pharmacology in addition to expectancies, potentially explaining its blunting of more intense but not less intense aspects of subjective response.
It is worth noting that the effect of treatment condition on craving did not exceed the SESOI. In addition, the zero-order correlation between treatment condition and craving was very small and in the opposite direction of hypotheses (r=−.02). One potential explanation for these findings is that the current study focused only on drinking days. It is possible that naltrexone reduces overall desire to drink, with lower craving on non-drinking days relative to drinking days, an effect that could contribute to reduced frequency of drinking. To capture such effects, analyses would need to include non-drinking as well as drinking days, which future research should examine. It is also possible that craving differs as a function of the amount of time in treatment. Although outside the scope of this study, future research should use time-dependent models to determine whether effects of naltrexone on subjective response and craving differ as a function of time in treatment.
The current study also found an effect exceeding the SESOI, contrary to hypotheses, of taking 2 (vs.1) pills on feeling more “buzzed” and heavier drinking, compared to an effect of taking 1 (vs. 0) pills on lighter drinking. None of these effects differed by treatment condition. Although surprising, it is likely that these findings were influenced by the instructions participants were given about medication dosing. Because participants were instructed to take a targeted dose in anticipation of alcohol consumption, or if they had started drinking, it makes sense that taking 2 pills (daily + targeted) would be associated with higher levels of drinking, potentially leading to a stronger subjective response and craving. Thus, taking 2 (vs 1) pills may be a proxy for the current study’s instructions to participants to take a daily + targeted pill when anticipating drinking, which would naturally be associated with heavier drinking. In contrast, the association between taking 1 (vs. 0) pills and lighter drinking may be due to unplanned drinking on days when only 1 pill was taken. In line with the previous comment, participants may have only taken one pill when they were not planning to drink, as they were directly instructed to take two pills when they anticipated drinking. Recent studies suggest that unplanned drinking days are associated with lower daily drinking quantity (Lauher et al., 2020) and less day-level intentions to get drunk/buzzed (Stevens et al., 2020). Thus, if days when only 1 pill was taken were unplanned, it would make sense that taking 2 (vs. 1) pills was associated with heavier drinking days and taking 1 (vs. 0) pills was associated with lighter drinking days. However, motives for taking or not taking both pills is outside the scope of the current study. Possibilities may include participants forgetting to take a pill, participants engaging in unplanned drinking, and participants not wanting their subjective state to be affected by taking two pills. Future research is needed to examine the motivation for medication adherence in naltrexone studies of heavy drinking.
Contrary to hypotheses, we did not detect an interaction between treatment condition and medication dose predicting subjective response/craving at the daily level, though this may have been due to lower power to detect a cross-level interaction than main effects. Nevertheless, future research is needed with larger samples to examine dose as a potential moderator of naltrexone medication effects at the daily level.
In terms of the blunting effect of naltrexone on impaired and “intense” subjective response, it is worth noting that the small but meaningful effect sizes observed (bs=.14-.17) were consistent with the effect size of naltrexone on stimulant subjective response found in the meta-analysis by Ray et al. (2019) (hedges g=.23; b=.15), and exceeded our SESOI. Given that naltrexone is a low- burden medication with minimal side effects (e.g., O’Malley et al., 2015), the medication’s small effect sizes are nonetheless clinically meaningful and a contribution to the literature on the mechanisms of naltrexone’s treatment efficacy. In addition to direct effects, we observed stable, robust indirect blunting effects of naltrexone on subjective measures (buzzed, drunk, and impaired) via lighter drinking and indirect effects on craving that were non-negligable (standardized beta = −.11). Thus, it is also possible that naltrexone is indirectly associated with blunted subjective effects/craving by reducing drinking, which reinforces lighter future drinking due to a lack of reward/reinforcement when drinking. Temporally sequenced momentary designs are needed to test such hypotheses. The current study is consistent with that of Miranda et al. (2014) in that it accounted for levels of drinking (e.g., drinking quantity at the day level, eBAC at a momentary level). However, other EMA studies (e.g., Tidey et al., 2008) did not control for levels of drinking, which could lead to an overestimation of naltrexone’s effects on subjective response and craving. Thus, future research should test whether drinking levels mediate the effects of naltrexone on subjective response and craving. In sum, we believe that the current findings advance the literature on mechanisms of naltrexone treatment, informing our understanding of the mechanisms of naltrexone’s efficacy.
Although the current study yielded novel findings, they must be interpreted in light of several limitations. First, although our analyses were more ecologically valid than many, data were collected at the daily rather than momentary level. Although next-day retrospective reports of subjective response are subject to recall bias, several other studies have used daily dairies to test overall past-day subjective response (e.g., Fairlie et al., 2021; Linden-Carmichael et al., 2020; Lipperman-Kreda et al., 2017). Nonetheless, it was not possible in the current study to establish temporal precedence for drinking and subjective responses/craving and medication ingestion. In addition, the current study did not model subjective response and craving together, which did not allow us to examine the possibility that subjective response may mediate the effect of condition/medication on craving; future research using momentary assessments is needed to establish temporal precedence. Our measurement of subjective response was also a limitation. We did not explicitly measure stimulant/rewarding effects, and the valence/arousal of items like “buzzed” and “impaired” is ambiguous. Future EMA research should include more specific items that operationalize stimulation and sedation.
Another limitation was that our sample was mostly young adult men (69%), limiting generalizability to heavy drinking samples of women. In addition, the current study had little variability in days when 0 pills were taken (5.5% of days). However, despite this lack of variability, there were consistent effects of taking 1 (vs. 0) pills on lighter drinking. Thus, future research is needed to test medication dosing in samples with more variability. Finally, although research has found that naltrexone is most effective at reducing heavier drinking (e.g., O’Malley et al., 2015), future research should investigate whether naltrexone dosing is associated with the frequency of drinking at the day level, particularly among treatment seekers who are motivated to abstain from drinking.
Despite these limitations, the current study builds upon several foundational efficacy studies by conducting daily diary analyses of variables that may mediate naltrexone’s efficacy. This is one of the first studies to test the effects of naltrexone on subjective response and craving using daily diary methods in young adults, in which within- and between-person effects were disaggregated. We found effects exceeding our SESOI of naltrexone on intense subjective response. Thus, naltrexone blunted alcohol effects that are likely to occur at higher BACs and are readily differentiated from expectancy effects. In addition, we found indirect effects of naltrexone on subjective response via drinking quantity, such that naltrexone blunted all subjective effects by reducing drinking. Considering that only one of two past EMA studies of naltrexone’s effects controlled for drinking levels, these findings suggest that naltrexone’s direct effects on subjective measures may have been overestimated and/or are mediated through levels of drinking. Future research is needed to validate this pattern of results in larger, more diverse samples, and with multiple daily assessments and broader subjective response measures.
Acknowledgments
This study was supported by grant R01 AA016621 from the National Institute on Alcoholism and Alcohol Abuse to Stephanie S. O’Malley. Authors JTW, WRC, RFL, and DPM have no conflicts of interest. Authors SSO and HRK are members of the American Society of Clinical Psychopharmacology’s workgroup, the Alcohol Clinical Trials Initiative, which was supported in the past three years by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor Pharmaceuticals, and Amygdala Neurosciences, Inc. SSO also reports the following activities for the past 12 months: Donated study medications from Astra Zeneca and Novartis; Consultant/Advisory Board Member for Amydala, Alkermes and Dicerna; provisional patent application with Novartis; DSMB member for NIDA Clinical Trials Network, Emmes Corporation. HRK is also named as an inventor on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018.
Footnotes
The use of an SESOI precluded the use of random slopes, as Mplus does not provide standardized estimates when random slopes are specified.
Models were also run with (1) only theoretical (but not statistical/data/driven) covariates (i.e., sex, family history, weekend vs weekday, and day of treatment) and 2) no covariates. For the craving model, effect sizes and significance levels were very similar in both models, and no paths moved from significant to non-significant or vice versa. For the “buzzed” model, effect sizes were identical in all models and there were no changes in significant effects. For the “drunk” model, the size of the naltrexone between-person effect on feeling “drunk” moved from b=−.14, p=.055 (in-text) to b=−.13, p =.065 (only theoretical covariates model). For the “impaired” model, the effect size of naltrexone on “impaired” moved from b=−.18, p=.027 (in-text) to b=−.17, p=.031 (only theoretical covariates model).
Models were also run with only theoretical covariates (i.e., sex, family history, weekend vs weekday, and day of treatment) and no covariates. In both models, effect sizes and significance levels remained nearly identical, the only difference being that the between-person effect of naltrexone on intense subjective response moved from b=−.16, p=.029 (in-text) to b=−.15, p=.054 (only theoretical covariates model).
References:
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, & Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharm. 2004;173:32–40. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, & Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alc Clin Exp Res 1995;19: 600–606. [DOI] [PubMed] [Google Scholar]
- Bold KW, Fucito LM, DeMartini KS, Leeman RF, Kranzler HR, Corbin WR, & O’Malley SS. Urgency traits moderate daily relations between affect and drinking to intoxication among young adults. Drug Alcohol Depen. 2017;170:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges CR. Effect size guidelines, sample size calculations, and statistical power in gerontology. Innovation in Aging, 2019; 3(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Henson JM, Carey MP, & Maisto SA. Which heavy drinking college students benefit from a brief motivational intervention? J Consult Clin Psych. 2007;75:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin WR, Scott C, Boyd SJ, Menar KR, & Enders CK. Contextual influences on subjective and behavioral responses to alcohol. Exper Clin Psychopharm. 2015;30:59–70. [DOI] [PubMed] [Google Scholar]
- DeMartini KS, Gueorguieva R, Leeman RF, Corbin WR, Fucito LM, Kranzler HR, & O’Malley SS. Longitudinal findings from a randomized clinical trial of naltrexone for young adult heavy drinkers. J Consult Clin Psych. 2016;84:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimeff LA, Baer JS, Kivlahan DR, & Marlatt GA. Brief Alcohol Screening and Intervention for College Students (BASICS): a harm reduction approach. 1999; New York: Guilford Press. [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment seeking alcoholics and social drinkers. Alc Clin Exp Res. 2004;28:1362–1370. [DOI] [PubMed] [Google Scholar]
- Epler AJ, Sher KJ, Loomis TB, & O’Malley SS, College student receptiveness to various alcohol treatment options. J Am Coll Health. 2009;58:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairlie AM, Graupensperger S, Duckworth JC, Patrick ME, Lee CM. Unplanned versus planned simultaneous alcohol and marijuana use in relation to substance use and consequences: Results from a longitudinal daily study. Psych Addict Behav. 2021. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzino TL, Harder VS, Rose GL, & Helzer JE. A daily process examination of the bidirectional relationship between craving and alcohol consumption as measured via interactive voice response. Alc Clin Exp Res. 2014;37;2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George WH, Gilmore AK, & Stappenbeck CA. Balanced placebo design: Revolutionary impact on addictions research and theory. Addict Res Theory. 2012;20:186–203. [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiat. 2017;74:911–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueorguieva R, Wu R, Pittman B, Cramer J, Rosenheck RA, O’Malley SS, & Krystal JH. New insights into the efficacy of naltrexone based on trajectory-based reanalyses of two negative clinical trials. Biol Psychiat, 2007;61:1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Samokhvalov AV, & Rehm J. Effects of naltrexone on alcohol self-administration and craving: meta-analysis of human laboratory studies. Addict Biol. 2017;22:1515–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struc Eq Modeling. 1999;6;1–55. [Google Scholar]
- Huh D, Mun EY, Larimer ME, White HR, Ray AE, Rhew IC, Kim SY, Jiao Y, Atkins DC. Brief motivational interventions for college student drinking may not be as powerful as we think: An individual participant-level data meta-analysis. Alcohol Clin Exp Res. 2015; 39:919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, & Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiat. 2014;75:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit J, McNamara PJ, & Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psych, 2011;68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Tennen H, Armeli A, Chan G, Covault J, Arias A, & Oncken C. Targeted naltrexone for problem drinkers. J Clin Psychopharm. 2014;29:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Cooper ML. Drinking to have fun and to get drunk: Motives as predictors of weekend drinking over and above usual drinking habits. Drug and alcohol dependence. 2010;110(3):259–62. [DOI] [PubMed] [Google Scholar]
- Lauher ML, Merrill JE, Boyle HK, & Carey KB. The relationship between unplanned drinking and event-level alcohol-related outcomes. Psych Add Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Palmer RS, Corbin WR, Romano DM, Meandzija B, & O’Malley SS. A pilot study of naltrexone and BASICS for heavy drinking young adults. Addict Behav. 2008;33:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden-Carmichael AN, Van Doren N, Masters LD, Lanza ST. Simultaneous alcohol and marijuana use in daily life: Implications for level of use, subjective intoxication, and positive and negative consequences. Psych Addict Behav. 2020. Advanced Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipperman-Kreda S, Gruenewald PJ, Grube JW, Bersamin M. Adolescents, alcohol, and marijuana: context characteristics and problems associated with simultaneous use. Drug and Alcohol Dependence, 2017;179:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful. Addiction. 2013;108:275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Roos CR, Hoffmann S, Nakovics H, Leménager T, Heinz A, & Witkiewitz K. Precision medicine in alcohol dependence: a controlled trial testing pharmacotherapy response among reward and relief drinking phenotypes. Neuropsychopharm, 2018;43:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Baer JS, Kivlahan DR, Dimeff LA, Larimer ME, Quigley LA, Somers JM, Williams E Screening and brief intervention for high-risk college student drinkers: results from a 2-year follow-up assessment. J Consult Clin Psych. 1998;66:604–615. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, & Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharm. 2000;22:480–492. [DOI] [PubMed] [Google Scholar]
- Miranda R, Ray L, Blanchard A, Reynolds EK, Monti PM, Chun T, Justus A, Swift RM, Tidey J, Gwaltney CJ, Ramirez J. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19:941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, Treat TA. The Subjective Effects of Alcohol Scale: Development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychological assessment. 2013;3:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcoholism and Alcohol Abuse. Alcohol’s Impact on Health 2018. [Google Scholar]
- O’Malley SS, Corbin WR, Leeman RF, DeMartini KS, Fucito LM, Ikomi J, Romano DM, Wu R, Toll BA, Sher KJ, Gueorguieva R. Reduction of alcohol drinking in young adults by naltrexone: a double-blind, placebo-controlled, randomized clinical trial of efficacy and safety. J Clin Psychiat, 2015;76:e207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS. Naltrexone and coping skills for alcohol dependence: a controlled study. Arch Gen Psych; 1992;49:881–887. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, & Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates hypothalamo-pituitary-adrenocortical axis. Psychopharm. 2002;160:19–29. [DOI] [PubMed] [Google Scholar]
- Perkins HW. Surveying the damage: A review of research on consequences of alcohol misuse in college populations. J Stud Alcohol Supp. 2002;14:91–100. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Wood PK, Shiffman S, Sher KJ, & Heath AC. Responses to alcohol and cigarette use during ecologically assessed drinking episodes. Psychopharm. 2012;223:331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, & Hutchison KE. A double-blind placebo-controlled study of the effects of naltrexone on alcohol sensitivity and genetic moderators of medication response. Arch Gen Psych. 2007;64:1069–1077. [DOI] [PubMed] [Google Scholar]
- Ray LA, Green R, Roche DJ, Magill M, & Bujarski S. Naltrexone effects on subjective responses to alcohol in the human laboratory: A systematic review and meta-analysis. Addict Bio. 2019;24:1138–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alc Clin Exp Res. 1995;19:1018–1023. [DOI] [PubMed] [Google Scholar]
- Roos CR, Bold KW, Witkiewitz K, Leeman RF, DeMartini KS, Fucito LM, Corbin WR, Mann K, Kranzler HR, O’Malley SS. Reward drinking and naltrexone treatment response among young adult heavy drinkers. Addiction 2021;116:2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SML, Gianoulakis C, Palmour RM, Benkelfat C, & Leyton M. The effect of naltrexone on alcohol’s stimulant properties and self-administration behavior in social drinkers: influence of gender and genotype. Alc Clin Exp Res, 2011;35:1134–1141. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, & Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropharmacol. 2005; 8:267–280. [DOI] [PubMed] [Google Scholar]
- Stevens AK, Haikalis M, & Merrill JE. Unplanned vs. planned drinking: Event-level influences of drinking motives and affect. Addict Behav. 2020;112:106592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BC, Fidell LS, & Ullman JB. Using multivariate statistics (Vol. 5, pp. 481–498). Boston, MA: Pearson. 2007. [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, McGeary JE. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alc Clin Exp Res, 2008;32:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi D, & MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela CJ, Hayes AW, Bartholow BD, Sher KJ, Heath AC, & Piasecki TM. Moderation of alcohol craving reactivity to drinking-related contexts by individual differences in alcohol sensitivity: An ecological investigation. Exper Clin Psychopharm. 2018;26:354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrisi R, Larimer ME, Mallett KA, Kilmer JR, Ray AE, Mastroleo NR., Geisner IM, Grossbard J, Tollison S, Lostutter TW, Montoya H A randomized trial evaluating a combined alcohol intervention for high-risk college students. J Stud Alcohol Drugs. 2009;70:555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, & O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psych,1992; 49:876–880. [DOI] [PubMed] [Google Scholar]
- Waddell JT. Hierarchical and mediated relations between internalizing symptoms, alcohol and cannabis co-use, and AUD. 2021. Addict Res Theory:1–7. [Google Scholar]
- Waddell JT, Blake AJ, Chassin L. Relations between impulsive personality traits, alcohol and cannabis co-use, and negative alcohol consequences: A test of cognitive and behavioral mediators. Drug Alc Dep. 2021. 225:108780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell JT, Corbin WR, Chassin L, Anderson SF. The prospective interactive effects of alcohol expectancies and subjective response on future drinking behavior. Exp Clin Psychopharm, 2020, Advanced Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, & Castillo S. Health and behavioral consequences of binge drinking in college: A national survey of students at 140 campuses. JAMA. 2004;272;1672–1677. [PubMed] [Google Scholar]
- Witkiewitz K, Roos CR, Mann K, & Kranzler HR. Advancing Precision Medicine for Alcohol Use Disorder: Replication and Extension of Reward Drinking as a Predictor of Naltrexone Response. Alc Clin Exp Res. 2019;43:2395–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]


