Abstract
Pneumococcal surface protein A (PspA) is a surface-exposed protein virulence factor for Streptococcus pneumoniae. In this study, no significant depletion of serum complement was observed for the serum of mice infected with pneumococci that express PspA. In contrast, in mice infected with an isogenic strain of pneumococci lacking PspA, significant activation of serum complement was detected within 30 min after infection. Also, the PspA-deficient strain but not the PspA-expressing strain was cleared from the blood within 6 h. The contribution of PspA to pneumococcal virulence was further investigated by using mice deficient for C5, C3, or factor B. In mice deficient for C3 or factor B, PspA-negative pneumococci became fully virulent. In contrast, in C5-deficient mice as in wild-type mice, PspA-deficient pneumococci were avirulent. These in vivo data suggest that, in nonimmune mice infected with pneumococci, PspA interferes with complement-dependent host defense mechanisms mediated by factor B. Immunoblots of pneumococci opsonized in vitro suggested that more C3b was deposited on PspA-negative than on PspA-positive pneumococci. This was observed with and without anticapsular antibody. Furthermore, processing of the α chain of C3b was reduced in the presence of PspA. We propose that PspA exerts its virulence function by interfering with deposition of C3b onto pneumococci and/or by inhibiting formation of a fully functional alternative pathway C3 convertase. By blocking recruitment of the alternative pathway, PspA reduces the amount of C3b deposited onto pneumococci, thereby reducing the effectiveness of complement receptor-mediated pathways of clearance.
Streptococcus pneumoniae is a major cause of morbidity and mortality worldwide. Annually in the United States, there are at least 40,000 deaths due to pneumococcal infection, primarily among the elderly (10). S. pneumoniae causes otitis media in children and sepsis in patients infected with the human immunodeficiency virus (14). One of the virulence factors associated with this organism is pneumococcal surface protein A (PspA). The role of PspA in virulence has been demonstrated elsewhere with strains of pneumococci in which pspA genes have been either deleted or inactivated (22). Mutant strains not able to make PspA are cleared more rapidly from the blood of nonimmunized mice than are PspA-producing strains (8, 22). In accordance with its role in virulence, PspA has been found to elicit immune responses that can protect mice against infection with S. pneumoniae (21, 25). Although PspA is variable in structure (12), antibodies to PspA are highly cross-protective (25). Based on their structure and serology, PspAs are currently divided into six clades which make up three families (18).
The specific mechanism by which PspA confers virulence on pneumococci is not known. However, using a bystander complement fixation assay P. C. Aerts and H. van Dijk showed that heat-killed pneumococci lacking PspA fixed more complement than did heat-killed pneumococci possessing PspA (5, 7). In the current study, PspA+ and PspA− strains of pneumococci were used to examine the effect of PspA on bacterial virulence and complement activation in vivo and the influence of PspA on opsonization of pneumococci in vitro. The virulence of wild-type pneumococci and their PspA− counterparts was compared between normal and complement-deficient mice. Our results suggest that PspA functions as an inhibitor of factor B-mediated complement activation in vivo and as an inhibitor of C3b deposition and/or α-chain processing in vitro. These findings are consistent with an ability of PspA to inhibit the formation and/or function of the alternative pathway C3 convertase and provide insights into the role of PspA in disease and the mechanism of action of protective anti-PspA antibodies.
MATERIALS AND METHODS
Pneumococcal strains.
S. pneumoniae strains were grown as described elsewhere (2) in Todd-Hewitt broth supplemented with 0.5% yeast extract or on blood agar plates containing 3% defibrinated sheep erythrocytes. Bacterial stocks were stored frozen at −80°C in Todd-Hewitt broth containing 10% glycerol. The capsular type 3 strain WU2 (PspA+) and the isogenic strain JY1119 (PspA−) were used (6, 30). Derivation of the PspA− mutant has been fully described elsewhere (30). To ensure the purity of each strain, WU2 and JY1119 were grown on blood agar plates containing gentamicin (4 μg/ml) and erythromycin (0.3 μg/ml), respectively (2, 30).
Mice.
CBA/CAHN-XID/J mice (XID mice) from Jackson Laboratories (Bar Harbor, Maine) were used in the initial experiments. These mice carry an X-linked immunodeficiency mutation and are not able to produce antibodies to most polysaccharides (1, 6). DBA/2J mice (C5− mice) carry a spontaneous mutation in exon 7 of the murine C5 gene which renders them deficient in serum complement C5 (28). C3-deficient mice (C3− mice) produce no serum complement C3 due to targeted disruption of the C3 gene (11), while factor B-deficient mice (FB− mice) produce no serum complement factor B due to targeted disruption of the factor B gene (20). C5− mice lack the ability to generate C5 convertases through any complement pathway and thus are unable to produce C5a, C5b, and the cell-lytic membrane attack complex. C3− mice lack the ability to generate C3 convertases through the alternative pathway and do not produce the anaphylotoxin C3a nor the opsonin C3b. They also have no serum complement lytic activity. FB− mice are unable to form the alternative pathway C3 convertases; thus, they have no alternative complement pathway activity and have reduced classical pathway activity (20). C3− mice and phenotypically normal littermates carrying one C3 allele were produced by mating C3− mice with C57BL/6J partners and intercrossing the F1 hybrids. The same breeding system was used to produce FB− and C5− mice and normal littermates. All mice were 6 to 12 weeks old when used.
Infections of mice.
Pneumococci from frozen stock cultures were thawed and plated on blood agar plates the day prior to infection to verify the concentration of bacteria (2). On the day of infection, separate aliquots of the same stock were diluted with Ringer’s solution to the required cell density and 2 × 105 CFU of WU2 or JY1119 was used to infect XID mice. We used 107 CFU for intravenous infection of complement-deficient mice, since this dose was sufficient to cause sublethal infection in all normal littermates. Mice were injected with pneumococci through the lateral tail vein and bled retro-orbitally with sterile disposable micropipettes (Fisher Scientific). Whole blood was collected serially just prior to and at various times up to 12 h after infection. Blood (75 μl) was added to 425 μl of phosphate-buffered saline (PBS) (0.03 M NaCl, 0.3 mM KH2PO4, 2 mM Na2HPO4, and 0.5 mM KCl) to prevent clotting and then placed on ice. Half the mixture was serially diluted and used to inoculate blood agar plates for determination of bacteremia. The remaining half of the diluted blood sample was mixed with 250 μl of PBS containing 10 mM EDTA (pH 8.0) and centrifuged at 5,585 × g to remove erythrocytes, and the supernatant was transferred to a fresh tube and kept frozen at −20°C.
In vitro complement fixation.
Pooled blood from XID mice with normal complement levels was allowed to clot on ice for 30 min and then was immediately spun in a microcentrifuge to isolate the serum. This serum was aliquoted, stored at −80°C, and, since it contains no antibodies against pneumococci (1, 6), used as the complement source for in vitro opsonization. For the complement fixation assay, freshly thawed XID mouse serum was diluted 2.5-fold in gelatin Veronal buffer (Sigma). To five 1-ml aliquots of the diluted serum was added 150 μl of WU2 or JY1119 (9.5 × 108 CFU) or 150 μl of sterile Veronal buffer. To determine complement fixation due to classical pathway activation, bacteria were pretreated with immunoglobulin G3 anti-type 3 capsule monoclonal antibody 16.3 for 5 min (4, 6) prior to exposure to XID mouse serum. The mixtures were incubated at 37°C, and at various time points, a 50-μl sample was removed, diluted with an equal volume of PBS containing 10 mM EDTA, and centrifuged for 2 min at 4°C to pellet pneumococci. The supernatant was removed, and the bacterial pellet was washed twice in PBS containing 10 mM EDTA, resuspended in 50 μl of reducing sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and loaded onto SDS-polyacrylamide gels for electrophoresis. Western blotting was performed according to standard procedures. To monitor the accumulation of C3 on bacteria, immunoblots were developed with peroxidase-conjugated goat anti-mouse C3 polyclonal antibody (ICN Pharmaceuticals, Inc.). This antibody recognizes the intact α chain (110 kDa) and β chain (65 kDa) of mouse serum C3 and also reacts with the various α-chain fragments (∼46 kDa) that remain bound to pneumococci after C3b deposition. Protein bands were quantitated by densitometry with the Bio-Rad gel documentation system.
C3 enzyme-linked immunosorbent assay.
C3 enzyme-linked immunosorbent assay was used to monitor the disappearance of serum C3 during opsonization of bacteria in vivo. Microtiter plates were coated overnight at 4°C with 25 μg of polyclonal goat anti-mouse C3 (Cappel) per ml diluted in 15 mM Na2CO3–30 mM NaHCO3, pH 9.6. The next day, the antibody mixture was removed and the plate was rinsed and blocked for 1 h with 100 μl of 1% bovine serum albumin (Sigma) in PBS containing 10 mM EDTA, pH 7.5. Plates were subsequently washed with the same buffer containing 0.05% Tween 20. Normal mouse serum (200 ng of mouse C3/ml) and sera collected from infected mice were diluted in washing buffer containing 0.05% bovine serum albumin and added to the wells. The plates were then incubated at room temperature for 2 h. At the end of this time, wells were washed and 50 μl of goat anti-mouse C3 peroxidase-conjugated antibody was added to each well. The plates were incubated for 1 h and washed twice in wash buffer, and 100 μl of ABTS (Sigma) substrate buffer was added. After 30 min of substrate conversion, the plates were scanned at 405 nm with a Labsystems Multiskan MS scanner (Scientific Consultant Inc.).
RESULTS AND DISCUSSION
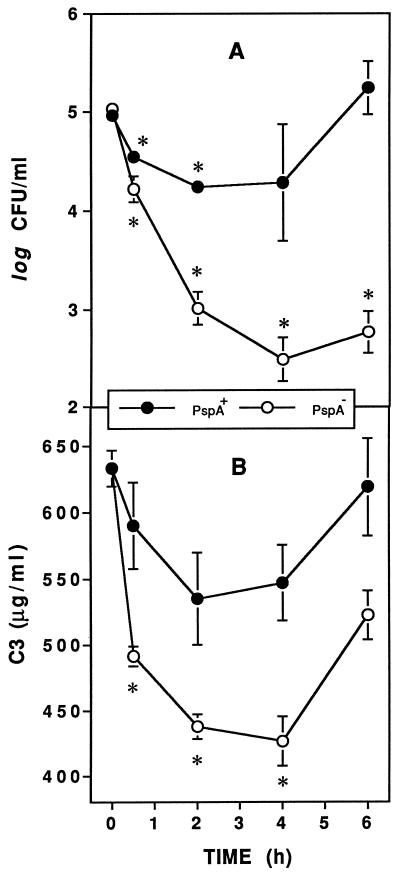
The influence of PspA on complement activation in vivo was revealed by comparing consumption of serum C3 in XID mice after infection with WU2 (PspA+) and JY1119 (PspA−). After intravenous infection with 105 CFU of PspA− pneumococci, clearance of bacteria was significant within 2 h and virtually complete by 6 h (Fig. 1A). In contrast, with the PspA+ strain only marginal clearance of bacteria was observed and the infection was fully established by 6 h (Fig. 1A). Within 30 min after infection with the PspA− strain, the concentration of serum C3 decreased significantly and consumption of serum C3 continued until 4 h (Fig. 1B). In contrast, there was less activation of complement in response to the PspA+ strain. In fact, no statistically significant consumption of C3 (Fig. 1B) was measured, even in mice receiving up to 107 CFU of PspA+ bacteria (data not shown). Beyond 6 h postinfection, there was a net increase in serum C3 in mice infected with either strain of pneumococci, likely due to the C3 acute-phase response (24). These data suggest that an ability of PspA to inhibit complement activation during the early phase of infection allows pneumococci to evade host defense mechanisms otherwise responsible for their clearance.
FIG. 1.
PspA inhibits complement activation and clearance of pneumococci. XID mice were infected intravenously with approximately 105 CFU of PspA+ or PspA− pneumococci, and blood was collected to quantitate bacteremia (A) and serum C3 (B). The data are presented as the means ± standard errors of the means from a total of 15 mice per group (two experiments). Asterisks indicate significant decreases in bacteremia or serum C3 concentrations compared to 0-h (preinfection) values (P < 0.05, paired t test).
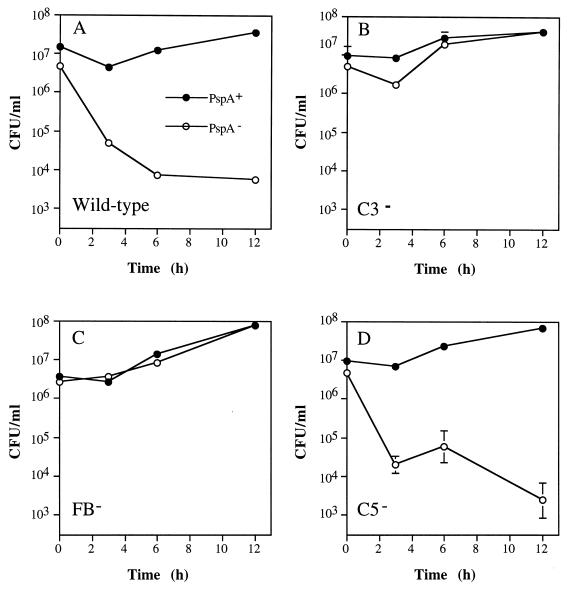
To investigate the point along the complement activation cascade at which PspA exerts its influence, C3−, FB−, and C5− mice and their wild-type littermates were infected with PspA+ and with PspA− pneumococci. As in XID mice (Fig. 1A), PspA− pneumococci were cleared rapidly from the blood of wild-type mice (Fig. 2A). In stark contrast, PspA− pneumococci were not cleared by C3− or FB− mice (Fig. 2B and C). Moreover, in both C3− and FB− mice, infection with PspA− pneumococci led to bacteremia as intense as that caused by PspA+ pneumococci. These data are in agreement with our initial hypothesis that PspA increases virulence of pneumococci through inhibition of complement function and suggest that the inhibitory step targets factor B-mediated activation. In fact, unlike deficiencies of C3 and factor B, deficiency in the late-acting component C5 had no influence on the virulence of the PspA− strain (Fig. 2D). Thus, PspA’s role in virulence does not extend to events that require C5 cleavage or membrane attack complex assembly. The associated mortality data, though limited, clearly demonstrate the effectiveness of PspA as a virulence factor. Of the mice shown in Fig. 2 that were infected with the PspA+ strain, all but a single wild-type mouse died within 24 h. In contrast, for mice infected with the PspA− strain no deaths were observed in the wild-type and C5− groups, but all C3− mice and half of the FB− mice died.
FIG. 2.
PspA+ and PspA− pneumococci (closed and open circles, respectively) have equal virulence in C3− and FB− mice. Mice were infected intravenously with approximately 107 CFU of PspA+ or PspA− pneumococci and bled at various times thereafter to quantitate bacteremia. The data are presented as the means ± standard errors of the means for groups of two to five mice.
The various pathways leading to complement activation and its proteolytic cascade that ensues have been reviewed in detail elsewhere (26). Briefly, when complement activation is initiated the α chain of serum C3 binds covalently to the activating surface. The covalently bound α/β dimer, termed C3b, composed of an α and a β chain, is then recognized and bound by factor B to form C3bB. Upon cleavage of C3b-bound factor B by the enzyme factor D, the C3 convertase C3bBb is formed. This complex is short-lived and therefore is not an effective amplifier of the alternative pathway. However, it is stabilized by the binding of properdin to form the alternative pathway amplification convertase. The properdin-stabilized convertase catalyzes the hydrolysis of additional C3 molecules, and more C3b is deposited onto the activating surface. More C3 convertases are formed, and the alternative pathway is amplified. Amplification is limited by the complement regulatory enzymes factor H and factor I, which together can cleave the α chain of C3b into smaller fragments. The resulting molecule, termed iC3b, no longer supports the amplification process, but like C3b, it serves as a recognition molecule for complement receptors on phagocytes.
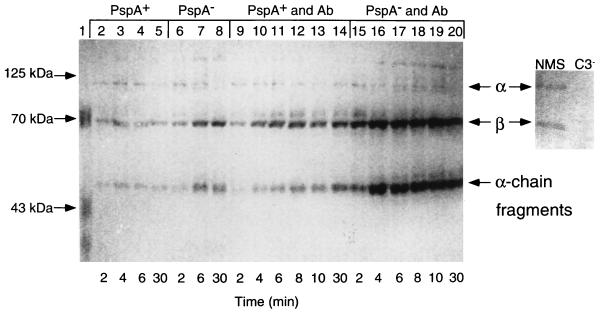
To determine more directly if PspA increases virulence through interference with complement-mediated opsonization of pneumococci, we compared the amounts of C3b deposited onto PspA+ and PspA− pneumococci opsonized in vitro. Control immunoblots of normal mouse serum revealed discrete α and β chains of serum C3 (110 and 65 kDa, respectively) that were absent in C3− mouse sera (Fig. 3). On serum-opsonized pneumococci (Fig. 3, lanes 2 to 8), α and β chains of C3b and variable amounts of ∼46-kDa α-chain fragments of iC3b were consistently observed. Compared to PspA+ bacteria, more C3b was deposited onto the PspA− strain, as indicated by increased levels of β-chain and α-chain fragments (Fig. 3, compare lanes 4 and 5 to 7 and 8). Although treatment of pneumococci with anticapsular antibody prior to opsonization with XID mouse serum substantially increased C3b deposition on both the PspA+ and the PspA− pneumococci (Fig. 3, compare lanes 9 to 14 with 15 to 20), again more C3b was deposited on the PspA− strain. By using the Bio-Rad gel documentation system, we compared the relative densities of the C3b α chain, β chain, and α-chain fragments deposited on PspA+ and PspA− pneumococci. These bands accounted for 27, 39, and 34% of the total integrated density value for C3b on the PspA+ strain (lane 9) compared to 20, 41, and 39% on the PspA− strain (lane 15), respectively. Thus, despite the overall increase in C3b deposited onto antibody-sensitized PspA− pneumococci, the relative strength of the α-chain signal is reduced by 7% compared to that on the PspA+ strain. Although these data are limited, the reduced contribution of the α chain to the total C3b signal detected on PspA− pneumococci 2 min after exposure to serum, with concomitant strengthening (by 5%) of the ∼46-kDa α-chain fragments, does suggest that processing of C3b on PspA− pneumococci may be more rapid than on PspA+ pneumococci. Although the precise mechanism is not yet known, these combined data indicate that the virulence function of PspA involves inhibition of deposition and processing of surface-bound C3b.
FIG. 3.
Western blot analysis of C3 deposition onto PspA+ and PspA− pneumococci. Pneumococci were exposed to XID mouse serum in the absence (lanes 2 to 8) or presence (lanes 9 to 20) of anticapsular antibody (Ab). Pneumococci were collected after exposure to XID mouse serum for 2 to 30 min (see Materials and Methods), boiled in reducing buffer, electrophoresed in SDS-polyacrylamide gels, transferred to nitrocellulose, and visualized with anti-C3 antibody. The right panel shows intact α and β chains of C3 present in normal mouse serum (NMS) and absent in C3− serum.
For gram-positive bacteria, opsonization with C3b and/or iC3b is an important prerequisite for their destruction by phagocytic cells (9, 16), and S. pneumoniae has evolved a variety of mechanisms to avoid opsonophagocytosis. For example, on group A streptococci, the surface M protein inhibits phagocyte complement receptor binding of deposited C3b and iC3b (27). Also, it had been suggested earlier (29) that since C3b is fixed to the cell wall of pneumococci, deep in the capsule, the capsule therefore interferes with recognition of C3b by phagocytes. The capsules of some group B streptococci contain sialic acid, which enhances the binding of factor H to C3b, thereby preventing complement activation (13). The M protein binds factor H (15) and another complement regulatory protein termed C4 binding protein (19), thus blocking both alternative and classical pathway activation. Taken together, our combined in vivo and in vitro data suggest that the mechanism by which PspA increases virulence of pneumococci is interference with deposition of C3b and/or prevention of formation of alternative pathway amplification C3 convertases. This process leads to an anticipated reduction in opsonophagocytosis and explains the increased rate of clearance of PspA− bacteria from mice. The cumulative effects of PspA on complement deposition would be expected to be greater in vivo than in vitro since the amount of complement available and the time available for activation are much greater. This model thus explains why PspA+ strains elicit minimal complement activation during the early phase of infection. Circulating C3 is significantly consumed only in mice infected with PspA− bacteria. Furthermore, although no remarkable difference in the total amount of C3b initially bound by PspA+ and PspA− pneumococci opsonized in the absence of antibody was apparent, C3b bound to PspA+ pneumococci appears to be less readily processed during progression of complement activation. In C3− mice, PspA− pneumococci become highly virulent, while in C5− mice PspA− bacteria are cleared from the blood as effectively as in wild-type mice, indicating that complement activation events proximal to C3 cleavage are targeted by PspA. In FB− mice, PspA− bacteria are able to avoid clearance from the blood, suggesting that PspA acts specifically on the alternative pathway of complement activation. Thus, like the proposed function of M protein of group A streptococci (3), PspA likely inhibits alternative pathway activation. Interestingly, PspA also reduces deposition of C3b initiated by the classical pathway, as indicated by our results with anticapsular antibody. Perhaps, PspA has an inhibitory effect on the classical pathway C3 convertase, or it blocks recruitment of the alternative pathway amplification loop.
In conclusion, we propose that PspA functions like the membrane regulators of complement activation decay-accelerating factor, complement receptor type 1, and/or factors H and I, i.e., by blocking formation of or accelerating the dissociation of the alternative pathway C3 convertase. Since PspA is one of the more variable gene products of pneumococci, understanding its precise mechanism of pathogenesis will lead to the development of a more effective pneumococcal vaccine. The complement-inhibitory function of PspA is probably superimposed on the capacity of certain capsules to block phagocyte receptor access to cell wall-bound C3b, and so PspA+ pneumococci are predicted to be somewhat more virulent than PspA− pneumococci even in mice producing anticapsular antibody. Furthermore, the serologic variation in PspA may be an evolutionary attempt by pneumococci to avoid recognition by antibodies that can neutralize the anticomplement function of PspA. Recently, a protein called PspC (also known as CbpA and SpsA) has been identified (17, 23) and shown to elicit a protective immune response. The similarity of this molecule to PspA suggests that it might also interfere with complement activation.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI-07051, AI-21548 (D.E.B.), and AI-42183 (A.J.S.).
REFERENCES
- 1.Amsbaugh D F, Hansen C T, Prescott B, Stashak P W, Barthold D R, Baker P J. Genetic control of the antibody response to type III pneumococcal polysaccharide in mice. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972;136:931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton K A, Paton J C, Briles D E. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect Immun. 1997;65:1237–1244. doi: 10.1128/iai.65.4.1237-1244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisno A L. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect Immun. 1979;26:1172–1176. doi: 10.1128/iai.26.3.1172-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briles D E, Forman C, Horowitz J C, Volanakis J E, Benjamin W H, Jr, McDaniel L S, Eldridge J, Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989;57:1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briles, D. E., S. K. Hollingshead, E. Swiatlo, A. Brooks-Walter, A. Szalai, A. Virolainen, L. S. McDaniel, K. A. Benton, P. C. Aerts, H. van Dijk, and M. J. Crain. Pneumococcal proteins PspA and PspC: their potential for use as vaccines. In A. Tomasz and R. Austrian (ed.), Molecular biology of pneumococci and pneumococcal diseases, in press. Mary Ann Liebert Inc., Larchmont, N.Y. [DOI] [PubMed]
- 6.Briles D E, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles D E, Tart R C, Swiatlo E, Dillard J P, Smith P, Benton K A, Ralph B A, Brooks-Walter A, Crain M J, Hollingshead S K, McDaniel L S. Pneumococcal diversity: considerations for new vaccine strategies with an emphasis on pneumococcal surface protein A (PspA) Clin Microbiol Rev. 1998;11:645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briles D E, Yother J, McDaniel L S. Role of pneumococcal surface protein A in the virulence of Streptococcus pneumoniae. Rev Infect Dis. 1988;10:S372–S374. doi: 10.1093/cid/10.supplement_2.s372. [DOI] [PubMed] [Google Scholar]
- 9.Brown E J. Interaction of Gram-positive microorganisms with complement. Curr Top Microbiol Immunol. 1985;121:159–187. doi: 10.1007/978-3-642-45604-6_8. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. Pneumococcal polysaccharide vaccine, recommendations of the immunization practices. Morbid Mortal Weekly Rep. 1989;38:64–68. [PubMed] [Google Scholar]
- 11.Circolo A, Garnier G, Fukuda W, Wang X, Hidvegi T, Szalai A J, Briles D E, Volanakis J E, Wetsel R A, Colten H R. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extra-hepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 12.Crain M J, Waltman II W D, Turner J S, Yother J, Talkington D E, McDaniel L M, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards M S, Kasper D L, Jennings H J, Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway of type III, group B streptococci. J Immunol. 1982;128:1278–1283. [PubMed] [Google Scholar]
- 14.Farley J J, King J C, Nair P, Hines S E, Tressler R L, Vink P E. Invasive pneumococcal disease among infected and uninfected children of mothers with immunodeficiency virus infection. J Pediatr. 1994;124:853–858. doi: 10.1016/s0022-3476(05)83170-1. [DOI] [PubMed] [Google Scholar]
- 15.Fischetti V A, Horstmann R D, Pancholi V. Location of the complement factor H binding site on streptococcal M6 protein. Infect Immun. 1995;63:149–153. doi: 10.1128/iai.63.1.149-153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon D L, Rice J, Finlay-Jones J J, McDonald P J, Hostetter M K. Analysis of C3 deposition and degradation on bacterial surfaces after opsonization. J Infect Dis. 1988;157:697–704. doi: 10.1093/infdis/157.4.697. [DOI] [PubMed] [Google Scholar]
- 17.Hammerschmidt S, Talay S R, Brandtzaeg P, Chhatwal G S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 18.Hollingshead, S. K., R. S. Becker, and D. E. Briles. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Unpublished data. [DOI] [PMC free article] [PubMed]
- 19.Johnsson E, Andersson G, Lindahl G, Heden L-O. Identification of the IgA-binding region in streptococcal protein Arp. J Immunol. 1994;153:3557–3564. [PubMed] [Google Scholar]
- 20.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin D D, Colten H R. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci USA. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDaniel L S, Scott G, Kearney J F, Briles D E. Monoclonal antibodies against protease sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J Exp Med. 1984;160:386–397. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDaniel L S, Yother J, Vijayakumar M, McGarry L, Guild W R, Briles D E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987;165:381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 24.Taktak Y S, Stenning B. Solid phase enzyme immunoassay for quantification of serum amyloid P (SAP) and complement component 3 (C3) proteins in acute-phase mouse sera. Horm Metab Res. 1992;24:371–374. doi: 10.1055/s-2007-1003338. [DOI] [PubMed] [Google Scholar]
- 25.Tart R C, McDaniel L S, Ralph B A, Briles D E. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis. 1996;173:380–386. doi: 10.1093/infdis/173.2.380. [DOI] [PubMed] [Google Scholar]
- 26.Volanakis J E. The human complement system in health and disease. In: Volanakis J E, Frank M M, editors. Overview of the complement system. New York, N.Y: Marcel Dekker, Inc.; 1998. pp. 9–32. [Google Scholar]
- 27.Weiss J J, Law S K, Levine R P, Cleary P P. Resistance to phagocytosis by group A streptococci: failure of deposited complement opsonins to interact with cellular receptors. J Immunol. 1985;134:500–505. [PubMed] [Google Scholar]
- 28.Wetsel R A, Fleischer D T, Haviland D L. Deficiency of the murine fifth complement component (C5). A 2-base pair gene deletion in a 5′-exon. J Biol Chem. 1990;265:2435–2440. [PubMed] [Google Scholar]
- 29.Winkelstein J A, Abramovitz A S, Tomasz A. Activation of C3 via the alternative complement pathway results in fixation of C3b to the pneumococcal cell wall. J Immunol. 1980;124:2502–2506. [PubMed] [Google Scholar]
- 30.Yother J, Handsome G L, Briles D E. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]