Abstract
Although frequently used, venous catheters are often associated with serious complications such as infection and thrombosis. Lock solution therapies are clinically used to deter these issues but generally address only infection or thrombosis with limited success. Here, we report the development of a dual-functional lock therapy using nitric oxide (NO) donor molecule, S-nitrosoglutathione (GSNO). NO is a potent, broad-spectrum antimicrobial agent that also temporarily inhibits platelet activation, preventing thrombosis. Furthermore, NO has antibiofilm actions, an ability that traditional antibiotic lock solutions lack, thus limiting their efficacy. In this work, different concentrations of GSNO were characterized via NO analysis to determine a range of NO-releasing lock solution (NOreLS) concentrations to investigate and to demonstrate prolonged potential efficacy. Tested against clinically used vancomycin and gentamicin lock solutions, GSNO-based NOreLS repeatedly outperformed in models of different stages of catheter infections. NOreLS also prevented clot formation when exposed to whole blood, showing increased efficacy compared to a heparin lock solution. Moreover, NOreLS was demonstrated to be biocompatible via hemolysis and cytotoxicity assays. NOreLS has excellent potential for safely and effectively preventing infection and thrombosis related to catheter usage.
Keywords: nitric oxide, infection, thrombosis, S-nitrosoglutathione, biofilm, lock solution, central venous catheter
Graphical Abstract
1. INTRODUCTION
Over 5 million central venous catheters (CVCs) are used each year for cancer treatments, hemodialysis, parenteral nutrition support, etc. in the United States alone.1 Despite their frequency of use, complications such as infection and thrombosis are unfortunately common and contribute significantly to increases in morbidity, mortality, length of hospital stay, and healthcare costs. An estimated 250 000 catheter-related bloodstream infections (CRBSIs) occur annually in the US,2 costing the healthcare system an estimated 670 million to 2.68 billion dollars per year.3 CRBSIs are acknowledged to be among the most expensive medical device-associated complications4 and the most common form of nosocomial bacteremia.2 Additionally, these infections are associated with considerable mortality rates ranging from 12.24% to 25.96%.5,6 CRBSIs can be caused by a wide range of pathogens, the most common culprits being coagulase-negative staphylococci, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans.2,7 With the alarming emergence of antibiotic-resistant pathogens, particularly in healthcare settings, the prevention of infections such as CRSBIs has become more important and treatments more difficult.8–11
Antimicrobial lock therapies are often utilized to combat CRSBIs. Current commonly used antimicrobial lock therapies contain high concentrations of antibiotics such as vancomycin, gentamicin, or cefazolin.12 However, the use of prophylactic antibiotics is undesirable due to concerns for the emergence of resistant pathogens.13 Some antiseptic lock solutions are also available, but they are generally considered less effective than antibiotic-based lock solutions14 or have been associated with adverse effects (e.g., ethanol locks).12 While antimicrobial lock solutions have been used to prevent catheter infection with moderate success,15 their efficacy is limited by their inability to penetrate biofilms.12 Biofilms are self-secreted, jellylike structures that protect the encased microbes from antibiotics, host immune cells, etc.16,17 They also provide excellent environments for proliferation and horizontal gene transfer, which is one of the most common mechanisms of acquiring antibiotic resistance mechanisms.18,19 Even highly concentrated antibiotic lock solutions are relatively ineffective against biofilms, as they are unable to penetrate the biofilm matrix.20 Moreover, attempted and ineffective treatments of biofilms with antibiotics can lead to the rapid development of resistance, exacerbating the situation.21 As biofilms are notoriously difficult to disperse or eradicate, biofilm formation on catheters necessitates removal to prevent the onset of CRBSIs and subsequent recurring infections.20 Thus, there is a need for a broad spectrum, biofilm-dispersing lock solution for improved preventative therapies.
Thrombosis, or blood clotting, is another common complication associated with the use of CVCs and can result in minor to life-threatening issues.22,23 Catheter-related thrombosis (CRT) has been found to occur in 14–16% of patients, usually within the first 100 days of CVC use,24 but the frequency increases greatly with long-term CVC use (occurrences of up to 50% in children and 60% in adults).22 Thrombi can cause vascular and catheter obstruction and provide a surface to which bacteria can easily adhere, increasing the risk of infection and sepsis.25,26 Additionally, detached thrombi have been seen to cause pulmonary embolism in 10–15% of patients with CRT and can be lethal.23,27
The prevention of thrombotic complications is preferred to treatment, but no preventative method is consistently or significantly effective.23,28 Typical prophylactic strategies include the incorporation of anticoagulants (commonly heparin) into lock solutions or systemic administration at low doses. However, there is little evidence for the efficacy of heparin lock solutions compared to normal saline in preventing thrombotic occlusions,22,29 and similarly, there is no conclusive evidence that systemic warfarin or heparin administration significantly reduces CRT.23 Moreover, these therapies are associated with serious adverse effects such as major bleeding.22,28 As such, there is a need for effective anticoagulant prophylaxis strategies to prevent CRT.
To combat the onset of CRBSIs and CRT, herein, we have investigated the use of a nitric oxide-releasing lock solution (NOreLS). Nitric oxide-releasing technologies have been incorporated into numerous promising antimicrobial and antithrombotic materials among others.30–34 Nitric oxide (NO) can kill a wide range of pathogens including those most commonly seen in CRBSIs (coagulase-negative staphylococci, P. aeruginosa, S. aureus, and C. albicans) and multidrug-resistant (MDR) strains.35,36 As NO has a nonspecific mechanism of action, it does not promote the generation of new resistant strains,35,37 a growing concern associated with the use of traditional antibiotics. Additionally, NO has been shown to disperse biofilms and prevent their formation,38,39 an ability most antimicrobial therapies lack. NO release can also help mitigate medical device-associated thrombosis as it temporarily inhibits platelet activation.40–44
Previous work has shown the potential that NO-releasing technologies have in lock therapies for effects on microbial viability,45 but their prevention of CRT has yet to be explored, and their ability to disperse catheter biofilms has yet to be demonstrated. Here, we describe a thorough in vitro investigation of NOreLS using S-nitrosoglutathione (GSNO). GSNO is an established NO donor molecule46,47 but has yet to be applied to mitigation of CRBSIs and CRT in a lock solution. GSNO has far-reaching potential applications and has been investigated in numerous clinical trials.46,48 It has demonstrated antimicrobial efficacy when incorporated into various materials (polymers,42 fibers,49 creams,50 etc.), and its solubility in aqueous solutions supports its suitability in a lock solution. Furthermore, GSNO has demonstrated antithrombotic effects via temporary inhibition of platelet activation.42,49 NOreLS’s potential to deter CRBSIs is evaluated at different time points in catheter infection progression including effects against planktonic bacteria, treatment of an established infection on medical-grade tubing, and biofilm dispersal of a 3-d growth model. CRT prevention is shown through exposure to whole blood and quantification of reduced clot area. Moreover, the biocompatibility of NOreLS is demonstrated via hemolysis and cytotoxicity assays with human umbilical vein endothelial cells (HUVECs).
2. MATERIALS AND METHODS
2.1. Materials.
Sodium nitrite, Mueller-Hinton broth (MHB) and agar (MHA), Cell Counting Kit-8 (CCK-8), heparin (HEP), and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO 63103). Tygon non-DEHP Surgical and Hospital Tubing (nd-100–65) was purchased from Thomas Scientific (Swedesboro, NJ). Acetone was purchased from VWR (Radnor, PA). Phosphate-buffered saline (PBS), pH 7.4, which was used for all in vitro experiments, contained 138 mM NaCl, 2.7 mM KCl, and 10 mM sodium phosphate. The bacterial strains MDR P. aeruginosa (ATCC BAA 2110) and methicillin resistant S. aureus (ATCC BAA 41) were purchased from American Type Culture Collection (ATCC). Crystal violet was obtained from Fisher Scientific (Waltham, MA). Reduced glutathione, Gentamicin (GEN), and Vancomycin (VAN) were purchased from GoldBio (Saint Louis, MO). Drabkin’s reagent was purchased from Ricca Chemical Company (Arlington, TX). Trypsin–EDTA was purchased from Corning (Corning, NY). Fetal bovine serum (FBS) and penicillin–streptomycin (Pen–Strep) were purchased from Gibco-Life Technologies (Grand Island, NY). Porcine blood was purchased from Animal Technologies (Tyler, TX).
2.2. Preparation of NOreLS.
GSNO was synthesized as reported previously.51 Briefly, reduced glutathione (5 g/16 mmol) was dissolved in 12 M HCl and DI water and cooled in an ice bath. Excess NaNO2 (1.2 g/17 mmol) was added to the mixture, which remained in the ice bath for 40 min. Cold acetone was added to precipitate GSNO, which was collected via vacuum filtration and washed with additional cold acetone and DI water. Once dried overnight under vacuum, GSNO was kept in the dark at −20 °C until used. All NOreLS’s tested consisted of GSNO dissolved in PBS buffer, pH 7.4.
2.3. Nitric Oxide Release Analysis.
NO release measurements were conducted using a Sievers chemiluminescence Nitric Oxide Analyzer (NOA) model 280i (Boulder, CO). A baseline was recorded with 3 mL of PBS with 100 mM EDTA before being replaced with 3 mL of NOreLS. The samples were measured at 37 °C to simulate physiological environments. Nitrogen was bubbled into the solution to continuously carry NO into the chemiluminescence detection chamber. The NO release measurements were normalized using the volume of the samples. Unless otherwise indicated, samples were analyzed directly after dissolution. Where applicable, the samples were kept in the dark in an incubator at 37 °C between measurements.
2.4. Antibacterial Assessment.
MRSA strain ATCC BAA 41 and MDR P. aeruginosa strain ATCC BAA 2110 with MHB, MHA, or PBS were used for all antibacterial experiments. Plating of colony forming units (CFU) was aided by an Eddy Jet W2 spiral plater (IUL, Farmingdale, NY), and the viable colonies on the resulting plates were counted via a SphereFlash automated colony counter (IUL, Farmingdale, NY) after overnight incubation at 37 °C. The limit of detection of the spiral plater is noted to be 2 × 102 CFU/mL.
2.4.1. Time Kill Assay.
Time kill assays were conducted with slight modifications to a previously reported procedure.52 Inoculum cultures were grown until reaching the log phase in MHB before washing with PBS and diluting to a starting OD 600 of 0.05. Growth controls and samples were incubated at 37 °C in a 48-well plate and shaken at 150 rpm. Samples included varying concentrations of GSNO (1, 5, 10, and 20 mg/mL), 5 mg/mL VAN, or 5 mg/mL GEN all dissolved in MHB. VAN and GEN are commonly used antibiotic lock solutions53,54 and were included for comparison purposes. Aliquots were taken at specific time points (T = 2, 8, and 24 h) and serially diluted 10-fold in PBS. Only plates with individual colonies able to be detected by the SphereFlash automated colony counter were used. In the event that repeated plating of nondiluted aliquots showed no bacteria growth, the CFU was considered to be 0. These data points are denoted with an asterisk. Data are reported as time-kill curves, plotting the log10 CFU/mL versus time.
2.4.2. Treatment of 24 h Infected Tubing.
Sterile, medical-grade tubing was filled with 0.3 OD 600 MRSA or MDR P. aeruginosa in MHB after inoculums were grown until log phase and washed with PBS. The infected tubing was incubated at 37 °C for 24 h before being gently rinsed with sterile PBS. The tubing was then locked with PBS, VAN (5 mg/mL in PBS), GEN (5 mg/mL in PBS), or NOreLS (1, 5, 10, or 20 mg/mL) for 2 h at 37 °C and in the dark. After the 2 h treatment period, the tubing was gently flushed with PBS before the attached bacteria were removed via homogenization in PBS. The bacterial solutions were serial diluted 10-fold in PBS before plated. Following overnight incubation at 37 °C, the viable CFU were counted and reported as CFU/cm2. N = 5 per sample type is reported.
2.4.3. 72 h Anti-Biofilm Assessment.
A crystal violet assay was performed to evaluate the biofilm dispersing abilities of the lock solutions, similar to a previously reported procedure.55 Sterile, medical-grade tubing was filled with 0.1 OD 600 MRSA or MDR P. aeruginosa in MHB and shaken at 150 rpm at 37 °C for 72 h. The indwelling solution was removed and replaced with fresh MHB approximately every 12 h. After 72 h of growth, the tubing samples with the resulting biofilms were gently rinsed with PBS to remove unadhered bacteria before being locked with the NOreLS or antibiotic lock therapies for 24 h at 37 °C in the dark. The growth control or treated tubing samples were gently rinsed with PBS after 72 h of growth or 24 h of treatment, respectively. After washing, the samples were then filled with 0.1% crystal violet for 15 min before being repeatedly flushed with PBS to remove unbound dye. The tubing was cut into 1 cm segments and placed into 300 μL of 30% acetic acid to dissolve the stained biofilm. The absorbance of the resulting solution was read at 550 nm. The amount of remaining biofilm relative to the growth control was calculated using eq 1. N = 3 per sample type is reported:
| (1) |
Viability of bacteria within the biofilms after treatment was assessed. The 72 h biofilms were grown in medical grade tubing as described above. After 72 h (for the growth controls) or the 24 h treatments, the tubings were cut into 1 cm sections and homogenized in PBS. The samples were then serially diluted and plated. Following overnight incubation at 37 °C, the viable CFU were counted and reported as CFU/cm2. N = 6 per sample type is reported.
2.5. Antithrombotic Assessment.
Locked medical-grade tubing samples containing PBS, HEP (10k units/mL), or NOreLS (1, 5, 10, or 20 mg/mL) were incubated in porcine whole blood to model clotting on the tip of a CVC. Calcium chloride was added to fresh porcine blood with sodium citrate to reverse anticoagulation. Tubing containing lock solution was plugged at one end, leaving the other end open to resemble the tip of a CVC. Each sample was individually placed in 1 mL of whole blood for 20 min before rinsed in CMF-PBS. The surface areas of attached clots were quantified using ImageJ. Data are presented as relative surface area of attached clots compared to those of the PBS control. Final values are reported as the mean ± standard deviation (n = 3).
2.6. Biocompatibility Assessment.
2.6.1. Hemolysis.
Hemolysis assessments were conducted according to the NAMSA ASTM F756 protocol. Briefly, fresh porcine whole blood was diluted with calcium and magnesium-free phosphate-buffered saline (CMF-PBS) to a total hemoglobin concentration of 10 ± 1.0 mg/mL. One milliliter of diluted whole blood was incubated with 7 mL of CMF-PBS as a negative control, sterile DI water as a positive control, or CMF-PBS containing sample lock solutions. Varying blood/lock solution ratios were tested to simulate full leakage over time and specific leakage events (0.06–0.2% lock solution in volume). The samples were incubated at 37 °C for 3 h with periodic inversions before centrifuging at 800g for 15 min. The supernatant containing any freed hemoglobin was combined 1:1 with Drabkin’s reagent and allowed to stand for 15 min before the absorbance at 540 nm was quantified. In the event that an experimental hemolytic activity was found to be less than zero, it is reported here to be 0.0. Percent hemolysis was calculated via eq 2:
| (2) |
2.6.2. Cytocompatibility.
Cellular biocompatibility was assessed via 24 h in vitro cytotoxicity assays with HUVECs. Cryopreserved stocks of HUVECs were revived and cultured in Clonetics EGM-2 Bulletkit containing endothelial cell growth basal medium-2 (EBM-2) supplemented with human Epidermal Growth Factor, Vascular Endothelial Growth Factor, R3-Insulin-like Growth Factor, ascorbic acid, hydrocortisone, human Fibroblast Growth Factor-Beta, heparin, fetal bovine serum, and Gentamicin/Amphotericin-B per the manufacturer’s instructions. HUVECs were subcultured for no more than ten passages between experiments. Cells were grown to ~70% monolayer confluency before being detached with 0.05% trypsin supplemented with 5 mM EDTA. Detached cells were centrifuged down, resuspended in supplemented EBM-2, stained with trypan blue dye, and counted for the total number of viable cells using an EVE cell counting system. In all cytotoxicity tests, 10 000 cells were seeded per well in 96-well plates and grown for 24 h to achieve >70% confluency before testing. Cells were then treated with 0.06%, 0.09%, or 0.2% solutions of vancomycin, gentamicin, heparin, and NOreLS in fresh media to mimic any potential leaking of the lock solution. Cells were incubated for an additional 24 h, and afterward, the media in each well was replaced once more with fresh media supplemented with 10% Cell Counting Kit-8 (CCK-8) reagent. Cells were incubated with CCK-8 for 1 h, during which time dehydrogenase reactions of nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate converted the water-soluble tetrazolium dye to its formazan derivative. The presence of the formazan derivative was then quantified via plate reading (OD 450 nm) with baseline reading (OD 650 nm). The final relative cellular viability for each dilution was calculated according to eq 3, with respect to the viability of untreated control wells. Final values are reported as the mean ± standard deviation (n = 3 treatment groups from independent passages):
| (3) |
2.7. Statistical Analysis.
Averages and standard deviations were calculated using Graphpad Prism 8. Statistical significance was determined using a one-way analysis of variance (ANOVA) on the same software. A P value < 0.05 was considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. NO Release Studies.
When in the presence of heat, light, or metal ions, NO is released from GSNO, which then forms a dithiol bond with another glutathione molecule to create glutathione disulfide (GSSG)46 (Figure 1A). To determine an appropriate range of NO-releasing solutions to investigate, several concentrations of GSNO in PBS were analyzed via NOA (Figure 1B). Although the trend is not linear, there is a positive correlation between GSNO concentration and NO flux. NO flux steeply increased with increasing GSNO concentrations until 25 mg/mL. No statistically significant difference was observed in NO release between 20 and 25 mg/mL solutions, though 25 mg/mL took considerably longer to prepare (i.e., ~25 min to dissolve versus 1 min for 20 mg/mL). Since this extended preparation time may limit the potential clinical application of NOreLS, 20 mg/mL GSNO preparations in PBS were chosen as the maximum concentration for further analysis.
Figure 1.
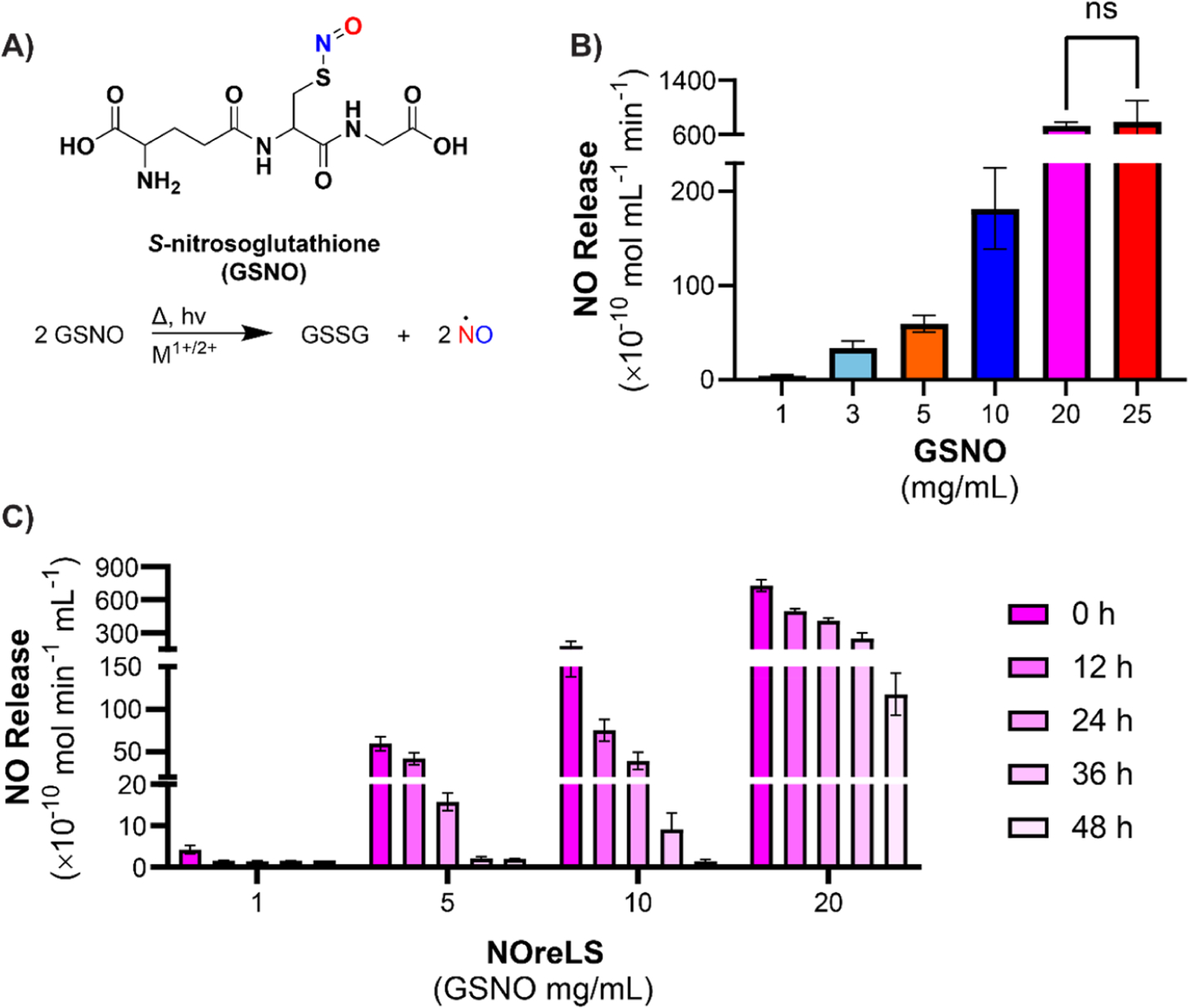
(A) GSNO releases NO when exposed to heat, light, or metal ions. (B) NO release of solutions containing varying concentrations of GSNO quantified via NOA at 37 °C. GSNO is dissolved in PBS, pH 7.4. ns indicates no statistically significant difference. (C) NO release of NOreLS at various time points quantified via NOA at 37 °C. Data are shown as the mean ± standard deviation (n = 3 repeats tested per formulation).
The dwell time of lock solutions is recommended to be 8 to 24 h,54 but realistically can be 2 min to 48 h.56 Thus, the NO release of NOreLS over 48 h was measured to demonstrate prolonged potential efficacy (Figure 1C). Statistical analysis is reported in Table S1. All GSNO concentrations tested showed release for at least 48 h, which is notably longer than the previous NO-releasing lock solution reported (24 h).45 This indicates that NOreLS can sustain NO release levels for clinically relevant durations, an issue that has limited the clinical use of other NO-releasing compounds.57
3.2. Antibacterial Evaluations.
To demonstrate the antibacterial effects of NOreLS, it was tested against MRSA and MDR P. aeruginosa to represent Gram-positive and Gram-negative bacteria, respectively, and drug-resistant strains. S. aureus and P. aeruginosa are also two of the most common causes of CRBSIs.7 PBS was used as a control, and two antibiotics commonly used in clinical lock solutions, gentamicin (GEN) and vancomycin (VAN), were included for comparison. The antibiotics were used at concentrations of 5 mg/mL as this is one of the highest concentrations used clinically considering solubility and precipitation concerns.54
Time kill assays were used to simulate the start of a potential CRBSI, that is, the effects of lock solutions on planktonic bacteria. Decreases in viable MRSA CFUs were seen over time for the antibiotics and 5, 10, and 20 mg/mL NOreLS (Figure 2A). The 1 mg/mL NOreLS did not affect the viability of MRSA. No viable CFUs were observed after 2 h treatments with 20 mg/mL NOreLS and GEN, 8 h treatment with 10 mg/mL NOreLS, and 24 h treatment with VAN. Similar trends were seen in MDR P. aeruginosa treatment (Figure 2B). No viable CFUs were seen after 2 h with GEN, 10, and 20 mg/mL NOreLS, 8 h with 5 mg/mL NOreLS, and 24 h with VAN. Statistical analyses are reported in Tables S2 and S3. These results indicate that GEN and 20 mg/mL NOreLS are most effective at quickly preventing both Gram-positive and -negative bacterial colonization.
Figure 2.
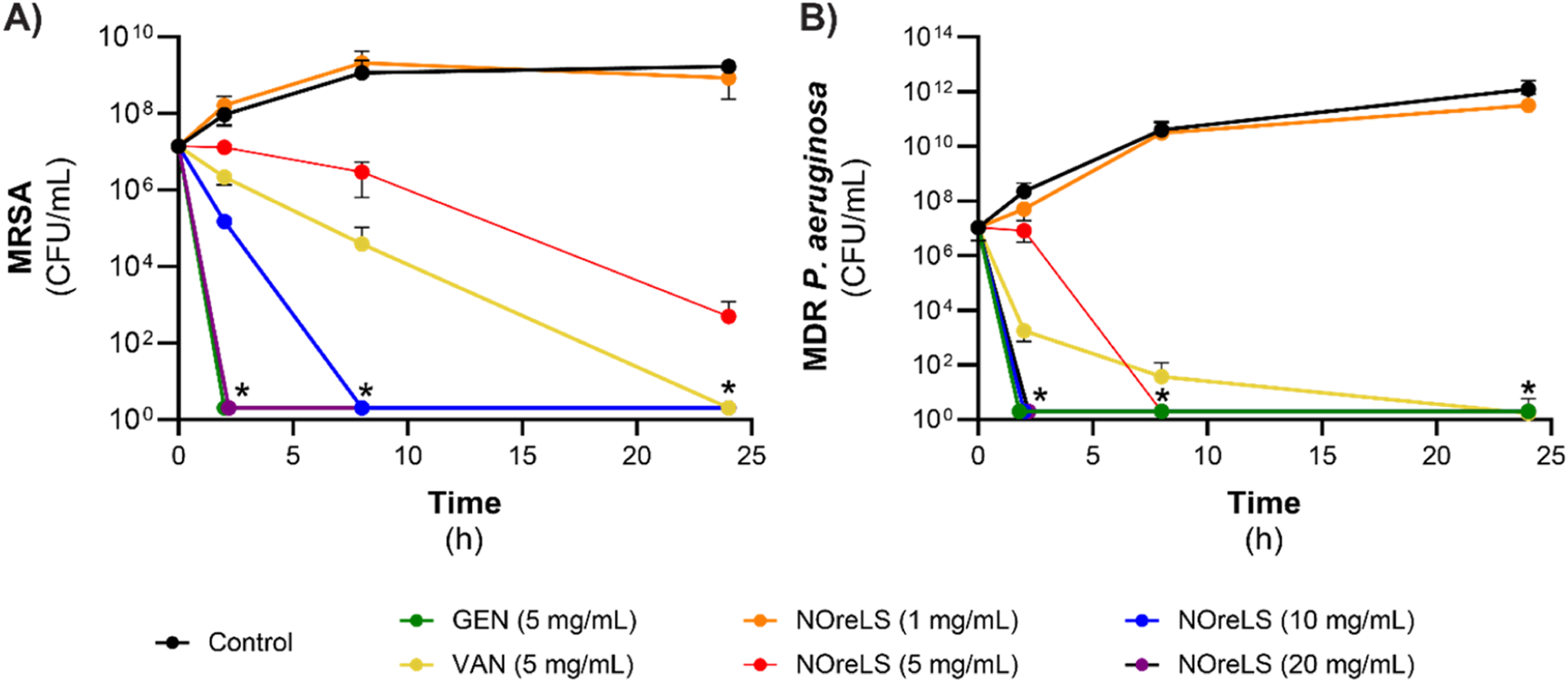
Time kill curves of (A) MRSA and (B) MDR P. aeruginosa. *No viable CFU detected. Data are shown as the mean ± standard deviation (n = 3 repeats tested per formulation).
Once infected, current inabilities to eradicate adhered bacteria necessitate the catheter’s removal to prevent CRBSIs.3 However, removal and replacement are not always options for critical patients who may have multiple catheters or pediatric patients with limited access sites.12 Therefore, it is highly desirable to have more effective antimicrobial lock therapies to prevent infected catheters leading to CRBSIs. To model an infected catheter, medical-grade tubing was filled with bacterial solutions and incubated for 24 h prior to 2-h lock therapies. Statistically significant decreases in viable MRSA CFUs on tubing were seen after treatment with GEN, 10, and 20 mg/mL NOreLS with 2.5-, 3.2-. and 4.4-fold reductions, respectively (Figure 3A). Moreover, 20 mg/mL NOreLS significantly decreased viable CFUs (p < 0.05) compared to GEN and VAN, as well as 1 and 5 mg/mL NOreLS, which did not have significant effects on the established MRSA infections. Similar to the time kill assays, MDR P. aeruginosa was more susceptible to NOreLS compared to MRSA when treating colonies adhered to tubing (Figure 3B). GEN as well as 5, 10, and 20 mg/mL NOreLS showed significant reductions (p < 0.05) in viable MDR P. aeruginosa. However, treatment with GEN, which only had a decrease slightly above 1-fold, was significantly (p < 0.01) outperformed by 5, 10, and 20 mg/mL NOreLS, which had 4.1-, 4.9-, and 5.2-fold reductions, respectively.
Figure 3.
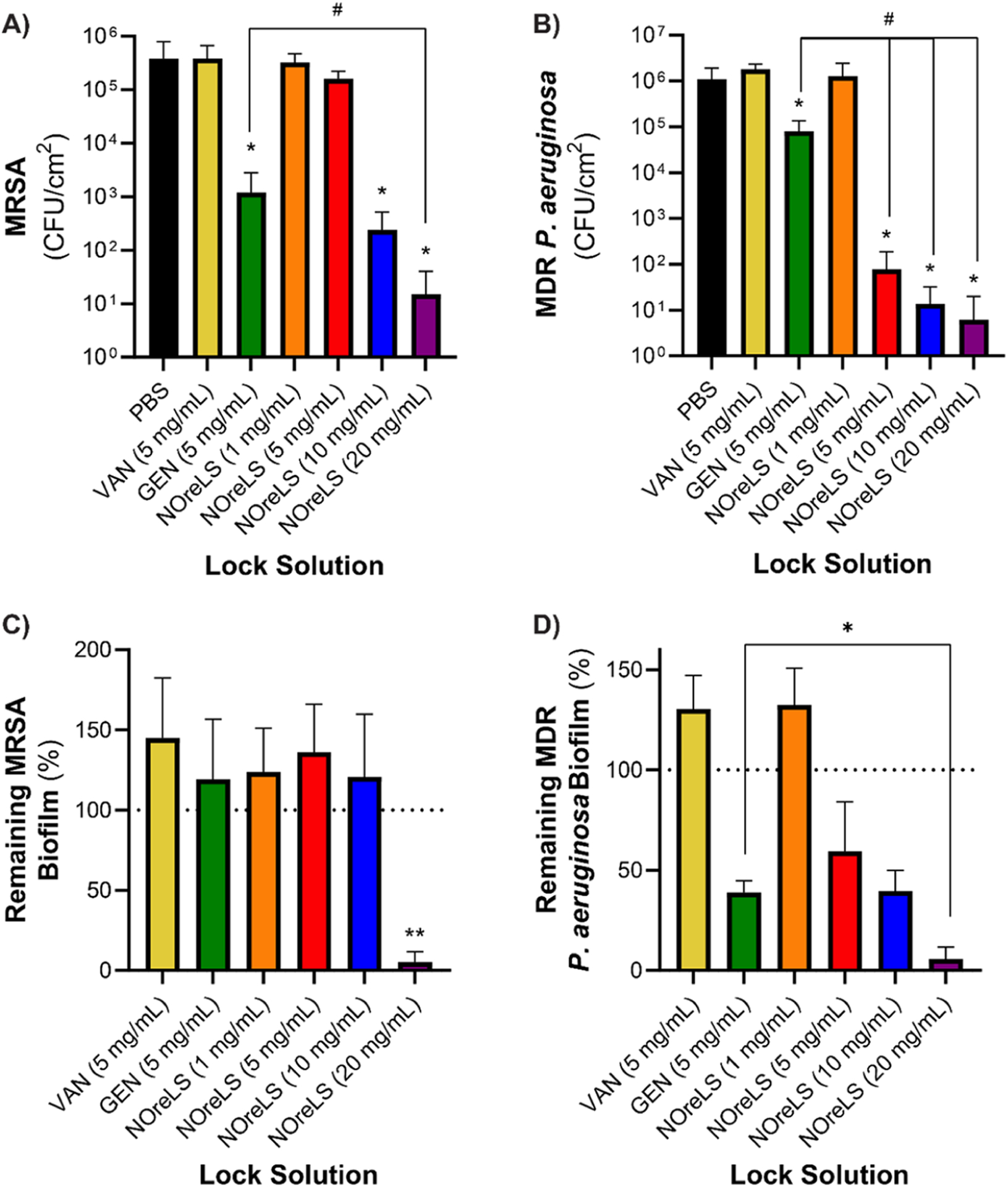
(A) MRSA and (B) MDR P. aeruginosa adherence to medical grade tubing after 24 h bacterial exposure and 2 h lock therapy. *p < 0.05 compared to control; # p < 0.01 compared to GEN. Remaining (C) MRSA and (D) MDR P. aeruginosa biofilm on medical grade tubing after 24 h lock treatments compared to a growth control. **p < 0.01 compared to all samples; *p < 0.05 compared to GEN. Data are shown as the mean ± standard deviation.
Biofilms are a formidable adversary in combatting device-associated infections, including CRBSI. Biofilm penetration or dispersion are two major challenges for antimicrobial therapies.58 The 72-h biofilms were grown within medical grade tubing as a model of severely infected CVCs. The 24-h lock therapies within these samples demonstrated the greatly improved biofilm-mitigating capabilities of NOreLS compared to clinically used antibiotic lock solutions. Compared to 72-h growth controls, 24-h biofilm treatments with VAN, GEN, as well as 1, 5, and 10 mg/mL NOreLS did not decrease the amount of MRSA biofilm present on the tubing (Figure 3C). This is consistent with previous studies with treatments of VAN, GEN, and low levels of NO with S. aureus biofilms.59–61 However, VAN, GEN, and 5 and 10 mg/mL NOreLS did decrease the number of viable bacteria within the biofilms even if they were unable to disperse it (Figure S1a). Treatment with 20 mg/mL NOreLS reduced the adhered biofilms by 94.73 ± 6.51%, which is statistically significant (p < 0.01) compared to all other treatments. As with all other antibacterial assessments, MDR P. aeruginosa biofilms were more susceptible to treatment with NOreLS compared to MRSA (Figure 3D). Compared to the 72-h growth controls, 5, 10, and 20 mg/mL NOreLS decreased adhered biofilms by 40.55 ± 24.66%, 60.41 ± 10.29%, and 94.21 ± 5.90%, respectively. GEN also reduced the amount of biofilm adhered to the tubing by 61.09 ± 5.92%, but it was significantly (p < 0.05) outperformed by 20 mg/mL NOreLS. VAN and 1 mg/mL NOreLS treatments did not deter the MDR P. aeruginosa biofilm, but VAN (and all other lock solutions excepting 1 mg/mL NOreLS) was able to reduce the viable bacteria within it (Figure S1b).
These combined results indicate that 20 mg/mL NOreLS is a fast-acting, broad-spectrum antimicrobial therapy that has the potential as a lock therapy to prevent the onset of CRBSIs at many stages of bacteria colonization of CVCs. Compared to the commonly used clinical antibiotic lock therapies (VAN and GEN), only GEN matched 20 mg/mL NOreLS for efficiency in the time-kill assay, and 20 mg/mL NOreLS continually outperformed both antibiotics in all assessments involving established infections.
3.3. Antithrombotic Evaluation.
Antithrombotic lock solutions aim to prevent thrombosis at the tip of the catheter, where clots commonly form.62 Locked medical-grade tubing that contained PBS, HEP, or NOreLS (1, 5, 10, or 20 mg/mL) and was open at one end was incubated in whole blood to observe prevention of thrombi formation on the samples (Figure 4). Compared to the PBS controls, all HEP and NOreLS samples reduced clot formation on the surface of the tubing. HEP only reduced the clot area by 44.0 ± 11.7%. There is a trend of further area reduction with increasing NOreLS concentrations with both 10 and 20 mg/mL being statistically significant compared to HEP, reducing the clot areas by 73.5 ± 5.0% and 81.2 ± 7.8%, respectively. This supports that NOreLS has excellent potential to address efficacy issues currently seen in clinically used antithrombotic lock solutions.
Figure 4.
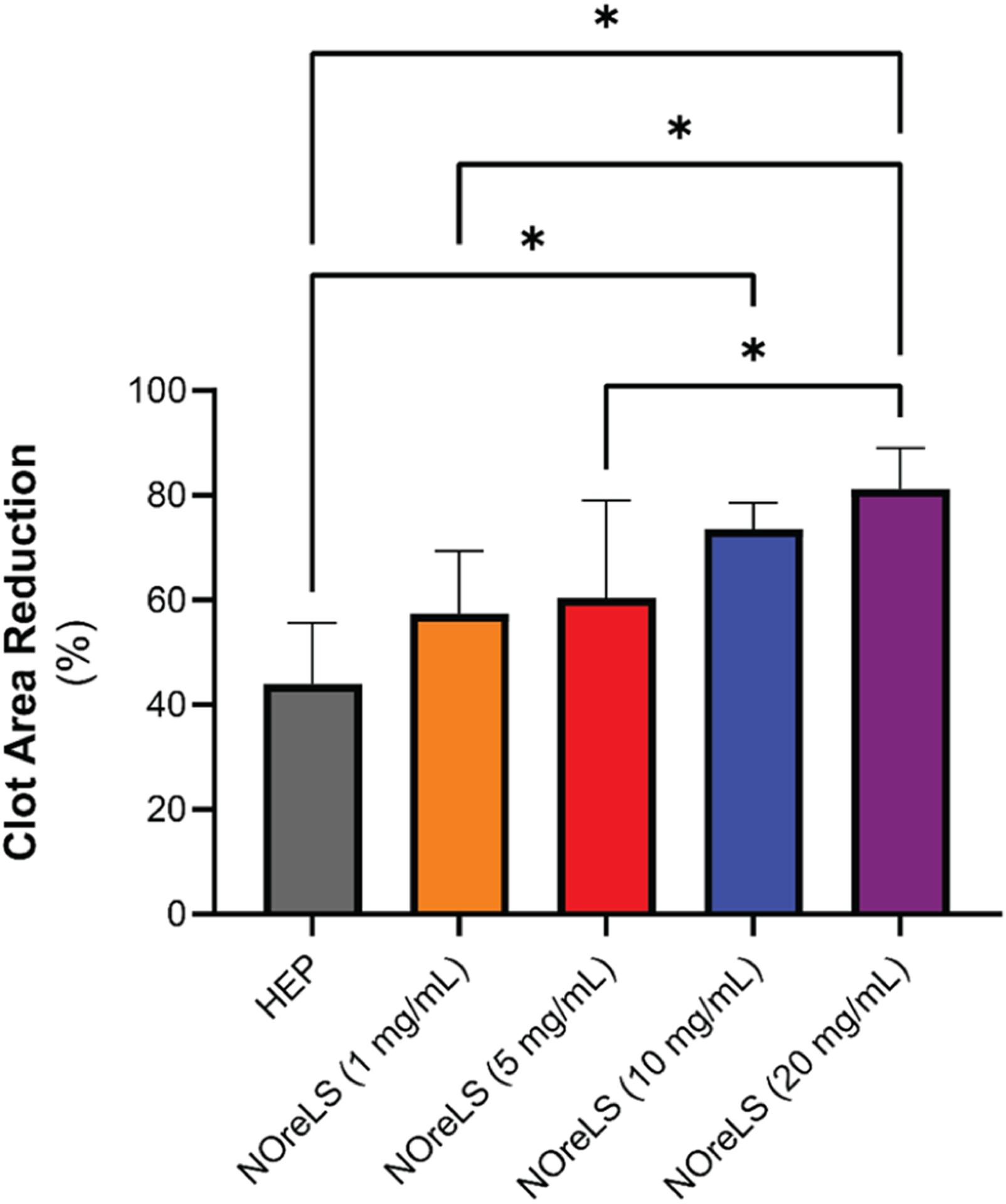
Percent reduction in clot area on medical grade tubing with lock therapy after 20 min exposure in porcine whole blood. Data are shown as mean ± standard deviation (n = 4). *p < 0.05 between compared groups.
3.4. Biocompatibility Evaluations.
The small volumes of lock solution that may leak from a CVC are generally not considered to be a major toxicity concern,54 particularly because catheter tips are commonly positioned within the superior vena cava,63 a place of rapid, high-volume blood flow. However, as 10–15% of the lock solution may leak into circulation within the first 10 min of administration,64 the effects of concentrated lock solutions should be considered. The volume per lumen and number of lumens vary between devices. Even for larger devices, ≤ 3 mL of lock solution will sufficiently fill the lumen, but it has been reported that 5–10 mL of lock solution may be used clinically.62 A range of leaked lock solution volumes was thus tested. The 3 mL, 4.5 mL, and 10 mL of leaked lock solution into 5 L of blood correspond to the 0.06%, 0.09%, and 0.2% lock solution (calculated using eq 4) used in biocompatible evaluations:
| (4) |
As they are primarily in contact with blood, the potential hemolytic effects of clinically used lock solutions (PBS, VAN, GEN, and HEP) and NOreLS were evaluated (Table 1). According to the NAMSA ASTM F756 protocol, samples that have a 2–5% hemolytic activity are considered slightly hemolytic and >5% are hemolytic.65 All NOreLS, antibiotic lock solutions, and heparin lock solution at all lock-solution-to-blood ratios were therefore found to be nonhemolytic.
Table 1.
Hemolytic Activities of Clinically Used Lock Solutions and NOreLSa
| Lock Solution | 0.06% LS | 0.09% LS | 0.2% LS |
|---|---|---|---|
| VAN (5 mg/mL) | 0.0 | 0.0 | 0.0 |
| GEN (5 mg/mL) | 0.0 | 0.0 | 0.001 ± 0.023 |
| HEP (10k unit/mL) | 0.005 ± 0.023 | 0.0 | 0.0 |
| NOreLS (1 mg/mL) | 0.005 ± 0.023 | 0.0 | 0.0 |
| NOreLS (5 mg/mL) | 0.0 | 0.0 | 0.0 |
| NOreLS (10 mg/mL) | 0.0 | 0.0 | 0.0 |
| NOreLS (20 mg/mL) | 0.0 | 0.0 | 0.0 |
Data are shown as the mean ± standard deviation (n = 3 repeats tested per formulation).
Further cytocompatibility evaluation was conducted with HUVECs to explore any potential cytotoxic effects of the clinically used lock solutions and NOreLS based on potential percent by volume leaking of the lock solutions into the physiological environment. In parallel to blood studies, confluent monolayers of HUVECs were treated with 0.06%, 0.09%, and 0.20% solutions of VAN, GEN, HEP, and NOreLS prepared in culture media to evaluate any cytotoxic effects from lock solution leaking. As shown in Figure 5, leaking of the lock solution biocidal/anticoagulant agents did not induce any cytotoxic response in HUVECs, with each treatment resulting in >80% cellular viability with respect to untreated controls.
Figure 5.
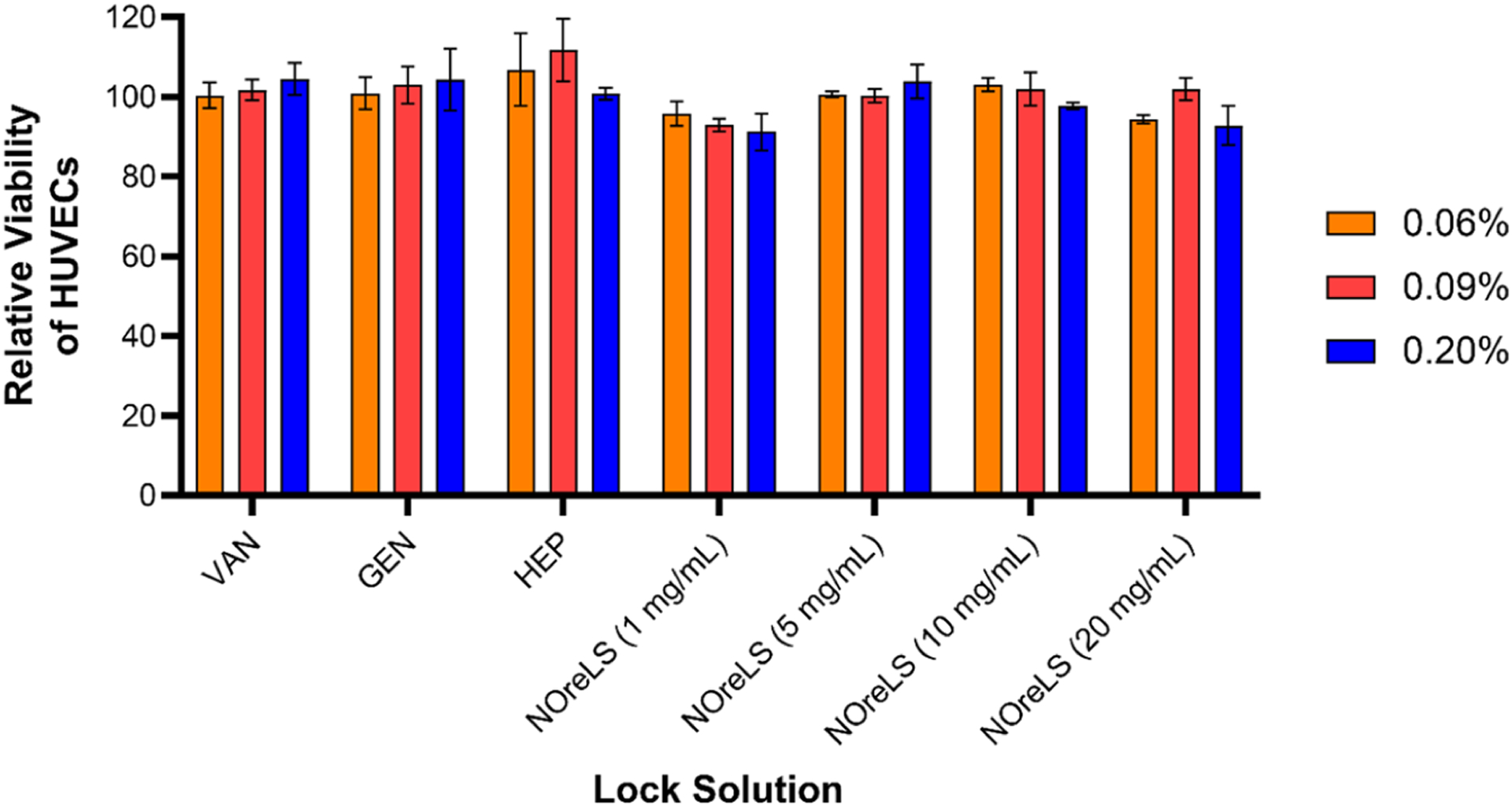
Cellular cytocompatibility evaluation of lock solutions with HUVECs over 24 h studies with 0.06%, 0.09%, and 0.20% solutions of the antimicrobial/anticlotting agents. Lock solution dilutions reflect possible concentrations of antibiotic/anticlotting agents that may leak into the physiological environment. Data are shown as mean ± standard deviation (n = 3).
4. CONCLUSION
CVC use is often complicated by CRBSIs and CRT, leading to exacerbated healthcare issues. In this work, NOreLSs containing the NO donor molecule GSNO are shown to have the potential to combat these complications. Varying concentrations of NOreLS characterized via NOA all showed NO release for at least 48 h, the maximum dwell time for a lock solution. NOreLS consistently outperformed clinically used antibiotic lock solutions (VAN and GEN) at different stages of bacterial colonization of medical-grade tubing. Notably, 20 mg/mL NOreLS was able to reduce adhered MRSA and MDR P. aeruginosa biofilms by 94.73 ± 6.51% and 94.21 ± 5.90%, respectively. The 20 mg/mL NOreLS was also able to reduce the area of clot formation on medical-grade tubing by 81.2 ± 7.8%, surpassing the effects of a clinically relevant HEP lock solution. All concentrations of NOreLS were established to be nonhemolytic and noncytotoxic to HUVECs. In conclusion, this work demonstrates the potential of NOreLS in deterring CRBSIs and CRT and, thus, improving the medical outcome and quality of life of patients with CVCs.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this work was supported by the National Institutes of Health Grant R01HL134899 and the National Science Foundation Graduate Research Fellowship under Grant No. 1842396.
ABBREVIATIONS
- CVCs
central venous catheters
- CRSBIs
catheter-related bloodstream infections
- CRT
catheter-related thrombosis
- NOreLS
nitric oxide-releasing lock solution
- NO
nitric oxide
- GSNO
S-nitrosoglutathione
- HUVECs
human umbilical vein endothelial cells
- MHB
Mueller-Hinton broth
- MHA
Mueller-Hinton agar
- HEP
heparin
- PBS
phosphate-buffered saline
- MRSA
methicillin-resistant Staphylococcus aureus
- MDR
multi-drug resistant
- GEN
gentamicin
- VAN
vancomycin
- FBS
fetal bovine serum
- Pen-Strep
penicillin–streptomycin
- NOA
nitric oxide analyzer
- CFU
colony forming unit
- CMF-PBS
calcium and magnesium-free phosphate-buffered saline
- CCK-8
cell counting kit-8
- ANOVA
analysis of variance
- GSSG
glutathione disulfide
- EBM-2
endothelial cell growth basal medium-2
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsabm.1c01272.
Calculated P values for NO release over time; calculated P values for MRSA and MDR P. aeruginosa time kill assays; MRSA and MDR P. aeruginosa adherence to medical grade tubing after 24 h bacterial exposure and 2 h lock therapy (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsabm.1c01272
The authors declare no competing financial interest.
Contributor Information
Morgan Ashcraft, Pharmaceutical and Biomedical Sciences Department, College of Pharmacy, University of Georgia, Athens, Georgia 30602, United States.
Megan Douglass, School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
Mark Garren, School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
Arnab Mondal, School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
Lori Estes Bright, School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
Yi Wu, School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
Hitesh Handa, Pharmaceutical and Biomedical Sciences Department, College of Pharmacy and School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
REFERENCES
- (1).Kornbau C; Lee KC; Hughes GD; Firstenberg MS Central line complications. Int. J. Crit Illn Inj Sci 2015, 5 (3), 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gahlot R; Nigam C; Kumar V; Yadav G; Anupurba S Catheter-related bloodstream infections. Int. J. Crit Illn Inj Sci 2014, 4 (2), 162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Shah H; Bosch W; Thompson KM; Hellinger WC Intravascular catheter-related bloodstream infection. Neurohospitalist 2013, 3 (3), 144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Anand P; Kranker K; Chen AY Estimating the hospital costs of inpatient harms. Health Serv Res 2019, 54 (1), 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zhong Y; Zhou L; Liu X; Deng L; Wu R; Xia Z; Mo G; Zhang L; Liu Z; Tang J Incidence, Risk Factors, and Attributable Mortality of Catheter-Related Bloodstream Infections in the Intensive Care Unit After Suspected Catheters Infection: A Retrospective 10-year Cohort Study. Infect Dis Ther 2021, 10 (2), 985–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ziegler MJ; Pellegrini DC; Safdar N Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection 2015, 43 (1), 29–36. [DOI] [PubMed] [Google Scholar]
- (7).Goede MR; Coopersmith CM Catheter-related bloodstream infection. Surg Clin North Am 2009, 89 (2), 463–74. [DOI] [PubMed] [Google Scholar]
- (8).Mielke M [The role of infection prevention in the control of antimicrobial resistance: Any avoided infection contributes to the reduction of antibiotic use]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018, 61 (5), 553–561. [DOI] [PubMed] [Google Scholar]
- (9).Roca I; Akova M; Baquero F; Carlet J; Cavaleri M; Coenen S; Cohen J; Findlay D; Gyssens I; Heuer OE; Kahlmeter G; Kruse H; Laxminarayan R; Liebana E; Lopez-Cerero L; MacGowan A; Martins M; Rodriguez-Bano J; Rolain JM; Segovia C; Sigauque B; Tacconelli E; Wellington E; Vila J The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect 2015, 6, 22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ventola CL The antibiotic resistance crisis: part 1: causes and threats. P T 2015, 40 (4), 277–83. [PMC free article] [PubMed] [Google Scholar]
- (11).Whitelaw AC Role of infection control in combating antibiotic resistance. S Afr Med. J 2015, 105 (5), 421. [DOI] [PubMed] [Google Scholar]
- (12).Kim EY; Saunders P; Yousefzadeh N Usefulness of anti-infective lock solutions for catheter-related bloodstream infections. Mt Sinai J. Med 2010, 77 (5), 549–58. [DOI] [PubMed] [Google Scholar]
- (13).Srinivasan A Antibiotic stewardship: Why we must, how we can. Cleve Clin J. Med 2017, 84 (9), 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Arechabala MC; Catoni MI; Claro JC; Rojas NP; Rubio ME; Calvo MA; Letelier LM Antimicrobial lock solutions for preventing catheter-related infections in haemodialysis. Cochrane Database Syst. Rev 2018, 4, CD010597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zhang J; Li RK; Chen KH; Ge L; Tian JH Antimicrobial lock solutions for the prevention of catheter-related infection in patients undergoing haemodialysis: study protocol for network meta-analysis of randomised controlled trials. BMJ. Open 2016, 6 (1), No. e010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Flemming HC; Wingender J; Szewzyk U; Steinberg P; Rice SA; Kjelleberg S Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol 2016, 14 (9), 563–75. [DOI] [PubMed] [Google Scholar]
- (17).Lebeaux D; Ghigo JM; Beloin C Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol. Biol. Rev 2014, 78 (3), 510–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Madsen JS; Burmolle M; Hansen LH; Sorensen SJ The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med. Microbiol 2012, 65 (2), 183–95. [DOI] [PubMed] [Google Scholar]
- (19).Steenackers HP; Parijs I; Dubey A; Foster KR; Vanderleyden J Experimental evolution in biofilm populations. FEMS Microbiol Rev 2016, 40 (3), 373–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Gominet M; Compain F; Beloin C; Lebeaux D Central venous catheters and biofilms: where do we stand in 2017? APMIS 2017, 125 (4), 365–375. [DOI] [PubMed] [Google Scholar]
- (21).Penesyan A; Nagy SS; Kjelleberg S; Gillings MR; Paulsen IT Rapid microevolution of biofilm cells in response to antibiotics. NPJ. Biofilms Microbiomes 2019, 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Baskin JL; Pui CH; Reiss U; Wilimas JA; Metzger ML; Ribeiro RC; Howard SC Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374 (9684), 159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wall C; Moore J; Thachil J Catheter-related thrombosis: A practical approach. J. Intensive Care Soc 2016, 17 (2), 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kamphuisen PW; Lee AY Catheter-related thrombosis: lifeline or a pain in the neck? Hematology Am. Soc. Hematol Educ Program 2012, 2012, 638–44. [DOI] [PubMed] [Google Scholar]
- (25).Nakazawa N Infectious and thrombotic complications of central venous catheters. Semin Oncol Nurs 2010, 26 (2), 121–31. [DOI] [PubMed] [Google Scholar]
- (26).Rooden CJ; Tesselaar ME; Osanto S; Rosendaal FR; Huisman MV Deep vein thrombosis associated with central venous catheters - a review. J. Thromb Haemost 2005, 3 (11), 2409–19. [DOI] [PubMed] [Google Scholar]
- (27).Burns KE; McLaren A Catheter-related right atrial thrombus and pulmonary embolism: a case report and systematic review of the literature. Can. Respir J 2009, 16 (5), 163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lee AY; Kamphuisen PW Epidemiology and prevention of catheter-related thrombosis in patients with cancer. J. Thromb Haemost 2012, 10 (8), 1491–9. [DOI] [PubMed] [Google Scholar]
- (29).Mitchell MD; Anderson BJ; Williams K; Umscheid CA Heparin flushing and other interventions to maintain patency of central venous catheters: a systematic review. J. Adv. Nurs 2009, 65 (10), 2007–21. [DOI] [PubMed] [Google Scholar]
- (30).Liang H; Nacharaju P; Friedman A; Friedman JM Nitric oxide generating/releasing materials. Future Sci. OA 2015, 1 (1). DOI: 10.4155/fso.15.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Rong F; Tang Y; Wang T; Feng T; Song J; Li P; Huang W Nitric Oxide-Releasing Polymeric Materials for Antimicrobial Applications: A Review. Antioxidants (Basel) 2019, 8 (11), 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ashcraft M; Douglass M; Chen Y; Handa H Combination strategies for antithrombotic biomaterials: an emerging trend towards hemocompatibility. Biomaterials Science 2021, 9, 2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Shen Z; Zheng S; Xiao S; Shen R; Liu S; Hu J Red-Light-Mediated Photoredox Catalysis Enables Self-Reporting Nitric Oxide Release for Efficient Antibacterial Treatment. Angew. Chem., Int. Ed 2021, 60 (37), 20452–20460. [DOI] [PubMed] [Google Scholar]
- (34).Tao S; Cheng J; Su G; Li D; Shen Z; Tao F; You T; Hu J Breathing Micelles for Combinatorial Treatment of Rheumatoid Arthritis. Angew. Chem., Int. Ed 2020, 59 (49), 21864–21869. [DOI] [PubMed] [Google Scholar]
- (35).Schairer DO; Chouake JS; Nosanchuk JD; Friedman AJ The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3 (3), 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Jones ML; Ganopolsky JG; Labbe A; Wahl C; Prakash S Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol 2010, 88 (2), 401–7. [DOI] [PubMed] [Google Scholar]
- (37).Privett BJ; Broadnax AD; Bauman SJ; Riccio DA; Schoenfisch MH Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26 (3), 169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Barraud N; Kelso MJ; Rice SA; Kjelleberg S Nitric oxide: a key mediator of biofilm dispersal with applications in infectious diseases. Curr. Pharm. Des 2014, 21 (1), 31–42. [DOI] [PubMed] [Google Scholar]
- (39).Xiong Y; Liu Y Biological control of microbial attachment: a promising alternative for mitigating membrane biofouling. Appl. Microbiol. Biotechnol 2010, 86 (3), 825–37. [DOI] [PubMed] [Google Scholar]
- (40).Devine R; Singha P; Handa H Versatile biomimetic medical device surface: hydrophobin coated, nitric oxide-releasing polymer for antimicrobial and hemocompatible applications. Biomater Sci 2019, 7 (8), 3438–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Mondal A; Douglass M; Hopkins SP; Singha P; Tran M; Handa H; Brisbois EJ Multifunctional S-Nitroso-N-acetylpenicillamine-Incorporated Medical-Grade Polymer with Selenium Interface for Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 11 (38), 34652–34662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Douglass ME; Goudie MJ; Pant J; Singha P; Hopkins S; Devine R; Schmiedt CW; Handa H Catalyzed Nitric Oxide Release via Cu Nanoparticles Leads to an Increase in Antimicrobial Effects and Hemocompatibility for Short-Term Extracorporeal Circulation. ACS Applied Bio Materials 2019, 2 (6), 2539–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Goudie MJ; Singha P; Hopkins SP; Brisbois EJ; Handa H Active Release of an Antimicrobial and Antiplatelet Agent from a Nonfouling Surface Modification. ACS Appl. Mater. Interfaces 2019, 11 (4), 4523–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Hopkins SP; Pant J; Goudie MJ; Schmiedt C; Handa H Achieving Long-Term Biocompatible Silicone via Covalently Immobilized S-Nitroso- N-acetylpenicillamine (SNAP) That Exhibits 4 Months of Sustained Nitric Oxide Release. ACS Appl. Mater. Interfaces 2018, 10 (32), 27316–27325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kumar R; Massoumi H; Chug MK; Brisbois EJ S-Nitroso-N-acetyl-l-cysteine Ethyl Ester (SNACET) Catheter Lock Solution to Reduce Catheter-Associated Infections. ACS Appl. Mater. Interfaces 2021, 13 (22), 25813–25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Broniowska KA; Diers AR; Hogg N S-nitrosoglutathione. Biochim. Biophys. Acta 2013, 1830 (5), 3173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Corpas F; Alché J; Barroso J Current overview of S-nitrosoglutathione (GSNO) in higher plants. Frontiers in Plant Science 2013, 4 (126). DOI: 10.3389/fpls.2013.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Garren MR; Ashcraft M; Qian Y; Douglass M; Brisbois EJ; Handa H Nitric oxide and viral infection: Recent developments in antiviral therapies and platforms. Applied Materials Today 2021, 22, 100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Douglass M; Hopkins S; Pandey R; Singha P; Norman M; Handa H S-Nitrosoglutathione-Based Nitric Oxide-Releasing Nano-fibers Exhibit Dual Antimicrobial and Antithrombotic Activity for Biomedical Applications. Macromol. Biosci 2021, 21 (1), 2000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Doverspike JC; Zhou Y; Wu J; Tan X; Xi C; Meyerhoff ME Nitric oxide releasing two-part creams containing S-nitrosoglutathione and zinc oxide for potential topical antimicrobial applications. Nitric Oxide 2019, 90, 1–9. [DOI] [PubMed] [Google Scholar]
- (51).Douglass M; Hopkins S; Pandey R; Singha P; Norman M; Handa H S-Nitrosoglutathione-Based Nitric Oxide-Releasing Nano-fibers Exhibit Dual Antimicrobial and Antithrombotic Activity for Biomedical Applications. Macromolecular Bioscience n/a 2021, 21 (1), 2000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Sim J-H; Jamaludin NS; Khoo C-H; Cheah Y-K; Halim SNBA; Seng H-L; Tiekink ERT In vitro antibacterial and time-kill evaluation of phosphanegold(I) dithiocarbamates, R3PAu[S2CN(iPr)-CH2CH2OH] for R = Ph, Cy and Et, against a broad range of Gram-positive and Gram-negative bacteria. Gold Bulletin 2014, 47 (4), 225–236. [Google Scholar]
- (53).Bleyer AJ Use of Antimicrobial Catheter Lock Solutions to Prevent Catheter-Related Bacteremia. Clinical Journal of the American Society of Nephrology 2007, 2 (5), 1073. [DOI] [PubMed] [Google Scholar]
- (54).Justo JA; Bookstaver PB Antibiotic lock therapy: review of technique and logistical challenges. Infect Drug Resist 2014, 7, 343–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).O’Toole GA Microtiter dish biofilm formation assay. J. Vis Exp 2011, 30 (47), 2437 DOI: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Zhang J; Wang B; Wang J; Yang Q Ethanol locks for the prevention of catheter-related infection in patients with central venous catheter: A systematic review and meta-analysis of randomized controlled trials. PLoS One 2019, 14 (9), No. e0222408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Yang T; Zelikin AN; Chandrawati R Progress and Promise of Nitric Oxide-Releasing Platforms. Advanced Science 2018, 5 (6), 1701043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Fux CA; Stoodley P; Hall-Stoodley L; Costerton JW Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev. Anti Infect Ther 2003, 1 (4), 667–83. [DOI] [PubMed] [Google Scholar]
- (59).He X; Yuan F; Lu F; Yin Y; Cao J Vancomycin-induced biofilm formation by methicillin-resistant Staphylococcus aureus is associated with the secretion of membrane vesicles. Microb Pathog 2017, 110, 225–231. [DOI] [PubMed] [Google Scholar]
- (60).Hess DJ; Henry-Stanley MJ; Wells CL Gentamicin promotes Staphylococcus aureus biofilms on silk suture. J. Surg Res 2011, 170 (2), 302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Jardeleza C; Foreman A; Baker L; Paramasivan S; Field J; Tan LW; Wormald PJ The effects of nitric oxide on Staphylococcus aureus biofilm growth and its implications in chronic rhinosinusitis. Int. Forum Allergy Rhinol 2011, 1 (6), 438–44. [DOI] [PubMed] [Google Scholar]
- (62).Goossens GA Flushing and Locking of Venous Catheters: Available Evidence and Evidence Deficit. Nurs Res. Pract 2015, 2015, 985686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Silverstein DM; Trerotola SO; Clark T; James G; Ng W; Dwyer A; Florescu MC; Shingarev R; Ash SR; Kidney Health Initiative, H. D. F. W. Clinical and Regulatory Considerations for Central Venous Catheters for Hemodialysis. Clin J. Am. Soc. Nephrol 2018, 13 (12), 1924–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).McGah PM; Gow KW; Aliseda A Leakage of Central Venous Catheter Locking Fluid by Hemodynamic Transport. ASAIO Journal 2014, 60 (4), 443. [DOI] [PubMed] [Google Scholar]
- (65).Brisbois EJ; Major TC; Goudie MJ; Meyerhoff ME; Bartlett RH; Handa H Attenuation of thrombosis and bacterial infection using dual function nitric oxide releasing central venous catheters in a 9day rabbit model. Acta Biomaterialia 2016, 44, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


