Abstract
Background
according to the revised sarcopenia definition proposed by the European Working Group on Sarcopenia in Older People (EWGSOP2) and revised definition of the Asian Working Group for Sarcopenia (AWGS2019), handgrip strength (HGS) and chair stand test (CST) can be used interchangeably as initial diagnostic measures.
Objective
to assess the agreement between sarcopenia prevalence, using either HGS or CST, and their association with adverse outcomes in geriatric rehabilitation inpatients.
Methods
REStORing health of acutely unwell adulTs is an observational, longitudinal cohort of geriatric rehabilitation inpatients. Cohen’s kappa (κ) was used to assess the agreement between sarcopenia prevalence (no, probable and confirmed and severe sarcopenia) according to EWGSOP2 and AWGS2019 using either HGS or CST. Associations between HGS and CST and readmission, institutionalisation and mortality were assessed by binomial regression.
Results
patients (n = 1,250, 57% females) had a median age of 83.1 years (interquartile range: [77.5–88.3]). There was no agreement between probable sarcopenia prevalence using HGS or CST for EWGSOP2 and AWGS2019, respectively (HGS: 70.9% and 76.2%; CST: 95.5% and 98.4%; κ = 0.08 and 0.02). Agreement between confirmed and severe sarcopenia prevalence using either HGS or CST was strong to almost perfect. HGS was associated with 3-month institutionalisation and 3-month and 1-year mortality, whereas CST was not associated.
Conclusions
HGS and CST cannot be used interchangeably as diagnostic measures for probable sarcopenia in geriatric rehabilitation inpatients. CST is not useful to predict adverse outcomes in geriatric rehabilitation inpatients.
Keywords: rehabilitation, sarcopenia, diagnosis, muscle strength, aged, older people
Key Points
HGS and CST cannot be used interchangeably to diagnose probable sarcopenia in geriatric rehabilitation.
HGS should be used to diagnose sarcopenia in geriatric rehabilitation and not CST.
HGS is predictive of adverse outcomes while CST is not.
Introduction
Sarcopenia, characterised by low muscle strength, muscle mass and physical performance [1], is prevalent in >50% of geriatric rehabilitation patients [2] and is associated with worse functional outcomes at discharge from rehabilitation [3] as well as mortality [4]. Sarcopenia may be a reversible cause of disability and patients may benefit from early intervention with resistance training and protein supplementation [5]. However, different diagnostic criteria result in a large variation in sarcopenia prevalence [6, 7], which hampers diagnosis in clinical practice.
The revised sarcopenia definition proposed by the European Working Group on Sarcopenia in Older People (EWGSOP2) sets muscle strength at the forefront of the algorithm, which is assessed with handgrip strength (HGS) or chair stand test (CST) [1]. Similarly, the revised definition of the Asian Working Group for Sarcopenia (AWGS2019) recommends to use either HGS or CST as a first step [8] but defines CST as a physical performance rather than muscle strength assessment. Both sarcopenia definitions use muscle mass to confirm the diagnosis of sarcopenia and comprise stages: no sarcopenia, probable sarcopenia (HGS or CST below cut-off points), confirmed sarcopenia and severe sarcopenia. Although HGS and CST are suggested to be both used as a first step to diagnose probable sarcopenia, there is a low agreement between HGS and CST in community-dwelling older people [9], which affects sarcopenia prevalence [10, 11]. This finding is of importance in geriatric rehabilitation patients as upper and lower limb muscle strengths might be differently affected after a period of acute disease [12].
The aims of this study were to determine (i) the agreement between sarcopenia prevalence (EWGSOP2 and AWGS2019) using either HGS or CST; and (ii) the association between HGS and CST using EWGSOP2 and AWGS2019 cut-offs and adverse outcomes (readmission, institutionalisation and mortality) in a large inception cohort of geriatric rehabilitation inpatients.
Material and Methods
Study design and population
REStORing health of acutely unwell adulTs (RESORT) is an observational, longitudinal inception cohort of geriatric rehabilitation inpatients admitted to the department of aged care at the Royal Melbourne Hospital (Melbourne, Victoria, Australia). The physical, cognitive and physiological health statuses of the patient were assessed using standardised assessment tools as part of a Comprehensive Geriatric Assessment (CGA) [13] within 48 hours of admission by physicians, nurses, physiotherapists, occupational therapists and dietitians. RESORT was approved by the Melbourne Health Human Research Ethics Committee (HREC/17/MH/103) and was conducted in accordance with the Declaration of Helsinki [14].
Patients were included at admission to geriatric rehabilitation wards and were excluded if they had no capacity to consent and had no nominated proxy to consent, or if the patients were palliative at admission. For the analysis, patients admitted from 16 October 2017 and discharged by 18 March 2020 (Waves 1–3) were eligible for inclusion. Of the 2,692 patients admitted, 446 patients were excluded, and 356 patients refused consent, which resulted in the inclusion of 1,890 patients. A total of 640 patients were excluded from the present analysis due to missing sarcopenia diagnostic measures (patient characteristics are shown in Supplementary Appendix 1), which left 1,250 patients. Institutionalisation data were available for 1,052 patients, and readmission and mortality data were available for all patients.
Patient characteristics
Age, sex, primary reason for admission and length of stay in geriatric rehabilitation were retrieved from medical records. Ethnicity data were collected through a patient survey. Disease burden was documented by a physician using the 56-point Cumulative Illness Rating Scale (CIRS) [15] and the 37-point Charlson Comorbidity Index (CCI) [16] in which higher points indicate higher morbidity. Frailty was measured by a physician using the Clinical Frailty Scale (CFS) on a scale from 1 (very fit) to 9 (terminally ill) [17]. Cognitive impairment was assessed by the diagnosis of dementia or by a cognitive score below cut-off values for one of the following tests: Mini-Mental State Examination (MMSE) <24 point [18], Montreal Cognitive Assessment (MoCA) <26 points [19] or the Rowland Universal Dementia Assessment Scale (RUDAS) <23 points [20]. Anthropometric measurements were performed by a nurse. Standing height without footwear was measured when the patient was able to stand, up to the nearest 0.1 cm. If the patient was unable to stand, knee height was measured using a sliding calliper between knee and ankle joints positioned at 90°, and height was estimated with the Chumlea equation for Caucasians [21]. Weight was measured on a calibrated standing weighing scale, weighing chair or hoist without shoes or heavy clothes, measured to the nearest 0.1 kg. Body mass index (BMI) was calculated by dividing the body weight by height squared (kg/m2). The risk of malnutrition was assessed by a nurse with the Malnutrition Screening Tool (MST), ranging from 0 to 5 points, with higher scores indicating a higher risk of malnutrition [22]. Risk of malnutrition was defined by an MST score ≥2. Functional independence status was assessed by an occupational therapist using the Katz index for Activities of Daily Living (ADL) [23] and the Lawton and Brody scale for Instrumental Activities of Daily Living (IADL) [24]. ADL and IADL scores range between 0 and 6 and 0 and 8 points, respectively, with higher scores indicating higher levels of independence.
Sarcopenia diagnosis
Muscle strength and physical performance were assessed by a physiotherapist. HGS was assessed using a handheld hydraulic dynamometer (JAMAR; Sammons Preston, Inc., Boling-brook, IL, USA) in a sitting position, elbow bent at 90° to the body, exerting maximum force. HGS was measured six times, alternating for both hands, and the maximum value was used for analysis [25] and was expressed in kilogrammes. Physical performance was assessed with the Short Physical Performance Battery (SPPB) with a score from 0 to 12 points, where a higher score indicates better physical performance [26]. The SPPB consists of three tests: standing balance test, CST and 4-m walk test (gait speed). For the CST, patients were asked to rise from a chair five times with their arms folded across their chest, and time was recorded in seconds from the beginning of the first rise until seated again after the fifth rise [26]. Gait speed, expressed in m/s, was measured twice at usual pace with or without walking aid and the fastest time was used for analysis.
Muscle mass was measured by direct-segmental multi-frequency bio-electrical impedance analysis (DSM-BIA, InBody S10, Biospace Co., Ltd, Seoul, South Korea). DSM-BIA has been validated for assessing segmental and whole-body composition against dual energy X-ray absorptiometry [27]. Patients were measured in a supine position. DSM-BIA was not performed in patients with (i) an electronic internal medical device or implant such as a pacemaker; (ii) plasters or bandages that interfered with the placement of the electrodes; (iii) an amputation or (iv) admission under contact isolation/precautions. Muscle mass was expressed appendicular lean mass (ALM) in kilogrammes and ALM index (ALMI, kg/m2) was calculated by dividing ALM by height squared (m2) [28].
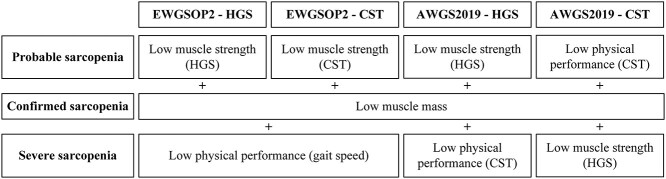
EWGSOP2 and AWGS2019 definitions and cut-offs were used for sarcopenia diagnosis [1, 8]. The EWGSOP2 algorithm includes (i) low muscle strength: HGS <27 kg for males and <16 kg for females or CST >15 s or failing the pre-test (not able to rise from the chair without using the arms); (ii) low muscle mass: ALMI <7.0 kg/m2 and <5.5 kg/m2 for males and females, respectively; (iii) low physical performance: gait speed ≤0.8 m/s or inability to walk. AWGS2019 includes: (i) low muscle strength: HGS <28 kg for males and <18 kg for females; (ii) low muscle mass: ALMI <7.0 kg/m2 and <5.7 kg/m2 for males and females, respectively, and (iii) low physical performance: CST ≥12 s or failing the pre-test. Sarcopenia stages were determined for both definitions, once using HGS and once using CST cut-offs, as shown in Figure 1.
Figure 1.
Flowchart of EWGSOP2 and AWGS2019 algorithms for diagnosis of sarcopenia.
Readmission, institutionalisation and mortality
Unplanned 3-month readmissions to the Royal Melbourne Hospital were obtained from the hospital administrative system. Three-month readmissions to other hospitals were obtained during a follow-up phone call with the patient or caregiver. Planned admissions after discharge were excluded, including elective admission for follow-up surgical or medical treatments such as scheduled dialysis or chemotherapy. Three-month institutionalisation was obtained during a follow-up phone call with the patient or caregiver. Patients already institutionalised before admission, deceased in hospital or at follow-up were excluded. All-cause mortality was assessed at 3-month and 1-year post-discharge from geriatric rehabilitation through the Registry of Births, Deaths and Marriages Victoria and through medical records.
Statistical analysis
Patient characteristics were reported with descriptive statistics. Continuous variables were reported as mean with standard deviation (SD) when normally distributed and else as median with interquartile range (IQR). Categorical variables were reported as frequency (n) with percentages (%). Cohen’s Kappa (κ) was used to determine the level of agreement between sarcopenia stages using either HGS or CST according to EWGSOP2 and AWGS2019. Coefficients were interpreted as follows: 0.00–0.20 representing no agreement, 0.21–0.39 representing minimal, 0.40–0.59 representing weak, 0.60–0.79 representing moderate, 0.80–0.90 representing strong and >0.90 representing almost perfect agreement [29]. Binomial logistic regression analyses of the association between HGS and CST, dichotomised as normal or low/abnormal values by EWGSOP2 and AWGS2019 cut-offs, and readmission, institutionalisation and mortality were performed. Analyses were adjusted for age and sex. Additionally, analyses were adjusted for co-morbidity (CCI score) and cognitive impairment as they are associated with both muscle strength [30, 31] and readmission, institutionalisation and mortality [32, 33]. Effect modification of sex was tested by introducing interaction terms. Results were presented as odds ratios (ORs) and 95% confidence intervals (CIs). P-values < 0.05 were considered to be statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences (IBM SPSS Advanced Statistics 26.0, Armonk, NY: IBM Corp.).
Results
Table 1 shows the patient characteristics at admission. Median age was 83.1 years (IQR: 77.5–88.3); 56.6% were females; median length of stay was 19.7 days (IQR: 13.0–30.0). Prevalence of cognitive impairment was 64.4%; median frailty score was 6 (IQR: 5–7). Mean HGS was 13.4 ± 7.8 and 21.6 ± 7.8 kg for females and males, respectively; 7.4% was unable to perform the test. Median CST time was 20.5 s (IQR: 16.1–27.6_; 76.8% was unable to perform the test.
Table 1.
Patient characteristics at admission to geriatric rehabilitation and adverse outcomes at 3-month and 1-year post discharge (n = 1,250)
| Characteristics | n | Total |
|---|---|---|
| Age, years | 1,250 | 83.1 [77.5–88.3] |
| Female, n (%) | 1,250 | 707 (56.6) |
| Primary reason for acute admission, n (%) | 1,250 | |
| Musculoskeletal | 586 (46.9) | |
| Neurological | 207 (16.6) | |
| Cardiac | 90 (7.2) | |
| Respiratory | 84 (6.7) | |
| Infection | 75 (6.0) | |
| Gastrointestinal | 65 (5.2) | |
| Other | 143 (11.4) | |
| Ethnicity, n (%) | 1,217 | |
| Caucasian | 1,059 (87.0) | |
| Asian | 68 (5.6) | |
| Other | 90 (7.4) | |
| Length of stay in geriatric rehabilitation, days | 1,250 | 19.7 [13.0–30.0] |
| CIRS, score | 1,249 | 12 [9–15] |
| CCI, score | 1,250 | 2 [1–4] |
| CFS, score | 1,151 | 6 [5–7] |
| Cognitive impairment, n (%)a | 1,250 | 805 (64.4) |
| BMI, kg/m2 | 1,250 | 26.1 [22.7–30.4] |
| At risk of malnutrition (MST ≥ 2), n (%) | 1,243 | 504 (40.5) |
| Katz-ADL, score | 1,235 | 2 [1–3] |
| Lawton-IADL, score | 1,235 | 1 [0–2] |
| Muscle and physical performance measures | ||
| HGS, kg, mean ± SD | 1,158 | 16.9 ± 7.8 |
| Female | 661 | 13.4 ± 5.8 |
| Male | 497 | 21.6 ± 7.8 |
| Unable, n (%) | 1,250 | 92 (7.4) |
| CST, s | 290 | 20.5 [16.1–27.6] |
| Unable, n (%) | 1,250 | 960 (76.8) |
| SPPB, score | 1,242 | 1 [0–4] |
| Gait speed, m/s | 788 | 0.43 [0.30–0.59] |
| Unable, n (%) | 1,250 | 462 (37.0) |
| ALMI, kg/m2, mean ± SD | 1,248 | 7.27 ± 1.56 |
| Female | 706 | 6.84 ± 1.49 |
| Male | 542 | 7.82 ± 1.47 |
| Adverse outcomes | ||
| 3-month readmission, n (%) | 1,250 | 274 (21.9) |
| 3-month institutionalisation, n (%) | 1,052 | 249 (24.2) |
| 3-month mortality, n (%) | 1,210 | 82 (6.6) |
| 1-year mortality, n (%) | 1,210 | 227 (18.2) |
Data presented as median (IQR) unless otherwise indicated. aPresence of dementia or abnormal sMMSE score <24 points or MoCA <26 points or RUDAS <23 point.
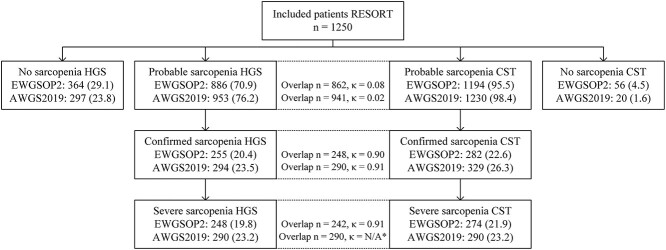
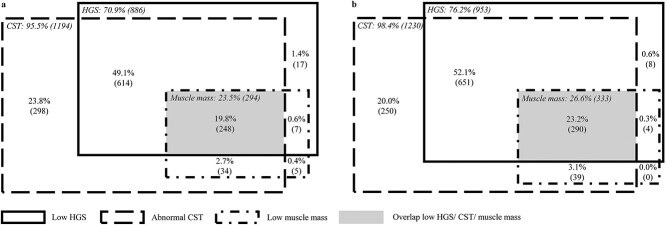
Prevalence of probable sarcopenia was 70.9 and 76.2% using HGS and was 95.5 and 98.4% using CST for EWGSOP2 and AWGS2019, respectively, indicating no agreement (EWGSOP2: κ = 0.08, 95% CI = 0.04–0.12; AWGS2019: κ = 0.02, 95% CI = −0.01 to 0.05, Figure 2). Agreement between the prevalence of confirmed sarcopenia using HGS (20.4%) or CST (22.6%) was strong according to EWGSOP2 (κ = 0.90, 95% CI = 0.87–0.93) and was almost perfect according to AWGS2019 (prevalence with HGS: 23.5% and with CST: 26.3%; κ = 0.91, 95% CI = 0.88–0.94). A total of 12.1 and 11.9% of the patients diagnosed with confirmed sarcopenia using CST were not diagnosed using HGS for EWSGOP2 and AWGS2019, respectively. The difference in confirmed sarcopenia prevalence for EWGSOP2 and AWGS2019 stratified per ethnicity is shown in Supplementary Appendix 2. Agreement between severe sarcopenia prevalence using HGS or CST was almost perfect for EWGSOP2 (κ = 0.91, 95% CI = 0.88–0.94). Low muscle mass without low HGS occurred in 3.1% of the patients for EWGSOP2 and AWGS2019, while low muscle mass without abnormal CST occurred in 1.0 and 0.3% of the patients for EWGSOP2 and AWGS2019, respectively (Figure 3). Overall, 19.8 and 23.2% of the patients had low HGS, abnormal CST and low muscle mass for EWGSOP2 and AWGS2019, respectively (Figure 3).
Figure 2.
Agreement between sarcopenia stages prevalence using either HGS or CST according to EWGSOP2 and AWGS2019, which was assessed with Cohen’s kappa (n = 1,250). Data presented as n (%). κ = Cohen’s kappa coefficient. Figure adapted from Johansson et al. [10]. *Agreement analysis was not performed as the diagnosis of severe sarcopenia for AWGS2019 relies on both HGS and CST.
Figure 3.
Number of patients with low HGS and/or abnormal CST and/or low muscle mass according to EWGSOP2 (n = 1,223 out of 1,250 participants) (a) and AWGS2019 (n = 1,242 out of 1,250 participants) (b).
Table 2 shows the association between HGS and CST and adverse outcomes. There was no effect modification for sex. After adjustments, low HGS was associated with higher odds for 3-month institutionalisation (EWGSOP2: OR = 1.59, 95% CI = 1.12–2.24; AWGS2019: OR = 1.53, 95% CI = 1.06–2.21), 3-month mortality (EWGSOP2: OR = 2.12, 95% CI = 1.12–4.04; AWGS2019: OR = 2.51, 95% CI = 1.18–5.35) and 1-year mortality (EWGSOP2: OR = 1.67, 95% CI = 1.14–2.44; AWGS2019: OR = 1.62, 95% CI = 1.08–2.44) compared to normal HGS. HGS and CST were not associated with 3-month readmission. No association was found between abnormal CST and institutionalisation or mortality.
Table 2.
Association between HGS and CST, according to the EWSGOP2 and AWGS2019 cut-offs, and adverse outcomes in geriatric rehabilitation inpatients
| EWSGOP2 | AWGS2019 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusteda | Crude | Adjusteda | ||||||||
| n | OR (95% CI) | P | OR (95% CI) | P | n | OR (95% CI) | P | OR (95% CI) | P | ||
| 3-month readmission | |||||||||||
| HGS | Normal | 364 | 1 | 1 | 297 | 1 | 1 | ||||
| Low | 886 | 1.09 (0.81–1.47) | 0.569 | 1.11 (0.81–1.51) | 0.517 | 953 | 1.21 (0.87–1.67) | 0.254 | 1.23 (0.88–1.71) | 0.230 | |
| CST | Normal | 56 | 1 | 1 | 20 | 1 | 1 | ||||
| Abnormal | 1,194 | 0.93 (0.49–1.75) | 0.811 | 0.95 (0.50–1.82) | 0.884 | 1,230 | 1.60 (0.47–5.51) | 0.455 | 1.76 (0.50–6.20) | 0.377 | |
| 3-month institutionalisation | |||||||||||
| HGS | Normal | 314 | 1 | 1 | 259 | 1 | 1 | ||||
| Low | 738 | 1.78 (1.27–2.50) | <0.001 | 1.59 (1.12–2.24) | 0.009 | 793 | 1.70 (1.19–2.45) | 0.004 | 1.53 (1.06–2.21) | 0.024 | |
| CST | Normal | 49 | 1 | 1 | 16 | 1 | 1 | ||||
| Abnormal | 1,003 | 1.22 (0.60–2.48) | 0.583 | 1.12 (0.54–2.32) | 0.755 | 1,036 | 0.93 (0.30–2.91) | 0.900 | 0.76 (0.24–2.45) | 0.644 | |
| 3-month mortality | |||||||||||
| HGS | Normal | 353 | 1 | 1 | 290 | 1 | 1 | ||||
| Low | 857 | 2.53 (1.35–4.72) | 0.004 | 2.12 (1.12–4.04) | 0.022 | 920 | 3.08 (1.47–6.47) | 0.003 | 2.51 (1.18–5.35) | 0.017 | |
| CST | Normal | 56 | 1 | 1 | 20 | 1 | 1 | ||||
| Abnormal | 1,154 | 0.94 (0.33–2.67) | 0.911 | 0.69 (0.24–2.05) | 0.509 | 1,190 | 0.40 (0.12–1.40) | 0.154 | 0.26 (0.07–0.97) | 0.045 | |
| 1-year mortality | |||||||||||
| HGS | Normal | 353 | 1 | 1 | 290 | 1 | 1 | ||||
| Low | 857 | 1.97 (1.38–2.82) | <0.001 | 1.67 (1.14–2.44) | 0.008 | 920 | 1.92 (1.30–2.83) | <0.001 | 1.62 (1.08–2.44) | 0.021 | |
| CST | Normal | 56 | 1 | 1 | 20 | 1 | 1 | ||||
| Abnormal | 1,154 | 1.65 (0.74–3.69) | 0.224 | 1.40 (0.60–3.26) | 0.438 | 1,190 | 0.92 (0.31–2.79) | 0.886 | 0.66 (0.21–2.15) | 0.494 | |
Bold values indicate statistical significance P < 0.05. a Adjusted for age, sex, CCI score and cognitive impairment.
Discussion
In a large inception cohort of geriatric rehabilitation inpatients, there was no agreement between the prevalence of probable sarcopenia using HGS or CST for both EWGSOP2 and AWGS2019 definitions. Strong to perfect agreement was found between confirmed as well as severe sarcopenia prevalence using either HGS or CST. Low HGS was associated with higher odds for 3-month institutionalisation and 3-month and 1-year mortality; no associations were observed between CST and adverse outcomes.
Agreement between sarcopenia prevalence using either HGS or CST
Probable sarcopenia prevalence was higher using CST compared to HGS resulting in no agreement between definitions. This implies that the interchangeability of both measures as a first step to diagnose sarcopenia in geriatric rehabilitation inpatients suggested by EWGSOP2 and AWGS2019 is not adequate in this population. Studies in community-dwelling older adults found inconsistent results: higher probable sarcopenia prevalence using CST than using HGS [10, 34], higher prevalence using HGS than using CST [11] and no difference in prevalence [35]. In community-dwelling older adults, HGS was reported not to be a proxy measure of lower extremity strength [36]. The higher probable sarcopenia prevalence in this cohort using CST compared to HGS is explained by the inability of three-fourth of the patients to perform the CST, while HGS assessment in a seated or supine position was feasible for most patients. The CST is a measure of overall physical performance rather than only muscle strength and is influenced by multiple factors including trunk stability, balance and pain [37–39], which are hampered in geriatric rehabilitation patients who experience mobility and function loss after a period of acute disease. In clinical rehabilitation practice, CST assessment at admission may therefore not be representative of the patients’ muscle strength.
As the diagnosis of confirmed sarcopenia mostly relies on low muscle mass when using CST as first step in geriatric rehabilitation inpatients, the agreement in confirmed and severe sarcopenia using either HGS or CST was strong to perfect for both definitions. In clinical practice, the need to measure muscle mass in almost all geriatric rehabilitation patients when using CST compared to 7 patients out of 10 when using HGS may affect the feasibility of diagnosis implementation. As low muscle mass appears to rarely occur without low HGS in this population, it is advised to first assess HGS and then muscle mass as described in the EWSGOP2 and AWGS2019 algorithms [1, 8]. In community-dwelling older adults, confirmed sarcopenia prevalence was found to be significantly higher when using HGS compared to CST [11, 35, 40] except for one study showing a higher prevalence when using CST [10]. These conflicting findings highlight the need to assess the adequacy of diagnostic criteria with respect to the target population.
Association between HGS and CST and adverse outcomes
HGS is known to be a good predictor of mortality in various populations, including healthy individuals [41, 42], older adults [43] and older hospitalised patients [44]. Similarly, HGS has shown to be associated with institutionalisation in older patients [45, 46]. Although poorly studied, CST was associated with long-term mortality in older adults [47]. In healthy older females, both HGS and CST were predictors for all-cause mortality, with comparable ORs for both measures [48]. The present study confirmed the association between low HGS and higher odds for institutionalisation and mortality. CST, on the other hand, was not associated with institutionalisation and mortality as very few patients scored above the EWSGOP2 and AWGS2019 cut-off points, leading to an important floor effect of the test in this population. CST performance at admission is therefore not useful as predictor of adverse outcomes in geriatric rehabilitation inpatients. Contrary to our expectations, neither HGS nor CST were associated with readmission. A meta-analysis in hospitalised older patients showed a higher risk of readmission in patients with sarcopenia, who were assessed with HGS and muscle mass [49]. Moreover, CST was associated with 26-week readmission in older patients [50]. Whereas, in hip fracture patients, HGS at admission to hospital was not associated with 1-year readmission [45]. The discrepancy between populations highlights the need for specific cut-off points in certain populations, such as hospitalised and geriatric rehabilitation patients, based on their predictive value for adverse outcomes.
Strengths and limitations
To the best of our knowledge, this is the first study investigating the impact of using either HGS or CST on sarcopenia prevalence using both EWGSOP2 and AWGS2019 in a large cohort of geriatric rehabilitation inpatients. Moreover, all measurements were conducted by a multidisciplinary team as part of a CGA with validated and standardised assessments appropriate to older patients. A limitation of this study is the assessment of muscle mass using BIA, which can be influenced by the hydration status of the patient [27] and could not be performed in patients with amputations or pacemakers and other electronic implants. Furthermore, this was a single-site study, which could limit generalisability to other hospitals.
Conclusion
HGS and CST are not interchangeable as initial diagnostic measures of sarcopenia in geriatric rehabilitation given the low agreement in probable sarcopenia prevalence using either HGS or CST. CST is not predictive of adverse outcomes in this population while HGS is. As low muscle mass rarely occurs with normal HGS, it is advised to first assess HGS and subsequently muscle mass to diagnose sarcopenia in geriatric rehabilitation inpatients. Further research is needed to find adequate alternative(s) to the CST to measure lower extremity strength in this population.
Supplementary Material
Acknowledgements
The authors thank the multidisciplinary team members of the Royal Melbourne Hospital, Royal Park Campus, who were involved in the RESORT cohort for their clinical work and the @AgeMelbourne team for their role in the data collection and data curation, especially Dr EM Reijnierse.
Contributor Information
Laure M G Verstraeten, Department of Human Movement Sciences, @AgeAmsterdam, Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam 1081BT, The Netherlands.
Nina J de Haan, Department of Human Movement Sciences, @AgeAmsterdam, Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam 1081BT, The Netherlands.
Eline Verbeet, Department of Human Movement Sciences, @AgeAmsterdam, Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam 1081BT, The Netherlands.
Janneke P van Wijngaarden, Danone Nutricia Research, Uppsalalaan 12, Utrecht 3584 CT, The Netherlands.
Carel G M Meskers, Department of Rehabilitation Medicine, Amsterdam University Medical Center, Amsterdam Movement Sciences, Amsterdam 1081HZ, The Netherlands.
Andrea B Maier, Department of Human Movement Sciences, @AgeAmsterdam, Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam 1081BT, The Netherlands; Department of Medicine and Aged Care, @AgeMelbourne, The Royal Melbourne Hospital, The University of Melbourne, Parkville, Victoria 3050, Australia; Healthy Longevity Translational Research Program, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 119228, Singapore; Centre for Healthy Longevity, @AgeSingapore, National University Health System, Singapore 119228, Singapore.
Declaration of Conflicts of Interest
A. B. Maier reports grants from Danone Nutricia Research, outside the submitted work; J. P. van Wijngaarden reports that she is an employee of Danone Nutricia Research.
Declaration of Sources of Funding
This work was supported by an unrestricted grant of the University of Melbourne received by Prof. Andrea B. Maier and the Medical Research Future Fund provided by the Melbourne Academic Centre for Health. This work is also part of a collaboration project co-funded by the PPP Allowance made available by Health~Holland (grant number TKI-LSHM19069-H049), Top Sector Life Sciences & Health, to stimulate public-private partnerships, and Top Sector Agri & Food (grant number LWV19287). The collaboration project also includes an in-cash and in-kind contribution from Danone Nutricia Research.
References
- 1. Cruz-Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2018; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Churilov I, Churilov L, MacIsaac RJ, Ekinci EI. Systematic review and meta-analysis of prevalence of sarcopenia in post acute inpatient rehabilitation. Osteoporos Int 2018; 29: 805–12. [DOI] [PubMed] [Google Scholar]
- 3. Morandi A, Onder G, Fodri L et al. The association between the probability of sarcopenia and functional outcomes in older patients undergoing in-hospital rehabilitation. J Am Med Dir Assoc 2015; 16: 951–6. [DOI] [PubMed] [Google Scholar]
- 4. Xu J, Reijnierse EM, Pacifico J, Wan CS, Maier AB. Sarcopenia is associated with three-month and one-year mortality in geriatric rehabilitation inpatients: RESORT. Age Ageing 2021; 50: 2147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lauretani F, Russo CR, Bandinelli S et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003; 95: 1851–60. [DOI] [PubMed] [Google Scholar]
- 6. Van Ancum JM, Alcazar J, Meskers CGM, Nielsen BR, Suetta C, Maier AB. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: a clinical perspective. Arch Gerontol Geriatr 2020; 90: 104125. 10.1016/j.archger.2020.104125. [DOI] [PubMed] [Google Scholar]
- 7. Reijnierse EM, Buljan A, Tuttle CSL et al. Prevalence of sarcopenia in inpatients 70 years and older using different diagnostic criteria. Nurs Open 2019; 6: 377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L, Woo J, Assantachai P et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21: 300–7.e2. [DOI] [PubMed] [Google Scholar]
- 9. Yeung SSY, Reijnierse EM, Trappenburg MC et al. Handgrip strength cannot be assumed a proxy for overall muscle strength. J Am Med Dir Assoc 2018; 19: 703–9. [DOI] [PubMed] [Google Scholar]
- 10. Johansson J, Strand BH, Morseth B, Hopstock LA, Grimsgaard S. Differences in sarcopenia prevalence between upper-body and lower-body based EWGSOP2 muscle strength criteria: the Tromsø study 2015–2016. BMC Geriatr 2020; 20: 461. 10.1186/s12877-020-01860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chew J, Yeo A, Yew S et al. Muscle strength definitions matter: prevalence of sarcopenia and predictive validity for adverse outcomes using the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) criteria. J Nutr Health Aging 2020; 24: 614–8. [DOI] [PubMed] [Google Scholar]
- 12. Aarden JJ, Reijnierse EM, van der Schaaf M et al. Longitudinal changes in muscle mass, muscle strength, and physical performance in acutely hospitalized older adults. J Am Med Dir Assoc 2021; 22: 839–45.e1. [DOI] [PubMed] [Google Scholar]
- 13. Ellis G, Gardner M, Tsiachristas A et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017; 9: CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Medical Association Declaration of Helsinki . Ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–4. [DOI] [PubMed] [Google Scholar]
- 15. Miller MD, Paradis CF, Houck PR et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. J Psychiatr Res 1992; 41: 237–48. [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 17. Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. CAMJ 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. ``Mini-mental state''. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- 19. Nasreddine ZS, Phillips NA, Bédirian V et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- 20. Storey JE, Rowland JT, Basic D, Conforti DA, Dickson HG. The Rowland Universal Dementia Assessment Scale (RUDAS): a multicultural cognitive assessment scale. Int Psychogeriatr 2004; 16: 13–31. [DOI] [PubMed] [Google Scholar]
- 21. Chumlea WC, Roche AF, Steinbaugh ML. Estimating stature from knee height for persons 60 to 90 years of age. J Am Geriatr Soc 1985; 33: 116–20. [DOI] [PubMed] [Google Scholar]
- 22. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999; 15: 458–64. [DOI] [PubMed] [Google Scholar]
- 23. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970; 10: 20–30. [DOI] [PubMed] [Google Scholar]
- 24. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–86. [PubMed] [Google Scholar]
- 25. Reijnierse EM, de Jong N, Trappenburg MC et al. Assessment of maximal handgrip strength: How many attempts are needed? J Cachexia Sarcopenia Muscle 2017; 8: 466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guralnik JM, Simonsick EM, Ferrucci L et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–94. [DOI] [PubMed] [Google Scholar]
- 27. Ling CH, de Craen AJ, Slagboom PE et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr 2011; 30: 610–5. [DOI] [PubMed] [Google Scholar]
- 28. Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: What are we really estimating? J Cachexia Sarcopenia Muscle 2017; 8: 187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012; 22: 276–82. [PMC free article] [PubMed] [Google Scholar]
- 30. Veronese N, Smith L, Cereda E et al. Multimorbidity increases the risk for sarcopenia onset: longitudinal analyses from the English Longitudinal Study of Ageing. Exp Gerontol 2021; 156: 111624. 10.1016/j.exger.2021.111624. [DOI] [PubMed] [Google Scholar]
- 31. Dodds RM, Murray JC, Granic A et al. Prevalence and factors associated with poor performance in the 5-chair stand test: findings from the Cognitive Function and Ageing Study II and proposed Newcastle protocol for use in the assessment of sarcopenia. J Cachexia Sarcopenia Muscle 2021; 12: 308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graversen SB, Pedersen HS, Sandbaek A, Foss CH, Ribe AR. Factors associated with 30-day rehospitalization and mortality in older patients after a pneumonia admission. J Am Med Dir Assoc 2020; 21: 1869–78.e10. 10.1016/j.jamda.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 33. Fogg C, Meredith P, Culliford D, Bridges J, Spice C, Griffiths P. Cognitive impairment is independently associated with mortality, extended hospital stays and early readmission of older people with emergency hospital admissions: a retrospective cohort study. Int J Nurs Stud 2019; 96: 1–8. 10.1016/j.jamda.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 34. Phu S, Vogrin S, Zanker J, Bani Hassan E, Al Saedi A, Duque G. Agreement between initial and revised european working group on sarcopenia in older people definitions. J Am Med Dir Assoc 2019; 20: 382–3.e1. [DOI] [PubMed] [Google Scholar]
- 35. Yee XS, Ng YS, Allen JC et al. Performance on sit-to-stand tests in relation to measures of functional fitness and sarcopenia diagnosis in community-dwelling older adults. Eur Rev Aging Phys Act 2021; 18: 1. 10.1186/s11556-020-00255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harris-Love MO, Benson K, Leasure E, Adams B, McIntosh V. The influence of upper and lower extremity strength on performance-based sarcopenia assessment tests. J Funct Morphol Kinesiol 2018; 3: 53. 10.3390/jfmk3040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bohannon RW, Magasi SR, Bubela DJ, Wang Y, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012; 46: 555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCarthy EK, Horvat MA, Holtsberg PA, Wisenbaker JM. Repeated chair stands as a measure of lower limb strength in sexagenarian women. J Gerontol A Biol Sci Med Sci 2004; 59: 1207–12. [DOI] [PubMed] [Google Scholar]
- 39. Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci 2002; 57: M539–43. [DOI] [PubMed] [Google Scholar]
- 40. Kim M, Won CW. Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: findings from the Korean Frailty and Aging Cohort Study. Age Ageing 2019; 48: 910–6. [DOI] [PubMed] [Google Scholar]
- 41. Leong DP, Teo KK, Rangarajan S et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015; 386: 266–73. [DOI] [PubMed] [Google Scholar]
- 42. García-Hermoso A, Cavero-Redondo I, Ramírez-Vélez R et al. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: a systematic review and meta-analysis of data from approximately 2 million men and women. Arch Phys Med Rehabil 2018; 99: 2100–13.e5. [DOI] [PubMed] [Google Scholar]
- 43. Ling CH, Taekema D, de Craen AJ, Gussekloo J, Westendorp RG, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ 2010; 182: 429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scheerman K, Meskers CGM, Verlaan S, Maier AB, Maier AB. Sarcopenia, Low handgrip strength and low absolute muscle mass predict long-term mortality in older hospitalized patients: an observational inception cohort study. J Am Med Dir Assoc 2021; 22: 816–20.e2. [DOI] [PubMed] [Google Scholar]
- 45. Pérez-Rodríguez P, Rabes-Rodríguez L, Sáez-Nieto C et al. Handgrip strength predicts 1-year functional recovery and mortality in hip fracture patients. Maturitas 2020; 141: 20–5. [DOI] [PubMed] [Google Scholar]
- 46. Verlaan S, Van Ancum JM, Pierik VD et al. Muscle measures and nutritional status at hospital admission predict survival and independent living of older patients - the EMPOWER Study. J Frailty Aging 2017; 6: 161–6. [DOI] [PubMed] [Google Scholar]
- 47. Arnau A, Espaulella J, Méndez T et al. Lower limb function and 10-year survival in population aged 75 years and older. Fam Pract 2016; 33: 10–6. [DOI] [PubMed] [Google Scholar]
- 48. Karlsen T, Nauman J, Dalen H, Langhammer A, Wisløff U. The combined association of skeletal muscle strength and physical activity on mortality in older women: the HUNT2 Study. Mayo Clini Proc 2017; 92: 710–8. [DOI] [PubMed] [Google Scholar]
- 49. Zhao Y, Zhang Y, Hao Q, Ge M, Dong B. Sarcopenia and hospital-related outcomes in the old people: a systematic review and meta-analysis. Aging Clin Exp Res 2019; 31: 5–14. [DOI] [PubMed] [Google Scholar]
- 50. Nielsen LM, Maribo T, Kirkegaard H, Bjerregaard MK, Oestergaard LG. Identifying elderly patients at risk of readmission after discharge from a short-stay unit in the emergency department using performance-based tests of daily activities. BMC Geriatr 2020; 20: 217. 10.1186/s12877-020-01591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.