Abstract
Background
Emerging evidence indicates the potential clinical significance of specific microbial signatures as diagnostic and prognostic biomarkers, in multiple cancers. However, to date, no studies have systematically interrogated circulating metagenome profiling in oesophageal adenocarcinoma (EAC) patients, particularly as novel non-invasive, early detection, surveillance and prognostic classifiers.
Methods
Metagenome sequencing was performed on 81 serum specimens collected across EAC spectrum, with sequencing reads classified using Bracken and MetaPhlAn3. Followed by the Linear Discriminant Analysis effect size (LEfSe) method to identify microbial profiles between groups. Logistic regression and Kaplan–Meier analyses were used to build classifiers.
Results
A significant loss of alpha and beta diversity was identified in serum specimens from EAC patients. We observed a shift in microbial taxa between each group—at the phylum, genus, and species level—with Lactobacillus sakei as the most prominent species in gastroesophageal reflux (GERD) vs other patient groups. Interestingly, LEfSe analysis identified a complete loss of Lactobacillus (L. Sakei and L. Curvatus), Collinsella stercoris and Bacteroides stercoris but conversely a significant increase in Escherichia coli in patients with EAC. Finally, we developed a metagenome panel that discriminated EAC from GERD patients with an AUC value of 0.89 (95% CI: 0.78–0.95; P < 0.001) and this panel in conjunction with the TNM stage was a robust predictor of overall survival (≥24 months; AUC = 0.84 (95% CI: 0.66–0.92; P = 0.006)).
Conclusion
This study firstly describes unique blood-based microbial profiles in patients across EAC carcinogenesis, that are further utilised to establish a novel circulating diagnostic and prognostic metagenomic signature for EAC.
Translational relevance
Accumulating data indicates the clinical relevance of specific microbial signatures as diagnostic and prognostic biomarkers, in multiple cancers. However, to date, no studies have systematically interrogated circulating metagenome profiling in patients with oesophageal adenocarcinoma (EAC). Herein, we performed metagenome sequencing in serum specimens from EAC patients 81 collected across EAC spectrum and observed a significant loss of alpha and beta diversity, with a shift in microbial taxa between each group—at the phylum, genus, and species level—with Lactobacillus sakei as the most prominent species in gastroesophageal reflux (GERD) vs other patient groups. Interestingly, LEfSe analysis identified a complete loss of Lactobacillus (L. Sakei and L. Curvatus), Collinsella stercoris and Bacteroides stercoris but conversely a significant increase in Escherichia coli in patients with EAC. Finally, we developed a metagenome panel that discriminated EAC from GERD patients with an AUC value of 0.89 and this panel, in conjunction with the TNM stage, was a robust predictor of overall survival. This study for the first time describes unique blood-based microbial profiles in patients across EAC carcinogenesis, that are further utilised to establish a novel circulating diagnostic and prognostic metagenomic signature for EAC.
Subject terms: Oncology, Biomarkers
Introduction
Oesophageal cancer (EC) is the seventh most common type of malignancy and the sixth leading cause of cancer-related death worldwide. EC has two major histological subtypes, oesophageal adenocarcinoma (EAC) and oesophageal squamous cell carcinoma (ESCC), which relate to different disease etiologies and geographical distributions [1]. Among these, EAC remains the most predominant subtype with a high prevalence in western populations, resulting in a dramatic increase of incidence in the last few years [2]. Unfortunately, most of these cases are detected at advanced stages, hence contributing to the high morbidity and mortality rates associated with this malignancy [3–5].
The evolution and development of EAC is often intimately linked with a cascade of dietary and lifestyle risk factors, including obesity, alcohol abuse, tobacco consumption or imbalanced diets [6–8]. Gastroesophageal reflux disease (GERD) is a well-defined risk factor for the development of EAC due to chronic and persistent inflammation. It causes injury of oesophageal squamous epithelial lining as a result of constant exposure to acidic stomach content [9, 10], with 5–15% of cases progressing to Barret’s oesophagus (BE)—a bona fide precursor lesion in patients with EAC [11]. Malignant transformation of BE to high-grade dysplasia (HGD), and further progression to EAC involves increased accumulation of various genetic and epigenetic alterations, dysregulated inflammatory processes, altered immune response, and the adverse influence of various environmental factors [12, 13]. In this context, while the presence of Gram-positive commensal microbiota is often associated with a healthy oesophagus, the shift to a Gram-negative anaerobic microbial environment has been reported to exist in patients with premalignant BE, along with the altered composition of specific microbes that are potentially pathogenic and associate with HGD and EAC [14, 15]. Accumulating studies in recent years have unequivocally highlighted a potentially causal link between dysbiosis and neoplastic progression in multiple cancers, including EC, which is orchestrated via a complex interplay between inflammatory factors, DNA damage, tumour-inducing metabolites and dysregulated activity in key intercellular signalling pathways [16–18]. With the advances in molecular biology and sequencing techniques in the microbiome field, these findings highlight the importance of oesophageal microbiota and its link to not only causal implications for tumorigenesis, but also an opportunity and rationale for their potential application as diagnostic, predictive and therapeutic targets in cancer.
Previously, multiple studies have assessed microbiota signatures of Barrett’s carcinogenesis, with disease progression generally correlating with reduced biodiversity and an increase in Gram-negative bacterial composition in the oral cavity and/or oesophageal mucosal tissue samples [19]. These studies have demonstrated enrichment of Akkermansia muciniphila, Enterobacteriaceae, Lactobacillus fermentum, Leptotrichia and Prevotella and depletion of Planctomycetes, Crenarchaeota and Streptococci in EAC patients [20–23]. In addition, a lower risk of developing EAC is correlated with the presence of Neisseria, Streptococcus pneumoniae and Helicobacter pylori [24, 25]. While the presence of Tanerella forsythia was associated with a higher risk of developing EAC. However, inconsistent findings have been reported across multiple studies due to small sample size of analysed datasets and due to limitation of variation in sample acquisition techniques [19].
Recently, circulating microbial DNA has shown promise as a non-invasive cancer detection tool in multiple solid tumours using microbial DNA (mbDNA) sequencing, including amplicon sequencing and metagenomic sequencing [26, 27]. For gastric cancer, the circulating microbial profiles have correlated with pathological staging and are significantly different from healthy controls [28]. Moreover, circulating microbial DNA in colorectal cancer patients has demonstrated reduced biodiversity that was consistent between blood and stool sample profiles [29]. Hence, establishing the value of circulating microbial DNA as a novel liquid biopsy approach for cancer detection, disease monitoring and prognosis; alone or in combination with widely adopted ctDNA assays, is of utmost importance. These techniques at minimum can augment existing ctDNA-based detection assays that have a host of limitations, such as low sensitivity for early-stage disease and contamination with noncancerous circulating cell-free DNA [30].
Therefore, unlike conventional microbial studies derived from mucosal samples [22], this exploratory study characterised the oesophageal microbiota composition from the systemic circulation, using serum samples obtained from patients with GERD, BE, dysplasia, and EAC to identify specific microbial profiles with a potential precursor role in tumour development. We hypothesised that serum might have higher sensitivity and specificity as a biomarker considering the low biomass of microbiome in the circulation [26, 31]. This is the first study to identify and validate the differential profile of microbial DNA in circulation using a shotgun metagenomic sequencing approach and to identify its potential as a non-invasive early diagnostic and prognostic biomarker for EAC.
Materials and methods
Study design and sample collection
This study included an analysis of serum specimens collected from a cohort of 81 patients, which included 51 with EAC, 10 with dysplasia, 10 patients with BE and 10 with GERD. All were enrolled at the Oesophageal and Lung Institute, Allegheny Health Network (Pittsburgh, PA) between 2016 and 2018 as part of an observational study. The patients with GERD were diagnosed by a combination of clinical presentation, oesophageal pH monitoring and esophagogastroduodenoscopy (EGD). Likewise, patients with BE, HGD, and EAC were diagnosed using EGD and histological evaluation on biopsy specimens. All research subjects were patients who were being diagnosed and/or treated at the Oesophageal and Lung Institute and the Allegheny Health Network (AHN) with pre-defined oesophageal benign and neoplastic conditions. All subjects were male or female and over the age of 18. No exclusions were made based on race, ethnicity, gender, or Human Immunodeficiency Virus (HIV) status. The racial, sex and ethnic characteristics proposed for recruitment were reflective of the demographic patient population in the institutional neighbourhood. Patients with known blood coagulation disorders who had excessive pre-operative bleeding, as indicated by the coagulation factors in the pre-operative blood work, were excluded. The exclusion criteria were as follows: platelet count less than 150,000/mm; PTT of 50 s or above; or PT INR of 1.8 or higher.
Written informed consent was given to all patients according to the Declaration of Helsinki and demographic and clinical outcomes were collected from all patients. The study was approved by the Institutional Review Board at Allegheny Health Network under protocol #12-036 “Oesophageal Disease Tissue Bank for the Oesophageal and Lung Institute”, and samples and clinical data were retrospectively analysed according to IRB #19-177. The clinical characteristics of the study subjects are summarised in Supplementary Table 1.
DNA extraction and library preparation
The serum from total blood was collected from each patient and aliquoted into microcentrifuge tubes and stored at −80 °C until use. The DNA from serum was extracted using PowerSoil Pro Kit (Qiagen, Hilden, Germany) automated for high throughput on the Qiacube HT (Qiagen) using Powerbead Pro Plates (Qiagen). The DNA quantity was measured using Quant-iT Picogreen dsDNA Assay (Invitrogen, Waltham, MA). Sequencing libraries were prepared using a procedure adapted from the Nextera Library Prep kit (Illumina, San Diego, CA). For BoosterShot® (shallow metagenome sequencing, 2 M reads/sample), libraries were sequenced on the Illumina NextSeq platform using paired-end 2 × 150 reads with a NextSeq 500/550 High Output v2 kit (Illumina), and this sequencing work was performed at Diversigen (Houston, TX, USA).
Analysis of microbial composition
DNA sequences were filtered for low quality (Q-Score <30) and length (<50), and adapter sequences were trimmed using cutadapt V1.1.6. [32] FastQ files were converted to a single fasta file using shi7 [33]. Sequences were trimmed to a maximum length of 100 bp prior to alignment by aligning them to a curated database containing all representative bacterial genomes in RefSeq [34]. Alignments were made at 97% identity against all reference genomes. Sequences were placed into unique Operational Taxonomic Units (OTUs) and taxonomy was assigned based on the lowest common ancestor, which was consistent with at least 80% of all reference sequences tied for best hit. The number of counts for each OTU was normalised to the average genome length. OTUs accounting for less than one-millionth of all species-level markers and those with less than 0.01% of their unique genome regions covered (and <1% of the whole genome) were discarded. Samples with fewer than 10,000 sequences were also discarded. Count data was then converted to relative abundance for each sample. A total of 18 samples did not meet this criterion and were removed from the analysis. This included 3 GERD samples, 5 BE samples, 5 HGD samples and 5 EAC samples.
Alpha and beta microbial DNA diversity analysis
Biodiversity and composition of mbDNA within each group were analysed via alpha and beta diversity values. For alpha-diversity, the Shannon and Chao-1 index was calculated using a rarefied OTU table set to the minimum depth allowed for a sample (10,000) using Quantitative Insights into Microbial Ecology (QIIME) version 1.9.1 and analysed by principal coordinates analysis (PCoA). While distinct beta diversity was characterised using PCoA of the Bray–Curtis index with a comparative analysis between subgroups performed within QIIME 1.9.1.
Development of metagenome panel as a diagnostic and prognostic biomarker for EAC
For classification using MetaPhlAn3 [35], reads were trimmed using Trimmomatic [36] to remove adapters and low-quality bases and reads. Samples that passed quality were taxonomically profiled, and output was merged to retain species-level assignments. Hierarchical clustering was performed by Bray–Curtis distance analysis on pairs of various clinical groups (e.g., EAC vs. GERD) using hclust2 (https://bitbucket.org/nsegata/hclust2). Linear Discriminant Analysis Effect Size (LEfSe) was used to identify taxa that discriminated clinical sample types [35]. The Linear Discriminant Analysis (LDA) threshold was set to 1.0.
Other statistical analyses
For clinicopathologic characteristics of patient cohorts, relevant characteristics were compared between each group using the t test for continuous variables and chi-square Yate's analysis for categorical data. Kruskal–Wallis H test was used to determine the statistical significance for alpha-diversity. Survival performance was estimated using the Kaplan–Meier methods. Analyses were performed using NCSS 11 software 2021 (NCSS, LLC, Kaysville, UT, USA, ncss.com/software/ncss), MedCalc software (MedCalc Software Ltd, Ostend, Belgium) and the GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
Results
Loss of microbial diversity in the blood specimens of patients with EAC is a unique and previously unrecognised clinical feature
The mbDNA biodiversity and composition of the circulating microbiome between patients with EAC patients were compared to the ones with dysplasia, BE, and GERD (Fig. 1 and Table 1). Alpha-diversity analysis revealed that dysbiosis was significantly associated with EAC according to the Shannon index, Chao-1 index, and observed OTUs. Based on the Shannon index, which estimates the diversity of the microbiota, more significant loss was observed when all groups were compared (P = 0.01), and these findings were even more prominent in patients with GERD vs. EAC (P = 0.01) and GERD vs. BE (P = 0.004; Fig. 1a). There was no further loss of mbDNA diversity observed between patients with dysplasia and EAC. The expected species richness of the microbiota was compared based on both the Chao-1 index and OTUs, with the result illustrating a higher richness of microbial diversity in patients with GERD compared to those with EAC (Chao 1: P = 0.01, OTUs: P = 0.01; Fig. 1b, c). There were no significant differences observed between all groups or in patients with BE and dysplasia. Beta diversity was analysed according to the clustering analysis using PCoA methods based on species Bray–Curtis distances, which revealed significant discrimination of microbial profiles between EAC and the other groups (Fig. 1d). These results confirm that a state of dysbiosis occurs during the malignant disease progression from GERD to BE to EAC, and mbDNA profiles have important implications for the clinical management of these patients.
Fig. 1. Alpha and beta diversity analysis between patients with GERD, BE, dysplasia and EAC.
a Shannon index estimates the diversity of the microbiota, where a more significant loss was observed when comparing all groups (P = 0.01), and a more prominent effect between patients with GERD vs. EAC and GERD vs. BE. The expected species richness of the microbiota was compared based on both (b) the Chao-1 index and (c) OTUs showing higher richness in GERD compared to EAC. d Principal component analysis (PCA) plot showing beta diversity between each group, with visible separation of microbiome profile between EAC and other groups. Weighted and unweighted-Unifranc distance were used to determine the relative abundance of species shared between the samples and the presence and absence information, respectively.
Table 1.
Alpha-diversity analysis between each group.
| Parameter of alpha-diversity | GERD | BE | Dysplasia | EAC | P value |
|---|---|---|---|---|---|
| (mean ± SD) | (n = 10) | (n = 10) | (n = 10) | (n = 51) | |
| Shannon index | 4.92 (±0.36) | 4.93 (±0.35) | 4.64(±0.43) | 4.34 (±0.72) | All groups (P = 0.01) |
| GERD vs. EAC (P = 0.01) | |||||
| BE vs. EAC (P = 0.004) | |||||
| Dysplasia vs. EAC (P = 0.27) | |||||
| Chao-1 index | 231.5 (±48.38) | 162.9 (±70.68) | 155.6 (±92.14) | 160.8 (±67.79) | All groups (P = 0.10) |
| GERD vs. EAC (P = 0.01) | |||||
| BE vs. EAC (P = 0.86) | |||||
| Dysplasia vs. EAC (P = 0.95) | |||||
| Observed OTUs | 201.3 (±30.10) | 153.1 (±59.36) | 143.6 (±78.25) | 149.6 (±62.88) | All groups (P = 0.22) |
| GERD vs. EAC (P = 0.01) | |||||
| BE vs. EAC (P = 0.75) | |||||
| Dysplasia vs. EAC (P = 0.95) |
Bold values represent the ones which are significantly different between comparison groups – p > 0.05.
OTU Operational taxonomic unit.
Distinct circulating microbial metagenomic profiles in GERD, BE and EAC
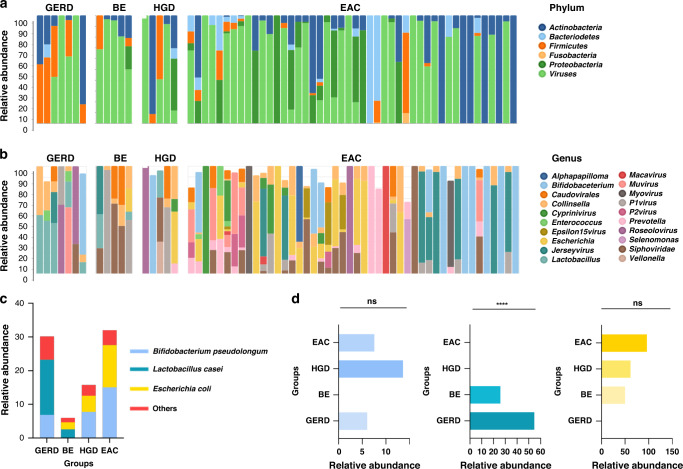
We observed changes of the microbial circulating metagenomics profile at the phylum, genus and species level that corresponded to distinct stages of progression in EAC. At the phylum level, Actinobacteria, Bacteriodetes, Firmicutes, and Proteobacteria were the most predominant bacterial phyla in this cohort of patients with EAC. Firmicutes were lower in EAC group while Actinobacteria and Proteobacteria were significantly more abundant (Fig. 2a). At the genus level, Bifidobacterium, Escherichia, Jerseyvirus, and Prevotella were more prominent in serum specimens of EAC patients compared to the other groups. In contrast, Lactobacillus was significantly more abundant in EAC, and in contrast, it was significantly less abundant in patients with BE, dysplasia and EAC (Fig. 2b).
Fig. 2. Relative abundance of microbial taxa at the phylum, genus and species level.
Descriptive visual representation of microbial taxa at (a) phylum and (b) genus level showing the distinctive profile of microbes between patients with GERD, BE, dysplasia and EAC. c At the species level, Bifidobacterium pseudolongum, Lactobacillus sakei and Escherichia coli were the most abundant taxa between groups. d Bar plot illustrates the mean relative abundances of B. pseudolongum, L. sakei and E. coli between groups. L. sakei is significantly more prominent in GERD, as determined by one-way ANOVA.
At the species level, among the microbial compositions between each group (Supplementary Fig. 1), we identified the three most abundant profiles of bacteria in all groups, which included Bifidobacterium pseudolongum, Lactobacillus sakei and Escherichia coli (Fig. 2c). We calculated the mean abundance of all samples from each group and performed Kruskal–Wallis test for each significant microbe (Fig. 2d). Our data revealed that the relative abundance of L. sakei was significantly more prominent in GERD, accounting for 55% of mean relative abundance of the total population, ~25% of mean relative abundance in patients with BE, but was not present in patients with HGD and EAC (P = <0.0001). In contrast, E. coli was not present in GERD but had the highest abundance in patients with EAC (P = 0.53). There was no trend observed for B. pseudolongum.
Metagenome panel as a novel non-invasive diagnostic biomarker for patients with EAC
Next, we performed LEfSe analysis to further compare the microbial features of each disease group. Firstly, we performed a pairwise analysis at all taxonomic levels and plotted a cladogram to depict the differences in the relative abundance of each taxa when comparing each disease group (Fig. 3a). Whenever the log10 transformed values (LDA score) were greater than 3.5, we observed consistent differential abundances of taxonomic significance in patients with GERD, which included Bacilli (P < 0.001), Lactobacillales (P < 0.001), Lactobacillaceae (P < 0.0001) and Lactobacillus being the most significant group at the class, order, family, and genus level, respectively.
Fig. 3. Linear Discriminant Analysis (LDA) effect size (LEfSe) and logistic regression analysis showing the potential of metagenome panel as a diagnostic biomarker.
a Cladogram representation of the significantly different taxa features from class (inner level) to genus (outer level) between GERD and other groups. The different significant bacterial taxa between (b) GERD vs. EAC, c GERD and other groups, and (d) BE vs. other groups were identified according to LDA score. e Receiver operating characteristics (ROC) curves showing the diagnostic potential of metagenome panel consists of Lactobacillus sakei, Lactobacillus curvatus, Collinsella stercoris, Bacteroides stercoris and Escherichia coli to predict EAC from GERD. ROC curve was created according to the logit formula from logistic regression analysis.
We further performed LEfSe analysis at the species level and identified that the significant differences were driven primarily by the changes of several species of Lactobacillus when the LDA score cut-off was greater than 4 (Table 2). We observed that L. sakei was more significant in GERD compared to the other three groups (P < 0.00001; Fig. 3b). When comparing GERD with only EAC, the significance of L. sakei (P < 0.00001), Lactobacillus curvatus (P < 0.001), Collinsella stercoris (P < 0.02) and Bacteroides stercoris (P < 0.02) were important in GERD; while in contrast, Escherichia coli (P < 0.02) was more significant in patients with EAC (Fig. 3c). Comparison between BE with EAC revealed three species of Lactobacillus consisting of L. sakei (P < 0.02), L. curvatus (P < 0.02), and Lactobacillus animalis (P < 0.02), were remarkably more significant in the BE group (Fig. 3d). Taken together, we observed that Lactobacillus was the most dominant genus associated in patients with GERD and BE, and the loss of Lactobacillus in systemic circulation was significantly associated with disease progression to EAC.
Table 2.
Linear Discriminant Analysis (LDA) effect size (LEfSe) significance between study variables in species level.
| Organism | GERD vs. BE vs. dysplasia vs EAC | GERD vs. EAC | BE vs. EAC | Overall Survival | ||||
|---|---|---|---|---|---|---|---|---|
| Group | P value | Group | P value | Group | P value | Group | P value | |
| Lactobacillus sakei | GERD | <0.00001 | GERD | <0.0000001 | BE | 0.02 | ||
| Lactobacillus animalis | BE | 0.02 | ||||||
| Lactobacillus curvatus | GERD | 0.001 | BE | 0.02 | ||||
| Bacteroides stercoris | GERD | 0.02 | ||||||
| Collinsella stercoris | GERD | 0.02 | ||||||
| Escherichia coli | EAC | 0.02 | ||||||
| Escherichia virus P1 | ≥24 months | 0.04 | ||||||
| Salmonella virus phage | ≥24 months | 0.03 | ||||||
LDA score cut-off value ≥4.
To validate this observation, we proceeded to develop a metagenome mbDNA panel according to the highest LDA-scoring microbes which comprised L. sakei, L. curvatus, C. stercoris, B. stercoris and E. coli—leading to the establishment of a metagenome panel for early diagnosis of EAC. Using a one-way stepwise logistic regression model, our metagenome panel could achieve impressive diagnostic performance with the area under the curve (AUC) value for the receiver operating characteristic (ROC) curve of 0.89 (95% CI, 0.78–0.95) with an optimal sensitivity of 60% and specificity of 95% (P < 0.001) under a logit formula: 1.48 – (7.6 × L. sakei) + (8.65 × C. stercoris) – (20.95 × B. stercoris) + (22.19 × L. curvatus) + (3.04 × E. coli) (Fig. 3e). Accordingly, these data reveal, to the best of our knowledge, the first report of the successful identification of a robust mbDNA panel in blood, which could serve as a standalone adjunctive diagnostic assay for the early detection of patients with EAC.
The mbDNA panel in blood predicts overall survival in patients with EAC
In view of the encouraging results for the potential clinical application of a mbDNA panel for the detection of EAC, we next questioned whether this panel also possesses any prognostic significance for predicting survival in patients with EAC. For these analyses, we performed a LEfSe analysis according to the overall survival (OS) in patients with EAC patients with a survival time frame of <24 months vs. those with an OS of ≥24 months. Based on the LDA cut-off score of >4, we identified two bacteriophages, Salmonella phage jersey (P = 0.03) and Escherichia virus P1 (P = 0.04) were significant in terms of their association with OS of ≥24 months (Fig. 4a).
Fig. 4. Metagenome panel as a predictive biomarker of overall survival (OS) in patients with EAC.
a Linear Discriminant Analysis (LDA) showing two significant microbial species in patients with OS ≥ 24 months. b Receiver operating characteristics (ROC) curves showing the prognostic potential of metagenome panel with clinical TNM stage to predict OS ≥ 24 months. c Kaplan–Meier survival curve showing that metagenome panel with clinical TNM stage is able to predict longer OS compared to TNM alone with the hazard ratio (HR) of 6.23 (95% CI, 2.65–14.46, P < 0.001).
We subsequently performed univariate and multivariate cox regression analysis, which revealed the significance of Clinical TNM stage (univariate: HR: 1.69 (95% CI, 1.03–2.79), P = 0.03; multivariate: HR: 4.25 (95% CI, 1.16–15.58), P = 0.03) and our OS metagenome panel (univariate: HR: 0.10 (95% CI, 0.006–0.55), P = 0.009; multivariate: HR: 0.05 (95% CI, 0.03–0.81), P = 0.03) with patient survival (Supplementary Table 2). Accordingly, we developed a prognostic panel using these two microbes along with E. coli due to its prominent presence in EAC patients. Using the metagenome panel alone, we were able to achieve an AUC of 0.70 (95% CI, 0.48–0.83) to distinguish EAC patients with OS ≥24 months. A significant improvement was achieved when we combined our panel with the patient’s clinical TNM stage, which resulted in a superior predictive AUC of 0.84 (95% CI, 0.66–0.92) and a corresponding sensitivity of 86% and specificity of 65% (P = 0.006) with a logit formula: 5.01 – (1.77 × clinical TNM stage) – (0.02 × E. coli) + (0.53 × Salmonella phage jersey) + (0.02 × Escherichia virus P1) (Fig. 4b and Supplementary Table 3).
Based on the median follow-up period of 54 months, where 38 patients died and the median OS of all patients was 28 months, we stratified the patients into high- and low-risk groups, based upon our logistic regression model and performed Kaplan–Meier analysis according to the OS ≥ 24 months. Figure 4c illustrates that our metagenome panel was able to improve the hazard ratio (HR) to 6.23 (95% CI, 2.65–14.46, P < 0.001) for predicting OS < 24 months compared to clinical TNM stages alone (HR: 4.23, 95% CI 1.80–9.94, P = 0.004), hence, highlighting that a non-invasive mbDNA panel can also be used for predicting survival outcomes in patients with EAC.
Discussion
In recent years, the gut microbiome has become an emerging research field in oncology due to the potential involvement of various microbes in carcinogenesis, as well as their potential to be used as a novel class of analytes for development as disease biomarkers. Physiologically, it was described that the oesophagus contains a diverse set of resident microbiota, with dysbiosis (loss of alpha- and beta-diversity) and changes in oesophageal microbiota, has been frequently observed in patients with GERD and BE [10, 15, 37]. For instance, the high relative abundance of the genus Escherichia was observed in patients with reflux esophagitis and BE and certain strains of E. coli were associated with chronic inflammation, oncogenesis, and cancer cell survival [38, 39]. Growing evidence has revealed that the oesophageal microbiota is involved with several key factors, such as the expression of a DNA damage-inducing bacterial toxin, changes in metabolism, chronic inflammation, and manipulation of host immune response that could facilitate carcinogenesis via activation of several key oncogenic pathways such as toll-like receptors (TLRs) and the modulation of Warburg effect [40–42]. Moreover, interfering with epithelial and systemic immune barriers, translocation from mucosal tissue to blood might occur and constituently play a role in carcinogenesis [43]. To date, several studies have characterised the profile of oesophageal microbiome signatures from oesophageal cells and mucosal tissues in relation to GERD, BE and EAC. However, to the best of our knowledge, none of the previous studies has yet characterised circulating microbial DNA (mbDNA) profiles in this setting despite its mounting diagnostic and prognostic relevance in other types of diseases, including various human cancers [44]. We believe our present study is the first to describe the circulating microbiome profiles among patients with GERD, BE, dysplasia and EAC, and highlight their potential as a diagnostic and prognostic marker, and potential therapeutic targets.
In this pilot study, we successfully performed metagenomic sequencing from serum samples and discovered several clinically relevant milestones. Firstly, we observed a significant loss of mdDNA diversity and an absence of specific bacterial communities in EAC patients, which is in line with the previous result from Dong et al. where they observed a prominent reduction of bacterial diversity in serum samples in patients with gastric cancer patients [44]. Even though it remains unclear, these results might suggest that under a certain pathological condition, loss of integrity of the epithelial barrier might enable microbial translocation into systemic circulation, which could play a significant pathogenic role in tumour development. However, further studies on the underlying mechanism(s), if any, are necessary to confirm this hypothesis. Secondly, at the genus level, our results revealed a significant loss of Lactobacillus abundance in EAC when compared to GERD and to a lesser extent in patients with BE, which reflects the possible protective role of Lactobacillus in preventing the development of EAC [45, 46]. Several species of Lactobacillus have been widely known as probiotics, with a proven mechanism of action to improve host immune response, and as anti-cancer agents primarily due to their role as antioxidants, promoting apoptosis, and their epigenetic-targeting properties [47–49]. In our study, L. sakei was found to be the most significant and differently abundant species between GERD and EAC. Therefore, these data suggest a potential antitumorigenic role of L. sakei considering that recent in vitro and in vivo studies have described that this bacterium, commonly found in traditional Korean fermented foods such as kimchi, has strong immunostimulatory and anti-inflammatory properties [50, 51].
From a clinical standpoint, our study has unravelled the potential of a circulating mbDNA panel with a robust diagnostic and prognostic potential in patients with EAC, which was subsequently verified by its predictive efficacy through the LEfSe analysis combined with the logistic regression and Cox proportional hazard model. Outside of Lactobacillus, several studies have previously identified the association between each microbe in our diagnostic and prognostic panel with carcinogenesis. E. coli and C. stercoris were traced in blood from cancer patients [52], with recent evidence suggesting the involvement of E. coli as a modulator of several cancer-related pathways [53–55]. In colorectal cancer, pathogenic E. coli strains lead to carcinogenesis through production of toxins, such as Colibactin and cytotoxic necrotising factor type 1 (CNF-1). These toxins induce cancer development by causing angiogenesis, inflammation, direct epithelial DNA damage or dysregulation of apoptosis, differentiation, proliferation and cancer cell motility [56]. In addition, B. stercoris is associated with antitumor immunity and better response to immune checkpoint blockade inhibitors. Even though the exact mechanism remains poorly understood [48, 57].
Ours is the first study in patients with EAC, which highlights that a biomarker based on the circulating metagenomic mbDNA profile may help tackle two main issues: predicting the risk of EAC in patients who have GERD, as well as its ability to predict the survival of patients suffering from EAC. These findings are important for two reasons, chronic GERD is strongly related with poor quality of life and it is also considered a major risk for the development of EAC, hence, adding a potential value to our panel as a possible surveillance marker. Moreover, our prognostic panel can also add significant value to improving patient risk stratification and treatment strategies for better survival for locally advanced EAC.
Although this pilot study has yielded an intriguing result, several limitations remain. An important limitation is the small sample size that consequently limited the effect size and statistical power of our discovery; hence, highlighting the need for further follow-up validation study in a larger cohort. We also acknowledge the retrospective design of our study, thus there were no strict controls and stratification in the patient profile that might confound the microbiome profile of each patient. Furthermore, there were no inversely proportional data observed between sequencing quantity and total sample input mass across samples. Due to the low concentration of mbDNA in the blood [58], the detection rate from our sequencing data was low compared to other biological samples. This is due to the expected decrease in signal-to-noise in the blood compartment, which can potentially impact its robustness as a biomarker. Lastly, even though we have identified significant microbes related to diagnosis and prognosis, the precise mechanistic role of these microbes in EAC development remains unclear. Therefore, in-depth studies of each microbe are essential to confirm our discovery.
In conclusion, our study is the first of its kind that describes the alterations in microbial diversity and profiles between patients with GERD, BE and EAC from systemic circulation using a shotgun metagenomic sequencing pipeline. It also enables the discrimination and identification of several microbes that allowed the development of a non-invasive diagnostic and predictive biomarker panel for patients afflicted with a lethal malignancy such as EAC.
Supplementary information
Acknowledgements
We would like to thank Dr. John Gillece and Dr. Sarah Highlander for their expertise and hard work in data analysis and for providing their critical insights into the analysis of microbial metagenome sequencing data.
Author contributions
AHZ (conceptualisation: lead; funding acquisition: equal; methodology: equal; writing—review and editing: equal). MYP (data curation: lead; formal analysis: equal; methodology: equal; writing—original draft: lead; writing—review and editing: equal). ANO (data curation: equal; formal analysis: supporting; validation: supporting; writing—original draft: lead; writing—review and editing: supporting). AG (resources: supporting; writing—review and editing: supporting). RM (resources: supporting; writing—review and editing: supporting). RM-K (resources: supporting; writing—review and editing: supporting). BAJ (resources: supporting; validation: supporting; writing—review and editing: supporting). PLW (resources: supporting; validation: supporting; writing—review and editing: supporting). RJK (resources: supporting; validation: supporting; writing—review and editing: supporting). AG (conceptualisation: lead; funding acquisition: lead; methodology: equal; writing—review and editing: equal). All authors read and approved the final manuscript.
Funding
This work was supported by CA72851, CA181572, CA184792, CA202797 and CA227602 grants from the National Cancer Institute, National Institutes of Health.
Data availability
The datasets used for this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients, and the study was approved by the institutional review boards of the participating institutions.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01974-5.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, Lemmens VEPP, van Heijningen EB, Aragonés N, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and The Netherlands. Am J Gastroenterol. 2014;109:336–44. doi: 10.1038/ajg.2013.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. J Am Med Assoc. 2013;310:627–36. doi: 10.1001/jama.2013.226450. [DOI] [PubMed] [Google Scholar]
- 4.Kambhampati S, Tieu AH, Luber B, Wang H, Meltzer SJ. Risk factors for progression of Barrett’s esophagus to high grade dysplasia and esophageal adenocarcinoma. Sci Rep. 2020;10:4899. doi: 10.1038/s41598-020-61874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Then EO, Lopez M, Saleem S, Gayam V, Sunkara T, Culliford A, et al. Esophageal cancer: an updated surveillance epidemiology and end results database analysis. World J Oncol. 2020;11:55–64. doi: 10.14740/wjon1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Y, Yuan J-M, Wang R, Gao Y-T, Yu MC. Alcohol, tobacco and diet in relation to esophageal cancer: The Shanghai Cohort Study. Nutr Cancer. 2008;60:354–63. doi: 10.1080/01635580701883011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandre L, Long E, Beales IL. Pathophysiological mechanisms linking obesity and esophageal adenocarcinoma. World J Gastrointest Pathophysiol. 2014;5:534–49. doi: 10.4291/wjgp.v5.i4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu X, Chen J, Jiang W, Zhang D. Alcohol, alcoholic beverages and risk of esophageal cancer by histological type: a dose-response meta-analysis of observational studies. Alcohol Alcohol. 2020;55:457–67. doi: 10.1093/alcalc/agaa047. [DOI] [PubMed] [Google Scholar]
- 9.Chang JT, Katzka DA. Gastroesophageal reflux disease, Barrett esophagus, and esophageal adenocarcinoma. Arch Intern Med. 2004;164:1482–8. doi: 10.1001/archinte.164.14.1482. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande NP, Riordan SM, Castaño-Rodríguez N, Wilkins MR, Kaakoush NO. Signatures within the esophageal microbiome are associated with host genetics, age, and disease. Microbiome. 2018;6:227. doi: 10.1186/s40168-018-0611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. JNCI: J Natl Cancer Inst. 2011;103:1049–57. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22:1119–29. doi: 10.1093/carcin/22.8.1119. [DOI] [PubMed] [Google Scholar]
- 13.Kaz AM, Grady WM, Stachler MD, Bass AJ. Genetic and epigenetic alterations in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am. 2015;44:473–89. doi: 10.1016/j.gtc.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajayi TA, Cantrell S, Spann A, Garman KS. Barrett’s esophagus and esophageal cancer: links to microbes and the microbiome. PLoS Pathog. 2018;14:e1007384. doi: 10.1371/journal.ppat.1007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv J, Guo L, Liu J-J, Zhao H-P, Zhang J, Wang J-H. Alteration of the esophageal microbiota in Barrett’s esophagus and esophageal adenocarcinoma. World J Gastroenterol. 2019;25:2149–61. doi: 10.3748/wjg.v25.i18.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong H, Bording-Jorgensen M, Dijk S, Wine E. The complex interplay between chronic inflammation, the microbiome, and cancer: understanding disease progression and what we can do to prevent it. Cancers. 2018;10:83. doi: 10.3390/cancers10030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quante M, Graham TA, Jansen M. Insights into the pathophysiology of esophageal adenocarcinoma. Gastroenterology. 2018;154:406–20. doi: 10.1053/j.gastro.2017.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smet A, Kupcinskas J, Link A, Hold GL, Bornschein J. The role of microbiota in gastrointestinal cancer and cancer treatment: chance or curse? Cell Mol Gastroenterol Hepatol. 2022;13:857–74. doi: 10.1016/j.jcmgh.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snider EJ, Compres G, Freedberg DE, Khiabanian H, Nobel YR, Stump S, et al. Alterations to the esophageal microbiome associated with progression from Barrett's esophagus to esophageal adenocarcinoma. Cancer Epidemiol, Biomark Prev. 2019;28:1687–93. doi: 10.1158/1055-9965.EPI-19-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott DRF, Walker AW, O’Donovan M, Parkhill J, Fitzgerald RC. A non-endoscopic device to sample the oesophageal microbiota: a case-control study. lancet Gastroenterol Hepatol. 2017;2:32–42. doi: 10.1016/S2468-1253(16)30086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopetuso LR, Severgnini M, Pecere S, Ponziani FR, Boskoski I, Larghi A, et al. Esophageal microbiome signature in patients with Barrett’s esophagus and esophageal adenocarcinoma. PLoS ONE. 2020;15:e0231789. doi: 10.1371/journal.pone.0231789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peter S, Pendergraft A, VanDerPol W, Wilcox CM, Kyanam Kabir Baig KR, Morrow C, et al. Mucosa-associated microbiota in Barrett’s esophagus, dysplasia, and esophageal adenocarcinoma differ similarly compared with healthy controls. Clin Transl Gastroenterol. 2020;11:e00199–e00199. doi: 10.14309/ctg.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gall A, Fero J, McCoy C, Claywell BC, Sanchez CA, Blount PL, et al. Bacterial composition of the human upper gastrointestinal tract microbiome is dynamic and associated with genomic instability in a Barrett’s esophagus cohort. PLoS ONE. 2015;10:e0129055. doi: 10.1371/journal.pone.0129055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters BA, Wu J, Pei Z, Yang L, Purdue MP, Freedman ND, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017;77:6777–87. doi: 10.1158/0008-5472.CAN-17-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579:567–74. doi: 10.1038/s41586-020-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zozaya-Valdes E, Wong SQ, Raleigh J, Hatzimihalis A, Ftouni S, Papenfuss AT, et al. Detection of cell-free microbial DNA using a contaminant-controlled analysis framework. Genome Biol. 2021;22:187. doi: 10.1186/s13059-021-02401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong Z, Chen B, Pan H, Wang D, Liu M, Yang Y, et al. Detection of microbial 16S rRNA gene in the serum of patients with gastric cancer. Front Oncol. 2019;9:608. doi: 10.3389/fonc.2019.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Q, Lu W, Kong X, Shao YW, Hu Y, Wang A, et al. Alterations of circulating bacterial DNA in colorectal cancer and adenoma: a proof-of-concept study. Cancer Lett. 2021;499:201–8. doi: 10.1016/j.canlet.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Chin RI, Chen K, Usmani A, Chua C, Harris PK, Binkley MS, et al. Detection of solid tumor molecular residual disease (MRD) using circulating tumor DNA (ctDNA) Mol diagnosis Ther. 2019;23:311–31. doi: 10.1007/s40291-019-00390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adlung L, Elinav E, Greten TF, Korangy F. Microbiome genomics for cancer prediction. Nat Cancer. 2020;1:379–81. doi: 10.1038/s43018-020-0059-x. [DOI] [PubMed] [Google Scholar]
- 32.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 33.Al-Ghalith GA, Hillmann B, Ang K, Shields-Cutler R, Knights D. SHI7 is a self-learning pipeline for multipurpose short-read DNA quality control. mSystems 2018;3: e00202-00217. [DOI] [PMC free article] [PubMed]
- 34.O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beghini F, McIver LJ, Blanco-Míguez A, Dubois L, Asnicar F, Maharjan S, et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife. 2021;10:e65088. doi: 10.7554/eLife.65088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.May M, Abrams JA. Emerging insights into the esophageal microbiome. Curr Treat Options Gastroenterol. 2018;16:72–85. doi: 10.1007/s11938-018-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi HJ, Kim J, Do KH, Park S-H, Moon Y. Enteropathogenic Escherichia coli-induced macrophage inhibitory cytokine 1 mediates cancer cell survival: an in vitro implication of infection-linked tumor dissemination. Oncogene. 2013;32:4960–9. doi: 10.1038/onc.2012.508. [DOI] [PubMed] [Google Scholar]
- 39.Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE. Gastric microbiota is altered in oesophagitis and Barrett’s oesophagus and further modified by proton pump inhibitors. Environ Microbiol. 2014;16:2905–14. doi: 10.1111/1462-2920.12285. [DOI] [PubMed] [Google Scholar]
- 40.Zaidi AH, Kelly LA, Kreft RE, Barlek M, Omstead AN, Matsui D, et al. Associations of microbiota and toll-like receptor signaling pathway in esophageal adenocarcinoma. BMC Cancer. 2016;16:52. doi: 10.1186/s12885-016-2093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Han D, Li L, Luo W, Liu J, Qian L. EphA5 silencing increases the radiosensitivity of ESCC cells through ATM-dependent pathway. Cancer Manag Res. 2020;12:9539–49. doi: 10.2147/CMAR.S261182. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Gillespie MR, Rai V, Agrawal S, Nandipati KC. The role of microbiota in the pathogenesis of esophageal adenocarcinoma. Biology. 2021;10:697. doi: 10.3390/biology10080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouaknine Krief J, Helly de Tauriers P, Dumenil C, Neveux N, Dumoulin J, Giraud V, et al. Role of antibiotic use, plasma citrulline and blood microbiome in advanced non-small cell lung cancer patients treated with nivolumab. J Immunother Cancer. 2019;7:176. doi: 10.1186/s40425-019-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong Z, Chen B, Pan H, Wang D, Liu M, Yang Y, et al. Detection of microbial 16S rRNA gene in the serum of patients with gastric cancer. Front Oncol. 2019;9:608. [DOI] [PMC free article] [PubMed]
- 45.Kohata Y, Nakahara K, Tanigawa T, Yamagami H, Shiba M, Watanabe T, et al. Rebamipide alters the esophageal microbiome and reduces the incidence of Barrett’s esophagus in a rat model. Digestive Dis Sci. 2015;60:2654–61. doi: 10.1007/s10620-015-3662-4. [DOI] [PubMed] [Google Scholar]
- 46.Kaakoush NO, Morris MJ. The oesophageal microbiome: an unexplored link in obesity-associated oesophageal adenocarcinoma. FEMS Microbiol Ecol. 2016;92. [DOI] [PubMed]
- 47.Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterology: WJG. 2014;20:7878–86. doi: 10.3748/wjg.v20.i24.7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–80. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Legesse Bedada T, Feto TK, Awoke KS, Garedew AD, Yifat FT, Birri DJ. Probiotics for cancer alternative prevention and treatment. Biomedicine Pharmacother. 2020;129:110409. doi: 10.1016/j.biopha.2020.110409. [DOI] [PubMed] [Google Scholar]
- 50.Rather IA, Bajpai VK, Ching LL, Majumder R, Nam G-J, Indugu N, et al. Effect of a bioactive product SEL001 from Lactobacillus sakei probio65 on gut microbiota and its anti-colitis effects in a TNBS-induced colitis mouse model. Saudi J Biol Sci. 2020;27:261–70. doi: 10.1016/j.sjbs.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S-Y, Shin J-S, Chung K-S, Han H-S, Lee H-H, Lee J-H, et al. Immunostimulatory effects of live Lactobacillus sakei K040706 on the CYP-induced immunosuppression mouse model. Nutrients. 2020;12:E3573. doi: 10.3390/nu12113573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ota A, Morita S, Matsuoka A, Shimokata T, Maeda O, Mitsuma A, et al. Detection of bacteria in blood circulation in patients receiving cancer chemotherapy. Int J Clin Oncol. 2020;25:210–5. doi: 10.1007/s10147-019-01521-y. [DOI] [PubMed] [Google Scholar]
- 53.Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE. 2013;8:e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fabbri A, Travaglione S, Ballan G, Loizzo S, Fiorentini C. The cytotoxic necrotizing factor 1 from E. Coli: a Janus toxin playing with cancer regulators. Toxins. 2013;5:1462–74. doi: 10.3390/toxins5081462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature. 2020;580:269–73. doi: 10.1038/s41586-020-2080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fabbri A, Bracci L. Immunomodulatory properties of CNF1 toxin from E. coli: implications for colorectal carcinogenesis. Am J Cancer Res. 2022;12:651–60. [PMC free article] [PubMed] [Google Scholar]
- 57.Polimeno L, Barone M, Mosca A, Viggiani MT, Di Leo A, Debellis L, et al. Gut microbiota imbalance is related to sporadic colorectal neoplasms. A Pilot Study. Appl Sci. 2019;9:5491. doi: 10.3390/app9245491. [DOI] [Google Scholar]
- 58.Castillo DJ, Rifkin RF, Cowan DA, Potgieter M. The healthy human blood microbiome: fact or fiction? Front Cell Infect Microbiol. 2019;9:148. doi: 10.3389/fcimb.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used for this study are available from the corresponding author on reasonable request.