Abstract
Background
The efficacy and added benefit of platinum-based chemotherapy (PtCT) for metastatic breast cancer (MBC) remain unclear in patients with and without germline BRCA1 or BRCA2 mutations (gBRCA1/2m and gBRCA1/2wt, respectively).
Methods
We selected from the French national real-world multicentre ESME cohort (2008–2016) all patients with HER2-negative MBC with known gBRCA1/2 status at first-line chemotherapy initiation. Using multivariable Cox models, we compared the outcome (progression-free (PFS) and overall survival (OS)) of first-line PtCT and non-PtCT regimens based on the patients’ gBRCA1/2 status and tumour subtype.
Results
Patients who received PtCT had more aggressive tumour features. In the multivariable analysis, first-line PtCT was associated with better adjusted PFS and OS in gBRCA1/2m carriers (N = 300), compared with non-PtCT (HR 0.54, 95% CI 0.4–0.73, P < 0.001, and HR 0.70, 95% CI 0.49–0.99, P = 0.047, respectively). Conversely, outcomes were similar in gBRCA1/2wt patients (N = 922) treated with PtCT and non-PtCT, whatever the tumour subtype. Landmark analyses at months 3 and 6 post treatment initiation supported these results.
Conclusions
In this pre-PARP inhibitor real-world cohort, PFS and OS were better after PtCT than non-PtCT in patients with gBRCA1/2m, but not in those with gBRCA1/2wt. These results emphasise the need of early gBRCA1/2 testing in patients with MBC.
Clinical trial number
Subject terms: Breast cancer, Predictive markers, Cancer genetics
Introduction
Breast cancer is the second most common cancer worldwide and the most frequent cancer in women [1]. The breast cancer susceptibility genes BRCA1 and 2 represent the main inherited risk factor for breast cancer [2]. Although germline BRCA1/2 mutations (gBRCA1/2m) remain a rare event (2.7–4.3%) in patients with metastatic/advanced breast cancer (MBC) [3], their implication in the DNA damage response suggests that DNA-damaging agents, such as platinum-based chemotherapy regimens (PtCT), should be more effective in this population.
Triple-negative breast cancers (TNBC), defined by the absence of oestrogen receptors (ER), progesterone receptors (PR), and HER2 overexpression/amplification, represent 15% of all breast cancers (BCs) [4, 5] but 50–60% of all breast cancers caused by hereditary BRCA1 pathogenic variants [6–8]. Despite their good chemosensitivity, patients with TNBC have a poorer prognosis, at all disease stages [4, 9, 10]. It has been reported that a significant part of sporadic TNBCs display a ‘BRCAness’ phenotype, defined by phenotypic similarities with tumours in BRCA1 or BRCA2 mutation carriers [11], particularly DNA-repair alterations. DNA-repair capacity upregulation is a common mechanism used by cancer cells to survive DNA-damaging therapy [12]. Lack of efficient major DNA-repair capability by simultaneous loss or inhibition of two DNA-repair pathways causes synthetic lethality and cell death, thus representing an attractive approach for cancer therapy [13]. It also suggests that DNA-damaging agents (e.g., PtCT) could be effective in TNBC. Indeed, in early TNBC settings, it has been consistently shown that PtCT increases the pathologic complete response and disease-free survival when combined with neoadjuvant standard of care treatment [14–16]. Paradoxically, such effect was not observed in gBRCA1/2m carriers with TNBC in whom platinum does not seem to increase the efficacy of standard anthracycline-, alkylator- and paclitaxel-based chemotherapy [17, 18]. Moreover, the efficacy of platinum agents has not been clearly demonstrated in patients with metastatic TNBC [19–21]. Preclinical data and the results of a single randomised clinical trial suggest that PtCT could be more efficient than non-PtCT in gBRCA1/2m carriers, unlike what observed in patients with gBRCA1/2wt [22]. Data also lack on the role of platinum compounds in the management of patients with ER-positive/HER2-negative MBC, independently of their BRCA1/2 status. Although the published data remain controversial on this topic [20], the Advanced Breast Cancer consensus guidelines consider PtCT as the preferred chemotherapy regimen for patients with gBRCA1/2m-associated advanced TNBC or endocrine-resistant MBC previously treated with anthracycline with or without taxane (in the adjuvant and/or metastatic setting) [23].
In 2014, the French UNICANCER group (i.e., 18 French Comprehensive Cancer Centres that manage more than 30% of all patients with breast cancer nationwide) launched the Epidemiological Strategy and Medical Economics (ESME) initiative to investigate real-world data on the management of solid tumours. Real-world data allow assessing retrospectively the activity of specific drugs outside the framework of clinical trials. The primary objective of the present study was to determine the effect of first-line chemotherapy regimens (PtCT versus non-PtCT) on progression-free survival (PFS) and overall survival (OS) among MBC patients with and without pathogenic germline BRCA1/2 mutations included in the ESME-MBC cohort between 2008 and 2016.
Methods
Study design
The ESME-MBC cohort is a national population-based registry that collects data on all consecutive patients treated for MBC in the 18 French Comprehensive Cancer Centres between January 1, 2008 and December 31, 2016. For this study, data were collected until the cut-off date (October 13, 2018). Eligible patients were older than 18 years of age with newly diagnosed MBC, and who initiated treatment during the registry enrolment period [10]. Patients with HER2-negative MBC and known hormone receptor (HR) expression status who received first-line chemotherapy for MBC, with or without previous endocrine therapy in the metastatic setting, were selected. Germline BRCA1/2 testings were performed in clinical practice, following each centre's guidelines and the French guidelines in use at this time [24]. Patients were classified as untested for predisposing germinal mutations, and tested and found to be carriers of a germline BRCA1/2 mutation (gBRCA1/2m) or not (wild-type, gBRCA1/2wt). Patients with gBRCA1/2wt and germinal alterations of other genes were considered gBRCA1/2wt for the present study. Data included the patients’ demographic and tumour characteristics, outcomes and treatment strategies. The ESME initiative is managed by UNICANCER in accordance with the current best practice guidelines and rules. It is supervised by a scientific independent steering committee that approved the present study. This study was authorised by the French data protection authority ([Registration ID 1704113 and authorisation N_DE-2013.-117], NCT03275311). Moreover, in compliance with the applicable European regulations, a complementary authorisation was obtained for the ESME Data Warehouse on 14 October 2019. All data were obtained retrospectively, and no procedure was taken to recover unavailable data by contacting healthcare providers or patients. The present analysis was approved by an independent French ethics committee (Comité de Protection des Personnes Sud-Est II-2015–79).
Study objectives and population
The primary objective was to assess the adjusted effect of the first-line chemotherapy type (PtCT vs. non-PtCT) on PFS in the function of the gBRCA1/2 status. The secondary objectives were to assess the effect on OS in the function of the gBRCA1/2 status, and on OS and PFS in the function of the breast cancer subtype (TNBC and HR+/HER2− MBC).
The collected variables were previously described [10]. Patients were not included in the study if they had uncertain tumour histology, HER2-positive (HER2+) tumour, or unknown HER2 status. HER2 and HR status were determined using the existing results obtained in MBC tissue samples when available, or from the last early disease tissue sample. Tumours were defined as HR-positive if ER or PR expression was >10% (by immunohistochemistry, IHC) [25, 26]. As detailed in the current version of the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) criteria [27], cancer was considered HER2+ if the HER2 IHC score was ≥2 with positive fluorescence in situ hybridisation (FISH) or chromogenic in situ hybridisation (CISH). All cancers with IHC score 0–1 or 2 and negative FISH/CISH test and all tumours with negative FISH/CISH test without any IHC information were considered as HER2-negative (HER2−). Cancers with IHC score 2 and without FISH/CISH information were considered as HER2 indeterminate and excluded from the analysis.
To avoid guarantee-time bias related to the time between treatment initiation and genetic test availability, analyses were performed with gBRCA1/2 status defined at different time points. The main analysis was performed at baseline (chemotherapy initiation), and supportive analyses were performed 3 and 6 months after treatment initiation. Thus, patients were classified in function of the genetic test result availability at each time point in three groups: gBRCA1/2m (presence of a deleterious gBRCA1/2 mutation), gBRCA1/2wt (absence of deleterious gBRCA1/2 mutation, or mutations in other genes), or gBRCA1/2 untested.
Data were collected until the cut-off date (October 13, 2018), the date of death or of the last contact if lost to follow-up.
Study endpoints
The primary endpoint was PFS defined as the time between the start of the first chemotherapy treatment for MBC and the date of first disease progression or death in patients with known gBRCA1/2 status. Patients without known gBRCA1/2 status were excluded from the analysis. Patients still alive and without progression at the time of analysis were censored at the date of the last contact. The main secondary endpoint was OS, defined as the time between the date of the first-line chemotherapy initiation and the date of death due to any cause or the date of the last contact. Disease progression was defined as the occurrence of a new metastatic site, progression of existing metastases, local or locoregional recurrence of the primary tumour, discontinuation of chemotherapy and/or targeted therapy due to metastatic progression, or death from any cause.
Statistical considerations
Categorical variables were described using frequencies and percentages, and quantitative variables using medians and range (min; max). Groups were compared using the Chi-square or Fisher’s exact test for categorical variables and the Kruskal–Wallis test for quantitative variables. The gBRCA1/2 status was defined at different time points to prevent guarantee-time bias: at chemotherapy initiation (baseline) and at 3 and 6 months after chemotherapy initiation (landmark analyses). For each analysis, the gBRCA1/2 status was defined on the basis of the genetic testing performed before that time point, and patients who died, progressed, or were censored before that time point were excluded. Patients with untested gBRCA1/2 status at a given time point were excluded from the analyses at that time point. Survival curves were generated with the Kaplan–Meier method. In univariable analyses, groups were compared using the log-rank test, and unadjusted hazard ratios (HR) with their 95% confidence intervals (CI) were estimated with an univariable Cox proportional hazard model. Adjusted HRs and their 95% CIs were estimated using multivariable Cox proportional hazard models. Subgroup analyses were performed in function of the patients’ gBRCA1/2 status (gBRCA1/2m and gBRCA1/2wt) and tumour subtype (TNBC and HR+/HER2− tumours). All statistical tests were two-sided, and a P value <0.05 was considered statistically significant. No adjustment was made for multiple comparisons. Statistical analyses were performed using the Stata software version 16. All reported results are based on the gBRCA1/2 status defined at CT initiation, and on multivariable analyses adjusted for tumour subtype (TNBC vs. HR+/HER2−), age at MBC diagnosis, the interval between primary diagnosis and MBC diagnosis (de novo MBC status: <6 months vs. 6–24 months vs. >24 months), interval from MBC diagnosis to chemotherapy initiation (≤1 month vs. >1 month), metastasis localisation (visceral vs. non-visceral), and number (<3 vs. ≥3) of metastases.
Results
Patients’ characteristics
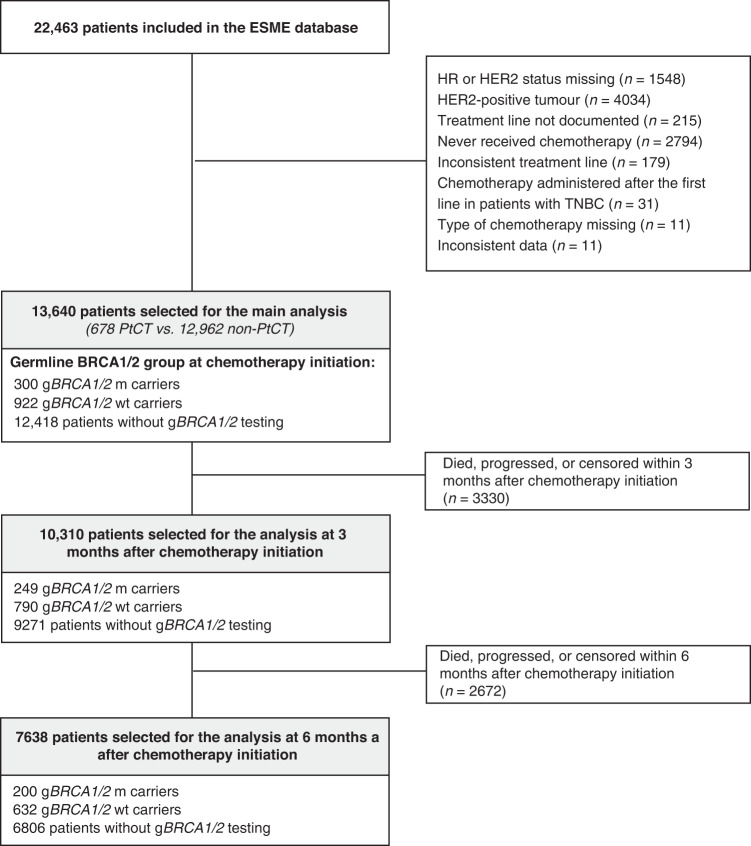
Among the 22,463 patients with MBC included in the ESME-MBC cohort 2008–2016, 13,640 were selected based on their tumour HER2 and HR status and administration of first-line chemotherapy for MBC. The gBRCA1/2 status at the time of first-line chemotherapy initiation (baseline) was known for 1222 of these patients. The patient sample composition at baseline and at the 3-month and 6-month time points are summarised in Fig. 1. HR+ patients in the Platinum-based chemotherapy received less frequently an endocrine therapy before first-line chemotherapy, compared to patients in the non-platinum group (73.7% vs. 86%, P = 0.0125). Due to the considered period of inclusion, only one patient received concomitant anti-CDK4/6 treatment.
Fig. 1.
Study flowchart.
The characteristics of the patients with known gBRCA1/2 status at baseline are detailed in Table 1. Their median follow-up was 39 months [95% CI: 35.6–42.7]. Patients who received first-line PtCT (n = 186) were younger, more often gBRCA1/2m carriers, had a shorter interval between initial diagnosis and MBC diagnosis, higher histological grade and/or TNBC, with more frequent visceral and central nervous system metastases than those who received non-PtCT (n = 1036). In the PtCT group, treatment was initiated faster, and platinum agents were more often combined with other chemotherapy agents than with targeted therapies (mainly bevacizumab). The characteristics of all patients at baseline (n = 13,640, including patients still without genetic testing) are in Supplementary Table S1. Due to the indications of oncogenetic testings used during this period, the untested population was older, and less frequently affected by Grade III tumours or TNBC, compared to the tested population.
Table 1.
Population’s characteristics in function of the first-line chemotherapy regimen.
| Total | Non-platinum | Platinum-based | ||
|---|---|---|---|---|
| (N = 1222) | (N = 1036) | (N = 186) | P value | |
| Germline BRCA1/2 group | <0.0001 | |||
| gBRCA1/2wt | 922 (75.5%) | 817 (78.9%) | 105 (56.5%) | |
| gBRCA1/2m | 300 (24.5%) | 219 (21.1%) | 81 (43.5%) | |
| Age (years) at MBC diagnosis: median (range) | 48 (23;85) | 48.0 (24;85) | 46 (23;80) | 0.0002 |
| Age at MBC diagnosis | 0.0002 | |||
| <50 years | 668 (54.7%) | 543 (52.4%) | 125 (67.2%) | |
| ≥50 years | 554 (45.3%) | 493 (47.6%) | 61 (32.8%) | |
| Sex | 0.0372 | |||
| Men | 22 (1.8%) | 22 (2.1%) | 0 (0.0%) | |
| Women | 1200 (98.2%) | 1014 (97.9%) | 186 (100.0%) | |
| Primary tumour histological grade (n = 1043) | <0.0001 | |||
| Grade I/II | 568 (54.5%) | 513 (58.7%) | 55 (32.5%) | |
| Grade III | 475 (45.5%) | 361 (41.3%) | 114 (67.5%) | |
| Primary tumour histological type (n = 1217) | <0.0001 | |||
| Not specified/IDC | 988 (81.2%) | 816 (79.1%) | 172 (92.5%) | |
| ILC | 94 (7.7%) | 90 (8.7%) | 4 (2.2%) | |
| IDC+ILC | 12 (1.0%) | 12 (1.2%) | 0 (0.0%) | |
| Other | 123 (10.1%) | 113 (11.0%) | 10 (5.4%) | |
| Breast cancer subtype* | <0.0001 | |||
| HR+/HER2− | 821 (67.2%) | 762 (73.6%) | 59 (31.7%) | |
| TNBC | 401 (32.8%) | 274 (26.4%) | 127 (68.3%) | |
| Time from the primary tumour to MBC diagnosis (months) median (range) | 42.3 (−1.2; 516.2) | 47.3 (−1.2; 516.2) | 23.6 (−0.3; 404.4) | <0.0001 |
| <6 months | 89 (7.3%) | 80 (7.7%) | 9 (4.8%) | <0.0001 |
| 6–24 months | 290 (23.7%) | 204 (19.7%) | 86 (46.2%) | |
| >24 months | 843 (69.0%) | 752 (72.6%) | 91 (48.9%) | |
| Number of metastatic sites | 0.0015 | |||
| <3 sites | 909 (74.4%) | 788 (76.1%) | 121 (65.1%) | |
| ≥3 sites | 313 (25.6%) | 248 (23.9%) | 65 (34.9%) | |
| Metastasis type | 0.0015 | |||
| Visceral | 767 (62.8%) | 631 (60.9%) | 136 (73.1%) | |
| Non-visceral | 455 (37.2%) | 405 (39.1%) | 50 (26.9%) | |
| CNS/CSF metastases | 140 (11.5%) | 97 (9.4%) | 43 (23.1%) | <0.0001 |
| Bone metastases | 621 (50.8%) | 551 (53.2%) | 70 (37.6%) | <0.0001 |
| Lung metastases | 337 (27.6%) | 259 (25.0%) | 78 (41.9%) | <0.0001 |
| Lymph node metastases | 453 (37.1%) | 356 (34.4%) | 97 (52.2%) | <0.0001 |
| Liver metastases | 353 (28.9%) | 299 (28.9%) | 54 (29.0%) | 0.9622 |
| Bone metastases only | 222 (18.2%) | 209 (20.2%) | 13 (7.0%) | <0.0001 |
| Time from MBC diagnosis to chemotherapy initiation (n = 1222) | 0.0059 | |||
| 0–1 month | 512 (41.9%) | 417 (40.3%) | 95 (51.1%) | |
| >1 month | 710 (58.1%) | 619 (59.7%) | 91 (48.9%) | |
| Multi-treatment (n = 1222) | 636 (52.0%) | 572 (55.2%) | 64 (34.4%) | <0.0001 |
| First-line polychemotherapy (n = 1222) | 479 (39.2%) | 301 (29.1%) | 178 (95.7%) | <0.0001 |
CNS/CSF central nervous system/cerebrospinal fluid, gBRCA1/2 m germline BRCA1/2 deleterious mutation, gBRCA1/2wt germline BRCA1/2 wild-type, HER2− HER2 negative, HR+ hormone receptor-positive, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, MBC metastatic breast cancer, multi-treatment first-line treatment in which chemotherapy is combined with targeted therapy, TNBC triple-negative breast cancer.
Bold values represent statistical significance p < 0.05.
*See definition of tumour subtype in “Methods”.
Progression-free and overall survival in gBRCA1/2m population at baseline and in function of their breast cancer subtype
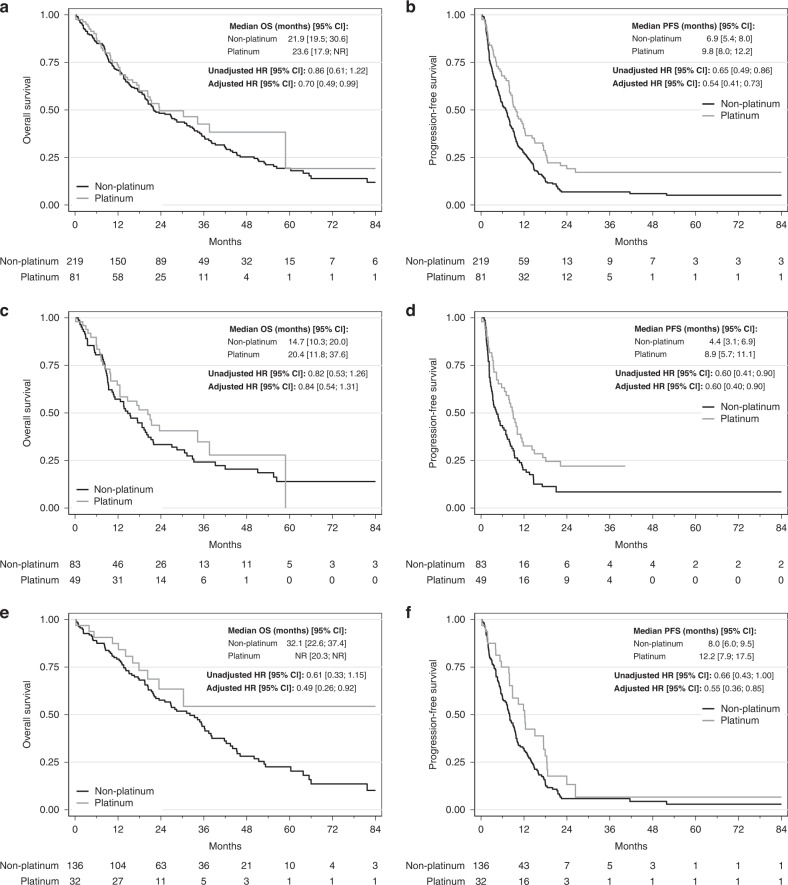
In the univariable analyses of patients with gBRCA1/2m at baseline (n = 300), PFS was increased in the PtCT group compared with the non-PtCT group (median PFS: 9.8, 95% CI 8.0–12.2, vs. 6.9, 95% CI 5.4–8.0 months, HR = 0.65, 95% CI 0.49–0.86, P = 0.021, Fig. 2a), but not OS (median OS: 23.6, 95% CI 17.9–NR, vs. 21.9, 95% CI 19.5–30.6 months, HR = 0.86, 95% CI 0.61–1.22, P = 0.39, Fig. 2b). Similar results were obtained when patients were divided in function of the tumour type (TNBC and HR+/HER2− subgroups) (Fig. 2c–f).
Fig. 2. Progression-free survival (PFS) and overall survival (OS) in function of the chemotherapy regimen (PtCT and non-PtCT) in the gBRCA1/2m population, and in function of the tumour subtype (TNBC and HR+/HER2−).
a PFS in the gBRCA1/2m group. b OS in the gBRCA1/2m group. c PFS in the gBRCA1/2m TNBC group. d OS in the gBRCA1/2m TNBC group. e PFS in the gBRCA1/2m HR+/HER2− group. f OS in the gBRCA1/2m HR+/HER2− group.
In the multivariable analysis of gBRCA1/2m carriers, PtCT remained significantly associated with better PFS compared with non-PtCT (HR 0.54, 95% CI 0.41–0.73, P < 0.001) (Table 2 and Fig. 2a) and also with better OS (HR 0.70, 95% CI 0.49–0.99, P = 0.047) (Table 2 and Fig. 2b).
Table 2.
Multivariable analysis of factors predicting progression-free and overall survival of patients with gBRCA1/2m at chemotherapy initiation.
| PFS | OS | |||
|---|---|---|---|---|
| HR [95% CI] | P | HR [95% CI] | P | |
| Chemotherapy type (first line) | ||||
| Non-platinum | 1 | 1 | ||
| Platinum | 0.54 [0.41; 0.73] | <0.001 | 0.70 [0.49; 0.99] | 0.047 |
| Age at MBC diagnosis | ||||
| <50 years | 1 | 1 | ||
| ≥50 years | 1.02 [0.78; 1.34] | 0.857 | 1.40 [1.03; 1.91] | 0.033 |
| Metastasis type | ||||
| Visceral | 1 | 1 | ||
| Non-visceral | 0.76 [0.56; 1.03] | 0.082 | 0.61 [0.43; 0.88] | 0.009 |
| Number of metastatic sites | ||||
| <3 sites | 1 | 1 | ||
| ≥3 sites | 1.80 [1.34; 2.41] | <0.001 | 1.39 [0.99; 1.94] | 0.055 |
| Breast cancer subtype* | ||||
| HR+/HER2− | 1 | 1 | ||
| TNBC | 1.13 [0.87; 1.48] | 0.345 | 1.49 [1.11; 2.00] | 0.009 |
| Time from the primary tumour to MBC diagnosis | ||||
| <6 months | 1 | 1 | ||
| 6–24 months | 3.36 [1.92; 5.88] | <0.001 | 3.65 [1.72; 7.75] | 0.001 |
| >24 months | 2.17 [1.29; 3.67] | 0.004 | 1.89 [0.92; 3.90] | 0.083 |
| Time from MBC diagnosis to chemotherapy initiation | ||||
| 0–1 month | 1 | 1 | ||
| >1 month | 0.91 [0.71; 1.16] | 0.450 | 0.80 [0.60; 1.06] | 0.125 |
gBRCA1/2m germline BRCA1/2 deleterious mutation, HER2− HER2 negative, HR+ hormone receptors positive, MBC metastatic breast cancer, PFS progression-free survival, OS overall survival, TNBC triple-negative breast cancer.
Bold values represent statistical significance p < 0.05.
*See definition of tumour subtype in “Methods”.
In patients with gBRCA1/2m and TNBC (N = 132), PtCT again was independently associated with better PFS compared with non-PtCT (HR = 0.60, 95% CI 0.4–0.9, P = 0.014), without any significant difference in OS (HR = 0.84, 95% CI 0.54–1.31, P = 0.44) (Supplementary Table S2 and Fig. 2c, d).
In patients with gBRCA1/2m and HR+/HER2− breast cancer (N = 168), PtCT was significantly associated with better PFS and OS compared with non-PtCT regimens (HR 0.55, 95% CI 0.36–0.85, P = 0.007 and HR 0.49, 95% CI 0.26–0.92, P = 0.028, respectively) (Supplementary Table S2 and Fig. 2e, f).
The landmark analyses at the 3- and 6-month time points supported these results (Supplementary Figs. S1 and S2). However, the HR values progressively increased in favour of non-PtCT. For example, for PFS in the whole gBRCA1/2m population, the HR value was 0.54 at baseline, 0.59 at 3 months, and 0.65 at 6 months (Supplementary Fig. S1). Lastly, the exclusion of nine patients having received a PARP inhibitor before first metastatic chemotherapy did not modify these results (data not shown).
Progression-free and overall survival in the gBRCA1/2 wild-type population
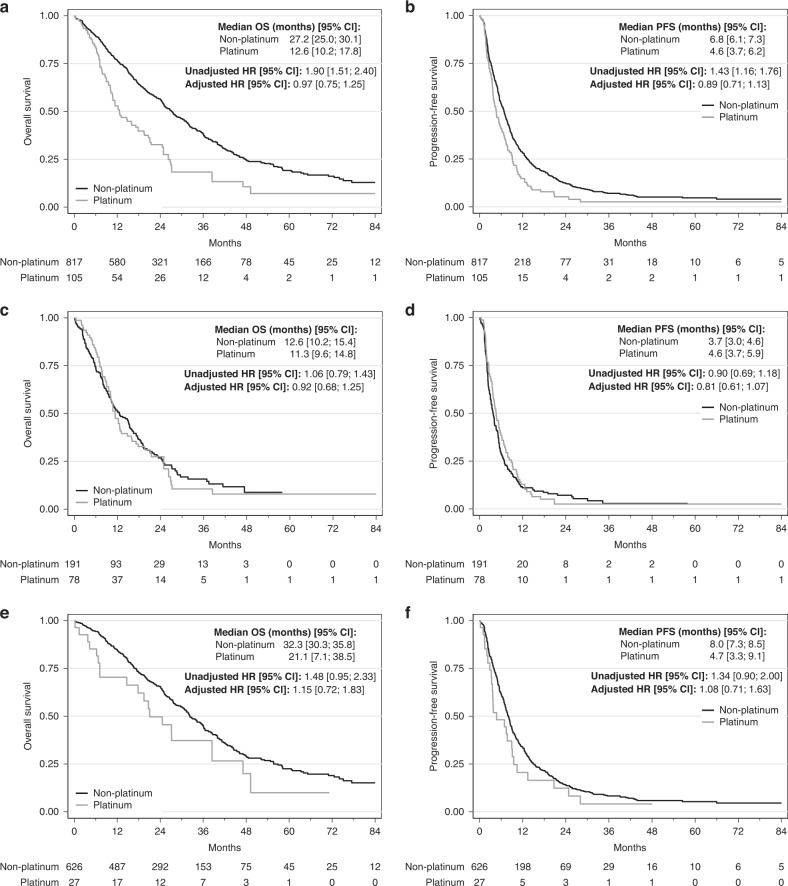
In the univariable analyses of patients with gBRCA1/2wt at baseline, PtCT was associated with significantly worse PFS (median PFS: 4.6 [3.7; 6.2] vs. 6.8 [6.1; 7.3] months, HR = 1.43 [1.16; 1.76], P = 0.0008, Fig. 3a) and also OS compared with non-PtCT (median OS: 12.6 [10.2;17.8] vs. 27.2 [25.0; 30.1] months, HR = 1.90 [1.51; 2.40], P < 0.0001, Fig. 3b). In the tumour subgroup analyses, PFS and OS were not significantly different between PtCT and non-PtCT in the TNBC gBRCA1/2wt subgroup (Fig. 3c, d). Conversely, PFS and OS were shorter after PtCT in the HR+/HER2− gBRCA1/2wt subgroup (median PFS: 4.7 [3.3; 9.1] vs. 8 months [7.3; 8.5], HR = 1.34 [0.90; 2.00], P = 0.15; median OS: 21.1 [7.1; 38.5] vs. 32.3 [30.3; 35.8] months, HR = 1.48 [0.95; 2.33], P = 0.08) (Fig. 3e, f).
Fig. 3. Progression-free survival (PFS) and overall survival (OS) in function of the chemotherapy regimen (PtCT and non-PtCT) in the gBRCA1/2wt population, and in function of the tumour subtype (TNBC and HR+/HER2−).
a PFS in the gBRCA1/2wt group. b OS in the gBRCA1/2wt group. c PFS in the gBRCA1/2wt TNBC group. d OS in the gBRCA1/2wt TNBC group. e PFS in the gBRCA1/2wt HR+/HER2− group. f OS in the gBRCA1/2wt HR+/HER2− group.
In multivariable analyses, no significant association was found between chemotherapy type and PFS and OS neither in the whole gBRCA1/2wt population (N = 922, Table 3), nor in the TNBC (N = 269) and HR+/HER2− (N = 653) subgroups (Supplementary Table S3 and Fig. 3). Similar results were obtained at the 3- and 6-month landmark analyses (Supplementary Figs. S1 and S2), in which no significant difference was observed among subgroups. Lastly, the exclusion of nine patients having received a PARP inhibitor before the first metastatic chemotherapy did not modify these results (data not shown).
Table 3.
Multivariable analysis of factors predicting progression-free and overall survival in patients with gBRCA1/2wt at CT initiation.
| PFS | OS | |||
|---|---|---|---|---|
| HR [95% CI] | P | HR [95% CI] | P | |
| Chemotherapy type (first line) | ||||
| Non-platinum | 1 | 1 | ||
| Platinum | 0.89 [0.71; 1.13] | 0.339 | 0.97 [0.75; 1.25] | 0.812 |
| Age at MBC diagnosis | ||||
| <50 years | 1 | 1 | ||
| ≥50 years | 1.00 [0.87; 1.15] | 0.976 | 1.26 [1.07; 1.49] | 0.006 |
| Metastasis type | ||||
| Visceral | 1 | 1 | ||
| Non-visceral | 0.77 [0.66; 0.90] | 0.001 | 0.72 [0.59; 0.87] | 0.001 |
| Number of metastatic sites (cl.) | ||||
| <3 sites | 1 | 1 | ||
| ≥3 sites | 1.00 [0.84; 1.20] | 0.984 | 1.07 [0.87; 1.32] | 0.519 |
| Breast cancer subtype* | ||||
| HR+/HER2− | 1 | 1 | ||
| TNBC | 1.52 [1.27; 1.83] | <0.001 | 2.20 [1.78; 2.70] | <0.001 |
| Time from the primary tumour to MBC diagnosis | ||||
| <6 months | 1 | 1 | ||
| 6–24 months | 1.94 [1.41; 2.67] | <0.001 | 2.37 [1.57; 3.58] | <0.001 |
| >24 months | 1.22 [0.91; 1.64] | 0.18 | 1.30 [0.89; 1.92] | 0.178 |
| Time from MBC diagnosis to chemotherapy initiation | ||||
| 0–1 month | 1 | 1 | ||
| >1 month | 0.99 [0.86; 1.15] | 0.916 | 0.93 [0.78; 1.10] | 0.373 |
gBRCA1/2wt germline BRCA1/2 wild-type, HER2− HER2 negative, HR+ hormone receptors positive, MBC metastatic breast cancer, PFS progression-free survival, OS overall survival, TNBC triple-negative breast cancer.
Bold values represent statistical significance p < 0.05.
*See definition of tumour subtype in “Methods”.
Results of the multivariate analyses remain consistent while considering the overall population and evaluating the oncogenetic groups in three (gBRCAm, gBRCAwt and untested populations, data not shown), the untested population display HRs extremely consistent with the gBRCAwt population.
Progression-free and overall survival in patients receiving first-line anthracycline-based chemotherapy
In order to evaluate the impact of anthracyclines in this population, as DNA-damaging agents, we performed a second analysis using three treatment modalities: Platinum-based chemotherapy, Anthracyclines-based chemotherapy and other chemotherapies. In multivariate analyses, Anthracyclines-based chemotherapy and Platinum-based chemotherapy groups presented significantly better PFS and OS compared to the other chemotherapy group for the gBRCA1/2m population (data not shown). However, the Anthracycline-based chemotherapy group displayed a significantly higher proportion of de novo metastatic cancers compared to the Platinum-based and other chemotherapy groups (28% vs. 4.8 and 5.1%, respectively).
Discussion
We report here the results of the analysis of data from a very large retrospective series on the real-world efficacy of first-line PtCT among patients affected by MBC, with and without pathogenic germline BRCA1/2 mutations. In gBRCA1/2m carriers, PtCT was significantly and independently associated with better PFS and OS compared with non-PtCT regimens (HR 0.54, 95% CI 0.41–0.73, P < 0.001, and HR 0.70, 95% CI 0.49–0.99, P = 0.047, respectively). Of note, PtCT was more frequently prescribed in patients with negative prognostic factors, such as younger age, shorter metastasis-free interval, TNBC phenotype, and visceral spreading (Table 1). PtCT had a positive effect on both PFS and OS in patients with luminal-type (HR+/HER2−) cancer, whereas only PFS was significantly improved in patients with gBRCA1/2m-linked TNBC. Conversely, the use of platinum did not have any positive effect in the gBRCA1/2wt population. In these patients, PtCT was associated with a significantly worse prognosis in the univariable analysis, but not in the multivariable analysis that took into account the adverse prognostic factors associated with PtCT prescription (Table 1).
These results are in accordance with those of the TNT trial, the only randomised clinical trial in this setting [22] that compared first-line carboplatin and docetaxel in patients with HER2-negative MBC. Overall, carboplatin was not more active than docetaxel, whatever the tumour HR status. However, in the pre-specified analysis of the gBRCA1/2m population, carboplatin induced more responses (overall response rate: 68% for carboplatin vs. 33% for docetaxel) and longer PFS (median PFS: 6.8 months for carboplatin vs. 4.4 months for docetaxel; P = 0.002). In the BROCADE-3 study, the combination of carboplatin and paclitaxel in patients with gBRCA1/2m-linked MBC gave a response rate of 74%, and a median PFS of 12.6 months (95% CI 10.6–14.4). As the efficacy of platinum salts in gBRCA1/2m carriers is not limited to patients with TNBC, the gBRCA1/2 status appears to be more relevant than the tumour IHC for predicting PtCT efficacy. Indeed, our results and those of these two trials show consistent benefits of PtCT also in gBRCA1/2m carriers with HR+/HER2− breast cancer.
PARP1 inhibitors, the main current available therapy to target the DNA-repair machinery in gBRCA1/2m carriers, outperform PtCT, although no direct comparison has been performed yet. As observed for PtCT, two (EMBRACA and BROCADE 3) of the three published PARP inhibitor Phase III trials in advanced breast cancer demonstrated consistent benefit across subtypes, and one (OlympiaD) reported less significant results in patients with luminal cancer [28–31].
The prominent role of the gBRCA1/2m status, rather than the IHC profile, on PtCT impact might indirectly explain the results of the meta-analyses on PtCT effect in MBC without specific identification of the gBRCA1/2 status. They reported some effect in the TNBC subgroup [20, 32], with no strong evidence of an additional benefit in the HR+/HER2− population [32]. Thus, our results strengthen the concept of PtCT usefulness specifically in gBRCA1/2m MBC, whatever the tumour IHC profile (HR+/HER2− or TNBC), and its limited or no additional efficacy in gBRCA1/2wt carriers. This supports early gBRCA1/2 status testing in patients with MBC. Indeed, our analyses at different time points show a progressive reduction of the beneficial effect over time, with the highest effect observed in the population with known gBRCA1/2m at CT initiation.
As expected, our results are in disagreement with studies showing that neoadjuvant PtCT does not bring any added value in gBRCA1/2m carriers. For example, in the BrighTNess trial, a matched cohort analysis did not find any difference in pathological complete response rates between gBRCA1/2m and gBRCA1/2wt carriers after neoadjuvant carboplatin+/− veliparib [33]. This might be linked to the backbone therapy used in the neoadjuvant setting, because alkylating agents and topoisomerase II inhibitors can induce single- and double-strand DNA breaks that mimic the damage induced by platinum salts. Conversely, chemotherapy regimens frequently used as first-line treatment of MBC include taxanes and capecitabine that do not induce direct DNA damage. However, in an exploratory analysis, we evaluated the impact of the first-line use of anthracyclines-based chemotherapies as another group of DNA-damaging chemotherapies. While highly biased, as this regimen was, as expected, strongly associated with de novo metastatic breast cancers, the results suggest that gBRCA1/2m display closely related sensitivities to Platinum- and Anthracyclines-based chemotherapies, when compared to the other chemotherapies. While interesting, and consistent with the results of the TBCRC 031 trial [34], these results must remain hypothesis generating and needs to be validated in an independent cohort. In the present study, despite the large size of the whole cohort, the limited number of gBRCA1/2m carriers and the heterogeneity of comparator chemotherapy regimens precluded the evaluation of PtCT regimen impact compared with a given comparator drug. Similarly, sub-analyses in the function of the mutation type (gBRCA1m or gBRCA2m) were not performed due to a lack of statistical power.
Our national, real-life ESME-MBC cohort has several major strengths described in previous publications [35, 36], particularly the nationwide, unbiased inclusion of all new patients with MBC treated in one of the 18 participating comprehensive cancer centres, the low number of missing data considering the cohort size, the standardised procedures for the homogeneous collection of the variables of interest, and the development of robust statistical methodologies to reduce the analysis limitations. Moreover, the large and free access to genetic testing performed at the National Cancer Institute platforms allowed germline BRCA1/2 mutation testing in all patients with a family history of breast/ovarian cancer. However, the present study was carried out in the pre-PARP inhibitor era, when theranostic genetic testing was not routinely done, and the number of carriers identified at baseline remains limited. Other limitations are inherent to this type of real-life cohort. They include the heterogeneity of treatment regimens. The PtCT and non-PtCT populations were unbalanced in terms of polychemotherapies (95.7% vs. 29.1%, respectively), and non-PtCT regimens were more frequently used in association with targeted therapies (55.2% vs. 34.4% for PtCT), mainly bevacizumab. The precise impact of combination and single-agent treatments could not be assessed due to the limited number of evaluable patients; however, one might expect increased efficacy of combination regimens [36, 37]. Our cohort could robustly capture efficacy data (e.g., PFS and OS), but not toxicity and quality of life data, thus precluding a fine evaluation of the toxicity–efficacy ratio. Indeed, platinum salts are associated with specific toxicities (i.e., renal and haematopoietic toxicities) that could reduce the benefit associated with the crude increase in survival [32]. In addition, chemotherapy combinations have been consistently associated with an increase in toxicities compared with single-agent treatments [37]. Thus, a comprehensive evaluation of the efficacy/toxicity profile of PtCT and non-PtCT regimens is necessary to optimise the care of patients with MBC.
Finally, we cannot completely rule out biases linked to gBRCA testing and its potential association with specific cancer features and behaviours, as well as survival bias. These biases have been widely described in retrospective studies that assessed the prognosis of patients with localised breast cancer in the function of their gBRCA status [38]. In the MBC setting of the present study, prognosis (PFS) was assessed in function the baseline gBRCA1/2 status, thus avoiding most of these biases. Furthermore, time-varying covariates included both new mutant and wild-type status. The sensitivity analyses and the analyses at different time points limited these potential biases.
Lastly, while the use of Platinum salts in the neoadjuvant/adjuvant setting was rare during the period considered by this study, the recent results of the BrighTNess [39] and KEYNOTE-522 [40] trials are currently practice changing on this point. Thus, considering our results, one major, and currently unanswered, the question will be the activity of Platinum salts in the metastatic setting, after previous exposure in the early breast cancer setting.
In conclusion, this large-scale real-world analysis conducted in the pre-PARP inhibitor era strongly suggests that first-line PtCT for MBC is associated with increased PFS and OS in patients with gBRCA1/2m but not in patients with gBRCA1/2wt. These results indicate that PtCT should be proposed only to gBRCA1/2m carriers and support the very early gBRCA1/2 testing in MBC.
Supplementary information
Acknowledgements
We thank the 18 French Comprehensive Cancer Centers (Institut Curie, Paris and Saint-Cloud; Gustave Roussy, Villejuif; Institut Cancerologie de l’Ouest, Angers and Nantes; Centre Francois Baclesse, Caen; Institut du Cancer de Montpellier, Montpellier; Centre Leon Berard, Lyon; Centre Georges-Francois Leclerc, Dijon; Centre Henri Becquerel, Rouen; Institut Claudius Regaud, Toulouse; Centre Antoine Lacassagne, Nice; Institut de Cancerologie de Lorraine, Nancy; Centre Eugene Marquis, Rennes; Institut Paoli-Calmettes, Marseille; Centre Jean Perrin, Clermont Ferrand; Institut Bergonie, Bordeaux; Centre Paul Strauss, Strasbourg; Institut de Cancerologie Jean-Godinot, Reims; and Centre Oscar Lambret, Lille) for providing the data and each ESME contact for coordinating the project at the local level. Moreover, we thank the central coordination team of Unicancer and the ESME strategic and scientific committee members for their ongoing support.
Author contributions
WJ: conceptualisation, formal analysis, investigation, methodology, visualisation, writing—original draft, writing—review and editing. AL: conceptualisation, data curation, formal analysis, methodology, visualisation, funding acquisition, writing—original draft, writing—review and editing. CV: investigation, writing—review and editing. AM: investigation, writing—review and editing. TDLMR: investigation, writing—review and editing. LC: investigation, writing—review and editing. CL: investigation, writing—review and editing. AP: investigation, writing—review and editing. ID: investigation, writing—review and editing. LU: investigation, writing—review and editing. JCT: investigation, writing—review and editing. MR: investigation, writing—review and editing. OC: investigation, writing—review and editing. OT: investigation, writing—review and editing. TF: conceptualisation, data curation, investigation, writing—review and editing. JSF: conceptualisation, investigation, writing—review and editing. SD: conceptualisation, investigation, writing—review and editing.
Funding
The ESME-MBC database receives financial support from an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai, and Daiichi Sankyo). Data collection, analysis and publication are managed entirely by UNICANCER, independently of the industrial consortium.
Data availability
The data presented in this study are available on reasonable request from the corresponding author.
Ethics approval and consent to participate
This study was authorised by the French data protection authority ([Registration ID 1704113 and authorisation N_DE-2013.-117], NCT03275311). Moreover, in compliance with the applicable European regulations, a complementary authorisation was obtained for the ESME Data Warehouse on 14 October 2019. All data were obtained retrospectively, and no procedure was taken to recover unavailable data by contacting healthcare providers or patients. The present analysis was approved by an independent French ethics committee (Comité de Protection des Personnes Sud-Est II-2015–79).
Consent to publish
Not applicable.
Competing interests
Dr. TDlMR reports personal fees and non-financial support from AstraZeneca, personal fees from Clovis oncology, personal fees from GSK, grant and personal fees from MSD, grant, personal fees and non-financial support from Pfizer, personal fees from Tesaro, personal fees and non-financial support from Roche Genentech, grant from SeattleGenetics, grant from Novartis, personal fees from Eisai, grant from Novartis, outside the submitted work. Dr. SD reports grants and non-financial support from Pfizer, grants from Novartis, grants and non-financial support from AstraZeneca, grants and non-financial support from Roche Genentech, grants from Lilly, grants from Puma, grants from Myriad, grants from Orion, grants from Amgen, grants from Sanofi, grants from Genomic Health, grants from GE, grants from Servier, grants from MSD, grants from BMS, grants from Pierre Fabre, grants from Seagen, grants from Exact Sciences, grants from Rappta, grants from Besins, grants from European Commission, grants from French government grants, grants from Fondation ARC, outside the submitted work. Dr. TF reports fees to its institution from Cellectis, grants from BMS, outside the submitted work. Dr. JSF reports personal fees from Roche Genentech, personal fees and non-financial support from SeattleGenetics, personal fees and non-financial support from Novartis, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Lilly, personal fees and non-financial support from Novartis, personal fees and non-financial support from GSK, personal fees and non-financial support from Clovis oncoloy, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Daiichi Sankyo, personal fees and non-financial support from Gilead, personal fees and non-financial support from MSD, personal fees and non-financial support from Pierre Fabre, personal fees and non-financial support from Amgen, outside the submitted work. Dr. WJ reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Eisai, personal fees and non-financial support from Novartis, personal fees and non-financial support from Roche, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Eli Lilly, personal fees from MSD, personal fees from BMS, personal fees and non-financial support from Chugai, personal fees from Seagen, grants and personal fees from Daiichi Sankyo, outside the submitted work. Dr. CL reports personal fees from Roche, non-financial support from MSD, grants from Lilly, other from DAIICHI, other from Pfizer, other from SANDOZ, outside the submitted work. Dr. AP reports other from Lilly, other from Pfizer, other from Pierre Fabre, others from Daiichi Sankyo, outside the submitted work. Dr. MR reports non-financial support from Roche, outside of the submitted work. Dr. JCT reports personal fees and non-financial support from PFIZER, personal fees from MSD, personal fees from ASTRA ZENECA, non-financial support from NOVARTIS, outside the submitted work. Dr. OT reports grants and personal fees from Roche, grants and personal fees from MSD-Merck, grants from BMS, personal fees from Novartis-Sandoz, personal fees from Pfizer, personal fees from Lilly, personal fees from AstraZeneca, personal fees from Daiichi Sankyo, personal fees from Eisai, personal fees from Pierre Fabre, personal fees from Seagen, personal fees from Gilead, outside the submitted work. Dr. CV reports personal fees from AstraZeneca, grants from BMS, non-financial support from IPSEN, personal fees from Novartis, personal fees and non-financial support from Pfizer, personal fees from Daiichi Sankyo, outside the submitted work. Drs. LC, OC, ID, AL, AM and LU have nothing to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02003-1.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Engel C, Fischer C. Breast cancer risks and risk prediction models. Breast Care. 2015;10:7–12. doi: 10.1159/000376600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong N, Ryder S, Forbes C, Ross J, Quek RG. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543–61. doi: 10.2147/CLEP.S206949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol. 2010;33:637–45. doi: 10.1097/COC.0b013e3181b8afcf. [DOI] [PubMed] [Google Scholar]
- 6.Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20:2310–8. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Stevens KN, Vachon CM, Couch FJ. Genetic susceptibility to triple-negative breast cancer. Cancer Res. 2013;73:2025–30. doi: 10.1158/0008-5472.CAN-12-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y, Barry WT, Seah DS, Tung NM, Garber JE, Lin NU. Patterns of recurrence and metastasis in BRCA1/BRCA2-associated breast cancers. Cancer. 2020;126:271–80. doi: 10.1002/cncr.32540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 10.Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–19. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Palma J, Kinders R, Shi Y, Donawho C, Ellis PA, et al. An enzyme-linked immunosorbent poly(ADP-ribose) polymerase biomarker assay for clinical trials of PARP inhibitors. Anal Biochem. 2008;381:240–7. doi: 10.1016/j.ab.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 14.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 16.Loibl S, Weber KE, Timms KM, Elkin EP, Hahnen E, Fasching PA, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018;29:2341–7. doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 17.Hahnen E, Lederer B, Hauke J, Loibl S, Krober S, Schneeweiss A, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the geparsixto randomized clinical trial. JAMA Oncol. 2017;3:1378–85. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poggio F, Bruzzone M, Ceppi M, Ponde NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497–508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Feng J, Xu B. A meta-analysis of platinum-based neoadjuvant chemotherapy versus standard neoadjuvant chemotherapy for triple-negative breast cancer. Future Oncol. 2019;15:2779–90. doi: 10.2217/fon-2019-0165. [DOI] [PubMed] [Google Scholar]
- 20.Pandy JGP, Balolong-Garcia JC, Cruz-Ordinario MVB, Que FVF. Triple negative breast cancer and platinum-based systemic treatment: a meta-analysis and systematic review. BMC Cancer. 2019;19:1065. doi: 10.1186/s12885-019-6253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garutti M, Pelizzari G, Bartoletti M, Malfatti MC, Gerratana L, Tell G, et al. Platinum salts in patients with breast cancer: a focus on predictive factors. Int J Mol Sci. 2019;20:3390. [DOI] [PMC free article] [PubMed]
- 22.Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–37. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–49. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesueur F, Mebirouk N, Jiao Y, Barjhoux L, Belotti M, Laurent M, et al. GEMO, a national resource to study genetic modifiers of breast and ovarian cancer risk in BRCA1 and BRCA2 pathogenic variant carriers. Front Oncol. 2018;8:490. doi: 10.3389/fonc.2018.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 26.Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–66. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 27.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 28.Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee KH, Goncalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31:1526–35. doi: 10.1016/j.annonc.2020.08.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 30.Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–66. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:1269–82. doi: 10.1016/S1470-2045(20)30447-2. [DOI] [PubMed] [Google Scholar]
- 32.Egger SJ, Willson ML, Morgan J, Walker HS, Carrick S, Ghersi D, et al. Platinum-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2017;6:CD003374. doi: 10.1002/14651858.CD003374.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metzger-Filho O, Collier K, Asad S, Ansell PJ, Watson M, Bae J, et al. Matched cohort study of germline BRCA mutation carriers with triple negative breast cancer in brightness. npj Breast Cancer. 2021;7:142. doi: 10.1038/s41523-021-00349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tung N, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, et al. TBCRC 031: randomized phase II study of neoadjuvant cisplatin versus doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial) J Clin Oncol. 2020;38:1539–48. doi: 10.1200/JCO.19.03292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacot W, Heudel PE, Fraisse J, Gourgou S, Guiu S, Dalenc F, et al. Real-life activity of eribulin mesylate among metastatic breast cancer patients in the multicenter national observational ESME program. Int J Cancer. 2019;145:3359–69. doi: 10.1002/ijc.32402. [DOI] [PubMed] [Google Scholar]
- 36.Delaloge S, Perol D, Courtinard C, Brain E, Asselain B, Bachelot T, et al. Paclitaxel plus bevacizumab or paclitaxel as first-line treatment for HER2-negative metastatic breast cancer in a multicenter national observational study. Ann Oncol. 2016;27:1725–32. doi: 10.1093/annonc/mdw260. [DOI] [PubMed] [Google Scholar]
- 37.Carrick S, Parker S, Thornton CE, Ghersi D, Simes J, Wilcken N. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2009;CD003372: 10.1002/14651858.CD003372.pub3. [DOI] [PubMed]
- 38.Vos JR, Hsu L, Brohet RM, Mourits MJ, de Vries J, Malone KE, et al. Bias correction methods explain much of the variation seen in breast cancer risks of BRCA1/2 mutation carriers. J Clin Oncol. 2015;33:2553–62. doi: 10.1200/JCO.2014.59.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O’Shaughnessy J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol. 2022;33:384–94. doi: 10.1016/j.annonc.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556–67. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.