Abstract
The hallmark of Legionnaires’ disease is replication of Legionella pneumophila within cells in the alveolar spaces. The mechanisms by which L. pneumophila replicates intracellularly and kills the host cell are largely not understood. We have recently shown that within 3 h of initiation of the infection and prior to intracellular replication, L. pneumophila induces apoptosis in macrophages, alveolar epithelial cells, and peripheral blood monocytes, which correlates with cytopathogenicity (L.-Y. Gao and Y. Abu Kwaik, Infect. Immun. 67:862–870, 1999). In this report, we show that the ability of L. pneumophila to induce apoptosis is, largely, not growth phase regulated. We demonstrate that the induction of apoptosis by L. pneumophila in macrophages is mediated through the activation of caspase 3. The enzymatic activity of caspase 3 to cleave a specific synthetic substrate in vitro is detected in L. pneumophila-infected macrophages at 2 h after infection and is maximal at 3 h, with over 900% increase in activity. The activity of caspase 3 to cleave a specific substrate [poly(ADP-ribose) polymerase, or PARP] in vivo is also detected at 2 h and is maximal at 3 h postinfection. The activity of caspase 3 to cleave the synthetic substrate in vitro and PARP in vivo is blocked by a specific inhibitor of caspase 3. The kinetics of caspase 3 activation correlates with that of L. pneumophila-induced nuclear apoptosis. Inhibition of caspase 3 activity blocks L. pneumophila-induced nuclear apoptosis and cytopathogenicity during early stages of the infection. Consistent with the ability to induce apoptosis, extracellular L. pneumophila also activates caspase 3. Three dotA/icmWXYZ mutants of L. pneumophila that are defective in inducing apoptosis do not induce caspase 3 activation, suggesting that expression and/or export of the apoptosis-inducing factor(s) is regulated by the dot/icm virulence system. This is the first description of the role of caspase 3 activation in induction of nuclear apoptosis in the host cell infected by a bacterial pathogen.
Apoptosis is a strictly regulated genetic and biochemical suicide program that plays critical roles during development and tissue homeostasis and in modulating pathogenesis of a variety of diseases (54). The expanding family of cysteine proteases (caspases) that specifically cleave proteins next to aspartate (Asp) residues has been demonstrated to include crucial components of the apoptotic pathways (9). A cascade mechanism for transmission of diverse apoptotic signals into a common apoptotic effector pathway by networks of caspases has been well demonstrated (36, 38, 48). Among the 11 caspases that have been identified so far, caspase 3 plays a central role in driving the apoptotic effector pathway (36, 37). Activated caspase 3 cleaves and inactivates the inhibitor for caspase-activated DNase (ICAD), allowing CAD to enter the nucleus and degrade chromosomal DNA (17, 47). Activation of caspase 3 has been observed in various types of cells undergoing apoptosis induced by a variety of stimuli. In immune system-responsive cells, such as macrophages, neutrophils, and lymphocytes, activation of caspase 3 has been shown to be required for apoptosis induced by Fas-FasL or tumor necrosis factor alpha (TNF-α)–TNF receptor (TNFR) interactions (42).
A number of bacterial pathogens are capable of manipulating host cell apoptotic pathways, although whether these manipulations are to the advantage of the host or of the bacteria may vary among pathogens. The obligate intracellular pathogens, such as Chlamydia trachomatis and Rickettsia rickettsii, inhibit host cell apoptosis, which may allow these organisms to grow and persist intracellularly (15, 19). Many facultative intracellular bacteria, such as Mycobacterium avium (21), Shigella flexneri (61), Salmonella typhimurium (39), Legionella pneumophila (22, 40), and Yersinia pseudotuberculosis (45), have been shown to induce apoptosis in the host cell. Salmonella- and Shigella-induced apoptosis in macrophages is mediated through direct binding and activation of ICE (caspase 1) (14, 29, 31, 60). Although caspase 3 plays a central role in driving the apoptotic pathways triggered by a variety of stimuli, its role in apoptosis induced by bacterial pathogens is not known.
L. pneumophila is a parasite of protozoa in the environment and is the causative agent of Legionnaires’ disease, a potentially fatal pneumonia (1, 7). The ability of L. pneumophila to cause pneumonia is dependent on its capacity to invade and replicate within alveolar macrophages, monocytes, and potentially alveolar epithelial cells (1, 23). Initial bacterial attachment to the host cells is mediated, at least in part, by type IV pili (52), the heat shock protein Hsp60 (26), and the major outer membrane protein opsonized by complement (59). Following entry into the host cell, L. pneumophila replicates within a phagosome that does not fuse to lysosomes (see references 1 and 7 for recent reviews). This alteration in endocytic trafficking has been recently shown to be mediated by L. pneumophila proteins encoded by the pmi, mil, dot, and icm loci (24, 25, 49, 57). During the intracellular infection, the bacteria exhibit dramatic alterations in gene expression, which are thought to play major roles in bacterial adaptation to the intracellular microenvironment (2–4, 6, 8, 20) and possibly in killing the host cell upon termination of intracellular replication (13, 22).
Induction of necrosis and apoptosis plays roles in killing of the host cell by L. pneumophila. Necrosis in L. pneumophila-infected macrophages occurs within 20 to 60 min of infection at a high multiplicity of infection (MOI), 500 (32, 34). The induction of necrosis is mediated by a cell-associated pore-forming toxin, and evidence for this mediation has been recently provided (34). Interestingly, L. pneumophila is not cytotoxic to host cells during the exponential phase of growth but becomes cytotoxic upon entering the postexponential phase (13), indicating that expression of the pore-forming toxin is growth phase regulated. We have recently shown that L. pneumophila induces apoptosis in U937 human macrophages, human peripheral blood monocytes, and alveolar epithelial cells within 2 to 3 h postinfection in a dose-dependent manner and that the induction of apoptosis correlates with cytopathogenicity (22). We proposed a biphasic model by which L. pneumophila kills the host cell. The first phase is mediated by induction of apoptosis during early stages of the infection (22), and it is followed by rapid necrosis upon termination of intracellular bacterial replication concomitant with the phenotypic transition of the bacteria into the cytotoxic phenotype (13).
In this investigation, we continued our studies on characterization of the mechanisms by which L. pneumophila induces apoptosis (22). Our data clearly demonstrate that L. pneumophila-induced apoptosis in macrophages is mediated through the activation of caspase 3.
MATERIALS AND METHODS
Bacterial strains and growth media.
The virulent strain AA100 of L. pneumophila has been described previously (5). Isolation and characterization of the pmi mutants and the mil mutants of L. pneumophila have been described previously (24, 25). L. pneumophila strains were grown on buffered charcoal yeast extract agar plates or, for the mutant strains, in buffered yeast extract (BYE) broth supplemented with 50 μg of kanamycin/ml.
Cytopathogenicity of L. pneumophila to U937 macrophages.
The human macrophage-like cell line U937 was maintained and differentiated into macrophage-like cells by phorbol 12-myristate 13-acetate (Sigma, St. Louis, Mo.), as previously described (24). Infection was performed, in triplicate, in 96-well plates containing 105 cells/well at an MOI of 5 or 50 for 1 h at 37°C, followed by three washes with the culture medium to remove unattached extracellular bacteria and subsequent incubation at 37°C. Cytopathogenicity (loss of cell viability) was determined by Alamar blue assay and expressed as we described previously (22). To examine the effect of caspase inhibitor on the cytopathogenicity of L. pneumophila to the host cell, macrophages were pretreated for 90 min with 50 μmol of the caspase 3-specific inhibitor Z-DEVD-FMK (Oncogene Research Products, Cambridge, Mass.) (18). The monolayers were then infected by strain AA100 for 1 h in the presence of the inhibitor, followed by washes to remove unattached bacteria and subsequent incubation in the presence of the inhibitor. An additional 50 μmol of Z-DEVD-FMK was added to the monolayers at 12 h postinfection to replenish any inhibitor potentially degraded during this period.
DNA fragmentation analysis.
Differentiated U937 cells were plated in six-well plates (2 × 106 cells/well) and were infected with strains of L. pneumophila at an MOI of 50, as described above. At several intervals after the 1-h infection period, the cells in each well were lysed with 500 μl of lysis buffer (22), and the DNA was extracted, electrophoresed in 1.8% agarose gel, and stained with ethidium bromide, exactly as we described previously (22). To examine inhibition of DNA fragmentation by the caspase 3-specific inhibitor, U937 macrophages were preincubated with or without the inhibitor, infected by strain AA100, and incubated in the presence or absence of the inhibitor. The monolayers were lysed at 3 h after the 1-h infection period, and the DNA samples were processed exactly as described above.
TUNEL assays.
Differentiated U937 macrophages on glass coverslips were infected by strain AA100 at an MOI of 5 or 50, exactly as described above. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were performed as we described previously (22). Briefly, at 2 and 3 h after the 1-h infection period, the monolayers were fixed, permeabilized, and blocked with 2% bovine serum albumin. For labeling of L. pneumophila, the monolayers were incubated with a rabbit polyclonal antiserum raised against strain AA100 (22), followed by a goat anti-rabbit immunoglobulin G secondary antibody conjugated to Alexa Red (Molecular Probes, Inc., Eugene, Oreg.). Apoptotic nuclei were labeled with a cell death detection kit based on TUNEL, according to the instructions of the manufacturer (Boehringer Mannheim Corporation, Indianapolis, Ind.). To examine inhibition of nuclear apoptosis in the presence of the caspase 3-specific inhibitor, the monolayers were preincubated with or without the inhibitor, infected by strain AA100 at an MOI of 50, and incubated in the presence or absence of the inhibitor. The monolayers were fixed at 8 h after the 1-h infection period and labeled, exactly as described above. To demonstrate changes in plasma membrane permeability during inhibition of nuclear apoptosis by the caspase 3-specific inhibitor, cells in the monolayers at 8 h postinfection were stained with the nuclear dye propidium iodide (PI) (molecular mass, 668 Da) (22), which does not penetrate intact plasma membranes. Labeled cells were examined with a Leica model TCS NT confocal laser scanning microscope, and a minimum of 100 cells per sample were counted. Apoptosis or necrosis was quantitated as the percentage of TUNEL-positive or PI-positive cells, respectively, in the total number of cells examined.
Assays for caspase 3 enzymatic activities.
U937 macrophages in six-well plates (2 × 106 cells per well) were infected with strains of L. pneumophila or the DH5α strain of E. coli at an MOI of 50, exactly as described above. At several intervals after the 1-h infection period, the cells in each well were lysed with 300 μl of lysis buffer (5 mM EDTA [pH 8.0], 2 mM dithiothreitol, 20 mM Tris-HCl [pH 7.5], 2 μg of aprotinin/ml, and 2 μg of leupeptin/ml) for 30 min at 4°C. Cell lysates were centrifuged for 5 min at 15,000 × g and 4°C to remove insoluble cellular contents. Supernatants equivalent to 2 × 105 cells were diluted in reaction buffer (100 mM Tris-HCl [pH 7.5], 10 mM dithiothreitol, 0.1% CHAPS, 2 μg of aprotinin/ml, and 2 μg of leupeptin/ml) to a total suspension volume of 100 μl. For detection of caspase 3 activity, the suspensions were incubated in the presence or absence of 50 μmol of a fluorogenic substrate, Z-DEVD-AMC (Oncogene Research Products), which is specific for caspase 3 (43). For detection of the inhibition of caspase 3 activity, the suspensions were treated with or without 1 μmol of the caspase 3-specific inhibitor prior to addition of the fluorogenic substrate. Release of AMC from Z-DEVD-AMC by the activity of caspase 3 was measured on an LS50B luminescence spectrometer (Perkin Elmer, Norwalk, Conn.) at excitation and emission wavelengths of 380 and 460 nm, respectively.
To examine the activation of caspase 3 in macrophages by extracellular L. pneumophila, infection was carried out in the presence of 1 μg of cytochalasin D (CytD) per ml, as we described previously (22, 25). Inhibition of bacterial uptake was confirmed by complete sterilization of the infected monolayers with 50 μg of gentamicin/ml after the infection period, as we described previously (22, 25). To examine whether a factor secreted to the culture supernatant of L. pneumophila induces apoptosis, caspase 3 activity was examined in U937 macrophages that had been incubated for 4 h in tissue culture medium containing 5% bacterial culture supernatant, which is equivalent to the ratio of bacterial suspension added during inoculation at an MOI of 50. The bacterial culture supernatant was prepared from stationary-phase cultures (optical density at 550 nm [OD550], 2.3) that were filter sterilized through a 0.2-μm-pore-size low-protein-binding filter (Millipore, Bedford, Mass.). Incubation of control monolayers with 10 μM actinomycin D (ActD) was used as a positive control for induction of caspase 3 activation.
Immunoblot analyses.
U937 cells (5 × 106) uninfected or infected by strain AA100 at an MOI of 50 in the presence or absence of the caspase 3-specific inhibitor were resuspended, at several intervals after the 1-h infection period, in cold phosphate-buffered saline containing protease inhibitors (2 μg of leupeptin/ml and 2 μg of aprotinin/ml) and phosphatase inhibitors (5 mM NaF and 1 mM Na3VO4) (56). Cells were pelleted by low-speed centrifugation at 1,000 × g for 2 min and lysed in 50 μl of cold lysis buffer (56). Equivalent amounts of proteins were resolved on sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrotransferred onto Immobilon-P (Millipore) membranes that were probed with a rabbit polyclonal anti-poly(ADP-ribose) polymerase (PARP) antiserum according to the recommendations of the manufacturer (Upstate Biotechnology, Lake Placid, N.Y.). The blots were subsequently stripped in 62.5 mM Tris-HCl [pH 6.8]–2% SDS–100 mM β-mercaptoethanol for 45 min at 65°C and reprobed with a mouse monoclonal antiactin antibody clone AC-15 (Sigma Co.), as we described previously (55).
RESULTS
L. pneumophila-induced apoptosis is not growth phase regulated.
L. pneumophila exhibits rapid cytotoxicity to the host cells only upon entry into the postexponential growth phase, which seems to be due to necrosis mediated by a potent cell-associated pore-forming toxin (13, 32, 34). We have recently shown that L. pneumophila induces a dose- and time-dependent apoptosis in U937 macrophages, peripheral blood monocytes, and alveolar epithelial cells during early stages of infection (22). Therefore, we first examined whether, similar to necrosis, L. pneumophila-induced apoptosis was growth phase regulated.
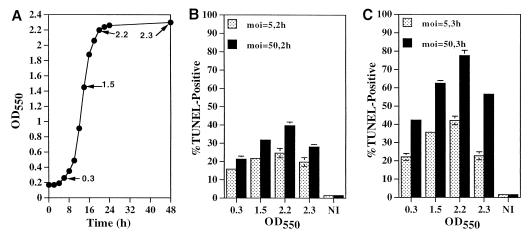
L. pneumophila grown in BYE broth to different growth phases was pelleted, washed with tissue culture medium, and then used to infect U937 macrophages at an MOI of 5 or 50 for 1 h. TUNEL assays were performed at 2 and 3 h after the 1-h infection period, and the percent apoptotic cells was determined (see the Materials and Method section). The data showed that, at all the growth phases examined, L. pneumophila induced apoptosis (Fig. 1 and 2). However, there was a slight trend towards a gradual increase in the induction of apoptosis by the bacteria from the early exponential (OD550 = 0.3) to mid-exponential (OD550 = 1.5) and postexponential (OD550 = 2.2) growth phases, at either MOI and either time point examined (Fig. 1 and 2). However, compared to the enhanced gradual increase in the induction of apoptosis by L. pneumophila at different growth phases, the induction of necrosis is strictly growth phase regulated and is not detectable during the exponential phase but is detectable only upon entry into the postexponential phase (13). Bacteria grown to late stationary phase (OD550 > 2.3, 24 h after termination of replication) exhibited a slight reduction in the ability to induce apoptosis, which correlated with the reduction of bacterial viability by threefold (Fig. 1 and data not shown). Heat-killed bacteria did not induce apoptosis (data not shown).
FIG. 1.
Induction of apoptosis in macrophages by L. pneumophila grown to different growth phases. (A) Growth kinetics of L. pneumophila in BYE broth. The arrows are accompanied by OD550 values and indicate bacterial cultures used for infection of U937 macrophages at MOI of 5 and 50. (B and C) Quantitation of apoptosis (examined by TUNEL assays) in macrophages infected at MOI of 5 and 50 at 2 h (panel B) and 3 h (panel C) postinfection. OD550 was the OD of the bacterial cultures used in the infections. At least 100 cells were examined for each sample. Error bars represent standard deviations, some of which are too small to be presented. Representative images of the infection at an MOI of 50 are shown in Fig. 2.
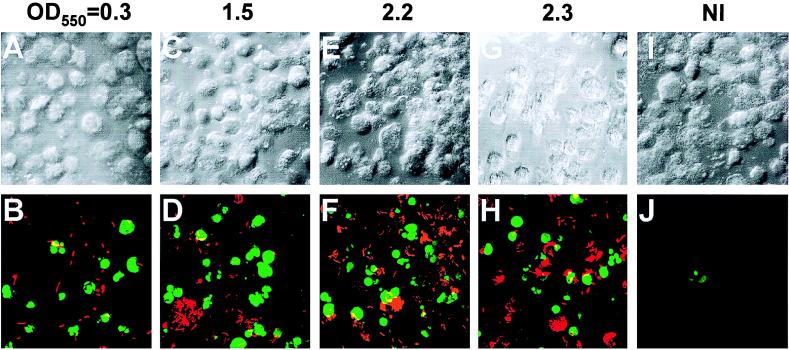
FIG. 2.
Representative confocal laser scanning microscopy images of L. pneumophila-infected macrophages. Apoptosis was examined by TUNEL assays at 3 h after the 1-h period of infection at an MOI of 50. The apoptotic nuclei appear in green. L. pneumophila was labeled by an L. pneumophila-specific polyclonal antiserum followed by an Alexa Red conjugate, which is shown in red. Panels B, D, F, and H are images of cells infected by bacterial cultures at the indicated values of OD550, and for comparison noninfected cells (NI) are shown in panel J. The panels on the top are the phase-contrast images corresponding to the ones on the bottom.
Since L. pneumophila grown to the postexponential phase is highly cytotoxic (13), it is possible that some of the cells infected by the bacteria at this growth stage may have detached from the monolayers, which may have resulted in underestimation of the actual percent apoptotic cells. Therefore, we examined detachment of cells from the monolayers that were infected, at different MOI, by strain AA100 grown to the postexponential phase (OD550 = 2.2). Although about 30% of the cells detached at 3 h postinfection from the monolayers infected at an MOI of 50, loss of cells from the monolayers infected at an MOI of 5 was minimal (8%). The gradual increase in the induction of apoptosis by bacteria grown to the postexponential phase may be due to the dramatic increase in invasiveness of the bacteria at this growth stage (13). This is supported by our recent findings that although extracellular L. pneumophila can induce apoptosis, bacterial invasion enhances this process (22). Therefore, L. pneumophila is capable of induction of apoptosis at all growth phases, with an approximately twofold increase upon exiting the exponential phase. This is in contrast to the growth phase-regulated induction of necrosis, which is not detectable during exponential growth and is exhibited only upon entry into the postexponential phase.
Inhibition of caspase 3 activation blocks L. pneumophila-induced nuclear apoptosis.
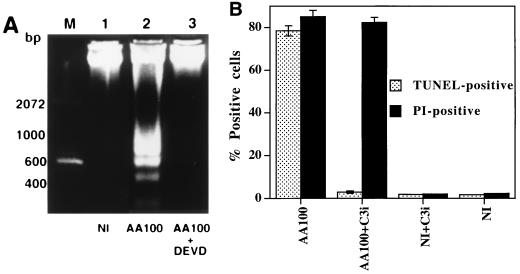
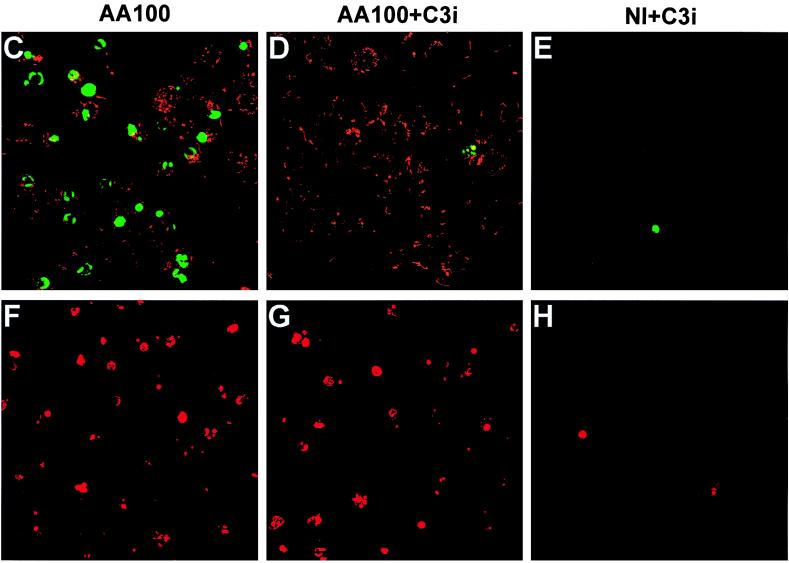
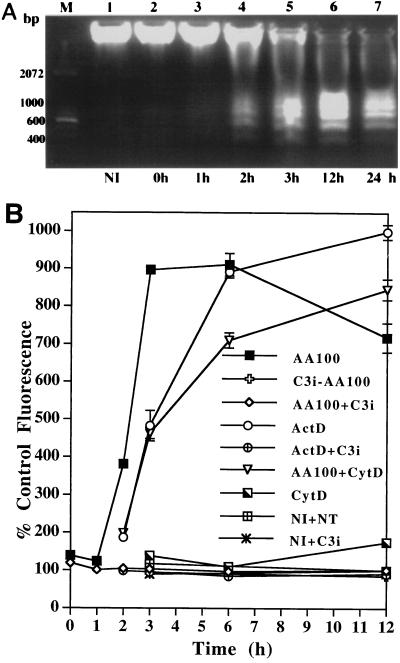
Caspase 3 activation is essential for DNA fragmentation to occur in apoptosis induced by a variety of stimuli (9, 47, 58). The synthetic tetrapeptide derivative Z-DEVD-FMK has been extensively used as a specific inhibitor of caspase 3 (18). As shown in Fig. 3A, this inhibitor completely blocked DNA fragmentation in U937 macrophages induced by L. pneumophila (Fig. 3A, lane 3). Complete inhibition of nuclear apoptosis in L. pneumophila-infected macrophages by this inhibitor was further confirmed by TUNEL assays performed at 3 h postinfection (data not shown) and 8 h postinfection (Fig. 3B through E).
FIG. 3.
Inhibition of caspase 3 activation blocks L. pneumophila-induced nuclear apoptosis. (A) Agarose gel electrophoresis of U937 macrophage DNA prepared at 3 h after the 1 h period of infection at an MOI of 50. The monolayers were infected in the presence (AA100 + DEVD) (lane 3) or absence (AA100) (lane 2) of the caspase 3-specific inhibitor, Z-DEVD-FMK, or were neither infected nor treated (NI) (lane 1). M indicates the 100-bp molecular size markers. (B) Quantitation of TUNEL-positive and PI-positive cells. U937 macrophages were infected, at an MOI of 50, by strain AA100 in the presence (AA100+C3i) or absence (AA100) of the caspase 3-specific inhibitor (C3i). At 8 h after the 1-h infection period, one portion of the monolayers was fixed and labeled by TUNEL. The other portion of the monolayers was labeled, in parallel to TUNEL, with PI without fixation. As negative controls, noninfected monolayers were treated with (NI+C3i) or without (NI) the inhibitor. At least 100 cells were counted for each sample. Error bars represent standard deviations, some of which are too small to be presented. (C through H) Representative confocal laser scanning microscopy images of U937 macrophages infected in the presence or absence of the caspase 3-specific inhibitor and examined by TUNEL (upper panels) or PI staining (lower panels). For TUNEL assay, the apoptotic nuclei are shown in green, and the L. pneumophila cells are shown in red (see legend to Fig. 2). For PI staining, the nuclei of the cells with changes in plasma membrane permeability are shown in red. The images of TUNEL and PI staining of noninfected monolayers treated with the inhibitor, as negative controls, are shown in panels E and H, respectively.
Activity of caspase 3 in L. pneumophila-infected cells to cleave a synthetic substrate in vitro.
To further confirm that caspase 3 was enzymatically active in L. pneumophila-infected macrophages, cell lysates were incubated with the fluorogenic substrate specific for caspase 3 (Z-DEVD-AMC) (43). Compared to the noninfected cells, a 380% increase in fluorescence was detected in the lysate of infected cells at 2 h postinfection, and the increase culminated at 3 h postinfection, when there was a 900% increase in fluorescence (Fig. 4B). ActD, which was used as a positive control for induction of apoptosis in U937 macrophages (22), induced caspase 3 activation (Fig. 4B). Heat-killed L. pneumophila or E. coli cells, used as negative controls, did not induce activation of caspase 3 (see Fig. 7). Importantly, the kinetics of caspase 3 activation in L. pneumophila-infected macrophages correlated with the kinetics of nuclear apoptosis (Fig. 4A).
FIG. 4.
Kinetics of caspase 3 activity in L. pneumophila-infected U937 macrophages to cleave a synthetic substrate and its correlation with nuclear apoptosis. (A) Kinetics of nuclear apoptosis in macrophages examined by DNA fragmentation at several intervals after the 1-h period of infection at an MOI of 50 and, for comparison, in noninfected cells (NI). M indicates the 100-bp molecular size markers. (B) Kinetics of caspase 3 enzymatic activity to cleave the fluorogenic substrate, Z-DEVD-AMC, which is specific for caspase 3. Some of the monolayers were infected in the presence (AA100+CytD) or absence (AA100) of 1 μg of CytD/ml. ActD indicates incubation of the noninfected macrophages with 10 μg of ActD/ml. C3i-AA100 indicates infection in the presence of the caspase 3 inhibitor (C3i), Z-DEVD-FMK. NI and NT indicate noninfected and nontreated cells, respectively. Cell lysates were prepared at the indicated time points after the 1-h period of infection at an MOI of 50. The caspase 3-specific inhibitor was added to the lysates of the infected (AA100+C3i) or ActD-treated (ActD+C3i) cells to demonstrate the specificity of the enzymatic activity of caspase 3. Relative enzymatic activity of caspase 3 was calculated as the percent increase in fluorescence compared to that for NI+NT and expressed as percent control fluorescence. Error bars represent standard deviations, some of which are too small to be presented.
FIG. 7.
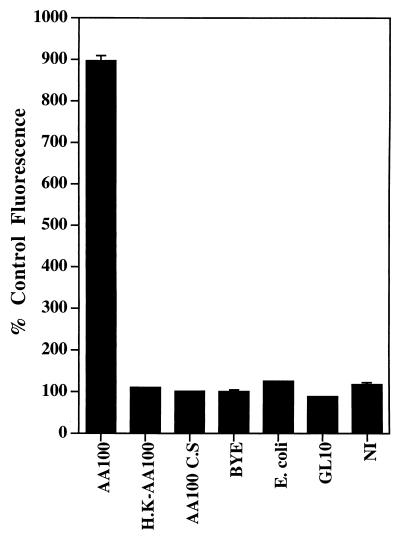
Dependence of caspase 3 activation in macrophages on live L. pneumophila macrophage contact. Caspase 3 activity to cleave Z-DEVD-AMC was determined for cells infected by live (AA100) or heat-killed (H.K-AA100) strain AA100, live dot/icm mutant bacteria (only data for GL10 are shown), and E. coli. C.S and BYE indicate cells treated with cell-free bacterial culture supernatant of strain AA100 and BYE broth, respectively. Relative enzymatic activity of caspase 3 was determined at 3 h after the 1-h period of infection or 4 h after the initiation of the treatment with C.S or BYE and was expressed as percent control fluorescence (percent compared to the fluorescence of noninfected cells [NI]). Error bars represent standard deviations, some of which are too small to be presented.
Cleavage of Z-DEVD-AMC was specifically due to the activity of caspase 3, since the reaction was completely blocked, in the lysates of both L. pneumophila-infected and ActD-treated cells, by the caspase 3-specific inhibitor, Z-DEVD-FMK (Fig. 4B). Furthermore, L. pneumophila-induced caspase 3 activation in macrophages was completely abolished, up to 12 h postinfection, by the caspase 3-specific inhibitor (Fig. 4B). Pretreatment of the bacteria with the caspase 3 inhibitor did not affect the ability to induce apoptosis in nontreated macrophages (data not shown), indicating that inhibition of apoptosis by this inhibitor was indeed due to inhibition of caspase 3 activity in the host cells but not that of the bacterial apoptotic factor.
Activity of caspase 3 in L. pneumophila-infected cells to cleave a natural substrate in vivo.
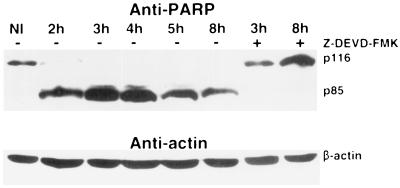
To further demonstrate caspase 3 activity in vivo, we examined the kinetics of cleavage of one of its natural substrates, PARP, by immunoblot analysis of cell lysates (Fig. 5). Cleavage of PARP p116 into the signature fragment p85 in L. pneumophila-infected macrophages was not detected at 1 h postinfection (data not shown), was very prominent at 2 h, and was complete at 3 to 4 h. Reduction in the amount of p85 at 5 and 8 h postinfection may be due to partial degradation. Importantly, cleavage of PARP was completely blocked in cells infected in the presence of the caspase 3-specific inhibitor for at least 8 h postinfection, confirming that cleavage of PARP was specifically due to the activation of caspase 3 (Fig. 5). Interestingly, although the caspase 3 inhibitor blocked L. pneumophila-induced nuclear apoptosis in U937 macrophages, it failed to block the surface exposure of phosphatidylserine induced by the bacteria (reference 22 and data not shown), which indicates that these two events in the apoptotic pathway are independent. This finding is consistent with previous observations of apoptosis in macrophages induced by Yersinia spp. (45).
FIG. 5.
Caspase 3 activity in L. pneumophila-infected U937 macrophages to cleave the natural specific substrate (PARP) for caspase 3 in vivo. The in vivo caspase 3 activity in L. pneumophila-infected macrophages was detected by cleavage of PARP in immunoblots of cell lysates prepared at the indicated time points after the 1-h period of infection at an MOI of 50 and probed with a rabbit polyclonal anti-PARP antibody. The blots were stripped and reprobed with a mouse anti-actin monoclonal antibody. Caspase 3 activity is demonstrated by cleavage of p116 PARP to its signature fragment p85.
Role of caspase 3 in L. pneumophila-mediated cytopathogenicity.
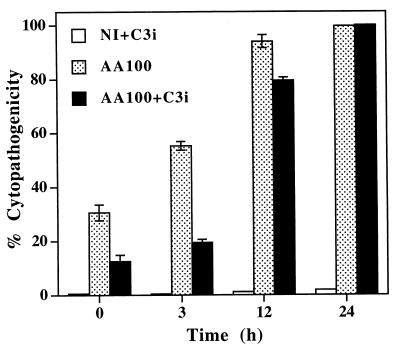
Inhibition of caspase 3 activity by Z-DEVD-FMK significantly reduced cytopathogenicity of L. pneumophila to U937 macrophages during the first 3 h after the 1-h infection period (Fig. 6). After 12 h postinfection, inhibition of caspase 3 activity was no longer sufficient to protect the cells from L. pneumophila-induced cytopathogenicity (Fig. 6), although caspase 3 activity was completely blocked during these periods (Fig. 4B and 5). Pretreatment of the bacteria with the caspase 3 inhibitor did not have any detectable effect on their cytopathogenic capacities (data not shown). The data indicated that inhibition of caspase 3 activity delayed but did not completely block the L. pneumophila-induced cell death. This may not be surprising since inhibition of nuclear apoptosis by the caspase 3 inhibitor may not block all of the apoptotic pathways induced by L. pneumophila. Our data may also suggest that at later times of the infection necrotic cell death could have occurred due to the presence of a large number of post-exponential-phase cytotoxic bacteria (13). To test this possibility, cells were stained with PI, in parallel with TUNEL, to examine whether cytopathogenicity at later time points in the presence of the caspase 3 inhibitor was due to necrotic damage. The data showed that the plasma membrane of macrophages infected at an MOI of 50 in the presence of the caspase 3 inhibitor became permeable to PI at 8 h postinfection, although nuclear apoptosis was completely blocked by this inhibitor during this period (Fig. 3B through H). This increased permeability in the plasma membrane could be due to apoptotic pathways independent of nuclear apoptosis or to a direct action of the pore-forming toxin expressed by a small proportion of bacteria that have already entered the postexponential phase.
FIG. 6.
Inhibition of the activity of caspase 3 blocks cytopathogenicity of L. pneumophila to U937 macrophages during early stages of the infection. Macrophages were infected at an MOI of 50 in the presence or absence of the caspase 3-specific inhibitor (C3i), Z-DEVD-FMK, and cytopathogenicity was determined at the indicated time points by Alamar blue assay and compared to that for noninfected cells (NI). Error bars represent standard deviations, some of which are too small to be presented.
Viable extracellular L. pneumophila induces caspase 3 activation in macrophages.
We have recently shown that extracellular L. pneumophila induces nuclear apoptosis in U937 macrophages, but bacterial invasion enhances the apoptotic process (22). In this study, we examined the activation of caspase 3 by extracellular bacteria. U937 macrophages were preincubated with CytD for 30 min and infected by strain AA100 for 30 min in the presence of CytD, extracellular bacteria were killed with gentamicin, and U937 macrophages were further incubated in the presence of CytD. We confirmed complete blockage of bacterial uptake by CytD-treated macrophages by sterilization of the infected monolayers following gentamicin treatment. Cell lysates were prepared at different time points to examine caspase 3 activity in cleaving the synthetic substrate. As shown in Fig. 4B, caspase 3 was activated by extracellular L. pneumophila: the activity increased by approximately 460 and 710% at 3 and 6 h postinfection, respectively. CytD by itself did not have any detectable effect on caspase 3 activation during this period. Activation of caspase 3 by extracellular bacteria correlated with the induction of nuclear apoptosis (22), indicating that the activation of caspase 3 is associated with induction of nuclear apoptosis by extracellular L. pneumophila. The reduced level of caspase 3 activity induced by extracellular bacteria is consistent with our finding that bacterial invasion enhances apoptosis (22). A live L. pneumophila macrophage contact was required for the induction of apoptosis in macrophages, since neither the heat-killed bacteria nor the bacterial culture supernatant induced activation of caspase 3 (Fig. 7). E. coli, which was used as a negative control, had no detectable effect on caspase 3 activation (Fig. 7). We have recently shown that three dotA/icmWXYZ mutants (GG105, GL10, and GS95) (24) are defective in inducing nuclear apoptosis, indicating that the apoptosis-inducing factor is regulated and/or exported by the Dot/Icm type V-like secretion apparatus (22, 49, 57). Since DotA is a cytoplasmic membrane protein, it is most likely that these three mutants are defective in export but not in expression of the apoptosis-inducing factor. We further confirmed the dependence of L. pneumophila-induced apoptosis on the Dot/Icm potential secretion apparatus by demonstrating that the three dotA/icmWXYZ mutants were also defective in inducing caspase 3 activation in infected macrophages (Fig. 7 and data not shown) (22).
DISCUSSION
We have previously shown that L. pneumophila induces apoptosis in macrophages, alveolar epithelial cells, and peripheral blood monocytes during the first few hours of the infection (22). In addition, we have also shown that the induction of apoptosis is dose dependent and is detectable at 3 h after infection at an MOI of 0.5 (22). Therefore, it is very clear that the induction of apoptosis occurs very early in the interaction between L. pneumophila and mammalian cells. We have proposed a biphasic model by which L. pneumophila kills the host cell (22). Killing is mediated through the induction of apoptosis during the early stages of the infection (22, 40) followed by an independent and rapid induction of necrosis upon entry into the postexponential phase (13).
In this study, we have used an MOI of 50 to ensure maximal activation of the apoptotic pathway to examine the activity of caspase 3 during L. pneumophila-induced apoptosis. We have demonstrated that activation of caspase 3 in macrophages by L. pneumophila is essential for nuclear apoptosis. To our knowledge, this is the first report demonstrating an involvement of caspase 3 activity in induction of apoptosis by a bacterial pathogen. Yersinia spp.-induced nuclear apoptosis in macrophages has been shown to require the activity of the family of caspases, since it is blocked by a broad-spectrum caspase inhibitor, Z-VAD-FMK (45); this finding is similar to our previous findings in L. pneumophila-induced apoptosis in macrophages (22). However, which caspase(s) is activated in Yersinia spp.-induced apoptosis has not been reported. On the other hand, S. typhimurium and S. flexneri are the only two bacterial species known to activate a particular member of the caspase family, i.e., caspase 1/ICE (14, 29, 60). Inhibition of caspase 1 activity by a specific inhibitor blocks S. typhimurium- and S. flexneri-induced apoptosis in macrophages and prevents cell death induced by the bacteria (29, 30). Furthermore, it has recently been shown that caspase 3 is not required for S. flexneri-induced apoptosis in macrophages (31), which indicates that the caspase 1- and caspase 3-mediated apoptotic pathways are distinct. In contrast to the distinct caspase activation-mediated nuclear apoptosis by L. pneumophila and Shigella, host cell DNA fragmentation by Mycoplasma penetrans is mediated through a bacterial endonuclease (10). These differences add to the diversity of mechanisms utilized by bacterial pathogens to induce nuclear apoptosis in the host cell.
The primary function of caspase 1 is generally believed to be proinflammatory, whereas the function of caspase 3 and most other caspases is mostly proapoptotic (27, 28, 48). Activation of caspase 1 in S. flexneri-infected macrophages leads to cleavage of the precursor interleukin (IL) 1β into mature IL-1 that may subsequently initiate an intense host inflammatory response, which is evident in infected patients (30, 62). Therefore, it has been proposed that activation of caspase 1 in S. flexneri-infected macrophages converts a proapoptotic event into a proinflammatory one (62). Release of proinflammatory cytokines, such as IL-1 and TNF-α, by L. pneumophila-infected macrophages has been reported (12, 53). However, our preliminary data suggest that caspase 1 is not activated in L. pneumophila-infected macrophages (unpublished data), which is consistent with other observations that caspase 1- and caspase 3-mediated apoptotic pathways are distinct (31). Therefore, in contrast to S. flexneri-induced apoptosis in macrophages, which is primarily proinflammatory, apoptosis induced by L. pneumophila in macrophages may be primarily proapoptotic.
Although cytopathogenicity of L. pneumophila to macrophages has been well documented, the mechanisms of cell death are not well understood. We have recently shown that L. pneumophila induces a dose- and time-dependent apoptosis during early stages of infection (22). We have also provided genetic and biochemical evidence that apoptosis plays an important role in cytopathogenicity of L. pneumophila to the host cells (22). Here we further demonstrate that inhibition of nuclear apoptosis in macrophages by a caspase 3-specific inhibitor reduces cytopathogenicity of L. pneumophila to these cells during early stages of the infection. Our data do not exclude the possibility that changes in plasma membrane permeability at later stages of the infection may have occurred due to the presence of a large number of post-exponential-phase cytotoxic bacteria (13). Nevertheless, our data show that caspase 3-mediated apoptosis plays an important role in cytopathogenicity of L. pneumophila to the host cell during early stages of the infection.
Extracellular L. pneumophila induces caspase 3-mediated apoptosis. However, entry of the bacteria into the host cell enhances but is not required for the activation of caspase 3. In contrast, entry of S. flexneri into macrophages and subsequent bacterial escape into the cytoplasm are essential for S. flexneri to induce caspase 1-mediated apoptosis (14, 30). Similarly, S. typhimurium entry into macrophages is required for activation of the apoptotic pathway (39). Thus, L. pneumophila is the first documented example of an intracellular pathogen that induces caspase 3-mediated apoptosis in macrophages upon contact and prior to entry.
Our data demonstrate that the Dot/Icm type V-like secretion apparatus is required for the induction of apoptosis. We propose three possibilities for how extracellular L. pneumophila activates the caspase cascade. First, upon contact with the host cell, L. pneumophila translocates, through the Dot/Icm secretion machinery, the apoptotic factor(s) into the host cell cytosol, which results in activation of caspase 3 either directly or by activation of caspases upstream of caspase 3. Second, upon contact with the host cell, L. pneumophila secretes, through the Dot/Icm secretion machinery, the apoptotic factor(s) in close proximity of attachment, which binds to a death receptor on the host cell surface. This receptor may be Fas (35, 46), TNFR (42), or other death receptors. Third, L. pneumophila translocates the apoptotic factor(s), through the Dot/Icm secretion machinery, to the bacterial surface, which may then mediate bacterial binding to a death receptor on the host cell surface. It is also possible that more than one of these proposed strategies are exploited by L. pneumophila. Nevertheless, our preliminary data show that caspase 8, which is the most upstream caspase that interacts with the cytoplasmic domains of many death receptors (11, 41), is activated in L. pneumophila-infected macrophages, suggesting that the apoptotic signal is generated upon binding to a death receptor. Therefore, it should be of great interest to investigate whether L. pneumophila binds a known or a novel death receptor to induce apoptosis.
Why does L. pneumophila induce apoptosis from an extracellular location at such an early stage of the infection of the macrophages and alveolar epithelial cells (22) that subsequently support intracellular replication of the bacteria? First, it is possible that extracellular L. pneumophila, upon contact with the cells, induces host cell apoptotic pathways to facilitate alteration of trafficking of the bacterium-containing phagosome to block its fusion to endocytic vesicles that allow phagosomal maturation through the endosomal-lysosomal pathway (1, 44). It has been shown that induction of apoptosis results in blockage of endocytic fusion, due to cleavage of Rabaptin 5 by the caspase cascade (16). Rabaptin 5 stabilizes the Rab5-GTP complex (50), which stimulates endocytic fusion, and thus cleavage of Rabaptin-5 is thought to result in a Rab5-GDP complex that blocks endocytic fusion (16, 51). Interestingly, the three dotA/icmwxyz mutants that are defective in induction of apoptosis are also targeted into a phagosome that is trafficked through the endosomal-lysosomal pathway, which culminates in fusion to the lysosomes (reference 44 and our unpublished data). Second, since L. pneumophila has been shown to suppress the oxidative burst in monocytes (33), induction of apoptosis may down-regulate the bacteriocidal activity of these cells to enable the bacteria to survive in what is otherwise considered a harsh environment for microorganisms. Third, induction of apoptosis may play an important role in killing the host cells for subsequent release of the intracellular bacteria from the host cell. Fourth, host cell death by apoptosis may reduce inflammation at the foci of infection (compared to that resulting from necrosis), which would enhance bacterial proliferation at the sites of infection. During this study we observed that apoptotic macrophages harboring L. pneumophila are taken up by other, uninfected macrophages (unpublished data). It would be intriguing to examine whether this process results in elimination of the bacteria through lysosomal degradation of the engulfed infected macrophage or whether the bacteria are able to escape this fatal fate and replicate in the new host cell.
ACKNOWLEDGMENTS
We thank Charles E. Snow, Vivek Rangnekar, and members of Abu Kwaik laboratory for their helpful suggestions and comments.
Y.A.K. is supported by Public Health Service Award R29AI-38410.
REFERENCES
- 1.Abu Kwaik Y. Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol. 1998;30:689–696. doi: 10.1046/j.1365-2958.1998.01092.x. [DOI] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during intracellular infection. Infect Immun. 1998;66:203–212. doi: 10.1128/iai.66.1.203-212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Kwaik Y, Engleberg N C. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol Microbiol. 1994;13:243–251. doi: 10.1111/j.1365-2958.1994.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Abu Kwaik Y, Fields B S, Engleberg N C. Protein expression by the protozoan Hartmannella vermiformis upon contact with its bacterial parasite Legionella pneumophila. Infect Immun. 1994;62:1860–1866. doi: 10.1128/iai.62.5.1860-1866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu Kwaik Y, Gao L-Y, Harb O S, Stone B J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- 7.Abu Kwaik Y, Gao L-Y, Stone B J, Venkataraman C, Harb O S. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl Environ Microbiol. 1998;64:3127–3133. doi: 10.1128/aem.64.9.3127-3133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu Kwaik Y, Pederson L L. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol Microbiol. 1996;21:543–556. doi: 10.1111/j.1365-2958.1996.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderson P. Kinase cascades regulating entry into apoptosis. Microbiol Mol Biol Rev. 1997;61:33–46. doi: 10.1128/mmbr.61.1.33-46.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendjennat M, Blanchard A, Loutfi M, Montagnier L, Bahraoui E. Purification and characterization of Mycoplasma penetrans Ca2+/Mg2+-dependent endonuclease. J Bacteriol. 1997;179:2210–2220. doi: 10.1128/jb.179.7.2210-2220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/Apo-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 12.Brieland J K, Remick D G, Freeman P T, Hurley M C, Fantone J C, Engleberg N C. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous tumor necrosis factor alpha and nitric oxide. Infect Immun. 1995;63:3253–3258. doi: 10.1128/iai.63.9.3253-3258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by directly binding ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 15.Clifton D R, Goss R A, Sahni S K, Antwerp D V, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosulich S C, Horiuchi H, Zerial M, Clarke P R, Woodman P G. Cleavage of Rabaptin-5 blocks endosome fusion during apoptosis. EMBO J. 1997;16:6182–6191. doi: 10.1093/emboj/16.20.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 18.Enari M, Talanian R V, Wong W W, Nagata S. Sequential activation of ICE-like and cpp32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–736. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 19.Fan T, Lu H, Shi L, MaClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez R C, Logan S, Lee S H S, Hoffman P S. Elevated levels of Legionella pneumophila stress protein Hsp60 early in infection of human monocytes and L929 cells correlated with virulence. Infect Immun. 1996;64:1968–1976. doi: 10.1128/iai.64.6.1968-1976.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fratazzi C, Arbeit R D, Carini C, Remold H G. Programmed cell death of Mycobacterium avium serovar 4-infected macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158:4320–4327. [PubMed] [Google Scholar]
- 22.Gao L-Y, Abu Kwaik Y. Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity. Infect Immun. 1999;67:862–870. doi: 10.1128/iai.67.2.862-870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L-Y, Gutzman M, Brieland J K, Abu Kwaik Y. Different fates of Legionella pneumophila pmi and mil mutants within human-derived macrophages and alveolar epithelial cells. Microb Pathog. 1998;25:291–306. doi: 10.1006/mpat.1998.0237. [DOI] [PubMed] [Google Scholar]
- 24.Gao L-Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao L-Y, Harb O S, Abu Kwaik Y. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garduno R A, Garduno E, Hoffman P S. Surface-associated Hsp60 chaperonin of Legionella pneumophila mediates invasion in a HeLa cell model. Infect Immun. 1998;66:4602–4610. doi: 10.1128/iai.66.10.4602-4610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 28.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 29.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1β-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilbi H, Moss J E, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell R A, Yuan J, Sansonetti P J, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 32.Husmann L K, Johnson W. Cytotoxicity of extracellular Legionella pneumophila. Infect Immun. 1994;62:2111–2114. doi: 10.1128/iai.62.5.2111-2114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob T, Escallier J C, Sanguedolce M V, Chicheportiche C, Bongrand P, Capo C, Mege J L. Legionella pneumophila inhibits superoxide generation in human monocytes via the down-modulation of α and β protein kinase C isotypes. J Leukoc Biol. 1994;55:310–312. doi: 10.1002/jlb.55.3.310. [DOI] [PubMed] [Google Scholar]
- 34.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 35.Li B, Bassiri H, Rossman M D, Kramer P, Eyuboglu A F, Torres M, Sada E, Imir T, Carding S R. Involvement of Fas/Fas ligand pathway in activation-induced cell death of Mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol. 1998;161:1558–1567. [PubMed] [Google Scholar]
- 36.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 37.MacFarlane M, Cain K, Sun X-M, Alnemri E S, Cohen G M. Processing/activation of at least four interleukin-1β converting enzyme-like proteases occurs during the execution phase of apoptosis in human monocytic tumor cells. J Cell Biol. 1997;137:469–479. doi: 10.1083/jcb.137.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monack D M, Raupach B, Hromocky A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi D, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE-CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 42.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 43.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A. Identification and inhibition of ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 44.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 45.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudi J, Luck D, Strand S, von Herbay A, Mariani S M, Krammer P H, Galle P R, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Investig. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 48.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1998;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 49.Segal G, Purcell M, Shuman H A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila chromosome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stenmark H, Parton R G, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M. Inhibition of Rab5 GTPase stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 52.Stone B J, Abu Kwaik Y. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun. 1998;66:1768–1775. doi: 10.1128/iai.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Susa M, Ticac T, Rukavina T, Doric M, Marre R. Legionella pneumophila infection in intratracheally inoculated T cell depleted or non-depleted A/J mice. J Immunol. 1998;160:316–321. [PubMed] [Google Scholar]
- 54.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 55.Venkataraman C, Gao L-Y, Bondada S, Abu Kwaik Y. Identification of putative cytoskeletal protein homologues in the protozoan Hartmannella vermiformis as substrates for induced tyrosine phosphatase activity upon attachment to the Legionnaires’ disease bacterium, Legionella pneumophila. J Exp Med. 1998;188:505–514. doi: 10.1084/jem.188.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venkataraman C, Haack B J, Bondada S, Abu Kwaik Y. Identification of a Gal/GalNAc lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion by the Legionnaires’ disease bacterium, Legionella pneumophila. J Exp Med. 1997;186:537–547. doi: 10.1084/jem.186.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 58.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 59.Zuckman D M, Hung J B, Roy C R. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular replication. Mol Microbiol. 1999;32:990–1001. doi: 10.1046/j.1365-2958.1999.01410.x. [DOI] [PubMed] [Google Scholar]
- 60.Zychlinsky A, Kenny B, Menard R, Prevost M C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 61.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 62.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what we can learn from bacteria-induced cell death. Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]