Abstract
Blindness is a common sequela after stroke affecting the primary visual cortex, presenting as a contralesional, homonymous, visual field cut. This can occur unilaterally or, less commonly, bilaterally. While it has been widely assumed that after a brief period of spontaneous improvement, vision loss becomes stable and permanent, accumulating data show that visual training can recover some of the vision loss, even long after the stroke. Here, we review the different approaches to rehabilitation employed in adult-onset cortical blindness (CB), focusing on visual restoration methods. Most of this work was conducted in chronic stroke patients, partially restoring visual discrimination and luminance detection. However, to achieve this, patients had to train for extended periods (usually many months), and the vision restored was not entirely normal. Several adjuvants to training such as noninvasive, transcranial brain stimulation, and pharmacology are starting to be investigated for their potential to increase the efficacy of training in CB patients. However, these approaches are still exploratory and require considerably more research before being adopted. Nonetheless, having established that the adult visual system retains the capacity for restorative plasticity, attention recently turned toward the subacute poststroke period. Drawing inspiration from sensorimotor stroke rehabilitation, visual training was recently attempted for the first time in subacute poststroke patients. It improved vision faster, over larger portions of the blind field, and for a larger number of visual discrimination abilities than identical training initiated more than 6 months poststroke (i.e., in the chronic period). In conclusion, evidence now suggests that visual neuroplasticity after occipital stroke can be reliably recruited by a range of visual training approaches. In addition, it appears that poststroke visual plasticity is dynamic, with a critical window of opportunity in the early postdamage period to attain more rapid, more extensive recovery of a larger set of visual perceptual abilities.
BACKGROUND
Cortical blindness (CB) is a common cause of morbidity in humans resulting from damage to the primary visual cortex (V1) or its immediate afferents (Duncan et al., 2005; Zhang et al., 2006; Pollock et al., 2019). Here, “cortical blindness” is used as an umbrella term, to include all homonymous visual field defects resulting from postchiasmal lesions. This includes left- and right-sided homonymous hemianopias, as well as superior and inferior homonymous quadrantanopias and scotomas of the right and left visual hemifields. While most cases of cortical blindness are unilateral, they can also present bilaterally. Demographic studies are few for this condition. The Blue Mountains Eye Study in Australia revealed CB to afflict approximately 0.8% of the noninstitutionalized population over 49 years of age (Gilhotra et al., 2002). In the United Kingdom, two studies found visual impairments to occur in a majority of new stroke survivors, with 28–52% exhibiting visual field loss (Rowe et al., 2013, 2019b). Similarly, in the United States, Pollock and colleagues (Pollock et al., 2019) estimated a 27–57% incidence of visual field defects following ischemic brain injury. Since the 2009 National Hospital Discharge Survey reported the incidence of stroke in the United States as approaching 1 million per year, this equates to approximately a quarter to half a million new individual cases of stroke-induced CB annually. Together with other possible causes of CB—traumatic brain injury, brain tumors, demyelinating diseases, and congenital conditions (Pollock et al., 2019)—this amounts to a substantial disease burden on three different continents, and likely worldwide. We should also note here that in this chapter, our discussions will focus on adult-acquired CB, rather than childhood cortical visual impairment. In addition, we will deal only with stroke-induced CB, both because it represents the most common etiology of this disease (Pollock et al., 2019; Rowe et al., 2019b) and because most rehabilitation approaches to date have been tested in this particular group of patients.
Patients with cortical blindness are impaired in many activities of daily living, including reading, navigating, driving, and recognizing people and objects (Dombovy et al., 1986; Jongbloed, 1986; Jones and Shinton, 2006; Papageorgiou et al., 2007; Gall et al., 2009; Goodwin,2014). As such, the burden of disease from this condition is high, causing a significant and measurable impact on quality of life (Warren, 2009; Rowe et al., 2019a). Patients report that their cortical blindness is very distressing (Kischka, 2019; Falkenberg et al., 2020; Hanna et al., 2020), which is compounded by the paucity of vision care that they receive (Falkenberg et al., 2020; Hanna et al., 2020). With the incidence of stroke rising among younger people (Béjot et al., 2016), this situation should only be expected to worsen in coming years.
APPROACHES TO VISUAL REHABILITATION FOR CORTICAL BLINDNESS
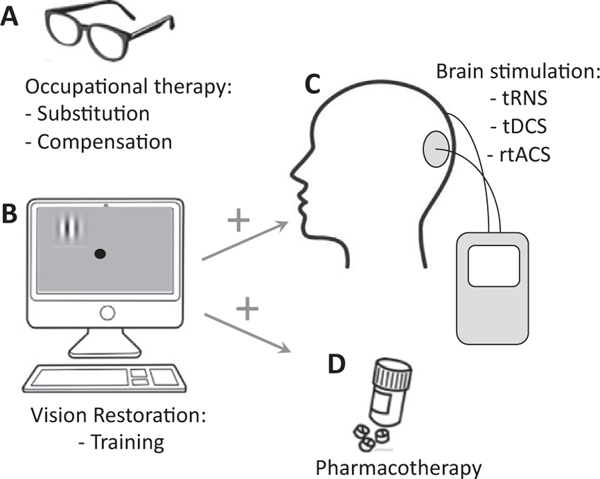
In contrast to physical therapy for motor stroke, there are currently no standardized, validated, or widely accepted vision restoration treatments for cortically blind patients (Pollock et al., 2019). Therapies typically focus on aspects of occupational therapy that involve teaching compensatory strategies or using substitution, whereby the goal is to teach patients to maximize the use of their remaining vision (Fig. 25.1A). This is similar to how a patient with an amputated limb might use occupational therapy or, in some cases, a prosthesis, to learn to adapt and accomplish activities of daily living such as getting dressed.
Fig. 25.1.
Main experimental and clinical therapies attempted to date for vision rehabilitation after occipital stroke. (A) Occupational therapy includes both substitution (prisms or other lenses) and compensation (teaching eye and head movement) strategies. These therapies may improve quality of life but do not restore lost vision. (B) Visual restoration approaches may be accomplished through home computer-based vision training using a range of psychophysical tasks. The effectiveness of restitution therapy may depend on the timing poststroke, the specific training task used, and patient compliance, especially for fixation control. (C) Visual training could potentially be enhanced with noninvasive electric brain stimulation, of which there are many strategies currently under investigation as a potential adjuvant to training. tRNS, transcranial random noise stimulation; tDCS, transcranial direct current stimulation, rtACS, repeated transorbital alternating current stimulation. (D) Finally, visual training could also potentially be paired with pharmacotherapy to promotegreater recovery, although this approach remains to be vetted both experimentally and clinically.
Visual substitution therapy aims to reposition the visual world so that objects that normally fall within the blind field instead fall within intact portions of the visual field. While work is being done on developing digital, wearable, “augmented reality” technology for this purpose (Sayed et al., 2020), the most common current methods involve the use of eyeglasses with prisms attached to one lens (Rossi et al., 1990; Bainbridge and Reding, 1994; Peli, 2000; Szlyk et al., 2005; Bowers et al., 2014; Rowe et al., 2017; Houston et al., 2018). The prisms shift images so that what would normally project onto the hemianopic retina is instead seen by portions of the retina projecting to the intact visual cortex. Although most patients must be trained to interpret the shifted visual world, the use of prism lenses allows them to capture visual information they might otherwise miss, improving mobility (Bowers et al., 2014), visual functioning, and quality of life (O’Neill et al., 2011). Unfortunately, many patients are never able to learn to properly integrate this information or disambiguate it from the image directly in front of them, thus failing to benefit from these lenses (Rowe et al., 2017; Pollock et al., 2019). In particular, users encounter challenges when attempting tasks exercising near vision, walking downstairs, or gazing through the prisms themselves (Giorgi et al., 2009). The optical quality of a particular set of prisms may also limit its utility, and some patients may experience adverse effects such as headaches and diplopia (Pollock et al., 2019). Overall, only a third to a half of patients who begin a rehabilitation program with prisms end up continuing to wear their lenses long-term (Bowers et al., 2008; Giorgi et al., 2009).
Compensation therapy similarly aims to reposition the visual world, but instead of using lenses, patients are taught to capture the visual information that would normally fall in the deficit area by moving their eyes and head to more systematically sample their blind field (Weinberg et al., 1977; Zihl, 1980; Kerkhoff et al., 1992; Kerkhoff, 1999; Kerkhoff, 2000; Nelles et al., 2001; Pambakian et al., 2004; Spitzyna et al., 2007; Roth et al., 2009; Mannan et al., 2010; Trauzettel-Klosinski, 2010; Ong et al., 2015; Sahraie et al., 2016). Strategies include increasing the number of saccades toward the impaired visual field, varying the amplitude of saccades to increase sampling efficiency, developing organized patterns of scanning, and increasing the number and amplitude of head movements (Lane et al., 2010; Schuett et al., 2012; Aimola et al., 2014; Goodwin, 2014). This can be particularly helpful for reading, visual search, and obstacle avoidance (Zihl, 1995; Bouwmeester et al., 2007; Goodwin, 2014; de Haan et al., 2015). Interestingly, without coaching, many patients do not learn to make appropriate, compensatory eye and head movements toward their blind field on their own. In fact, cortically-blind patients, who lack feedback from their visual periphery on the affected side, can exhibit very different visual search strategies compared to visually intact participants; this includes ineffective scanning, abnormal saccade patterns, and trouble estimating how far they need to scan to capture relevant visual information (Ishiai et al., 1987; Zangemeister et al., 1995; Zihl, 1995; Hildebrandt et al., 1999; Kerkhoff, 1999; Pambakian et al., 2000; Martin et al., 2007; Iorizzo et al., 2011). As such, CB patients can benefit from explicit instruction and repetitive training, for instance, on how to use physical landmarks, in order to properly sample different areas of their visual field defect (Bowers et al., 2014).
Both substitution and compensation techniques improve quality of life and activities of daily living by teaching patients how best to use their remaining vision (Weinberg et al., 1977; Spitzyna et al., 2007; Roth et al., 2009; Rowe et al., 2017; Pollock et al., 2019). However, they are neither designed nor aim to recover any of the vision lost (Campion et al., 1983; Pollock et al., 2019). Restoring vision and shrinking the size of the visual field defect have been holy grails of the field for many decades. The remainder of this chapter will provide an updated review of vision restoration approaches to CB (Fig. 25.1B–D), and will critically evaluate their relative merits. First, however, we will start with a short discussion of methods employed to measure visual field defects in CB, followed by an outline of the natural history of this condition.
MEASURING VISION LOSS AFTER OCCIPITAL STROKE
To appropriately target rehabilitation efforts, stroke survivors must have an accurate diagnosis of their visual sequelae, including accurate and reliable measurements of the size and borders of areas of vision loss. Also, key to ultimately developing individualized treatment plans is the ability to define what specific aspects of visual perception are impaired, and the extent of impairment across different regions of the visual field (i.e., across different retinotopic locations). These deficits can vary dramatically between patients, across retinotopic locations, and they also often appear in combination, including impaired central vision, eye movement abnormalities, visual field loss, visual inattention/neglect, and other visual “perceptual” disorders (Rowe et al., 2019b). Many of these abnormalities are first identified at the bedside or in neurology/ophthalmology/optometry clinics. However, some of these determinations require more sophisticated, detailed testing than is possible in the clinic. Both procedural and technologic limitations currently relegate such testing to basic research laboratories, which can perform psychophysical measurements under controlled stimulus conditions and with controlled fixation.
Nonetheless, the first assessment of visual field loss after occipital stroke is often confrontation, which involves the clinician using hand gestures to ascertain the presence and general location of visual field deficits. During typical confrontation testing, the examiner asks the patient to focus on the examiner’s nose or face, and to make a series of judgments monocularly and/or binocularly, such as counting fingers presented in each quadrant of the visual field or indicating when the examiner has moved their finger(s) into the field of view (Elliott et al., 1997; Pandit et al., 2001; Kerr et al., 2010).
Once confrontation confirms a suspected visual field defect, the clinical gold standard for measuring the extent of visual field loss in adult-onset CB is automated perimetry, most commonly using the Humphrey perimeter (Townend et al., 2007; Hepworth and Rowe, 2018). The test presents small stationary lights of varying luminance monocularly to determine detection thresholds at retinotopically determined test points throughout the visual field. Patients fixate centrally, and their head position is controlled using a forehead/chin rest. Eye-tracking in the Humphrey is optional, but whether or not it is used, the gaze is interrogated by presenting some of the lights to be detected inside the optic disc representation (which should normally lack visual sensation, and thus represents a means of assessing false-positive responses). Patients indicate visual detection by pressing a handheld button; false positives (pressing the button during a blind spot check), false negatives (failing to detect light of previously-detected threshold brightness or lower), and fixation losses are monitored throughout testing to estimate test reliability and fixation accuracy. While standard static automated perimetry is limited to the central visual field (typically a radius of the central 10, 24, or 30 degrees of vision), it is more sensitive for detecting poststroke homonymous visual field loss than confrontation (Pandit et al., 2001; Townend et al., 2007; Kerr et al., 2010; Hanna et al., 2017). The device measurement error for Humphrey perimetry is reported by the manufacturer to be within 3 dB at individual test spots. However, test–retest variability can be influenced by many factors, including patient motivation and focus, time of day, season, as well as the technician’s experience, instruction of the patient, and monitoring, along with adjustments and feedback during testing (Montolio et al., 2012). In addition to Humphrey static perimetry, several kinetic perimetry options are available, in which small lights are moved, usually from the visual periphery toward the center, and patients indicate when they first detect each light. These options include the Goldmann manual kinetic perimeter, which outputs hand-drawn visual fields (Hepworth and Rowe, 2018; Pollock et al., 2019), the Tubingen kinetic perimeter (Pollock et al., 2019), and the Octopus kinetic perimeter, which can be viewed as an automated version of the Goldmann test (Hepworth and Rowe, 2018; Pollock et al., 2019). Although there are few guidelines for exactly how visual fields should be assessed in neurologic conditions (Hepworth and Rowe, 2018), in common practice, kinetic perimetry appears to be used less often than Humphrey static perimetry for stroke patients.
As mentioned earlier, in addition to luminance detection deficits assessed by perimetric methods, many other visual abilities are impaired within cortically-blinded fields, although their full extent is rarely captured clinically. Laboratory psychophysical measures across a range of different tasks (including detection, identification, discrimination, comparison, etc.) and incorporating fixation control with eye trackers, are generally needed to properly characterize impairments across the spectrum of visual abilities. Such studies have revealed specific deficits in motion direction discrimination, motion integration, and other, more complex motion computations (Hess and Pointer, 1989; Azzopardi and Cowey, 2001; Huxlin et al., 2009; Das et al., 2014; Vaina et al., 2014; Saionz et al., 2020).In the visual form domain,V1damage impairs simple functions such as orientation discrimination of static shapes (Hess and Pointer, 1989; Das et al., 2014;Saionz et al., 2020), as well as higher-order functions such as face and object recognition (Riddoch, 1917; Zeki and Ffytche, 1998; Stoerig, 2006; Cowey, 2010).
Despite a deficit of conscious vision in cortically-blinded fields, it has been noted since Riddoch’s original observations in soldiers with battlefield wounds (Riddoch, 1917) that some patients with occipital damage retain partial visual processing within their perimetrically-defined visual field defect. Weiskrantz further detailed this phenomenon, dubbing it “blindsight” (Weiskrantz et al., 1974). Blindsight is broadly defined as a deficit in conscious vision with concomitant, limited, weak visual processing abilities. It is most commonly elicited during forced-choice behavioral testing when upon presentation of a stimulus inside the blind field, the participant is asked to “guess” the response even if they are uncertain that they can perform the task. In most cases of blindsight, this results in better-than-chance performance (i.e., better than pure guessing). Blindsight also appears to require large, coarse, moving, orflickering visualstimuli (Weiskrantz et al., 1995), containing high luminance contrasts, low spatial frequencies, and high temporal frequencies (Sahraie et al., 2008)—e.g., a very large, well-defined object moving or flickering at a moderate to high speed/rate, on a clear background. Patients with bilateral CB can use blindsight to perform such remarkable feats as successfully navigating an obstacle course in a hallway (de Gelder et al., 2008). It is also possible for them to recognize emotions (de Gelder et al., 1999; Tamietto and de Gelder, 2010) and even to exhibit fear conditioning using visual cues (Hamm et al., 2003). Yet, while blindsight has been documented for many facets of visual processing (Stoerig, 2006; Cowey, 2010), it is not always reliably elicited, even within the same individual, and it captures only a subset of normal human vision (Campbell and Robson, 1968; Robson, 1993; Azzopardi and Cowey, 1997). In more common cases, when the visual field defect poststroke is unilateral, the intact hemifield provides information that is so much more salient to the person than information processed in the blind field, that the functional relevance of blindsightis questionable (Weiskrantz, 2009; Sahraie et al., 2013). Thus, it may be safe to assume that patients with unilateral CB place little reliance on blindsight for visual functioning in daily life.
NATURAL HISTORY OF OCCIPITAL STROKE: RECENT INSIGHTS INTO THE DYNAMICS OF VISION LOSS
Few studies to date have characterized the natural history of vision loss after occipital stroke, and the extant literature has focused on deficits in luminance detection as measured by clinical perimetry tests. Two large, longstanding, prospective studies indicated that spontaneous improvements in the size of visual fields are most likely to occur within the first 2 weeks after stroke; the first used confrontation assessment (Gray et al., 1989), and the second used manual kinetic perimetry (Tiel and Kolmel, 1991). A third study, also using manual kinetic perimetry, followed patients over 3 years after stroke and found that the majority experienced improvements, which were restricted to the first 6 months poststroke (Messing and Ganshirt, 1987). The largest prospective study that utilized automated perimetry examined a mixed group of stroke, trauma, and tumor patients with damage to the occipital lobe (Zhang et al., 2006); the authors found that roughly 40% of their participants exhibited some spontaneous improvement. However, the probability of improvement was highest (affecting approximately 60% of patients) in the first 3 months postdamage, dropping precipitously from 3 to 6 months (only about 20% penetrance during this time frame). Beyond 6 months postdamage, the likelihood of any further improvement was essentially null (Zhang et al., 2006). The time course of spontaneous visual recovery in perimetrically defined blind fields after occipital stroke matches nicely the reported time course for motor recovery after motor cortex stroke (Duncan et al., 1992, 1994). Together, these studies have informed the general belief among both clinicians and scientists that, after a brief period of possible spontaneous improvement in the visual field defect in the first 6 months after adult-onset occipital damage, vision loss becomes stable and permanent.
However, recent work using more stringent approaches to patient selection and more refined, quantitative analyses of visual defects (both perimetrically and psychophysically defined) suggest that the natural history of stroke-induced CB may differ from current dogma. Psychophysical testing of a small group of subacute occipital stroke patients within the first 3 months of their stroke revealed that half or more retained conscious visual discrimination abilities for some classes of targets presented fully within their perimetrically-defined blind field (Saionz et al., 2019, 2020). Preserved abilities included fine direction discrimination, coarse direction discrimination, and contrast sensitivity, but they all disappeared by the onset of the chronic poststroke period, i.e., 6 months poststroke (Saionz et al., 2019, 2020).
Notably, preserved vision in subacute blind fields was distinct from blindsight in that it was consciously accessible. The strength of these visual discriminations varied across the blind field, signifying that vision loss immediately after occipital stroke is neither complete nor uniform. Instead, V1 damage appears to initiate a process of gradual vision loss, beginning with small-spot luminancedetection (such asused duringperimetry) and progressing to include larger targets, and more complex, higher-order visual functions. The precise timescale of loss for different visual functions initially preserved in the early poststroke period is currently unknown. However, in all cases examined to date, by 6 months poststroke, preserved visual discrimination abilities within the field defect degraded and blindness became profound (Saionz et al., 2019, 2020). Importantly, in contrast with perimetrically-defined blind fields, which spontaneously shrank in a large proportion of patients in the first few months poststroke, discrimination abilities inside the blind field never recovered spontaneously (Saionz et al., 2020). Thus, there are major differences in the types of “vision” measured by visual perimetry (whether static, kinetic, automated, or manual), as opposed to psychophysical approaches (which rely on forced-choice tasks and threshold levels of performance). These differences are key to properly characterize both residual vision in the blind field, as well as the properties of vision that can be recovered with therapeutic interventions such as visual training.
Beyond the subacute poststroke period, new research also challenges the notion that chronic blind fields are stable in the absence of an intervention. In one small, but detailed, prospective study, chronic patients who received standard-of-care treatment after occipital stroke actually showed slight worsening of their perimetrically-defined visual field defect, measured by Humphrey automated perimetry (Cavanaugh and Huxlin, 2017). This finding was confirmed by a larger retrospective review of stroke-induced CB, which noted that from 6 months poststroke onward, the visual field defect tends to worsen slowly over time (Wang et al., 2017; Saionz, 2020). These studies identified worsening of the perimetric visual field defect in the chronic period by using a novel, quantitative approach to the analysis of Humphrey automated perimetry, combined with rigorous inclusion/exclusion criteria for patient type and reliability indices of the perimetric fields themselves (Cavanaugh and Huxlin, 2017). It is hoped that future studies will continue to apply rigorous inclusion criteria, as well as quantitative and modeling approaches to the analysis of visual perimetry data so as to attain greater precision and sensitivity for changes in visual function, both spontaneous and following therapeutic interventions.
PRESERVED VISUAL PATHWAYS: SUBSTRATES FOR VISUAL REHABILITATION
Insights into the natural history of vision loss after V1 stroke, including the new perspectives outlined in the previous section, can be gained from reviewing the anatomic and physiologic consequences of V1 damage. In the classical, image-forming pathway (Kandel et al., 2012), information is first collected and processed by the retina, the visual sensory organ, whose only output is via the axons of retinal ganglion cells. These axons carry information through the optic nerve to the rest of the brain. At the optic chiasm, retinal ganglion cell axons originating from each nasal retina decussate, so that information reorganizes from segregation by eye to segregation by visual hemifield. Retro-chiasmatic fibers thus carry information about the contralateral visual hemifield, from both eyes, and form their first synapse with neurons in the dorsal lateral geniculate nucleus (dLGN) of thet halamus, where 6 cell layers sort, process, and ultimately transmit the information further up the system. The optic radiations carry this feedforward visual information to a second, postretinal synapse in the primary or striate visual cortex (V1), the major target of the early, classical, image-forming pathway. V1 further processes information and distributes it to multiple extra-striate visual cortical areas, each of which performs specialized processing of the received visual information. While lesions to any of the retro-chiasmatic structures up to and including V1 can cause CB, visual information is also relayed to extra-striate visual areas through pathways that bypass V1. These include projections from the retina to the superior colliculus to the pulvinar of the thalamus and on to the extra-striate cortex, and from the dLGN directly to the extra-striate cortex (Tamietto et al., 2010; Das et al., 2014; Ajina et al., 2015a; Tamietto and Morrone, 2016; Ajina and Bridge, 2019).
Another important consideration is that when V1 is damaged by a stroke (or other means), neuronal death is not limited to cells in V1. The damage initiates a process of trans-synaptic retrograde degeneration of afferent neurons—something that has been observed in both humans and nonhuman primates (Porrello and Falsini, 1999; Jindahra et al., 2009; Bridge et al., 2011; Cowey et al., 2011; Jindahra et al., 2012; Patel et al., 2016; Millington et al., 2017; Rajanala et al., 2019; Schneider et al., 2019). This occurs sequentially, with neurons in the dLGN being the first to die (Mihailović et al., 1971; Wong-Riley, 1972), followed by retinal ganglion cells in the lesion projection zone (Cowey et al., 2011). The time course of retrograde degeneration has been most carefully documented in animal studies (Mihailović et al., 1971; Wong-Riley, 1972; Atapour et al., 2017). In humans, our information is less precise, with best estimates coming from imaging studies—magnetic resonance imaging of the brain and optical coherence tomography of the retina. Data obtained with both of these techniques suggest that early retinogeniculate portions of the visual system begin to show measurable signs of retrograde degeneration by 6 months after injury (Jindahra et al., 2009; Bridge et al., 2011; Cowey et al., 2011; Jindahra et al., 2012; Patel et al., 2016; Schneider et al., 2019). These anatomic findings support the psychophysical observation that the evolution of vision loss after V1 stroke is gradual—at least for many visual discrimination abilities. The residual vision preserved in the subacute poststroke period (Saionz et al., 2020) may well be mediated by regions of the striate cortex that survive the incident stroke (indeed such regions have been proposed to underlie blindsight/residual visual processing in the chronic period (Kleiser et al., 2001; Morland et al., 2004; Radoeva et al., 2008; Das and Huxlin, 2010; Papanikolaou et al., 2014; Barbot et al., 2021)). The disappearance of subacute residual vision by the onset of the chronic period could be caused by some of the surviving V1 regions becoming quiescent due to progressing atrophy from a persistent lack of normal feedforward and feedback input (Fendrich et al., 1992; Wessinger et al., 1997; Das and Huxlin, 2010). The psychophysical properties of subacute residual vision—namely, preservation of some but not all visual abilities across regions of the blind field (Saionz, 2020; Saionz et al., 2020)—are also consistent with the notion of possible “spared islands of visual cortex” (Albrecht and Hamilton, 1982; Tootell et al., 1995; Boynton et al., 1999; Ajina et al., 2015b; Niemeyer et al., 2017; Pawar et al., 2019). However, emerging data suggest that these islands lose the ability to mediate conscious visual perception sometime within the first 6 months poststroke. We posit here that this could occur through a combination of progressing retrograde degeneration and lack of normal feed forward and feedback inputs. Infact, this combination of factors could lead to a form of “visual disuse atrophy” (Saionz et al., 2020), where by the activity of weak, surviving connections becomes down-weighted over time, as patients learn to ignore the abnormal visual signals originating from their blind field. Many questions naturally emerge from this, but the ones we will focus on in the remainder of this chapter are (1) can vision be recovered in stroke-induced cortical blindness and (2) when is the most optimal time to administer treatment?
USE OF VISION RESTORATION TRAINING IN CORTICAL BLINDNESS (FIGS. 25.1B AND 25.2)
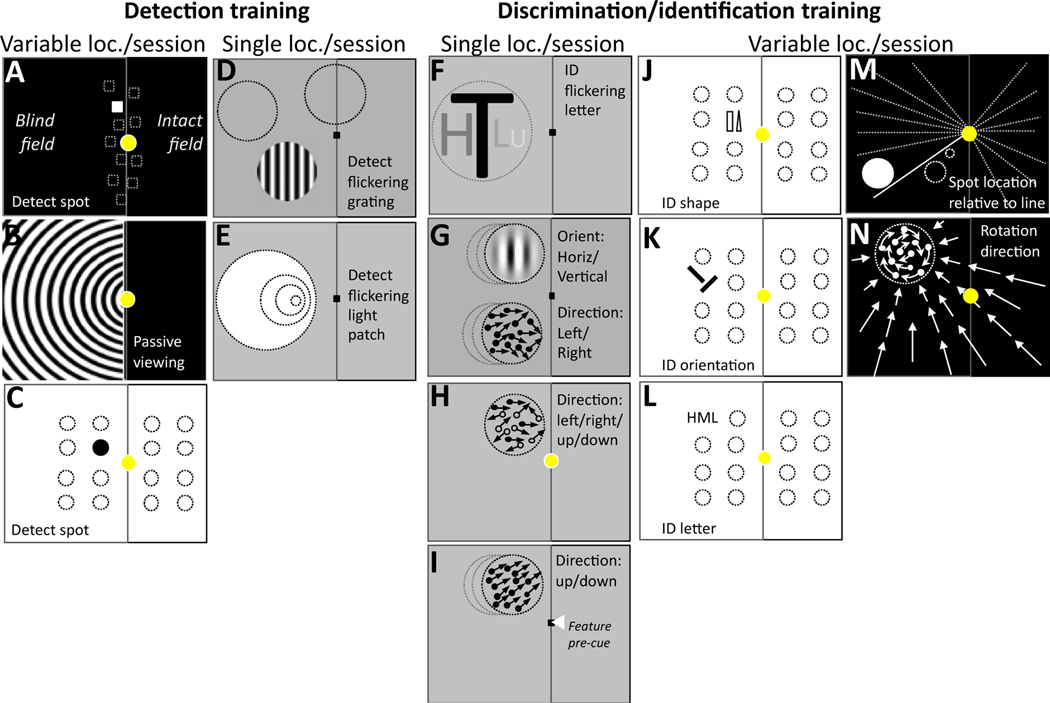
Fig.25.2.
Simplified, schematic representation of visual training tasks administered to restore vision in cortically-blinded portions of the visual field after occipital stroke. Each rectangular aperture represents the computer display in front of the putative patient, with a fixation spot (yellow in paradigms that did not use an eye tracker, and a small black square in paradigms that used an eye tracker for fixation enforcement at least during pre-and posttests). For illustrative purposes, the putative blind field is considered to be on the left side of the vertical centerline in each aperture. Tasks the patients were asked toperform are notedin each aperture and are separated according to whether they involved stimulus detection or discrimination/identification (ID), as well as according to whether they stimulated single or variable/multiple locations in a given training session. (A) Original VRT task, Kasten et al. (1998). (B) Extrastriate VRT task, Jobke et al. (2009). (C). Spot detection task, Chokron et al. (2008). (D) Detection of 10 Hz-flickering, contrast-varying sinusoidal grating, Sahraie et al. (2006, 2010), Trevethan et al. (2012). (E) Detection of flickering light patch of different sizes, Raninen et al. (2007). (F) Identify flickering letters (4 possibilities) of different sizes, Raninen et al. (2007). (G) Discriminate the orientation (horizontal/vertical) of high-contrast, static, nonflickering Gabors, or the global motion direction (left/right) and integration of random-dot stimuli, Huxlin et al. (2009), Das et al. (2014). (H) Discriminate the direction of motion (4 possibilities: up/down/right/left) of noisy, random-dot stimuli, Vaina et al. (2014). (I) Fine direction discrimination with feature-based attentional pre-cues, Cavanaugh et al. (2019). (J) Shape identification task (rectangle/triangle), Chokron et al. (2008). (K) Orientation identification task (2 choices: left/right of vertical), Chokron et al. (2008). (L) Letter identification task (3 choices: H, M or L), Chokron et al. (2008). (M) Spot location is relative to the line (2 choices: clockwise/ anticlockwise), Elshout et al. (2016). (N) Rotation direction of circular target at the center of flow-field (2 choices: clockwise/anti-clockwise), Elshout et al. (2016).
Despite evidence of residual visual processing in cortically blind fields, it has been repeatedly suggested that the adult visual system may not be capable of functional recovery after V1 stroke in adulthood (Horton, 2005a, 2005b). Yet an abundance of basic research into poststroke visual plasticity has shown that visual training can recover certain visual functions in long-standing, chronic, CB (reviewed in Melnick et al., 2016). Because these original studies were conducted beyond the window of spontaneous visual recovery, the investigators were able to establish that vision improvements were exclusively the result of training and not spontaneous plasticity. These training studies were based on principles of perceptual learning, an umbrella term for a form of neuroplasticity within sensory systems whereby repeated stimulation improves processing of stimuli across the lifespan (Ahissar and Hochstein, 1997; Dosher and Lu, 1998; Gilbert et al., 2009; Lu et al., 2011; Sagi, 2011; Lu et al., 2016; Dosher and Lu, 2017). A prevailing hypothesis about the mechanism of visual perceptual learning is that it is a form of Hebbian neuroplasticity (Dosher and Lu, 2009). The evidence we detail here suggests that this mechanism is functioning quite effectively in the residual visual system of V1-damaged patients, both in the chronic and subacute poststroke periods.
As mentioned earlier, most of the data demonstrating the efficacy of visual perceptual training as a vision restoration tool has come from research studies performed in chronic occipital stroke patients (summarized in Fig. 25.2). Few of these scientific research studies have been validated and published as randomized, placebo-controlled, double-blinded trials (Pollock et al., 2019) and this remains a gap in the field. Kasten and Sabel’s visual restitution therapy (VRT, Fig. 25.2A) was claimed to improve luminance detection in CB fields (Kasten et al., 1998) and became clinically available in Europe and the United States for vision restitution, marketed by NovaVision. However, it was later shown to suffer from insufficient fixation control (Reinhard et al., 2005), light scatters (Pelak et al., 2007), and problems induced by using the same task for both training and evaluation of vision restoration. Sabel’s group later tested many variants on VRT (Kasten et al., 1998; Poggel et al., 2004; Kasten et al., 2007; Jobke et al., 2009; Poggel et al., 2010; Gall et al., 2014; Turco et al., 2015; Alber et al., 2017). These included a high-contrast, moving spiral that occupied the entire blind field (Fig. 25.2B) and was dubbed an “extrastriate” stimulus (Jobke et al., 2009). However, fixation remained un-enforced and it was unclear if patients were required to perform a motion task or just passively view this moving stimulus. Unfortunately, the high-profile failure of VRT in clinical circles, together with the general lack of randomized, controlled, clinical trials for most of the other visual restoration approaches developed by other research groups (see below), have thrown significant doubt on the feasibility and usefulness of visual restitution in adult-onset CB (Horton, 2005a, 2005b; Pollock et al., 2019). Thankfully, however, efforts to attain solutions for this patient population have persisted.
Fundamentally, such efforts should start with a better understanding of the basic science of training-induced plasticity and perceptual learning in CB patients. Below, we will review the basic, neuroscientific approaches used to date in this population. As shown graphically in Fig. 25.2C–N, these can be subdivided into the following categories: (1) training to detect the presence of a visual target, (2) training to discriminate or identify specific aspects of a visual target, (3) repeated presentation of a single stimulus at the same blind-field location in each training session, until improvement is attained (Fig. 25.2D–H), or (4) presentation of stimuli at different locations on each trial within a session, either in the intact or blind field (Fig. 25.2A–C, J–N). In (3), stimulus location is predictable from trial to trial; in (4), the patient needs to deal with location uncertainty for the stimulus to be detected or discriminated.
Chokron and colleagues (Chokron et al., 2008) attained encouraging results with a multilocation spot detection task that resembled VRT but seemed to better address light scatter and task-specificity issues (Fig. 25.2C). Subsequent attempts to improve upon this shifted the field toward visual psychophysics performed with online fixation control using an eye tracker (thus ensuring gaze-contingent stimulus presentation), as well as manipulations of stimulus and task difficulty (pushing the visual system to its perceptual limits and allowing measurement of threshold performance in the blind field).
These studies demonstrated recovery of a range of visual abilities within cortically-blinded fields, including detection of flickering sinusoidal gratings and detection contrast sensitivity (Sahraie et al., 2006, 2010; Trevethan et al., 2012), flicker sensitivity (Raninen et al., 2007), relative target localization (Chokron et al., 2008; Elshout et al., 2016), letter identification (Raninen et al., 2007; Chokron et al., 2008), direction integration (Huxlin et al., 2009; Das et al., 2014), motion coherence (Vaina et al., 2014), coarse and fine [translational] direction discrimination (Cavanaugh et al., 2015, 2019), rotational direction discrimination (Elshout et al., 2016), and contrast sensitivity for both static orientation and motion direction discriminations (Das et al., 2014). Most of the psychophysical training regimens listed above also caused reductions in the size of visual field defects measured with clinical perimetry (Sahraie et al., 2006; Chokron et al., 2008; Huxlin et al., 2009; Bergsma et al., 2012; Trevethan et al., 2012; Vaina et al., 2014; Elshout et al., 2016; Cavanaugh and Huxlin, 2017). Importantly, the improved/restored vision appeared to be consciously accessible (Sahraie et al., 2013; Saionz et al., 2020) and recovery did not always seem to represent a simple enhancement of blindsight (Das et al., 2014). Indeed, in many cases, the stimuli used to retrain vision could not elicit blindsight—e.g., static nonflickering Gabors with slow temporal cosine envelopes (Hess and Pointer, 1989; Weiskrantz et al., 1991; Morland et al., 1996; Sahraie et al., 2008) or random-dot stimuli with complex motion (Azzopardi and Cowey, 2001).
In summary, patients with longstanding, adult-onset CB retain the potential for visual neuroplasticity and this potential can be recruited by intensive, repeated detection or discrimination training to recover conscious visual perception in the blind field. Fixation control during stimulus presentation appears to be important for training to be most effective, but this is beyond the capacity of most current systems to incorporate. However, what is more realistic to implement with current technology is real-time fixation control using eye-trackers during pre- and post-training tests that are usually performed in laboratories or clinics. This is key for the accurate assessment of changes in visual functions, whether measured with perimetry or psychophysics. As such, it would be good to see this implemented routinely for visual testing in this patient population.
LIMITATIONS OF TRAINING IN THE CHRONIC PERIOD AFTER OCCIPITAL STROKE
While visual training does recover chronic stroke patients’ ability to perform a range of detection and discrimination tasks in their blind field, recovery generally requires several weeks to months of daily practice at each blind field location (Huxlin et al., 2009; Sahraie et al., 2010; Das et al., 2014; Cavanaugh et al., 2015; Larcombe et al., 2018; Cavanaugh et al., 2019). Indeed, several studies have shown that the amount of improvement in chronic CB (both psychophysically-measured and perimetrically-derived) is directly proportional to the number of training sessions performed (Cavanaugh et al., 2015; Cavanaugh and Huxlin, 2017). Even so, recovered vision in chronic CB patients is not as accurate or sensitive as normal vision, appearing coarser and exhibiting lower contrast (Huxlin et al., 2009; Das et al., 2014; Cavanaugh et al., 2015). At some level, the inability of therapy to restore normal vision in CB is not entirely surprising. After all, V1 damage kills a large portion of neurons selective for fundamental visual attributes, such as orientation and direction. In addition, it has been postulated that V1 lesions shift the excitation/ inhibition balance in the residual visual circuitry toward excessive inhibition (Spolidoro et al., 2009). High levels of intracortical inhibition can limit plasticity and would be expected to increase the threshold for activation of relevant circuits. This may explain why visual training started more than 6 months after stroke is reported by patients to be arduous and slow, something that was confirmed empirically in a recent study comparing training-induced recovery in chronic and subacute CB (Saionz et al., 2020).
MOUNTING EVIDENCE FOR BENEFITS OF EARLIER INTERVENTION AFTER V1 STROKE
The approach to rehabilitation used in vision stroke research to date has largely involved delaying intervention until the chronic poststroke period, when visual fields have stabilized, making it easier to isolate the impact of the intervention from spontaneous improvements. This stands in stark contrast to sensorimotor stroke therapy, which has been systematically investigated as early as the first week poststroke (Bernhardt et al., 2017). Current treatment guidelines for sensorimotor stroke recommend initiating early rehabilitation to promote faster, greater recovery (Krakauer, 2006; Winstein et al., 2016; Bernhardt et al., 2017; Dromerick et al., 2021). These clinical observations are supported by scientific evidence suggesting that the damaged brain may have increased neuroplastic potential early after injury (Rossini et al., 2003; Bavelier et al., 2010; Hensch and Bilimoria, 2012; Seitz and Donnan, 2015). The early poststroke period is characterized by resolution of peri-lesional inflammation and edema, variability in vascular perfusion, upregulation of growth and injury-response factors (especially brain-derived neurotrophic factor), changes in neurotransmitter modulation (especially GABA, glutamate, and acetylcholine), an overall shift in the excitation/inhibition balance to favor excitation, and possibly even the re-emergence of acritical-period-likestate (Rossini et al., 2003; Bavelier et al., 2010; Hensch and Bilimoria, 2012; SeitzandDonnan,2015).Moreover, structural barriers to plasticity in the form of myelin-related proteins inhibiting axonal sprouting, perineuronal nets of chondroitin sulfate proteoglycans, and fibrotic tissue have yet to form (Rossini et al., 2003; Bavelier et al., 2010; Hensch and Bilimoria, 2012). It is conceivable that these processes underlie the spontaneous improvements in detection perimetry observed in the first few months after an occipital stroke. Recent work has now begun to investigate whether these processes can also be recruited by early administration of visual training to facilitate greater and faster recovery of visual discriminations (not just detection) in cortically blind fields.
In addition to enhanced neuroplastic potential early poststroke, the specter of trans-synaptic retrograde degeneration further motivates administering training during the subacute period. Degeneration is likely well underway by 6 months poststroke (Bridge et al., 2011; Bridge and Plant, 2012; Jindahra et al., 2012; Schneider et al., 2019) and, once it happens, it will deprive chronic patients of important sensory substrates, and can only limit training-induced visual recovery. For instance, loss of parvocellular neurons, which are especially susceptible to retrograde degeneration (Cowey et al., 1989; Yu et al., 2018), will impair responses of the remaining visual circuitry (and of the person) to fine detail (mediated by sensitivity to high spatial frequencies), tasks involving form recognition or discrimination (mediated by neural orientation and shape preferences) and low luminance contrasts (due to loss of the sufficient number of sensitive neurons), among others. As detailed above, preserved, blind-field contrast sensitivity and orientation discrimination are key differences between early subacute and chronic CB patients (Saionz et al., 2020).
With these motivations, the question becomes: does identical training initiated in the subacute poststroke period lead to greater recovery than training in the chronic period? In one of the first, systematic, psychophysical studies of subacute vision rehabilitation (Saionz et al., 2020), patients recovered visual discriminations in a fraction of the number of training sessions needed by chronic patients. Subacute patients also showed transfer of learning to deeper, untrained areas of the blind field and they exhibited substantial enhancements of Humphrey perimetry, even greater than those attained spontaneously. Early data also suggest that subacute patients may be able to train on a wider range of stimuli than chronic patients; in particular, they can recover the ability to discriminate low-contrast, stationary stimuli—something very few chronic patients are able to do (Saionz et al., 2019; Saionz, 2020). Importantly, in these studies, untrained subacute patients, who served as a control group, experienced the expected spontaneous improvements in their perimetrically-measured [Humphrey] visual fields, but they did not show any spontaneous improvements in visual discrimination abilities. This observation highlighted a dissociation between the type of vision that could improve spontaneously poststroke (luminance detection, a.k.a. perimetry) and that which required deliberate training (discriminationtasks).Spontaneous improvements in perimetry are hypothesized to be the result of subsiding peri-lesional edema and inflammation, restoration of blood flow to the penumbra, and some degree of spontaneous reorganization (Krakauer, 2006). In contrast, subacute improvements in visual discrimination abilities seemed to occur only as a result of specific, training-dependent neuroplasticity. The dissociation between luminance detection and discrimination tasks also suggests a need to develop more comprehensive clinical assessments of vision loss after stroke, beyond perimetry (Saionz et al., 2020)—current clinical assessments fail to capture so many key aspects of visual function, including those which are preserved in the first few months after a stroke, or which are recovered following directed blindfield training using complex visual stimuli/tasks.
All in all, results of psychophysical testing and training in subacute patients support the notion that occipital stroke may reopen a sensitive or maybe even critical period for vision (Bavelier et al., 2010; Hensch and Bilimoria, 2012; Voss, 2013). In this poststroke sensitive period, the neuroplastic potential is enhanced but environmental input is required to guide optimal, functional tuning of relevant circuits. If it turns out that the early poststroke period is actually a critical period (i.e., a period with the exclusive potential to develop specific functions), this will have important implications for the field. As is true of all critical periods, it would mean that if residual circuits are not appropriately stimulated during this limited period of time, then their function could be permanently and negatively altered. It is therefore key for future research to determine whether the early period after an occipital stroke is a sensitive or a critical period, and for what visual functions. It will also be important to better define the temporal extent of this early sensitive/critical period and to identify factors that control its closing—factors that could perhaps be manipulated to prolong its duration.
SAFETY OF VISION TRAINING EARLY AFTER STROKE
In sensorimotor stroke, where more research has been conducted on early rehabilitation, concerns have emerged that very early rehabilitation may actually be harmful. Animal studies have found that exercise beginning less than 24 hours after motor cortex stroke led to an increase in inflammatory cytokines (Li et al., 2017) and enlargement of the ischemic lesions (Risedal et al., 1999), but neither study found behavioral consequences. Two animal studies initiating exercise at 24hours did find worse outcomes, along with tissue-level effects such as reduced neuronal proliferation (Kozlowski et al., 1996; Komitova et al., 2005). In humans, a large multicenter, randomized, control trial (AVERT) reported that patients who received very early (within 24hours poststroke) mobilization had worse outcomes than patients who received standard of care, of which the majority were mobilized 24–48hours poststroke (Bernhardt et al., 2015). Another smaller trial found similar results (Sundseth et al., 2012), but other studies of early rehabilitation beyond the first 24-hour window did not find significant harm or worse outcomes (reviewed in Coleman et al., 2017; Dromerick et al., 2021). An important caveat here is that the AVERT trial focused on out-of-bed mobilization. This is relevant because negative results seen with very early mobilization could be due to hemodynamic effects of upright posture on the brain areas at risk for further ischemic damage, and may not be relevant for interventions that can be done in-bed (Ward and Kitago, 2016).
In studies of early visual restoration training (Saionz et al., 2019; Saionz, 2020; Saionz et al., 2020), participants started training no less than 14 days poststroke, which falls well beyond the window for safety concerns in sensorimotor stroke rehabilitation; none of the trained patients reported adverse events, and none experienced worsening of vision. Moreover, while a motor stroke patient may avoid using an affected limb, a vision stroke patient cannot selectively refrain from exposing the affected visual field to light or other visual stimulation; the only recourse would be visual deprivation, which is not the current standard of care. As such, it is in fact universal practice that vision stroke patients receive immediate, constant exposure to visual sensory stimulation in the affected hemifield (except while sleeping). This prompts the consideration that it may be possible to enhance recovery potential—even spontaneous recovery in untrained individuals—with a short (less than24hour) period of vision deprivation immediately after an occipital stroke. Indeed, animal studies have shown that dark adaptation can re-open a critical period window (He et al., 2006; Montey and Quinlan, 2011; Duffy and Mitchell, 2013; Mitchell et al., 2016), and this approach has even been considered for augmenting treatment of adult human amblyopes (He et al., 2007; Eaton et al., 2016). Thus, while subacute visual training is not likely to cause additional harm over no training, it may be worth investigating whether very early poststroke visual exposure can be manipulated to further improve recovery.
ADJUVANTS TO BEHAVIORAL TRAINING FOR VISION REHABILITATION
An important question is whether pharmacologic or other tools can be used to enhance rehabilitation after vision stroke, either alone or in combination with a visual training program. The goal of such an adjuvant is to aid neuroplasticity and speed learning, perhaps by prolonging the duration of the poststroke sensitive/critical period or reopening a sensitive period after it has closed (Bavelier et al., 2010; Ng et al., 2015).
Noninvasive brain stimulation hasbeen used by many research groups as an adjuvant to training for vision recovery, albeit primarily in the chronic poststroke period. Transcranial random noise stimulation (tRNS) applies a current of defined amplitude and randomly variable direction within a frequency range. tRNS has been shown to enhance cortical excitability in the motor cortex (Terney et al., 2008) and improve perception in the visual cortex (Pirulli et al., 2013; Campana et al., 2014; van der Groen and Wenderoth, 2016).When administered during vision training sessions, it was found to improve learning in both visually intact and chronic cortically-blind adults (Herpich et al., 2019). Chronic, cortically-blind participants receiving tRNS were able to recover motion discriminations far faster than would normally be expected from training alone. Transcranial direct current stimulation (tDCS) applies unidirectional current and can cause excitatory or inhibitory responses depending on electrode positioning and, thus, the current direction (Nitsche et al., 2003). Investigators using NovaVision’s VRT have also reported success combining their program with tDCS in a small number of chronic cortically blind patients, showing that VRT in conjunction with tDCS improves perimetric visual fields and activities of daily living (Plow et al., 2011, 2012) as well as blind field motion perception (Olma et al., 2013) more than sham stimulation. A follow-up study suggested that tDCS may be safe to use in subacutes, and may lead to greater improvement in visual fields than sham stimulation (Alber et al., 2017). However, these results have not been replicated by others using visual training other than VRT (Herpich et al., 2019), suggesting that this approach needs further investigation before its efficacy can be reliably claimed. Investigators have also studied the use of transorbital alternating current stimulation (rtACS) for CB, with case reports indicating possible improvement in perimetric sensitivity of the spared visual field (Gall et al., 2014) and increased area of BOLD activation in fMRI (Sabel et al., 2020). As further evidence emerges, noninvasive brain stimulation may become a promising tool for enhancing visual restoration in the chronic period, warranting further investigations into its mechanisms of action, safety, and efficacy if administered in the subacute period.
The use of pharmacology in vision recovery poststroke has not yet been systematically evaluated (although one pilot trial is currently ongoing: NCT02737930). Animal studies have suggested that serotonin reuptake inhibitors such as fluoxetine, as a stand-alone agent, could improve plasticity in the adult visual system by reopening a critical period (Maya Vetencourt et al., 2008). Results from a rodent model (Ng et al., 2015) suggested that serotonin reuptake inhibitors may improve recovery and may prolong the transient period of increased plasticity poststroke. However, human studies have shown mixed results. The FLAME trial, a moderately sized randomized controlled trial, found that 20mg of fluoxetine for 3 months poststroke enhanced motor recovery. Unfortunately, several large-scale, follow-up trials (FOCUS, EFFECTS, and AFFINITY) failed to confirm these results (Dennis et al., 2019; Hankey et al., 2020; Lundström et al., 2020). One reason for this may be that these larger trials had less selective inclusion criteria than FLAME and did not ensure that all participants received physical therapy during the treatment period (which may be relevant if the beneficial effects of fluoxetine are activity-dependent). However, all three of the larger trials also found a significantly increased risk of bone fractures in the fluoxetine group. As such, fluoxetine is unlikely to become routinely recommended for augmenting poststroke recovery. It remains to be seen if other agents may have the potential to modulate neuro-recovery without significant side effects.
CONCLUSIONS
The intact adult visual system is capable of neuroplasticity throughout the lifespan, as evidenced by visual perceptual learning and its ability to dynamically process ever-changing visual inputs. An expanding body of research now shows this potential to persist even after strokes that damage large regions of the primary visual cortex. Emerging evidence suggests that aspects of vision loss immediately after stroke are neither sudden nor complete. Instead, vision loss is a gradual, spatially, and functionally uneven process that, without intervention, continues well into the chronic poststroke period. Recent work suggests that early visual restoration training can interrupt this process, preserving and then enhancing residual vision within the perimetric blind field. The stroke may even open up a new sensitive period of visual plasticity, in which deliberate visual stimulation provided by training (possibly enhanced by adjuvants such as noninvasive brain stimulation or pharmacology) is required to optimize the function of residual visual circuits. Early training likely takes advantage of enhanced neuroplasticity and a visual system that has yet to suffer the consequences of retrograde degeneration. Results to date suggest that when training is administered in the first few months’ poststroke, subacute CB patients are able to recover vision much faster than chronic patients. In addition, they are able to recover a larger range of visual discrimination abilities, over larger regions of their blind field, than patients who begin identical training in the chronic, poststroke period. However, even when interventions are not administered until the chronic poststroke period, benefits in terms of visual recovery—albeit more limited—can still be elicited. In conclusion, recent advances in visual restoration research suggest that, paralleling sensorimotor stroke, visual neuroplasticity is dynamic after visual cortex stroke. Importantly, research highlights the benefits of using psychophysical approaches to probe the complexities of visual processing in CB fields, and the importance offixation control for attaining accurate measures of the magnitude of change in visual perception whether spontaneously or as a function of rehabilitation. Finally, it remains true, as recommended by the latest Cochrane review (Pollock et al., 2019), that the basic scientific studies described presently still need to be validated by randomized, placebo-controlled, double-blinded trials, contrasting the interventions proposed with the standard of care (which sadly remains doing nothing), as well as appropriate “control” training interventions. Nonetheless, this body of work provides both new hope and opportunity to revise our theoretic and therapeutic approach in this growing clinical population, employing accumulating evidence from basic neuroscientific studies that vision can indeed be restored after permanent damage to the primary visual cortex.
REFERENCES
- Ahissar M, Hochstein S (1997). Task difficulty and the specificity of perceptual learning. Nature 387: 401–406. [DOI] [PubMed] [Google Scholar]
- Aimola L, Lane AR, Smith DT et al. (2014). Efficacy and feasibility of home-based training for individuals with homonymous visual field defects. Neurorehabil Neural Repair 28: 207–218. [DOI] [PubMed] [Google Scholar]
- Ajina S, Bridge H (2019). Subcortical pathways to extrastriate visual cortex underlie residual vision following bilateral damage to V1. Neuropsychologia 128: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajina S, Pestilli F, Rokem A et al. (2015a). Human blindsight is mediated by an intact geniculo-extrastriate pathway. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajina S, Rees G, Kennard C et al. (2015b). Abnormal contrast responses in the Extrastriate cortex of blindsight patients. J Neurosci 35: 8201–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alber R, Moser H, Gall C et al. (2017). Combined transcranial direct current stimulation and vision restoration training in subacute stroke rehabilitation: a pilot study. PM and R 9: 787–794. [DOI] [PubMed] [Google Scholar]
- Albrecht DG, Hamilton DB (1982). Striate cortex of monkey and cat: contrast response function. J Neurophysiol 48:217–237. [DOI] [PubMed] [Google Scholar]
- Atapour N, Worthy KH, Lui LL et al. (2017). Neuronal degeneration in the dorsal lateral geniculate nucleus following lesions of primary visual cortex:comparison of young adult and geriatric marmoset monkeys. Brain Structure and Function 222: 3283–3293. [DOI] [PubMed] [Google Scholar]
- Azzopardi P, Cowey A (1997). Is blindsight like normal, near-threshold vision? Proc Natl Acad Sci U S A 94: 14190–14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi P, Cowey A (2001). Motion discrimination in cortically blind patients. Brain 124: 30–46. [DOI] [PubMed] [Google Scholar]
- Bainbridge W, Reding M (1994). Full-field prisms for hemifield visual impairments following stroke: a controlled trial. Neurology 44: A312–A313. [DOI] [PubMed] [Google Scholar]
- Barbot A, Das A, Melnick MD et al. (2021). Spared perilesional V1 activity underlies training-induced recovery of luminance detection sensitivity in cortically-blind patients. Nat Commun 12: 6102. 10.1038/s41467-021-26345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW et al. (2010). Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 30: 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béjot Y, Delpont B, Giroud M (2016). Rising stroke incidence in young adults: more epidemiological evidence. More Questions to Be Answered Journal of the American Heart Association 5: e003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma DP, Elshout JA, van der Wildt GJ et al. (2012). Transfer effects of training-induced visual field recovery in patients with chronic stroke. Top Stroke Rehabil 19: 212–225. [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Langhorne P, Lindley RI et al. (2015). Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. The Lancet 386: 46–55. [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Godecke E, Johnson L et al. (2017). Early rehabilitation after stroke. Curr Opin Neurol 30: 48–54. [DOI] [PubMed] [Google Scholar]
- Bouwmeester L, Heutink J, Lucas C (2007). The effect of visual training for patients with visual field defects due to brain damage: a systematic review. J Neurol Neurosurg Psychiatry 78: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers AR, Keeney K, Peli E (2008). Community-based trial of a peripheral prism visual field expansion device for hemianopia. Arch Ophthalmol 126: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers AR, Keeney K, Peli E (2014). Randomized crossover clinical trial of real and sham peripheral prism glasses for hemianopia. JAMA Ophthalmology 132: 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH et al. (1999). Neuronal basis of contrast discrimination. Vision Res 39: 257–269. [DOI] [PubMed] [Google Scholar]
- Bridge H, Plant GT (2012). Conclusive evidence of human transneuronal retrograde degeneration in the visual system. Journal of Clinical & Experimental Ophthalmology S3. 10.4172/2155-9570.S3-003. [DOI] [Google Scholar]
- Bridge H, Jindahra P, Barbur J et al. (2011). Imaging reveals optic tract degeneration in hemianopia. Investigative Ophthalmology and Visual Science 52: 382–388. [DOI] [PubMed] [Google Scholar]
- Campana G, Camilleri R, Pavan A et al. (2014). Improving visual functions in adult amblyopia with combined perceptual training and transcranial random noise stimulation (tRNS): a pilot study. Front Psychol 5: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Robson JG (1968). Application of fourier analysis to the visibility of gratings. J Physiol 197: 551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion J, Latto R, Smith YM (1983). Is blindsight an effect of scattered light, spared cortex, and near-threshold vision? Behavioral and Brain Sciences 6: 423–448. [Google Scholar]
- Cavanaugh MR, Huxlin KR (2017). Visual discrimination training improves Humphrey perimetry in chronic cortically induced blindness. Neurology 88: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh MR, Zhang R, Melnick MD et al. (2015). Visual recovery in cortical blindness is limited by high internal noise. J Vision 15: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh MR, Barbot A, Carrasco M et al. (2019). Feature-based attention potentiates recovery of fine direction discrimination in cortically blind patients. Neuropsychologia 128: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokron S, Perez C, Obadia M et al.(2008).From blindsight to sight: cognitive rehabilitation of visual field defects. Restor Neurol Neurosci 26: 305–320. [PubMed] [Google Scholar]
- Coleman ER, Moudgal R, Lang K et al. (2017). Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep 19: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A (2010). The blindsight saga. Exp Brain Res 200: 3–24. [DOI] [PubMed] [Google Scholar]
- Cowey A, Stoerig P, Perry VH (1989). Transneuronal retrograde degeneration of retinal ganglion cells after damage to striate cortex in macaque monkeys: selective loss of Pβ cells. Neuroscience 29: 65–80. [DOI] [PubMed] [Google Scholar]
- Cowey A, Alexander I, Stoerig P (2011). Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain 134: 2149–2157. [DOI] [PubMed] [Google Scholar]
- Das A, Huxlin KR (2010). New approaches to visual rehabilitation for cortical blindness: outcomes and putative mechanisms. Neuroscientist 16: 374–387. [DOI] [PubMed] [Google Scholar]
- Das A, Tadin D, Huxlin KR (2014). Beyond blindsight: properties of visual relearning in cortically blind fields. J Neurosci 34: 11652–11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Pourtois G et al. (1999). Nonconscious recognition of affect in the absence of striate cortex. Neuroreport 10: 3759–3763. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Tamietto M, van Boxtel G et al. (2008). Intact navigation skills after bilateral loss of striate cortex. Curr Biol 18: R1128–R1129. [DOI] [PubMed] [Google Scholar]
- de Haan GA, Melis-Dankers BJM, Brouwer WH et al. (2015). The effects of compensatory scanning training on mobility in patients with homonymous visual field defects: a randomized controlled trial. PLOS ONE 10: e0134459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Mead G, Forbes J et al. (2019). Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. The Lancet 393: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombovy ML, Sandok BA, Basford JR (1986). Rehabilitation for stroke: a review. Stroke 17: 363–369. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu Z-L (1998). Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc Natl Acad Sci 95:13988–13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL (2009). Hebbian reweighting on stable representations in perceptual learning. Learning & Perception 1: 37–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu Z-L (2017). Visual perceptual learning and models. Annual Review of Vision Science 3: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromerick AW, Geed S, Barth J (2021). Critical Period After Stroke Study (CPASS): A phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc Natl Acad Sci 118 (39): e20266761118. 10.1073/pnas.20266761118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KR, Mitchell DE (2013). Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Curr Biol 23: 382–386. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Goldstein LB, Matchar D et al. (1992). Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 23: 1084–1089. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Goldstein LB, Horner RD et al. (1994). Similar motor recovery of upper and lower extremities after stroke. Stroke 25: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Zorowitz R, Bates B et al. (2005). Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke 36: e100–e143. [DOI] [PubMed] [Google Scholar]
- Eaton NC, Sheehan HM, Quinlan EM (2016). Optimization of visual training for full recovery from severe amblyopia in adults. Learning and Memory 23: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott BD, North I, Flanagan J (1997). Confrontation visual field tests. Ophthal Physiol Opt 17 (suppl): 17–24. [DOI] [PubMed] [Google Scholar]
- Elshout JA, van Asten F, Hoyng CB et al. (2016). Visual rehabilitation in chronic cerebral blindness: a randomized controlled crossover study. Front Neurol 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg HK, Mathisen TS, Ormstad H et al. (2020). “invisible” visual impairments. A qualitative study of stroke survivors’ experience of vision symptoms, health services and impact of visual impairments. BMC Health Serv Res 20: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich R, Wessinger CM, Gazzaniga MS (1992). Residual vision in a scotoma: implications for blindsight. Science 258: 1489–1491. [DOI] [PubMed] [Google Scholar]
- Gall C, Lucklum J, Sabel BA et al. (2009). Vision- and health-related quality of life in patients with visual field loss after postchiasmatic lesions. Investigative Ophthalmology and Visual Science 50: 2765–2776. [DOI] [PubMed] [Google Scholar]
- Gall C, Rossini PM, Tatlisumak T et al. (2014). O26: non-invasive alternating current stimulation to improve visual impairment after post-chiasmatic lesions. Clin Neurophysiol 125: S36. [Google Scholar]
- Gilbert CD, Li W, Piech V (2009). Perceptual learning and adult cortical plasticity. J Physiol 587: 2743–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhotra JS,Mitchell P, Healey PR et al.(2002). Homonymous visual field defects and stroke in an older population. J Am Heart Assoc 33: 2417–2420. [DOI] [PubMed] [Google Scholar]
- Giorgi RG, Woods RL, Peli E (2009). Clinical and laboratory evaluation of peripheral prism glasses for hemianopia. Optom Vis Sci 86: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D(2014).Homonymous hemianopia: challenges and solutions. Clinical Ophthalmology 8: 1919–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CS, French JM, Bates D et al. (1989). Recovery of visual fields in acute stroke: homonymous hemianopia associated with adverse prognosis. Age Ageing 18: 419–421. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI, Schupp HT et al. (2003). Affective blindsight: intact fear conditioning to a visual cue in a cortically blind patient. Brain 126: 267–275. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, Hackett ML, Almeida OP et al. (2020). Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. The Lancet Neurology 19: 651–660. [DOI] [PubMed] [Google Scholar]
- Hanna KL, Hepworth LR, Rowe FJ (2017).Screening methods for post-stroke visual impairment: a systematic review. Disabil Rehabil 39: 2531–2543. [DOI] [PubMed] [Google Scholar]
- Hanna K, Mercer D, Rowe F (2020). A qualitative exploration of the sociology of poststroke visual impairments and the associated health inequalities. Brain Behav 10: e01738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM (2006). Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci 26: 2951–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K et al. (2007). Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci 10: 1134–1136. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Bilimoria PM (2012). Re-opening windows: manipulating critical periods for brain development. Cerebrum: the Dana forum on brain science 2012: 11. [PMC free article] [PubMed] [Google Scholar]
- Hepworth LR, Rowe FJ(2018). Programme choice for perimetry in neurological conditions (PoPiN): a systematic review of perimetry options and patterns of visual field loss. BMC Ophthalmol 18: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpich F, Melnick MD, Agosta S et al. (2019). Boosting learning efficacy with noninvasive brain stimulation in intact and brain-damaged humans. J Neurosci 39: 5551–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Pointer JS (1989). Spatial and temporal contrast sensitivity in hemianopia. Brain 112: 871–894. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Giesselmann H, Sachsenheimer W (1999). Visual search and visual target detection in patients with infarctions of the left or right posterior or the right middle brain artery. J Clin Exp Neuropsychol 21: 94–107. [DOI] [PubMed] [Google Scholar]
- Horton JC (2005a). Disappointing results from Nova Vision’s visual restoration therapy. Br J Ophthalmol 89: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC (2005b). Vision restoration therapy: confounded by eye movements. Br J Ophthalmol 89: 792–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston KE, Bowers AR, Peli E et al. (2018). Peripheral prisms improve obstacle detection during simulated walking for patients with left Hemispatial neglect and hemianopia. Optom Vis Sci 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxlin KR, Martin T, Kelly K et al.(2009). Perceptual relearning of complex visual motion after V1 damage in humans. J Neurosci 29: 3981–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorizzo DB, Riley ME, Hayhoe M et al. (2011). Differential impact of partial cortical blindness on gaze strategies when sitting and walking—an immersive virtual reality study. Vision Res 51: 1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiai S, Furukawa T, Tsukagoshi H (1987). Eye-fixation patterns in homonymous hemianopia and unilateral spatial neglect. Neuropsychologia 25: 675–679. [DOI] [PubMed] [Google Scholar]
- Jindahra P, Petrie A, Plant GT (2009). Retrograde transsynaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 132: 628–634. [DOI] [PubMed] [Google Scholar]
- Jindahra P, Petrie A, Plant GT (2012). The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain 135: 534–541. [DOI] [PubMed] [Google Scholar]
- Jobke S, Kasten E, Sabel BA (2009). Vision restoration through Extrastriate stimulation in patients with visual field defects: a double-blind and randomized experimental study. Neurorehabil Neural Repair 23: 246–255. [DOI] [PubMed] [Google Scholar]
- Jones SA, Shinton RA (2006). Improving outcome in stroke patients with visual problems. Age Ageing 35: 560–565. [DOI] [PubMed] [Google Scholar]
- Jongbloed L (1986). Prediction of function after stroke: a critical review. Stroke 17: 765–776. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM et al. (2012). Principles of neural science, Fifth Edition, McGraw-Hill Education. [Google Scholar]
- Kasten E, Wust S, Behrens-Baumann W et al. (1998). Computer-based training for the treatment of partial blindness. Nat Med 4: 1083–1087. [DOI] [PubMed] [Google Scholar]
- Kasten E, Bunzenthal U, Müller-Oehring EM et al. (2007). Vision restoration therapy does not benefit from costimulation: a pilot study. J Clin Exp Neuropsychol 29: 569–584. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G (1999). Restorative and compensatory therapy approaches in cerebral blindness - a review. Restor Neurol Neurosci 15: 255–271. [PubMed] [Google Scholar]
- Kerkhoff G (2000). Neurovisual rehabiliation: recent developments and future directions. J Neurol Neurosurg Psychiatry 68: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff G, Münsinger U, Haaf E et al. (1992). Rehabilitation of homonymous scotomata in patients with postgeniculate damage of the visual system: saccadic compensation training. Restor Neurol Neurosci 4: 245–254. [DOI] [PubMed] [Google Scholar]
- Kerr NM, Chew SSL, Eady EK et al. (2010). Diagnostic accuracy of confrontation visual field tests. Neurology 74: 1184–1190. [DOI] [PubMed] [Google Scholar]
- Kischka U (2019). Stroke: the doctor as patient. The Lancet 394: 1984–1985. [DOI] [PubMed] [Google Scholar]
- Kleiser R, Wittsack J, Niedeggen M et al. (2001). Is V1 necessary for conscious vision in areas of relative cortical blindness? Neuroimage 13: 654–661. [DOI] [PubMed] [Google Scholar]
- Komitova M, Zhao LR, Gidö G et al. (2005). Postischemic exercise attenuates whereas enriched environment has certain enhancing effects on lesion-induced subventricular zone activation in the adult rat. Eur J Neurosci 21:2397–2405. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, James DC, Schallert T (1996). Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci 16: 4776–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW (2006). Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol 19: 84–90. [DOI] [PubMed] [Google Scholar]
- Lane AR, Smith DT, Ellison A et al. (2010). Visual exploration training is no better than attention training for treating hemianopia. Brain 133: 1717–1728. [DOI] [PubMed] [Google Scholar]
- Larcombe SJ, Kulyomina Y, Antonova N et al. (2018). Visual training in hemianopia alters neural activity in the absence of behavioural improvement: a pilotstudy. Ophthalmic and Physiological Optics 38: 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Pendy JT, Ding JN et al. (2017). Exercise rehabilitation immediately following ischemic stroke exacerbates inflammatory injury. Neurol Res 39: 530–537. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Hua T, Huang C-B et al. (2011). Visual perceptual learning. Neurobiol Learn Mem 95: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z-L, Lin Z, Dosher BA (2016). Translating perceptual learning from the laboratory to applications. Trends Cogn Sci 20: 561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström E, Isaksson E, Näsman P et al. (2020). Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. The Lancet Neurology 19: 661–669. [DOI] [PubMed] [Google Scholar]
- Mannan SK, Pambakian ALM, Kennard C (2010). Compensatory strategies following visual search training in patients with homonymous hemianopia: an eye movement study. J Neurol 257: 1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Riley ME, Kelly KN et al. (2007). Visually-guided behavior of homonymous hemianopes in a naturalistic task. Vision Res 47: 3434–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A et al. (2008). The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 319: 1490–1492. [DOI] [PubMed] [Google Scholar]
- Melnick MD, Tadin D, Huxlin KR (2016). Relearning to see in cortical blindness. Neuroscientist 22: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing B, Ganshirt H (1987). Follow-up of visual field defects with vascular damage of the geniculostriate visual pathway. J Neuroophthalmol 7: 231–242. [Google Scholar]
- Mihailović LT, Čupić D, Dekleva N (1971). Changes in the numbers of neurons and glial cells in the lateral geniculate nucleus of the monkey during retrograde cell degeneration. J Comp Neurol 142: 223–229. [DOI] [PubMed] [Google Scholar]
- Millington RS, James-Galton M, Maia Da Silva MN et al. (2017). Lateralized occipital degeneration in posterior cortical atrophy predicts visual field deficits. NeuroImage: Clinical 14: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE, Macneill K, Crowder NA et al. (2016). Recovery of visual functions in amblyopic animals following brief exposure to total darkness. J Physiol 594: 149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montey KL, Quinlan EM (2011). Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nat Commun 2: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montolio FGJ, Wesselink C, Gordijn M et al. (2012). Factors that influence standard automated perimetry test results in glaucoma: test reliability, technician experience, time of day, and season. Investigative Ophthalmology and Visual Science 53: 7010–7017. [DOI] [PubMed] [Google Scholar]
- Morland AB, Ogilvie JA, Ruddock KH et al. (1996). Orientation discrimination is impaired in the absence of the striate cortical contribution to human vision. Proceedings of the Royal Society B: Biological Sciences 263: 633–640. [DOI] [PubMed] [Google Scholar]
- Morland AB, Lê S, Carroll E et al. (2004). The role of spared Calcarine cortex and lateral occipital cortex in the responses of human Hemianopes to visual motion. J Cogn Neurosci 16:204–218. [DOI] [PubMed] [Google Scholar]
- Nelles G, Esser J, Eckstein A et al. (2001). Compensatory visual field training for patients with hemianopia after stroke. Neurosci Lett 306: 189–192. [DOI] [PubMed] [Google Scholar]
- Ng KL, Gibson EM, Hubbard R et al. (2015). Fluoxetine maintains a state of heightened responsiveness to motor training early after stroke in a mouse model. Stroke 46: 2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer JE, Paradiso MA, Paradiso MA (2017). Contrast sensitivity, V1 neural activity, and natural vision. J Neurophysiol 117: 492–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N et al.(2003). Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15: 619–626. [DOI] [PubMed] [Google Scholar]
- Olma MC, Dargie RA, Behrens JR et al. (2013). Long-term effects of serial anodal tDCS on motion perception in subjects with occipital stroke measured in the unaffected visual hemifield. Front Hum Neurosci 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill EC, Connell PP, O’Connor JC et al. (2011). Prism therapy and visual rehabilitation in homonymous visual field loss. Optom Vis Sci 88: 263–268. [DOI] [PubMed] [Google Scholar]
- Ong YH, Jacquin-Courtois S, Gorgoraptis N et al. (2015). Eye-search: a web-based therapy that improves visual search in hemianopia. Ann Clin Transl Neurol 2: 74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pambakian ALM, Wooding DS, Patel N et al. (2000). Scanning the visual world: a study of patients with homonymous hemianopia. J Neurol Neurosurg Psychiatry 69: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pambakian ALM, Mannan SK, Hodgson TL et al. (2004). Saccadic visual search training: a treatment for patients with homonymous hemianopia. J Neurol Neurosurg Psychiatry 75: 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit RJ, Gales K, Griffiths PG (2001). Effectiveness of testing visual fields by confrontation. Lancet 358: 1339–1340. [DOI] [PubMed] [Google Scholar]
- Papageorgiou E, Hardiess G, Schaeffel F et al. (2007). Assessment of vision-related quality of life in patients with homonymous visual field defects. Graefes Arch Clin Exp Ophthalmol 245: 1749–1758. [DOI] [PubMed] [Google Scholar]
- Papanikolaou A, Keliris GA, Papageorgiou TD et al. (2014). Population receptive field analysis of the primary visual cortex complements perimetry in patients with homonymous visual field defects. Proc Natl Acad Sci 111: E1656–E1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Ramsey LE, Metcalf NV et al. (2016). Early diffusion evidence of retrograde transsynaptic degeneration in the human visual system. Neurology 87: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar AS, Gepshtein S, Savel’ev S et al. (2019). Mechanisms of spatiotemporal selectivity in cortical area MT. Neuron 101: e512: 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelak VS, Dubin M, Whitney E (2007). Homonymous hemianopia: a critical analysis of optical devices, compensatory training, and NovaVision. Curr Treat Options Neurol 9: 41–47. [DOI] [PubMed] [Google Scholar]
- Peli E (2000). Field expansion for homonymous hemianopia by optically induced peripheral exotropia. Optom Vis Sci 77: 453–464. [DOI] [PubMed] [Google Scholar]
- Pirulli C, Fertonani A, Miniussi C (2013). The role of timing in the induction of neuromodulation in perceptual learning by transcranial electric stimulation. Brain Stimul 6: 683–689. [DOI] [PubMed] [Google Scholar]
- Plow EB, Obretenova SN, Halko MA et al. (2011). Combining visual rehabilitative training and noninvasive brain stimulation to enhance visual function in patients with hemianopia: a comparative case study. PM and R 3: 825–835. [DOI] [PubMed] [Google Scholar]
- Plow EB, Obretenova SN, Fregni F et al. (2012). Comparison of visual field training for hemianopia with active versus sham transcranial direct cortical stimulation. Neurorehabil Neural Repair 26: 616–626. [DOI] [PubMed] [Google Scholar]
- Poggel DA, Kasten E, Sabel BA (2004). Attentional cueing improves vision restoration therapy in patients with visual field defects. Neurology 63: 2069. [DOI] [PubMed] [Google Scholar]
- Poggel DA, Mueller I, Kasten E et al. (2010). Subjective and objective outcome measures of computer-based vision restoration training. NeuroRehabilitation 27: 173–187. [DOI] [PubMed] [Google Scholar]
- Pollock A, Hazelton C,Rowe FJ et al. (2019). Interventionsfor visual field defects in people with stroke. Cochrane Database Syst Rev 5: CD008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello G, Falsini B (1999). Retinal ganglion cell dysfunction in humans following post-geniculate lesions: specific spatio-temporal losses revealed by pattern ERG. Vision Res 39: 1739–1748. [DOI] [PubMed] [Google Scholar]
- Radoeva PD, Prasad S, Brainard DH et al. (2008). Neural activity within area V1 reflects unconscious visual performance in a case of blindsight. J Cogn Neurosci 20:1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanala AP, Shariati MA, Liao YJ (2019). Long distance retrograde degeneration of the retino-geniculo-cortical pathway in homonymous hemianopia. bioRxiv. 10.1101/750208. [DOI] [Google Scholar]
- Raninen A, Vanni S, Hyvarinen L et al. (2007). Temporal sensitivity in a hemianopic visual field can be improved by long-term training using flicker stimulation. J Neurol Neurosurg Psychiatry 78: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Schreiber A, Schiefer U et al. (2005). Does visual restitution training change absolute homonymous visual field defects? A fundus controlled study. Br J Ophthalmol 89: 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch G (1917). On the relative perceptions of movement and a stationary object in certain visual disturbances due to occipital injuries. Proc R Soc Med 10: 13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risedal A, Zeng J, Johansson BB (1999). Early training may exacerbate brain damage after focal brain ischemia in the rat. J Cereb Blood Flow Metab 19: 997–1003. [DOI] [PubMed] [Google Scholar]
- Robson JG (1993). In: Shapley R, Lam DM-K (Eds.), Contrast sensitivity: one hundred years of clinical measurement. MIT Press, Boston, MAA. [Google Scholar]
- Rossi PW, Kheyfets S, Reding MJ (1990). Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect. Neurology 40: 1597–1599. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Calautti C, Pauri F et al. (2003). Post-stroke plastic reorganisation in the adult brain. The Lancet: Neurology 2: 493–502. [DOI] [PubMed] [Google Scholar]
- Roth T, Sokolov AN, Messias A et al. (2009). Comparing explorative saccade and flicker training in hemianopia: a randomized controlled study. Neurology 72: 324–331. [DOI] [PubMed] [Google Scholar]
- Rowe FJ, Wright D, Brand D et al. (2013). A prospective profile of visual field loss following stroke: prevalence, type, rehabilitation, and outcome. Biomed Res Int 2013: 719096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe FJ, Conroy EJ, Bedson E et al. (2017). A pilot randomized controlled trial comparing effectiveness of prism glasses, visual search training and standard care in hemianopia. Acta Neurol Scand 136: 310–321. [DOI] [PubMed] [Google Scholar]
- Rowe FJ, Hepworth LR, Conroy EJ et al. (2019a). Visual function questionnaire as an outcome measure for homonymous hemianopia: subscales and supplementary questions, analysis from the VISION trial. Eye (Lond) 33: 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe FJ, Hepworth LR, Howard C et al. (2019b). High incidence and prevalence of visual problems after acute stroke: an epidemiology study with implications for service delivery. PLoS One 14: e0213035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel BA, Hamid AIA, Borrmann C et al. (2020). Transorbital alternating current stimulation modifies BOLD activity in healthy subjects and in a stroke patient with hemianopia: a 7 tesla fMRI feasibility study. Int J Psychophysiol 154: 80–92. [DOI] [PubMed] [Google Scholar]
- Sagi D (2011). Perceptual learning in vision research. 51: 1552–1566. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod MJ et al.(2006).Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. Proc Natl Acad Sci 103: 14971–14976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod MJ (2008). Temporal properties of spatial channel of processing in hemianopia. Neuropsychologia 46: 879–885. [DOI] [PubMed] [Google Scholar]
- Sahraie A, MacLeod MJ, Trevethan CT et al.(2010).Improved detection following neuro-eye therapy in patients with post-geniculate brain damage. Exp Brain Res 206: 25–34. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod MJ et al. (2013). The continuum of detection and awareness of visual stimuli within the blindfield: from blindsight to the sighted-sight. Invest Ophthalmol Vis Sci 54: 3579–3585. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Smania N, Zihl J (2016). Use of Neuro Eye Coach to improve eye movement efficacy in patients with homonymous visual field loss. Biomed Res Int 2016: 5186461. 10.1155/2016/5186461. Epub 2016 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saionz EL (2020). Time is Vision: Properties of Vision Early after Occipital Stroke and Capacity for Recovery. [Google Scholar]
- Saionz EL, Melnick MD, Tadin D et al. (2019). Use it before you lose it: greater efficacy of visual training for recovering contrast sensitivity in subacute cortical blindness. J Vis 19: 33. [Google Scholar]
- Saionz EL, Tadin D, Melnick MD et al. (2020). Functional preservation and enhanced capacity for visual restoration in subacute occipital stroke. Brain 143: 1857–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed AM, Kashem R, Abdel-Mottaleb M et al. (2020). Toward improving the mobility of patients with peripheral visual field defects with novel digital spectacles. Am J Ophthalmol 210: 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CL, Prentiss EK, Busza A et al. (2019). Survival of retinal ganglion cells after damage to the occipital lobe in humans is activity dependent. Proceedings of the Royal Society B: Biological Sciences 286: 1–9. 10.1098/rsb.2018.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuett S, Heywood CA, Kentridge RW et al. (2012). Rehabilitation of reading and visual exploration in visual field disorders: transfer or specificity? Brain 135: 912–921. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Donnan GA (2015). Recovery potential after acute stroke. Front Neurol 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzyna GA, Wise RJS, McDonald SA et al. (2007). Optokinetic therapy improves text reading in patients with hemianopic alexia: a controlled trial. Neurology 68: 1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolidoro M, Sale A, Berardi N et al. (2009). Plasticity in the adult brain: lessons from the visual system. Exp Brain Res 192: 335–341. [DOI] [PubMed] [Google Scholar]
- Stoerig P (2006). Blindsight, conscious vision, and the role of primary visual cortex. Prog Brain Res 155: 217–234. [DOI] [PubMed] [Google Scholar]
- Sundseth A, Thommessen B, Rønning OM (2012). Outcome after mobilization within 24 hours of acute stroke: a randomized controlled trial. Stroke 43: 2389–2394. [DOI] [PubMed] [Google Scholar]
- Szlyk JP, Fau SW, Stelmack J et al. (2005). Use of prisms for navigation and driving in hemianopic patients. Ophthal Physiol Opt 25: 128–135. [DOI] [PubMed] [Google Scholar]
- Tamietto M, de Gelder B (2010). Neural bases of the nonconscious perception of emotional signals. Nat Rev Neurosci 11: 697–709. [DOI] [PubMed] [Google Scholar]
- Tamietto M, Morrone MC (2016). Visual plasticity: blindsight bridges anatomy and function in the visual system. Curr Biol 26: R70–R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M, Cauda F, Corazzini LL et al. (2010). Collicular vision guides nonconscious behavior. J Cogn Neurosci 22: 888–902. [DOI] [PubMed] [Google Scholar]
- Terney D, Chaieb L, Moliadze V et al. (2008). Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci 28: 14147 LP-14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiel K, Kolmel HW (1991). Patterns of recovery from homonymous hemianopia subsequent to infarction in the distribution of the posterior cerebral artery. J Neuroophthalmol 11: 33–39. [Google Scholar]
- Tootell RBH, Reppas JB, Kwong KK et al. (1995). Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15: 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townend BS, Sturm JW, Petsoglou C et al. (2007). Perimetric homonymous visual field loss post-stroke. J Clin Neurosci 14: 754–756. [DOI] [PubMed] [Google Scholar]
- Trauzettel-Klosinski S (2010). Rehabilitation for visual disorders. J Neuroophthalmol 30: 73–84. [DOI] [PubMed] [Google Scholar]
- Trevethan CT, Urquhart J, Ward R et al. (2012). Evidence for perceptual learning with repeated stimulation after partial and total cortical blindness. Advances in Cognitive Psychology 8: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco S, Albamonte E, Ricci D et al. (2015). Bernhard Sabel and ‘residual vision activation theory’: a history spanning three decades. Multisens Res 28: 309–330. [DOI] [PubMed] [Google Scholar]
- Vaina L, Soloviev S, Calabro FJ et al. (2014). Reorganization of retinotopic maps after occipital lobe infarction. J Cogn Neurosci 26: 1266–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Groen O, Wenderoth N (2016). Transcranial random noise stimulation of visual cortex: stochastic resonance enhances central mechanisms of perception. J Neurosci 36: 5289–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P (2013). Sensitive and critical periods in visual sensory deprivation. Front Psychol 4: 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang V, Saionz E, Cavanaugh M et al. (2017). Natural progression of perimetric visual field defects after V1 stroke. J Vis 17: 51–52. [Google Scholar]
- Ward NS, Kitago T (2016). Getting the right prescription for rehabilitation after stroke. Neurology 86: 2120. [DOI] [PubMed] [Google Scholar]
- Warren M (2009). Pilot study on activities of daily living limitations in adults with Hemianopsia. Am J Occup Ther 63: 626–633. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Diller L, Gordon WA et al. (1977). Visual scanning training effect on reading-related tasks in acquired right brain damage. Arch Phys Med Rehabil 58: 479–486. [PubMed] [Google Scholar]
- Weiskrantz L (2009). Is blindsight just degraded normal vision? Exp Brain Res 192: 413–416. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK, Sanders MD et al. (1974). Visual capacity in the hemianopic field following a restricted occipital ablation the relationship between attention and visual experience. Brain 97: 709–728. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Harlow A, Barbur JL (1991). Factors affecting visual sensitivity in a hemianopic subject. Brain 114: 2269–2282. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Barbur JL, Sahraie A (1995). Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (V1). Proc Natl Acad Sci U S A 92: 6122–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessinger CM, Fendrich R, Gazzaniga MS (1997). Islands of residual vision in hemianopic patients. J Cogn Neurosci 9: 203–221. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Stein J, Arena R et al. (2016).Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke 47 (6): e98–e169. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT (1972). Changes in the dorsal lateral geniculate nucleus of the squirrel monkey after unilateral ablation of the visual cortex. J Comp Neurol 146: 519–547. [DOI] [PubMed] [Google Scholar]
- Yu H-H, Atapour N, Chaplin TA et al. (2018). Robust visual responses and normal retinotopy in primate lateral geniculate nucleus following long-term lesions of striate cortex. J Neurosci 38: 3955–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangemeister WH, Oechsner U, Freksa C (1995). Short-term adaptation of eye movements in patients with visual Hemifield defects indicates high level control of human Scanpath. Optom Vis Sci 72: 467–477. [PubMed] [Google Scholar]
- Zeki S, Ffytche DH (1998). The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain 121: 25–45. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kedar S, Lynn MJ et al. (2006). Natural history of homonymous hemianopia. Neurology 66: 901–905. [DOI] [PubMed] [Google Scholar]
- Zihl J (1980). “blindsight”: improvement of visually guided eye movements by systematic practice in patients with cerebral blindness. Neuropsychologia 18: 71–78. [DOI] [PubMed] [Google Scholar]
- Zihl J (1995). Visual scanning behavior in patients with homonymous hemianopia. Neuropsychologia 33: 287–303. [DOI] [PubMed] [Google Scholar]