Abstract
Background:
Previous studies have shown that transcatheter aortic valve implantation is the best alternative therapy to surgical aortic valve replacement in high-risk surgical patients with aortic stenosis. However, it is not clear whether transcatheter aortic valve implantation can be utilized in low-risk surgical patients with aortic stenosis. This study aimed to evaluate the safety and efficacy of transcatheter aortic valve implantation in low-risk patients.
Methods:
From the outset of our initiative until April 2022, PubMed, EMBASE, and the Cochrane database were thoroughly searched, yielding the selection of 3 randomized controlled trials including 2644 patients with aortic stenosis, to assess outcome measures at distinct follow-up time.
Results:
The mean Society of Thoracic Surgeons Predicted Risk of Mortality score of patients was 2.2. At the 30-day and 1-year follow-up, transcatheter aortic valve implantation was associated with a lower incidence of all-cause mortality, cardiovascular mortality, acute kidney injury (stage 2 or 3), life-threatening or significant bleeding, and new atrial fibrillation but an increased risk of permanent pacemaker implantation. At the 2-year follow-up, transcatheter aortic valve implantation only had an advantage in new atrial fibrillation (relative risk, 0.27; 95% CI, 0.14-0.51; P < .0001), with no significant difference in all-cause mortality or cardiovascular mortality.
Conclusions:
For low-risk surgical patients with aortic stenosis, compared to surgical aortic valve replacement, transcatheter aortic valve implantation was associated with lower all-cause mortality at 30-day follow-up and lower cardiovascular mortality at 1-year follow-up. Except for the advantages in new atrial fibrillation, transcatheter aortic valve implantation had no significant impact on mortality at 2-year follow-up.
Keywords: TAVI, SAVR, aortic stenosis, meta-analysis, low risk
Highlights
In low-risk surgical patients with aortic stenosis, compared to surgical aortic valve replacement (SAVR), transcatheter aortic valve implantation (TAVI) is associated with lower all‐cause mortality at 30-day follow-up and lower cardiovascular mortality at 1-year follow-up.
Except for advantages in new atrial fibrillation, TAVI had no significant differences in mortality at 2-year follow-up, compared to SAVR.
In lieu of 2-year follow-up results and potential valve degradation risks, the decision to use TAVI in patients with a longer life expectancy is yet to be recommended.
Introduction
Aortic stenosis (AS) is a common heart valve disorder in the elderly with increasing incidence in the aging population.1 Currently, there is no effective therapy for this condition as valve replacement is the standard of care. Historically, surgical aortic valve replacement (SAVR) is regarded as the gold standard for patients with severe AS.2 As a novel modality, transcatheter aortic valve implantation (TAVI) has garnered significant support for its use over the years since its first application in 2002,3 and it is currently the best alternative to SAVR in high-risk surgical patients with AS.4
The PARTNER II trial shows that the efficacy of TAVI is non-inferior to that of SAVR in intermediate-risk patients with AS,5 prompting the American College of Cardiology to recommend TAVI for intermediate-risk patients (class IIa).6 However, complications due to TAVI, such as paravalvular leakage and inadequate durability, are still a cause for concern.7 Industry experts are debating whether TAVI can be widely used in low-risk surgical patients with AS. Several randomized controlled trials (RCTs) have been conducted on this matter,8,9 but the results from these experiments and meta-analyses are not consistent. The latest 2020 guideline still lists only SAVR as a class I treatment for low-risk surgical patients without recommending TAVI for this patient subset.10 The 2-year follow-up results published in the PARTNER III and EVOLUT study did provide some evidence to suggest that further investigation of the efficacy of TAVI in low-risk surgical patients with AS—versus that of SAVR—would be prudent.11,12 As a result, we conducted a new meta-analysis to compare TAVI with SAVR to clearly delineate their performance based on different time frames and patient risk stratification.
Methods
Eligibility Criteria
The research follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines-P guidelines and is based on those guidelines.13,14 The inclusion criteria were as follows: (1) populations of low-risk surgical patients (Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) <4%); (2) comparison of TAVI; (3) SAVR as a control; (4) primary outcome—measured over a 2-year period—as all‐cause mortality and secondary outcomes as cardiovascular mortality, stroke, transient ischemic attack (TIA), myocardial infarction (MI), acute kidney injury (stage 2 or 3), life-threatening or significant bleeding, permanent pacemaker implantation (PPI), and new atrial fibrillation (NAF)15; and (5) study designs as RCTs.
Literature Search
From the outset to April 21, 2022, we conducted a comprehensive, systematic search of PubMed, EMBASE, and the Cochrane database. ClinicalTrials.gov trial registries were also reviewed to determine if the available results were reported from ongoing or completed studies. Our supplement details the study strategy.
Data Analyses
Two authors separately collected the required, relevant data—any discrepancies between them were resolved by group consultation. The 2 authors used the Cochrane collaborative risk of bias tool to assess the risk of bias independently in 5 aspects and used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) to estimate the quality of evidence for each outcome.16 The results of each RCT were converted to dichotomous data, analyzed using the Mantel-Haenszel method, and presented as relative risk (RR). The summary RR and 95% CI of the survey results were calculated using a random-effect model.17 P ≤ .5 was considered statistically significant, and heterogeneity was assessed through I‐squared (I2) and Q statistics; I2 > 50% was considered substantial.18,19 Because fewer than 10 studies were included, we performed neither Egger’s nor Begg’s tests to evaluate the publication bias of studies.20
Results
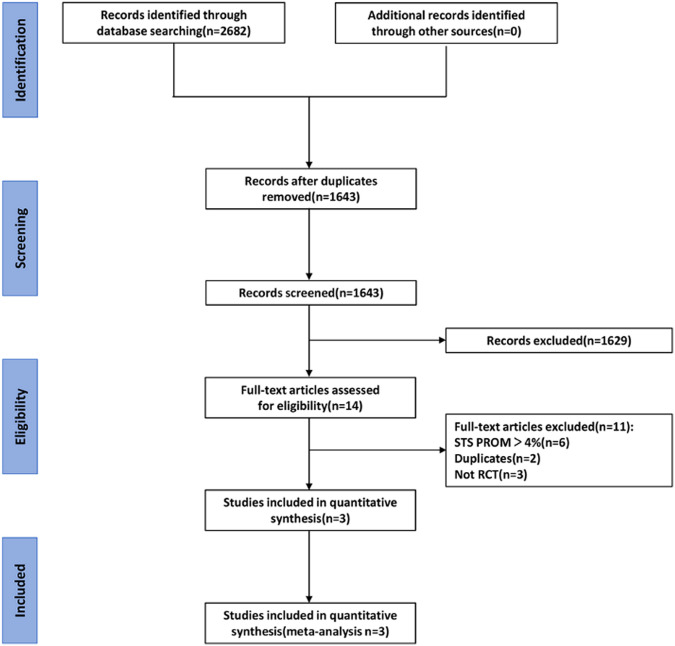
Figure 1 details the study selection process, illustrating a total of 2682 retrieved articles with 1039 duplicates, which were deleted by the Endnote X9 software. After reviewing the titles and abstracts, 1629 repetitive literature reviews, case reports, meta-analyses, and unrelated articles were excluded. Eleven items were further excluded based on the inclusion criteria, resulting in the final 3 articles. Table 1 comprises the details of the included studies; 2633 patients with AS across the 3 cohorts were enrolled (EVOLUT,12 NOTION,15 and PARTNER III11). In the assessment of deviation risk, due to specific study designs, it is impossible to blind operators or patients (Supplement Figure 1A and B.). The summary of findings and strength of evidence (GRADE) are shown in the supplement (Supplement Tables 1A-D.); the quality of evidence for the most results was evaluated to be high.
Figure 1.
Flowchart for screening and study selection process.
Table 1.
Characteristics of Studies and Patients
| Study | NOTION | PARTNER III | EVOLUT | |
|---|---|---|---|---|
| Number of centers | 3 | 71 | 86 | |
| Recruitment period | 2011-2013 | 2012-2016 | 2016-2018 | |
| Valve type | CoreValve, Evolut R, or Evolut PRO | Sapien 3 | CoreValve | |
| Sample size | TAVI | 145 | 496 | 725 |
| SAVR | 135 | 454 | 678 | |
| Male, no. (%) | TAVI | 78 (53.8) | 335 (67.5) | 464 (64.0) |
| SAVR | 71 (52.6) | 323 (71.1) | 449 (66.2) | |
| Mean year | TAVI | 79.2 ± 4.9 | 73.3 ± 5.8 | 74.1 ± 5.8 |
| SAVR | 79 ± 4.7 | 73.6 ± 6.1 | 73.6 ± 5.9 | |
| Mean STS-PROM score | TAVI | 2.9 ± 1.6 | 1.9 ± 0.7 | 1.9 ± 0.7 |
| SAVR | 3.1 ± 1.7 | 1.9 ± 0.6 | 1.9 ± 0.7 | |
| Prior cerebrovascular accident, n (%) | TAVI | 24 (16.6) | 17 (3.4) | 74 (10.2) |
| SAVR | 22 (16.3) | 23 (5.1) | 80 (11.8) | |
| Prior myocardial infarction, n (%) | TAVI | 8 (5.5) | 28 (5.7) | 48 (6.6) |
| SAVR | 6 (4.4) | 26 (5.8) | 33 (4.9) | |
| Peripheral vascular disease, n (%) | TAVI | 6 (4.1) | 34 (6.9) | 54 (7.5) |
| SAVR | 9 (6.7) | 33 (7.3) | 56 (8.3) | |
| Chronic lung disease, n. (%) | TAVI | 17 (11.7) | 25 (5.1) | 104 (15.0) |
| SAVR | 16 (11.9) | 28 (6.2) | 117 (18.0) | |
| Diabetes mellitus, n. (%) | TAVI | 26 (17.9) | 155 (31.2) | 228 (31.4) |
| SAVR | 28 (20.7) | 137 (30.2) | 207 (30.5) | |
| Creatinine level >2 mg/dL, no. (%) | TAVI | 2 (1.4) | 1 (0.2) | 3 (0.4) |
| SAVR | 1 (0.7) | 1 (0.2) | 1 (0.1) |
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement.
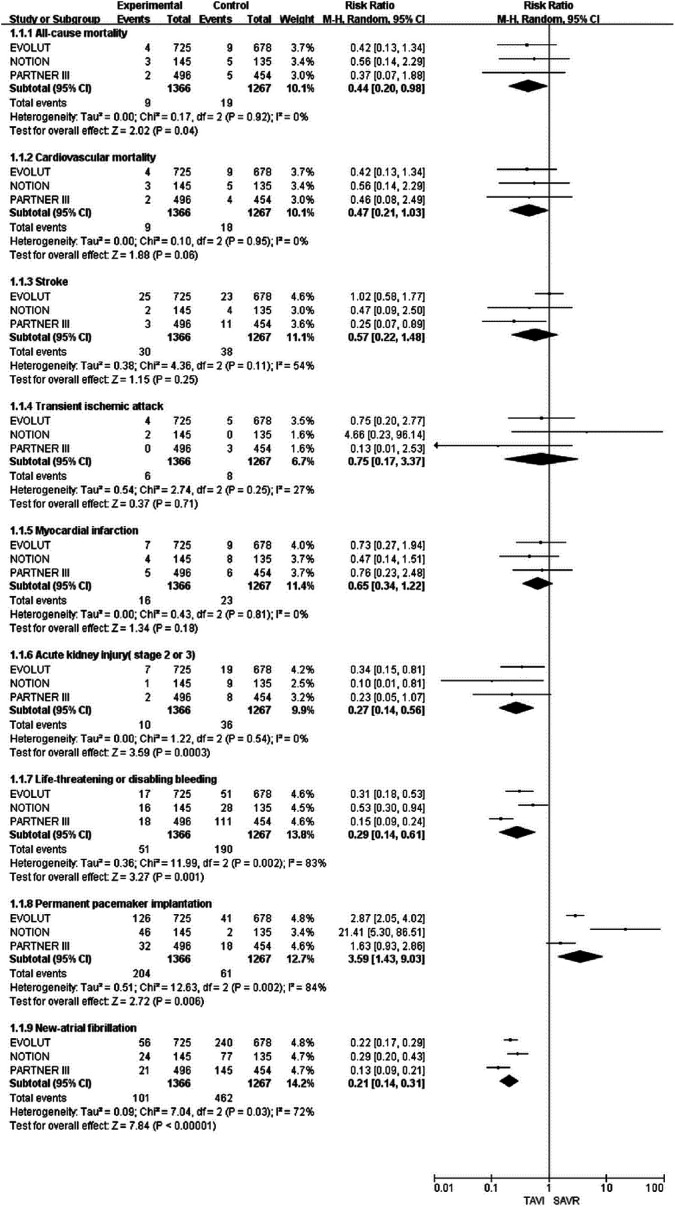
The results of the 30-day, 1-year, and 2-year follow-ups are shown in Figures 2, 3, and 4, respectively. There were several patients enrolled at sites in Japan later in the EVOLUT trial who are included in this analysis at the 2-year baseline; thus, the population in the second year of the EVOLUT trial is different from that before. At the 30-day follow-up of the low-risk surgical patients with AS, TAVI was associated with a lower incidence of all-cause mortality (RR: 0.44; 95% CI: 0.20-0.98; P = .04), acute kidney injury (stage 2 or 3) (RR: 0.27; 95% CI: 0.14-0.56; P = .0003), life-threatening or significant bleeding (RR: 0.29; 95% CI: 0.14-0.61; P = .001), and NAF (RR: 0.21; 95% CI: 0.14-0.31; P < .00001) but showed an increased risk of PPI (RR: 3.59; 95% CI, 1.43-9.03; P = .006).
Figure 2.
Forest plot for incidence of all-cause mortality, cardiovascular mortality, stroke, transient ischemic attack, myocardial infarction, acute kidney injury, life-threatening or disabling bleeding, permanent pacemaker implantation, and new-atrial fibrillation at the 30-day follow-up.
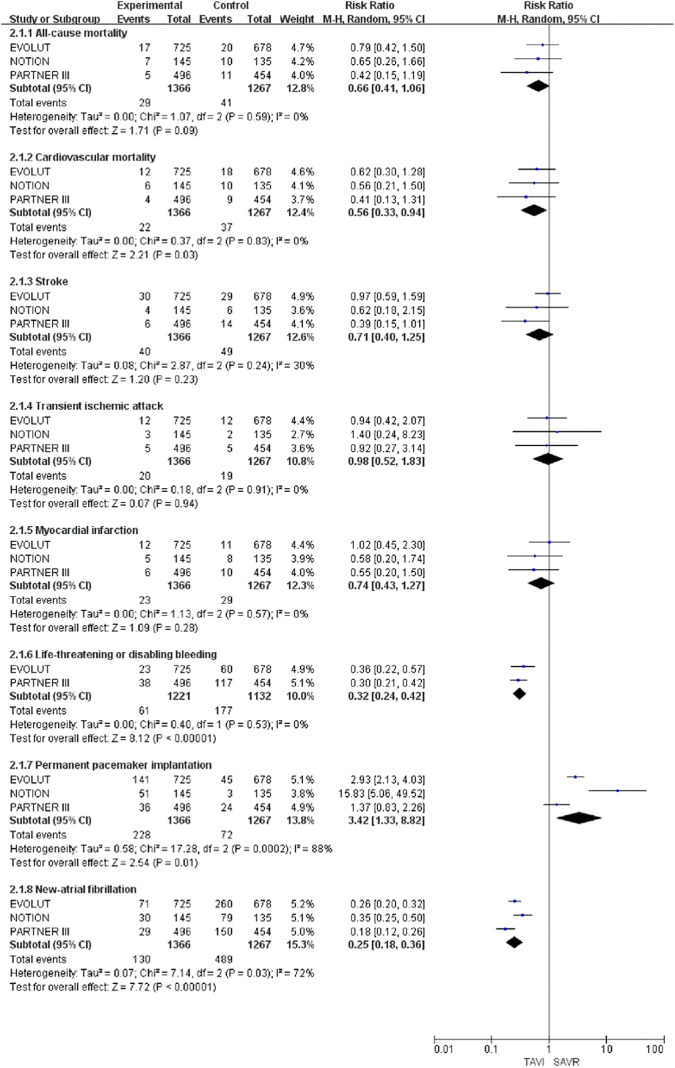
Figure 3.
Forest plot for incidence of all-cause mortality, cardiovascular mortality, stroke, transient ischemic attack, myocardial infarction, life-threatening or disabling bleeding, permanent pacemaker implantation, and new-atrial fibrillation at the 1-year follow-up.
At the 1-year follow-up of the low-risk surgical patients with AS, the cardiovascular mortality (RR: 0.56; 95% CI: 0.33-0.94; P = .03), presence of life-threatening or significant bleeding (RR: 0.32; 95% CI: 0.24-0.42; P < .00001), and the NAF (RR: 0.25; 95% CI, 0.18-0.36; P < .00001) results in the TAVI group were significantly decreased compared to those in the SAVR group. However, the incidence of PPI in the TAVI group was significantly increased when compared to that of the SAVR group (RR: 3.42; 95% CI: 1.33-8.82; P = .01).
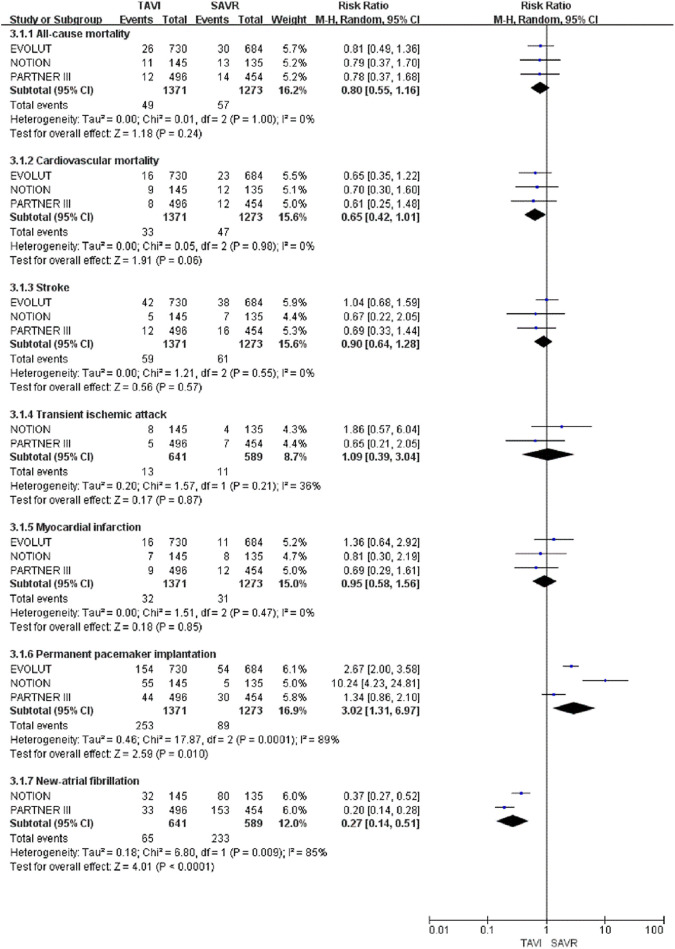
At the 2-year follow-up of low-risk surgical patients with AS, only the NAF results in the TAVI group were significantly decreased (RR: 0.27; 95% CI: 0.14 to 0.51; P < .0001), compared to those in the SAVR group. Transcatheter aortic valve implantation was also associated with a higher incidence of PPI (RR: 3.02; 95% CI: 1.31-6.97; P = .01). The differences in all-cause mortality, cardiovascular mortality, stroke, TIA, and MI between the TAVI and SAVR groups were not statistically significant.
Discussion
Since currently established guidelines do not recommend the use of TAVI in low-risk surgical patients with AS, our study aimed to evaluate the efficacy and effectiveness of TAVI in this patient subset by comparing the clinical outcomes of TAVI and SAVR at 30-day, 1-year, and 2-year follow-up time frames. This study included 3 RCTs, comprising 2644 patients, and used a meta-analysis to compare the aforementioned outcomes. Kolte et al21 reported that TAVI was associated with a lower risk of cardiovascular and all-cause mortality at 1 year. Our 1-year follow-up had similar results; however, their study did not report outcomes at other follow-up time intervals. In reviewing the 2-year results of the newly released PARTNER III and EVOLUT trial, we found that the low-risk patients who underwent TAVI at the 30-day and 1-year follow-up outperformed those who underwent SAVR in cardiovascular mortality, acute kidney injury (stage 2 or 3), NAF, and life-threatening or significant bleeding. However, TAVI resulted in a higher risk of PPI during the same time period. Compared with SAVR at the 2-year follow-up, there was no significant difference in cardiovascular and all-cause mortality for patients who underwent TAVI. Therefore, TAVI can reduce mortality and complications at the 30-day and 1-year follow-up; however, at the 2-year follow-up, most of the results demonstrated no significant difference. Most notably, the 5-year follow-up of the PARTNER II trial noted that patients who underwent TAVI had a higher risk of death or disabling strokes.22,23 Furthermore, Barili et al24 performed time-interval modeling, incorporating 3 RCTs (including the PARTNER II trial), and found that TAVI was associated with better survival in the first few months after implantation but was a risk factor for all-cause mortality after 40 months. Although these trials were conducted with patients at intermediate and high risk, the results still have important significance to our research conclusions. It reminds us that, over time, the risk of mortality and complications after TAVI may increase rapidly, which corresponds to our discovery in the 2-year clinical results.
The PARTNER III trial using the SAPIEN 3 valve has achieved superior results. According to the analysis of Deharo, the design of SAPIEN 3 is easier to fit the landing zone, which reduces the risk of cardiovascular complications after TAVI.25,26 This may also be the reason for the large heterogeneity of TIA and PPI in our findings. Different valves used in various experiments affect the heterogeneity of the analysis. Although the new generation of the valve reduces the incidence of PPI, compared to SAVR, the incidence of PPI after TAVI is still higher. Recent studies have shown that PPI is associated with late all-cause mortality and increased risk of hospitalization due to cardiac failure.27 Therefore, reducing the incidence of PPI after TAVI is an important issue to be considered and an interesting area for valve improvement.
Valve degeneration is another TAVI-associated complication that should be considered. Once it occurs, valve-in-valve implantation is indicated,28,29 and it is a complex operative procedure. Postoperatively, device malposition and ostial coronary obstruction are also common TAVI-associated complications. Only the NOTION trial reports data on valve conditions in low-risk surgical patients with AS undergoing TAVI for more than 5 years30; therefore, there are insufficient data to analyze this problem. Moreover, most of the patients undergoing TAVI in the current RCTs are over 75 years old; therefore, their life expectancy is much less than the expected valve use time, hindering the valve durability study. Randomized controlled trials need to be conducted among relatively younger patients to assess long-term follow-up, providing more effective data for future meta-analyses.
Finally, based on the optimal performance of TAVI at the 30-day and the 1-year clinical follow-up and the continuous replacement of the operative valve, TAVI appears to be a very promising procedure in low-risk surgical patients with AS. The eventual use of TAVI in older patients with a shortened life expectancy is reasonable. However, we should also note the changes at the 2-year TAVI follow-up and the potential clinical complications of PPI and valve degeneration. In lieu of these results, the decision to use TAVI in patients with a longer life expectancy is yet to be recommended.
Study Limitations
First, study omissions occurred due to their non-inclusion in the search database, resulting in eventual publication bias. Second, some inevitable differences in baseline characteristics between studies affect the accuracy of the results. Third, there is significant variability in the literature of the definitions for valve type, surgical risk, and outcomes, leading to possible discrepancies in the results.
Conclusions
In low-risk surgical patients with AS, compared to SAVR, TAVI was associated with lower all-cause mortality at 30-day follow-up and lower cardiovascular mortality at 1-year follow-up. At the 2-year follow-up, with the exception of decreased NAF risk, there was no significant difference in all-cause mortality, cardiovascular mortality, and mi between TAVI and SAVR. However, potential late TAVI-associated complications, such as valvular degeneration and PPI, are important clinical concerns that must be considered when weighing treatment options for AS.
Figure 4.
Forest plot for incidence of all-cause mortality, cardiovascular mortality, stroke, transient ischemic attack, myocardial infarction, permanent pacemaker implantation, and new-atrial fibrillation at the 2-year follow-up.
Supplement Figure 1.
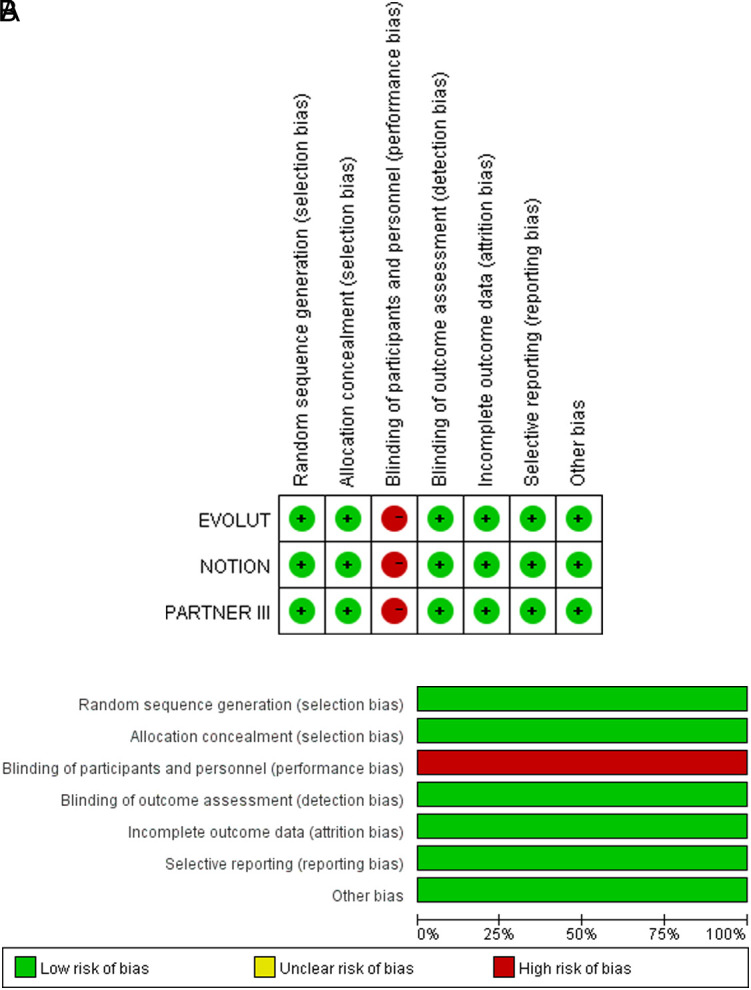
A. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study. B. Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Supplement Table 1A.
Search Strategy
| PubMed | ||
|---|---|---|
| 1 | “Aortic Valve Stenosis”[Mesh] | 48093 |
| 2 | (((((((Aortic Valve Stenoses[Title/Abstract]) OR (Stenoses, Aortic Valve[Title/Abstract])) OR (Stenosis, Aortic Valve[Title/Abstract])) OR (Valve Stenoses, Aortic[Title/Abstract])) OR (Valve Stenosis, Aortic[Title/Abstract])) OR (Aortic Stenosis[Title/Abstract])) OR (Stenoses, Aortic[Title/Abstract])) OR (Stenosis, Aortic[Title/Abstract]) | 20267 |
| 3 | 1 OR 2 | 53638 |
| 4 | “Transcatheter Aortic Valve Replacement”[Mesh] | 9162 |
| 5 | ((((((((((((((((((((((percutaneous aortic valve implantation[Title/Abstract]) OR (percutaneous aortic valve replacement[Title/Abstract])) OR (TAVI[Title/Abstract])) OR (trans-apical aortic valve implantation[Title/Abstract])) OR (trans-apical aortic valve replacement[Title/Abstract])) OR (trans-arterial aortic valve implantation[Title/Abstract])) OR (trans-arterial aortic valve replacement[Title/Abstract])) OR (trans-catheter aortic valve implantation[Title/Abstract])) OR (trans-catheter aortic valve replacement[Title/Abstract])) OR (trans-cutaneous aortic valve implantation[Title/Abstract])) OR (trans-cutaneous aortic valve replacement[Title/Abstract])) OR (trans-femoral aortic valve implantation[Title/Abstract])) OR (trans-femoral aortic valve replacement[Title/Abstract])) OR (transapical aortic valve implantation[Title/Abstract])) OR (transapical aortic valve replacement[Title/Abstract])) OR (transarterial aortic valve implantation[Title/Abstract])) OR (transarterial aortic valve replacement[Title/Abstract])) OR (transcatheter aortic valve replacement[Title/Abstract])) OR (transcutaneous aortic valve implantation[Title/Abstract])) OR (transcutaneous aortic valve replacement[Title/Abstract])) OR (transfemoral aortic valve implantation[Title/Abstract])) OR (transfemoral aortic valve replacement[Title/Abstract])) OR (TAVR[Title/Abstract]). | 12828 |
| 6 | (((((((((aorta valve replacement[Title/Abstract]) OR (aorta valve transplantation[Title/Abstract])) OR (aortic valve transplantation[Title/Abstract])) OR (aortic valve xenotransplantation[Title/Abstract])) OR (heart valve transplantation, aortic valve[Title/Abstract])) OR (transplantation, aortic valve[Title/Abstract])) OR (surgical aortic valve replacement)) OR (surgical aortic valve implantation)) OR (SAVR)) OR (surgical AVR) | 36581 |
| 7 | 4 OR 5 OR 6 | 38222 |
| 8 | randomized controlled trial[Publication Type] OR randomized[Title/Abstract] OR placebo[Title/Abstract] | 945899 |
| 9 | 3 AND 7 AND 8 | 943 |
| Embase | ||
| 1 | “aortic valve stenosis”/exp | 20578 |
| 2 | “aortic valve stenoses”:ab,ti OR “stenoses, aortic valve”:ab,ti OR “stenosis, aortic valve”:ab,ti OR “valve stenoses, aortic”:ab,ti OR “valve stenosis, aortic”:ab,ti OR “aortic stenosis”:ab,ti OR “stenoses, aortic”:ab,ti OR “stenosis, aortic”:ab,ti | 31812 |
| 3 | 1 OR 2 | 44747 |
| 4 | “transcatheter aortic valve implantation”/exp | 27560 |
| 5 | “percutaneous aortic valve implantation”:ab,ti OR “percutaneous aortic valve replacement”:ab,ti OR tavi:ab,ti OR “trans-apical aortic valve implantation”:ab,ti OR “trans-apical aortic valve replacement”:ab,ti OR “trans-arterial aortic valve implantation”:ab,ti OR “trans-arterial aortic valve replacement”:ab,ti OR “trans-catheter aortic valve implantation”:ab,ti OR “trans-catheter aortic valve replacement”:ab,ti OR “trans-cutaneous aortic valve implantation”:ab,ti OR “trans-cutaneous aortic valve replacement”:ab,ti OR “trans-femoral aortic valve implantation”:ab,ti OR “trans-femoral aortic valve replacement”:ab,ti OR “transapical aortic valve implantation”:ab,ti OR “transapical aortic valve replacement”:ab,ti OR “transarterial aortic valve implantation”:ab,ti OR “transarterial aortic valve replacement”:ab,ti OR “transcatheter aortic valve replacement”:ab,ti OR “transcutaneous aortic valve implantation”:ab,ti OR “transcutaneous aortic valve replacement”:ab,ti OR “transfemoral aortic valve implantation”:ab,ti OR “transfemoral aortic valve replacement”:ab,ti | 22178 |
| 6 | “aorta valve replacement”:ab,ti OR “aorta valve transplantation”:ab,ti OR “aortic valve transplantation”:ab,ti OR “aortic valve xenotransplantation”:ab,ti OR “heart valve transplantation, aortic valve”:ab,ti OR “transplantation, aortic valve”:ab,ti OR “surgical aortic valve replacement”:ab,ti OR “surgical aortic valve implantation”:ab,ti OR savr:ab,ti OR “surgical avr”:ab,ti | 5186 |
| 7 | 4 OR 5 OR 6 | 31214 |
| 8 | “randomized controlled trial”:ab,ti OR “randomized”:ab,ti OR “placebo”:ab,ti | 1038314 |
| 9 | 3 AND 7 AND 8 | 822 |
| Cochrane CENTRAL | ||
| 1 | MeSH descriptor: [Aortic Valve Stenosis] explode all trees | 975 |
| 2 | (Aortic Valve Stenoses):ti,ab,kw OR (Stenoses, Aortic Valve):ti,ab,kw OR (Stenosis, Aortic Valve):ti,ab,kw OR (Valve Stenoses, Aortic):ti,ab,kw OR (Valve Stenosis, Aortic):ti,ab,kw OR (Aortic Stenosis):ti,ab,kw OR (Stenoses, Aortic):ti,ab,kw OR (Stenosis, Aortic):ti,ab,kw | 1898 |
| 3 | 1 OR 2 | 2111 |
| 4 | MeSH descriptor: [Transcatheter Aortic Valve Replacement] explode all trees | 203 |
| 5 | (percutaneous aortic valve implantation):ti,ab,kw OR (percutaneous aortic valve replacement):ti,ab,kw OR (TAVI):ti,ab,kw OR (trans-apical aortic valve implantation):ti,ab,kw OR (trans-apical aortic valve replacement):ti,ab,kw OR (trans-arterial aortic valve implantation):ti,ab,kw OR (trans-arterial aortic valve replacement):ti,ab,kw OR (trans-catheter aortic valve implantation):ti,ab,kw OR (trans-catheter aortic valve replacement):ti,ab,kw OR (trans-cutaneous aortic valve implantation):ti,ab,kw OR (trans-cutaneous aortic valve replacement):ti,ab,kw OR (trans-femoral aortic valve implantation):ti,ab,kw OR (trans-femoral aortic valve replacement):ti,ab,kw OR (transapical aortic valve implantation):ti,ab,kw OR (transapical aortic valve replacement):ti,ab,kw OR (transarterial aortic valve implantation):ti,ab,kw OR (transarterial aortic valve replacement):ti,ab,kw OR (transcutaneous aortic valve implantation):ti,ab,kw OR (transcatheter aortic valve replacement):ti,ab,kw OR (transcutaneous aortic valve replacement ):ti,ab,kw OR (transfemoral aortic valve implantation):ti,ab,kw OR (transfemoral aortic valve replacement):ti,ab,kw | 1094 |
| 6 | (aorta valve replacement):ti,ab,kw OR (aorta valve transplantation):ti,ab,kw OR (aortic valve transplantation):ti,ab,kw OR (aortic valve xenotransplantation):ti,ab,kw OR (heart valve transplantation, aortic valve):ti,ab,kw OR (transplantation, aortic valve):ti,ab,kw OR (surgical aortic valve replacement):ti,ab,kw OR (surgical aortic valve implantation):ti,ab,kw OR (SAVR ):ti,ab,kw OR (surgical AVR):ti,ab,kw | 1152 |
| 7 | 4 OR 5 OR 6 | 1706 |
| 8 | 3 AND 7 | 917 |
Supplement Table 1B.
Summary of Findings and Strength of Evidence (GRADE) for 30-Day Results
| TAVI Compared to SAVR for Low-Risk Surgical Patients with Aortic Stenosis | ||||||
|
Patient or population: Low-risk surgical patients with aortic stenosis Settings: Intervention: TAVI1 Comparison: SAVR2 | ||||||
| Outcomes | Illustrative Comparative Risks* (95% CI) | Relative Effect (95% CI) | No. of Participants (Studies) | Quality of the Evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
| SAVR | TAVI | |||||
|
All-cause mortality
Follow-up: 30 days |
Study population | RR 0.44 (0.2-0.98) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 15 per 1000 | 7 per 1000 (3-15) | |||||
| Moderate | ||||||
| 13 per 1000 | 6 per 1000 (3-13) | |||||
|
Cardiovascular mortality
Follow-up: 30 days |
Study population | RR 0.47 (0.21-1.03) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 14 per 1000 | 7 per 1000 (3-15) | |||||
| Moderate | ||||||
| 13 per 1000 | 6 per 1000 (3-13) | |||||
|
Stroke
Follow-up: 30 days |
Study population | RR 0.57 (0.22-1.48) | 2633 (3 studies) | ⊕⊕⊕⊝ Moderate3 | ||
| 30 per 1000 | 17 per 1000 (7-44) | |||||
| Moderate | ||||||
| 30 per 1000 | 17 per 1000 (7-44) | |||||
|
Transient ischemic attack
Follow-up: 30 days |
Study population | RR 0.75 (0.17-3.37) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 6 per 1000 | 5 per 1000 (1-21) | |||||
| Moderate | ||||||
| 7 per 1000 | 5 per 1000 (1-24) | |||||
|
Myocardial infarction
Follow-up: 30 days |
Study population | RR 0.65 (0.34-1.22) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 18 per 1000 | 12 per 1000 (6-22) | |||||
| Moderate | ||||||
| 13 per 1000 | 8 per 1000 (4-16) | |||||
|
Acute kidney injury (stage 2 or 3)
Follow-up: 30 days |
Study population | RR 0.27 (0.14-0.56) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 28 per 1000 | 8 per 1000 (4-16) | |||||
| Moderate | ||||||
| 28 per 1000 | 8 per 1000 (4-16) | |||||
|
Life-threatening or disabling bleeding
Follow-up: 30 days |
Study population | RR 0.29 (0.14-0.61) | 2633 (3 studies) | ⊕⊕⊕⊝ Moderate3 | ||
| 150 per 1000 | 43 per 1000 (21-91) | |||||
| Moderate | ||||||
| 207 per 1000 | 60 per 1000 (29-126) | |||||
|
Permanent pacemaker implantation
Follow-up: 30 days |
Study population | RR 3.59 (1.43-9.03) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 48 per 1000 | 173 per 1000 (69-435) | |||||
| Moderate | ||||||
| 40 per 1000 | 144 per 1000 (57-361) | |||||
|
New-atrial fibrillation
Follow-up: 30 days |
Study population | RR 0.21 (0.14-0.31) | 2633 (3 studies) | ⊕⊕⊕⊕ High3,4 | ||
| 365 per 1000 | 77 per 1000 (51-113) | |||||
| Moderate | ||||||
| 354 per 1000 | 74 per 1000 (50-110) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). RR, risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1Transcatheter aortic valve implantation; 2surgical aortic valve replacement; 3inconsistency;4large effect. | ||||||
Supplement Table 1C.
Summary of Findings and Strength of Evidence (GRADE) for 1-Year Results
| TAVI Compared to SAVR for Low-Risk Surgical Patients with Aortic Stenosis | ||||||
|
Patient or population: low-risk surgical patients with aortic stenosis Settings: Intervention: TAVI1 Comparison: SAVR2 | ||||||
| Outcomes | Illustrative Comparative Risks* (95% CI) | Relative Effect (95% CI) | No of Participants (Studies) | Quality of the Evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
| SAVR | TAVI | |||||
|
All-cause mortality
Follow-up: 1 year |
Study population | RR 0.66 (0.41-1.06) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 32 per 1000 | 21 per 1000 (13-34) | |||||
| Moderate | ||||||
| 30 per 1000 | 21 per 1000 (12-32) | |||||
|
Cardiovascular mortality
Follow-up: 1 year |
Study population | RR 0.56 (0.33-0.94) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 29 per 1000 | 16 per 1000 (10-27) | |||||
| Moderate | ||||||
| 27 per 1000 | 15 per 1000 (9-25) | |||||
|
Stroke
Follow-up: 1 year |
Study population | RR 0.71 (0.4-1.25) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 39 per 1000 | 27 per 1000 (15-48) | |||||
| Moderate | ||||||
| 43 per 1000 | 31 per 1000 (17-54) | |||||
|
Transient ischemic attack
Follow-up: 1 year |
Study population | RR 0.98 (0.52-1.83) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 15 per 1000 | 15 per 1000 (8-27) | |||||
| Moderate | ||||||
| 15 per 1000 | 15 per 1000 (8-27) | |||||
|
Myocardial infarction
Follow-up: 1 year |
Study population | RR 0.74 (0.43-1.27) | 2633 (3 studies) | ⊕⊕⊕⊕ High | ||
| 23 per 1000 | 17 per 1000 (10-29) | |||||
| Moderate | ||||||
| 22 per 1000 | 16 per 1000 (9-28) | |||||
|
Life-threatening or disabling bleeding
Follow-up: 1 year |
Study population | RR 0.32 (0.24-0.42) | 2353 (2 studies) | ⊕⊕⊕⊕ High | ||
| 156 per 1000 | 50 per 1000 (38-66) | |||||
| Moderate | ||||||
| 173 per 1000 | 55 per 1000 (42-73) | |||||
|
Permanent pacemaker implantation
Follow-up: 1 year |
Study population | RR 3.42 (1.33-8.82) | 2633 (3 studies) | ⊕⊕⊕⊝ Moderate3,4 | ||
| 57 per 1000 | 194 per 1000 (76-501) | |||||
| Moderate | ||||||
| 53 per 1000 | 181 per 1000 (70-467) | |||||
|
New-atrial fibrillation
Follow-up: 1 year |
Study population | RR 0.25 (0.18-0.36) | 2633 (3 studies) | ⊕⊕⊕⊕ High3,4 | ||
| 386 per 1000 | 96 per 1000 (69-139) | |||||
| Moderate | ||||||
| 384 per 1000 | 96 per 1000 (69-138) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). RR, risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1Transcatheter aortic valve implantation; 2surgical aortic valve replacement; 3inconsistency; 4large effect. | ||||||
Supplement Table 1D.
Summary of Findings and Strength of Evidence (GRADE) for 2-Year Results
| TAVI Compared to SAVR for Low-Risk Surgical Patients with Aortic Stenosis | ||||||
|
Patient or population: Low-risk surgical patients with aortic stenosis Settings: Intervention: TAVI1 Comparison: SAVR2 | ||||||
| Outcomes | Illustrative Comparative Risks* (95% CI) | Relative Effect (95% CI) | No. of Participants (Studies) | Quality of the Evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
| SAVR | TAVI | |||||
|
All-cause mortality
Follow-up: 2 years |
Study population | RR 0.8 (0.55-1.16) | 2644 (3 studies) | ⊕⊕⊕⊕ High | ||
| 45 per 1000 | 36 per 1000 (25-52) | |||||
| Moderate | ||||||
| 44 per 1000 | 35 per 1000 (24-51) | |||||
|
Cardiovascular mortality
Follow-up: 2 years |
Study population | RR 0.65 (0.42-1.01) | 2644 (3 studies) | ⊕⊕⊕⊕ High | ||
| 37 per 1000 | 24 per 1000 (16-37) | |||||
| Moderate | ||||||
| 34 per 1000 | 22 per 1000 (14-34) | |||||
|
Stroke
Follow-up: 2 years |
Study population | RR 0.9 (0.64-1.28) | 2644 (3 studies) | ⊕⊕⊕⊕ High | ||
| 48 per 1000 | 43 per 1000 (31-61) | |||||
| Moderate | ||||||
| 52 per 1000 | 47 per 1000 (33-67) | |||||
|
Transient ischemic attack
Follow-up: 2 years |
Study population | RR 1.09 (0.39-3.04) | 1230 (2 studies) | ⊕⊕⊕⊕ High | ||
| 19 per 1000 | 20 per 1000 (7-57) | |||||
| Moderate | ||||||
| 23 per 1000 | 25 per 1000 (9-70) | |||||
|
Myocardial infarction
Follow-up: 2 years |
Study population | RR 0.95 (0.58-1.56) | 2644 (3 studies) | ⊕⊕⊕⊕ High | ||
| 24 per 1000 | 23 per 1000 (14-38) | |||||
| Moderate | ||||||
| 26 per 1000 | 25 per 1000 (15-41) | |||||
|
Permanent pacemaker implantation
Follow-up: 2 years |
Study population | RR 3.02 (1.31-6.97) | 2644 (3 studies) | ⊕⊕⊕⊝ Moderate3 | ||
| 70 per 1000 | 211 per 1000 (92-487) | |||||
| Moderate | ||||||
| 66 per 1000 | 199 per 1000 (86-460) | |||||
|
New atrial fibrillation
Follow-up: 2 years |
Study population | RR 0.27 (0.14-0.51) | 1230 (2 studies) | ⊕⊕⊕⊕ High3,4 | ||
| 396 per 1000 | 107 per 1000 (55-202) | |||||
| Moderate | ||||||
| 465 per 1000 | 126 per 1000 (65-237) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). RR, risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1Transcatheter aortic valve implantation; 2surgical aortic valve replacement; 3inconsistency; 4large effect. | ||||||
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Union Hospital, Fujian Medical University, (Approval No: 2020KJT091).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – C.G.C., B.B.X.; Design – C.G.C., Q.F.D.; Supervision – L.W.C., Z.H.Q.; Fundings – L.W.C., Z.H.Q.; Materials – C.G.C., B.B.X.; Data collection &/or processing –X.Y.Z., W.C.L.; Analysis &/or interpretation – C.G.C., B.B.X.; Literature search – Z.H.Q.; Writing –C.G.C., B.B.X.; Critical review – L.W.C., Z.H.Q.
Acknowledgments: None.
Declaration of Interests: The authors have nothing to disclose.
Funding: This work was funded by the National Natural Science Foundation of China (U2005202), the Fujian Province Major Science and Technology Program (2018YZ001-1), the Natural Science Foundation of Fujian Province (2020J02056), and Fujian Provincial Health Technology Project (2019-ZQN-50).
References
- 1. Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373(9667):956 966. 10.1016/S0140-6736(09)60211-7) [DOI] [PubMed] [Google Scholar]
- 2. Holmes DR, Mack MJ, Kaul S.et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(13):1200 1254. 10.1016/j.jacc.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 3. Cribier A, Eltchaninoff H, Bash A.et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006 3008. 10.1161/01.cir.0000047200.36165.b8) [DOI] [PubMed] [Google Scholar]
- 4. Mack MJ, Leon MB, Smith CR.et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477 2484. 10.1016/S0140-6736(15)60308-7) [DOI] [PubMed] [Google Scholar]
- 5. Leon MB, Smith CR, Mack MJ.et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609 1620. 10.1056/NEJMoa1514616) [DOI] [PubMed] [Google Scholar]
- 6. Nishimura RA, Otto CM, Bonow RO.et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2017;135(25):e1159-e1195. 10.1161/CIR.0000000000000503) [DOI] [PubMed] [Google Scholar]
- 7. Faroux L, Chen S, Muntané-Carol G.et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J. 2020;41(29):2771 2781. 10.1093/eurheartj/ehz924) [DOI] [PubMed] [Google Scholar]
- 8. Popma JJ, Deeb GM, Yakubov SJ.et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706 1715. 10.1056/NEJMoa1816885) [DOI] [PubMed] [Google Scholar]
- 9. Thyregod HG, Steinbrüchel DA, Ihlemann N.et al. Transcatheter Versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results From the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65(20):2184 2194. 10.1016/j.jacc.2015.03.014) [DOI] [PubMed] [Google Scholar]
- 10. Otto CM, Nishimura RA, Bonow RO.et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2021;143(5):e72 e227. 10.1161/CIR.0000000000000923) [DOI] [PubMed] [Google Scholar]
- 11. Leon MB, Mack MJ, Hahn RT.et al. Outcomes 2 years After transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77(9):1149 1161. 10.1016/j.jacc.2020.12.052) [DOI] [PubMed] [Google Scholar]
- 12. Forrest JK, Deeb GM, Yakubov SJ.et al. 2-year outcomes After transcatheter Versus surgical aortic valve replacement in low-risk patients. J Am Coll Cardiol. 2022;79(9):882 896. 10.1016/j.jacc.2021.11.062) [DOI] [PubMed] [Google Scholar]
- 13. Shamseer L, Moher D, Clarke M.et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. 10.1136/bmj.g7647) [DOI] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J.et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65 W94. 10.7326/0003-4819-151-4-200908180-00136) [DOI] [PubMed] [Google Scholar]
- 15. Søndergaard L, Steinbrüchel DA, Ihlemann N.et al. Two-year outcomes in patients With severe aortic valve stenosis randomized to transcatheter Versus surgical aortic valve replacement: the all-comers Nordic aortic valve intervention randomized clinical trial. Circ Cardiovasc Interv. 2016;9(6):e003665. 10.1161/CIRCINTERVENTIONS.115.003665) [DOI] [PubMed] [Google Scholar]
- 16. Guyatt GH, Oxman AD, Vist GE.et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924 926. 10.1136/bmj.39489.470347.AD) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ades AE, Lu G, Higgins JPT. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646 654. 10.1177/0272989X05282643) [DOI] [PubMed] [Google Scholar]
- 18. Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914 916. 10.1136/bmj.39343.408449.80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557 560. 10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629 634. 10.1136/bmj.315.7109.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kolte D, Vlahakes GJ, Palacios IF.et al. Transcatheter Versus surgical aortic valve replacement in low-risk patients. J Am Coll Cardiol. 2019;74(12):1532 1540. 10.1016/j.jacc.2019.06.076) [DOI] [PubMed] [Google Scholar]
- 22. Makkar RR, Thourani VH, Mack MJ.et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382(9):799 809. 10.1056/NEJMoa1910555) [DOI] [PubMed] [Google Scholar]
- 23. Van Belle E. TAVR at 5 years — rematch or swan song for surgery? N Engl J Med. 2020;382(9):867 868. 10.1056/NEJMe2000240) [DOI] [PubMed] [Google Scholar]
- 24. Barili F, Freemantle N, Pilozzi Casado A.et al. Mortality in trials on transcatheter aortic valve implantation versus surgical aortic valve replacement: a pooled meta-analysis of Kaplan–Meier-derived individual patient data. Eur J Cardiothorac Surg. 2020;58(2):221 229. 10.1093/ejcts/ezaa087) [DOI] [PubMed] [Google Scholar]
- 25. Deharo P, Bisson A, Herbert J.et al. Impact of sapien 3 balloon-expandable Versus Evolut R self-expandable transcatheter aortic valve implantation in patients with aortic stenosis: data From a nationwide analysis. Circulation. 2020;141(4):260 268. 10.1161/CIRCULATIONAHA.119.043971) [DOI] [PubMed] [Google Scholar]
- 26. Schymik G, Schröfel H, Heimeshoff M, Luik A, Thoenes M, Mandinov L. How to adapt the implantation technique for the new SAPIEN 3 transcatheter heart valve design. J Interv Cardiol. 2015;28(1):82 89. 10.1111/joic.12165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jørgensen TH, De Backer O, Gerds TA, Bieliauskas G, Svendsen JH, Søndergaard L. Mortality and heart failure hospitalization in patients with conduction abnormalities after transcatheter aortic valve replacement. JACC Cardiovasc Intv. 2019;12(1):52 61. 10.1016/j.jcin.2018.10.053) [DOI] [PubMed] [Google Scholar]
- 28. Dvir D, Webb J, Brecker S.et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation. 2012;126(19):2335 2344. 10.1161/CIRCULATIONAHA.112.104505) [DOI] [PubMed] [Google Scholar]
- 29. Bidar E, Folliguet T, Kluin J.et al. Postimplant biological aortic prosthesis degeneration: challenges in transcatheter valve implants. Eur J Cardiothorac Surg. 2019;55(2):191 200. 10.1093/ejcts/ezy391) [DOI] [PubMed] [Google Scholar]
- 30. Thyregod HGH, Ihlemann N, Jørgensen TH.et al. Five-year clinical and echocardiographic outcomes from the Nordic aortic valve intervention (NOTION) randomized clinical trial in lower surgical risk patients. Circulation. 2019;139:2714 2723. 10.1161/CIRCULATIONAHA.118.036606) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a