Abstract
Animals must adapt their dietary choices to meet their nutritional needs. How these needs are detected and translated into nutrient-specific appetites that drive food-choice behaviours is poorly understood. Here we show that enteroendocrine cells of the adult female Drosophila midgut sense nutrients and in response release neuropeptide F (NPF), which is an ortholog of mammalian neuropeptide Y-family gut-brain hormones. Gut-derived NPF acts on glucagon-like adipokinetic hormone (AKH) signalling to induce sugar satiety and increase consumption of protein-rich food, and on adipose tissue to promote storage of ingested nutrients. Suppression of NPF-mediated gut signalling leads to overconsumption of dietary sugar while simultaneously decreasing intake of protein-rich yeast. Furthermore, gut-derived NPF has a female-specific function in promoting consumption of protein-containing food in mated females. Together, our findings suggest that gut NPF-to-AKH signalling modulates specific appetites and regulates food choice to ensure homeostatic consumption of nutrients, providing insight into the hormonal mechanisms that underlie nutrient-specific hungers.
Subject terms: Endocrine system and metabolic diseases, Drosophila, Metabolism, Feeding behaviour, Gastrointestinal hormones
Malita, Kubrak et al. show that the gut-derived hormone neuropeptide F suppresses sugar intake and increases the consumption of protein-rich food in Drosophila. This gives insight into the regulation of nutrient-specific appetite that ensures appropriate food choices to meet nutritional demands.
Main
Animals must be able to select the specific nutrients they need to consume. Food selection is governed by appetites for specific nutrients to ensure adequate ingestion of macronutrients needed to maintain nutritional homeostasis and optimal fitness1,2. This has given rise to the hypothesis that organisms can feel specific hungers or appetites for the type of nutrients they need3,4. Nutrient-specific appetite has been demonstrated in many organisms, including humans3–6. Such homeostatic nutrient consumption requires sensors that detect the internal nutritional state and mechanisms that translate this information into changes in feeding decisions. Food consumption is controlled by nutritional signals from the periphery, such as the adipokine leptin and a variety of gut hormones, that act together with circulating nutrients on the brain7. However, the hormones and mechanisms that govern nutrient-specific appetites that drive appropriate food choices to maintain or restore homeostasis are poorly defined.
The fruit fly Drosophila, like mammals, regulates feeding behaviours according to internal state1,6,8,9. The gut is one of the largest endocrine organs, releasing a number of different hormones from specialized enteroendocrine cells (EECs) in both flies and mammals10,11. Gut-to-brain signalling conveys important information about the nutritional nature of the intestinal contents, and enteric nutrient-sensing and signalling play key roles in regulating food intake12,13. For example, in the nutrient-deficient state, orexigenic or hunger signals from the mammalian gut such as the hormone ghrelin drive appetite to promote food consumption. Conversely, in response to food consumption, the mammalian gut releases glucagon-like peptide 1 (GLP-1), which acts as a satiety signal that reduces further food intake. Such satiety signals prevent excess nutrient intake, which can lead to the development of obesity and associated metabolic disorders, and GLP-1 therapy is effective in reducing body weight by lowering appetite14. The fly gut is structurally similar to the mammalian gastrointestinal tract, and many gut-derived hormones are evolutionarily conserved15,16, making Drosophila an attractive model for unravelling the signals by which the gut controls feeding decisions and sex differences in feeding behaviour. Indeed, a great deal has been learned about gut-derived hormonal signalling in this system17–21.
Although substantial progress has been made in understanding the gut-hormonal signalling that controls metabolism18,20,21, much less is known about how the gut communicates the presence or absence of specific nutrients to adjust food choice, and gut-derived signals that regulate appetite towards specific nutrients have not been described. Here, we show that EECs in the adult female Drosophila gut sense sugar and in response release neuropeptide F (NPF), an ortholog of mammalian neuropeptide Y (NPY) hormones. NPF acts via several routes of tissue crosstalk to suppress sugar appetite and promote intake of protein-rich food in mated females, suggesting that NPF is important for regulation of food choices and prevention of excessive sugar consumption, which has been linked to obesity.
Results
Midgut NPF suppresses sugar intake and energy breakdown
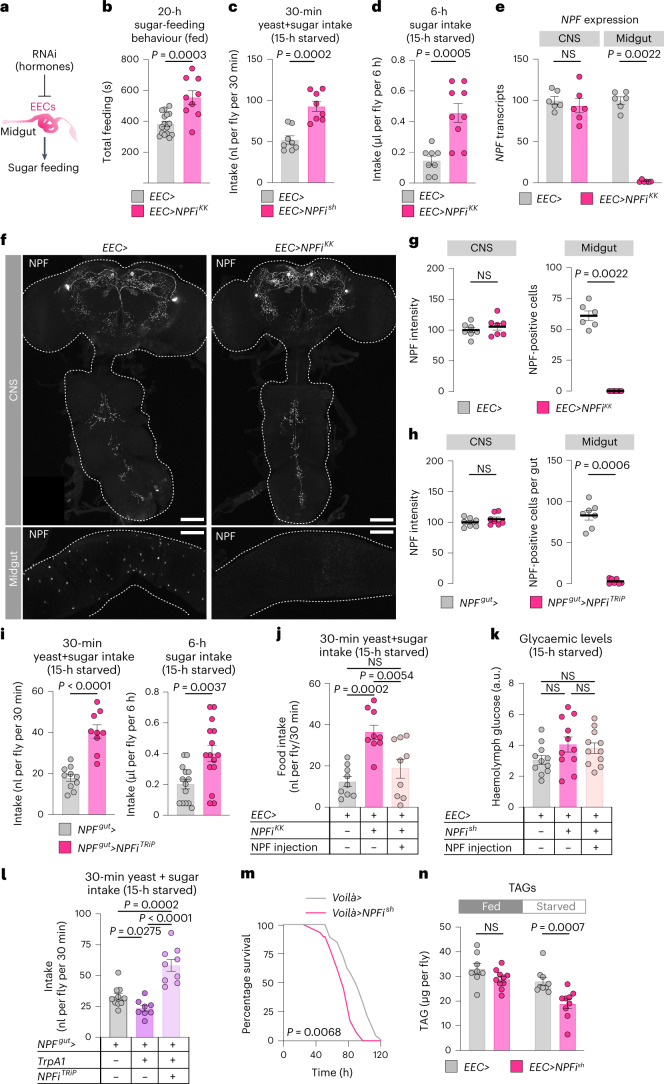
To identify gut-derived hormones and nutrient-sensing mechanisms that regulate feeding, we performed an in vivo RNA-interference screen of secreted factors and receptors in adult Drosophila. We focused on the EECs, which produce a variety of factors that play key roles in the coordination of food intake and metabolism12,18,20,21. We examined the effect of adult-restricted, EEC-specific gene knockdown on the sugar-water feeding behaviour of fed males and females (Fig. 1a) using the fly liquid-food interaction counter (FLIC) system, which allows automated monitoring of Drosophila feeding behaviours22. We used the driver voilà-GAL4 to target the RNAi effect to the EECs, in combination with ubiquitously expressed temperature-sensitive GAL80 (Tub-GAL80TS), together referred to as EEC> hereafter, which allowed us to induce gene silencing only in the adult stage18,20. Among our hits was the peptide NPF, knockdown of which increased the feeding time of mated females on sugar-only food while decreasing males’ sugar-interaction time (Fig. 1b and Extended Data Fig. 1a). To rule out contributions of the UAS transgene itself to the phenotype, we crossed the UAS-NPF-RNAiKK (NPFiKK) line to the control w1118 background. This genotype showed results similar to those seen with the driver control (Extended Data Fig. 1b). This suggests that lack of NPF production in the EECs of mated females enhances their interest in or motivation to feed on sugar.
Fig. 1. Gut-derived NPF regulates sugar intake and metabolism in mated females.
a, Sugar feeding in mated females with RNAi-mediated knockdown of hormones and transporters in the EECs of the midgut. b, Total time feeding using FLIC; n = 16 EEC>, n = 9 EEC > NPFiKK. c,d, Consumption (c) of sugar+yeast food (9% sugar and 8% yeast) determined by dye assay, and of 10% sugar (d) measured by CAFÉ assay; c, n = 8 EEC> and EEC>NPFish; d, n = 8 EEC>, n = 9 EEC>NPFiKK. e, Conditional NPF knockdown with EEC> affects the EECs but not the CNS (brain and VNC); n = 6 biological replicates from tissues pooled from six animals for each condition. f,g, NPF immunostaining of CNS and midgut, quantified in g; n = 7 CNS, n = 6 midguts. Scale bars, 50 μm. h, Quantification of images represented in Extended Data Fig. 1i, immunostaining of NPF knockdown using the NPF> driver with pan-neuronal GAL80 (R57C10-GAL80; NPF>– together, NPFgut>) of midgut EECs and CNS; n = 7 tissues each. i, Intake measured by dye-consumption and CAFÉ assays. Left n = 10 NPFgut>, n = 9 NPFgut>NPFiTRiP; right n = 14 NPFgut>, n = 15 NPFgut>NPFiTRiP. j,k, Consumption and glycaemic levels after injection of NPF peptide into the haemolymph. j, n = 9 each. k, n = 11 each. l, Thirty-minute food intake measured by dye assay during activation of NPF+ EECs using the heat-sensitive TrpA1 channel; n = 10 NPFgut>, n = 8 NPFgut>TrpA1, n = 9 NPFgut>TrpA1, NPFiTRiP. m, Survival under starvation. n, TAG levels; n = 8 fed EEC>, n = 10 fed EEC>NPFiKK, n = 9 starved EEC>, n = 9 starved EEC>NPFiKK. All animals were mated females. Bars represent mean ± s.e.m. NS, not significant. b–d,i, Two-tailed unpaired Student’s t-test. e,g,h,n, Two-tailed unpaired Mann–Whitney U-test. j–l, One-way ANOVA with Tukey’s multiple-comparisons test. m, Kaplan–Meier log-rank tests.
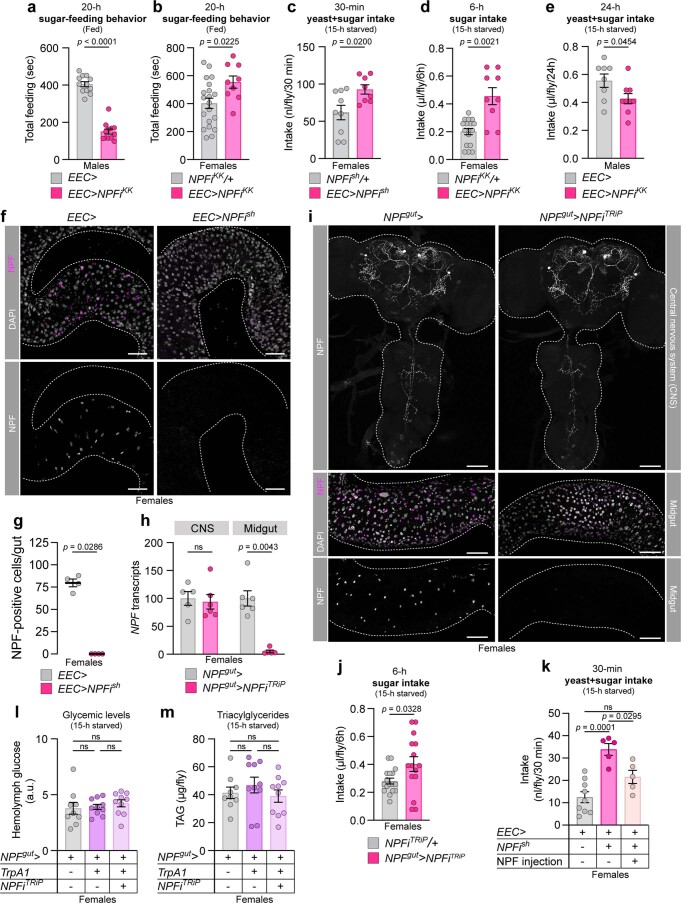
Extended Data Fig. 1. NPF knockdown efficiently depletes NPF specifically in the midgut EECs and affects food intake.
(a) Total time feeding using FLIC; n = 12. (b) Time spent feeding determined by FLIC; n = 20 NPFiKK/+, n = 9 EEC > NPFiKK. (c) Amount of sugar+yeast solid food (9% sugar + 8% yeast) consumed, by dye assay; n = 9 NPFish/+; n = 8 EEC > NPFish. (d) Consumption of 10% sugar measured by CAFÉ assay; n = 17 NPFiKK/+ and n = 9 EEC > NPFiKK. (e) Consumption of sugar+yeast liquid food (5% sugar + 5% yeast extract) measured by CAFÉ assay; n = 8 EEC > , n = 9 EEC > NPFiKK. (f,g) NPF immunostaining in the midgut of mated females, quantified in (g); n = 4 guts. Scale bars, 50 μm. (h) Knockdown using the R57C10-GAL80, NPF > (NPFgut > ) driver with NPFiTRiP affects NPF transcripts in mated female guts but not the CNS (brain and ventral nerve cord), n = 5 NPFgut > CNS samples, n = 6 NPFgut > NPFiTRiP CNS samples, n = 6 NPFgut > midgut samples, n = 5 NPFgut > NPFiTRiP midgut samples, each replicate containing tissues from 6 animals. (i) NPF immunostaining of CNS and midguts from mated females, quantified in Fig. 1 h. Scale bars, 50 μm. (j) Consumption of 10% sugar-water measured by CAFÉ assay; n = 17 NPFiTRiP/+, n = 15 NPFgut > NPFiTRiP. (k) Food intake after injection of NPF peptide into the haemolymph measured by dye assay; n = 9 EEC > , n = 5 EEC > NPFish, n = 5 EEC > NPFish with NPF injection. (l,m) Hemolymph glucose (l) and whole-body TAG (m) levels after 30-minute incubation at 29 °C for TrpA1 activation; n = 9 NPFgut > , n = 10 NPFgut > TrpA1, n = 10 NPFgut > TrpA1 + NPFi. All females were mated. Bars represent mean±SEM. ns, non-significant. a, b, c, e, j: Two-tailed unpaired Student’s t test. d, g, h: Two-tailed unpaired Mann–Whitney U test. k: One-way ANOVA with Tukey’s multiple-comparisons test. l, m: Kruskal-Wallis nonparametric ANOVA with Dunn’s multiple-comparisons test.
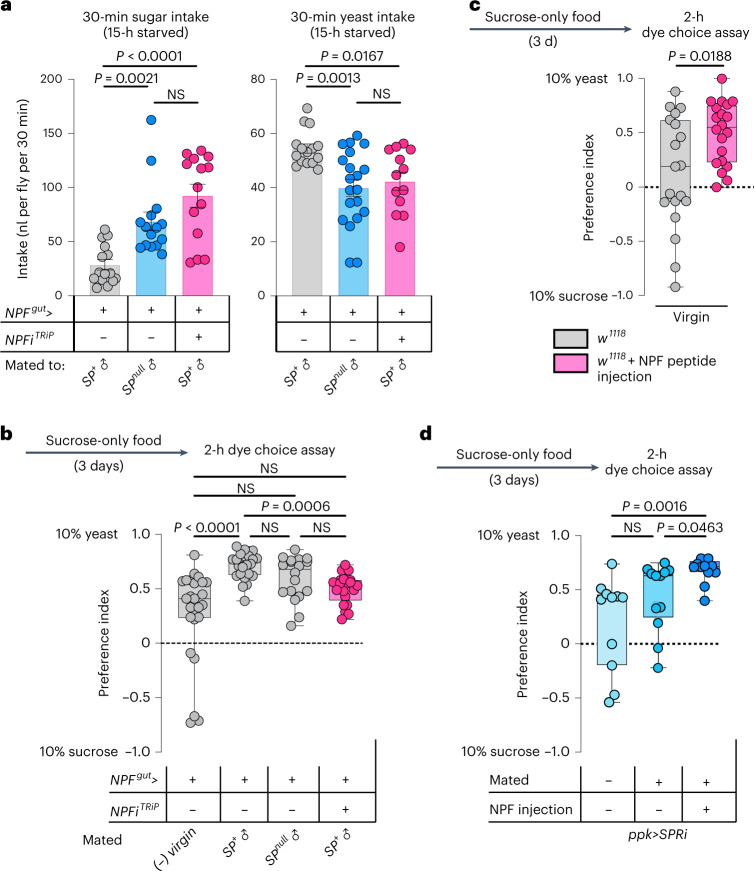
Knockdown of gut NPF throughout development has recently been associated with increased consumption of food containing both sugar and yeast in virgin female adults21. We therefore analyzed whether EEC-derived NPF also regulates intake of sugar+yeast food in mated females. We measured short-term (30-minute) food intake using a dye-consumption assay with standard adult fly food23 containing both sugar (9%) and yeast (8%). To measure short-term intake, we preconditioned animals by fasting them for 15 hours to increase consumption. We confirmed that, like virgins, mated females with adult-restricted EEC knockdown of NPF also consumed significantly more sugar+yeast food than controls (Fig. 1c and Extended Data Fig. 1c). We also applied the capillary feeder (CAFÉ) assay24 to quantify sugar intake over a longer period. Before this assay as well, we exposed animals to a 15-hour period of fasting to enhance consumption. Mated females with adult-restricted EEC knockdown of NPF consumed significantly more sugar over the 6-hour period than controls (Fig. 1d and Extended Data Fig. 1d). Since the sugar-feeding phenotype was observed in animals whether they were fully fed or preconditioned by 15-hour fasting (Fig. 1b,c,d), we chose to use animals that were 15-hour fasted for consistency in the following feeding assays, since this allows robust measurements in short-term food intake assays as well as longer-term feeding assays. Conversely, in males, loss of NPF in the EECs led to reduced food consumption over an even longer period of 24 hours (Extended Data Fig. 1e). Together, these results indicate that gut-derived NPF suppresses sugar intake in females.
To attribute these effects specifically to EEC-derived NPF, we measured the expression of NPF in dissected midguts and central nervous systems (CNS; brain and ventral nerve cord, VNC). NPF transcript levels were strongly reduced in the female midgut when EEC> was used to drive knockdown of NPF, whereas expression in the CNS was unaltered, which was confirmed by immunostainings (Fig. 1e,f,g and Extended Data Fig. 1f,g). To further support this, we used a second driver, NPF::2A::GAL4 (NPF>), a CRISPR-mediated insertion of T2A::GAL4 into the native NPF locus that drives GAL4 expression in only NPF-producing cells25. Since NPF is expressed in both neurons and EECs, we used pan-neuronal R57C10-GAL80, an optimized nSyb-GAL80 variant that suppresses neuronal GAL4 (ref. 18), to suppress the GAL4 activity of NPF> in the nervous system. We confirmed that this driver combination (R57C10-GAL80; NPF>), referred to hereafter as NPFgut>, efficiently knocks NPF down in the midgut without affecting CNS expression (Fig. 1h and Extended Data Fig. 1h,i). Knockdown of NPF using this gut NPF-specific driver caused a marked increase in intake of sugar+yeast food measured over 30 minutes and in consumption of sugar-only medium measured over 6 hours after 15-hour starvation (Fig. 1i and Extended Data Fig. 1j). Taken together, these data indicate that EEC-specific loss of NPF is responsible for the observed feeding phenotypes and indicate that gut NPF acts as a satiety signal that inhibits sugar consumption.
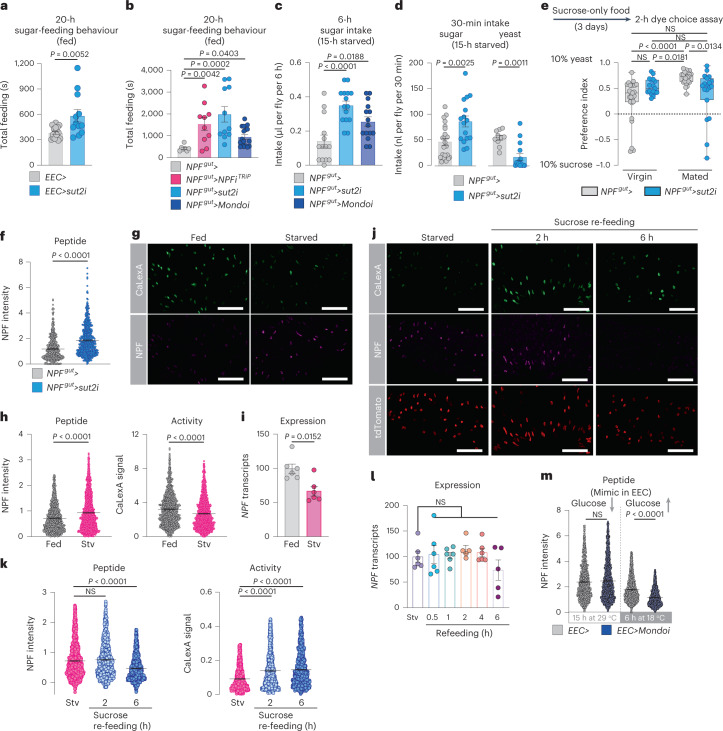
To examine the ability of NPF to promote satiety, we injected synthetic NPF peptide into circulation in mated females. EEC-specific knockdown of NPF induced hyperphagia, which was blocked by NPF injection (Fig. 1j and Extended Data Fig. 1k). NPF injection did not affect haemolymph sugar levels (Fig. 1k), indicating that the observed feeding effect was not a consequence of alterations in glycaemic levels. Next, we expressed the thermosensitive Transient receptor potential A1 (TrpA1) cation channel26 in the NPF+ EECs to enable induction of NPF release. Incubation at 29 °C, which induces TrpA1-mediated peptide release, inhibited food intake, an effect that was abolished by simultaneous NPF knockdown (Fig. 1l). These NPF-induced changes in food intake were likewise not associated with altered triacylglyceride (TAG) or circulating sugar levels (Extended Data Fig. 1l,m), supporting a direct role for gut-derived NPF in governing feeding behaviour, rather than effects of NPF on metabolism that then lead secondarily to altered behaviour. Together, these results indicate that NPF from these EECs is both necessary and sufficient to inhibit food intake and prevent food overconsumption.
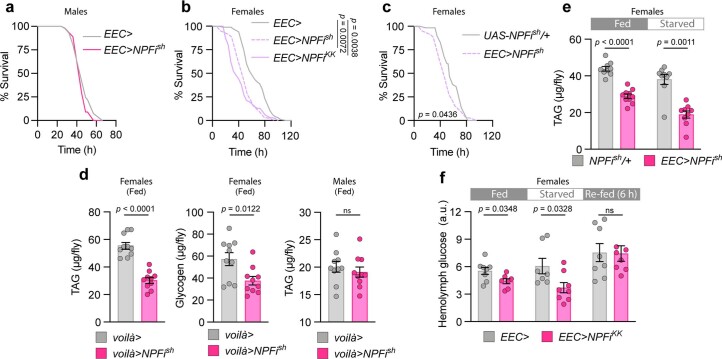
Feeding behaviours are tightly coordinated with physiology to maintain metabolic balance. Our findings indicate that NPF acts as a satiety signal, which suggests that it should act after a meal. In this scenario NPF would be expected to promote storage and inhibit mobilization of energy. As an indirect measure of energy storage and mobilization, we first assessed animals’ starvation resistance. NPF knockdown in the EECs throughout development (voilà> without Tub-GAL80TS (Fig. 1m)), as well as adult-restricted RNAi (Extended Data Fig. 2a,b,c), led to a decrease in starvation resistance in females but not males, in line with a recent study linking gut NPF to metabolic programs associated with energy storage21. Consistent with their shortened starvation survival, we found that females with constitutive (Extended Data Fig. 2d) or adult-restricted (Fig. 1n and Extended Data Fig. 2e) EEC knockdown of NPF showed a decrease in both TAG and glycogen levels, whereas TAG levels were not affected by EEC-specific NPF loss in males. These observations suggest that although NPF does affect metabolism in the adult stage, it also regulates early life history in ways that affect the adult. We found that haemolymph glucose levels increased more after re-feeding in animals with EEC suppression of NPF, consistent with their increased sugar consumption (Extended Data Fig. 2f), and showing that the mechanisms of sugar absorption and transport into circulation are functional. Together our findings indicate that, in addition to the metabolic findings described recently21, a main function of EEC-derived NPF in the adult stage is the regulation of feeding, particularly the inhibition of sugar intake in mated females.
Extended Data Fig. 2. Enteroendocrine NPF regulates systemic metabolism and resistance to nutritional stress.
(a-c) Survival during starvation; in (a), n = 41 EEC > males, n = 45 EEC > NPFish males; in (b), n = 130 EEC > , n = 122 EEC > NPFish, n = 94 EEC > NPFiKK; in (c), n = 50 UAS-NPFish/+, n = 122 EEC > NPFish. (d) TAG and glycogen levels in animals with gut NPF knockdown during development; n = 10. (e) TAG levels in the fed condition and following 24-hour starvation; n = 9 fed NPFiKK/+, n = 10 fed EEC > NPFiKK, n = 9 starved NPFiKK/+, n = 9 starved EEC > NPFiKK. (f) Hemolymph sugar levels in the fed condition, after 24 hours’ starvation, and after 6 hours of re-feeding from the 24-hour-starved condition; n = 8 fed EEC > , n = 7 fed EEC > NPFiKK, n = 7 starved EEC > , n = 8 starved EEC > NPFiKK, n = 8 re-fed EEC > , n = 7 re-fed EEC > NPFiKK. All females were mated. Bars represent mean±SEM. ns, non-significant. a, b, c: compared using Gehan-Breslow-Wilcoxon test. d, e (left), f: Two-tailed unpaired Student’s t test. e (right): Two-tailed unpaired Mann-Whitney U test.
Gut NPF suppresses sugar intake and regulates food choice
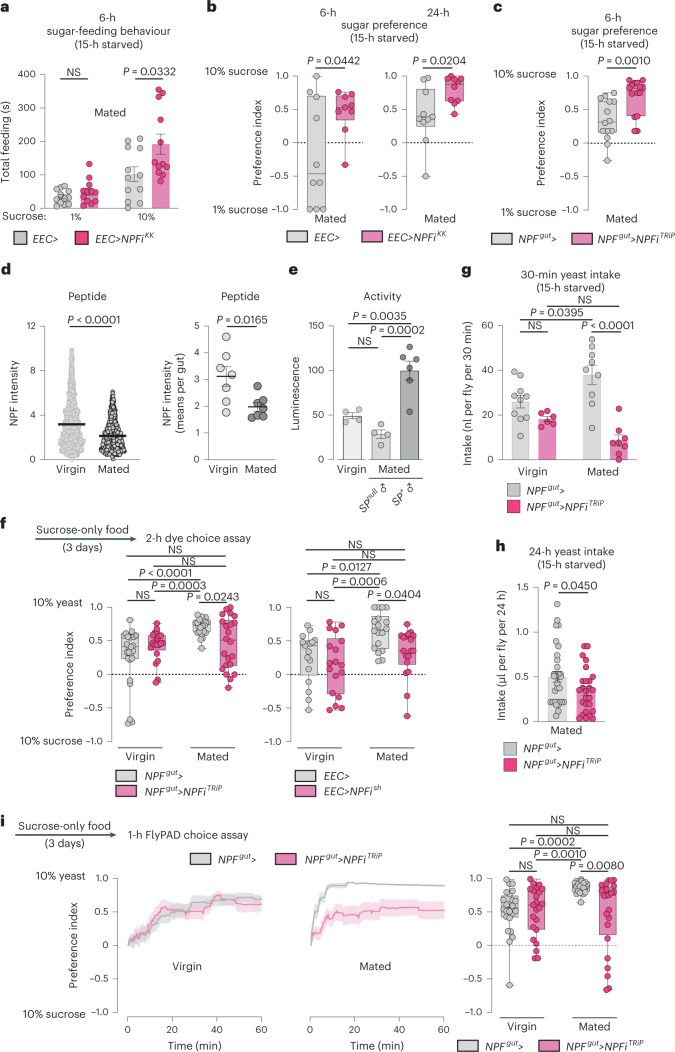
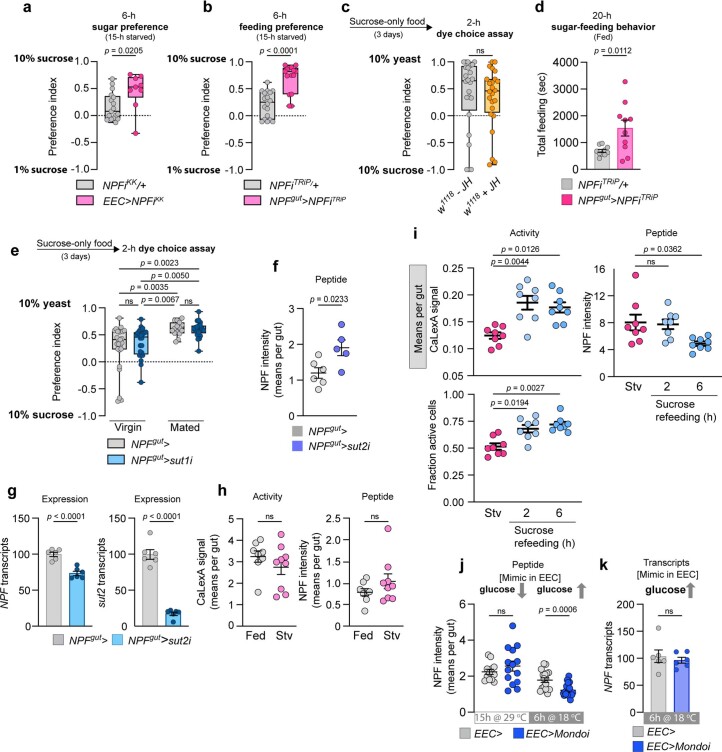
Our findings suggest that NPF acts as a sugar-satiety signal. To test this hypothesis directly, we examined whether gut NPF affected animals’ preference for dietary sugar when they were given the choice between two different sucrose concentrations (1 and 10%). NPF knockdown in the EECs increased feeding and preference for 10% sugar in mated females (Fig. 2a,b,c and Extended Data Fig. 3a,b). These results indicate that NPF is part of a postingestion sugar-sensing mechanism required to decrease sugar appetite.
Fig. 2. Mating induces gut NPF that suppresses sugar appetite and promotes intake of protein-rich yeast food in females.
a, Time spent feeding on 1 or 10% sucrose using FLIC; all n = 12 animals. b,c, Preference between 1 versus 10% sugar measured over 6 or 24 hours by CAFÉ assay. b, 6-hour preference: n = 10 EEC>, n = 9 EEC>NPFiKK. 24-hour preference: n = 10 each. c, n = 14 NPFgut>, n = 15 NPFgut>NPFiTRiP. d, Midgut NPF staining intensity in fed females on a per-cell basis and on a per-gut basis; n = 703 cells from seven guts for virgins, n = 883 cells from seven guts from mated females. e, NPF-cell activity measured in dissected midguts (two midguts per replicate) using a luciferase-based CaLexA calcium-reporter system (NPF>LexA::NFAT::VP16; LexAop-luciferase); n = 4 from fed virgins, n = 4 from fed females mated to SP-deficient males (SP0/Df(3L)delta130), n = 6 from fed females mated to SP+ males. f, Consumption preference for 10% sucrose versus 10% yeast after 3 days of yeast deprivation (3 days on sucrose-only medium) using two-choice dye assay; n = 25 NPFgut> virgins, n = 23 NPFgut>NPFiTRiP virgins, n = 24 NPFgut> mated females, n = 22 NPFgut>NPFiTRiP mated females, n = 17 EEC> virgins, n = 18 EEC>NPFish virgins, n = 18 EEC> mated females, n = 18 EEC>NPFish mated females. g, Yeast consumption determined by dye assay; virgins n = 10 NPFgut> virgins, n = 6 NPFgut>NPFiTRiP virgins, n = 9 NPFgut> mated females, n = 8 NPFgut>NPFiTRiP mated females. h, Yeast intake determined by CAFÉ; n = 32 NPFgut>, n = 26 NPFgut>NPFiTRiP. i, Cumulative behavioural preference of females for sucrose versus yeast monitored using flyPAD. Lines represent means, and shading indicates s.e.m.; n = 24 NPFgut> virgins, n = 23 NPFgut>NPFiTRiP virgins, n = 24 NPFgut> mated females, n = 24 NPFgut>NPFiTRiP mated females. Bars represent mean ± s.e.m. Box plots indicate minimum, 25th percentile, median, 75th percentile and maximum values. NS, not significant. a,c,d (left), Two-tailed unpaired Mann–Whitney U-test. b,d (right), h, Two-tailed unpaired Student’s t-test. e,f (right), g, One-way ANOVA with Tukey’s post hoc test. f (left), i, Kruskal–Wallis nonparametric ANOVA with Dunn’s multiple-comparisons test.
Extended Data Fig. 3. EEC-specific loss of NPF affects feeding and is influenced by sugar sensing.
(a,b) Consumption preference for 1% vs. 10% sugar-water, measured by CAFÉ assay; n = 17 NPFiKK/+ animals, n = 9 EEC > NPFiKK, n = 18 NPFiTRiP/+, n = 15 NPFgut > NPFiTRiP. (c) Consumption preference of virgin female flies with or without JH treatment (methoprene); n = 24 each. (d) Feeding time on 10% sucrose measured by FLIC; n = 10 NPFiTRiP/+ animals, n = 10 NPFgut > NPFiTRiP. (e) Preference of virgin and mated females, measured by two-choice dye consumption assay; n = 25 NPFgut > virgins, n = 24 NPFgut > sut1i virgins, n = 15 NPFgut > mated females, n = 22 NPFgut > sut1i mated females. (f) Midgut NPF staining intensity with sut2 knockdown in NPF+ EECs on a per-gut basis; n = 6 guts for NPFgut > , n = 5 guts for NPFgut > sut2i. (g) Transcript levels of NPF (left) and sut2 (right) in midguts from fed mated females; n = 6 replicates containing five tissues each. (h) NPF intensity and NPF-cell activity (CaLexA) in fed and 24-h-starved animals on a per-gut basis; n = 8 for fed, n = 9 for 24-hours starved. (i) NPF intensity, NPF-cell activity (CaLexA), and fraction of CaLexA-active cells in 24-h-starved and 2-h- and 6-h-sugar-re-fed mated females measured by calcium-LexA reporter system, aggregated on a per-gut basis; n = 8 guts per condition. (j) NPF staining intensity in midguts of mated females with 15 hours’ EEC knockdown of Mondo (29 oC inactivation of GAL80TS) and following 6-hour re-activation of Mondo expression (18 oC to renature GAL80TS) on a per-gut basis; 15 h at 29 oC: n = 12 EEC > , n = 14 EEC > Mondoi; 6 h at 18 oC: n = 17 EEC > , n = 18 EEC > Mondoi. (k) Transcript levels of NPF in midguts from fed mated females; n = 6 samples of five tissues each. All animals were mated females, except in (e). Bars represent mean±SEM. Box plots indicate minimum, 25th percentile, median, 75th percentile, and maximum values. ns, non-significant. a, b, c, h, j, k: Two-tailed unpaired Mann-Whitney U test. d, f, g: Two-tailed unpaired Student’s t test. e, i: One-way Kruskal-Wallis ANOVA with Dunn’s multiple-comparisons test.
Given that females increase their preference for protein-rich food after mating to meet the metabolic requirements of egg production6, we speculated that gut NPF might be important to reduce sugar appetite and increase intake of protein-rich food in mated females. Postmating sex peptide (SP) signalling within the female induces an increased preference for yeast6, and this peptide has also been shown to potentiate NPF release from the midgut17. We confirmed that mating induces NPF secretion from the midgut by measuring EEC NPF protein levels. After mating, NPF peptide levels were reduced in the midgut, consistent with increased release (Fig. 2d). Using a luciferase-based CaLexA reporter27, in which calcium induces the expression of luciferase, we found that NPF+ EECs showed increased calcium-reporter activity after mating in an SP-dependent manner (Fig. 2e). We therefore proposed that gut-derived NPF might be involved in mediating the SP-induced increase in protein consumption in mated females. To test this possibility, we investigated whether NPF affects yeast preference by using a two-choice dye-based consumption assay to measure preference between sugar or protein-rich yeast food6. Animals were deprived of protein for 3 days before the experiment by keeping them on sucrose-only food to increase their preference for yeast food6, making any reduction in this preference easier to observe. We observed that mating increased control females’ preference for yeast food after this treatment (Fig. 2f), consistent with previous findings6. However, animals with EEC-specific NPF loss displayed a reduced preference for yeast food that did not significantly increase after mating. This indicates that EEC-derived NPF is required to inhibit sugar intake in mated females, thereby promoting consumption of protein-rich food. Consistent with this, control animals increased their yeast consumption after mating, whereas mating did not significantly increase yeast consumption in females lacking gut NPF that consumed less yeast (Fig. 2g,h). To further test our conjecture that gut NPF is involved in mating-induced yeast consumption, we used a second automated behaviour-monitoring apparatus, the flyPAD28, to measure feeding preference in a two-choice assay. Behavioural results obtained with this assay indicate that gut NPF is important for the mating-induced increase in preference for yeast (Fig. 2i). Together, these data indicate that NPF from the midgut is involved in promoting yeast intake in mated females, an effect triggered by SP signalling6. Consistent with this notion, females with EEC-specific NPF knockdown mated to SP+ males displayed a consumption pattern similar to that of control females mated to SP-mutant males: both consumed more sugar and less protein than control females mated to SP+ males (Fig. 3a). Control females mated to SP-mutant males showed a yeast-preference phenotype that was intermediate between those of virgin females and females mated to SP+ males, as previously reported6. Females with EEC-specific NPF knockdown mated to SP+ males displayed lower yeast preference than control females mated to SP+ males, and their yeast preference was not significantly different from that of virgin females (Fig. 3b). Thus, females upregulate their protein intake after mating in a partially SP-dependent manner, and our results indicate that NPF from the EECs is involved in mediating this SP-induced shift in food choice, independently of juvenile hormone (Extended Data Fig. 3c), which is known to affect gut remodelling after mating29. We therefore rationalized that injection of NPF into virgin females should induce an increase in their yeast preference. As expected, virgin females injected with NPF peptide exhibited an increased preference for dietary yeast (Fig. 3c).
Fig. 3. NPF regulates food choice downstream of SP signalling in mated females, and exogenous NPF promotes yeast preferences in virgins.
a, Intake of sucrose or yeast measured by dye assay in females mated to SP-producing or SP-deficient males (SP0/Df(3L)delta130). Sucrose, n = 15 NPFgut> females mated to SP+ males, n = 15 NPFgut> females mated to SP-mutant (SPnull) males, n = 14 NPFgut>NPFiTRiP females mated to SP+ males. Yeast, n = 15 NPFgut> females mated to SP+ males, n = 20 NPFgut> females mated to SP-mutant males, n = 13 NPFgut>NPFiTRiP females mated to SP+ males. b, Consumption preference using two-choice dye assay; n = 25 NPFgut> virgins, n = 24 NPFgut> females mated to SP+ males, n = 18 NPFgut> females mated to SP-null males, n = 21 NPFgut>NPFiTRiP females mated to SP+ males. c,d, Consumption preference of w1118 virgin females with or without NPF injection (c) and virgin and mated females (d) (with and without NPF injections) with knockdown of SPR in Ppk+ neurons (ppk>SPRi) using two-choice dye assay. c, n = 19 w1118 virgins, n = 20 w1118 virgins with NPF injection. d, n = 11 virgin ppk>SPRi females, n = 12 mated ppk>SPRi females, n = 12 mated ppk>SPRi females with NPF injection. Bars represent mean ± s.e.m. Box plots indicate minimum, 25th percentile, median, 75th percentile and maximum values. NS, not significant. a (left), b,d, One-way Kruskal–Wallis ANOVA with Dunn’s multiple-comparisons test. a, Right, one-way ANOVA with Dunnett’s multiple-comparisons test. c, Two-tailed unpaired Mann–Whitney U-test.
The induction of yeast preference and the stimulation of gut NPF release after mating are both triggered by SP receptor (SPR) activity in reproductive-tract Ppk+ neurons6,17. We found that mating did not significantly upregulate yeast preference in females with SPR knockdown in the Ppk+ neurons (Fig. 3d), confirming that SP/SPR signalling is important for the preference change. However, injection of NPF was still able to increase the yeast preference of these mated females (Fig. 3d), suggesting that gut NPF acts downstream of SP-SPR signalling in mated females to regulate food choice.
Sut2 in NPF+ EECs regulates NPF release and food choice
Given these results indicating that NPF acts as a mediator of sugar satiety, we asked whether NPF-producing EECs might be activated by sucrose ingestion. In our initial analysis of a collection of RNAi lines (Fig. 1a), we found that knockdown of sugar transporter 2 (sut2), a member of the glucose-transporter class of solute carrier (SLC) proteins, in the EECs of fed mated females increased their sugar-feeding behaviour (Fig. 4a). Loss of sut2 in NPF+ EECs increased animals’ sugar-feeding behaviour and sugar intake, similar to the effects observed in animals with knockdown of NPF itself (Fig. 4b,c and Extended Data Fig. 3d), suggesting that Sut2 might be required as part of a mechanism governing NPF production or release.
Fig. 4. Sugar transporter 2 in the EECs regulates glucose-stimulated NPF secretion in mated females.
a,b, Feeding time measured using FLIC. a, n = 16 EEC>, n = 12 EEC>sut2i. b, n = 7 NPFgut>, n = 10 NPFgut>NPFiTRiP, n = 11 NPFgut>sut2i, n = 17 NPFgut>Mondoi. c, Sugar consumption measured by CAFÉ assay; n = 14 NPFgut>, n = 15 NPFgut>sut2i, n = 15 NPFgut>Mondoi. d, Consumption of sucrose or yeast measured by dye assay. Sucrose n = 22 NPFgut>, n = 16 NPFgut>sut2i. Yeast n = 10 NPFgut>, n = 15 NPFgut>sut2i. e, Consumption preference measured by two-choice dye-consumption assay. Virgins, n = 25 NPFgut>, n = 17 NPFgut>sut2i; mated n = 24 NPFgut>, n = 19 NPFgut>sut2i. f, Midgut NPF staining with sut2 knockdown; n = 571 cells from six guts for NPFgut>, n = 625 cells (five guts) for NPFgut>sut2i. g,h, Representative images of midgut NPF-cell activity (g), quantified in h; left shows n = 1,167 cells from eight fed guts, n = 1,394 cells from nine starved (stv) guts; right shows n = 1,209 cells from eight fed guts, n = 1,405 cells from nine starved guts. i, Midgut NPF expression; n = 6 for stv, 0.5 h, 1 h, 4 h and n = 5 for 2 h, 6 h, each samples of five guts. j, Representative images of NPF staining and cell activity in 24-h starved, 2-h sugar re-fed and 6-h sugar re-fed mated females. All measured cells are marked with tdTomato. k, Quantification of j. Eight guts per condition. Left, n = 1,297, 1,038 and 1,216 cells from starved, 2-h and 6-h re-fed animals. Right, n = 1,141, 954 and 1,151 cells from starved, 2-h re-fed and 6-h re-fed animals. l, Midgut NPF expression in mated females after re-feeding following 24-hour starvation (Stv, no re-feeding); n = 6 replicates of six midguts except n = 5 replicates for 2 and 6 h. m, NPF staining in mated females’ midguts after 15 hours’ knockdown of Mondo (29 °C inactivation of GAL80TS) and following subsequent 6-h derepression of Mondo (18 °C). 15 h at 29 °C: n = 1,595 cells/12 guts for EEC>, n = 1,840 cells/14 guts for EEC>Mondoi; 6 h at 18 °C: n = 1,948 cells/17 guts for EEC>, n = 2,194 cells/18 guts for EEC>Mondoi. All animals were mated females, except in e. Bars represent mean ± s.e.m. Box plots indicate minimum, 25th percentile, median, 75th percentile and maximum values. NS, not significant. a,d (left), Two-tailed unpaired Student’s t-test. b,e,k, One-way Kruskal–Wallis ANOVA with Dunn’s multiple-comparisons test. c,l, One-way ANOVA with Dunnett’s multiple-comparisons test. d (right), f,h,i,m, Two-tailed unpaired Mann–Whitney U-test. Scale bars, 50 μm.
In Drosophila and mammals, the sugar-responsive transcription factor Mondo/ChREBP (carbohydrate-responsive-element-binding protein) contributes to many of the cellular responses to sugar30. To probe the molecular sugar-sensing mechanisms regulating NPF, we silenced Mondo specifically in NPF+ EECs of mated females and found that this manipulation increased both sugar-feeding behaviour and sugar intake, although not as dramatically as the loss of NPF or sut2 (Fig. 4b,c). This suggests that although other mechanisms are probably involved, Mondo/ChREBP-mediated sugar sensing may contribute to NPF regulation in EECs.
Because NPF loss led to increased sugar intake and decreased protein feeding, we investigated whether Sut2 also affects sugar versus protein intake in mated females. We found that knockdown of sut2 in NPF+ EECs led to a strongly increased intake of sucrose and a marked decrease in consumption of yeast when animals were presented with these foods separately (Fig. 4d). Similarly, when given a choice between these two foods, mated females with knockdown of sut2 in NPF+ EECs displayed a reduced preference for dietary yeast (Fig. 4e). Although Sut1 has been linked to NPF secretion in virgins21, we did not observe significant changes in yeast preference in virgins or mated females with sut1 knockdown in NPF+ EECs (Extended Data Fig. 3e). These results indicate that loss of sut2 in NPF+ EECs shifts consumption towards sugar-rich food, similar to knockdown of NPF in these same gut cells, consistent with a role for Sut2 in regulating NPF production or release. Knockdown of sut2 in NPF+ EECs led to a strong intracellular accumulation of NPF peptide, even though NPF transcript levels were reduced (Fig. 4f and Extended Data Fig. 3f,g), suggesting that sut2 loss in NPF+ EECs leads to NPF retention and thus that Sut2 is required for normal NPF expression and release. We also found that sut2 transcript levels in the entire midgut were strongly reduced by knockdown targeted only at the NPF+ gut endocrine cells (Extended Data Fig. 3g), demonstrating that sut2 is predominantly expressed in NPF+ EECs.
To assess more directly whether sugar regulates NPF+ EECs, we exposed mated females to different nutritional conditions and observed their calcium-signalling history, using the CaLexA reporter system, in which green fluorescent protein (GFP) expression reflects calcium signalling31. After 24 hours of starvation, we observed decreased calcium-induced GFP signal and increased NPF peptide staining in the NPF+ EECs when measured on a per-cell basis, even though NPF transcript levels were reduced (Fig. 4g–i), indicating NPF retention. Although these measures were higher in starved animals on a per-cell basis (Fig. 4g,h), they were not significantly altered when analyzed on a one-mean-per-gut basis (Extended Data Fig. 3h). This might possibly reflect the inhibition of only a subpopulation of the NPF+ EECs by starvation, which could be masked by averaging all the cells, or that starvation longer than 24 hours is required for strong inhibition, as also found by a recent report showing that NPF release is reduced after 48 hours’ starvation21. Re-feeding with sucrose after starvation elicited a strong increase in calcium signalling within 2 hours in the NPF+ EECs, associated with a decrease in NPF peptide staining within 6 hours of re-feeding (Fig. 4j,k and Extended Data Fig. 3i), as also reported by an independent study21. Since midgut NPF transcript levels were unaltered under these conditions (Fig. 4l), these results indicate that midgut NPF+ cells are activated by dietary sugar, leading to their secretion of NPF peptide.
We then used genetic methods to mimic sugar sensing occurring in the EECs following a meal. We first induced RNAi-mediated silencing of Mondo/ChREBP by switching flies to 29 °C to inactivate GAL80TS for 15 hours, to reduce sugar sensing in the EECs. We then reactivated Mondo/ChREBP signalling, to mimic sugar-sensing occurring after a meal, by switching animals from 29 back to 18 °C to renature GAL80TS and thereby inactivate the RNAi effect. Reactivation of Mondo/ChREBP signalling in the EECs caused a decrease in NPF peptide levels in these cells without altering NPF expression (Fig. 4m and Extended Data Fig. 3j,k), indicating increased NPF secretion, consistent with the notion that sugar sensing in the EECs is associated with NPF release. Taken together, our findings indicate that, in mated females, sugar intake leads to EEC NPF release through a process requiring glucose-transporter-family protein Sut2 and involving Mondo/ChREBP-mediated sugar sensing.
NPF suppresses energy mobilization in adipose tissues
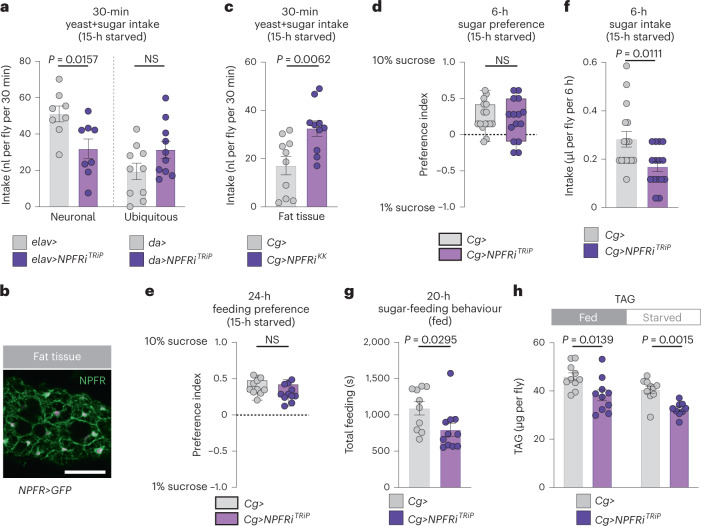
We then asked which target tissues might be involved in NPF-mediated appetite regulation by knocking down the NPF receptor (NPFR). Whereas EEC-specific NPF knockdown induced overfeeding (Fig. 1d), adult females with neuronal knockdown of NPFR driven by elav-GAL4 (elav>) showed decreased food intake (Fig. 5a), consistent with previous reports that neuronal NPF/NPFR signalling promotes feeding32 and indicating that other tissues mediate the downregulation of sugar intake induced by gut NPF. Global knockdown of NPFR driven by daughterless-GAL4 (da>) led to a feeding phenotype intermediate between those observed with gut or neuronal NPF signalling loss (Fig. 5a), probably reflecting opposing effects on feeding of NPF signalling within different organs.
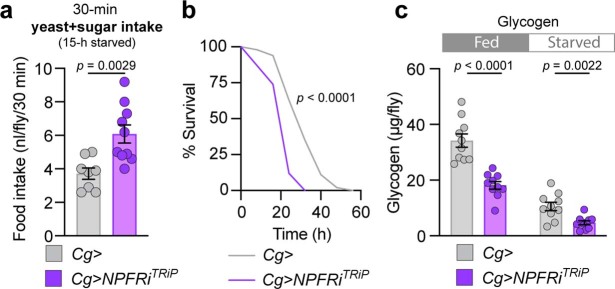
Fig. 5. Loss of NPFR in the fat affects metabolism but does not increase preference for dietary sugar in mated females.
a, Food intake measured by dye in animals with knockdown of NPFR in the nervous system (elav>) or the entire body (da>) measured by dye assay; n = 8 elav>, n = 8 elav>NPFRiTRiP, n = 10 da>, n = 10 da>NPFRiTRiP. b, Fat-body immunostaining of NPFR>mCD8::GFP reporter. Scale bar, 50 μm c, Food intake determined by dye assay in fat-body NPFR-knockdown animals; both n = 10. d, Consumption preference for 1 versus 10% sucrose, measured by CAFÉ assay; n = 16 Cg>, n = 15 Cg>NPFRiTRiP. e, Behavioural preference for interacting with 1 versus 10% dietary sucrose measured by FLIC; n = 10 Cg>, n = 11 Cg>NPFRiTRiP. f, Sucrose intake by CAFÉ assay; n = 16 Cg>, n = 15 Cg>NPFRiTRiP. g, Time spent feeding on sucrose using FLIC; n = 10 Cg>, n = 11 Cg>NPFRiTRiP. h, Whole-body TAG levels in fed and 15-hour-starved fat-body NPFR-knockdown animals. All n = 10 except n = 9 starved Cg>NPFRiTRiP. All animals were mated females. Bars represent mean ± s.em. Box plots indicate minimum, 25th percentile, median, 75th percentile and maximum values. NS, not significant. a,c–e,h (left), Two-tailed unpaired Student’s t-test. f–h (right), Two-tailed unpaired Mann–Whitney U-test.
To assess receptor expression in other target tissues, we used a CRISPR-mediated knock-in of T2A::GAL4 into the native NPFR locus (NPFR::T2A::GAL4, hereafter NPFR>)33 to express UAS-mCD8::GFP. We observed reporter expression in the fat body (Fig. 5b), a tissue analogous to adipose tissue and liver in mammals. Although fat-body-specific NPFR knockdown driven by Cg-GAL4 (Cg>) in adult females did lead to increased short-term intake of food containing both sugar and yeast, it did not increase preference for sugar (Fig. 5c–e and Extended Data Fig. 4a). Indeed, suppression of NPFR in the fat body led to decreased sucrose intake and sugar-feeding behaviour (Fig. 5f,g). Thus, fat-body NPFR signalling does not appear to underlie the specific feeding phenotypes observed with gut NPF loss. We next asked whether fat-body NPFR mediates the effects of gut NPF on metabolism. Like animals with gut-specific NPF knockdown, fat-body NPFR knockdown animals were more sensitive to starvation and displayed metabolic phenotypes similar to those seen with loss of gut NPF (Fig. 5h and Extended Data Fig. 4b,c). These findings indicate that gut-derived NPF acts on NPFR in the fat body as part of a metabolic pathway that maintains energy homeostasis.
Extended Data Fig. 4. Knockdown of NPFR in the fat body leads to increased food intake after starvation, reduced starvation resistance, and lower glycogen stores.
(a) Food intake measured by dye assay (medium containing 9% sugar and 8% yeast); n = 8 Cg > , n = 10 Cg > NPFRiTRiP. (b) Survival during starvation (n = 100 Cg > , n = 50 Cg > NPFRiTRiP). (c) Glycogen levels in fed and 15-hour-starved females; all n = 10. All animals were mated females. Bars represent mean±SEM. ns, non-significant. a, c: Two-tailed unpaired Student’s t test. b: Gehan-Breslow-Wilcoxon test.
NPF regulates food choice through glucagon-like signalling
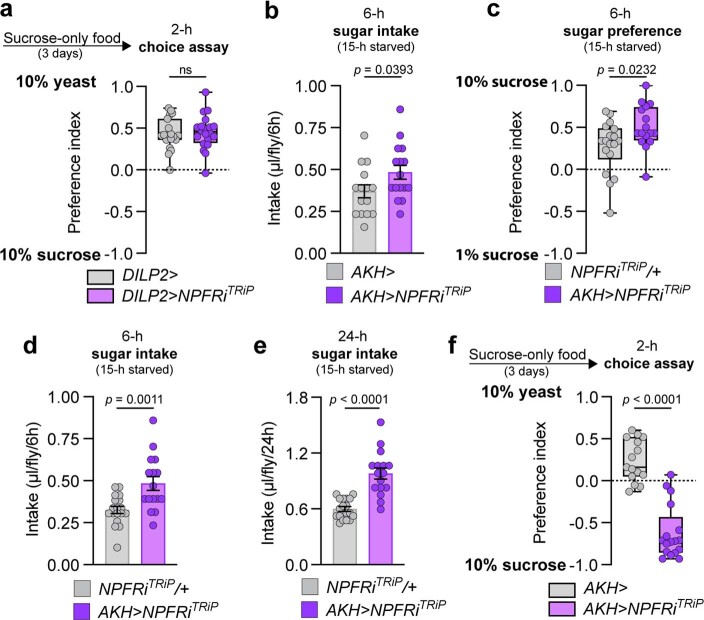
Our experiments indicate that gut NPF signalling regulates sugar appetite via tissues other than the CNS and fat body. In Drosophila, the brain cells that produce insulin express NPFR21 and these cells also regulate aspects of feeding and satiety34. However, knockdown of NPFR in the insulin-producing cells (IPCs) did not change preference for yeast versus sugar in mated females (Extended Data Fig. 5a), suggesting that gut-derived NPF does not act through insulin to modulate preference for dietary sugar and protein.
Extended Data Fig. 5. Knockdown of NPFR in the AKH-producing cells leads to increased preference for and intake of dietary sugar.
(a) Consumption preference using two-choice dye assay; n = 19 DILP2 > , n = 20 DILP2 > NPFRiTRiP. (b) Consumption of 10% sucrose measured by CAFÉ assay; n = 15 AKH > , n = 16 AKH > NPFRiTRiP. (c) Consumption preference for 1% vs. 10% sucrose solution measured by CAFÉ assay; n = 18 NPFRiTRiP/+, n = 16 AKH > NPFRiTRiP. (d,e) Consumption of 10% sucrose measured over 6 hours and 24 hours by CAFÉ assay; n = 18 NPFRiTRiP/+, n = 16 AKH > NPFRiTRiP. (f) Consumption preference using two-choice dye assay; n = 15 AKH > , n = 17 AKH > NPFRiTRiP. All animals were mated females. Bars represent mean±SEM. Box plots indicate minimum, 25th percentile, median, 75th percentile, and maximum values. ns, non-significant. a, d, e, f: Two-tailed unpaired Mann-Whitney U test. b, c: Two-tailed unpaired Student’s t test.
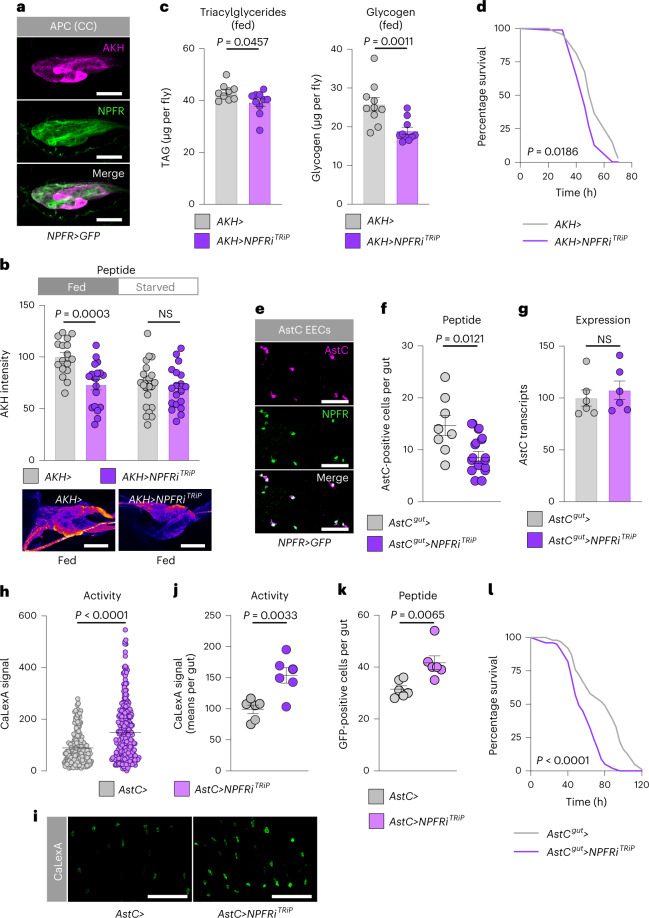
To identify the tissue mediating this effect, we examined NPFR expression in other tissues, which revealed expression of the receptor in the cells producing the glucagon-like factor AKH (Fig. 6a). AKH is released from the AKH-producing cells (APCs) during starvation and acts through its receptor, AkhR, on the fat body to promote the mobilization of stored energy, and it is also thought to act as a hunger signal to drive feeding behaviours18,31,35–37. However, whether AKH regulates sugar- or protein-specific feeding is unknown. We proposed that gut-derived NPF, released in response to sugar feeding, might suppress AKH release from the APCs in the fed state. Consistent with a recent report21, we found that knocking down NPFR in the APCs using AKH-GAL4 (AKH>) resulted in decreased AKH peptide levels within these cells in fed mated females (Fig. 6b), indicating that NPFR is required to suppress AKH release when the animal has ingested food. AKH promotes lipid and glycogen breakdown, and we therefore tested whether NPFR activity in the APCs regulates metabolism. As with knockdown of NPF in the midgut, adult females with knockdown of NPFR in the APCs showed reduced TAG and glycogen levels and increased susceptibility to starvation, as also reported recently21 and consistent with an increase in AKH signalling (Fig. 6c,d).
Fig. 6. NPFR regulates metabolism through inhibition of AKH signalling in mated females.
a, Immunohistochemistry of APCs shows NPFR>GFP reporter expression in APCs of mated females. Scale bars 20 μm. b, Quantification of AKH levels within the APCs of fed and 15-hour-starved mated females with and without NPFR knockdown in these cells; n = 17 fed AKH>, n = 19 fed AKH>NPFRiTRiP, n = 22 starved AKH>, n = 19 starved AKH>NPFRiTRiP. Representative images are shown below. Scale bars 20 μm. c, Metabolite levels in fed mated females. TAG n = 9 AKH>, n = 10 AKH>NPFRiTRiP; glycogen n = 10 AKH>, n = 10 AKH>NPFRiTRiP. d, Survival during starvation of mated females; n = 96 AKH>, n = 96 AKH>NPFRiTRiP. e, Immunohistochemistry of guts from mated female NPFR>GFP flies showing expression of NPFR reporter in AstC+ cells. Scale bars, 25 μm. f, Quantification of the number of midgut cells per gut that showed detectable AstC staining with and without NPFR knockdown in AstC+ EECs of fed mated females; n = 8 AstCgut> guts, n = 11 AstCgut>NPFRiTRiP. g, Midgut AstC transcript levels in fed mated females; each n = 6. h–k, AstC+ EEC-cell activity levels (h) with representative images (i) quantified on a per-gut basis (j) and the number of AstC+ EEC cells showing detectable GFP (k), measured by calcium-reporter system (AstC>LexA::NFAT::VP16; LexAop-GFP, denoted by AstC> in the figure) with or without NPFR knockdown in the AstC+ EECs in the midgut. h, n = 182 AstC> cells, n = 255 AstC>NPFR cells; j, each n = 6 guts; k, each n = 6 guts. Scale bars, 50 μm. l, Survival during starvation of mated females; n = 153 AstCgut> animals, n = 198 AstCgut>NPFRiTRiP. All animals were mated females. Bars represent mean ± s.e.m. Box plots indicate minimum, 25th percentile, median, 75th percentile and maximum values. NS, not ignificant. b,c (left), f,g,j, Two-tailed unpaired Student’s t-test. d,l, Gehan–Breslow–Wilcoxon test. c (right), h,k, Two-tailed unpaired Mann–Whitney U-test.
We recently reported that the peptide hormone AstC released by midgut EECs promotes AKH release during starvation conditions18. We wondered whether NPF signalling might also inhibit AstC release from the midgut to suppress the activation of the AKH axis at multiple hierarchical levels. We found expression of NPFR reporter in AstC+ EECs (Fig. 6e), consistent with single-cell RNA-sequencing data38. We silenced NPFR expression specifically in AstC+ EECs using AstC-GAL4 (AstC>) with pan-neuronal GAL80 (R57C10-GAL80, AstC>–AstCgut> hereafter) to suppress nervous system GAL4 activity. This did not alter AstC expression, but it did lead to a reduction in the number of cells containing detectable AstC peptide (Fig. 6f,g), suggesting that EEC loss of NPFR cell-autonomously increases AstC release. Knockdown of NPFR with AstCgut> led to an increased number of active midgut AstC cells and to higher overall calcium-reporter activity (Fig. 6h–k), indicating that NPFR knockdown promotes AstC+ EEC activation. This indicates that the decreased number of AstC immune-positive cells in adult females with NPFR knockdown in the AstC+ EECs (Fig. 6f) is due to increased release of AstC peptide, which would be expected to promote AKH release, leading to more rapid depletion of energy stores and therefore reduced capacity to survive starvation18. Consistent with this, NPFR knockdown targeted to the AstC+ EECs led to a clear reduction in the capacity of these animals to survive starvation (Fig. 6l). Taken together, our results indicate that in response to sugar intake, gut-derived NPF inhibits the AKH axis at three levels: blocking the release of adipokineticotropic AstC from midgut EECs, blocking the release of AKH itself from the APCs and counteracting AKH’s effects on the fat body.
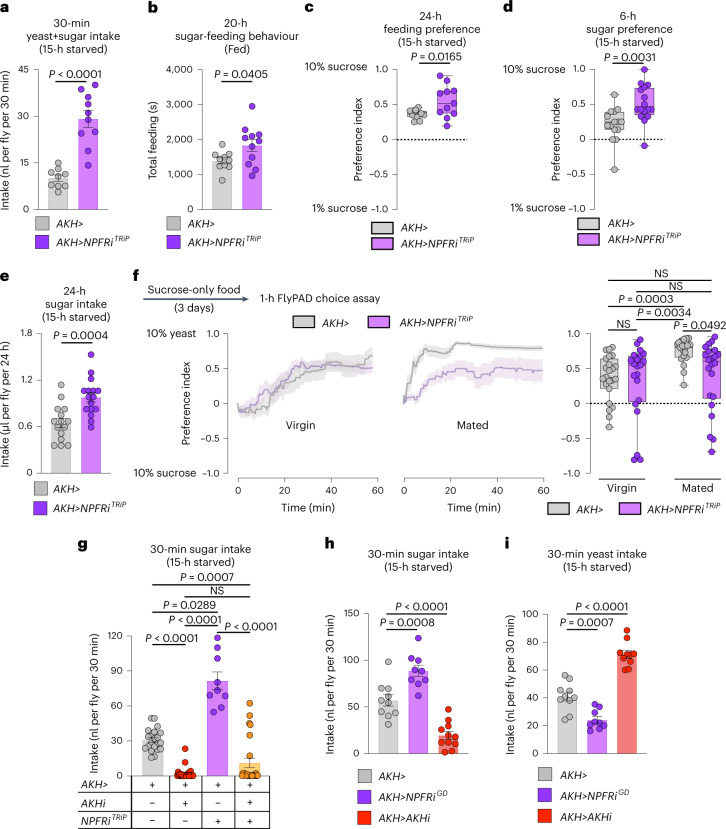
NPFR knockdown in the APCs was recently linked to increased consumption of food in virgin females21, an effect we confirmed in mated females (Fig. 7a). Next, we tested whether NPF regulates sugar- versus protein-specific feeding through NPFR in the APCs. Mated females with APC-specific NPFR knockdown exhibited elevated sugar-directed feeding behaviour, sugar consumption and preference for dietary sugar (Fig. 7b–e and Extended Data Fig. 5b–e), similar to animals with loss of NPF in the midgut. Next, we examined whether NPFR in the APCs might be involved in promoting yeast preference in mated females. Indeed, whereas APC knockdown of NPFR in virgin females did not detectably alter feeding preference, this manipulation had a strong effect in mated females (Fig. 7f and Extended Data Fig. 5f), similar to animals with knockdown of NPF in the gut, indicating that NPFR in the APCs is an important element for promoting consumption of protein-rich food in mated females. To determine whether AKH mediates the effects of NPFR loss on feeding, we examined the ability of AKH knockdown to rescue NPFR-RNAi’s sugar-overeating phenotype. We found that knockdown of AKH completely abolished this effect (Fig. 7g), suggesting that AKH is the primary factor mediating the feeding effects of NPFR signalling. Consistent with NPFR’s suppression of AKH release, AKH loss and NPFR knockdown induced opposite effects on sugar intake and yeast consumption (Fig. 7h,i). Together, our results indicate that, in mated females, gut-derived NPF acts on the APCs via NPFR to inhibit AKH release after a sugar-rich meal, to suppress further sugar feeding while promoting protein intake.
Fig. 7. Loss of NPFR in the APCs phenocopies the gut NPF-loss feeding phenotypes in mated females.
a, Food intake measured by dye assay; n = 9 AKH>, n = 10 AKH>NPFRiTRiP. b, Time spent feeding on sucrose measured by FLIC; n = 10 AKH>, n = 11 AKH>NPFRiTRiP. c, Behavioural preference for 1 versus 10% sugar solution measured by FLIC; both n = 11. d, Consumption preference for 1 versus 10% sucrose measured by CAFÉ assay; n = 15 AKH>, n = 16 AKH>NPFRiTRiP. e, Sucrose consumption measured by CAFÉ assay; n = 15 AKH>, n = 16 AKH>NPFRiTRiP. f, Behavioural preference using flyPAD. Lines represent means, and shading indicates s.e.m.; virgins n = 23 AKH>, n = 21 AKH>NPFRiTRiP; mated n = 19 AKH>, n = 22 AKH>NPFRiTRiP. g, Rescue of sugar overconsumption induced by NPFR knockdown in the APCs through simultaneous AKH knockdown, by dye assay; n = 21 AKH>, n = 24 AKH>AKHiKK, n = 9 AKH>NPFRiTRiP, n = 24 AKH>AKHiKK, NPFRiTRiP. h,i, Sucrose (h) and yeast (i) intake measured over 30 min by dye assay. h, n = 10 AKH>, n = 9 AKH>NPFRiGD, n = 11 AKH>AKHiKK. i, n = 10 AKH>, n = 9 AKH>NPFRiGD, n = 10 AKH>AKHiKK. All animals were mated females, except in f. Bars represent mean ± s.e.m. Box plots indicate minimum, 25th percentile, median, 75th percentile and maximum values. NS, not significant. a–d, Two-tailed unpaired Student’s t-test. e, Two-tailed unpaired Mann–Whitney U-test. f,g, One-way Kruskal–Wallis ANOVA with Dunn’s multiple-comparisons test. h,i, One-way ANOVA with Dunnett’s multiple-comparisons test.
AKH regulates appetites for sugar and protein-rich food
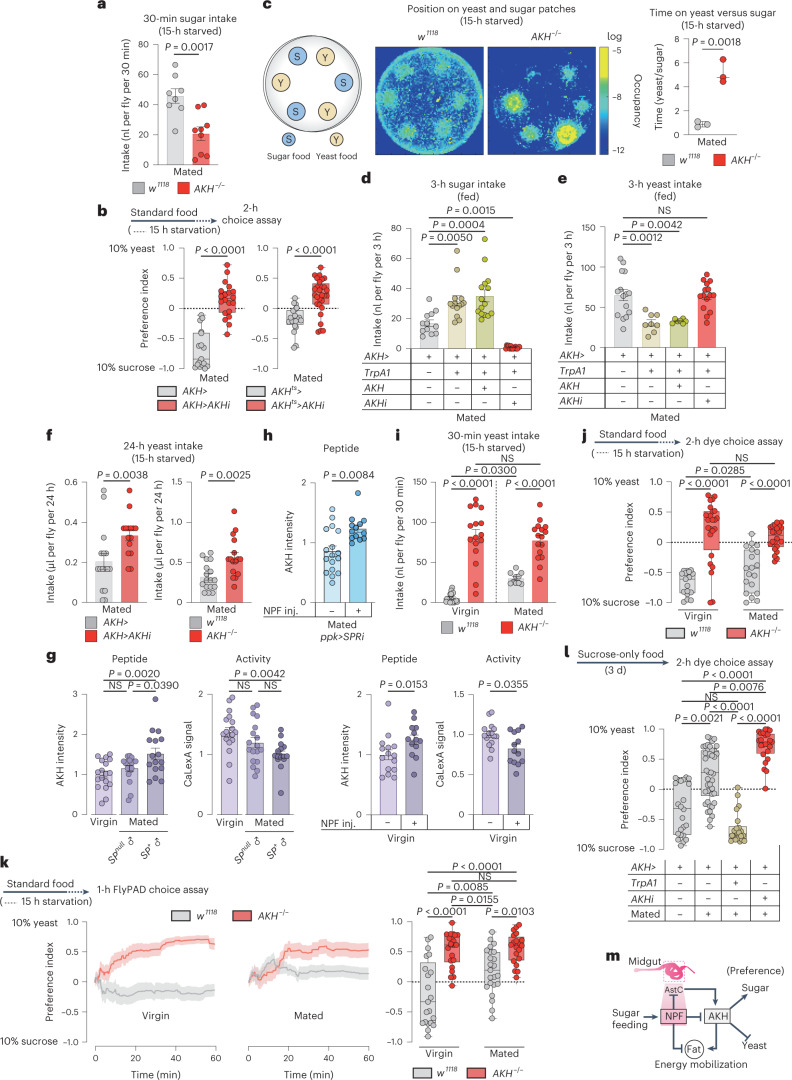
AKH is described as a generic hunger hormone released during nutritional deprivation18,35,39. However, our findings indicate that AKH regulates food choice. To confirm this effect, we examined the feeding behaviour of mated AKH mutant females and found that these animals exhibited significantly reduced sugar intake (Fig. 8a), suggesting that AKH promotes sugar preference. On this basis, this we expected an increase in yeast preference with loss of AKH, so we used assay conditions in which animals do not normally exhibit strong yeast preference: 15-hour starvation rather than 3-day yeast deprivation. Loss of AKH, including adult-restricted APC-specific knockdown (with GAL80TS; AKH>, together referred to as AKHts>), led to a striking shift in preference towards yeast using the two-choice dye assay in mated females (Fig. 8b). Consistent with their increased intake of and preference for yeast food, mated AKH mutant females displayed a strong increase in the amount of time spent exploring patches of yeast food compared to sugar patches (Fig. 8c). This further supports a role for AKH in controlling feeding decisions, biasing behaviour towards sugar intake. Consistent with this, activation of the APCs to induce AKH release caused increased sugar intake while decreasing yeast intake, effects that were blocked by simultaneous AKH knockdown (Fig. 8d,e), indicating that they were mediated by AKH. Circulating sugar levels and whole-body TAG levels were not altered (Extended Data Fig. 6a,b), suggesting that the observed AKH-induced feeding phenotypes are direct effects that precede detectable metabolic changes. Together, these findings indicate that AKH is a hormone that controls selective feeding decisions by increasing appetite for sugar and reducing intake of protein food.
Fig. 8. AKH promotes sugar feeding and suppresses protein intake in mated females.
a, Sugar intake by dye assay. n = 8 w1118, n = 9 AKH−/−. b, Consumption preference, n = 17 AKH>, n = 20 AKH>AKHiKK, n = 20 AKHts>, n = 28 AKHts>AKHiKK. c, Heat map of 30 min tracking of 12 females per genotype. Ratio of time spent on yeast versus sugar patches, n = 3. d,e, Sugar and yeast intake using dye assay, at 29 °C for TrpA1 activation. d, n = 12 AKH>, n = 14 AKH>TrpA1, n = 15 AKH>TrpA1+AKH, n = 15 AKH>TrpA1+AKHi. e, n = 15 AKH>, n = 8 AKH>TrpA1, n = 7 AKH>TrpA1+AKH, n = 15 AKH>TrpA1+AKHi. f, Yeast intake measured by CAFÉ assay. n = 6 AKH>, n = 15 AKH>AKHiKK, n = 17 w1118, n = 16 AKH−/−. g, APCs staining and cell activity, left two panels show n = APCs from 18 w1118 virgins, n = 18 w1118 females mated to SP-deficient males (SP0/Df(3L)delta130), n = 16 w1118 females mated to SP+ males. Right two panels show n = 15 w1118 virgins without NPF injection, n = 13 w1118 virgins with NPF injection. h, AKH staining intensity in mated females with SPR knockdown in the ppk+ neurons with or without NPF injection, n = 17 ppk>SPRi mated females, n = 13 ppk>SPRi mated females with NPF injection. i, Yeast consumption measured by dye assay, n = 18 w1118 virgins, n = 16 AKH−/− virgins, n = 10 mated w1118 females, n = 16 mated AKH−/− females. j, Consumption preference using the two-choice dye assay; n = 17 w1118 virgins, n = 24 AKH−/− virgins, n = 19 mated w1118 females, n = 22 mated AKH−/− females. k, Cumulative behavioural preference using flyPAD. Lines represent means, and shading indicates s.e.m., n = 21 w1118 virgins, n = 22 AKH−/− virgins, n = 23 mated w1118 females, n = 20 mated AKH−/− females. l, Preference measured using the two-choice dye assay at 29 °C for TrpA1 activation, n = 23 AKH> virgins, n = 42 AKH>, n = 22 AKH>TrpA1, n = 24 AKH>AKHi mated females. m, A model of the NPF-AKH axis. Bars represent mean ± s.e.m. Box plots indicate minimum, 25th percentile, media, 75th percentile and maximum. Inj., injection; NS, not significant. a,b (right), c,f, Two-tailed unpaired Student’s t-test. b (left), g (right), h, Two-tailed unpaired Mann–Whitney U-test. d,e,g (left), i,k, One-way ANOVA with Tukey’s post hoc test. j, One-way ANOVA with Dunnett’s multiple comparison test or Kruskal–Wallis with Dunn’s multiple-comparisons test. l, One-way Kruskal–Wallis ANOVA with Dunn’s multiple-comparisons test.
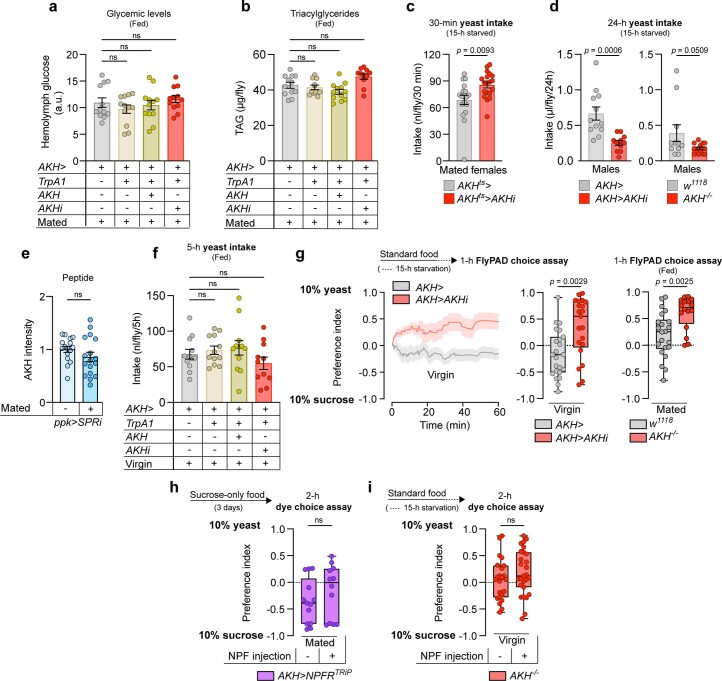
Extended Data Fig. 6. Alteration of AKH signaling leads to increased yeast intake in females, not males, without metabolic effects, indicating sexual dimorphism in AKH effects on feeding preferences.
(a,b). Hemolymph glucose and TAG levels in mated females at 29 °C for TrpA1 activation; (a) all n = 12, (b) n = 12 AKH > , n = 11 AKH > TrpA1, n = 12 AKH > TrpA1 + AKH, n = 11 AKH > TrpA1 + AKHi. (c) Yeast consumption measured by dye assay in mated female flies; n = 18 AKHts > , n = 19 AKHts > AKHiKK. (d) Yeast intake of males measured over 24 hours by CAFÉ assay. Left panel, AKH knockdown; right panel, AKH mutant; n = 12 AKH > , n = 11 AKH > AKHiKK, n = 11 w1118, n = 15 AKH-/-. (e) AKH staining intensity in virgin and mated females with SPR knockdown in the ppk+ neurons; n = 16 ppk > SPRi virgins, n = 17 ppk > SPRi mated females. Note that animals were injected with hemolymph-like buffer without NPF peptide. (f) Yeast consumption measured in fed virgin females using dye assay, at 29 °C for TrpA1 activation; n = 12 AKH > , n = 12 AKH > TrpA1, n = 12 AKH > TrpA1 + AKH, n = 11 AKH > TrpA1 + AKHi). (g) Cumulative behavioural preference of virgin females after 15 hours of starvation or of fed mated females using flyPAD. Lines represent the mean and shading indicates SEM. Virgins: n = 23 AKH > , n = 21 AKH > AKHiKK. Mated females: n = 22 w1118, n = 16 AKH-/-. (h,i) Injection of NPF peptide into the hemolymph of 3-days-yeast-deprived mated females with NPFR knockdown in the APCs (h) or 15-h starved AKH mutant virgin females (i) using two-choice dye assay; n = 13 AKH > NPFRiTRiP, n = 12 AKH > NPFRiTRiP with NPF injection, n = 23 AKH-/-, n = 27 AKH-/- with NPF injection. Bars represent mean±SEM. Box plots indicate minimum, 25th percentile, median, 75th percentile, and maximum values. ns, non-significant. a, b, f: One-way ANOVA with Tukey’s multiple-comparisons test. c, e, g, h, i: Two-tailed unpaired Mann-Whitney U test. d: Two-tailed unpaired Student’s t test.
AKH has recently emerged as a key factor in sex-specific metabolic regulation40. Unlike mated females, in which AKH loss enhanced yeast intake, males lacking AKH exhibited a decrease in yeast intake, indicating that AKH plays a sexually dimorphic role in feeding decisions (Fig. 8f and Extended Data Fig. 6c,d). Together these findings indicate that, in mated females, gut-derived NPF inhibits AKH secretion, and this inhibition suppresses sugar appetite and increases the consumption of protein-rich food. In this scenario, increased AKH signalling in virgins promotes preference for dietary sugar. We therefore examined whether mating reduces AKH release. Mating increased AKH peptide levels and reduced the calcium activity of the APCs, indicating repression of AKH release, and the effect on AKH was dependent on SP signalling (Fig. 8g). These findings suggest that SP signalling, through activation of gut NPF, contributes to the suppression of AKH release after mating. Injecting NPF peptide into virgin females increased AKH peptide levels within the APCs and reduced their calcium activity, indicating that NPF is sufficient to repress AKH secretion (Fig. 8g). Mating did not lead to AKH retention in females with knockdown of SPR in the Ppk+ neurons (Extended Data Fig. 6e), further indicating that SP-SPR signalling is required to repress AKH signalling after mating. To test whether NPF functions downstream of SP-SPR signalling in the AKH-regulatory hierarchy, we injected NPF into mated females with reduced SP-SPR signalling (ppk>SPR-RNAi) and found that this led to increased AKH peptide levels in the APCs (Fig. 8h). This suggests that NPF is sufficient downstream of the SP pathway to repress AKH signalling after mating, which increases yeast intake. We therefore conjectured that loss of AKH would increase yeast intake in virgin females, whereas increasing AKH signalling in virgin females would have little effect on feeding behaviour. To assess the effect of loss of AKH, we examined behaviour under starved conditions, under which AKH signalling is normally high. As expected, we found that while control females increased their yeast consumption in response to mating, similarly conditioned AKH mutant virgins displayed a striking overconsumption of yeast food that was not significantly altered by mating (Fig. 8i). Activation of the APCs to induce AKH release in the fed state, in which AKH signalling is generally lower, did not alter yeast intake in fed virgin females (Extended Data Fig. 6f), presumably because of the already higher AKH signalling in the virgin state, whereas as mentioned above this treatment did reduce the yeast intake of fed mated females (Fig. 8e). Consistent with these findings, virgin AKH mutant females exhibited a strong preference for yeast food that did not increase significantly in response to mating as it did in control females (Fig. 8j,k and Extended Data Fig. 6g). Together, this suggests that, in mated females, NPF acts through NPFR in the APCs to repress AKH signalling, which increases yeast intake. Consistent with this, we found that inducing AKH release was sufficient to block the high yeast preference exhibited by mated females after 3 days of yeast deprivation (Fig. 8l). To demonstrate that NPF regulates food choice via AKH, we injected NPF into mated females with APC-specific NPFR knockdown. This manipulation did not increase yeast preference after 3 days’ yeast deprivation (Extended Data Fig. 6h), suggesting that NPFR in the APCs is required for NPF to promote yeast preference. Furthermore, injection of NPF into AKH mutant virgin females also did not alter sugar versus yeast preference, indicating that AKH is required for mediating the effects of NPF-NPFR on food preference (Extended Data Fig. 6i). We propose that sugar-induced AKH-repressive NPF signalling from the gut constitutes a hormonal axis involved in suppressing sugar appetite and promoting intake of protein-rich food in mated females (Fig. 8m).
Discussion
To maintain nutritional homeostasis, animals need to match their ingestion of specific nutrients to their needs. This is achieved by modulating appetite towards the specific nutrients needed. A number of factors, including gut hormones, that regulate food consumption have been identified in both flies and mammals, and reports have also described central brain mechanisms that induce ingestion of protein food in response to amino-acid deprivation, that sense amino acids and promote food consumption and that reject food lacking essential amino acids1,6,12,41,42. However, little is known about the hormonal mechanisms that regulate nutrient-specific appetite, and gut hormones that regulate selective food intake are completely unknown. Our findings indicate that, in mated female Drosophila, gut-derived NPF is a selective driver of sugar satiety and protein consumption, providing a basis for understanding these mechanisms. Hormone-based therapies that inhibit appetite offer promising new directions for weight-loss treatment14. For example, Fibroblast growth factor 21 (FGF21) is a liver-derived hormone that promotes protein consumption, and it is emerging as a promising target for metabolic disorders43. Uncovering appetite-regulatory hormones such as gut-derived NPF that specifically inhibit sugar consumption while promoting the intake of protein-rich foods could provide effective new weight-management strategies by promoting healthier food choices.
The SLC2-family sugar transporter Sut2 is the closest Drosophila homologue of human SLC2A7 (GLUT7), a transporter expressed mainly in the intestine whose function is poorly defined44. In flies, GLUT1 is important for Bursicon secretion from the EECs, and Sut1, another SLC2-family sugar transporter protein, was recently shown to be involved in midgut NPF release in virgin females20,21. Our results implicate Sut2 in the release of NPF from EECs in mated females and thus link it to the mechanism by which NPF-mediated gut signalling controls feeding decisions. This indicates that both Sut1 and Sut2 sugar transporters are involved in glucose-stimulated NPF secretion from the gut. In mammals, several mechanisms also regulate glucose-stimulated GLP-1 secretion from intestinal endocrine cells, which involves sodium-glucose cotransporter 1 (SGLT1), the glucose transporter GLUT2 and sweet taste receptors45. Targeting of these intestinal glucose-sensing mechanisms therefore has become a focus of weight-management therapies because of its potential in regulating appetite and incretin effects46. Future studies should investigate whether GLUT7, like its Drosophila homologue Sut2, affects appetite-regulatory mechanisms in the mammalian gut.
NPF is orthologous with the mammalian NPY family of gut-brain peptides, including peptide YY (PYY), pancreatic polypeptide and NPY itself, that regulate food-seeking behaviours and metabolism47,48. Like mammalian NPY-family hormones, Drosophila NPF is expressed in both the nervous system and the gut. While NPY is abundant in the nervous system and, like brain NPF, promotes food intake, PYY is mainly produced by endocrine cells of the gut as a satiety factor. Gut-expressed PYY is homologous to NPY, and both act through specific G-protein coupled receptors, called NPY receptors (NPYRs), that are orthologous with Drosophila NPFR49. Thus, in mammals, multiple NPY-family peptides from different tissues sources exert their functions on target organs through several related NPYRs, while in Drosophila, these functions may be regulated through the single peptide–receptor pair of NPF and NPFR47.
Our results indicate that gut-derived Drosophila NPF fulfils the function of mammalian PYY. PYY is produced by the endocrine l-cells of the gut, which, like the EECs of Drosophila, produce a context-dependent combination of multiple hormones49. The physiological role of PYY in feeding regulation has been difficult to clarify, but it is believed to act through different NPYRs on tissues including the hypothalamus and the pancreatic islets to suppress appetite. Our findings show that, in flies, NPF injection strongly reduces the intake of sugar-containing food and promotes the ingestion of protein-rich food. In humans, PYY infusion also been shown to strongly reduce food intake. Although the satiety function of human PYY has made it a prime therapeutic target for potential weight management, it is not clear whether PYY regulates nutrient-specific appetite, which would be important from a therapeutic perspective. Our results indicate that Drosophila gut NPF, perhaps filling the role of mammalian gut PYY, acts to mediate sugar-specific satiety, illustrating a key hormonal mechanism that underlies selective hunger by which animals adjust their intake of specific nutrients.
Feeding decisions are based on internal state and exhibit sexual dimorphism. In Drosophila, males and females differ in their preference for and intake of dietary sugar and protein6. Our findings define a complex interorgan communication system through which mating influences food choices in females. We have found that midgut NPF is involved in mediating SP-induced postmating responses in females, inhibiting sugar appetite and promoting the ingestion of protein-rich yeast food, and we have further shown that AKH is required for mediating the effects of NPF. When mated females consume dietary carbohydrates, NPF is released from the EECs and inhibits the AKH axis by directly suppressing AKH release from the APCs as well as by inhibiting the release of midgut AstC, a factor that stimulates AKH secretion18. Furthermore, NPF acts directly on the fat body through NPFR to inhibit energy mobilization, thereby antagonizing AKH-mediated signalling in the adipose tissue. Likewise, mammalian NPY-family peptides also regulate metabolism by direct actions on adipose tissue via NPYR50. Although a number of studies have demonstrated that AKH is a regulator of metabolism (reviewed in ref. 2), our findings uncover a key role of AKH in governing nutrient-specific feeding decisions. It is becoming clear that the APCs integrate many signals that affect AKH release18,20,21,36,51,52, and these signals may therefore also affect food choice. The APCs therefore seem to function as a signal-integration hub, similar to the IPCs, which receive many different inputs to control insulin production and release. AstC, Bursicon and NPF from the gut control AKH expression and secretion, indicating that multiple signals, even from the same organ, converge on the APCs. These signals presumably convey different aspects of nutritional status and may act with different dynamics to regulate AKH production and/or release, or even in a redundant manner to regulate AKH signalling. Likewise, many signals released from the fat body convey similar and seemingly redundant nutritional information to the IPCs2,53,54.
Recent work has also revealed a sex-specific role of AKH, with lower activity in females underlying differences in male and female metabolism40. Consistent with this notion, our results indicate that in mated females the midgut NPF system inhibits AKH signalling, suppressing intake of sugar-rich food. Furthermore, we recently showed that in mated females, midgut-derived AstC acts in a sex-specific manner through AKH to coordinate metabolism and food intake under nutritional stress18. Our work here shows that NPF also works sex-specifically to sustain physiological requirements in mated females by signalling from the gut to control AKH, suggesting that the gut-AKH axis occupies a central link in the hormonal relays underlying sex-specific regulation of physiology. A recent report showed that female germline cells modulate sugar appetite, but this effect is not induced by mating and does not affect yeast feeding55 as we have found here for gut NPF and AKH, suggesting that it is an independent mechanism.
How nutrient signals from the gut modulate feeding is key to understanding how nutritional needs are translated into specific feeding actions to maintain balance. We have identified a homeostatic circuit triggered by gut-derived NPF that limits sugar consumption. Similar mechanisms for sugar-induced satiety that promote protein consumption may also enable mammals to balance their intake of different nutrients with their metabolic needs. Explaining how nutrient-responsive gut hormones such as NPF affect dietary choice is important to better understand hunger and cravings for specific nutrients that may ultimately lead to obesity.
Methods
Drosophila stocks and husbandry
Flies were reared on a standard cornmeal diet (82 g l−1 cornmeal, 60 g l−1 sucrose, 34 g l−1 yeast, 8 g l−1 agar, 4.8 ml l−1 propionic acid and 1.6 g l−1 Tegosept/methyl-4-hydroxybenzoate) at 25 °C and 60% humidity with a 12-h light:12-h dark daily cycle. Flies were transferred after eclosion to an adult-optimized cornmeal-free diet (90 g l−1 sucrose, 80 g l−1 yeast, 10 g l−1 agar, 5 ml l−1 propionic acid and 15 ml l−1 of 10% methyl-4-hydroxybenzoate in ethanol)23 and aged for 4–7 d before experiments. Virgin female flies were collected within 3–5 h of eclosion, whereas mated flies were sorted by sex 1 d before experiments. Genotypes that contained temperature-sensitive Tubulin-GAL80TS were raised at 18 °C and kept on adult food for 3–4 d posteclosion, after which they were incubated at 29 °C for 5 d to induce RNAi effects before experiments began. The animals were transferred to fresh food every third day. The following lines were obtained from the University of Indiana Bloomington Drosophila Stock Center (BDSC): AKH-GAL4 (no. 25684); AstC::2A::GAL4 (no. 84595)25; CaLexA system (no. 66542: LexAop-CD8::GFP::2A::CD8::GFP; UAS-LexA::VP16::NFAT, LexAop-CD2::GFP/TM6B, Tb)56; Cg-GAL4 (no. 7011); da-GAL4 (no. 55850); elav-GAL4 (no. 458); NPF::2A::GAL4 (no. 84671)25; SP0 mutant (no. 77892); Tub-GAL80TS (no. 7108); UAS-mCD8::GFP (no. 5137); UAS-NPF-RNAiTRiP (no. 27237); UAS-NPFR-RNAiTRiP (no. 25939); 10xUAS-IVS-myr::tdTomato[su(Hw)attP8] (no. 32223); UAS-sut1-RNAi (no. 65964) and UAS-TrpA1 (no. 26263). Other lines were obtained from the Vienna Drosophila Resource Center: the control line w1118 (no. 60000, isogenic with the Vienna Drosophila Resource Center RNAi lines) as well as several UAS-RNAi lines including ones targeting AKH (no. 105063), Mondo (no. 109821), NPF (NPFiKK, no. 108772 and NPFish, no. 330277), NPFR (NPFRiGD, no. 9605), SPR (no. 106804) and sut2 (no. 102028). A second UAS-TrpA1 insertion, into attP2, was a kind gift from C. Wegener (University of Würzburg). voilà-GAL4 (ref. 57) was kindly given by A. Scopelliti (University of Glasgow). R57C10-GAL80-6 (refs. 58–63) on the X chromosome was a kind gift from R. Niwa (University of Tsukuba). AKH mutant64 and NPFR::T2A::GAL433 were kind gifts from S. Kondo (Tokyo University of Science). Df(3L)delta130 was a kind gift from A. von Philipsborn (Aarhus University). UAS-LexA::VP16::NFAT; LexAop-luciferase was a kind gift from M. Rosbash (Brandeis University). The fly lines used are listed in Supplementary Table 1. No ethical approval is needed for the use of the fruit fly Drosophila. For standardizing the genetic background and generating controls with proper genetic background, all GAL4 lines and GAL80 lines used this study were backcrossed for several generations to the same w1118 genetic background population before they were used in a final outcross with the genetic background of the RNAi lines and used as controls18.
Starvation-survival assay
Flies were transferred without anaesthesia to vials containing starvation medium (1% agar in water) and kept either at 29 or 25 °C, depending on whether they carried GAL80TS. Forty to 150 animals, at 10–15 flies per vial, were assayed for each genotype/sex. Dead animals were counted every 4–8 h. The statistical significance of survival differences was determined by using the Kaplan–Meier log-rank survival test or Gehan–Breslow–Wilcoxon survival test in the Prism software package (GraphPad v.9).
Feeding assays
Short-term food consumption was measured by using a spectrophotometric dye-feeding assay65,66, and all food intake experiments were performed during the time when animals have their morning meal (1 h after lights on; 12/12 h light/dark cycle). During the morning meal (after lights on), flies were transferred without anaesthesia to adult-optimized food containing 0.5% erioglaucine dye (brilliant blue R, FD&C Blue No. 1, Sigma-Aldrich, no. 861146) and allowed to feed for 30 min, if the flies had been 15 h starved to stimulate food intake or for 2–3 h if not. Another set of flies was fed with undyed food to measure the baseline absorbance of fly lysates. For two-choice assays, the protocol of Ribeiro and Dickson6 was used with some modifications. Briefly, 25 flies were lightly anaesthetized with CO2 before being placed into a 60-mm Petri dish with a chequerboard array of 20-μl patches of alternative diets containing either 100 g l−1 of sucrose and dyed red with 0.5% amaranth (Sigma no. A1016), or 100 g l−1 yeast (dyed with 0.5% erioglaucine) and allowed to eat for 2 h in the dark. For each genotype, 10–25 samples of 1–2 flies each were homogenized in 100 μl of phosphate buffer, pH 7.5, using a TissueLyser LT (Qiagen) bead mill with 5-mm stainless-steel beads (Qiagen, no. 69989). Homogenates were centrifuged at 16,000g for 5 min and 50 μl of each cleared supernatant was loaded into a 384-well plate. Sample absorbance was measured at 520 nm (amaranth) and at 629 nm (erioglaucine) on an Ensight multimode plate reader (PerkinElmer). Standard curves for erioglaucine and amaranth were used to convert absorbance values to food consumption amounts.
Long-term food intake was monitored using the CAFÉ capillary-feeding assay24. For one-choice consumption assays, assay tubes were constructed by inserting a 5-μl microcapillary (Hirschmann) through a hole in the lid of a 2-ml Eppendorf tube. The capillary was filled with a liquid sugar or yeast-extract medium24 containing 100 g l−1 sucrose or 100 g l−1 yeast extract, with 1 ml l−1 propionic acid and 1 g l−1 methyl-4-hydroxybenzoate preservatives, before the start of the experiment. For sugar-preference assays, two capillaries were inserted into each tube, one filled with 10 g l−1 sucrose solution and the other filled with 100 g l−1 sucrose solution. Individual flies were briefly anaesthetized on ice and placed into assay tubes, and the tubes were placed inside a moist chamber within a standard fly incubator. The level of the meniscus in each tube was measured at intervals. Tubes containing no flies were used as controls for evaporation; the amount of meniscus movement in these tubes was subtracted from the other measurements.
To monitor feeding behaviour, interactions with food were measured over a 20–24 h period using the FLIC assay22. Drosophila feeding monitors (DFMs) (Sable Systems) were installed in an incubator (25 or 29 °C if GAL80TS was present; 70% humidity, 12/12-h light/dark cycle). Feeding wells were filled with a 10% sucrose solution, and individual flies were placed in each of the 12 chambers of the DFMs in the afternoon (after the morning meal) and left to acclimate for several hours, after which evening feeding data were recorded. The next morning, at lights on, fresh sugar solution was added to the DFMs and morning meal data were recorded. In two-choice sugar-preference experiments, half of the DFM wells were filled with 1% sugar solution and the other half with 10% sugar solution. The data were recorded using the manufacturer’s software and analyzed in R Studio using the published package, available at https://github.com/PletcherLab/FLIC_R_Code.
For flyPAD28 two-choice behavioural experiments, 4–7-day-old female flies, mated or virgin, were either starved for 15 h on 1% agar (to establish a low yeast-preference baseline for experiments in which a manipulation was anticipated to increase this preference) or yeast-deprived for 3 days by keeping them on medium containing 10% sucrose and 1% agar (to establish a higher baseline yeast preference, for experiments in which yeast preference was expected to decrease). Flies were briefly immobilized on ice and transferred with a brush into flyPAD behavioural arenas. They were left to acclimate for several minutes before data acquisition was started. The flies were allowed to choose between food droplets containing 1% agarose and either 10% sucrose or 10% yeast. The food was aliquoted into 1.5-ml tubes and kept frozen at −20 °C. Before each experiment, the tubes were placed into a heat block for melting at 90 °C and 3 µl of food was loaded into each well. A package created by the developer for the Bonsai data-stream processing program was used to acquire the data. Data processing was done by using the developer’s software, which is available at http://www.flypad.pt.
Video recording of feeding behaviour
Behaviour chambers (40 mm in diameter) were coated with fluon on the top and sides to prevent flies’ walking on these surfaces. Fifteen-microlitre patches of either 10% sucrose or 10% yeast (with no dyes) were placed in a circular pattern within the arena. Twelve 15-hour-starved animals per genotype were introduced into the chamber and were allowed to acclimate in darkness for a few minutes. The behaviour chambers were placed on an infrared-light transilluminator viewed by a Basler camera, and half-hour videos were recorded at 15 Hz using an imaging setup described elsewhere67. Flies were tracked using the Ctrax software68, and locomotion data were analyzed using custom MATLAB code (Code availability statement).
Immunohistochemistry and confocal imaging
Adult midguts, CNSs and fat bodies were dissected in cold PBS and fixed for 1 h in 4% paraformaldehyde at room temperature with agitation. Anterior parts of guts, containing APCs (CCs) were first fixed for 30 min, finely dissected and fixed for a further 30 min. Fixed tissues were quickly rinsed once with PBST (PBS with 0.1% Triton X-100, Merck no. 12298) and washed in PBST three times for 15 min each. Washed tissues were incubated in blocking solution (PBST containing 5% normal goat serum (Sigma)) for 30 minutes at room temperature and incubated with primary antibodies diluted in blocking solution overnight (or 2 d for CNS samples) at 4 °C with gentle agitation. Primary-antibody solution was removed, and the tissues were rinsed once and washed three times, 20 minutes each, with PBST. Tissues were incubated with secondary antibodies diluted in PBST overnight at 4 °C, washed three times with PBST and mounted in Vectashield mounting medium containing 4,6-diamidino-2-phenylindole (Vector Laboratories, no. H-1200) on slides treated with poly-l-lysine (Sigma, no. P8920). Tissues were scanned on a Zeiss LSM-900 confocal microscope using a 20× air objective using the Zen software package. Image analysis was carried out using the open-source program ImageJ69. For quantification of NPF, AstC, AKH and GFP (CaLexA reporter) staining intensity, samples to be compared were stained simultaneously using the same reagent preparations and imaged with the same settings. Relevant midgut regions (the entire NPF-expressing region or the AstC-positive region) were tiled at 20× with 10–20 Z-stacks of at least 100 planes separated by 1 μm. Tiled stacks were stitched into a single large stack for each gut using the Stitching function of Zeiss Zen Blue v.3.1. For quantification of NPF and CaLexA staining, a binary mask containing identified cells was created using local thresholding in ImageJ. This mask was manually curated in ImageJ by comparison with the raw image data, and incorrectly joined cells were manually resegmented. In a custom MATLAB script (Code availability statement), this mask was applied to the image data to segment out each cell. Staining intensity within each cell was summed, and the local background of each cell was removed by measuring the signal around the circumference of each cell. For AstC quantifications, stacks were Z-projected using the sum method. Cells were manually segmented, and their intensity was measured using ImageJ with local background subtraction. We integrated a UAS-tdTomato transgene into the CaLexA system to normalize calcium-dependent GFP fluorescence. Antibodies used included a rabbit antibody against the processed AKH peptide37, a kind gift of J. Park, University of Tennessee, 1:500; rabbit anti-NPF (Ray BioTech, no. RB-19-0001-20), 1:500; rabbit anti-AstC70, kindly given by J. Veenstra, University of Bordeaux and M. Zandawala, Brown University, 1:500; mouse anti-GFP (ThermoFisher, no. A11120), 1:500; rat anti-mCherry (used against tdTomato; ThermoFisher, no. M11217), 1:2,000; mouse anti-Prospero (University of Iowa Developmental Studies Hybridoma Bank, no. MR1A), 1:20; Alexa Fluor 488-conjugated goat anti-mouse (ThermoFisher, no. A32723), 1:500; Alexa Fluor 555-conjugated goat anti-rabbit (ThermoFisher, no. A32732), 1:500; Alexa Fluor 555-conjugated goat anti-rat (ThermoFisher, no. A21434), 1:500 and Alexa Fluor 405-conjugated goat anti-rabbit (ThermoFisher, no. 31556), 1:500.
Injection and methoprene-treatment experiments
Synthetic amidated NPF peptide (SNSRPPRKNDVNTMADAYKFLQDLDTYYGDRARVRFamide) was a kind gift from F. Hauser (University of Copenhagen). Peptide was dissolved at 25 μM in a synthetic haemolymph-like buffer71 containing 5 mM glucose, 5 mM trehalose and 110 mM sucrose (inert osmolyte). Flies were reared at 18 °C as described above. After 4 days at 29 °C to permit GAL4 activity, flies were starved on 1% agar for 15 h or protein-deprived on sugar-agar for 3 days. Flies were immobilized on ice and 50 nl of haemolymph-like solution with or without NPF was injected into each animal at the lateral mid-thorax ventral to the wings using a Nanoject II injector (Drummond Scientific). Assuming each injected animal contained 1 μl of haemolymph, the final NPF concentration in the injected animals was increased by 1.25 μM, a level that should strongly activate NPFR (IC50 roughly 60 nM, ref. 72). Animals were allowed to recover for 30 min at 29 °C before use in dye-feeding assays as described above.
Because the juvenile hormone analogue methoprene (Sigma-Aldrich no. 33375) is not thermally stable, the working solution (0.01 µg µl−1 in acetone) or vehicle (pure acetone) was applied to the surface of cooled, solidified fly medium instead of being mixed into melted medium before solidification. We applied 32 µl of hormone or vehicle solution to the surface of 2 ml of fly medium in 25-mm diameter plastic vials (in total, 0.32 µg per vial), an amount that should be effective while also being well tolerated by the animals73. Treated media were kept at room temperature for roughly 12 h to allow acetone evaporation before flies were added.
Metabolite measurements
Triglyceride and glycogen levels were measured using established protocols23,74. For each genotype, ten batches of three flies each were homogenized in PBS containing 0.05% Tween-20 (Sigma no. 1379) in a TissueLyser LT (Qiagen) bead mill with 5-mm stainless-steel beads. Glycogen was measured by hydrolysing glycogen into glucose by using amyloglucosidase (Sigma, no. A7420) followed by colorimetric glucose measurement (Sigma, no. GAGO20). TAG levels were assayed by cleaving their ester bonds using Triglyceride Reagent (Sigma, no. T2449) to obtain free glycerol, the level of which was then colorimetrically measured using the Free Glycerol Reagent (Sigma, no. F6428). For determination of circulating glucose concentration, haemolymph was extracted as described previously23 and glucose was measured using the colorimetric assay (Sigma, no. GAGO20). Each sample’s absorbance at 540 nm was measured in a 384-well plate using an Ensight multimode plate reader (PerkinElmer) and converted to metabolite concentrations using glycerol and glucose standard curves. Measurements are reported on a per-fly basis.
Luciferase assay
Female guts were dissected into lysis buffer (Promega, no. E2920). For each condition, 4–7 replicates with two guts in each were homogenized in 50 µl of lysis buffer in 2-ml round-bottomed Eppendorf tubes using a TissueLyser LT (Qiagen) bead mill with 5-mm stainless-steel beads (Qiagen, no. 69989). Homogenates were centrifuged at 21,000g for 5 min, and the supernatant was transferred into new tubes and centrifuged a second time. Ten microlitres of the cleared supernatant were loaded into a 384-well plate, and 10 µl of Dual Glo Stop & Glo Reagent (Promega) was added. The plate was left to incubate for 15 min at room temperature to allow for the reaction to pass from the burst phase into the glow phase, after which luciferase activity was measured using the luminescence mode of an Ensight multimode plate reader (PerkinElmer).
Transcript measurement using quantitative PCR
Six tissue replicates (each containing five CNSs, five midguts or five CC-containing anterior parts of guts) per condition or genotype were homogenized in 2-ml Eppendorf tubes containing lysis buffer with 1% beta-mercaptoethanol using a TissueLyser LT bead mill (Qiagen) and 5-mm stainless-steel beads (Qiagen no. 69989). RNA purification was performed using the NucleoSpin RNA kit (Macherey-Nagel, no. 740955) according to the manufacturer’s instructions. Complementary DNA was synthesized using the High-Capacity cDNA Synthesis kit (Applied Biosystems, no. 4368814). Quantitative PCR was done using RealQ Plus 2× Master Mix Green (Ampliqon, no. A324402) on a QuantStudio 5 (Applied Biosystems) machine. Results were normalized against the housekeeping gene Rp49 using the delta-delta-Ct method. The oligos used are listed in Supplementary Table 2.
Statistics
All statistics were computed using the Prism analysis package (GraphPad v.9). Starvation-survival curves were analyzed using Kaplan–Meier log-rank tests or Gehan–Breslow–Wilcoxon test. Other data were assessed for normality before comparisons were performed. For normally distributed data, pairwise comparisons were made using two-tailed unpaired Student’s t-tests and multiple samples were compared using one-way analysis of variance (ANOVA) with post hoc multiple-comparisons tests. Other data were compared using two-tailed unpaired Mann–Whitney U-tests or one-way Kruskal–Wallis ANOVA followed by multiple-comparisons tests. Bar plots show the mean plus or minus the standard error of the mean (s.e.m.). Box plots that show the median and the first and third quartile, with whiskers indicating the full range of values. No data were excluded. Sample size was chosen on the basis of similar previously published studies of Drosophila behaviour and metabolism17,18,20,55. No sample-size calculations were performed.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Supplementary Tables 1–2
Acknowledgements
Anti-Akh was a kind gift from J. Park (University of Tennessee). Anti-AstC was a generous gift from J. Veenstra (University of Bordeaux) and M. Zandawala (Brown University). R57C10-GAL80 was kindly given by R. Niwa (University of Tsukuba). AKH mutant and NPFR::2A::GAL4 flies were kindly provided by S. Kondo (Tokyo University of Science). UAS-TrpA1[attP2] was kindly given by C. Wegener (University of Würzburg). voilà-GAL4 (ref. 57) was kindly given by A. Scopelliti (University of Glasgow). Df(3L)delta130 (SP deletion) was a kind gift from A. von Philipsborn (Aarhus University). UAS-LexA::VP16::NFAT; LexAop-luciferase was a kind gift from M. Rosbash (Brandeis University). We thank the Vienna Drosophila Resource Center and the University of Indiana Bloomington Drosophila Stock Center for fly lines, and we also thank the University of Iowa Developmental Studies Hybridoma bank for providing anti-Prospero. This work was supported by Novo Nordisk Foundation grant no. NNF19OC0054632 and Lundbeck Foundation grant no. 2019-772 to K.R. T.K. and K.V.H. were supported by funding from the Villum Foundation (grant no. 15365) and Danish Council for Independent Research Natural Sciences (grant no. 9064-00009B) to K.V.H. The Zeiss LSM 900 confocal microscope was purchased with a generous grant from the Carlsberg Foundation (grant no. CF19-0353).
Extended data
Source data
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Numerical chart data.
Author contributions
A.M., O.K. and K.R. conceived and designed the study. A.M., O.K., T.K., N.A., M.J.T., S.N., K.V.H., and K.R. designed, performed and analyzed experiments. A.M., O.K., M.J.T. and K.R. wrote the manuscript.
Peer review
Peer review information
Nature Metabolism thanks Herbert Herzog and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Data availability
All data generated or analyzed during this study are available as Source Data files, which are provided with this paper.
Code availability
The custom MATLAB scripts used for image analysis and for locomotion data analysis in this study are publicly available at 10.5281/zenodo.6641933 and 10.5281/zenodo.6641957.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alina Malita, Olga Kubrak.
Extended data
is available for this paper at 10.1038/s42255-022-00672-z.
Supplementary information
The online version contains supplementary material available at 10.1038/s42255-022-00672-z.
References
- 1.Itskov PM, Ribeiro C. The dilemmas of the gourmet fly: the molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front Neurosci. 2013;7:12. doi: 10.3389/fnins.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koyama T, Texada MJ, Halberg KA, Rewitz K. Metabolism and growth adaptation to environmental conditions in Drosophila. Cell. Mol. Life Sci. 2020;77:4523–4551. doi: 10.1007/s00018-020-03547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter CP. Increased salt appetite in adrenalectomized rats. Am. J. Physiol. 1936;115:155–161. [Google Scholar]
- 4.Richter CP. Total self regulatory functions of animals and human beings. Harvey Lect. Ser. 1943;38:63–103. [Google Scholar]
- 5.Khan, M. S. et al. Protein appetite at the interface between nutrient sensing and physiological homeostasis. Nutrients10.3390/nu13114103 (2021). [DOI] [PMC free article] [PubMed]
- 6.Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 2010;20:1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Rebello CJ, O’Neil CE, Greenway FL. Gut fat signaling and appetite control with special emphasis on the effect of thylakoids from spinach on eating behavior. Int J. Obes. 2015;39:1679–1688. doi: 10.1038/ijo.2015.142. [DOI] [PubMed] [Google Scholar]
- 8.Dus M, et al. Nutrient sensor in the brain directs the action of the brain-gut axis in Drosophila. Neuron. 2015;87:139–151. doi: 10.1016/j.neuron.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, et al. Branch-specific plasticity of a bifunctional dopamine circuit encodes protein hunger. Science. 2017;356:534–539. doi: 10.1126/science.aal3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001;12:S63–S68. doi: 10.1093/annonc/12.suppl_2.s63. [DOI] [PubMed] [Google Scholar]
- 11.Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu Rev. Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- 12.Perry B, Wang Y. Appetite regulation and weight control: the role of gut hormones. Nutr. Diabetes. 2012;2:e26. doi: 10.1038/nutd.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasoamanana R, Darcel N, Fromentin G, Tome D. Nutrient sensing and signalling by the gut. Proc. Nutr. Soc. 2012;71:446–455. doi: 10.1017/S0029665112000110. [DOI] [PubMed] [Google Scholar]
- 14.Finer N. Future directions in obesity pharmacotherapy. Eur. J. Intern. Med. 2021;93:13–20. doi: 10.1016/j.ejim.2021.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Miguel-Aliaga I, Jasper H, Lemaitre B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics. 2018;210:357–396. doi: 10.1534/genetics.118.300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, et al. Physiological and pathological regulation of peripheral metabolism by gut-peptide hormones in Drosophila. Front. Physiol. 2020;11:577717. doi: 10.3389/fphys.2020.577717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ameku T, et al. Midgut-derived neuropeptide F controls germline stem cell proliferation in a mating-dependent manner. PLoS Biol. 2018;16:e2005004. doi: 10.1371/journal.pbio.2005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubrak O, et al. The gut hormone Allatostatin C/Somatostatin regulates food intake and metabolic homeostasis under nutrient stress. Nat. Commun. 2022;13:692. doi: 10.1038/s41467-022-28268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, H. H. et al. A nutrient-specific gut hormone arbitrates between courtship and feeding. Nature10.1038/s41586-022-04408-7 (2022). [DOI] [PMC free article] [PubMed]
- 20.Scopelliti A, et al. A neuronal relay mediates a nutrient responsive gut/fat body axis regulating energy homeostasis in adult Drosophila. Cell Metab. 2019;29:269–284 e210. doi: 10.1016/j.cmet.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshinari Y, et al. The sugar-responsive enteroendocrine neuropeptide F regulates lipid metabolism through glucagon-like and insulin-like hormones in Drosophila melanogaster. Nat. Commun. 2021;12:4818. doi: 10.1038/s41467-021-25146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ro J, Harvanek ZM, Pletcher SD. FLIC: high-throughput, continuous analysis of feeding behaviors in Drosophila. PLoS ONE. 2014;9:e101107. doi: 10.1371/journal.pone.0101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tennessen JM, Barry WE, Cox J, Thummel CS. Methods for studying metabolism in Drosophila. Methods. 2014;68:105–115. doi: 10.1016/j.ymeth.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ja WW, et al. Prandiology of Drosophila and the CAFE assay. Proc. Natl Acad. Sci. USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng B, et al. Chemoconnectomics: mapping chemical transmission in Drosophila. Neuron. 2019;101:876–893 e874. doi: 10.1016/j.neuron.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 26.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo F, Chen X, Rosbash M. Temporal calcium profiling of specific circadian neurons in freely moving flies. Proc. Natl Acad. Sci. USA. 2017;114:E8780–E8787. doi: 10.1073/pnas.1706608114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itskov PM, et al. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 2014;5:4560. doi: 10.1038/ncomms5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiff T, et al. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. eLife. 2015;4:e06930. doi: 10.7554/eLife.06930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havula E, Hietakangas V. Sugar sensing by ChREBP/Mondo-Mlx-new insight into downstream regulatory networks and integration of nutrient-derived signals. Curr. Opin. Cell Biol. 2018;51:89–96. doi: 10.1016/j.ceb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Yu, Y. et al. Regulation of starvation-induced hyperactivity by insulin and glucagon signaling in adult Drosophila. eLife10.7554/eLife.15693 (2016). [DOI] [PMC free article] [PubMed]
- 32.Chung BY, et al. Drosophila neuropeptide F signaling independently regulates feeding and sleep-wake behavior. Cell Rep. 2017;19:2441–2450. doi: 10.1016/j.celrep.2017.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo S, et al. Neurochemical organization of the Drosophila brain visualized by endogenously tagged neurotransmitter receptors. Cell Rep. 2020;30:284–297 e285. doi: 10.1016/j.celrep.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Sudhakar SR, et al. Insulin signalling elicits hunger-induced feeding in Drosophila. Dev. Biol. 2020;459:87–99. doi: 10.1016/j.ydbio.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Inagaki HK, Panse KM, Anderson DJ. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron. 2014;84:806–820. doi: 10.1016/j.neuron.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koyama T, et al. A nutrient-responsive hormonal circuit mediates an inter-tissue program regulating metabolic homeostasis in adult Drosophila. Nat. Commun. 2021;12:5178. doi: 10.1038/s41467-021-25445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung RJ, et al. A cell atlas of the adult Drosophila midgut. Proc. Natl Acad. Sci. USA. 2020;117:1514–1523. doi: 10.1073/pnas.1916820117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jourjine N, Mullaney BC, Mann K, Scott K. Coupled sensing of hunger and thirst signals balances sugar and water consumption. Cell. 2016;166:855–866. doi: 10.1016/j.cell.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wat, L. W., Chowdhury, Z. S., Millington, J. W., Biswas, P. & Rideout, E. J. Sex determination gene transformer regulates the male-female difference in Drosophila fat storage via the adipokinetic hormone pathway. eLife10.7554/eLife.72350 (2021). [DOI] [PMC free article] [PubMed]
- 41.Bjordal M, Arquier N, Kniazeff J, Pin JP, Leopold P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 2014;156:510–521. doi: 10.1016/j.cell.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z, et al. A post-ingestive amino acid sensor promotes food consumption in Drosophila. Cell Res. 2018;28:1013–1025. doi: 10.1038/s41422-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tillman EJ, Rolph T. FGF21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front. Endocrinol. 2020;11:601290. doi: 10.3389/fendo.2020.601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheeseman C. GLUT7: a new intestinal facilitated hexose transporter. Am. J. Physiol. Endocrinol. Metab. 2008;295:E238–E241. doi: 10.1152/ajpendo.90394.2008. [DOI] [PubMed] [Google Scholar]
- 45.Sun EW, et al. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes. 2017;66:2144–2149. doi: 10.2337/db17-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams DM, Nawaz A, Evans M. Drug therapy in obesity: a review of current and emerging treatments. Diabetes Ther. 2020;11:1199–1216. doi: 10.1007/s13300-020-00816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fadda M, et al. Regulation of feeding and metabolism by neuropeptide F and short neuropeptide F in invertebrates. Front Endocrinol. 2019;10:64. doi: 10.3389/fendo.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh K, Herzog H, Shi YC. Regulation of energy homeostasis by the NPY system. Trends Endocrinol. Metab. 2015;26:125–135. doi: 10.1016/j.tem.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Lafferty RA, Flatt PR, Irwin N. Established and emerging roles peptide YY (PYY) and exploitation in obesity-diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2021;28:253–261. doi: 10.1097/MED.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Cline MA, Gilbert ER. Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutr. Metab. 2014;11:27. doi: 10.1186/1743-7075-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh Y, et al. A glucose-sensing neuron pair regulates insulin and glucagon in Drosophila. Nature. 2019;574:559–564. doi: 10.1038/s41586-019-1675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pauls D, et al. Endocrine signals fine-tune daily activity patterns in Drosophila. Curr. Biol. 2021;31:4076–4087 e4075. doi: 10.1016/j.cub.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Okamoto N, Watanabe A. Interorgan communication through peripherally derived peptide hormones in Drosophila. Fly. 2022;16:152–176. doi: 10.1080/19336934.2022.2061834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Texada MJ, Koyama T, Rewitz K. Regulation of body size and growth control. Genetics. 2020;216:269–313. doi: 10.1534/genetics.120.303095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carvalho-Santos Z, et al. Cellular metabolic reprogramming controls sugar appetite in Drosophila. Nat. Metab. 2020;2:958–973. doi: 10.1038/s42255-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 56.Masuyama K, Zhang Y, Rao Y, Wang JW. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J. Neurogenet. 2012;26:89–102. doi: 10.3109/01677063.2011.642910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balakireva M, Gendre N, Stocker RF, Ferveur JF. The genetic variant Voila causes gustatory defects during Drosophila development. J. Neurosci. 2000;20:3425–3433. doi: 10.1523/JNEUROSCI.20-09-03425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl Acad. Sci. USA. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shirangi TR, Stern DL, Truman JW. Motor control of Drosophila courtship song. Cell Rep. 2013;5:678–686. doi: 10.1016/j.celrep.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfeiffer BD, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. Proc. Natl Acad. Sci. USA. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris, R. M., Pfeiffer, B. D., Rubin, G. M. & Truman, J. W. Neuron hemilineages provide the functional ground plan for the Drosophila ventral nervous system. eLife10.7554/eLife.04493 (2015). [DOI] [PMC free article] [PubMed]
- 64.Hentze JL, Carlsson MA, Kondo S, Nassel DR, Rewitz KF. The neuropeptide allatostatin A regulates metabolism and feeding decisions in Drosophila. Sci. Rep. 2015;5:11680. doi: 10.1038/srep11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong R, Piper MDW, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS ONE. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagy S, et al. AMPK signaling linked to the schizophrenia-associated 1q21.1 deletion is required for neuronal and sleep maintenance. PLoS Genet. 2018;14:e1007623. doi: 10.1371/journal.pgen.1007623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat. Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veenstra JA, Agricola HJ, Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- 71.Feng Y, Ueda A, Wu CF. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J. Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- 72.Garczynski SF, Brown MR, Shen P, Murray TF, Crim JW. Characterization of a functional neuropeptide F receptor from Drosophila melanogaster. Peptides. 2002;23:773–780. doi: 10.1016/s0196-9781(01)00647-7. [DOI] [PubMed] [Google Scholar]
- 73.Wilson TG, Ashok M. Insecticide resistance resulting from an absence of target-site gene product. Proc. Natl Acad. Sci. USA. 1998;95:14040–14044. doi: 10.1073/pnas.95.24.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hildebrandt A, Bickmeyer I, Kuhnlein RP. Reliable Drosophila body fat quantification by a coupled colorimetric assay. PLoS ONE. 2011;6:e23796. doi: 10.1371/journal.pone.0023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–2
Data Availability Statement
All data generated or analyzed during this study are available as Source Data files, which are provided with this paper.
The custom MATLAB scripts used for image analysis and for locomotion data analysis in this study are publicly available at 10.5281/zenodo.6641933 and 10.5281/zenodo.6641957.