Abstract
The purpose of this study was to elucidate the roles of peptidoglycan-associated lipoprotein (Pal protein) in the proliferation of Brucella in macrophage and bacterial virulence, and to evaluate the immune effect of Pal protein to Salmonella enteritidis. Murine macrophage-like cell line Raw264.7 was stimulated by recombinant Pal protein, and the expression of TNF-α and IFN-γ were up-regulated, but not it of IL-1β and IL-6. The macrophages infection and in vitro simulated stress assays showed that deletion of pal gene reduced the proliferation of Brucella in macrophages, the survival in acidic, oxidative and polymyxin B-contained environment. The mice infection assay showed that mice challenged with the pal mutant strain were found to have more severe splenomegaly, but less bacterial load. After oral immunization of mice, Pal protein induced a higher titer of mucosal and humoral antibody (IgA and IgG) against heat-killed Salmonella enteritidis, and a stronger Th1 cellular immune response. The challengte experiments showed Pal protein elevated the survival rate and reduced the bacterial load of spleens in immunized mice. In conclusion, our results revealed the important roles of pal gene in Brucella virulence, and Pal protein was a potentially valuable adjuvant against mucosal pathogens, such as Salmonella enteritidis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00284-022-03107-w.
Introduction
Brucella is a facultative intracellular pathogen, causing Brucellosis, which spreads widely worldwide, especially in developing countries [1]. The ability to survive within macrophages and subsequently traffic the whole body is an important mechanism of Brucella virulence. Still and all, macrophages play an important role in the body’s defense against Brucella infection [2, 3]. First, macrophages can engulf and kill pathogens, and present antigens. A number of strategies, such as acidic pH, oxidizing substance and antimicrobial peptides, were adopted to kill the internalized bacteria [4, 5]. Previously researched showed that the deletion of pal gene resulted in the reduced survival rate in macrophages of some intracellular bacterium, including Brucella suis (B. suis), Salmonella and Legionella pneumoniae (L. pneumoniae) [6–9]. While, the function of pal gene in defensing against macrophage killing was not fully elucidated.
Second, by secreting a variety of cytokines, macrophages play a role in promoting inflammatory response and immune activation and regulation [10]. In Burkholderia cenocepacia, Klebsiella pneumoniae (K. pneumoniae) and Escherichia coli (E. coli), Pal can activate macrophages and induce them to secrete a variety of cytokines, and play a key role in the virulence of these bacteria [11–13]. In addition, Pal protein from Brucella can activate dendritic cells, leading to the release of cytokine TNF-α [6]. However, the effect of Pal protein on the expression of cytokines in macrophages has not been fully elucidated.
It has been reported that Brucella Pal protein was a new bacterial Pathogen-Associated Molecular Pattern that activated dendritic cells in vivo, induced a Th1 immune response, and was a promising self-adjuvanting vaccine against systemic and oral acquired Brucellosis [6]. In another studies, researchers constructed a novel recombinant Lactobacillus casei-OMP16-PEDVS strain expressing S protein of PEDV and Pal protein of Brucella abortus, and the recombinant strains could induce higher levels of humoral immunity, cellular immunity, and mucosal immunity [14]. The potential of Pal from Brucella as an adjuvant is worth further exploring.
Salmonella infection can cause a variety of livestock and poultry Salmonellosis, including systemic extra-intestinal infection and intestinal infection, clinical manifestations of sepsis and enteritis, can also lead to abortion of pregnant animals, a serious threat to the health of young animals and breeding livestock and poultry. There are many serotypes of Salmonella, and more than 2000 serotypes have been identified without complete statistics. Among these serotypes, Salmonella enteritis (S. enteritis) is a typical zoonotic pathogen with high infection rate [15–17]. Vaccine is an important means to control Salmonellosis.
Taking into account these previous results, in this study, we screened the pal gene of Brucella, and analyzed its effect on cytokine expression in macrophages. Then, simulated stress and polymyxin B tolerance assays, the macrophage survival assay, and mice challenge experiment were performed, aimed to evaluate the role of pal gene in Brucella virulence. Finally, the immune and challenge protection experiments of mice using inactivated S. enteritis and Pal protein was performed to explore the potential value of Pal protein in Salmonella vaccine development.
Materials and Methods
Strains, Cells and Mice
E. coli and Salmonella enteritidis C50336 (Table S1) was routinely cultured on LB broth or agar at 37 °C, and were added at the following concentrations: ampicillin, 100 μg/mL, when required. All of the Brucella strains (Table S1), include B. melitensis 16 M, 16MΔpal and 16MΔpal + pal. were routinely cultured in or on tryptic soy broth (TSB), tryptic soy agar(TSA) or minimal medium (0.5% lactic acid, 3% glycerol, 0.75% NaCl, 1% K2HO4, 0.01% Na2S2O3·5H2O, 10 μg/mL Mg2+, 0.1 μg/mL Fe2+, 0.1 μg/mL Mn2+, 0.21 μg/mL thiamine HCl, 0.2 μg/mL nicotinic acid, 0.04 μg/mL calcium pantothenate, 0.001 μg/mL biotin, 5 mg/mL glutamate; pH 6.8–7.0 with NaOH). All work with live Brucella strains was performed at a biosafety level three laboratory in China Institute of Veterinary Drug Control.
Murine macrophage-like cell line Raw264.7 was gained from Brucellosis Laboratory, China Agricultural University, and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 100 U/mL penicillin, 100 μg/mL streptomycin, 10% fetal bovine serum and 0.1 mmol/L nonessential amino acids. The cells were used until the third generation after cell resuscitation and discarded until 15th generation.
Female BALB/c mice (aged 4–6 weeks) were purchased from the Wei Tong Li Hua Laboratory Animal Services Centre (Beijing, China).
Sequence Analysis of Pal Gene
The comparison of nucleotide and amino acid sequence similarity were performed in Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The basic Physical and chemical properties were analyzed by DNASTAR Bioinformatics Software. The signal sequence was checked by LipoP 1.0 (http://www.cbs.dtu.dk/services/LipoP/) and SignalP 5.0 (https://services.healthtech.dtu.dk/service.php?SignalP-5.0). The protein domains, families and functional sites as well as associated patterns and profiles was analyzed by Domains & Structures (https://www.ncbi.nlm.nih.gov/guide/domains-structures/). The phylogenetic tree was constructed by MegAlign workspace of DNASTAR.
The Prokaryotic Expression and Purification of Pal Protein
The pal gene fragment was subcloned into the digested plasmid pET32a, and transformed into the expression strain BL21(DE3). After indued expression, identified, purified and endotoxin removal, rPal was used to carry out the cell stimulation experiment.
Cells Stimulation by rPal Protein and the Detection of Cytokines Production
The mice macrophage-like cell line Raw 264.7 were cultured by rPal for 24 h in a 6-well plate at a plating density of 4 × 105 CFU each well. After stimulation, the cells were harvested to extract mRNA and perform real-time PCR analysis.
Construction of B. melitensis 16 M Pal Mutant and the Complementation Strain
The pal mutant and complemented strain was constructed, according to the previous research [18], and the subsequent mutant were named as 16MΔpal and 16MΔpal + pal.
Intra-Macrophage Proliferation and the Mice Infection Assays
The intracellular proliferation in RAW 264.7 cells and BALB/c mice infection were performed as previous description [18].
In Vitro Simulated Stress and Polymyxin B Tolerance Assays
To investigate the effect of the pal gene deletion on B. melitensis, in vitro stress assays and polymyxin B (Code No. 1405-20-5, Sigma) tolerance assays were performed by 16 M, 16MΔpal and 16MΔpal + pal [5, 18]. The percent survival was calculated by dividing the live bacterial CFUs by those under physiological condition (0.85% NaCl, pH 7.0).
Preparation of Heat-Killed S. enteritidis C50336
S. enteritidis C50336 was heat-killed for preparation of heat-killed Salmonella enteritidis (HKSE). The protein concentration of HKSE was determined using the BCA Protein Assay Kit (Code No. CW0014, CWBIO).
Mice Immunization and Samples Collection
As described with reference [19], mice were immunized by intragastric administration with HKSE (5 × 108 CFU) alone or with HKSE and rPal (200 μg) on day 0, 7 and 17. Two weeks after immunization, blood and fecal extracts was collected for IgG and IgA detection.
Antibody Level Detection
The serum IgG and IgA in feces was measured by indirect enzyme-linked immunosorbent assay (ELISA). Briefly, ELISA plates were coated with HKSE (200 μg/well) overnight at 4 °C, and blocked with 2% bovine serum albumin at 37 °C for 1 h. After washed with PBS containing 0.05% Tween-80, serum (IgG, 1:100 dilution) or fecal extracts (IgA) were incubated for 2 h at room temperature. Then, the plates were washing and anti-mouse IgG-HRP (1:5000 dilution, Code No. A-10685, Invitrogen) or anti-mouse IgA-HRP IgA (1:5000 dilution, Code No. ab97235, Abcam) were added for 1 h at room temperature. After the reaction was terminated by 2 mol/L H2SO4, OD450 nm of the well was measured.
Delayed Type Hypersensitivity Assay (DTH)Test
Two weeks after immunization, five mice were subcutaneous injected into one foot pad with 20 μg of HKSE, and equal volume of PBS was injected into the other foot pad. After 72 h, the thickness of foot pad was measured using a digital caliper. The increase of footpad thickness (mm) was calculated as the footpad thickness of mice injected by HKSE minus it of mice injected by PBS.
The Lymphocyte Proliferation Assay and Cytokines Test After Immunization
The lymphocyte proliferation ability and cytokines were determined by the methods of MTT and ELISA, according to the previous research, according to the previous research [7].
Protection of S. enteritidis C50336 Challenge
The bacterial challenge assay was performed as previous description [20]. Two weeks after the final immunization, the mice in three immunized groups, were challenged by intragastric administration with S. enteritidis C50336 at a dose of 1 × 108 CFU/mouse. The number of surviving mice (ten mice) and bacterial load in spleens (five mice, 4 days p.i.) were determined.
Results
In Silico Analysis of the Brucella Pal Gene
The pal (encoding Peptidoglycan-Associated Lipoprotein, Pal) gene, carrying 507 nt, was located on B. melitensis 16 M chromosome I (Locus-tag = BMEI0340), and the accession number was NC_003317.1. The Pal protein contained 168 amino acids and had a molecular weight of 18.233 kDa. The isolectric point and charge at pH 7.0 was 9.931 and 6.994. The lipoprotein signal peptide was located at N-terminus, rather than other signal peptides and N-terminal membrane helices, and the cleavage site was between pos. 24 and 25(Fig.S1A). The Phylogenetic tree suggested that the rest domain of Pal was very close (at least 65.9% in protein sequence) to Pal proteins from Escherichia coli (HBP1323627.1), Salmonella Typhi (CQC26716.1), Haemophilus influenzae (WP_046067759.3), Legionella pneumophila (WP_003636618.1), Klebsiella pneumoniae (WP_130952229.1), Helicobacter pylori (EJB93082.1) and Pseudomonas putida (WP_150060284.1) (Fig. S1B).
The rPal Protein Stimulated the Expression of TNF-a and IFN-γ in Raw264.7
After induced expression, the rPal protein was identified by SDS-PAGE and western blot using anti His-Tag mice monoclonal antibody and HRP conjugated goat anti-mice IgG, and furtherly purified (Fig. S2). The purified rPal was used for follow-up experiments.
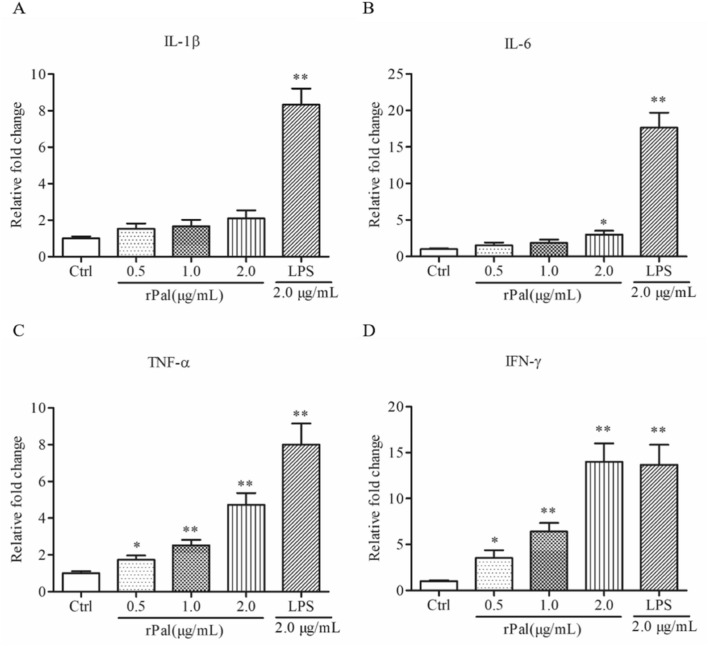
Cytokines such as IL-1β, IL-6, TNF-a and IFN-γ, have important roles in provoking the direction of immune responses, either cellular and/or humoral immunity. Some reports suggested rPal was a stronger inducer of cytokines in some kinds of bacteria [7, 21]. The above-mentioned information prompted us to conduct the protein stimulation experiments by the mice macrophage-like cell line Raw 264.7, with LPS as positive control. As shown in Fig. 1, with expected, LPS stimulated the expression of cytokines, IL-1β, IL-6, TNF-a and IFN-γ. Interestingly, the Brucella rPal protein increased the expression of TNF-a and IFN-γ in dose-dependent, but didn’t change the expression of IL-1β and IL-6. What’s more, the expression of IFN-γ was markedly increased, even when the concentration was raised to 2.0 μg/mL, the rPal stimulating effect was similar with LPS.
Fig.1.
The rPal protein elevated the cytokines expression in Raw 264.7 macrophages. Cells were incubated with rPal and LPS for 24 h, and the cytokines expression, IL-1β (A), IL-6 (B), TNF-α (C) and IFN-γ (D), were analyzed using real-time PCR. Error bars represent the standard deviation of three independent biological repeats.*P < 0.05 and **P < 0.01, indicate comparison with the untreated control(Ctrl)
Deletion of Pal Gene Reduced the Proliferation of Brucella in Macrophages, the Survival in Acidic, Oxidative and Polymyxin B-Contained Environment
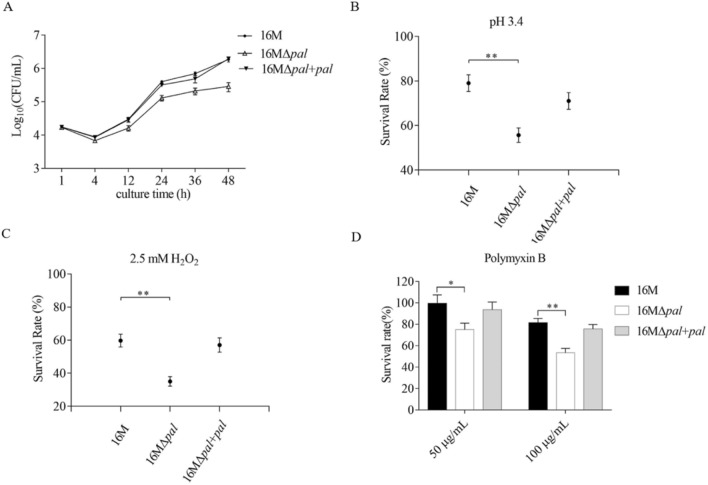
The intracellular survival ability in macrophages is the key of Brucella virulence. Thus, we conducted the pal mutant of B. melitnesis 16 M (Fig. S3A) and assessed the intracellular survival ability of the pal mutant 16MΔpal. As shown in Fig. S3B, deletion of pal gene did not affect the growth of Brucella in minimal medium. Interestingly, as shown in Fig. 2A, compared to 16 M, 16MΔpal showed lower bacterial counts after 4 h p.i. The results suggested that deletion of pal gene reduced proliferation ability of Brucella in macrophages.
Fig. 2.
The multiplication curve in macrophages (A) and survival rates curves of B.menlitensis 16 M, 16 M∆pal and 16 M∆pal + pal under acidic (B), and oxidative (C) conditions, and in different concentrations of polymyxin B (D). The intracellular bacteria count in macrophages, at different time points, was determined by serially dilution. In the stress assays, after a 1 h exposure to pH 3.4, 2.5 mM H2O2 and 50 or 100 μg/mL polymyxin B, the survival rates of each strain were calculated. The presented values represent the means of three experiments performed in duplicate, and the error bars indicate the SD. Significant differences between the strains are indicated by *(P < 0.05) and **(P < 0.01)
In macrophages, the internalized bacteria will encounter acidic pH, oxidizing substance and antimicrobial peptides, resulting in a decrease in intracellular proliferation. Therefore, we performed in vitro acidic and oxidative stress and polymyxin B tolerance assay. After 1 h incubated in pH 3.4, 2.5 mM H2O2, and 50 or 100 μg/mL polymyxin B, 16MΔpal showed a reduced survival rate in different degrees, compared to 16 M (Fig. 2B, C and D). These results indicated that pal gene was involved in the resistance of Brucella to acid and oxidative stress, and antimicrobial peptides, benefit to the bacterial intra-macrophage proliferation.
The Pal Gene was Important in Virulence to Mice
Considering that rPal protein stimulated the expression of TNF-a and IFN-γ and the pal gene plays the key role in the proliferation in macrophages, we assessed in vivo the effects on the colonization of the wild and mutant strain.
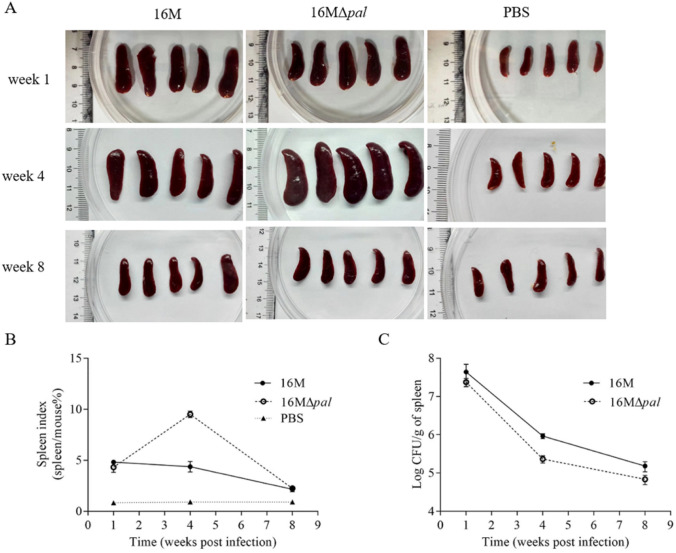
In the fourth week after bacterial challenge, strain 16 M and 16MΔpal all caused splenomegaly and a large spleen index, while 16MΔpal unexpectedly caused the more severe splenomegaly and a larger spleen index (Fig. 3A and B). Still and all, when it comes to the bacterial load in the spleen per unit mass (often used to evaluate the virulence of Brucella), 16MΔpal showed a slight decrease in the fourth and eighth week after the bacterial challenge, compared to 16 M (Fig. 3C). Hence, it can be concluded that the deletion of pal gene of Brucella resulted in a slight decline in virulence to mice, despite of more severe splenomegaly and larger spleen index.
Fig. 3.
Deletion of pal gene resulted in a decline in virulence to mice. The spleen (A) and spleen index (B) in mice (the proportion of body weight of the spleen) after challenge. The mice were injected intraperitoneally with B. melitensis 16 M and 16MΔpal at a dose of 1 × 106 CFU/mouse and the spleen of the mice was weighed, on week 1, 4 and 8. The mice, which were injected intraperitoneally with PBS, were regarded as control. C Bacterial load in spleen of mice after challenge. The isolated spleens were homogenized to further bacterial counting by plate method. The graphs represent the results of three independent trials
rPal Induced a Mucosal, Humoral and Cellular Immune Response Against HKSE
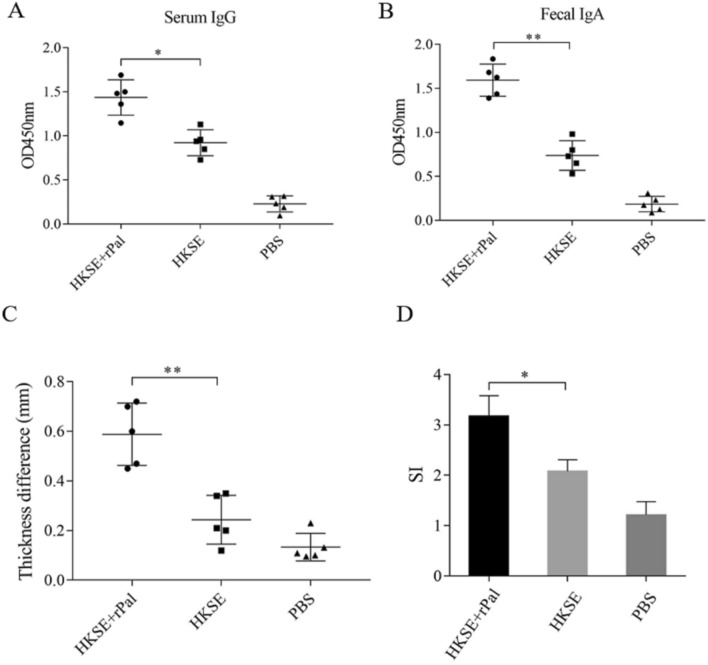
To determine the immune effect, mice was immunized with HKSE and HKSE Plus rPal, and the serum IgG and fecal IgA was obtained for ELISA. Compared to the mice immunized with HKSE alone, HKSE-specific IgA in fecal extracts and IgG in serum from those mice immunized with HKSE Plus rPal was significantly increased (Fig. 4A and B). These results indicated that rPal induced a mucosal antibody immune response, and elevated a stronger humoral immune against HKSE.
Fig. 4.
rPal increased an increase in serum IgG (A), fecal IgA (B) and cellular immune response (C, D and E) against HKS. After two weeks post finally immunization, HKSE-specific IgG in serum and HKSE-specific IgA in fecal extracts were detected by ELISA. The mice, immunized with HKSE and rPal, HKSE and PBS, was foot pad challenged by HKSE on one footpad and PBS on the contralateral footpad. After 72 h, the thickness difference of foot pad from each mouse was measured with callipers (C). The spleen lymphocyte of immunized mice was separated to perform MTT assays in the presence or absent of 10 μg/mL of HKSE, and the stimulation indices was represented by SI (D). Error bars represent the standard deviation of three independent biological repeats. Significant differences was indicated by *(P < 0.05) and **(P < 0.01)
Delayed type hypersensitivity (DTH) assays (by foot pad challenge) and the lymphocyte proliferation assay was usually used to assess the cellular immune response. The mice immunized with HKSE Plus rPal displayed a stronger TDH response than HKSE immunized mice (Fig. 4C). And, the proliferative activity (or SI) of spleen cells from the mice immunized with HKSE Plus rPal was higher than those with HKSE and PBS (Fig. 4D). Furthermore, IL-2, IL-4 and INF-γ is important cytokines participated in the immune response to restrict the proliferation of pathogens. At two weeks after final immunization, the spleen cells was separated and the cultured splenocyte supernatant was harvested to test the concentration of these cytokines. As shown in Fig. S4, compared with the HKSE immunized mice, the concentration of IL-2, IL-4 and INF-γ from HKSE Plus rPal immunized mice was increased. Of note, the concentration of IL-2 was more markedly increased than it of IL-4. These figures suggested that rPal induced a stronger Th1 cellular immune response.
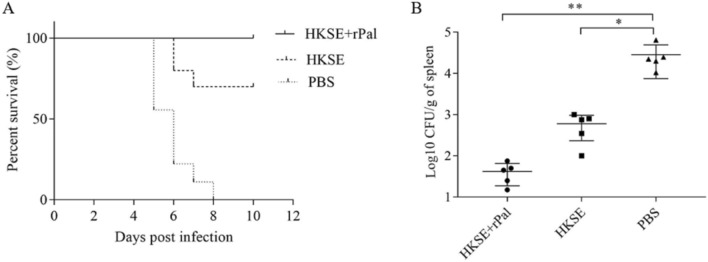
rPal Combined with HKSE Elevated the Survival Rate and Reduced the Bacterial Load of Spleens in Immunized Mice
To determine the immune efficacy, the challenge experiment was performed using the live wild strain S. enteritidis C50336 and the mice, and the protection effect was marked by the survival rate and the bacterial load of spleen. After oral administration, ten mice immunized with rPal plus HKSE all survived, seven mice immunized with HKSE only survived, and all mice administered with PBS died within 8 days (Fig. 5A). The mice, before death, showed various clinical symptoms, including anorexia, diarrhea and depression. The remaining five immunized mice were sacrificed 4 days after challenge with S. enteritidis C50336, and the spleens was homogenized and bacterial counts were performed. Compared to PBS-administered mice, the mice immunized with rPal plus HKSE, or HKSE only, carried much lower bacterial load. And, of particular note was that the bacterial load of spleens from the mice immunized with rPal plus HKSE was the lowest (Fig. 5B). These data suggested that rPal enhanced the immune protection effect of HKSE to S. enteritidis in mice. Please confirm the section headings are correctly identified.Thanks for your help!I have checked carefully and confirmed that the section headings were correct.
Fig. 5.
Oral immunization of mice with rPal plus HKSE elevated the survival rate (A) and reduced the bacterial load of spleens (B) in immunized mice. The error bars indicate the SD, and significant differences between the strains are indicated by *(P < 0.05) and **(P < 0.01)
Discussion
Pal protein is an important outer membrane protein, belonging to Tol/Pal complex, which mainly contains five proteins including Pal, and plays an important role in the transport of outer membrane proteins and other substances [22, 23]. B. mlitensis 16 M carries a predicted pal gene (Locus-tag was BMEI0340),whose encoded product has a typical N-terminal lipoprotein signal peptide, which guides Pal protein to transport to cell membrane and bind to peptidoglycan. The amino acid homology of the remaining functional regions of Pal was more than 65% compared with other bacteria. These findings indicated that BMEI0340 encoded protein was peptidoglycan associated lipoprotein (Pal protein).
Due to its location on the surface of bacteria, Pal protein is easily recognized by host cells during infection, and some bacteria can even secrete Pal protein directly to induce the immune response of the host [24]. As facultative intracellular pathogens, Brucella can invade and survive in macrophages, and macrophages can secrete a variety of cytokines to regulate host immune responses to defend against its infection, although Brucella has evolved a variety of defense mechanisms [25, 26]. In this study, macrophages were incubated with the prokaryotic expressed Pal protein of Brucella, and Pal up-regulated the expression of TNF-α and IFN-γ, but did not IL-1β and IL-6. Similar with our results, it was reported that, in Brucella suis infected macrophages, Pal protein by tetracycline-induced expression could inhibit the expression of IL-1β and IL-6 [8]. However, inconsistent with our findings, Pal proteins of E. coli, L. pneumoniae and Aggregatibacter actinomycetemcomitans, up-regulated the secretion of IL-1β, IL-6, and TNF-α. These studies indicated that there were some differences roles in stimulating macrophage to secrete cytokines between Brucella Pal protein and other Pal proteins. The reasons for these functional differences require further experimental confirmation.
Brucella can survive in professional phagocytes, such as macrophages, and internalized Brucella traffics along with macrophages to other sites of hosts [2, 27]. Hence, we constructed the pal mutant by homologous recombination technique, and analyzed the proliferation in macrophages. Similar to previous researched, deletion of pal gene resulted in reduced proliferation of Brucella in macrophages. In macrophage, Brucella adopts a variety of strategies to resist acidic and oxidative stress and antimicrobial peptide, to benefit its survival and the following proliferation [2, 5, 18, 27]. So, stress and resistance assay of polymyxin B were performed to analyze the role of pal gene in pathogenesis. Interestingly, compared to the wild type strain, B. melitensis 16 M, the pal mutant 16MΔpal showed a reduced survival rate in different degrees in a low pH 3.5, H2O2 and polymyxin B-contained saline. These results suggested that pal gene was involved in the resistance of Brucella to acid and oxidative stress, and antimicrobial peptides, benefit to the bacterial intra-macrophage proliferation.
Furthermore, the virulence evaluation of the pal mutant to BALB/c mice was conducted. 4 weeks after challenge, it was found that the spleen of mice challenged with 16MΔpal was significantly enlarged, even higher than that of wild strain 16 M. However, the bacteria load in spleen of 16MΔpal was lower than that of 16 M. It has been reported that Pal of Brucella can inhibit the secretion of IL-1β and IL-6 by macrophages, but when the gene is knocked out, the expression of IL-1β and IL-6 is up-regulated, and the virulence of the mutant was decreased[8, 28]. Meanwhile, we have confirmed that Pal up-regulated the expression of TNF-α and IFN-γ. There is an explanation, maybe, that up-regulated IL-1β and IL-6 followed by the deletion of pal gene, leading to a more significant inflammatory response and splenomegaly, and counteracting the effect of down-regulation of TNF-α and IFN-γ. Consequently, the enhancing inflammatory response makes it easier for clearing of Brucella. This hypothesis needs to be further confirmed by subsequent studies.
Despite of its roles in down-regulating IL-1β and IL-6, Pal have an ability to up-regulate TNF-α and IFN-γ, which play an important role in the body's defense against Brucella [29, 30]. Moreover, Pal of Brucella was verified that could activate dendritic cells in vivo, induces a Th1 immune response, and was a promising self-adjuvanting vaccine [6]. And other researchers constructed a novel recombinant Lactobacillus casei-Pal-PEDVS strain expressing S protein of porcine epidemic diarrhea virus and Pal protein of Brucella, and found that this strain could induce higher levels of humoral immunity, cellular immunity, and mucosal immunity [14]. According to related reports, Brucella also carries another antigen that has the properties of an adjuvant. Oral vaccination with U-Omp19 plus Salmonella antigens conferred protection against virulent challenge with Salmonella Typhimurium, with a significant reduction in bacterial loads [19]. It prompted us to immunize the mice, by rPal and heat-killed Salmonella enteritidis, a fecal-oral-transmitted pathogen. Similar to these researched, after intragastric administration of mice, rPal induced a stronger humoral, mucosal and Th1 cellular immune response. The challenge experiments showed rPal elevated the survival rate and reduced the bacterial load of spleens in immunized mice.
Conclusion
In conclusion, Pal protein from Brucella was able to in vitro induce the expression of TNF-α and IFN-γ in macrophages, and the pal mutant showed reduced proliferation, stress resistance and virulence to mice. And, rPal induced a stronger mucosal, humoral antibody response and Th1 cellular response against HKSE, and enhanced the immune protection effect of HKSE to S. enteritidis in mice. These findings revealed the mechanism of Pal affecting Brucella virulence to some extent, and provide basic data for rPal as a suitable adjuvant in oral vaccine formulations against mucosal pathogens, such as S. enteritidis. However, there are still some problems that need to be further solved. For example, as mucosal adjuvants, what’s the mechanism of rPal? Anyway, our findings suggested that rPal from Burcella was a potentially valuable adjuvant for the prevention and control of mucosal pathway infections.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks to many students of Hebei Normal University of Science & Technology and peers of China Institute of Veterinary Drug Control for their support.
Author Contributions
TW conceptualized and designed the study. Material preparation, data collection and analysis were performed by YCh, LK and FW. The first draft of the manuscript was written by YC, and all authors critically revised all versions of the manuscript. Resources and supervision were provided by TW and QW. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 31902310) and Hebei Key Research and Development Projects (No. 19226629D).
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical Approval
The mice and were handled in strict accordance with the Experimental Animal Regulation Ordinances defined by the China National Science and Technology Commission. The study was approved by the animal ethics committee of China Institute of Veterinary Drug Control under permit number (CIVDC 2019–000672).
Consent to Participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saez D, Guzman I, Andrews E, Cabrera A, Onate A. Evaluation of Brucella abortus DNA and RNA vaccines expressing Cu-Zn superoxide dismutase (sod) gene in cattle. Vet Microbiol. 2008;129(3–4):396–403. doi: 10.1016/j.vetmic.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Celli J. Surviving inside a macrophage: the many ways of Brucella. Res Microbiol. 2006;157(2):93–98. doi: 10.1016/j.resmic.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Ferrero MC, Alonso PI, Munoz GF, Baldi PC. Pathogenesis and immune response in Brucella infection acquired by the respiratory route. Microbes Infect. 2020;22(9):407–415. doi: 10.1016/j.micinf.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Liu W, Wu T, Bie P, Wu Q. RNA-seq reveals the critical role of CspA in regulating Brucella melitensis metabolism and virulence. Sci China Life Sci. 2016;59(4):417–424. doi: 10.1007/s11427-015-4981-6. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Bie P, Cheng J, Lu L, Cui B, Wu Q. The abc transporter YejABEF is required for resistance to antimicrobial peptides and the virulence of Brucella melitensis. Sci Rep. 2016;6:31876. doi: 10.1038/srep31876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasquevich KA, Garcia SC, Coria LM, Estein SM, Zwerdling A, Ibanez AE, Barrionuevo P, Oliveira FS, Carvalho NB, Borkowski J, Oliveira SC, Warzecha H, Giambartolomei GH, Cassataro J. The protein moiety of Brucella abortus outer membrane protein 16 is a new bacterial pathogen-associated molecular pattern that activates dendritic cells in vivo, induces a Th1 immune response, and is a promising self-adjuvanting vaccine against systemic and oral acquired brucellosis. J Immunol. 2010;184(9):5200–5212. doi: 10.4049/jimmunol.0902209. [DOI] [PubMed] [Google Scholar]
- 7.Mobarez AM, Rajabi RA, Salmanian AH, Khoramabadi N, Hosseini DS. Induction of protective immunity by recombinant peptidoglycan associated lipoprotein (rPAL) protein of Legionella pneumophila in a BALB/c mouse model. Microb Pathog. 2019;128:100–105. doi: 10.1016/j.micpath.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhi F, Zhou D, Li J, Tian L, Zhang G, Jin Y, Wang A. Omp16, a conserved peptidoglycan-associated lipoprotein, is involved in Brucella virulence in vitro. J Microbiol. 2020;58(9):793–804. doi: 10.1007/s12275-020-0144-y. [DOI] [PubMed] [Google Scholar]
- 9.Masilamani R, Cian MB, Dalebroux ZD. Salmonella Tol-Pal reduces outer membrane glycerophospholipid levels for envelope homeostasis and survival during bacteremia. Infect Immun. 2018 doi: 10.1128/IAI.00173-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao H, Li B, Zheng Z, Zhou Z, Li W, Gu G, Liu J, Luo Y, Shuai X, Zhao Y, Liu Y, Wang Y, Wang X, Hu X, Wu L, Chen J, Huang Q. Transcriptome landscape of intracellular Brucella ovis surviving in RAW264.7 macrophage immune system. Inflammation. 2020;43(5):1649–1666. doi: 10.1007/s10753-020-01239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh PF, Liu JY, Pan YJ, Wu MC, Lin TL, Huang YT, Wang JT. Klebsiella pneumoniae peptidoglycan-associated lipoprotein and murein lipoprotein contribute to serum resistance, antiphagocytosis, and proinflammatory cytokine stimulation. J Infect Dis. 2013;208(10):1580–1589. doi: 10.1093/infdis/jit384. [DOI] [PubMed] [Google Scholar]
- 12.Dennehy R, Romano M, Ruggiero A, Mohamed YF, Dignam SL, Mujica TC, Callaghan M, Valvano MA, Berisio R, Mcclean S. The Burkholderia cenocepacia peptidoglycan-associated lipoprotein is involved in epithelial cell attachment and elicitation of inflammation. Cell Microbiol. 2017 doi: 10.1111/cmi.12691. [DOI] [PubMed] [Google Scholar]
- 13.Liang MD, Bagchi A, Warren HS, Tehan MM, Trigilio JA, Beasley-Topliffe LK, Tesini BL, Lazzaroni JC, Fenton MJ, Hellman J. Bacterial peptidoglycan-associated lipoprotein: a naturally occurring toll-like receptor 2 agonist that is shed into serum and has synergy with lipopolysaccharide. J Infect Dis. 2005;191(6):939–948. doi: 10.1086/427815. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Zhang B, Zhang D, Liu S, Ren J. The construction of recombinant Lactobacillus casei vaccine of PEDV and its immune responses in mice. Bmc Vet Res. 2021;17(1):184. doi: 10.1186/s12917-021-02885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz JR, Goggins JA, Mclachlan JB. Salmonella infection: interplay between the bacteria and host immune system. Immunol Lett. 2017;190:42–50. doi: 10.1016/j.imlet.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knodler LA, Elfenbein JR. Salmonella enterica . Trends Microbiol. 2019;27(11):964–965. doi: 10.1016/j.tim.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Galan JE. Salmonella typhimurium and inflammation: a pathogen-centric affair. Nat Rev Microbiol. 2021;19(11):716–725. doi: 10.1038/s41579-021-00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Wang S, Wu Q. Cold shock protein plays an important role in the stress adaptation and virulence of brucella melitensis. Fems Microbiol Lett. 2014;354(1):27–36. doi: 10.1111/1574-6968.12430. [DOI] [PubMed] [Google Scholar]
- 19.Risso GS, Carabajal MV, Bruno LA, Ibanez AE, Coria LM, Pasquevich KA, Lee SJ, Mcsorley SJ, Briones G, Cassataro J. U-omp19 from Brucella abortus is a useful adjuvant for vaccine formulations against Salmonella infection in mice. Front Immunol. 2017;8:171. doi: 10.3389/fimmu.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Z, Tang P, Jiao Y, Kang X, Li Q, Xu X, Sun J, Pan Z, Jiao X. Immunogenicity and protective efficacy of a Salmonella enteritidis sptP mutant as a live attenuated vaccine candidate. BMC Vet Res. 2017;13(1):194. doi: 10.1186/s12917-017-1115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero-Saavedra F, Laverde D, Wobser D, Michaux C, Budin-Verneuil A, Bernay B, Benachour A, Hartke A, Huebner J. Identification of peptidoglycan-associated proteins as vaccine candidates for enterococcal infections. PLoS ONE. 2014;9(11):e111880. doi: 10.1371/journal.pone.0111880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duche D, Houot L. Similarities and differences between colicin and filamentous phage uptake by bacterial cells. EcoSal Plus. 2019 doi: 10.1128/ecosalplus.ESP-0030-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szczepaniak J, Press C, Kleanthous C. The multifarious roles of tol-pal in gram-negative bacteria. FEMS Microbiol Rev. 2020;44(4):490–506. doi: 10.1093/femsre/fuaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellman J, Roberts JJ, Tehan MM, Allaire JE, Warren HS. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J Biol Chem. 2002;277(16):14274–14280. doi: 10.1074/jbc.M109696200. [DOI] [PubMed] [Google Scholar]
- 25.Billard E, Dornand J, Gross A. Brucella suis prevents human dendritic cell maturation and antigen presentation through regulation of tumor necrosis factor alpha secretion. Infect Immun. 2007;75(10):4980–4989. doi: 10.1128/IAI.00637-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao H, Zhou Z, Li B, Xiao Y, Li M, Zeng H, Guo X, Gu G. The mechanism of facultative intracellular parasitism of Brucella. Int J Mol Sci. 2021 doi: 10.3390/ijms22073673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo X, Zhang X, Wu X, Yang X, Han C, Wang Z, Du Q, Zhao X, Liu SL, Tong D, Huang Y. Brucella downregulates tumor necrosis factor-α to promote intracellular survival via OMP25 regulation of different micrornas in porcine and murine macrophages. Front Immunol. 2017;8:2013. doi: 10.3389/fimmu.2017.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhi F, Fang J, Zheng W, Li J, Zhang G, Zhou D, Jin Y, Wang A. A Brucella OMP16 conditional deletion strain is attenuated in BALB/c mice. J Microbiol Biotechnol. 2022;32(1):6–14. doi: 10.4014/jmb.2107.07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hop HT, Reyes A, Huy T, Arayan LT, Min W, Lee HJ, Rhee MH, Chang HH, Kim S. Activation of NF-kB-mediated TNF-induced antimicrobial immunity is required for the efficient Brucella abortus clearance in RAW 264.7 cells. Front Cell Infect Microbiol. 2017 doi: 10.3389/fcimb.2017.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. Interferon-γ is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology. 2001;103(4):511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.