Abstract
Gamma interferon (IFN-γ)-secreting CD4+ T cells have long been established as an essential component of the protective immune response against Mycobacterium tuberculosis. It is now becoming evident from studies with the murine model of tuberculosis that an important role also exists for major histocompatibility complex (MHC) class I-restricted CD8+ T cells. These cells are capable of acting as both IFN-γ secretors and cytotoxic T lymphocyte (CTL) effectors; however, their exact role in immunity against tuberculosis remains unclear. This study demonstrates the presence of Mycobacterium bovis BCG-reactive CD8+ T cells in healthy BCG-vaccinated donors and that these CD8+ T cells are potent cytokine producers as well as cytotoxic effector cells. Using FACScan analysis, we have shown that restimulation with live M. bovis BCG induced more CD8+-T-cell activation than the soluble antigen purified protein derivative and that these cells are actively producing the type 1 cytokines IFN-γ and tumor necrosis factor alpha (TNF-α). These CD8+ T cells also contain the cytolytic granule perforin and are capable of acting as potent CTLs against M. bovis BCG-infected macrophages. The mycobacterial antigens 85A and B (Ag85A and Ag85B, respectively), and to a lesser extent the 19- and 38-kDa proteins, are major antigenic targets for these mycobacterium-specific CD8+ T cells, while whole-M. bovis BCG activated effector cells from these BCG-vaccinated donors, as expected, failed to recognize the 6-kDa ESAT-6 protein. The use of metabolic inhibitors and blocking antibodies revealed that the CD8+ T cells recognize antigen processed and presented via the classical MHC class I pathway. These data suggest that CD8+ T cells may play a critical role in the human immune response to tuberculosis infection.
Mycobacterium tuberculosis, the etiologic agent of human tuberculosis, is a weakly gram-positive acid-fast bacillus, capable of surviving within cells of the mononuclear-phagocyte lineage. Estimates suggest one-third of the world’s population is latently infected with M. tuberculosis, causing 8 to 12 million new cases of active tuberculosis and 3 million deaths each year (3, 41, 54). This has made tuberculosis the leading cause of death from an infectious agent.
Protective immune responses towards tuberculosis infection remain poorly understood. Cell-mediated immunity is known to be critical for restricting M. tuberculosis infection. The interaction between infected macrophages and gamma interferon (IFN-γ)-secreting CD4+ T cells has been shown to be essential for protection against tuberculosis in both murine (1, 7, 17, 27) and human (4, 42, 47) studies.
Major histocompatibility complex (MHC) class I-restricted CD8+ T cells, which are capable of acting as cytotoxic T lymphocyte (CTL) effectors and type 1 cytokine producers, secreting IFN-γ and tumor necrosis factor alpha (TNF-α), have only recently been linked with protective immunity against tuberculosis. Gene-disrupted mice lacking β2 microglobulin which express no MHC class I proteins and possess no functional CD8+ T cells show increased susceptibility to challenge with M. tuberculosis and Mycobacterium bovis bacillus Calmette-Guérin (BCG) (16, 27). Vaccination of mice with recombinant vaccinia virus or DNA plasmids expressing Ag85A (12, 22), the 38-kDa protein (56, 57), or the heat shock protein hsp-65 (48) generated antigen-specific CD8+ CTLs that conferred protection against subsequent challenge with M. tuberculosis.
CD8+-T-cell-mediated CTL activity against M. tuberculosis has been shown in both murine and human studies; however, the importance of this action in protective immunity against tuberculosis is unclear. Perforin, granzyme, and CD95-CD95L pathway knockout mice show no change in the early course of M. tuberculosis infection (8, 29), suggesting that another mode of action by CD8+ T cells is important. Indeed, the transfer of protection against M. tuberculosis infection in mice has been shown to be dependent upon the capacity of CD8+ T cells to produce IFN-γ (49). Alternatively, granulysin, another component of secreted granules which possesses anti-mycobacterial activity, has been shown to be of importance in CD8+-T-cell-mediated immunity to tuberculosis (46).
Human in vitro studies have shown that CD8+ T cells become activated and show cytolytic activity when stimulated with live M. bovis BCG or M. tuberculosis (15, 50). Human IFN-γ-secreting and CTL CD8+ T cells have been demonstrated in response to M. tuberculosis infection (5, 30, 47). However, little is known about the mechanisms by which antigens from M. tuberculosis gain access to the MHC class I pathway, since the bacillus resides primarily within the phagosome (6), a site inaccessible to the MHC class I processing pathway. A possible mechanism for mycobacterial antigens to reach the cytosol and enter the class I pathway is through pore-forming molecules such as a recently identified M. tuberculosis hemolysin (55).
This study demonstrates the existence of M. bovis BCG-reactive CD8+ T cells in BCG-vaccinated subjects. These cells are capable of producing the type 1 cytokines IFN-γ and TNF-α, in addition to possessing potent CTL activity against mycobacterial antigens. Purified CD8+ T cells showed CTL activity against target cells infected with recombinant vaccinia virus (rVV) expressing the mycobacterial antigens Ag85A and Ag85B and, to a lesser extent, the 19- and 38-kDa proteins. This CTL activity could be blocked by the addition of metabolic inhibitors against phagocytosis, proteosome activity, or Golgi-endoplasmic reticulum (ER) trafficking, in addition to anti-HLA-A, -B, or -C antibody, thus demonstrating a classical MHC class I antigen-processing and presentation pathway for M. bovis BCG-reactive CD8+ T cells.
MATERIALS AND METHODS
Human subjects.
Healthy laboratory donors who had previously received BCG vaccination were recruited from the London School of Hygiene & Tropical Medicine. Blood samples were taken after gaining written permission from the individuals participating in the study. Ethical permission was obtained from the Ethics Committee at the London School of Hygiene & Tropical Medicine.
Growth and quantification of M. bovis BCG stocks.
Cultures of M. bovis BCG (GlaxoEvans strain; Evans Medical, Leatherhead, United Kingdom) were grown in sterile Middlebrook 7H9 medium supplemented with 10% Middlebrook ADC enrichment and 0.2% glycerol (Sigma Chemical Co., Poole, United Kingdom). After preparation of stock cultures, aliquots were frozen and stored at −70°C and titers were subsequently determined in complete Middlebrook 7H9 medium. On the day of experiments, aliquots were thawed, washed in RPMI 1640 (Gibco-BRL, Paisley, United Kingdom), and sonicated for 10 s in a sonicating water bath (Grant XB2; Fisons, Leicester, United Kingdom) prior to use.
rVV construction.
rVVs expressing the Ag85A, Ag85B, and ESAT-6 antigens from M. tuberculosis were constructed by A. Malin, as previously described (20, 28). rVVs expressing the 19- and 38-kDa antigens were kindly provided by J. Ivanyi and M. Vordermier (53, 56). Briefly, the coding sequences of the Ag85A, Ag85B, and ESAT-6 proteins were cloned into the nonessential thymidine kinase locus of wild-type vaccinia virus by using the transfer plasmid p1108; a negative control rVV was constructed by using the p1108 plasmid with an irrelevant coding sequence; the coding sequences of the 19,000- and 38,000-MW proteins had been inserted into the SmaI site of the vaccinia virus recombinant plasmid pSC11. rVV were produced by transfection of the plasmid into the osteosarcoma cell line 143 (TK−143), with the thymidine gene disrupted, coinfected with wild-type vaccinia virus, followed by selection for recombinant viruses. The resulting recombinant viruses were plaque purified, and protein expression was confirmed by PCR and Western blot analysis of infected TK−143 cells.
PBMC separation.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by density gradient centrifugation over Ficoll-Histopaque (Sigma). Isolated PBMC were washed three times in Hanks balanced salt solution (Gibco-BRL) and resuspended in complete medium.
Autologous monocyte-derived-macrophage target cells were isolated by incubating PBMC in RPMI 1640 alone for 1 h at 37°C on 96-well U-bottom plastic tissue culture plates (Nunclon, Roskilde, Denmark); the nonadherent cells were then removed, leaving only adherent monocytes, which were then cultured in complete medium (as described below) for 7 days to give monocyte-derived macrophages.
Expansion of antigen-specific T cells.
PBMC (2 × 106 per well) were cultured in 24-well tissue culture plates (Nunclon) with either live M. bovis BCG (1 bacterium/macrophage), purified protein derivative (PPD) (10 μg/ml; batch RT49; Statens Seruminstitut, Copenhagen, Denmark), midterm culture filtrate protein (CFP) (10 μg/ml; J. Belisle, Colorado State University), or no antigen. The culture medium consisted of RPMI 1640 (Gibco-BRL) supplemented with 10% autologous plasma, 2 mM l-glutamine (Gibco-BRL), and 50 μg of ampicillin (Sigma)/ml. After 6 to 7 days of culture at 37°C in the presence of 5% CO2, the cells were harvested.
Isolation of CD8+ T cells.
Antigen-specific CD8+ T cells were prepared by positive enrichment with the MACS system (Miltenyi Biotech, Bergisch-Gladbach, Germany). In brief, PBMC were labelled with CD8 microbeads (20 μl/107 cells; Miltenyi Biotech) in incubation buffer (phosphate-buffered saline [PBS]–0.5% bovine serum albumin–2 mM EDTA; 80 μl/107 cells) for 15 min at 4°C. After one washing step, the cells were resuspended in incubation buffer (1 ml of buffer/107 cells) and enrichment was performed with LS+ columns and the MidiMACS magnet according to the manufacturer’s instructions. The resulting CD8+-T-cell population was >95% pure as determined by flow cytometric analysis for the surface markers αβ T-cell receptors, CD3, CD4, CD8, and CD56.
Flow cytometric detection of intracellular cytokine and perforin production.
Flow cytometric detection of intracellular cytokine was performed by modification of methods previously described (18, 23, 24, 40). Briefly, intracellular cytokine and perforin production by purified antigen-specific CD8+ T cells was assessed by two-color flow cytometry. Cytokine and perforin secretion was blocked by incubating PBMC in the presence of 3 μg of brefeldin A (Sigma)/ml for 16 h at 37°C. The cells were stained for surface CD8 with fluorescein isothiocyanate (FITC)-conjugated antibody (Becton Dickinson, Oxford, United Kingdom) in incubation buffer (PBS–1% fetal calf serum [FCS]–0.1% Na azide) for 30 min at 4°C. Phycoerythrin (PE)-conjugated anti-CD25 (Becton Dickinson) was used with FITC-conjugated anti-CD8 when surface CD25 expression was determined. The cells were washed twice in cold PBS–1% FCS and fixed with PBS–4% paraformaldehyde (Sigma) at 4°C for 30 min. Fixation was followed by permeabilization with PBS–1% FCS–0.3% saponin (Fluka, Gillingham, Dorset, United Kingdom)–0.1% Na azide at 4°C for 10 min. The cells were then washed twice in cold PBS–1% FCS. Staining of intracellular cytokines or perforin was performed by incubation of fixed permeabilized cells with either PE-labelled anti-IFN-γ, -TNF-α, -interleukin-4 (IL-4), or -perforin antibodies (PharMingen, Oxford, United Kingdom) in incubation buffer (PBS–1% FCS–0.3% saponin–0.1% Na azide) at 4°C for 30 min. The cells were analyzed with Lysis II and CellQuest software and a Becton Dickinson FACScan flow cytometer; 50,000 events were counted.
51Cr release cytotoxicity assay.
Monocyte-derived-macrophage target cells were infected with BCG at an infection ratio of five/macrophage or rVV at 10/macrophage by incubation for 2 h at 37°C; cells were simultaneously loaded with 2 μCi of Na2 51Cr4/well and incubated overnight at 37°C. All experiments were set up in triplicate. The cells were then washed with RPMI 1640 (Gibco-BRL) and incubated in growth medium with M. bovis BCG-specific CTL. Effector cells from whole-PBMC, purified CD8+-T-cell, or CD8−-T-cell populations were added at effector-to-target cell ratios of 15:1 (shown in preliminary experiments to give optimum cytotoxicity), and the plate was incubated at 37°C for 6 h. The supernatants were harvested, and 51Cr release was measured with a gamma counter. The remaining cells (pellet) were lysed with 5% sodium dodecyl sulfate (Sigma) and harvested.
Spontaneous release was measured in wells containing target cells alone. The percent specific 51Cr release was calculated for each experiment by the following equations: percent isotope release = [counts per minute of supernatant/counts per minute of supernatant + counts per minute of pellet)] × 100 and percent specific lysis = percent isotope release test wells − percent isotope release control wells. For inhibition of presentation molecules, the target cells were incubated with anti-MHC class I antibody W6/32 (Sigma), anti-CD1a, -b, and -c antibodies (S. A. Porcelli, Harvard Medical School, Boston, Mass.), or an immunoglobulin IgG2a isotype control antibody (Sigma) at 5-μg/ml final concentration for 1 h before the addition of effector cells.
Metabolic inhibition of antigen presentation.
One hour before the addition of M. bovis BCG or rVV to monocyte-derived-macrophage antigen-presenting cells, brefeldin A (10 μg/ml; Sigma), chloroquine (100 mM; Sigma), N-Cbz-Leu-Leu-leucinal (10 μg/ml; Sigma), or cytochalasin D (10 μg/ml; Sigma) was added to the medium. After 18 h of coincubation with M. bovis BCG, monocyte-derived macrophages were washed thoroughly with RPMI 1640 before purified CD8+ T cells were added and a standard 6-h CTL assay was performed.
Statistical analysis.
Data are presented as mean values from replicate samples and assays. The Student t test was used to determine statistical significance between groups of data, and where paired results were available, the paired Student t test was used.
RESULTS
CD8+ T cells produce IFN-γ and TNF-α in response to a live M. bovis BCG infection.
PBMC from healthy BCG-vaccinated donors (n = 10) were stimulated with either a live M. bovis BCG (1:1 BCG/macrophage infection ratio) infection or the soluble antigen PPD (10 μg/ml) to generate a recall CD8+-T-cell response to each antigen. These two antigens were chosen for their different antigen-processing properties; PPD, being a soluble antigen, should be processed via the MHC class II pathway and therefore should not activate MHC class I-dependent CD8+ T cells. In contrast, a live infection of M. bovis BCG may allow antigens to gain access to the MHC class I presentation pathway. In each case PBMC were stimulated for 6 days in the presence of antigen, either live M. bovis BCG (1:1 BCG/macrophage ratio) or PPD (10 μg/ml), or without antigen; CD8+ T cells were then separated by MACS and analyzed by flow cytometry.
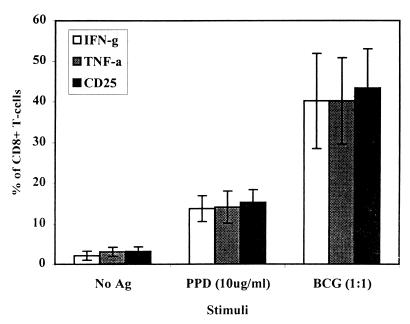
Both antigens proved capable of stimulating and activating type 1 CD8+ T cells (Tc1) capable of producing the cytokines IFN-γ and TNF-α but not IL-4. However, the percentage of cells staining positive for CD25, IFN-γ, and TNF-α was significantly higher (P < 0.001) in the population of CD8+ T cells stimulated with live M. bovis BCG (Fig. 1 and 2), thus demonstrating the potential for antigens released during a live infection to gain access to the MHC class I presentation pathway. The percentages of CD8+ T cells staining positive for both IFN-γ and TNF-α were directly comparable; indeed, analysis of the larger granular blast cell population (>350 U of SSC) of CD8+ T cells revealed that >95% of these cells stained positive for both cytokines, thus demonstrating that the same cells are producing both cytokines. These data show that BCG-vaccinated individuals possess circulating CD8+ T cells capable of recognizing and responding to M. bovis BCG.
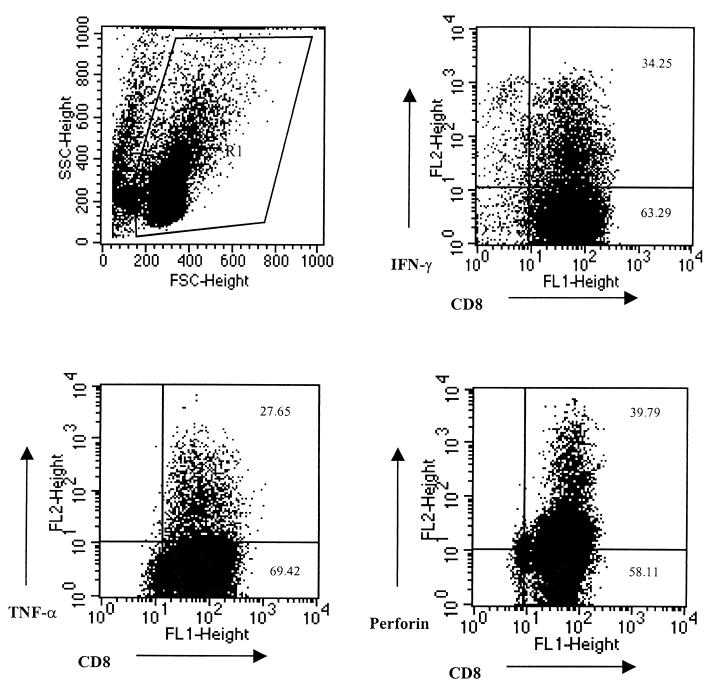
FIG. 1.
Flow cytometric detection of type 1 cytokine and perforin synthesis by CD8+ T cells. PBMC were stimulated for 6 days in the presence of live M. bovis BCG. CD8+ T cells were positively selected from the PBMC by the MACS separation system and incubated for 16 h in the presence of brefeldin A. Intracellular cytokine and perforin staining was performed before FACScan analysis.
FIG. 2.
Intracellular measurement of cytokines produced by CD8+ T cells. PBMC were stimulated for 6 days in the presence of either no antigen, PPD, or a live M. bovis BCG infection. After stimulation, CD8+ T cells were positively selected by MACS separation and incubated for 16 h in the presence of brefeldin A. Cells were simultaneously stained for CD8-FITC in combination with PE-conjugated anti-CD25 (IL-2R), anti-IFN-γ, or anti-TNF-α antibodies. The results are expressed as the means of 10 donors ± standard deviations.
CD8+ T cells induced by a live M. bovis BCG infection express perforin.
Our data thus far demonstrated that CD8+ T cells stimulated with M. bovis BCG are capable of acting as potent cytokine producers. To further characterize the CD8+-T-cell response to mycobacterial antigens, PPD, CFP, and M. bovis BCG-reactive CD8+ T cells were stained for intracellular perforin in conjunction with intracellular cytokine staining to determine whether these cells possess cytolytic potential.
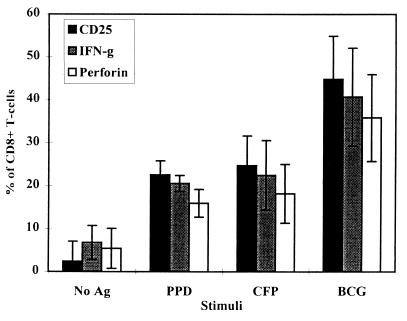
PBMC from healthy BCG-vaccinated donors (n = 10) were cultured in the presence of PPD, CFP, or a live M. bovis BCG infection for 6 days. Isolated CD8+ T cells were analyzed for the presence of intracellular perforin and IFN-γ. As before, over 40% of CD8+ T cells contained IFN-γ, while over 30% stained positive for perforin when stimulated with live M. bovis BCG (Fig. 3). The percentage of perforin-positive cells followed the same patterns as for IFN-γ staining, with live M. bovis BCG being the best stimulus. Blast cell analysis again revealed that >95% of CD8+ T cells were producing IFN-γ while >75% of blast cells also contained perforin. These results indicate that M. bovis BCG-reactive CD8+ T cells possess cytolytic potential in addition to their cytokine-producing function.
FIG. 3.
Intracellular detection of perforin production by CD8+ T cells. PBMC were stimulated for 6 days in the presence of either no antigen, PPD, CFP, or a live M. bovis BCG infection. CD8+ T cells were positively selected for by MACS and incubated with brefeldin A. After being surface stained for CD8 and CD25, the cells were fixed and permeabilized, and the intracellular accumulation of IFN-γ and perforin was measured. The results are expressed as the means of 10 donors ± standard deviations.
CD8+ T cells are potent antigen-specific CTLs.
To assess the role of CD8+ T cells as CTLs for mycobacterial antigen-pulsed monocyte-derived macrophages, PBMC from 10 healthy BCG-vaccinated donors were stimulated for 7 days with a live M. bovis BCG infection. To directly prove that CD8+ T cells exerted antigen-specific CTL activity against mycobacterial antigens, CD8+ T cells were purified by positive selection from PBMC. The CD8+ T-cell-enriched, CD8+ T-cell-depleted, and whole-PBMC populations were compared for CTL activity against target cells infected with either M. bovis BCG or rVV expressing one of the mycobacterial antigens Ag85A, Ag85B, 19- or 38-kDa protein, or ESAT-6.
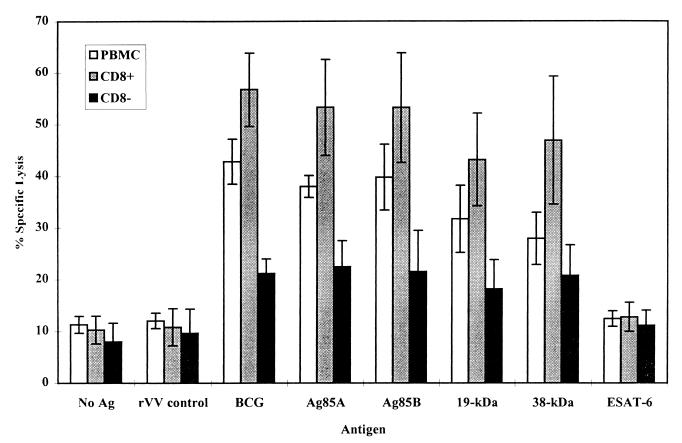
The CD8+ T-cell-enriched and whole-PBMC effectors showed CTL activity against all antigens tested except ESAT-6, with the greatest specific lysis against live M. bovis BCG-, Ag85A-, and Ag85B-infected target cells (Fig. 4). Strong CTL activity was also demonstrated towards the 19- and 38-kDa antigens of M. tuberculosis. The greatest CTL activity was mediated by the CD8+ T-cell-enriched population; in contrast, the CTL activity was significantly reduced in the CD8+ T-cell-depleted population (P < 0.001). These results demonstrate that CD8+ T cells induced by a live M. bovis BCG infection possess potent CTL activity and that the CD8− T-cell population showed only limited CTL activity.
FIG. 4.
CTL activity against mycobacterial antigens. PBMC were incubated for 7 days in the presence of M. bovis BCG. The CTL activity of whole PBMC was compared to that of CD8+ T-cell-enriched and CD8+ T-cell-depleted populations separated by MACS. Effector cells were incubated with autologous, antigen-pulsed target cells for 6 h at a 15:1 ratio. CTL activity was measured by specific 51Cr release. The results shown are the means of 10 donors ± standard deviations.
CD8+-T-cell-mediated CTL activity requires classical MHC class I antigen presentation.
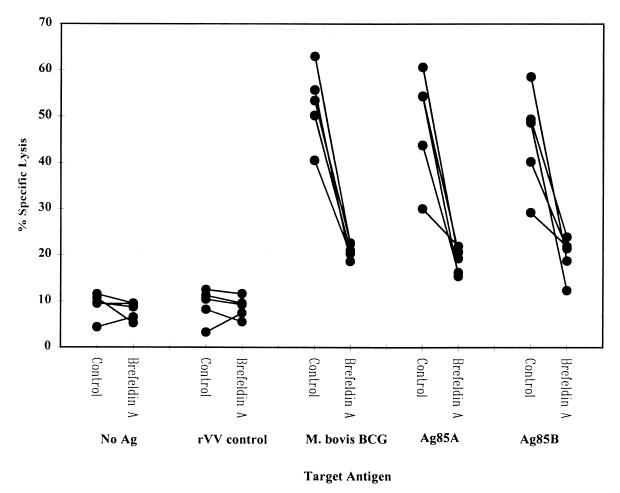
To define the mechanism by which antigen is processed and presented to these mycobacterium-specific CD8+ T cells by monocyte-derived macrophages, inhibitors known to interfere with discrete stages of antigen processing were used. As shown in Fig. 5 and 6, the metabolic inhibitor brefeldin A (an inhibitor of the Golgi-ER processing and presentation pathway and thus of MHC class I expression) significantly reduced the CD8+-CTL activity mediated against mycobacterial antigen-pulsed target cells (P < 0.001). Approximately 80% of the CTL activity was blocked with brefeldin A treatment, reducing lysis almost to that demonstrated against uninfected target cells or target cells infected with the control vaccinia virus. Figure 6 demonstrates that the addition of the phagocytosis inhibitor cytochalasin D or the proteosome inhibitor N-Cbz-Leu-Leu-leucinal to M. bovis BCG-infected target cells resulted in complete inhibition of lysis, reducing the levels of cytotoxicity observed to that of uninfected targets. In contrast, the addition of chloroquine (an inhibitor of phagolysosomal acidification and thus of MHC class II presentation) had no significant effect upon the levels of cytotoxicity against M. bovis BCG-infected antigen-presenting cells; cells treated with chloroquine (100 mM) before infection with BCG demonstrated 37.95% lysis (±3.35 standard deviation for five donors) compared to untreated cells, which showed 41.48% lysis (±4.61 standard deviation). The addition of anti-MHC class I antibody (W6/32) but not anti-CD1 or an isotype-matched control antibody inhibited lysis, again reducing it to levels observed for uninfected target cells. These results show that M. bovis BCG-reactive CD8+ T cells predominantly recognize mycobacterial antigens in a classical MHC class I-dependent manner.
FIG. 5.
Effect of brefeldin A on presentation of mycobacterial antigens. PBMC were stimulated for 7 days in the presence of M. bovis BCG. Antigen-specific CD8+ T cells were positively selected for by MACS. CD8+ T cells were added to target cells pulsed with antigen and treated or untreated with brefeldin A. The results show the responses of five individual donors.
FIG. 6.
Antigen processing of M. bovis BCG by monocyte-derived macrophages. Monocyte-derived macrophages were treated with either N-Cbz-Leu-Leu-leucinal (CLLL; 10 μg/ml), cytochalasin D (CYT D; 10 μg/ml), brefeldin A (BFA; 10 μg/ml), or antibodies against HLA-A, -B, or -C (W6/32; 5 μg/ml), CD1 a, b, or c (5 μg/ml), or an isotype control antibody (IC; 5 μg/ml) before MACS-purified antigen-specific CD8+ T cells were added and cellular cytotoxicity was measured by a 6-h 51Cr release assay. The results are expressed as mean values for 10 donors ± standard deviations.
DISCUSSION
Little is known about the mechanisms involved in the protective immune response against M. tuberculosis infection or about the virulence mechanisms used by this organism to evade the defenses. Cellular immunity is known to be the major protective response, involving the interaction between activated infected macrophages and antigen-specific CD4+ T cells. This model of defense has long been established as essential to mount a strong protective immune response against infection with M. tuberculosis (25).
However, recent studies with murine models have also demonstrated the importance of CD8+ T cells in the protective immune response against mycobacteria, showing that mice lacking functional CD8+ T cells are more susceptible to mycobacterial infection (16, 27). Human studies have also begun to demonstrate a capacity for CD8+ T cells to become activated in response to antigen-presenting cells infected with mycobacteria and to act as both cytokine producers and CTLs (5, 15, 28, 30, 47, 50).
This study demonstrates that BCG-vaccinated individuals possess CD8+ T cells capable of expansion upon in vitro restimulation with live M. bovis BCG and that these activated CD8+ T cells are capable of acting as both type 1 cytokine-producing cells and CTLs. Furthermore, we provide evidence that human CD8+ T cells are capable of recognizing protein antigens from mycobacteria presented by monocyte-derived-macrophage antigen-presenting cells and that this recognition is dependent upon antigen processing through the Golgi-ER.
The presence of CD8+ T cells capable of recognizing mycobacterial antigens upon stimulation with a live M. bovis BCG infection suggests that mycobacterial antigens are able to enter the MHC class I presentation pathway. Studies with the murine model have shown the capacity for exogenous antigens to gain access to the MHC class I processing and presentation pathway (26, 37–39); however, the efficiency of these processes remains controversial. Alternatively, M. tuberculosis has recently been shown to possess a hemolysin similar to that of Listeria monocytogenes which may allow the bacterial antigens to actively escape the phagosome and gain access to the cytosol (55).
The CD8+ T-cell responses to an infection with live M. bovis BCG and to the soluble mycobacterial antigen PPD were compared. It has long been established that a Th1 CD4+-T-cell response characterized by the presence of the cytokine IFN-γ is required for an effective immune response against M. tuberculosis. Evidence is now emerging that CD8+ T cells can be divided into type 1 and type 2 phenotypes (Tc1 and Tc2) characterized by the presence or absence of the cytokines IFN-γ, TNF-α, and IL-4, rather like subsets of CD4+ T-cells (10, 31, 36, 44).
When the CD8+-T-cell response to these two antigens was compared by intracellular cytokine staining and flow cytometry, significantly more CD8+ T cells were activated and produced the cytokines IFN-γ and TNF-α in response to live M. bovis BCG than in response to PPD. These results agree with those of several other studies demonstrating a CD8+-T-cell response to live mycobacterial stimulation (15, 21, 30, 50). However, the findings of this study further characterized the reactive CD8+ T cells as Tc1 cytokine producers by the presence of IFN-γ and TNF-α, with no detectable IL-4.
Some CD8+ T cells became activated by the soluble antigen PPD, which should be processed and presented via the MHC class II pathway and not presented to CD8+ T cells. This may be explained in two ways: PPD consists of highly degraded mycobacterial protein antigens, and thus, peptide fragments could bind directly to membrane MHC class I molecules without the need for processing; or these antigens may be taken up by macropinocytosis and enter an alternative TAP-independent MHC class I pathway (11, 13, 37, 38).
Evidence exists suggesting that there may be some dichotomy in the CD8+-T-cell population, with some cells making cytokines while others act as CTLs (45). To investigate whether this was the case for the M. bovis BCG-reactive CD8+ T cells, intracellular perforin staining was performed alongside cytokine staining. These experiments found no such dichotomy within the majority of the CD8+-T-cell population with regard to cytokine production and cytotoxic potential.
Previous studies by other groups have shown CD8+ T cells to be capable of responding to a number of secreted and cell wall antigens of mycobacteria, including ESAT-6 (28, 51), Ag85 (1, 9, 12), and the 38-kDa protein (52, 53, 56, 57) and the 19-kDa protein in humans (34) but not in the mouse (14). To further define the CD8+-T-cell response to mycobacterial infection, we investigated CTL activity against target cells infected with rVV expressing mycobacterial antigens. The findings of this study clearly demonstrate that CD8+ CTLs are induced by M. bovis BCG infection and that these cells strongly recognize the antigens Ag85A and Ag85B, demonstrating these molecules to be major antigenic targets for CTLs. Other groups have already demonstrated Ag85A and -B to be major antigenic targets recognized by CD4+ T cells. Studies with the murine model have shown that T cells from immune mice are able to recognize Ag85B (1, 9); furthermore, vaccination studies of mice and guinea pigs have shown that DNA vaccines expressing Ag85A induce CD8+ and CD4+ T cells and afford protection against M. tuberculosis challenge (2, 12). Our study enhances the evidence that Ag85A is an antigenic target for CD8+ T cells, since CTLs can be generated against Ag85A in the blood of BCG-vaccinated donors (35). However, the present study characterizes this CTL activity as being CD8+ T cell mediated, something previous studies have not proven.
The CTL response to target cells infected with rVV expressing ESAT-6 showed no significant increase in specific lysis over the uninfected controls or rVV control targets. This lack of response was not due to the absence of the ESAT-6 protein, since the rVV had previously been shown to produce the protein (20). Instead, this result can be explained by the recent findings that the gene for ESAT-6 is absent from the vaccine strain of M. bovis BCG (19). Since the healthy BCG-vaccinated donors in theory might have been exposed only to M. bovis BCG and not to M. tuberculosis or the other environmental mycobacteria, such as Mycobacterium kansasii or Mycobacterium marinium, known to express ESAT-6 (19), they would possess no memory immune cells capable of recognizing ESAT-6. Also, since M. bovis BCG was used to stimulate the CD8+ T cells, no ESAT-6-reactive cells should have been generated. An increase in the number of ESAT-6-reactive CD8+ T cells might be expected in patients with M. tuberculosis infection (28).
Metabolic inhibition was used to define the antigen-processing and presentation pathways involved in CD8+ T-cell activation. Metabolic inhibitors of phagocytosis, proteosome activity, and Golgi-ER transport and antibodies against MHC class I all significantly reduced the CTL activity against M. bovis BCG-infected target cells, while the phagolysosomal acidification inhibitor chloroquine and anti-CD1 antibodies showed no inhibition of cytotoxicity. These data suggest that M. bovis BCG is phagocytosed and antigens from the bacilli gain access to the cytosol, where they are presented to antigen-specific CD8+ T cells via the classical MHC class I pathway. Lewinsohn et al. and Canaday et al. found that brefeldin A treatment of antigen-presenting cells infected with M. tuberculosis showed no decrease in CD8+-T-cell-mediated CTLs and concluded that a nonclassical MHC class I presentation pathway might be involved in M. tuberculosis infection (5, 30). This could be explained by the fact that the dendritic cells used as antigen-presenting cells by Lewinsohn et al. may express other presentation molecules which could present antigen to CD8+ T cells in an MHC class I-independent manner. Other groups have suggested that CD1 might be a presentation molecule for CD8+ T cells in interactions with dendritic cells (43); however, Lewinsohn et al. found this not to be the case. Also, both Canaday et al. and Lewinsohn et al. studied presentation of M. tuberculosis, a more virulent mycobacterium than M. bovis BCG, and therefore a virulence mechanism associated with M. tuberculosis may be its ability to bypass conventional MHC class I presentation. Indeed, Liebana et al. showed presentation of M. bovis to bovine CD8+ T cells to be brefeldin A sensitive, suggesting that nonclassical MHC class I presentation may be a characteristic of M. tuberculosis infection (32).
The presence of an MHC class I-restricted response to mycobacterial infection has been demonstrated by several groups. However, the exact function of this cell population has yet to be defined (5, 28, 30, 33, 47). CD8+ T cells act as cytolytic effector cells lysing infected macrophages, thus releasing bacteria and allowing uptake by activated macrophages better equipped for microbial killing. CD8+ T cells are good cytokine producers, secreting IFN-γ and TNF-α, which are essential for macrophage activation and antimycobacterial immunity. The results of this study clearly demonstrate that CD8+ T cells are capable of responding to a live mycobacterial infection, as well as to specific target antigens from mycobacteria. The activated CD8+ T cells produce type 1 cytokines, primarily IFN-γ and TNF-α, and possess potent CTL activity capable of lysing macrophages infected with live M. bovis BCG, as well as rVV expressing mycobacterial antigens. In addition, Ag85A and -B have been identified as major antigenic targets for CD8+ T cells induced by M. bovis BCG, and to a lesser extent, the 19- and 38-kDa proteins are also recognized as target antigens. These findings support a potential role for CD8+ T cells reactive to secreted antigens from mycobacteria in protective immunity against M. tuberculosis.
ACKNOWLEDGMENTS
We thank P. Andersen for providing the constructs for the preparation of the rVVs expressing the ESAT-6 protein and X. Zhu, M. Vordermeier, and J. Ivanyi for providing us with the rVVs expressing 19- and 38-kDa proteins. We also thank O. Denis for critical review of the manuscript.
This work was supported by a grant from the European Commission (IC 18*CT 970236). Steven Smith is the recipient of an MRC Glaxo-Wellcome Collaborative Studentship (G78/5415).
REFERENCES
- 1.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 2.Baldwin S L, D’Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D M, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom B R, Murray C J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 4.Bonecini-Almeida M G, Chitale S, Boutsikakis I, Geng J, Doo H, He S, Ho J L. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-γ and primed lymphocytes. J Immunol. 1998;160:4490–4499. [PubMed] [Google Scholar]
- 5.Canaday D H, Ziebold C, Noss E H, Chervenak K A, Harding C V, Boom W H. Activation of human CD8+ αβ TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J Immunol. 1999;162:327–379. [PubMed] [Google Scholar]
- 6.Clemens D L, Horwitz M A. Characterisation of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper A M, D’Souza C, Frank A A, Orme I M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper A M, Callahan J E, Keen M, Belisle J T, Orme I M. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 10.Croft M, Carter L, Swain S L, Dutton R W. Generation of polarised antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis O, Lozes E, Huygen K. Induction of cytotoxic T-cell responses against culture filtrate antigens in Mycobacterium bovis bacillus Calmette-Guérin-infected mice. Infect Immun. 1997;65:676–684. doi: 10.1128/iai.65.2.676-684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denis O, Tanghe A, Palfliet K, Jurion F, van der Berg T-P, Vanonckelen A, Ooms J, Saman E, Ulmer J B, Content J, Huygen K. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denis O, Huygen K. Characterisation of the culture filtrate-specific cytotoxic T lymphocyte response induced by bacillus Calmette-Guerin vaccination in H-2b mice. Int Immunol. 1999;11:209–216. doi: 10.1093/intimm/11.2.209. [DOI] [PubMed] [Google Scholar]
- 14.Erb K J, Kirman J, Woodfield L, Wilson T, Collins D M, Watson J D, LeGros G. Identification of potential CD8+ T-cell epitopes of the 19 kDa and AhpC proteins from Mycobacterium tuberculosis. No evidence for CD8+ T-cell priming against the identified peptides after DNA-vaccination of mice. Vaccine. 1998;16:692–697. doi: 10.1016/s0264-410x(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 15.Esin S, Batoni G, Kallenius G, Gaines H, Campa M, Svenson S B. Proliferation of distinct human T cell subsets in response to live, killed or soluble extracts of Mycobacterium tuberculosis and M. avium. Clin Exp Immunol. 1996;104:419–425. doi: 10.1046/j.1365-2249.1996.d01-691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn J-A L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn J-A L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamann D, Baars P A, Rep M H G, Hooibrink B, Kerkhof-Garde S R, Klein M R, van Lier R A W. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harboe M, Oettinger T, Winker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harboe M, Malin A S, Dockrell H M, Winker H G, Ulvund G, Holm A, Jorgensen M C, Andersen P. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect Immun. 1998;66:717–723. doi: 10.1128/iai.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard A D, Zwilling B S. Cytokine production by CD4 and CD8 T cells during the growth of Mycobacterium tuberculosis in mice. Clin Exp Immunol. 1998;113:443–449. doi: 10.1046/j.1365-2249.1998.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–897. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 23.Jason J, Larned J. Single-cell cytokine profiles in normal humans: comparison of flow cytometric reagents and stimulation protocols. J Immunol Methods. 1997;207:13–22. doi: 10.1016/s0022-1759(97)00079-3. [DOI] [PubMed] [Google Scholar]
- 24.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 26.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock K L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladel C H, Daugelat S, Kaufmann S H E. Immune response to Mycobacterium bovis bacille Calmette Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 28.Lalvani A, Brookes R, Wilkinson R J, Malin A S, Pathan A A, Andersen P, Dockrell H, Pasvol G, Hill A V S. Human cytolytic and interferon γ-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laochumroonvorapong P, Wang J, Liu C-C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewinsohn D M, Alderson M R, Briden A L, Riddell S R, Reed S G, Grabstein K H. Characterisation of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Sad S, Kagi D, Mosmann T R. CD8Tc1 and Tc2 cells secrete distinct cytokine patterns in vitro and in vivo but induce similar inflammatory reactions. J Immunol. 1997;158:4152–4161. [PubMed] [Google Scholar]
- 32.Liebana E, Girvin R M, Welsh M, Neill S D, Pollock J M. Generation of CD8+ T-cell responses to Mycobacterium bovis and mycobacterial antigen in experimental bovine tuberculosis. Infect Immun. 1999;67:1034–1044. doi: 10.1128/iai.67.3.1034-1044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzaccaro R J, Gedde M, Jensen E R, van Santen H M, Ploegh H L, Rock K L, Bloom B R. Major histocompatibility class I presentation of soluble antigen facilitated by Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1996;93:11786–11791. doi: 10.1073/pnas.93.21.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohagheghpour N, Gammon D, Kawamura L M, van Vollenhoven A, Benike C J, Engleman E G. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161:2400–2406. [PubMed] [Google Scholar]
- 35.Munk M E, De Bruyn J, Gras H, Kaufmann S H E. The Mycobacterium bovis 32-kilodalton protein antigen induces human cytolytic T-cell responses. Infect Immun. 1994;62:726–728. doi: 10.1128/iai.62.2.726-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noble A, Macary P A, Kemeny D M. IFN-γ and IL-4 regulate the growth and differentiation of CD8+ T cells into subpopulations with distinct cytokine profiles. J Immunol. 1995;155:2928–2937. [PubMed] [Google Scholar]
- 37.Norbury C C, Hewlett L J, Prescott A R, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 38.Norbury C C, Chambers B J, Prescott A R, Ljunggren H-G, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–288. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 39.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 40.Prussin C, Metcalfe D D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 41.Raviglione M C, Snider D E J, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 42.Ravn P, Boesen H, Pedersen B K, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis BCG. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 43.Rosat J-P, Grant E P, Beckman E M, Dascher C C, Sieling P A, Frederique D, Modlin R L, Porcelli S A, Furlong S T, Brenner M B. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ αβ T cell pool. J Immunol. 1999;162:366–371. [PubMed] [Google Scholar]
- 44.Sad S, Marcotte R, Mosmann T R. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 45.Sad S, Kagi D, Mosmann T R. Perforin and Fas killing by CD8+ T cells limits their cytokine synthesis and proliferation. J Exp Med. 1996;184:1543–1547. doi: 10.1084/jem.184.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stenger S, Hanson D A, Teitelbaum R, Dewan P, Niazi K R, Froelich C J, Ganz T, Thomas-Uszynski S, Melian A, Bogdan C, Porcelli S A, Bloom B R, Krensky A M, Modlin R L. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 47.Tan J S, Canaday D H, Boom W H, Balaji K N, Schwander S K, Rich E A. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J Immunol. 1997;159:290–297. [PubMed] [Google Scholar]
- 48.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 49.Tascon R E, Stavropoulos E, Lukacs K V, Colston M J. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner J, Dockrell H M. Stimulation of peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996;87:339–342. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulrichs T, Munk M E, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro M L, Kaufmann S H E. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–3958. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 52.Vordermeier H M, Zhu X, Harris D P. Induction of CD8+ CTL recognising mycobacterial peptides. Scand J Immunol. 1997;45:521–526. doi: 10.1046/j.1365-3083.1997.d01-432.x. [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson R J, Zhu X, Wilkinson K A, Lalvani A, Ivanyi J, Pasvol G, Vordermeier H M. 38,000 MW antigen-specific major histocompatibility complex class I restricted interferon-γ-secreting CD8+ T cells in healthy contacts of tuberculosis. Immunology. 1998;95:585–590. doi: 10.1046/j.1365-2567.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. Global tuberculosis control. W. H. O. Report 1998. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 55.Wren B W, Stabler R A, Das S S, Butcher P D, Mangan J A, Clarke J D, Casali N, Parish T, Stoker N G. Characterisation of a haemolysin from Mycobacterium tuberculosis with homology to a virulence factor of Serpulina hyodysenteriae. Microbiology. 1998;144:1205–1211. doi: 10.1099/00221287-144-5-1205. [DOI] [PubMed] [Google Scholar]
- 56.Zhu X, Venkataprasad N, Ivanyi J, Vordermeier H M. Vaccination with recombinant vaccinia virus protects mice against Mycobacterium tuberculosis infection. Immunology. 1997;92:6–9. doi: 10.1046/j.1365-2567.1997.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu X, Stauss H J, Ivanyi J, Vordermeier H M. Specificity of CD8+ T cells from subunit-vaccinated and infected H-2b mice recognising the 38 kDa antigen of Mycobacterium tuberculosis. Int Immunol. 1997;9:1669–1676. doi: 10.1093/intimm/9.11.1669. [DOI] [PubMed] [Google Scholar]