Abstract
Obstructive sleep apnoea (OSA) is associated with cardiovascular and metabolic comorbidities, including hypertension, dyslipidaemia, insulin resistance and atherosclerosis. Strong evidence suggests that OSA is associated with an altered lipid profile including elevated levels of triglyceride-rich lipoproteins and decreased levels of high-density lipoprotein (HDL). Intermittent hypoxia; sleep fragmentation; and consequential surges in the sympathetic activity, enhanced oxidative stress and systemic inflammation are the postulated mechanisms leading to metabolic alterations in OSA. Although the exact mechanisms of OSA-associated dyslipidaemia have not been fully elucidated, three main points have been found to be impaired: activated lipolysis in the adipose tissue, decreased lipid clearance from the circulation and accelerated de novo lipid synthesis. This is further complicated by the oxidisation of atherogenic lipoproteins, adipose tissue dysfunction, hormonal changes, and the reduced function of HDL particles in OSA. In this comprehensive review, we summarise and critically evaluate the current evidence about the possible mechanisms involved in OSA-associated dyslipidaemia.
Keywords: obstructive sleep apnoea, OSA, dyslipidaemia, lipid, metabolic dysfunction
1. Introduction
Obstructive sleep apnoea (OSA) is common disorder which is characterised by recurrent collapses of the upper airways during sleep. Intermittent hypoxia (IH) and sleep fragmentation are the most important factors in the pathomechanism of OSA resulting in sympathetic overdrive, oxidative stress and systemic inflammation. These derangements lead to cardiovascular and metabolic alterations, such as atherosclerosis, hypertension, insulin resistance and dyslipidaemia, ultimately contributing to cardiovascular morbidity and mortality [1].
Dyslipidaemia is an independent risk factor for cardiovascular morbidity [2]. There is also strong evidence supporting the role of OSA with altered lipid profile: elevated triglyceride (TG), total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) concentrations with a corresponding reduction in high-density lipoprotein cholesterol (HDL-C) levels were commonly found in patients with OSA [3,4]. Understanding the mechanisms linking OSA to lipid abnormalities is of major clinical importance, as they could represent treatable traits (i.e., choosing the right lipid-lowering medication and lifestyle changes), and also highlights the importance of active screening of dyslipidaemia in patients with OSA.
The aim of this review is to summarise and critically evaluate the current evidence about the possible mechanisms involved in OSA-associated dyslipidaemia. Naturally, we focus on human studies; however, we briefly discuss animal models and highlight if the research was conducted in humans or animals.
2. Overview of Physiological Lipid Metabolism
2.1. The Physiological Role of Chylomicrons
Dietary TGs are hydrolysed by several lipases (for example pancreatic and gastric lipases) to FFAs and monoacylglycerol (MAG) to be absorbed by the enterocytes in the small intestine [5]. FFAs can be transported by passive diffusion or fatty acid transporters, such as cluster determinant 36 (CD36) or fatty acid-transport protein 4 (FATP4). Dietary cholesterol esters (CEs) are hydrolysed to FFAs and free cholesterol (FC). Several FC transporters were identified on the enterocytes, such as the Niemann–Pick C1-like protein (NPC1L1), ATP-binding cassette protein G5 (ABCG5) and G8 (ABCG8) and scavenger receptor class B type I (SR-BI). They are also expressed in the apical membrane of hepatocytes [6].
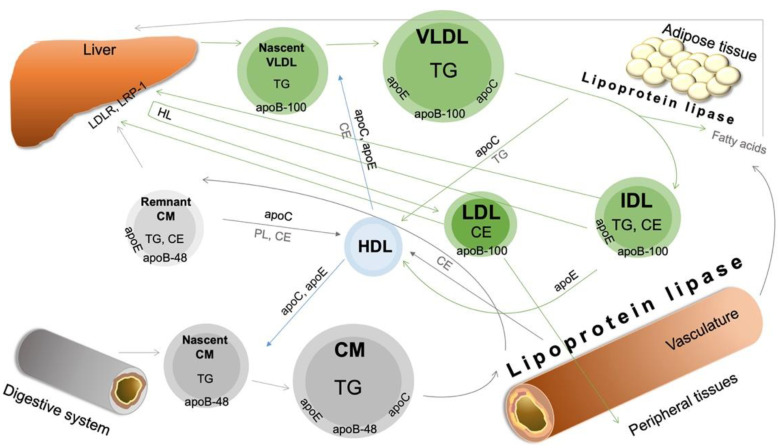
The chylomicrons (CMs) are large TG-rich lipoproteins containing apolipoprotein B-48 (apoB-48) which are usually formed by dietary FFA absorbed from the small intestine [7]. The most common role of CMs is to transport dietary cholesterol and TGs to the peripheral tissues and to the liver; the process is called “exogenous lipid transport” (Figure 1). CMs have also an active role in enterohepatic cholesterol transport. Around 1000 g of biliary cholesterol is secreted to the intestine every day. Thus, the majority of CM-transported cholesterol derives from the reabsorption of biliary cholesterol [8].
Figure 1.
Overview of physiological lipoprotein metabolism. Apo—apolipoprotein; CE—cholesteryl ester; CM—chylomicron; HDL—high-density lipoprotein; HL—hepatic lipase; IDL—intermediate-density lipoprotein; LDLR—low-density lipoprotein receptor; LRP-1—LDL receptor-related protein 1; PL—phospholipid; TG—triglyceride; VLDL—very-low-density lipoprotein. For description and references, please see the text.
CMs are synthetised in the endoplasmic reticulum (ER) and Golgi apparatus of the enterocytes. First, in the ER, the previously hydrolysed FFAs, FC and MAG are resynthesised. FATP4 converts FFAs to fatty acyl-CoA (FFA-CoA) [9], and thus FFA-CoA and MAG can be converted to diacylglycerol (DAG) by monoacylglycerol acyl transferase 2 (MGAT2) [10,11]. Then, diacylglycerol acyl transferase 1 (DGAT1) further converts DAG with FFA-CoA to triacylglycerol (TAG) [12], which is the main component of the nascent CM [10]. TAG can leave the ER to form cytosolic lipid droplets or chylomicrons. FC is transformed to CEs by the acyl-coenzyme A:cholesterol acyltransferase (ACAT).
Apolipoprotein B-48 (apoB-48) is a unique marker of CMs, and each CM particle contains one apoB-48 molecule [13]. ApoB-48 is a truncated form of the hepatic apolipoprotein B-100 (apoB-100), and it has 48% of the initial length of apoB-100 after a post-transcriptional mRNA modification in the intestine. The scaffolding of apoB-48 with TAG, CE and phospholipids is mediated by microsomal triglyceride transfer protein (MTP), resulting in primordial CMs. Primordial CMs can be expanded with further TG or CM by MTP or after the fusion with lipid droplets [14]. After the core expansion, apolipoprotein A-IV (apoA-IV) is also assembled into the nascent CM surface [15]. Nascent CMs are transported to the Golgi in pre-CM transport vesicles (PCTVs) [16,17] where apolipoprotein A-I (apoA-I) is also incorporated. Finally, a pre-CM leaves the enterocyte through its basolateral membrane by exocytosis.
In the circulation, a nascent CM gains further apolipoproteins, such as apoC-I, apoC-II, apoC-III and apoE from HDL [18], resulting in a mature CM. After hydrolysis of TAGs from CMs by lipoprotein lipase (LpL), the core of CMs is decreased. The remnant CM particles are taken up mainly by LDL receptors (LDLRs) of hepatocytes [19].
2.2. The Physiological Role of VLDL
Very-low-density lipoprotein (VLDL) delivers lipids from the liver to the peripheral tissues; this process is called “endogenous lipid transport” (Figure 1). VLDL is synthetised in the liver and regulated by the FFA influx. The FFAs originate from adipocytes, CM remnants and the intestine via the portal vein [20]. VLDL is composed of a TG-rich core (synthetised from FFAs) surrounded by FC, CE, phospholipids (PLs) and apolipoproteins (apoB-100, C, E).
ApoB-100 expressed by the liver is the essential component of VLDL, IDL and LDL particles. ApoB-100 is lipidated in the ER by MTP, resulting in primordial VLDL and then pre-VLDL particles [21]. Under inadequate TG availability, apoB-100 can be degraded in several ways (such as ER-associated degradation or post-ER presecretory proteolysis). Primordial VLDL can also fuse with microsomal lipid droplets associated with apoC-III [22]. Pre-VLDLs are transported to the Golgi in specialised vesicles (VLDL transport vesicles). During the Golgi-associated maturation of VLDL, apoB-100 undergoes conformational changes and VLDL is expanded by lipoproteins [23], and VLDL leaves the Golgi by exocytosis.
VLDL is metabolised by LpL to produce intermediate-density lipoproteins (IDLs) which can be taken up by the liver via apoE receptors [24]. IDLs can transfer apoE to HDL particles, avoiding hepatic clearance, and their TG content is hydrolysed by hepatic lipase (HL), resulting in CE-rich LDL particles. VLDL receptors (VLDLRs) can be found in several tissues such as adipose tissue, muscle and heart and recognise apoB-100 and apoE. They can bind VLDL, IDL or CM but not LDL particles [25].
2.3. The LDL Metabolism
LDL particles are the most important cholesterol carriers in the circulation (Figure 1). LDL consists of mainly CE, FC, TG, PL and a single molecule of apoB-100 [26]. The size, composition and density of HDL are mainly influenced by LpL and cholesteryl ester transfer protein (CETP) functions [27]. Several modified LDL particles were identified, such as oxidised LDL (oxLDL), small dense LDL (sdLDL) and desialylated LDL, which are strongly atherogenic [28,29,30]. Further subclasses can be identified by gel electrophoresis: large (LDL 1–2) and small (LDL 3–7) subfractions [31]. Circulating LDL particles are absorbed by the liver (70%) and peripheral tissues (30%), mainly by the LDL receptor (LDLR) [32].
LDLR binds apoB-100 and apoE with high affinity and is thus responsible for the uptake of VLDL, IDL and LDL particles [6]. The expression of LDLR is regulated via a negative feedback mechanism mediated by the complex of sterol-regulated transcription protein-2 (SREBP-2) and SREBP cleavage activating protein (SCAP) [33]. In cholesterol-depleted cells, the SREBP-2/SCAP complex is proteolytically cleaved in the Golgi, resulting in SREBP-2. SREBP-2 activates HMG-CoA reductase and LDLR, leading to cholesterol uptake [34,35]. On the contrary, high cellular cholesterol levels lead to conformational changes of SCAP in the ER not allowing the transport of the SREBP-2/SCAP complex to the Golgi [36]. LDLR can be regulated at the posttranscriptional level by proprotein convertase subtilisin/kexin type 9 (PCSK9) which degrades LDLR [37].
Other receptors can also eliminate LDL from the circulation. Low-density lipoprotein receptor-related protein-1 (LRP-1; also known as cluster of differentiation 91 (CD91)) is a multifunctional receptor expressed in several tissues (hepatocytes, adipocytes, muscle cells, macrophages and endothelial cells). Its expression is strongly regulated by metabolic and inflammatory processes [38]. LRP-1 receptors mediate the clearance of apoE-containing lipoproteins (VLDL and CM remnants) mainly in the absence of LDLR. In the lack of hepatic LRP-1, CM clearance is decreased [39]. Moreover, it also binds lipases, such as HL and LpL: they enhance the binding of apoE-containing lipoproteins to LRP [40,41]. LRP-1 plays a role in the HDL metabolism by enhancing the recycled apoE accumulation in early endosomes [42]. The presence of macrophages with LRP-1 deletion was associated with elevated plasma CE and TG levels resulting in the accumulation of circulating TG-rich lipoproteins [43]. Other studies demonstrated the atherogenic effects of LRP-1 as it can also mediate the accumulation of cholesterol in macrophages [43] or in cardiomyocytes [44]. Besides the lipid metabolism, LRP-1 internalises more than 100 ligands, including proteinases and proteinase-inhibitor complexes (tissue plasminogen activator (tPA), urokinase PA (uPA), matrix metalloproteases (MMPs)), coagulation factors, growth factors and matrix proteins (fibronectin, thrombospondin). LRP-1 is able to regulate several transcription factors (such as nuclear factor-κB (NF-κB)) affecting immune responses and tissue survival. In pathophysiological circumstances, LRP-1 can be shed by proteases, resulting in its soluble form (sLRP-1). During inflammation, sLRP-1 stimulates the expression of further pro-inflammatory cytokines, such as TNF-α [38]. However, other studies reported its anti-inflammatory function. sLRP1 mediates the internalisation of αMβ2 integrin, resulting in the inhibition of its adhesion properties [45]. In addition, sLRP-1 decreased the expression of TNF-α and IL-1 [46].
2.4. The Role of Lipoprotein Lipase
LpL hydrolyses the VLDL- and CM-associated TAGs to FFAs and MAGs which are taken up by the target cells. This enzyme is mainly produced by adipose tissue and skeletal and cardiac muscle and transported to the luminal surface of endothelial cells by the glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) [47]. This endothelial LpL pool is referred to as the functional LpL [48]. LpL activity is regulated by different physiological stimuli in a tissue-specific manner. In white adipose tissue, LpL activity is increased by the postprandial state and decreased by fasting [49]. On the contrary, fasting activates the myocardial LpL [50]. Finally, in skeletal muscle, LpL activity is promoted by acute exercise [51]. ApoC-II is the main cofactor of LpL activity [18], whereas apoC-I and apoC-III have been shown to inhibit LpL activity [52]. Moreover, some members of the family of angiopoietin-like proteins, such as ANGPTL3 (hepatocyte), ANGPTL4 (adipocyte) and ANGPTL8, also promote the inhibition of LpL [53,54]. Several hormones, such as insulin, glucocorticoids and adrenalin, stimulate LpL activity in the adipose tissue [6].
2.5. The Physiological Role of HDL
HDL is a major mediator in reverse cholesterol transport (RCT). RCT is termed as a cholesterol transport from the peripheral cells (including macrophages) back to the hepatocytes for further metabolism [55]. In general, HDL particles comprise a hydrophobic core with CE and TG covered by PL, FC and apolipoproteins (apoA-I, A-II, A-IV, A-V, C-I, C-II, C-III, E, F, J, M). Various HDL particles highly differ in their size, shape, proportion of proteins and lipids and biological activities [56]. The two main forms of HDL are the small and poorly lipidated discoid HDL (also known as preβ-HDL) and the larger and CE/TG-containing spherical HDL (also known as α-HDL) [56,57]. Spherical particles represent the majority of HDL particles in the circulation [57]. HDL2 particles are larger and lipid-rich but less dense, and HDL3 particles are smaller, lipid-poor and dense [58]. These can further be divided into HDL3c, HDL3b, HDL3a, HDL2a and HDL2b fractions [57]. Further subclasses can be identified by gel electrophoresis: large (HDL 1–3), intermediate (HDL 4–7) and small (HDL 8–10) subfractions [31]. The small HDL 8-10 particles are atherogenic through easy penetration to the endothelium and low recognition by HDL receptors [59,60].
The main structural apolipoproteins of HDL are apoA-I (70%) and apoA-II (20%) [61]. ApoA-I plays role in activating LCAT and also has anti-inflammatory and antioxidant effects [62,63]. ApoA-II is an important inhibitor of LpL directly and indirectly by replacing apoC-II in VLDL. Moreover, it also has a cofactor activity for LCAT and CETP [64]. ApoM accounts for approximately 5% of HDL proteins. It plays role in lipid transfer into nascent HDL [65] and enhances the cholesterol efflux from foam cells [66]. Noteworthily, apoM is a carrier of sphingosine-1-phosphate (S1P) mentioned below [67]. Other apolipoproteins constitute a minor amount of HDL, such as apoA-IV, V, C-I, C-II, C-III, D, E, J and L [68]. It is important to note that apoJ or clusterin has anti-apoptotic, anti-atherogenic and anti-inflammatory properties and is involved in lipid transport forming HDL particles [69].
ApoA-I is mainly produced by the liver (70%) [70] and partly by the intestine (30%) [71]. Lipid-poor apoA-I binds ATP-binding cassette transporter A1 (ABCA1) on peripheral cells (such as hepatocytes and macrophages [72]), resulting in FC and PL transport from the cells to apoA-I [73]. Two apoA-I molecules with FC and PL form a discoidal HDL formation [57]. Noteworthily, these particles can also be produced from surface components of the catabolism of TG-rich lipoproteins after the LpL hydrolysis [56]. The discoidal HDL formation reacts quickly with lecithin cholesterol acyltransferase (LCAT) which transports free acid from lecithin to FC, resulting in CE. After esterification and incorporation of more apoA-I by LCAT, the HDL particle becomes a mature spherical form (small HDL3, large HDL2) [55,57] which is dynamically modified in the RCT. Phospholipid transfer protein (PLTP) transfers more PL and FC from VLDL to HDL, enhancing the LCAT reaction and resulting in HDL2 with increased size [74]. PLTP can lead to the fusion of HDL particles with a consequential production of small lipid-poor apoA-I/PL complexes [75].
The mature HDL particles can be cleared from the circulation by two main pathways: (1) The main receptor in the RCT is SR-BI, which is expressed on hepatocytes and steroidogenic cells. SR-BI has an affinity for CE and apoA-I content in HDL particles [76,77]. The hepatic HDL uptake is stimulated by HL [78]. (2) The other mechanism is the indirect pathway in which spherical HDL particles are modified by CETP. CETP is mainly produced by hepatocytes and adipocytes and circulates with HDL [79]. CETP transports CE from HDL towards apoB-containing lipoproteins (mainly LDL, but also VLDL and CM) in exchange for TG in the opposite direction. The transfer activity of CETP is regulated by the triglyceride levels [80]: in the physiological state, predominantly CEs are transported from HDL to apoB-containing lipoproteins with a minor transfer of TG in the opposite direction. In hypertriglyceridaemia, increased concentrations of apoB-containing lipoproteins are available as potential acceptors for CEs. Moreover, CETP also transports TG from TG-rich lipoproteins (VLDL, CM) to LDL and HDL, resulting in small, dense and TG-rich particles [80]. The TG and PL content of these HDL2 particles can be further hydrolysed by HL, resulting in lipid-poor small HDL3 particles which interact with ABCA1 for the next HDL circle [56,78]. The CE content of the apoB-containing particles is taken up by the hepatic LDLR.
It is important to mention that by taking cholesterol from foam cells, HDL has a protective role against atherosclerosis [81,82,83]. HDL also inhibits LDL oxidation. Small HDL3 particles are more resistant to oxidative damage than HDL2 particles and inactivate the products of LDL lipid peroxidation [84,85]. Several HDL-associated apolipoproteins [56] and HDL-bound paraoxonase-1 (PON-1) possess antioxidant properties [86]. HDL displays anti-inflammatory effects by decreasing the expression of inflammatory cytokines and adhesion molecules and inhibiting inflammatory cell activation [87,88].
3. Current Knowledge of The Pathophysiological Lipid Metabolism in OSA
3.1. Animal Models
Animal models allow experimental investigation of OSA-related processes in isolation and have been extensively used to explore the relationship between dyslipidaemia and OSA. The effects of IH were the most widely investigated [89]. These models allow researchers to precisely define the major parameters of IH, such as the frequency or the severity of the hypoxic events [90]. However, it is important to consider that experimental IH episodes cause hypoxia in animals that is significantly more severe than that experienced in humans. For a realistic stimulation of IH, SaO2 should be much lower in mice than the SaO2 observed in patients [90]. Most of the experimental studies investigated whether IH regulates the expression of different transcription factors involved in the lipid metabolism. The regulation of hypoxia-inducible factor-1 (HIF-1), SREBP-1 and stearoyl-coenzyme A desaturase 1 (SCD-1) was investigated in detail in rat and mouse models [91,92,93]. The consequences of dyslipidaemia, such as atherosclerotic lesions associated with IH, can also be studied more precisely in animal models [94].
In humans, IH and sleep fragmentation are closely interrelated [95], and animal models could better separate these entities. On the other hand, dyslipidaemia in humans is complicated by genetic factors, diet, exercise, abdominal obesity, the presence of comorbidities and medications. Therefore, complex animal models which study numerous heterogeneous processes simultaneously are warranted [94].
3.2. Calorie Intake in OSA
Excessive calorie intake is a main cause of aberrant obesity, which is the most important risk factor for OSA. Indeed, patients with OSA tend to consume high-calorie diets [96]. Hunger and food intake are controlled by the balance of a number of hormones, such as leptin, ghrelin, insulin, cholecystokinin, glucagon-like peptide 1 (GLP-1) and peptide YY [97]. However, increased levels of GLP-1 and gastric inhibitory polypeptide/glucose-dependent insulinotropic polypeptide were found in patients with OSA [98]. Moreover, IH seemed to upregulate the expression of peptide YY, GLP-1 and neurotensin in enteroendocrine cells [99]. These hormones have an anorexigenic influence on the enteric nervous system. As a vicious circle, sleep fragmentation in OSA attenuates hypothalamic leptin receptors, resulting in cravings for high-energy foods [100]. The consequences of this leptin resistance are an increase in fat mass and gaining weight, worsening obesity [100]. The ingested fat is the main drive of CM production [101] leading to further alterations in the lipid profile.
3.3. Intestinal Lipid Absorption in OSA
OSA is associated with postprandial hyperlipidaemia [102]. Indeed, using oral retinyl-palmitate, the retinyl-esters incorporated into CM had an earlier peak under IH than under normoxia [103]. Although patients with OSA have higher postprandial TG levels, experimental IH in this group did not result in a further increase in TG levels [104].
High postprandial TG levels could be due to accelerated intestinal absorption. For instance, the FFA transporter CD36 is upregulated by HIF-1 [105]. However, CD36 expression is also upregulated by the peroxisome proliferator-activated receptor-gamma (PPAR-γ), the expression of which was reported to be decreased in OSA [106]. Nevertheless, intrahepatic CD36 was increased in mice exposed to IH [107].
Bile acids act as natural detergents: they emulsify lipid dietary fat into smaller lipid droplets, making the digestion by lipases easier. Cytochrome P450 7A1 (CYP7A1), an important enzyme in bile acid synthesis, was repressed by HIF-1α under hypoxia, suggesting altered bile acid production [108]. However, the effects of IH on bile acid synthesis and absorption have not been investigated. Similarly, gastric and pancreatic lipases were not studied in OSA.
3.4. Impaired Intravascular Lipolysis and Uptake by the Periphery: Lpl Dysfunction in OSA
A well-described mechanism for OSA-associated hyperlipidaemia is the impaired clearance of circulating lipoproteins by LpL (Figure 2). Drager et al. showed that the functional clearance rate of CEs and TGs was significantly lower among patients with OSA compared to controls [103]. This delayed clearance was correlated with the depth of nocturnal hypoxaemia (MinSatO2) and disease severity (apnoea–hypopnoea index (AHI)) [109]. In human preadipocytes exposed to 24 hours of hypoxia in vitro, a 6-fold decrease in LpL activity was detected [110]. Serum LpL concentrations were lower in patients with OSA compared to controls and negatively correlated with disease severity [111].
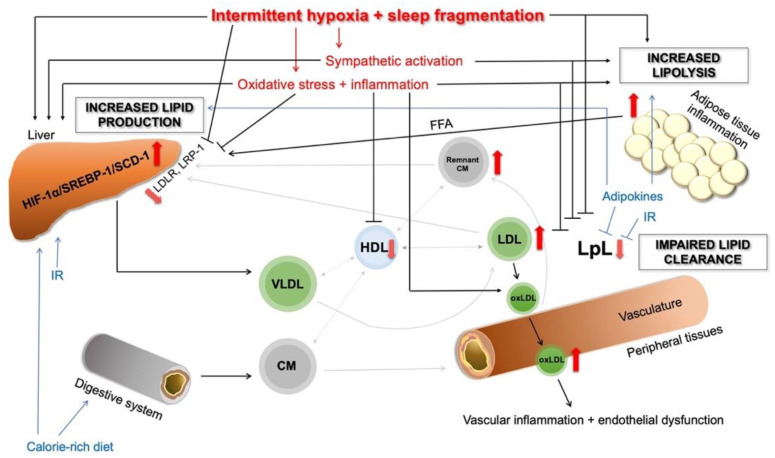
Figure 2.
Overview of the main pathophysiological pathways in OSA-related dyslipidaemia. The red arrows show the altered pathways and dysregulations. CM—chylomicron; FFA—free fatty acid; HDL—high-density lipoprotein; HIF-1α—hypoxia-inducible factor 1 alpha; IR—insulin resistance; LDL—low-density lipoprotein; LDLR—low-density lipoprotein receptor; LpL—lipoprotein lipase; LRP-1—LDL receptor-related protein 1; oxLDL—oxidised-LDL; SCD-1—stearoyl-coenzyme A desaturase 1; SREBP-1—sterol regulatory element-binding protein 1; TG—triglyceride; VLDL—very-low-density lipoprotein. For description and references, please see the text.
Several OSA-associated mechanisms can lead to the altered function of LpL, including IH, oxidative stress, inflammation, catecholamines and hormones. IH itself is a potent inhibitor of LpL [103], and the degree of hypoxia correlates with the delay in TG clearance [112,113]. Serum LpL concentrations correlated with markers of nocturnal hypoxia, such as the oxygen desaturation index (ODI) [114] and nocturnal SpO2 [111]. In animal models of OSA, CIH increased the levels of adipose ANGPTL4 in an HIF-1α-dependent manner [103], and ANGPTL4 levels correlated with the severity of nocturnal desaturation [115]. Moreover, the antibody against ANGPTL4 increased the activity of LpL in the adipose tissue and the lung [112]. However, Mahat et al. failed to demonstrate any differences in postprandial LpL activity or ANGPTL4 expression between normoxia and IH [110], suggesting other, ANGPTL-4-independent, regulatory mechanisms during CIH [112]. In vivo, higher concentrations of plasma ANGPTL4 and ANGPTL8 were measured in patients with OSA compared to the controls [116]. Higher serum levels of ANGPTL3 were detected in patients with OSA and coronary artery disease (CAD) compared to the patients having OSA alone [117].
PPAR-γ is a main regulator of several genes associated with lipid metabolism, including LpL [118], and it is downregulated by hypoxia in an HIF-1α-dependent manner [119]. Jun et al. detected that acute hypoxia decreased the PPAR-γ expression, resulting in downregulated LpL in mice [113]. However, hypoxia had no effect on the expression of GPIHBP1, which is the carrier of LpL [113].
Inflammation was also found to impair the function of LpL in several ways. Interleukin-1 (IL-1) and tumour necrosis factor-α (TNF-α) decrease the activity of LpL in vitro [120,121] and in vivo [122], at transcriptional [123] and post-transcriptional levels [124]. Circulating LpL levels inversely correlated with CRP levels, emphasising the inhibitory role of inflammation in LpL function [111].
OSA is characterised by increased sympathetic activity [125]. Early studies indicate that catecholamines reduce LpL activity directly [126,127] and indirectly through the activation of ANGPLT4 [128].
Insulin activates LpL in the adipose tissue [129] and downregulates the expression ANGPTL3 [130]. However, insulin resistance (IR) decreases the activity of LpL. In line with this, HOMA-IR, the marker of IR, negatively correlated with LpL [111]. Leptin decreases the activity of LpL directly [131] and indirectly by decreasing the expression of ANGPTL3 [132]. Leptin levels were elevated in OSA [133]. Decreased levels of adiponectin, detected in OSA [134], were associated with lower LPL activity independently of systemic inflammation [135].
In conclusion, impaired function of LpL in OSA leads to decreased lipid uptake of the peripheral tissues resulting in an increase in circulating CM and VLDL-C levels.
3.5. Alternative Ways Leading to Decreased Lipid Uptake
In OSA, LRP-1 can be downregulated by SREBP-1 [136] in an FFA- [137] or IH-dependent fashion [138]. Moreover, vitamin D [139] and klotho [140], which both increase LRP-1 expression, are decreased in OSA [141,142]. The shedding of LRP-1 is facilitated by pro-inflammatory cytokines [38] or atherogenic lipoproteins [143], resulting in its soluble form (sLRP-1) which can be measured in the circulation; sLRP-1 was decreased in OSA and correlated with disease severity [144].
3.6. Increased Lipid Production in the Liver
The lipid production in the liver is influenced by three main mechanisms: (1) de novo lipogenesis of the hepatocytes, (2) FFA delivery and uptake from the periphery and (3) availability of lipids and carbohydrates.
Previous evidence suggested that IH activates SREPB-1, the key transcriptional factor involved in lipid biosynthesis, through HIF-1α activation [91,92]. SREBP-1 upregulates SCD-1. SCD-1 is responsible for the synthesis of monosaturated FAs (MUFAs) [93], which are substrates for PL, TG and CE synthesis [145]. As mentioned above, the HIF-1α/SREBP-1/SCD-1 pathway was widely investigated in OSA (Figure 2). Mice with partial HIF-1α-deficiency exhibited lower hepatic mRNA and protein levels of SCAP and SCD, lower hepatic protein levels of SREBP-1 and lower hepatic fat accumulation compared to the wild-type mice [92]. In a SCAP-deficient mouse model, 5 days of IH did not influence the levels of serum and hepatic lipids and expression of SREBP-1, SCD-1 and HMG-CoA-reductase [138]. Furthermore, SCD-1 deficiency in mice abolished the IH-induced increased hepatic SCD-1 and plasma VLDL-C levels and atherosclerosis in the ascending aorta [146].
The duration of IH seems to influence lipid production in OSA. Five days of IH exposure increased the serum levels of total cholesterol, HDL-C, PL, TG, hepatic TG and SREBP-1 and the protein and mRNA levels of SCD-1 [91]. However, the genetically obese leptin-deficient rats that had higher baseline lipid values did not show changes in serum lipid profile after 5 days of IH compared to the lean rats. The authors concluded that short-term IH upregulates lipid biosynthesis but does not affect it in the presence of pre-existing lipid alterations [91]. On the contrary, genetically obese rats exposed to 12 weeks of IH experienced elevated TG and PL levels as well as SREBP-1 and SCD-1 transcription [147].
The severity of IH may also affect lipid production. The ubiquitination of HIF-1α leads to the proteasomal degradation of HIF-1α protein and depends on the O2 tension [148,149]. In the study of Li et al., only severe IH (oxygen nadir of 5% compared to 10%) increased the hepatic SCD-1 levels [3]. The authors hypothesised that moderate IH did not prevent HIF-1α from proteasomal degradation [3]. In addition, oxidative stress contributes to hepatic lipid overproduction in two ways. Firstly, reactive oxygen species (ROS) stabilise HIF-1α [150]. Secondly, ROS induce lipid peroxidation in the liver [3]. Lipid peroxidation leads to hepatic inflammation and fibrosis resulting in nonalcoholic steatohepatitis (NASH) [151]. The pathomechanism of NASH in OSA was reviewed in detail previously by Mesarwi et al. [151].
IH also enhances hepatic lipid production through the increased sympathetic tone which has a stimulatory effect on VLDL secretion [152].
However, IH alone did not seem to be enough to cause dyslipidaemia in animal models. In atherosclerosis-resistant mice (C57BL/6J), atherosclerosis was observed only in those exposed to both IH and cholesterol-rich diet, but not in those exposed to cholesterol-rich diet or to IH alone [94]. Moreover, the combination of IH and a cholesterol-rich diet was associated with a marked progression of dyslipidaemia. The authors suggested that the presence of dyslipidaemia due to genetic or environmental factors is required for atherogenic consequences of CIH [94].
In line with this, twin studies showed genetic susceptibility to the development of dyslipidaemia [153] and OSA too [154]. In our previous twin study, we detected a heritable relationship between TG levels and sleep parameters (AHI, ODI, TST90%), suggesting a common genetic background [155]. The genetic link between OSA and TG levels has recently been confirmed in a genome-wide association study [156]. Most notably, dyslipidaemia and OSA share common genetic loci, such as PPAR-γ [157,158] or APOE polymorphism [159].
The hepatic lipid accumulation and hepatic insulin resistance can enhance the lipid alterations in OSA. The hepatic lipid accumulation is the consequence of the FFA overload from the periphery due to adipose tissue dysfunction with increased lipolysis and altered lipid clearance by LpL. The coexistence of insulin resistance may also increase VLDL production. In insulin resistance, insulin loses the ability to promote the degradation of apoB [160]. The accumulated lipid content undergoes lipid peroxidation under IH leading to NASH [151]. Moreover, the lipid overproduction leads to increased VLDL production and export to the circulation.
3.7. Abnormal Modifications of LDL in OSA
LDL modification is one of the most important consequences of oxidative stress and inflammation. LDL can be modified in the extracellular space or in the lysosome of macrophages [161] by enzymatic (such as myeloperoxidase (MPO)) and non-enzymatic (such as desialylation, glycosylation, interaction directly with ROS) mechanisms. Not only the lipids but also the protein components of LDL can be modified [162]. Small dense LDL (sdLDL) particles associated with hypertriglyceridaemia are often desialylated, which is the most frequent modification of LDL. Due to their decreased affinity for LDL-R, their longer circulation time makes them susceptible to other modifications [163], including glycosylation [164] and oxidation [165]. Oxidised LDL (oxLDL) particles were found to have pro-inflammatory and atherogenic potential contributing to atherosclerosis (Figure 3). OxLDL particles can be hydrolysed by PON-1 associated with HDL [166].
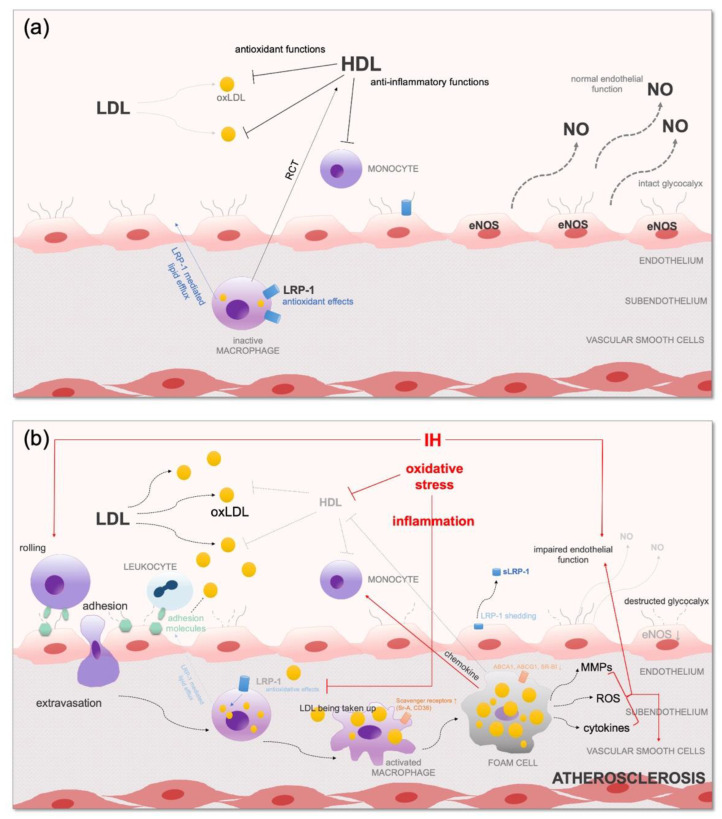
Figure 3.
(a) Antioxidant effects of HDL and LRP-1 and normal endothelial function; (b) impaired antioxidant mechanisms with consequential oxidative stress, inflammation and endothelial dysfunction in OSA. ABCA1—ATP-binding cassette transporter A1; ABCG1—ATP-binding cassette transporter G1; CD36—cluster determinant 36; CM—chylomicron; eNOS—endothelial nitric oxide synthase; HDL—high-density lipoprotein; IH—intermittent hypoxia; IL—interleukin; LDL—low-density lipoprotein; MMP—matrix metalloproteinase; NO—nitric oxide; oxLDL—oxidised-LDL; RCT—reverse cholesterol transport; ROS—reactive oxygen species; sLRP-1—soluble LDL receptor-related protein 1; Sr-A—macrophage scavenger receptor; SR-BI—scavenger receptor class B type I; VLDL—very-low-density lipoprotein. For description and references, please see the text.
Pro-atherogenic sdLDL3–7 subfractions were significantly higher in the OSA group [31]. SdLDL particles were independently associated with OSA in non-obese participants [167]. LDL size was independently associated with metabolic syndrome in OSA [168]. However, Liu et al. did not detect a correlation between OSA severity measures and sdLDL [169].
Only a few studies investigated oxLDL in OSA; oxLDL levels were found to be increased in OSA in most [170,171,172,173] but not all studies [174,175]. A recent meta-analysis concluded that oxLDL levels are increased in OSA [176]. However, studies that matched in age or BMI between patients with OSA and controls showed no significant difference in oxLDL levels [176]. Furthermore, endothelial lectin-like oxidised low-density lipoprotein receptor-1 (LOX-1) was upregulated in OSA [172]. LOX-1 is the main receptor for oxLDL on endothelial cells and orchestrates the expression of adhesion molecules and may induce atherosclerosis in OSA [177].
3.8. HDL Dysfunction in OSA
HDL is converted to a dysfunctional form with impaired physiological effects due to IH, oxidative stress and inflammation [178] (Figure 3). The dysfunctional HDL comprises lower CE, oxidised PL, increased TG and decreased apoA-I content, serum amyloid A (SAA) and several inflammatory proteins, such as complement C3 [178,179].
There is some evidence that IH and inflammation [180] downregulate molecules in the RCT, such as ABCA1 [181] and SR-BI [91]. Short-term IH (5 days) decreased liver SR-BI protein levels independent of obesity in a mouse model. However, obese mice had lower baseline SR-B1 levels than lean mice [91]. On the contrary, long-term IH (4 weeks) did not cause a change in hepatic SR-B1 levels [3].
Oxidative stress enzymes associated with OSA [182], such as MPO, excessively oxidise HDL. The oxidative modification of apoA-I leads to its inability to interact with ABCA-1, resulting in decreased premature HDL and impaired cholesterol efflux [183,184]. Other oxidised components of HDL, such as oxidised PLs [185] or FFAs [186], can also impair the functions of apoA-I by destroying its structure [187]. Although the functionality of apoA-I seems to be altered, its levels were not affected in OSA [171]. Decreased activity of PON-1 is also associated with HDL dysfunction [188]. Circulating levels of PON-1 were lower in subjects with OSA than in controls [189,190,191,192,193].
Modified apoA-I is also not able to activate LCAT, leading to impaired RCT [194]. Moreover, oxidised HDL, through activating the NF-κB pathway [195], increases the expression of pro-inflammatory molecules, such as the adhesion molecule vascular cell adhesion molecule-1 (VCAM-1) [177]. Circulating SAA, the levels of which were elevated in OSA [196], dislocates apoA-I from HDL [197]. This SAA-rich HDL is unable to interact with ABCA-1 [198]. High calorie intake also attenuates the anti-inflammatory functions of HDL [199].
The higher levels of apoJ or clusterin in OSA [200,201] may suggest its protective function in the HDL metabolism.
Several studies evaluated the circulating HDL-C concentrations in OSA and reported decreased HDL-C levels in most [202,203] but not all cases [31]. In the study of Tan et al., OSA-associated HDL dysfunction was measured as reduced LDL oxidation by HDL [171]. Patients with OSA presented a higher degree of HDL dysfunction with a consequential higher concentration of oxLDL independent of cardiovascular comorbidities. HDL dysfunction was more strongly correlated with disease severity than HDL-C concentration [171]. In another study, HDL2 and HDL3 levels were correlated with IR, but not with OSA severity or the degree of hypoxia. The authors concluded that IR plays a role in OSA-related dyslipidaemia [169]. In a recent study, despite similar HDL-C levels between the OSA and control groups, the participants with OSA had higher pro-atherogenic small HDL 8-10 subfractions and decreased anti-atherogenic large HDL 1-3 subfractions [31]. Moreover, not only OSA severity but also sleep fragmentation was inversely correlated with HDL-C and HDL 1-3 subfractions [31].
The atherogenic index of plasma (AIP) is a biomarker of atherosclerosis and coronary heart disease which is calculated as log(TG/HDL-C) [204] and reflects the dysregulation between anti- and pro-atherogenic lipoproteins. Previous studies found significantly higher AIP values among participants with OSA compared to the controls [205,206,207,208,209]. AIP was higher in patients with OSA and associated with disease severity [206,207,209] and daytime sleepiness in some [209] but not all studies [208].
3.9. Increased Intracellular Lipolysis in Adipose Tissue
Fatty acids are mainly stored in the form of TAG in adipocytes [210]. This storage can be mobilised in three main steps: (1) Adipocyte triglyceride lipase (ATGL) catalyses the hydrolysis of TAG to DAG and FFAs [211]. (2) The hydrolysis of DAG is catalysed by hormone-sensitive lipase (HSL), resulting in MAG and FFAs [212]. (3) Finally, monoacylglycerol lipase (MGL) completes the hydrolysis, producing FFAs and glycerol [213].
Dysregulated peripheral lipolysis has been associated with OSA (Figure 2). IH leads to increased sympathetic activity [214], and elevated levels of catecholamines are major activators of lipolysis [215]. In healthy subjects, increased sympathetic tone with consequential higher HSL expression was detected after two weeks of IH [216]. In mice, IH-induced lipolysis and decreased adipocyte size were detected [217]. In line with this, IH resulted in an increase in lipolysis rate by 211% and a decrease in intracellular lipid stores by 37% in human adipocytes too [218]. However, IH did not seem to affect postprandial lipolysis in lean healthy men [110].
Oxidative stress stimulates both HSL [219] and ATGL [220]. Moreover, several lipolysis-stimulating cytokines, such as TNF-α [221] and IL-6 [222], are detected in increased concentrations in OSA [223,224].
Endothelin-1 (ET-1) is upregulated by IH and induces lipolysis through the phosphorylation of HSL [225]. Fatty acid binding protein-4 (FABP-4) facilitates lipolysis by binding HSL [226], and its levels were detected in elevated concentration in OSA [227,228,229]. FABP-4 also interacts with a co-activator of ATGL, enhancing TAG hydrolysis [230].
Obesity is associated with higher basal levels of lipolysis [231]. Leptin exerts lipolytic activity [232], whilst adiponectin has an inhibitory effect on catecholamine-induced lipolysis [233]. In line with this, increased levels of leptin [234] and decreased levels of adiponectin [235] were reported in OSA.
Insulin is the main negative regulator of lipolysis. Insulin resistance is associated with the loss of the suppressive effects of insulin [236]. Moreover, the anti-lipolytic effect of insulin depends on the O2 tension of adipose tissue [237]; in hypoxia, it seems to be inhibited [238].
It is important to note that fragmented sleep leads to the nocturnal secretion of adrenocorticotropin and cortisol [239], which enhance lipolysis [240].
4. Further Mechanisms in OSA-Associated Dyslipidaemia
4.1. Adipose Tissue Dysfunction
Obesity is the most important risk factor for OSA. At least 30% of obese patients have OSA, and 60% of the patients with OSA are obese [241,242]. The dysfunction of adipose tissue is an important contributor to the metabolic consequences of OSA [243]. White adipose tissue (WAT) is the most important energy storage. High levels of circulating FFAs force WAT to store lipids via two mechanisms: through increases in the number (hyperplasia) and the size (hypertrophy) of the adipocytes [244]. In contrast to hyperplasia, hypertrophy induces pathological changes in the adipose tissue by activating stress pathways, such as endoplasmic reticulum stress, oxidative stress and inflammation [245]. IH induces specific changes in WAT even in the absence of obesity [246]. However, adipocyte hypertrophy and hyperplasia are not always present in IH-induced adipose tissue dysfunction. Some previous studies detected shrunken adipocytes in the WAT of non-obese mice exposed to IH [247,248]. Moreover, IH reduced fat mass by inducing lipolysis [217]. Whereas the morphological changes of WAT are different between IH and obesity, they share the consequential abnormalities.
4.1.1. Inflammation in Adipose Tissue
The larger size of adipocytes reduces the vascularity of hypertrophic adipose tissue, resulting in lower oxygen tension and hypoxic damage. The consequential hypoxia contributes to inappropriate angiogenesis mediated by vascular endothelial growth factor (VEGF) [249]. Furthermore, IH activates HIF-1α and NF-κB, consequently resulting in an increased production of cytokines and adipokines [243].
In contrast to the healthy state characterised by anti-inflammatory immune cells, such as M2 type macrophages, T-helper 2 (Th2) cells, regulatory T cells and anti-inflammatory mediators (IL-10 or adiponectin), hypertrophic WAT is infiltrated by pro-inflammatory immune cells, mainly by CD8+ cytotoxic T cells and Th1 cells leading to the production of pro-inflammatory cytokines (TNF-α, IL-6) [246]. Moreover, hypoxic and inflammatory changes result in macrophage polarisation from M2 type to M1 type. In lean mice exposed to IH, reduced M2-type and increased M1-type macrophage infiltration were also detected in adipocytes [250]. M1-type macrophages enhance the inflammation, producing further cytokines, such as monocyte chemoattractant protein-1 (MCP-1). MCP-1 is an important regulator of macrophage tissue infiltration and chemotaxis of monocytes [251]. Moreover, it is secreted from adipose tissue to the circulation and may increase the hepatic expression of SREBP-1 [251]. Increased plasma levels of MCP-1 were detected in patients with OSA irrespective of obesity and correlated with ODI [252,253]. Furthermore, in the presence of IH, human adipocytes have a higher sensitivity to express pro-inflammatory genes [254].
4.1.2. Role of Adipokines
Leptin is a master regulator of food intake and body energy balance, and its levels were shown to be increased in obesity [255], diabetes [256] and cardiovascular diseases [257,258]. Leptin levels were widely investigated in OSA and found to be increased [133,259,260,261,262,263] even after adjustment for obesity [261]. OSA-associated hyperleptinaemia was related to disease severity measures, such as AHI [133,235,259,260], TST90% [235] and MinSatO2 [261,264]. However, high levels of leptin contribute to leptin resistance by downregulating its cellular responses [265]. Leptin resistance with the loss of physiological functions of leptin also plays a role in OSA-associated metabolic alterations [266]. In a recent animal model, leptin injection did not decrease the food intake of rats exposed to IH [267]. Moreover, IH resulted in a reduced expression of leptin receptors, suggesting the role of leptin resistance in OSA [267,268]. Sleep fragmentation attenuates leptin signalling in the hypothalamus, resulting in consequential high-calorie food intake enhancing obesity [100]. However, sleep fragmentation itself was not found to influence circulating leptin levels [269]. Obese patients with OSA have dysfunctional adipose tissue with adipocyte hyperplasia which increases leptin production [270]. Independently of obesity, IH can itself induce leptin secretion via activating the sympathetic nervous system, renin–angiotensin system and hypothalamic–pituitary–adrenal axis [246,266]. Moreover, leptin gene expression is induced by HIF-1α [271].
Leptin may contribute to lipid alterations in OSA. Leptin activates hepatic lipid production [152] and peripheral lipolysis [232] through the activation of the sympathetic nervous system and by increasing the expression of SREBP-1 and SCD-1 [272]. Moreover, it decreases the activity of LpL [131]. The dissociation between high leptin levels and its action is caused by leptin resistance and attenuated leptin signalling in the liver [273]. A recent study found that leptin levels in OSA correlated positively with TG and negatively with HDL-C concentrations [274]. Leptin can lead to oxidative stress by activating the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [275].
Adiponectin is another important adipokine with anti-inflammatory and antioxidant properties, and its levels are inversely correlated with various disorders, such as obesity [276] and hypertension [277]. Lower adiponectin levels were detected in patients with OSA compared to controls [134,278] and were correlated with disease severity independently of obesity [279]. However, some studies found comparable [280] or even higher [274] adiponectin levels in patients compared to controls. IH suppresses adiponectin expression directly and indirectly by increased sympathetic activation [281]. Adiponectin increases the production of apoA-I and ABCA1 and induces HDL assembly [282,283]. It positively correlates with HDL-C levels independent of obesity [284]. Adiponectin enhances the catabolism of VLDL by activating LpL [285]. Moreover, it increases the mRNA levels of the VLDL-R in skeletal muscle cells [286]. In line with this, there is a negative correlation between VLDL-C and adiponectin levels [287].
Another anti-inflammatory and antioxidant adipokine is omentin, the levels of which were detected in lower concentrations and correlated positively with HDL levels in OSA [280].
4.2. Altered Hormone Production
Several other hormones have an impact on the lipid metabolism, such as cortisol [288], growth hormone (GH) [289] and insulin [290]. GH deficiency is known to be associated with lipid alterations [291], and GH levels were decreased in OSA [292]. Cortisol overproduction is strongly associated with dyslipidaemia [293], and its levels were detected in high concentrations in OSA [294]. Insulin activates LpL in the adipose tissue [129] and inhibits lipolysis [236]. Moreover, it promotes the degradation of apoB [160], leading to decreased hepatic production of apoB-containing lipoproteins. As OSA is associated with insulin resistance, these effects are mitigated.
4.3. Sleep Stages
It is known that rapid eye movement (REM) sleep is associated with higher sympathetic tone [295]. REM and non-REM (NREM) sleep influence the production of several hormones, such as cortisol [296] and GH [297]. GH is mainly produced during N3 sleep [297]. Some patients have a disproportionally higher burden of obstructive events in REM than in non-REM sleep. These patients have a higher risk for hypertension, diabetes and cardiovascular disease [298].
Only a few studies investigated the association between sleep stages and OSA. Interestingly, AHI measured in the REM phase (AHIREM) correlated with TG levels only in one study [299], and it did not have any correlation with lipid parameters in another study [300]. Xu et al. found an independent association between AHIREM and increasing levels of TG, HDL-C and apoE. However, this association became insignificant after analysing only the patients who had an AHINREM or AHIREM < 5/h [301]. In contrast, AHINREM correlated with TG, apoB [299,301], HDL-C, apoA-I [299], LDL-C and cholesterol levels [301]. Slow wave sleep duration and REM latency were independently and inversely associated with cholesterol and LDL-C levels [302]. In conclusion, it could be postulated that NREM sleep may have the greatest impact on lipid alterations in OSA.
5. Direct Consequences of Dyslipidaemia
5.1. Endothelial Dysfunction
Endothelial dysfunction is defined as an impairment in the vasodilatory ability of the vessels (mainly due to the compromised nitric oxide (NO) availability) leading to altered oxygenation, oxidative stress, vascular inflammation and consequential atherosclerosis. IH has a direct detrimental effect on endothelial function [303,304,305,306,307]. OxLDL particles also impair eNOS function by decreasing its expression [308], decreasing L-arginine availability [309]. Moreover, oxLDL increases iNOS expression and ROS generation [308].
5.2. Systemic Inflammation and Consequential Atherosclerosis
OxLDL particles increase the levels of adhesion molecules (VCAM-1, P-selectin) on the endothelium, resulting in enhanced leukocyte recruitment [310]. In OSA, these molecules are also overexpressed by IH and oxidative stress in an NF-κB-dependent fashion [311,312,313]. This leads to increased adhesion between leukocytes and endothelium cells, resulting in the adhesion of circulating leukocytes to the endothelium and slowing down the rolling of leukocytes, thus facilitating their extravasation [314]. Moreover, the oxLDLs have a greater affinity for scavenger receptors, such as LOX-1 on endothelial and smooth muscle cells [315] and CD36 on macrophages [316]. Thus, the activated macrophages increase their CD36 expression, facilitating uncontrolled oxLDL uptake [317], and release pro-inflammatory cytokines (IL-1, TNF-α) [318]. This activation of innate immunity is a key mechanism in foam cell formation in atherosclerosis. It is important to know that adaptive immune cells, such as B-cell-derived plasma cells, are also activated and produce antibodies against oxLDL, and antigen-specific T cells produce further cytokines, resulting in enhanced inflammation [319].
HDL dysfunction in OSA also contributes to atherosclerosis [87,88]. The anti-inflammatory and anti-atherogenic effects of HDL are mainly mediated by sphingosine-1-phosphate (S1P). S1P decreases the expression of several inflammatory cytokines (such as TNF-α) and increases the expression of eNOS [320], improving endothelial function [321]. Elevated S1P enrichment was found in HDL3 particles [322]. HDL particles also enhance the eNOS function by binding to SR-BI expressed on endothelial cells [323]. HDL is an important inhibitor of platelet activation and aggregation as well as of coagulation factors, such as factor X and tissue factor [324].
5.3. Insulin Resistance
Dyslipidaemia can cause insulin resistance. Increased FFA levels due to increased lipolysis reduce insulin-mediated glucose uptake in skeletal muscle by interrupting insulin signalling [325]. Moreover, FFAs activate the NF-κB pathway, resulting in the production of pro-inflammatory cytokines such as TNF-α, IL1β and IL6 in the peripheral tissues. Systemic low-grade inflammation reduces the responsiveness of the peripheral tissues to insulin, leading to insulin resistance [326].
6. The Effect of OSA Therapy on the Lipid Metabolism
6.1. The Effect of CPAP Therapy
Continuous positive airway pressure (CPAP) is the gold standard treatment for OSA [327]. Several studies investigated the effect of CPAP on plasma or serum lipid profile in OSA. Various duration of CPAP (i.e., from 8 weeks to 6 months) effectively decreased TG, TC, LDL-C and apoB and increased HDL-C levels [328,329,330,331,332]. However, these effects depended on sufficient therapy adherence in some cases [331]. On the contrary, some studies failed to demonstrate improvement in lipid levels; the TG, TC and HDL-C levels did not change after 6 weeks to 4 months of CPAP therapy [333,334,335,336].
The effect of CPAP therapy on the lipid profile was also investigated in meta-analyses. Nadeem et al. evaluated 29 articles including 1958 participants with therapy durations ranging from 2 days to 1 year [337]. They concluded that there was a significant reduction in TC (−5.66 mmol/L) and LDL-C (−0.49 mmol/L) levels; however, TG levels did not change (−0.05 mmol/L). HDL-C levels increased after the therapy (+0.21 mmol/L) [337]. Xu et al. analysed the results of six studies including 456 subjects with therapy durations of 2–24 weeks [338]. CPAP therapy sufficiently reduced only the TC levels (−0.15 mmol/L). TG (0.00 mmol/L), LDL-C (−0.04 mmol/L) and HDL-C (−0.02 mmol/L) levels were not different between CPAP and the sham CPAP/control groups [338]. According to their subgroup analysis, younger subjects, more obese patients and patients with a longer duration of CPAP showed a significant decrease in TC concentrations (−0.27, −0.24 and −0.20 mmol/L). The authors postulated that CPAP therapy may not have any clinical effect on circulating lipid levels [338]. In the meta-analysis of Lin et al., six studies with 699 subjects met the inclusion criteria [339]. The time of the therapy was 4-24 weeks. Significant improvements in TC (−6.23 mg/dL), TG (−12.60 mg/dL) and HDL-C (−1.05 mg/dL) levels were detected but LDL-C concentrations did not decrease (−1.01 mg/dL) after CPAP therapy. Moreover, moderate-to-severe OSA, daytime sleepiness, CPAP treatment with short-term duration and good compliance were associated with the changes in lipid profile [339]. In a recent paper by Chen et al., 14 studies with 1792 subjects were included [340]. The therapy duration was 4-48 weeks. The CPAP therapy significantly decreased the TC levels (−0.09 mmol/L); however, it failed to change the levels of TG (0.07 mmol/L), LDL-C (−0.06 mmol/L) or HDL-C (−0.03 mmol/L). The authors did not find any confounders of CPAP treatment effect on lipid profile changes [340].
CPAP may improve some aspects of dyslipidaemia. For example, CPAP decreases the levels of several inflammatory molecules by mitigating hypoxia [341], reduces sympathetic activity [342], decreases the levels of cortisol [343] and improves insulin sensitivity [344]. CPAP increased the LpL concentrations after 3–6 months in patients with OSA [111,114]. The fractional clearance rate (FCR) of TG showed a 5-fold increase after 3-month CPAP therapy, but the FCR of CE was unchanged [109]. Circulating FFAs, which are the markers of increased lipolysis, were decreased after CPAP [345]. In line with this, CPAP withdrawal dynamically increased nocturnal FFA levels [346]. CPAP reduced the markers of lipid peroxidation, such as malondialdehyde levels [347], and decreased the endothelial LOX-1 expression [348]. However, it did not influence the oxLDL levels after 1 year of therapy in patients with OSA having comorbidities [170].
In summary, the previous studies investigating the effect of CPAP on lipid profiles were inconclusive. The studies were heterogeneous with different designs and sample sizes. The negative results of some studies may suggest that CPAP treatment alone does not improve lipid profiles in patients with OSA. Dyslipidaemia in OSA is strongly associated with comorbidities, such as obesity, insulin resistance and cardiovascular diseases, which also need to be addressed with pharmacological interventions. Furthermore, the differences between CPAP trials could be due to differences in diet, which was often uncontrolled in these studies. Most importantly, the effect of CPAP on triglyceride levels was more pronounced and more sustainable when it was combined with weight loss [349].
6.2. The Effect of MAD Therapy
A mandibular advancement device (MAD) is an alternative therapy option for OSA [350]. Only a few studies evaluated the impact of MAD on lipid profile in OSA. Interestingly, Recoquillon et al. detected a significant increase in TG levels after 2 months of effective MAD therapy, whilst the other investigated lipid parameters (TC, LDL-C, HDL-C) were unchanged [351]. There was no improvement in lipid profile after 12 months of MAD therapy in the study of Venema et al. [352]. Silva et al. compared the effectiveness of MAD on the metabolic profile with CPAP: CPAP was more effective in reducing TC and LDL-C levels compared to MAD therapy after 12 months [353].
6.3. The Effect of Upper Airway Surgery
The effect of upper airway surgery on the lipid profile in OSA has been poorly investigated. Li et al. investigated the postoperative lipid profile in patients with OSA who underwent uvulopalatopharyngoplasty (UPPP) or nasal surgery [354]. In patients who underwent UPPP, serum TC and HDL-C levels were significantly improved. In patients who underwent nasal surgery, these values did not change. Patients with isolated hypertriglyceridaemia showed significant improvements in serum TG and HDL-C levels [354]. Another study detected a UPPP-induced decrease in TG and TC levels after a 3-year follow-up [355].
7. Discussion of Major Findings and Further Research Directions
As outlined above, intermittent hypoxia, oxidative stress and consequential systemic inflammation may result in lipid alterations in OSA. Although most of the studies investigating these pathways were performed in vitro or in animal models, the results were also confirmed in humans. Although large population-based studies are concordant in OSA-related dyslipidaemia, they usually did not control for diet, regular exercise or lipid-lowering medications, which could contribute to bias. Clinical studies on large groups of patients are warranted to control for these factors. Furthermore, multiple mediators that are involved in dyslipidaemia (see Section 2) have not been investigated in OSA yet.
Coexistent disorders, such as obesity, insulin resistance and nonalcoholic steatohepatitis, may also lead to systemic inflammation and dyslipidaemia. This could be a reason for inconclusive results with CPAP on lipid profile. CPAP treatment alone may not be able to improve the lipid profiles in patients with OSA. Thus, parallel treatment of these comorbidities is essential to improve dyslipidaemia. Studies should also focus on which patients benefit the most from an intervention with CPAP.
As dyslipidaemia is strongly linked to OSA, patients should actively be screened for lipid abnormalities and cardiovascular complications. The detailed lipid profile of the patients with OSA should be measured at the screening visit and later under the CPAP therapy. Patients with lipid abnormalities detected during OSA management should be also referred to the appropriate specialty. Compared to single lipid components, the use of lipid components in combination with measures of abdominal obesity could better select those patients who are at higher cardiovascular risk [356].
8. Conclusions
In summary, OSA is associated with altered lipid metabolism and results in elevated circulating lipid levels. Intermittent hypoxia, oxidative stress and inflammatory mechanisms lead to altered lipid profiles in OSA. Dyslipidaemia promotes endothelial dysfunction and consequential atherosclerosis leading to increased cardiovascular morbidity and mortality. However, OSA-associated comorbidities might enhance these alterations. Further well-designed studies investigating potential causative associations between dyslipidaemia and OSA and involving CPAP treatment are warranted. The studies in the future should also take into consideration the role of OSA-related comorbidities in the pathomechanism of OSA-related dyslipidaemia. We strongly advocate measuring blood lipids in patients with OSA to estimate and ultimately reduce cardiovascular risk in clinical practice.
Acknowledgments
Andras Bikov is supported by the NIHR Manchester BRC.
Author Contributions
The manuscript was drafted by both authors and was critically reviewed and approved by M.M. and A.B. The figures were created by M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This manuscript received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gileles-Hillel A., Kheirandish-Gozal L., Gozal D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat. Rev. Endocrinol. 2016;12:290–298. doi: 10.1038/nrendo.2016.22. [DOI] [PubMed] [Google Scholar]
- 2.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur. Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 3.Li J., Savransky V., Nanayakkara A., Smith P.L., O’Donnell C.P., Polotsky V.Y. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J. Appl. Physiol. 2007;102:557–563. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 4.Gündüz C., Basoglu O.K., Hedner J., Zou D., Bonsignore M.R., Hein H., Staats R., Pataka A., Barbe F., Sliwinski P., et al. Obstructive sleep apnoea independently predicts lipid levels: Data from the European Sleep Apnea Database. Respirology. 2018;23:1180–1189. doi: 10.1111/resp.13372. [DOI] [PubMed] [Google Scholar]
- 5.Pan X., Hussain M.M. Gut triglyceride production. Biochim. Biophys. Acta. 2012;1821:727–735. doi: 10.1016/j.bbalip.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramasamy I. Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 2014;52:1695–1727. doi: 10.1515/cclm-2013-0358. [DOI] [PubMed] [Google Scholar]
- 7.Zilversmit D.B. Formation and transport of chylomicrons. Fed. Proc. 1967;26:1599–1605. [PubMed] [Google Scholar]
- 8.Redgrave T.G. Chylomicron metabolism. Biochem. Soc. Trans. 2004;32:79–82. doi: 10.1042/bst0320079. [DOI] [PubMed] [Google Scholar]
- 9.Milger K., Herrmann T., Becker C., Gotthardt D., Zickwolf J., Ehehalt R., Watkins P.A., Stremmel W., Füllekrug J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J. Cell Sci. 2006;119:4678–4688. doi: 10.1242/jcs.03280. [DOI] [PubMed] [Google Scholar]
- 10.Black D.D. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: Cellular events in chylomicron assembly and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G519–G524. doi: 10.1152/ajpgi.00189.2007. [DOI] [PubMed] [Google Scholar]
- 11.Yen C.L., Farese R.V., Jr. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 2003;278:18532–18537. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- 12.Buhman K.K., Smith S.J., Stone S.J., Repa J.J., Wong J.S., Knapp F.F., Jr., Burri B.J., Hamilton R.L., Abumrad N.A., Farese R.V., Jr. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J. Biol. Chem. 2002;277:25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 13.Ko C.W., Qu J., Black D.D., Tso P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:169–183. doi: 10.1038/s41575-019-0250-7. [DOI] [PubMed] [Google Scholar]
- 14.Hussain M.M., Kancha R.K., Zhou Z., Luchoomun J., Zu H., Bakillah A. Chylomicron assembly and catabolism: Role of apolipoproteins and receptors. Biochim. Biophys. Acta. 1996;1300:151–170. doi: 10.1016/0005-2760(96)00041-0. [DOI] [PubMed] [Google Scholar]
- 15.Kumar N.S., Mansbach C.M., 2nd Prechylomicron transport vesicle: Isolation and partial characterization. Am. J. Physiol. 1999;276:G378–G386. doi: 10.1152/ajpgi.1999.276.2.G378. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqi S., Saleem U., Abumrad N.A., Davidson N.O., Storch J., Siddiqi S.A., Mansbach C.M., 2nd A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J. Lipid Res. 2010;51:1918–1928. doi: 10.1194/jlr.M005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqi S.A., Mahan J., Siddiqi S., Gorelick F.S., Mansbach C.M., 2nd Vesicle-associated membrane protein 7 is expressed in intestinal ER. J. Cell Sci. 2006;119:943–950. doi: 10.1242/jcs.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havel R.J., Kane J.P., Kashyap M.L. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J. Clin. Investig. 1973;52:32–38. doi: 10.1172/JCI107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willnow T.E. Mechanisms of hepatic chylomicron remnant clearance. Diabet. Med. 1997;14((Suppl. S3)):S75–S80. doi: 10.1002/(SICI)1096-9136(199708)14:3+<S75::AID-DIA449>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Tiwari S., Siddiqi S.A. Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shelness G.S., Ingram M.F., Huang X.F., DeLozier J.A. Apolipoprotein B in the rough endoplasmic reticulum: Translation, translocation and the initiation of lipoprotein assembly. J. Nutr. 1999;129:456s–462s. doi: 10.1093/jn/129.2.456S. [DOI] [PubMed] [Google Scholar]
- 22.Qin W., Sundaram M., Wang Y., Zhou H., Zhong S., Chang C.-C., Manhas S., Yao E.F., Parks R.J., McFie P.J., et al. Missense mutation in APOC3 within the C-terminal lipid binding domain of human ApoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins: Evidence that ApoC-III plays a major role in the formation of lipid precursors within the microsomal lumen. J. Biol. Chem. 2011;286:27769–27780. doi: 10.1074/jbc.M110.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gusarova V., Seo J., Sullivan M.L., Watkins S.C., Brodsky J.L., Fisher E.A. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J. Biol. Chem. 2007;282:19453–19462. doi: 10.1074/jbc.M700475200. [DOI] [PubMed] [Google Scholar]
- 24.Soutar A.K., Myant N.B., Thompson G.R. The metabolism of very low density and intermediate density lipoproteins in patients with familial hypercholesterolaemia. Atherosclerosis. 1982;43:217–231. doi: 10.1016/0021-9150(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi S., Sakai J., Fujino T., Hattori H., Zenimaru Y., Suzuki J., Miyamori I., Yamamoto T.T. The Very Low-density Lipoprotein (VLDL) Receptor: Characterization and Functions as a Peripheral Lipoprotein Receptor. J. Atheroscler. Thromb. 2004;11:200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- 26.Esterbauer H., Gebicki J., Puhl H., Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic. Biol. Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-F. [DOI] [PubMed] [Google Scholar]
- 27.Shelness G.S., Sellers J.A. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 2001;12:151–157. doi: 10.1097/00041433-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Orekhov A.N., Tertov V.V., Mukhin D.N., Mikhailenko I.A. Modification of low density lipoprotein by desialylation causes lipid accumulation in cultured cells: Discovery of desialylated lipoprotein with altered cellular metabolism in the blood of atherosclerotic patients. Biochem. Biophys. Res. Commun. 1989;162:206–211. doi: 10.1016/0006-291X(89)91982-7. [DOI] [PubMed] [Google Scholar]
- 29.Quinn M.T., Parthasarathy S., Fong L.G., Steinberg D. Oxidatively modified low density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. USA. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobenin I.A., Tertov V.V., Orekhov A.N. Characterization of chemical composition of native and modified low density lipoprotein occurring in the blood of diabetic patients. Int. Angiol. 1994;13:78–83. [PubMed] [Google Scholar]
- 31.Kollar B., Siarnik P., Hluchanova A., Klobucnikova K., Mucska I., Turcani P., Paduchova Z., Katrencikova B., Janubova M., Konarikova K., et al. The impact of sleep apnea syndrome on the altered lipid metabolism and the redox balance. Lipids Health Dis. 2021;20:175. doi: 10.1186/s12944-021-01604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khosravi M., Hosseini-Fard R., Najafi M. Circulating low density lipoprotein (LDL) Horm. Mol. Biol. Clin. Investig. 2018;35 doi: 10.1515/hmbci-2018-0024. [DOI] [PubMed] [Google Scholar]
- 33.McPherson R., Gauthier A. Molecular regulation of SREBP function: The Insig-SCAP connection and isoform-specific modulation of lipid synthesis. Biochem. Cell Biol. 2004;82:201–211. doi: 10.1139/o03-090. [DOI] [PubMed] [Google Scholar]
- 34.Brown M.S., Goldstein J.L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nohturfft A., DeBose-Boyd R.A., Scheek S., Goldstein J.L., Brown M.S. Sterols regulate cycling of SREBP cleavage-activating protein (SCAP) between endoplasmic reticulum and Golgi. Proc. Natl. Acad. Sci. USA. 1999;96:11235–11240. doi: 10.1073/pnas.96.20.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams C.M., Goldstein J.L., Brown M.S. Cholesterol-induced conformational change in SCAP enhanced by Insig proteins and mimicked by cationic amphiphiles. Proc. Natl. Acad. Sci. USA. 2003;100:10647–10652. doi: 10.1073/pnas.1534833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maxwell K.N., Breslow J.L. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl. Acad. Sci. USA. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorovoy M., Gaultier A., Campana W.M., Firestein G.S., Gonias S.L. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J. Leukoc. Biol. 2010;88:769–778. doi: 10.1189/jlb.0410220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laatsch A., Merkel M., Talmud P.J., Grewal T., Beisiegel U., Heeren J. Insulin stimulates hepatic low density lipoprotein receptor-related protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis. 2009;204:105–111. doi: 10.1016/j.atherosclerosis.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 40.Beisiegel U., Weber W., Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA. 1991;88:8342–8346. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kounnas M.Z., Chappell D.A., Wong H., Argraves W.S., Strickland D.K. The Cellular Internalization and Degradation of Hepatic Lipase Is Mediated by Low Density Lipoprotein Receptor-related Protein and Requires Cell Surface Proteoglycans. J. Biol. Chem. 1995;270:9307–9312. doi: 10.1074/jbc.270.16.9307. [DOI] [PubMed] [Google Scholar]
- 42.Laatsch A., Panteli M., Sornsakrin M., Hoffzimmer B., Grewal T., Heeren J. Low density lipoprotein receptor-related protein 1 dependent endosomal trapping and recycling of apolipoprotein E. PLoS ONE. 2012;7:e29385. doi: 10.1371/journal.pone.0029385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lillis A.P., Muratoglu S.C., Au D.T., Migliorini M., Lee M.J., Fried S.K., Mikhailenko I., Strickland D.K. LDL receptor-related protein-1 (LRP1) regulates cholesterol accumulation in macrophages. PLoS ONE. 2015;10:e0128903. doi: 10.1371/journal.pone.0128903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samouillan V., Dandurand J., Nasarre L., Badimon L., Lacabanne C., Llorente-Cortés V. Lipid loading of human vascular smooth muscle cells induces changes in tropoelastin protein levels and physical structure. Biophys. J. 2012;103:532–540. doi: 10.1016/j.bpj.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranganathan S., Cao C., Catania J., Migliorini M., Zhang L., Strickland D.K. Molecular basis for the interaction of low density lipoprotein receptor-related protein 1 (LRP1) with integrin alphaMbeta2: Identification of binding sites within alphaMbeta2 for LRP1. J. Biol. Chem. 2011;286:30535–30541. doi: 10.1074/jbc.M111.265413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaultier A., Arandjelovic S., Li X., Janes J., Dragojlovic N., Zhou G.P., Dolkas J., Myers R.R., Gonias S.L., Campana W.M. A shed form of LDL receptor-related protein-1 regulates peripheral nerve injury and neuropathic pain in rodents. J. Clin. Investig. 2008;118:161–172. doi: 10.1172/JCI32371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beigneux A.P., Davies B.S.J., Gin P., Weinstein M.M., Farber E., Qiao X., Peale F., Bunting S., Walzem R.L., Wong J.S., et al. Glycosylphosphatidylinositol-Anchored High-Density Lipoprotein-Binding Protein 1 Plays a Critical Role in the Lipolytic Processing of Chylomicrons. Cell Metab. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kersten S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2014;1841:919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Doolittle M.H., Ben-Zeev O., Elovson J., Martin D., Kirchgessner T.G. The response of lipoprotein lipase to feeding and fasting. Evidence for posttranslational regulation. J. Biol. Chem. 1990;265:4570–4577. doi: 10.1016/S0021-9258(19)39601-2. [DOI] [PubMed] [Google Scholar]
- 50.Borensztajn J., Robinson D.S.T. The effect of fasting on the utilization of chylomicron triglyceride fatty acids in relation to clearing factor lipase (lipoprotein lipase) releasable by heparin in the perfused rat heart. J. Lipid Res. 1970;11:111–117. doi: 10.1016/S0022-2275(20)43001-9. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton M.T., Etienne J., McClure W.C., Pavey B.S., Holloway A.K. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am. J. Physiol. 1998;275:E1016–E1022. doi: 10.1152/ajpendo.1998.275.6.E1016. [DOI] [PubMed] [Google Scholar]
- 52.Larsson M., Vorrsjö E., Talmud P., Lookene A., Olivecrona G. Apolipoproteins C-I and C-III Inhibit Lipoprotein Lipase Activity by Displacement of the Enzyme from Lipid Droplets*. J. Biol. Chem. 2013;288:33997–34008. doi: 10.1074/jbc.M113.495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quagliarini F., Wang Y., Kozlitina J., Grishin N.V., Hyde R., Boerwinkle E., Valenzuela D.M., Murphy A.J., Cohen J.C., Hobbs H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 2012;109:19751. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Müller M., Kersten S. The Fasting-induced Adipose Factor/Angiopoietin-like Protein 4 Is Physically Associated with Lipoproteins and Governs Plasma Lipid Levels and Adiposity. J. Biol. Chem. 2006;281:934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 55.Tosheska Trajkovska K., Topuzovska S. High-density lipoprotein metabolism and reverse cholesterol transport: Strategies for raising HDL cholesterol. Anatol. J. Cardiol. 2017;18:149–154. doi: 10.14744/AnatolJCardiol.2017.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rached F.H., Chapman M.J., Kontush A. HDL particle subpopulations: Focus on biological function. Biofactors. 2015;41:67–77. doi: 10.1002/biof.1202. [DOI] [PubMed] [Google Scholar]
- 57.von Eckardstein A., Kardassis D. High Density Lipoproteins: From Biological Understanding to Clinical Exploitation. Springer; Berlin/Heidelberg, Germany: 2015. p. 694. [Google Scholar]
- 58.Camont L., Chapman M.J., Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends. Mol. Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Oravec S., Dukat A., Gavornik P., Caprnda M., Kucera M., Ocadlik I. Contribution of the atherogenic lipoprotein profile to the development of arterial hypertension. Bratisl Lek Listy. 2011;112:4–7. [PubMed] [Google Scholar]
- 60.Oravec S., Dostal E., Dukát A., Gavorník P., Kucera M., Gruber K. HDL subfractions analysis: A new laboratory diagnostic assay for patients with cardiovascular diseases and dyslipoproteinemia. Neuro. Endocrinol. Lett. 2011;32:502–509. [PubMed] [Google Scholar]
- 61.Tailleux A., Duriez P., Fruchart J.C., Clavey V. Apolipoprotein A-II, HDL metabolism and atherosclerosis. Atherosclerosis. 2002;164:1–13. doi: 10.1016/S0021-9150(01)00751-1. [DOI] [PubMed] [Google Scholar]
- 62.Klimov A.N., Gurevich V.S., Nikiforova A.A., Shatilina L.V., Kuzmin A.A., Plavinsky S.L., Teryukova N.P. Antioxidative activity of high density lipoproteins in vivo. Atherosclerosis. 1993;100:13–18. doi: 10.1016/0021-9150(93)90063-Z. [DOI] [PubMed] [Google Scholar]
- 63.Frank P.G., Marcel Y.L. Apolipoprotein A-I: Structure-function relationships. J. Lipid Res. 2000;41:853–872. doi: 10.1016/S0022-2275(20)32028-9. [DOI] [PubMed] [Google Scholar]
- 64.Dominiczak M.H., Caslake M.J. Apolipoproteins: Metabolic role and clinical biochemistry applications. Ann. Clin. Biochem. 2011;48:498–515. doi: 10.1258/acb.2011.011111. [DOI] [PubMed] [Google Scholar]
- 65.Mulya A., Seo J., Brown A.L., Gebre A.K., Boudyguina E., Shelness G.S., Parks J.S. Apolipoprotein M expression increases the size of nascent pre beta HDL formed by ATP binding cassette transporter A1. J. Lipid Res. 2010;51:514–524. doi: 10.1194/jlr.M002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christoffersen C., Nielsen L.B., Axler O., Andersson A., Johnsen A.H., Dahlbäck B. Isolation and characterization of human apolipoprotein M-containing lipoproteins. J. Lipid Res. 2006;47:1833–1843. doi: 10.1194/jlr.M600055-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Christoffersen C., Obinata H., Kumaraswamy S.B., Galvani S., Ahnström J., Sevvana M., Egerer-Sieber C., Muller Y.A., Hla T., Nielsen L.B., et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Vorst E.P.C. High-Density Lipoproteins and Apolipoprotein A1. Subcell Biochem. 2020;94:399–420. doi: 10.1007/978-3-030-41769-7_16. [DOI] [PubMed] [Google Scholar]
- 69.Pereira R.M., Mekary R.A., da Cruz Rodrigues K.C., Anaruma C.P., Ropelle E.R., da Silva A.S.R., Cintra D.E., Pauli J.R., de Moura L.P. Protective molecular mechanisms of clusterin against apoptosis in cardiomyocytes. Heart Fail. Rev. 2018;23:123–129. doi: 10.1007/s10741-017-9654-z. [DOI] [PubMed] [Google Scholar]
- 70.Timmins J.M., Lee J.-Y., Boudyguina E., Kluckman K.D., Brunham L.R., Mulya A., Gebre A.K., Coutinho J.M., Colvin P.L., Smith T.L., et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Investig. 2005;115:1333–1342. doi: 10.1172/JCI200523915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brunham L.R., Kruit J.K., Iqbal J., Fievet C., Timmins J.M., Pape T.D., Coburn B.A., Bissada N., Staels B., Groen A.K., et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Investig. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langmann T., Klucken J., Reil M., Liebisch G., Luciani M.F., Chimini G., Kaminski W.E., Schmitz G. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): Evidence for sterol-dependent regulation in macrophages. Biochem. Biophys. Res. Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 73.Oram J.F., Vaughan A.M. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr. Opin. Lipidol. 2000;11:253–260. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Bailey D., Ruel I., Hafiane A., Cochrane H., Iatan I., Jauhiainen M., Ehnholm C., Krimbou L., Genest J. Analysis of lipid transfer activity between model nascent HDL particles and plasma lipoproteins: Implications for current concepts of nascent HDL maturation and genesis. J. Lipid Res. 2010;51:785–797. doi: 10.1194/jlr.M001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jauhiainen M., Metso J., Pahlman R., Blomqvist S., van Tol A., Ehnholm C. Human plasma phospholipid transfer protein causes high density lipoprotein conversion. J. Biol. Chem. 1993;268:4032–4036. doi: 10.1016/S0021-9258(18)53575-4. [DOI] [PubMed] [Google Scholar]
- 76.Acton S., Rigotti A., Landschulz K.T., Xu S., Hobbs H.H., Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 77.Xu S., Laccotripe M., Huang X., Rigotti A., Zannis V.I., Krieger M. Apolipoproteins of HDL can directly mediate binding to the scavenger receptor SR-BI, an HDL receptor that mediates selective lipid uptake. J. Lipid Res. 1997;38:1289–1298. doi: 10.1016/S0022-2275(20)37413-7. [DOI] [PubMed] [Google Scholar]
- 78.Thuren T. Hepatic lipase and HDL metabolism. Curr. Opin. Lipidol. 2000;11:277–283. doi: 10.1097/00041433-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Chapman M.J., Le Goff W., Guerin M., Kontush A. Cholesteryl ester transfer protein: At the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur. Heart J. 2010;31:149–164. doi: 10.1093/eurheartj/ehp399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barter P.J., Brewer H.B., Jr., Chapman M.J., Hennekens C.H., Rader D.J., Tall A.R. Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003;23:160–167. doi: 10.1161/01.ATV.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 81.Silver D.L., Wang N., Xiao X., Tall A.R. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type 1 results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J. Biol. Chem. 2001;276:25287–25293. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]
- 82.Pagler T.A., Rhode S., Neuhofer A., Laggner H., Strobl W., Hinterndorfer C., Volf I., Pavelka M., Eckhardt E.R., van der Westhuyzen D.R., et al. SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux. J. Biol. Chem. 2006;281:11193–11204. doi: 10.1074/jbc.M510261200. [DOI] [PubMed] [Google Scholar]
- 83.Daniil G., Phedonos A.A., Holleboom A.G., Motazacker M.M., Argyri L., Kuivenhoven J.A., Chroni A. Characterization of antioxidant/anti-inflammatory properties and apoA-I-containing subpopulations of HDL from family subjects with monogenic low HDL disorders. Clin. Chim. Acta. 2011;412:1213–1220. doi: 10.1016/j.cca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Kontush A., Chantepie S., Chapman M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23:1881–1888. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 85.Shuhei N., Söderlund S., Jauhiainen M., Taskinen M.R. Effect of HDL composition and particle size on the resistance of HDL to the oxidation. Lipids Health Dis. 2010;9:104. doi: 10.1186/1476-511X-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aviram M., Rosenblat M., Bisgaier C.L., Newton R.S., Primo-Parmo S.L., La Du B.N. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Investig. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicholls S.J., Dusting G.J., Cutri B., Bao S., Drummond G.R., Rye K.A., Barter P.J. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–1550. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 88.Yvan-Charvet L., Wang N., Tall A.R. Role of HDL, ABCA1, and ABCG1 Transporters in Cholesterol Efflux and Immune Responses. Arterioscler. Thromb. Vasc. Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Almendros I., Basoglu Ö.K., Conde S.V., Liguori C., Saaresranta T. Metabolic dysfunction in OSA: Is there something new under the sun? J. Sleep Res. 2022;31:e13418. doi: 10.1111/jsr.13418. [DOI] [PubMed] [Google Scholar]
- 90.Farré R., Montserrat J.M., Gozal D., Almendros I., Navajas D. Intermittent Hypoxia Severity in Animal Models of Sleep Apnea. Front. Physiol. 2018;9:1556. doi: 10.3389/fphys.2018.01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J., Thorne L.N., Punjabi N.M., Sun C.K., Schwartz A.R., Smith P.L., Marino R.L., Rodriguez A., Hubbard W.C., O’Donnell C.P., et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 92.Li J., Bosch-Marce M., Nanayakkara A., Savransky V., Fried S.K., Semenza G.L., Polotsky V.Y. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol. Genom. 2006;25:450–457. doi: 10.1152/physiolgenomics.00293.2005. [DOI] [PubMed] [Google Scholar]
- 93.Tabor D.E., Kim J.B., Spiegelman B.M., Edwards P.A. Identification of conserved cis-elements and transcription factors required for sterol-regulated transcription of stearoyl-CoA desaturase 1 and 2. J. Biol. Chem. 1999;274:20603–20610. doi: 10.1074/jbc.274.29.20603. [DOI] [PubMed] [Google Scholar]
- 94.Savransky V., Nanayakkara A., Li J., Bevans S., Smith P.L., Rodriguez A., Polotsky V.Y. Chronic intermittent hypoxia induces atherosclerosis. Am. J. Respir. Crit. Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tamisier R., Gilmartin G.S., Launois S.H., Pépin J.L., Nespoulet H., Thomas R., Lévy P., Weiss J.W. A new model of chronic intermittent hypoxia in humans: Effect on ventilation, sleep, and blood pressure. J. Appl. Physiol. 2009;107:17–24. doi: 10.1152/japplphysiol.91165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dobrosielski D.A., Papandreou C., Patil S.P., Salas-Salvadó J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur. Respir. Rev. 2017;26 doi: 10.1183/16000617.0110-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giel K.E., Bulik C.M., Fernandez-Aranda F., Hay P., Keski-Rahkonen A., Schag K., Schmidt U., Zipfel S. Binge eating disorder. Nat. Rev. Dis. Primers. 2022;8:16. doi: 10.1038/s41572-022-00344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsumoto T., Harada N., Azuma M., Chihara Y., Murase K., Tachikawa R., Minami T., Hamada S., Tanizawa K., Inouchi M., et al. Plasma Incretin Levels and Dipeptidyl Peptidase-4 Activity in Patients with Obstructive Sleep Apnea. Ann. Am. Thorac. Soc. 2016;13:1378–1387. doi: 10.1513/AnnalsATS.201510-697OC. [DOI] [PubMed] [Google Scholar]
- 99.Shobatake R., Itaya-Hironaka A., Yamauchi A., Makino M., Sakuramoto-Tsuchida S., Uchiyama T., Ota H., Takahashi N., Ueno S., Sugie K., et al. Intermittent Hypoxia Up-Regulates Gene Expressions of Peptide YY (PYY), Glucagon-like Peptide-1 (GLP-1), and Neurotensin (NTS) in Enteroendocrine Cells. Int. J. Mol. Sci. 2019;20:1849. doi: 10.3390/ijms20081849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hakim F., Wang Y., Carreras A., Hirotsu C., Zhang J., Peris E., Gozal D. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and PTP1b-mediated leptin resistance in male mice. Sleep. 2015;38:31–40. doi: 10.5665/sleep.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dash S., Xiao C., Morgantini C., Lewis G.F. New Insights into the Regulation of Chylomicron Production. Annu. Rev. Nutr. 2015;35:265–294. doi: 10.1146/annurev-nutr-071714-034338. [DOI] [PubMed] [Google Scholar]
- 102.Phillips C.L., Yee B.J., Marshall N.S., Liu P.Y., Sullivan D.R., Grunstein R.R. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: A randomized, placebo-controlled crossover trial. Am. J. Respir. Crit. Care Med. 2011;184:355–361. doi: 10.1164/rccm.201102-0316OC. [DOI] [PubMed] [Google Scholar]
- 103.Drager L.F., Li J., Shin M.K., Reinke C., Aggarwal N.R., Jun J.C., Bevans-Fonti S., Sztalryd C., O’Byrne S.M., Kroupa O., et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur. Heart J. 2012;33:783–790. doi: 10.1093/eurheartj/ehr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morin R., Mauger J.F., Amaratunga R., Imbeault P. The effect of acute intermittent hypoxia on postprandial triglyceride levels in humans: A randomized crossover trial. J. Transl. Med. 2021;19:268. doi: 10.1186/s12967-021-02933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mwaikambo B.R., Yang C., Chemtob S., Hardy P. Hypoxia up-regulates CD36 expression and function via hypoxia-inducible factor-1- and phosphatidylinosi.itol 3-kinase-dependent mechanisms. J. Biol. Chem. 2009;284:26695–26707. doi: 10.1074/jbc.M109.033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flores J.J., Klebe D., Rolland W.B., Lekic T., Krafft P.R., Zhang J.H. PPARγ-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiol. Dis. 2016;87:124–133. doi: 10.1016/j.nbd.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rey E., Del Pozo-Maroto E., Marañón P., Beeler B., García-García Y., Landete P., Isaza S.C., Farré R., García-Monzón C., Almendros I., et al. Intrahepatic Expression of Fatty Acid Translocase CD36 Is Increased in Obstructive Sleep Apnea. Front. Med. 2020;7:450. doi: 10.3389/fmed.2020.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moon Y., Park B., Park H. Hypoxic repression of CYP7A1 through a HIF-1α- and SHP-independent mechanism. BMB Rep. 2016;49:173–178. doi: 10.5483/BMBRep.2016.49.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drager L.F., Tavoni T.M., Silva V.M., Santos R.D., Pedrosa R.P., Bortolotto L.A., Vinagre C.G., Polotsky V.Y., Lorenzi-Filho G., Maranhao R.C. Obstructive sleep apnea and effects of continuous positive airway pressure on triglyceride-rich lipoprotein metabolism. J. Lipid Res. 2018;59:1027–1033. doi: 10.1194/jlr.M083436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahat B., Chassé É., Mauger J.-F., Imbeault P. Effects of acute hypoxia on human adipose tissue lipoprotein lipase activity and lipolysis. J. Transl. Med. 2016;14:212. doi: 10.1186/s12967-016-0965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iesato K., Tatsumi K., Saibara T., Nakamura A., Terada J., Tada Y., Sakao S., Tanabe N., Takiguchi Y., Kuriyama T. Decreased lipoprotein lipase in obstructive sleep apnea syndrome. Circ. J. 2007;71:1293–1298. doi: 10.1253/circj.71.1293. [DOI] [PubMed] [Google Scholar]
- 112.Yao Q., Shin M.K., Jun J.C., Hernandez K.L., Aggarwal N.R., Mock J.R., Gay J., Drager L.F., Polotsky V.Y. Effect of chronic intermittent hypoxia on triglyceride uptake in different tissues. J. Lipid Res. 2013;54:1058–1065. doi: 10.1194/jlr.M034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jun J.C., Shin M.K., Yao Q., Bevans-Fonti S., Poole J., Drager L.F., Polotsky V.Y. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am. J. Physiol. Endocrinol. Metab. 2012;303:E377–E388. doi: 10.1152/ajpendo.00641.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li J., Zhang Y., Wang J., Feng P., Chen R., Cao Y., Liu C. Association between serum lipoprotein lipase level and dyslipidemia in patients with obstructive sleep apnea syndrome. Zhonghua Yi Xue Za Zhi. 2014;94:403–407. [PubMed] [Google Scholar]
- 115.Drager L.F., Yao Q., Hernandez K.L., Shin M.K., Bevans-Fonti S., Gay J., Sussan T.E., Jun J.C., Myers A.C., Olivecrona G., et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am. J. Respir. Crit. Care Med. 2013;188:240–248. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Terki A., Abu-Farha M., AlKhairi I., Cherian P.T., Sriraman D., Shyamsundar A., Ali S., Almulla F., Tuomilehto J., Abubaker J.A. Increased Level of Angiopoietin Like Proteins 4 and 8 in People With Sleep Apnea. Front. Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J., Yang Y., Jiao X., Yu H., Du Y., Zhang M., Hu C., Wei Y., Qin Y. The Clinical Role of Angiopoietin-Like Protein 3 in Evaluating Coronary Artery Disease in Patients with Obstructive Sleep Apnea. Cardiovasc. Drugs. 2020;34:773–780. doi: 10.1007/s10557-020-06991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schoonjans K., Peinado-Onsurbe J., Lefebvre A.M., Heyman R.A., Briggs M., Deeb S., Staels B., Auwerx J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. Embo J. 1996;15:5336–5348. doi: 10.1002/j.1460-2075.1996.tb00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yun Z., Maecker H.L., Johnson R.S., Giaccia A.J. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of adipogenesis by hypoxia. Dev. Cell. 2002;2:331–341. doi: 10.1016/S1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 120.Mackay A.G., Oliver J.D., Rogers M.P. Regulation of lipoprotein lipase activity and mRNA content in rat epididymal adipose tissue in vitro by recombinant tumour necrosis factor. Biochem. J. 1990;269:123–126. doi: 10.1042/bj2690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feingold K.R., Marshall M., Gulli R., Moser A.H., Grunfeld C. Effect of endotoxin and cytokines on lipoprotein lipase activity in mice. Arterioscler. Thromb. 1994;14:1866–1872. doi: 10.1161/01.ATV.14.11.1866. [DOI] [PubMed] [Google Scholar]
- 122.Grunfeld C., Gulli R., Moser A.H., Gavin L.A., Feingold K.R. Effect of tumor necrosis factor administration in vivo on lipoprotein lipase activity in various tissues of the rat. J. Lipid Res. 1989;30:579–585. doi: 10.1016/S0022-2275(20)38349-8. [DOI] [PubMed] [Google Scholar]
- 123.Zechner R., Newman T.C., Sherry B., Cerami A., Breslow J.L. Recombinant human cachectin/tumor necrosis factor but not interleukin-1 alpha downregulates lipoprotein lipase gene expression at the transcriptional level in mouse 3T3-L1 adipocytes. Mol. Cell. Biol. 1988;8:2394. doi: 10.1128/mcb.8.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu G., Brouckaert P., Olivecrona T. Rapid downregulation of adipose tissue lipoprotein lipase activity on food deprivation: Evidence that TNF-alpha is involved. Am. J. Physiol. Endocrinol. Metab. 2004;286:E711–E717. doi: 10.1152/ajpendo.00257.2003. [DOI] [PubMed] [Google Scholar]
- 125.Somers V.K., Dyken M.E., Clary M.P., Abboud F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Raynolds M.V., Awald P.D., Gordon D.F., Gutierrez-Hartmann A., Rule D.C., Wood W.M., Eckel R.H. Lipoprotein Lipase Gene Expression in Rat Adipocytes Is Regulated by Isoproterenol and Insulin through Different Mechanisms. Mol. Endocrinol. 1990;4:1416–1422. doi: 10.1210/mend-4-9-1416. [DOI] [PubMed] [Google Scholar]
- 127.Chiappe de Cingalani G.E., Goers J.W., Giannotti M., Caldiz C.I. Comparative effects of insulin and isoproterenol on lipoprotein lipase in rat adipose cells. Am. J. Physiol.-Cell Physiol. 1996;270:C1461–C1467. doi: 10.1152/ajpcell.1996.270.5.C1461. [DOI] [PubMed] [Google Scholar]
- 128.Dijk W., Heine M., Vergnes L., Boon M.R., Schaart G., Hesselink M.K., Reue K., van Marken Lichtenbelt W.D., Olivecrona G., Rensen P.C., et al. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. Elife. 2015;4 doi: 10.7554/eLife.08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ong J.M., Kirchgessner T.G., Schotz M.C., Kern P.A. Insulin increases the synthetic rate and messenger RNA level of lipoprotein lipase in isolated rat adipocytes. J. Biol. Chem. 1988;263:12933–12938. doi: 10.1016/S0021-9258(18)37651-8. [DOI] [PubMed] [Google Scholar]
- 130.Inukai K., Nakashima Y., Watanabe M., Kurihara S., Awata T., Katagiri H., Oka Y., Katayama S. ANGPTL3 is increased in both insulin-deficient and -resistant diabetic states. Biochem. Biophys. Res. Commun. 2004;317:1075–1079. doi: 10.1016/j.bbrc.2004.03.151. [DOI] [PubMed] [Google Scholar]
- 131.Picard F., Richard D., Huang Q., Deshaies Y. Effects of leptin adipose tissue lipoprotein lipase in the obese ob/ob mouse. Int. J. Obes. 1998;22:1088–1095. doi: 10.1038/sj.ijo.0800732. [DOI] [PubMed] [Google Scholar]
- 132.Shimamura M., Matsuda M., Ando Y., Koishi R., Yasumo H., Furukawa H., Shimomura I. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochem. Biophys. Res. Commun. 2004;322:1080–1085. doi: 10.1016/j.bbrc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 133.Phillips B.G., Kato M., Narkiewicz K., Choe I., Somers V.K. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 134.Zeng F., Wang X., Hu W., Wang L. Association of adiponectin level and obstructive sleep apnea prevalence in obese subjects. Medicine. 2017;96:e7784. doi: 10.1097/MD.0000000000007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.von Eynatten M., Schneider J.G., Humpert P.M., Rudofsky G., Schmidt N., Barosch P., Hamann A., Morcos M., Kreuzer J., Bierhaus A., et al. Decreased plasma lipoprotein lipase in hypoadiponectinemia: An association independent of systemic inflammation and insulin resistance. Diabetes Care. 2004;27:2925–2929. doi: 10.2337/diacare.27.12.2925. [DOI] [PubMed] [Google Scholar]
- 136.Costales P., Castellano J., Revuelta-López E., Cal R., Aledo R., Llampayas O., Nasarre L., Juarez C., Badimon L., Llorente-Cortés V. Lipopolysaccharide downregulates CD91/low-density lipoprotein receptor-related protein 1 expression through SREBP-1 overexpression in human macrophages. Atherosclerosis. 2013;227:79–88. doi: 10.1016/j.atherosclerosis.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 137.Qin S., Yin J., Huang K. Free Fatty Acids Increase Intracellular Lipid Accumulation and Oxidative Stress by Modulating PPARα and SREBP-1c in L-02 Cells. Lipids. 2016;51:797–805. doi: 10.1007/s11745-016-4160-y. [DOI] [PubMed] [Google Scholar]
- 138.Li J., Nanayakkara A., Jun J., Savransky V., Polotsky V.Y. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol. Genom. 2007;31:273–280. doi: 10.1152/physiolgenomics.00082.2007. [DOI] [PubMed] [Google Scholar]
- 139.Guo Y.X., He L.Y., Zhang M., Wang F., Liu F., Peng W.X. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Aβ1–40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. 2016;322:28–38. doi: 10.1016/j.neuroscience.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 140.Zhao Y., Zeng C.Y., Li X.H., Yang T.T., Kuang X., Du J.R. Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer’s disease. Aging Cell. 2020;19:e13239. doi: 10.1111/acel.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bozkurt N.C., Cakal E., Sahin M., Ozkaya E.C., Firat H., Delibasi T. The relation of serum 25-hydroxyvitamin-D levels with severity of obstructive sleep apnea and glucose metabolism abnormalities. Endocrine. 2012;41:518–525. doi: 10.1007/s12020-012-9595-1. [DOI] [PubMed] [Google Scholar]
- 142.Pákó J., Kunos L., Mészáros M., Tárnoki D.L., Tárnoki Á D., Horváth I., Bikov A. Decreased Levels of Anti-Aging Klotho in Obstructive Sleep Apnea. Rejuvenation Res. 2020;23:256–261. doi: 10.1089/rej.2019.2183. [DOI] [PubMed] [Google Scholar]
- 143.de Gonzalo-Calvo D., Cenarro A., Martínez-Bujidos M., Badimon L., Bayes-Genis A., Ordonez-Llanos J., Civeira F., Llorente-Cortés V. Circulating soluble low-density lipoprotein receptor-related protein 1 (sLRP1) concentration is associated with hypercholesterolemia: A new potential biomarker for atherosclerosis. Int. J. Cardiol. 2015;201:20–29. doi: 10.1016/j.ijcard.2015.07.085. [DOI] [PubMed] [Google Scholar]
- 144.Meszaros M., Kunos L., Tarnoki A.D., Tarnoki D.L., Lazar Z., Bikov A. The Role of Soluble Low-Density Lipoprotein Receptor-Related Protein-1 in Obstructive Sleep Apnoea. J. Clin. Med. 2021;10:1494. doi: 10.3390/jcm10071494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ntambi J.M., Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004;43:91–104. doi: 10.1016/S0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 146.Savransky V., Jun J., Li J., Nanayakkara A., Fonti S., Moser A.B., Steele K.E., Schweitzer M.A., Patil S.P., Bhanot S., et al. Dyslipidemia and atheroscleros.sis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ. Res. 2008;103:1173–1180. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J., Grigoryev D.N., Ye S.Q., Thorne L., Schwartz A.R., Smith P.L., O’Donnell C.P., Polotsky V.Y. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J. Appl. Physiol. 2005;99:1643–1648. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 148.Salceda S., Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 149.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., von Kriegsheim A., Hebestreit H.F., Mukherji M., Schofield C.J., et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 150.Goyal P., Weissmann N., Grimminger F., Hegel C., Bader L., Rose F., Fink L., Ghofrani H.A., Schermuly R.T., Schmidt H.H., et al. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic. Biol. Med. 2004;36:1279–1288. doi: 10.1016/j.freeradbiomed.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 151.Mesarwi O.A., Loomba R., Malhotra A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir. Crit. Care Med. 2019;199:830–841. doi: 10.1164/rccm.201806-1109TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bruinstroop E., Pei L., Ackermans M.T., Foppen E., Borgers A.J., Kwakkel J., Alkemade A., Fliers E., Kalsbeek A. Hypothalamic neuropeptide Y (NPY) controls hepatic VLDL-triglyceride secretion in rats via the sympathetic nervous system. Diabetes. 2012;61:1043–1050. doi: 10.2337/db11-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jermendy G., Horváth T., Littvay L., Steinbach R., Jermendy A.L., Tárnoki A.D., Tárnoki D.L., Métneki J., Osztovits J. Effect of genetic and environmental influences on cardiometabolic risk factors: A twin study. Cardiovasc. Diabetol. 2011;10:96. doi: 10.1186/1475-2840-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guilleminault C., Partinen M., Hollman K., Powell N., Stoohs R. Familial aggregates in obstructive sleep apnea syndrome. Chest. 1995;107:1545–1551. doi: 10.1378/chest.107.6.1545. [DOI] [PubMed] [Google Scholar]
- 155.Meszaros M., Tarnoki A.D., Tarnoki D.L., Kovacs D.T., Forgo B., Lee J., Sung J., Vestbo J., Müller V., Kunos L., et al. Obstructive sleep apnea and hypertriglyceridaemia share common genetic background: Results of a twin study. J. Sleep Res. 2020;29:e12979. doi: 10.1111/jsr.12979. [DOI] [PubMed] [Google Scholar]
- 156.Tang H., Zhou Q., Zheng F., Wu T., Tang Y.D., Jiang J. The Causal Effects of Lipid Profiles on Sleep Apnea. Front. Nutr. 2022;9:910690. doi: 10.3389/fnut.2022.910690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun J., Hu J., Tu C., Zhong A., Xu H. Obstructive sleep apnea susceptibility genes in Chinese population: A field synopsis and meta-analysis of genetic association studies. PLoS ONE. 2015;10:e0135942. doi: 10.1371/journal.pone.0135942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li Q., Chen R., Bie L., Zhao D., Huang C., Hong J. Association of the variants in the PPARG gene and serum lipid levels: A meta-analysis of 74 studies. J. Cell Mol. Med. 2015;19:198–209. doi: 10.1111/jcmm.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Uyrum E., Balbay O., Annakkaya A.N., Gulec Balbay E., Silan F., Arbak P. The relationship between obstructive sleep apnea syndrome and apolipoprotein E genetic variants. Respiration. 2015;89:195–200. doi: 10.1159/000369560. [DOI] [PubMed] [Google Scholar]
- 160.Fisher E.A., Pan M., Chen X., Wu X., Wang H., Jamil H., Sparks J.D., Williams K.J. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J. Biol. Chem. 2001;276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 161.Wen Y., Leake D.S. Low Density Lipoprotein Undergoes Oxidation Within Lysosomes in Cells. Circ. Res. 2007;100:1337–1343. doi: 10.1161/CIRCRESAHA.107.151704. [DOI] [PubMed] [Google Scholar]
- 162.Itabe H., Obama T., Kato R. The Dynamics of Oxidized LDL during Atherogenesis. J. Lipids. 2011;2011:418313. doi: 10.1155/2011/418313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Poznyak A.V., Nikiforov N.G., Markin A.M., Kashirskikh D.A., Myasoedova V.A., Gerasimova E.V., Orekhov A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharm. 2020;11:613780. doi: 10.3389/fphar.2020.613780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Younis N.N., Soran H., Pemberton P., Charlton-Menys V., Elseweidy M.M., Durrington P.N. Small dense LDL is more susceptible to glycation than more buoyant LDL in Type 2 diabetes. Clin. Sci. 2013;124:343–349. doi: 10.1042/CS20120304. [DOI] [PubMed] [Google Scholar]
- 165.Ivanova E.A., Myasoedova V.A., Melnichenko A.A., Grechko A.V., Orekhov A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxidative Med. Cell Longev. 2017;2017:1273042. doi: 10.1155/2017/1273042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.White C.R., Anantharamaiah G.M. Cholesterol reduction and macrophage function: Role of paraoxonases. Curr. Opin. Lipidol. 2017;28:397–402. doi: 10.1097/MOL.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Luyster F.S., Kip K.E., Drumheller O.J., Rice T.B., Edmundowicz D., Matthews K., Reis S.E., Strollo P.J., Jr. Sleep apnea is related to the atherogenic phenotype, lipoprotein subclass B. J. Clin. Sleep Med. 2012;8:155–161. doi: 10.5664/jcsm.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sopkova Z., Berneis K., Rizzo M., Spinas G.A., Dorkova Z., Tisko R., Tkacova R. Size and Subclasses of Low-Density Lipoproteins in Patients With Obstructive Sleep Apnea. Angiology. 2012;63:617–621. doi: 10.1177/0003319711433811. [DOI] [PubMed] [Google Scholar]
- 169.Liu A., Cardell J., Ariel D., Lamendola C., Abbasi F., Kim S.H., Holmes T.H., Tomasso V., Mojaddidi H., Grove K., et al. Abnormalities of lipoprotein concentrations in obstructive sleep apnea are related to insulin resistance. Sleep. 2015;38:793–799. doi: 10.5665/sleep.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feres M.C., Fonseca F.A., Cintra F.D., Mello-Fujita L., de Souza A.L., De Martino M.C., Tufik S., Poyares D. An assessment of oxidized LDL in the lipid profiles of patients with obstructive sleep apnea and its association with both hypertension and dyslipidemia, and the impact of treatment with CPAP. Atherosclerosis. 2015;241:342–349. doi: 10.1016/j.atherosclerosis.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 171.Tan K.C., Chow W.S., Lam J.C., Lam B., Wong W.K., Tam S., Ip M.S. HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 2006;184:377–382. doi: 10.1016/j.atherosclerosis.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 172.Kizawa T., Nakamura Y., Takahashi S., Sakurai S., Yamauchi K., Inoue H. Pathogenic role of angiotensin II and oxidised LDL in obstructive sleep apnoea. Eur. Respir. J. 2009;34:1390–1398. doi: 10.1183/09031936.00009709. [DOI] [PubMed] [Google Scholar]
- 173.Tauman R., Lavie L., Greenfeld M., Sivan Y. Oxidative stress in children with obstructive sleep apnea syndrome. J. Clin. Sleep Med. 2014;10:677–681. doi: 10.5664/jcsm.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee S.D., Ju G., Choi J.A., Kim J.W., Yoon I.Y. The association of oxidative stress with central obesity in obstructive sleep apnea. Sleep Breath. 2012;16:511–517. doi: 10.1007/s11325-011-0536-7. [DOI] [PubMed] [Google Scholar]
- 175.Svatikova A., Wolk R., Lerman L.O., Juncos L.A., Greene E.L., McConnell J.P., Somers V.K. Oxidative stress in obstructive sleep apnoea. Eur. Heart J. 2005;26:2435–2439. doi: 10.1093/eurheartj/ehi440. [DOI] [PubMed] [Google Scholar]
- 176.Fadaei R., Safari-Faramani R., Rezaei M., Ahmadi R., Rostampour M., Moradi N., Khazaie H. Circulating levels of oxidized low-density lipoprotein in patients with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. 2020;24:809–815. doi: 10.1007/s11325-020-02089-y. [DOI] [PubMed] [Google Scholar]
- 177.Undurti A., Huang Y., Lupica J.A., Smith J.D., DiDonato J.A., Hazen S.L. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J. Biol. Chem. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rosenson R.S., Brewer H.B., Jr., Ansell B.J., Barter P., Chapman M.J., Heinecke J.W., Kontush A., Tall A.R., Webb N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016;13:48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cabana V.G., Lukens J.R., Rice K.S., Hawkins T.J., Getz G.S. HDL content and composition in acute phase response in three species: Triglyceride enrichment of HDL a factor in its decrease. J. Lipid Res. 1996;37:2662–2674. doi: 10.1016/S0022-2275(20)37469-1. [DOI] [PubMed] [Google Scholar]
- 180.Song D., Fang G., Mao S.Z., Ye X., Liu G., Gong Y., Liu S.F. Chronic intermittent hypoxia induces atherosclerosis by NF-κB-dependent mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012;1822:1650–1659. doi: 10.1016/j.bbadis.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 181.Chen M., Li W., Wang N., Zhu Y., Wang X. ROS and NF-kappaB but not LXR mediate IL-1beta signaling for the downregulation of ATP-binding cassette transporter A1. Am. J. Physiol. Cell Physiol. 2007;292:C1493–C1501. doi: 10.1152/ajpcell.00016.2006. [DOI] [PubMed] [Google Scholar]
- 182.Hanikoglu F., Huseyinoglu N., Ozben S., Cort A., Ozdem S., Ozben T. Increased plasma soluble tumor necrosis factor receptor-1 and myeloperoxidase activity in patients with obstructive sleep apnea syndrome. Int. J. Neurosci. 2015;125:655–662. doi: 10.3109/00207454.2014.960521. [DOI] [PubMed] [Google Scholar]
- 183.Bergt C., Pennathur S., Fu X., Byun J., O’Brien K., McDonald T.O., Singh P., Anantharamaiah G.M., Chait A., Brunzell J., et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zheng L., Settle M., Brubaker G., Schmitt D., Hazen S.L., Smith J.D., Kinter M. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J. Biol. Chem. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 185.Gao D., Ashraf M.Z., Zhang L., Kar N., Byzova T.V., Podrez E.A. Cross-linking modifications of HDL apoproteins by oxidized phospholipids: Structural characterization, in vivo detection, and functional implications. J. Biol. Chem. 2020;295:1973–1984. doi: 10.1074/jbc.RA119.008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pruzanski W., Stefanski E., de Beer F.C., de Beer M.C., Vadas P., Ravandi A., Kuksis A. Lipoproteins are substrates for human secretory group IIA phospholipase A2: Preferential hydrolysis of acute phase HDL. J. Lipid Res. 1998;39:2150–2160. doi: 10.1016/S0022-2275(20)32470-6. [DOI] [PubMed] [Google Scholar]
- 187.Kar S., Patel M.A., Tripathy R.K., Bajaj P., Suvarnakar U.V., Pande A.H. Oxidized phospholipid content destabilizes the structure of reconstituted high density lipoprotein particles and changes their function. Biochim. Biophys. Acta. 2012;1821:1200–1210. doi: 10.1016/j.bbalip.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 188.Jaouad L., Milochevitch C., Khalil A. PON1 paraoxonase activity is reduced during HDL oxidation and is an indicator of HDL antioxidant capacity. Free Radic. Res. 2003;37:77–83. doi: 10.1080/1071576021000036614. [DOI] [PubMed] [Google Scholar]
- 189.Lavie L., Dyugovskaya L., Golan-Shany O., Lavie P. Heat-shock protein 70: Expression in monocytes of patients with sleep apnoea and association with oxidative stress and tumour necrosis factor-alpha. J. Sleep Res. 2010;19:139–147. doi: 10.1111/j.1365-2869.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 190.Kotani K., Kimura S., Tsuzaki K., Sakane N., Komada I., Schulze J., Gugliucci A. Reduced paraoxonase 1/arylesterase activity and its post-therapeutic increase in obstructive sleep apnea syndrome: A preliminary study. Clin. Chim. Acta. 2008;395:184–185. doi: 10.1016/j.cca.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 191.Lavie L., Vishnevsky A., Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–128. [PubMed] [Google Scholar]
- 192.Baysal E., Taysi S., Aksoy N., Uyar M., Celenk F., Karatas Z.A., Tarakcioglu M., Bilinç H., Mumbuç S., Kanlikama M. Serum paraoxonase, arylesterase activity and oxidative status in patients with obstructive sleep apnea syndrome (OSAS) Eur. Rev. Med. Pharm. Sci. 2012;16:770–774. [PubMed] [Google Scholar]
- 193.Płóciniczak A., Baszczuk A., Ludziejewska A., Winiarska H., Michalak S., Kasprzak G., Formanowicz D., Cofta S., Wysocka E. Paraoxonase 1 gene L55M polymorphism and paraoxonase 1 activity in obstructive sleep apnea patients. Adv. Exp. Med. Biol. 2019;1150:17–24. doi: 10.1007/5584_2018_267. [DOI] [PubMed] [Google Scholar]
- 194.Shao B., Cavigiolio G., Brot N., Oda M.N., Heinecke J.W. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc. Natl. Acad. Sci. USA. 2008;105:12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Huang Y., DiDonato J.A., Levison B.S., Schmitt D., Li L., Wu Y., Buffa J., Kim T., Gerstenecker G.S., Gu X., et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014;20:193–203. doi: 10.1038/nm.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Svatikova A., Wolk R., Shamsuzzaman A.S., Kara T., Olson E.J., Somers V.K. Serum amyloid a in obstructive sleep apnea. Circulation. 2003;108:1451–1454. doi: 10.1161/01.CIR.0000089091.09527.B8. [DOI] [PubMed] [Google Scholar]
- 197.Whitehead A.S., de Beer M.C., Steel D.M., Rits M., Lelias J.M., Lane W.S., de Beer F.C. Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. J Biol. Chem. 1992;267:3862–3867. doi: 10.1016/S0021-9258(19)50605-6. [DOI] [PubMed] [Google Scholar]
- 198.Wroblewski J.M., Jahangiri A., Ji A., de Beer F.C., van der Westhuyzen D.R., Webb N.R. Nascent HDL formation by hepatocytes is reduced by the concerted action of serum amyloid A and endothelial lipase. J. Lipid Res. 2011;52:2255–2261. doi: 10.1194/jlr.M017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nicholls S.J., Lundman P., Harmer J.A., Cutri B., Griffiths K.A., Rye K.A., Barter P.J., Celermajer D.S. Consumption of saturated fat impairs the anti-in.n.nflamma.atory properties of high-density lipoproteins and endothelial function. J. Am. Coll. Cardiol. 2006;48:715–720. doi: 10.1016/j.jacc.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 200.Meszaros M., Horvath P., Kis A., Kunos L., Tarnoki A.D., Tarnoki D.L., Lazar Z., Bikov A. Circulating levels of clusterin and complement factor H in patients with obstructive sleep apnea. Biomark. Med. 2021;15:323–330. doi: 10.2217/bmm-2020-0533. [DOI] [PubMed] [Google Scholar]
- 201.Peng Y., Zhou L., Cao Y., Chen P., Chen Y., Zong D., Ouyang R. Relation between serum leptin levels, lipid profiles and neurocognitive deficits in Chinese OSAHS patients. Int. J. Neurosci. 2017;127:981–987. doi: 10.1080/00207454.2017.1286654. [DOI] [PubMed] [Google Scholar]
- 202.Roche F., Sforza E., Pichot V., Maudoux D., Garcin A., Celle S., Picard-Kossovsky M., Gaspoz J.M., Barthélémy J.C. Obstructive sleep apnoea/hypopnea influences high-density lipoprotein cholesterol in the elderly. Sleep Med. 2009;10:882–886. doi: 10.1016/j.sleep.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 203.Drager L.F., Lopes H.F., Maki-Nunes C., Trombetta I.C., Toschi-Dias E., Alves M.J., Fraga R.F., Jun J.C., Negrão C.E., Krieger E.M., et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS ONE. 2010;5:e12065. doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cai G., Shi G., Xue S., Lu W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine. 2017;96:e8058. doi: 10.1097/MD.0000000000008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shimizu Y., Yoshimine H., Nagayoshi M., Kadota K., Takahashi K., Izumino K., Inoue K., Maeda T. Serum triglyceride levels in relation to high-density lipoprotein cholesterol (TG-HDL) ratios as an efficient tool to estimate the risk of sleep apnea syndrome in non-overweight Japanese men. Environ. Health Prev. Med. 2016;21:321–326. doi: 10.1007/s12199-016-0532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wu W.-T., Tsai S.-S., Shih T.-S., Lin M.-H., Chou T.-C., Ting H., Wu T.-N., Liou S.-H. The association between obstructive sleep apnea and metabolic markers and lipid profiles. PLoS ONE. 2015;10:e0130279. doi: 10.1371/journal.pone.0130279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cao B., Fan Z., Zhang Y., Li T. Independent association of severity of obstructive sleep apnea with lipid metabolism of atherogenic index of plasma (AIP) and apoB/apoAI ratio. Sleep Breath. 2020;24:1507–1513. doi: 10.1007/s11325-020-02016-1. [DOI] [PubMed] [Google Scholar]
- 208.Silva L.O.E., Guimarães T.M., Luz G.P., Coelho G., Badke L., Almeida I.R., Millani-Carneiro A., Tufik S., Bittencourt L., Togeiro S.M. Metabolic profile in patients with mild obstructive sleep apnea. Metab. Syndr. Relat. Disord. 2018;16:6–12. doi: 10.1089/met.2017.0075. [DOI] [PubMed] [Google Scholar]
- 209.Bikov A., Meszaros M., Kunos L., Negru A.G., Frent S.M., Mihaicuta S. Atherogenic Index of Plasma in Obstructive Sleep Apnoea. J. Clin. Med. 2021;10:417. doi: 10.3390/jcm10030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bays H.E., González-Campoy J.M., Bray G.A., Kitabchi A.E., Bergman D.A., Schorr A.B., Rodbard H.W., Henry R.R. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev. Cardiovasc. Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 211.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 212.Reynisdottir S., Dauzats M., Thörne A., Langin D. Comparison of hormone-sensitive lipase activity in visceral and subcutaneous human adipose tissue. J. Clin. Endocrinol. Metab. 1997;82:4162–4166. doi: 10.1210/jc.82.12.4162. [DOI] [PubMed] [Google Scholar]
- 213.Fredrikson G., Tornqvist H., Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta. 1986;876:288–293. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- 214.Chalacheva P., Thum J., Yokoe T., O’Donnell C.P., Khoo M.C. Development of autonomic dysfunction with intermittent hypoxia in a lean murine model. Respir. Physiol. Neurobiol. 2013;188:143–151. doi: 10.1016/j.resp.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Carpéné C., Bousquet-Mélou A., Galitzky J., Berlan M., Lafontan M. Lipolytic effects of beta 1-, beta 2-, and beta 3-adrenergic agonists in white adipose tissue of mammals. Ann. N. Y. Acad. Sci. 1998;839:186–189. doi: 10.1111/j.1749-6632.1998.tb10756.x. [DOI] [PubMed] [Google Scholar]
- 216.Netchitaïlo M., Fabre F., Briançon A., Belaidi-Corsat E., Arnaud C., Borel A.-L., Lévy P., Pépin J.-L., Tamisier R. Two weeks of intermittent hypoxic exposure induce lipolysis at the fat tissue level in healthy human subjects. Eur. Respir. J. 2017;50:PA2968. [Google Scholar]
- 217.Weiszenstein M., Shimoda L.A., Koc M., Seda O., Polak J. Inhibition of Lipolysis Ameliorates Diabetic Phenotype in a Mouse Model of Obstructive Sleep Apnea. Am. J. Respir. Cell Mol. Biol. 2016;55:299–307. doi: 10.1165/rcmb.2015-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Musutova M., Weiszenstein M., Koc M., Polak J. Intermittent hypoxia stimulates lipolysis, but inhibits differentiation and de novo lipogenesis in 3T3-L1 cells. Metab. Syndr. Relat. Disord. 2020;18:146–153. doi: 10.1089/met.2019.0112. [DOI] [PubMed] [Google Scholar]
- 219.Krawczyk S.A., Haller J.F., Ferrante T., Zoeller R.A., Corkey B.E. Reactive oxygen species facilitate translocation of hormone sensitive lipase to the lipid droplet during lipolysis in human differentiated adipocytes. PLoS ONE. 2012;7:e34904. doi: 10.1371/journal.pone.0034904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang H., Bell M., Sreenivasan U., Sreenevasan U., Hu H., Liu J., Dalen K., Londos C., Yamaguchi T., Rizzo M.A., et al. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ryden M., Dicker A., van Harmelen V., Hauner H., Brunnberg M., Perbeck L., Lonnqvist F., Arner P. Mapping of early signaling events in tumor necrosis factor-alpha -mediated lipolysis in human fat cells. J. Biol. Chem. 2002;277:1085–1091. doi: 10.1074/jbc.M109498200. [DOI] [PubMed] [Google Scholar]
- 222.van Hall G., Steensberg A., Sacchetti M., Fischer C., Keller C., Schjerling P., Hiscock N., Møller K., Saltin B., Febbraio M.A., et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J. Clin. Endocrinol. Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- 223.Cao Y., Song Y., Ning P., Zhang L., Wu S., Quan J., Li Q. Association between tumor necrosis factor alpha and obstructive sleep apnea in adults: A meta-analysis update. BMC Pulm. Med. 2020;20:215. doi: 10.1186/s12890-020-01253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Imani M.M., Sadeghi M., Khazaie H., Emami M., Sadeghi Bahmani D., Brand S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020;11:1343. doi: 10.3389/fimmu.2020.01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Briançon-Marjollet A., Monneret D., Henri M., Hazane-Puch F., Pepin J.L., Faure P., Godin-Ribuot D. Endothelin regulates intermittent hypoxia-induced lipolytic remodelling of adipose tissue and phosphorylation of hormone-sensitive lipase. J. Physiol. 2016;594:1727–1740. doi: 10.1113/JP271321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shen W.J., Sridhar K., Bernlohr D.A., Kraemer F.B. Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc. Natl. Acad. Sci. USA. 1999;96:5528–5532. doi: 10.1073/pnas.96.10.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Català R., Cabré A., Hernández-Flix S., Ferré R., Sangenís S., Plana N., Texidó A., Masana L. Circulating FABP4 and FABP5 levels are differently linked to OSA severity and treatment. Sleep. 2013;36:1831–1837. doi: 10.5665/sleep.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lam D.C., Xu A., Lam K.S., Lam B., Lam J.C., Lui M.M., Ip M.S. Serum adipocyte-fatty acid binding protein level is elevated in severe OSA and correlates with insulin resistance. Eur. Respir. J. 2009;33:346–351. doi: 10.1183/09031936.50075408. [DOI] [PubMed] [Google Scholar]
- 229.Balci M.M., Arslan U., Firat H., Kocaoğlu I., Vural M.G., Balci K.G., Maden O., Gürbüz O.A., Ardiç S., Yeter E. Serum levels of adipocyte fatty acid-binding protein are independently associated with left ventricular mass and myocardial performance index in obstructive sleep apnea syndrome. J. Investig. Med. 2012;60:1020–1026. doi: 10.2310/JIM.0b013e31826868f2. [DOI] [PubMed] [Google Scholar]
- 230.Hofer P., Boeszoermenyi A., Jaeger D., Feiler U., Arthanari H., Mayer N., Zehender F., Rechberger G., Oberer M., Zimmermann R., et al. Fatty Acid-binding Proteins Interact with Comparative Gene Identification-58 Linking Lipolysis with Lipid Ligand Shuttling *. J. Biol. Chem. 2015;290:18438–18453. doi: 10.1074/jbc.M114.628958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gordon E.S. Non-Esterified Fatty Acids in the Blood of Obese and Lean Subjects. Am. J. Clin. Nutr. 1960;8:740–747. doi: 10.1093/ajcn/8.5.740. [DOI] [Google Scholar]
- 232.Frühbeck G., Gómez-Ambrosi J., Salvador J. Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J. 2001;15:333–340. doi: 10.1096/fj.00-0249com. [DOI] [PubMed] [Google Scholar]
- 233.Wedellová Z., Dietrich J., Siklová-Vítková M., Kološtová K., Kováčiková M., Dušková M., Brož J., Vedral T., Stich V., Polák J. Adiponectin inhibits spontaneous and catecholamine-induced lipolysis in human adipocytes of non-obese subjects through AMPK-dependent mechanisms. Physiol. Res. 2011;60:139–148. doi: 10.33549/physiolres.931863. [DOI] [PubMed] [Google Scholar]
- 234.Kapsimalis F., Varouchakis G., Manousaki A., Daskas S., Nikita D., Kryger M., Gourgoulianis K. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186:209–217. doi: 10.1007/s00408-008-9082-x. [DOI] [PubMed] [Google Scholar]
- 235.Tokuda F., Sando Y., Matsui H., Koike H., Yokoyama T. Serum levels of adipocytokines, adiponectin and leptin, in patients with obstructive sleep apnea syndrome. Intern. Med. 2008;47:1843–1849. doi: 10.2169/internalmedicine.47.1035. [DOI] [PubMed] [Google Scholar]
- 236.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pasarica M., Rood J., Ravussin E., Schwarz J.-M., Smith S.R., Redman L.M. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J. Clin. Endocrinol. Metab. 2010;95:4052–4055. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rooney K., Trayhurn P. Lactate and the GPR81 receptor in metabolic regulation: Implications for adipose tissue function and fatty acid utilisation by muscle during exercise. Br. J. Nutr. 2011;106:1310–1316. doi: 10.1017/S0007114511004673. [DOI] [PubMed] [Google Scholar]
- 239.Späth-Schwalbe E., Gofferje M., Kern W., Born J., Fehm H.L. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol. Psychiatry. 1991;29:575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 240.Brindley D.N., McCann B.S., Niaura R., Stoney C.M., Suarez E.C. Stress and lipoprotein metabolism: M odulators and mechanisms. Metabolism. 1993;42:3–15. doi: 10.1016/0026-0495(93)90255-M. [DOI] [PubMed] [Google Scholar]
- 241.Young T., Palta M., Dempsey J., Skatrud J., Weber S., Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 242.Peppard P.E., Young T., Palta M., Dempsey J., Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. Jama. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 243.Ryan S., Arnaud C., Fitzpatrick S.F., Gaucher J., Tamisier R., Pépin J.-L. Adipose tissue as a key player in obstructive sleep apnoea. Eur. Respir. Rev. 2019;28:190006. doi: 10.1183/16000617.0006-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jo J., Gavrilova O., Pack S., Jou W., Mullen S., Sumner A.E., Cushman S.W., Periwal V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cildir G., Akıncılar S.C., Tergaonkar V. Chronic adipose tissue inflammation: All immune cells on the stage. Trends Mol. Med. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 246.Ryan S. Adipose tissue inflammation by intermittent hypoxia: Mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J. Physiol. 2017;595:2423–2430. doi: 10.1113/JP273312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Poulain L., Thomas A., Rieusset J., Casteilla L., Levy P., Arnaud C., Dematteis M. Visceral white fat remodelling contributes to intermittent hypoxia-induced atherogenesis. Eur. Respir. J. 2014;43:513. doi: 10.1183/09031936.00019913. [DOI] [PubMed] [Google Scholar]
- 248.Poulain L., Richard V., Lévy P., Dematteis M., Arnaud C. Toll-like receptor-4 mediated inflammation is involved in the cardiometabolic alterations induced by intermittent hypoxia. Mediat. Inflamm. 2015;2015:620258. doi: 10.1155/2015/620258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Briançon-Marjollet A., Pépin J.L., Weiss J.W., Lévy P., Tamisier R. Intermittent hypoxia upregulates serum VEGF. Sleep Med. 2014;15:1425–1426. doi: 10.1016/j.sleep.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 250.Murphy A.M., Thomas A., Crinion S.J., Kent B.D., Tambuwala M.M., Fabre A., Pepin J.L., Roche H.M., Arnaud C., Ryan S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur. Respir. J. 2017;49:1601731. doi: 10.1183/13993003.01731-2016. [DOI] [PubMed] [Google Scholar]
- 251.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kim J., Lee C.H., Park C.S., Kim B.G., Kim S.W., Cho J.H. Plasma levels of MCP-1 and adiponectin in obstructive sleep apnea syndrome. Arch. Otolaryngol. Head Neck Surg. 2010;136:896–899. doi: 10.1001/archoto.2010.142. [DOI] [PubMed] [Google Scholar]
- 253.Fanfulla F., Rotondi M., Morrone E., Coperchini F., Lodigiani S., Trentin R., Maccabruni V., Chiovato L. Sleep hypoxia and not obesity is the main determinant of the increasing monocyte chemoattractant protein-1 (MCP-1) in patients with obstructive sleep apnoea. ERJ Open Res. 2017;3:P70. [Google Scholar]
- 254.Taylor C.T., Kent B.D., Crinion S.J., McNicholas W.T., Ryan S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem. Biophys Res. Commun. 2014;447:660–665. doi: 10.1016/j.bbrc.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 255.Mishra S., Harris T.B., Hue T., Miljkovic I., Satterfield S., de Rekeneire N., Mehta M., Sahyoun N.R. Hyperleptinemia, adiposity, and risk of metabolic syndrome in older adults. J. Nutr. Metab. 2013;2013:327079. doi: 10.1155/2013/327079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Welsh P., Murray H.M., Buckley B.M., de Craen A.J.M., Ford I., Jukema J.W., Macfarlane P.W., Packard C.J., Stott D.J., Westendorp R.G.J., et al. Leptin predicts diabetes but not cardiovascular disease: Results from a large prospective study in an elderly population. Diabetes Care. 2009;32:308–310. doi: 10.2337/dc08-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sierra-Johnson J., Romero-Corral A., Lopez-Jimenez F., Gami A.S., Sert Kuniyoshi F.H., Wolk R., Somers V.K. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am. J. Cardiol. 2007;100:234–239. doi: 10.1016/j.amjcard.2007.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ku I.A., Farzaneh-Far R., Vittinghoff E., Zhang M.H., Na B., Whooley M.A. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2011;217:503–508. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schäfer H., Pauleit D., Sudhop T., Gouni-Berthold I., Ewig S., Berthold H.K. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–839. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 260.Ozturk L., Unal M., Tamer L., Celikoglu F. The association of the severity of obstructive sleep apnea with plasma leptin levels. Arch. Otolaryngol. Head Neck Surg. 2003;129:538–540. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 261.Tatsumi K., Kasahara Y., Kurosu K., Tanabe N., Takiguchi Y., Kuriyama T. Sleep oxygen desaturation and circulating leptin in obstructive sleep apnea-hyp.p.p.popnea syndrome. Chest. 2005;127:716–721. doi: 10.1378/chest.127.3.716. [DOI] [PubMed] [Google Scholar]
- 262.McArdle N., Hillman D., Beilin L., Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: A matched controlled study. Am. J. Respir. Crit. Care Med. 2007;175:190–195. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 263.Hirotsu C., Albuquerque R.G., Nogueira H., Hachul H., Bittencourt L., Tufik S., Andersen M.L. The relationship between sleep apnea, metabolic dysfunction and inflammation: The gender influence. Brain Behav. Immun. 2017;59:211–218. doi: 10.1016/j.bbi.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 264.Arnardottir E.S., Maislin G., Jackson N., Schwab R.J., Benediktsdottir B., Teff K., Juliusson S., Pack A.I., Gislason T. The role of obesity, different fat compartments and sleep apnea severity in circulating leptin levels: The Icelandic Sleep Apnea Cohort study. Int. J. Obes. 2013;37:835–842. doi: 10.1038/ijo.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Knight Z.A., Hannan K.S., Greenberg M.L., Friedman J.M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Berger S., Polotsky V.Y. Leptin and leptin resistance in the pathogenesis of obstructive sleep apnea: A possible link to oxidative stress and cardiovascular complications. Oxidative Med. Cell Longev. 2018;2018:5137947. doi: 10.1155/2018/5137947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ciriello J., Moreau J.M., Caverson M.M., Moranis R. Leptin: A Potential Link Between Obstructive Sleep Apnea and Obesity. Front. Physiol. 2022;12:767318. doi: 10.3389/fphys.2021.767318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pan W., Kastin A.J. Leptin: A biomarker for sleep disorders? Sleep Med. Rev. 2014;18:283–290. doi: 10.1016/j.smrv.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gonnissen H.K., Hursel R., Rutters F., Martens E.A., Westerterp-Plantenga M.S. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br. J. Nutr. 2013;109:748–756. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 270.Framnes S.N., Arble D.M. The Bidirectional Relationship Between Obstructive Sleep Apnea and Metabolic Disease. Front. Endocrinol. 2018;9:440. doi: 10.3389/fendo.2018.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Grosfeld A., Andre J., Hauguel-De Mouzon S., Berra E., Pouyssegur J., Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J. Biol. Chem. 2002;277:42953–42957. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 272.Gallardo N., Bonzón-Kulichenko E., Fernández-Agulló T., Moltó E., Gómez-Alonso S., Blanco P., Carrascosa J.M., Ros M., Andrés A. Tissue-specific effects of central leptin on the expression of genes involved in lipid metabolism in liver and white adipose tissue. Endocrinology. 2007;148:5604–5610. doi: 10.1210/en.2007-0933. [DOI] [PubMed] [Google Scholar]
- 273.Brabant G., Müller G., Horn R., Anderwald C., Roden M., Nave H. Hepatic leptin signaling in obesity. FASEB J. 2005;19:1048–1050. doi: 10.1096/fj.04-2846fje. [DOI] [PubMed] [Google Scholar]
- 274.Sertogullarindan B., Komuroglu A.U., Ucler R., Gunbatar H., Sunnetcioglu A., Cokluk E. Betatrophin association with serum triglyceride levels in obstructive sleep apnea patients. Ann. Thorac. Med. 2019;14:63–68. doi: 10.4103/atm.ATM_52_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Morawietz H., Bornstein S.R. Leptin, Endothelin, NADPH Oxidase, and Heart Failure. Hypertension. 2006;47:e20–e21. doi: 10.1161/01.HYP.0000218452.18010.fb. [DOI] [PubMed] [Google Scholar]
- 276.Pilz S., Horejsi R., Möller R., Almer G., Scharnagl H., Stojakovic T., Dimitrova R., Weihrauch G., Borkenstein M., Maerz W., et al. Early atherosclerosis in obese juveniles is associated with low serum levels of adiponectin. J. Clin. Endocrinol. Metab. 2005;90:4792–4796. doi: 10.1210/jc.2005-0167. [DOI] [PubMed] [Google Scholar]
- 277.Kim D.H., Kim C., Ding E.L., Townsend M.K., Lipsitz L.A. Adiponectin levels and the risk of hypertension: A systematic review and meta-analysis. Hypertension. 2013;62:27–32. doi: 10.1161/HYPERTENSIONAHA.113.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lu M., Fang F., Wang Z., Wei P., Hu C., Wei Y. Association between serum/plasma levels of adiponectin and obstructive sleep apnea hypopnea syndrome: A meta-analysis. Lipids Health Dis. 2019;18:30. doi: 10.1186/s12944-019-0973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Domagała-Kulawik J., Osińska I., Piechuta A., Bielicki P., Skirecki T. T, B, and NKT Cells in Systemic Inflammation in Obstructive Sleep Apnoea. Mediat. Inflamm. 2015;2015:161579. doi: 10.1155/2015/161579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhang D.-M., Pang X.-L., Huang R., Gong F.-Y., Zhong X., Xiao Y. Adiponectin, Omentin, Ghrelin, and Visfatin Levels in Obese Patients with Severe Obstructive Sleep Apnea. Biomed. Res. Int. 2018;2018:3410135. doi: 10.1155/2018/3410135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Gonzalez F.J., Xie C., Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat. Rev. Endocrinol. 2018;15:21–32. doi: 10.1038/s41574-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Oku H., Matsuura F., Koseki M., Sandoval J.C., Yuasa-Kawase M., Tsubakio-Yamamoto K., Masuda D., Maeda N., Ohama T., Ishigami M., et al. Adiponectin deficiency suppresses ABCA1 expression and ApoA-I synthesis in the liver. FEBS Lett. 2007;581:5029–5033. doi: 10.1016/j.febslet.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 283.Matsuura F., Oku H., Koseki M., Sandoval J.C., Yuasa-Kawase M., Tsubakio-Yamamoto K., Masuda D., Maeda N., Tsujii K., Ishigami M., et al. Adiponectin accelerates reverse cholesterol transport by increasing high density lipoprotein assembly in the liver. Biochem. Biophys. Res. Commun. 2007;358:1091–1095. doi: 10.1016/j.bbrc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 284.Matsubara M., Maruoka S., Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J. Clin. Endocrinol. Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 285.Kobayashi J., Kusunoki M., Murase Y., Kawashiri M., Higashikata T., Miwa K., Katsuda S., Takata M., Asano A., Nohara A., et al. Relationship of lipoprotein lipase and hepatic triacylglycerol lipase activity to serum adiponectin levels in Japanese hyperlipidemic men. Horm. Metab. Res. 2005;37:505–509. doi: 10.1055/s-2005-870318. [DOI] [PubMed] [Google Scholar]
- 286.Qiao L., Zou C., van der Westhuyzen D.R., Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes. 2008;57:1824–1833. doi: 10.2337/db07-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yoshida H., Hirowatari Y., Kurosawa H., Tada N. Implications of decreased serum adiponectin for type IIb hyperlipidaemia and increased cholesterol levels of very-low-density lipoprotein in type II diabetic patients. Clin. Sci. 2005;109:297–302. doi: 10.1042/CS20040353. [DOI] [PubMed] [Google Scholar]
- 288.Møller N., Gjedsted J., Gormsen L., Fuglsang J., Djurhuus C. Effects of growth hormone on lipid metabolism in humans. Growth Horm. IGF Res. 2003;13((Suppl. SA)):S18–S21. doi: 10.1016/S1096-6374(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 289.Brindley D.N. Role of glucocorticoids and fatty acids in the impairment of lipid metabolism observed in the metabolic syndrome. Int. J. Obes. Relat. Metab. Disord. 1995;19((Suppl. S1)):S69–S75. [PubMed] [Google Scholar]
- 290.Williamson D.H. Role of insulin in the integration of lipid metabolism in mammalian tissues. Biochem. Soc. Trans. 1989;17:37–40. doi: 10.1042/bst0170037. [DOI] [PubMed] [Google Scholar]
- 291.Christ E.R., Cummings M.H., Russell-Jones D.L. Dyslipidaemia in Adult Growth Hormone (GH) Deficiency and the Effect of GH Replacement Therapy: A Review. Trends Endocrinol. Metab. 1998;9:200–206. doi: 10.1016/S1043-2760(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 292.Lanfranco F., Motta G., Minetto M.A., Ghigo E., Maccario M. Growth hormone/insulin-like growth factor-I axis in obstructive sleep apnea syndrome: An update. J. Endocrinol. Investig. 2010;33:192–196. doi: 10.1007/BF03346580. [DOI] [PubMed] [Google Scholar]
- 293.Whitworth J.A., Williamson P.M., Mangos G., Kelly J.J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk Manag. 2005;1:291–299. doi: 10.2147/vhrm.2005.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kritikou I., Basta M., Vgontzas A.N., Pejovic S., Fernandez-Mendoza J., Liao D., Bixler E.O., Gaines J., Chrousos G.P. Sleep apnoea and the hypothalamic–pituitary–adrenal axis in men and women: Effects of continuous positive airway pressure. Eur. Respir. J. 2016;47:531. doi: 10.1183/13993003.00319-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Somers V.K., Dyken M.E., Mark A.L., Abboud F.M. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 296.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 297.Takahashi Y., Kipnis D.M., Daughaday W.H. Growth hormone secretion during sleep. J. Clin. Investig. 1968;47:2079–2090. doi: 10.1172/JCI105893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Alzoubaidi M., Mokhlesi B.O.O. Obstructive sleep apnea during rapid eye movement sleep: Clinical relevance and therapeutic implications. Curr. Opin. Pulm. Med. 2016;22:545–554. doi: 10.1097/MCP.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bikov A., Lazar Z., Horvath P., Tarnoki D.L., Tarnoki A.D., Fesus L., Horvath M., Meszaros M., Losonczy G., Kunos L. Association Between Serum Lipid Profile and Obstructive Respiratory Events During REM and Non-REM Sleep. Lung. 2019;197:443–450. doi: 10.1007/s00408-019-00195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Uchida T., Nishimura A., Kasai T., Kikuno S., Nagasawa K., Okubo M., Narui K., Mori Y. Relationship between obstructive sleep apnoea during rapid eye movement sleep and metabolic syndrome parameters in patients with type 2 diabetes mellitus. Sleep Breath. 2021;25:309–314. doi: 10.1007/s11325-020-02129-7. [DOI] [PubMed] [Google Scholar]
- 301.Xu H., Xia Y., Li X., Qian Y., Zou J., Fang F., Yi H., Wu H., Guan J., Yin S. Association between obstructive sleep apnea and lipid metabolism during REM and NREM sleep. J. Clin. Sleep Med. 2020;16:475–482. doi: 10.5664/jcsm.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Martínez-Cerón E., Casitas R., Galera R., Sánchez-Sánchez B., Zamarrón E., Garcia-Sanchez A., Jaureguizar A., Cubillos-Zapata C., Garcia-Rio F. Contribution of sleep characteristics to the association between obstructive sleep apnea and dyslipidemia. Sleep Med. 2021;84:63–72. doi: 10.1016/j.sleep.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 303.Coulet F., Nadaud S., Agrapart M., Soubrier F. Identification of hypoxia-response element in the human endothelial nitric-oxide synthase gene promoter. J. Biol. Chem. 2003;278:46230–46240. doi: 10.1074/jbc.M305420200. [DOI] [PubMed] [Google Scholar]
- 304.Wang B., Yan B., Song D., Ye X., Liu S.F. Chronic intermittent hypoxia down-regulates endothelial nitric oxide synthase expression by an NF-κB-dependent mechanism. Sleep Med. 2013;14:165–171. doi: 10.1016/j.sleep.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 305.Kuzkaya N., Weissmann N., Harrison D.G., Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 306.Vásquez-Vivar J., Kalyanaraman B., Martásek P., Hogg N., Masters B.S., Karoui H., Tordo P., Pritchard K.A. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc. Natl. Acad. Sci. USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jelic S., Padeletti M., Kawut S.S.S.S.S.M., Higgins C., Canfield S.M., Onat D., Colombo P.C., Basner R.C., Factor P., LeJemtel T.H. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lee W.J., Ou H.C., Hsu W.C., Chou M.M., Tseng J.J., Hsu S.L., Tsai K.L., Sheu W.H. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J. Vasc. Surg. 2010;52:1290–1300. doi: 10.1016/j.jvs.2010.04.085. [DOI] [PubMed] [Google Scholar]
- 309.Wang W., Hein T.W., Zhang C., Zawieja D.C., Liao J.C., Kuo L. Oxidized low-density lipoprotein inhibits nitric oxide-mediated coronary arteriolar dilation by up-regulating endothelial arginase I. Microcirculation. 2011;18:36–45. doi: 10.1111/j.1549-8719.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kattoor A.J., Pothineni N.V.K., Palagiri D., Mehta J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017;19:42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 311.Williams A., Scharf S.M. Obstructive sleep apnea, cardiovascular disease, and inflammation--is NF-kappaB the key? Sleep Breath. 2007;11:69–76. doi: 10.1007/s11325-007-0106-1. [DOI] [PubMed] [Google Scholar]
- 312.Horváth P., Lázár Z., Gálffy G., Puskás R., Kunos L., Losonczy G., Mészáros M., Tárnoki Á D., Tárnoki D.L., Bikov A. Circulating P-Selectin Glycoprotein Ligand 1 and P-Selectin Levels in Obstructive Sleep Apnea Patients. Lung. 2020;198:173–179. doi: 10.1007/s00408-019-00299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Pak V.M., Keenan B.T., Jackson N., Grandner M.A., Maislin G., Teff K., Schwab R.J., Arnardottir E.S., Júlíusson S., Benediktsdottir B., et al. Adhesion molecule increases in sleep apnea: Beneficial effect of positive airway pressure and moderation by obesity. Int. J. Obes. 2015;39:472–479. doi: 10.1038/ijo.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Galkina E., Ley K. Leukocyte influx in atherosclerosis. Curr. Drug Targets. 2007;8:1239–1248. doi: 10.2174/138945007783220650. [DOI] [PubMed] [Google Scholar]
- 315.Kattoor A.J., Goel A., Mehta J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants. 2019;8:218. doi: 10.3390/antiox8070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Oh J., Riek A.E., Weng S., Petty M., Kim D., Colonna M., Cella M., Bernal-Mizrachi C. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J. Biol. Chem. 2012;287:11629–11641. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Tontonoz P., Nagy L., Alvarez J.G., Thomazy V.A., Evans R.M. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/S0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 318.Tall A.R., Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kobiyama K., Ley K. Atherosclerosis: A Chronic Inflammatory Disease with an Autoimmune Component. Circ. Res. 2018;123:1118–1120. doi: 10.1161/CIRCRESAHA.118.313816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Nofer J.-R., van der Giet M., Tölle M., Wolinska I., von Wnuck Lipinski K., Baba H.A., Tietge U.J., Gödecke A., Ishii I., Kleuser B., et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Investig. 2004;113:569–581. doi: 10.1172/JCI200418004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kimura T., Sato K., Kuwabara A., Tomura H., Ishiwara M., Kobayashi I., Ui M., Okajima F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J. Biol. Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 322.Kontush A., Therond P., Zerrad A., Couturier M., Négre-Salvayre A., de Souza J.A., Chantepie S., Chapman M.J. Preferential Sphingosine-1-Phosphate Enrichment and Sphingomyelin Depletion Are Key Features of Small Dense HDL3 Particles. Arterioscler. Thromb. Vasc. Biol. 2007;27:1843–1849. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 323.Li X.A., Titlow W.B., Jackson B.A., Giltiay N., Nikolova-Karakashian M., Uittenbogaard A., Smart E.J. High density lipoprotein binding to scavenger receptor, Class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner. J. Biol. Chem. 2002;277:11058–11063. doi: 10.1074/jbc.M110985200. [DOI] [PubMed] [Google Scholar]
- 324.Nofer J.R., Brodde M.F., Kehrel B.E. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin. Exp. Pharm. Physiol. 2010;37:726–735. doi: 10.1111/j.1440-1681.2010.05377.x. [DOI] [PubMed] [Google Scholar]
- 325.Kim J.K., Fillmore J.J., Chen Y.Y., Yu C., Moore I.K., Pypaert M., Lutz E.P., Kako Y., Velez-Carrasco W., Goldberg I.J., et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl. Acad. Sci. USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Boden G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Patil Susheel P., Ayappa Indu A., Caples Sean M., Kimoff R.J., Patel Sanjay R., Harrod Christopher G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019;15:335–343. doi: 10.5664/jcsm.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ip M.S., Lam K.S., Ho C., Tsang K.W., Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 329.Robinson G.V., Pepperell J.C., Segal H.C., Davies R.J., Stradling J.R. Circulating cardiovascular risk factors in obstructive sleep apnoea: Data from randomised controlled trials. Thorax. 2004;59:777–782. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Börgel J., Sanner B.M., Bittlinsky A., Keskin F., Bartels N.K., Buechner N., Huesing A., Rump L.C., Mügge A. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur. Respir. J. 2006;27:121–127. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- 331.Dorkova Z., Petrasova D., Molcanyiova A., Popovnakova M., Tkacova R. Effects of Continuous Positive Airway Pressure on Cardiovascular Risk Profile in Patients With Severe Obstructive Sleep Apnea and Metabolic Syndrome. Chest. 2008;134:686–692. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 332.Barceló A., Barbé F., de la Peña M., Martinez P., Soriano J.B., Piérola J., Agustí A.G. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63:946–950. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- 333.Coughlin S.R., Mawdsley L., Mugarza J.A., Wilding J.P., Calverley P.M. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur. Respir. J. 2007;29:720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 334.Lattimore J.L., Wilcox I., Skilton M., Langenfeld M., Celermajer D.S. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial func.c.ction in the systemic circulation. Thorax. 2006;61:491–495. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kitahara Y., Hattori N., Yokoyama A., Nakajima M., Kohno N. Effect of CPAP on brachial-ankle pulse wave velocity in patients with OSAHS: An open-labelled study. Respir. Med. 2006;100:2160–2169. doi: 10.1016/j.rmed.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 336.Drager L.F., Bortolotto L.A., Figueiredo A.C., Krieger E.M., Lorenzi G.F. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 337.Nadeem R., Singh M., Nida M., Kwon S., Sajid H., Witkowski J., Pahomov E., Shah K., Park W., Champeau D. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: A meta-regression analysis. J. Clin. Sleep Med. 2014;10:1295–1302. doi: 10.5664/jcsm.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Xu H., Yi H., Guan J., Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: A meta-analysis of randomized controlled trials. Atherosclerosis. 2014;234:446–453. doi: 10.1016/j.atherosclerosis.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 339.Lin M.T., Lin H.H., Lee P.L., Weng P.H., Lee C.C., Lai T.C., Liu W., Chen C.L. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: A meta-analysis. Sleep Breath. 2015;19:809–817. doi: 10.1007/s11325-014-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Chen B., Guo M., Peker Y., Salord N., Drager L.F., Lorenzi-Filho G., Tang X., Li Y. Effect of Continuous Positive Airway Pressure on Lipid Profiles in Obstructive Sleep Apnea: A Meta-Analysis. J. Clin. Med. 2022;11:596. doi: 10.3390/jcm11030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Cholidou K.G., Kostakis I.D., Manali E.D., Perrea D., Margeli A., Vougas K., Markozannes E., Koulouris N., Alchanatis M. Calprotectin: A protein related to cardiovascular risk in adult patients with obstructive sleep apnea. Cytokine. 2013;61:917–923. doi: 10.1016/j.cyto.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 342.Chen B., Somers V.K., Tang X., Li Y. Moderating Effect of BMI on the Relationship Between Sympathetic Activation and Blood Pressure in Males with Obstructive Sleep Apnea. Nat. Sci. Sleep. 2021;13:339–348. doi: 10.2147/NSS.S297707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Schmoller A., Eberhardt F., Jauch-Chara K., Schweiger U., Zabel P., Peters A., Schultes B., Oltmanns K.M. Continuous positive airway pressure therapy decreases evening cortisol concentrations in patients with severe obstructive sleep apnea. Metabolism. 2009;58:848–853. doi: 10.1016/j.metabol.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 344.Shang W., Zhang Y., Wang G., Han D. Benefits of continuous positive airway pressure on glycaemic control and insulin resistance in patients with type 2 diabetes and obstructive sleep apnoea: A meta-analysis. Diabetes Obes. Metab. 2021;23:540–548. doi: 10.1111/dom.14247. [DOI] [PubMed] [Google Scholar]
- 345.Koenig A.M., Koehler U., Hildebrandt O., Schwarzbach H., Hannemann L., Boneberg R., Heverhagen J.T., Mahnken A.H., Keller M., Kann P.H., et al. The Effect of Obstructive Sleep Apnea and Continuous Positive Airway Pressure Therapy on Skeletal Muscle Lipid Content in Obese and Nonobese Men. J. Endocr. Soc. 2021;5:bvab082. doi: 10.1210/jendso/bvab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Chopra S., Rathore A., Younas H., Pham L.V., Gu C., Beselman A., Kim I.Y., Wolfe R.R., Perin J., Polotsky V.Y., et al. Obstructive Sleep Apnea Dynamically Increases Nocturnal Plasma Free Fatty Acids, Glucose, and Cortisol During Sleep. J. Clin. Endocrinol. Metab. 2017;102:3172–3181. doi: 10.1210/jc.2017-00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Fadaei R., Koushki M., Sharafkhaneh A., Moradi N., Ahmadi R., Rostampour M., Khazaie H. The impact of continuous positive airway pressure therapy on circulating levels of malondialdehyde: A systematic review and meta-analysis. Sleep Med. 2020;75:27–36. doi: 10.1016/j.sleep.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 348.Akinnusi M.E., Laporta R., El-Solh A.A. Lectin-like oxidized low-density lipoprotein receptor-1 modulates endothelial apoptosis in obstructive sleep apnea. Chest. 2011;140:1503–1510. doi: 10.1378/chest.11-0302. [DOI] [PubMed] [Google Scholar]
- 349.Chirinos J.A., Gurubhagavatula I., Teff K., Rader D.J., Wadden T.A., Townsend R., Foster G.D., Maislin G., Saif H., Broderick P., et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 2014;370:2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ramar K., Dort Leslie C., Katz Sheri G., Lettieri Christopher J., Harrod Christopher G., Thomas Sherene M., Chervin Ronald D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015;11:773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Recoquillon S., Pépin J.L., Vielle B., Andriantsitohaina R., Bironneau V., Chouet-Girard F., Fleury B., Goupil F., Launois S., Martinez M.C., et al. Effect of mandibular advancement therapy on inflammatory and metabolic biomarkers in patients with severe obstructive sleep apnoea: A randomised controlled trial. Thorax. 2019;74:496–499. doi: 10.1136/thoraxjnl-2018-212609. [DOI] [PubMed] [Google Scholar]
- 352.Venema J., Vries G.E.K., van Goor H., Westra J., Hoekema A., Wijkstra P.J. Cardiovascular and metabolic effects of a mandibular advancement device and continuous positive airway pressure in moderate obstructive sleep apnea: A randomized controlled trial. J. Clin. Sleep Med. 2022;18:1547–1555. doi: 10.5664/jcsm.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Silva L., Guimarães T.M., Pontes G., Coelho G., Badke L., Fabbro C.D., Tufik S., Bittencourt L., Togeiro S. The effects of continuous positive airway pressure and mandibular advancement therapy on metabolic outcomes of patients with mild obstructive sleep apnea: A randomized controlled study. Sleep Breath. 2021;25:797–805. doi: 10.1007/s11325-020-02183-1. [DOI] [PubMed] [Google Scholar]
- 354.Li L., Zhan X., Wang N., Pinto J.M., Ge X., Wang C., Tian J., Wei Y. Does airway surgery lower serum lipid levels in obstructive sleep apnea patients? A retrospective case review. Med. Sci. Monit. 2014;20:2651–2657. doi: 10.12659/MSM.892230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.She W., Wang J., Qian X., Hang M. Long-term follow-up of patients with obstructive sleep apnea syndrome treated with uvulopalatopharyngoplasty. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2001;36:227–230. [PubMed] [Google Scholar]
- 356.Bikov A., Frent S., Reisz D., Negru A., Gaita L., Breban Schwarzkopf D., Mihaicuta S. Comparison of Composite Lipid Indices in Patients with Obstructive Sleep Apnoea. Nat. Sci. Sleep. 2022;14:1333–1340. doi: 10.2147/NSS.S361318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.