Abstract
Simple Summary
The Greater Mekong Subregion (GMS) has a diverse geographic landscape, and due to its varied environmental conditions, it harbors numerous florae, fauna, and microorganisms. Thus, the biodiversity in this region is exceptionally high. Over recent decades, the number of studies on microfungal diversity in the GMS increased rapidly. However, in the GMS the fungi of terrestrial habitats such as woody litter is still poorly researched. This paper introduces one monotypic genus, five novel species, and two new host records in Didymosphaeriaceae-inhabiting woody plant litter from the GMS and provides morpho-molecular justifications.
Abstract
The Greater Mekong Subregion (GMS) is known as a diverse geographic landscape and one of the richest biodiversity hotspots in the world with a high fungal diversity. Collections were carried out in terrestrial habitats to determine the diversity of woody litter fungi in the GMS, with an emphasis on northern Thailand and the Yunnan Province of China. Morphological characteristics and multigene phylogenetic analyses of combined SSU, LSU, ITS, and tef1-α supported the placement of the new isolates in the family Didymosphaeriaceae. The phylogenetic affinities of our isolates are illustrated through maximum likelihood and Bayesian inference analyses. Seven species of woody litter fungi were identified, comprising a new monotypic genus, Septofusispora; five novel species (Chromolaenicola sapindi, Dictyoarthrinium thailandicum, Karstenula lancangensis, Septofusispora thailandica, and Spegazzinia jinghaensis); and new host records of two species (Austropleospora archidendri, and Montagnula donacina). Furthermore, this study provides a synopsis of the Montagnula aff. donacina species based on their morphological characteristics, which can be useful in the species-level identifications in this genus.
Keywords: new taxa, Ascomycota, new genus, saprobic, taxonomy, phylogenetic
1. Introduction
The Greater Mekong Subregion (GMS) is a global biodiversity hotspot with a 2.5 million km2 land area [1], including Cambodia, Lao PDR, Myanmar, Thailand, the People’s Republic of China, and Vietnam. Due to its varied environmental conditions, the GMS harbors abundant biodiversity [2,3]. Numerous studies have shown that China (Yunnan Province) and Thailand have the potential to support a high diversity of macro- and micro-fungi, many yet to be discovered [3,4,5,6,7,8,9]. For instance, many saprobic taxa have been discovered on woody litter in this region [8,10,11,12,13,14,15,16,17,18,19,20,21]. Leaf litter and freshwater taxa have been well-studied in the GMS [5,7,22,23], but less attention has been given to saprobic fungi on woody litter in terrestrial habitats.
The Didymosphaeriaceae [24] is a diverse family of Pleosporales, comprising 33 genera [25]. Its species occur on a wide range of hosts in various habitats worldwide [26,27]. Didymosphaeriaceae species include endophytes, pathogens (plants and occasionally humans), and saprobes on woody branches, herbaceous stems, leaves, pods, and soil [28,29,30]. Didymosphaeriaceae comprises economically important fungi, such as the Austropleospora and Barria species, which have potential agricultural and medical applications, or the species of Deniquelata, which cause plant disease [29,31].
The sexual morphs of Didymosphaeriaceae are characterized by globose to sub-globose, central ostiolate ascomata; a peridium with several layers of lightly pigmented to dark brown or black cells of textura angularis; cellular or trabeculate pseudoparaphyses; 2–4-spored or 8-spored, bitunicate, fissitunicate, cylindric or oblong, pedicellate asci; and 1–2-seriate, overlapping, ellipsoid or oblong, 1–3-septate or muriform ascospores [29,30]. The asexual morphs are diverse, i.e., camarosporium-like, diplodia, fusicladium, pithomyces, phoma, and spegazzinia-like [32]. Out of the Didymosphaeriaceae genera, ten (Alloconiothyrium, Cylindroaseptospora, Dictyoarthrinium, Neptunomyces, Paraconiothyrium, Paracamarosporium, Pseudocamarosporium, Pseudopithomyces, Spegazzinia, and Xenocamarosporium) were introduced based on their asexual morphs characters only. Alloconiothyrium has pycnidial conidiomata with a single cavity and olivaceous-brown conidia [28]. Cylindroaseptospora has hyaline, cylindrical, aseptate conidia [33]. Dictyoarthrinium has square-to-spherical, subspherical or oblong, pale-to-dark brown, often four-celled conidia [34]. Neptunomyces has aseptate, golden yellow, subcylindrical conidia [29]. Paraconiothyrium has eustromatic conidiomata and hyaline-to-brown conidia [29]. Paracamarosporium has pale brown-to-brown, ellipsoid-to-ovoid, with obtuse ends, and 1–3 transversely septate conidia [35]. Pseudocamarosporium has oblong, muriform, brown-to-dark-brown conidia, with transverse, longitudinal, and oblique septa [36]. Pseudopithomyces has fusiform, verruculose dark conidia, producing brown-to-black colonies on the host [37]. Spegazzinia produces two types of conidia in the same mycelium: α conidia which are composed of 4–8 subglobose, dark cells with very long spines, while β conidia are subspherical or broadly ellipsoid conidia, in general flattened in one plane, crucially septate or muriform, pale brown and smooth [38]. Finally, Xenocamarosporium has ellipsoidal-to-subcylindrical, golden-brown, and verruculose conidia with (1–)3-septa [35].
On the other hand, twenty-three genera of Didymosphaeriaceae were introduced with their sexual morphs. Austropleospora has clavate-to-cylindrical, 6–8-spored asci and dictyosporous, ellipsoidal, and yellowish-brown ascospores [30,33]. Barria has short, knob-like pedicellate asci and brown, muriform ascospores [29]. Bimuria has fissitunicate, 2-spored asci with muriform, dark brown, and verrucose ascospores [29]. Chromolaenicola has cylindrical asci with an ocular chamber, and ellipsoid-to-broadly fusiform, muriform ascospores with three transverse septa and one vertical septum [39]. Curreya has small, sclerotial cells of its peridium, and narrower, thinner-walled asci [29]. Deniquelata has bitunicate asci and brown, muriform ascospores [31]. Didymocrea has unitunicate asci and two-celled, brown ascospores [31]. Julella has cylindric or oblong, 2-spored asci, and oblong-to-narrowly oblong, muriform ascospores [31]. Didymosphaeria has paraphyses richly anastomosing above the asci, and brown, thin, distoseptate ascospores [40]. Kalmusia has clavate asci, with narrowly ovoid to clavate, pale brown, and 3-septate ascospores [29]. Kalmusibambusa has multi-loculate ascostromata and cylindrical asci [41]. Karstenula has cylindrical-to-cylindro-clavate asci, with short, furcate pedicel, ellipsoid-to-fusoid, reddish-brown to dark brown, muriform ascospores [29]. Laburnicola has ellipsoidal-to-fusoid ascospores, with 6–8 transverse septa and 1–2 longitudinal septa [42]. Letendraea has obclavate-to-cylindrical asci and fusoid-to-oblong, 1-septate ascospores [31]. Lineostroma has trabeculate pseudoparaphyses asci with a short pedicel and 1-septate ascospores [31]. Montagnula has claviform asci, fusoid-or-ellipsoid ascospores with transverse septa and one or more longitudinal septa [31]. Neokalmusia has cylindric-clavate, 4–8-spored asci, and fusiform, yellowish-brown-to-reddish-brown, 3–5-septate ascospores with a sheath [31]. Paramassariosphaeria has cylindrical-clavate asci with a long pedicel, and curved-fusoid, asymmetrical ascospores with a mucilaginous sheath. [31] Paraphaeosphaeria has bitunicate asci with a short pedicel and multi-septate, broadly elliptical, yellowish-brown ascospores [42]. Phaeodothis has a sparse hamathecium, consisting of cellular pseudoparaphyses and 1-septate ascospores [31]. Tremateia has fissitunicate, clavate asci, and ellipsoid, muriform ascospores [29]. Verrucoconiothyrium has one-septate or aseptate, brown, subcylindrical-to-narrowly ellipsoid conidia [35]. Finally, Vicosamyces forms orange-brown wounds and 2-celled apiospores [29].
In our survey of the diversity of woody litter fungi in the GMS, the field collections were carried out within the Yunnan Province (China) and northern Thailand. This study aimed to (1) look for novel species and new host records supported by morphological illustrations and multi-gene phylogenetic analyses based on combined SSU, LSU, ITS, and tef1-α sequence data, and (2) provide a synopsis of the Montagnula species based on phylogeny and morphology.
2. Materials and Methods
2.1. Sample Collection, Morphological Observation, and Fungal Isolation
Decayed woody samples were collected from mixed forest areas located in Thailand (Chiang Mai, Chiang Rai, and Tak Provinces) during the wet season (August and September 2019), and in China (Yunnan Province) during the dry season (March 2020). The woody litter was cut into no more than 20 cm pieces. Collected samples were placed in separate zip-lock plastic bags and transported to the laboratory.
Specimens were examined using a stereomicroscope (Olympus SZ61, Tokyo, Japan). Micro-morphological characteristics were photographed using a Canon EOS 600D (Tokyo, Japan) digital camera mounted on a Nikon ECLIPSE 80i (Tokyo, Japan) compound microscope. All microscopic measurements were taken using the Tarosoft (R) Image Frame Work v.09 program, and the measurements were reported as minimum–maximum values and average values. Images were processed with Adobe Photoshop CS6 software v.13 (Adobe Systems, San Jose, CA, USA).
Single-spore isolation was used to obtain pure cultures. The ascomata containing ascospores were transferred using a sterile needle to a drop of sterile water on a flamed microscope slide. The spore suspension was spread over a few square centimeters of a Petri plate containing water agar (WA) or potato dextrose agar (PDA). Germinating spores were photographed, transferred to PDA media, and incubated at room temperature for seven days. Cultures were then photographed, and their characters recorded. After another week, hyphal tips were transferred into PDA plates and grown at 25 °C in the daylight [43]. Herbarium materials were deposited at the herbarium of Mae Fah Luang University, Chiang Rai Province, Thailand (MFLU), the Cryptogams Kunming Institute of Botany, Academia Sinica (HKAS), Kunming Institute of Botany, Chinese Academy of Sciences, China, and living cultures were deposited at the Culture Collection of Mae Fah Luang University (MFLUCC), Mae Fah Luang University, Thailand, and Kunming Institute of Botany Culture Collection (KUMCC), Kunming Institute of Botany, Chinese Academy of Sciences, China. Faces of fungi [44] and Index Fungorum [45] numbers were obtained for the new taxa, and the details were added to the Greater Mekong Subregion’s webpage [8].
2.2. DNA Extraction, PCR Amplification, and Sequencing
Fungal mycelia were scraped from the 14-day-old colonies grown on PDA at 25–30 °C, and the DNA was isolated using the Biospin Fungus Genomic DNA Extraction Kit (BioFlux® Hangzhou, China). Polymerase chain reactions (PCRs) were conducted to amplify parts of the small nuclear ribosomal subunit rDNA (SSU), internal transcribed spacer region (ITS), large nuclear ribosomal subunit rDNA (LSU), and translation elongation factor 1-alpha gene (tef1-α) using primer pairs NS1/NS4 [46], ITS5/ITS4 [46], LR0R/LR5 [47], and EF1-983F/EF1-2218R [48], respectively. PCR was carried out in a 25 μL reaction volume containing 12.5 μL 2X PCR MasterMix (TIANGEN Co., Bejing, China), 8.5 μL double distilled water, 2 μL genomic DNA, and 1 μL of each primer. PCR thermal cycles for SSU, LSU, ITS, and tef1-α gene regions were conducted following Tennakoon et al. [49]. PCR products were sequenced at the Qingke Company, Yunnan Province, China.
2.3. Phylogenetic Analyses
Phylogenetic analyses were performed as described in Dissanayake et al. [50]. Each newly generated sequence was assembled using BioEdit 7.0.9.0 [51] and subjected to BLAST searches against the NCBI nucleotide non-redundant database (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 August 2021)) for selection of the closest matching taxa. Based on BLAST search results and recently published data, sequences of representative taxa were downloaded and used for comparison [30,33,34,52] (Table 1). Individual gene regions were aligned using MAFFT v.7 (http://mafft.cbrc.jp/alignment/server/ (accessed on 20 May 2022)) [53], and the uninformative gaps and ambiguous regions were manually removed and different gene regions were concatenated using BioEdit 7.0.9.0.
The ML analysis was performed on the CIPRES Science Gateway v.3.3 (http://www.phylo.org/portal2/ (accessed on 21 May 2022), [54]) using RAxML-HPC2 on XSEDE v.8.2.12 [55] with parameters adjusted for 1000 bootstrap iterations and the GTRGAMMA substitution model. Gaps were treated as missing data, and the branches of zero length were collapsed [56]. Bayesian inference was performed in MrBayes v.3.2.2 using Markov chain Monte-Carlo sampling (BMCMC) [57] to determine posterior probabilities (PPs) [58,59]. The model of evolution was estimated using MrModeltest v.2.3 [60] via PAUP v.4.0b10 [61]. Six simultaneous Markov chains were run for 2,000,000 generations, with trees sampled every 200 generations, until it was stopped when the standard deviation of split frequencies between the two simultaneous runs dropped below 0.01. The first 25% of sampled trees was discarded as part of the burn-in procedure, and the remaining 7501 trees were used to calculate posterior probabilities in the consensus tree. Phylogenetic trees were visualized with FigTree v.1.4.0 [62] and edited using Microsoft PowerPoint and Adobe Illustrator® CS6 v.26.0 (Adobe Systems, San Jose, CA, USA). The newly produced sequences were deposited in the GenBank nucleotide database (Table 1).
Table 1.
Taxa used in the phylogenetic analysis, species voucher/culture numbers, and GenBank accession numbers for the sequences.
| Taxon | Strain Number | GenBank Accession Numbers | Reference | |||
|---|---|---|---|---|---|---|
| SSU | LSU | ITS | tef1-α | |||
| Alloconiothyrium aptrootii | CBS 980.95 T | JX496121 | JX496234 | NA | NA | [28] |
| A. aptrootii | CBS 981.95 T | JX496122 | JX496235 | NA | NA | [28] |
| A. camelliae | NTUCC 17-032-1 T | MT112294 | MT071270 | MT071221 | MT232967 | [63] |
| A. camelliae | NTUCC 17-032-2 | MT112295 | MT071271 | MT071222 | MT232965 | [63] |
| A. camelliae | NTUCC 17-032-3 | MT112296 | MT071272 | MT071223 | MT232966 | [63] |
| A. encephalarti | CPC: 35980 | MN562102 | MN567610 | NA | NA | [63] |
| Austropleospora archidendri | MFLUCC 17-2429 | MK347757 | MK347974 | MK347863 | MK360044 | [33] |
| A. archidendri | CBS 168.77 | NA | JX496162 | JX496049 | NA | [28] |
| A. archidendri | KUMCC 21-0680 | OP059006 | OP059055 | OP058964 | OP135941 | This study |
| A. keteleeriae | MFLUCC 18-1551 T | MK347802 | MK348021 | MK347910 | MK360045 | [33] |
| A. ochracea | KUMCC 20-0020 T | MT799859 | MT799860 | MT808321 | MT872714 | [30] |
| A. osteospermi | BRIP51628 | FJ481946 | NA | NA | NA | [64] |
| Bambusistroma didymosporum | MFLU 15-0057 T | KP761733 | KP761730 | KP761737 | KP761727 | [65] |
| B. didymosporum | MFLU 15-0058 | KP761734 | KP761731 | KP761738 | KP761728 | [65] |
| Bimuria novae-zelandiae | CBS 107.79 T | MH861181 | AY016356 | AY016338 | DQ471087 | [66] |
| Chromolaenicola chiangraiensis | MFLUCC 17-1493 T | MN325017 | MN325005 | MN325011 | MN335650 | [39] |
| C. clematidis | MFLUCC 17-2075 T | MT310601 | MT214554 | MT226671 | NA | [67] |
| C. lampangensis | MFLUCC 17-1462 T | MN325016 | MN325004 | MN325010 | MN335649 | [39] |
| C. thailandensis | MFLUCC 17-1510 T | MN325018 | MN325006 | MN325012 | MN335651 | [39] |
| C. thailandensis | MFLUCC 17-1475 | MN325019 | MN325007 | MN325013 | MN335652 | [39] |
| C. nanensis | MFLUCC 17-1473 T | MN325015 | MN325003 | MN325009 | MN335648 | [39] |
| C. nanensis | MFLUCC 17-1477 | MN325014 | MN325002 | MN325008 | MN335647 | [39] |
| C. siamensis | MFLUCC 17-2527 T | NR_163337 | NG_066311 | NA | NA | [33] |
| C. sapindi | KUMCC 21-0564 T | OP059009 | OP059058 | OP058967 | OP135943 | This study |
| C. sapindi | KUMCC 21-0594 | OP059010 | OP059059 | OP058968 | OP135944 | This study |
| Cylindroaseptospora leucaenae | MFLUCC 17-2424 T | NR_163333 | NG_066310 | MK347856 | MK360047 | [33] |
| C. siamensis | MFLUCC 17-2527 T | MK347760 | MK347976 | MK347866 | MK360048 | [33] |
| Deniquelata barringtoniae | MFLUCC 11-0422 T | NR_111779 | NG_042696 | JX254656 | NA | [68] |
| D. vittalii | NFCCI4249 T | MF406218 | MF182395 | MF622059 | MF182398 | [69] |
| Dictyoarthrinium hydei | SQUCC 13296 T | MW077145 | NA | MW077161 | MW075771 | [26] |
| D. musae | MFLUCC 20-0105 T | MT482323 | MT482320 | MT482326 | MT495602 | [52] |
| D. musae | MFLUCC 20-0106 T | MT482324 | MT482321 | MT482327 | MT495603 | [52] |
| D. sacchari | MFLUCC 20-0107 | MT482325 | MT482322 | MT482328 | NA | [52] |
| D. sacchari | CBS 529.73 | NA | MH872479 | NA | NA | [70] |
| D. thailandicum | KUMCC 21-0664 T | OP059007 | OP059056 | OP058965 | NA | This study |
| D. thailandicum | KUMCC 21-0665 | OP059008 | OP059057 | OP058966 | OP135942 | This study |
| Didymocrea sadasivanii | CBS 438.65 T | MH858658 | DQ384103 | NA | NA | [70] |
| Didymosphaeria rubi-ulmifolii | MFLUCC 14-0023 T | NA | KJ436586 | NG_063557 | NA | [40] |
| D. rubi-ulmifolii | MFLUCC 14-0024 | NA | KJ436585 | KJ436587 | NA | [40] |
| Kalmusia italica | MFLUCC 14-0560 T | KP325440 | KP325441 | KP325442 | NA | [71] |
| K. variispora | CBS 121517 T | MH863113 | MH874668 | NA | NA | [28] |
| K. ebuli | CBS 123120 T | KF796674 | JN644073 | JN851818 | NA | [72] |
| Kalmusibambusa triseptata | MFLUCC 13-0232 T | KY682697 | KY682695 | KY682696 | NA | [41] |
| Karstenula lancangensis | KUMCC 21-0670 T | OP059011 | OP059060 | OP058969 | NA | This study |
| K. lancangensis | KUMCC 21-0677 | OP059012 | OP059061 | OP058970 | NA | This study |
| K. rhodostoma | CBS 690.94 | NA | GU301821 | GU296154 | GU349067 | [73] |
| K. rhodostoma | CBS 691.94 | LC014559 | AB807531 | AB797241 | AB808506 | [73] |
| Laburnicola hawksworthii | MFLUCC 13-0602 T | KU743194 | KU743195 | KU743196 | NA | [42] |
| L. muriformis | MFLUCC 14-0921 T | KU743200 | KU743201 | KU743202 | NA | [42] |
| Letendraea cordylinicola | MFLUCC 11-0150 | KM213996 | KM213999 | KM214002 | NA | [40] |
| L. cordylinicola | MFLUCC 11-0148 T | NR_154118 | NG_059530 | KM214001 | NA | [40] |
| Montagnula aloes | CPC 19671 T | JX069863 | JX069847 | NA | NA | [74] |
| M. aloes | CBS 132531 T | NR_111757 | NG_042676 | NA | NA | [74] |
| M. appendiculata | CBS 109027 T | DQ435529 | AY772016 | NA | NA | [75] |
| M. bellevaliae | MFLUCC 14-0924 T | KT443906 | KT443902 | KT443904 | NA | [76] |
| M. camporesii | MFLUCC 16-1369 T | MN401746 | NG_070946 | NG_068418 | MN397908 | [77] |
| M. chiangraiensis | MFLUCC 17-1420 T | NR_168864 | NG_068707 | NG_070155 | NA | [39] |
| M. chromolaenae | MFLUCC 17-1435 T | NR_168865 | NG_068708 | NG_070156 | NA | [39] |
| M. chromolaenicola | MFLUCC 17-1469 T | NR_168866 | NG_070948 | NG_070157 | MT235773 | [39] |
| M. cirsii | MFLUCC 13-0680 | KX274242 | KX274249 | KX274255 | KX284707 | [78] |
| M. cylindrospora | UTHSC: DI16-208 T | LT796834 | LN907351 | NA | LT797074 | [74] |
| M. donacina | HFG07004 | MF967419 | MF183940 | NA | NA | [79] |
| M. donacina | HVVV01 | KJ628375 | KJ628377 | KJ628376 | NA | [80] |
| M. donacina | KUMCC 21-0653 | OP059003 | OP059052 | OP058961 | OP135938 | This study |
| M. donacina | KUMCC 21-0579 | OP059005 | OP059054 | OP058963 | OP135940 | This study |
| M. donacina | KUMCC 21-0631 | OP059004 | OP059053 | OP058962 | OP135939 | This study |
| M. graminicola | MFLUCC 13-0352 T | KM658314 | KM658315 | KM658316 | NA | [81] |
| M. jonesii | MFLUCC 16-1448 T | KY313619 | KY273276 | KY313618 | KY313620 | [49] |
| M. krabiensis | MFLUCC 16-0250 T | NR168179 | NG068826 | NG068385 | MH412776 | [82] |
| M. puerensis | KUMCC 20-0225 T | MW567739 | MW575866 | MW575864 | MW575859 | [83] |
| M. puerensis | KUMCC 20-0331 | MW567740 | MW575867 | MW575865 | MW575860 | [83] |
| M. saikhuensis | MFLUCC 16-0315 T | KU743209 | KU743210 | KU743211 | NA | [42] |
| M. scabiosae | MFLUCC 14-0954 T | KT443907 | KT443903 | KT443905 | NA | [76] |
| M. thailandica | MFLUCC 17-1508 T | MT214352 | NG070949 | NG070158 | MT235774 | [39] |
| Neokalmusia brevispora | KT 1466 T | LC014573 | AB524600 | AB524459 | AB539112 | [73] |
| N. scabrispora | KT 1023 | LC014575 | AB524593 | AB524452 | AB539106 | [73] |
| Neptunomyces aureus | CMG12 T | MK912121 | NA | NA | MK948000 | [84] |
| N. aureus | CMG13 | MK912122 | NA | NA | MK948001 | [84] |
| Paraconiothyrium cyclothyrioides | CBS 972.95 T | JX496119 | JX496232 | AY642524 | NA | [28] |
| P. cyclothyrioides | CBS 432.75 | MH860933 | MH872689 | NA | NA | [28] |
| P. estuarinum | CBS 109850 | MH862842 | MH874432 | NA | NA | [28] |
| Paracamarosporium fagi | CPC 24890 | KR611886 | KR611904 | NA | NA | [35] |
| P. fagi | CPC 24892 T | KR611887 | KR611905 | NA | NA | [35] |
| Paramassariosphaeria anthostomoides | CBS 615.86 | MH862005 | GU205223 | GU205246 | NA | [28] |
| P. anthostomoides | MFLU 16-0172 T | KU743206 | KU743207 | KU743208 | NA | [42] |
| Paraphaeosphaeria rosae | MFLUCC 17-2547 | MG828935 | MG829044 | MG829150 | MG829222 | [85] |
| P. rosae | MFLUCC 17-2549 T | MG828937 | MG829046 | MG829152 | MG829223 | [85] |
| P. rosicola | MFLUCC 15-0042 T | NR_157528 | MG829047 | MG829153 | NA | [85] |
| Phaeodothis winteri | CBS 182.58 | NA | GU301857 | GU296183 | NA | [86] |
| Pseudocamarosporium propinquum | MFLUCC 13-0544 | KJ747049 | KJ813280 | KJ819949 | NA | [36] |
| P. pteleae | MFLUCC 17-0724 T | NR_157536 | MG829061 | MG829166 | MG829233 | [85] |
| Pseudopithomyces entadae | MFLUCC 17-0917 T | NA | NG_066305 | MK347835 | MK360083 | [33] |
| P. rosae | MFLUCC 15-0035 T | MG828953 | MG829064 | MG829168 | NA | [85] |
| Septofusispora thailandica | KUMCC 21-0647 T | OP059013 | OP059062 | OP058971 | OP135945 | This study |
| S. thailandica | KUMCC 21-0652 | OP059014 | OP059063 | OP058972 | NA | This study |
| Spegazzinia bromeliacearum | URM 8084 T | MK804501 | MK809513 | NA | NA | [87] |
| S. deightonii | MFLUCC 20-0002 T | MN956768 | MN956772 | MN956770 | MN927133 | [73] |
| S. intermedia | CBS 249.89 T | MH862171 | MH873861 | NA | NA | [70] |
| S. jinghaensis | KUMCC 21-0495 T | OP059015 | OP059064 | OP058973 | OP135946 | This study |
| S. jinghaensis | KUMCC 21-0496 | OP059016 | OP059065 | OP058974 | OP135947 | This study |
| S. lobulata | CBS 361.58 T | MH857812 | MH869344 | NA | NA | [70] |
| S. musae | MFLUCC 20-0001 T | MN930512 | MN930514 | MN930513 | MN927132 | [52] |
| S. neosundara | MFLUCC 15-0456 T | KX965728 | KX954397 | KX986341 | NA | [41] |
| S. radermacherae | MFLUCC 17-2285 T | MK347740 | MK347957 | MK347848 | MK360088 | [33] |
| S. tessarthra | SH 287 | JQ673429 | AB807584 | AB797294 | AB808560 | [73] |
| Tremateia arundicola | MFLU 16-1275 T | KX274241 | KX274248 | KX274254 | KX284706 | [49] |
| T. guiyangensis | GZAAS01 T | KX274240 | KX274247 | KX274253 | KX284705 | [49] |
| T. murispora | GZCC 18-2787 T | NR_165916 | MK972751 | MK972750 | MK986482 | [88] |
| Verrucoconiothyrium nitidae | CBS: 119209 | EU552112 | EU552112 | NA | NA | [89] |
| Xenocamarosporium acaciae | CBS: 139895 T | NR_137982 | NG_058163 | NA | NA | [35] |
| X. acaciae | MFLUCC 17-2432 | MK347766 | MK347983 | MK347873 | MK360093 | [33] |
The newly generated sequences are indicated in bold. T refers to ex-type strains and NA refers to “no data in GenBank”.
3. Results
3.1. Phylogeny
The combined dataset of SSU, LSU, ITS, and tef1-α comprised 109 strains of Didymosphaeriaceae and 2 strains of Periconia didymospora (MFLU 15-0057 and MFLU 15-0058), the latter 2 strains from Periconiaceae as the outgroup taxa (Table 1). The final concatenated aligned data matrix had 2939 characters (SSU: 909 bp, LSU: 725 bp, ITS: 382 bp, and tef1-α: 923 bp), including alignment gaps. The RAxML analysis of the combined dataset yielded the best-scoring tree with a final ML optimization likelihood value of −18,003.440225. The matrix had 891 distinct alignment patterns with 24.94% undetermined characters or gaps. The estimated base frequencies were as follows: A = 0.235809, C = 0.252488, G = 0.274923, T = 0.236780; substitution rates: AC = 1.104424, AG = 2.404211, AT = 1.383490, CG = 1.027352, CT = 6.936114, GT = 1.00; and gamma distribution shape parameter: α = 0.187954 and tree-length = 2.009896.
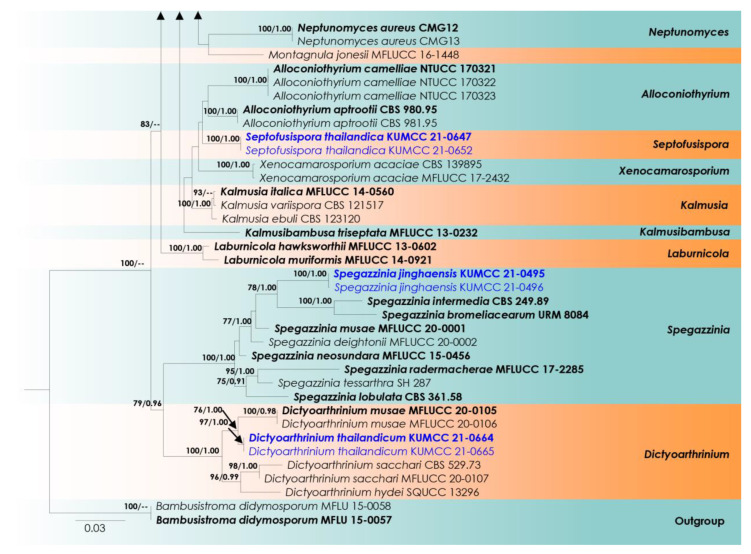
Didymosphaeriaceae comprises 33 genera, but molecular data are available only for 28 of them. Thus, sequence data representing 28 genera were used in the phylogenetic analyses. Phylogenetic trees resulting from ML and BI (Figure 1) analyses have similar overall topologies compared to the trees illustrated in Dissanayake et al. [30], Jayasiri et al. [33], and Samarakoon et al. [34]. These results show that the KUMCC 21-0647 and KUMCC 21-0652 isolates formed a monophyletic clade independent from all others (Alloconiothyrium, Kalmusia, and Xenocamarosporium) (Figure 1), and is thus introduced as a new genus, Septofusispora, with Septofusispora thailandica as the type species. Karstenula lancangensis (KUMCC 21-0670, KUMCC 21-0677) was clustered sister to the type species of this genus, K. rhodostoma (CBS 690.94, CBS 691.94), with 100% ML bootstrap and 1.00 BYPP statistical support (Figure 1). Chromolaenicola sapindi (KUMCC 21-0564, KUMCC 21-0594) was grouped in an independent lineage inside Chromolaenicola with 84% ML bootstrap and 0.91 BYPP support (Figure 1). Spegazzinia jinghaensis (KUMCC 21-0495, KUMCC 21-0496) has a sister affiliation to S. bromeliacearum (URM 8084) and S. intermedia (CBS 249.89) with 78% ML bootstrap and 1.00 BYPP support (Figure 1). Dictyoarthrinium thailandicum (KUMCC 21-0664, KUMCC 21-0665) was nested with D. musae (MFLUCC 20-0105 and MFLUCC 20-0106) with 76% ML bootstrap and 1.00 BYPP support (Figure 1). The samples of Montagnula donacina (KUMCC 21-0579, KUMCC 21-0653, and KUMCC 21-0631) were grouped with eight Montagnula species, viz., M. chromolaenicola (MFLUCC 17-1469), M. donacina (HFG07004 and HVVV01), M. puerensis (KUMCC 20-0225 and KUMCC 20-0331), M. saikhuensis (MFLUCC 16-0315), M. thailandica (MFLUCC 17-1508), and M. graminicola (MFLUCC 13-0352) in a monophyletic clade (Figure 1), while A. archidendri (KUMCC 21-0680) formed a well-supported clade with other strains of this genus with 80% ML bootstrap and 0.91 BYPP support. Based on the nucleotide base pair comparisons of LSU and ITS, our new strains are identical to the type strain of Austropleospora archidendri (CBS 168.77) with 100% similarity (Figure 1), but they appear in a different branch, perhaps due to the lack of homologous SSU and tef1-α sequences from the type collection.
Figure 1.
Phylogram generated from ML analysis based on the combined SSU, LSU, ITS, and tef1-α dataset. Bootstrap support values for ML equal to or higher than 75%, and BYPP equal to or greater than 0.90 are shown above the nodes. The ex-type strains are in bold, and new isolates are in blue. The tree is rooted with Periconia didymospora (MFLU 15-0057 and MFLU 15-0058).
3.2. Taxonomy
Septofusispora G.C. Ren and K.D. Hyde, gen. nov.
Index Fungorum number: IF559804; FacesofFungi number: FoF 10702.
Etymology: Epithet refers to the fusiform and septate spores of this genus.
Saprobic on decaying wood. Sexual morph: Ascomata solitary or gregarious, erumpent to immersed, globose-to-subglobose, black. Ostiole central. Peridium thick, comprising pale-to-brown cells of textura angularis. Hamathecium of septate, branched, cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical-to-clavate, with or without an ocular chamber, with a pedicel. Ascospores overlapping, brown, fusiform, with 4–5 transverse septa, smooth-walled, guttulate. Asexual morph: undetermined.
Notes: In our phylogenetic analysis, Septofusispora is seemingly nested near Alloconiothyrium, Kalmusibambusa, Kalmusia, and Xenocamarosporium (Figure 1), but it is well-separated from these genera. Septofusispora has uni-loculate ascomata, clavate asci, fusiform, guttulate ascospores with 4–5 transverse septa, whereas the Kalmusia species have ovoid-to-clavate asci, ovoid-to-clavate, 3-septate ascospores (sometimes muriform), with a mucilaginous sheath [81,90,91]. Kalmusibambusa has multi-loculate, elongate ascostromata, cylindrical asci, ellipsoidal-to-fusiform, 3-septate ascospores with round-to-acute ends and a wide mucilaginous sheath [41]. Alloconiothyrium and Xenocamarosporium are known only from their asexual morphs [28,63]. Most studies used molecular data to delimit species boundaries, which is not possible using morphological characters. Therefore, considering the morphological differences and phylogenetic support, we introduce Septofusispora as a new genus.
Type species: Septofusispora thailandica G.C. Ren and K.D. Hyde.
Septofusispora thailandica G.C. Ren and K.D. Hyde, sp. nov. Figure 2.
Figure 2.
Septofusispora thailandica (MFLU 22-0043, holotype). (a,b) Appearance of ascomata on host substrate; (c) section of ascoma; (d) peridium; (e) hamathecium; (f) asci; (g–j) ascospores; (k) germinated ascospore; (l,m) culture characters on PDA (l from above, m from below). Scale bars, (c) 50 μm; (d,e,k) 20 μm; (f) 30 μm; (g–j) 10 μm; (l,m) 30 mm.
Index Fungorum number: IF559805. FacesofFungi number: FoF 10703.
Etymology: The epithet reflects Thailand, where this species was collected.
Holotype: MFLU 22-0043.
Saprobic on dead woody twigs of Castanopsis sp. Sexual morph: Ascomata 140–190 × 155–210 μm ( = 165 × 180 μm, n = 5), solitary, scattered, erumpent-to-immersed, uni-loculate, globose-to-sub-globose, black. Ostiole central. Peridium 20–35 μm wide, thick, comprising 3–5 layers of light-brown-to-brown cells of textura angularis. Hamathecium of sparse, 1–2 μm wide, cylindrical, septate, branched, cellular pseudoparaphyses. Asci 60–80 × 12–16 μm ( = 73.9 × 14.9 μm, n = 20), 8-spored, bitunicate, fissitunicate, clavate, slightly broad at center, apically rounded, with short, rounded pedicel. Ascospores 24–26.5 × 5–6 μm ( = 25.5 × 5.3 μm, n = 30), overlapping uni- to bi-seriate, pale brown, narrowly fusiform, cell above median septum slightly wider than below, tapering towards ends, slightly acute at both ends, with 4–5 transverse septa, constricted at the septa, smooth-walled, guttulate, without a mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: The colonies on PDA, reaching 15–20 mm diam. at 14 days at room temperature (25–30 °C), superficial, entire margin, umbonate at center, rough surface, with dense mycelia, velvety, raised, gray at the center, white at the edge; reverse atrovirens, darkening towards center and white at the edge.
Material examined: Thailand, Tak Province, Mogro Amphoe Umphang, on dead woody twigs of Castanopsis sp., 20 August 2019, G.C. Ren, T213 (MFLU 22-0043 holotype), ex-type culture KUMCC 21-0647; ibid., T214 (MFLU 22-0044, isotype), living culture KUMCC 21-0652.
Austropleospora R.G. Shivas and L. Morin, Fungal Diversity 40: 70 (2010).
Austropleospora was introduced by Morin et al. [64], with A. osteospermi as the type species. Ariyawansa et al. [37] transferred Austropleospora from Pleosporaceae to Didymosphaeriaceae, and four taxa are currently accepted [45]. Austropleospora osteospermi was introduced with both sexual and asexual morphs, but A. archidendri and A. keteleeriae were introduced with only their asexual morphs, while A. ochracea was introduced with only its sexual morph [30,33,64,92]. The Austropleospora species have been reported from Australia, China, Myanmar, and Thailand [28,30,33,64,92]. The species in the genus are saprobic on Archidendron bigeminum, Leucaena sp., Keteleeria forturei, and pathogenic on stems of Chrysanthemoides monilifera [28,33].
Austropleospora archidendri (Verkley, Göker, and Stielow) Ariyaw. and K.D. Hyde, Fungal Diversity 75: 64 (2015) Figure 3.
Figure 3.
Austropleospora archidendri (MFLU 22-0042). (a,b) Conidiomata on the natural wood surface; (c) section through a conidioma; (d) ostiolar neck; (e) pycnidial wall; (f,g) conidiogenous cells and developing conidia; (h–k) conidia; (l) germinated conidia; (m,n) culture characters on PDA (n from the bottom). Scale bars, (c) 100 μm; (d) 50 μm; (e) 25 μm; (f,g,l) 10 μm; (h–k) 5 μm; (m,n) 30 mm.
≡ Paraconiothyrium archidendri Verkley, Göker and Stielow, Persoonia 32: 37 (2014).
Index Fungorum number: IF551419; FacesofFungi number: FoF 00936.
Saprobic on dead woody twigs of Euphoria longana. Sexual morph: Undetermined. Asexual morph: Coelomycetous. Conidiomata 165–225 µm high × 115–165 µm diam. ( = 190 × 140 µm, n = 10), scattered, immersed, unilocular, coriaceous, globose-to-sub-globose, brown-to-dark-brown with a central ostiole. Ostiole 60–75 × 40–50 μm ( = 70 × 45 μm, n = 5), short papillate, black. Conidiomatal wall 15–25 µm thick, 3–4-layered, composed of brown outer and hyaline inner layers, thin-walled cells of textura angularis. Conidiophores reduced into conidiogenous cells. Conidiogenous cells 3.7–6 × 2.8–3.8 μm ( = 4.2 × 3.4 μm, n = 10), enteroblastic, phialidic, determinate, discrete, doliiform-to-ampulliform, hyaline, smooth-walled, arising from stratum. Conidia 4.8–5.8 × 3–3.6 μm ( = 5.4 × 3.4 μm, n = 30), straight, initially hyaline, guttulate, becoming brown at maturity, sub-globose to ovate, one-celled, rounded ends, thick-walled.
Culture characteristics: The colonies reached 70–80 mm diam. on PDA at 14 days at room temperature (25–30 °C), superficial, flat, circular, medium-dense, rough, fluffy, zonate, raised between margin and center, gray at the margin, white at the center; reverse, zonate, pale gray at the margin, dark gray at the center and zonate.
Material examined: Thailand, Chiang Mai Province, Yang Piang Omkoi, on dead woody twigs of Euphoria longana, 25 August 2019, G.C. Ren, YP03 (MFLU 22-0042), living culture KUMCC 21-0680.
Known distribution: on leaf spot in Archidendron bigeminum (Myanmar), decaying pod of Leucaena sp. (Thailand) [28,33].
Notes: Austropleospora archidendri was introduced by Ariyawansa et al. [92] as a new combination of Paraconiothyrium archidendri based on the combined phylogeny of LSU, SSU, β-tubulin, and ITS sequence data. In the present study, the multi-gene phylogenetic analyses indicated that our new strain, KUMCC 21-0680, formed a sister clade with A. archidendri (MFLUCC 17-2429) with 100% ML bootstrap and 0.95 BYPP support (Figure 1). Since the type species lacks SSU and tef1-α sequences, the nucleotide base pair comparisons of LSU and ITS demonstrated that our new strains are identical to the type of the Austropleospora archidendri strain and other species (Table 1). Our strain, KUMCC 21-0680, is similar to A. archidendri (CBS 168.77, MFLUCC 17-2429) in having doliiform conidiogenous cells and sub-globose-to-ovate, brown, aseptate conidia [28,33]. Austropleospora archidendri was reported as a pathogen on A. bigeminum leaves in Thailand and a saprobe on the pods of a Leucaena sp. in Myanmar [28,33]. Therefore, we report our strain KUMCC 21-0680 as a new record of A. archidendri on woody litter of Euphoria longana in Thailand.
Chromolaenicola Mapook and K.D. Hyde, Fungal Diversity 101: 20 (2020).
Chromolaenicola was introduced in Didymospheriaceae by Mapook et al. [39], with C. nanensis as the type species. Currently, Chromolaenicola comprises six species [67]: Chromolaenicola chiangraiensis, C. clematidis, C. lampangensis, and C. siamensis, which were reported from their asexual morphs, while C. nanensis and C. thailandensis were reported from their sexual morphs. The taxa of Chromolaenicola have only been reported so far from Thailand as saprobes on dead stems of Chromolaena odorata, Clematis subumbellata, and Leucaena sp. [33,39,67]. Here, we introduce a new sexual morph, C. sapindi, from China based on phylogenetic analyses and morphological evidence.
Chromolaenicola sapindi G.C. Ren and K.D. Hyde, sp. nov. Figure 4.
Figure 4.
Chromolaenicola sapindi (HKAS 122789, holotype). (a,b) Appearance of ascomata on host substrate; (c) section of ascoma; (d) peridium; (e) hamathecium; (f–i) asci; (j–o) ascospores; (p) germinated ascospore; (q,r) culture characters on PDA (q from above, r from below). Scale bars, (c) 200 μm; (d,f–i) 50 μm; (e,j–p) 10 μm; (q,r) 30 mm.
Index Fungorum number: IF559806. FacesofFungi number: FoF 10704.
Etymology: The epithet refers to the host genus Sapindus.
Holotype: HKAS 122789.
Saprobic on dead woody twigs of Sapindus rarak. Sexual morph: Ascomata 420–530 µm high × 270–350 µm diam. ( = 480 × 300 µm, n = 5), immersed-to-erumpent, solitary or scattered, coriaceous, ampulliform or obovoid, dark brown. Ostiole central. Peridium 15–25 µm thick, 4–7-layered, comprising pale-brown-to-brown cells of textura angularis. Hamathecium 1.5–3 µm wide, comprising cylindrical, septate, branching pseudoparaphyses, embedded in a hyaline, gelatinous matrix. Asci 125–155 × 12–16 µm ( = 138 × 13 µm, n = 20), bitunicate, 8-spored, cylindrical-clavate, straight, slightly curved at the end, apically rounded, with a pedicel (7–10 µm long). Ascospores 16–23 × 6.5–9.5 µm ( = 18.9 × 8 µm, n = 30), overlapping 1-seriate, ellipsoidal, initially hyaline-to-pale-brown and aseptate or 1-septate, guttulate, becoming reddish-brown-to-brown, and 1-septate at maturity, slightly constricted at the central septum, with or without guttules, thick and smooth-walled, without a gelatinous sheath. Asexual morph: undetermined.
Culture characteristics: The colonies on PDA reached 20–30 mm diam. after 14 days at room temperature (25–30 °C), superficial, circular, umbonate at the center, with dense mycelia, smooth, downy, velvety, fimbriate, white; reverse white at the margin, dark brown at the center.
Material examined: China, Yunnan Province, Lancang, Lahu Autonomous Prefecture, Hani (22°24.381′ N, 100°06.647′ E, elevation 900 m), on dead woody twigs of S. rarak, 23 March 2020, G.C. Ren, LGY32 (HKAS 122789, holotype), ex-type culture KUMCC 21-0564; ibid., LGY33 (HKAS 122876, isotype), living culture KUMCC 21-0594.
Notes: Chromolaenicola sapindi is introduced as a newly discovered species based on its distinct morphology and analysis of a combined SSU, LSU, ITS, and tef1-α dataset. Our samples (KUMCC 21-0564 and KUMCC 21-0594) were clustered with other Chromolaenicola species with 84% ML bootstrap and 0.91 BYPP support (Figure 1). Our species can be distinguished from C. nanensis and C. thailandensis in having 2-celled, guttulate ascospores. Both C. nanensis and C. thailandensis have muriform ascospores with 3-transverse septa and 1-vertical septum when mature [39]. We did not obtain the asexual morph from C. sapindi. Therefore, the morphological comparison between our new species and other Chromolaenicola species known only in their asexual morph was not possible. However, based on the phylogenetic distinctiveness, C. sapindi is introduced as a new species.
Dictyoarthrinium S. Hughes, Mycological Papers 48: 29 (1952).
Dictyoarthrinium was introduced by Hughes [93], with D. quadratum as the type species. The genus is characterized by basauxic conidiogenous cell development, conidiophores that are minutely verruculose, subhyaline and transversely septate, conidiophore mother cells which are often hyaline or pale brown and cup-shaped, and conidia of square-to-spherical, subspherical or oblong, pale-to-dark-brown, often 4-celled, and sometimes 16-celled [34,93,94]. Previous studies have accommodated Dictyoarthrinium in Apiosporaceae, Sordariomycetes [91,95,96]. Subsequent studies transferred Dictyoarthrinium to Didymosphaeriaceae, Dothideomycetes based on morphological and molecular evidence [34,70]. Currently, ten species are accepted in Dictyoarthrinium [26].
Dictyoarthrinium thailandicum G.C. Ren and K.D. Hyde, sp. nov. Figure 5.
Figure 5.
Dictyoarthrinium thailandicum (MFLU 22-0040, holotype). (a,b) Conidia on the host; (c–e) conidia with conidiophores on stalk; (f,g) developmental stage of an immature lateral conidium; (h) four-celled terminal conidium; (i–l) warted four-celled mature conidia; (m) germinated conidia; (n,o) culture characters on PDA. Scale bars, (c,e) 100 μm; (d) 50 μm; (f–h) 15 μm; (i–m) 10 μm; (n,o) 30 mm.
Index Fungorum number: IF559807. FacesofFungi number: FoF 10705.
Etymology: The epithet “thailandicum” refers to Thailand, where the species was first collected.
Holotype: MFLU 22-0040.
Saprobic on dead woody twigs of Castanopsis sp. Sexual morph: undetermined. Asexual morph: Colonies solitary, irregular, black. Mycelium superficial, septate, branched, anastomosing hyphae. Conidiophores 130–220 × 4–5 μm ( = 180 × 4.5 μm, n = 25), erect, macronematous, basauxic, cylindrical, straight or flexuous, sub-hyaline-to-pale-brown, the transverse septa partly brown with distances of 3–7 μm, rough-walled. Conidiophore mother cells 4–4.5 × 3.8–4.1 μm ( = 4.5 × 4 μm, n = 10), cup-shaped, pale brown. Conidiogenous cells 3–7 × 3–5 μm ( = 5 × 4 μm, n = 20), blastic, integrated, terminal and intercalary, cylindrical, sub-hyaline. Conidia 9–11 × 8.5–10.5 μm ( = 10 × 9.7 μm, n = 30), solitary, holoblastic, spherical, 1-celled and sub-hyaline-to-pale-brown when young, cruciate-septate with four cells, constricted at the septa, rounded at the ends, spherical or subspherical, brown-to-dark-brown at maturity, verrucose, mature conidia split along one line of the septa, arising from the lateral or apical part of conidiophores.
Culture characteristics: The colonies on PDA reached 15–20 mm diam. after 14 days at room temperature (25–30 °C), superficial, circular, umbonate at the center, rough surface, with dense mycelia, velvety, flat, and white.
Material examined: Thailand, Chiang Mai Province, Yang Piang Omkoi, on dead woody twigs of Castanopsis sp., 25 August 2019, G.C. Ren, YP01 (MFLU 22-0040, holotype), ex-type culture KUMCC 21-0664; ibid, Tak Province, Moe Wa Luang Tha Song Yang, on dead woody twigs of Castanopsis sp., 17 October 2019, G.C. Ren, TSY02 (MFLU 22-0041, paratype), ex-paratype culture KUMCC 21-0665.
Notes: Dictyoarthrinium thailandicum is introduced as a new species based on its distinct morphology and the phylogeny of the combined SSU, LSU, ITS, tef1-α dataset. This species is phylogenetically distinct from other Dictyoarthrinium species and formed a clade sister to D. musae with 76% ML bootstrap and 1.00 BYPP support (Figure 1). This species is similar to D. musae in having black colonies, cup-shaped conidiophore mother cells, and cylindrical conidiogenous cells. However, the size of the conidiophores and conidia of D. thailandicum (180 × 4.5 µm, 10 × 9.7 μm) is comparatively larger than those of D. musae (81.5 × 1.6 μm, 8.7 × 7.9 µm) [34].
Karstenula Speg., Decades Mycologicae Italicae 7–12: no. 94 (in sched.) (1879).
Karstenula was introduced with K. rhodostoma as the type species [97]. The genus is characterized by globose or sub-globose, black ascomata with flattened apices, and rounded pore-like ostioles. Pseudoparaphyses are cellular and septate; asci are 8-spored, bitunicate, fissitunicate, and cylindrical with short furcate pedicels; ascospores are muriform, ellipsoid-to-fusoid, reddish-brown-to-dark-brown and constricted at the septa [31]. The asexual morph was described by Constantinescu [98] as pycnidial, globose conidioma; enteroblastic, phialidic, determinate, ampulliform-to-doliiform or cylindric-to-ampulliform conidiogenous cells; cylindric, yellow-to-golden-brown conidia with one septate. Twenty-two taxa are listed in species Fungorum [45]; however, molecular data are available only for K. rhodostoma. Herein, we introduced another novel Karstenula species based on morphology and molecular data.
Karstenula lancangensis G.C. Ren and K.D. Hyde, sp. nov. Figure 6.
Figure 6.
Karstenula lancangensis (HKAS 122790, holotype). (a–c) Conidiomata on the natural wood surface; (d,e) sections through conidiomata; (f) conidioma wall; (g) conidiogenous cells and developing conidia; (h–l) conidia; (m) germinated conidium; (n,o) culture characters on PDA. Scale bars, (d) 200 μm; (e) 40 μm; (f) 20 μm; (g) 10 μm; (h–m) 5 μm; (n,o) 30 mm.
Index Fungorum number: IF559808; FacesofFungi number: FoF 10706.
Etymology: The species epithet “lancangensis” refers to Lancang (Yunnan, China) where the species was collected.
Holotype: HKAS 122790.
Saprobic on dead woody twigs of Cinnamomum glanduliferum. Sexual morph: undetermined. Asexual morph: Conidiomata 270–480 µm high × 240–430 µm diam. ( = 410 × 350 µm, n = 5), pycnidial, solitary, immersed, unilocular or bilocular, obpyriform, black conidiomata formed under the bark, with broadly rounded apex, and a broad pore opening. Conidioma wall 30–40 µm wide, 4–6-layered, composed of an outer layer of brown cells and an inner layer of hyaline cells of textura angularis. Conidiophores reduced into conidiogenous cells. Conidiogenous cells 4–6.5 × 4.4–6.4 μm ( = 5.3 × 5.4 µm, n = 15), holoblastic, ampulliform-to-doliiform, determinate, hyaline with conspicuous periclinal thickening. Conidia 8–10 × 3–4 μm ( = 8.8 × 3.6 µm, n = 30), oval-to-ellipsoid, straight, aseptate or 1-septate, initially hyaline, becoming brown, cylindrical at maturity, 1-septate (median), partly dark brown septum at median, not constricted at the septum, apex and base rounded, thick-, and smooth-walled.
Culture characteristics: The colonies on PDA reached 45–50 mm diam. after 14 days at room temperature (25–30 °C), superficial, with sparse mycelia, circular, rough, granular, gray-white; reverse dark brown.
Material examined: China, Yunnan Province, Lancang, Lahu Autonomous Prefecture, Hani (22°24.381′ N, 100°06.647′ E, elevation 900 m), on dead woody twigs of Cinnamomum glanduliferum, 23 March 2020, G.C. Ren, W07 (HKAS 122790, holotype), ex-type culture KUMCC 21-0670; ibid., W08 (HKAS 122888, isotype), living culture KUMCC 21-0677.
Notes: Karstenula lancangensis is introduced as a new species based on its distinct morphology and its phylogenetic position. In the phylogenetic analyses, K. lancangensis formed a sister clade to K. rhodostoma with 100% ML bootstrap and 1.00 BYPP support (Figure 1). Karstenula lancangensis shows similar morphological features to K. rhodostoma in having cylindric, 1-septate, brown conidia. However, the size of the conidia of K. lancangensis (8–10 × 3–4 μm) is comparatively smaller than those of K. rhodostoma (10–) 11–13 (–14) × (4–) 4.5–5 (5.5) µm). In addition, the conidiomata of K. lancangensis are unilocular or bilocular, obpyriform with a broadly rounded apex and broad pore, while they are unilocular, globose with a 100 × 80 μm ostiolate in K. rhodostoma [98].
Montagnula Berl., Icon. fung. (Abellini) 2: 68 (1896).
Montagnula was introduced by Berlese [99], with M. infernalis as the type species. Currently, 39 Montagnula species are accepted [45], with cosmopolitan distribution [27]. The present paper identified five Montagnula isolates from woody plant litter in the GMS. Montagnula species are characterized by globose or spherical, immersed ascomata with a clypeus, claviform asci, and fusoid or ellipsoid ascospores with transverse septa and one or more longitudinal septa [31].
Montagnula donacina (Niessl) Wanas., E.B.G. Jones and K.D. Hyde Index Fungorum 319: 1 (2017) Figure 7.
Figure 7.
donacina (HKAS 122782). (a,b) Appearance of ascomata on host substrate; (c) section of ascoma; (d) ostiolar neck; (e) peridium; (f) hamathecium; (g–j) asci; (k,l) immature ascospores; (m–q) mature ascospores; (r) germinated ascospore; (s,t) culture characters on PDA (s from above, t from below). Scale bars, (c) 200 μm; (d,h–j) 50 μm; (e,g) 20 μm; (f,k–r) 10 μm; (s,t) 30 mm.
≡ Microthelia donacina Niessl, Instituto de Coimbra 28: 366 (1881).
≡ Didymosphaeria donacina (Niessl) Sacc., Syll. fung. (Abellini) 1: 715 (1882).
≡ Didymosphaerella donacina (Niessl) Cooke, Grevillea 18 (no. 86): 29 (1889).
≡ Munkovalsaria donacina (Niessl) Aptroot, Nova Hedwigia 60 (3–4): 346 (1995).
Index Fungorum number: IF552762; FacesofFungi number: FoF 04638.
Saprobic on decaying wood. Sexual morph: Ascomata 320–400 µm high × 350–440 µm diam. ( = 350 × 400 µm, n = 5), immersed-to-erumpent, solitary or scattered, coriaceous, black, with a central ostiole. Ostiole short papillate, 150–190 × 70–90 µm ( = 170 × 80 µm, n = 5), protruding from substratum. Peridium 15–25 µm wide, comprising 4–6 layers of thin-walled, pale-brown-to-brown cells of textura angularis. Hamathecium comprising 1–2 µm wide, hyaline, cylindrical-to-filiform, septate, branching pseudoparaphyses. Asci 70–100 × 10–11 µm ( = 87 × 10.7 µm, n = 15), bitunicate, fissitunicate, 8-spored, elongate-clavate, slightly curved, with a long pedicel (30–50 µm long; = 40 μm, n = 10). Ascospores 14–16 × 4.5–6 µm ( = 14.5 × 5 µm, n = 30), overlapping uni- to bi-seriate, hyaline or yellowish, straight-to-slightly curved, aseptate or 1-septate, guttulate when immature and becoming brown-to-dark-brown when mature, 2-celled, fusiform, rounded ends, 1-septate, constricted at the septum, with slightly pointed upper cell and rounded lower cell, straight to slightly curved, smooth-walled, guttulate, without sheaths or appendages. Asexual morph: undetermined.
Culture characteristics: Colonies on PDA, reaching 90 mm diam. at 14 days at room temperature (25–30 °C), superficial, circular, rough surface, with sparse mycelia, velvety, flat, zonate, white at the margin and center, light brown between margin and center.
Material examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Jinghong, Xishuangbanna Tropical Botanical Garden (21°55.19′ N, 101°15.24′ E), on dead woody twigs of Ehretia acuminata, 4 March 2020, G.C. Ren, JH27 (HKAS 122782), living culture KUMCC 21-0579; Thailand, Chiang Rai Province, Mae Yao District, on dead woody twigs of Betula sp., 23 September 2019, G.C. Ren, MY22 (MFLU 22-0045), living culture KUMCC 21-0631; Thailand, Tak Province, near Mae Jun river and police school Ban Mea Junta, on dead woody twigs of Betula sp., 21 August 2019, G.C. Ren, T404 (MFLU 22-0046), living culture KUMCC 21-0653.
The known hosts are: Acacia reficiens, Acacia sp., Adhatoda vasica, Ailanthus altissima, Annona squamosa, Arundo donax, Bambusoideae sp., Cajanus cajan, Calamus australis, Careya arborea, Citrus aurantiifolia, Clerodendrum infortunatum, C. multiflorum, Coffea arabica, C. robusta, Dioscorea dumetorum, Duranta repens, Ficus glomerata, Funtumia africana, Hibiscus sp., Ipomoea carnea, Lantana camara, Mallotus philippinensis, Morus alba, Nephelium litchi, Nerium odorum, Phyllostachys bambusoides, Pistacia indica, Platanus sp., Premna cumingiana, Pseudosasa japonica, Saccharum officinarum, Strophanthus eminii, Tectona grandis, Terminalia tomentosa, Trachycarpus fortunei, Wikstroemia sp., and Zea mays [27].
The known distribution is: Australia, Brazil, Central African Republic, China, Colombia, France, Georgia, Hawaii, India, Japan, Louisiana, Myanmar, Namibia, Nigeria, Papua New Guinea, Paraguay, Philippines, Portugal, Sierra Leone, and Tanzania [27].
Notes: Wanasinghe et al. [42] synonymized Munkovalsaria donacina and M. appendiculata under Montagnula based on the phylogenetic analyses of the combined LSU, SSU, and ITS sequence data. Generally, M. donacina is characterized by immersed-to-erumpent, single, or gregarious ascomata with a single ostiole, bitunicate, clavate or cylindrical asci with a pedicel and an ocular chamber, ellipsoid, unicellular, 1-septate ascospores strongly constricted at the septum with the upper cell wider and the lower cell rounded [80,100]. The characters of these new isolates (KUMCC 21-0653, KUMCC 21-0579, and KUMCC 21-0631) are similar to M. donacina [80]. The multi-gene phylogenetic analysis based on the combined SSU, LSU, ITS, and tef1-α sequences showed that our collections (KUMCC 21-0653, KUMCC 21-0579, and KUMCC 21-0631) form a monophyletic group with M. thailandica (MFLUCC 17-1508), M. puerensis (KUMCC 20-0225, KUMCC 20-0331), M. donacina (HFG07004, HVVV01), M. chromolaenicola (MFLUCC 17-1469), M. saikhuensis (MFLUCC 16-0315), and M. graminicola (MFLUCC 13-0352). Based on morphological characteristics and phylogenetic analysis, we report our isolations as the first records of M. donacina from decaying wood of E. acuminata and Betula sp. in Thailand. However, our phylogenetic analyses suggest the presence of a possible complex for M. donacina. Hence, extensive studies combining morphology and multi-gene phylogeny of additional samples are necessary.
Spegazzinia Sacc., Michelia 2 (6): 37 (1880).
Spegazzinia was introduced by Saccardo [101] with S. ornata as the type species. Hyde et al. [95] accommodated Spegazzinia in Sordariomycetes (Apiosporaceae), and based on morphological and molecular evidence, Tanaka et al. [73] transferred Spegazzinia to Didymosphaeriaceae in Dothideomycetes. This was supported by Jayasiri et al. [33], Samarakoon et al. [52], and Thambugala et al. [41]. Currently, 14 taxa are listed in Species Fungorum [45]. Spegazzinia is a widely distributed genus with species reported as saprobes on decaying leaves, wood, fruit, and bambusae from Australia, Brazil, China, Cuba, Ghana, and Thailand [38,41,52,102,103,104,105], and endophytes from lichen and leaves in Brazil and India [87,106]. The Spegazzinia species have also been reported from the soil in Congo and estuarine sediment [94,107]. Morphologically, most species of Spegazzinia have two types of conidia in the same mycelium: α conidia are composed of 4–8 subglobose, very dark cells with very long spines, while β conida are subspherical or broadly ellipsoid in general, flattened in one plane, cruciately septate or muriform, almost always pale brown and smooth [38]. This paper introduces two new isolates of the Spegazzinia species observed from decaying wood in terrestrial habitats in China and Thailand.
Spegazzinia jinghaensis G.C. Ren and K.D. Hyde, sp. nov. Figure 8.
Figure 8.
Spegazzinia jinghaensis (HKAS 122787, holotype). (a,b) Fungal colonies on the host surface; (c–e) conidiophore of α conidia and α conidia; (f,g) α conidia; (h–l) β conidia; (m,n) germinated conidia (m α conidium, n β conidium); (o,p) culture characters on PDA. Scale bars, (c–e) 20 μm; (f,g) 15 μm; (h–l) 10 μm; (m,n) 20 μm; (o,p) 30 mm.
Index Fungorum number: IF559809; FacesofFungi number: FoF 10707.
Etymology: The species epithet “jinghaensis” refers to Jingha (Yunnan, China), the location where the holotype was collected.
Holotype: HKAS 122787.
Saprobic on dead woody twigs Myristica yunnanensis. Sexual morph: undetermined. Asexual morph: Hyphomycetous. Sporodochia dark, dense, dry, powdery, velvety, 2–3 mm in diameter. Conidiogenous cells basauxic, ampulate, 5–6 μm high × 4–5 μm wide ( = 5.5 × 4.5 μm; n = 10), subspherical, hyaline-to-light-brown. Conidiophores of α conidia up to 80–120 × 1.4–2.0 μm ( = 100 × 1.7 μm, n = 10), erect or flexuous, unbranched, dark brown. Conidiophores of β conidia 3.5–8 × 2.5–3.5 μm ( = 5.2 × 3 μm, n = 10) short, erect, unbranched, sub-hyaline or light brown. α conidia 16–20 × 15–19 μm ( = 17.9 × 17.5 μm; n = 20), 4-celled, stellate-shaped, brown-to-dark-brown, each cell globose to subglobose with dark brown warts on the surface of the cells, conspicuous spines 3.5–8 × 1–2 μm ( = 6 × 1.4 μm; n = 15), deeply constricted at the septa. β conida 12–16 × 13–17.5 μm ( = 14.3 × 15 μm; n = 30), 4-celled, disc-shaped, quadrangular or subspherical, initially pale brown, becoming brown-to-dark-brown at maturity, each cell turbinate, crossed septate, the cross-septate partly brown, smooth to verrucose, sometimes cells have raised verrucose around their edges, deeply constricted at the septa, flat from the side view, frequently with attached conidiogenous cells when splitting from the conidiophores.
Culture characteristics: The colonies on PDA reached 30–40 mm diam. at 14 days at room temperature (25–30 °C), superficial, circular, rough surface, gray on the base, with sparse white mycelia on the surface; reverse black.
Material examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Jinghong, Jingha (21°78.06′ N, 101°05.61′ E), on dead woody twigs of Myristica yunnanensis, 4 March 2020, G. C. Ren, JHD24 (HKAS 122787, holotype), ex-type culture, KUMCC 21-0495; ibid., JHD25 (HKAS 122878, isotype), living culture, KUMCC 21-0496.
Notes: Spegazzinia jinghaensis is introduced as a new species based on its distinct morphology and the combined phylogeny of SSU, LSU, ITS, and tef1-α. In the phylogenetic analyses, S. jinghaensis is distinct from other sequenced species within this genus and closely related to S. bromeliacearum (URM 8084) and S. intermedia (CBS 249.89) with strong statistical support (78% ML bootstrap and 1.00 BYPP, Figure 1). Spegazzinia jinghaensis differs from S. bromeliacearum and S. intermedia in having two types of conidia (stellate-shaped conidia: 17.9 × 17.5 μm and disc-shaped conidia: 14.3 × 15 μm). In contrast, S. bromeliacearum has globose conidia (26.5–28 μm diam.) with spines, and S. intermedia has disc-shaped conidia (18–28 μm diam.), which are dentate at the margin [87,108].
4. Discussion
Didymosphaeriaceae contains a wide range of taxa occurring on diverse hosts worldwide [26,29,81]. It has been relatively well-studied in recent years, and numerous genera and species are accepted in this family based on phylogenetic studies [30,33,34,42,63,67,77,78,85,109]. Out of 33 genera, Kalmusia, Montagnula, Paraphaeosphaeria, Paraconiothyrium, Phaeodothis, Pseudocamarosporium, Pseudopithomyces, and Spegazzinia are well studied compared to other genera in Didymosphaeriaceae, but many species are likely awaiting discovery [77]. Barria, Cylindroaseptospora, Kalmusibambusa, Lineostroma, Neptunomyces, Vicosamyces, and Xenocamarosporium are still monotypic [45], and new species discovery is expected [77]. In this study, we added taxonomic novelties from the GMS to better understand the morphological and phylogenetic relationships of Didymosphaeriaceae.
Septofusispora, typified by S. thailandica, is introduced to accommodate terrestrial dothideomycetes species with a characteristic morphology compared to the extant genera (Alloconiothyrium, Kalmusibambusa, Kalmusia, and Xenocamarosporium) in Didymosphaeriaceae. This genus is characterized by its clavate asci, fusiform, guttulate ascospores with 4–5 transverse septa, whereas Kalmusia has ovoid-to-clavate, 3-septate ascospores (sometimes muriform), with a mucilaginous sheath. Kalmusibambusa differs from Septofusispora by having ellipsoidal-to-fusiform, 3-septate ascospores with a wide mucilaginous sheath [41,81,90,91]. Alloconiothyrium and Xenocamarosporium are known only from their asexual morphs [28,63]; therefore, they cannot be morphologically compared with the teleomorph of Septofusispora. The phylogenetic analyses showed that Septofusispora is distinctly separated from its closely related taxa in this family. Therefore, based on morphological characters and the SSU, LSU, ITS and tef1-α sequence data, we recognize Septofusispora as a new genus in the family Didymosphaeriaceae.
Alloconiothyrium was introduced by Verkley and coauthors [28] with A. aptrootii as the type species, which is characterized by having pycnidial or eustromatic conidiomata, holoblastic, annellidic conidiogenous cells, olivaceous-brown and irregularly outlined conidia with a rough surface [28]. However, Ariyawansa and coauthors [63] introduced Alloconiothyrium camelliae as a new species with uni-loculate, globose-to-subglobose conidiomata, ampulliform-to-doliiform or cylindrical conidiogenous cells and smooth-walled conidia. Furthermore, the multi-gene phylogenies of Ariyawansa and coauthors [63] and our multi-gene phylogenies show that Alloconiothyrium aptrootii is well-separated from A. camelliae. Therefore, we suggest that A. camelliae is a monotypic genus of Didymosphaeriaceae. Further studies are needed for a better understanding of the morphological and phylogenetic relationships of Alloconiothyrium.
Karstenula is an ambiguous genus that exhibits morphological similarities with different families [29,31]. Usually, the sexual morph of Karstenula was thought to be characterized by having cylindrical or clavate asci, and brown ascospores with transverse septa and sparse longitudinal septate as dominant characters [31]. For instance, Karstenula adenocarpi has oblong ascospores with three transverse septa and 1–several longitudinal septa [110]; Karstenula calligoni has clavate asci, and fusoid ascospores with 5–7 transverse septa and a longitudinal septum [111]; Karstenula guttulata has cylindrical asci, and ellipsoid, oblong-to-oval ascospores with 4–6 transverse septa and 1–2 longitudinal septa [112]; Karstenula rhodostoma has cylindrical asci, and ellipsoid ascospores with three transverse septa and a vertical septum in one or two central cells [31]. As mentioned by Constantinescu [98], the anamorph of Karstenula rhodostoma is identical to the coelomycete Microdiplodia frangulae, characterized by ampulliform-to-doliiform or cylindric-to-ampulliform conidiogenous cells, and cylindrical, yellow-to-golden-brown conidia with one septum. Karstenula lancangensis is similar to the asexual morph of Karstenula rhodostoma in having cylindric, 1-septate, brown conidia. However, in our phylogenetic analysis, Karstenula lancangensis forms a well-supported clade sister to K. rhodostoma in Didymosphaeriaceae (Figure 1). Our study provides a reference for further understanding the asexual morphology of Karstenula.
Montagnula donacina is a prevalent species distributed almost all over the world. It has been isolated from 38 plant species within 24 families [27,80], from which 20 hosts have been from India. However, M. donacina has been rarely reported in the GMS, with only two hosts (Althaea rosea and Trachycarpus fortunei) recorded from China and one host (Nephelium litchi) from Myanmar [100,113,114]. The present study reports two M. donacina collections from the hosts E. acuminata and Betula sp. in China and Thailand (the first report of this species in this country). The morphological comparisons between species of Montagnula, putatively related to M. donacina (Table 2), showed that the ascospores of M. graminicola are light brown, verruculose, and have a mucilaginous sheath without guttules. In contrast, all the other species in this clade have no significant apomorphic morphological traits, except for slight differences in the size of ascomata, asci, or ascospores (Table 2), which can be due to ecological factors [115]. In addition, as mentioned in the notes of M. donacina, these species are not significantly distinct in phylogeny. Therefore, based on the current morphological data and phylogenetic analyses, we suggest that M. chromolaenicola, M. puerensis, M. saikhuensis, and M. thailandica can be considered conspecific with M. donacina. However, even though M. donacina is widely reported from different hosts, molecular data from only two collections are available in GenBank. Therefore, more extensive studies are needed, applying a combination of different species delimitation criteria to more sequence data obtained from additional samples [116,117] in order to resolve and define the species boundaries in the Montagnula donacina complex. Finally, Montagnula jonesii was introduced by Tennakoon et al. [49] based on morphology coupled with the analysis of the combined LSU, SSU, ITS, and tef1-α sequence data. Despite the fact that our phylogenetic tree based on multigene data showed that Montagnula jonesii is not monophyletic with the taxa in Montagnula s. str., single-gene analyses (not shown) showed that LSU, SSU, and tef1-α data support that M. jonesii belongs in Montagnula, and only ITS suggests a different placement. Therefore, we believe that the published ITS sequence of M. jonesii may be incorrect and needs to be conducted again.
Table 2.
Synopsis of the morphological characteristics of Montagnula species.
| Species Name | Ascomata | Asci | Ascospores | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Color | Shape | Size | Rows of Ascospores in Asci | Septation | Surface | ||||
| M. chromolaenicola | Uni-loculate, 310 × 275 μm diam. | 90 × 12 μm, bitunicate, elongate-clavate, 8-spored, long pedicellate | Brown to dark brown | Broadly fusiform to ellipsoid |
15.5 × 6 μm | Overlapping 1–2-seriate |
1 | Guttulate | [39] |
| M. donacina | Multi-loculate, 500 μm diam. |
90–100 × 12–13 μm, bitunicate, clavate, 8-spored, long pedicellate | Brown | Ellipsoid | 14.8–15.2 × 7.5–7.7 μm | Irregularly biseriate | 1 | Guttulate | [80,100] |
| M. donacina (HKAS 122778) | Uni-loculate, 490 × 410 µm diam. |
110 × 13 µm, bitunicate, elongate-clavate, slightly curved, 8-spored, long pedicellate |
Pale brown to brown | Broadly fusiform | 15 × 5 µm | Overlapping 1–2-seriate |
1 | Guttulate | This study |
|
M. donacina
graminicola |
Uni-loculate, 37–117.22 μm diam. |
81.3 × 10.1 μm, bitunicate, cylindrical to clavate, 8-spored, long pedicellate | Brown | Ellipsoid | 11.3 × 4.9 μm | Biseriate | 1 | Verruculose, mucilaginous sheath |
[81] |
| M. puerensis | Uni-loculate, 300–600 × 230–380 μm diam. |
92 × 11 μm, bitunicate, elongate-clavate, 8-spored, long, furcate pedicellate |
Brown to dark brown |
Ellipsoid | 14 × 6 μm | Biseriate | 1 | Guttulate | [83] |
| M. saikhuensis | Uni-loculate, 411.7 × 460.5 μm diam. |
84.2 × 11.2 μm, bitunicate, elongate-clavate to short cylindrical, 8-spored, long pedicellate | Brown to blackish |
Ellipsoid | 14.6 × 5.1 μm | Overlapping 1–2-seriate | 1 | Guttulate | [42] |
| M. thailandica | Uni-loculate, 380 × 340 μm diam. |
90 × 11 μm, bitunicate, elongate-clavate, slightly curved, 8-spored, long pedicellate |
Brown to reddish-brown |
Broadly fusiform to ellipsoid |
15 × 5.5 μm | Overlapping 1–2-seriate |
1 | Guttulate | [39] |
Acknowledgments
We acknowledge the Molecular Biology Experiment Center, Germplasm Bank of Wild Species in Southwest China (Kunming Institute of Botany, Chinese Academy of Sciences) for providing the laboratories and instruments for molecular work.
Author Contributions
Conceptualization, G.R., H.G., A.R.G.d.F. and K.W.T.C.; resources, K.D.H., J.X. and H.G.; writing—original draft preparation, G.R.; writing—review and editing, D.N.W., A.R.G.d.F., E.Y., A.B., K.D.H. and K.W.T.C.; supervision, A.R.G.d.F. and K.W.T.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study can be found in the GenBank, NCBI and the accession numbers are given in Table 1. Newly introduced fungal names were registered at the Index Fungorum and the identification numbers are shown in their respective entries.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Science Foundation of China (Grant No.: 32001296), the Youth Innovation Promotion Association of CAS, China (Grant No.: 2022396), the Thailand Research Fund (Grant No. RDG6130001), the High-End Foreign Experts in the High-Level Talent 318 Recruitment Plan of Yunnan Province, 2021, CAS President’s International Fellowship Initiative (Grant No. 2021FYB0005), the National Science Foundation of China (NSFC) (Grant No. 32150410362), the Postdoctoral Fund from Human Resources and Social Security Bureau of Yun-nan Province, the Onsite Visiting Scholars for World Class Research Collaboration Programme under the Reinventing University System Project sponsored by the Ministry of Higher Education, Science, Research, and Innovation, Thailand, and the National Research Council of Thailand—NRCT (Grant No. N42A650547).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hapuarachchi K.K., Karunarathna S.C., Phengsintham P., Yang H.D., Kakumyan P., Hyde K.D., Wen T.C. Ganodermataceae (Polyporales): Diversity in Greater Mekong Subregion countries (China Laos Myanmar Thailand and Vietnam) Mycosphere. 2019;10:221–309. doi: 10.5943/mycosphere/10/1/6. [DOI] [Google Scholar]
- 2.Costenbader J., Varns T., Vidal A., Stanley L., Broadhead J. Drivers of Deforestation in the Greater Mekong Subregion Regional Report. USAID Lowering Emissions in Asia’s Forests (USAID LEAF); Bangkok, Thailand: 2015. pp. 1–38. [DOI] [Google Scholar]
- 3.Li H., Guo J., Karunarathna S.C., Ye L., Xu J., Hyde K.D., Mortimer P.E. Native forests have a higher diversity of macrofungi than comparable plantation forests in the Greater Mekong Subregion. Forests. 2018;9:402. doi: 10.3390/f9070402. [DOI] [Google Scholar]
- 4.Feng B., Yang Z. Studies on diversity of higher fungi in Yunnan southwestern China: A review. Plant Divers. 2018;40:165–171. doi: 10.1016/j.pld.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Z., Hyde K.D., Bhat D.J., Jeewon R., Maharachchikumbura S.S.N., Bao D.F., Li W.L., Su X.J., Yang X.Y., Su H.Y. Morphological and molecular taxonomy of novel species Pleurotheciaceae from freshwater habitats in Yunnan China. Mycol. Prog. 2018;17:511–530. doi: 10.1007/s11557-018-1377-6. [DOI] [Google Scholar]
- 6.Ye L., Li H., Mortimer P.E., Xu J., Gui H., Karunarathna S.C., Kumar A., Hyde K.D., Shi L. Substrate preference determines macrofungal biogeography in the Greater Mekong sub-region. Forests. 2019;10:824. doi: 10.3390/f10100824. [DOI] [Google Scholar]
- 7.Dong W., Wang B., Hyde K.D., McKenzie E.H.C., Raja H.A., Tanaka K., Abdel-Wahab M.A., Abdel-Aziz F.A., Doilom M., Phookamsak R., et al. Freshwater Dothideomycetes. Fungal Divers. 2020;105:319–575. doi: 10.1007/s13225-020-00463-5. [DOI] [Google Scholar]
- 8.Chaiwan N., Tibpromma S., Jayawardena R.S., Mapook A., Wanasinghe D.N., Mortimer P.E., Lumyong S., Hyde K.D. Colletotrichum dracaenigenum, a new species on Dracaena fragrans. Phytotaxa. 2021;491:143–157. doi: 10.11646/phytotaxa.491.2.4. [DOI] [Google Scholar]
- 9.Hyde K.D., Norphanphoun C., Chen J., Dissanayake A.J., Doilom M., Hongsanan S., Jayawardena R.S., Jeewon R., Perera R.H., Thongbai B., et al. Thailand’s amazing diversity—Up to 96 % of fungi in northern Thailand are novel. Fungal Divers. 2018;93:215–239. doi: 10.1007/s13225-018-0415-7. [DOI] [Google Scholar]
- 10.Kodsueb R., McKenzie E.H.C., Lumyong S., Hyde K.D. Diversity of saprobic fungi on Magnoliaceae. Fungal Divers. 2008;30:37–53. [Google Scholar]
- 11.Kodsueb R., McKenzie E.H.C., Lumyong S., Hyde K.D. Fungal succession on woody litter of Magnolia liliifera (Magnoliaceae) Fungal Divers. 2008;30:55–72. [Google Scholar]
- 12.Seephueak P., Phongpaichit S., Hyde K.D., Petcharat V. Diversity of saprobic fungi on decaying branch litter of the rubber tree (Hevea brasiliensis) Mycosphere. 2011;2:307–330. [Google Scholar]
- 13.Monkai J., Boonmee S., Ren G.C., Wei D.P., Phookamsak R., Mortimer P.E. Distoseptispora hydei sp. nov. (Distoseptisporaceae) a novel lignicolous fungus on decaying bamboo in Thailand. Phytotaxa. 2020;459:093–107. doi: 10.11646/phytotaxa.459.2.1. [DOI] [Google Scholar]
- 14.Ren G.C., Wanasinghe D.N., Wei D.P., Monkai J., Yasanthika E., Gui H., Mortimer P.E., Xu J.C., Hyde K.D. Loculosulcatispora thailandica gen. et sp. nov. (Sulcatisporaceae) saprobic on woody litter in Thailand. Phytotaxa. 2020;475:067–078. doi: 10.11646/phytotaxa.475.2.1. [DOI] [Google Scholar]
- 15.Ren G.C., Wanasinghe D.N., Monkai J., Hyde K.D., Mortimer P.E., Xu J.C., Pang A., Gui H. Introduction of Neolophiotrema xiaokongense gen. et sp. nov. to the poorly represented Anteagloniaceae (Pleosporales, Dothideomycetes) Phytotaxa. 2021;482:25–35. doi: 10.11646/phytotaxa.482.1.3. [DOI] [Google Scholar]
- 16.Ren G.C., Wanasinghe D.N., Monkai J., Mortimer P.E., Hyde K.D., Xu J.C., Pang A., Gui H. Novel saprobic Hermatomyces species (Hermatomycetaceae Pleosporales) from China (Yunnan Province) and Thailand. MycoKeys. 2021;82:57–79. doi: 10.3897/mycokeys.82.67973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren G.C., Wanasinghe D.N., Jeewon R., Monkai J., Mortimer P.E., Hyde K.D., Xu J.C., Gui H. Taxonomy and phylogeny of the novel rhytidhysteron-like collections in the Greater Mekong Subregion. MycoKeys. 2021;86:65–85. doi: 10.3897/mycokeys.86.70668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanasinghe D.N., Wijayawardene N.N., Xu J.C., Cheewangkoon R., Mortimer P.E. Taxonomic novelties in Magnolia-associated pleosporalean fungi in the Kunming Botanical Gardens (Yunnan China) PLoS ONE. 2020;15:e0235855. doi: 10.1371/journal.pone.0235855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wanasinghe D.N., Mortimer P.E., Xu J. Insight into the systematics of microfungi colonizing dead woody twigs of Dodonaea viscosa in Honghe (China) J. Fungi. 2021;7:180. doi: 10.3390/jof7030180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanasinghe D.N., Ren G.C., Xu J.C., Cheewangkoon R., Mortimer P.E. Insight into the Taxonomic Resolution of the Pleosporalean Species Associated with Dead Woody Litter in Natural Forests from Yunnan, China. J. Fungi. 2022;8:375. doi: 10.3390/jof8040375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortimer P.E., Jeewon R., Xu J.C., Lumyong S., Wanasinghe D.N. Morpho-phylo taxonomy of novel dothideomycetous fungi associated with dead woody twigs in Yunnan Province China. Front. Microbiol. 2021;12:654683. doi: 10.3389/fmicb.2021.654683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabon M.S., Jones E.B.G., Boonmee S., Doilom M., Lumyong S., Hyde K.D. Five novel freshwater ascomycetes indicate high undiscovered diversity in lotic habitats in Thailand. J. Fungi. 2021;7:117. doi: 10.3390/jof7020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabon M.S., Hyde K.D., Jones E.B.G., Luo Z.L., Dong W., Hurdeal V.G., Gentekaki E., Rossi W., Leonardi M., Thiyagaraja V., et al. Freshwater fungal numbers. Fungal Divers. 2022;114:3–235. doi: 10.1007/s13225-022-00503-2. [DOI] [Google Scholar]
- 24.Munk A. The system of the pyrenomycetes. A contribution to a natural classification of the group Sphaeriales sensu Lindau. Dan. Bot. Ark. 1953;15:1–163. [Google Scholar]
- 25.Wijayawardene N.N., Hyde K.D., Dai D.Q., Sánchez-García M., Goto B.T., Saxena R.K., Erdoğdu M., Selçuk F., Rajeshkumar K.C., Aptroot A., et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere. 2022;13:53–453. doi: 10.5943/mycosphere/13/1/2. [DOI] [Google Scholar]
- 26.Maharachchikumbura S.S.N., Wanasinghe D.N., Cheewangkoon R., Al-Sadi A.M. Uncovering the hidden taxonomic diversity of fungi in Oman. Fungal Divers. 2021;106:229–268. doi: 10.1007/s13225-020-00467-1. [DOI] [Google Scholar]
- 27.Farr D.F., Rossman A.Y. Fungal Databases U.S. National Fungus Collections ARS USDA. [(accessed on 20 July 2022)]; Available online: http://nt.ars-grin.gov/fungaldatabases/
- 28.Verkley G.J.M., Dukik K., Renfurm R., Göker M., Stielow J.B. Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota) Persoonia. 2014;32:25–51. doi: 10.3767/003158514X679191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hongsanan S., Hyde K.D., Phookamsak R., Wanasinghe D.N., McKenzie E.H.C., Sarma V.V., Boonmee S., Lücking R., Bhat D.J., Liu N.G., et al. Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere. 2020;11:1553–2107. doi: 10.5943/mycosphere/11/1/13. [DOI] [Google Scholar]
- 30.Dissanayake L.S., Wijayawardene N.N., Samarakoon M.C., Hyde K.D., Kang J.C. The taxonomy and phylogeny of Austropleospora ochracea sp. nov. (Didymosphaeriaceae) from Guizhou China. Phytotaxa. 2021;491:217–229. doi: 10.11646/phytotaxa.491.3.2. [DOI] [Google Scholar]
- 31.Ariyawansa H.A., Tanaka K., Thambugala K.M., Phookamsak R., Tian Q., Camporesi E., Hongsanan S., Monkai J., Wanasinghe D.N., Mapook A., et al. A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae) Fungal Divers. 2014;68:69–104. doi: 10.1007/s13225-014-0305-6. [DOI] [Google Scholar]
- 32.Wijayawardene N.N., Hyde K.D., Al-Ani L.K.T., Tedersoo L., Haelewaters D., Rajeshkumar K.C., Zhao R.L., Aptroot A., Leontyev D.V., Saxena R.K., et al. Outline of Fungi and fungilike taxa. Mycosphere. 2020;11:1060–1456. doi: 10.5943/mycosphere/11/1/8. [DOI] [Google Scholar]
- 33.Jayasiri S.C., Hyde K.D., Jones E.B.G., McKenzie E.H.C., Jeewon R., Phillips A.J.L., Bhat D.J., Wanasinghe D.N., Liu J.K., Lu Y.Z., et al. Diversity morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere. 2019;10:1–186. doi: 10.5943/mycosphere/10/1/1. [DOI] [Google Scholar]
- 34.Samarakoon B.C., Wanasinghe D.N., Samarakoon M.C., Phookamsak R., McKenzie E.H.C., Chomnunti P., Hyde K.D., Lumyong S., Karunarathna S.C. Multi-gene phylogenetic evidence suggests Dictyoarthrinium belongs in Didymosphaeriaceae (Pleosporales, Dothideomycetes) and Dictyoarthrinium musae sp. nov. on Musa from Thailand. MycoKeys. 2020;71:101–118. doi: 10.3897/mycokeys.71.55493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crous P.W., Schumacher R.K., Wingfeld M.J., Lombard L., Giraldo A., Christensen M., Gardiennet A., Nakashima C., Pereira O., Smith A.J., et al. Fungal systematics and evolution: FUSE 1. Sydowia. 2015;67:81–118. doi: 10.12905/0380. [DOI] [Google Scholar]
- 36.Wijayawardene N.N., Hyde K.D., Bhat D.J., Camporesi E., Schumacher R.K., Chethana K.W.T., Wikee S., Bahkali A.H., Wang Y. Camarosporium-like species are polyphyletic in Pleosporales; introducing Paracamarosporium and Pseudocamarosporiumgen. nov. in Montagnulaceae. Cryptogam. Mycol. 2014;35:177–198. doi: 10.7872/crym.v35.iss2.2014.177. [DOI] [Google Scholar]
- 37.Ariyawansa H.A., Thambugala K.M., Manamgoda D.S., Jayawardena R., Camporesi E., Boonmee S., Wanasinghe D.N., Phookamsak R., Hongsanan S., Singtripop C., et al. Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers. 2015;71:85–139. doi: 10.1007/s13225-015-0323-z. [DOI] [Google Scholar]
- 38.Mena-Portales J., Cantillo-Pérez T., Minter D.W. A new species of the conidial fungal genus Spegazzinia (Pleosporales Didymosphaeriaceae) collected on sugarcane in Cuba. Phytotaxa. 2017;331:295–298. doi: 10.11646/phytotaxa.331.2.14. [DOI] [Google Scholar]
- 39.Mapook A., Hyde K.D., McKenzie E.H.C., Jones E.B.G., Bhat D.J., Jeewon R., Stadler M., Samarakoon M.C., Malaithong M., Tanunchai B., et al. Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed) Fungal Divers. 2020;101:1–175. doi: 10.1007/s13225-020-00444-8. [DOI] [Google Scholar]
- 40.Ariyawansa H.A., Camporesi E., Thambugala K.M., Mapook A., Kang J.C., Alias S.A., Chukeatirote E., Thines M., McKenzie E.H.C., Hyde K.D. Confusion surrounding Didymosphaeria–phylogenetic and morphological evidence suggest Didymosphaeriaceae is not a distinct family. Phytotaxa. 2014;176:102–119. doi: 10.11646/phytotaxa.176.1.12. [DOI] [Google Scholar]
- 41.Thambugala K.M., Wanasinghe D.N., Phillips A.J.L., Camporesi E., Bulgakov T.S., Phukhamsakda C., Ariyawansa H.A., Goonasekara I.D., Phookamsak R., Dissanayake A., et al. Mycosphere notes 1–50: Grass (Poaceae) inhabiting Dothideomycetes. Mycosphere. 2017;8:697–796. doi: 10.5943/mycosphere/8/4/13. [DOI] [Google Scholar]
- 42.Wanasinghe D.N., Jones E.G., Camporesi E., Dissanayake A.J., Kamolhan S., Mortimer P.E., Xu J.C., Hyde K.D. Taxonomy and phylogeny of Laburnicola gen. nov. and Paramassariosphaeria gen. nov. (Didymosphaeriaceae, Massarineae Pleosporales) Fungal Biol. 2016;120:1354–1373. doi: 10.1016/j.funbio.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Senanayake I.C., Rathnayaka A.R., Marasinghe D.S., Calabon M.S., Gentekaki E., Lee H.B., Hurdeal V.G., Pem D., Dissanayake L.S., Wijesinghe S.N., et al. Morphological approaches in studying fungi: Collection examination isolation sporulation and preservation. Mycosphere. 2020;11:2678–2754. doi: 10.5943/mycosphere/11/1/20. [DOI] [Google Scholar]
- 44.Jayasiri S.C., Hyde K.D., Ariyawansa H.A., Bhat J., Buyck B., Cai L., Dai Y.C., Abd-Elsalam K.A., Ertz D., Hidayat I., et al. The Faces of Fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 2015;74:3–18. doi: 10.1007/s13225-015-0351-8. [DOI] [Google Scholar]
- 45.Index Fungorum. [(accessed on 1 August 2022)]. Available online: http://www.indexfungorum.org/names/Names.asp.
- 46.White T.J., Bruns T., Lee S., Taylor J.W. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego, CA, USA: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [DOI] [Google Scholar]
- 47.Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- 49.Tennakoon D.S., Hyde K.D., Wanasinghe D.N., Bahkali A.H., Camporesi E., Khan S., Phookamsak R. Taxonomy and phylogenetic appraisal of Montagnula jonesii sp. nov. (Didymosphaeriaceae, Pleosporales) Mycosphere. 2016;7:1346–1356. doi: 10.5943/mycosphere/7/9/8. [DOI] [Google Scholar]
- 50.Dissanayake A.J., Bhunjun C.S., Maharachchikumbura S.S.N., Liu J.K. Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere. 2020;11:2652–2676. doi: 10.5943/mycosphere/11/1/18. [DOI] [Google Scholar]
- 51.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 52.Samarakoon B.C., Phookamsak R., Wanasinghe D.N., Chomnunti P., Hyde K.D., McKenzie E.H.C., Promputtha I., Xu J.C., Li Y.J. Taxonomy and phylogenetic appraisal of Spegazzinia musae sp. nov. and S. deightonii (Didymosphaeriaceae Pleosporales) on Musaceae from Thailand. MycoKeys. 2020;70:19–37. doi: 10.3897/mycokeys.70.52043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: Multiple sequence alignment interactive sequence choice and visualization. Brief. Bioinform. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller M.A., Pfeiffer W., Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; Proceedings of the 2010 Gateway Computing Environments Workshop (GCE); New Orleans, LA, USA. 14 November 2010; pp. 1–8. [DOI] [Google Scholar]
- 55.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hillis D.M., Bull J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993;42:182–192. doi: 10.1093/sysbio/42.2.182. [DOI] [Google Scholar]
- 57.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rannala B., Yang Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996;43:304–311. doi: 10.1007/BF02338839. [DOI] [PubMed] [Google Scholar]
- 59.Zhaxybayeva O., Gogarten J.P. Bootstrap, Bayesian probability and maximum likelihood mapping: Exploring new tools for comparative genome analyses. BMC Genom. 2002;3:4. doi: 10.1186/1471-2164-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nylander J.A.A., Wilgenbusch J.C., Warren D.L., Swofford D.L. AWTY: A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
- 61.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 62.Rambaut A. FigTree Version 1.4.0. 2012. [(accessed on 30 May 2022)]. Available online: http://tree.bio.ed.ac.uk/software/figtree.
- 63.Ariyawansa H.A., Tsai I., Thambugala K.M., Chuang W.Y., Lin S.R., Hozzein W.N., Cheewangkoon R. Species diversity of Pleosporalean taxa associated with Camellia sinensis (L.) Kuntze in Taiwan. Sci. Rep. 2020;10:12762. doi: 10.1038/s41598-020-69718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morin L., Shivas R.G., Piper M.C., Tan Y.P. Austropleospora osteospermi gen. et sp. nov. and its host specificity and distribution on Chrysanthemoides monilifera ssp. rotundata in Australia. Fungal Divers. 2010;40:65–74. doi: 10.1007/s13225-009-0007-7. [DOI] [Google Scholar]
- 65.Adamčík S., Cai L., Chakraborty D., Chen X.H., Cotter H.V.T., Dai D.Q., Dai Y.C., Das K., Deng C.Y., Ghobad-Nejhad M., et al. Fungal biodiversity profiles 1–10. Cryptogamie Mycologie. 2015;36:121–166. doi: 10.7872/crym/v36.iss2.2015.121. [DOI] [Google Scholar]
- 66.Lumbsch H.T., Hindemith R. Major lineages of Dothideomycetes (Ascomycota) inferred from SSU and LSU rDNA sequences. Mycol. Res. 2001;105:901–908. doi: 10.1016/S0953-7562(08)61945-0. [DOI] [Google Scholar]
- 67.Phukhamsakda C., McKenzie E.H.C., Phillips A.J.L., Jones E.B.G., Bhat D.J., Marc S., Bhunjun C.S., Wanasinghe D.N., Thongbai B., Camporesi E., et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020;102:1–203. doi: 10.1007/s13225-020-00448-4. [DOI] [Google Scholar]
- 68.Ariyawansa H.A., Maharachchikumbura S.S.N., Karunarathne S.C., Chukeatirote E., Bahkali A.H., Kang J.C., Bhat J.B., Hyde K.D. Deniquelata barringtoniae gen. et sp. nov. associated with leaf spots of Barringtonia asiatica. Phytotaxa. 2013;105:11–20. doi: 10.11646/phytotaxa.105.1.2. [DOI] [Google Scholar]
- 69.Devadatha B., Sarma V.V., Ariyawansa H.A., Gareth J.E.B. Deniquelata vittalii sp.nov. a novel indian saprobic marine fungus on Suaeda monoica and two new records of marine fungi from Muthupet mangroves East coast of India. Mycosphere. 2018;9:565–582. doi: 10.5943/mycosphere/9/3/8. [DOI] [Google Scholar]
- 70.Vu D., Groenewald M., De Vries M., Gehrmann T., Stielow B., Eberhardt U., Al-Hatmi A., Groenewald J.Z., Cardinali G., Houbraken J., et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019;92:135–154. doi: 10.1016/j.simyco.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thambugala K.M., Hyde K.D., Tanaka K., Tian Q., Wanasinghe D.N., Ariyawansa H.A., Jayasiri S.C., Boonmee S., Camporesi E., Hashimoto A., et al. Towards a natural classifcation and backbone tree for Lophiostomataceae Floricolaceae and Amorosiaceae fam. nov. Fungal Divers. 2015;74:199–266. doi: 10.1007/s13225-015-0348-3. [DOI] [Google Scholar]
- 72.Zhang Y., Zhang J., Wang Z., Fournier J., Crous P.W., Zhang X., Li W., Hyde K.D. Neotypification and phylogeny of Kalmusia. Phytotaxa. 2014;176:164–173. doi: 10.11646/phytotaxa.176.1.16. [DOI] [Google Scholar]
- 73.Tanaka K., Hirayama K., Yonezawa H., Sato G., Toriyabe A., Kudo H., Hashimoto A., Matsumura M., Harada Y., Kurihara Y., et al. Revision of the Massarineae (Pleosporales, Dothideomycetes) Stud. Mycol. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crous P.W., Wingfield M.J., Chooi Y.H., Gilchrist C.L.M., Lacey E., Pitt J.I., Roets F., Swart W.J., Cano-Lira J.F., Valenzuela-Lopez N., et al. Fungal planet description sheets: 1042–1111. Persoonia. 2020;44:301–459. doi: 10.3767/persoonia.2020.44.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aptroot A. Two new ascomycetes with long gelatinous appendages collected from monocots in the tropics. Stud. Mycol. 2004;50:307–311. [Google Scholar]
- 76.Hongsanan S., Hyde K.D., Bahkall A.H., Camporesi B.E., Chomnunti P., Ekanayaka H., Gomes A.A.M., Hofstetter V., Jones E.B.G., Pinho D.B., et al. Fungal biodiversity profiles 11–20. Cryptogam. Mycol. 2015;36:355–380. doi: 10.7872/crym/v36.iss3.2015.355. [DOI] [Google Scholar]
- 77.Hyde K.D., Dong Y., Phookamsak R., Jeewon R., Bhat D.J., Jones E.B.G., Liu N.G., Abeywickrama P.D., Mapook A., Wei D.P., et al. Fungal diversity notes 1151–1276: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2020;100:5–277. doi: 10.1007/s13225-020-00439-5. [DOI] [Google Scholar]
- 78.Hyde K.D., Hongsanan S., Jeewon R., Bhat D.J., McKenzie E.H.C., Jones E.B.G., Phookamsak R., Ariyawansa H.A., Boonmee S., Zhao Q., et al. Fungal diversity notes 367–490: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;80:1–270. doi: 10.1007/s13225-016-0373-x. [DOI] [Google Scholar]
- 79.Zhao Z.Z., Zhao K., Chen H.P., Bai X., Zhang L., Liu J.K. Terpenoids from the mushroom-associated fungus Montagnula donacina. Phytochemistry. 2017;147:21–29. doi: 10.1016/j.phytochem.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Pitt W., Úrbez-Torres J.R., Trouillas F.P. Munkovalsaria donacina from grapevines and Desert Ash in Australia. Mycosphere. 2014;5:656–661. doi: 10.5943/mycosphere/5/5/6. [DOI] [Google Scholar]
- 81.Liu J.K., Hyde K.D., Jones E.B.G., Ariyawansa H.A., Bhat D.J., Boonmee S., Maharachchikumbura S., McKenzie E.H.C., Phookamsak R., Phukhamsakda C., et al. Fungal Diversity notes 1–110: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015;72:1–197. doi: 10.1007/s13225-015-0324-y. [DOI] [Google Scholar]
- 82.Tibpromma S., Hyde K.D., McKenzie E.H.C., Bhat J.D., Phillips A.J.L., Wanasinghe D.N., Samarakoon M.C., Jayawardena R.S., Dissanayake A.J., Tennakoon D.S., et al. Fungal diversity notes 840–928: Micro-fungi associated with Pandanaceae. Fungal Divers. 2018;93:1–160. doi: 10.1007/s13225-018-0408-6. [DOI] [Google Scholar]
- 83.Du T., Hyde K.D., Mapook A., Mortimer P.E., Xu J.C., Karunarathna S.C., Tibpromma S. Morphology and phylogenetic analyses reveal Montagnula puerensis sp. nov. (Didymosphaeriaceae, Pleosporales) from southwest China. Phytotaxa. 2021;514:1–25. doi: 10.11646/phytotaxa.514.1.1. [DOI] [Google Scholar]
- 84.Goncalves M.F.M., Vicente T.F.L., Esteves A.C., Alves A. Neptunomyces aureus gen. et sp. nov. (Didymosphaeriaceae Pleosporales) isolated from algae in Ria de Aveiro Portugal. MycoKeys. 2019;60:31–44. doi: 10.3897/mycokeys.60.37931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wanasinghe D.N., Phukhamsakda C., Hyde K.D., Jeewon R., Lee H.B., Jones E.B.G., Tibpromma S., Tennakoon D.S., Dissanayake A.J., Jayasiri S.C., et al. Fungal diversity notes 709–839: Taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018;89:1–236. doi: 10.1007/s13225-018-0395-7. [DOI] [Google Scholar]
- 86.Schoch C.L., Crous P.W., Groenewald J.Z., Boehm E.W.A., Burgess T.I., de Gruyter J., de Hoog G.S., Dixon L.J., Grube M., Gueidan C., et al. A class-wide phylogenetic assessment of Dothideomycetes. Stud. Mycol. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crous P.W., Carnegie A.J., Wingfield M.J., Sharma R., Mughini G., Noordeloos M.E., Santini A., Shouche Y.S., Bezerra J.D.P., Dima B., et al. Fungal planet description sheets: 868–950. Persoonia. 2019;42:291–473. doi: 10.3767/persoonia.2019.42.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feng Y., Zhang S.N., Liu Z.Y. Tremateia murispora sp. nov. (Didymosphaeriaceae Pleosporales) from Guizhou China. Phytotaxa. 2019;416:79–87. doi: 10.11646/phytotaxa.416.1.10. [DOI] [Google Scholar]
- 89.Marincowitz S., Crous P.W., Groenewald J.Z., Wingfield M.J. CBS Biodiversity Series. CBS Fungal Biodiversity Centre; Utrecht, The Netherlands: 2008. Microfungi occurring on Proteaceae in the fynbos; p. 166. No. 7. [Google Scholar]
- 90.Niessl G. Beiträge zur Kenntniss der Pilze. Beschreibung neuerund wenig bekannter Pilze. Verh Nat. Ver. Brünn. 1872;10:153–217. [Google Scholar]
- 91.Hyde K.D., Norphanphoun C., Maharachchikumbura S.S.N., Bhat D.J., Jones E.B.G., Bundhun D., Chen Y.J., Bao D.F., Boonmee S., Calabon M.S., et al. Refined families of Sordariomycetes. Mycosphere. 2020;11:305–1059. doi: 10.5943/mycosphere/11/1/7. [DOI] [Google Scholar]
- 92.Ariyawansa H.A., Hyde K.D., Jayasiri S.C., Buyck B., Chethana K.W.T., Dai D.Q., Dai Y.C., Daranagama D.A., Jayawardena R.S., Lücking R., et al. Fungal diversity notes 111–252—Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015;75:27–274. doi: 10.1007/s13225-015-0346-5. [DOI] [Google Scholar]
- 93.Hughes S.J. Fungi from the Gold Coast. I. Mycol. Pap. 1952;48:1–91. [Google Scholar]
- 94.Ellis M.B. Dematiaceous Hyphomycetes. Commonwealth Mycological Institute; Kew, UK: 1971. p. 608. [Google Scholar]
- 95.Hyde K.D., Fröhlich J., Taylor J.E. Fungi from palms XXXVI Reflections on unitunicate ascomycetes with apiospores. Sydowia. 1998;50:21–80. [Google Scholar]
- 96.Wijayawardene N.N., Hyde K.D., Lumbsch T., Liu J.K., Maharachchikumbura S.S.N., Ekanayaka A.H., Tian Q., Phookamsak R. Outline of Ascomycota—2017. Fungal Divers. 2018;88:167–263. doi: 10.1007/s13225-018-0394-8. [DOI] [Google Scholar]
- 97.Barr M.E. North American Flora Series II Part. Volume 13. NYBG Press; New York, NY, USA: 1990. Melanommatales (Loculoascomycetes) pp. 1–129. [Google Scholar]
- 98.Constantinescu O. Teleomorph-anamorph connections in ascomycetes: Microdiplodia anamorph of Karstenula rhodostoma. Mycol. Res. 1993;97:377–380. doi: 10.1016/S0953-7562(09)81141-6. [DOI] [Google Scholar]
- 99.Berlese A.N. Icones fungorum. Pyrenomycetes. 1896;2:1–216. [Google Scholar]
- 100.Aptroot A. Redisposition of some species excluded from Didymosphaeria (Ascomycotina) Nova Hedwig. 1995;60:325–379. [Google Scholar]
- 101.Saccardo P.A. Conspectus generum fungorum Italiae inferorium. Michelia. 1880;2:1–38. [Google Scholar]
- 102.Tianyu Z. Flora Fungorum Sincorum: 26 Genera of Dematiaceous Dictyosporous Hyphomycetes Excluding Alternaria. Volume 31. Science Press; Beijing, China: 2009. pp. 97–101. [Google Scholar]
- 103.Leão-Ferreira S.M., Gusmão L.F.P. Conidial fungi from the semi-arid Caatinga biome of Brazil. New species of Endophragmiella, Spegazzina and new records for Brazil South America and Neotropica. Mycotaxon. 2010;111:1–10. doi: 10.5248/111.1. [DOI] [Google Scholar]
- 104.Whitton S.R., McKenzie E.H.C., Hyde K.D. Fungi associated with Pandanaceae. Fungal Divers. Res. Ser. 2012;21:1–458. [Google Scholar]
- 105.Tennakoon D.S., Kuo C.H., Maharachchikumbura S.S.N., Thambugala K.M., Gentekaki E., Phillips A.J.L., Bhat D.J., Wanasinghe D.N., de Silva N.I., Promputtha I., et al. Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi. Fungal Divers. 2021;108:1–215. doi: 10.1007/s13225-021-00474-w. [DOI] [Google Scholar]
- 106.Manish T., Gupta R.C., Yogesh J. Spegazzinia tessarthra isolated as a true endophyte from lichen Heterodermia flabellate. Indian Phytopathol. 2014;67:109–110. [Google Scholar]
- 107.Borut S.Y., Johnson T.W. Some biological observations on fungi in estuarine sediments. Mycologia. 1962;54:181–193. doi: 10.1080/00275514.1962.12024990. [DOI] [Google Scholar]
- 108.Ellis M.B. More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute; Kew, UK: 1976. p. 507. [Google Scholar]
- 109.Chethana K.W.T., Niranjan M., Dong W., Samarakoon M.C., Bao D.F., Calabon M.S., Chaiwan N., Chuankid B., Dayarathne M.C., de Silva N.I., et al. AJOM new records and collections of fungi: 101–150. Asian J. Mycol. 2021;4:113–260. doi: 10.5943/ajom/4/1/8. [DOI] [Google Scholar]
- 110.Urríes M.J. El Museo Canaria. Años XVII-XVIII; Las Palmas De Gran Canaria, Espana: 1957. [(accessed on 20 August 2022)]. Hongos microscópicos de Canarias; pp. 43–44. Available online: http://www.elmuseocanario.com/images/documentospdf/revistaelmuseo/Revistas/1956-1957.pdf. [Google Scholar]
- 111.Komarovii V.L. Novitates Systematicae Plantarum Non Vasculariul VII. Academia Scientiarum Urss Institutum Botanicum; Leningrad, Russia: 1970. [(accessed on 20 August 2022)]. Species Novae Ascomycetum Turkomania; pp. 189–197. Available online: https://www.binran.ru/files/journals/NSNR/1970_7/NSNR_1970_7_Frolov_2.pdf. [Google Scholar]
- 112.Komarovii V.L. Novitates Systematicae Plantarum Non Vasculariul. Academia Scientiarum Urss Institutum Botanicum; Leningrad, Russia: 1967. [(accessed on 20 August 2022)]. Species Novae Ascomycetum Turkomania; pp. 233–234. Available online: https://www.binran.ru/files/journals/NSNR/1967_4/NSNR_1967_4_Frolov.pdf. [Google Scholar]
- 113.Hyde K.D., Aptroot A., Frohlich J., Taylor J.E. Fungi from palms. XLII. Didymosphaeria and similar ascomycetes from palms. Nova Hedwig. 1999;69:449–471. doi: 10.1127/nova.hedwigia/69/1999/449. [DOI] [Google Scholar]
- 114.Thaung M.M. Pathologic and taxonomic analysis of leaf spot and tar spot diseases in a tropical dry to wet monsoon ecosystem of lowland Burma. Australas. Plant Pathol. 2008;37:180–197. doi: 10.1071/AP08007. [DOI] [Google Scholar]
- 115.Francisco C.S., Ma X., Zwyssig M.M., Mcdonald B.A., Palma-Guerrero J. Morphological changes in response to environmental stresses in the fungal plant pathogen Zymoseptoria tritici. Sci. Rep. 2019;9:9642. doi: 10.1038/s41598-019-45994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chethana K.W.T., Manawasinghe I.S., Hurdeal V.G., Bhunjun C.S., Appadoo M.A., Gentekaki E., Raspé O., Promputtha I., Hyde K.D. What are fungal species and how to delineate them? Fungal Divers. 2021;109:1–25. doi: 10.1007/s13225-021-00483-9. [DOI] [Google Scholar]
- 117.Pem D., Jeewon R., Chethana K.W.T., Hongsanan S., Doilom M., Suwannarach N., Hyde K.D. Species concepts of Dothideomycetes: Classification, phylogenetic inconsistencies and taxonomic standardization. Fungal Divers. 2021;109:283–319. doi: 10.1007/s13225-021-00485-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study can be found in the GenBank, NCBI and the accession numbers are given in Table 1. Newly introduced fungal names were registered at the Index Fungorum and the identification numbers are shown in their respective entries.