Abstract
Simple Summary
Colorectal cancer (CRC) is one of the most prevalent cancers worldwide. Due to the absence of specific early symptoms, most of CRC patients are often diagnosed at late stages. Different screening and diagnostic biomarkers are currently used for risk stratification and early detection of CRC, which might prolong the overall survival. High-throughput technologies have witnessed rapid advancements in the last decade. Consequently, the development of multiple omics technologies, such as genomics, transcriptomics, proteomics, metabolomics, microbiomics, and lipidomics, has been widely applied to develop novel biomarkers that could contribute to the clinical management of CRC. In this paper, we aim to summarize the recent advances and future perspectives in using multi-omics technologies in CRC research, and reveal the potential implications of multi-omics for discovering novel biomarkers and enhancing clinical evaluations.
Abstract
Colorectal cancer (CRC) is common Cancer as well as the third leading cause of mortality around the world; its exact molecular mechanism remains elusive. Although CRC risk is significantly correlated with genetic factors, the pathophysiology of CRC is also influenced by external and internal exposures and their interactions with genetic factors. The field of CRC research has recently benefited from significant advances through Omics technologies for screening biomarkers, including genes, transcripts, proteins, metabolites, microbiome, and lipidome unbiasedly. A promising application of omics technologies could enable new biomarkers to be found for the screening and diagnosis of CRC. Single-omics technologies cannot fully understand the molecular mechanisms of CRC. Therefore, this review article aims to summarize the multi-omics studies of Colorectal cancer, including genomics, transcriptomics, proteomics, microbiomics, metabolomics, and lipidomics that may shed new light on the discovery of novel biomarkers. It can contribute to identifying and validating new CRC biomarkers and better understanding colorectal carcinogenesis. Discovering biomarkers through multi-omics technologies could be difficult but valuable for disease genotyping and phenotyping. That can provide a better knowledge of CRC prognosis, diagnosis, and treatments.
Keywords: colorectal cancer, multi-omics, biomarkers
1. Introduction
Colorectal cancer (CRC) is the third most common cancer accounting for 10.2% of new cases and 9.2% of Cancer-related mortality, thus accounting for the second most deadly cancer globally [1]. It has been reported that the overall survival rate of metastatic CRC (mCRC) at 5 years over the first examination lowers from 87–90% in stages I–II, and 68–72% in stages III; in stage IV, the rate drops to 11–14% [2]. Most CRC treatment options currently rely on cancer staging, patient performance status, RAS, BRAF, ERBB2, and mismatch repair (MMR) status assessments using tumour samples taken during surgery or core biopsy [3,4]. At present, for patients with mCRC, it is recommended to determine KRAS/NRAS and BRAF mutation status, as well as HER2 amplification and microsatellite instability high (MSI)/mismatch repair (MMR) status (if not performed already) [4]. Recent studies have demonstrated that immune checkpoint inhibitor therapy is effective in treating dMMR/MSI-H mCRC tumours at advanced stages of the disease [5]. The discovery of new molecular biomarkers in CRC and other cancers has begun to follow the approval of tumour-agnostic drugs, including NTRK1-3 translocations and high tumour mutational burdens (TMBs) [6,7]. As opposed to metastatic cancer, there are still no validated biomarkers indicating which patients are more likely to benefit from adjuvant cytotoxic therapy in stage II or III CRC, except for microsatellite instability (MSI) [8]. Additionally, postoperative treatments are often administered following metastatic resections, despite the absence of predictive biomarkers [3,4]. Currently, Colonoscopy, tissue biopsy, and fecal occult blood test (FOBT) are the major techniques used in CRC screening and detection. However, in the case of Colonoscopy or biopsy, these techniques are invasive, causing discomfort for the patient, or in the case of FOBT, they may also have low sensitivity [9,10,11]. Therefore, it is demonstrated that a less invasive test with higher sensitivity is needed in clinical practice.
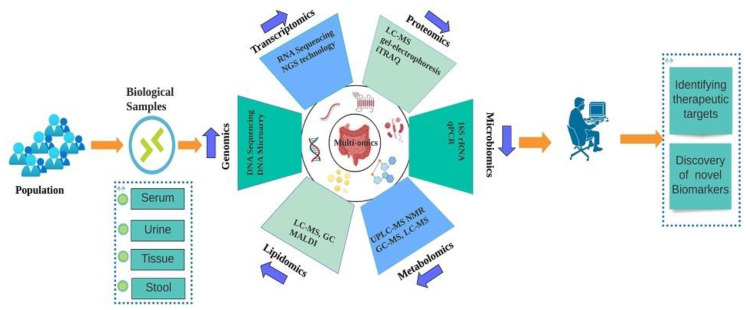
In particular, high throughput “multi-omics” technologies, including genomics, transcriptomics, proteomics, microbiomics, and metabolomics, provide less or noninvasive approaches for diagnosing CRC. Each method offers a unique advantage for the discovery of novel diagnostic cancer biomarkers, such as Genomics, which is incredibly efficient for evaluating CRC vulnerability and the disease’s genetic risk. However, it has little diagnostic potential since DNA sequences seldom translate directly to phenotype due to epigenetic, post-transcriptional, and post-translational alterations [12]. Transcriptomics and proteomics have great therapeutic potential as they are more closely tied to organisms’ physiological states. Still, their diagnostic power is not as good as that of metabolomics, which enables quick and precise phenotypic characterization of the organism and its metabolic pathways as well as the potential to evaluate how host and gut bacterial metabolites interact, which is a crucial step in the CRC progression [13]. Additionally, a number of recent research have shown that the gut microbial community and microbial metabolites play a crucial role in the emergence of CRC [14,15]. Recent years have seen the emergence of lipidomics as a research tool and a multi-omics technology that holds great promise. As a result, this tool has been demonstrated to be useful for both the quantification of cellular lipids and their characterization. This is not only for disease diagnosis but also for other mechanistic studies [16,17]. The mechanism of CRC initiation and progression has remained largely enigmatic despite the discovery of more diagnostic methods and potential therapies; many challenges remain unresolved due to the lack of new biomarkers and the heterogeneity of tumours. After the completion of the human genome project, omics science has revolutionized CRC research [18]. In order to enable personalized medicine and to define CRC treatment, the identification of novel biomarkers has become an essential part of molecular diagnosis and treatment [19]. The use of new biomarkers in clinical practice is still challenging despite developments in the molecular analysis [20]. However, genomic advances have made significant contributions to understanding cancer biology over the past few years [21]. In oncology, the structure and functions of the genome, as well as mechanisms governing genes’ expression, have been extensively investigated since the completion of the Human Genome Project and the development of next-generation sequencing (NGS) techniques [21,22]. A constantly expanding understanding of genomic hallmarks of malignant transformation provides a new perspective on pathogenesis and targeted treatment of particular tumours [23,24]. Genomics, transcriptomics, proteomics, metabolomics, microbiomics, and lipidomics, make a significant contribution to a fundamental change toward a multiparametric, innovative, immunological, and stromal model, which helps us to understand how CRC develops and categorizes it into various molecular subtypes for clinical diagnosis as well as the emergence of new biomarkers and therapeutic strategies [25], (Figure 1).
Figure 1.
Graphical representation of different multi-omics-based approaches in discovering novel CRC biomarkers and therapeutic targets.
The aim of this review is to summarize the recent developments in multiple multi-omics technologies in the exploration of CRC biomarkers signatures via genomics, transcriptomics, proteomics, microbiomics, metabolomics, and lipidomics. These promising multi-omics base CRC biomarkers could be useful for clinical research.
2. Genomics of CRC
Genomic science comprises the study of an individual’s entire set of DNA (including all of their genes) [26]. An individual’s genomes are a comprehensive collection of information that enables them to grow and develop [27]. Using genomic analysis, researchers may better understand gene interactions, environmental effects, and how several conditions, such as cancer and diabetes, develop [28]. The development of these new approaches may facilitate disease diagnosis, treatment, and prevention [29]. During carcinogenesis, genetic and epigenetic changes occur that contribute to the identification of ideal biomarkers of CRC [30]. There is growing evidence that genetic changes play a key role in tumorigenesis. Due to this, genomics is becoming a powerful tool for finding genetic markers that can be used to diagnose and prognosis cancer, as well as improving our understanding of the disease. High-throughput next-generation sequencing is a genomic technique for sequencing an organism’s DNA [31]. Multiple- biomarker panels are usually more sensitive than single biomarkers, as demonstrated by many research studies over the last few years [32]. For illustration, Ghatak S et al. used differential gene expression analysis in five independent in silico CRC cohorts and immunohistochemistry in one clinical cohort to validate their results. The authors developed a novel biomarker for early diagnosis and prognosis of cancer based on a five-panel gene signature [33]. All five in silico datasets showed that four genes (PTGS2, BDNF, CTNNB1, and GSK3B) were highly upregulated. One gene (HPGD) was substantially downregulated in primary tumour tissues compared to neighbouring normal tissues. Based on independent clinical validation cohorts, this five-gene signature was significantly associated with poor overall survival (AUC = 0.82) among colon cancer patients.
An epigenetic change happens when modified nucleotide sequences in the genome appear to be altered beyond their original form [34]. Gene expression is regulated by epigenetic mechanisms such as DNA methylation, histone modification, and nucleosome positioning Inhibitions in these regulatory processes promote malignant transformation by impairing gene function [35,36]. There is abnormal methylation of the CpG promoter during CRC, which leads to promoter hypermethylation in the promoters of tumour suppressor genes and the silencing of the transcriptional activity of DNA repair genes, which is accompanied by a loss of methylation (hypomethylation) that contributes to oncogene activation, chromosome instability, and microsatellite instability [37,38]. There is evidence that CRC biomarkers such as methylation in cfDNA and CTCs may be useful for the noninvasive diagnosis of CRC [39,40]. In addition, the epigenetic modification of 5-methylcytosine (5mC) has been associated with the emergence of several disorders, including CRC. An increasing number of studies suggest that 5mC can be used in diagnoses and prognosis of colorectal Cancer [41,42,43,44,45,46,47]. Furthermore, members of the ten-eleven translocation family catalyze the production of 5-hydroxymethylcytosine (5hmC), a persistent byproduct of DNA epigenetic regulation. The change of 5hmC, a new epigenetic biomarker, is linked to several disorders, particularly Cancer [48,49,50,51,52,53]. There is evidence that 5hmC plays an important role in the progression of CRC [47,53]. However, it has rarely been studied as a potential diagnostic marker for the early detection of CRC. The potential genomics biomarkers are shown in Table 1.
Table 1.
Potential multi-omics base Genomics biomarkers in CRC.
| Biomarker | Sample Type | Change | Application | References |
|---|---|---|---|---|
| CBX8, CD96 | datasets | downregulated | diagnostic | [54] |
| MTUS1 | tissue | downregulated | diagnostic and prognostic | [55] |
| SDC2, NDRG4 | stool | upregulated | Screening | [56] |
| SOX21 | stool | upregulated | diagnostic | [57] |
| BDNF, PTGS2, GSK3B and CTNNB1 | tissue | upregulated | prognostic and diagnostic | [33] |
| HPGD | tissue | downregulated | prognostic and diagnostic | [33] |
| YWHAB, MCM4, and FBXO46 | datasets | overexpress | prognostic | [58] |
| DPP72 | datasets | lower expression | prognostic | [58] |
| SDC2, TFPI2 | stool | hypermethylated | screening | [59] |
| SNORD15B, SNORA5C | tissue | upregulated | diagnostic and prognostic | [60] |
| GALR1 | tissue | hypermethylation | screening | [61] |
| LRRC19 | datasets | downregulated | prognosis | [62] |
| KRAS, BRAF, PIK3CA | tissue | mutation | detection | [63] |
3. Transcriptomics of CRC
A transcriptomic study analyzes an organism’s entire RNA content. Transcription represents an overview of the cell’s activity at a particular moment due to the information in DNA [64]. In recent years, transcriptomics has made unprecedented progress in molecular genetics [21,65]. At certain developmental stages and under certain physiological or pathological conditions, transcriptomes represent all RNA molecules produced in a cell from the genome [66,67]. It consists of protein-coding RNAs (pcRNAs), also known as messenger RNAs (mRNAs), and non-coding RNAs (ncRNAs), of which each molecule exhibits a wide range of cellular functions and responses to external stimuli [68,69,70,71]. As a result of epigenetic changes and genomic instability, transcriptome changes may occur in CRC. In CRC, ncRNAs play an important role in angiogenesis, migration, differentiation, and apoptosis. Therefore, the study of ncRNAs is one of the most prominent areas of RNA research. Numerous studies have provided evidence that ncRNA expression is abnormal in CRCs. A study of ncRNA stability in stool, plasma, and serum may provide new possibilities for developing new methods of detecting ncRNAs, and it has been demonstrated that among ncRNAs, microRNAs have significant impacts on CRC [72,73]. By using next-generation sequencing, deep sequencing of CRC tumours was performed to examine the miRNA transcriptome results demonstrating that CRC patients had increased levels of miRNA-615-3p and miRNA-10b-5p expression in both the right and left side of the colons correspondingly. Additionally, five miRNAs were found to be significantly elevated in CRC patients in the study, including miR-143-3p, miR-22-3p, miR-192-5p, miR-21-5p and miR-10a-5p [74]. Several studies have been conducted to identify novel miRNA as biomarkers, and several studies have demonstrated an important role for miR-92a and miR-429 in CRC pathogenesis [75,76]. In contrast, several miRNA molecules have been demonstrated to have significant diagnostic value for advanced neoplasia, including miR-17-92, miR-135, miR-143, and miR-145.
Moreover, a recent study improved and facilitated exosome-miRNA identification in blood using SHERLOCK-based miRNA detection. It revealed that miR-23a, miR 126, miR-940 and miR-1290, are the best good prognostic indicators for the initial stages of CRC [77]. Several miRNAs including MiR-192a, miR-29a, miR-19a-3p, miR-92a-3p, miR-125b, miR-422a and miR-223-3p, have been considering significant CRC marker. However, miR-21 has been studied extensively for diagnosing CRC [78]. In another study, miR-429 was found to reside at the centre of a miRNA-target gene network, indicating that it plays a critical role in cancer development. The miRNA samples from 28 patients with CRC markedly showed an increase in miR-32 levels. It has been determined that miR-32 expression and CRC lymphatic invasion and metastasis are correlated by the cancer genome atlas (TCGA), and a negative association was also observed between miR-32 and bone morphogenetic protein 5 (BMP5) [79]. By inhibiting EPST11 activation, BMP5 acts as a tumour suppressor. Alteration in BMP5 levels triggers the epithelial-mesenchymal transition, which stimulates tumorigenesis. Sporadic CRC tissues show a positive correlation between BMP5 expression and E-cadherin expression. Yamada et al. identified four lncRNAs, including CRCAL-1, CRCAL-2, CRCAL-3, and CRCAL-4, which differ in expression among normal mucosa and CRC patients through RNA sequencing [80]. The findings of this research highlight the implication of RNA-Seq for identifying new lncRNAs in colorectal cancer. CRC tissue also showed downregulation of NONHSAT074176.2 under GO and KEGG analysis, which may serve as a valuable diagnostic biomarker [81].
A fusion transcript (FT) is a chimeric RNA that comes from a single gene product or the trans-splicing of a transcript made by two gene products. FTs play an important role in the regulation of cancerous cells. It has been reported that transcripts from COMMD10-AP3S1, CTB-35F21.1-PSD2, and AKAP13-PDE8A are the most frequently reported transcripts in CRC. According to another study, higher levels of NFATC3-PLA2G15 fusion transcript were detected in 19 pairs of CRC tumours and adjacent normal tissue samples. As a result of the knockdown of NFATC3-PLA2G15, invasion and proliferation are inhibited in cancer cells, suggesting that NFATC3-PLA2G15 FTs may influence CRC progression; these impact findings show that this fusion transcript can serve as a novel biomarker for CRC [82] TGFRN-NOTCH2 fusion transcripts were the only transcripts detected in CRC and adjacent normal tissues from deep transcriptome sequencing. RT-PCR analysis confirmed the findings, suggesting that PTGFRN-NOTCH2 may be an FT gene in CRCs and may serve as a potential biomarker [83].
Furthermore, single-cell RNA sequencing (scRNA-seq) assesses the transcriptomic status of specific populations of single cells compared with RNA sequencing (RNA-seq) in which transcript levels are measured across different cell types [84,85]. In microdroplets and microwells, thousands of single cells can be simultaneously barcoded and handled at the same time [84]. Several technologies have been developed that measure mRNAs that are isolated from a single cell, including Quartz-Seq, Smart-seq, Smart-seq2, and CEL-seq [84,86,87]. These different types of mRNA sequencing technologies with distinct purposes. Smart-seq, for example, detects full-length transcripts. During Quartz-Seq, samples are analyzed and pooled according to the 30 end of transcripts and the CEL-Seq barcodes before linear amplification of mRNA [86]. In a recent study, the transcriptional profiles of 371,223 cells from colorectal cancer and neighbouring normal tissues were taken from 28 tumours with mismatch repair proficiency, and 34 tumours with mismatch repair deficient [88]. a significant finding of this study is that there is a structured arrangement of T cells within a tumour. In summary, the authors have provided a large number of individuals with colorectal cancer with datasets that contain information about cellular states, gene networks, and tumour transformations [88,89]. The results of scRNA-seq studies are promising because, for each cell type in a tumour, alterations may be associated with patient characteristics, diagnostic methods, therapeutic approaches, and prognosis. In the near future, scRNA-seq could be used clinically to develop customized treatment regimens for each patient based on their genetic information [90]. The potential transcriptomics biomarker is shown in Table 2.
Table 2.
Potential multi-omics base transcriptomics biomarkers in CRC.
| Biomarker | Sample Type | Change | Application | References |
|---|---|---|---|---|
| miR-92a, miR-21 | serum | upregulated | diagnostic and prognostic | [91] |
| hsa_circ_0000567 | CRC tissue and cell lines | downregulated | diagnostic | [92] |
| hsa-circ-0006282 | plasma | upregulated | Diagnostic | [93] |
| hsa_circ_000592, hsa_circ_0001900 and hsa_circ_0001178 | plasma | upregulated | diagnostic | [94] |
| miR-129-1-3p mmiR-566 |
urine | upregulated | detection | [95] |
| GPR55 | CRC tissue and cell lines | downregulated | prognostic | [96] |
| miR-1290 | plasma | upregulated | prognostic | [97] |
| miR-320d | plasma | downregulated | diagnostic | [98] |
| miR-103a-3p, miR-127-3p, miR-17-5p, miR151a5p, miR-181a-5p, miR-18a-5p and miR-18b-5p |
plasma | upregulated | diagnostic | [99] |
| CCAT2, CCAT1, H19, MALAT1, MEG3, HULC, HOTAIR, PCAT1, PTENP1 and TUSC7 |
stool | upregulated | detection | [100] |
| miR-214, miR-199a-3p, miR-196a, miR-106a, miR-183, miR-134, miR-92a, miR-96, miR-20a, miR-21, miR-17, miR-7. |
stool | upregulated | screening | [101] |
| miR-138, miR-143, miR-29b, miR-9, miR-146a, miR-127-5p, miR-938, miR-222. |
stool | downregulated | screening | [101] |
4. Proteomics of CRC
Proteins regulate many biological processes, and gene mutations could alter their expression. As well as serving as a source of potential biomarkers, the proteome is also the functional translation of the genome. Compared to the normal proteome, cancer proteome biomarkers are up- or downregulated; Thus, researchers have recently focused their attention on identifying differences between cancerous and normal cells in terms of their expression characteristics. To develop new classification tools for CRC, diagnostic, prognostic, and predictive biomarkers must be developed to detect proteins involved in its development and progression and observe the effects of protein perturbations and modifications.
Many proteomic techniques have been employed in order to find putative diagnostic biomarkers. According to Ghazanfar et al. [102], protein expression in fresh freeze samples of colorectal cancer tissue (12 individuals) was analyzed using gel electrophoresis in combination with mass spectrometry, demonstrating that number of proteins has been upregulated in colorectal Cancer. These include actin beta-like 2 (ACTBL2). Another study by Hao et al. [103], using high-resolution Fourier transform mass spectrometry, revealed that colorectal tumour tissue overexpressed dipeptidase 1 (DPEP1) Based on the examination of 22 pairs of normal tissues adjacent to cancerous tissue. Yamamoto and colleagues used a global proteomic approach to study formalin-fixed and paraffin-embosted (FFPE) CRC tissue with liquid chromatography (LC)/mass spectrometry (MS). They found a higher concentration of cyclophilin A, annexin A2, and aldolase A in cancerous tissues versus non-cancer tissues [104]. Similarly, in another study, fibroblasts associated with cancer progression were identified from human and mouse tissue. As a result of this study, it has been demonstrated that the proteins LTBP2, OLFML3, CDH11, CDH11, CALU, and FSTL1 play an important role in the migration and invasion of CRCs and have been implicated as stromal biomarkers [105].
Among the potential biomarkers of colorectal Cancer, blood-based markers are some of the most promising for performing early detection and surveillance of CRC because obtaining the specimens is relatively easy and noninvasive with minimal risk [106,107]. A targeted liquid chromatography-tandem mass spectrometry analysis was performed on 213 healthy subjects and 50 colorectal cancer patients by Ivancic et al. [108]. This study identified five proteins, including inter-alpha-trypsin inhibitor heavy-chain family member 4, leucine-rich alpha-2-glycoprotein 1, EGFR, hemopexin, and superoxide dismutase 3, that play a significant role in detecting CRC with 89% specificity and over 70%sensitivity. Furthermore, A protein panel for early detection of CRC was discovered by Bhardwaj et al. [109], by using liquid chromatography/multiple reaction monitoring-mass spectrometry in plasma samples from 96 CRC patients, and 94 controls, using a blood-based profile of five markers, osteopontin, serum paraoxonase lactonase 3, transferrin receptor protein 1, mannan-binding lectin serine protease 1, and amphiregulin. Demonstrated promising performance in screening for colorectal Cancer. Additionally, a number of members of the Serpin family including SERPINC1 (antithrombin-3, AT-III), SERPINA3 (alpha-1 antichymotrypsin, AACT), and SERPINA1 (alpha-1 antitrypsin, A1AT), have been identified as potential biomarkers for colorectal carcinoma and adenomatous polyps by using multiplexed quantification isobaric tags for absolute and relative quantitation (iTRAQ), [110]. The importance of CC chemokines (CCL15, CCL4 and CCL2) has also been assessed in CRC however further research is needed for their utility as diagnostic and clinical markers [111].
Numerous LC-MS-based research has been conducted demonstrating different CRC biomarkers. For instance, Quesada-Calvo et al. [112] suggested KNG1, Sec24C, and OLFM4 as diagnostic biomarkers out of 561 proteins with different expression levels. One other study demonstrated that ACTBL2, Annexin A2, Aldose A, DPEP1, and cyclophilin A could also serve as a biomarker for the early detection and treatment of CRC and provide new therapeutic targets [102,103,104]. As a biomarker source, circulating proteins are widely accepted as a better diagnostic tool for many diseases, particularly CRC [113]. Western blot (WB) and ELISA verification studies demonstrate that MRC1 and S100A9 are higher in CRC patients’ serum compared to healthy individuals [114]. Furthermore, Ivancic et al. demonstrated that serum samples containing LRG1, EGFR, ITIH4, HPX, and SOD3 could detect CRC with 89% specificity and 70% sensitivity. According to these findings, GC, CRP, CD44, and ITIH3 proteins may be able to differentiate CRC depending on its stage [108]. Additionally, Bhardwaj and colleagues [109] showed five protein signatures, including MASP1, AREG, PON3, TR, and OPN, compared with FDA-approved biomarkers derived from blood superior diagnostic performance. CXCL-1 (C-X-C motif ligand 1) and CXCL-8 (C-X-C motif ligand 8) and their receptors have also demonstrated a potential role as biomarkers for CRC prognosis and diagnosis [115], Pczek S et al. conducted a study in which increased levels of CXCL-8 were found in CRC patients when compared to normal subjects. The findings of their research revealed enhanced versatility of CXCL-8 as compared to CEA in CRC diagnosis [116]. The potential proteomics biomarkers are shown in Table 3.
Table 3.
Potential multi-omics based proteomics biomarkers in CRC.
| Biomarker | Sample Type | Change | Application | References |
|---|---|---|---|---|
| CHD 9 | tissue | upregulated | prognostic | [117] |
| ACTBL2 | tissue | upregulated | diagnostic | [102] |
| CDK3, CDK5, and CDK8 | tissue | upregulated | diagnostic | [118] |
| STK4 or MST1 | serum | downregulated | detection | [119] |
| MRC1 and S100A90 | serum | upregulated | diagnostic | [114] |
| CEACAM-7 | tissue | downregulated | predictive | [120] |
| CEA | plasma | upregulated | predictive and prognostic | [121] |
| SPG20 and STK31 | blood | upregulated | diagnostic | [122] |
| TPM3 | tissue/plasma | upregulated | detection | [123] |
| FJX1 | serum | upregulated | prognostic and diagnostic | [124] |
| NOP14 | datasets | upregulated | Prognosis | [125] |
| SPARCL1 | datasets | Downregulated | diagnosis | [126] |
5. Microbiomics of CRC
Microbiomics is an emerging field of omics technologies that examines a symbiotic or pathological relationship between microbial communities [127]. Many microorganisms exist in the human microbiota (microbiome), such as bacteria, viruses, fungi, etc. [128,129,130,131,132]. An individual’s gut microbiome is composed of microorganisms and their genetic materials. Over 3 million genes exist in the gastrointestinal tract, which is 150 times more than the human genome. In the gastrointestinal tract, 1013 to 1014 different microorganisms live, and over 30 million genes exist. Approximately 7000 different strains of bacteria comprise the gut microbiome in adults [133]. Gut microbiome signatures in CRC were studied using different approaches by various researchers. Various methods enrich 16S rRNA for variable regions in stool DNA, from amplifying and sequencing the V1, V2, and V4 regions to shot-gun metagenomic sequencing. Here are various methods to enrich 16S rRNA for variable regions in stool DNA, from amplification and sequencing of the V1, V2, and V4 regions to shot-gun metagenomic sequencing [134,135,136]. Various qPCR methods have been used to quantitate the abundance of target microbial genes in samples of interest [137,138,139].
Gut microbiomes have shown a significant role in the treatment of CRC; As an example, the gut microbiome may be able to be used for screening, diagnosis, prediction and/or predictive biomarkers. Alternatively, it might be a changeable factor affecting systemic CRC treatment efficacy or prevention [127,140]. The gut microbiota is a screening marker among asymptomatic individuals with high-risk adenomas or CRC. The Fusobacterium nucleatum bacteria, for example, can be examined in faecal samples from patients with adenomas and CRC to serve as a screening biomarker. Detecting and screening for CRC early may also be possible based on metabolic markers and genotoxic metabolites of specific strains [139].
Recent research published in a nature journal examined 33 cancer patients’ blood and tissue samples. It revealed that the blood contained specific gut-derived pathogenic bacterial DNA that may be used to distinguish various types of tumours [141]. Therefore, the authors concluded that further research should be undertaken on this possible microbiome-based tumour diagnosis tool. In addition, the study of pathogenic bacteria (intestinal flora), and their metabolites have been linked to CRC, and the correlation analysis of gut-microbiome and metabolomics have shown a promising role in CRC prevention, treatment and diagnosis [142]. This common gastrointestinal malignancy has also become a hotspot of research in recent years [143,144]. Chen F et al. investigated the macro genomic and metabolomic compositions of serum collected from normal patients, colorectal adenomas, and CRC patients. A total of 885 differential metabolites were found in the serum associated with intestinal bacteria. This led to the Identification of eight serum metabolites that were reproducible and were used to develop categorical diagnostic models for healthy/colorectal adenoma (AUC = 0.84) and healthy/colorectal Cancer (AUC = 0.93) [145].
Some common metabolites of intestinal bacteria in the blood, bile acids, such as short-chain fatty acids and oxotrimethylamine, could be biomarkers for early CRC detection [146,147,148]. According to research by Huang Y et al. [149], Fusobacterium nucleatum plays a major role in promoting colorectal carcinogenesis by increasing tumour-associated metabolites, including 12a hydroxy3oxycholic acid and phosphorylcholine in the serum. Another area of active research is the discovery of biomarkers from microbial metabolomes, as some metabolites derived from the microbiota are associated with colorectal cancer. Microbial metabolites have been identified in several studies as potential biomarkers for CRC; for example, using GC-MS, an analysis of stool metabolites was conducted for CRC patients using a GC-MS technique with the result that there was a higher concentration of acetate and a lower concentration of butyrate and ursodeoxycholic, acid (UDCA) in their stool [150]. Another GC-MS metabolomic study was conducted in CRC tissue in which 19 differentiating metabolites were identified, along with pathway enrichment analyses that demonstrated that CRC patients exhibit a significant disruption of several metabolism pathways, including short-chain fatty acid metabolism, secondary bile acid metabolism, and carbohydrate metabolism [151]. Using NMR, a combined examination of tumour tissue and feces revealed a decrease in butyrate levels in patients with CRC; Fecal and tissue samples had AUCs of 0.692 and 0.717, respectively for diagnosing CRC from normal subjects, An AUC of 0.843 was reported for fecal acetate, which was the strongest indicator of diagnostic performance [152]. According to an MS-based metabolomic analysis in CRC cohorts, polyamine-based metabolites also showed a significant upregulation (N1-acetylspermidine, citrulline, arginine and ornithine) [153]. Integrating microbiome and metabolome data has demonstrated that fecal abundances of microbial-associated polyamines (cadaverine and putrescine) may play a role in colorectal cancer diagnosis [154]. CRC screening can benefit from metabolic markers, as demonstrated in these examples. The potential microbiomics and metabolomics biomarkers are shown in the Table 4.
Table 4.
Potential multi-omics base microbiomics and metabolomics biomarkers in CRC.
| Biomarker | Sample | Change | Application | References |
|---|---|---|---|---|
|
F.nucleatum, P. anaerobius and P. Micra |
stool | increase | detection | [155] |
| P. micra, Streptococcus anginosus |
stool | increase | diagnosis | [156] |
| P. Micra F. nucleatum |
stool | increase | diagnosis | [157] |
| norvaline and myristic acid | stool | upregulated | diagnosis | [158] |
| menaquinone-10 | stool | upregulated | diagnosis | [159] |
| F. nucleatum | stool | upregulated | detection | [160] |
| Oleic acid | stool | Upregulated | screening | [161] |
| Succinate, Butyrate, Lactate, Glutamate, and Alanine. |
tumour tissue/feces | Upregulated (excluding Butyrate downregulated) |
detection | [152] |
| Cholesteryl esters, Sphingomyelins | stool | Upregulated | diagnosis | [134] |
| Fusobacterium, Parvimonas and Staphylococcus |
stool | increase | diagnosis | [134] |
| Pyruvic acid, lysine, glycolic acid, fumaric acid, ornithine | blood | upregulated | detection | [162] |
| tryptophan, Palmitoleic acid, lysine, 3hydroxyisovaleric acid | blood | decrease | detection | [162] |
| octadecanoic acid, citric acid, hexadecanoic acid, and propanoic acid-2-methyl-1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl este | urine | downregulated | screening | [163] |
| Hydroxyproline dipeptide, tyrosine, tryptophan, pseudouridine, glucuronic acid, glycine, histidine, glucose, 5-oxoproline, threonic acid, and isocitric acid | urine | upregulated | screening | [163] |
6. Metabolomics of CRC
Metabolomics is a new research area in the omics arena. Refers to an in-depth investigation of low molecular weight substances formed by metabolism in a biological fluid, including metabolic substrates and products, small peptides, lipids, vitamins, and other protein cofactors. In biomarker discovery, metabolomics is one of the fast-growing fields [164,165]. Furthermore, unlike genomics, transcriptomics, and proteomics, it represents the connections between genes and the environment, which allows it to be more precise in describing multifactorial diseases [164,166]. Many biological specimens can be used for metabolomics, most of which can be obtained using noninvasive techniques. Although biomarkers and metabolites can vary from study to study and even between specimens and colorectal cancer levels, they remain useful for diagnosing colorectal cancer [167]. Targeted metabolomics involves quantifying the metabolites linked with particular pathways associated with a specific state of disease [168]. In contrast, the untargeted approach was used in many samples and did not undergo any bias; it often measures as many metabolites as possible [169]. Due to its unique insight into disease origin and development processes, the metabolome remains a key component of disease research. Metabolomics may provide valuable information about the pathology of CRC, identify predictive biomarkers, and evaluate the severity of the disease by examining the metabolomic fingerprint in detail [170]. The metabolomics approach based on urine metabolites can be used to identify cancer biomarkers to distinguish patients with early-stage and advanced-stage colorectal cancer [171]. Lactosylceramide has also been identified as a key metabolite distinguishing Crohn’s disease from ulcerative colitis in untargeted metabolomics [172].
Several metabolomic research studies have been carried out in a small cohort of colorectal cancer patients using several biological samples such as blood, urine, stool, and tissue [173,174]. A comparison was made between metabolic profiles of healthy individuals, as well as of individuals with benign polypoid pathology [175] employing nuclear magnetic resonance (NMR) or gas- and liquid-chromatography coupled to mass spectrometry (GC-MS, LC-MS) as analytical tools. Several studies have shown a negative correlation between stool and urine metabolites in patients with advanced colon cancer. The study’s authors conducted a comparison of plasma, stool, and urine metabolic profiles [176]. There have been studies conducted that identified 154 different metabolites, including those that are produced during the tricarboxylic acid (TCA) cycle, urea cycle, polyamine pathways, glycolysis and amino acids, among others. With the progression of Cancer, the concentrations of these metabolites increased, with the greatest difference found in stage IV. Moreover, polyps and CRC samples were discriminated by metabolite analysis [177]. Ning et al. carried out a research study that revealed 11 upregulated and four downregulated metabolites in urine samples collected from CRC patients and healthy subjects, as shown in Table 4. Patients with CRC who were examined for pathways involved in these metabolites showed increased glycolysis, and amino acid metabolism while showing a decrease in lipid metabolism [163].
Another research has been conducted; they studied the relationships between metabolites and health status in healthy individuals and CRC patients using GC-MS analysis based on a metabolomics-based approach. This study identified several polyamines (putrescine, cadaverine) as potential biomarkers for cancer prognosis [154]. By observing metabolomic alterations in patients with CRC, another study utilizing gas chromatography-mass spectrometry (GC-MS) found that stool fatty acids, particularly increased oleic acid, may be used to screen CRC [161]. UHPLC-MS analysis of stool samples from CRC patients revealed different sphingolipid and cholesteryl esters levels [134]. A recent study of CRC tissues and stools conducted through the proton nuclear magnetic resonance (1H NMR) technology showed that butyrate was downregulated in CRC tissue and stools. At the same time, alanine, lactate, glutamate, and succinate were upregulated [152]. As metabolomics have been made a great contribution to drug discovery, UPLC-MS base metabolites biomarkers from natural compounds have also played an essential role in disease treatments [178], for instance our recent pharmacodynamic metabolomics base study using mice serum revealed that flavonoids and anthraquinones have a role in CRC treatment [179]. A combination of several multi-omics technologies could provide a powerful strategy for making valid conclusions about biomarker signatures for Colorectal Cancer. So far, no single Omics technology offers enough information to demonstrate the detailed molecular mechanism and validation of biomarker signatures.
7. Lipidomics of CRC
The field of lipidomics is one of the newest branches of multi-omics technologies. With the help of various analytical techniques, this technology can classify and analyze almost all cellular lipids, to understand their role and characteristics within biological systems. It has been studied on a larger scale for lipid species molecules. Several kinds of disease-specific biomarkers have been found through lipidomics, and the lipid species are linked to disease severity [180,181]. Regarding CRC, A very recent study, by Zaytseva et al. suggested that fatty acid metabolism might be used as a strong predisposition in CRC. It emphasized the significance of targeting lipid dysregulation in future therapeutic strategies [182]. Many studies have been conducted to examine the complex lipid profile of serum tissue samples; Consequently, a specific CRC lipidome has been the subject of ongoing discussions that may have implications for clinical treatment. The elevated levels of VLCFA (Very Long-chain Fatty acids) and lower levels of LCFA (long-chain fatty acids) have been observed in CRC patients’ serum. It was explained that ELOVLs (Elongation of Very Long-chain Fatty acids Protein) may increase VLCFA elongation by increasing saturated or monosaturated fatty acids [183,184]. Based on LC-MS analysis, it was found that saturated triacylglycerols accounted for the majority of perturbations that occurred in CRC progression. The authors attributed these perturbations to LD (lipid droplet) accumulation [185]. According to another study, glycolipids, glycerophosphocholine, and acylcarnitines serum concentrations decreased in CRC patients [186]. In a recent study, Ecker et al. also found an independent prognostic marker in triglyceride lipidomic tissue signatures capable of discriminating against patients and can be used as a prognostic indicator. Through quantitative lipidomics analysis, the author demonstrated altered levels of glycerol, glycerophospholipids and sphingolipids in matched tumour samples. It has been shown that glycerol and sphingolipids can discriminate among patients with distinct mismatch repair proficient and deficient statuses, oncogenic mutations (KRAS/BRAF), or grading [187]. Several diseases have multidimensions and networks of lipid molecules fused with genes and proteins in the molecular mechanism. Lipidomics platforms can be used to analyze and characterize these compounds. Furthermore, common and traditional disease diagnoses can be more difficult to identify therapeutic targets. However, lipidomics technology offers the possibility of easier diagnosis for certain types of diseases. A diagnosis of the disease can be made by lipidomics based on existing biomarkers as therapeutic agents [188,189]. It is also possible to study various protein-lipid interactions, lipid-lipid interactions, and lipid-gene interactions, enabling the development of better diagnostic procedures for advanced diseases. A lipidomics approach has been considered better than traditional approaches for disease investigation because it provides an understanding of systemic metabolisms and their mechanisms and precisely identifies therapeutic targets and diagnostic biomarkers [190].
8. Future Perspectives and Conclusions
It is well acknowledged that early cancer diagnosis would enhance patient prognosis and provide a greater knowledge of the disease, decrease mortality, and increase patient satisfaction. There has been a significant advancement in identifying new biomarkers in recent years, paving the way for a more personalized approach to the clinical diagnosis and treatment of CRC [19]. Several DNA, RNA, and protein-based cancer biomarkers have been developed recently through high-throughput research in cancer biomarkers that can be discovered from readily available biological samples such as blood, serum, urine, stool, and tissues. Technological advancements have improved the sensitivity and specificity of cancer-specific biomarkers in CRCs. However, traditional biomarkers in clinical practice do not have high specificities and sensitivity. Therefore, in order to develop an accurate and clinically useful test, it is recommended to discover multiple biomarker panels instead of a single biomarker. By identifying prospective new therapeutic intervention targets that might contribute to the diagnosis of CRC, it is possible to develop an alternative to conventional methods of early detection of cancer.
With the recent developments in high-throughput sequencing technologies, Increasingly, cancer researchers are relying on “multi-omics” data sets. Multi-omics combines a range of omics data sets, including genomics, proteomics, transcriptomics, metabolomics, and microbiomes, for analysis [191,192]. By combining quantitative analyses of multi-omics data and clinical features, we can get insight into alterations at the molecular level and gain a more comprehensive, systemic comprehension of various biological pathways [192,193]. By integrating multi-omics approaches, we can simultaneously uncover how information flows between different levels of omics. It will, therefore, help us to bridge and close the gap between genotypes and phenotypes data. With the advent of this Technology, colorectal cancer can be diagnosed, prognosed, treated, and prevented with greater accuracy in the future. Due to the huge amount of data available, multi-omics and big data analytics are required to interconnect all available information. In particular, integrating patient demographic, genomic, transcriptomic, proteomic, metabolomic, lipidomic, and microbiota data could assist in developing new biomarkers discovery and clinical outcomes prediction.
Another emerging field of multi-omics technologies is microbiomics which offers a non-traditional tool with potential applications in more significant comprehension of tumour biology. Identifying microbial metabolites correlated with the development of colorectal tumours has significant implications for identifying new treatment targets and possible biomarkers for disease screening [194]. There has been significant progress in recently using intestinal bacteria and their metabolites as early detection markers for CRC. The association between CRC and gut bacteria and their metabolites has received much attention recently. Moreover, the gut microbiota’s microbial metabolite composition is frequently renewed and changes depending on the diet, making it more amenable to therapeutic intervention in developing CRC. New paradigms in CRC diagnosis, prevention, and treatment will be provided by elucidating the role of microbial metabolites [15]. CRC prevention, comprehensive treatment planning, and minimizing adverse effects of treatment will be significantly impacted by the gut microbiome in the near future. Individuals’ gut microbiomes vary according to their geographic location, ethnicity, dietary habits, and lifestyles. In the future, clinical research will need to include several factors that contribute to the microbiota of patients, including geography, race, sex, and diet, as well as how systemic cancer treatment affects the microbiome, especially chemotherapy and immunotherapy [195].
In recent years, lipidomics has been actively used in the research community and regarded as a cutting-edge example of multi-Omics Technology. Particularly useful in analyzing the structure and function of lipid molecules to analyze changes in their dynamic composition during certain pathological and physiological changes. The alterations in lipid metabolism have also been linked to several kinds of cancer development and progression. Despite this, there is a limited understanding of the metabolic changes of lipids in cancer due to their structural diversity and characteristics, distinct from those of other biomolecules. Several analytical tools in cancer research have been used in lipidomic analysis to determine lipid composition at a large scale. Additionally, in cohort studies, glycero-, glycerophospho-, and sphingolipid levels have been significantly changed between tumours and normal tissue. A marked difference between cancerous and non-diseased tissue in sphingomyelin and triacylglycerol (TG) species [187]. Recent research demonstrated that GZMs (granzymes) proteins have a significant role in carcinogenesis, their role as new biomarkers for CRC prognosis and diagnosis will need further exploration [196]. Furthermore, it is imperative to emphasize the importance of lipidomics and proteomics research for discovering novel biomarkers and diagnosing CRCs. Integrating lipidomics with other omics, such as metabolomics, microbiomics, proteomics, etc., would provide a powerful tool that could help researchers identify novel therapeutic targets and biomarkers.
Many studies have been conducted using different multi-omics techniques and clinical samples to discover novel biomarkers. However, clearing up the mechanism of how markers are generated and their diagnostic value, a critical factor in drug discovery, remains a challenge. Combining multiple experimental approaches and then integrating the results is a valuable strategy to generalize human cancer’s complexity from experimental models [197]. The integration of multiple omics, such as genomics, proteomics, and metabolomics, will help us understand tumours and advance antitumor drug developments [198,199,200]. In addition, numerous studies have confirmed that developing high-throughput sequencing technologies has revolutionized multi-omics research. It is expected that multi-omics applications will increase in scope with the optimization and maturity of various technologies, making it possible to develop novel biomarkers for CRC due to multi-omics research.
Although multi-omics methods have great potential, there are still limitations and challenges to overcome. The first problem is that omics methods are expensive and require specialized equipment and high-level data analysis skills. There can also be problems in the collection of data and verification of the data because of unreliable data quality, inaccurate data sources, and nonstandard sampling. Currently, there are no standard research platforms or bioinformatics methods for the processing of large-scale omics datasets. Data processing and analysis are the biggest challenges in metabolomics studies because biological organisms contain thousands of metabolites. Additionally, numerous obstacles will need to be overcome in order to translate biomarker discoveries into clinical applications for CRC. It is still difficult to evaluate the specificity and sensitivity of candidate biomarkers due to the absence of strategies and selection panels. Due to the fact that most patient data come from different laboratories, it is also difficult to validate biomarker candidates in large cohorts of patients. To establish potential diagnostic biomarkers, further validation may be obtained through meta-analysis. Another obstacle to overcome is the heterogeneity of the patient population and their sporadic cancers. By performing advanced MS at single-cell resolution, this problem may be tackled by understanding the biological and molecular heterogeneity of disease states.
Abbreviations
| CBX8 | Chromobox 8 |
| CD96 | CD96 Molecule |
| 8MTUS1 | Microtubule Associated Scaffold Protein 1 |
| SDC2 | Syndecan 2 |
| NDRG4 | NDRG Family Member 4 |
| SOX21 | SRY-Box Transcription Factor 21 |
| BDNF | Brain-Derived Neurotrophic Factor |
| PTGS2 | Prostaglandin–Endoperoxide Synthase 2 |
| GSK3B | Glycogen Synthase Kinase 3 Beta |
| CTNNB1 | Catenin Beta 1 |
| HPGD | 15-Hydroxyprostaglandin Dehydrogenase |
| YWHAB | Tyrosine 3–Monooxygenase/Tryptophan 5–Monooxygenase Activation Protein Beta |
| MCM4, | Minichromosome Maintenance Complex Component 4 |
| FBXO46 | F-Box Protein 46 |
| DPP7/2 | Dipeptidyl Peptidase 7 |
| SDC2 | Syndecan 2 |
| TFPI2 | Tissue Factor Pathway Inhibitor 2 |
| SNORD15B | Small Nucleolar RNA, C/D Box 15B |
| SNORA5C | Small Nucleolar RNA, H/ACA Box 5C |
| GALR1 | Galanin Receptor 1 |
| LRRC19 | Leucine-rich repeat-containing protein 19 |
| GPR55 | G protein-coupled receptor 55 |
| CCAT2 | Colon Cancer Associated Transcript 2 |
| CCAT1 | Colon Cancer Associated Transcript 1 |
| H19 | H19 Imprinted Maternally Expressed Transcript |
| MALAT1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 |
| MEG3 | Maternally Expressed 3 |
| HULC | Hepatocellular Carcinoma Up-Regulated Long Non-Coding RNA |
| HOTAIR | HOX Transcript Antisense RNA |
| PCAT1 | Prostate Cancer Associated Transcript 1 |
| PTENP1 | Phosphatase And Tensin Homolog Pseudogene 1 |
| TUSC7 | Tumour Suppressor Candidate 7 |
| CHD 9 | Chromodomain Helicase DNA Binding Protein 9 |
| ACTBL2 | Actin Beta Like 2 |
| CDK3, | Cyclin Dependent Kinase 3 |
| CDK5 | Cyclin Dependent Kinase 5 |
| CDK8 | Cyclin-dependent kinase 8 |
| STK4 or MST1 | serine/threonine kinase 4 or Macrophage Stimulating 1 |
| MRC1 | Mannose Receptor C-Type 1 |
| S100A90 | S100 Calcium Binding Protein A9 |
| CEACAM-7 | CEA Cell Adhesion Molecule 7 |
| CEA | Carcinoembryonic antigen |
| SPG20 | spastic paraplegia 20 |
| STK31 | Serine/Threonine Kinase 31 |
| TPM3 | Tropomyosin 3 |
| FJX1 | Four-Jointed Box Kinase 1 |
| NOP14 | Nucleolar protein 14 |
| SPARCL1 | Secreted protein acidic and rich in cysteine-like 1 |
Author Contributions
Conceptualization, I.U. and X.-J.W.; writing—original draft preparation, I.U.; review and editing X.-J.W., L.Y. and Y.S.; literature search, F.-T.Y., X.-H.L. and J.L.; supervision, X.-J.W., funding acquisition, X.-J.W. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 81830110, 81903847, 32141005). And UNPYSCT-2020226 Heilongjiang province regular undergraduate higher education institution youth innovation talent training program project.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Yoshino T., Arnold D., Taniguchi H., Pentheroudakis G., Yamazaki K., Xu R.H., Kim T.W., Ismail F., Tan I.B., Yeh K.H., et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018;29:44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 4.Benson A.B., Venook A.P., Al-Hawary M.M., Arain M.A., Chen Y.J., Ciombor K.K., Cohen S., Cooper H.S., Deming D., Farkas L., et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:329–359. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 5.Iyer P., Deng M., Handorf E.A., Nakhoda S., Dotan E. Assessing Oncologists’ Adoption of Biomarker Testing in Metastatic Colorectal Cancer Using Real World Data. JNCI Cancer Spectr. 2022:pkac065. doi: 10.1093/jncics/pkac065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amatu A., Sartore-Bianchi A., Bencardino K., Pizzutilo E.G., Tosi F., Siena S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019;30((Suppl. S8)):viii5–viii15. doi: 10.1093/annonc/mdz383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marabelle A., Fakih M., Lopez J., Shah M., Shapira-Frommer R., Nakagawa K., Chung H.C., Kindler H.L., Lopez-Martin J.A., Miller W.H., Jr., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 8.Argilés G., Tabernero J., Labianca R., Hochhauser D., Salazar R., Iveson T., Laurent-Puig P., Quirke P., Yoshino T., Taieb J., et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1291–1305. doi: 10.1016/j.annonc.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Testing for Colorectal Cancer. How Is Colorectal Cancer Diagnosed? [(accessed on 12 September 2022)]; Available online: https://www.cancer.gov/types/colorectal/screening-fact-sheet.
- 10.Elsafi S.H., Alqahtani N.I., Zakary N.Y., Al Zahrani E.M. The sensitivity, specificity, predictive values, and likelihood ratios of fecal occult blood test for the detection of colorectal cancer in hospital settings. Clin. Exp. Gastroenterol. 2015;8:279–284. doi: 10.2147/CEG.S86419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan S.C.H., Liang J.Q. Advances in tests for colorectal cancer screening and diagnosis. Expert Rev. Mol. Diagn. 2022;22:449–460. doi: 10.1080/14737159.2022.2065197. [DOI] [PubMed] [Google Scholar]
- 12.Tan K.C., Ipcho S.V., Trengove R.D., Oliver R.P., Solomon P.S. Assessing the impact of transcriptomics, proteomics and metabolomics on fungal phytopathology. Mol. Plant Pathol. 2009;10:703–715. doi: 10.1111/j.1364-3703.2009.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalal N., Jalandra R., Sharma M., Prakash H., Makharia G.K., Solanki P.R., Singh R., Kumar A. Omics technologies for improved diagnosis and treatment of colorectal cancer: Technical advancement and major perspectives. Biomed. Pharmacother. 2020;131:110648. doi: 10.1016/j.biopha.2020.110648. [DOI] [PubMed] [Google Scholar]
- 14.Jahani-Sherafat S., Alebouyeh M., Moghim S., Ahmadi Amoli H., Ghasemian-Safaei H. Role of gut microbiota in the pathogenesis of colorectal cancer; a review article. Gastroenterol. Hepatol. Bed Bench. 2018;11:101–109. [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y., Nie Y., Yu J., Wong C.C. Microbial Metabolites in Colorectal Cancer: Basic and Clinical Implications. Metabolites. 2021;11:159. doi: 10.3390/metabo11030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontush A., Chapman M.J. Lipidomics as a tool for the study of lipoprotein metabolism. Curr. Atheroscler. Rep. 2010;12:194–201. doi: 10.1007/s11883-010-0100-0. [DOI] [PubMed] [Google Scholar]
- 17.Lam S.M., Shui G. Lipidomics as a principal tool for advancing biomedical research. J. Genet. Genom. 2013;40:375–390. doi: 10.1016/j.jgg.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Mato-Abad V., Pazos A., Munteanu C.R., Liñares-Blanco J., Alvarez-Gonzalez S., Vázquez-Naya J.M., Pedreira N., Amigo J., Fernandez-Lozano C. Foundations of Colorectal Cancer. Elsevier; Amsterdam, The Netherlands: 2022. Bioinformatic tools for research in CRC; pp. 231–247. [Google Scholar]
- 19.Lopez G., Boggio F., Ferrero S., Fusco N., Del Gobbo A. Molecular and Immunohistochemical Markers with Prognostic and Predictive Significance in Liver Metastases from Colorectal Carcinoma. Int. J. Mol. Sci. 2018;19:3014. doi: 10.3390/ijms19103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loomans-Kropp H.A., Umar A. Increasing Incidence of Colorectal Cancer in Young Adults. J. Cancer Epidemiol. 2019;2019:9841295. doi: 10.1155/2019/9841295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casamassimi A., Federico A., Rienzo M., Esposito S., Ciccodicola A. Transcriptome Profiling in Human Diseases: New Advances and Perspectives. Int. J. Mol. Sci. 2017;18:1652. doi: 10.3390/ijms18081652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler D.A., Wang L. From human genome to cancer genome: The first decade. Genome Res. 2013;23:1054–1062. doi: 10.1101/gr.157602.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Lázaro M. A new view of carcinogenesis and an alternative approach to cancer therapy. Mol. Med. 2010;16:144–153. doi: 10.2119/molmed.2009.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Supplitt S., Karpinski P., Sasiadek M., Laczmanska I. Current Achievements and Applications of Transcriptomics in Personalized Cancer Medicine. Int. J. Mol. Sci. 2021;22:1422. doi: 10.3390/ijms22031422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sardo E., Napolitano S., Della Corte C.M., Ciardiello D., Raucci A., Arrichiello G., Troiani T., Ciardiello F., Martinelli E., Martini G. Multi-Omic Approaches in Colorectal Cancer beyond Genomic Data. J. Pers. Med. 2022;12:128. doi: 10.3390/jpm12020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paleri P. Revisiting National Security: Prospecting Governance for Human Well-Being. Springer Nature; Berlin/Heidelberg, Germany: 2022. [Google Scholar]
- 27.Bunnik E.M., Le Roch K.G. An Introduction to Functional Genomics and Systems Biology. Adv. Wound Care. 2013;2:490–498. doi: 10.1089/wound.2012.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Przybyla L., Gilbert L.A. A new era in functional genomics screens. Nat. Rev. Genet. 2022;23:89–103. doi: 10.1038/s41576-021-00409-w. [DOI] [PubMed] [Google Scholar]
- 29.Definition of genomics. NCI Dictionary of Cancer Terms—National Cancer Institute. [(accessed on 12 September 2022)]; Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/genomics.
- 30.Bosch L.J., Carvalho B., Fijneman R.J., Jimenez C.R., Pinedo H.M., van Engeland M., Meijer G.A. Molecular tests for colorectal cancer screening. Clin. Colorectal. Cancer. 2011;10:8–23. doi: 10.3816/CCC.2011.n.002. [DOI] [PubMed] [Google Scholar]
- 31.Escobar-Zepeda A., Vera-Ponce de León A., Sanchez-Flores A. The Road to Metagenomics: From Microbiology to DNA Sequencing Technologies and Bioinformatics. Front. Genet. 2015;6:348. doi: 10.3389/fgene.2015.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gan X., Wang T., Chen Z.Y., Zhang K.H. Blood-derived molecular signatures as biomarker panels for the early detection of colorectal cancer. Mol. Biol. Rep. 2020;47:8159–8168. doi: 10.1007/s11033-020-05838-0. [DOI] [PubMed] [Google Scholar]
- 33.Ghatak S., Mehrabi S.F., Mehdawi L.M., Satapathy S.R., Sjölander A. Identification of a Novel Five-Gene Signature as a Prognostic and Diagnostic Biomarker in Colorectal Cancers. Int. J. Mol. Sci. 2022;23:793. doi: 10.3390/ijms23020793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Essa H.Y.S., Kusaf G., Yuruker O., Kalkan R. Epigenetic Alteration in Colorectal Cancer: A Biomarker for Diagnostic and Therapeutic Application. Glob. Med. Genet. 2022;9:258–262. doi: 10.1055/s-0042-1757404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 36.Singh M.P., Rai S., Suyal S., Singh S.K., Singh N.K., Agarwal A., Srivastava S. Genetic and epigenetic markers in colorectal cancer screening: Recent advances. Expert Rev. Mol. Diagn. 2017;17:665–685. doi: 10.1080/14737159.2017.1337511. [DOI] [PubMed] [Google Scholar]
- 37.Zamani M., Hosseini S.V., Mokarram P. Epigenetic biomarkers in colorectal cancer: Premises and prospects. Biomarkers. 2018;23:105–114. doi: 10.1080/1354750X.2016.1252961. [DOI] [PubMed] [Google Scholar]
- 38.Berdasco M., Esteller M. Clinical epigenetics: Seizing opportunities for translation. Nat. Rev. Genet. 2019;20:109–127. doi: 10.1038/s41576-018-0074-2. [DOI] [PubMed] [Google Scholar]
- 39.Galanopoulos M., Tsoukalas N., Papanikolaou I.S., Tolia M., Gazouli M., Mantzaris G.J. Abnormal DNA methylation as a cell-free circulating DNA biomarker for colorectal cancer detection: A review of literature. World J. Gastrointest. Oncol. 2017;9:142–152. doi: 10.4251/wjgo.v9.i4.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamurthy N., Spencer E., Torkamani A., Nicholson L. Liquid Biopsies for Cancer: Coming to a Patient near You. J. Clin. Med. 2017;6:3. doi: 10.3390/jcm6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belkhiri A., El-Rifai W. 5-Methylcytosine hydroxylation-mediated LINE-1 hypomethylation: A novel mechanism of proto-oncogenes activation in colorectal cancer? Gut. 2014;63:538–539. doi: 10.1136/gutjnl-2013-305176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichimura N., Shinjo K., An B., Shimizu Y., Yamao K., Ohka F., Katsushima K., Hatanaka A., Tojo M., Yamamoto E., et al. Aberrant TET1 Methylation Closely Associated with CpG Island Methylator Phenotype in Colorectal Cancer. Cancer Prev. Res. 2015;8:702–711. doi: 10.1158/1940-6207.CAPR-14-0306. [DOI] [PubMed] [Google Scholar]
- 43.Hur K., Cejas P., Feliu J., Moreno-Rubio J., Burgos E., Boland C.R., Goel A. Hypomethylation of long interspersed nuclear element-1 (LINE-1) leads to activation of proto-oncogenes in human colorectal cancer metastasis. Gut. 2014;63:635–646. doi: 10.1136/gutjnl-2012-304219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng R., Gao D., He T., Zhang M., Zhang X., Linghu E., Wei L., Guo M. Methylation of DIRAS1 promotes colorectal cancer progression and may serve as a marker for poor prognosis. Clin. Epigenetics. 2017;9:50. doi: 10.1186/s13148-017-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., He T., Herman J.G., Linghu E., Yang Y., Fuks F., Zhou F., Song L., Guo M. Methylation of ZNF331 is an independent prognostic marker of colorectal cancer and promotes colorectal cancer growth. Clin. Epigenetics. 2017;9:115. doi: 10.1186/s13148-017-0417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He T., Zhang M., Zheng R., Zheng S., Linghu E., Herman J.G., Guo M. Methylation of SLFN11 is a marker of poor prognosis and cisplatin resistance in colorectal cancer. Epigenomics. 2017;9:849–862. doi: 10.2217/epi-2017-0019. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y., Lu H., Zhang D., Li M., Sun X., Wan L., Yu D., Tian Y., Jin H., Lin A., et al. Integrated analyses of multi-omics reveal global patterns of methylation and hydroxymethylation and screen the tumor suppressive roles of HADHB in colorectal cancer. Clin. Epigenetics. 2018;10:30. doi: 10.1186/s13148-018-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasanthakumar A., Godley L.A. 5-hydroxymethylcytosine in cancer: Significance in diagnosis and therapy. Cancer Genet. 2015;208:167–177. doi: 10.1016/j.cancergen.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Su Y., Tian Y., Ding Y., Wang X. Characterization of DNA hydroxymethylation profile in cervical cancer. Artif. Cells Nanomed. Biotechnol. 2019;47:2706–2714. doi: 10.1080/21691401.2019.1634578. [DOI] [PubMed] [Google Scholar]
- 50.Hlady R.A., Sathyanarayan A., Thompson J.J., Zhou D., Wu Q., Pham K., Lee J.H., Liu C., Robertson K.D. Integrating the Epigenome to Identify Drivers of Hepatocellular Carcinoma. Hepatology. 2019;69:639–652. doi: 10.1002/hep.30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharyya S., Pradhan K., Campbell N., Mazdo J., Vasantkumar A., Maqbool S., Bhagat T.D., Gupta S., Suzuki M., Yu Y., et al. Altered hydroxymethylation is seen at regulatory regions in pancreatic cancer and regulates oncogenic pathways. Genome Res. 2017;27:1830–1842. doi: 10.1101/gr.222794.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomson J.P., Ottaviano R., Unterberger E.B., Lempiäinen H., Muller A., Terranova R., Illingworth R.S., Webb S., Kerr A.R., Lyall M.J., et al. Loss of Tet1-Associated 5-Hydroxymethylcytosine Is Concomitant with Aberrant Promoter Hypermethylation in Liver Cancer. Cancer Res. 2016;76:3097–3108. doi: 10.1158/0008-5472.CAN-15-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang A.C., Buckingham L., Barbanera W., Korang A.Y., Bishesari F., Melson J. LINE-1 is preferentially hypomethylated within adenomatous polyps in the presence of synchronous colorectal cancer. Clin. Epigenetics. 2017;9:25. doi: 10.1186/s13148-017-0325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song X., Tang T., Li C., Liu X., Zhou L. CBX8 and CD96 Are Important Prognostic Biomarkers of Colorectal Cancer. Med. Sci. Monit. 2018;24:7820–7827. doi: 10.12659/MSM.908656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng L.Y., Huang M.S., Zhong H.G., Ru H.M., Mo S.S., Wei C.Y., Su Z.J., Mo X.W., Yan L.H., Tang W.Z. MTUS1 is a promising diagnostic and prognostic biomarker for colorectal cancer. World J. Surg. Oncol. 2022;20:257. doi: 10.1186/s12957-022-02702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin S., Ye Q., Hong Y., Dai W., Zhang C., Liu W., Guo Y., Zhu D., Zhang Z., Chen S., et al. A systematic evaluation of stool DNA preparation protocols for colorectal cancer screening via analysis of DNA methylation biomarkers. Clin. Chem. Lab. Med. 2020;59:91–99. doi: 10.1515/cclm-2020-0300. [DOI] [PubMed] [Google Scholar]
- 57.Moradi K., Babaei E., Hosseinpour Feizi M.A., Safaralizadeh R., Rezvani N. Quantitative detection of SRY-Box 21 (SOX21) gene promoter methylation as a stool-based noninvasive biomarker for early diagnosis of colorectal cancer by MethyLight method. Indian J. Cancer. 2021;58:217–224. doi: 10.4103/ijc.IJC_37_19. [DOI] [PubMed] [Google Scholar]
- 58.Ahluwalia P., Mondal A.K., Bloomer C., Fulzele S., Jones K., Ananth S., Gahlay G.K., Heneidi S., Rojiani A.M., Kota V., et al. Identification and Clinical Validation of a Novel 4 Gene-Signature with Prognostic Utility in Colorectal Cancer. Int. J. Mol. Sci. 2019;20:3818. doi: 10.3390/ijms20153818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W., Yang C., Wang S., Xiang Z., Dou R., Lin Z., Zheng J., Xiong B. SDC2 and TFPI2 Methylation in Stool Samples as an Integrated Biomarker for Early Detection of Colorectal Cancer. Cancer Manag. Res. 2021;13:3601–3617. doi: 10.2147/CMAR.S300861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen L., Lu W., Huang Y., He J., Wang Q., Zheng X., Wang Z. SNORD15B and SNORA5C: Novel Diagnostic and Prognostic Biomarkers for Colorectal Cancer. Biomed Res. Int. 2022;2022:8260800. doi: 10.1155/2022/8260800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu S., Qian S., Lin S., Ye D., Li Q., Yang J., Ying X., Li Z., Tang M., Wang J., et al. Promoter hypermethylation of GALR1 acts as an early epigenetic susceptibility event in colorectal carcinogenesis. J. Hum. Genet. 2022;67:519–525. doi: 10.1038/s10038-022-01038-9. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y.J., Liu M., Jiang H.Y., Yu Y.W. Downregulation of LRRC19 Is Associated with Poor Prognosis in Colorectal Cancer. J. Oncol. 2022;2022:5848823. doi: 10.1155/2022/5848823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alizadeh-Sedigh M., Mahmoodzadeh H., Fazeli M.S., Haddadi-Aghdam M., Teimoori-Toolabi L. The potential of PIK3CA, KRAS, BRAF, and APC hotspot mutations as a non-invasive detection method for colorectal cancer. Mol. Cell. Probes. 2022;63:101807. doi: 10.1016/j.mcp.2022.101807. [DOI] [PubMed] [Google Scholar]
- 64.Mantione K.J., Kream R.M., Kuzelova H., Ptacek R., Raboch J., Samuel J.M., Stefano G.B. Comparing bioinformatic gene expression profiling methods: Microarray and RNA-Seq. Med. Sci. Monit. Basic Res. 2014;20:138–142. doi: 10.12659/msmbr.892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowe R., Shirley N., Bleackley M., Dolan S., Shafee T. Transcriptomics technologies. PLoS Comput. Biol. 2017;13:e1005457. doi: 10.1371/journal.pcbi.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z., Gerstein M., Snyder M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacquier A. The complex eukaryotic transcriptome: Unexpected pervasive transcription and novel small RNAs. Nat. Rev. Genet. 2009;10:833–844. doi: 10.1038/nrg2683. [DOI] [PubMed] [Google Scholar]
- 68.Popov D.V., Makhnovskii P.A., Shagimardanova E.I., Gazizova G.R., Lysenko E.A., Gusev O.A., Vinogradova O.L. Contractile activity-specific transcriptome response to acute endurance exercise and training in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2019;316:E605–E614. doi: 10.1152/ajpendo.00449.2018. [DOI] [PubMed] [Google Scholar]
- 69.Iwata M., Yuan L., Zhao Q., Tabei Y., Berenger F., Sawada R., Akiyoshi S., Hamano M., Yamanishi Y. Predicting drug-induced transcriptome responses of a wide range of human cell lines by a novel tensor-train decomposition algorithm. Bioinformatics. 2019;35:i191–i199. doi: 10.1093/bioinformatics/btz313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jaeger P.A., Doherty C., Ideker T. Modeling transcriptome dynamics in a complex world. Cell. 2012;151:1161–1162. doi: 10.1016/j.cell.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 71.Mallardo M., Poltronieri P., D’Urso O.F. Non-protein coding RNA biomarkers and differential expression in cancers: A review. J. Exp. Clin. Cancer Res. 2008;27:19. doi: 10.1186/1756-9966-27-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bashiardes S., Zilberman-Schapira G., Elinav E. Use of Metatranscriptomics in Microbiome Research. Bioinform. Biol. Insights. 2016;10:19–25. doi: 10.4137/BBI.S34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slaby O., Svoboda M., Michalek J., Vyzula R. MicroRNAs in colorectal cancer: Translation of molecular biology into clinical application. Mol. Cancer. 2009;8:102. doi: 10.1186/1476-4598-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schee K., Lorenz S., Worren M.M., Günther C.C., Holden M., Hovig E., Fodstad O., Meza-Zepeda L.A., Flatmark K. Deep Sequencing the MicroRNA Transcriptome in Colorectal Cancer. PLoS ONE. 2013;8:e66165. doi: 10.1371/journal.pone.0066165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun Y., Shen S., Tang H., Xiang J., Peng Y., Tang A., Li N., Zhou W., Wang Z., Zhang D., et al. miR-429 identified by dynamic transcriptome analysis is a new candidate biomarker for colorectal cancer prognosis. Omics. 2014;18:54–64. doi: 10.1089/omi.2012.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen E., Li Q., Wang H., Yang F., Min L., Yang J. MiR-92a promotes tumorigenesis of colorectal cancer, a transcriptomic and functional based study. Biomed Pharmacother. 2018;106:1370–1377. doi: 10.1016/j.biopha.2018.07.098. [DOI] [PubMed] [Google Scholar]
- 77.Durán-Vinet B., Araya-Castro K., Calderón J., Vergara L., Weber H., Retamales J., Araya-Castro P., Leal-Rojas P. CRISPR/Cas13-Based Platforms for a Potential Next-Generation Diagnosis of Colorectal Cancer through Exosomes Micro-RNA Detection: A Review. Cancers. 2021;13:4640. doi: 10.3390/cancers13184640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallardo-Gómez M., De Chiara L., Álvarez-Chaver P., Cubiella J. Colorectal cancer screening and diagnosis: Omics-based technologies for development of a non-invasive blood-based method. Expert Rev. Anticancer. Ther. 2021;21:723–738. doi: 10.1080/14737140.2021.1882858. [DOI] [PubMed] [Google Scholar]
- 79.Chen E., Li Q., Wang H., Zhang P., Zhao X., Yang F., Yang J. MiR-32 promotes tumorigenesis of colorectal cancer by targeting BMP5. Biomed. Pharmacother. 2018;106:1046–1051. doi: 10.1016/j.biopha.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 80.Yamada A., Yu P., Lin W., Okugawa Y., Boland C.R., Goel A. A RNA-Sequencing approach for the identification of novel long non-coding RNA biomarkers in colorectal cancer. Sci. Rep. 2018;8:575. doi: 10.1038/s41598-017-18407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Z., Jia H., Gu T., Hu Q., Yu J., Zang D., Song N., Wang H. RNA sequencing and bioinformatics analysis of the long noncoding RNA-mRNA network in colorectal cancer. J. Cell. Biochem. 2018;119:9957–9966. doi: 10.1002/jcb.27319. [DOI] [PubMed] [Google Scholar]
- 82.Jang J.E., Kim H.P., Han S.W., Jang H., Lee S.H., Song S.H., Bang D., Kim T.Y. NFATC3-PLA2G15 Fusion Transcript Identified by RNA Sequencing Promotes Tumor Invasion and Proliferation in Colorectal Cancer Cell Lines. Cancer Res. Treat. 2019;51:391–401. doi: 10.4143/crt.2018.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Y., Wang X., Wu F., Huang R., Xue F., Liang G., Tao M., Cai P., Huang Y. Transcriptome profiling of the cancer, adjacent non-tumor and distant normal tissues from a colorectal cancer patient by deep sequencing. PLoS ONE. 2012;7:e41001. doi: 10.1371/journal.pone.0041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kashima Y., Sakamoto Y., Kaneko K., Seki M., Suzuki Y., Suzuki A. Single-cell sequencing techniques from individual to multiomics analyses. Exp. Mol. Med. 2020;52:1419–1427. doi: 10.1038/s12276-020-00499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haque A., Engel J., Teichmann S.A., Lönnberg T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017;9:75. doi: 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee J., Hyeon D.Y., Hwang D. Single-cell multiomics: Technologies and data analysis methods. Exp. Mol. Med. 2020;52:1428–1442. doi: 10.1038/s12276-020-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Picelli S. Single-cell RNA-sequencing: The future of genome biology is now. RNA Biol. 2017;14:637–650. doi: 10.1080/15476286.2016.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pelka K., Hofree M., Chen J.H., Sarkizova S., Pirl J.D., Jorgji V., Bejnood A., Dionne D., Ge W.H., Xu K.H., et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021;184:4734–4752.e4720. doi: 10.1016/j.cell.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis R.T., Blake K., Ma D., Gabra M.B.I., Hernandez G.A., Phung A.T., Yang Y., Maurer D., Lefebvre A., Alshetaiwi H., et al. Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat. Cell Biol. 2020;22:310–320. doi: 10.1038/s41556-020-0477-0. [DOI] [PubMed] [Google Scholar]
- 90.Shalek A.K., Benson M. Single-cell analyses to tailor treatments. Sci. Transl. Med. 2017;9:eaan4730. doi: 10.1126/scitranslmed.aan4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu G.H., Zhou Z.G., Chen R., Wang M.J., Zhou B., Li Y., Sun X.F. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour. Biol. 2013;34:2175–2181. doi: 10.1007/s13277-013-0753-8. [DOI] [PubMed] [Google Scholar]
- 92.Wang J., Li X., Lu L., He L., Hu H., Xu Z. Circular RNA hsa_circ_0000567 can be used as a promising diagnostic biomarker for human colorectal cancer. J. Clin. Lab. Anal. 2018;32:e22379. doi: 10.1002/jcla.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohammadi D., Zafari Y., Estaki Z., Mehrabi M., Moghbelinejad S. Evaluation of plasma circ_0006282 as a novel diagnostic biomarker in colorectal cancer. J. Clin. Lab. Anal. 2022;36:e24147. doi: 10.1002/jcla.24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li J., Song Y., Wang J., Huang J. Plasma circular RNA panel acts as a novel diagnostic biomarker for colorectal cancer detection. Am. J. Transl. Res. 2020;12:7395–7403. [PMC free article] [PubMed] [Google Scholar]
- 95.Iwasaki H., Shimura T., Kitagawa M., Yamada T., Nishigaki R., Fukusada S., Okuda Y., Katano T., Horike S.I., Kataoka H. A Novel Urinary miRNA Biomarker for Early Detection of Colorectal Cancer. Cancers. 2022;14:461. doi: 10.3390/cancers14020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ismail H.T.H., AbdelMageed M., Lindmark G., Hammarström M.L., Hammarström S., Sitohy B. Prognostic Significance of GPR55 mRNA Expression in Colon Cancer. Int. J. Mol. Sci. 2022;23:4556. doi: 10.3390/ijms23094556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang E., Jung S.C., Nam S.K., Park Y., Seo S.H., Park K.U., Oh H.K., Kim D.W., Kang S.B., Lee H.S. Tissue miR-200c-3p and circulating miR-1290 as potential prognostic biomarkers for colorectal cancer. Sci. Rep. 2022;12:2295. doi: 10.1038/s41598-022-06192-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu X., Xu X., Pan B., He B., Chen X., Zeng K., Xu M., Pan Y., Sun H., Xu T., et al. Circulating miR-1290 and miR-320d as Novel Diagnostic Biomarkers of Human Colorectal Cancer. J. Cancer. 2019;10:43–50. doi: 10.7150/jca.26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang H., Zhu M., Shan X., Zhou X., Wang T., Zhang J., Tao J., Cheng W., Chen G., Li J., et al. A panel of seven-miRNA signature in plasma as potential biomarker for colorectal cancer diagnosis. Gene. 2019;687:246–254. doi: 10.1016/j.gene.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 100.Gharib E., Nazemalhosseini-Mojarad E., Baghdar K., Nayeri Z., Sadeghi H., Rezasoltani S., Jamshidi-Fard A., Larki P., Sadeghi A., Hashemi M., et al. Identification of a stool long non-coding RNAs panel as a potential biomarker for early detection of colorectal cancer. J. Clin. Lab. Anal. 2021;35:e23601. doi: 10.1002/jcla.23601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahmed F.E., Ahmed N.C., Gouda M.M., Vos P.W., Bonnerup C. RT-qPCR for Fecal Mature MicroRNA Quantification and Validation. Methods Mol. Biol. 2018;1765:203–215. doi: 10.1007/978-1-4939-7765-9_13. [DOI] [PubMed] [Google Scholar]
- 102.Ghazanfar S., Fatima I., Aslam M., Musharraf S.G., Sherman N.E., Moskaluk C., Fox J.W., Akhtar M.W., Sadaf S. Identification of actin beta-like 2 (ACTBL2) as novel, upregulated protein in colorectal cancer. J. Proteom. 2017;152:33–40. doi: 10.1016/j.jprot.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 103.Hao J.J., Zhi X., Wang Y., Zhang Z., Hao Z., Ye R., Tang Z., Qian F., Wang Q., Zhu J. Comprehensive Proteomic Characterization of the Human Colorectal Carcinoma Reveals Signature Proteins and Perturbed Pathways. Sci. Rep. 2017;7:42436. doi: 10.1038/srep42436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamamoto T., Kudo M., Peng W.X., Takata H., Takakura H., Teduka K., Fujii T., Mitamura K., Taga A., Uchida E., et al. Identification of aldolase A as a potential diagnostic biomarker for colorectal cancer based on proteomic analysis using formalin-fixed paraffin-embedded tissue. Tumour. Biol. 2016;37:13595–13606. doi: 10.1007/s13277-016-5275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Torres S., Bartolomé R.A., Mendes M., Barderas R., Fernandez-Aceñero M.J., Peláez-García A., Peña C., Lopez-Lucendo M., Villar-Vázquez R., de Herreros A.G., et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin. Cancer Res. 2013;19:6006–6019. doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 106.Alnabulsi A., Murray G.I. Proteomics for early detection of colorectal cancer: Recent updates. Expert Rev. Proteom. 2018;15:55–63. doi: 10.1080/14789450.2018.1396893. [DOI] [PubMed] [Google Scholar]
- 107.Ganepola G.A., Nizin J., Rutledge J.R., Chang D.H. Use of blood-based biomarkers for early diagnosis and surveillance of colorectal cancer. World J. Gastrointest. Oncol. 2014;6:83–97. doi: 10.4251/wjgo.v6.i4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ivancic M.M., Megna B.W., Sverchkov Y., Craven M., Reichelderfer M., Pickhardt P.J., Sussman M.R., Kennedy G.D. Noninvasive Detection of Colorectal Carcinomas Using Serum Protein Biomarkers. J. Surg. Res. 2020;246:160–169. doi: 10.1016/j.jss.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhardwaj M., Gies A., Weigl K., Tikk K., Benner A., Schrotz-King P., Borchers C.H., Brenner H. Evaluation and Validation of Plasma Proteins Using Two Different Protein Detection Methods for Early Detection of Colorectal Cancer. Cancers. 2019;11:1426. doi: 10.3390/cancers11101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peltier J., Roperch J.P., Audebert S., Borg J.P., Camoin L. Quantitative proteomic analysis exploring progression of colorectal cancer: Modulation of the serpin family. J. Proteom. 2016;148:139–148. doi: 10.1016/j.jprot.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 111.Zajkowska M., Dulewicz M., Kulczyńska-Przybik A., Safiejko K., Juchimiuk M., Konopko M., Kozłowski L., Mroczko B. The Significance of Selected C-C Motif Chemokine Ligands in Colorectal Cancer Patients. J. Clin. Med. 2022;11:1794. doi: 10.3390/jcm11071794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quesada-Calvo F., Massot C., Bertrand V., Longuespée R., Blétard N., Somja J., Mazzucchelli G., Smargiasso N., Baiwir D., De Pauw-Gillet M.C., et al. OLFM4, KNG1 and Sec24C identified by proteomics and immunohistochemistry as potential markers of early colorectal cancer stages. Clin. Proteom. 2017;14:9. doi: 10.1186/s12014-017-9143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chantaraamporn J., Champattanachai V., Khongmanee A., Verathamjamras C., Prasongsook N., Mingkwan K., Luevisadpibul V., Chutipongtanate S., Svasti J. Glycoproteomic Analysis Reveals Aberrant Expression of Complement C9 and Fibronectin in the Plasma of Patients with Colorectal Cancer. Proteomes. 2020;8:26. doi: 10.3390/proteomes8030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fan N.J., Chen H.M., Song W., Zhang Z.Y., Zhang M.D., Feng L.Y., Gao C.F. Macrophage mannose receptor 1 and S100A9 were identified as serum diagnostic biomarkers for colorectal cancer through a label-free quantitative proteomic analysis. Cancer Biomark. 2016;16:235–243. doi: 10.3233/CBM-150560. [DOI] [PubMed] [Google Scholar]
- 115.Łukaszewicz-Zając M., Pączek S., Mroczko P., Kulczyńska-Przybik A. The Significance of CXCL1 and CXCL8 as Well as Their Specific Receptors in Colorectal Cancer. Cancer Manag. Res. 2020;12:8435–8443. doi: 10.2147/CMAR.S267176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pączek S., Łukaszewicz-Zając M., Gryko M., Mroczko P., Kulczyńska-Przybik A., Mroczko B. CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. Int. J. Mol. Sci. 2020;21:2040. doi: 10.3390/ijms21062040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu L., Peng H., Huang X.X., Xia Y.B., Hu K.F., Zhang Z.M. Decreased expression of chromodomain helicase DNA-binding protein 9 is a novel independent prognostic biomarker for colorectal cancer. Braz. J. Med. Biol. Res. 2018;51:e7588. doi: 10.1590/1414-431x20187588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang D., Zhou Y., Hua L., Li J., Zhu N., Liu Y. CDK3, CDK5 and CDK8 Proteins as Prognostic and Potential Biomarkers in Colorectal Cancer Patients. Int. J. Gen. Med. 2022;15:2233–2245. doi: 10.2147/IJGM.S349576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu J., Zhai X., Li X., Zhong C., Guo C., Yang F., Yuan Y., Zheng S. Identification of MST1 as a potential early detection biomarker for colorectal cancer through a proteomic approach. Sci. Rep. 2017;7:14265. doi: 10.1038/s41598-017-14539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tu C., Mojica W., Straubinger R.M., Li J., Shen S., Qu M., Nie L., Roberts R., An B., Qu J. Quantitative proteomic profiling of paired cancerous and normal colon epithelial cells isolated freshly from colorectal cancer patients. Proteom. Clin. Appl. 2017;11:1600155. doi: 10.1002/prca.201600155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H., Zhao S., Jing Z., Li J., Shuanying Y., Zhang N. Combination of D-dimer and carcinoembryonic antigen levels as a predictive and prognostic biomarker in advanced colorectal cancer patients. J. Cell. Biochem. 2018;120:8086–8092. doi: 10.1002/jcb.28087. [DOI] [PubMed] [Google Scholar]
- 122.Hassan N.A., Idriss N.K., Gaber N., Ibrahim A., Tawfeek M.A., Mossad E., Mosa A.A., Ahmed E.H., Sayed S.A., Ahmed H.A., et al. Spastic Paraplegia 20 and Serine/Threonine Protein Kinase 31 Expression for the Detection of Colorectal Cancer. Cell. Physiol. Biochem. 2022;56:138–149. doi: 10.33594/000000509. [DOI] [PubMed] [Google Scholar]
- 123.Watany M.M., Elmashad N.M., Badawi R., Hawash N. Serum FBLN1 and STK31 as biomarkers of colorectal cancer and their ability to noninvasively differentiate colorectal cancer from benign polyps. Clin. Chim. Acta. 2018;483:151–155. doi: 10.1016/j.cca.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 124.Liu L., Huang Y., Li Y., Wang Q., Hao Y., Liu L., Yao X., Yao X., Wei Y., Sun X., et al. FJX1 as a candidate diagnostic and prognostic serum biomarker for colorectal cancer. Clin. Transl. Oncol. 2022;24:1964–1974. doi: 10.1007/s12094-022-02852-5. [DOI] [PubMed] [Google Scholar]
- 125.Lu C., Liao W., Huang Y., Huang Y., Luo Y. Increased expression of NOP14 is associated with improved prognosis due to immune regulation in colorectal cancer. BMC Gastroenterol. 2022;22:207. doi: 10.1186/s12876-022-02286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang H.P., Wu J., Liu Z.F., Gao J.W., Li S.Y. SPARCL1 Is a Novel Prognostic Biomarker and Correlates with Tumor Microenvironment in Colorectal Cancer. Biomed Res. Int. 2022;2022:1398268. doi: 10.1155/2022/1398268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y., Cheuk-Hay Lau H., Cheng W.Y., Yu J. Gut microbiome in colorectal cancer: Clinical diagnosis and treatment. Genom. Proteom. Bioinform. 2022 doi: 10.1016/j.gpb.2022.07.002. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong S.H., Yu J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019;16:690–704. doi: 10.1038/s41575-019-0209-8. [DOI] [PubMed] [Google Scholar]
- 129.Ternes D., Karta J., Tsenkova M., Wilmes P., Haan S., Letellier E. Microbiome in Colorectal Cancer: How to Get from Meta-omics to Mechanism? Trends Microbiol. 2020;28:401–423. doi: 10.1016/j.tim.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 130.Inamura K. Colorectal Cancers: An Update on Their Molecular Pathology. Cancers. 2018;10:26. doi: 10.3390/cancers10010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grenham S., Clarke G., Cryan J.F., Dinan T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holmes E., Li J.V., Marchesi J.R., Nicholson J.K. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16:559–564. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 134.Clos-Garcia M., Garcia K., Alonso C., Iruarrizaga-Lejarreta M., D’Amato M., Crespo A., Iglesias A., Cubiella J., Bujanda L., Falcón-Pérez J.M. Integrative Analysis of Fecal Metagenomics and Metabolomics in Colorectal Cancer. Cancers. 2020;12:1142. doi: 10.3390/cancers12051142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zeller G., Tap J., Voigt A.Y., Sunagawa S., Kultima J.R., Costea P.I., Amiot A., Böhm J., Brunetti F., Habermann N., et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 2014;10:766. doi: 10.15252/msb.20145645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zackular J.P., Rogers M.A., Ruffin M.T.t., Schloss P.D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 2014;7:1112–1121. doi: 10.1158/1940-6207.CAPR-14-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang J., Li D., Yang Z., Dai W., Feng X., Liu Y., Jiang Y., Li P., Li Y., Tang B., et al. Establishing high-accuracy biomarkers for colorectal cancer by comparing fecal microbiomes in patients with healthy families. Gut Microbes. 2020;11:918–929. doi: 10.1080/19490976.2020.1712986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liang J.Q., Li T., Nakatsu G., Chen Y.X., Yau T.O., Chu E., Wong S., Szeto C.H., Ng S.C., Chan F.K.L., et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut. 2020;69:1248–1257. doi: 10.1136/gutjnl-2019-318532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guo S., Li L., Xu B., Li M., Zeng Q., Xiao H., Xue Y., Wu Y., Wang Y., Liu W., et al. A Simple and Novel Fecal Biomarker for Colorectal Cancer: Ratio of Fusobacterium Nucleatum to Probiotics Populations, Based on Their Antagonistic Effect. Clin. Chem. 2018;64:1327–1337. doi: 10.1373/clinchem.2018.289728. [DOI] [PubMed] [Google Scholar]
- 140.McQuade J.L., Daniel C.R., Helmink B.A., Wargo J.A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20:e77–e91. doi: 10.1016/S1470-2045(18)30952-5. [DOI] [PubMed] [Google Scholar]
- 141.Poore G.D., Kopylova E., Zhu Q., Carpenter C., Fraraccio S., Wandro S., Kosciolek T., Janssen S., Metcalf J., Song S.J., et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579:567–574. doi: 10.1038/s41586-020-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li J., Zhang A.H., Wu F.F., Wang X.J. Alterations in the Gut Microbiota and Their Metabolites in Colorectal Cancer: Recent Progress and Future Prospects. Front. Oncol. 2022;12:841552. doi: 10.3389/fonc.2022.841552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiao Q., Lu W., Kong X., Shao Y.W., Hu Y., Wang A., Bao H., Cao R., Liu K., Wang X., et al. Alterations of circulating bacterial DNA in colorectal cancer and adenoma: A proof-of-concept study. Cancer Lett. 2021;499:201–208. doi: 10.1016/j.canlet.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 144.Wang X.Q., Zhang A.H., Miao J.H., Sun H., Yan G.L., Wu F.F., Wang X.J. Gut microbiota as important modulator of metabolism in health and disease. RSC Adv. 2018;8:42380–42389. doi: 10.1039/C8RA08094A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen F., Dai X., Zhou C.C., Li K.X., Zhang Y.J., Lou X.Y., Zhu Y.M., Sun Y.L., Peng B.X., Cui W. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut. 2022;71:1315–1325. doi: 10.1136/gutjnl-2020-323476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jian H., Qinglian Z. Correlation between plasma trimethylamine oxide levels and colorectal neoplastic lesions. J. Dig. Oncol. 2020;12:275–278. [Google Scholar]
- 147.Haixia Z. Serum Folate and Fecal Short-Chain Fatty Acid Levels and Expression of Tissue Nuclear Stem Factor and Proliferating Cell Nuclear Antigen in Patients with Colorectal Cancer. Third Military Medical University; Chongqing, China: 2011. [Google Scholar]
- 148.Peinan L. Study on the Correlation between Left and Right Colon Cancer and Bile Acid Metabolism. Nanjing Medical University; Nanjing, China: 2018. [Google Scholar]
- 149.Yu H. The Role of Intestinal Flora in the Pathogenesis of Colorectal Cancer and Its Clinical Diagnostic Value Based on Multi-Omics. Chinese People’s Liberation Army Naval Medical University; Beijing, China: 2019. [Google Scholar]
- 150.Weir T.L., Manter D.K., Sheflin A.M., Barnett B.A., Heuberger A.L., Ryan E.P. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS ONE. 2013;8:e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brown D.G., Rao S., Weir T.L., O’Malia J., Bazan M., Brown R.J., Ryan E.P. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016;4:11. doi: 10.1186/s40170-016-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin Y., Ma C., Bezabeh T., Wang Z., Liang J., Huang Y., Zhao J., Liu X., Ye W., Tang W., et al. (1) H NMR-based metabolomics reveal overlapping discriminatory metabolites and metabolic pathway disturbances between colorectal tumor tissues and fecal samples. Int. J. Cancer. 2019;145:1679–1689. doi: 10.1002/ijc.32190. [DOI] [PubMed] [Google Scholar]
- 153.Manna S.K., Tanaka N., Krausz K.W., Haznadar M., Xue X., Matsubara T., Bowman E.D., Fearon E.R., Harris C.C., Shah Y.M., et al. Biomarkers of coordinate metabolic reprogramming in colorectal tumors in mice and humans. Gastroenterology. 2014;146:1313–1324. doi: 10.1053/j.gastro.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Y., Misra B.B., Liang L., Bi D., Weng W., Wu W., Cai S., Qin H., Goel A., Li X., et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics. 2019;9:4101–4114. doi: 10.7150/thno.35186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wong S.H., Kwong T.N.Y., Chow T.C., Luk A.K.C., Dai R.Z.W., Nakatsu G., Lam T.Y.T., Zhang L., Wu J.C.Y., Chan F.K.L., et al. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut. 2017;66:1441–1448. doi: 10.1136/gutjnl-2016-312766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shah M.S., DeSantis T.Z., Weinmaier T., McMurdie P.J., Cope J.L., Altrichter A., Yamal J.M., Hollister E.B. Leveraging sequence-based faecal microbial community survey data to identify a composite biomarker for colorectal cancer. Gut. 2018;67:882–891. doi: 10.1136/gutjnl-2016-313189. [DOI] [PubMed] [Google Scholar]
- 157.Xu J., Yang M., Wang D., Zhang S., Yan S., Zhu Y., Chen W. Alteration of the abundance of Parvimonas micra in the gut along the adenoma-carcinoma sequence. Oncol. Lett. 2020;20:106. doi: 10.3892/ol.2020.11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Coker O.O., Liu C., Wu W.K.K., Wong S.H., Jia W., Sung J.J.Y., Yu J. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome. 2022;10:35. doi: 10.1186/s40168-021-01208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu Y., Jiao N., Zhu R., Zhang Y., Wu D., Wang A.J., Fang S., Tao L., Li Y., Cheng S., et al. Identification of microbial markers across populations in early detection of colorectal cancer. Nat. Commun. 2021;12:3063. doi: 10.1038/s41467-021-23265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eklöf V., Löfgren-Burström A., Zingmark C., Edin S., Larsson P., Karling P., Alexeyev O., Rutegård J., Wikberg M.L., Palmqvist R. Cancer-associated fecal microbial markers in colorectal cancer detection. Int. J. Cancer. 2017;141:2528–2536. doi: 10.1002/ijc.31011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Song E.M., Byeon J.S., Lee S.M., Yoo H.J., Kim S.J., Lee S.H., Chang K., Hwang S.W., Yang D.H., Jeong J.Y. Fecal Fatty Acid Profiling as a Potential New Screening Biomarker in Patients with Colorectal Cancer. Dig. Dis. Sci. 2018;63:1229–1236. doi: 10.1007/s10620-018-4982-y. [DOI] [PubMed] [Google Scholar]
- 162.Nishiumi S., Kobayashi T., Kawana S., Unno Y., Sakai T., Okamoto K., Yamada Y., Sudo K., Yamaji T., Saito Y., et al. Investigations in the possibility of early detection of colorectal cancer by gas chromatography/triple-quadrupole mass spectrometry. Oncotarget. 2017;8:17115–17126. doi: 10.18632/oncotarget.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ning W., Qiao N., Zhang X., Pei D., Wang W. Metabolic profiling analysis for clinical urine of colorectal cancer. Asia Pac. J. Clin. Oncol. 2021;17:403–413. doi: 10.1111/ajco.13591. [DOI] [PubMed] [Google Scholar]
- 164.Troisi J., Cavallo P., Colucci A., Pierri L., Scala G., Symes S., Jones C., Richards S. Metabolomics in genetic testing. Adv. Clin. Chem. 2020;94:85–153. doi: 10.1016/bs.acc.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 165.Zhang A., Sun H., Yan G., Wang P., Wang X. Metabolomics for Biomarker Discovery: Moving to the Clinic. Biomed Res. Int. 2015;2015:354671. doi: 10.1155/2015/354671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dunn W.B., Broadhurst D.I., Atherton H.J., Goodacre R., Griffin J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011;40:387–426. doi: 10.1039/B906712B. [DOI] [PubMed] [Google Scholar]
- 167.Ivanisevic J., Want E.J. From Samples to Insights into Metabolism: Uncovering Biologically Relevant Information in LC-HRMS Metabolomics Data. Metabolites. 2019;9:308. doi: 10.3390/metabo9120308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vignoli A., Ghini V., Meoni G., Licari C., Takis P.G., Tenori L., Turano P., Luchinat C. High-Throughput Metabolomics by 1D NMR. Angew Chem. Int. Ed. Engl. 2019;58:968–994. doi: 10.1002/anie.201804736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Commisso M., Strazzer P., Toffali K., Stocchero M., Guzzo F. Untargeted metabolomics: An emerging approach to determine the composition of herbal products. Comput. Struct. Biotechnol. J. 2013;4:e201301007. doi: 10.5936/csbj.201301007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Johnson C.H., Ivanisevic J., Siuzdak G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim E.R., Kwon H.N., Nam H., Kim J.J., Park S., Kim Y.H. Urine-NMR metabolomics for screening of advanced colorectal adenoma and early stage colorectal cancer. Sci. Rep. 2019;9:4786. doi: 10.1038/s41598-019-41216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Daniluk U., Daniluk J., Kucharski R., Kowalczyk T., Pietrowska K., Samczuk P., Filimoniuk A., Kretowski A., Lebensztejn D., Ciborowski M. Untargeted Metabolomics and Inflammatory Markers Profiling in Children With Crohn’s Disease and Ulcerative Colitis-A Preliminary Study. Inflamm. Bowel Dis. 2019;25:1120–1128. doi: 10.1093/ibd/izy402. [DOI] [PubMed] [Google Scholar]
- 173.Wang H., Tso V.K., Slupsky C.M., Fedorak R.N. Metabolomics and detection of colorectal cancer in humans: A systematic review. Future Oncol. 2010;6:1395–1406. doi: 10.2217/fon.10.107. [DOI] [PubMed] [Google Scholar]
- 174.Nannini G., Meoni G., Amedei A., Tenori L. Metabolomics profile in gastrointestinal cancers: Update and future perspectives. World J. Gastroenterol. 2020;26:2514–2532. doi: 10.3748/wjg.v26.i20.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gu J., Xiao Y., Shu D., Liang X., Hu X., Xie Y., Lin D., Li H. Metabolomics Analysis in Serum from Patients with Colorectal Polyp and Colorectal Cancer by (1)H-NMR Spectrometry. Dis. Markers. 2019;2019:3491852. doi: 10.1155/2019/3491852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Erben V., Poschet G., Schrotz-King P., Brenner H. Comparing Metabolomics Profiles in Various Types of Liquid Biopsies among Screening Participants with and without Advanced Colorectal Neoplasms. Diagnostics. 2021;11:561. doi: 10.3390/diagnostics11030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Udo R., Katsumata K., Kuwabara H., Enomoto M., Ishizaki T., Sunamura M., Nagakawa Y., Soya R., Sugimoto M., Tsuchida A. Urinary charged metabolite profiling of colorectal cancer using capillary electrophoresis-mass spectrometry. Sci. Rep. 2020;10:21057. doi: 10.1038/s41598-020-78038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang A., Sun H., Wang X. Mass spectrometry-driven drug discovery for development of herbal medicine. Mass Spectrom. Rev. 2018;37:307–320. doi: 10.1002/mas.21529. [DOI] [PubMed] [Google Scholar]
- 179.Yin F.T., Zhou X.H., Kang S.Y., Li X.H., Li J., Ullah I., Zhang A.H., Sun H., Wang X.J. Prediction of the mechanism of Dachengqi Decoction treating colorectal cancer based on the analysis method of “ into serum components -action target-key pathway”. J. Ethnopharmacol. 2022;293:115286. doi: 10.1016/j.jep.2022.115286. [DOI] [PubMed] [Google Scholar]
- 180.Wang R., Li B., Lam S.M., Shui G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020;47:69–83. doi: 10.1016/j.jgg.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 181.Kyle J.E. Extracting Biological Insight from Untargeted Lipidomics Data. Methods Mol. Biol. 2020;2104:121–137. doi: 10.1007/978-1-0716-0239-3_7. [DOI] [PubMed] [Google Scholar]
- 182.Zaytseva Y. Lipid Metabolism as a Targetable Metabolic Vulnerability in Colorectal Cancer. Cancers. 2021;13:301. doi: 10.3390/cancers13020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pakiet A., Kobiela J., Stepnowski P., Sledzinski T., Mika A. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Health Dis. 2019;18:29. doi: 10.1186/s12944-019-0977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kondo Y., Nishiumi S., Shinohara M., Hatano N., Ikeda A., Yoshie T., Kobayashi T., Shiomi Y., Irino Y., Takenawa T., et al. Serum fatty acid profiling of colorectal cancer by gas chromatography/mass spectrometry. Biomark Med. 2011;5:451–460. doi: 10.2217/bmm.11.41. [DOI] [PubMed] [Google Scholar]
- 185.Liu T., Peng F., Yu J., Tan Z., Rao T., Chen Y., Wang Y., Liu Z., Zhou H., Peng J. LC-MS-based lipid profile in colorectal cancer patients: TAGs are the main disturbed lipid markers of colorectal cancer progression. Anal. Bioanal. Chem. 2019;411:5079–5088. doi: 10.1007/s00216-019-01872-5. [DOI] [PubMed] [Google Scholar]
- 186.Tevini J., Eder S.K., Huber-Schönauer U., Niederseer D., Strebinger G., Gostner J.M., Aigner E., Datz C., Felder T.K. Changing Metabolic Patterns along the Colorectal Adenoma-Carcinoma Sequence. J. Clin. Med. 2022;11:721. doi: 10.3390/jcm11030721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ecker J., Benedetti E., Kindt A.S.D., Höring M., Perl M., Machmüller A.C., Sichler A., Plagge J., Wang Y., Zeissig S., et al. The Colorectal Cancer Lipidome: Identification of a Robust Tumor-Specific Lipid Species Signature. Gastroenterology. 2021;161:910–923.e919. doi: 10.1053/j.gastro.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 188.Dehairs J., Derua R., Rueda-Rincon N., Swinnen J.V. Lipidomics in drug development. Drug Discov. Today Technol. 2015;13:33–38. doi: 10.1016/j.ddtec.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 189.Moreno L.O., Sánchez P.N., Abalo R. Lipidomics as Tools for Finding Biomarkers of Intestinal Pathology: From Irritable Bowel Syndrome to Colorectal Cancer. Curr. Drug Targets. 2022;23:636–655. doi: 10.2174/1389450122666210707122151. [DOI] [PubMed] [Google Scholar]
- 190.Lv J., Zhang L., Yan F., Wang X. Clinical lipidomics: A new way to diagnose human diseases. Clin. Transl. Med. 2018;7:12. doi: 10.1186/s40169-018-0190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Subramanian I., Verma S., Kumar S., Jere A., Anamika K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights. 2020;14:1177932219899051. doi: 10.1177/1177932219899051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yan J., Risacher S.L., Shen L., Saykin A.J. Network approaches to systems biology analysis of complex disease: Integrative methods for multi-omics data. Brief Bioinform. 2018;19:1370–1381. doi: 10.1093/bib/bbx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.O’Connor J.P., Aboagye E.O., Adams J.E., Aerts H.J., Barrington S.F., Beer A.J., Boellaard R., Bohndiek S.E., Brady M., Brown G., et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2017;14:169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Koyande N., Gangopadhyay M., Thatikonda S., Rengan A.K. The role of gut microbiota in the development of colorectal cancer: A review. Int. J. Colorectal. Dis. 2022;37:1509–1523. doi: 10.1007/s00384-022-04192-w. [DOI] [PubMed] [Google Scholar]
- 195.Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. 2021;21:1325. doi: 10.1186/s12885-021-09054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pączek S., Łukaszewicz-Zając M., Mroczko B. Granzymes-Their Role in Colorectal Cancer. Int. J. Mol. Sci. 2022;23:5277. doi: 10.3390/ijms23095277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Eylem C.C., Yilmaz M., Derkus B., Nemutlu E., Camci C.B., Yilmaz E., Turkoglu M.A., Aytac B., Ozyurt N., Emregul E. Untargeted multi-omic analysis of colorectal cancer-specific exosomes reveals joint pathways of colorectal cancer in both clinical samples and cell culture. Cancer Lett. 2020;469:186–194. doi: 10.1016/j.canlet.2019.10.038. [DOI] [PubMed] [Google Scholar]
- 198.Nam A.S., Chaligne R., Landau D.A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet. 2021;22:3–18. doi: 10.1038/s41576-020-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vasaikar S.V., Straub P., Wang J., Zhang B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46:D956–D963. doi: 10.1093/nar/gkx1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Correa-Aguila R., Alonso-Pupo N., Hernández-Rodríguez E.W. Multi-omics data integration approaches for precision oncology. Mol. Omics. 2022;18:469–479. doi: 10.1039/D1MO00411E. [DOI] [PubMed] [Google Scholar]