Abstract
Although cardiac tumor formation is rare, accumulating evidence suggests that the two leading causes of deaths, cancers, and cardiovascular diseases are similar in terms of pathogenesis, including angiogenesis, immune responses, and fibrosis. These similarities have led to the creation of new exciting field of study called cardio-oncology. Here, we review the similarities between cancer and cardiovascular disease from the perspective of microRNAs (miRNAs). As miRNAs are well-known regulators of translation by binding to the 3′-untranslated regions (UTRs) of messenger RNAs (mRNAs), we carefully dissect how a specific set of miRNAs are both oncomiRs (miRNAs in cancer) and myomiRs (muscle-related miRNAs). Furthermore, from the standpoint of similar pathogenesis, miRNAs categories related to the similar pathogenesis are discussed; namely, angiomiRs, Immune-miRs, and fibromiRs.
Keywords: cancer, cardiovascular disease, miRNA
1. Introduction
Cancer and cardiovascular disease are the leading causes of death across the globe accounting for one in six deaths [1] and 32% of all deaths worldwide [2], respectively, according to World Health Organization (WHO). Both cancer and cardiovascular disease are the umbrella terms commonly used to describe several disease etiologies. Each etiology of cancer and cardiovascular disease (e.g., lung cancer and ischemic heart disease, respectively) has its own distinct cause and progression pattern. However, recent research suggests that many aspects of cancer and cardiovascular disease are similar in terms of pathogenesis [3,4,5], leading to the development of specific field of study called cardio-oncology [6,7]. For example, both diseases involve dysregulated functionalities in vasculature, where abnormal vasculature (called, tumor vasculature [8]) occurs in cancer, while coronary artery disease is a type of cardiovascular disease caused by the narrowing or blockage of coronary arteries [9]. Another example is the involvement of immune responses, where prolonged or chronic inflammation is a hallmark of cancer [10,11,12] as well as cardiovascular disease [13,14,15]. The activation of immune responses often leads to the deposition of excessive extracellular matrices [16,17], which are another hallmark of cancer [17] and cardiac fibrosis as the end-stage of heart failure [18].
MicroRNAs (miRNAs) are evolutionary-conserved, regulatory short [~22 nucleotides (nt)] non-protein-coding RNAs that function as translational inhibitors by binding to the 3′-untranslated regions (3′-UTRs) of messenger RNAs (mRNAs) [19,20]. As one miRNA is predicted to bind hundreds of mRNAs due to its very short seed sequence (~6 nt) [21,22,23], it is speculated and experimentally shown for some miRNAs to regulate cascades of signaling pathways and their downstream targets. Due to their versatilities, dysregulation in miRNAs is linked to a variety of diseases, including cancer [24,25] and cardiovascular disease [26,27,28]. As the regulatory importance of miRNAs is experimentally proven, the therapeutic silencing of miRNAs is being explored [29,30,31,32,33,34]. However, due to their biodistributions (e.g., including their presence in the circulation [35,36,37]) and the presence of many target mRNAs for one miRNA, the precise mechanistic elucidation of each miRNA is urgently needed to advance into clinics. Since a specific miRNA is highly dependent on which target mRNAs are present in a specific biological context, it must be taken into consideration that the same miRNA can yield different biological outcomes depending on the specific cell or tissue [38]. This is especially important when considering miRNAs as potential therapeutic targets.
As cancer and cardiovascular disease share several aspects of disease causes and progressions, it is no surprise that many miRNAs are shown to be involved in pathogeneses of both cancer and cardiovascular disease. Because the heart is the least likely organ to harbor tumor growth [39], the communication between researchers working in miRNAs for either cancer or cardiovascular biology is scarce, although many miRNAs are found to be dysregulated in both diseases. To fill this gap in knowledge, here, we summarize the current status of miRNA research from the perspective of shared disease progression mechanisms in cancer and cardiovascular disease.
2. OncomiRs vs. MyomiRs
According to the latest annotation provided by the GENCODE consortium (Release 41; https://www.gencodegenes.org/human/stats_41.html; accessed on 3 October 2022), there are 1879 human miRNAs. Due to the intensive miRNA research in the last three decades [40], many (but not all) miRNAs have been studied functionally and some mechanistically. To date, miRNAs have been categorized based on their functionalities. These categories include oncomiRs and (cardiac) myomiRs to describe cancer- and striated muscle-related miRNAs, respectively. Although the heart consists of cell types other than cardiac muscle (cardiomyocytes), for simplicity, here, we will compare oncomiRs and myomiRs to understand the possible overlaps of the functional miRNAs in both cancer and cardiovascular disease.
As there are many different types of cancer, the list of oncomiRs is growing rapidly due to the availability of next generation sequencing (i.e., small RNA sequencing) to identify miRNAs overexpressed in tumor samples. As such, there are several dedicated databases for oncomiRs available, including miRCancer [41], OncomiR [42,43,44], and the OncoMir Cancer Database (OMCD) [45]. Compared to oncomiRs, the list of myomiRs is small, including miR-1, miR-133a/b, miR-206, miR-208a/b, miR-302, miR-367, miR-486, and miR-499 [46,47]. Not surprisingly, all myomiRs are involved in tumorigenesis.
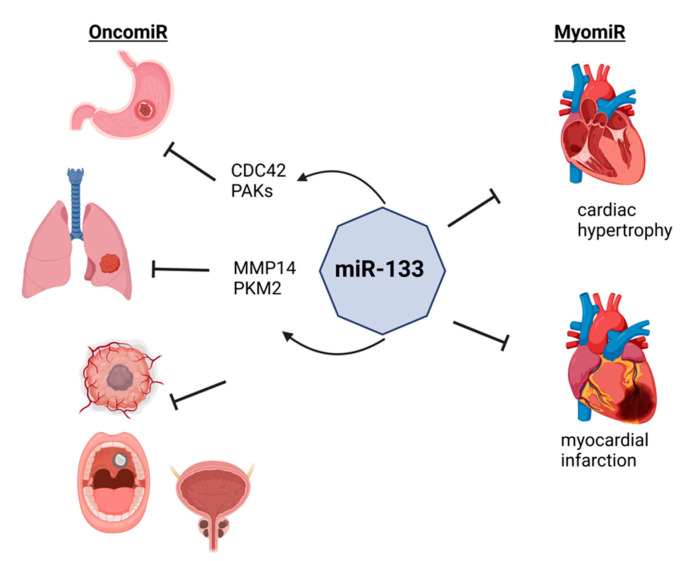
One of the most abundant miRNAs in the heart [48], miR-133, is a regulator of cardiac hypertrophy [49] and its down-regulation was observed in patients with myocardial infarction [50] (Figure 1). In gastric cancer, miR-133 is down-regulated in gastric cancer patients and negatively associated with tumor size, invasion depth, and peripheral organ metastasis [51]. Mechanistically, miR-133 targets 3′-UTR of the cell division cycle 42 (CDC42) gene to regulate the downstream effectors of CDC42, P21-activated kinases (PAKs). Similarly, an overexpression of miR-133a in the lung cancer cell lines, A549 and NCI-H1299, results in the suppression of cell proliferation, migration, and invasion by targeting matrix metallopeptidase 14 (MMP14) [52]. Another study shows that the overexpression of miR-133b in the lung cancer cell line, A549, re-sensitized the radioresistant A549 cells by targeting pyruvate kinase M1/2 (PKM, also known as PKM2) to regulate glycolysis [53]. Besides gastric and lung cancers, functions of miR-133 are also reported in glioblastoma [54], oral cancer [55], and prostate cancer [56]. All other miRNAs are also shown to be functionally important for tumorigenesis, suggesting the importance of examining miRNAs in onco-cardiology.
Figure 1.
OncomiRs and myomiRs. MyomiRs have a dual function and are involved in tumorigenesis. miR-133 regulates the cardiac hypertrophy and improves the myocardial function after infarction, while it is associated with multiple tumors. miR-133 targets 3′-UTR of CDC42 and regulates PAKs, thus preventing the growth and metastasis of gastric cancer. Similarly, miR-133 prevents the proliferation, migration, and invasion of lung cancer by targeting MMP14 and PKM2. Furthermore, miR-133 is involved also in the pathogenesis of glioblastoma and oral and prostate cancer. Figure created with BioRender.com, accessed on 24 October 2022.
3. Angiogenesis: AngiomiRs
Angiogenesis is the process of new blood vessel formation through the migration, growth, and differentiation of endothelial cells [57,58]. In cancer, angiogenesis allows for a tumor to grow as new vessels provide nutrients and oxygen to malignant cells [59,60]. In cardiovascular disease, therapeutic angiogenesis aims to provide the blood flow to the ischemic heart tissue [61,62]. Thus, in both diseases, angiogenesis is an important therapeutic target, although the opposite effects are observed. During angiogenesis, several miRNAs are functionally involved, which has created a specific term to describe these angiogenesis-related miRNAs called, angiomiRs (Figure 2). AngiomiRs include miR-15/16, miR-17~92 cluster, miR-18a, miR-19, miR-21, miR-23b, miR-27a/b, miR-29b, miR-30, miR-34a, miR-57, miR-125b, miR-126, miR-128, miR-143, miR-145, miR-155, miR-192, miR-194, miR-199a, miR-200 family, miR-204, miR-210, miR-217, miR-296, miR-378, miR-484, miR-494, miR-497, miR-542-3p, miR-573, miR-642, and let-7b [63,64], which some are discussed below.
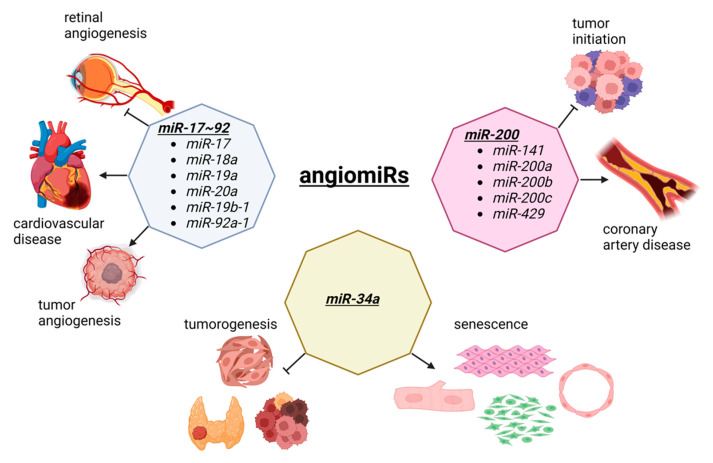
Figure 2.
The dual role of angiomiRs in cancer and cardiac pathophysiology. The miR-17~92 cluster is involved in tumorigenesis and tumor vascularization. This cluster is also involved in retinal angiogenesis and the progression of cardiovascular disease. The members of the miR-200 family prevent the tumor initiation and malignant transformation, although they are upregulated in coronary artery disease. MiR-34a is a tumor suppressor involved in the development of thyroid cancer, head and neck squamous cell carcinoma, and cancer stem cells division. The overexpression of miR-34a suppress the proliferation and induces senescence in cardiomyocytes, fibroblasts, smooth muscle, and endothelial cells, by inhibiting sirtuin 1 (SIRT1). Figure created with BioRender.com, accessed on 24 October 2022.
The miR-17~92 cluster was first reported in tumorigenesis [65] and is one of the most well-studied miRNA clusters [66,67]. By crossing miR-17~92 floxed mice with an inducible vascular endothelial cell specific Cre driver (Cdh5-cre/ERT2), Chamorro-Jorganes et al. demonstrated that retinal angiogenesis was reduced during the development of these mice [68]. Furthermore, the vascular endothelial growth factor (VEGF)-induced ear and tumor angiogenesis were reduced, suggesting that VEGF regulates miR-17~92 cluster expression leading to the regulation of angiogenesis. The involvement of the miR-17~92 cluster is well documented in various diseases, including cardiovascular disease [69,70]. Since the miR-17~92 cluster consists of miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1, each miRNA in this cluster is also shown to be important for angiogenesis, including tumorigenesis and cardiovascular disease. For example, miR-92a is dysregulated in many forms of cancer, suggesting it is a potential diagnostic biomarker as well as a therapeutic target [71]. In the cardiovascular system, Bonauer et al. demonstrated that overexpression of miR-92a in endothelial cells inhibited angiogenesis in murine models of limb ischemia and myocardial infarction, while the silencing of miR-92a via antagomiR resulted in enhanced angiogensis and the functional recovery of the damaged tissues in murine disease models, suggesting miR-92a as a potential therapeutic target for ischemia diseases [72].
The miR-200 family is another well studied miRNA family that includes miR-141, miR-200a, miR-200b, miR-200c, and miR-429 [73]. In cancer, the miR-200 family is shown to play functional roles in cell malignant transformation and preventing tumor initiation [74]. By profiling epicardial adipose tissue from coronary artery disease (CAD) patients and non-CAD atherosclerotic patients, Zhang et al. demonstrated that the expressions of miR-141-3p, miR-200b, miR-200c-3p, and miR-429 are up-regulated in CAD patients compared to non-CAD patients [75]. By performing a series of experiments in vitro, the authors demonstrated that the overexpression of miR-200b-3p in human umbilical vein endothelial cells (HUVECs) resulted in increased apoptosis under oxidative stress. Mechanistically, miR-300b-3p targets histone deacetylase 4 (HDAC4) as the overexpression of HDAC4 reduced the increased apoptosis induced by inhibiting miR-200b-3p, suggesting that miR-200b-3p is a potential therapeutic target for atherosclerosis.
MiR-34a is a tumor suppressor and considered as a diagnostic and prognostic biomarker as well as a therapeutic target in various cancers, including head and neck squamous cell carcinoma, thyroid cancer, and cancer stem cells [76,77]. Interestingly, the expression of miR-34a is increased in senescent HUVECs and in the heart and spleen of older mice [78]. When overexpressed, miR-34a suppressed cell cycle and proliferation by inhibiting sirtuin 1 (SIRT1). Because ageing is a hot topic to be investigated, subsequent research shows the functional importance of miR-34a in cell types other than endothelial cells in the heart, including in cardiomyocytes [79,80], fibroblasts [81], and smooth muscle cells [82,83]. This is not an isolated case as many other angiomiRs (and other miRNAs) are expressed rather ubiquitously, suggesting that examining miRNAs as a common mechanism of action for cardio-oncology is not a big surprise.
4. Immune Responses: Immuno-miRs
Prolonged inflammation is a hallmark of cancer [10] that immune systems can have both positive and negative effects on regarding the development of tumors and prognostics of cancer patients [84]. Indeed, immunotherapy is a type of treatment using one’s own immune system to fight cancer, but the success rates of immunotherapy drugs vary between 15–30% in most tumor types, while 50–80% in melanoma [85]. As the immune system is a complex system involving many different cell types (e.g., basophils, eosinophils, lymphocytes, macrophages, monocytes, and neutrophils) to fight against infection [86,87], understanding the immune system is also important in cardiovascular disease [88,89,90]. For example, myocardial infarction leads to the loss of cardiomyocytes, which are replaced by non-contracting scar tissue [91,92]. The immune system is a double-edged sword in the remodeling process of the infarcted heart as macrophages are necessary for repair in the acute phase as their systemic depletion results in impaired scar formation and the rupture of the left ventricle of the heart. However, the accumulation of macrophages in non-infarcted regions of the left ventricle leads to progressive myocyte attrition, collagen deposition, and loss of the pump function of the heart in a chronic phase of remodeling of the infarcted heart. As miRNAs are expressed in many immune cells and finetune the important signaling pathways, the list of immuno-miRs is growing rapidly [93,94]. As such, specialized databases for immune-miRs are available, including IRNdb [95], RNA2Immune [96], and RNAimmuno [97]. In the following, examples of immune-miRs are explained in cancer and cardiovascular disease.
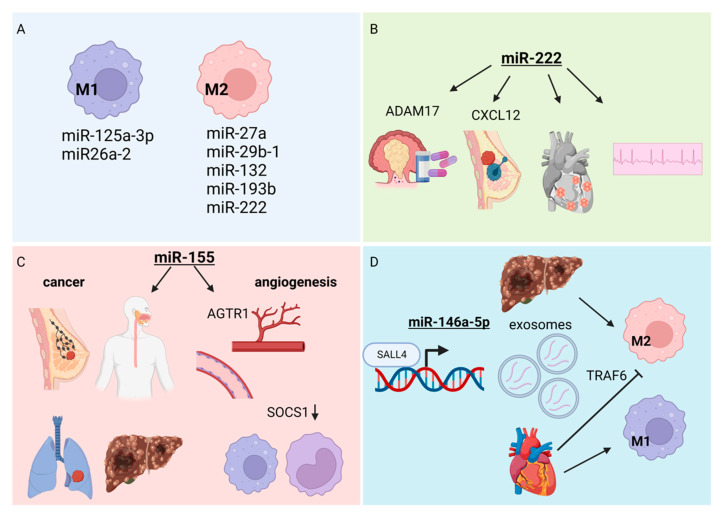
Monocytes are a type of white blood cells (leukocytes) that can differentiate into macrophages and dendritic cells [98]. Furthermore, monocyte-derived macrophages can be polarized into inflammatory subtype, M1, and anti-inflammatory subtype, M2 [99]. The cascade of differentiation and polarizations are controlled by the coordinate actions of cytokines, which can be regulated at the transcriptional and post-transcriptional levels, where miRNAs can regulate the translation of transcription factors responsible for cytokine gene expressions. These microRNAs include miR-125a-3p and miR-26a-2 in M1 macrophages, while miR-27a, miR-29b-1, miR-132, miR-193b, and miR-222 constitute the M2 macrophages [100] (Figure 3A). For example, miR-222 targets ADAM metallopeptidase domain 17 (ADAM17) to modulate multidrug resistance in colorectal carcinoma [101] (Figure 3B). In breast cancer, the overexpression of miR-222 inhibits the chemotaxis of tumor-associated macrophages by targeting C-X-C motif chemokine ligand 12 (CXCL12) [102]. In the serum, the level of miR-222 is independently associated with atrial fibrillation (irregular heart rhythm) in patients with degenerative valvular heart disease [103]. In addition, the level of miR-222 is elevated in acute viral myocarditis caused by Coxsackievirus B3 [104]. Although the functions of miR-222 are mainly reported in cardiomyocytes [104,105,106] and cardiac fibroblasts [107,108], it is clear that immune-miRs in monocytes and macrophages are important regulators of immune responses in cancer and cardiovascular disease.
Figure 3.
Immuno-miRs. (A) MiRNAs responsible for regulation of cytokine gene expressions leading to the differentiation of two types of monocytes-derived macrophages—inflammatory subtype M1 and anti-inflammatory subtype M2. (B) The role of miR-222 in tumorigenesis and cardiovascular disease. MiR-222 targets ADAM17 to prevent multidrug resistant colorectal carcinoma. The inhibitory effect on the chemotaxis of tumor associated macrophages in breast cancer is mediated by targeting CXCL12. The overexpression of miR-222 is associated with atrial fibrillation and Coxsackie virus caused myocarditis. (C) The overexpression of miR-155 is associated lymph node metastasis in breast cancer and advance of esophageal, liver, and lung cancer. Mir-155 regulates angiogenesis by controlling the expression of AGTR1 in endothelial cells and SOCS1 in monocytes/macrophages. (D) The extracellular RNA, miR-146a-5p, is highly presented in hepatocellular carcinoma derived exosomes and regulates the polarization of macrophages into M2 tumor-associated macrophages. On the contrary, the cardiomyocytes-derived miR-146a-5p inhibits the M2 macrophage polarization by targeting TRAF6 while promoting M1 macrophage polarization. Figure created with BioRender.com, accessed on 24 October 2022.
Enriched in immune cells, miR-155 is a master regulator of immune responses [109] (Figure 3C). In breast cancer, the increased expression of miR-155 is associated with high tumor grade, advanced stage, and lymph node metastasis [110]. Similarly, miR-155 is overexpressed in other forms of cancer, including esophageal cancer [111], liver cancer [112], and lung cancer [113], which calls for miR-155 as a diagnostic and prognostic cancer biomarker [114] as well as therapeutic target [115]. As shown in the previous section, miR-155 is an angiomiR so its function is well known in the endothelial cells and atherosclerosis [116,117]. Besides endothelial cells, miR-155 is highly expressed in monocytes and macrophages, which Pankratz et al. used in knockout mice to elegantly demonstrate that miR-155 regulates angiogenesis and arteriogenesis by controlling their target genes, angiotensin II receptor type 1 (AGTR1) and suppressor of cytokine signaling 1 (SOCS1) in endothelial and monocyte/macrophages, respectively [118].
Extracellular RNAs (exRNAs) are a type of cell–cell communication that are produced by a donor cell and are released into the extracellular environment (e.g., body fluid, circulation) [119]. They are contained in the lipid particles, such as extracellular vehicles (EVs), including exosomes. ExRNAs include proteins and RNAs, including miRNAs. For example, miR-146a-5p is enriched in the hepatocellular carcinoma-derived exosomes [120] (Figure 3D). The transcription factor, spalt like transcription factor 4 (SALL4), binds to the promoter of miR-146a-5p to directly control its expression in exosomes, thereby regulates the polarization of macrophages into M2 tumor-associated macrophages. In contrast, cardiomyocyte-derived exosomal miR-146a-5p promotes M1 macrophage polarization while inhibiting M2 macrophage polarization by targeting TNF receptor associated factor 6 (TRAF6) [121]. This is just of many miRNAs contained in exosomes.
The studying of immunology has intensified in recent years due to the rise of coronavirus disease 2019 (COVID-19) [122,123,124,125]. As there is a substantial risk of heart problems associated with COVID-19 and mRNA vaccines [126,127,128,129], it is likely that more and more miRNAs will be identified in the heart, which may have been studied in cancer previously to expand the list of immune-miRs in the cardio-oncology field.
5. Fibrosis: fibromiRs
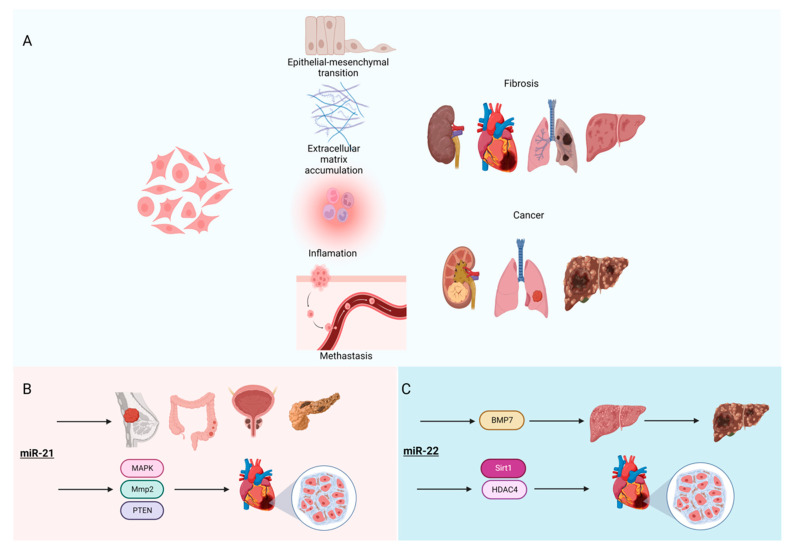
Fibrosis is a process in which fibroblasts and other mesenchymal cells are activated to become myofibroblasts to secrete an excess number of extracellular matrices (ECM; e.g., collagens, glycosaminoglycans, and glycoproteins) [130] (Figure 4A). It is the end stage of many diseases, including cardiovascular disease [18]. In cancer, cancer-associated fibroblasts (CAFs) promote tumorigenic features, including ECM deposition, epithelial-to-mesenchymal transition (EMT), and metastasis [131]. To understand fibrosis, many screening studies have been performed to identify differentially expressed genes and miRNAs, which are collectively called fibromiRs [132,133,134,135]. For example, miR-21 is the most studied fibromiR [136,137,138,139] (Figure 4B). Not only is it highly expressed in many forms of cancer and suggested as potential diagnostic biomarkers of cancer types (breast, pancreatic, colorectal, and prostate cancer) [140], miR-21 stimulates MAP kinase signaling in cardiac fibroblasts, thereby contributing to myocardial disease [141]. Furthermore, miR-21 targets matrix metallopeptidase 2 (Mmp2) in cardiac fibroblasts of the infarcted heart via phosphatase and the tensin homolog (PTEN) pathway [142], suggesting the important signaling roles of miR-21 in both cancer and cardiovascular disease.
Figure 4.
FibromiRs. (A) The core mechanisms of fibrosis and carcinogenesis. Multiple cell types (e.g., fibroblasts, myofibroblasts, epithelial cells, and macrophages) are involved. The pathophysiological mechanisms include inflammation, epithelial to mesenchymal transition, extracellular matrix accumulation, and metastasis. (B) MiR-21 is a diagnostic biomarker for multiple cancers, including breast, pancreatic, colorectal, and prostate. MiR-21 stimulates cardiac fibroblasts by targeting Mmp2 and the PTEN pathway, leading to the progression of myocardial disease. (C) MiR-22 induces the liver fibrosis through BMP7 leading to progression into hepatocellular carcinoma. In the heart, miR-22 promotes cardiac fibrosis by targeting Sirt1 and HDAC4. Figure created with BioRender.com, accessed on 24 October 2022.
Multiple reports show that another oncomiR, miR-22, is highly involved in tumor progression in multiple tumors, including breast cancer [143], acute myeloid leukemia (AML) [144], and hepatocellular carcinoma (HCC) [145,146]. The effect of miR-22 on HCC seems to be related to the early effect of miR-22 on liver fibrosis through its regulation of bone morphogenic protein 7 (BMP7) [147] (Figure 4C), which starts from a degenerative process and ultimately leads to HCC developing. Interestingly, miR-22 is reported to have the same effect on cardiac fibrosis [148] via the regulation of Sirt1 and HDAC4. As the role of miR-22 in fibrosis is conserved in multiple diseases and tissues, this miRNA could serve as a potential therapeutic target in liver [149] and cardiac fibrosis [150].
Although fibroblasts can be found throughout the human body, they are heterogeneous populations of cells without any single cell surface marker that is specific for fibroblasts as many markers are expressed in other cell types, including epithelial and immune cells [151,152]. In this regard, microRNAs are involved in activating fibroblasts, which contribute to the heterogeneity of fibroblasts [153]. For example, the members of the miR-200 family, miR-141 and miR-200a, target C-X-C motif chemokine ligand 12 (CXCL12; also known as CXCL12β) to regulate the immunosuppressive activity of a subtype of carcinoma-associated fibroblasts in ovarian cancer [154]. Besides the miR-200 family being angiomiRs as written in above subsection, miR-200b is negatively regulated by the epigenetic factor, DNA methyltransferase 3 alpha (Dnmt3a), to control autophagy in rat cardiac fibroblasts [155]. In addition, small RNA-seq experiment using rat cardiac fibroblasts induced with transforming growth factor-β1 (TGF-β1) showed 3 up- (miR-325-3p, miR-325-5p, and miR-210-5p) and 21 down-regulated miRNAs (e.g., miR-19a-3p, miR-19b-3p, miR-144-3p, and miR-200b-3p), potentially targeting genes involved in calcium and glutamatergic synapse signaling pathways [156]. Taken together, there are many shared fibromiRs between CAFs and cardiac fibroblasts.
6. Conclusions
To maintain the homeostasis of the tissues and remodeling of the tissues upon damages, angiogenesis, immune responses, and fibrosis are interconnected. As such, miRNAs are identified to be involved in each cellular activity as angiomiRs, immuno-miRs, and fibromiRs, respectively. Not surprisingly, some miRNAs (e.g., miR-17~92 cluster, miR-34a, and miR-200 family) are involved in all three cellular activities, which some overlapping miRNAs are responsible for such cellular activities and responses. This is particularly interesting as cancer is considered as a complex adaptive ecosystem [157], in which cancer cells and the stromal cells transform, cooperate, and even co-evolve with each other over time and space [158]. Thus, it will be interesting to further investigate miRNAs from the perspective of the ecosystem in cancer and possibly in cardiovascular disease.
As cancer and cardiovascular disease are two of the leading causes of death worldwide, it will be exciting to find a common disease mechanism. As reviewed above, miRNAs are shared between these life-threatening diseases. Given that miRNAs are investigated as potential therapeutic targets, increased communication between researchers working with cancer and cardiovascular disease is necessary to find a potential cure for these diseases. To this end, the rise of the cardio-oncology field should facilitate a further understanding of the pathogeneses of these two diseases, possibly through miRNAs.
Author Contributions
M.I., R.P. and S.U. wrote the manuscript, generated figures, and approved the final version of this manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1. [(accessed on 3 October 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2. [(accessed on 3 October 2022)]. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1.
- 3.Seton-Rogers S. Cardiovascular disease and cancer communicate. Nat. Rev. Cancer. 2020;20:552. doi: 10.1038/s41568-020-0294-6. [DOI] [PubMed] [Google Scholar]
- 4.Knisely J.P.S., Henry S.A., Saba S.G., Puckett L.L. Cancer and cardiovascular disease. Lancet. 2020;395:1904. doi: 10.1016/S0140-6736(20)30238-5. [DOI] [PubMed] [Google Scholar]
- 5.De Boer R.A., Meijers W.C., van der Meer P., van Veldhuisen D.J. Cancer and heart disease: Associations and relations. Eur. J. Heart Fail. 2019;21:1515–1525. doi: 10.1002/ejhf.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Wang Y., Han X., Sun J., Li C., Adhikari B.K., Zhang J., Miao X., Chen Z. Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer. Front. Cardiovasc. Med. 2022;9:727487. doi: 10.3389/fcvm.2022.727487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koutsoukis A., Ntalianis A., Repasos E., Kastritis E., Dimopoulos M.A., Paraskevaidis I. Cardio-oncology: A Focus on Cardiotoxicity. Eur. Cardiol. Rev. 2018;13:64–69. doi: 10.15420/ecr.2017:17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruoslahti E. Specialization of tumour vasculature. Nat. Rev. Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 9.Malakar A.K., Choudhury D., Halder B., Paul P., Uddin A., Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019;234:16812–16823. doi: 10.1002/jcp.28350. [DOI] [PubMed] [Google Scholar]
- 10.Hiam-Galvez K.J., Allen B.M., Spitzer M.H. Systemic immunity in cancer. Nat. Rev. Cancer. 2021;21:345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon-Weeks A., Yuzhalin A.E. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers. 2020;12:3331. doi: 10.3390/cancers12113331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorriento D., Iaccarino G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019;20:3879. doi: 10.3390/ijms20163879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Candales A., Hernandez Burgos P.M., Hernandez-Suarez D.F., Harris D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017;3:e341. [PMC free article] [PubMed] [Google Scholar]
- 15.Mason J.C., Libby P. Cardiovascular disease in patients with chronic inflammation: Mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur. Heart J. 2015;36:482–489. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd D.F., Thomas P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine. 2017;98:79–86. doi: 10.1016/j.cyto.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickup M.W., Mouw J.K., Weaver V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichiki T., Schirger J.A., Huntley B.K., Brozovich F.V., Maleszewski J.J., Sandberg S.M., Sangaralingham S.J., Park S.J., Burnett J.C., Jr. Cardiac fibrosis in end-stage human heart failure and the cardiac natriuretic peptide guanylyl cyclase system: Regulation and therapeutic implications. J. Mol. Cell. Cardiol. 2014;75:199–205. doi: 10.1016/j.yjmcc.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 20.Gebert L.F.R., MacRae I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y., Wang X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127–D131. doi: 10.1093/nar/gkz757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paraskevopoulou M.D., Georgakilas G., Kostoulas N., Vlachos I.S., Vergoulis T., Reczko M., Filippidis C., Dalamagas T., Hatzigeorgiou A.G. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41:W169–W173. doi: 10.1093/nar/gkt393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S., Wang Y., Li D., Wang H., Zhao X., Yang J., Chen L., Guo M., Zhao J., Chen C., et al. Mechanisms Controlling MicroRNA Expression in Tumor. Cells. 2022;11:2852. doi: 10.3390/cells11182852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding L., Gu H., Xiong X., Ao H., Cao J., Lin W., Yu M., Lin J., Cui Q. MicroRNAs Involved in Carcinogenesis, Prognosis, Therapeutic Resistance and Applications in Human Triple-Negative Breast Cancer. Cells. 2019;8:1492. doi: 10.3390/cells8121492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kansakar U., Varzideh F., Mone P., Jankauskas S.S., Santulli G. Functional Role of microRNAs in Regulating Cardiomyocyte Death. Cells. 2022;11:983. doi: 10.3390/cells11060983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song R., Hu X.Q., Zhang L. Mitochondrial MiRNA in Cardiovascular Function and Disease. Cells. 2019;8:1475. doi: 10.3390/cells8121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirna M., Paar V., Rezar R., Topf A., Eber M., Hoppe U.C., Lichtenauer M., Jung C. MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future? Cells. 2019;8:1352. doi: 10.3390/cells8111352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forterre A., Komuro H., Aminova S., Harada M. A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers. 2020;12:1852. doi: 10.3390/cancers12071852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szczepanek J., Skorupa M., Tretyn A. MicroRNA as a Potential Therapeutic Molecule in Cancer. Cells. 2022;11:1008. doi: 10.3390/cells11061008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Momin M.Y., Gaddam R.R., Kravitz M., Gupta A., Vikram A. The Challenges and Opportunities in the Development of MicroRNA Therapeutics: A Multidisciplinary Viewpoint. Cells. 2021;10:3097. doi: 10.3390/cells10113097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill C.P., Dwyer R.M. Nanoparticle-Based Delivery of Tumor Suppressor microRNA for Cancer Therapy. Cells. 2020;9:521. doi: 10.3390/cells9020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo H.A., Moeng S., Sim S., Kuh H.J., Choi S.Y., Park J.K. MicroRNA-Based Combinatorial Cancer Therapy: Effects of MicroRNAs on the Efficacy of Anti-Cancer Therapies. Cells. 2019;9:29. doi: 10.3390/cells9010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quemener A.M., Centomo M.L., Sax S.L., Panella R. Small Drugs, Huge Impact: The Extraordinary Impact of Antisense Oligonucleotides in Research and Drug Development. Molecules. 2022;27:536. doi: 10.3390/molecules27020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui M., Wang H., Yao X., Zhang D., Xie Y., Cui R., Zhang X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019;10:626. doi: 10.3389/fgene.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y., Li Q., Zhang R., Dai X., Chen W., Xing D. Circulating microRNAs: Biomarkers of disease. Clin. Chim. Acta. 2021;516:46–54. doi: 10.1016/j.cca.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Creemers E.E., Tijsen A.J., Pinto Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 38.Murakami Y., Yasuda T., Saigo K., Urashima T., Toyoda H., Okanoue T., Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–2545. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 39.Tyebally S., Chen D., Bhattacharyya S., Mughrabi A., Hussain Z., Manisty C., Westwood M., Ghosh A.K., Guha A. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. Cardio Oncol. 2020;2:293–311. doi: 10.1016/j.jaccao.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 41.Xie B., Ding Q., Han H., Wu D. miRCancer: A microRNA-cancer association database constructed by text mining on literature. Bioinformatics. 2013;29:638–644. doi: 10.1093/bioinformatics/btt014. [DOI] [PubMed] [Google Scholar]
- 42.Sarver A.L., Subramanian S. Competing endogenous RNA database. Bioinformation. 2012;8:731–733. doi: 10.6026/97320630008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarver A.L., Phalak R., Thayanithy V., Subramanian S. S-MED: Sarcoma microRNA expression database. Lab. Investig. 2010;90:753–761. doi: 10.1038/labinvest.2010.53. [DOI] [PubMed] [Google Scholar]
- 44.Sarver A.L., French A.J., Borralho P.M., Thayanithy V., Oberg A.L., Silverstein K.A., Morlan B.W., Riska S.M., Boardman L.A., Cunningham J.M., et al. Human colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states. BMC Cancer. 2009;9:401. doi: 10.1186/1471-2407-9-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarver A.L., Sarver A.E., Yuan C., Subramanian S. OMCD: OncomiR Cancer Database. BMC Cancer. 2018;18:1223. doi: 10.1186/s12885-018-5085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zilahi E., Adamecz Z., Bodoki L., Griger Z., Poliska S., Nagy-Vincze M., Danko K. Dysregulated expression profile of myomiRs in the skeletal muscle of patients with polymyositis. EJIFCC. 2019;30:237–245. [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy J.J. MicroRNA-206: The skeletal muscle-specific myomiR. Biochim. Biophys. Acta. 2008;1779:682–691. doi: 10.1016/j.bbagrm.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leptidis S., El Azzouzi H., Lok S.I., de Weger R., Olieslagers S., Kisters N., Silva G.J., Heymans S., Cuppen E., Berezikov E., et al. A deep sequencing approach to uncover the miRNOME in the human heart. PLoS ONE. 2013;8:e57800. doi: 10.1371/annotation/e33f9763-3385-42c7-b31e-d433dc8e499a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Care A., Catalucci D., Felicetti F., Bonci D., Addario A., Gallo P., Bang M.L., Segnalini P., Gu Y., Dalton N.D., et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 50.Bostjancic E., Brandner T., Zidar N., Glavac D., Stajer D. Down-regulation of miR-133a/b in patients with myocardial infarction correlates with the presence of ventricular fibrillation. Biomed. Pharmacother. 2018;99:65–71. doi: 10.1016/j.biopha.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 51.Cheng Z., Liu F., Wang G., Li Y., Zhang H., Li F. miR-133 is a key negative regulator of CDC42-PAK pathway in gastric cancer. Cell. Signal. 2014;26:2667–2673. doi: 10.1016/j.cellsig.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Xu M., Wang Y.Z. miR133a suppresses cell proliferation, migration and invasion in human lung cancer by targeting MMP14. Oncol. Rep. 2013;30:1398–1404. doi: 10.3892/or.2013.2548. [DOI] [PubMed] [Google Scholar]
- 53.Liu G., Li Y.I., Gao X. Overexpression of microRNA-133b sensitizes non-small cell lung cancer cells to irradiation through the inhibition of glycolysis. Oncol. Lett. 2016;11:2903–2908. doi: 10.3892/ol.2016.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu F., Li F., Zhang W., Jia P. Growth of glioblastoma is inhibited by miR-133-mediated EGFR suppression. Tumour Biol. 2015;36:9553–9558. doi: 10.1007/s13277-015-3724-4. [DOI] [PubMed] [Google Scholar]
- 55.Jung J.E., Lee J.Y., Park H.R., Kang J.W., Kim Y.H., Lee J.H. MicroRNA-133 Targets Phosphodiesterase 1C in Drosophila and Human Oral Cancer Cells to Regulate Epithelial-Mesenchymal Transition. J. Cancer. 2021;12:5296–5309. doi: 10.7150/jca.56138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tao J., Wu D., Xu B., Qian W., Li P., Lu Q., Yin C., Zhang W. microRNA-133 inhibits cell proliferation, migration and invasion in prostate cancer cells by targeting the epidermal growth factor receptor. Oncol. Rep. 2012;27:1967–1975. doi: 10.3892/or.2012.1711. [DOI] [PubMed] [Google Scholar]
- 57.Potente M., Carmeliet P. The Link Between Angiogenesis and Endothelial Metabolism. Annu. Rev. Physiol. 2017;79:43–66. doi: 10.1146/annurev-physiol-021115-105134. [DOI] [PubMed] [Google Scholar]
- 58.Chung A.S., Ferrara N. Developmental and pathological angiogenesis. Annu. Rev. Cell. Dev. Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 59.Lugano R., Ramachandran M., Dimberg A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eelen G., Treps L., Li X., Carmeliet P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020;127:310–329. doi: 10.1161/CIRCRESAHA.120.316851. [DOI] [PubMed] [Google Scholar]
- 61.Yla-Herttuala S., Bridges C., Katz M.G., Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: Dream or vision? Eur. Heart J. 2017;38:1365–1371. doi: 10.1093/eurheartj/ehw547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korpela H., Jarvelainen N., Siimes S., Lampela J., Airaksinen J., Valli K., Turunen M., Pajula J., Nurro J., Yla-Herttuala S. Gene therapy for ischaemic heart disease and heart failure. J. Intern. Med. 2021;290:567–582. doi: 10.1111/joim.13308. [DOI] [PubMed] [Google Scholar]
- 63.Salinas-Vera Y.M., Marchat L.A., Gallardo-Rincon D., Ruiz-Garcia E., Astudillo-De La Vega H., Echavarria-Zepeda R., Lopez-Camarillo C. AngiomiRs: MicroRNAs driving angiogenesis in cancer (Review) Int. J. Mol. Med. 2019;43:657–670. doi: 10.3892/ijmm.2018.4003. [DOI] [PubMed] [Google Scholar]
- 64.Wang S., Olson E.N. AngiomiRs--key regulators of angiogenesis. Curr. Opin. Genet. Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ota A., Tagawa H., Karnan S., Tsuzuki S., Karpas A., Kira S., Yoshida Y., Seto M. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–3095. doi: 10.1158/0008-5472.CAN-03-3773. [DOI] [PubMed] [Google Scholar]
- 66.Mogilyansky E., Rigoutsos I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20:1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mendell J.T. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chamorro-Jorganes A., Lee M.Y., Araldi E., Landskroner-Eiger S., Fernandez-Fuertes M., Sahraei M., Quiles Del Rey M., van Solingen C., Yu J., Fernandez-Hernando C., et al. VEGF-Induced Expression of miR-17-92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ. Res. 2016;118:38–47. doi: 10.1161/CIRCRESAHA.115.307408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu H., Liu Z., Zhou L. Roles of miR-17-92 Cluster in Cardiovascular Development and Common Diseases. Biomed. Res. Int. 2017;2017:9102909. doi: 10.1155/2017/9102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Danielson L.S., Park D.S., Rotllan N., Chamorro-Jorganes A., Guijarro M.V., Fernandez-Hernando C., Fishman G.I., Phoon C.K., Hernando E. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013;27:1460–1467. doi: 10.1096/fj.12-221994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li M., Guan X., Sun Y., Mi J., Shu X., Liu F., Li C. miR-92a family and their target genes in tumorigenesis and metastasis. Exp. Cell Res. 2014;323:1–6. doi: 10.1016/j.yexcr.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 72.Bonauer A., Carmona G., Iwasaki M., Mione M., Koyanagi M., Fischer A., Burchfield J., Fox H., Doebele C., Ohtani K., et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 73.Cavallari I., Ciccarese F., Sharova E., Urso L., Raimondi V., Silic-Benussi M., D’Agostino D.M., Ciminale V. The miR-200 Family of microRNAs: Fine Tuners of Epithelial-Mesenchymal Transition and Circulating Cancer Biomarkers. Cancers. 2021;13:5874. doi: 10.3390/cancers13235874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Humphries B., Yang C. The microRNA-200 family: Small molecules with novel roles in cancer development, progression and therapy. Oncotarget. 2015;6:6472–6498. doi: 10.18632/oncotarget.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang F., Cheng N., Du J., Zhang H., Zhang C. MicroRNA-200b-3p promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis. BMC Cardiovasc. Disord. 2021;21:172. doi: 10.1186/s12872-021-01980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li W.J., Wang Y., Liu R., Kasinski A.L., Shen H., Slack F.J., Tang D.G. MicroRNA-34a: Potent Tumor Suppressor, Cancer Stem Cell Inhibitor, and Potential Anticancer Therapeutic. Front. Cell Dev. Biol. 2021;9:640587. doi: 10.3389/fcell.2021.640587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalfert D., Ludvikova M., Pesta M., Ludvik J., Dostalova L., Kholova I. Multifunctional Roles of miR-34a in Cancer: A Review with the Emphasis on Head and Neck Squamous Cell Carcinoma and Thyroid Cancer with Clinical Implications. Diagnostics. 2020;10:563. doi: 10.3390/diagnostics10080563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ito T., Yagi S., Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem. Biophys. Res. Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 79.Yang Y., Cheng H.W., Qiu Y., Dupee D., Noonan M., Lin Y.D., Fisch S., Unno K., Sereti K.I., Liao R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015;117:450–459. doi: 10.1161/CIRCRESAHA.117.305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boon R.A., Iekushi K., Lechner S., Seeger T., Fischer A., Heydt S., Kaluza D., Treguer K., Carmona G., Bonauer A., et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 81.Huang Y., Qi Y., Du J.Q., Zhang D.F. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin. Ther. Targets. 2014;18:1355–1365. doi: 10.1517/14728222.2014.961424. [DOI] [PubMed] [Google Scholar]
- 82.Chen Q., Yang F., Guo M., Wen G., Zhang C., Zhu J., Xiao Q., Zhang L. miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. J. Mol. Cell. Cardiol. 2015;89:75–86. doi: 10.1016/j.yjmcc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 83.Badi I., Mancinelli L., Polizzotto A., Ferri D., Zeni F., Burba I., Milano G., Brambilla F., Saccu C., Bianchi M.E., et al. miR-34a Promotes Vascular Smooth Muscle Cell Calcification by Downregulating SIRT1 (Sirtuin 1) and Axl (AXL Receptor Tyrosine Kinase) Arterioscler. Thromb. Vasc. Biol. 2018;38:2079–2090. doi: 10.1161/ATVBAHA.118.311298. [DOI] [PubMed] [Google Scholar]
- 84.Janssen L.M.E., Ramsay E.E., Logsdon C.D., Overwijk W.W. The immune system in cancer metastasis: Friend or foe? J. Immunother. Cancer. 2017;5:79. doi: 10.1186/s40425-017-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Esfahani K., Roudaia L., Buhlaiga N., Del Rincon S.V., Papneja N., Miller W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020;27:S87–S97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marshall J.S., Warrington R., Watson W., Kim H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018;14:49. doi: 10.1186/s13223-018-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicholson L.B. The immune system. Essays Biochem. 2016;60:275–301. doi: 10.1042/EBC20160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fernandez-Ruiz I. Immune system and cardiovascular disease. Nat. Rev. Cardiol. 2016;13:503. doi: 10.1038/nrcardio.2016.127. [DOI] [PubMed] [Google Scholar]
- 89.Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lafuse W.P., Wozniak D.J., Rajaram M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells. 2020;10:51. doi: 10.3390/cells10010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duncan S.E., Gao S., Sarhene M., Coffie J.W., Linhua D., Bao X., Jing Z., Li S., Guo R., Su J., et al. Macrophage Activities in Myocardial Infarction and Heart Failure. Cardiol. Res. Pract. 2020;2020:4375127. doi: 10.1155/2020/4375127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lavine K.J., Pinto A.R., Epelman S., Kopecky B.J., Clemente-Casares X., Godwin J., Rosenthal N., Kovacic J.C. The Macrophage in Cardiac Homeostasis and Disease: JACC Macrophage in CVD Series (Part 4) J. Am. Coll. Cardiol. 2018;72:2213–2230. doi: 10.1016/j.jacc.2018.08.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kroesen B.J., Teteloshvili N., Smigielska-Czepiel K., Brouwer E., Boots A.M., van den Berg A., Kluiver J. Immuno-miRs: Critical regulators of T-cell development, function and ageing. Immunology. 2015;144:1–10. doi: 10.1111/imm.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davidson-Moncada J., Papavasiliou F.N., Tam W. MicroRNAs of the immune system: Roles in inflammation and cancer. Ann. N. Y. Acad. Sci. 2010;1183:183–194. doi: 10.1111/j.1749-6632.2009.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Denisenko E., Ho D., Tamgue O., Ozturk M., Suzuki H., Brombacher F., Guler R., Schmeier S. IRNdb: The database of immunologically relevant non-coding RNAs. Database. 2016;2016:baw138. doi: 10.1093/database/baw138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J., Li S., Wang T., Xu S., Wang X., Kong X., Lu X., Zhang H., Li L., Feng M., et al. RNA2Immune: A database of experimentally supported data linking non-coding RNA regulation to the immune system. Genom. Proteom. Bioinform. 2022 doi: 10.1016/j.gpb.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 97.Olejniczak M., Galka-Marciniak P., Polak K., Fligier A., Krzyzosiak W.J. RNAimmuno: A database of the nonspecific immunological effects of RNA interference and microRNA reagents. RNA. 2012;18:930–935. doi: 10.1261/rna.025627.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Italiani P., Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yunna C., Mengru H., Lei W., Weidong C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877:173090. doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 100.Gombozhapova A., Rogovskaya Y., Shurupov V., Rebenkova M., Kzhyshkowska J., Popov S.V., Karpov R.S., Ryabov V. Macrophage activation and polarization in post-infarction cardiac remodeling. J. Biomed. Sci. 2017;24:13. doi: 10.1186/s12929-017-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu K., Liang X., Shen K., Sun L., Cui D., Zhao Y., Tian J., Ni L., Liu J. MiR-222 modulates multidrug resistance in human colorectal carcinoma by down-regulating ADAM-17. Exp. Cell Res. 2012;318:2168–2177. doi: 10.1016/j.yexcr.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 102.Li Y., Zhao L., Shi B., Ma S., Xu Z., Ge Y., Liu Y., Zheng D., Shi J. Functions of miR-146a and miR-222 in Tumor-associated Macrophages in Breast Cancer. Sci. Rep. 2015;5:18648. doi: 10.1038/srep18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou H., Lin S., Li X., Guo D., Wang Y., Hu Y. Serum miR-222 is independently associated with atrial fibrillation in patients with degenerative valvular heart disease. BMC Cardiovasc. Disord. 2021;21:98. doi: 10.1186/s12872-021-01909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Corsten M.F., Heggermont W., Papageorgiou A.P., Deckx S., Tijsma A., Verhesen W., van Leeuwen R., Carai P., Thibaut H.J., Custers K., et al. The microRNA-221/-222 cluster balances the antiviral and inflammatory response in viral myocarditis. Eur. Heart J. 2015;36:2909–2919. doi: 10.1093/eurheartj/ehv321. [DOI] [PubMed] [Google Scholar]
- 105.Liu X., Xiao J., Zhu H., Wei X., Platt C., Damilano F., Xiao C., Bezzerides V., Bostrom P., Che L., et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–595. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knyrim M., Rabe S., Grossmann C., Gekle M., Schreier B. Influence of miR-221/222 on cardiomyocyte calcium handling and function. Cell Biosci. 2021;11:160. doi: 10.1186/s13578-021-00676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verjans R., Peters T., Beaumont F.J., van Leeuwen R., van Herwaarden T., Verhesen W., Munts C., Bijnen M., Henkens M., Diez J., et al. MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload-Induced Heart Failure. Hypertension. 2018;71:280–288. doi: 10.1161/HYPERTENSIONAHA.117.10094. [DOI] [PubMed] [Google Scholar]
- 108.Wang Z., Wang Z., Gao L., Xiao L., Yao R., Du B., Li Y., Wu L., Liang C., Huang Z., et al. miR-222 inhibits cardiac fibrosis in diabetic mice heart via regulating Wnt/beta-catenin-mediated endothelium to mesenchymal transition. J. Cell. Physiol. 2020;235:2149–2160. doi: 10.1002/jcp.29119. [DOI] [PubMed] [Google Scholar]
- 109.Alivernini S., Gremese E., McSharry C., Tolusso B., Ferraccioli G., McInnes I.B., Kurowska-Stolarska M. MicroRNA-155-at the Critical Interface of Innate and Adaptive Immunity in Arthritis. Front. Immunol. 2017;8:1932. doi: 10.3389/fimmu.2017.01932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mattiske S., Suetani R.J., Neilsen P.M., Callen D.F. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol. Biomark. Prev. 2012;21:1236–1243. doi: 10.1158/1055-9965.EPI-12-0173. [DOI] [PubMed] [Google Scholar]
- 111.Nariman-Saleh-Fam Z., Saadatian Z., Daraei A., Mansoori Y., Bastami M., Tavakkoli-Bazzaz J. The intricate role of miR-155 in carcinogenesis: Potential implications for esophageal cancer research. Biomark. Med. 2019;13:147–159. doi: 10.2217/bmm-2018-0127. [DOI] [PubMed] [Google Scholar]
- 112.Xin X., Lu Y., Xie S., Chen Y., Jiang X., Song S., Wang L., Pu H., Gui X., Li T., et al. miR-155 Accelerates the Growth of Human Liver Cancer Cells by Activating CDK2 via Targeting H3F3A. Mol. Ther. Oncolytics. 2020;17:471–483. doi: 10.1016/j.omto.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shao C., Yang F., Qin Z., Jing X., Shu Y., Shen H. The value of miR-155 as a biomarker for the diagnosis and prognosis of lung cancer: A systematic review with meta-analysis. BMC Cancer. 2019;19:1103. doi: 10.1186/s12885-019-6297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hosseini Mojahed F., Aalami A.H., Pouresmaeil V., Amirabadi A., Qasemi Rad M., Sahebkar A. Clinical Evaluation of the Diagnostic Role of MicroRNA-155 in Breast Cancer. Int. J. Genom. 2020;2020:9514831. doi: 10.1155/2020/9514831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bayraktar R., Van Roosbroeck K. miR-155 in cancer drug resistance and as target for miRNA-based therapeutics. Cancer Metastasis Rev. 2018;37:33–44. doi: 10.1007/s10555-017-9724-7. [DOI] [PubMed] [Google Scholar]
- 116.Bruen R., Fitzsimons S., Belton O. miR-155 in the Resolution of Atherosclerosis. Front. Pharmacol. 2019;10:463. doi: 10.3389/fphar.2019.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wei Y., Nazari-Jahantigh M., Neth P., Weber C., Schober A. MicroRNA-126, -145, and -155: A therapeutic triad in atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2013;33:449–454. doi: 10.1161/ATVBAHA.112.300279. [DOI] [PubMed] [Google Scholar]
- 118.Pankratz F., Bemtgen X., Zeiser R., Leonhardt F., Kreuzaler S., Hilgendorf I., Smolka C., Helbing T., Hoefer I., Esser J.S., et al. MicroRNA-155 Exerts Cell-Specific Antiangiogenic but Proarteriogenic Effects During Adaptive Neovascularization. Circulation. 2015;131:1575–1589. doi: 10.1161/CIRCULATIONAHA.114.014579. [DOI] [PubMed] [Google Scholar]
- 119.Gruner H.N., McManus M.T. Examining the evidence for extracellular RNA function in mammals. Nat. Rev. Genet. 2021;22:448–458. doi: 10.1038/s41576-021-00346-8. [DOI] [PubMed] [Google Scholar]
- 120.Yin C., Han Q., Xu D., Zheng B., Zhao X., Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology. 2019;8:1601479. doi: 10.1080/2162402X.2019.1601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shimada B.K., Yang Y., Zhu J., Wang S., Suen A., Kronstadt S.M., Jeyaram A., Jay S.M., Zou L., Chao W. Extracellular miR-146a-5p Induces Cardiac Innate Immune Response and Cardiomyocyte Dysfunction. Immunohorizons. 2020;4:561–572. doi: 10.4049/immunohorizons.2000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 123.Diamond M.S., Kanneganti T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022;23:165–176. doi: 10.1038/s41590-021-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhardwaj A., Sapra L., Saini C., Azam Z., Mishra P.K., Verma B., Mishra G.C., Srivastava R.K. COVID-19: Immunology, Immunopathogenesis and Potential Therapies. Int. Rev. Immunol. 2022;41:171–206. doi: 10.1080/08830185.2021.1883600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mortaz E., Tabarsi P., Varahram M., Folkerts G., Adcock I.M. The Immune Response and Immunopathology of COVID-19. Front. Immunol. 2020;11:2037. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Urban S., Fulek M., Blaziak M., Iwanek G., Jura M., Fulek K., Guzik M., Garus M., Gajewski P., Lewandowski L., et al. COVID-19 Related Myocarditis in Adults: A Systematic Review of Case Reports. J. Clin. Med. 2022;11:5519. doi: 10.3390/jcm11195519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuehn B.M. Cardiac Complications More Common After COVID-19 Than Vaccination. JAMA. 2022;327:1951. doi: 10.1001/jama.2022.8061. [DOI] [PubMed] [Google Scholar]
- 128.Abbasi J. The COVID Heart-One Year After SARS-CoV-2 Infection, Patients Have an Array of Increased Cardiovascular Risks. JAMA. 2022;327:1113–1114. doi: 10.1001/jama.2022.2411. [DOI] [PubMed] [Google Scholar]
- 129.Verma A.K., Lavine K.J., Lin C.Y. Myocarditis after COVID-19 mRNA Vaccination. N. Engl. J. Med. 2021;385:1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Plikus M.V., Wang X., Sinha S., Forte E., Thompson S.M., Herzog E.L., Driskell R.R., Rosenthal N., Biernaskie J., Horsley V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell. 2021;184:3852–3872. doi: 10.1016/j.cell.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Asif P.J., Longobardi C., Hahne M., Medema J.P. The Role of Cancer-Associated Fibroblasts in Cancer Invasion and Metastasis. Cancers. 2021;13:4720. doi: 10.3390/cancers13184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smolarz B., Durczynski A., Romanowicz H., Szyllo K., Hogendorf P. miRNAs in Cancer (Review of Literature) Int. J. Mol. Sci. 2022;23:2805. doi: 10.3390/ijms23052805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S., Lv T., Chen Q., Yang Y., Xu L., Zhang X., Wang E., Hu X., Liu Y. Transcriptome sequencing and lncRNA-miRNA-mRNA network construction in cardiac fibrosis and heart failure. Bioengineered. 2022;13:7118–7133. doi: 10.1080/21655979.2022.2045839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banerjee M., Ferragut Cardoso A., Al-Eryani L., Pan J., Kalbfleisch T.S., Srivastava S., Rai S.N., States J.C. Dynamic alteration in miRNA and mRNA expression profiles at different stages of chronic arsenic exposure-induced carcinogenesis in a human cell culture model of skin cancer. Arch. Toxicol. 2021;95:2351–2365. doi: 10.1007/s00204-021-03084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y., Wang D., Peng M., Tang L., Ouyang J., Xiong F., Guo C., Tang Y., Zhou Y., Liao Q., et al. Single-cell RNA sequencing in cancer research. J. Exp. Clin. Cancer Res. 2021;40:81. doi: 10.1186/s13046-021-01874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Surina S., Fontanella R.A., Scisciola L., Marfella R., Paolisso G., Barbieri M. miR-21 in Human Cardiomyopathies. Front. Cardiovasc. Med. 2021;8:767064. doi: 10.3389/fcvm.2021.767064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jenike A.E., Halushka M.K. miR-21: A non-specific biomarker of all maladies. Biomark. Res. 2021;9:18. doi: 10.1186/s40364-021-00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feng Y.H., Tsao C.J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016;5:395–402. doi: 10.3892/br.2016.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng Y., Zhang C. MicroRNA-21 in cardiovascular disease. J. Cardiovasc. Transl. Res. 2010;3:251–255. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bautista-Sanchez D., Arriaga-Canon C., Pedroza-Torres A., De La Rosa-Velazquez I.A., Gonzalez-Barrios R., Contreras-Espinosa L., Montiel-Manriquez R., Castro-Hernandez C., Fragoso-Ontiveros V., Alvarez-Gomez R.M., et al. The Promising Role of miR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Mol. Ther. Nucleic Acids. 2020;20:409–420. doi: 10.1016/j.omtn.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 142.Roy S., Khanna S., Hussain S.R., Biswas S., Azad A., Rink C., Gnyawali S., Shilo S., Nuovo G.J., Sen C.K. MicroRNA expression in response to murine myocardial infarction: MiR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Song S.J., Poliseno L., Song M.S., Ala U., Webster K., Ng C., Beringer G., Brikbak N.J., Yuan X., Cantley L.C., et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154:311–324. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song S.J., Ito K., Ala U., Kats L., Webster K., Sun S.M., Jongen-Lavrencic M., Manova-Todorova K., Teruya-Feldstein J., Avigan D.E., et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell. 2013;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang R., Deng L., Zhao L., Li X., Zhang F., Xia Y., Gao Y., Wang X., Sun B. miR-22 promotes HBV-related hepatocellular carcinoma development in males. Clin. Cancer Res. 2011;17:5593–5603. doi: 10.1158/1078-0432.CCR-10-1734. [DOI] [PubMed] [Google Scholar]
- 146.Zhang L., Yang P., Wang J., Liu Q., Wang T., Wang Y., Lin F. MiR-22 regulated T cell differentiation and hepatocellular carcinoma growth by directly targeting Jarid2. Am. J. Cancer Res. 2021;11:2159–2173. [PMC free article] [PubMed] [Google Scholar]
- 147.Ji D., Li B., Shao Q., Li F., Li Z., Chen G. MiR-22 Suppresses BMP7 in the Development of Cirrhosis. Cell. Physiol. Biochem. 2015;36:1026–1036. doi: 10.1159/000430276. [DOI] [PubMed] [Google Scholar]
- 148.Huang Z.P., Wang D.Z. miR-22 in cardiac remodeling and disease. Trends Cardiovasc. Med. 2014;24:267–272. doi: 10.1016/j.tcm.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu Y., Liu H.X., Jena P.K., Sheng L., Ali M.R., Wan Y.Y. miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction. JHEP Rep. 2020;2:100093. doi: 10.1016/j.jhepr.2020.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang R., Xu Y., Zhang W., Fang Y., Yang T., Zeng D., Wei T., Liu J., Zhou H., Li Y., et al. Inhibiting miR-22 Alleviates Cardiac Dysfunction by Regulating Sirt1 in Septic Cardiomyopathy. Front. Cell Dev. Biol. 2021;9:650666. doi: 10.3389/fcell.2021.650666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chang H.Y., Chi J.T., Dudoit S., Bondre C., van de Rijn M., Botstein D., Brown P.O. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Muhl L., Genove G., Leptidis S., Liu J., He L., Mocci G., Sun Y., Gustafsson S., Buyandelger B., Chivukula I.V., et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat. Commun. 2020;11:3953. doi: 10.1038/s41467-020-17740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.LeBleu V.S., Neilson E.G. Origin and functional heterogeneity of fibroblasts. FASEB J. 2020;34:3519–3536. doi: 10.1096/fj.201903188R. [DOI] [PubMed] [Google Scholar]
- 154.Givel A.M., Kieffer Y., Scholer-Dahirel A., Sirven P., Cardon M., Pelon F., Magagna I., Gentric G., Costa A., Bonneau C., et al. miR200-regulated CXCL12beta promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat. Commun. 2018;9:1056. doi: 10.1038/s41467-018-03348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhao X.D., Qin R.H., Yang J.J., Xu S.S., Tao H., Ding X.S., Shi K.H. DNMT3A controls miR-200b in cardiac fibroblast autophagy and cardiac fibrosis. Inflamm. Res. 2018;67:681–690. doi: 10.1007/s00011-018-1159-2. [DOI] [PubMed] [Google Scholar]
- 156.Liu S., Ke W., Liu Y., Zhao Z., An L., You X., Yang F., Yang X., Wang G., Zhao X. Function analysis of differentially expressed microRNAs in TGF-beta1-induced cardiac fibroblasts differentiation. BioSci. Rep. 2019;39:BSR20182048. doi: 10.1042/BSR20182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Willis A.J. The Ecosystem: An Evolving Concept Viewed Historically. Funct. Ecol. 1997;11:268–271. doi: 10.1111/j.1365-2435.1997.00081.x. [DOI] [Google Scholar]
- 158.Luo W. Nasopharyngeal Carcinoma Ecology Theory: Cancer as Multidimensional Spatiotemporal “Unity of Ecology and Evolution” Pathological Ecosystem. Preprints. 2022:2022100226. doi: 10.20944/preprints202210.0226.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]