Abstract
In areas where Plasmodium falciparum is endemic, pregnant women are at increased risk for malaria, and this risk is greatest during the first pregnancy. The placenta sequesters parasites that are able to cytoadhere to chondroitin sulfate A (CSA), a molecule expressed by the placental syncytiotrophoblast, while parasites from a nonpregnant host do not bind to CSA. Cytoadherence is mediated by the expression of variants of the P. falciparum-erythrocyte membrane protein 1 family. Each member of this molecule family induces antibodies that specifically agglutinate infected erythrocytes and inhibit their cytoadherence ability. We investigated whether the higher susceptibility of primigravidae was related to the lack of immune response towards CSA-binding parasites. In a cross-sectional study, primigravidae delivering with a noninfected placenta were less likely to have antibodies agglutinating CSA-binding parasites than multigravidae (P < 0.01). In contrast, parasites from nonpregnant hosts were as likely to be recognized by the sera from women of various parities. In a longitudinal study, at 6 months of pregnancy, antibodies against CSA-binding parasites were present in 31.8% of primigravidae and in 76.9% of secundigravidae (P = 0.02). The antibodies against CSA-binding parasites inhibited the cytoadherence of a CSA-adherent parasite strain to the human placental trophoblast. Our data support the idea that the higher susceptibility of primiparae is related to a lack of a specific immune response to placental parasites.
Plasmodium falciparum, a parasite responsible for the most severe forms of malaria, is a major cause of morbidity and mortality in the world. In areas where it is endemic, most people are frequently infected but the main part of the clinical burden is borne by young children and pregnant women, while few adults are usually clinically affected. Interestingly, among pregnant women, primiparae are more susceptible to malaria than multiparae (16). Our work aims at understanding the rationale for the higher susceptibility of primigravidae to malaria, in order to help in targeting and performing oriented public health programs.
It has long been known that repeated and continuous exposure to malaria parasites during infancy leads to premunition, an immune state defined as the symptomless persistence of a low number of parasites. The development of premunition has been extensively studied, and there is now good evidence that it is related to the acquisition of a repertoire of protective antibodies reactive against polymorphic molecules prominently exposed at the surface of the infected erythrocyte (2, 12). The best characterized of these molecules are collectively referred to as P. falciparum-erythrocyte membrane protein 1 (PfEMP1). Although the P. falciparum genome is able to encode around one hundred PfEMP1 molecules, these molecules are expressed not simultaneously but one at a time. In addition to their antigenic properties, PfEMP1 molecules mediate the cytoadherence of infected erythrocytes to a variety of endothelium cells that express receptors. Receptors potentially bound by P. falciparum-infected erythrocytes include CD36, intercellular adhesion molecule 1 (ICAM-1), thrombospondin, and chondroitin sulfate A (CSA), which are bound in vitro by some PfEMP1 members (1, 19). In addition, E selectin, vascular cell adhesion molecule 1 (VCAM-1), and platelet endothelial cell adhesion molecule 1 (PECAM1) are bound by P. falciparum-infected erythrocytes, but their properties of binding to PfEMP1 remain to be assessed.
In pregnant women, a large amount of mature parasites is sequestered in the maternal space of the placenta (22). It has been suggested that parasites could cytoadhere to the syncytiotrophoblast, the outermost line of the placenta in contact with maternal blood (6). It has been demonstrated that the syncytiotrophoblast expresses a large amount of CSA (13). It has also been shown that erythrocytes parasitized by mature parasites from pregnant women bind to the syncytiotrophoblast by adhering to CSA, while parasites from nonpregnant hosts do not usually bind to CSA (6, 13). Consequently, parasites from pregnant women may express a PfEMP1 variant not expressed in parasites from nonpregnant subjects. We hypothesized that women are not immune to placental parasites before being pregnant and that a first pregnancy allows them to develop an immune response against the CSA-parasite binding receptor that protects them from malaria during subsequent pregnancies.
In a cross-sectional study carried out in Yaoundé, Cameroon, we assessed the ability of sera from pregnant women to agglutinate parasite isolates from pregnant women and a P. falciparum strain that binds only to CSA (and was therefore used to mimic placental parasites). We next described the acquisition of immunity against pregnancy-associated parasites (PAPs) in women longitudinally monitored in Ebolowa, Cameroon, during their first two pregnancies. Finally we present evidence that antibodies directed against PAPs acquired during the first infected pregnancy inhibit the cytoadherence of placental parasites to the human syncytiotrophoblast and may account for the lower frequency of malaria in multigravidae.
MATERIALS AND METHODS
Samples from Yaoundé.
In this study, we enrolled all women delivering babies in the maternity wards of Nkolndongo, Yaoundé, Cameroon, from June 1996 to April 1997, after they gave their oral informed consent. Women delivering during weekends were excluded. After the women had delivered, blood samples were taken by puncture and plasma was frozen. A crush smear was made from an excised piece of placenta. Placental blood thick films were air dried, Giemsa stained, read by microscopy over 50 fields at a ×1,000 magnification, and considered positive when P. falciparum parasites or malarial pigments were observed. Peripheral blood parasites were cryopreserved. Nonpregnant subjects (women and men) were recruited in the dispensaries of Nkolndongo and Messa, in the same town. Plasma samples from all participants were frozen, and parasites, if any were isolated, were cryopreserved.
Serum samples from Ebolowa.
To study the evolution of P. falciparum-reactive antibodies during pregnancy, we used plasma samples collected in a longitudinal study conducted from 1991 to 1992 in Ebolowa, Cameroon (5). In this study, 50 women were monitored from their first to second pregnancies. Blood samples were drawn in the 6th month of first pregnancies, at first delivery, six months after delivery, in the 6th month of second pregnancies (if any), and then at second delivery. Cord blood samples were also collected. All sera were kept frozen at −20°C until being tested.
Parasite cultures.
The RP5 P. falciparum line (a gift from J. Gysin, Laboratoire de Génétique et d’Immunologie, IMTSSA, Parc du Pharo, Marseille, France) binds to CSA and not to the other known receptors of P. falciparum (8) and consequently binds to the human syncytiotrophoblast (14). In our laboratory, the binding phenotype was maintained by a fortnight flotation on plasmagel (18). Three parasite isolates from pregnant women, four from nonpregnant women, and the RP5 strain were thawed and cultivated in candle jars according to standard procedures (21) at a 5% hematocrit with 10% heat-inactivated human AB serum added to RPMI 1640-HEPES (25 mM). All tests were performed when parasites were in the late stage (from late trophozoite to young schizont). Parasites from isolates were used during the first life cycle.
Agglutination test.
Serum antibodies to infected erythrocytes (IEs) were detected by a modification of the antibody-mediated agglutination assay (11). Serum (2.5 μl) was deposited in a 96-well microtitration plate (U bottom). A parasite culture at the mature stage was washed and resuspended in phosphate-buffered saline, pH 7.4, at an 11% hematocrit, and 22.5 μl of this suspension containing 0.01% acridine orange was added into each well (final hematocrit, 10%; final serum concentration, 10%). After a 90-min rotation at room temperature on a Coulter mixer (a 45° inclination on a 22-round-per-minute rotating dish), 50 μl of phosphate-buffered saline was added and 20 μl of the suspension was examined between an examination slide and a 22- by 22-mm cover slide. Agglutinates were examined under UV and bright-field illumination. The assay result was considered positive when at least five agglutinates of at least three IEs were counted, and the result was quantified by the geometric mean of the five biggest agglutinates.
Inhibition of the cytoadherence to human trophoblast by immune-phase sera.
The effect of sera on the cytoadherence of the RP5 strain was assessed by using a modification of the cytoadherence assay previously described (14). Briefly, cytotrophoblasts were purified from a human placenta by negative immunoselection for CD9 (24). Cells were seeded in microwells on plastic dishes coated with parafilm (15) and cultivated for 7 to 15 days before the cytoadherence assay was performed. The RP5 strain was used when presenting with a majority of mature stages. Parasite culture at a 10% hematocrit was incubated with RP5-agglutinating sera and RP5-nonagglutinating sera (randomly chosen from the Yaoundé study) at a 1/10 dilution for 1 h with one shake at the midpoint. The parasite culture was then incubated with the trophoblast culture for 40 min before nonadherent IEs were removed by gentle washing. Preparations were glutaraldehyde fixed and observed microscopically. The number of IEs adhering to 1 mm2 of trophoblast culture, divided by the parasite density used in the assay, was counted (15).
Statistical analyses.
Proportions of agglutination were compared by contingency table analysis (chi-square test or Fisher’s exact test, when appropriate). Differences of means of agglutinating indices between groups were analyzed by nonparametric methods (Mann-Whitney U test). The evolution of paired agglutination indices in the longitudinal study was analyzed with Wilcoxon’s test for paired data. The significance limit (P) was 0.05, with a two-tailed risk. Statistics were computerized with StatView 4.5 (Abacus Concepts, Berkeley, Calif.).
RESULTS AND DISCUSSION
Cross-sectional study in Yaoundé.
A total of 664 women giving birth to live, single-born, term-delivered children were enrolled. Mothers’ ages and parities and birth weights of infants are reported in Table 1. A mean of 23.5% of parturients had parasitized placentas, but primigravidae were more often infected, and susceptibility to malaria decreased with parity (Table 1).
TABLE 1.
Mothers’ ages and parities, placental malaria infection, and birth weight of newborns in Yaoundé (n = 664)
| Characteristic | Mean ± SD (range) |
|---|---|
| Age (yr) | 26.8 ± 5.9 (14–45) |
| Parity | 3.3 ± 2.3 (1–14) |
| Birth weight (g) of newborn | 3,163 ± 454 (1,050–4,360) |
| Placental infection (%)a | 23.5 |
| Parity 1 (n = 170) | 34.7 |
| Parity 2–3 (n = 239) | 22.2 |
| Parity >3 (n = 255) | 17.3 |
Total infections for all women.
Serum ability to agglutinate IEs was assessed by using three isolates obtained from nonpregnant subjects, three isolates from pregnant women, and the RP5 strain. The four latter parasites will be collectively referred to as PAPs. All serum samples were tested with the RP5 strain, but only a subset of samples were tested with all isolates, because of ABO blood group incompatibility and limited amounts of some isolates. For pregnant women, we first compared primiparae to women with parities equal to or higher than 4. Data from men and nonpregnant women were similar and were pooled in a single group referred to as nonpregnant subjects.
The frequency of agglutinating antibodies against any of the seven tested parasites was higher in delivering women than in nonpregnant hosts (all P values < 0.03; Table 2). This may be related to the switch away from type 1 cytokines and towards type 2 cytokines that affects pregnancy and may favor antibody production (23). However, the effect of pregnancy was more pronounced against PAPs than against parasites from nonpregnant hosts. This is likely because the immune system is preferentially stimulated by PAPs during pregnancy and is in line with the fact that parasites from nonpregnant subjects bind rarely to CSA, while PAPs bind only to CSA (6, 13).
TABLE 2.
Proportions of serum samples agglutinating P. falciparum parasites from the RP5 strain and from isolates obtained from pregnant or nonpregnant subjectsa
| Isolate | % (No.) of serum samples agglutinating parasites
|
||||
|---|---|---|---|---|---|
| Nonpregnant subjects | Women delivering babies
|
||||
| Nonparasitized placenta
|
Parasitized placenta
|
||||
| Parity = 1 | Parity ≥ 4 | Parity = 1 | Parity ≥ 4 | ||
| Nonpregnant subjects | |||||
| 501 | 38.3 (47) | 73.0 (37) | 78.3 (60) | 73.9 (23) | 70.0 (10) |
| 543 | 22.9 (48) | 38.9 (36) | 35.6 (59) | 39.1 (23) | 30.0 (10) |
| 538 | 54.2 (48) | 63.9 (36) | 69.5 (59) | 91.3 (23) | 100 (10) |
| Pregnant women | |||||
| 1237 | 5.4 (56) | 5.2 (38) | 27.0 (63)* | 46.2 (25) | 40.0 (15) |
| 1396 | 20 (10) | 15.4 (13) | 44.0 (25) | 80.0 (10) | 100 (7) |
| 1492 | 6.6 (15) | 23.1 (13) | 52.9 (17) | ND | ND |
| RP5 | 7.8 (64) | 22.3 (76) | 50.0 (143)* | 61.0 (41) | 75.9 (29) |
P. falciparum isolates and the RP5 strain were cultured in vitro until the culture presented a majority of mature stages. Acridine orange-labelled parasites were rotated for 90 min in a 1/10 dilution of serum, and the level of agglutination was determined under UV microscopy. The result was considered as negative if fewer than five agglutinates of at least three IEs were counted. *, between parity 1 and parity ≥4, P < 0.05 (chi-square test or Fisher’s exact test). ND, not determined.
Among parturients, responses against PAPs were less frequent in primiparae than in multiparae, especially in the absence of placental infection; in noninfected pregnant women, the proportion of responses to the RP5 strain and isolate 1237 increased significantly between primiparae and multiparae (P = 0.0001 and 0.008, respectively). A similar increase was observed with isolates 1396 and 1492, although it was only of borderline significance (P = 0.08 and 0.10, respectively), likely because of a lack of power of the analysis (Table 2). Conversely, responses to parasites from nonpregnant subjects were similar in primiparae and multiparae, whether the placenta was infected or not (Table 2). We then extended our study of anti-RP5 antibody to pregnant women with parities of 2 and 3 (Table 3). In the absence of placental infection, sera from primiparae agglutinated RP5 less frequently than sera from parity 4 women and than sera from women with a parity of ≤2 (22.3 versus 41.5%; P = 0.002). We conclude that primiparae lack antibodies against PAPs. Among pregnant women, primiparae with noninfected placentas have the higher likelihood not to have been infected with placental parasites. However, even in these women, a past infection may have occurred (3), explaining why some of them had raised anti-PAP antibodies at delivery. The higher proportion of multiparae that recognize PAPs reflects the PAP-specific preimmunization of women during a previous parasitized pregnancy.
TABLE 3.
Serum ability to agglutinate CSA-adherent parasite line RP5 in pregnant women of Yaoundé at delivery
| Group of pregnant women | % (No.) of sera agglutinating RP5 for women at a parity of:
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ≥6 | |
| Noninfected | 22.3 (76) | 34.4 (64) | 31.7 (41) | 40.9 (44) | 51.6 (31) | 50 (68) |
| Infected | 61.0 (41) | 55.6 (18) | 80.0 (10) | 75.9 (29)a | ||
This value represents a parity of 4 or more.
Few sera from nonpregnant hosts agglutinated PAPs, while they did have antibodies to parasites from nonpregnant hosts: against each of the three tested parasites, the proportion of agglutinating sera was always higher than 22% (Table 2). In men, the low frequency of antibodies against PAPs is explained by the rarity of CSA-adherent parasites in nonpregnant hosts (4, 6). However, this frequency was also low in nonpregnant women, although half of them had previously delivered at least once and around a quarter had had more than four children (data not shown). This low frequency might be related to the fact that, in cases of placental infection, most antigens are locally presented to effector cells by macrophages from the placenta. These macrophages are likely to be impaired by high levels of corticosteroids, interleukin 10 (7), malarial pigment (20), and other local immunosuppressive molecules. This may induce an incomplete immune response, with a local but fugitive production of agglutinating antibodies, which would decrease rapidly to a nondetectable level when the antigen is not present anymore, i.e., after delivery.
There was a strong cross-reactivity between strain RP5 and the three isolates from pregnant women, as subjects with antibodies to PAPs usually had a high level of RP5 agglutination. Conversely, there was no evidence of cross-agglutination between the three isolates from nonpregnant subjects (data not shown), suggesting that parasites from pregnant women are more homogeneous than those from nonpregnant hosts.
Longitudinal study in Ebolowa.
The development during pregnancy of specific antibody to PAPs was confirmed in a longitudinal study in Ebolowa (5), which demonstrated that pregnant women were more susceptible to malaria during first pregnancies than second pregnancies. During first pregnancies, P. falciparum-infected erythrocytes were present in 46.7% of women at the 6th month and in 65.2% at first delivery. Conversely, during second pregnancies, infection rates at the 6th month and at delivery dramatically dropped to 7.7% (1 of 13) and 0 of 5, respectively. The infection rates at 6 months of first and second pregnancies differed significantly (Fisher’s exact test [P = 0.01]; Table 4). Compared to women from Yaoundé, pregnant women from Ebolowa were more often infected by P. falciparum and presented a stronger protective effect of parity, likely because Plasmodium transmission and exposure are higher (62 infective bites/year/person versus 15) (9, 10).
TABLE 4.
Evolution of the susceptibility to malaria and the ability of sera to agglutinate a CSA-adherent parasite and an ICAM-1-adherent isolate in 45 primigravidae from Ebolowa monitored during two consecutive pregnancies
| Parameter | Result for:
|
Pb | ||||
|---|---|---|---|---|---|---|
| First pregnancy
|
Six mo after delivery | Second pregnancy
|
||||
| Sixth mo | Delivery | Sixth mo | Delivery | |||
| No. of women | 45 | 23 | 33 | 13 | 5 | |
| Infection (%)a | 46.7 | 65.2 | 6.1 | 7.7 | 0 | 0.005 |
| Agglutination of a CSA-adherent parasite (RP5) | ||||||
| Positive tests (%) | 31.8 | 66.7 | 54.5 | 76.9 | 80 | 0.002 |
| Agglutination index (mean ± SD) | 16.3 ± 4.0 | 23.0 ± 4.3 | 15.5 ± 1.9 | 13.0 ± 3.8 | 9.7 ± 1.6 | |
| Agglutination of an ICAM-1-adherent parasite | ||||||
| Positive tests (%) | 20.5 | 40.9 | 31.0 | 30.8 | 20 | NSc |
| Agglutination index (mean ± SD) | 5.8 ± 2.5 | 5.8 ± 1.9 | 6.1 ± 1.4 | 4.1 ± 0.2 | 7.5 | |
Peripheral infection at the 6th month of pregnancy and 6 months after delivery; placental infection at delivery.
Sixth month of first pregnancy versus 6th month of second pregnancy (Fisher’s exact test).
NS, not significant.
At 6 months of pregnancy, the proportion of RP5-agglutinating sera was lower during first pregnancies than second pregnancies (31.8 versus 76.9%; P = 0.004). The mean agglutination index was raised between the 6th month and delivery time (Wilcoxon’s test; P = 0.006), then decreased after delivery (P = 0.008) (as expected from the rarity of response to RP5 in nonpregnant multiparae from Yaoundé), and increased again at 6 months of the second pregnancy (P = 0.06). The same sera were tested for agglutination against a parasite isolate collected from a nonpregnant host (T548), which bound to ICAM-1 but not to CSA (15). The frequency and intensity of agglutination of this isolate did not vary during the follow-up.
Mother to fetus passage of antibodies agglutinating RP5.
As agglutination of IEs is mainly mediated by immunoglobulin G and parasite-reactive antibodies should cross the maternofetal barrier, we assessed the agglutinating capacity of 42 pairs of mother and cord blood serum samples against both RP5 and an isolate from a nonpregnant woman. Antibodies against RP5 were detected in 90.3% (28 of 31) of cord blood samples which paired maternal blood and agglutinated RP5 and in none of the 11 cord blood samples which paired maternal blood and did not agglutinate RP5. Among paired agglutinating sera, agglutination indices were correlated in mother and offspring (P < 0.01).
Inhibition of cytoadherence of RP5 to syncytiotrophoblast by RP5-agglutinating serum samples.
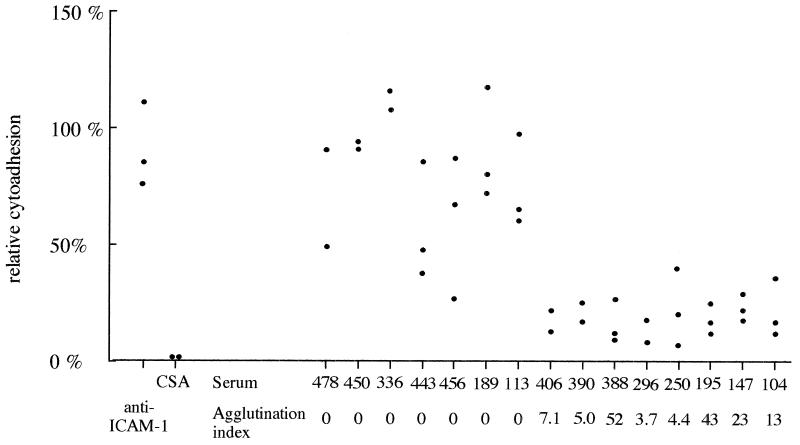
Figure 1 demonstrates that RP5 binds to the trophoblast by using only CSA. Indeed, exogenous CSA almost totally inhibited this binding, while anti-ICAM-1 monoclonal antibody did not affect cytoadherence. The cytoadherence of RP5 to the trophoblast culture was inhibited by RP5-agglutinating sera and not by nonagglutinating sera (81% ± 4% versus 20% ± 3%; Mann-Whitney U test [P = 0.001]). Cytoadherence inhibition by agglutinating sera was only partial, as some agglutinates of RP5-infected erythrocytes bound to the trophoblast, but the mean binding intensity was consistently lower in the presence of agglutinating sera than with nonagglutinating sera. Among RP5-agglutinating sera, the agglutination index and cytoadherence inhibition rate were not related.
FIG. 1.
Effect of sera on the cytoadhesion of RP5 to human syncytiotrophoblast. The ability of RP5 to bind to the human syncytiotrophoblast was tested after parasites were supplemented with CSA (1 mg/ml), after the trophoblast was incubated with 84H10 (a monoclonal antibody against ICAM-1), or after parasites were incubated with RP5-agglutinating sera and RP5-nonagglutinating sera (for details see Materials and Methods). Each point represents one measure.
In vivo, antibodies against PAPs may inhibit the sequestration of these parasites in the placenta and protect the mother against placental malaria. However, the efficacy of this inhibition is not complete, as a high proportion of women with antibodies against RP5 had a parasitized placenta and as, in our model, the inhibition of cytoadherence was uncomplete. In vivo, agglutinates could be trapped in the intervillous spaces and could be an alternative mechanism for placental sequestration. We recently observed agglutinates of IEs in maternal placental blood (data not shown).
Understanding the mechanism able to prevent P. falciparum sequestration in the placenta and the subsequent disorders that can be induced may have consequences in public health management, in terms of both drug-based control measures and malaria-preventing vaccine strategies. Indeed, in order to limit health expenses some countries have been advised to limit chemoprophylaxis against malaria during pregnancy only to primiparae. As our data suggest that the lower susceptibility of multiparae may rely on the anterior immunization of primiparae against placental parasites, a total inhibition of placental colonization during the first pregnancy should inhibit the development of antibodies against PAPs. Secundiparae would then be as likely as primiparae not treated by chemoprophylaxis to develop placental malaria. However, Menendez found no difference for the second pregnancies of women who during their first pregnancies had or had not been treated by prophylaxis (17). This discrepancy may be explained by the fact that prophylaxis is rarely initiated before the 5th or 6th month of pregnancy and is frequently impaired by drug resistance. Thus, chemoprophylaxis may limit placental colonization but may not inhibit the raising of a specific immunity. The second consequence of our findings is related to the design of a malaria vaccine. Bull and colleagues recently reported that disease protection is dependent on a variant-specific immune protection directed against a variety of members of the PfEMP1 family (2). This supports the idea that PfEMP1 molecules could be the targets of an antidisease vaccine, but the high number of members of the PfEMP1 family raises concern about the feasibility of this work. Conversely, in the case of malaria during pregnancy, the number of antigenic molecules is likely to be small (maybe only one), and this molecule could be an easy-to-obtain vaccine candidate against pregnancy-associated malaria.
ACKNOWLEDGMENTS
We acknowledge the strong support of the staff of the maternity clinic of Nkolndongo and the Ebolowa Hospital, who were responsible for the collection of all biological samples and parameters. We are grateful to the mothers. We thank Francis Louis, Pascal Ringwald, and Timoleon Tchuinkam (OCEAC, Yaoundé, Cameroon) for laboratory facilities and continuous support. We also thank Jean-Yves Le Hesran for providing data from women of Ebolowa.
This work was supported by grants from the French Ministry of Research and Space (92S0034), AUPELF/UREF, and from the French Ministry of Cooperation and Development. Bertrand Maubert was the recipient of a grant from the French Ministry of High Education and Research.
REFERENCES
- 1.Baruch D I, Gormley J A, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bull P C, Lowe B S, Kortok M, Molyneux C S, Newbold C I, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulmer J N, Rasheed F N, Francis N, Morrison L, Greenwood B M. Placental malaria. I. Pathological classification. Histopathology. 1993;22:211–218. doi: 10.1111/j.1365-2559.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 4.Chaiyaroj S C, Angkasekwinai P, Buranakiti A, Looareesuwan S, Rogerson S J, Brown G V. Cytoadherence characteristics of Plasmodium falciparum isolates from Thailand—evidence for chondroitin sulfate A as a cytoadherence receptor. Am J Trop Med Hyg. 1996;55:76–80. doi: 10.4269/ajtmh.1996.55.76. [DOI] [PubMed] [Google Scholar]
- 5.Fievet N, Cot M, Ringwald P, Bickii J, Dubois B, Le Hesran J Y, Migot F, Deloron P. Immune response to Plasmodium falciparum antigens in Cameroonian primigravidae: evolution after delivery and during second pregnancy. Clin Exp Immunol. 1997;107:462–467. doi: 10.1046/j.1365-2249.1997.d01-966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried M, Duffy P E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 7.Fried M, Muga R O, Misore A O, Duffy P E. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–2530. [PubMed] [Google Scholar]
- 8.Gay F, Robert C, Pouvelle B, Peyrol S, Scherf A, Gysin J. Isolation and characterization of brain microvascular endothelial cells from Saimiri monkeys. An in vitro model for sequestration of Plasmodium falciparum-infected erythrocytes. J Immunol Methods. 1995;184:15–28. doi: 10.1016/0022-1759(95)00070-q. [DOI] [PubMed] [Google Scholar]
- 9.Le Goff G, Toto J C. Rapport préliminaire sur la dynamique de la transmission du paludisme dans la ville d’Ebolowa et ses environs. Doc. Tech. 891/LPR/OCEAC. 1994. Ed. OCEAC, Yaoundé, Cameroon. [Google Scholar]
- 10.Manga L, Robert V, Messi J, Desfontaine M, Carnevale P. Le paludisme urbain à Yaoundé, Cameroun. 1. Etude entomologique dans deux quartiers centraux. Mem Soc R Belge Entomol. 1992;35:155–162. [Google Scholar]
- 11.Marsh K, Howard R J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 12.Marsh K, Otto L, Hayes R J, Carson D C, Greenwood B M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 13.Maubert, B., T. Fievet, G. Tami, C. Boudin, and P. Deloron. Cytoadhesion of Plasmodium falciparum-infected erythrocytes in the human placenta. Parasite Immunol., in press. [DOI] [PubMed]
- 14.Maubert B, Guilbert L J, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun. 1997;65:1251–1257. doi: 10.1128/iai.65.4.1251-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maubert B, Riche D, Deloron P. An in vitro microassay to assess the ability of Plasmodium falciparum-infected erythrocytes to bind the human syncytiotrophoblast. Am J Reprod Immunol. 1998;40:401–407. doi: 10.1111/j.1600-0897.1998.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 16.McGregor I A. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg. 1984;33:517–525. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 17.Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;11:178–193. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 18.Pasvol G, Wilson R J, Smalley M E, Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 19.Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, Lanzer M. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzer E, Turrini F, Ulliers D, Giribaldi G, Ginsburg H, Arese P. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992;176:1033–1041. doi: 10.1084/jem.176.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.Walter P R, Garin Y, Blot P. Placental pathologic changes in malaria. Am J Pathol. 1982;109:330–342. [PMC free article] [PubMed] [Google Scholar]
- 23.Wegmann T G, Lin H, Guilbert L, Mosmann T R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 24.Yui J, Garcia-Lloret M, Brown A J, Berdan R C, Morrish D W, Wegmann T G, Guilbert L J. Functional, long-term cultures of human term trophoblasts purified by column-elimination of CD9 expressing cells. Placenta. 1994;15:231–246. doi: 10.1016/0143-4004(94)90015-9. [DOI] [PubMed] [Google Scholar]