Abstract
Chiggers are the larval stage of Trombiculidae and Leeuwenhoekiidae mites of medical and veterinary importance. Some species in the genus Leptotrombidium and Herpetacarus vector Orientia species, the bacteria that causes scrub typhus disease in humans. Scrub typhus is a life-threatening, febrile disease. Chigger bites can also cause dermatitis. There were 248 chigger species reported from the US from almost every state. However, there are large gaps in our knowledge of the life history of other stages of development. North American wide morphological keys are needed for better species identification, and molecular sequence data for identification are minimal and not clearly matched with morphological data. The role of chiggers in disease transmission in the US is especially understudied, and the role of endosymbionts in Orientia infection are suggested in the scientific literature but not confirmed. The most common chiggers in the eastern United States were identified as Eutrombicula alfreddugesi but were likely misidentified and should be replaced with Eutrombicula cinnabaris. Scrub typhus was originally believed to be limited to the Tsutsugamushi Triangle and the chigger genus, Leptotrombidium, but there is increasing evidence this is not the case. The potential of Orientia species establishing in the US is high. In addition, several other recognized pathogens to infect humans, namely Hantavirus, Bartonella, Borrelia, and Rickettsia, were also detected in chiggers. The role that chiggers play in these disease transmissions in the US needs further investigation. It is possible some of the tick-borne diseases and red meat allergies are caused by chiggers.
Keywords: chiggers, biology, systematics, microbiome, pathogen transmission, control
1. Introduction
Chiggers are the larval stage of the families Trombiculidae and Leeuwenhoekiidae mites (Phylum Arthropoda, Class Arachnida, Order Trombidiformes, Superfamily Trombidioidea, and Family Trombiculidae, Ewing, 1929 and Family Leeuwenhoekiidae, Womersley, 1945. They are also called red bugs, harvest mites, berry bugs, scrub-itch mites in English-speaking countries, and sand mites in China. Chiggers are of medical and veterinary importance. Some species in the genus Leptotrombidium and Herpetacarus [1,2] are known to vector a Gram-negative bacterium, Orientia species (formerly in the genus Rickettsia), causing the disease, scrub typhus, in humans. Scrub typhus is a life-threatening, febrile disease. During the Second World War, a large number of scrub typhus patients were found in the Asia-Pacific region [3], which resulted in greater attention and research on all aspects of the disease. Humans are only an occasional host for chiggers. Chiggers parasitize many different vertebrates, including reptiles, amphibians, birds, and mammals, and were reported to be significant pests for free-range turkeys in the US [4]. Their bite frequently results in dermatitis (trombiculosis or trombiculiasis).
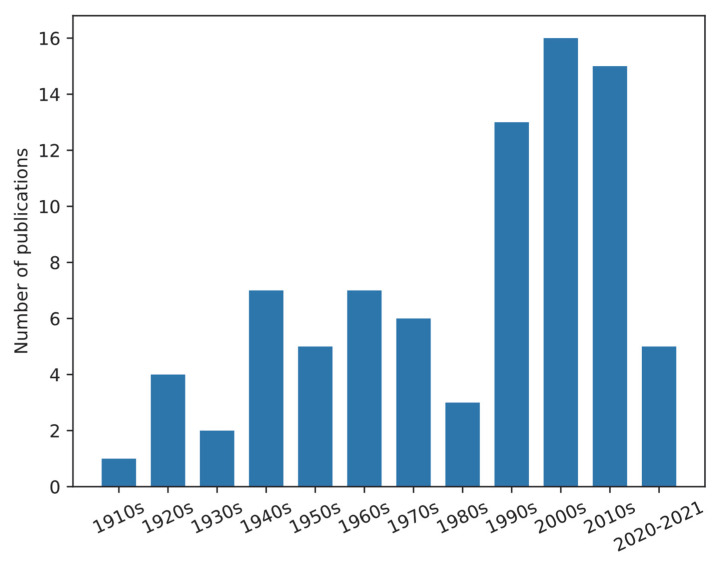
Publications of chigger studies in the US were searched on the Web of Science on 3 March 2022 (Table S1), showing 115 papers of which 83 were directly on chiggers. The number of publications on chiggers in the US is shown in Figure 1. Publications were summarized and shown in Table S2. The publications mainly focused on taxonomy (identification methods), ecology, and reports of the discovery of chiggers on various hosts in the US. Chigger research in the US started in the 1910s. The number of publications gradually increased from 1920 to 1979 followed by a drop in the 1980s. From 1990 till now, the number of publications per decade on this topic was 13–16. However, these data do not include all of the existing published papers, especially the older papers. For example, as stated in the world checklist of Trombiculidae and Leeuwenhoekiidae from Nielsen et al. [5], H.E. Ewing described 64 new chigger species from 1913 to 1950. Nielsen et al. listed 23 Ewing’s publications and five were marked as cited by other researchers. Based on our search results, there were only 10 papers published by Ewing that were available on the Web of Science, but additional papers were available at our institutional library.
Figure 1.
Bar chart of publications of chiggers in the US. Publications were searched through the Web of Science on 3 May 2022. The search strategy is in Table S1).
It is challenging to find papers about chigger studies in the US published 50–90 years ago. Even for the publications that can be found on the Web of Science, some are not easily accessible for review. Publications after the 1990s were better documented. It is clear that chiggers in the US are greatly understudied in general and especially as compared to other vector important arthropods like mosquitoes, ticks, lice, fleas, and filth flies. The goal of this paper was to review the biology, systematics, microbiome, pathogen transmission and control of chiggers with emphasis when possible on what we know about them in the US.
2. Chigger Biology
2.1. Life Cycle
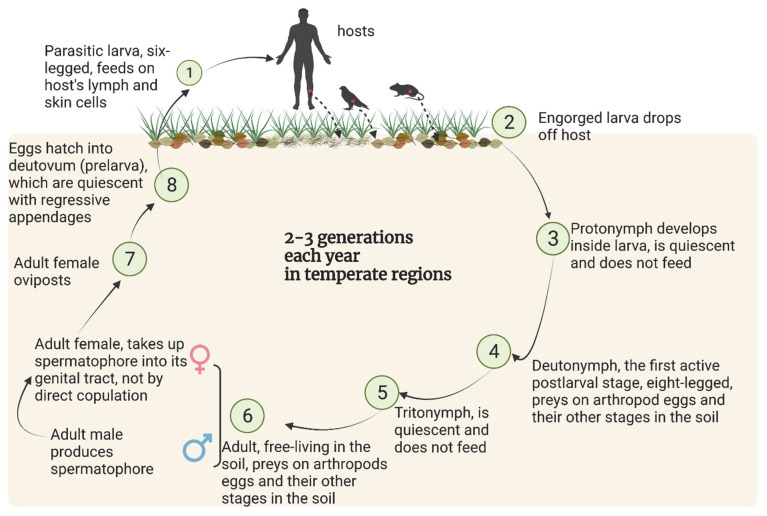
The life cycle of chigger mites is comprised of seven stages: egg, quiescent prelarva (also called deutovum), parasitic larva, quiescent protonymph, soil free-living deutonymph, quiescent tritonymph and soil free-living adult (Figure 2). The two active post larval stages, deutonymph and adult, have eight legs, live in soil, and are predators feeding on arthropod eggs and other life stages of small, soil-dwelling insects and other arthropods. The parasitic larval stage, so-called chiggers, have six legs. Unengorged chiggers are about 0.2 mm long and deep-red in color, while engorged chiggers are about 0.4 mm long and pale yellow [6,7].
Figure 2.
The typical life cycle of chiggers. Created with BioRender.com. Data sources: [11,13,14].
Chiggers do not burrow into skin and feed on blood as many may think. Instead, chiggers feed on saliva digested lymph and skin cells. They insert their chelicerae into the skin and secrete saliva containing proteolytic enzymes into the skin. The epidermal and cutaneous host tissue is liquefied, and the liquid is consumed. As the feeding progresses, a hard tube-like structure, called a stylostome, is formed by the salivary secretions and the host immune system responds to the foreign enzymes in the chigger saliva [8]. The stylostome functions as a straw, allowing the chiggers to consume the exo-digested skin. The saliva secretion and stylostome formation from feeding cause an inflammatory dermal reaction in the host [9], which is called trombiculiasis. Chiggers will attach to a host and feed for 1–12 d if not scratched off [10,11,12], the duration variable depending on the host species. After engorgement, chiggers drop naturally from the host to the soil and become quiescent.
Overall, the available data for the developmental time for each stage both in the field and lab are limited. In optimal lab conditions (25–30 °C, 100% relative humidity), a generation of L. deliense is 59–135 days, with an 89 days average [13]. The development time of a complete generation for boreal species can take anywhere from 150 to 400 days [14]. In the field, local climate conditions will obviously affect the developmental time since chiggers are ectotherms. Wharton and Fuller [15] reported E. alfreddugesi produced one to two generations a year in Ohio, three generations in North Carolina and developed year-round in Florida, US. Direct copulation between males and females has never been observed. The adult female is inseminated by making contact with a spermatophore deposited by males in the habitat that they share [16]. The male spermatophore is made up of a flexible stalk (about 5 µm thick and 45 µm tall) that is linked to the substrate and bears a sperm sac at the distal end [11,16]. When the female encounters the spermatophore, she will walk over the spermatophore with her genital plates fully opened and pick up the sperm sac. Lipovsky et al. [16] observed that chigger females could acquire multiple spermatophores. Females normally undergo one to three oviposition cycles with gaps between them throughout their lives [14].
2.2. Hosts and Habitat
The host spectrum of chiggers is broad. For example, E. alfreddugesi, the most widespread chigger species in the US, were found to parasitize reptiles, amphibians, birds and mammals. Walters et al. [17] listed that 145 species of reptiles and amphibians, 129 species of birds, and 225 species of mammals in the US were reported to be parasitized by chiggers. Some chigger species or genera were only reported parasitizing a particular host group. For example, almost all Neoschoengastia americana parasitize birds [17] and this species was reported to be a significant pest of turkeys in the US [4]. Chiggers in the genus Hannemania primarily fed on amphibians [17]. However, the preference of chiggers to a specific host species is low. Chiggers are habitat-specific rather than host-specific. Chiggers are known to have preferences for a certain local habitat, where they attack and feed on all or the majority of the vertebrate species in the same location [14,17,18,19]. The low host specificity of the chigger and broad host range may enhance their chances of contacting humans, as well as the spread of scrub typhus across diverse hosts. Scrub typhus so far has not been reported in the US (discussed in more detail later).
Chiggers prefer moist areas with overgrown vegetation [15], like abandoned plantations, tall grass, and river banks. Chiggers are found to be most abundant in early summer in temperate regions when the vegetation is heaviest. Clopton and Gold [20] reported the abundance of E. alfreddugesi peaked in late June to early July. In the summer, adults were most common near the soil surface, and in the fall and winter, they were found deeper in the soil [15]. Shatrov and Kudryashova [14] reported the eggs of some boreal chigger species can diapause for up to 400 d. This suggests they have temperature preferences likely related to their life cycle and survival when environmental conditions are not favorable for development.
2.3. Collecting Methods
2.3.1. Trapping Animals
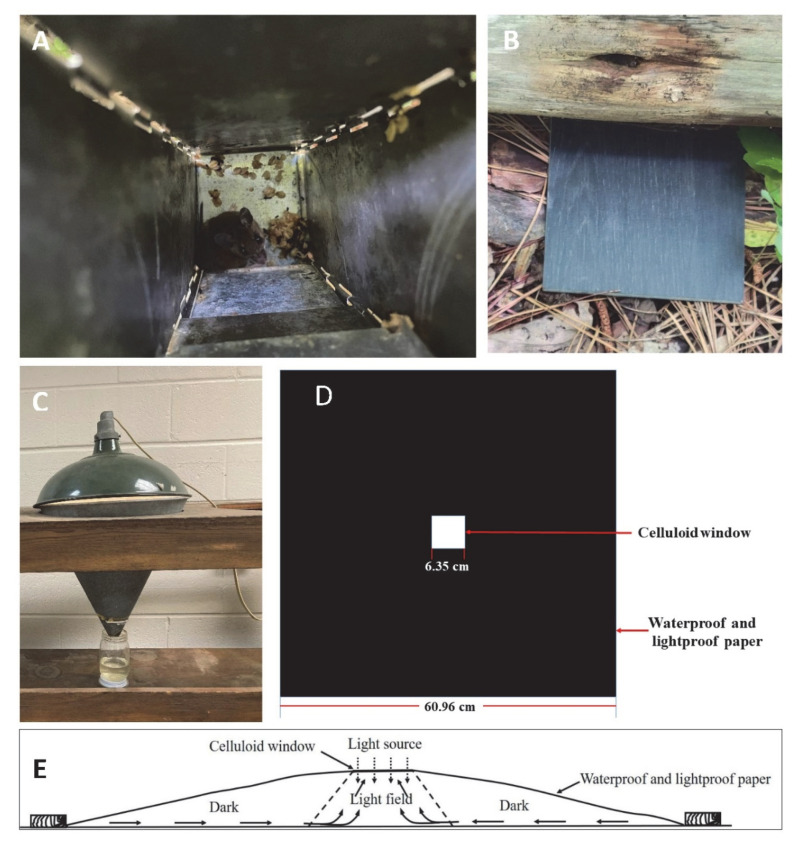
This method of collecting requires approval from Institutional Animal Care and Use Committees (IACUCs). Rodents can be live-trapped with Sherman traps (Figure 3A) placed on the ground overnight at any place where chiggers and potential hosts are expected to be found [21]. Chiggers then can be collected from rodents the next day. Sherman traps are usually baited with bird seed [22] or a peanut butter and oats mixture [23]. Ketamine hydrochloride and xylazine sulphate (1:10) are given intramuscularly to anesthetize the captured rodents [22]. Captured rodents are examined thoroughly for the presence of chiggers with particular attention to the ears, groin, foot, belly and area surrounding the genitals [23]. Removed chiggers are preserved in a vial containing 70% ethanol [22]. Similar approaches may be used for other types of animals like birds, lizards, or larger mammals. One advantage of this approach, the rodents are traveling throughout their natural geographical range foraging for food, obtaining nesting materials and mating and have a greater chance of acquiring chiggers from their habitat than other collection methods (discussed later). Additionally, the chiggers are easy to collect from the animal after trapping, since they are attached to specific locations, e.g., in the ears of mice. This approach is only effective for obtaining feeding chiggers. Since they are feeding on animals that could be infected with a human pathogen, for example, their microbiomes might reflect that of the host and not necessarily that of a free-living chigger.
Figure 3.
Chigger collecting methods: (A) live-trapped rodents in Sherman traps (photo credit to Reuben Garshong); (B) black tile on the ground; (C) Berlese funnel (photo credit to Steve Denning); (D) light trap (modified from [29]); (E) horizontal view of light trap, arrows inside the trap show the paths taken by chiggers (modified from [29]).
2.3.2. Plate Method
Unengorged chiggers can be attracted to foreign objects of almost any kind [24], for example, a porcelain plate, plastic disk, or oilcloth [21,24]. Black plates (Figure 3B) are attractive to unfed chiggers when heated by the sun, and the black color makes it easy to see the reddish color of chiggers on the plate, especially when they are crawling. Thus, black plates were commonly used to collect unengorged, free-living chiggers in rodent runways and near nests [25,26,27,28]. A set of black plates (10 cm by 10 cm) are laid at a possible breeding site at intervals. Each black plate is observed for any movement after a certain amount of time (e.g., 10 min). Chiggers can be collected with an ethanol-dipped pipette or small camel hair or equivalent brush and then transfer to a tube containing 70% ethanol. A hand lens helps ensure a successful transfer. Another collecting method is using an inverted white tray placed on the ground in a chigger infested area overnight; when the tray is righted the next day, free-living chiggers can be collected from what was the undersurface of the tray [21,24].
The advantages of the plate method, free-living chiggers are collected alive and in good viable condition, in case the researcher wanted to develop a laboratory colony or study the live biology, physiology or microbiome of the chiggers. The challenge with this method is finding an exact location where the chiggers are found. This is typically a random approach within a potential geographical location where chiggers are expected. Sometimes, picking a place to collect can be enhanced by incidental reports of chigger problems, frequently provided by park rangers or comments from visitors to a particular area.
2.3.3. Berlese Funnel
A Berlese funnel (Figure 3C) can be used to collect chigger mites from soil simply by placing the litter sample into the inverted funnel. The mites will crawl downward from the light shining into the top, large opening of the funnel, as the soil dries and they eventually fall into a bottom collection chamber filled with ethanol (to kill and preserve the mites) positioned below the end of the inverted funnel [18]. The challenge with this approach would be the need to collect enough soil samples to find the mites and have enough Berlese funnels to process the soil samples, since the free-living chiggers are not typically widely and evenly distributed in the landscape. Additionally, other soil animals will drop into the ethanol and will need to be separated from the free-living chiggers. If there is an interest in the external microbiome of the chigger, there would be cross contamination between animals. The chiggers collected by this method are killed by the ethanol.
2.3.4. Light Trapping
Johns [29] developed a light trap to collect unengorged chiggers in vegetation using the natural tendency for the unengorged chiggers to be positively phototropic with a preference to climb higher. The trap was made of a piece of 60.96 cm by 60.96 cm waterproof and lightproof paper with a 7.62 cm by 7.62 cm hole cut in the middle. A celluloid window measuring 8.26 cm by 8.26 cm was placed over the hole and attached to the paper with adhesive tape to make a central window frame about 6.35 cm by 6.35 cm (Figure 3D). The trap is laid in the desired location with the celluloid window in contact with the soil surface or the vegetation so the chiggers could climb from the dark field to the celluloid window (Figure 3E). The edges of the trap are secured by bricks, stones or wood pieces to hold the sheet to the ground and assure the only light entering the trap is was from the top celluloid window. When collecting the chiggers, the trap is turned over, and the chigger transferred by a wet brush onto a moistened filter paper in a collecting jar to extend the life of the chigger. This approach to keep the free living chiggers alive could also be used for the plate method of collecting (discussed earlier).
2.4. Chigger Lab Rearing
A chigger colony can be started from field-collected engorged larvae taken from trapped hosts. The larvae are allowed to detach from the host naturally to avoid killing them [30]. This will require a different IUCUC protocol for holding the wild-caught host longer than typically needed for collecting chiggers from a trapped host which are released the same day. Collecting chiggers for starting a lab colony is more challenging because the wild animals have to be maintained in environmental conditions to preserve the health and well-being of the host. For biosecurity reasons, wild hosts cannot be held in a rearing facility with other research animals because of the threat of introducing a disease.
Chiggers throughout their life cycle are maintained in 3.5 cm diameter plastic containers, 1/3 filled at the bottom with hardened plaster of Paris: charcoal (9:1), saturated with water [31]. Michener [32] also reported using fruit jars with the inner surface coated with 1/8 to 1/4 inch thickness of plaster of Paris to rear Eutrombicula batatas in the lab. Eggs of collembola, e.g., Sinella curviseta [33] or of mosquitoes, e.g., Culex pipiens [18], are provided ad libitum to nymphs and adults as food. Eggs before hatching are separated from the other stages by flooding the container; the eggs remain at the bottom [30]. The unfed chigger larvae are placed on a laboratory mouse in a tight-fitting wire-mesh cage where the activity of the mouse is restrained. After the chiggers have attached to the mouse for feeding, they (mouse and chiggers) are transferred to a loose-fitting cage [30]. Michener [32] also reported to use domestic chickens to be used as host of lab rearing chiggers. A tray (slightly bigger than the cage) filled with water and the cage are held above the surface of water and used to catch engorged larvae when they drop from the mouse [30]. For chiggers that do not parasitize mammals, snakes and turtles can be used to feed larvae [34]. The temperature for rearing L. deliense and L. fletcheri is 27 ± 3 °C [31]. The optimal relative humidity is 80–100% [14,21]. In addition, Audy and Nadchatram [35] reported method of rearing chigger larvae collected from the field in glass tube fitted with wet strip of paper. This method was for only taxonomic study purpose.
One challenge of starting a colony from the field is the potential for the engorged chiggers to be infected with human pathogens, especially if the chiggers were collected from known endemic areas of disease. Additionally, there are areas where the status of chigger pathogens have not been investigated nor the transmission potential. These unknown concerns can complicate starting a laboratory colony, since BSL level III containment that involves the use of vertebrate animals would be needed if human pathogens are involved or potentially involved as part of the rearing effort.
3. Systematics Status
3.1. Taxonomy
Three classification systems for chiggers have been suggested: (i) all chiggers are in the family Trombiculidae Ewing, 1929 with three subfamilies, Trombiculinae, Ewing, 1929, Leeuwenhoekiinae, Womersley, 1944 and Apoloniinae Wharton, 1947 [36,37,38,39]; (ii) all chiggers are in two families, Trombiculidae and Leeuwenhoekiidae Womersley, 1945 [5,17,40]; and (iii) all chiggers are in in three families, Trombiculidae, Leeuwenhoekiidae and Walchiidae [41,42,43]. There is disagreement on whether the taxa should be included in the family Trombiculidae [44]. In this review, we are using the classification that chiggers are the larval mites in the families Trombiculidae and Leeuwenhoekiidae.
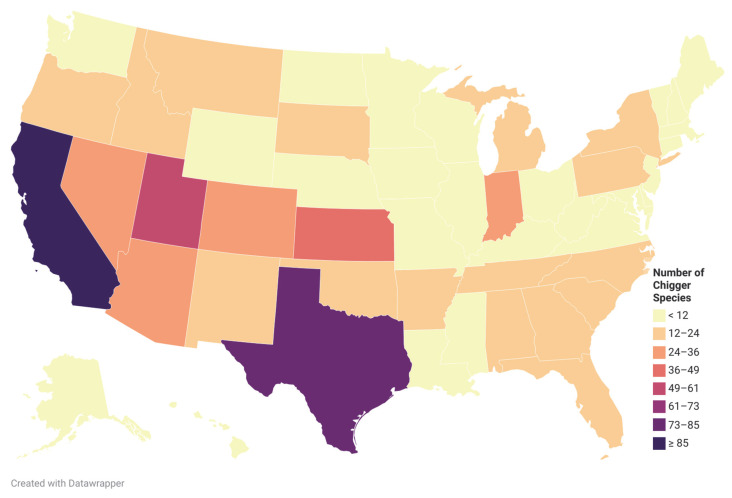
Walters et al. [17] summarized all publications from 1923 to 2009 that reported chiggers in North America, the chigger species reported in each publication, the hosts that the chiggers were found, and the US state or Canadian province where they were associated. The list contained 42 species in Leeuwenhoekiidae and 205 species in Trombiculidae in the US [17]. In 2015, Crossley and Clement [45] reported a new species, Hoffmanniella solickiana, from the Rafinesque’s Big-Eared Bat in Georgia, US, making a total of 248 chigger species from the US, containing 42 species in Leeuwenhoekiidae and 206 species in Trombiculidae. The chigger species richness by state in the US is diverse (Figure 4).
Figure 4.
Chigger species richness in the US. Created with Datawrapper. Data sources: [17,45].
Chiggers are reported in every state except Hawaii in the United States. The states that reported more than 30 chigger species were California (97 species), Texas (74), Utah (52), Kansas (47), Colorado (30) and Nevada (30). The chigger species were more diverse in the Southwest. Some species in the genus Leptotrombidium were found in the US, i.e., Leptotrombidium myotis, L. panamensis, and L. peromysci. The genus Leptotrombidium is known as the vector of O. tsutsugamushi, the causative agent of scrub typhus.
Based on the list from Walters et al. [17], E. alfreddugesi (Oudemans 1910) was the most widely spread chigger in the US, found in 22 states from 179 host species, including reptiles, amphibians, birds and mammals. However, Bennett et al. [46] argued that the most common chigger in the eastern US, E. alfreddugesi, was possibly misidentified and should be replaced with E. cinnabaris. Loomis and Wrenn [47] investigated the systematics of the genus Eutrombicula and found that this genus in the Eastern US needed revision since some of the records were misidentified. This species was named by Oudemans as Microthrombidium alfreddugesi in 1910 based on four specimens collected from Temascaltepec, close to Tejupilco, Mexico, which was the type species for the genus Eutrombicula, Ewing, 1938 [48]. Fuller [49] revisited the two representative specimens of Oudemans’ collection and found these two specimens were shrunken and not transparent enough for study. W. J. Wrenn argued that it was unlikely that the two improperly mounted specimens belonged to the common Eutrombicula species in the Eastern US, where there is a large number of species in the genus Eutrombicula in the US [46,47]. Another synonym on Fuller’s list for this species is Trombicula cinnabaris, Ewing, 1920 [49]. D. A. Crossley rechecked Ewing’s specimens named Trombicula cinnabaris and found several morphological characteristics were in accordance with the illustrations of E. alfreddugesi [46]. Thus W. J. Wrenn concluded that E. cinnabaris was the most suitable name for the species that was formerly classified as E. alfreddugesi [46,47]. Wrenn continued to use E. cinnabaris for his publications [50,51]. In the world checklist [5] and the North American checklist [17] of Trombiculidae and Leeuwenhoekiidae, E. alfreddugesi and E. cinnabaris were listed as two species. The possible reason for this could be the new name for this species that was proposed, but others treated it as another species and still used the old name. It was suggested by Loomis and Wrenn [47] that the genus Eutrombicula needed revision, and this revision is greatly overdue.
3.2. Identification
The taxonomy of chiggers almost exclusively relies on larval morphological characteristics [52,53,54,55]. A chigger’s body is divided into two sections, the gnathosoma and idiosoma. The gnathosoma is the chigger mouthparts comprised of four parts: the cheliceral blades, palps, cheliceral base and gnathobase [36]. The shape of the scutum (usually the only sclerotized plate on the idiosoma, next to the gnathosoma), the number and arrangement of dorsal setae, chaetotaxy of legs, leg segmentation, and different forms or length of the gnathosoma (the cheliceral blades, the galeal setae, the palpal setae and the palpal claw), etc. are used for chigger identification.
Chiggers typically were cleared in lactophenol for them to be transparent and easy to observe under the microscope for classification [56,57,58]. After clearing, chiggers are then mounted dorsal-ventrally in mounting media like Hoyer’s medium [59] or Berlese fluid [60] on glass slides and dried at 50 °C before identification under a high magnification microscope using differential interference contrast [61]. More recently methods to clear and mount ectoparasites has improved, e.g., lactic acid, phenol and iodine and water are used for the preparation of rapid clearing solution for chiggers [62].
The identification relies on published dichotomous keys based on morphological characteristics. Some of the published keys are specific for a particular region. For example, Nadchatram and Dohany [63] provided keys for Southeast Asian chiggers to the subfamily, genus and subgenus; Brennan and Goff [64] provided a key to the western hemisphere (Nearctic and Neotropical regions) to the genus level; and Fernandes and Kulkarni [35] provided a key of Indian chiggers to the subfamily, tribe, genus and species. There is also separate guidance for species-level identification. For example, Vercammen-Grandjean and Langston [65] and Stekolnikov [66] provided keys to Leptotrombidium which can be used to identify Leptotrombidium chiggers into species.
Identifying chiggers using morphological characteristics is complicated and also time-consuming as chiggers are tiny and require days for sample preparation before identifying them under a high magnification microscope. Another challenge is to find a suitable key to guide the identification process, and sometimes no keys are available for certain species since the keys are not up to date. This identification largely relies on personal experiences. Researchers often need to send samples to experts to verify their identification [67,68]. Current knowledge of intraspecific variance is not sufficient, and some species are described from only a few specimens [46]. For measurements of body parts of chiggers, different researchers will likely have different results depending on how the measurements were made. In the future, chigger identification should consider not only morphological characteristics but also molecular tools to improve accuracy.
The molecular data for chigger identification are limited, especially in the US. The nucleotide data on chiggers in GenBank (Access date: 23 March 2022) was examined and summarized in Table 1. There were 10,465 entries in total and molecular data for 47 chigger species. Data for 36 species were published in a peer-reviewed journal while 11 species have not been published. Given the number of species of chiggers worldwide, the molecular data in GenBank is greatly limited, only making up 1.5% (47/3013) of the total number of species. This was surprising considering the significant health risks to humans from these mites. We categorized the published chigger data available in GenBank by country. The molecular data were mostly found in Southeast Asian and South American countries. The only species with molecular data available from the US was for E. splendens [69,70] with a complete sequence provided of the small and large subunit ribosomal RNA gene.
Table 1.
Summary of chigger molecular data in GenBank in peer-reviewed publications.
| Country (Continent) | Different Chigger Species in GenBank | % * | Peer Reviewed Publications | Chigger Species Reported | Target Genes * |
|---|---|---|---|---|---|
| Laos (Asia) | 11 | 30.56 | [71] | Ascoschoengastia indica | COI |
| Blankaartia acuscutellaris | COI | ||||
| Leptotrombidium deliense | 18S, COI, whole genome shotgun | ||||
| Microtrombicula chamlongi | COI | ||||
| Schoengastia kanhaensis | COI | ||||
| Walchia alpestris | COI | ||||
| Walchia ewingi ewingi | COI | ||||
| Walchia ewingi lupella | COI | ||||
| Walchia kritochaeta | COI | ||||
| Walchia micropelta | COI | ||||
| Walchia minuscuta | COI | ||||
| Thailand (Asia) | 1 | 2.78 | [72] | Leptotrombidium deliense | Mitochondrial (mt) DNA complete genome |
| China (Asia) | 1 | 2.78 | [73] | Neoschoengastia gallinarum | small subunit rRNA, large subunit rRNA, 5.8S, internal transcribed spacer 2, large subunit rRNA, COI |
| Korea (Asia) | 2 | 5.56 | [74] | Helenicula miyagawai | 18S |
| Leptotrombidium scutellare | mt DNA complete genome, COI, 18S, 5.8S | ||||
| Japan (Asia) | 1 | 2.78 | [75] | Leptotrombidium pallidum | COI, mt DNA complete genome, 5.8S, whole genome shotgun |
| Malaysia (Asia) | 1 | 2.78 | [76] | Leptotrombidium fletcheri | mt DNA complete genome |
| Canada (North America) | 1 | 2.78 | [77] | Neotrombicula microti | COI |
| US (North America) | 1 | 2.78 | [69,70] | Eutrombicula splendens | large subunit rRNA, small subunit rRNA, 28S, 18S, Ef1-α, Srp54, Hsp70, COI |
| Mexico (North America) | 1 | 2.78 | [70] | Acomatacarus arizonensis | 28S, 18S, COI, Ef1-α, Srp54, Hsp70 |
| Brazil (South America) | 12 | 32.33 | [78,79] | Herpetacarus(Arisocerus) hertigi | small subunit rRNA |
| Blankaartia sinnamaryi | small subunit rRNA | ||||
| Eutrombicula daemoni | small subunit rRNA | ||||
| Eutrombicula goeldii | small subunit rRNA | ||||
| Eutrombicula tinami | small subunit rRNA | ||||
| Fonsecia ewingi | 18S | ||||
| Hannemania hepatica | 18S | ||||
| Hannemania yungicola | 18S | ||||
| Quadraseta brasiliensis | small subunit rRNA, 18S | ||||
| Quadraseta pazca | small subunit rRNA | ||||
| Quadraseta trapezoides | small subunit rRNA | ||||
| Trombewingia bakeri | small subunit rRNA | ||||
| Poland (Europe) | 3 | 8.33 | [80,81] | Hirsutiella zachvatkini | COI |
| Miyatrombicula muris | COI | ||||
| Neotrombicula inopinata | COI | ||||
| Spain (Europe) | 1 | 2.78 | [82] | Morelacarus sp. | COI |
| Madagascar (Africa) | 1 | 2.78 | [83] | Schoutedenichia microcebi | small subunit rRNA |
* %: Percent of different chigger species from each country out of the total 36 different species in GenBank in peer-reviewed publications. * Gene abbreviations: COI, cytochrome c oxidase I; 18S, 18S ribosomal RNA gene; 5.8S, 5.8S ribosomal RNA gene; Ef1-α, elongation factor 1alpha100E; Srp54, signal recognition particle protein 54k gene; Hsp70, Hsc70-5 heat shock protein cognate 5 gene.
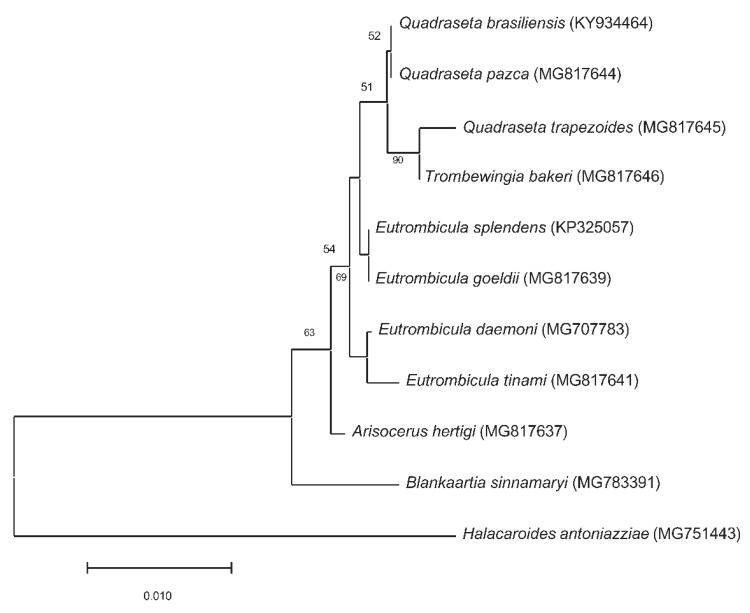
We constructed a Neighbor-Joining tree of the small subunit rRNA sequences for chiggers collected from American countries (Figure 5). The chigger small subunit rRNA sequences data were downloaded from GenBank only when the data were also published in peer-reviewed journals. Eutrombicula tinami, Quadraseta trapezoides and Trombewingia bakeri representing different genera, were clustered together in our analysis (Figure 5). Herpetacarus hertigi, Blankaartia sinnamaryi, and E. daemoni are more closely related to each other than they were E. splendens and E. goeldi in the tree. These results suggest that using the current available sequences in GenBank to identify chiggers would most likely lead to incorrect identifications.
Figure 5.
Neighbor-Joining tree of small subunit rRNA sequences of chiggers collected from North and South American countries. The chigger small subunit rRNA sequences data were downloaded from GenBank only from peer-reviewed journal publications. The Halacaroides antoniazziae (MG751443) was used as an outgroup. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown below the branches. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. This analysis involved 10 nucleotide sequences. All positions containing gaps and missing data were eliminated (complete deletion option). There were a total of 403 positions in the final dataset. Evolutionary analyses were conducted in MEGA11.
Obtaining chigger molecular data for the US is long overdue. One of the reasons this is lacking is the difficulty in making a morphological identification and at the same time obtaining molecular data. The sample preparation process for chiggers requires the use of lactophenol or acidic phenol for clearing and heat drying (discussed earlier). This process destroys the DNA. There are two solutions available for researchers to resolve this issue. Lee et al. [84] modified the method from Dohany et al. [85] and Ree et al. [86] for the detection of O. tsutsugamushi from chiggers using a fluorescent antibody test. Lee et al. [84] used a fine needle to puncture the dorsal-posterior part of the chigger and squeezed out internal tissue for DNA extraction while the exoskeleton was retained mostly intact for morphological identification. Kumlert et al. [71] argued that this method affects the morphological identification, and the DNA extraction process is challenging because of the limited amount of tissue obtained by this method. Kumlert et al. [71] alternatively used the autofluorescent characteristics of the chigger exoskeleton and developed an identification method using a combination of autofluorescent and brightfield microscopy imaging. Chiggers were mounted in sterile water or ethanol to prevent DNA damaged by clearing agents and saved for molecular identification later.
Having a database containing the sequences of mitochondrial cytochrome oxidase subunit I gene (COI) and nuclear genome fragments at a minimum is essential to building a reliable molecular identification method for chiggers. A substantial systematics investment is needed to achieve this objective for chiggers in the US. There is also an increasing interest of using high resolution, three channel imaging coupled with artificial intelligence using multi-layered neural networks to classify closely related species of almost identical-looking moth eggs (Roe, in review) that could be applied to chigger classification. This approach is especially useful for classifying small objects and because the system is automated, has the potential for high throughput.
Chiggers are a poorly understood group of arthropods with a significant importance to human health that in toto, are understudied at many levels including systematics and classification. Molecular tools not only could be used to identify the chiggers to species, but also be applicable for classification of the free-living deutonymph, adults and eggs.
4. Chigger Microbiome
Ponnusamy et al. [87] extracted DNA from Orientia tsutsugamushi infected and uninfected Leptotrombidium imphalum laboratory colonies at different life stages: larvae (chiggers), deutonymph, male adults and female adults from Thailand. The 16S rRNA V3-V4 region amplicons were amplified from the extracted DNA and sequenced using Illumina MiSeq. They reported Orientia, Ralstonia and Propionibacterium were the only three taxa that represented >5% of the relative bacteria abundance for O. tsutsugamushi infected chiggers. For O. tsutsugamushi uninfected chiggers, the Ralstonia, Propionibacterium, Streptococcus and Corynebacterium genera represented >5% the bacteria relative abundance [87]. Notably, unidentified taxa of Amoebophilaceae were found predominantly in O. tsutsugamushi infected female L. imphalum mites but with low relative abundance in larvae (chiggers) and male adult mites. This suggested a mutualistic relationship between Amoebophilaceae and O. tsutsugamushi in L. imphalum mites that needs further study [87].
Alghamdi [68] extracted DNA from ten species of chiggers collected from rodents in southwestern Saudi Arabia: Ascoschoengastia browni, Ericotrombidium caucasicum, Ericotrombidium kazeruni, Microtrombicula microscuta, Pentidionis agamae Schoutedenichia saudi, Gahrliepia lawrencei, Schoutedenichia thracica, Schoutedenichia zarudnyi and Helenicula lukshumiae. Chigger species were identified using an autofluorescence method [71]. Alghamdi amplified the 16S rRNA V3-V4 region from the extracted DNA and sequenced the 16S rRNA libraries using Illumina. Corynebacterium, Mycobacterium, Staphylococcus, Candidatus Cardinium, Burkholderiaceae and Wolbachia were found to be dominant in all the chigger samples. Especially, Wolbachia had a high relative abundance in P. agamae. Alghamdi [68] reported that Orientia OTUs were observed from E. kazeruni and P. agamae with a low relative abundance and had 98.88% similarity with the Orientia chuto sequence in NCBI. Alghamdi suggested that scrub typhus might exist in Saudi Arabia. More work is needed to confirm the Orientia sequence in chiggers.
Chaisiri et al. [88] studied the microbiome of nine chigger species that were collected on small mammals from 11 provinces in Thailand: Ascoshoengastia indica, Blankaartia acuscutellaris, Helenicula kohlsi, Helenicula pilosa, Leptotrombidium deliense, Schoengastiella ligula, Walchia micropelta, Walchia minuscuta, and Walchia kritochaeta. The chiggers were identified by autoflorescent imaging [71]. The 16S rRNA gene V4 region was amplified by PCR and sequenced by Illumina MiSeq. They reported that the dominant taxa across all the chigger samples were Sphingobium, Mycobacterium, Neisseriaceae and Bacillales. Geobacillus was found to be a potential arthropod endosymbiont. Cardinium, Pseudonocardia and Rickettsiella were presented in 20–45% of the pooled chigger samples while Wolbachia was detected in 3.18% of the pooled samples. O. tsutsugamushi and Borrelia spp. were detected in L. deliense. Potential human pathogens, Staphylococcus and Haemophilus parainfluenzae, were found in several chigger species.
The microbiome of chiggers from a Thailand lab colony and field samples from Thailand and Saudi Arabia was highly diverse. Not surprisingly, O. tsutsugamushi was detected in the Thailand chiggers. However, the finding of Borrelia spp. in chiggers [88] suggested they could be the vector for Borreliosis (Lyme disease), currently believed to be transmitted only by ticks. Chiggers might also harbor other human and animal pathogens like Staphylococcus. More work is needed to characterize the microbiome of different chigger species from different habitats to better understand if bacteria will affect the vector competence of chiggers, and to better understand if there are any other pathogens that chiggers can vector other than Orientia.
5. Do Chiggers Transmit Rickettsia and Orientia in the US?
5.1. Summary of Zoonotic Bacteria and Viruses Transmitted by Chiggers
Leptotrombidium spp. are vectors of Orientia tsutsugamushi that cause Scrub typhus, a life-threatening disease with up to a 50% fatality rate if left untreated [89]. There are 46 chigger species reported to be infected with O. tsutsugamushi [90]. Eleven Leptotrombidium species were proven to be vectors of scrub typhus [12]. Leptotrombidium spp. are both the vector and reservoir of O. tsutsugamushi [91]. The high transovarial and transstadial transmission rates among chigger species play an important role in maintaining the disease in nature, since chiggers only feed on an animal host once [92]. Whether rodents also serve as the reservoir for O. tsutsugamushi needs further investigation; rodents could only be dead-end hosts [93].
Scrub typhus was originally endemic in the tsutsugamushi triangle. This traditional triangle area extends north to Japan and Russia, south to Northern Australia, and west to Afghanistan and Pakistan [94]. Recent studies suggest scrub typhus is not limited to this triangle. Scrub typhus cases were reported in the United Arab Emirates, and a new Orientia species O. chuto was isolated from a patient in 2006 [95]. Scrub typhus cases reported in Chile [96,97] and serologic evidence in Peru [98] suggested scrub typhus is endemic in South America. Furthermore, Orientia sequences were detected in rodents from France [99], Senegal [99] and Kenya [100]. Besides some Leptotrombidium species, Schoengastiella ligula has been implicated as a vector of scrub typhus in India [101]. Thus, a larger geographic distribution of scrub typhus, potential new Orientia species and new vector chigger species should be taken into account. The potential of Orientia species establishing in the US is high given the number of chigger species found throughout the US but has never been seriously studied [17].
Chiggers are also reported to play a role in Hantavirus transmission, a disease previously thought to be transmitted through aerosol exposure to rodent saliva, urine and feces (CDC https://www.cdc.gov/hantavirus/, accessed on 19 May 2022). Hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) are the two clinical syndromes caused by hantavirus when transmitted from rodents to humans [102]. In the United States, hantavirus cause hantavirus pulmonary syndrome (HPS) instead of HFRS, and HPS is a severe and sometimes life-threatening respiratory disease [103]. Wu et al. [104] studied the role of L. scutellare in the transmission of hantavirus that cause HFRS in China. They reported hantavirus were isolated from (i) rats trapped in the HFRS endemic areas of Shaanxi Province, China and also from chiggers on the rats; (ii) from eight previously hantavirus negative mice placed in metal cages in the field to lure chiggers; (iii) from mice bitten by virus-positive chiggers; and (iv) from larvae hatched from eggs collected from endemic areas. Wu et al. [104] concluded that L. scutellare could be the vector of hantavirus and able to transmit the virus to its offspring and rodent hosts by biting. A review of the recent studies of mites and hantavirus in China also indicated that the hantavirus can be maintained in chiggers by transovarial transmission [105]. Rodent ectoparasite screening in Texas detected two chiggers collected from these rodents and one free-living chigger collected from the soil that were positive for hantavirus specific RNA [106]. Although cumulative cases in the US have occurred predominately in the western states, only 10 states have been immune to its presence (https://www.cdc.gov/hantavirus/surveillance/index.html, accessed on 19 May 2022). The possible role of chiggers in the transmission of hantavirus in the US needs further investigation.
Bartonella tamiae was isolated for the first time from patients to determine the etiology of three febrile cases in Thailand [107]. In another study, chiggers in the genus Leptotrombidium, Schoengastia, and Blankarrtia were collected from rodents in Thailand, and the 16S–23S intergenic spacer region and citrate synthase gene (gltA) region were targeted by PCR to screen for B. tamiae DNA in these chiggers [108]. In this work, 29 of the 40 chigger samples tested positive for B. tamiae DNA, and there were 97.7–100% sequence homology between the Bartonella DNA detected in this study and the Bartonella species isolated from three patients in Thailand. This suggested that chiggers play a potential role in the transmission of Bartonella to humans.
Rickettsia species have also been found in chiggers. Rickettsia spp.TwKM02 and TwKM03 were detected in Leptotrombidium chiggers from rodents in two islets in Taiwan; the two Rickettsia species were close to R. australis and R. felis URRWXCal2 based on a phylogenetic tree constructed on partial gltA gene sequences [109]. R. australis, R. japonica, R. felis, R. typhi, R. akari, Rickettsia sp. TwKM02, R. conorii and Rickettsia sp. Cf15 were detected in chiggers collected from rodents in South Korea [110]. A new Rickettsia species found in L. scutellare from rodents in Shandong, China was close to R. australis and R. akari [111]. Rickettsia spp. were detected in chiggers, Herpetacarus hertigi, Quadraseta trapezoides and Trombewingia bakeri, collected from rodents in Brazil and was identical to “Candidatus Rickettsia colombianensi” based on the amplified gltA and ompA genes [79]. Rickettsia spp. found from soil-inhabiting unfed L. scutellare chiggers in Japan were close to R. akari, R. aeschlimannii, R. felis and R. australis [112]. Since chiggers in this study were unfed, this suggested Rickettsia spp. were transstadially and/or transovarially transmitted in chigger mites [112].
Except for O. tsutsugamushi that cause scrub typhus, chiggers mostly are not considered as vectors for rickettsioses. Given that some Rickettsia spp. found in chiggers were similar to species in the spotted fever group (SFG), e.g., R. aeschlimannii, R. felis and R. australis (recognized because of their infectivity and pathogenicity as Rickettsia spp.), the possible role chiggers might play in the transmission of Rickettsia to humans deserves further investigation. A well, a pool of chiggers with Rickettsia spp. TwKm02 were positive for O. tsutsugamushi in Taiwan [109]. Therefore, the possibility of coinfection of SFGR and scrub typhus in chiggers needs further study. Additionally, R. felis and other Rickettsia species were detected in chiggers collected from rodent in the US [23]. It is possible that rickettsioses in the US attributed to ticks might also originate from chiggers. Since the chigger microbiomes in the US have been minimally studied, this remains a possibility.
Borrelia species have also been found in chiggers. Borrelia burgdorferi sensu lato (s.l.) was found in 634 Neotrombicula autumnalis chiggers collected on 100 wild-caught rodents along with 1380 questing chiggers on vegetation, using PCR and DNA hybridization in Germany and Borrelia DNA was detected from a chigger collected from a greater white-tooted shrew [113]. An infection experiment also was conducted by Kampen et al. [113] by placing 305 questing unfed chiggers on B. garinii infected laboratory mice and on B. afzelii infected laboratory Mongolian gerbils. Borrelia DNA was detected on a pool of four chiggers that fed on B. garinii infected laboratory mice, and a nymph kept from a larva that was previously fed on B. afzelii infected laboratory Mongolian gerbils. Results suggested a possible transmission of Borrelia spp. from N. autumnalis to mice and gerbils. Borrelia spp. DNA was also screened on 509 chiggers in the genus Neotrombicula that were collected from 1080 wild birds in Certak, Czech Republic [114]. One sample pooled with five Neotrombicula chiggers removed from one Eurasian blackcap (Sylvia atricapilla) tested positive for B. burgdorferi s.l. by PCR and a reverse line blotting assay [114].
5.2. Role of Endosymbionts in Orientia Infection
Takahashi et al. [115] reported that the adults in O. tsutsugamushi infected Leptotrombidium fletcheri, L. arenicola and L. deliense colonies were almost entirely made up of females, and this female-biased sex ratio represents a feminization. After treating the O. tsutsugamushi infected L. fletcheri colonies with antibiotics, a high ratio of adult males occurred. Takahashi et al. [115] suggested O. tsutsugamushi inhibits the development of males. Recent microbiome studies detected Wolbachia and Cardinum sequences in chiggers [68,88]. Wolbachia and Cardinum are facultative bacterial endosymbionts of arthropods, commonly maternally inherited and associated with reproductive manipulation such as feminization and parthenogenesis [116]. It is possible that Wolbachia and Cardinum can also cause feminization in some chigger species infected with O. tsutsugamushi.
Feminization also occurred in O. tsutsugamushi infected F1 generation adults of L. imphalum [117]. A microbiome study of a L. imphalum laboratory colony found an unidentified species, Amoebophilaceae, which dominated in O. tsutsugamushi infected female adults and was at a very low relative abundance in O. tsutsugamushi uninfected mites [87]. The results suggested a mutualistic relationship between O. tsutsugamushi and the unidentified species in the Amoebophilaceae [87]. It is possible that this unidentified species in Amoebophilaceae was responsible for the feminization of L. imphalum adults. Further characterization and pathogenicity of this Amoebophilaceae species is needed including understanding their possible contributing role in pathogenesis with other bacteria.
6. Possible Misdiagnosis of Tick-Borne Diseases and Allergies
Tick-borne diseases in the US include spotted fever rickettsioses, Lyme disease, ehrlichiosis, anaplasmosis, tularemia, babesiosis, Colorado tick fever, and relapsing fever [118]. Until recently, most patients with tick-associated rickettsiosis were considered to have Rocky Mountain spotted fever (RMSF), caused by R. rickettsii. Serological techniques like indirect the immunofluorescence assay (IFA) was commonly used for the routine diagnosis of RMSF [119,120]. However, other rickettsia species in the Spotted Fever Group (SFG) can also elicit antibodies that are cross-reactive with R. rickettsia (serological cross-reactions) [118]. Additionally, other SFG rickettsia species, including R. parkeri and Rickettsia species 364D [121] can cause illness that was diagnosed as RMSF. SFG rickettsia species have been detected in chiggers [79,109,110,111,112,122], suggesting that reported tick-associated rickettsiosis may have some level of chigger associated.
Borrelia spp. DNA was detected from Neotrombicula autumnalis collected from wild birds in the Czech Republic [114]. Additionally, a recent microbiome study of L. deliense collected from rodents in Thailand showed that Borrelia spp. were found in 49.2% of the chigger samples [88]. Larval ticks, sometimes known as “seed ticks,” bite humans and are nearly impossible for non-professionals to distinguish from chigger bites. In a sero-epidemiological study conducted on France forestry workers, a large number of people who were seropositive to B. burgdorferi were unable to recall a tick bite [123]. The reasons could be those workers were bitten by larval ticks or chiggers.
It is now well recognized that the carbohydrate galactose-α-1,3-galactose (alpha-gal) which is present in some tick species can elicit an alpha-gal specific IgE immune response [124]. People who were reported to be bitten by ticks have developed a hypersensitivity to red meats, including beef and lamb, called red meat allergy [125]. The symptoms of red meat allergy range from mild to severe, some can be acute and fatal [124]. Notably, people in three cases who had severe red meat allergy later reported having pruritic bites from chiggers either on their lower legs or at the waistband area [126]. It is possible that chiggers could also contribute to the prevalence of red meat allergy. The searching for alpha-gal antigens in chiggers should be investigated to determine if chiggers can also cause red meat allergy.
7. Chigger Control
Chiggers are frequently difficult to control chemically [127]. For successful management, large areas would have to be sprayed numerous times [127]. The best way to prevent chigger bites is to avoid them [128]. The methods of avoiding chigger bites are summarized in Table 2.
Table 2.
| Scenarios | Methods of Avoiding Chigger Bites |
|---|---|
| When venturing into chigger habitat | Wear permethrin-treated clothing |
| Apply repellents like DEET | |
| Wear tightly woven long sleeves, long pants, and closed-toe shoes | |
| Tuck shirts into pants, tuck pants into socks | |
| Stay in the middle of the trail, avoid high bushes and tall grass | |
| Avoid sitting directly on the ground | |
| After you come inside | Remove clothing immediately and launder it in hot water |
| Wipe down shoes and boots | |
| Take a hot and soapy shower with a vigorous skin massage | |
| Yard | Try to keep small mammals out of the yard by putting up a fence and keeping trash can lids secured |
| keep turf short and vegetation trimmed |
When venturing into chigger habitat, wearing permethrin-treated clothing and applying repellents containing DEET (diethyltoluamide) is effective against chiggers [129]. Wearing tightly woven long sleeves, long pants, closed-toe shoes and tucking shirts into pants and tucking pants into socks can keep chiggers on the outside of your clothing. There are also non-insecticidal garments available commercially like Rynoskin Total that claim to protect from chiggers, mainly based on preventing access under the garments.
When outdoors, stay in the middle of trails and avoid high bushes and tall grass that might have chiggers. It is also suggested to avoid sitting directly on the ground to reduce chigger encounters [130]. Upon return, it is recommended to remove clothing and wash clothing immediately. It is also helpful to wipe down shoes and boots to avoid bringing chiggers into the home. Taking a hot and soapy shower with a vigorous skin massage will help remove chiggers attached to your body [128].
To reduce chigger populations in the yard, keep turf grass short and vegetation trimmed, since high temperature and low humidity conditions are inhospitable for chiggers. Another approach is to keep small mammals out of the yard using fencing and keeping trash can lids secured to make the trash less attractive [129].
8. Conclusions
Overall, chiggers have been understudied world-wide but especially so for the US. The published research mainly focuses on taxonomy (identification methods), ecology, and reports of the discovery of chiggers on various hosts in the US. However, the life history of other stages other than larvae needs further study.
There are 248 chigger species reported from the U.S. to date. Chiggers are reported in every state except Hawaii in the United States. The taxonomy of chiggers almost exclusively relies on larval morphological characteristics; molecular data on chiggers are limited. It is likely that the most common chigger in North America, E. alfreddugesi, has been misidentified and should be replaced with E. cinnabaris. Building a reliable molecular identification method for chiggers correlated with morphological explicit characters is greatly needed. The molecular data are needed to better identify and study not only the parasitic stage but also free-living deutonymphs and adults correlated to what we already know about the larval stage. Three microbiome studies of chiggers were conducted from Thailand L. imphalum lab colonies, ten chigger species collected from rodents in Saudi Arabia, and nine chigger species collected from small mammals in Thailand. More work is needed in this area to characterize the microbiome of different chigger species from different habitats to better understand if bacteria will affect the vector competence of chiggers, and to better understand if pathogens in addition to Orientia are transmitted by these mites. Thus far, data suggests pathogen competence in chiggers for scrub typhus might be associated with co-infection with other bacteria.
Scrub typhus was reported outside of the Tsutsugamushi Triangle. In addition to Leptotrombidium species, new chigger species were reported to vector the bacteria that causes scrub typhus. The potential of Orientia species establishing in the US is high given the number of species found throughout the US but has been minimally investigated. Chiggers are reported to harbor Hantavirus, Bartonella, Rickettsia and Borrelia suggesting a need for further study in North America. The role that chiggers play in disease transmission dynamics in the US is essentially unknown. It is possible some of the tick-borne diseases and red meat allergies are caused by chiggers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192215147/s1, Table S1: Search strategy used for Figure 1 was #1 AND #2 from Web of Science Core Collection; Table S2: Summary of publications of chiggers in the US. Publications were searched through the Web of Science on 3 May 2022; Table S3: Number of chigger species reported in each US states. References [5,16,17,20,22,26,34,40,45,46,48,52,53,54,55,56,57,58,64,67,106,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, K.C., R.M.R. and L.P.; writing—original draft preparation, K.C.; writing—review and editing, K.C., R.M.R. and L.P.; supervision, R.M.R. and L.P.; funding acquisition, R.M.R. and L.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by a grant from NIH, the National Institute of Allergy and Infectious Diseases (1R03AI166406-01) to L.P (PI) and a grant from the Southeast Center for Agricultural Health and Injury Prevention (SCAHIP) to L.P (PI). The authors thank the NCSU NSF Center for Integrated Pest Management; the Institute of Plant Protection, Department of International Cooperation, and Graduate School in the Chinese Academy of Agricultural Sciences (CAAS); the Chinese Scholarship Council; and the NCSU-CAAS (China) traineeship program for the support of K.C. as a Ph.D. student at NCSU. Special thanks to Dr. Frank Louws and Dr. Yulu Xia for establishing the NCSU-CAAS traineeship program. K.C. was also supported as a Ph.D. student by the Department of Entomology and Plant Pathology at NCSU. L.P and R.M.R were supported by the NC Ag. Res. Station. All of the authors on this paper were involved in editing of the submitted draft. The authors do not have a conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weitzel T., Silva-de la Fuente M.C., Martínez-Valdebenito C., Stekolnikov A.A., Pérez C., Pérez R., Vial C., Abarca K., Acosta-Jamett G. Novel vector of scrub typhus in sub-Antarctic Chile: Evidence from human exposure. Clin. Infect. Dis. 2022;74:1862–1865. doi: 10.1093/cid/ciab748. [DOI] [PubMed] [Google Scholar]
- 2.Watt G., Parola P. Scrub typhus and tropical rickettsioses. Curr. Opin. Infect. Dis. 2003;16:429–436. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Philip C.B. Tsutsugamushi disease (scrub typhus) in World War II. J. Parasitol. 1948;34:169–191. doi: 10.2307/3273264. [DOI] [PubMed] [Google Scholar]
- 4.Kunz S.E., Price M.A., Graham O.H. Biology and economic importance of the chigger Neoschongastia americana on Turkeys. J. Econ. Entomol. 1969;62:872–875. doi: 10.1093/jee/62.4.872. [DOI] [Google Scholar]
- 5.Nielsen D.H., Robbins R.G., Rueda L.M. Annotated World Checklist of the Trombiculidae and Leeuwenhoekiidae (1758–2021) (Acari: Trombiculoidea), with notes on nomenclature, taxonomy, and distribution. Zootaxa. 2021;4967:1–243. doi: 10.11646/zootaxa.4967.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Pritt S., Cohen K., Sedlacek H. Chapter 15—Parasitic diseases. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. American College of Laboratory Animal Medicine; Academic Press; Boston, MA, USA: 2012. pp. 415–446. [Google Scholar]
- 7.Rashmir-Raven A.M. Chapter 18—Disorders of the skin. In: Reed S.M., Bayly W.M., Sellon D.C., Saunders W.B., editors. Equine Internal Medicine. 4th ed. Elsevier Inc.; Amsterdam, Netherlands: 2018. pp. 1159–1216. [Google Scholar]
- 8.Lee I.-Y., Yoon S.-S., Lee W.-J., Sin H.-K., Lee W.-K. Observation of stylostome formation in the striped-field mouse (Apodemus agrarius Pallas) skin by chigger feeding. Korean J. Soil Zool. 2006;11:1–6. [Google Scholar]
- 9.Shatrov A.B. Stylostome formation in Trombiculid mites (Acariformes: Trombiculidae) Exp. Appl. Acarol. 2009;49:261. doi: 10.1007/s10493-009-9264-0. [DOI] [PubMed] [Google Scholar]
- 10.Neal T.J., Barnett H.C. The life cycle of the scrub typhus chigger mite Trombicula akamushi. Ann. Entomol. Soc. Am. 1961;54:196–203. doi: 10.1093/aesa/54.2.196. [DOI] [Google Scholar]
- 11.Everett R.E., Price M.A., Kunz S.E. Biology of the chigger Neoschöngastia americana (Acarina: Trombiculidae) Ann. Entomol. Soc. Am. 1973;66:429–435. doi: 10.1093/aesa/66.2.429. [DOI] [Google Scholar]
- 12.Santibáñez P., Palomar A.M., Portillo A., Santibáñez S., Oteo J.A. The role of chiggers as human pathogens. In: Samie A., editor. An Overview of Tropical Diseases. I-Tech Education and Publishing; Vienna, Austria: 2015. [Google Scholar]
- 13.Lv Y., Guo X.-G., Jin D.-C. Research progress on Leptotrombidium deliense. Korean J. Parasitol. 2018;56:313–324. doi: 10.3347/kjp.2018.56.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shatrov A.B., Kudryashova N.I. Taxonomy, life cycles and the origin of parasitism in Trombiculid mites. In: Morand S., Krasnov B.R., Poulin R., editors. Micromammals and Macroparasites: From Evolutionary Ecology to Management. Springer Japan; Tokyo, Japan: 2006. pp. 119–140. [Google Scholar]
- 15.Wharton G.W., Fuller H.S. A Manual of the Chiggers: A manual of the chiggers: The biology, classification, distribution and importance to man of the larvae of the family Trombiculidae (Acarina) Mem. Entomol. Soc. Wash. 1952;4:185. [Google Scholar]
- 16.Lipovsky L.J., Byers G.W., Kardos E.H. Spermatophores: The mode of insemination of chiggers (Acarina: Trombiculidae) J. Parasitol. 1957;43:256–262. doi: 10.2307/3274343. [DOI] [PubMed] [Google Scholar]
- 17.Walters B.L., Whitaker J.O., Jr., Gikas N.S., Wrenn W.J. Host and Distribution Lists of Chiggers (Trombiculidae and Leeuwenhoekiidae), of North American Wild Vertebrates North of Mexico. Faculty Publications from the Harold W. Manter Laboratory of Parasitology No. 697, 2011. [(accessed on 19 May 2022)]. Available online: https://digitalcommons.unl.edu/parasitologyfacpubs/697/
- 18.Sasa M. Biology of chiggers. Annu. Rev. Entomol. 1961;6:221–244. doi: 10.1146/annurev.en.06.010161.001253. [DOI] [Google Scholar]
- 19.Zhan Y.-Z., Guo X.-G., Speakman J.R., Zuo X.-H., Wu D., Wang Q.-H., Yang Z.-H. Abundances and host relationships of chigger mites in Yunnan Province, China. Med. Vet. Entomol. 2013;27:194–202. doi: 10.1111/j.1365-2915.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- 20.Clopton R.E., Gold R.E. Distribution and seasonal and diurnal activity patterns of Eutrombicula alfreddugesi (Acari: Trombiculidae) in a forest edge ecosystem. J. Med. Entomol. 1993;30:47–53. doi: 10.1093/jmedent/30.1.47. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Wang D., Chen X. Trombiculid Mites of China: Studies on Vector and Pathogen of Tsutsugamushi Disease. Guangdong Science and Technology Publishing; Guangzhou, China: 1997. pp. 1–157. [Google Scholar]
- 22.Durden L.A., Hu R., Oliver J.H., Jr., Cilek J.E. Rodent ectoparasites from two locations in Northwestern Florida. J. Vector Ecol. 2000;25:222–228. [PubMed] [Google Scholar]
- 23.Ponnusamy L., Garshong R., McLean B.S., Wasserberg G., Durden L.A., Crossley D., Apperson C.S., Roe R.M. Rickettsia felis and other Rickettsia species in chigger mites collected from wild rodents in North Carolina, USA. Microorganisms. 2022;10:1342. doi: 10.3390/microorganisms10071342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krantz G.W., Walter D.E. A Manual of Acarology. 3rd ed. Texas Tech University Press; Lubbock, TX, USA: 2009. [Google Scholar]
- 25.Upham R.W., Jr., Hubert A.A., Phang O.W., Mat Y.B., Rapmund G. Distribution of Leptotrombidium (Leptotrombidium) arenicola (Acarina: Trombiculidae) on the ground in West Malaysia. J. Med. Entomol. 1971;8:401–406. doi: 10.1093/jmedent/8.4.401. [DOI] [PubMed] [Google Scholar]
- 26.Rohani I.B., Cromroy H.L. Taxonomy and distribution of chiggers (Acarina: Trombiculidae) in Northcentral Florida. Fla. Entomol. 1979;62:363–376. doi: 10.2307/3493993. [DOI] [Google Scholar]
- 27.Nadchatram M., Dohany A.L. Leptotrombidium (Leptotrombidium) umbricola, new species, a probable vector of scrub typhus in Peninsular Malaysia. Jpn. J. Med. Sci. Biol. 1980;33:277–282. doi: 10.7883/yoken1952.33.277. [DOI] [PubMed] [Google Scholar]
- 28.Tanskul P., Gingrich J.B. Two new species of Leptotrombidium (Acari: Actinedida: Trombiculidae), probable vectors of scrub typhus in Thailand. J. Med. Entomol. 1986;23:661–665. doi: 10.1093/jmedent/23.6.661. [DOI] [PubMed] [Google Scholar]
- 29.Jones B.M. A method for studying the distribution and bionomics of Trombiculid mites (Acarina: Trombidiidae) Parasitology. 1950;40:1–13. doi: 10.1017/S0031182000017819. [DOI] [PubMed] [Google Scholar]
- 30.Nadchatram M. A technique for rearing trombiculid mites (Acarina) developed in a tropical laboratory. J. Med. Entomol. 1968;5:465–469. doi: 10.1093/jmedent/5.4.465. [DOI] [PubMed] [Google Scholar]
- 31.Frances S.P., Khlaimanee N. Laboratory tests of arthropod repellents against Leptotrombidium Deliense noninfected and infected with Rickettsia tsutsugamushi and noninfected L. fletcheri (Acari: Trombiculidae) J. Med. Entomol. 1996;33:232–235. doi: 10.1093/jmedent/33.2.232. [DOI] [PubMed] [Google Scholar]
- 32.Michener C.D. A method of rearing chigger mites (Acarina, Trombiculinae) Am. J. Trop. Med. 1946;26:251–256. doi: 10.4269/ajtmh.1946.s1-26.251. [DOI] [PubMed] [Google Scholar]
- 33.Neal T.J., Lipvsky L.J. Techniques for rearing the chigger mite, Trombicula Akamushi (Brumpt) J. Econ. Entomol. 1959;52:824–826. doi: 10.1093/jee/52.5.824. [DOI] [Google Scholar]
- 34.Jenkins D.W. A laboratory method of rearing chiggers affecting man (Acarina: Trombiculidae) Ann. Entomol. Soc. Am. 1947;40:56–68. doi: 10.1093/aesa/40.1.56. [DOI] [Google Scholar]
- 35.Audy J.R., Nadchatram M. A Method of rearing individual Trombiculid mites in the field. Nature. 1954;174:1021–1022. doi: 10.1038/1741021b0. [DOI] [PubMed] [Google Scholar]
- 36.Goff M.L., Loomis R.B., Welbourn W.C., Wrenn W.J. A glossary of chigger terminology (Acari: Trombiculidae) J. Med. Entomol. 1982;19:221–238. doi: 10.1093/jmedent/19.3.221. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes S., Kulkarni S.M. Studies on the Trombiculid Mite Fauna of India. Zoological Survey of India; Kolkata, India: 2003. [Google Scholar]
- 38.Chaisiri K., Stekolnikov A.A., Makepeace B.L., Morand S. A revised checklist of chigger mites (Acari: Trombiculidae) from Thailand, with the description of three new species. J. Med. Entomol. 2016;53:321–342. doi: 10.1093/jme/tjv244. [DOI] [PubMed] [Google Scholar]
- 39.Stekolnikov A.A. A checklist of chigger mites (Acariformes: Trombiculidae) of Southeast Asia. Zootaxa. 2021;4913:1–163. doi: 10.11646/zootaxa.4913.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Reeves W.K., Durden L.A., Wrenn W.J. Ectoparasitic chiggers (Acari: Trombiculidae, Leeuwenhoekiidae), lice (Phthiraptera), and Hemiptera (Cimicidae and Reduviidae) from South Carolina, USA. Zootaxa. 2004;647:1–20. doi: 10.11646/zootaxa.647.1.1. [DOI] [Google Scholar]
- 41.Wen T.-H. New taxa and tentative rearrangement of Walchiidae Stat. n. with remarks on Trombiculoidea Nec Welbourn, 1991 (Acari: Acariformes) 1. Syst. Appl. Acarol. 1999;4:165–178. doi: 10.11158/saa.4.1.25. [DOI] [Google Scholar]
- 42.Wen T. Classification of the sand-mite Family Walchiidae (Acariformes: Trombiculoidea) Chin. J. Parasitol. Parasit. Dis. 2004;22:113–118. [PubMed] [Google Scholar]
- 43.Zhang Z.-Q., Fan Q.-H., Pesic V., Smit H., Bochkov A.V., Khaustov A.A., Baker A., Wohltmann A., Wen T., Amrine J.W., et al. Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness. Magnolia Press; Auckland, New Zealand: 2011. Order Trombidiformes Reuter, 1909; pp. 129–138. [Google Scholar]
- 44.Antonovskaia A.A. Using DNA Markers in Studies of Chigger Mites (Acariformes, Trombiculidae) Entmol. Rev. 2018;98:1351–1368. doi: 10.1134/S0013873818090130. [DOI] [Google Scholar]
- 45.Crossley D.A., Clement M.J. A new species of chigger (Acari: Trombiculidae) from Rafinesque’s Big-Eared bat (Chiroptera: Vespertilionidae) in Georgia, USA. J. Entomol. Sci. 2015;50:248–251. doi: 10.18474/JES14-40.1. [DOI] [Google Scholar]
- 46.Bennett S.G., Crossley D.A., Durden L.A., Goff M.L. Eutrombicula cinnabaris (Ewing, 1920) (Acari:Trombiculidae) is the common pest chigger mite of the Eastern United States. Entomol. Sci. 2014;49:413–414. doi: 10.18474/0749-8004-49.4.413. [DOI] [Google Scholar]
- 47.Loomis R.B., Wrenn W.J. Systematics of the Pest chigger genus Eutrombicula (Acari: Trombiculidae) In: Griffiths D.A., Bowman C.E., editors. Proceedings of the VI International Congress of Acarology. Ellis Horwood; Chichester, UK: 1984. [Google Scholar]
- 48.Ewing H.E. Notes on Trombiculid mites with descriptions of Walchiinae n. subf., Speotrombicula n. g., and Eutrombicula defecta n. sp. J. Parasitol. 1946;32:435–440. doi: 10.2307/3272913. [DOI] [PubMed] [Google Scholar]
- 49.Fuller H.S. The mite larvae of the family Trombiculidae in the Oudemans collection: Taxonomy and medical importance. Zool. Verh. 1952;18:1–261. [Google Scholar]
- 50.Wright S.M., Wikel S.K., Wrenn W.J. Host immune responsiveness to the chigger, Eutrombicula cinnabaris. Ann. Trop. Med. Parasitol. 1988;82:283–293. doi: 10.1080/00034983.1988.11812245. [DOI] [PubMed] [Google Scholar]
- 51.Tuegel M.A., Wrenn W.J. Sexual dimorphism in morphology and development of the pest chigger, Eutrombicula cinnabaris (Acari: Trombiculidae) Int. J. Acarol. 1998;24:199–211. doi: 10.1080/01647959808683585. [DOI] [Google Scholar]
- 52.Mertins J.W., Torrence S.M., Sterner M.C. Chiggers recently infesting Spea spp. in Texas, Usa, were Eutrombicula alfreddugesi, not Hannemania sp. J. Wildl. Dis. 2011;47:612–617. doi: 10.7589/0090-3558-47.3.612. [DOI] [PubMed] [Google Scholar]
- 53.Garvin S.D., Noden B.H., Dillwith J.W., Fox S.F., Payton M.E., Barker R.W. Sylvatic infestation of Oklahoma reptiles with immature Ixodes scapularis (Acari: Ixodidae) J. Med. Entomol. 2015;52:873–878. doi: 10.1093/jme/tjv100. [DOI] [PubMed] [Google Scholar]
- 54.Bakkegard K.A., Patton A.H., Ray C.H. Chigger mites (Hannemania Cf. Dunni) infest Northern Slimy Salamanders (Plethodon glutinosus) in Alabama, USA. Herpetol. Conserv. Biol. 2019;14:578–586. [Google Scholar]
- 55.McAllister C.T., Durden L.A., Greiman S.E. Euschoengastia pipistrelli (Acari: Trombiculidae) from American perimyotis, Perimyotis subflavus (Chiroptera: Vespertilionidae): Novel stereoscopic and scanning electron microscopy. J. Parasitol. 2021;107:125–128. doi: 10.1645/20-139. [DOI] [PubMed] [Google Scholar]
- 56.Durden L.A., Ellis B.A., Banks C.W., Crowe J.D., Oliver J.H. Ectoparasites of gray squirrels in two different habitats and screening of selected ectoparasites for Bartonellae. J. Parasitol. 2004;90:485–489. doi: 10.1645/GE-3299. [DOI] [PubMed] [Google Scholar]
- 57.McAllister C.T., Bursey C.R., Robison H.W., Connior M.B. Parasites of the Ozark zig-zag salamander, Plethodon angusticlavius (Caudata: Plethodontidae), from Northern Arkansas. Comp. Parasitol. 2013;80:69–79. doi: 10.1654/4600.1. [DOI] [Google Scholar]
- 58.McAllister C.T., Bursey C.R., Connior M.B., Trauth S.E., Durden L.A. New host and geographic distribution records for helminth and arthropod parasites of the southern toad, Anaxyrus terrestris (Anura: Bufonidae), from Florida. Southwest. Nat. 2015;14:641–649. doi: 10.1656/058.014.0401. [DOI] [Google Scholar]
- 59.Anderson L.E. Hoyer’s solution as a rapid permanent mounting medium for bryophytes. Bryologist. 1954;57:242–244. doi: 10.1639/0007-2745(1954)57[242:HSAARP]2.0.CO;2. [DOI] [Google Scholar]
- 60.Swan D.C. Berlese’s Fluid: Remarks upon its preparation and use as a mounting medium. Bull. Entomol. Res. 1936;27:389–391. doi: 10.1017/S0007485300058259. [DOI] [Google Scholar]
- 61.Stekolnikov A.A. A new genus and species of chigger mites (Acariformes: Trombiculidae) from Madagascar. Int. J. Acarol. 2021;47:301–307. doi: 10.1080/01647954.2021.1903555. [DOI] [Google Scholar]
- 62.Philip Samuel P., Govindarajan R., Krishnamoorthi R., Venkatesh A. A rapid protocol for clearing, staining, and mounting of Arthropoda: Trombiculidae, Pediculidae and Pulicidae. North-West. J. Zool. 2021;17:e201104. [Google Scholar]
- 63.Nadchatram M., Dohany A.L. A pictorial key to the subfamilies, genera and subgenera of Southeast Asian chiggers (Acari, Prostigmata, Trombiculidae) Bull. Inst. Med. Res. Malays. 1974;16:1–67. [Google Scholar]
- 64.Brennan J.M., Goff M.L. Keys to the genera of chiggers of the Western Hemisphere (Acarina: Trombiculidae) J. Parasitol. 1977;63:554–566. doi: 10.2307/3280021. [DOI] [PubMed] [Google Scholar]
- 65.Vercammen-Grandjean P.H., Langston R.L. The Chigger Mites of the World:(Acarina): Trombiculidae & Leeuwenhoekiidae. Volume 3 George Williams Hooper Foundation, University of California; San Francisco, CA, USA: 1976. [Google Scholar]
- 66.Stekolnikov A.A. Leptotrombidium (Acari: Trombiculidae) of the world. Zootaxa. 2013;3728:1–173. doi: 10.11646/zootaxa.3728.1.1. [DOI] [PubMed] [Google Scholar]
- 67.Pearce R.D., O’Shea T.J. Ectoparasites in an urban population of big brown bats (Eptesicus fuscus) in Colorado. J. Parasitol. 2007;93:518–530. doi: 10.1645/GE-973R.1. [DOI] [PubMed] [Google Scholar]
- 68.Alghamdi S. Ph.D. Thesis. The University of Liverpool (United Kingdom); Liverpool, UK: 2019. The Vector Biology and Microbiome of Parasitic Mites and Other Ectoparasites of Rodents. [Google Scholar]
- 69.Pepato A.R., Klimov P.B. Origin and higher-level diversification of acariform mites—Evidence from nuclear ribosomal genes, extensive taxon sampling, and secondary structure alignment. BMC Evol. Biol. 2015;15:178. doi: 10.1186/s12862-015-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klimov P.B., OConnor B.M., Chetverikov P.E., Bolton S.J., Pepato A.R., Mortazavi A.L., Tolstikov A.V., Bauchan G.R., Ochoa R. Comprehensive phylogeny of acariform mites (Acariformes) provides insights on the origin of the four-legged mites (Eriophyoidea), a long branch. Mol. Phylogenet. Evol. 2018;119:105–117. doi: 10.1016/j.ympev.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Kumlert R., Chaisiri K., Anantatat T., Stekolnikov A.A., Morand S., Prasartvit A., Makepeace B.L., Sungvornyothin S., Paris D.H. Autofluorescence microscopy for paired-matched morphological and molecular identification of individual chigger mites (Acari: Trombiculidae), the Vectors of Scrub Typhus. PLoS ONE. 2018;13:e0193163. doi: 10.1371/journal.pone.0193163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong X., Chaisiri K., Xia D., Armstrong S.D., Fang Y., Donnelly M.J., Kadowaki T., McGarry J.W., Darby A.C., Makepeace B.L. Genomes of trombidid mites reveal novel predicted allergens and laterally transferred genes associated with secondary metabolism. GigaScience. 2018;7:giy127. doi: 10.1093/gigascience/giy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Q., Wang Z.-X., Tao J.-M., Qin J.-P., Lu J.-P., Lin R.-Q., Wang L.-M., Weng Y.-B., Tan Z.-J. Characterization of Neoschoengastia gallinarum from subtropical China by rDNA and identification of two genotypes based on mitochondrial Cox1. Parasitol. Res. 2020;119:3339–3345. doi: 10.1007/s00436-020-06856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park S.-W., Ha N.-Y., Ryu B., Bang J.H., Song H., Kim Y., Kim G., Oh M., Cho N.-H., Lee J. Urbanization of scrub typhus disease in South Korea. PLOS Negl. Trop. Dis. 2015;9:e0003814. doi: 10.1371/journal.pntd.0003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao R., Mitani H., Barker S.C., Takahashi M., Fukunaga M. Novel mitochondrial gene content and gene arrangement indicate illegitimate inter-mtDNA recombination in the chigger mite, Leptotrombidium pallidum. J. Mol. Evol. 2005;60:764–773. doi: 10.1007/s00239-004-0226-1. [DOI] [PubMed] [Google Scholar]
- 76.Shao R., Barker S.C., Mitani H., Takahashi M., Fukunaga M. Molecular mechanisms for the variation of mitochondrial gene content and gene arrangement among chigger mites of the genus Leptotrombidium (Acari: Acariformes) J. Mol. Evol. 2006;63:251–261. doi: 10.1007/s00239-005-0196-y. [DOI] [PubMed] [Google Scholar]
- 77.Young M.R., Behan-Pelletier V.M., Hebert P.D.N. Revealing the hyperdiverse mite fauna of subarctic Canada through DNA Barcoding. PLoS ONE. 2012;7:e48755. doi: 10.1371/journal.pone.0048755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Castro Jacinavicius F., Bassini-Silva R., Muñoz-Leal S., Welbourn C., Ochoa R., Labruna M.B., Barros-Battesti D.M. Molecular detection of Rickettsia genus in chigger mites (Trombidiformes: Trombiculidae) collected on small mammals in Southeastern Brazilian. Rev. Bras. Parasitol. Vet. 2019;28:563–568. doi: 10.1590/s1984-29612019069. [DOI] [PubMed] [Google Scholar]
- 79.Ja M.-R., Dm B.-B. A new species of pit mite (Trombidiformes: Harpirhynchidae) from the South American rattlesnake (Viperidae): Morphological and molecular analysis. Entomol. Ornithol. Herpetol. 2017;6:8. [Google Scholar]
- 80.Moniuszko H., Zaleśny G., Mąkol J. Host-associated differences in morphometric traits of parasitic larvae Hirsutiella zachvatkini (Actinotrichida: Trombiculidae) Exp. Appl. Acarol. 2015;67:123–133. doi: 10.1007/s10493-015-9925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moniuszko H., Felska M., Mąkol J. Evidence for co-invasion events: Different chigger species (Actinotrichida, Trombidioidea: Trombiculidae) share a host. Exp. Appl. Acarol. 2018;76:29–39. doi: 10.1007/s10493-018-0293-4. [DOI] [PubMed] [Google Scholar]
- 82.Söller R., Wohltmann A., Witte H., Blohm D. Phylogenetic relationships within terrestrial mites (Acari: Prostigmata, Parasitengona) inferred from comparative DNA sequence analysis of the mitochondrial cytochrome oxidase subunit I gene. Mol. Phylogenet. Evol. 2001;18:47–53. doi: 10.1006/mpev.2000.0855. [DOI] [PubMed] [Google Scholar]
- 83.Kiene F., Andriatsitohaina B., Ramsay M.S., Rakotondramanana H., Rakotondravony R., Radespiel U., Strube C. Forest edges affect ectoparasite infestation patterns of small mammalian hosts in fragmented forests in Madagascar. Int. J. Parasitol. 2020;50:299–313. doi: 10.1016/j.ijpara.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Lee H.I., Shim S.K., Song B.G., Choi E.N., Hwang K.J., Park M.Y., Park C., Shin E.-H. Detection of Orientia tsutsugamushi, the causative agent of scrub typhus, in a novel mite species, Eushoengastia koreaensis, in Korea. Vector Borne Zoonotic Dis. 2011;11:209–214. doi: 10.1089/vbz.2009.0180. [DOI] [PubMed] [Google Scholar]
- 85.Dohany A.L., Shirai A., Robinson D.M., Ram S., Huxsoll D.L. Identification and antigenic typing of Rickettsia tsutsugamushi in naturally infected chiggers (Acarina: Trombiculidae) by direct immunofluorescence. Am. J. Trop. Med. Hyg. 1978;27:1261–1264. doi: 10.4269/ajtmh.1978.27.1261. [DOI] [PubMed] [Google Scholar]
- 86.Ree H.I., Lee I.Y., Cho M.K. Determination of the vector species of Tsutsugamushi disease in Korea. Korean J. Parasitol. 1991;29:87–92. doi: 10.3347/kjp.1991.29.1.87. [DOI] [PubMed] [Google Scholar]
- 87.Ponnusamy L., Willcox A.C., Roe R.M., Davidson S.A., Linsuwanon P., Schuster A.L., Richards A.L., Meshnick S.R., Apperson C.S. Bacterial microbiome of the chigger mite Leptotrombidium imphalum varies by life stage and infection with the scrub typhus pathogen Orientia tsutsugamushi. PLoS ONE. 2018;13:e0208327. doi: 10.1371/journal.pone.0208327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaisiri K., Gill A.C., Stekolnikov A.A., Hinjoy S., McGarry J.W., Darby A.C., Morand S., Makepeace B.L. Ecological and microbiological diversity of chigger mites, including vectors of scrub typhus, on small mammals across stratified habitats in Thailand. Anim. Microbiome. 2019;1:18. doi: 10.1186/s42523-019-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang J., Richards A.L. Scrub typhus: No longer restricted to the Tsutsugamushi Triangle. Trop. Med. Infect. Dis. 2018;3:11. doi: 10.3390/tropicalmed3010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elliott I., Pearson I., Dahal P., Thomas N.V., Roberts T., Newton P.N. Scrub typhus ecology: A systematic review of Orientia in vectors and hosts. Parasit. Vectors. 2019;12:513. doi: 10.1186/s13071-019-3751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu G., Walker D.H., Jupiter D., Melby P.C., Arcari C.M. A review of the global epidemiology of scrub typhus. PLOS Negl. Trop. Dis. 2017;11:e0006062. doi: 10.1371/journal.pntd.0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phasomkusolsil S., Tanskul P., Ratanatham S., Watcharapichat P., Phulsuksombati D., Frances S.P., Lerdthusnee K., Linthicum K.J. Transstadial and transovarial transmission of Orientia tsutsugamushi in Leptotrombidium imphalum and Leptotrombidium chiangraiensis (Acari: Trombiculidae) J. Med. Entomol. 2009;46:1442–1445. doi: 10.1603/033.046.0628. [DOI] [PubMed] [Google Scholar]
- 93.Paris D.H., Shelite T.R., Day N.P., Walker D.H. Unresolved problems related to scrub typhus: A seriously neglected life-threatening disease. Am. J. Trop. Med. Hyg. 2013;89:301–307. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelly D.J., Fuerst P.A., Ching W.-M., Richards A.L. Scrub typhus: The geographic distribution of phenotypic and denotypic variants of Orientia tsutsugamushi. Clin. Infect. Dis. 2009;48:S203–S230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- 95.Izzard L., Fuller A., Blacksell S.D., Paris D.H., Richards A.L., Aukkanit N., Nguyen C., Jiang J., Fenwick S., Day N.P. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010;48:4404–4409. doi: 10.1128/JCM.01526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balcells M.E., Rabagliati R., García P., Poggi H., Oddó D., Concha M., Abarca K., Jiang J., Kelly D.J., Richards A.L. Endemic scrub typhus–like illness, Chile. Emerg. Infect. Dis. 2011;17:1659. doi: 10.3201/eid1709.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weitzel T., Dittrich S., López J., Phuklia W., Martinez-Valdebenito C., Velásquez K., Blacksell S.D., Paris D.H., Abarca K. Endemic scrub typhus in South America. N. Engl. J. Med. 2016;375:954–961. doi: 10.1056/NEJMoa1603657. [DOI] [PubMed] [Google Scholar]
- 98.Kocher C., Jiang J., Morrison A.C., Castillo R., Leguia M., Loyola S., Ampuero J.S., Cespedes M., Halsey E.S., Bausch D.G. Serologic evidence of scrub typhus in the Peruvian Amazon. Emerg. Infect. Dis. 2017;23:1389. doi: 10.3201/eid2308.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cosson J.F., Galan M., Bard E., Razzauti M., Bernard M., Morand S., Brouat C., Dalecky A., Bâ K., Charbonnel N., et al. Detection of Orientia sp. DNA in rodents from Asia, West Africa and Europe. Parasit. Vectors. 2015;8:172. doi: 10.1186/s13071-015-0784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masakhwe C., Linsuwanon P., Kimita G., Mutai B., Leepitakrat S., Yalwala S., Abuom D., Auysawasi N., Gilbreath T., Wanja E., et al. Identification and characterization of Orientia chuto in Trombiculid chigger mites collected from wild rodents in Kenya. J. Clin. Microbiol. 2018;56:e01124-18. doi: 10.1128/JCM.01124-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tilak R., Kunwar R., Wankhade U.B., Tilak V.W. Emergence of Schoengastiella ligula as the vector of scrub typhus outbreak in Darjeeling: Has Leptotrombidium deliense been replaced? Indian J. Public Health. 2011;55:92. doi: 10.4103/0019-557X.85239. [DOI] [PubMed] [Google Scholar]
- 102.Peters C.J., Simpson G.L., Levy H. Spectrum of Hantavirus Infection: Hemorrhagic Fever with Renal Syndrome and Hantavirus Pulmonary Syndrome. Annu. Rev. Med. 1999;50:531–545. doi: 10.1146/annurev.med.50.1.531. [DOI] [PubMed] [Google Scholar]
- 103.CDC Hantavirus Pulmonary Syndrome (HPS)—Hantavirus. [(accessed on 11 April 2022)]; Available online: https://www.cdc.gov/hantavirus/hps/index.html.
- 104.Wu G., Zhang Y., Guo H., Jiang K., Zhang J., Gan Y. The role of Leptotrombidium scutellare in the transmission of human diseases. Chin. Med. J. 1996;109:670–673. doi: 10.5555/cmj.0366-6999.109.09.p670.01. [DOI] [PubMed] [Google Scholar]
- 105.Yu X., Tesh R.B. The role of mites in the transmission and maintenance of Hantaan Virus (Hantavirus: Bunyaviridae) J. Infect. Dis. 2014;210:1693–1699. doi: 10.1093/infdis/jiu336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Houck M.A., Qin H., Roberts H.R. Hantavirus transmission: Potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75–79. doi: 10.1089/153036601750137723. [DOI] [PubMed] [Google Scholar]
- 107.Kosoy M., Morway C., Sheff K.W., Bai Y., Colborn J., Chalcraft L., Dowell S.F., Peruski L.F., Maloney S.A., Baggett H. Bartonella Tamiae Sp. Nov., a newly recognized pathogen isolated from three human patients from Thailand. J. Clin. Microbiol. 2008;46:772–775. doi: 10.1128/JCM.02120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kabeya H., Colborn J.M., Bai Y., Lerdthusnee K., Richardson J.H., Maruyama S., Kosoy M.Y. Detection of Bartonella tamiae DNA in ectoparasites from rodents in Thailand and their sequence similarity with bacterial cultures from Thai patients. Vector Borne Zoonotic Dis. 2010;10:429–434. doi: 10.1089/vbz.2009.0124. [DOI] [PubMed] [Google Scholar]
- 109.Tsui P.-Y., Tsai K.-H., Weng M.-H., Hung Y.-W., Liu Y.-T., Hu K.-Y., Lien J.-C., Lin P.-R., Shaio M.-F., Wang H.-C. Molecular detection and characterization of spotted fever group Rickettsiae in Taiwan. Am. J. Trop. Med. Hyg. 2007;77:883–890. doi: 10.4269/ajtmh.2007.77.883. [DOI] [PubMed] [Google Scholar]
- 110.Choi Y.-J., Lee E.-M., Park J.-M., Lee K.-M., Han S.-H., Kim J.-K., Lee S.-H., Song H.-J., Choi M.-S., Kim I.-S., et al. Molecular detection of various Rickettsiae in mites (Acari: Trombiculidae) in Southern Jeolla Province, Korea. Microbiol. Immunol. 2007;51:307–312. doi: 10.1111/j.1348-0421.2007.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 111.Huang Y., Zhao L., Zhang Z., Liu M., Xue Z., Ma D., Sun X., Sun Y., Zhou C., Qin X., et al. Detection of a novel Rickettsia from Leptotrombidium scutellare mites (Acari: Trombiculidae) from Shandong of China. J. Med. Entomol. 2017;54:544–549. doi: 10.1093/jme/tjw234. [DOI] [PubMed] [Google Scholar]
- 112.Ogawa M., Takahashi M., Matsutani M., Takada N., Noda S., Saijo M. Obligate intracellular bacteria diversity in unfed Leptotrombidium scutellare larvae highlights novel bacterial endosymbionts of mites. Microbiol. Immunol. 2020;64:1–9. doi: 10.1111/1348-0421.12745. [DOI] [PubMed] [Google Scholar]
- 113.Kampen H., Schöler A., Metzen M., Oehme R., Hartelt K., Kimmig P., Maier W.A. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp. Appl. Acarol. 2004;33:93–102. doi: 10.1023/B:APPA.0000029975.92510.90. [DOI] [PubMed] [Google Scholar]
- 114.Literak I., Stekolnikov A.A., Sychra O., Dubska L., Taragelova V. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: A Field Study Including Birds from the Czech Carpathians as Hosts of Chiggers. Exp. Appl. Acarol. 2008;44:307–314. doi: 10.1007/s10493-008-9150-1. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi M., Urakami H., Yoshida Y., Furuya Y., Misumi H., Hori E., Kawamura A., Tanaka H. Occurrence of high ratio of males after introduction of minocycline in a colony of Leptotrombidium fletcheri infected with Orientia tsutsugamushi. Eur. J. Epidemiol. 1997;13:19–23. doi: 10.1023/A:1007341721795. [DOI] [PubMed] [Google Scholar]
- 116.Duron O., Bouchon D., Boutin S., Bellamy L., Zhou L., Engelstädter J., Hurst G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Phasomkusolsil S., Tanskul P., Ratanatham S., Watcharapichat P., Phulsuksombati D., Frances S.P., Lerdthusnee K., Linthicum K.J. Influence of Orientia tsutsugamushi infection on the developmental biology of Leptotrombidium imphalum and Leptotrombidium chiangraiensis (Acari: Trombiculidae) J. Med. Entomol. 2012;49:1270–1275. doi: 10.1603/ME12100. [DOI] [PubMed] [Google Scholar]
- 118.Rodino K.G., Theel E.S., Pritt B.S. Tick-borne diseases in the United States. Clin. Chem. 2020;66:537–548. doi: 10.1093/clinchem/hvaa040. [DOI] [PubMed] [Google Scholar]
- 119.Robinson M.T., Satjanadumrong J., Hughes T., Stenos J., Blacksell S.D. Diagnosis of spotted fever group Rickettsia infections: The Asian perspective. Epidemiol. Infect. 2019;147:e286. doi: 10.1017/S0950268819001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou C., Woods P., Abouzeid A., Brooks M.N. Case Report: Rocky Mountain Spotted Fever with adrenalectomy and a gard-to-find tick. Am. J. Case Rep. 2022;23:e934505-1–e934505-6. doi: 10.12659/AJCR.934505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shapiro M.R., Fritz C.L., Tait K., Paddock C.D., Nicholson W.L., Abramowicz K.F., Karpathy S.E., Dasch G.A., Sumner J.W., Adem P.V., et al. Rickettsia 364D: A newly recognized cause of eschar-Associated Illness in California. Clin. Infect. Dis. 2010;50:541–548. doi: 10.1086/649926. [DOI] [PubMed] [Google Scholar]
- 122.Kuo C.-C., Lee P.-L., Wang H.-C. Molecular identification of Rickettsia spp. in chigger mites in Taiwan. Med. Vet. Entomol. 2021:223–229. doi: 10.1111/mve.12560. [DOI] [PubMed] [Google Scholar]
- 123.Zhioua E., Rodhain F., Binet P.h., Perez-Eid C. Prevalence of antibodies to Borrelia burgdorferi in forestry workers of Ile de France, France. Eur. J. Epidemiol. 1997;13:959–962. doi: 10.1023/A:1007465305193. [DOI] [PubMed] [Google Scholar]
- 124.Crispell G., Commins S.P., Archer-Hartman S.A., Choudhary S., Dharmarajan G., Azadi P., Karim S. Discovery of Alpha-Gal-containing antigens in North American tick species believed to induce red meat allergy. Front. Immunol. 2019;10:1056. doi: 10.3389/fimmu.2019.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Commins S.P., Platts-Mills T.A.E. Tick bites and red meat allergy. Curr. Opin. Allergy Clin. Immunol. 2013;13:354–359. doi: 10.1097/ACI.0b013e3283624560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stoltz L.P., Cristiano L.M., Dowling A.P.G., Wilson J.M., Platts-Mills T.A.E., Traister R.S. Could chiggers be contributing to the prevalence of galactose-alpha-1,3-galactose sensitization and mammalian meat allergy? J. Allergy Clin. Immunol. Pract. 2019;7:664–666. doi: 10.1016/j.jaip.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chiggers. [(accessed on 7 April 2022)]. Available online: https://hortnews.extension.iastate.edu/chiggers.
- 128.Houseman R.M. Chiggers. [(accessed on 13 April 2022)]. Available online: https://extension.missouri.edu/publications/g7398.
- 129.Anderson R.R., Oi F.M. Chiggers. [(accessed on 13 April 2022)]. Available online: https://ssl.acesag.auburn.edu/pubs/docs/A/ANR-1109/ANR-1109-archive.pdf.
- 130.Moore G.C., Merchant M.E. Chiggers. [(accessed on 7 April 2022)]. Available online: https://agrilifeextension.tamu.edu/library/landscaping/chiggers/
- 131.Ewing H.E., Hartzell A. The chigger-mites affecting man and domestic animals. J. Econ. Entomol. 1918;11:256–264. doi: 10.1093/jee/11.2.256. [DOI] [Google Scholar]
- 132.Ewing H.E. Our only common North American chigger, its distribution and nomenclature. J. Agric. Res. 1923;26:401–403. [Google Scholar]
- 133.Ewing H.E. A contribution to our knowledge of the taxonomy of chiggers, including the descriptions of a new genus, six new species and a new variety. Am. J. Trop. Med. 1925;5:251–265. doi: 10.4269/ajtmh.1925.s1-5.251. [DOI] [Google Scholar]
- 134.Ewing H.E. The adult of our common North American chigger. Proceedings of the Biological Society of Washington. 1925 [Google Scholar]
- 135.Ewing H.E. Birds as hosts for the common chigger. Am. Nat. 1929;63:94–96. doi: 10.1086/280240. [DOI] [Google Scholar]
- 136.Ewing H.E. A catalogue of the Trombiculinae, or chigger mites, of the new world, with new genera and species and a key to the genera. Proc. United States Natl. Mus. 1931;80 doi: 10.5479/si.00963801.80-2908.1. [DOI] [Google Scholar]
- 137.Ewing H.E. A key to the genera of chiggers (mite larvae of the subfamily Trombiculinae) with descriptions of new genera and species. J. Wash. Acad. Sci. 1938;28:288–295. [Google Scholar]
- 138.Ewing H.E. Remarks on the taxonomy of some American chiggers (Trombiculinae), including the descriptions of New Genera and species. J. Parasitol. 1942;28:485–493. doi: 10.2307/3272909. [DOI] [Google Scholar]
- 139.Ewing H.E. The American chiggers (larvae of the Trombiculinae) of the genus Acariscus, new genus. Proc. Entomol. Soc. Wash. 1943;45:57–66. [Google Scholar]
- 140.Brennan J.M. A new genus and species of chigger, Chatia setosa (Trombiculidae, Acarina) from Northwestern United States. J. Parasitol. 1946;32:132–135. doi: 10.2307/3272585. [DOI] [PubMed] [Google Scholar]
- 141.Doetschman W., Furman D. A tropical chigger, Eutrombicula batatas (Linn) attacking man in California. Am. J. Trop. Med. 1949;29:605–608. doi: 10.4269/ajtmh.1949.s1-29.605. [DOI] [PubMed] [Google Scholar]
- 142.Jenkins D.W. Trombiculid mites affecting man. III. Trombicula (Eutrombicula) splendens Ewing in North America. J. Parasitol. 1949;35:201–204. doi: 10.2307/3273126. [DOI] [PubMed] [Google Scholar]
- 143.Brennan J.M., Wharton G.W. Studies on North American chiggers. No. 3. the subgenus Neotrombicula. Am. Midl. Nat. 1950;44:153–197. doi: 10.2307/2421759. [DOI] [Google Scholar]
- 144.Lipovsky L.J., Loomis R.B. A new chigger mite (genus Euschöngastia) from the Central United States. J. Parasitol. 1954;40:407–410. doi: 10.2307/3273886. [DOI] [PubMed] [Google Scholar]
- 145.Crossley D.A. A new species of chigger mite from Colorado (Acarina, Trombiculidae) J. Parasitol. 1955;41:289–291. doi: 10.2307/3274208. [DOI] [PubMed] [Google Scholar]
- 146.Hyland K.E. A new species of chigger mite, Hannemania hegeneri (Acarina; Trombiculidae) J. Parasitol. 1956;42:176–179. doi: 10.2307/3274733. [DOI] [PubMed] [Google Scholar]
- 147.Brennan J.M., White J.S. New records and descriptions of chiggers (Acarina: Trombiculidae) on Bats in Alabama. J. Parasitol. 1960;46:346–350. doi: 10.2307/3275497. [DOI] [PubMed] [Google Scholar]
- 148.Johnston D.E., DeGiusti D.L. Ecological and systematic notes on some chigger mites of the genera Gahrliepia, Trombicula, and Euschongastia (Acarina: Trombiculidae) in Michigan and Ontario. J. Parasitol. 1961;47:11–12. doi: 10.2307/3274965. [DOI] [Google Scholar]
- 149.Powder W.A., Loomis R.B. A new species and new records of chiggers (Acarina, Trombiculidae) from reptiles of Southern California. J. Parasitol. 1962;48:204–208. doi: 10.2307/3275564. [DOI] [PubMed] [Google Scholar]
- 150.Loomis R.B. The discovery of chiggers (Acarina, Trombiculidae) in the nasal passages of cricetid rodents from California, with descriptions of two new species. J. Parasitol. 1963;49:330–333. doi: 10.2307/3276008. [DOI] [Google Scholar]
- 151.Brennan J.M. Five new chiggers from Southwestern United States (Acarina: Trombiculidae) J. Parasitol. 1965;51:108–113. doi: 10.2307/3275656. [DOI] [PubMed] [Google Scholar]
- 152.Brennan J.M. New records of chiggers (Acarina: Trombiculidae) from Baja California and islands of the gulf of California. J. Parasitol. 1966;52:772–775. doi: 10.2307/3276453. [DOI] [PubMed] [Google Scholar]
- 153.Johnston D.E., Wharton G.W., Stoll R.J. On the occurrence of Fonsecia palmella Brennan and Loomis (Acari: Trombiculidae), a chigger mite of reptiles, in Ohio. J. Parasitol. 1966;52:966. doi: 10.2307/3276547. [DOI] [Google Scholar]
- 154.Webb J.P., Jr., Loomis R.B. A new subgenus of intranasal chiggers of the genus Microtrombicula from North America and Korea. J. Med. Entomol. 1970;7:655–663. doi: 10.1093/jmedent/7.6.655. [DOI] [PubMed] [Google Scholar]
- 155.Crossley D.A., Atyeo W.T. A new species of chigger, Blankaartia pauli (Acarina: Trombiculidae), from the Southeastern United States. J. Med. Entomol. 1972;9:253–254. doi: 10.1093/jmedent/9.3.253. [DOI] [PubMed] [Google Scholar]
- 156.Everett R.E., Price M.A., Kunz S.E. New host records of the chigger Neoschoengastia americana from Texas (Acarina: Trombiculidae) J. Med. Entomol. 1972;9:109–110. doi: 10.1093/jmedent/9.1.109. [DOI] [PubMed] [Google Scholar]
- 157.Easton E.R. Ectoparasites in two diverse habitats in Western Oregon. J. Med. Entomol. 1975;12:295–298. doi: 10.1093/jmedent/12.3.295. [DOI] [PubMed] [Google Scholar]
- 158.Goff M.L., Judd F.W. The first record of a chigger from the Texas tortoise, Gopherus berlandieri. Southwest. Nat. 1981;26:83–84. doi: 10.2307/3671346. [DOI] [Google Scholar]
- 159.Pomeroy L.V., Loomis R.B. A new genus of Trombiculine chiggers (Acari: Trombiculidae) from Western North America. J. Med. Entomol. 1984;21:268–273. doi: 10.1093/jmedent/21.3.268. [DOI] [PubMed] [Google Scholar]
- 160.Ludwig D.F., Crossley D.A., Jr., Hayes M.J., Mallow D., Wicht M.C., Jr. Pest chigger, Eutrombicula alfreddugesi infestation of small mammals in piedmont habitats of Georgia (Acarina: Trombiculidae) J. Entomol. Sci. 1985;20:1–8. doi: 10.18474/0749-8004-20.1.1. [DOI] [Google Scholar]
- 161.Durden L.A., Wilson N. Parasitic and phoretic arthropods of sylvatic and commensal white-footed mice (Peromyscus leucopus) in Central Tennessee, with notes on Lyme Disease. J. Parasitol. 1991;77:219–223. doi: 10.2307/3283085. [DOI] [PubMed] [Google Scholar]
- 162.Wilson N.A., Telford S.R., Jr., Forrester D.J. Ectoparasites of a population of urban gray squirrels in northern florida. J. Med. Entomol. 1991;28:461–464. doi: 10.1093/jmedent/28.3.461. [DOI] [PubMed] [Google Scholar]
- 163.Durden L.A. Parasitic arthropods of sympatric meadow voles and white-footed mice at Fort Detrick, Maryland. J. Med. Entomol. 1992;29:761–766. doi: 10.1093/jmedent/29.5.761. [DOI] [PubMed] [Google Scholar]
- 164.Goldberg S.R., Bursey C.R. Duration of attachment of the chigger, Eutrombicula lipovskyana (Trombiculidae) in mite pockets of Yarrow’s Spiny Lizard, Sceloporus jarrovii (Phrynosomatidae) from Arizona. J. Wildl. Dis. 1993;29:142–144. doi: 10.7589/0090-3558-29.1.142. [DOI] [PubMed] [Google Scholar]
- 165.Anthony C.D., Mendelson J.R., Simons R.R. Differential parasitism by sex on Plethodontid Salamanders and histological evidence for structural damage to the nasolabial groove. Am. Midl. Nat. 1994;132:302–307. doi: 10.2307/2426586. [DOI] [Google Scholar]
- 166.Goldberg S.R., Bursey C.R. Prevalence of ectoparasite infestation in neonate Yarrow’s spiny lizards, Sceloporus jarrovii (Phrynosomatidae), from Arizona. Great Basin Nat. 1994;54:189–190. doi: 10.5962/bhl.part.25746. [DOI] [Google Scholar]
- 167.Pung O.J., Durden L.A., Banks C.W., Jones D.N. Ectoparasites of opossums and raccoons in Southeastern Georgia. J. Med. Entomol. 1994;31:915–919. doi: 10.1093/jmedent/31.6.915. [DOI] [PubMed] [Google Scholar]
- 168.Forrester D.J., Mclaughlin G.S., Telford S.R., Jr., Foster G.W., Mccown J.W. Ectoparasites (Acari, Mallophaga, Anoplura, Diptera) of white-tailed deer, Odocoileus virginianus from Southern Florida. J. Med. Entomol. 1996;33:96–101. doi: 10.1093/jmedent/33.1.96. [DOI] [PubMed] [Google Scholar]
- 169.Durden L.A., Banks C.W., Clark K.L., Belbey B.V., Oliver J.H. Ectoparasite fauna of the eastern woodrat, Neotoma floridana: composition, origin, and comparison with ectoparasite faunas of Western woodrat species. J. Parasitol. 1997;83:374–381. doi: 10.2307/3284397. [DOI] [PubMed] [Google Scholar]
- 170.Goff M.L., Mckown R.D. The genus Hexidionis (Acari: Trombiculidae) with the description of a new species from Texas. J. Med. Entomol. 1997;34:438–440. doi: 10.1093/jmedent/34.4.438. [DOI] [PubMed] [Google Scholar]
- 171.Little S.E., Carmichael K.P., Rakich P.M. Trombidiosis-induced dermatitis in white-tailed deer (Odocoileus virginianus) Vet. Pathol. 1997;34:350–352. doi: 10.1177/030098589703400412. [DOI] [PubMed] [Google Scholar]
- 172.Spalding M.G., Wrenn W.J., Schwikert S.T., Schmidt J.A. Dermatitis in young Florida Sandhill cranes (Grus canadensis Pratensis) due to infestation by the chigger, Blankaartia sinnamaryi. J. Parasitol. 1997;83:768–771. doi: 10.2307/3284263. [DOI] [PubMed] [Google Scholar]
- 173.Pung O.J., Durden L.A., Patrick M.J., Conyers T., Mitchell L.R. Ectoparasites and gastrointestinal helminths of Southern flying squirrels in Southeast Georgia. J. Parasitol. 2000;86:1051–1055. doi: 10.1645/0022-3395(2000)086[1051:EAGHOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 174.Sladky K.K., Norton T.M., Loomis M.R. Trombiculid mites (Hannemania sp.) in canyon tree frogs (Hyla arenicolor) J. Zoo Wildl. Med. 2000;31:570–575. doi: 10.1638/1042-7260(2000)031[0570:TMHSIC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 175.Williams C.K., Davidson W.R., Lutz R.S., Applegate R.D. Health status of northern bobwhite quail (Colinus virginianus) in Eastern Kansas. Avian Dis. 2000;44:953–956. doi: 10.2307/1593071. [DOI] [PubMed] [Google Scholar]
- 176.Cunningham M.W., Phillips L.A., Welbourn C. Trombiculiasis in the Florida black bear. J. Wildl. Dis. 2001;37:634–639. doi: 10.7589/0090-3558-37.3.634. [DOI] [PubMed] [Google Scholar]
- 177.Klukowski M. Seasonal changes in abundance of host-seeking chiggers (Acari: Trombiculidae) and infestations on fence lizards, Sceloporus undulatus. J. Herpetol. 2004;38:141–144. doi: 10.1670/127-03N. [DOI] [Google Scholar]
- 178.Malone J.H., Paredes-Leon R. Characteristics of chigger mite (Hannemania sp.) parasitism on Eleutherodactylus marnockii (Amphibia: Leptodactylidae) Tex. J. Sci. 2005;57:345–358. [Google Scholar]
- 179.Kurta A., Whitaker J.O., Jr., Wrenn W.J., Soto-centeno J.A. Ectoparasitic assemblages on mormoopid bats (Chiroptera: Mormoopidae) from Puerto Rico. J. Med. Entomol. 2007;44:953–958. doi: 10.1093/jmedent/44.6.953. [DOI] [PubMed] [Google Scholar]
- 180.Torrence S.M., Smith L.M., McMurry S.T. Larval Hannemania sp. infestations of Spea spp. in the Southern High Plains, Texas, USA. J. Wildl. Dis. 2007;43:742–746. doi: 10.7589/0090-3558-43.4.742. [DOI] [PubMed] [Google Scholar]
- 181.Nims T.N., Durden L.A., Chandler C.R., Pung O.J. Parasitic and phoretic arthropods of the oldfield mouse (Peromyscus polionotus) from burned habitats with additional ectoparasite records from the eastern harvest mouse (Reithrodontomys humulis) and southern short-tailed shrew (Blarina carolinensis) Comp. Parasitol. 2008;75:102–106. doi: 10.1654/4273.1. [DOI] [Google Scholar]
- 182.Westfall M.C., Cecala K.K., Price S.J., Dorcas M.E. Patterns of Trombiculid mite (Hannemania dunni) parasitism among plethodontid salamanders in the western piedmont of North Carolina. J. Parasitol. 2008;94:631–634. doi: 10.1645/GE-1260.1. [DOI] [PubMed] [Google Scholar]
- 183.Bulté G., Plummer A.C., Thibaudeau A., Blouin-Demers G. Infection of Yarrow’s spiny lizards (Sceloporus jarrovii) by chiggers and malaria in the Chiricahua Mountains, Arizona. Southwest. Nat. 2009;54:204–207. doi: 10.1894/PAS-07.1. [DOI] [Google Scholar]
- 184.Mertins J.W., Hanson B.A., Corn J.L. Whartonacarus floridensis sp. nov. (Acari: Trombiculidae), with a taxonomic review and the first record of Whartonacarus chiggers in the continental United States. J. Med. Entomol. 2009;46:1260–1268. doi: 10.1603/033.046.0603. [DOI] [PubMed] [Google Scholar]
- 185.Corn J.L., Mertins J.W., Hanson B., Snow S. First reports of ectoparasites collected from wild-caught exotic reptiles in Florida. J. Med. Entomol. 2011;48:94–100. doi: 10.1603/ME10065. [DOI] [PubMed] [Google Scholar]
- 186.McCoy E.D., Styga J.M., Rizkalla C.E., Mushinsky H.R. Time since fire affects ectoparasite prevalence on lizards in the Florida scrub ecosystem. Fire Ecol. 2012;8:32–40. doi: 10.4996/fireecology.0803032. [DOI] [Google Scholar]
- 187.Ott-Conn C.N., Woods L.W., Clifford D.L., Branston T., Foley J. Histopathology and risk factors associated with Neotrombicula microti infestation in the endangered Amargosa vole (Microtus californicus scirpensis) J. Wildl. Dis. 2015;51:680–687. doi: 10.7589/2014-08-209. [DOI] [PubMed] [Google Scholar]
- 188.McAllister C.T., Bursey C.R., Connior M.B. New Host and Distributional records for parasites (Apicomplexa, Trematoda, Nematoda, Acanthocephala, Acarina) of Texas herpetofauna. Comp. Parasitol. 2017;84:42–50. doi: 10.1654/1525-2647-84.1.42. [DOI] [Google Scholar]
- 189.Milley C., Dryden M., Rosenkrantz W., Griffin J., Reeder C. Comparison of parasitic mite retrieval methods in a population of community cats. J. Feline Med. Surg. 2017;19:657–664. doi: 10.1177/1098612X16650717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Harrity E.J., Conway C.J. Novel ectoparasite infestation on Yuma Ridgway’s rails (Rallus obsoletus yumanensis) Wilson J. Ornithol. 2019;131:139–146. doi: 10.1676/18-55. [DOI] [Google Scholar]
- 191.Bassini-Silva R., Jacinavicius F.D.C., Welbourn C., Ochoa R., Barros-battesti D.M. Synonymy of the genus Delmohius Brennan and Goff, 1978 with Carebareia Goff and Brennan, 1977 (Trombidiformes: Trombiculidae) Syst. Appl. Acarol. 2020;25:1188–1196. doi: 10.11158/saa.25.7.3. [DOI] [Google Scholar]
- 192.Orton R.W., Kinsey C.T., McBrayer L.D. Mite load predicts the quality of sexual color and locomotor performance in a sexually dichromatic lizard. Ecol. Evol. 2020;10:3152–3163. doi: 10.1002/ece3.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McInnis A., Root K., Rizer C., Bradley D. Diagnosing a crisis: The conundrum of alpha-gal. J. Nurse Pract. 2021;17:1081–1084. doi: 10.1016/j.nurpra.2021.05.022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.