Abstract
A murine monoclonal antibody directed against Borrelia burgdorferi B31 outer surface protein C (OspC) antigen was generated by a method whereby borreliae were inoculated into the mouse via the natural transmission mode of tick feeding. Passive immunization with this antibody resulted in protection of C3H/HeJ and outbred mice from a tick-transmitted challenge infection. Immunofluorescence staining of borrelia cells indicated surface exposure of the OspC epitope reactive with the monoclonal antibody.
The outer surface protein C (OspC) of Borrelia burgdorferi, the causative agent of Lyme disease, elicits a protective immune response against infection in animals which have been actively immunized with the recombinant antigen (4, 9, 10). Anti-OspC activity in polyclonal serum samples from B. burgdorferi-infected, immune hosts has been implicated in protection (6, 7). Also, a polyclonal, monospecific anti-recombinant OspC has shown therapeutic properties when passively administered to chronically infected subjects (14). To our knowledge, however, no monoclonal antibodies (MAbs) against OspC have been documented to demonstrate protection upon passive transfer. This laboratory generated a panel of MAbs derived from a tick-borne B. burgdorferi antigen introduction method, rather than using an inoculum of borrelial antigens obtained from in vitro-cultivated organisms (5). One of these MAbs was directed against OspC. The present study was performed to determine if this anti-OspC MAb would passively protect experimental mice.
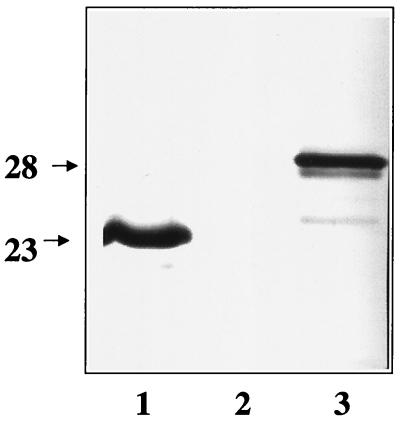
The generation of the MAb panel has been described elsewhere (5). Briefly, B. burgdorferi-infected nymphal ticks were allowed to feed on 8- to 12-week-old female BALB/cByJ mice. Infection of the mice with B. burgdorferi was confirmed by positive cultures derived from ear biopsy specimens (12). The mice were reinfested with B. burgdorferi-infected ticks 1 month later. At the end of this immunization schedule, serum samples were analyzed by enzyme-linked immunosorbent assay (ELISA) against B. burgdorferi whole-cell lysate, and the mouse with the highest titer was selected for hybridoma production. Three days before the cell fusion procedure, 105 strain B31 low-passage-number organisms (passage 1, cultured from ticks) were injected intravenously. This boost served to enhance existing, primed B-cell polyclonal populations prior to spleen harvesting, but only those populations common to antigens expressed in both cultured and tick-transmitted B. burgdorferi. Spleen cells were harvested and fused with cP3x63-Ag8.653 myeloma cells by use of polyethylene glycol 1000. The spleen cell/myeloma cell ratio was approximately 5:1. Fused cells were selected by using medium containing hypoxanthine-aminopterin-thymidine. Wells were screened by ELISA and Western blotting with low-passage-number B. burgdorferi B31 as the antigen. Cells from positive wells were expanded and cloned by limited dilution. The OspC specificity of one of the MAbs was determined by its immunoblot reactivity against recombinant OspC, and it was designated B5 (Fig. 1). MAb B5 was isotyped as an immunoglobulin G2a (IgG2a).
FIG. 1.
Western blot demonstrating MAb B5 reactivity against OspC antigens. Lanes: 1, B. burgdorferi B31 lysate; 2, lysate from Escherichia coli harboring the plasmid expression vector pBluescript (Stratagene, La Jolla, Calif.) only; 3, E. coli lysate expressing OspC from pBluescript. Molecular mass markers, in kilodaltons, are indicated on the left. The recombinant OspC migrates higher than the B. burgdorferi OspC due to the addition of a vector-encoded fusion partner. MAb B5 was used at a dilution of 1:5,000.
Anti-OspC IgG was purified from ascitic fluid by ammonium sulfate precipitation. Groups of test mice were injected intravenously with 100 μl containing either 200 to 300 μg of the anti-OspC antibody or the same amount of normal mouse IgG 1 day prior to tick infestation. Inbred mice (C3H/HeJ) and outbred mice (specific-pathogen-free mice maintained at the Division of Vector-Borne Infectious Diseases) were used in this study. One day following passive transfer of the antibody, each mouse was infested with 10 B. burgdorferi-infected nymphal Ixodes scapularis ticks, which were allowed to feed to repletion. The B31 strain-infected tick colony has been described previously (8). To assay for infectivity, ear skin biopsy specimens were cultured 4 weeks post-tick feeding as described previously (12); also, serological bleedings were taken between 2 and 4 weeks after tick drop-off and samples were assayed by Western blotting. One positive-control mouse was passively immunized with a polyclonal anti-OspA antibody, which was known to be protective (2, 3).
All mice passively immunized with the anti-OspC MAb were protected from infection (11 of 11), whereas each mouse inoculated with control antibody was not protected (Table 1). Following feeding, replete ticks were randomly collected from the OspC-immunized inbred mice and were surface sterilized (by serial washes in 70% ethanol for 2 min, 3% hydrogen peroxide for 2 min, and sterile water for 2 min), crushed, and inoculated into Barbour-Stoenner-Kelley modified culture medium (BSK II medium). Seven of nine ticks yielded viable borreliae in culture, reflecting the 70 to 80% infection rate of the tick colony. This result ensured that the ticks placed upon the mice were indeed harboring B. burgdorferi. It also corroborated a result previously seen in this laboratory, that a population of borreliae survives within the tick following feeding on OspC-immunized mice (4), unlike borreliae in ticks that feed on OspA-immunized mice (3).
TABLE 1.
Results of infectivity assay 4 weeks postchallenge
| Antibodya and mouse strain | No. of mouse samples positive for infection/no. testedb | % Protection |
|---|---|---|
| Normal mouse IgG | ||
| C3H/HeJ | 4/4 | 0 |
| Outbred | 7/7 | 0 |
| Anti-OspC MAb B5 IgG | ||
| C3H/HeJ | 0/4 | 100 |
| Outbred | 0/7 | 100 |
Antibody used for passive immunization.
Two assays, ear biopsy culture and Western blotting, were used, with identical results.
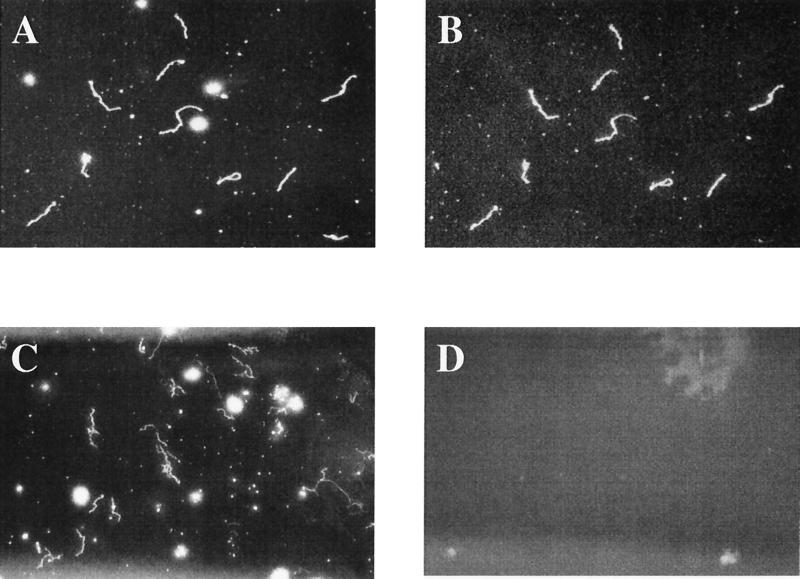
To assess the cell surface accessibility of the antibody, indirect immunofluorescence was performed on cultured B31 organisms as follows. Five microliters of a stationary-phase borrelial culture grown in BSK II medium was allowed to air dry on a microscope slide. The cells were heat fixed by a slight warming of the slide but were not acetone or methanol fixed. The organisms were overlaid with a 1:100 dilution of the anti-OspC B5 antibody and incubated for at least 1 h at room temperature. Following the primary incubation, the slide was washed three times in phosphate-buffered saline (PBS) and allowed to air dry, followed by a secondary incubation with a 1:50 dilution of fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) for 1 h at room temperature. The slide was washed three times with PBS, allowed to air dry, and overlaid with mounting medium (Kirkegaard & Perry Laboratories) and a coverslip. The finished slide was viewed under a dark-field microscope, and by epifluorescence with a fluorescein filter, at magnification of ×100 and ×400. The nonfixed borreliae fluoresced, indicating surface exposure of the OspC epitope reactive with this antibody (Fig. 2B). In addition, an indirect immunofluorescence assay using a MAb directed against flagellin, a non-surface-exposed antigen, gave a negative signal when nonfixed B. burgdorferi were used (data not shown). Normal mouse serum failed to cause the borrelia cells to fluoresce (Fig. 2D). Surface exposure of OspC epitopes in some B. burgdorferi strains has been speculated to correlate with protective capability (1), and the surface accessibility of the OspC epitope reactive with MAb B5 shown in Fig. 2B is consistent with that observation.
FIG. 2.
Indirect immunofluorescence staining of B. burgdorferi B31 cultured cells labeled with anti-OspC MAb B5 or normal mouse serum. (A) Representative field under dark-field microscopy; (B) the same field as in panel A labeled with anti-OspC MAb B5; (C) a second representative field under dark-field microscopy; (D) the same field as in panel C labeled with normal mouse serum. Magnification, ×400.
This study has demonstrated the protective efficacy of a MAb directed against the B. burgdorferi B31 OspC antigen. Because the protective properties of active immunization with OspC, and the fact that OspC expression is upregulated on the borrelial surface during tick feeding (11), have been well documented it is logical that a MAb generated by antigen inoculation via the natural route of tick bites would mirror the protective ability of the immunogen. Unexplained differences in the therapeutic effects of passively transferred and actively induced anti-OspC antibodies have been observed (13, 14). This MAb could be used to examine whether recognition of different epitopes is required for protection versus therapeutic clearance of infection.
Acknowledgments
We gratefully acknowledge the contributions of Rendi Murphree, Sarah Sullivan, and Steve Sviat. We express our thanks to Marc Dolan and Joe Piesman for providing ticks.
REFERENCES
- 1.Bockenstedt L K, Hodzic E, Feng S, Bourrel K W, de Silva A, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific Osp C-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fikrig E, Telford III S R, Barthold S W, Kantor F S, Spielman A, Flavell R A. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci USA. 1992;89:5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmore R D, Jr, Mbow M L. A monoclonal antibody generated by antigen inoculation via tick bite is reactive to the Borrelia burgdorferi Rev protein, a member of the 2.9 gene family locus. Infect Immun. 1998;66:980–986. doi: 10.1128/iai.66.3.980-986.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes C A, Engstrom S M, Coleman L A, Kodner C B, Johnson R C. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect Immun. 1993;61:5115–5122. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtti T J, Munderloh U G, Hughes C A, Engstrom S M, Johnson R C. Resistance to tick-borne spirochete challenge induced by Borrelia burgdorferi strains that differ in expression of outer surface proteins. Infect Immun. 1996;64:4148–4153. doi: 10.1128/iai.64.10.4148-4153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piesman J. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 1993;30:199–203. doi: 10.1093/jmedent/30.1.199. [DOI] [PubMed] [Google Scholar]
- 9.Preac-Mursic V, Wilske B, Patsouris E, Jauris S, Will G, Soutschek E, Rainhardt S, Lehnert G, Klockmann U, Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 10.Probert W S, LeFebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinsky R J, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong W, Gern L, Stehle T, Museteanu C, Kramer M, Wallich R, Simon M M. Resolution of experimental and tick-borne Borrelia burgdorferi infection in mice by passive, but not active immunization using recombinant OspC. Eur J Immunol. 1999;29:946–957. doi: 10.1002/(SICI)1521-4141(199903)29:03<946::AID-IMMU946>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 14.Zhong W, Stehle T, Museteanu C, Siebers A, Gern L, Kramer M, Wallich R, Simon M M. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc Natl Acad Sci USA. 1997;94:12533–12538. doi: 10.1073/pnas.94.23.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]