Abstract
Objective
This study investigated the effects of circadian misalignment (CM), induced by delaying meal times, independent of sleep timing and duration and eating window duration, on energy expenditure (EE), respiratory quotient (RQ), and substrate oxidation.
Methods
Healthy adults, age 20-49 y, participated in this randomized, crossover study under controlled feeding conditions. Eating window duration was identical in both conditions (CA: 0900-1900 h; CM: 1300-2300 h) and bedtimes were constant (2330-0800 h). EE, RQ, and substrate oxidation were obtained over 23 h in a metabolic chamber on days 3-4 and 14-15 in each condition. Twenty-four hour and post-meal outcomes were analyzed using a linear mixed effects model including condition, day, and day-by-condition interaction as main predictors, and sex as a covariate.
Results
Three men and four women (age 37.4±8.8 y, BMI 30.4±3.3 kg/m2) completed the study. Twenty-four-hour EE did not differ between conditions. Post-meal RQ for dinner and snack were higher in CM vs CA (both p<0.001) with correspondingly higher glucose oxidation (both p<0.01) and lower fat oxidation (dinner only p=0.0001).
Conclusions
CM, induced by delaying mealtimes by 4 h relative to CA, independently shifts nutrient metabolism towards greater carbohydrate and lower fat oxidation.
Keywords: circadian misalignment, meal timing, energy expenditure, respiratory quotient, substrate oxidation
INTRODUCTION
Eating patterns of U.S. adults have shifted towards breakfast skipping and later meal times over the past 40 y (1, 2). These eating behaviors can cause circadian misalignment (CM) (3), due to their discordance with natural day and night environmental cues that influence the central circadian clock (4). Disruptions in clock-regulated pathways resulting in CM, such as social and eating jetlag, are associated with obesity (3, 5–7). Accordingly, late meal timing is associated with greater body fat, body mass index (BMI), and odds of obesity (8–14). The observed increase in obesity risk with late meal consumption is hypothesized to occur through alterations in energy balance (3), however, the mechanisms are unclear. Experimental studies show that late meal timing results in metabolic dysfunction (15–17), while earlier meal timing improves energy metabolism (18, 19). Thus, changes in energy metabolism may be implicated in the increased obesity risk associated with late meal timing.
Energy metabolism follows circadian rhythms (20, 21), but studies investigating the influence of meal timing on energy metabolism show mixed results (15, 16, 18, 19, 22, 23). Simulated CM, induced by food intake during the biological night, has been shown to reduce 24-h energy expenditure (EE) (24). However, other modalities of simulated CM such as breakfast skipping (22), late evening meal consumption (15), and early time-restricted feeding (18) do not appear to affect 24-h EE. On the other hand, other research has demonstrated that consolidating meals to early in the day increases fat oxidation relative to an eating span lasting into the evening hours (18). Under controlled eating schedules, delaying the eating period to later times of the day decreases fat oxidation relative to similar eating periods occurring earlier in the day (19). Late dinner consumption has also been shown to decrease fat oxidation (17), although this has not been universally observed (15). These discrepancies among findings may be due to the following methodological differences which are known to influence metabolism: failure to standardize energy intakes, sleep duration, or eating window duration between conditions, factors which are known to influence energy metabolism.
To address the limitations highlighted above, we conducted a randomized controlled trial to investigate the effects of CM, induced by delaying meal times relative to awakening, on energy metabolism under controlled feeding and sleep conditions. The duration of the eating window was held constant in each study condition. We hypothesized that CM, induced by delaying meals by 5 h relative to wake time, would decrease EE and increase RQ relative to circadian alignment (CA), when the first meal was consumed within 1 h of awakening.
METHODS
Participants
Participants were recruited through internet and local advertisements (January 2019-March 2020). Eligible participants were men and premenopausal women, age 20-49 y, with a BMI of 25-34.9 kg/m2, who regularly slept 7-9 h/night and ate within 1 h of awakening at least 5 times per week. Participants were ineligible if they had more than minimal (0-13) or mild (14–19) depression based on the Beck Depression Inventory II (25), a high risk of obstructive sleep apnea on the Berlin Questionannire (26), a score ≥10 on the Epworth Sleepiness Scale (27), were either extreme evening (<31), or extreme morning (≥70) chronotypes on the Morningness and Eveningness in Human Circadian Rhythms Questionnaire (28), and had poor sleep quality (global score >5 on the Pittsburgh Sleep Quality Index) (29). Medical and health history questionnaires, the Sleep Disorder Inventory (30), and Three Factor Eating Questionnaire (31) were administered to exclude individuals with sleep, psychiatric, eating or any other medical disorders. Individuals with diabetes, taking beta blockers, excessive caffeine intake (32) current or past smoking history (<3 y), or recent participation in a weight loss program or weight change (>3%) in the last 3 mo were also excluded.
Sleep duration was ascertained during a 2-wk screening period using wrist actigraphy (Actigraph GT3X, ActiGraph LLC, Pensacola, FL). Participants were required to meet the following sleep criteria: 7 h of sleep at least 10/14 nights, less than 4 nights of sleep <6 h, and an average midpoint of sleep earlier than 0400 h. This study was approved by the Institutional Review Board at Columbia University Irving Medical Center (New York, NY) and registered on clinicaltrials.gov (NCT03663530). All participants were given the opportunity to ask questions about the protocol prior to providing informed consent.
Study Design
This was a randomized crossover study consisting of two 44-day study conditions separated by 4-6 wk washout. Each condition consisted of 15 days of controlled feeding with two inpatient assessments followed by 4 wk of ad libitum feeding. Both conditions were identical in sleep opportunity (8.5 h) and timing (2330-0800 h), as well as duration of eating window (10 h). The study conditions only differed in the timing of the eating window relative to sleep. In the circadian alignment (CA) condition, the eating window started 1 h after awaking. In the circadian misalignment (CM) condition, the eating window started 5 h after awakening. The present analyses focus on data obtained from days 3 and 14 of the 15-day controlled feeding segment, which were performed in the inpatient setting of a metabolic chamber, to assess both acute and longer-term effects of alignment status under fixed diet and sleep settings.
Two crossover sequences of the intervention conditions were block randomized to the participants in a 1:1 ratio, stratified by sex, using the random codes generated in R package by the study statistician (BC). Upon successful screening, the sequence of intervention condition was revealed. Participants were notified of their intervention condition (CA vs CM) at the first visit. Half of the participants underwent CA first and CM second; the other half underwent CM first and CA second.
Intervention
Days 3 and 14 of the the 15-day controlled feeding period consisted of inpatient stays at Columbia University Irving Medical Center, during which participants were provided calorically-tailored meals according to their individual energy requirements (33) tailored to their activity level with activity factor of 1.2 (sedentary; little to no exercise and desk job), 1.375 (lightly active; light exercise or sports 1-3 days a week), 1.55 (moderately active; moderate exercise or sports 3-5 days a week), 1.725 (very active; hard exercise or sports 6-7 days a week), or 1.9 (extremely active; hard daily exercise or sports and physical job). The macronutrient composition of the diet consisted of 30% of energy from fat, 54% from carbohydrates, and 16% from protein. The energy distribution across meals was 30% for breakfast, 30% for lunch, 30% for dinner, and 10% for snack. During CA, participants were instructed to consume breakfast, lunch, and dinner at 0900, 1300, and 1800 h, respectively, and a snack at 1900 h. During CM, corresponding meals and snack were consumed at 1300, 1700, 2200, and 2300 h. Meal times were modeled on data from normal and late sleepers from Baron et al. (8). Participants were asked to eat within 15 min of their prescribed meal times. Adherence was monitored via self-reported logs recording times of consumption throughout the 15-day controlled feeding period. During chamber stays, participants consumed the foods exactly at the scheduled times, with a reminder and verification from the research assistant.
Sleep
Sleep duration and timing were monitored using a triaxial accelerometer (Actigraph GT3X+) worn on the non-dominant wrist in conjunction with sleep diaries. For the week prior to the start of each study condition, participants were asked to follow assigned bedtimes between 2230-0000 h and wake times between 0700-0900 h daily based on average bedtimes and wake times from the 2-wk actigraphy screening and sleep diaries. Bed and wake times were fixed to the average bedtime and wake time assessed during screening to ensure similar time in bed for both study conditions. Wake time was no later than 0800 h. Adherence to daily sleep schedules was monitored weekly by actigraphy throughout each study condition and for the 2 wk during washout. During inpatient stays in the metabolic chamber on days 3 and 14, lights off was at 2330 h and lights on was at 0800 h. Participants remained in dim light conditions from 1700 h until lights off and wore blue-blocking lenses.
Energy expenditure, respiratory quotient, and substrate oxidation
EE and substrate oxidation were measured via indirect calorimetry in a pull through whole-room calorimetry metabolic chamber on days 3-4 and 14-15 in each study condition. The 23-h assessments began at 1600 h and ended at 1500 h the following day. The metabolic chamber at Columbia University Irving Medical Center consists of an air-tight, temperature controlled room with a pre-specified flow rate of 100 L/min and has an internal volume of 7,552 L. The rates of respiratory gases are analyzed using fuel cell oxygen and near infared carbon dioxide sensors (Model GA-3m2, Sable Systems International, Las Vegas, NV). Other features of the Sable Systems equipment includes subsampling, internal temperature control, baropmetric pressure compensations, background baselining and software controlled automate drift correction. All data are recorded and processed online by the manufacturer’s software Caloscreen and Expedata (Sable Systems International, Las Vegas NV). Prior to each data collection, a calibration and equilibration was perfomed and quarterly quality control consisted of n=10 propane burns to ensure sensor and room stability. EE, RQ, glucose oxidation (GluOx), and fat oxidation (FatOx) were calculated from minute-by-minute measurements of VO2 consumption and VCO2 production (34, 35). Measured rates of whole body VO2 and VCO2 were used to estimate disappearance rates (g/min) via oxidation for carbohydrate and lipid substrates. Protein oxidation (ProOx) rate was estimated at 66 mg/min based on previously published urinary urea excretion measurements made on 12 h post-absorptive men with normal carbohydrate reserves (36). The following formulas were used to estimate GluOx (37) and FatOx (38): Resting stoichiometric equations: GluOx = −3.226 VO2 + 4.585 VCO2 − 0.461 ProOx; FatOx = 1.695 VO2 − 1.701 VCO2 − 0.319 ProOx.
Available 23-h metabolic chamber data from each participant were compiled to investigate EE, RQ, and substrate oxidation outcomes at defined time intervals for 24-h, overnight, and post-meal periods. Twenty-four-hour data were collected as the average of minute-by-minute data over the 23-h chamber period multiplied by 1440 min. For the overnight period, minute-by-minute data were collected and averaged into 30-min bins. The overnight period was anchored at the end of the snack period for CA and thus spanned from 1930 to 0830 h. The same overnight period was used for CM. Post-meal EE was measured after breakfast, lunch, dinner, and snack. To ensure that the post-meal period was of equal duration in both conditions, average of 2-h data was available for breakfast and lunch, 1 h for dinner, and 30 min for post-dinner snack. Post-meal data for meals that anchored the eating window, i.e., breakfast, dinner, and snack, were our primary outcomes.
Power determination
The current analyses are part of a larger study related to body composition. Power and sample size were determined based on the primary hypothesis tests being based on Student’s t-test for outcomes of body weight and EE for which a sample size of ≥36 participants was calculated. A non-centrality parameter of 2.8839 is required for 80% power at the overall two-tailed 0.05 significance level. The non-centrality parameter for the treatment effect in a two-arm, two-period crossover study is given by the square root of the total sample size times the true treatment effect divided by the standard deviation (SD) of the pairwise differences. For the outcome of EE, we note that McHill et al. (24) found the SD of such pairwise differences was 15.8 kcal. Assuming similar SDs in our study, the detectable differences due to circadian misalignment in EE, was expected at 7.59 kcal with a full sample of ≥36 participants for the completers-only analysis.
Statistical analysis
Due to the nature of the study, neither study participants nor research staff were blinded to intervention condition (CA vs CM). However, the statistician performing the analyses (BC) was blind to intervention condition. A whole-group analysis for each outcome was conducted using a linear mixed effects model including condition, day, and day-by-condition interaction as predictor variables, and sex as a covariate. Random intercept effects were included, which correspond to compound symmetric correlation. The order in which conditions were administered was included in initial models and removed if not significant. Participant ID was included as a random effect variable. Outcomes included 24-h, overnight, and post-meal EE, RQ, and substrate oxidation. These analyses were performed by using Statistical Analysis Software (SAS) Version 9.4. Statistical significance was considered as p<0.05. Data are presented as raw means ± SD.
RESULTS
Participant characteristics
Of 8 participants accrued, 7 completed both study conditions (age 37.4 ± 8.8 y, BMI 30.4 ± 3.3 kg/m2) (Table 1). One participant voluntarily withdrew from the study during the second chamber stay during their first study intervention (CM) and was excluded from our analyses (Figure S1). Prior to entering the study, participants consumed breakfast at 0816 ± 1.4 h, lunch at 1319 ± 1.2 h, dinner at 1923 ± 1.3 h, and snack at 1430 ± 3.7 h. Their average eating window was 11.7 ± 1.6 h. Total sleep time during screening was 426 ± 64 min (Table 2).
Table 1.
Participant characteristics
| Characteristic | Male (n=3) | Female (n=5) | Total (n=8) |
|---|---|---|---|
| Age (y) | 30.7 ± 9.9 | 41.2 ± 3.8 | 37.3 ± 8.1 |
| Height (cm) | 173.6 ± 3.9 | 160.8 ± 5.3 | 165.6 ± 8.0 |
| Weight (kg) | 88.1 ± 10.9 | 80.7 ± 11.1 | 83.5 ± 10.9 |
| BMI (kg/m2) | 29.3 ± 3.7 | 31.1 ± 2.7 | 30.4 ± 3.0 |
| Systolic blood pressure (mmHg) | 119 ± 9 | 125 ± 4 | 121 ± 7 |
| Diastolic blood pressure (mmHg) | 86 ± 11 | 84 ± 3 | 85 ± 9 |
| Black or African American | 1 | 1 | 2 |
| White | 1 | 2 | 3 |
| Other | 1 | 2 | 3 |
| Hispanic or Latin | 3 | 2 | 5 |
Data presented as means ± SD and frequencies.
Table 2.
Sleep duration measured by actigraphy throughout the study.
| Actigraphy | Total sleep time (min/day) |
|---|---|
| Screening | 426 ± 64 |
| Pre-visit | 473 ± 43 |
| Circadian Alignment | 439 ± 65 |
| Circadian Misalignment | 428 ± 74 |
Data presented as means ± SD. Pre-visit data are average of 1 wk prior to each intervention condition when participants were asked to follow bedtimes between 2230-0000 h and wake times between 0700-0900 h. Data for circadian alignment and circadian misalignment are averages of the 15-d controlled feeding period.
Compliance with study conditions
During the study, average eating window was 10.0 ± 1.0 h in CA and 10.0 ± 0.3 h in CM (p=0.43). Adherence to scheduling of the first meal and last meal in CA was 90.2% and 80.0%, respectively. Adherence to the scheduling of the first meal and last meal in CM was 90.2% and 94.5%, respectively. No adverse events were reported. Weight (85.2 ± 10.9 vs. 85.7 ± 11.7 kg; p=0.94), and BMI (30.7 ± 2.9 vs. 30.9 ± 3.8 kg/m2; p=0.91) over the 15-day period did not differ between CA and CM. There were no differences in weight between day 3 and day 14 within CA (85.2 ± 10.7 vs. 85.1 ± 11.2 kg; p=0.61) or CM (85.8 ± 11.8 vs. 85.5 ± 11.7 kg; p=0.80).
Bedtimes were 2315 ± 1.4 h and waketimes were 0731 ± 1.1 h during the 15-day period in CA. Bedtimes and wake times were 2312 ± 1.1 and 0725 ± 0.9 h, respectively, in CM. There was no difference in actigraphy-measured total sleep time (439 ± 65 vs. 428 ± 74 min) between CA and CM (p=0.40) (Table 2).
Respiratory quotient and substrate oxidation
Twenty-four-hour
Twenty-four hour RQ was lower during CA relative to CM (Β=−0.040; p=0.02) with no effects of day (p=0.57) or day-by-condition interaction (p=0.37) (Table 3). Twenty-four hour GluOx was higher in CM relative to CA (Β=65.1; p=0.02) with no effect of day (p=0.59) or day-by-condition interaction (p=0.65). Twenty-four hour FatOx was lower in CM relative to CA (Β=29.4; p=0.002) with no effect of day (p=0.95) or day-by-condition interaction (p=0.38).
Table 3.
Energy expenditure and substrate oxidation measured in the metabolic chamber during circadian alignment and circadian misalignment.
| Circadian Alignment | Circadian Misalignment | P value | P value | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Day 3 | Day 14 | Day 3 | Day 14 | condition | day | day-by-condition | |
| 24-h | EE (kcal/day) | 2311±456.0 | 2229±326.2 | 2247±204.1 | 2228±248.3 | 0.56 | 0.26 | 0.47 |
| RQ | 0.78±0.03 | 0.79±0.04 | 0.83±0.05 | 0.81±0.05 | 0.002 | 0.57 | 0.37 | |
| GluOx (g/day) | 141±116 | 138±105 | 218±115 | 191±115 | 0.02 | 0.59 | 0.65 | |
| FatOx (g/day) | 136±25.5 | 129±28.9 | 99±38.7 | 107±41.3 | 0.002 | 0.95 | 0.38 | |
|
| ||||||||
| Overnight | EE (kcal/min) | 1.47±0.39 | 1.41±0.33 | 1.48±0.29 | 1.46±0.30 | 0.20 | 0.07 | 0.33 |
| RQ | 0.77±0.06 | 0.77±0.06 | 0.83±0.07 | 0.82±0.07 | <0.0001 | 0.007 | 0.19 | |
| GluOx (g/min) | 0.07±0.11 | 0.06±0.10 | 0.14±0.11 | 0.12±0.11 | <0.0001 | 0.01 | 0.70 | |
| FatOx (g/min) | 0.09±0.03 | 0.09±0.03 | 0.06±0.03 | 0.07±0.04 | <0.0001 | 0.21 | 0.14 | |
|
| ||||||||
| Breakfast | EE (kcal/min) | 1.81±0.28 | 1.66±0.30 | 1.73±0.18 | 1.79±0.25 | 0.07 | 0.36 | 0.03 |
| RQ | 0.81±0.04 | 0.79±0.03 | 0.83±0.05 | 0.82±0.02 | 0.06 | 0.31 | 0.96 | |
| GluOx (g/min) | 0.15±0.10 | 0.11±0.06 | 0.17±0.08 | 0.15±0.04 | 0.10 | 0.26 | 0.56 | |
| FatOx (g/min) | 0.10±0.02 | 0.10±0.02 | 0.08±0.02 | 0.09±0.03 | 0.21 | 0.51 | 0.48 | |
|
| ||||||||
| Lunch | EE (kcal/min) | 1.87±0.32 | 1.81±0.31 | 1.85±0.17 | 1.77±0.32 | 0.75 | 0.46 | 0.93 |
| RQ | 0.82±0.02 | 0.81±0.03 | 0.88±0.05 | 0.86±0.05 | 0.005 | 0.48 | 0.64 | |
| GluOx (g/min) | 0.16±0.07 | 0.16±0.23 | 0.35±0.23 | 0.24±0.12 | 0.02 | 0.27 | 0.35 | |
| FatOx (g/min) | 0.10±0.02 | 0.09±0.05 | 0.07±0.05 | 0.06±0.02 | 0.006 | 0.32 | 0.52 | |
|
| ||||||||
| Dinner | EE (kcal/min) | 1.92±0.40 | 1.81±0.33 | 1.81±0.25 | 1.75±0.23 | 0.22 | 0.24 | 0.77 |
| RQ | 0.79±0.04 | 0.82±0.06 | 0.86±0.08 | 0.87±0.08 | 0.0006 | 0.16 | 0.51 | |
| GluOx (g/min) | 0.14±0.10 | 0.18±0.12 | 0.23±0.13 | 0.24±0.13 | 0.004 | 0.31 | 0.54 | |
| FatOx (g/min) | 0.11±0.02 | 0.08±0.04 | 0.06±0.04 | 0.05±0.04 | 0.0002 | 0.047 | 0.37 | |
|
| ||||||||
| Snack | EE (kcal/min) | 1.90±0.53 | 1.79±0.37 | 1.93±0.29 | 1.88±0.18 | 0.59 | 0.45 | 0.79 |
| RQ | 0.82±0.03 | 0.84±0.06 | 0.90±0.06 | 0.88±0.05 | 0.0002 | 0.7 | 0.08 | |
| GluOx (g/min) | 0.18±0.11 | 0.22±0.12 | 0.31±0.11 | 0.28±0.11 | 0.006 | 0.88 | 0.22 | |
| FatOx (g/min) | 0.10±0.04 | 0.07±0.04 | 0.04±0.03 | 0.05±0.03 | 0.001 | 0.25 | 0.03 | |
Data are means ± SD, n=7. P values calculated with a linear mixed effects model testing condition (circadian alignment vs circadian misalignment), day (3 vs 14), and day-by-condition interaction as predictors with condition order as an adjusting covariate only when significant.
Abbreviations: EE, energy expenditure; FatOx, fat oxidation; GluOx, glucose oxidation; RQ, respiratory quotient.
Overnight period
Overnight RQ was higher in CM relative to CA (Β=0.056; p<0.0001) and on day 3 relative to day 14 (Β=0.010; p=0.007) (Figure 1, Table 3), with no effect of day-by-condition interaction (p=0.19). We observed main effects of condition (Β=0.070; p<0.0001) and day (Β=0.016; p=0.013) on overnight GluOx. Overnight GluOx was higher in CM relative to CA and on day 14 relative to day 3 with no effect of day-by-condition interaction (p=0.70). Overnight FatOx was generally lower in CM compared to CA (Β=−0.025; p<0.0001) with no effect of day (p=0.21) or day-by-condition interaction (p=0.14).
Figure 1.
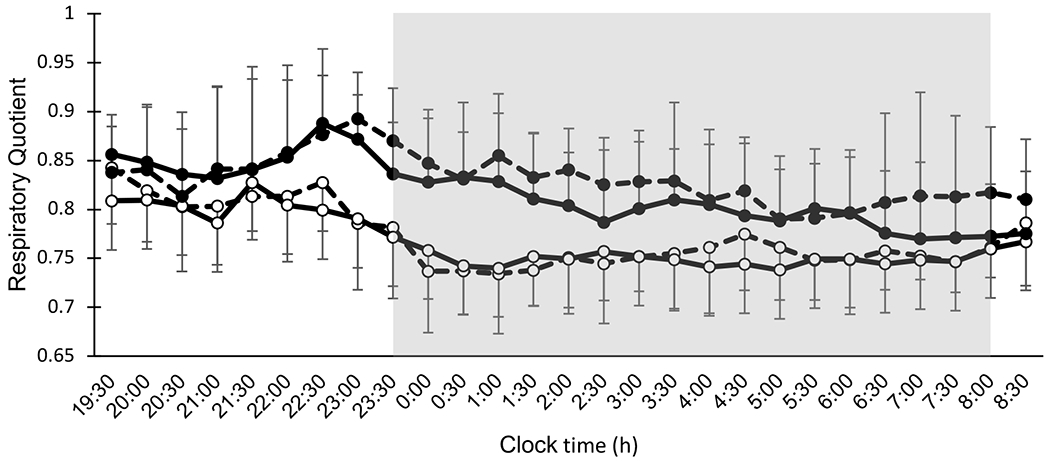
Thirteen-hour overnight respiratory quotient by whole-room indirect calorimetry (1930-0830 h) under circadian alignment (open circles) and circadian misalignment (filled circles). Data for d 3 and d 14 are indicated by dashed lines and solid lines, respectively. Shaded area represents sleep episode from 2330-0800 h. Dinner and snack were consumed at 2200 h and 2300 h, respectively, during circadian misalignment.
Breakfast
There were no effects of condition (Β=0.024; p=0.06), day (p=0.31) or day-by-condition interaction (p=0.96) on post-breakfast RQ (Table 3). There were no main effects of condition (p=0.10), day (p=0.26), or day-by-condition interaction (p=0.56) on post-breakfast GluOx. Similarly, there were no effects of condition (p=0.21), day (p=0.51), or day-by-condition interaction (p=0.48) on post-breakfast FatOx.
Lunch
Post-lunch RQ was lower in CA relative to CM (B=−0.050; p=0.005) with no effects of day (p=0.48) or day-by-condition interaction (p=0.64) (Table 3). Post-lunch GluOx was lower in CA relative to CM (B=−0.131; p=0.02) with no effect of day (p=0.27) or day-by-condition interaction (p=0.35). Post-lunch FatOx was higher in CA relative to CM (B=0.029; p=0.006) with no effect of day (p=0.32) or day-by-condition interaction (p=0.52).
Dinner
Post-dinner RQ was lower in CA relative to CM (Β=−0.058; p=0.0006) with no effects of day (p=0.16) or day-by-condition interaction (p=0.51) (Table 3). Post-dinner GluOx was lower in CA relative to CM (Β=−0.078; p=0.002) with no effect of day (p=0.29) or day-by-condition interaction (p=0.54). Post-dinner FatOx was higher in CA relative to CM (Β=0.040; p=0.0001) and higher on day 3 than day 14 (Β=0.018; p=0.04) with no effect of day-by-condition interaction (p=0.32).
Snack
Post-snack RQ was lower in CA relative to CM (Β=−0.059; p=0.0002). There was no effect of day (p=0.75) or day-by-condition interaction (p=0.08) (Table 3). Post-snack GluOx was lower in CA relative to CM (Β=−0.10; p=0.004) but did not differ by day (p=0.88) nor by day-by-condition interaction (p=0.22). Post-snack FatOx was higher in CA relative to CM (Β=0.05; p=0.002) on day 3, with no effect of day (Type 3 test: p=0.25). However, there was a significant effect of day-by-condition interaction (p=0.03) whereby post-snack FatOx increased over time in CA relative to CM.
Energy expenditure
Twenty-four-hour
There were no main effects of condition (p=0.56), day (p=0.26), or day-by-condition interaction (p=0.47) on 24-h EE (Table 3).
Overnight
There were no main effects of condition (p=0.20), day (p=0.07), or day-by-condition interaction (p=0.33) on overnight EE (Figure 2,Table 3).
Figure 2.
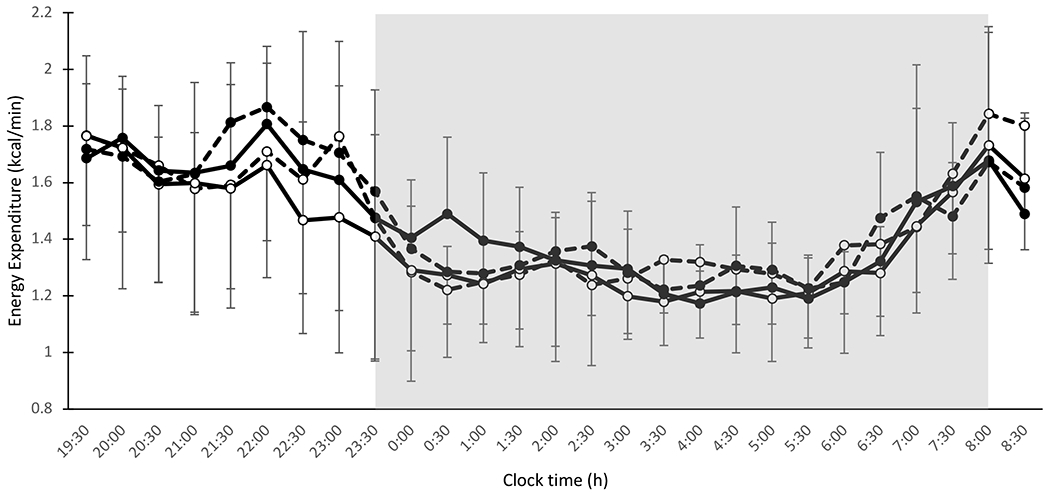
Thirteen-hour energy expenditure (kcal/min) by whole-room indirect calorimetry (19:30-0830 h) under circadian alignment (open circles) and circadian misalignment (filled circles). Data for d 3 and d 14 are indicated by dashed lines and solid lines, respectively. Shaded area represents sleep episode from 2330-0800 h. Dinner and snack were consumed at 2200 h and 2300 h, respectively, during circadian misalignment.
Breakfast
There was a significant day-by-condition interaction on EE (Β=0.22; p=0.03) (Table 3). Post-breakfast EE decreased over time in CA (B=−0.16; p=0.04) but there was no significant change over time in CM (p=0.43).
Lunch
There were no main effects of condition (p=0.75), day (p=0.46), or day-by-condition interaction (p=0.93) on post-lunch EE (Table 3).
Dinner
There were no main effects of condition (p=0.22), day (p=0.24), or day-by-condition interaction (p=0.77) on post-dinner EE (Table 3).
Snack
There were no main effects of condition (p=0.59), day (p=0.45), or day-by-condition interaction (p=0.79) on post-snack EE (Table 3).
DISCUSSION
Our results show that inducing CM by delaying meal times by 4 h relative to CA leads to preferential glucose utilization over fat. This is most marked after late-day meals (lunch, dinner and snack) and overnight. Importantly, our findings were observed in the context of identical food intake, served over the same length of time with equal inter-meal intervals and fasting periods. These findings suggest that meal timing has an independent impact on EE and substrate utilization.
Respiratory quotient and substrate oxidation
Our main observation was that CM increased RQ relative to CA under controlled feeding conditions. Similarly, a study by Allison et al. with a comparable eating window demonstrated that resting state RQ was higher in a delayed eating schedule compared to a daytime eating schedule (19). We further showed that a higher RQ as a result of delayed meal times was prolonged over a 24-h period. Ravussin and colleagues also found that early time-restricted feeding (0800-1400 h) increased FatOx compared to a control eating schedule spread across the day (0800-2000 h) (18). A trial by Gu et al. (17) comparing the effects of late dinner and routine dinner on postprandial metabolic parameters found that dinner consumption at 2200 h reduced postprandial FatOx relative to 1800 h dinner. By reversing the timing of the larger eating occasion, they demonstrated that a dinner consumed 4 h later in an isocaloric setting directly alters lipid metabolism (17). Further, we observed similar results under controlled energy intake on FatOx in the context of delaying meal times. However, in contrast to our study, Sato et al. (15) demonstrated that acute late evening meal consumption at 2230 h increased overall fat oxidation relative to an earlier evening meal consumed at 1900 h. They also observed a reduction in FatOx during sleep in the late evening meal condition (15), consistent with our observations of increased RQ in response to CM during the overnight period. Sato and colleagues delayed only the evening meal by 3.5 h (15) whereas we delayed all meals. It is possible that we observed a sustained reduction in FatOx as a result of this overall delayed pattern of intake. These studies, along with our current findings, imply that later meal timing directly influences metabolic responses to meals, reducing reliance on fat as a metabolic fuel.
Energy expenditure
We observed no differences in 24-h EE (Table 3) or overnight EE between CA and CM. In the context of overall EE, our observations are consistent with studies showing that meal timing does not affect 24-h EE (15, 18), total EE (23), or resting EE (18, 23). In contrast, a study by McHill et al. (24) demonstrated that CM, induced within a simulated shift-work protocol with controlled energy intake involving late-night meal times, reduced 24-h EE. However, circadian variation in EE (20, 39) has been shown to follow endogenous rhythms rather than behavioral influences (40). Accordingly, our study showed that shifting meal times as an intervention did not affect 24-h EE for the whole group. Taken altogether, our observations suggest that delaying meal times does not significantly impact overall EE.
Similarly, we observed no main effect of condition on post-meal EE. Interestingly, we observed significant day-by-condition interaction on post-breakfast EE, which decreased over time in CA. Consistent with our main effect observations, a study by Bandin et al. (16) comparing early and delayed eating conditions with fixed breakfast and dinner times observed no effect of delaying lunch consumption by 3.5 h on postprandial EE. In contrast to our study, Ravussin and colleagues (18) demonstrated that early time-restricted feeding increased EE following lunch and dinner consumption relative to a 12-h control eating window. However, previous studies (15, 16, 18) report acute effects of meal timing on post-prandial EE, whereas we observed differences over 15 days. Whether these changes persist over a longer period is unknown and studies extending this intervention beyond 2 wk are needed. Overall, our study and others (15, 18, 23) have shown that overall EE is unaffected by meal timing.
Study limitations and strengths
This study had some limitations. First, our sample size was small and power may have been a limiting factor in detecting higher-order interactions. However, each participant contributed 2 observations per condition and we showed significant findings. However, our observations should be reproduced in future studies. Second, we did not have a direct measurement of ProOx. Ravussin and colleagues attributed differences in ProOx to increased gluconeogenesis due to a prolonged fasting window in early time-restricted feeding relative to the control schedule (18). However, in our study, participants maintained the same eating and fasting windows in both conditions. Protein oxidation was therefore unlikely to contribute to between-condition differences in RQ. Third, post-meal data intervals were relatively short, which may have been a limiting factor in characterizing the post-prandial state. Our observations from 7 participants may have generalizability issues, which was mitigated by having a diverse sample of individuals.
The experimental design of our study allowed the investigation of the independent effect of delaying meal times while addressing potential differences in sleep and energy intake that previous studies have noted (17–19). We ensured that sleep duration did not differ between conditions. Because outcomes were obtained under controlled feeding conditions, differences between conditions were not influenced by alterations in diet quantity and quality, which are known to influence EE and RQ, isolating the impact of timing of food consumption. Additionally, maintaining constant sleep, fasting duration, and eating window duration isolated the effect of delaying meal times on outcomes.
Conclusion
Finally, we report herein findings that CM increases RQ, independent of sleep timing and duration, indicating a shift in nutrient oxidation towards greater reliance on carbohydrates and lower reliance on fat. Over 24 h, the difference between conditions amounts to an average of ~29.5 g, or 265.5 kcal. These shifts in substrate oxidation are clinically meaningful and may have implications for weight management and energy metabolism, indicative of increased propensity for weight gain, particularly in the context of excess energy intake (41). These findings may provide further insight behind the increased obesity risk associated with shift work and late-night eating aside from diet quality, exercise, and sleep duration. Further studies are needed to investigate the long-term effects of CM on energy metabolism and weight management.
Supplementary Material
Study Importance.
What is already known?
Circadian misalignment (CM) as a result of late meal timing increases the risk of obesity, but the mechanisms are not fully understood.
Circadian variation in energy metabolism may be influenced by meal timing.
What do these findings add?
Under controlled feeding and sleep conditions, delaying meal times increased carbohydrate oxidation and reduced fat oxidation compared to earlier meal times.
Eating later in the day, independent of duration of eating window and sleep times, shifts nutrient metabolism towards reduced fat oxidation, which has implications for weight management.
How might these results change the direction of research or the focus of clinical practice?
These results may in part, explain the increased obesity risk associated with late meal timing.
ACKNOWLEDGMENTS
We would like to thank our study participants for their contributions to our research. Data described in the manuscript, code book, and analytic code will be made available upon request in writing to the corresponding author (MPSO).
Funding:
MPSO is funded in part by R01HL142648 and R35HL155670. This research was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1TR001873 and by the New York Nutrition Obesity Research Center grant P30DK026687-41. KJW was supported by T32 DK07559-31.
Footnotes
Registered on clinicaltrials.gov #NCT03663530
This study is registered at clinicaltrials.gov (NCT03663530)
REFERENCES
- 1.Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet 2015;115(1):50–63. doi: 10.1016/j.jand.2014.06.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 2015;22(5):789–98. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boege HL, Bhatti MZ, St-Onge MP. Circadian rhythms and meal timing: impact on energy balance and body weight. Curr Opin Biotechnol 2020;70:1–6. doi: 10.1016/j.copbio.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buijs FN, Leon-Mercado L, Guzman-Ruiz M, Guerrero-Vargas NN, Romo-Nava F, Buijs RM. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology (Bethesda) 2016;31(3):170–81. doi: 10.1152/physiol.00037.2015. [DOI] [PubMed] [Google Scholar]
- 5.Mazri FH, Manaf ZA, Shahar S, Mat Ludin AF, Karim NA, Hazwari NDD, Kek QW, Abdul Basir SM, Arifin A. Do Temporal Eating Patterns Differ in Healthy versus Unhealthy Overweight/Obese Individuals? Nutrients 2021;13(11). doi: 10.3390/nu13114121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeron-Rugerio MF, Hernaez A, Porras-Loaiza AP, Cambras T, Izquierdo-Pulido M. Eating Jet Lag: A Marker of the Variability in Meal Timing and Its Association with Body Mass Index. Nutrients 2019;11(12). doi: 10.3390/nu11122980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mota MC, Silva CM, Balieiro LCT, Goncalves BF, Fahmy WM, Crispim CA. Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PLoS One 2019;14(2):e0212126. doi: 10.1371/journal.pone.0212126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–81. doi: oby2011100 [pii] 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 9.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FA, Klerman EB. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr 2017;106(5):1213–9. doi: 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culnan E, Reid KJ, Zee PC, Crowley SJ, Baron KG. Meal timing relative to DLMO: Associations with BMI and body fat. Sleep Health 2021;7(3):339–44. doi: 10.1016/j.sleh.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas EA, Zaman A, Cornier MA, Catenacci VA, Tussey EJ, Grau L, Arbet J, Broussard JL, Rynders CA. Later Meal and Sleep Timing Predicts Higher Percent Body Fat. Nutrients 2020;13(1). doi: 10.3390/nu13010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet 2014;27 Suppl 2:255–62. doi: 10.1111/jhn.12141. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Q, Garaulet M, Scheer F. Meal timing and obesity: interactions with macronutrient intake and chronotype. Int J Obes (Lond) 2019;43(9):1701–11. doi: 10.1038/s41366-018-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K, American Heart Association Obesity Committee of the Council on L, Cardiometabolic H, Council on Cardiovascular Disease in the Y, et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017;135(9):e96–e121. doi: 10.1161/CIR.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato M, Nakamura K, Ogata H, Miyashita A, Nagasaka S, Omi N, Yamaguchi S, Hibi M, Umeda T, Nakaji S, et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes Res Clin Pract 2011;5(3):e169–266. doi: 10.1016/j.orcp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Bandin C, Scheer FA, Luque AJ, Avila-Gandia V, Zamora S, Madrid JA, Gomez-Abellan P, Garaulet M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. International journal of obesity 2015;39(5):828–33. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 17.Gu C, Brereton N, Schweitzer A, Cotter M, Duan D, Borsheim E, Wolfe RR, Pham LV, Polotsky VY, Jun JC. Metabolic Effects of Late Dinner in Healthy Volunteers-A Randomized Crossover Clinical Trial. J Clin Endocrinol Metab 2020;105(8). doi: 10.1210/clinem/dgaa354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity (Silver Spring) 2019;27(8):1244–54. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allison KC, Hopkins CM, Ruggieri M, Spaeth AM, Ahima RS, Zhang Z, Taylor DM, Goel N. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr Biol 2020. doi: 10.1016/j.cub.2020.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitting KM, Vujovic N, Yuan RK, Isherwood CM, Medina JE, Wang W, Buxton OM, Williams JS, Czeisler CA, Duffy JF. Human Resting Energy Expenditure Varies with Circadian Phase. Curr Biol 2018;28(22):3685–90 e3. doi: 10.1016/j.cub.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rynders CA, Morton SJ, Bessesen DH, Wright KP Jr., Broussard JL. Circadian Rhythm of Substrate Oxidation and Hormonal Regulators of Energy Balance. Obesity (Silver Spring) 2020;28 Suppl 1:S104–S13. doi: 10.1002/oby.22816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi F, Ogata H, Omi N, Nagasaka S, Yamaguchi S, Hibi M, Tokuyama K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes Res Clin Pract 2014;8(3):e201–98. doi: 10.1016/j.orcp.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE, et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, Wright KP Jr. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences of the United States of America 2014;111(48):17302–7. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131(7):485–91. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 1976;4(2):97–110. [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 30.Tractenberg RE, Singer CM, Cummings JL, Thal LJ. The Sleep Disorders Inventory: an instrument for studies of sleep disturbance in persons with Alzheimer’s disease. J Sleep Res 2003;12(4):331–7. doi: 10.1046/j.0962-1105.2003.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of psychosomatic research 1985;29(1):71–83. [DOI] [PubMed] [Google Scholar]
- 32.Landrum RE. College students’ use of caffeine and its relationship to personality. College Student Journal 1992;26(2):151–5. [Google Scholar]
- 33.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc 2005;105(5):775–89. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Kelly LP, Basset FA. Acute Normobaric Hypoxia Increases Post-exercise Lipid Oxidation in Healthy Males. Front Physiol 2017;8:293. doi: 10.3389/fphys.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haman F, Legault SR, Weber JM. Fuel selection during intense shivering in humans: EMG pattern reflects carbohydrate oxidation. J Physiol 2004;556(Pt 1):305–13. doi: 10.1113/jphysiol.2003.055152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol 1990;258(3 Pt 1):E399–412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- 38.Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci 1991;16(1):23–9. [PubMed] [Google Scholar]
- 39.Bo S, Fadda M, Castiglione A, Ciccone G, De Francesco A, Fedele D, Guggino A, Parasiliti Caprino M, Ferrara S, Vezio Boggio M, et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. International journal of obesity 2015;39(12):1689–95. doi: 10.1038/ijo.2015.138. [DOI] [PubMed] [Google Scholar]
- 40.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FA. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring) 2015;23(10):2053–8. doi: 10.1002/oby.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990;259(5 Pt 1):E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


