Abstract
Chlamydospore formation of the fungal pathogen Candida albicans was found to depend on the Efg1 protein, which regulates the yeast-hyphal transition. Isogenic mutants lacking EFG1 or encoding T206A and T206E variants did not differentiate chlamydospores, while cek1, cph1, or tpk2 mutations had no effect. Furthermore, filamentation of efg1 cph1 double mutants in microaerophilic conditions suggests a novel Efg1p/Cph1p-independent filamentation pathway in C. albicans.
Among Candida species, the human fungal pathogen Candida albicans has the special ability to form thick-walled cells, termed chlamydospores, in certain environmental conditions (18). Chlamydospores arise on elongated suspensor cells situated on pseudohyphae or hyphae. To efficiently induce chlamydospores, cells are distributed at low cell concentrations on nutrient-poor media, which optimally is supplemented with detergents, such as Tween 80, and incubated at a low temperature (25 to 30°C) for several days (12, 17). Chlamydospore formation is especially abundant on solid media under glass coverslips providing microaerophilic conditions (Dalmau inoculation technique). The presence of glucose inhibits chlamydospore formation, while nitrogen levels have no major influence (5). Light inhibits chlamydospore formation (6). Although chlamydospore formation is used routinely to differentiate C. albicans from other Candida species (only rare isolates of Candida tropicalis also form chlamydospores [11]), the molecular mechanisms leading to this form of cellular differentiation are unknown. Also, the functions of chlamydospores for the biology and possibly for the virulence of C. albicans are unclear. Although chlamydospores can be efficiently induced in vitro, they are rarely detected in vivo (3). Chlamydospores are considered dormant cellular forms, but because they rapidly loose viability, they do not appear to allow long-term survival (19).
Another striking feature of C. albicans is its ability to switch growth forms in certain environments, between a unicellular yeast and a filamentous multicellular form (dimorphism). Among Candida species, C. albicans has the strongest tendency to form hyphae, which parallels its role as the most virulent Candida species (18). C. albicans mutants defective in hyphal formation have almost completely lost their virulence (16). In recent years, two major signalling pathways leading to hyphal growth have been defined. One pathway leads via a conserved mitogen-activated protein (MAP) kinase cascade to phosphorylation and activation of the transcription factor Cph1p (13, 14). A second pathway includes the transcription factor Efg1p (23); recent evidence suggests that protein kinase A (PKA) is situated functionally upstream of Efg1p (22). The Efg1p pathway appears more important than the Cph1p pathway for hyphal morphogenesis, because efg1 mutants are unable to form hyphae in most inducing conditions, e.g., in the presence of serum or GlcNAc as well as during growth on Spider medium (8, 16, 23), while cph1 mutants show only a morphogenetic block on Spider medium (13, 14). efg1 mutants have a strongly reduced virulence in the mouse model of infection (16), while cph1 mutants essentially retain virulence (16); nevertheless, efg1 cph1 double mutants show even a further attenuation in virulence than the efg1 single mutant (16), suggesting a definite but minor contribution of Cph1p to virulence. The ability to undergo GlcNAc-induced hyphal formation is not a prerequisite for chlamydospore formation, since monomorphic yeast mutants can still differentiate chlamydospores (24).
efg1 mutants are defective in chlamydospore formation.
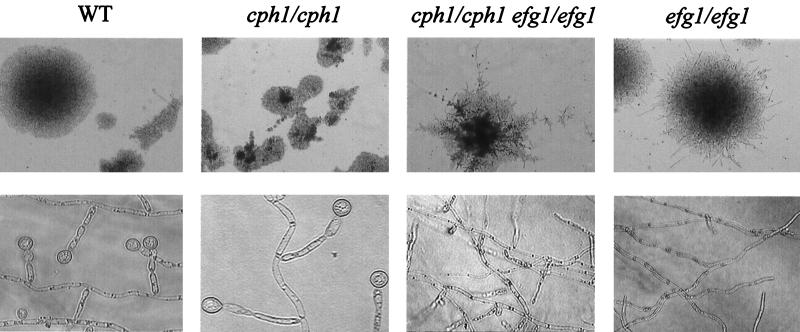
This study was performed to determine if members of signal transduction pathways leading to hyphal development also have a function in the formation of chlamydospores. Therefore, a wild-type strain, SC5314 (9), as well as an isogenic series of C. albicans strains derived from CAI4 (9) (containing a plasmid- or genomically borne URA3 gene to complement the ura3 mutation), was streaked out lightly on cornmeal agar (Difco)–0.33% Tween 80, covered by coverslips, and incubated at 25°C for 5 to 7 days. The strains and plasmids used in this study are listed in Table 1. Figure 1 shows that strain CAI4 (9) transformed with control plasmid pBI-1 (23) developed chlamydospores; the wild-type strain SC5314 had an identical phenotype (data not shown). Chlamydospores formed as terminal thick-walled cells on short side branches (suspensor cells) or as terminal cells on filaments. Similar to wild-type cells, a cph1 mutant (strain JKC19 [15]) lacking the Cph1p transcription factor activated by the Cek1p MAP kinase, as well as a tpk2 strain (22), which lacks an isoform of PKA, was competently able to form chlamydospores (data not shown). On the other hand, efg1/efg1 mutants, such as strain HLC67 (16) containing the control plasmid pBI-1 (Fig. 1) or strain HLC52 (16) (data not shown), were completely deficient for chlamydospore formation, although filamentous growth and occasionally short side branches resembling suspensor cells were detected. The double knockout strain HLC54 (efg1/efg1 cph1/cph1) (16) was as defective as strain HLC52 for chlamydospore formation. These results demonstrate that Efg1p is needed for chlamydospore formation. Because an efg1 mutant reconstituted with a single wild-type EFG1 gene (HLC74 [16]) was able to develop chlamydospores (data not shown), it appears that one functional allele for the EFG1 gene is sufficient for this morphogenetic process.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| C. albicans | ||
| SC5314 | Prototrophic | 9 |
| CAI4 | Δura3::imm434/Δura3::imm434 | 9 |
| HLC52 | Same as CAI4 but efg1::hisG/efg1::hisG-URA3-hisG | 16 |
| HLC67 | Same as CAI4 but efg1::hisG/efg1::hisG | 16 |
| HLC54 | Same as CAI4 but cph1::hisG/cph1::hisG efg1::hisG/efg1::hisG-URA3-hisG | 16 |
| HLC74 | HLC67 (EFG1) | 16 |
| JKC19 | Same as CAI4 but cph1::hisG/cph1::hisG-URA3-hisG | 16 |
| Plasmids | ||
| pBI-1 | URA3-marked control vector | 23 |
| pLJ19 | URA3-marked vector containing CEK1 | 4 |
| pCCa4 | URA3-marked vector containing CPH1 | 4 |
| pBI-HAHYD | URA3-marked vector containing PCK1p-EFG1 | A. Sonneborn |
| pDB1 | Same as pBI-HAHYD but encoding Efg1p(T206A) variant | D. P. Bockmühl |
| pDB2 | Same as pBI-HAHYD but encoding Efg1p(T206E) variant | D. P. Bockmühl |
FIG. 1.
Chlamydospore formation of C. albicans is defective in efg1 strains. All strains were streaked out lightly on chlamydospore induction medium (cornmeal agar [Difco]–0.33% Tween 80), covered by a coverslip, and incubated for 5 days at 20°C. Strains used were CAI4 (WT, wild type; EFG1/EFG1) complemented with empty plasmid pBI-1 (23), JKC19 (cph1/cph1) (15), and HLC67 (efg1/efg1) (16) complemented with pBI-1 and HLC54 (efg1/efg1 cph1/cph1) (16). Colonies were visualized by phase-contrast microscopy after 2 days of growth at 100-fold magnification (top row). Photographs of chlamydospores and filaments magnified 400-fold (across coverslips on plates) were obtained after 5 days of growth (bottom row).
Efg1p residue T206 is essential for chlamydospore formation.
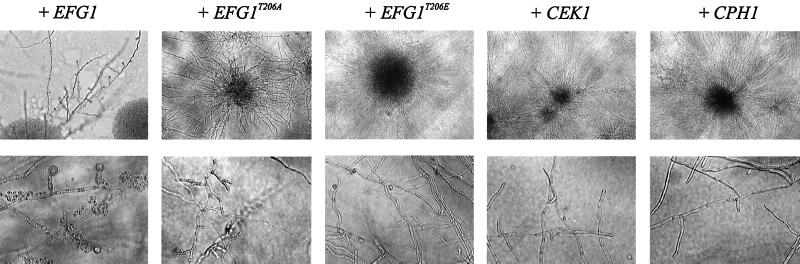
We sought to determine if the lack of chlamydospore formation in efg1 mutants could be suppressed by expression of several plasmid-borne genes. To perform this analysis, strain HLC67 (efg1/efg1 ura3/ura3 [16]) was used as the host for plasmids derived from pRC2312 (2). While strain HLC67 transformed with basic plasmid pBI-1 (23) did not develop chlamydospores, a transformant carrying the EFG1 expression plasmid pBI-HAHYD (23) was complemented (Fig. 2). In contrast, neither high expression of CPH1 carried by plasmid pLJ19 (4) nor high expression of the CEK1 gene encoding the MAP kinase Cek1p present on plasmid pCCa4 (4) was able to restore the formation of chlamydospores. Thus, a functional Efg1 protein cannot be substituted by extraneous levels of components of the parallel MAP kinase pathway. Efg1p contains a single potential site for PKA phosphorylation at T206 (sequence I R P R V T206 T T), which we had determined previously to be essential for hyphal development (22). To determine the effect of this residue on chlamydospore formation, we used expression plasmids encoding a T206A or T206E substitution (plasmid pDB1 or pDB2, respectively [22]) and tested transformants containing these plasmids. Transformants carrying pDB1 or pDB2 were completely deficient in chlamydospore formation, similar to the efg1 host strain (Fig. 2). These results demonstrate that the status of the single PKA phosphorylation site in Efg1p is crucial for chlamydospore differentiation to occur. In parallel experiments, we have determined that hyphal formation on Spider medium is blocked in strains producing the T206A but not the T206E mutant version of Efg1p (22). Since both Efg1p variants do not support chlamydospore formation, it appears that structural requirements for the Efg1 protein are different for its functions in hyphal development and in chlamydospore formation. Also, because tpk2 mutants are not affected in chlamydospore formation, it appears that the PKA isoform encoded by CaTPK2 is not involved in this process, unlike its important function in hyphal development (22). Possibly, a yet undefined isoform of A-type kinases has an essential function in chlamydospore formation.
FIG. 2.
Attempts to suppress the block of chlamydospore formation in efg1 mutants. The efg1/efg1 mutant strain HLC67 was transformed with an EFG1 expression vector (pBI-HAHYD) (+ EFG1), expression vectors for the T206A (pDB1) and T206E (pDB2) variants of Efg1p (+ EFG1T206A and + EFG1T206E) (22), the expression vector pLJ19 carrying CEK1 (pLJ19) (+ CEK1) (4), and the expression vector pCCa4 carrying CPH1 (+ CPH1) (4). Phase-contrast microscopy on colonies and on chlamydospores and filaments was performed after 5 days of growth at 100-fold (top row) or 400-fold (bottom row) magnification.
Filamentous growth of efg1 strains.
Deletions in EFG1 are known to block hyphal formation in a variety of standard inducing conditions, e.g., in the presence of serum or GlcNAc or during growth on Spider medium (16, 23); an efg1 cph1 double mutant was unable to form residual pseudohyphae as efg1 strains in some conditions (16). Therefore, our result demonstrating that the efg1 and cph1 single mutants, as well as the efg1 cph1 double mutant, were not blocked in filament formation under conditions of chlamydospore formation (Fig. 1 and 2) appeared surprising. Even more surprisingly, the efg1 mutant (but not the cph1 mutant) appeared to form even more filaments, which were more elongated than those of the EFG1 wild-type strains. The more extensive filamentation of the efg1 strains than of the EFG1 strains was most obvious after about 2 days of incubation on cornmeal agar–Tween 80 (Fig. 1). Filaments developed by the EFG1 wild-type strains can be qualified as pseudohyphae, since they carry constrictions at the site of cell separations and buds are observed occasionally at the tips of filaments. In the case of the efg1 mutant strains, many filaments appeared to be extremely elongated and it cannot be decided with certainty if such filaments are to be classified as abnormally elongated pseudohyphae or true hyphae. Regardless of the nature of the filaments as pseudohyphae or hyphae, the results demonstrate that in the conditions of chlamydospore induction, filaments are formed by hitherto unknown signal transduction pathways not including known components of hyphal induction pathways. The determining factor for activation of this novel pathway appears to be anaerobiosis, since growth of colonies on cornmeal agar (Difco) or on Spider medium in an anaerobic jar also induced filaments in an efg1 mutant strain (data not shown).
Efg1p belongs to a group of bHLH proteins termed APSES-proteins (1), which contain a highly homologous region of about 100 amino acids and which regulate morphogenetic processes in fungi. Specifically, all of these proteins are required for the interconversion between a spherical, yeast-like cell and an elongated filamentous cell. In asm-1 mutants of Neurospora crassa, the transition from filamentous to spherical growth is blocked and mutants are unable to form ascospores; similarly, in Aspergillus nidulans stuA mutants, the formation of conidiophores is blocked (7). In sok2 mutants of Saccharomyces cerevisiae (25) and in transformants overexpressing PHD1 (10), pseudohyphal growth is induced. Hyphal development in C. albicans efg1 mutants is blocked in standard induction conditions, e.g., in the presence of serum and other inducers (however, as discussed above, not in anaerobic conditions). Here we have demonstrated that the transition from a filamentous growth form to a spherical cell type, chlamydospores, also is blocked in efg1 mutants. Efg1p is the first defined component known to regulate chlamydospore formation. The results demonstrate that Efg1p, possibly in association with auxiliary proteins, has a dual role in C. albicans cell differentiation in that it regulates the spherical cell-filament interconversion in both directions. It has recently been uncovered (21) that EFG1 also regulates the spontaneous opaque white phenotypic switching in strain WO-1 (20). We found that overexpression of EFG1 forces the opaque white switch and that lowered expression of EFG1 induces an opaque-like state in cells of strain CAI8 (9). Thus, Efg1p emerges as a central regulator of morphogenetic processes in C. albicans, regulating dimorphism, phenotypic switching, and chlamydospore formation.
Acknowledgments
We thank C. Csank, G. Fink, and H. Liu for strains and plasmids. We acknowledge the excellent technical assistance of M. Gerads.
REFERENCES
- 1.Aramayo R, Peleg Y, Addison R, Metzenberg R. Asm-1+, a Neurospora crassa gene related to transcriptional regulators of fungal development. Genetics. 1996;144:991–1003. doi: 10.1093/genetics/144.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon R D, Jenkinson H F, Shepherd M G. Cloning and expression of Candida albicans ADE2 and proteinase genes on a replicative plasmid in C. albicans and in Saccharomyces cerevisiae. Mol Gen Genet. 1992;235:453–457. doi: 10.1007/BF00279393. [DOI] [PubMed] [Google Scholar]
- 3.Cole G T, Seshan K R, Phaneuf M, Lynn K T. Chlamydospore-like cells of Candida albicans in the gastrointestinal tract of infected, immunocompromised mice. Can J Microbiol. 1991;37:637–646. doi: 10.1139/m91-108. [DOI] [PubMed] [Google Scholar]
- 4.Csank C, Schröppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas D Y, Whiteway M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dujardin L, Walbaum S, Biguet J. Influence de la concentration du glucose et de l’azote sur la morphologie de Candida albicans et la formation de ses chlamydospores dans un milieu de culture synthétique. Mycopathologia. 1980;71:113–118. doi: 10.1007/BF00440617. [DOI] [PubMed] [Google Scholar]
- 6.Dujardin L, Walbaum S, Biguet J. Chlamydosporulation de Candida albicans: déroulement de la morphogenèse, influence de la lumière et de la densité dénsemencement. Ann Microbiol. 1980;131A:141–149. [PubMed] [Google Scholar]
- 7.Dutton J R, Johs S, Miller B L. StuA is a sequence-specific transcription factor that regulates developmental complexity in Aspergillus nidulans. EMBO J. 1997;16:5710–5721. doi: 10.1093/emboj/16.18.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, J. F. Regulation of dimorphism in Candida albicans. In J. F. Ernst and A. Schmidt (ed.), Contributions to microbiology. Karger AG, Basel, Switzerland, in press. [DOI] [PubMed]
- 9.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimeno C J, Fink G R. Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol Cell Biol. 1994;14:2100–2112. doi: 10.1128/mcb.14.3.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasenclever H F. The consistent formation of chlamydospores by Candida tropicalis. Sabouraudia. 1971;9:164–166. [PubMed] [Google Scholar]
- 12.Joshi K R, Solanki A, Prakash P. Morphological identification of Candida species on glucose agar, rice agar and corn meal agar with and without Tween-80. Indian J Pathol Microbiol. 1993;36:48–52. [PubMed] [Google Scholar]
- 13.Köhler J R, Fink G R. Candida albicans strains heterozygous and homozygous in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leberer E, Harcus D, Broadbent I D, Clark K L, Dignard D, Ziegelbauer K, Schmidt A, Gow N A R, Brown A J P, Thomas D Y. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 1996;93:13217–13222. doi: 10.1073/pnas.93.23.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Köhler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1725. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 16.Lo H-J, Köhler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 17.Montazeri M, Hedrick H G. Factors affecting spore formation in a Candida albicans strain. Appl Environ Microbiol. 1984;47:1341–1342. doi: 10.1128/aem.47.6.1341-1342.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odds F C. Candida and candidiosis. London, England: Baillière Tindall; 1988. [Google Scholar]
- 19.Raudonis B M, Smith A G. Germination of the chlamydospores of Candida albicans. Mycopathologia. 1982;78:87–91. doi: 10.1007/BF00442631. [DOI] [PubMed] [Google Scholar]
- 20.Soll D. Gene regulation during high-frequency switching in Candida albicans. Microbiology. 1997;143:279–288. doi: 10.1099/00221287-143-2-279. [DOI] [PubMed] [Google Scholar]
- 21.Sonneborn A, Tebarth B, Ernst J F. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect Immun. 1999;67:4655–4660. doi: 10.1128/iai.67.9.4655-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonneborn, A., D. Bockmühl, M. Gerads, and J. F. Ernst. Unpublished data.
- 23.Stoldt V R, Sonneborn A, Leuker C, Ernst J F. Efg1, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torosantucci A, Cassone A. Induction and morphogenesis of chlamydospores in an agerminative variant of Candida albicans. Sabouraudia. 1983;21:49–57. doi: 10.1080/00362178385380081. [DOI] [PubMed] [Google Scholar]
- 25.Ward M P, Gimeno C J, Fink G R, Garrett S. SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol Cell Biol. 1995;5:6854–6863. doi: 10.1128/mcb.15.12.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]