Abstract
Multidrug-resistant (MDR) superbugs can breach the blood–brain barrier (BBB), leading to a continuous barrage of pro-inflammatory modulators and induction of severe infection-related pathologies, including meningitis and brain abscess. Both broad-spectrum or species-specific antibiotics (β-lactamase inhibitors, polymyxins, vancomycin, meropenem, plazomicin, and sarecycline) and biocompatible poly (lactic-co-glycolic acid) (PLGA) nanoparticles have been used to treat these infections. However, new therapeutic platforms with a broad impact that do not exert off-target deleterious effects are needed. Membrane vesicles or extracellular vesicles (EVs) are lipid bilayer-enclosed particles with therapeutic potential owing to their ability to circumvent BBB constraints. Bacteria-derived EVs (bEVs) from gut microbiota are efficient transporters that can penetrate the central nervous system. In fact, bEVs can be remodeled via surface modification and CRISPR/Cas editing and, thus, represent a novel platform for conferring protection against infections breaching the BBB. Here, we discuss the latest scientific research related to gut microbiota- and probiotic-derived bEVs, and their therapeutic modifications, in terms of regulating neurotransmitters and inhibiting quorum sensing, for the treatment of neurodegenerative diseases, such as Parkinson’s and Alzheimer’s diseases. We also emphasize the benefits of probiotic-derived bEVs to human health and propose a novel direction for the development of innovative heterologous expression systems to combat BBB-crossing pathogens.
Keywords: blood–brain barrier, extracellular vesicles, gut microbiota, membrane vesicles, meningitis, probiotics, superbugs
1. Introduction
The blood–brain barrier (BBB) plays a central role in the unique and complex microenvironment of the central nervous system (CNS) [1]. In particular, it restricts the entry of drugs and other exogenous molecules, including host immune cells [2] and infectious pathogens [3]. Nevertheless, opportunistic pathogens can occasionally breach the BBB and cause serious illnesses, including meningitis and brain abscess [4]. Although the occurrence of CNS infection is relatively rare, chronic malignancies can result in serious neurological disorders [5]. Drug-resistant pathogens, including Acinetobacter baumannii, Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, and Streptococcus spp. can enter via the respiratory tract and mucosa and breach the BBB [6]. In the BBB, pathogens tightly regulate intrinsic virulence mechanisms via drug-resistance pumps [7] and biofilm formation [8]. Acinetobacter spp., Klebsiella, and S. aureus further modulate the expression of proinflammatory cytokines [9] and movement of immune cells, thereby destabilizing the endothelial lining and tight junctions of the BBB [10]. However, due to the complexity of the brain microenvironment and its associated endothelial tight junctions, transport of effective antimicrobials and therapies is challenging [11]. In fact, the physiological nature of the CNS environment prevents 90–95% of antimicrobials from progressing toward drug development [12]. Various nanoparticles (NPs), especially liposomal NPs [13,14], and their derivatives (e.g., polysaccharide and polyester NPs) [15,16] are considered effective and innovative drugs against pathogens that invade the BBB. However, NP-associated toxicity [17] and dose-dependent mortality [18] seriously limit their application. It is, therefore, necessary to consider alternatives, particularly those that can mimic non-immunogenic biological entities [19].
Membrane vesicles or extracellular vesicles (EVs) play crucial roles in polymicrobial interkingdom communication [20]. Microbial evolution involves the continuous transfer of metabolites via nanosized vesicles that carry important biomolecules, virulence factors, and membrane receptors of the cells from which they originate [21] to proximal and distant cells via blood and lymphatic systems. These vesicles range in size from 20 to 400 nm. The release of EVs is a general phenomenon performed by many cell types, including those of eukaryotes, Gram-negative/-positive bacteria, and archaea [22], as a means of communicating with other cells. In particular, bEVs have been characterized as the delivery vehicles of host–microbe interactions, responsible for the delivery of signaling molecules, such as autoinducers, virulence factors [23,24], and antibiotic genes [25,26]. In contrast to pathogen–host interactions, mucosal- or gut microbiota-derived bEVs contribute to homeostasis, immune system regulation, bowel movements, and the gut–brain axis [27]. Based on their immunomodulatory properties, gut microbiota-derived bEVs are currently employed in therapies aimed at promoting both humoral and cell-mediated responses [28]. Among them, tuning probiotic-derived bEVs, for interactions between interstitial cells and the gut–brain axis, represents a novel strategy for promoting immune responses during infectious disease [29]. Furthermore, this strategy can benefit from the ease of fermentation culture techniques, potential application of probiotics, and mucoadhesive encapsulation [30,31]. Moreover, combining functional biomaterials with active bEVs has the potential to target autoimmune inflammatory dispositions and treat severe chronic infections [32]. More specifically, beneficial gut microbiota-derived bEVs are a promising tool to regulate the gut–brain axis by reducing inflammation and restoring immunity [33], creating a benchmark for the targeted delivery of drugs to the CNS. However, currently, most EV-based drugs are derived from eukaryotic systems, including those for cancer [34], gastric disorders, and polymicrobial infections, due to the various challenges related to bEVs [35]. Nevertheless, genetically modifying bEVs via surface remodeling [36] to target neurotransmitters and quorum sensing (QS) inhibitors, and through CRISPR/Cas system-based modifications [37], has the potential to provide novel noninvasive therapies against BBB infections [38].
In this review, we introduce cutting-edge research on the mechanism of action and production of gut microbiota- and probiotic-derived bEVs against pathogens crossing the BBB. Hence, this article will serve as a valuable resource for future research aimed at enhancing the production of probiotic-derived bEVs in the context of antimicrobial research and designing novel heterologous expression systems.
2. Blood–Brain Barrier (BBB): A Roadblock to Invading Pathogens
The endothelial layer of the BBB selectively transports immune cells and other metabolites involved in maintaining the functional stability of the nervous system [39]. However, during the neonatal period, in some cases, the BBB can shield pathogens, resulting in a breach of the protective layer and subsequent serious disorders and infection [40]. Endogenous markers, such as pathogen-associated molecular patterns and small molecular motifs conserved within a class of microbes [41], are recognized by endothelial receptors of the BBB. This recognition results in an immunological burst at the target site [42] that can breach the endothelial lining. Moreover, the complicated structure of the CNS limits the access of several antimicrobial agents to the nervous system [43], however, facilitating the transport of lipophilic drugs with a molecular weight <400 Da [44] that form fewer than eight hydrogen bonds via lipid-mediated free diffusion [45], into the bloodstream via the transcellular route [46]. As efficient drugs, antiepileptics (e.g., diazepam and phenytoin) [47,48], PLGA-coated nanoparticles, and laser-assisted therapies (e.g., focused ultrasound and interstitial thermal therapy) are commercially available [49,50]. However, these therapies do not guarantee the long-term potency of drugs because the microbial flora is constantly evolving, either through horizontal gene transfer or cell-to-cell communication, resulting in reduced susceptibility to certain drugs [51].
3. Multidrug-Resistant (MDR) Superbugs: A Prominent Case Involving the BBB
Infections caused by MDR superbugs have emerged as a major threat to global health in the post-antibiotic era, especially in the 21st century [52]. The Centre for Disease Control and World Health Organization have predicted that there will be ~2 million cases of MDR infections and 27,000 related deaths per year by 2050 in Asia, Africa, and North America [53]. Carbapenem and colistin are the most widely used last-resort antibiotics against bacterial infections [54]; however, by the late 2000s, drug resistance exhibited an unexpected increase in mortality associated with hospital-acquired infections by 40–60% [55]. Pan-drug resistant A. baumannii is routinely reported in patients with meningitis [56,57,58] and has acquired resistance to most antibiotic therapies, including colistin and tetracycline [59,60]. Although combined treatment with gentamicin and meropenem is efficient, the reduction rate of infection is <17–19% [61] given that the BBB limits the permeability of drugs and the continuous administration of drugs further increases the probability of resistance [62,63]. Moreover, frequently screened drug-resistant pathogens (A. baumannii [64] and E. coli [65]), few routinely screened pathogens (N. meningitidis and Streptococcus spp.) [66,67], other neuroinvasive pathogens (Haemophilus influenzae) [68], and Chlamydophila pneumoniae [69] not only disrupt the tight junctions of the BBB but also induce leakage between tight junctions and vascular endothelial cells [70]. For example, Gram-positive L. monocytogenes, Staphylococcus spp., and Streptococcus pneumoniae elevate the levels of proinflammatory cytokines and disrupt the endothelial lining in the CNS [71], thus creating a path of invasion for opportunistic pathogens.
4. Bacteria-Derived EVs (bEVs): Nanoscale Vesicles
bEVs have been studied since the early 1960s when lipid-like structures released from E. coli were discovered as a means to transport secondary metabolites and intrinsic biomolecules to the communicating host [72]. After the discovery of bEV production from Gram-positive bacteria, such as Bacillus subtilis, Mycobacterium tuberculosis, S. aureus, and Streptococcus spp., bEV release is regarded as a general phenomenon carried out by bacteria that has an important role in cell-to-cell communication and disease progression during gastric cancers and tuberculosis [73,74]. Cell-to-cell communication by bEVs involves internalization via the endothelial layer, micropinocytosis, and endocytosis by utilizing invasion proteins at the host–pathogen interface [75]. Certain pathways, such as the stress induced network, cause bEVs to function as anti-phagocytosis bodies, evading phagocytosis and weakening the clearing mechanism via the host immune response [76]. M. tuberculosis is a classic example of pathogen evasion of the innate immune responses; that is, it infects phagocytic and inhibits phagosome maturation. Moreover, Athman et al. [77] discovered that Mycobacterium bEVs produce lipoglycans and lipoproteins that play an important role in regulating the host immune response and facilitating persistent infection. Further, it was found that S. aureus-derived bEVs contain super-antigens (protein A and lipase) that aid cells in phagocytosis evasion. Meanwhile, a proteomics study [78] found that immunoglobulin (IgG)-bound lipase and super-antigen (hydrogenated form of squalene; SQA) are presented in bEVs, thus highlighting the potential role of S. aureus in evading anti-phagocytic activity via super-antigens and lipase production. bEVs also have a basic role in exchange of genetic materials (DNA and RNA) through horizontal gene transfer, during which bEVs serve as a means of cell-to-cell communication within the same bacterial species [79]. Additionally, a study conducted on bEV cargo of A. baumannii reported the presence of a carbapenamse gene (blaOXA-24) that increases the antibiotic susceptibility pattern against β-lactam antibiotics [80]. Similar studies on bEVs derived from N. gonorrhoeae [81] and S. aureus [82] have identified the presence of the outer membrane (OM) protein PorB and alpha toxins that transfer genetic materials, inducing apoptosis and host cell death.
bEVs released from the cell envelope of Gram-negative bacteria are so-called outer membrane vesicles (OMVs). The envelope is made up of three layers: the OM, cytoplasmic membrane, and the periplasmic space in between, which contains a layer of peptidoglycan (PG) [83]. An inside leaflet of phospholipids and an outer leaflet of lipopolysaccharide (LPS; also known as endotoxin) constitute the OM. LPS causes inflammatory responses in host cells [84], whereas the OM has a porous structure that aids in waste removal and nutrition uptake, and the peptidoglycan (PG) layer maintains the osmotic pressure of the cell and regulates the hostile environment (antibiotic stress) [85]. Gram-positive, unlike Gram-negative, bEVs are produced from cytoplasmic constituents via a blebbing mechanism; their genetic composition is comparable to that of Gram-negative bEVs, with the exception of the lipoprotein structure [86]. Apart from the normal mechanism of blebbing, prophage-encoded endolysins have also facilitated bEV release from Gram-negative and -positive bacteria. Studies on Bacillus spp. and Staphylococcus spp. have revealed that the prophage-encoded endolysin generates holes in the peptidoglycan cell wall, thus highlighting the potential role of these enzymes in bacterial cell wall lysis during mass production of bEVs [87,88].
5. Nanoscale bEVs as Potential Therapeutic Platforms
Recently, bioinspired NPs such as host (eukaryotic) EVs (hEV) and bEVs have shown promising effects against chronic infections [89,90]. Compared with their nanomaterial counterparts (liposomal NPs), bEVs provide increased drug delivery and efficient antigen-presenting properties [91,92,93]. Various microbes including Helicobacter spp., Klebsiella pneumoniae, Lactobacillus spp., P. aeruginosa, S. aureus, and Streptococcus spp. are involved in the transfer of metabolites between species for intracellular communication and are used in novel adjuvant-associated therapeutics as well as nano-sized vaccine delivery platforms for various infections [94,95] (Table 1).
Table 1.
bEVs involved in the pathogenic infections and their roles.
| Origin of bEV | Infecting Pathogens | Role of bEV | Reference |
|---|---|---|---|
| Gram-positive bacteria | |||
| Bifidobacterium longum | Food-borne infections | Induction of progenitor cells | [96] |
| Burkholderia spp. | Activity against A. baumannii and S. aureus | N.D. | [97] |
| L. gasseri | Human Immunodeficiency Virus (HIV) | Change in susceptibility pattern of viral infection by regulation of toll-like receptor (TLR)-2 signaling | [98] |
| L. rhamnosus | Superficial infections | M2 Macrophage | [99] |
| S. aureus | Pneumococcal infection | TH1-mediated cell immunity | [100] |
| Streptococcus spp. | Streptococcal infection | Induction of dendritic cells | [101] |
| Tetragenococcus halophilus | Opportunistic pathogens | Anti-inflammatory factor interferon beta (IFN-β) | [102] |
| Gram-negative bacteria | |||
| Acinetobacter spp. | Pan-drug resistant A. baumannii | Activation of IgG and IgM | [103] |
| Borrelia burgdorferi | B. burgdorferi colonization | Stabilizing superoxide | [104] |
| Helicobacter pylori | H. pylori infection | Induction of TH2 immune cells | [105] |
| K. pneumoniae | K. pneumoniae infection | Humoral and cellular immunity | [106] |
| N. meningitis | Meningococcal disease | IgG-mediated response | [107] |
| Pertussis A | Bordetella pertussis infection | Induction of CD4 cells | [108] |
| P. aeruginosa | Lethal dose of P. aeruginosa | Mixed cellular response | [109] |
hEVs have shown complexity of the yield coefficient, a high production cost, and limited downstream process, all of which limit their biomedical applications [110,111] (Table 2). The continuous evaluation of EVs as potential tools against chronic infection has led to the development of bEVs derived from Clostridium butyricum [112] and L. paracasei [113]. Given that most chronic illnesses involve ‘dysbiosis’ of the gut microbiota, tuning the absorption capacity and nutrition digestion factors of the microbiome might influence the host–microbe physiological imbalance.
Table 2.
Current limitations of eukaryotic and bacterial EVs in biomedical applications.
| Eukaryotic (hEVs) | Bacterial (bEVs) | Common Limitations |
|---|---|---|
| Differentiation between cell surface markers | Lipopolysaccharide (LPS) toxicity | Immunomodulators outburst |
| Inefficient purification of vesicles | High inflammatory responses | Low viability and inefficient growth conditions |
| Lack of heterogeneity | High chance of infection (pathogen-derived bEVs) |
High cellular toxicity |
6. Unresolved Issues with Gut Microbiota-Derived bEVs in Modulating the Gut–Brain Axis: Old Is Gold
The continuous usage of antibiotics during BBB infections leads to prognosis of early psychosis and neurotoxicity [114]. Gut microbiota dysbiosis, a state where the physiological combinations of flora are transformed into pathological combinations [115] via continuous antibiotic administration, has been linked to neural abnormalities. This link is via the vagal nerve, which is associated with a lower response of neurotransmitters inducing systemic inflammation in the CNS [116]. These features highlight the importance of the gut–brain axis in modulating CNS homeostasis.
6.1. Gut–Brain Axis
The term ‘Gut–Brain axis’ refers to a bidirectional network that includes multiple connections such as the vagus nerve (nervous control), immune coordination (epithelial and mucosal barrier), and secondary metabolite generation from microbes [117]. The complex architecture of the gut–brain axis entails the constant transit of neurotransmitters within the gastrointestinal (GI) tract, which, in turn, modulates the immune system, including macrophages and mast cells [118]. These immune cells boost neuron excitability and regulate the host’s behavioral response. A recent study found that gut dysbiosis caused by a broad-spectrum antibiotic during traumatic brain injury (TBI) resulted in increased neuronal loss, suppressed neurogenesis, altered microglia and peripheral immune response, and modulated fear memory response, suggesting a role of gut microbiota in the recovery from TBI [119].
6.2. Gut Microbiota-Derived bEVs vs. Eukaryotic-Derived hEVs (Physiological Counterpart)
Generally, the use of hEVs is significantly limited by the yield coefficient and high-throughput screening. In addition, the current scenario for combating antibiotic resistance with chronic illness is not favored by the use of pathogen-derived bEVs, because the sudden release of pro-inflammatory factors by bacteria cannot be controlled [120]. In contrast, beneficial gut microbiota have shown the effective immune responses and efficient pathogen inhibition activity [121]. Moreover, bEVs from beneficial gut microbiota take a role in triggering inflammatory responses through LPS and lipoteichoic acid [122] and can cross the intestinal barrier, and have effective anti-inflammatory properties against chronic infections and gut dysbiosis [123]. The physiological features of hEVs differ significantly from gut microbiota-derived bEVs, as shown in Table 3.
Table 3.
Difference between eukaryotic-derived hEVs and gut-microbiota-derived bEVs.
| Category | Eukaryotic-Derived hEVs | Gut-Microbiota-Derived bEVs |
|---|---|---|
| Biogenesis | Generally produced from plasma membrane except exosomes, which originate from endocytic pathway | Gram-negative bacteria: decreased protein linkages between the OM and peptidoglycan, accumulation of unfolded proteins and/or peptidoglycan in the periplasmic space, and explosive cell lysis Gram-positive bacteria: turgor pressure by accumulation of bEVs and the action of cell-wall-degrading enzymes |
| Composition (Cargo) |
Multivesicular bodies composed of endosomal proteins; RNA and miRNA are regularly incorporated | Proteins, peptidoglycans, lipids, LPS, lipoteichoic acids (LTA), nucleic acids, and metabolites |
| Major functions | Intercellular communications (cell proliferation, matrix formation, and phagocytosis) | Innate and adaptive immunity, bacterial communications, interaction with host miRNA for movement across intestinal barrier |
| Size | 40–100 nm (exosomes) [124]; 500–2000 nm (apoptotic bodies) and 100–500 nm (microvesicles) [125] | 10–300 nm [126] |
6.3. Problems Related to Gut Microbiota-Derived bEVs on BBB-Associated Diseases
The ‘dysbiosis’ condition in the gut microbiota environment by antibiotic usage has also shown certain detrimental impacts such as Alzheimer’s disease, autism, and arthritis, all of which clearly demonstrate the mechanistic behavior and coordinated axis of mental health and intestinal mucosa [127]. A study by Lee et al. [128] showed that the release of bEVs from a gut pathogen Paenalcaligenes hominis, revealed movement of bEVs via the vagus nerve, producing cognitive impairment in the nervous system. Another study using Porphyromonas gingivalis, an oral pathogen, demonstrated the importance of LPS-coated bEVs in the onset of Alzheimer’s disease, emphasizing the role of protease and LPS in triggering the damage of collagen fibers, fibrinogen connective tissues, and induction of proinflammatory mediators in the transfer of bEVs that alter brain cognitive function [129]. The main drawback of bEVs derived from the gut microbiota is that they have a negative impact on memory, cognition, and neuroinflammation. Therefore, direct application of such bEVs may have both adverse and beneficial neurologic effects on CNS homeostasis.
6.4. Beneficial Roles of Probiotic-Derived bEVs on Gut–Brain-Axis Control
Considering the diverse array of gut microbiota from intestinal niches, probiotics including Bifidobacterium spp. and Lactobacillus spp. have been identified to create neurotransmitters (acetylcholine, gamma-aminobutyric acid (GABA), and serotonin), which continually control CNS homeostasis [130,131]. Overall, probiotics not only govern the bidirectional transit of biochemical signals, but also improve the host’s behavioral response such as anxiety [132], depression, and stroke [133]. Apart from periodontal and gut pathogens, probiotics such as Lactobacillus spp. have influence on the motor neuron complex (M-N complex). This M-N complex includes the enteric nervous system (endocrine functions and secretion from intestinal mucosa) and the vagus nerve. Lactobacillus spp. normally modulates the neurotransmitter signals via the vagus nerve (intestinal nerve), involving sensory transmission of neuronal signals via the enteric nervous system to the CNS [131]. Few bEVs derived from Lactobacillus spp. have also demonstrated the direct regulation of the gut–brain axis in CNS homeostasis (Table 4).
Table 4.
Beneficial roles of probiotic-derived bEVs on gut–brain-axis control.
| Origin of bEVs | Roles of bEVs | References |
|---|---|---|
| L. acidophilus | Changes in complex microbial communities | [134] |
| L. plantarum | Enhance the action of brain-derived neurotropic factor (BDNF), lowering the stress level in hippocampus neuron | [135] |
| L. reuteri DSM 17938 | Modulate intestinal and colon motility and enhance gut–brain intercommunication for CNS homeostasis | [136] |
| L. rhamnosus | Reduce the behavioral changes including anxiety and depression | [137] |
7. Filling Gaps with Probiotic-Derived bEVs against BBB-Breaching Pathogens
The fundamental issue with antibiotic therapy is the associated drug resistance, which causes a widespread distribution of MDR BBB-breaching bacteria, causing secondary neurological disorders that impair CNS homeostasis [138]. Moreover, the risks associated with the continuous use of antibiotics include seizure, neuromuscular blockade, cranial nerve toxicity, and intracranial hypertension [139]. Conventional therapeutic options for the treatment of MDR bacterial infections include β-lactamase inhibitors, aminoglycosides, fluoroquinolones, and last-resort polymyxins [140,141]. However, such therapies have limited efficacy in CNS infections caused by MDR bacteria, such as A. baumannii, K. pneumoniae, M. tuberculosis, L. monocytogenes, N. meningitidis, and Streptococcus spp. (Table 5). This is due to the BBB integrity as well as severe side-effects such as neurotoxicity and nonspecific targeting. The continuous administration of antibiotics, and its associated risk factors, often creates dysregulation between the gut microbiota and the cerebrospinal fluid of the CNS. Mucosal bacteria regulate the communication between the enteric nervous system and peripheral intestinal regulation. Meanwhile, the constant dysregulation caused by antibiotic overuse has created a gap between efficient metabolism of intestinal regulation and CNS modulation.
Table 5.
BBB-breaching pathogenic infections and associated immunomodulatory activity.
| Pathogen | Mode of Pathogenesis | Immunological Factors Contributing BBB Infection | References |
|---|---|---|---|
| A. baumannii | -Meningitis -Catheter-associated infection |
Increased inflammatory cell response, toll-like receptor (TLR) altered expression, and proinflammatory cytokine burst within 24 h of infection | [142,143] |
| E. coli | -Endothelial cells -Attenuation of transforming growth factor (TGF)-β 1 signaling |
Increased expression of endothelial-derived platelet-derived growth factor receptor (PDFGR) and intercellular adhesion molecule (ICAM), resulting in inflammation | [144] |
| H. influenzae | -Large amount of vascular endothelial growth factor receptor (VEGFR) -Adenosine receptor dysfunction |
Endothelial disruption and tight junction altered expression: downregulation of tumor necrosis factor (TNF-α); endothelial proliferation | [145,146] |
| K. pneumoniae | -Cerebrospinal infection -Intracranial infection |
Increased production of proinflammatory cytokines and chemokines; induction of hypoxia inducible factor (HIF)-1α | [147] |
| L. monocytogenes | -Vimentin-mediated infection Neuroinflammation |
In1F virulent factor-associated downregulation of tight junction and overexpression of PDFGR and ICAM, resulting in inflammation | [148] |
| N. meningitidis | -Secretion of IgA protease -Evasion of immune response |
Deformation of adherence junction, triggering IL-6 and IL-8 expression: leukocyte infiltration and infected phagocyte movement | [149,150] |
| P. aeruginosa | -Cerebrospinal infection -Meningitis |
Increased production of inflammatory cell response; overproduction of IL-1β and IL-6 | [151] |
| S. aureus | -Brain abscesses and endocarditis -Cytokine burst |
Stimulate immune invasion, T cell activation: burst of proinflammatory cytokines; TNF-α, IL-6, and IL-10 overproduction | [152] |
| S. pneumoniae | -Neonatal meningitis -Laminin receptor transcytosis |
TNF-α, IL-6, and IL-10 overproduction and increased permeability through anchored tight junction; cleavage of IgA through pneumococci IgA protease | [153,154] |
Several probiotics such as Bifidobacterium spp., L. lactis, and L. rhamanosus are being actively investigated for their therapeutic potential and are in the final stage of clinical trials [155]. bEVs originated from such species have gained attention as effective therapeutic platforms owing to their natural immunogenicity and self-adjuvating properties [156], which induce a better adaptive immune response and can transport diverse cargos across various cell types. In addition, such therapeutic platforms against antibiotic-resistance-related neurological disorders could be improved using genetic modification of gut microbiota with the CRISPR/Cas9 system [157]. The antimicrobial activity of probiotic-derived bEVs against pathogens has revealed a broader role of probiotics in enhancing the anti-inflammatory response during pathogen invasion (Table 6).
Table 6.
Probiotic-derived bEVs against BBB-invading pathogens.
| Origin of bEV | Physiological Roles | Invading Pathogen(s) | References |
|---|---|---|---|
| Burkholderia thailandensis with quinolone | Synergistic antibiofilm activity | Streptococcus spp. | [158] |
| E. coli Nissle 1917 | Increased anti-inflammatory properties, such as IL-10 and T helper (TH) cell-mediated cytotoxicity | E. coli and S. aureus | [159] |
| L. crispatus and L. jensenii | Antibiofilm and anti-inflammatory effect | Candida albicans | [160] |
| L. paracasei and L. plantarum | Decrease pro-inflammatory cytokine production | Enteroinvasive E. coli | [161] |
A proteomics study of probiotic L. plantarum BGAN8-derived bEVs that regulate brain function [136] revealed the enrichment in enzymes involved in central metabolic pathways and in membrane components with transporters [162]. Because such proteins are associated with transferring beneficial metabolites to pathogens or hosts, proteomics of probiotic-derived bEVs appears to be a potential tool to reveal underlying mechanisms of bEVs on escaping pathogen infection and the beneficial effect on brain function.
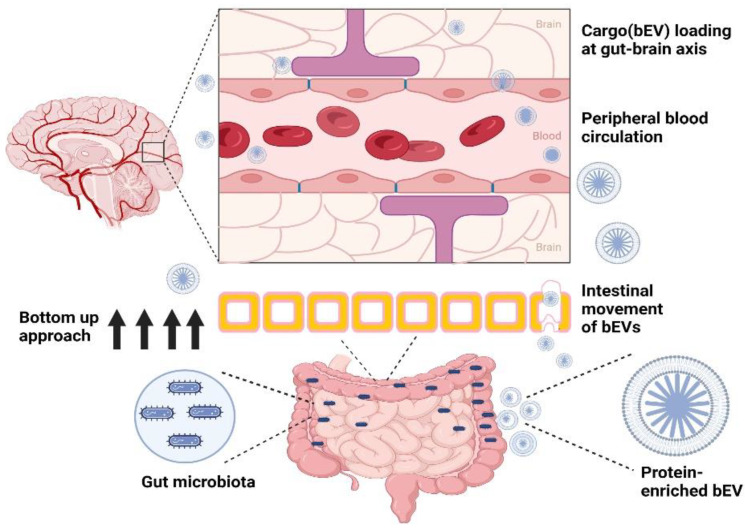
Vaccine-antigen-presenting probiotic-derived bEVs can also be employed as vehicles to transport antigens and potent antimicrobial agents to specific targets. Moreover, given that probiotics regulate various intrinsic signals, such as regulation of active short chain fatty acids, hormone metabolism, and neurotransmitters signaling and expression, they can also facilitate a wide range of interactions between the normal flora and host cognitive behavior [163]. Moreover, their role in regulating the gut–brain axis has highlighted the potential application of probiotic-derived bEVs for enhancing the neurodevelopment process [164]. For instance, studies with L. plantarum JB-1-derived bEVs highlighted the role of bEVs in regulating the neuron signaling system [165], demonstrating the direct role that probiotic-derived bEVs have in CNS development [166] (Figure 1). Moreover, unlike host immune cells, bEVs derived from immune cells can pass though the BBB and, thus, participate in the immunological regulation of the CNS.
Figure 1.
Bottom-up approach for the transport of probiotic-derived extracellular vesicles (bEVs). The figure was created using BioRender.com (https://app.biorender.com; accessed on 2 November 2022).
8. Remodeling of Probiotic-Derived bEVs against BBB-Invading Pathogens
NP-derived therapeutics show high efficacy against pathogens [167]. As it is possible to control the size and release of NP-derived therapeutics, modified NPs are potential candidates for conferring protection against drug-resistant pathogens [168]. Combination therapies with commercial antibiotics also exert beneficial effects against antibiotic-resistant pathogens; however, their selectivity and toxicity remain major concerns [169]. The modification of NPs with EVs has been driven by the aim to increase yield and reduce toxicity [170,171]. For instance, amalgamated nanocarriers with hEVs have shown promising results in cancer therapy [172]. However, to induce an efficient immunogenic response with low toxicity, bEVs from probiotics should be used in native or genetically modified form to protect against hospital-acquired infections [173]. Such an engineered, or remodeled, probiotic-derived bEV will have advantages over conventional drug delivery systems in terms of their bioavailability and targeted drug distribution.
8.1. Surface-Modified Proteins in Probiotic-Derived bEVs
Exosome-associated transmembrane proteins and their fusion to peptide domains have been investigated for their ability to confer protection against various pathological conditions in eukaryotes. For example, their role in tumor therapy, and the delivery of siRNAs and miRNAs targeting immune cells and neuronal junctions of the brain, have been evaluated [174]. However, targeting the efficiency of surface-modified probiotic-derived bEVs has not been evaluated in infections involving BBB breach. Among prokaryotes, a two-component signaling system in Gram-positive bacteria regulates diverse intracellular signals, including genetic transduction and bacteriocin production [175]. Studies conducted on Bifidobacterium spp., L. gasseri, and L. plantarum have demonstrated the role of the two-component system in the regulation of bacteriocin production [176]. For instance, surface-associated proteins of Lactobacillus spp., such as histidine protein kinase (HPK), and S-layer proteins (SlpA, B, and X) [177], have been explored in bEV studies for evaluating heterologous gene expression and enhancement of host–microbe interactions. HPK-associated recombinant protein expression is a novel approach for biotherapeutic delivery. Previous studies on bEVs revealed that the expression of heterologous antigens such as OmpA was in response to infection severity. The production of fusion proteins and hemolysin ClyA in E. coli bEVs elicited an immune response against green fluorescent (GFP) protein [178]. The concept may involve the association of a signal peptide with a reporter system that can trigger the robust secretion of target molecules for cell surface display. Similarly, the Slp system has been studied extensively in L. acidophilus and L. brevis against diarrhea and skin infections [179]. A system with strong transcription facilitated by promoter fusion [180] could increase protein production and provide a useful vaccine delivery platform. Strategies for protection against infections crossing the BBB may involve the addition of the Slp short peptide region to the upstream region of the targeted antigen or therapeutic gene, which can increase the secretion and efficacy of the therapeutic protein against infectious agents [181].
8.2. Regulation of Neurotransmitters across the BBB
BBB-associated infections are related to Parkinson’s and Alzheimer’s diseases, in which direct correlations between pathogens such as Staphylococcus spp. have been demonstrated to regulate the neurotransmitter-serotonin signaling mechanism [182]. To date, the interaction of probiotics with host miRNAs in regulating host cerebral inflammatory signaling is rare. Instead, the regulation of the bidirectional movement of miRNA by probiotic-derived bEVs in regulating the neuro-immune endocrine regulation was reported [183].
Only a few studies examining the relationship between probiotics and the serotonergic system, as well as the role of the GI tract in managing neuropsychotic disorders, have been reported. For instance, a previous study [30] reported that Akkermansia muciniphila, an intestinal symbiont colonizing the mucosal layer, increases the serotonin signaling pathway via the gut–brain axis in mice. More specifically, they showed that downregulation of Htr mediators (secreted metabolites in the colon) in the intestinal mucosa activates the bacterial colonization and, hence, increases the serotonin level and enteric neuronal activity. One classic study on probiotic supplements, including short-chain and long-chain oligosaccharides, showed that lower expression of Htr reduces anxiety behavior in mice, thus demonstrating the possible significance of probiotics in maintaining neurotransmitter signaling [184].
8.3. Quorum-Quenching Proteins
Studies of microbiome-associated neurological disorders have supported the systemic movement of quorum-sensing molecules [185] and their associated virulence factors. These factors penetrate tight junctions using the Trojan horse method and trigger nervous system connections [186]. Pathogens (e.g., Clostridium and Streptomyces species) can induce nervous system dysregulation and result in anxiety and stress-associated disorders [187]. The association between polymicrobial infections and common neurological disorders has been clarified; however, well-established tools to overcome chronic-infection-associated neurological disorders, such as bacterial meningitis and polymicrobial-associated multiple sclerosis, are needed [185]. Most neurological disorders are accompanied by a decreased abundance of beneficial, as well as commensal, microbes. Accordingly, it may be possible to express quorum-quenching-related proteins on the surface of probiotic-derived bEVs, as a targeted approach against microbes to reduce their virulence and chronicity.
8.4. bEVs as a Drug Delivery Platform to Prevent Degradation and Immune Elimination of Antimicrobials
Regarding CNS infections, most CNS-associated drugs have side-effects and lack the potential to cross the BBB. Additionally, the inefficient movement of neurotherapeutic drugs requires them to remain in the neural environment for a sufficient duration to exert the desired effect. The presence of phosphorylated glycoprotein (P-gp) in the endothelial lining of the BBB undoubtedly limits the entry of lipophilic drugs, thus increasing the risk of meningococcal infections [188]. Antibiotics such as vancomycin, meropenem, fluoroquinolones, β-lactams (occasionally), and cephalosporins are thought to be effective against CNS infection. However, inefficient administration and toxicity levels limit their usage [189]. In this case, bEVs can act as high specific loading cargos for antibiotics to provide a shielding effect [190], which will protect the antibiotics against various pathogen-derived enzymes and multi-antigen determinants on the surface will specifically target meningococcal infections [191].
8.5. CRISPR/Cas-Modified bEVs as Biotherapeutic Agents against BBB-Breach-Related Infections
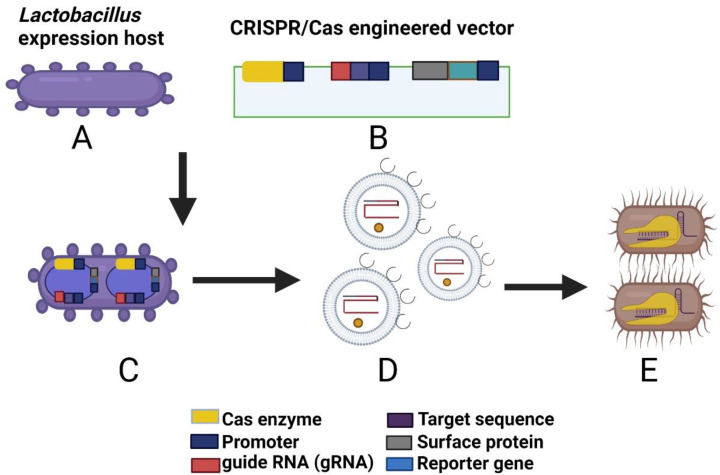
The CRISPR/Cas system is a novel gene editing approach that has been successfully employed to make opportunistic pathogens (e.g., E. coli and S. aureus) vulnerable to commercially available antibiotics, or to reverse their drug resistance [192] (Figure 2).
Figure 2.
Application of the CRISPR/Cas system for the development of biotherapeutic tools against infections crossing the BBB. (A) Lactobacillus expression host, (B) CRISPR/Cas expression vector, (C) expression of the engineered vector in Lactobacillus, (D) CRISPR/Cas-enriched Lactobacillus bEV, and (E) targeted therapy against pathogenic bacteria via surface protein receptors. The figure was created using BioRender.com (https://app.biorender.com; accessed on 2 November 2022).
This approach involves identification of the Cas system from Lactobacillus species (Type I or Type II system), constructing an engineered vector model and designing an expression system based on the surface modification of a targeted ligand using a reporter system [191] comprising an inducible promoter sequence, guide RNA, Cas9, selectable marker, surface protein with a reporter gene, and target DNA sequence. Using this approach, the entire vector can be transformed into the Lactobacillus via electroporation or microfluidic injection. bEVs from transformed Lactobacillus contain surface-expressed heterologous proteins that can be targeted to the specific host cell receptors for vaccine therapy. Studies on L. reuteri and L. sakei [193,194] have identified the presence of 20–25 CRISPR systems with varying degrees of polymorphism, conferring an evolutionary advantage against invasive pathogens. Recent examples of L. acidophilus and L. crispatus delivery mechanisms using the Slp system (S-layer membrane protein) (see Section 8.1) [180] have highlighted the utility of genome editing tools in probiotic species. Therefore, the CRISPR/Cas system can facilitate development of tools targeting drug-resistant pathogens and create avenues for designing potent and targeted therapeutic strategies against infections crossing the BBB.
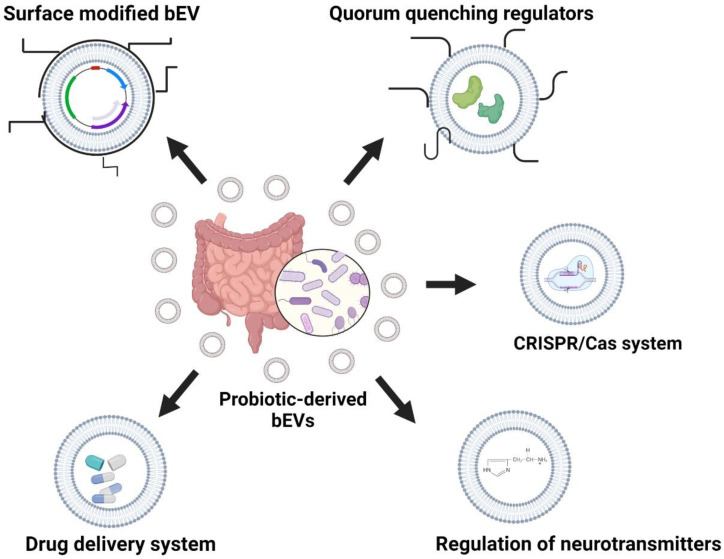
The potential role of probiotic-derived bEVs can provide numerous benefits against hospital-acquired infections. The fine tuning of probiotic-derived bEVs on parameters such as quorum-quenching enzymes and the CRISPR/Cas mechanism can provide a possible strategy to target secondary risk factors associated with dysbiosis in the gut–brain axis (Figure 3).
Figure 3.
Remodeling of probiotic-derived bEVs against BBB related anomalies. The figure was created using BioRender.com (https://app.biorender.com; accessed on 2 November 2022).
9. Potential for Application of Probiotic-Derived bEV Platforms against BBB-Associated CNS Infections
Infections with MDR bacteria, which secrete various virulence factors and toxic proteins that target sensitive regions of the brain, can readily cross the BBB’s endothelial barrier and cause serious neurological disorders. Furthermore, the robust movement of various immune cells at the site of injury promotes localized inflammatory responses and results in cytokine bursts, thus affecting CNS permeability and causing neurological imbalance.
Probiotic-derived bEVs represent safe therapeutic agents against a variety of infections and outperform conventional antibiotic therapy for BBB-associated CNS infections. However, the efficacy of bEVs derived from probiotics other than L. paracasei, in the treatment of CNS infections, is currently under evaluation in ongoing clinical trials [195]. Indeed, the presence of CNS inflammation can significantly impact bEV efficacy as it reduces the amount of drug crossing the CNS barrier, which is impeded by BBB-mediated exclusion. Nevertheless, certain drugs, including citalopram, doxepin, erythropoietin, and fluvoxamine, have demonstrated significant anti-neural anomaly activity [196]. However, these drugs are limited by their low membrane permeability, rapid clearance, and rapid degradation. Therefore, additional treatments are now being developed, such as nano-based drug delivery agents, liposomal NPs, and biomimetic NPs or nanocomposites with the potential to penetrate the BBB. However, studies using anti-seizure drug-loaded gold NPs revealed increased oxidative stress [195], necessitating a re-evaluation of the associated dosing regimen. Similarly, chitosan-based NPs exhibit minimal BBB absorption and are not currently used in clinical practice [196]. Meanwhile, for extended periods of usage, liposomal NPs outperformed metallic counterparts in post-stroke inflammatory responses. However, their instability, shorter lifetime, and restricted drug encapsulation capability limit their use as a drug delivery vehicle for nondegenerative disorders.
In contrast, bEVs outperform lipophilic and hydrophilic/hydrophobic drugs. In fact, a bEV derived from Chromobacterium violaceum—a facultative anaerobic, oxidase-positive, glucose-fermenting, non-lactose-fermenting, Gram-negative Bacillus—was successfully used to encapsulate violacein by enhancing its absorption coefficient [197]. Hence, due to their direct linkage with the gut–brain axis, as well as their movement via the autonomic nervous system, bEVs might represent an alternative drug-encapsulating vehicle for treatment of BBB infections; however, it is necessary to first address the issues regarding their bioavailability and surface modifications. In fact, probiotic-derived bEVs represent a useful platform for the development of new treatments as they have been shown to improve immunogenic responses to numerous pathogens that affect the gut–brain-axis function.
Genetic engineering of hEVs has recently been recognized as a paradigm shift in the treatment of CNS infections. Therefore, modified eukaryotic hEVs are regarded as effective delivery vehicles for hydrophobic and hydrophilic medicines. However, improving the ability of hEVs to invade the BBB has proven challenging. In this regard, probiotics with enhanced invading BBB activity might be viable therapeutic options against BBB-associated MDR pathogen infections. Most microbiota-related neurological disorders are associated with an imbalance of intestinal commensal bacteria, and probiotic-derived bEV-based platforms provide a successful therapy against CNS infections.
10. Future Research and Perspective
Strong efforts are required to improve the design of therapeutic agents that target MDR superbugs associated with the BBB. Unlike eukaryotic hEV biomarkers, proteins of probiotic-derived bEVs remain unidentified, thus limiting the utility of bEVs in BBB-associated therapy. Therefore, multiple omics approaches and in silico analysis are warranted. Additional high-throughput-scale functional analysis is required to identify potential therapeutic proteins of bEVs and design novel platforms for the selective and efficient targeting of BBB-associated infections that also elicit memory T cell responses to establish long-term immunity. Notably, most probiotic-derived bEVs exhibit antibacterial activity and enrichment of antibacterial metabolites. Thus, probiotic-derived bEVs can be used in combination with commercial antibiotics or repurposed drugs to increase their therapeutic efficacy against pathogens. With the aid of cheminformatics [197], formulated antimicrobial analogs can be designed to target pathogenic microbial factors. Indeed, this approach is expected to expand the current scope of antimicrobial use by generating probiotic-derived bEVs to effectively treat BBB-breaching infections.
11. Conclusions
The recent literature has demonstrated the effectiveness of EVs against various infectious pathogens. However, most research has largely focused on developing therapeutics or drug delivery vehicles by utilizing either NPs or hEVs (exosomes). Although these agents are clinically significant, their utilization is limited by long-term toxicity and the related mortality, low immunogenic response, stability issues, cost of scaling up, fermentation culture conditions, and downstream processing. In contrast to hEVs, there are only a few FDA-approved therapeutic bEVs, including a bEV vaccine (MeNZB) cleared for use against N. meningitidis. This is due to either failed trials or a low therapeutic efficiency. The concept of ‘postbiotics’ has recently been evaluated as a source of nonviable bacterial supplements capable of regulating the gut–brain axis. That is, the use of probiotics alone may be limited in scope; however, it can be enhanced by tuning the active components of postbiotics to initiate the release of probiotics-derived bEVs or -enriched bEVs. Meanwhile, limitations of combining NPs with antimicrobial compounds have hampered their application for the treatment of infections; moreover, this strategy does not address safety issues related to BBB breach. Collectively, the work summarized in this review provides insights into the efficacy of probiotic-derived bEVs and the novel concept of ‘postbiotics’ as a potential tool for the development of therapeutic platforms to overcome drug resistance in pathogens causing neurological disorders.
Author Contributions
P.S.: conceptualization, investigation, data collection, formal analysis, writing—original draft; K.-s.K.: conceptualization, supervision, writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant number NRF-2021R1A2C1007413).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gosselet F., Loiola R.A., Roig A., Rosell A., Culot M. Central nervous system delivery of molecules across the blood-brain barrier. Neurochem. Int. 2021;144:104952. doi: 10.1016/j.neuint.2020.104952. [DOI] [PubMed] [Google Scholar]
- 2.Muldoon L.L., Alvarez J.I., Begley D.J., Boado R.J., Del Zoppo G.J., Doolittle N.D., Engelhardt B., Hallenbeck J.M., Lonser R.R., Ohlfest J.R., et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J. Cereb. Blood Flow Metab. 2013;33:13–21. doi: 10.1038/jcbfm.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miner J.J., Diamond M.S. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr. Opin. Immunol. 2016;38:18–23. doi: 10.1016/j.coi.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cain M.D., Salimi H., Diamond M.S., Klein R.S. Mechanisms of Pathogen Invasion into the Central Nervous System. Neuron. 2019;103:771–783. doi: 10.1016/j.neuron.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Li S., Nguyen I.P., Urbanczyk K. Common infectious diseases of the central nervous system-clinical features and imaging characteristics. Quant. Imaging Med. Surg. 2020;10:2227–2259. doi: 10.21037/qims-20-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sousa S.A., Feliciano J.R., Pita T., Soeiro C.F., Mendes B.L., Alves L.G., Leitao J.H. Bacterial Nosocomial Infections: Multidrug Resistance as a Trigger for the Development of Novel Antimicrobials. Antibiotics. 2021;10:942. doi: 10.3390/antibiotics10080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loscher W., Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 8.Gaddy J.A., Actis L.A. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009;4:273–278. doi: 10.2217/fmb.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galea I. The blood-brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021;18:2489–2501. doi: 10.1038/s41423-021-00757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X., Hussain B., Chang J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021;27:36–47. doi: 10.1111/cns.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teleanu R.I., Preda M.D., Niculescu A.G., Vladacenco O., Radu C.I., Grumezescu A.M., Teleanu D.M. Current Strategies to Enhance Delivery of Drugs across the Blood-Brain Barrier. Pharmaceutics. 2022;14:987. doi: 10.3390/pharmaceutics14050987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhowmik A., Khan R., Ghosh M.K. Blood brain barrier: A challenge for effectual therapy of brain tumors. BioMed Res. Int. 2015;2015:320941. doi: 10.1155/2015/320941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morse S.V., Mishra A., Chan T.G., de Rosales R.T.M., Choi J.J. Liposome delivery to the brain with rapid short-pulses of focused ultrasound and microbubbles. J. Control. Release. 2022;341:605–615. doi: 10.1016/j.jconrel.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Juhairiyah F., de Lange E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies that Provide Mechanistic Insights Are Essential. AAPS J. 2021;23:114. doi: 10.1208/s12248-021-00648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curcio M., Cirillo G., Rouaen J.R.C., Saletta F., Nicoletta F.P., Vittorio O., Iemma F. Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective. Pharmaceutics. 2020;12:1183. doi: 10.3390/pharmaceutics12121183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., Mehta A., Tong Z., Esser L., Voelcker N.H. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv. Sci. 2021;8:2003937. doi: 10.1002/advs.202003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyes W.K., van Thriel C. Neurotoxicology of Nanomaterials. Chem. Res. Toxicol. 2020;33:1121–1144. doi: 10.1021/acs.chemrestox.0c00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daraee H., Etemadi A., Kouhi M., Alimirzalu S., Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016;44:381–391. doi: 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- 19.Fikatas A., Dehairs J., Noppen S., Doijen J., Vanderhoydonc F., Meyen E., Swinnen J.V., Pannecouque C., Schols D. Deciphering the Role of Extracellular Vesicles Derived from ZIKV-Infected hcMEC/D3 Cells on the Blood-Brain Barrier System. Viruses. 2021;13:2363. doi: 10.3390/v13122363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correa R., Caballero Z., De Leon L.F., Spadafora C. Extracellular Vesicles Could Carry an Evolutionary Footprint in Interkingdom Communication. Front. Cell. Infect. Microbiol. 2020;10:76. doi: 10.3389/fcimb.2020.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendrix A., De Wever O. Systemically circulating bacterial extracellular vesicles: Origin, fate, and function. Trends Microbiol. 2022;30:213–216. doi: 10.1016/j.tim.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Fang Y., Wang Z., Liu X., Tyler B.M. Biogenesis and Biological Functions of Extracellular Vesicles in Cellular and Organismal Communication with Microbes. Front. Microbiol. 2022;13:817844. doi: 10.3389/fmicb.2022.817844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Beek L.F., Surmann K., van den Berg van Saparoea H.B., Houben D., Jong W.S.P., Hentschker C., Ederveen T.H.A., Mitsi E., Ferreira D.M., van Opzeeland F., et al. Exploring metal availability in the natural niche of Streptococcus pneumoniae to discover potential vaccine antigens. Virulence. 2020;11:1310–1328. doi: 10.1080/21505594.2020.1825908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malekan M., Siadat S.D., Aghasadeghi M., Shahrokhi N., Afrough P., Behrouzi A., Ahmadi K., Mousavi S.F. Evaluation of protective immunity responses against pneumococcal PhtD and its C-terminal in combination with outer-membrane vesicles as adjuvants. J. Med. Microbiol. 2020;69:465–477. doi: 10.1099/jmm.0.001103. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero-Mandujano A., Hernandez-Cortez C., Ibarra J.A., Castro-Escarpulli G. The outer membrane vesicles: Secretion system type zero. Traffic. 2017;18:425–432. doi: 10.1111/tra.12488. [DOI] [PubMed] [Google Scholar]
- 26.Schaar V., Nordstrom T., Morgelin M., Riesbeck K. Moraxella catarrhalis outer membrane vesicles carry beta-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob. Agents Chemother. 2011;55:3845–3853. doi: 10.1128/AAC.01772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuesta C.M., Guerri C., Urena J., Pascual M. Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication. Int. J. Mol. Sci. 2021;22:4235. doi: 10.3390/ijms22084235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T., Fahrmann J.F., Hanash S.M., Vykoukal J. Extracellular Vesicles Mediate B Cell Immune Response and Are a Potential Target for Cancer Therapy. Cells. 2020;9:1518. doi: 10.3390/cells9061518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubio A.P.D., D’Antoni C.L., Piuri M., Perez O.E. Probiotics, Their Extracellular Vesicles and Infectious Diseases. Front. Microbiol. 2022;13:864720. doi: 10.3389/fmicb.2022.864720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaghoubfar R., Behrouzi A., Ashrafian F., Shahryari A., Moradi H.R., Choopani S., Hadifar S., Vaziri F., Nojoumi S.A., Fateh A., et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020;10:22119. doi: 10.1038/s41598-020-79171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heavey M.K., Durmusoglu D., Crook N., Anselmo A.C. Discovery and delivery strategies for engineered live biotherapeutic products. Trends Biotechnol. 2022;40:354–369. doi: 10.1016/j.tibtech.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn T., Koch M., Fuhrmann G. Probiomimetics-Novel Lactobacillus-Mimicking Microparticles Show Anti-Inflammatory and Barrier-Protecting Effects in Gastrointestinal Models. Small. 2020;16:e2003158. doi: 10.1002/smll.202003158. [DOI] [PubMed] [Google Scholar]
- 33.Shandilya S., Kumar S., Jha N.K., Kesari K.K., Ruokolainen J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022;38:223–244. doi: 10.1016/j.jare.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahan S., Mukherjee S., Ali S., Bhardwaj U., Choudhary R.K., Balakrishnan S., Naseem A., Mir S.A., Banawas S., Alaidarous M., et al. Pioneer Role of Extracellular Vesicles as Modulators of Cancer Initiation in Progression, Drug Therapy, and Vaccine Prospects. Cells. 2022;11:490. doi: 10.3390/cells11030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elsharkasy O.M., Nordin J.Z., Hagey D.W., de Jong O.G., Schiffelers R.M., Andaloussi S.E., Vader P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong J.P., Holme M.N., Stevens M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loureiro A., da Silva G.J. CRISPR-Cas: Converting A Bacterial Defence Mechanism into A State-of-the-Art Genetic Manipulation Tool. Antibiotics. 2019;8:18. doi: 10.3390/antibiotics8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y., Gao K., Yang H. CRISPR/Cas: A potential gene-editing tool in the nervous system. Cell Regen. 2020;9:12. doi: 10.1186/s13619-020-00044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhea E.M., Banks W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019;13:521. doi: 10.3389/fnins.2019.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Govic Y., Demey B., Cassereau J., Bahn Y.S., Papon N. Pathogens infecting the central nervous system. PLoS Pathog. 2022;18:e1010234. doi: 10.1371/journal.ppat.1010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Festoff B.W., Sajja R.K., Cucullo L. Proximate Mediators of Microvascular Dysfunction at the Blood-Brain Barrier: Neuroinflammatory Pathways to Neurodegeneration. BioMed Res. Int. 2017;2017:1549194. doi: 10.1155/2017/1549194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaheryar Z.A., Khan M.A., Adnan C.S., Zaidi A.A., Hanggi D., Muhammad S. Neuroinflammatory Triangle Presenting Novel Pharmacological Targets for Ischemic Brain Injury. Front. Immunol. 2021;12:748663. doi: 10.3389/fimmu.2021.748663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connell J.J., Chatain G., Cornelissen B., Vallis K.A., Hamilton A., Seymour L., Anthony D.C., Sibson N.R. Selective permeabilization of the blood-brain barrier at sites of metastasis. J. Natl. Cancer Inst. 2013;105:1634–1643. doi: 10.1093/jnci/djt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banks W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9((Suppl. 1)):S3. doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardridge W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dando S.J., Mackay-Sim A., Norton R., Currie B.J., John J.A.S., Ekberg J.A., Batzloff M., Ulett G.C., Beacham I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014;27:691–726. doi: 10.1128/CMR.00118-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novakova I., Subileau E.A., Toegel S., Gruber D., Lachmann B., Urban E., Chesne C., Noe C.R., Neuhaus W. Transport rankings of non-steroidal antiinflammatory drugs across blood-brain barrier in vitro models. PLoS ONE. 2014;9:e86806. doi: 10.1371/journal.pone.0086806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchi N., Betto G., Fazio V., Fan Q., Ghosh C., Machado A., Janigro D. Blood-brain barrier damage and brain penetration of antiepileptic drugs: Role of serum proteins and brain edema. Epilepsia. 2009;50:664–677. doi: 10.1111/j.1528-1167.2008.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Appelboom G., Detappe A., LoPresti M., Kunjachan S., Mitrasinovic S., Goldman S., Chang S.D., Tillement O. Stereotactic modulation of blood-brain barrier permeability to enhance drug delivery. Neuro-Oncology. 2016;18:1601–1609. doi: 10.1093/neuonc/now137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salehi A., Paturu M.R., Patel B., Cain M.D., Mahlokozera T., Yang A.B., Lin T.H., Leuthardt E.C., Yano H., Song S.K., et al. Therapeutic enhancement of blood-brain and blood-tumor barriers permeability by laser interstitial thermal therapy. Neurooncol. Adv. 2020;2:vdaa071. doi: 10.1093/noajnl/vdaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karmur B.S., Philteos J., Abbasian A., Zacharia B.E., Lipsman N., Levin V., Grossman S., Mansouri A. Blood-Brain Barrier Disruption in Neuro-Oncology: Strategies, Failures, and Challenges to Overcome. Front. Oncol. 2020;10:563840. doi: 10.3389/fonc.2020.563840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffman P.S. Antibacterial Discovery: 21st Century Challenges. Antibiotics. 2020;9:213. doi: 10.3390/antibiotics9050213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saha M., Sarkar A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. J. Xenobiotics. 2021;11:197–214. doi: 10.3390/jox11040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun H., Zhang Q., Wang R., Wang H., Wong Y.T., Wang M., Hao Q., Yan A., Kao R.Y., Ho P.L., et al. Resensitizing carbapenem- and colistin-resistant bacteria to antibiotics using auranofin. Nat. Commun. 2020;11:5263. doi: 10.1038/s41467-020-18939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nation R.L., Li J. Colistin in the 21st century. Curr. Opin. Infect. Dis. 2009;22:535–543. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim B.-N., Peleg A.Y., Lodise T.P., Lipman J., Li J., Nation R., Paterson D.L. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect. Dis. 2009;9:245–255. doi: 10.1016/S1473-3099(09)70055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Q., Zhang X., Jia A., Huang Q., Jiang Y., Xie L. The Pharmacokinetics/Pharmacodynamics and Neurotoxicity of Tigecycline Intraventricular Injection for the Treatment of Extensively Drug-Resistant Acinetobacter baumannii Intracranial Infection. Infect. Drug Resist. 2022;15:4809–4817. doi: 10.2147/IDR.S377772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu X.B., Huang Y.Y., Zhang X.S., Wang Y.Z., Shi D.W., Zhang C.H., Chen J., Wang X.R., Lin G.Y. Intraventricular colistin sulphate as a last resort therapy in a patient with multidrug-resistant Acinetobacter baumannii induced post-neurosurgical ventriculitis. Br. J. Clin. Pharmacol. 2022;88:3490–3494. doi: 10.1111/bcp.15238. [DOI] [PubMed] [Google Scholar]
- 59.Lin M.F., Lan C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases. 2014;2:787–814. doi: 10.12998/wjcc.v2.i12.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X., Wang L., Ye Y.Z., Yu H. Postoperative multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intravenous doxycycline and intraventricular gentamicin: A case report. World J. Clin. Cases. 2019;7:4342–4348. doi: 10.12998/wjcc.v7.i24.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider F., Gessner A., El-Najjar N. Efficacy of Vancomycin and Meropenem in Central Nervous System Infections in Children and Adults: Current Update. Antibiotics. 2022;11:173. doi: 10.3390/antibiotics11020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pimentel E., Sivalingam K., Doke M., Samikkannu T. Effects of Drugs of Abuse on the Blood-Brain Barrier: A Brief Overview. Front. Neurosci. 2020;14:513. doi: 10.3389/fnins.2020.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang Y.-Q., Zhan R.-C., Jia W., Zhang B.-Q., Wang J.-J. A case report of intraventricular tigecycline therapy for intracranial infection with extremely drug resistant Acinetobacter baumannii. Medicine. 2017;96:e7703. doi: 10.1097/MD.0000000000007703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z., An Y., Li L., Yi H. Intrathecal Injection of Tigecycline and Polymyxin B in the Treatment of Extensively Drug-Resistant Intracranial Acinetobacter baumannii Infection: A Case Report and Review of the Literature. Infect. Drug Resist. 2022;15:1411–1423. doi: 10.2147/IDR.S354460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong Y.M., Zhang X.H., Ma Z., Liu W.E. Prevalence of Escherichia coli ST1193 Causing Intracranial Infection in Changsha, China. Trop. Med. Infect. Dis. 2022;7:217. doi: 10.3390/tropicalmed7090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coureuil M., Join-Lambert O., Lécuyer H., Bourdoulous S., Marullo S., Nassif X. Mechanism of meningeal invasion by Neisseria meningitidis. Virulence. 2014;3:164–172. doi: 10.4161/viru.18639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cutting A.S., Del Rosario Y., Mu R., Rodriguez A., Till A., Subramani S., Gottlieb R.A., Doran K.S. The Role of Autophagy during Group B Streptococcus Infection of Blood-Brain Barrier Endothelium. J. Biol. Chem. 2014;289:35711–35723. doi: 10.1074/jbc.M114.588657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parisi D.N., Martinez L.R. Intracellular Haemophilus influenzae invades the brain. Virulence. 2014;5:645–647. doi: 10.4161/viru.36086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chacko A., Delbaz A., Walkden H., Basu S., Armitage C.W., Eindorf T., Trim L.K., Miller E., West N.P., John J.A.S., et al. Chlamydia pneumoniae can infect the central nervous system via the olfactory and trigeminal nerves and contributes to Alzheimer’s disease risk. Sci. Rep. 2022;12:2759. doi: 10.1038/s41598-022-06749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guttman J.A., Samji F.N., Li Y., Vogl A.W., Finlay B.B. Evidence that Tight Junctions Are Disrupted Due to Intimate Bacterial Contact and Not Inflammation during Attaching and Effacing Pathogen Infection In Vivo. Infect. Immun. 2006;74:6075–6084. doi: 10.1128/IAI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doran K.S., Engelson E.J., Khosravi A., Maisey H.C., Fedtke I., Equils O., Michelsen K.S., Arditi M., Peschel A., Nizet V. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Investig. 2005;115:2499–2507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deatherage B.L., Cookson B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turkina M.V., Olofsson A., Magnusson K.E., Arnqvist A., Vikstrom E. Helicobacter pylori vesicles carrying CagA localize in the vicinity of cell-cell contacts and induce histone H1 binding to ATP in epithelial cells. FEMS Microbiol. Lett. 2015;362:fnv076. doi: 10.1093/femsle/fnv076. [DOI] [PubMed] [Google Scholar]
- 74.Prados-Rosales R., Baena A., Martinez L.R., Luque-Garcia J., Kalscheuer R., Veeraraghavan U., Camara C., Nosanchuk J.D., Besra G.S., Chen B., et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Investig. 2011;121:1471–1483. doi: 10.1172/JCI44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volgers C., Savelkoul P.H.M., Stassen F.R.M. Gram-negative bacterial membrane vesicle release in response to the host-environment: Different threats, same trick? Crit. Rev. Microbiol. 2018;44:258–273. doi: 10.1080/1040841X.2017.1353949. [DOI] [PubMed] [Google Scholar]
- 76.de Figueiredo P., Ficht T.A., Rice-Ficht A., Rossetti C.A., Adams L.G. Pathogenesis and immunobiology of brucellosis: Review of Brucella-host interactions. Am. J. Pathol. 2015;185:1505–1517. doi: 10.1016/j.ajpath.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Athman J.J., Wang Y., McDonald D.J., Boom W.H., Harding C.V., Wearsch P.A. Bacterial Membrane Vesicles Mediate the Release of Mycobacterium tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages. J. Immunol. 2015;195:1044–1053. doi: 10.4049/jimmunol.1402894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee E.Y., Choi D.Y., Kim D.K., Kim J.W., Park J.O., Kim S., Kim S.H., Desiderio D.M., Kim Y.K., Kim K.P., et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 79.Domingues S., Nielsen K.M. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr. Opin. Microbiol. 2017;38:16–21. doi: 10.1016/j.mib.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 80.Rumbo C., Fernandez-Moreira E., Merino M., Poza M., Mendez J.A., Soares N.C., Mosquera A., Chaves F., Bou G. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: A new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011;55:3084–3090. doi: 10.1128/AAC.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deo P., Chow S.H., Hay I.D., Kleifeld O., Costin A., Elgass K.D., Jiang J.H., Ramm G., Gabriel K., Dougan G., et al. Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathog. 2018;14:e1006945. doi: 10.1371/journal.ppat.1006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thay B., Wai S.N., Oscarsson J. Staphylococcus aureus alpha-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS ONE. 2013;8:e54661. doi: 10.1371/journal.pone.0054661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S., Gao J., Wang Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019;11:e1523. doi: 10.1002/wnan.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones L.B., Kumar S., Bell C.R., Crenshaw B.J., Coats M.T., Sims B., Matthews Q.L. Lipopolysaccharide Administration Alters Extracellular Vesicles in Cell Lines and Mice. Curr. Microbiol. 2021;78:920–931. doi: 10.1007/s00284-021-02348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fabrega M.J., Aguilera L., Gimenez R., Varela E., Canas M.A., Antolin M., Badia J., Baldoma L. Activation of Immune and Defense Responses in the Intestinal Mucosa by Outer Membrane Vesicles of Commensal and Probiotic Escherichia coli Strains. Front. Microbiol. 2016;7:705. doi: 10.3389/fmicb.2016.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dean S.N., Thakur M., Spangler J.R. Extracellular vesicle production in Gram-positive bacteria. Microb. Biotechnol. 2022;15:1055–1057. doi: 10.1111/1751-7915.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X., Thompson C.D., Weidenmaier C., Lee J.C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 2018;9:1379. doi: 10.1038/s41467-018-03847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toyofuku M., Carcamo-Oyarce G., Yamamoto T., Eisenstein F., Hsiao C.C., Kurosawa M., Gademann K., Pilhofer M., Nomura N., Eberl L. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat. Commun. 2017;8:481. doi: 10.1038/s41467-017-00492-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raghav A., Jeong G.B. A systematic review on the modifications of extracellular vesicles: A revolutionized tool of nano-biotechnology. J. Nanobiotechnol. 2021;19:459. doi: 10.1186/s12951-021-01219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zou C., Zhang Y., Liu H., Wu Y., Zhou X. Extracellular Vesicles: Recent Insights into the Interaction Between Host and Pathogenic Bacteria. Front. Immunol. 2022;13:840550. doi: 10.3389/fimmu.2022.840550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pant S., Hilton H., Burczynski M.E. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 2012;83:1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brakhage A.A., Zimmermann A.-K., Rivieccio F., Visser C., Blango M.G. Host-derived extracellular vesicles for antimicrobial defense. microLife. 2021;2:uqab003. doi: 10.1093/femsml/uqab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seo M.K., Park E.J., Ko S.Y., Choi E.W., Kim S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018;101:8662–8671. doi: 10.3168/jds.2018-15014. [DOI] [PubMed] [Google Scholar]
- 95.Morishita M., Horita M., Higuchi A., Marui M., Katsumi H., Yamamoto A. Characterizing Different Probiotic-Derived Extracellular Vesicles as a Novel Adjuvant for Immunotherapy. Mol. Pharm. 2021;18:1080–1092. doi: 10.1021/acs.molpharmaceut.0c01011. [DOI] [PubMed] [Google Scholar]
- 96.Kim J.H., Jeun E.J., Hong C.P., Kim S.H., Jang M.S., Lee E.J., Moon S.J., Yun C.H., Im S.H., Jeong S.G., et al. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J. Allergy Clin. Immunol. 2016;137:507–516.e8. doi: 10.1016/j.jaci.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y., Hoffmann J.P., Chou C.-W., Bentrup K.H.Z., Fuselier J.A., Bitoun J.P., Wimley W.C., Morici L.A. Burkholderia thailandensis outer membrane vesicles exert antimicrobial activity against drug-resistant and competitor microbial species. J. Microbiol. 2020;58:550–562. doi: 10.1007/s12275-020-0028-1. [DOI] [PubMed] [Google Scholar]
- 98.Costantini P.E., Vanpouille C., Firrincieli A., Cappelletti M., Margolis L., Palomino R.A.N. Extracellular Vesicles Generated by Gram-Positive Bacteria Protect Human Tissues Ex Vivo From HIV-1 Infection. Front. Cell. Infect. Microbiol. 2021;11:822882. doi: 10.3389/fcimb.2021.822882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim W., Lee E.J., Bae I.H., Myoung K., Kim S.T., Park P.J., Lee K.H., Pham A.V.Q., Ko J., Oh S.H., et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles. 2020;9:1793514. doi: 10.1080/20013078.2020.1793514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tartaglia N.R., Breyne K., Meyer E., Cauty C., Jardin J., Chretien D., Dupont A., Demeyere K., Berkova N., Azevedo V., et al. Staphylococcus aureus Extracellular Vesicles Elicit an Immunostimulatory Response in vivo on the Murine Mammary Gland. Front. Cell. Infect. Microbiol. 2018;8:277. doi: 10.3389/fcimb.2018.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee H., Yun S.H., Hyon J.Y., Lee S.Y., Yi Y.S., Choi C.W., Jun S., Park E.C., Kim S.I. Streptococcus equi-derived extracellular vesicles as a vaccine candidate against Streptococcus equi infection. Vet. Microbiol. 2021;259:109165. doi: 10.1016/j.vetmic.2021.109165. [DOI] [PubMed] [Google Scholar]
- 102.Islam S.M.S., Ryu H.M., Sohn S. Tetragenococcus halophilus Alleviates Intestinal Inflammation in Mice by Altering Gut Microbiota and Regulating Dendritic Cell Activation via CD83. Cells. 2022;11:1903. doi: 10.3390/cells11121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang W., Zhang Q., Li W., Chen Y., Shu C., Li Q., Zhou J., Ye C., Bai H., Sun W., et al. Anti-outer Membrane Vesicle Antibodies Increase Antibiotic Sensitivity of Pan-Drug-Resistant Acinetobacter baumannii. Front. Microbiol. 2019;10:1379. doi: 10.3389/fmicb.2019.01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wawrzeniak K., Gaur G., Sapi E., Senejani A.G. Effect of Borrelia burgdorferi Outer Membrane Vesicles on Host Oxidative Stress Response. Antibiotics. 2020;9:275. doi: 10.3390/antibiotics9050275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez M.F., Diaz P., Sandoval-Borquez A., Herrera D., Quest A.F.G. Helicobacter pylori Outer Membrane Vesicles and Extracellular Vesicles from Helicobacter pylori-Infected Cells in Gastric Disease Development. Int. J. Mol. Sci. 2021;22:4823. doi: 10.3390/ijms22094823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee W.H., Choi H.I., Hong S.W., Kim K.S., Gho Y.S., Jeon S.G. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp. Mol. Med. 2015;47:e183. doi: 10.1038/emm.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cai W., Kesavan D.K., Wan J., Abdelaziz M.H., Su Z., Xu H. Bacterial outer membrane vesicles, a potential vaccine candidate in interactions with host cells based. Diagn. Pathol. 2018;13:95. doi: 10.1186/s13000-018-0768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bottero D., Gaillard M.E., Errea A., Moreno G., Zurita E., Pianciola L., Rumbo M., Hozbor D. Outer membrane vesicles derived from Bordetella parapertussis as an acellular vaccine against Bordetella parapertussis and Bordetella pertussis infection. Vaccine. 2013;31:5262–5268. doi: 10.1016/j.vaccine.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 109.Armstrong D.A., Lee M.K., Hazlett H.F., Dessaint J.A., Mellinger D.L., Aridgides D.S., Hendricks G.M., Abdalla M.A.K., Christensen B.C., Ashare A. Extracellular Vesicles from Pseudomonas aeruginosa Suppress MHC-Related Molecules in Human Lung Macrophages. Immunohorizons. 2020;4:508–519. doi: 10.4049/immunohorizons.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramirez M.I., Amorim M.G., Gadelha C., Milic I., Welsh J.A., Freitas V.M., Nawaz M., Akbar N., Couch Y., Makin L., et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906. doi: 10.1039/C7NR08360B. [DOI] [PubMed] [Google Scholar]
- 111.Turturici G., Tinnirello R., Sconzo G., Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014;306:C621–C633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y., Xie Q., Zhang Y., Ma W., Ning K., Xiang J.Y., Cui J., Xiang H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020;104:335–349. doi: 10.1007/s00253-019-10259-6. [DOI] [PubMed] [Google Scholar]
- 113.Choi J.H., Moon C.M., Shin T.S., Kim E.K., McDowell A., Jo M.K., Joo Y.H., Kim S.E., Jung H.K., Shim K.N., et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 2020;52:423–437. doi: 10.1038/s12276-019-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hurkacz M., Dobrek L., Wiela-Hojenska A. Antibiotics and the Nervous System-Which Face of Antibiotic Therapy Is Real, Dr. Jekyll (Neurotoxicity) or Mr. Hyde (Neuroprotection)? Molecules. 2021;26:7456. doi: 10.3390/molecules26247456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ou J., Wang Z., Liu X., Song B., Chen J., Li R., Jia X., Huang R., Xiang W., Zhong S. Regulatory effects of marine polysaccharides on gut microbiota dysbiosis: A review. Food Chem. X. 2022;15:100444. doi: 10.1016/j.fochx.2022.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shaik L., Kashyap R., Thotamgari S.R., Singh R., Khanna S. Gut-Brain Axis and its Neuro-Psychiatric Effects: A Narrative Review. Cureus. 2020;12:e11131. doi: 10.7759/cureus.11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rutsch A., Kantsjo J.B., Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020;11:604179. doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McVey Neufeld K.A., Mao Y.K., Bienenstock J., Foster J.A., Kunze W.A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013;25:183-e88. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 119.Celorrio M., Abellanas M.A., Rhodes J., Goodwin V., Moritz J., Vadivelu S., Wang L., Rodgers R., Xiao S., Anabayan I., et al. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol. Commun. 2021;9:40. doi: 10.1186/s40478-021-01137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Palomino R.A.N., Vanpouille C., Costantini P.E., Margolis L. Microbiota-host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog. 2021;17:e1009508. doi: 10.1371/journal.ppat.1009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng H.Y., Ning M.X., Chen D.K., Ma W.T. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019;10:607. doi: 10.3389/fimmu.2019.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaparakis-Liaskos M., Ferrero R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015;15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 123.Shen Q., Huang Z., Yao J., Jin Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J. Adv. Res. 2022;37:221–233. doi: 10.1016/j.jare.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Battistelli M., Falcieri E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology. 2020;9:21. doi: 10.3390/biology9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu Q.Y., Liu B.C., Ruan X.Z., Ma K.L. Intestinal microbiota-derived membrane vesicles and their role in chronic kidney disease. Biochim. Biophys. Acta Mol. Basis Dis. 2022;1868:166478. doi: 10.1016/j.bbadis.2022.166478. [DOI] [PubMed] [Google Scholar]
- 127.Liu S., Gao J., Zhu M., Liu K., Zhang H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020;57:5026–5043. doi: 10.1007/s12035-020-02073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee K.E., Kim J.K., Han S.K., Lee D.Y., Lee H.J., Yim S.V., Kim D.H. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8:107. doi: 10.1186/s40168-020-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singhrao S.K., Olsen I. Are Porphyromonas gingivalis Outer Membrane Vesicles Microbullets for Sporadic Alzheimer’s Disease Manifestation? J. Alzheimer’s Dis. Rep. 2018;2:219–228. doi: 10.3233/ADR-180080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pokusaeva K., Johnson C., Luk B., Uribe G., Fu Y., Oezguen N., Matsunami R.K., Lugo M., Major A., Mori-Akiyama Y., et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017;29:e12904. doi: 10.1111/nmo.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Neufeld K.-A.M., Kang N., Bienenstock J., Foster J.A. Effects of intestinal microbiota on anxiety-like behavior. Commun. Integr. Biol. 2014;4:492–494. doi: 10.4161/cib.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Derrien M., van Hylckama Vlieg J.E. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 134.Dean S.N., Rimmer M.A., Turner K.B., Phillips D.A., Caruana J.C., Hervey W.J.T., Leary D.H., Walper S.A. Lactobacillus acidophilus Membrane Vesicles as a Vehicle of Bacteriocin Delivery. Front. Microbiol. 2020;11:710. doi: 10.3389/fmicb.2020.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choi J., Kim Y.K., Han P.L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019;28:158–171. doi: 10.5607/en.2019.28.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.West C.L., Stanisz A.M., Mao Y.K., Champagne-Jorgensen K., Bienenstock J., Kunze W.A. Microvesicles from Lactobacillus reuteri (DSM-17938) completely reproduce modulation of gut motility by bacteria in mice. PLoS ONE. 2020;15:e0225481. doi: 10.1371/journal.pone.0225481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haas-Neill S., Iwashita E., Dvorkin-Gheva A., Forsythe P. Effects of Two Distinct Psychoactive Microbes, Lacticaseibacillus rhamnosus JB-1 and Limosilactobacillus reuteri 6475, on Circulating and Hippocampal mRNA in Male Mice. Int. J. Mol. Sci. 2022;23:9653. doi: 10.3390/ijms23179653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xiao M., Huang X. Unmasking antibiotic-associated neurological disorders: The underminer in Intensive Care Unit. J. Clin. Neurosci. 2021;91:131–135. doi: 10.1016/j.jocn.2021.06.040. [DOI] [PubMed] [Google Scholar]
- 139.Wanleenuwat P., Suntharampillai N., Iwanowski P. Antibiotic-induced epileptic seizures: Mechanisms of action and clinical considerations. Seizure. 2020;81:167–174. doi: 10.1016/j.seizure.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 140.Kim K.S. Investigating Bacterial Penetration of the Blood-Brain Barrier for the Pathogenesis, Prevention, and Therapy of Bacterial Meningitis. ACS Infect. Dis. 2020;6:34–42. doi: 10.1021/acsinfecdis.9b00319. [DOI] [PubMed] [Google Scholar]
- 141.Nau R., Sorgel F., Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010;23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jimenez-Mejias M.E., Pichardo-Guerrero C., Marquez-Rivas F.J., Martin-Lozano D., Prados T., Pachon J. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 2002;21:212–214. doi: 10.1007/s10096-001-0680-2. [DOI] [PubMed] [Google Scholar]
- 143.Cascio A., Conti A., Sinardi L., Iaria C., Angileri F.F., Stassi G., David T., Versaci A., Iaria M., David A. Post-neurosurgical multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intrathecal colistin. A new case and a systematic review of the literature. Int. J. Infect. Dis. 2010;14:e572–e579. doi: 10.1016/j.ijid.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 144.Fu J., Li L., Huo D., Yang R., Yang B., Xu B., Yang X., Dai M., Tan C., Chen H., et al. Meningitic Escherichia coli alpha-hemolysin aggravates blood-brain barrier disruption via targeting TGFbeta1-triggered hedgehog signaling. Mol. Brain. 2021;14:116. doi: 10.1186/s13041-021-00826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Caporarello N., Olivieri M., Cristaldi M., Scalia M., Toscano M.A., Genovese C., Addamo A., Salmeri M., Lupo G., Anfuso C.D. Blood-Brain Barrier in a Haemophilus influenzae Type a In Vitro Infection: Role of Adenosine Receptors A2A and A2B. Mol. Neurobiol. 2018;55:5321–5336. doi: 10.1007/s12035-017-0769-y. [DOI] [PubMed] [Google Scholar]
- 146.Wegele C., Stump-Guthier C., Moroniak S., Weiss C., Rohde M., Ishikawa H., Schroten H., Schwerk C., Karremann M., Borkowski J. Non-Typeable Haemophilus influenzae Invade Choroid Plexus Epithelial Cells in a Polar Fashion. Int. J. Mol. Sci. 2020;21:5739. doi: 10.3390/ijms21165739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ku Y.H., Chuang Y.C., Chen C.C., Lee M.F., Yang Y.C., Tang H.J., Yu W.L. Klebsiella pneumoniae Isolates from Meningitis: Epidemiology, Virulence and Antibiotic Resistance. Sci. Rep. 2017;7:6634. doi: 10.1038/s41598-017-06878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cassidy B.R., Zhang M., Sonntag W.E., Drevets D.A. Neuroinvasive Listeria monocytogenes infection triggers accumulation of brain CD8+ tissue-resident memory T cells in a miR-155-dependent fashion. J. Neuroinflammation. 2020;17:259. doi: 10.1186/s12974-020-01929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schubert-Unkmeir A., Konrad C., Slanina H., Czapek F., Hebling S., Frosch M. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: A role for MMP-8. PLoS Pathog. 2010;6:e1000874. doi: 10.1371/journal.ppat.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Orihuela C.J., Mahdavi J., Thornton J., Mann B., Wooldridge K.G., Abouseada N., Oldfield N.J., Self T., Ala’Aldeen D.A., Tuomanen E.I. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J. Clin. Investig. 2009;119:1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen H., Guo X., Xie D., Dong X., Niu J., Chen G. A Clinical Study on the Use of Intraventricular Polymyxin B Supplemented by Continuous External Ventricular Drainage in the Treatment of Drug-Resistant Gram-Negative Bacilli Intracranial Infection. Infect. Drug Resist. 2020;13:2963–2970. doi: 10.2147/IDR.S261510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McLoughlin A., Rochfort K.D., McDonnell C.J., Kerrigan S.W., Cummins P.M. Staphylococcus aureus-mediated blood-brain barrier injury: An in vitro human brain microvascular endothelial cell model. Cell. Microbiol. 2017;19:e12664. doi: 10.1111/cmi.12664. [DOI] [PubMed] [Google Scholar]
- 153.Anil A., Banerjee A. Pneumococcal Encounter with the Blood-Brain Barrier Endothelium. Front. Cell. Infect. Microbiol. 2020;10:590682. doi: 10.3389/fcimb.2020.590682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Iovino F., Seinen J., Henriques-Normark B., van Dijl J.M. How Does Streptococcus pneumoniae Invade the Brain? Trends Microbiol. 2016;24:307–315. doi: 10.1016/j.tim.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 155.Zocco M.A., dal Verme L.Z., Cremonini F., Piscaglia A.C., Nista E.C., Candelli M., Novi M., Rigante D., Cazzato I.A., Ojetti V., et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006;23:1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 156.Tsai Y.-L., Tsai W.-C., Qing Z., Chang C.-J. Dichotomous effects of microbial membrane vesicles on the regulation of immunity. Med. Microecol. 2020;3:100009. doi: 10.1016/j.medmic.2020.100009. [DOI] [Google Scholar]
- 157.Ramachandran G., Bikard D. Editing the microbiome the CRISPR way. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374:20180103. doi: 10.1098/rstb.2018.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y., Hoffmann J.P., Baker S.M., Bentrup K.H.Z., Wimley W.C., Fuselier J.A., Bitoun J.P., Morici L.A. Inhibition of Streptococcus mutans biofilms with bacterial-derived outer membrane vesicles. BMC Microbiol. 2021;21:234. doi: 10.1186/s12866-021-02296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hu R., Lin H., Li J., Zhao Y., Wang M., Sun X., Min Y., Gao Y., Yang M. Probiotic Escherichia coli Nissle 1917-derived outer membrane vesicles enhance immunomodulation and antimicrobial activity in RAW264.7 macrophages. BMC Microbiol. 2020;20:268. doi: 10.1186/s12866-020-01953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chee W.J.Y., Chew S.Y., Than L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020;19:203. doi: 10.1186/s12934-020-01464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qin H., Zhang Z., Hang X., Jiang Y.L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009;9:63. doi: 10.1186/1471-2180-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bajic S.S., Canas M.A., Tolinacki M., Badia J., Sanchez B., Golic N., Margolles A., Baldoma L., Ruas-Madiedo P. Proteomic profile of extracellular vesicles released by Lactiplantibacillus plantarum BGAN8 and their internalization by non-polarized HT29 cell line. Sci. Rep. 2020;10:21829. doi: 10.1038/s41598-020-78920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Han E.C., Choi S.Y., Lee Y., Park J.W., Hong S.H., Lee H.J. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood–brain barrier in mice. FASEB J. 2019;33:13412. doi: 10.1096/fj.201901575R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Al-Nedawi K., Mian M.F., Hossain N., Karimi K., Mao Y.K., Forsythe P., Min K.K., Stanisz A.M., Kunze W.A., Bienenstock J. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2015;29:684–695. doi: 10.1096/fj.14-259721. [DOI] [PubMed] [Google Scholar]
- 166.Haas-Neill S., Forsythe P. A Budding Relationship: Bacterial Extracellular Vesicles in the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2020;21:8899. doi: 10.3390/ijms21238899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mba I.E., Nweze E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021;37:108. doi: 10.1007/s11274-021-03070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rudramurthy G.R., Swamy M.K., Sinniah U.R., Ghasemzadeh A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules. 2016;21:836. doi: 10.3390/molecules21070836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 170.Van de Waterbeemd B., Streefland M., van der Ley P., Zomer B., van Dijken H., Martens D., Wijffels R., van der Pol L. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine. 2010;28:4810–4816. doi: 10.1016/j.vaccine.2010.04.082. [DOI] [PubMed] [Google Scholar]
- 171.Van der Pol L., Stork M., van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015;10:1689–1706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Meng W., He C., Hao Y., Wang L., Li L., Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhu Z., Antenucci F., Villumsen K.R., Bojesen A.M. Bacterial Outer Membrane Vesicles as a Versatile Tool in Vaccine Research and the Fight against Antimicrobial Resistance. mBio. 2021;12:e0170721. doi: 10.1128/mBio.01707-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu M., Feng T., Liu B., Qiu F., Xu Y., Zhao Y., Zheng Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926–8944. doi: 10.7150/thno.62330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tiwari S., Jamal S.B., Hassan S.S., Carvalho P., Almeida S., Barh D., Ghosh P., Silva A., Castro T.L.P., Azevedo V. Two-Component Signal Transduction Systems of Pathogenic Bacteria as Targets for Antimicrobial Therapy: An Overview. Front. Microbiol. 2017;8:1878. doi: 10.3389/fmicb.2017.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dobson A., Cotter P.D., Ross R.P., Hill C. Bacteriocin production: A probiotic trait? Appl. Environ. Microbiol. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jakava-Viljanen M., Avall-Jaaskelainen S., Messner P., Sleytr U.B., Palva A. Isolation of three new surface layer protein genes (slp) from Lactobacillus brevis ATCC 14869 and characterization of the change in their expression under aerated and anaerobic conditions. J. Bacteriol. 2002;184:6786–6795. doi: 10.1128/JB.184.24.6786-6795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim J.Y., Doody A.M., Chen D.J., Cremona G.H., Shuler M.L., Putnam D., DeLisa M.P. Engineered bacterial outer membrane vesicles with enhanced functionality. J. Mol. Biol. 2008;380:51–66. doi: 10.1016/j.jmb.2008.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Uchida H., Kinoshita H., Kawai Y., Kitazawa H., Miura K., Shiiba K., Horii A., Kimura K., Taketomo N., Oda M., et al. Lactobacilli binding human A-antigen expressed in intestinal mucosa. Res. Microbiol. 2006;157:659–665. doi: 10.1016/j.resmic.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 180.Klotz C., Barrangou R. Engineering Components of the Lactobacillus S-Layer for Biotherapeutic Applications. Front. Microbiol. 2018;9:2264. doi: 10.3389/fmicb.2018.02264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee S.H., Kim M.S., Jung H.C., Lee J., Lee J.H., Lee H.S., Kang S.G. Screening of a novel strong promoter by RNA sequencing and its application to H2 production in a hyperthermophilic archaeon. Appl. Microbiol. Biotechnol. 2015;99:4085–4092. doi: 10.1007/s00253-015-6444-1. [DOI] [PubMed] [Google Scholar]
- 182.Parker A., Fonseca S., Carding S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11:135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao L., Ye Y., Gu L., Jian Z., Stary C.M., Xiong X. Extracellular vesicle-derived miRNA as a novel regulatory system for bi-directional communication in gut-brain-microbiota axis. J. Transl. Med. 2021;19:202. doi: 10.1186/s12967-021-02861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Layunta E., Buey B., Mesonero J.E., Latorre E. Crosstalk Between Intestinal Serotonergic System and Pattern Recognition Receptors on the Microbiota-Gut-Brain Axis. Front. Endocrinol. 2021;12:748254. doi: 10.3389/fendo.2021.748254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wynendaele E., Verbeke F., Stalmans S., Gevaert B., Janssens Y., Van De Wiele C., Peremans K., Burvenich C., De Spiegeleer B. Quorum Sensing Peptides Selectively Penetrate the Blood-Brain Barrier. PLoS ONE. 2015;10:e0142071. doi: 10.1371/journal.pone.0142071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abib R.T., Gaman A., Dargel A.A., Tamouza R., Kapczinski F., Gottfried C., Leboyer M. Intracellular Pathogen Infections and Immune Response in Autism. Neuroimmunomodulation. 2018;25:271–279. doi: 10.1159/000491821. [DOI] [PubMed] [Google Scholar]
- 187.Garcia-Gutierrez E., Narbad A., Rodriguez J.M. Autism Spectrum Disorder Associated with Gut Microbiota at Immune, Metabolomic, and Neuroactive Level. Front. Neurosci. 2020;14:578666. doi: 10.3389/fnins.2020.578666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jodoin J., Demeule M., Fenart L., Cecchelli R., Farmer S., Linton K.J., Higgins C.F., Beliveau R. P-glycoprotein in blood-brain barrier endothelial cells: Interaction and oligomerization with caveolins. J. Neurochem. 2003;87:1010–1023. doi: 10.1046/j.1471-4159.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- 189.Grill M.F., Maganti R.K. Neurotoxic effects associated with antibiotic use: Management considerations. Br. J. Clin. Pharmacol. 2011;72:381–393. doi: 10.1111/j.1365-2125.2011.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fazal S., Lee R. Biomimetic Bacterial Membrane Vesicles for Drug Delivery Applications. Pharmaceutics. 2021;13:1430. doi: 10.3390/pharmaceutics13091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen G., Bai Y., Li Z., Wang F., Fan X., Zhou X. Bacterial extracellular vesicle-coated multi-antigenic nanovaccines protect against drug-resistant Staphylococcus aureus infection by modulating antigen processing and presentation pathways. Theranostics. 2020;10:7131–7149. doi: 10.7150/thno.44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu Y., Battalapalli D., Hakeem M.J., Selamneni V., Zhang P., Draz M.S., Ruan Z. Engineered CRISPR-Cas systems for the detection and control of antibiotic-resistant infections. J. Nanobiotechnol. 2021;19:401. doi: 10.1186/s12951-021-01132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Oh J.H., van Pijkeren J.P. CRISPR-Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res. 2014;42:e131. doi: 10.1093/nar/gku623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schuster J.A., Vogel R.F., Ehrmann M.A. Characterization and distribution of CRISPR-Cas systems in Lactobacillus sakei. Arch. Microbiol. 2019;201:337–347. doi: 10.1007/s00203-019-01619-x. [DOI] [PubMed] [Google Scholar]
- 195.Loo Y.S., Bose R.J., McCarthy J.R., Azmi I.D.M., Madheswaran T. Biomimetic bacterial and viral-based nanovesicles for drug delivery, theranostics, and vaccine applications. Drug Discov. Today. 2021;26:902–915. doi: 10.1016/j.drudis.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 196.Zambrowicz B.P., Sands A.T. Knockouts model the 100 best-selling drugs—Will they model the next 100? Nat. Rev. Drug Discov. 2003;2:38–51. doi: 10.1038/nrd987. [DOI] [PubMed] [Google Scholar]
- 197.Choi S.Y., Lim S., Cho G., Kwon J., Mun W., Im H., Mitchell R.J. Chromobacterium violaceum delivers violacein, a hydrophobic antibiotic, to other microbes in membrane vesicles. Environ. Microbiol. 2020;22:705–713. doi: 10.1111/1462-2920.14888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.