Abstract
Klotho (KL) was initially thought to be a typical "ageing suppressor" gene, but recent studies have suggested that KL is involved in the progression of several types of human cancer. This study aims to analyse whether the expression level of KL could impact patient prognosis, clinical parameters, and tumour immunity in different tumour patients. KL activity was utilized to determine differences between the KL transcript and KL protein. Expression levels were detected by single-sample gene enrichment analysis (GSEA). To explore the inherent mechanisms of KL in tumour immunotherapy, we investigated the possible impact of KL on the tumour microenvironment, immune processes and immune components. GO and KEGG analysis showed that KL was significantly involved in immune response, Inflammation, and calcium signaling pathway. We also found that KL was significantly correlated with multiple immunotherapeutic biomarkers (TMB, MSI, CD274, PDCD1, CTLA4 and TIGIT) in a variety of tumours. Furthermore, increased KL expression was closely associated with the non-response of anti-PD-1 immunotherapy, indicating that KL might affect the response sensitivity of tumour patients to anti-PD-1 immunotherapy. This study will provide a basis for further research on how KL regulates tumour immune cells and may lead to the development of more effective target tumour immunotherapy.
Keywords: Klotho, Pan-cancer, Immune, Immunotherapy, Biomarkers
Highlights
-
•
KL is highly associated with prognostic in some cancers, especially KIRC and KIRP.
-
•
KL was correlated with multiple immunotherapeutic biomarkers in some cancers.
-
•
KL was significantly correlated with various immune cells in a variety of tumors.
-
•
KL response the sensitivity of tumor patients of anti-PD-1 immunotherapy.
-
•
This study may lead to the development of more effective target tumor immunotherapy.
Klotho; Pan-Cancer; immune; immunotherapy; biomarkers.
1. Introduction
Cancer is recognized to be associated with age, as the incidence of many cancers increases with age [1]. Human malignancy is predicted to be the leading cause of death and the single most important obstacle to the increase of life expectancy in the 21st century [2]. The cancers with the highest incidences among men are Colorectal Cancer, Prostate Cancer and skin Melanoma, while Breast Cancer, Uterine Cancer, and Colorectal Cancer are the most prevalent among women [3]. Given the high mortality and tumour incidence, it is crucial to employ a pan-cancer expression analysis of any target gene of interest and assess its correlation with potential therapeutic methods. At present, the most common treatments for tumours are surgery, chemotherapy and radiotherapy, but the curative effects are not satisfactory [4]. Immunotherapy has led to considerable advances in cancer treatment in recent years. Several cancer immunotherapy drugs have been approved for clinical use by the United States Food and Drug Administration Cancer immunotherapy was ranked as the most important scientific breakthrough of the year by Science in 2013 due to its outstanding efficacy and innovation [5, 6]. These novel cancer immunotherapy approaches have attracted considerable attention and are thought to be able to cure various cancers [7, 8]. Long recognized for its role in antiaging, Klotho (KL) has been reported to be involved in the pathogenesis of numerous autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis and systemic sclerosis [9]. Recently a few studies have shown that KL participates in the progression of several types of human cancer [10, 11, 12, 13, 14]. The roles of Klotho in these human cancers are of significantly high interest. However, the role of KL in cancer immunotherapy and its underlying mechanisms have not yet been fully studied. Overall, the study examined the possible association of KL with immunotherapy in 33 different cancers.
In our study, we demonstrate the KL expression landscape of 33 kinds of tumour and examine the possible impact of KL on the tumour immune microenvironment. Therefore, we examined various biomarkers for immunotherapy, including tumour mutational burden (TMB), microsatellite instability (MSI) and CD274 molecule (CD274/PD-L1), PDCD1 programmed cell death 1 (PDCD1/PD-1), cytotoxic T-lymphocyte associated protein 4 (CTLA4) and T-cell immunoglobulin and ITIM domains (TIGIT). In addition, the correlativity between the KL expression and immune checkpoint blockade (ICB) therapy was also investigated. Collectively, our study provides evidence for elucidating the immunotherapeutic role of KL in tumours and provides a basis for further mechanistic studies.
2. Materials and methods
2.1. Data collection
Clinical and genomic information associated with 33 human tumours was extracted from The Cancer Genome Atlas database (TCGA database: https://portal.gdc.cancer.gov/) and UC Santa Cruz Xena Explorer (TCGA Pan-Cancer Cohort) [15]. In addition, through systematic studies, we identified groups of therapies that block immune checkpoints that are publicly reported and contain comprehensive clinical information. Finally, three immunotherapy cohorts were selected: advanced urothelial carcinoma treated with anti-PD-L1 checkpoint inhibition (Atezolizumab, IMvigor210 cohort) [16],PD-1 inhibitor therapy in metastatic melanoma and renal cell carcinoma (Pembrolizumab, cohort GSE78220; Nivolumab, cohort GSE67501). These therapeutic cohorts were downloaded from previously published research and the Gene Expression Omnibus database.
2.2. Correlation between KL expression and clinical prognosis in different cancers
To confirm whether KL expression differs between tumour and adjacent normal groups, we analysed differences in gene expression by using the R package "limma". We also investigated the possible correlation between KL expression and age, gender, and tumour stage. In addition, one-way Cox regression analysis using the R package (“survival”) and Kaplan–Meier survival method was carried out to examine the prognostic value of KL in 33 tumours. If the hazard ratio exceeds one (HR > 1), KL expression may lead to the occurrence of a positive event (death). When the Overall Survival test P < 0.05 in the univariate cox regression analysis and Kaplan–Meier chart met the difference simultaneously, we found that KL expression significantly affected tumour prognosis.
2.3. Generation and investigation of KL activity
A single-sample GSEA (ssGSEA) assay was used to detect KL protein levels in 33 cancer patients [17]. ssGSEA is an extension of the GSEA method to calculate the enrichment fraction of each sample paired with the gene set [18]. In our study, each ssGSEA enrichment score represented the degree to which the top 100 genes associated with KL in the tumor were harmoniously up-regulated or down-regulated as a specific gene set. In this way, ssGSEA converts the gene expression profile of a single sample into a gene-set enrichment profile. This transformation allows researchers to describe cell states based on the activity levels of biological processes and pathways rather than the expression levels of individual genes. Therefore, ssGESA can calculate the activity score of the KL at the protein level if it uses a gene set associated with the KL gene. The activity of KL in the tumour and adjacent normal groups was then compared. Subsequently, the potential association of KL expression with cancer types was explored by averaging KL expression and activity in 33 cancers.
2.4. Analysis of the potential association between KL expression and tumour purity, the infiltrating stromal and immune cells
The ESTIMATE software package was used to calculate the stromal score and immune score [19]. Based on single sample GSEA, tumour purity was calculated according to the stromal score and immune score. Furthermore, CIBERSORT algorithm was used to assess the incidence of immune cell invasion in low- and high-expressing KL groups [20]. In conclusion, the number of permutations was set to 1,000 and if they had a p value <0.05, the samples in the tumour-cohort were valuable for further investigation.
2.5. Analysis of potential association between KL expression and TMB, MSI, CD274, PDCD1, CTLA4 and TIGIT
Previous reports suggest that the biomarkers of ICB are significantly related to the immune response. For this reason, the relationship between the expression of KL and these indicators was explored. To calculate the TMB of each case, the total number of mutations was divided by the exome size (38 Mb). The MSI score for each case was derived from a previous report [21]. Moreover, the potential association between the expression of KL and immune checkpoint biomarkers (CD274, PDCD1, CTLA4 and TIGIT) and modulators (immunosuppressants, immunostimulants, and MHC molecules) was analysed through the TIMER website (http://timer.comp-genomics.org) and TISIDB website (http://cis.hku.hk/TISIDB/index.php). The most relevant results are presented in plots and displayed on the graph.
2.6. GO and KEGG analysis of KL in GSEA
To better understand the related gene product function and signalling pathways, we used GSEA to define the differences between the low- and high-expressing KL groups, which were defined and derived from the GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) databases. The results are shown in the figures if they met certain criteria (p < 0.05) and were within the five highest normalized enrichment scores.
2.7. Analysis of immunotherapeutic response
In this study, we finally selected and analysed three relatively independent immunotherapy cohorts. Patients who achieved complete response or partial response were considered as responders, while those who exhibited symptoms of stable disease or progressive disease were considered non-responders. We used the Wilcoxon test to determine differences in the expression of KL between responders and non-responders.
3. Results
3.1. Expression level of KL in 33 human cancers
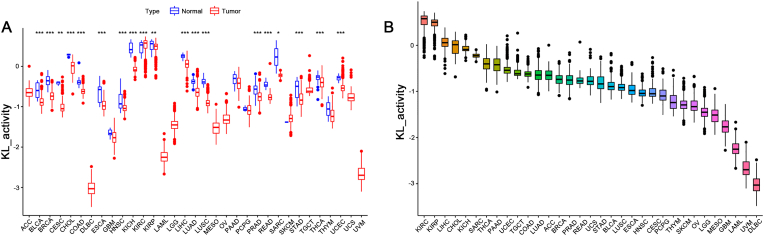
The abbreviations and full names of the various human cancers included in our study are shown in Table 1. As shown in Figure 1A, KL expression differed in 20 of 33 cancers. We ranked the 33 cancers based on the expression level of KL (Figure 1B).
Table 1.
The abbreviations and full names of the various human cancers.
| Abbreviation | Full Name | Abbreviation | Full Name |
|---|---|---|---|
| ACC | Adrenocortical carcinoma | LUSC | Lung squamous cell carcinoma |
| BLCA | Bladder Urothelial Carcinoma | MESO | Mesothelioma |
| BRCA | Breast invasive carcinoma | OV | Ovarian serous cystadenocarcinoma |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma | PAAD | Pancreatic adenocarcinoma |
| CHOL | Cholangiocarcinoma | PCPG | Pheochromocytoma and Paraganglioma |
| COAD | Colon adenocarcinoma | PRAD | Prostate adenocarcinoma |
| DLBC | Lymphoid Neoplasm Diffuse Large B-cell Lymphoma | READ | Rectum adenocarcinoma |
| ESCA | Esophageal carcinoma | SARC | Sarcoma |
| GBM | Glioblastoma multiforme | SKCM | Skin Cutaneous Melanoma |
| HNSC | Head and Neck squamous cell carcinoma | STAD | Stomach adenocarcinoma |
| KICH | Kidney Chromophobe | TGCT | Testicular Germ Cell Tumors |
| KIRC | Kidney renal clear cell carcinoma | THCA | Thyroid carcinoma |
| KIRP | Kidney renal papillary cell carcinoma | THYM | Thymoma |
| LAML | Acute Myeloid Leukemia | UCEC | Uterine Corpus Endometrial Carcinoma |
| LGG | Brain Lower Grade Glioma | UCS | Uterine Carcinosarcoma |
| LIHC | Liver hepatocellular carcinoma | UVM | Uveal Melanoma |
| LUAD | Lung adenocarcinoma |
Figure 1.
KL expression landscape in pan-cancer and its correlation with clinical parameters (A) indicates difference analysis of KL expression between tumour and adjacent normal groups in 33 cancers (B) shows the rank of the KL expression in 33 human cancers (C) represents the association between KL and age (D) demonstrates the association between KL and gender (E) shows the association between KL and tumour stage. P values were presented as: ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
3.2. Association between KL expression and age, gender and tumour stage in 33 human cancers
KL was very different in elderly patients in the ESCA and THYM groups, but weaker in the ACC, COAD, PRAD, SKCM, and THCA groups (Figure 1C). Furthermore, KL expression was significantly correlated with tumour stage in eight human tumours (Figure 1D). In addition, results did not show significant gender differences in KL expression in most tumours (Figure 1E).
3.3. Activity level of KL in 33 human cancers
Significantly increased KL activity was observed in the KIRC tumour group, whereas it was decreased in 18 tumour groups (Figure 2A). Similarly, the 33 cancers were classified according to the level of KL activity (Figure 2B). When combining the results from Figure 1A and 2A, 11 tumours showed relatively higher KL expression (p < 0.001) and activity (p < 0.001).
Figure 2.
Generation and investigation of KL activity (A) demonstrates different analyses of KL activity between the tumour and adjacent normal group in 33 cancers (B) shows the rank of the KL activity in 33 human cancers.
3.4. Prognostic value of KL in 33 human cancers
In addition, the results of Cox analysis and Kaplan–Meier analysis showed that the positive correlation was significant between KL expression and Overall Survival in ACC, HNSC, KIRC, KIRP, LUAD, PAAD and SARC (Figure 3A, S1A). With regard to KL and Disease Free Survival, an apparent positive association was observed in SARC (Figure 3B, S1B). For Disease Specific Survival, KL expression was protected in PAAD, LUAD, KIRP, KIRC and HNSC (Figure 3C, S1C). Progression Free Survival analysis indicated the protective role of KL expression in HNSC, KIRP, KIRC, PAAD and PRAD (Figure 3D, S1D).
Figure 3.
The relativity between the KL and the prognosis of patients in the forest plots.
3.5. Analysis of potential association between KL expression and tumour purity, the infiltrating stromal and immune cells
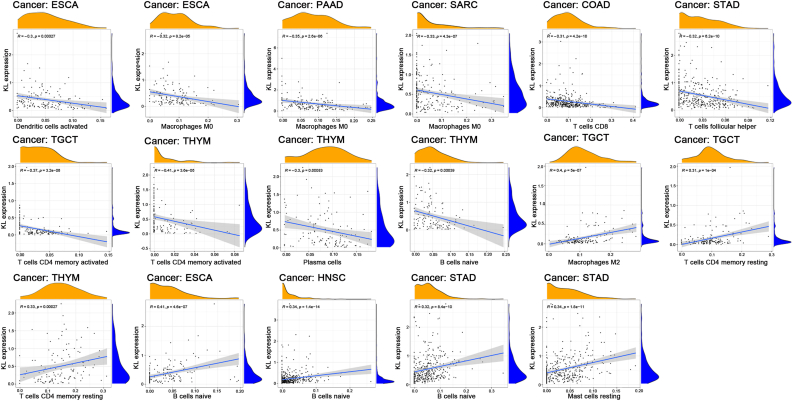
The expression of KL was strongly related to stromal scores in MESO, SKCM, STAD, TGCT and UCS, but it did not show a relationship with the immune score (p < 0.01 and |R| > 0.5, Figure 4). We further explored the relationship between KL expression and immune cell infiltration and the results showed that, elevated KL expression was inversely correlated with the infiltration levels of activated DCs in ESCA, M0 macrophages in ESCA, PAAD and SARC, CD8 T cells in COAD, follicular helper T cells in STAD, activated memory CD4 T cells in TGCT and THYM, and plasma cells and naïve B cells in THYM. However, increased KL expression was positively correlated with the infiltration levels of resting M2 macrophages and resting memory CD4 T cells in TGCT, resting memory CD4 T cells in THYM, naïve B cells in ESCA, HNSC and STAD, mast cells in STAD (p < 0.01 and |R| > 0.3, Figure 5).
Figure 4.
The relativity between the expression level of KL expression and the ESTIMATE score. The correlation plots were presented when R > 0.5 and p < 0.001.
Figure 5.
The relativity between the expression level of KL expression and the immune cell infiltration. The correlation plots were presented when R > 0.3 and p < 0.001.
3.6. Relativity between KL expression and immune modulators
Furthermore, we examined the association of KL expression with 24 immunosuppressants (Figure 6A). As shown in Figure 6D, the KL signature was closely related to KDR in most types of cancer. We also analysed the correlation of KL with 45 immune stimulators (Figure 6B) and demonstrated that KL expression was positively correlated with CXCL12 and ENTPD1 in STAD, ENTPD1 in MESO, and TMEM173 in TGCT (Figure 6E). Moreover, KL expression was positively correlated with HLA-DOA in LUSC as well as HLA-DOA and HLA-DMB in ESCA (Figure 6C), but negatively correlated with TAPBP in PAAD (Figure 6F).
Figure 6.
The relativity between KL expression and immune regulator (A) Relativity between KL expression and immune inhibitors (B) Relativity between KL expression and immune stimulators (C) Relativity between KL expression and MHC molecules (D) The top 4 strongest associations in immune inhibitors (E) The top 4 strongest associations in immune stimulators (F) The top 4 strongest associations in MHC molecules. Red demonstrates positive relativity whereas blue demonstrates negative relativity.
3.7. GO and KEGG analysis of KL in GSEA
Since previous results have revealed relationships between KL and BLCA, BRCA, CESC, HNSC, SKCM and UVM, GSEA was performed to investigate the potential KL-gene product functions and pathways involved in these cancers. The results showed that calcium signalling tended to be enriched in the highly expressed groups of BLCA, BRCA, CESC, HNSC, SKCM, and UVM and that the immune response related networks tended to be enriched in the highly expressed groups of HNSC, SKCM and UVM (Figure 7, S2).
Figure 7.
GO analysis of KL in GSEA. For each figure, the gene product function marked in the left were enriched in the high KL expression group, while in the right were enriched in the low expression group.
3.8. Analysis of potential association between KL expression and TMB, MSI, CD274, PDCD1, CTLA4 and TIGIT
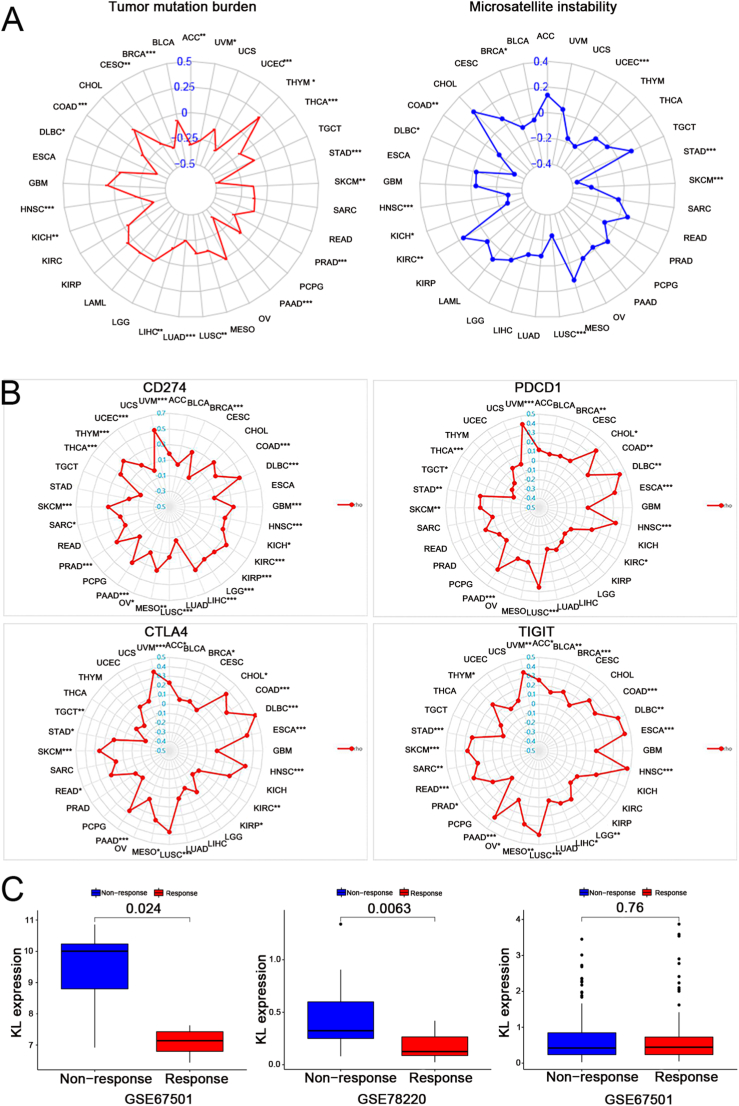
We investigated the relationship between KL and biomarkers of ICB. We found that the KL signature was positively correlated with MSI in KIRC; however, it was negatively related to MSI in BRCA, COAD, DLBC, HNSC, KICH, LUSC, SKCM, STAD and UCEC (Figure 8A). Similar results were identified in Figure 8B and Tables 2, 3, 4, and 5.
Figure 8.
The relativity between KL and TMB, MSI, CD274, PDCD1, CTLA4, TIGIT, the immunotherapeutic response. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
Table 2.
Exploring the correlation between KL gene with CD274 gene in various cancer types.
| cancer | infiltrates | rho | P | adj.p |
|---|---|---|---|---|
| ACC (n = 79) | CD274 | 0.182624 | 0.107201 | 0.142935 |
| BLCA (n = 408) | CD274 | 0.054871 | 0.268824 | 0.307228 |
| BRCA (n = 1100) | CD274 | 0.260198 | 1.76E-18 | 2.35E-17 |
| CESC (n = 306) | CD274 | -0.04794 | 0.40333 | 0.436032 |
| CHOL (n = 36) | CD274 | 0.308366 | 0.067284 | 0.09612 |
| COAD (n = 458) | CD274 | 0.213352 | 4.09E-06 | 1.09E-05 |
| DLBC (n = 48) | CD274 | 0.475358 | 0.000639 | 0.001279 |
| ESCA (n = 185) | CD274 | 0.090125 | 0.222459 | 0.261935 |
| GBM (n = 153) | CD274 | 0.323895 | 4.42E-05 | 0.000104 |
| HNSC (n = 522) | CD274 | 0.228772 | 1.26E-07 | 4.58E-07 |
| KICH (n = 66) | CD274 | 0.245089 | 0.047319 | 0.070102 |
| KIRC (n = 533) | CD274 | 0.380079 | 9.21E-20 | 1.84E-18 |
| KIRP (n = 290) | CD274 | 0.317481 | 3.26E-08 | 1.3E-07 |
| LGG (n = 516) | CD274 | 0.307774 | 8.77E-13 | 7.01E-12 |
| LIHC (n = 371) | CD274 | 0.375345 | 7.4E-14 | 7.4E-13 |
| LUAD (n = 515) | CD274 | -0.05921 | 0.179757 | 0.224697 |
| LUSC (n = 501) | CD274 | 0.146989 | 0.000968 | 0.001759 |
| MESO (n = 87) | CD274 | 0.331614 | 0.001703 | 0.002962 |
| OV (n = 303) | CD274 | 0.134867 | 0.018841 | 0.031402 |
| PAAD (n = 179) | CD274 | 0.360718 | 7.04E-07 | 2.35E-06 |
| PCPG (n = 181) | CD274 | 0.039777 | 0.59497 | 0.610226 |
| PRAD (n = 498) | CD274 | 0.311693 | 1.11E-12 | 7.39E-12 |
| READ (n = 166) | CD274 | 0.113967 | 0.143733 | 0.185462 |
| SARC (n = 260) | CD274 | 0.143277 | 0.020828 | 0.033325 |
| SKCM (n = 471) | CD274 | 0.284567 | 3.18E-10 | 1.82E-09 |
| STAD (n = 415) | CD274 | 0.027713 | 0.573456 | 0.603638 |
| TGCT (n = 150) | CD274 | -0.10016 | 0.222645 | 0.261935 |
| THCA (n = 509) | CD274 | 0.252614 | 7.5E-09 | 3.75E-08 |
| THYM (n = 120) | CD274 | 0.333738 | 0.000195 | 0.000411 |
| UCEC (n = 545) | CD274 | 0.144394 | 0.000722 | 0.001376 |
| UCS (n = 57) | CD274 | 0.005413 | 0.968123 | 0.968123 |
| UVM (n = 80) | CD274 | 0.503985 | 1.88E-06 | 5.78E-06 |
Note: Thirty-three types of cancers were selected to analyzed the correlation between KL and CD274. The higher the correlation coefficient(rho), the closer the correlation.
Abbreviations: rho: correlation coefficient; CD274: CD274 molecule; p: p-value.
Table 3.
Exploring the correlation between KL gene with PDCD1 gene in various cancer types.
| cancer | infiltrates | rho | P | adj.p |
|---|---|---|---|---|
| ACC (n = 79) | PDCD1 | 0.119637 | 0.2936342 | 0.3915122 |
| BLCA (n = 408) | PDCD1 | 0.084269 | 0.0891394 | 0.1550251 |
| BRCA (n = 1100) | PDCD1 | 0.097945 | 0.0011435 | 0.004574 |
| CESC (n = 306) | PDCD1 | 0.103021 | 0.0719327 | 0.1307868 |
| CHOL (n = 36) | PDCD1 | 0.364221 | 0.0289745 | 0.060999 |
| COAD (n = 458) | PDCD1 | 0.130997 | 0.0049864 | 0.0126777 |
| DLBC (n = 48) | PDCD1 | 0.434216 | 0.0020453 | 0.0068175 |
| ESCA (n = 185) | PDCD1 | 0.325882 | 5.99E-06 | 4.79E-05 |
| GBM (n = 153) | PDCD1 | 0.026008 | 0.749642 | 0.8819317 |
| HNSC (n = 522) | PDCD1 | 0.336707 | 2.65E-15 | 5.30E-14 |
| KICH (n = 66) | PDCD1 | 0.113333 | 0.3649066 | 0.4561333 |
| KIRC (n = 533) | PDCD1 | -0.08694 | 0.0448344 | 0.0896687 |
| KIRP (n = 290) | PDCD1 | -0.108 | 0.0662605 | 0.1262105 |
| LGG (n = 516) | PDCD1 | -0.06009 | 0.1728898 | 0.256133 |
| LIHC (n = 371) | PDCD1 | -0.01412 | 0.7862731 | 0.8934352 |
| LUAD (n = 515) | PDCD1 | -0.04846 | 0.2723645 | 0.3756751 |
| LUSC (n = 501) | PDCD1 | 0.350927 | 5.77E-16 | 2.31E-14 |
| MESO (n = 87) | PDCD1 | 0.093479 | 0.3891335 | 0.471677 |
| OV (n = 303) | PDCD1 | 0.09006 | 0.1177303 | 0.1962172 |
| PAAD (n = 179) | PDCD1 | 0.294955 | 6.12E-05 | 0.000408 |
| PCPG (n = 181) | PDCD1 | -0.0074 | 0.9212311 | 0.9448524 |
| PRAD (n = 498) | PDCD1 | 0.010966 | 0.807139 | 0.8934352 |
| READ (n = 166) | PDCD1 | 0.117241 | 0.1324993 | 0.2119989 |
| SARC (n = 260) | PDCD1 | 0.008143 | 0.896036 | 0.9431957 |
| SKCM (n = 471) | PDCD1 | 0.128935 | 0.0050711 | 0.0126777 |
| STAD (n = 415) | PDCD1 | 0.137863 | 0.0049012 | 0.0126777 |
| TGCT (n = 150) | PDCD1 | -0.20688 | 0.0110833 | 0.0246296 |
| THCA (n = 509) | PDCD1 | -0.15357 | 0.0005076 | 0.002538 |
| THYM (n = 120) | PDCD1 | -0.12874 | 0.1611146 | 0.2478686 |
| UCEC (n = 545) | PDCD1 | 0.009415 | 0.8264276 | 0.8934352 |
| UCS (n = 57) | PDCD1 | 0.003695 | 0.9782399 | 0.9782399 |
| UVM (n = 80) | PDCD1 | 0.410673 | 0.0001545 | 0.0008829 |
Note: Thirty-three types of cancers were selected to analyzed the correlation between KL and PDCD1. The higher the correlation coefficient(rho), the closer the correlation.
Abbreviations: rho: correlation coefficient; PDCD1: programmed cell death 1; p: p-value.
Table 4.
Exploring the correlation between KL gene with CTLA4 gene in various cancer types.
| cancer | infiltrates | rho | P | adj.p |
|---|---|---|---|---|
| ACC (n = 79) | CTLA4 | 0.228809 | 0.042532 | 0.070887 |
| BLCA (n = 408) | CTLA4 | 0.059694 | 0.228926 | 0.277486 |
| BRCA (n = 1100) | CTLA4 | 0.072622 | 0.015995 | 0.033673 |
| CESC (n = 306) | CTLA4 | 0.026662 | 0.642244 | 0.676046 |
| CHOL (n = 36) | CTLA4 | 0.359846 | 0.031109 | 0.055242 |
| COAD (n = 458) | CTLA4 | 0.237165 | 2.82E-07 | 2E-06 |
| DLBC (n = 48) | CTLA4 | 0.495332 | 0.000344 | 0.001148 |
| ESCA (n = 185) | CTLA4 | 0.348078 | 1.2E-06 | 6.02E-06 |
| GBM (n = 153) | CTLA4 | 0.01099 | 0.892746 | 0.892746 |
| HNSC (n = 522) | CTLA4 | 0.328913 | 1.23E-14 | 2.47E-13 |
| KICH (n = 66) | CTLA4 | 0.197501 | 0.111935 | 0.172208 |
| KIRC (n = 533) | CTLA4 | -0.12069 | 0.005272 | 0.014058 |
| KIRP (n = 290) | CTLA4 | -0.14734 | 0.012008 | 0.026683 |
| LGG (n = 516) | CTLA4 | 0.027892 | 0.52728 | 0.585867 |
| LIHC (n = 371) | CTLA4 | -0.06921 | 0.183452 | 0.244602 |
| LUAD (n = 515) | CTLA4 | 0.019137 | 0.664819 | 0.681866 |
| LUSC (n = 501) | CTLA4 | 0.367104 | 1.98E-17 | 7.93E-16 |
| MESO (n = 87) | CTLA4 | 0.254757 | 0.017253 | 0.034505 |
| OV (n = 303) | CTLA4 | 0.056532 | 0.326706 | 0.38436 |
| PAAD (n = 179) | CTLA4 | 0.269598 | 0.000263 | 0.000955 |
| PCPG (n = 181) | CTLA4 | -0.09495 | 0.203556 | 0.254445 |
| PRAD (n = 498) | CTLA4 | -0.0649 | 0.148147 | 0.211639 |
| READ (n = 166) | CTLA4 | 0.173419 | 0.025455 | 0.048486 |
| SARC (n = 260) | CTLA4 | 0.08456 | 0.174036 | 0.24005 |
| SKCM (n = 471) | CTLA4 | 0.249187 | 4.25E-08 | 4.25E-07 |
| STAD (n = 415) | CTLA4 | 0.105434 | 0.031764 | 0.055242 |
| TGCT (n = 150) | CTLA4 | -0.22463 | 0.005718 | 0.014296 |
| THCA (n = 509) | CTLA4 | -0.07612 | 0.086222 | 0.137955 |
| THYM (n = 120) | CTLA4 | -0.12 | 0.191718 | 0.247378 |
| UCEC (n = 545) | CTLA4 | 0.066392 | 0.121601 | 0.18015 |
| UCS (n = 57) | CTLA4 | 0.074735 | 0.580597 | 0.627672 |
| UVM (n = 80) | CTLA4 | 0.361721 | 0.000978 | 0.003009 |
Note: Thirty-three types of cancers were selected to analyzed the correlation between KL and CTLA4. The higher the correlation coefficient(rho), the closer the correlation.
Abbreviations: rho: correlation coefficient; CTLA4: cytotoxic T-lymphocyte associated protein 4; p: p-value.
Table 5.
Exploring the correlation between KL gene with TIGIT gene in various cancer types.
| cancer | infiltrates | rho | P | adj.p |
|---|---|---|---|---|
| ACC (n = 79) | TIGIT | 0.257762 | 0.021824 | 0.037954 |
| BLCA (n = 408) | TIGIT | 0.13941 | 0.004786 | 0.009901 |
| BRCA (n = 1100) | TIGIT | 0.182759 | 1.02E-09 | 6.81E-09 |
| CESC (n = 306) | TIGIT | 0.086867 | 0.129469 | 0.184956 |
| CHOL (n = 36) | TIGIT | 0.262291 | 0.122249 | 0.181109 |
| COAD (n = 458) | TIGIT | 0.221542 | 1.69E-06 | 5.95E-06 |
| DLBC (n = 48) | TIGIT | 0.415979 | 0.003276 | 0.00728 |
| ESCA (n = 185) | TIGIT | 0.434803 | 6.24E-10 | 4.99E-09 |
| GBM (n = 153) | TIGIT | 0.114153 | 0.160016 | 0.220712 |
| HNSC (n = 522) | TIGIT | 0.4635 | 3.67E-29 | 1.47E-27 |
| KICH (n = 66) | TIGIT | 0.163845 | 0.188659 | 0.251546 |
| KIRC (n = 533) | TIGIT | -0.00843 | 0.84606 | 0.84606 |
| KIRP (n = 290) | TIGIT | -0.02133 | 0.717519 | 0.755283 |
| LGG (n = 516) | TIGIT | 0.121012 | 0.005917 | 0.011271 |
| LIHC (n = 371) | TIGIT | 0.105258 | 0.042744 | 0.06576 |
| LUAD (n = 515) | TIGIT | 0.041399 | 0.348449 | 0.387166 |
| LUSC (n = 501) | TIGIT | 0.396027 | 2.92E-20 | 4.13E-19 |
| MESO (n = 87) | TIGIT | 0.298699 | 0.00495 | 0.009901 |
| OV (n = 303) | TIGIT | 0.067827 | 0.239141 | 0.298926 |
| PAAD (n = 179) | TIGIT | 0.354577 | 1.12E-06 | 4.47E-06 |
| PCPG (n = 181) | TIGIT | -0.09069 | 0.224696 | 0.28993 |
| PRAD (n = 498) | TIGIT | 0.094944 | 0.034155 | 0.054648 |
| READ (n = 166) | TIGIT | 0.254877 | 0.00092 | 0.0023 |
| SARC (n = 260) | TIGIT | 0.168749 | 0.006382 | 0.011604 |
| SKCM (n = 471) | TIGIT | 0.265851 | 4.63E-09 | 2.64E-08 |
| STAD (n = 415) | TIGIT | 0.229516 | 2.3E-06 | 7.09E-06 |
| TGCT (n = 150) | TIGIT | -0.08532 | 0.299224 | 0.362696 |
| THCA (n = 509) | TIGIT | -0.00915 | 0.836831 | 0.84606 |
| THYM (n = 120) | TIGIT | 0.200333 | 0.028245 | 0.047075 |
| UCEC (n = 545) | TIGIT | 0.040545 | 0.344785 | 0.387166 |
| UCS (n = 57) | TIGIT | 0.080373 | 0.552295 | 0.597076 |
| UVM (n = 80) | TIGIT | 0.355973 | 0.001193 | 0.002806 |
Note: Thirty-three types of cancers were selected to analyzed the correlation between KL and TIGIT. The higher the correlation coefficient(rho), the closer the correlation.
Abbreviations: rho: correlation coefficient; TIGIT: T cell immunoreceptor with Ig and ITIM domains; p: p-value.
3.9. Association between immunotherapeutic response and KL expression
As presented in Figure 8C, significant differences in KL expression were evident between the responder and non-responder groups in the GSE78220 (p = 0.0063) and GSE67501 (p = 0.024) cohorts. Based on the results of this study, patients with low KL expression respond better to immunotherapy.
4. Discussion
Klotho (KL) protein was initially recognized as an antiaging and inflammatory factor that plays an important role in the process of autoimmune diseases [9]. Several recent studies have shown that KL participates in the progression of several types of human cancer [22, 23, 24, 25, 26, 27, 28, 29, 30]. However, few studies have confirmed the possibility of KL in cancer immunotherapy. Our study performed an in-depth comparison of KL expression between tumour and adjacent normal tissues across 33 malignant tumours. The outcomes demonstrated a high potential immunotherapeutic value of KL in various tumours, which is contrary to what was initially believed and indicates that KL is not only an ageing and inflammatory-related transcription factor, but also plays a key role in the microenvironment of the tumour immune system. Therefore, further research on KL is necessary to explore the specific biological mechanism.
First, the correlation between KL and clinical parameters was analysed and significant differences in gender and tumour stage were identified in some cancer types. As previously mentioned, patients with low KL expression were seemingly more associated to the older and advanced stage; numerous studies have demonstrated that loss of KL expression widely exists in Breast Cancer [25], Gastric Cancer [24] and Colorectal Cancer [26]. Loss of Klotho is readily apparent during cancer progression and promotes tumour migration, invasion and growth [12]. In addition, our study shows that KL expression is highly associated with prognostic value in some cancers, especially KIRC and KIRP. Similarly, a number of previous studies have identified KL as a marker of good prognosis in various cancers, including gastric cancer, breast cancer, esophageal squamous cell carcinoma, LIHC, renal cell carcinoma, lung cancer and ovarian cancer [12]. Based on the above evidence, we confirmed the usefulness of KL in cancer prognosis and hypothesized that therapeutic regulation of KL activity in various human tumour types may be an effective strategy with clinical benefits.
Generally, KL expression is affected by posttranscriptional protein level modification or protein metabolism. Therefore, compared with the gene expression, the protein expression level is considered as a more accurate method to reflect the tissue activity of KL. However, the lack of relevant data in public databases makes it difficult toperform relevant analysis at the protein level. Alternatively, we analysed the KL activity score. Comparing the transcript level with the KL activity score, we found that the transcriptional level of KL in some cancers partially matched the overall KL activation, indicating that the transcription level of KL could represent KL activation in these cancers. Nevertheless, in some cancers (CHOL, KIRC, LIHC and THCA), inconsistency was observed between KL transcriptional level and activity.
In the present study, the data indicated that the signature of KL was closely related to the activation status of naïve B cells, M2 macrophages and memory CD4 T cells, while negatively associated with the DCs of resting cells, follicular helper T cells, M0 macrophages and mast cells. In previous studies, KL was found to be highly correlated with these immune cells in numerous autoimmune diseases [9]. Among various ICBs, the kinase insert domain receptor (KDR) showed the most significant positive association with KL in most cancers. KDR, also known as VEGFR-2, shows greater affinity towards VEGF. In pathological processes, KDR is often involved in tumoral angiogenesis [31]. Klotho deficient mice displayed significant impairment in angiogenesis. Klotho has also been reported to could also facilitate the ischemia-induced angiogenesis [32]. Based on these data, our study suggests the existence of an underlying mechanism linking KL, KDR, and angiogenesis.
For immune stimulators and MHC molecules, most of the modulators exhibited a positive correlation with KL and we found that KL was positively correlated with KDR in most cancers [33]; this interesting finding may lead to the discovery of a novel regulatory mechanism in immunotherapy. The obtained GO results indicate that genes with immune-relevant functions tend to be enriched. Moreover, in some cancers, calcium signalling is accumulated in the high-expressing group of KL. Ca2+ channels are cell membrane proteins with calcium-selective pores and their opening depends on the membrane voltage. Investigations in mice lacking KL have shown the diminished effect of renal calcium reabsorption by KL, resulting in severe hypercalciuria, since renal Ca2+ excretion is crucial for total body calcium homeostasis, which contributes to immunosuppression. Major cell membrane Ca2+ channels involved in the regulation of Ca2+ inflow pathways to replenish the internal calcium storage of cancer cells [34].
Additionally, in this study, six biomarkers of ICB showed a significant association with KL in some types of cancers. Previous studies showed TMB could provide a useful estimation of tumour-neoantigen load [35]. In contrast, MSI is defined as a robust mutator phenotype caused by deficient DNA mismatch repair and is a potential predictive marker for immunotherapy [36]. Our study found that KL was negatively associated with TMB and MSI in various cancers. CTLA4, PDCD1, CD274 and TIGIT are members of a family of immunoglobulin-related receptors and ligands that are responsible for various aspects of T-cell immune regulation. All these receptors and ligands play inhibitory roles in T-cell function and cause down-regulation of the adaptive anti-tumour immune response, resulting in poor prognosis of tumour patients [37]. Currently, all these receptors and ligands are considered as immunotherapeutic biomarkers. Interestingly, our study shows that KL was simultaneously and positively correlated with CD274, PDCD1, CTLA4 and TIGIT in BRCA, COAD, DLBC, HNSC, LUSC, PAAD, SKCM and UVM. All these results indicated that KL might have an indirect effect on the immunotherapeutic response of these cancers. Moreover, the association between KL and the immunotherapeutic response was investigated. KL expression differed significantly between the responder and non-responder groups in the GSE78220 (p = 0.0063) and GSE67501 (p = 0.024) cohorts. Based on these outcomes, we hypothesize that patients with low KL expression are more responsive to immunotherapy, although this hypothesis should be further verified in functional experiments. We hypothesize that although the three treatment-cohorts underwent and responded to anti-PD1 treatment, KL may affect the immunotherapeutic response by targeting other immune checkpoints such as CD274, PDCD1, CTLA4 and TIGIT. Additionally, only three relevant treatment-cohorts were analysed in our research, making it difficult to demonstrate the actual immunotherapeutic response of KL expression. Future studies should include more relevant immunotherapeutic cohorts.
Nevertheless, our study has several limitations. First, the population ethnicity in TCGA database is primarily limited to whites and blacks, and extrapolation of the findings to other ethnicities is needed. Second, our data comes from a public database, and potential selection bias cannot be ruled out. And it is still required to further verify them through experiments in the future.
5. Conclusion
As far as we know, this is the first study focusing on the value of KL in pan-cancer. Although not all cancers have demonstrated an association between the tumour immune microenvironment and KL, these findings highlight the immunological effect of KL on certain cancers, and provide valuable insights into the role of KL in cancer immunotherapy and its association with important immunological indicators. As we relied on the bioinformatics approaches in this study, further exploration on this topic is needed before the relationship between KL and cancer immunotherapy is clearly understood and widely accepted.
Declarations
Author contribution statement
Jinghao Liang: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xin Zhang: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xiuxiu Wang: Analyzed and interpreted the data; Wrote the paper.
Weiqiang Yin & Zhihua Guo: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Chigene (Beijing) Translational Medical Research Center Co., Ltd, which provided excellent assistance in the review and revision of the manuscript.
Contributor Information
Weiqiang Yin, Email: yinweiqiang88@163.com.
Zhihua Guo, Email: guozhihua84@126.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
The prognostic value of KL in the Kaplan–Meier survival estimation. (A) Overall survival. (B) Disease-specific survival. (C) Disease-free survival. (D) Progression-free survival.
KEGG analysis of KL in GSEA. For each figure, the pathways marked in the left were enriched in the high KL expression group, while in the right were enriched in the low expression group.
References
- 1.White M.C., Holman D.M., Boehm J.E., Peipins L.A., Grossman M., Henley S.J. Age and cancer risk: a potentially modifiable relationship. Am. J. Prev. Med. 2014;46:S7–S15. doi: 10.1016/j.amepre.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., Jemal A., Kramer J.L., Siegel R.L. Cancer treatment and survivorship statistics, 2019. CA: a cancer journal for clinicians. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 4.Gotwals P., Cameron S., Cipolletta D., Cremasco V., Crystal A., Hewes B., Mueller B., Quaratino S., Sabatos-Peyton C., Petruzzelli L. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 5.Shi Z., Shen J., Qiu J., Zhao Q., Hua K., Wang H. CXCL10 potentiates immune checkpoint blockade therapy in homologous recombination-deficient tumors. Theranostics. 2021;11:7175. doi: 10.7150/thno.59056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens D., Ingels J., Van Lint S., Vandekerckhove B., Vermaelen K. Dendritic cell-based immunotherapy in lung cancer. Front. Immunol. 2021;11:3881. doi: 10.3389/fimmu.2020.620374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Giglio A., Di Federico A., Nuvola G., Deiana C., Gelsomino F. The landscape of immunotherapy in advanced NSCLC: driving beyond PD-1/PD-L1 inhibitors (CTLA-4, LAG3, Ido, OX40, TIGIT, vaccines) Curr. Oncol. Rep. 2021;23:1–15. doi: 10.1007/s11912-021-01124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowshanravan B., Halliday N., Sansom D.M. CTLA-4: a moving target in immunotherapy, Blood. The Journal of the American Society of Hematology. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell D.L., Oates J.C., Markiewicz M. Association between the anti-aging gene klotho and selected rheumatologic autoimmune diseases. Am. J. Med. Sci. 2021;361:169–175. doi: 10.1016/j.amjms.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamal A., Salama M., Kamal A., Mohsen A., Siam, Klotho I. Gene variants and colorectal cancer risk. Turk. J. Gastroenterol. 2020;31:497. doi: 10.5152/tjg.2020.19235. rs1207568 and rs564481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mencke R., Olauson H., Hillebrands J.-L. Effects of Klotho on fibrosis and cancer: a renal focus on mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2017;121:85–100. doi: 10.1016/j.addr.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Xuan N.T., Van Hai N. Changes in expression of klotho affect physiological processes, diseases, and cancer. Iranian journal of basic medical sciences. 2018;21:3. [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdeva A., Gouge J., Kontovounisios C., Nikolaou S., Ashworth A., Lim K., Chong I. Klotho and the treatment of human malignancies. Cancers. 2020;12:1665. doi: 10.3390/cancers12061665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie B., Nie S., Hu G., Xiong L., Hu F., Li M., Peng T., Nie J., He Y. The involvement of NF-κB/Klotho signaling in colorectal cancer cell survival and invasion. Pathol. Oncol. Res. 2019;25:1553–1565. doi: 10.1007/s12253-018-0493-6. [DOI] [PubMed] [Google Scholar]
- 15.Shi Z., Zhao Q., Lv B., Qu X., Han X., Wang H., Qiu J., Hua K. Identification of biomarkers complementary to homologous recombination deficiency for improving the clinical outcome of ovarian serous cystadenocarcinoma. Clin. Transl. Med. 2021;11:e399. doi: 10.1002/ctm2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel E.E., III, Koeppen H., Astarita J.L., Cubas R. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo Z., Li P., Cao Z., Zhang S. A comprehensive pan-cancer analysis of 33 human cancers reveals the immunotherapeutic value of aryl hydrocarbon receptor. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.564948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbie D.A., Tamayo P., Boehm J.S., Kim S.Y., Moody S.E., Dunn I.F., Schinzel A.C., Sandy P., Meylan E., Scholl C. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature Nov 5. 2009;462(7269):108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshihara K., Shahmoradgoli M., Martínez E., Vegesna R., Kim H., Torres-Garcia W., Treviño V., Shen H., Laird P.W., Levine D.A. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013;4:1–11. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., Hoang C.D., Diehn M., Alizadeh A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonneville R., Krook M.A., Kautto E.A., Miya J., Wing M.R., Chen H.-Z., Reeser J.W., Yu L., Roychowdhury S. Landscape of microsatellite instability across 39 cancer types. JCO precision oncology. 2017;1:1–15. doi: 10.1200/PO.17.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X., Fan Z., Wang Y., Ji G., Wang M., Lin J., Huang S. Expression of klotho and β-catenin in esophageal squamous cell carcinoma, and their clinicopathological and prognostic significance. Dis. Esophagus. 2016;29:207–214. doi: 10.1111/dote.12289. [DOI] [PubMed] [Google Scholar]
- 23.He X.-J., Ma Y.-Y., Yu S., Jiang X.-T., Lu Y.-D., Tao L., Wang H.-P., Hu Z.-M., Tao H.-Q. Up-regulated miR-199a-5p in gastric cancer functions as an oncogene and targets klotho. BMC Cancer. 2014;14:1–11. doi: 10.1186/1471-2407-14-218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Jiang B., Gu Y., Chen Y. Identification of novel predictive markers for the prognosis of pancreatic ductal adenocarcinoma. Cancer Invest. 2014;32:218–225. doi: 10.3109/07357907.2014.905586. [DOI] [PubMed] [Google Scholar]
- 25.Dallol A., Buhmeida A., Merdad A., Al-Maghrabi J., Gari M.A., Abu-Elmagd M.M., Elaimi A., Assidi M., Chaudhary A.G., Abuzenadah A.M. Frequent methylation of the KLOTHO gene and overexpression of the FGFR4 receptor in invasive ductal carcinoma of the breast. Tumor Biol. 2015;36:9677–9683. doi: 10.1007/s13277-015-3733-3. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Chen Y., Guo X., Zhou L., Jia Z., Tang Y., Lin L., Liu W., Ren C. Inhibition of miR-15b decreases cell migration and metastasis in colorectal cancer. Tumor Biol. 2016;37:8765–8773. doi: 10.1007/s13277-015-4396-9. [DOI] [PubMed] [Google Scholar]
- 27.Aviel-Ronen S., Rubinek T., Zadok O., Vituri A., Avivi C., Wolf I., Barshack I. Klotho expression in cervical cancer: differential expression in adenocarcinoma and squamous cell carcinoma. J. Clin. Pathol. 2016;69:53–57. doi: 10.1136/jclinpath-2015-202929. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y., Xu L., Zhang J., Xu W., Liu Y., Yin H., Lv T., An H., Liu L., He H. K lotho suppresses tumor progression via inhibiting PI 3 K/A kt/GSK 3β/Snail signaling in renal cell carcinoma. Cancer Sci. 2013;104:663–671. doi: 10.1111/cas.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peshes-Yeloz N., Ungar L., Wohl A., Jacoby E., Fisher T., Leitner M., Nass D., Rubinek T., Wolf I., Cohen Z.R. Role of Klotho protein in tumor genesis, cancer progression, and prognosis in patients with high-grade glioma. World neurosurgery. 2019;130:e324–e332. doi: 10.1016/j.wneu.2019.06.082. [DOI] [PubMed] [Google Scholar]
- 30.Tang X., Wang Y., Fan Z., Ji G., Wang M., Lin J., Huang S., Meltzer S.J. Klotho: a tumor suppressor and modulator of the Wnt/β-catenin pathway in human hepatocellular carcinoma. Lab. Invest. 2016;96:197–205. doi: 10.1038/labinvest.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melincovici C.S., Boşca A.B., Şuşman S., Mărginean M., Mihu C., Istrate M., Moldovan I.-M., Roman A.L., Mihu C.M. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018;59:455–467. [PubMed] [Google Scholar]
- 32.Zhou X., Wang X. Klotho: a novel biomarker for cancer. J. Cancer Res. Clin. Oncol. 2015;141:961–969. doi: 10.1007/s00432-014-1788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuro-o M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019;15:27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 34.Shmulevich R., Nissim T.B.-K., Wolf I., Merenbakh-Lamin K., Fishman D., Sekler I., Rubinek T. Klotho rewires cellular metabolism of breast cancer cells through alteration of calcium shuttling and mitochondrial activity. Oncogene. 2020;39:4636–4649. doi: 10.1038/s41388-020-1313-5. [DOI] [PubMed] [Google Scholar]
- 35.Chan T.A., Yarchoan M., Jaffee E., Swanton C., Quezada S.A., Stenzinger A., Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto H., Imai K. Elsevier; 2019. An Updated Review of Microsatellite Instability in the Era of Next-Generation Sequencing and Precision Medicine, Seminars in Oncology; pp. 261–270. [DOI] [PubMed] [Google Scholar]
- 37.Li B., Chan H.L., Chen P. Immune checkpoint inhibitors: basics and challenges. Curr. Med. Chem. 2019;26:3009–3025. doi: 10.2174/0929867324666170804143706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The prognostic value of KL in the Kaplan–Meier survival estimation. (A) Overall survival. (B) Disease-specific survival. (C) Disease-free survival. (D) Progression-free survival.
KEGG analysis of KL in GSEA. For each figure, the pathways marked in the left were enriched in the high KL expression group, while in the right were enriched in the low expression group.
Data Availability Statement
Data will be made available on request.