Abstract
The development of effective prophylactic agents against gonorrhea and the study of adaptation by Neisseria gonorrhoeae to the urogenital mucosa are hindered by the lack of a well-established animal model of gonococcal genital tract infection. Here, a murine model of long-term gonococcal genital tract infection is described. Female BALB/c mice were treated with 17-β-estradiol and inoculated intravaginally with wild-type gonococcal strain FA1090 or MS11. N. gonorrhoeae was recovered from vaginal swabs for an average of 12 to 13 days following inoculation with 106 CFU of either strain. Inflammation occurred in over 80% of infected mice, and diplococci were associated with epithelial cells and neutrophils in stained vaginal smears. Ascended infection occurred in 17 to 20% of mice inoculated with strain FA1090. An outbred mouse strain (SLC:ddY) previously reported to be naturally susceptible to N. gonorrhoeae was also tested; however, as with BALB/c mice, estradiol was required for prolonged infection. Although piliation was not maintained during experimental murine infection, 46 to 100% of vaginal isolates from four of eight BALB/c mice and three of four SLC:ddY mice expressed one or more opacity (Opa) proteins within 4 days after inoculation with an Opa-negative variant of strain FA1090. The observed selection for and/or induction of gonococcal Opa protein expression during murine infection appears to parallel events that occur during experimental urethritis in volunteers.
Gonorrhea holds a high rank among reportable infectious diseases in the United States, second only to Chlamydia trachomatis infection (8). Neisseria gonorrhoeae most commonly infects the urethra in men and the endocervix and/or urethra in women. The hallmark feature of acute gonorrhea is the presence of a pyogenic response characterized by a frank, mucopurulent exudate containing polymorphonuclear leukocytes (PMNs) with intracellular gonococci. Asymptomatic infection can occur in both genders. Ascension of the pathogen to the upper reproductive tract results in more serious infections such as epididymitis, endometritis, salpingitis, and pelvic inflammatory disease (PID) (24). Women have the highest morbidity and mortality due to N. gonorrhoeae on account of the frequency of ascended infection and associated complications in females. N. gonorrhoeae is isolated from 40 to 50% of women with PID in populations where the incidence of gonorrhea is high (61). Over one million cases of PID occur in the United States annually, the health costs of which are staggering (58, 60).
The development of laboratory animal models of gonococcal infection is challenged by the human specificity of N. gonorrhoeae. Rodent models using the implantation of subcutaneous chambers to stimulate mucosal infection (36, 40) and intraperitoneal inoculation (44) or synovial injection (21, 22) to simulate disseminated gonococcal infection and gonococcal arthritis, respectively, are currently employed to study gonococcal pathogenesis. Gonococcal genital tract infection of chimpanzees and female mice has also been described. Male chimpanzees develop a disease that closely mimics gonococcal urethritis in humans, and natural transmission of N. gonorrhoeae from an experimentally infected male chimpanzee to female chimpanzees was documented (reviewed in reference 3). However, the use of chimpanzees as an experimental host for N. gonorrhoeae is no longer feasible due to the expense and limited availability of these animals. Female mice in the proestrus stage of the estrus cycle are susceptible to N. gonorrhoeae following intrauterine (7, 54) or intravaginal (28, 32) inoculation. However, with the exception of ddY mice, used by Kita et al. (32), colonization is short-term and gonococci are no longer recovered from mice upon transition into the luteal stage of the cycle. Taylor-Robinson et al. (57) treated germfree BALB/c mice with 17-β-estradiol to increase their susceptibility to N. gonorrhoeae. Positive vaginal cultures were obtained for up to 39 days, and gonococci were associated with epithelial cells in vaginal smears. No inflammatory response was detected. Gonococci were isolated from endometrial and ovarian tissue, suggesting that local dissemination to the upper reproductive tract occurred. Despite this report, however, the use of estradiol to promote murine infection has not been pursued, for reasons that are unclear.
In the absence of a well-established animal model of gonococcal genital tract infection, experimental urethral infection of male volunteers has been used to address certain questions concerning gonococcal pathogenesis (reviewed in reference 11). The human model is a valuable tool for ascertaining the requirement for certain gonococcal virulence factors in causing urethritis (12, 16). The population dynamics of gonococcal pilin (51, 55), lipooligosaccharide (LOS) (50), and opacity (Opa) protein (27, 56) expression have also been studied in male volunteers. Antigenic variation of these molecules in vivo may play a role in evasion of the specific immune response as well as confer adaptive advantages or provide functionally different phenotypes. For example, a high percentage of gonococci expressing one or more Opa proteins were isolated from volunteers who were inoculated with a predominantly Opa-negative population of N. gonorrhoeae. This observation suggests that there is a selective advantage to Opa protein expression in the male urethra, such as enhanced adherence to and invasion of host tissues (reviewed in reference 17) or protection from components of the innate immune response (6).
The human model is highly relevant for studying early events in gonococcal urethritis. Limitations to this model include the inability to fully study the effect of innate or acquired immune responses on gonococcal adaptation in vivo due to ethical restrictions against manipulating the immunological state of a volunteer or maintaining untreated experimental infection. Extrapolation of data from the human model to infection in females is also risky, since the microenvironment of the male urethra may differ substantially from that of the female reproductive tract. Gonococcal pathogenicity studies cannot be conducted with female volunteers due to the risk of serious complications, and therefore, the group that suffers the greatest mobidity from N. gonorrhoeae is virtually excluded from experimental research. Surrogate animal models for human infection may at least partially satisfy the presently unfulfilled need for a female model of infection, as well as provide intermediate steps towards eventual testing of predictions regarding gonococcal pathogenesis in male volunteers. Obviously, experimental murine infection cannot fully mimic human disease due to the absence in mice of several human-specific factors that are utilized by N. gonorrhoeae during infection. However, the genital tract of female mice shares many other features with that of humans, including similarities in oxygen tension, cervical pH, commensal flora, hormonally driven changes in mucus, and certain histological characteristics (7, 14). The murine immune response to infection is easily studied, and genetically defined strains of mice are available for evaluating the importance of host factors in infection. The increasing availability of transgenic mice further increases the appeal of the laboratory mouse as a research tool for studying N. gonorrhoeae. In summary, the physiologically balanced environment of the female mouse along with the presence of intact immune defenses may provide an experimental background against which critical survival mechanisms utilized by N. gonorrhoeae can be delineated. In this report, long-term gonococcal genital tract infection of estradiol-treated BALB/c mice is described as a first step towards developing murine models for studying specific aspects of gonococcal pathogenesis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
N. gonorrhoeae FA1090 and MS11 were used in this study. FA1090 is a serum-resistant strain originally isolated from a female with disseminated gonococcal infection (DGI) (11). Variant A21 is a predominantly piliated, Opa-negative variant of strain FA1090 that is capable of causing urethritis in male volunteers (11, 27). MS11 is a serum-sensitive strain originally isolated from a case of cervicitis (55, 56); an Opa-negative variant of the piliated MS11 variant, MS11A (18), was kindly provided by Hank Seifert (Northwestern University). MS11var.72 is a nonpiliated, mouse-passaged variant of MS11A with an uncharacterized Opa phenotype. N. gonorrhoeae was cultured on GC agar with Kellogg's supplements as described previously (27). GC agar with antibiotic selection contained VCNTS (vancomycin, colistin, nystatin, and trimethoprim sulfate) supplement (Becton Dickinson, Cockeysville, Md.) and 100 μg of streptomycin sulfate (Sigma, St. Louis, Mo.) per ml. Incubation conditions for N. gonorrhoeae and for commensal bacteria were at 37°C in a humid atmosphere containing 7% CO2. All bacteriological culture media used in this study were purchased from Difco Laboratories (Detroit, Mich.).
Mouse infection protocol.
Four- to 6-week-old female BALB/c mice (17 to 22 g) (National Cancer Institute, Bethesda, Md.) or SLC:ddy mice (25 to 30 g) (32) produced by a breeding colony originally imported from Japan (Japan SLC Inc., Hamamatsu, Japan) were utilized in these studies. Mice were housed in filter top isolator cages with autoclaved litter, food, and water. Following a week of acclimation to the animal facility, mice in the diestrus stage of the estrus cycle were identified by cytological examination of vaginal smears (53), and a 5-mg, 21-day controlled-release estradiol pellet (Research of America, Sarasota, Fla.) was implanted intradermally. To reduce the overgrowth of commensal flora that occurs under the influence of estradiol, antibiotic treatment was initiated upon pellet implantation (streptomycin sulfate [1.2 mg] and vancomycin HCl [0.6 mg] twice daily intraperitoneally and trimethoprim sulfate [0.04 g/100 ml of drinking water]). Two days after pellet implantation, mice were inoculated intravaginally with N. gonorrhoeae by first rinsing the vagina with 30 μl of 50 mM HEPES (pH 7.4), followed by inoculation with 20 μl of gonococci suspended in phosphate-buffered saline containing 0.5 mM CaCl2, 1 mM MgCl2, and 1% gelatin. Gonococci cultured on solid GC agar for 18 h were used in the inocula. The bacteria were harvested into the saline-based diluent with a cotton swab, and the suspension was passed through a sterile 1.2-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.) to remove bacterial aggregates and thereby increase the accuracy of the dose. Filtered suspensions were adjusted to an A600 of 1.0, and appropriate dilutions were made to obtain the desired dose by using a standard curve established by comparing the A600 versus CFU per milliliter of suspension. The number of gonococci in the inoculum was confirmed by a standard quantitative culture method in which serial dilutions of the inoculum were cultured on solid GC agar. Control (placebo) mice were inoculated intravaginally with the diluent.
Monitoring of murine infection.
Following inoculation, the vaginal mucosae of test mice were cultured each day by gently inserting a Dacron swab (PurFybr, Inc., Munster, Ind.) into the vagina. The specimen was resuspended in 100 μl of GC broth, and undiluted and diluted samples were cultured for N. gonorrhoeae on GC agar with antibiotic selection. Serial dilutions of the sample were made in GC broth containing 0.05% saponin (Sigma); this concentration of saponin was found to break up aggregated gonococci without decreasing viability and was therefore used to increase the accuracy of the quantitative culture method. Daily vaginal swabs were also cultured on Bacto heart infusion and Lactobacilli MRS agars to isolate facultatively anaerobic commensal bacteria. Preliminary identification of commensal bacteria was based on Gram staining, colony morphology, and growth characteristics on sheep blood, MRS, or MacConkey agar plates. The antibiotic treatment regimen suppressed colonization by facultatively anaerobic commensal flora in the majority of mice, except for lactobacilli, which were cultured from about 50% of the mice 5 to 14 days after inoculation. Only the presence of enteric gram-negative rods and catalase-positive, gram-positive cocci in clusters was associated with the clearance of gonococcal infection. Inhibition of N. gonorrhoeae by gram-negative rods and Staphylococcus spp. was reported by others (7, 54). Only 10 to 15% of mice were colonized with these particular commensals in each experiment, and these mice were excluded from the data analysis. In some experiments, mice were sacrificed by CO2 asphyxiation to obtain endometrial tissue for culture. The uterine horns were immediately removed and dissected longitudinally. The mucosal surface was rinsed three times with 300 μl of sterile saline, and the wash fluid was collected and quantitatively cultured for N. gonorrhoeae.
Measurement of inflammation.
Vaginal smears were prepared from both test and control mice each day following inoculation with N. gonorrhoeae or the inoculum diluent, respectively. Smears were stained with a modified Wright stain (Leukostat; Fisher Scientific, Pittsburg, Pa.) and viewed under light microscopy to determine the percentage of PMNs among 100 vaginal cells. The average percentage of PMNs plus one standard deviation among placebo-treated controls was used as a baseline for each day following inoculation; test mice that had a higher percentage of PMNs than this value on any two consecutive days following inoculation were considered positive for inflammation.
Opa protein analysis.
Strain FA1090 has the capacity to express eight antigenically different Opa proteins referred to as OpaA, -B, -C, -D, -E, -F, -K, and -I (4, 5, 13). The Opa protein phenotypes of FA1090 inoculum and murine isolates were determined by using Opa-specific monoclonal antibodies (MAbs) in a previously described immunoblot method. This method allows one to determine the predominant Opa phenotype within a colony following a single in vitro passage from the host (27). The MAbs utilized in this assay (H138, H4, H157, H164, and H156) are specific for epitopes within the hypervariable regions of seven of the eight Opa proteins of FA1090 and were kindly provided by Janne Cannon (University of North Carolina). MAbs H138, H157, and H156 recognize OpaA, OpaC, and OpaF in FA1090, respectively. MAb H4 and MAb H164 recognize an epitope present in OpaB and OpaD (MAb H4) and in OpaE and OpaK (MAb H164) (4, 5). Different Opa proteins within a strain confer different degrees of photo-opacity to a colony when viewed under a stereomicroscope, ranging from transparent to highly opaque. OpaD variants produce colonies that are markedly opaque compared to those of OpaB variants, and OpaD has a higher apparent molecular weight than OpaB when analyzed by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (4). Colony opacity and/or a difference in apparent molecular weight by SDS-PAGE was used to distinguish OpaB from OpaD expression among MAb H4-positive colonies. OpaE and OpaK variants both produce transparent colonies. Although there is a slight difference in the migration of OpaK and OpaE by SDS-PAGE (27), H164-positive colonies were not analyzed further. There is no MAb specific for the OpaI protein of FA1090. OpaI variants were identified by colony opacity, since the expression of OpaI confers a markedly opaque colony morphology which is highly predictive of OpaI expression (27). MAb 4B12 is specific for a conserved linear epitope present in all Opa proteins (2) and was a generous gift of Milan Blake (North American Vaccines, Inc., Beltsville, Md.). MAb 4B12 was used to confirm the expression of OpaI in randomly picked colonies with OpaI morphology and of OpaF in MAb H156-positive variants, since Mab H156 binds weakly to OpaF in the described immunoblot procedure. SDS-PAGE and Western immunoblotting of whole bacteria cultured from frozen primary isolates were performed as described previously (27).
Serum bactericidal assay.
Serum was obtained from pooled venous tail blood of estradiol-treated SLC:ddY mice and from a healthy female volunteer. Serum was separated from clotted blood within 30 min after collection and used immediately or frozen at −80°C before being tested for bactericidal activity. The bactericidal activity of human and murine sera against N. gonorrhoeae was tested essentially as described by Chen et al. (9). Briefly, serial dilutions of human or murine serum were made in Eagle's minimal essential medium (EMEM) (Biofluids, Inc., Rockville, Md.). Bacteria cultured for 18 to 22 h on GC agar were suspended in saline to an A600 of 0.46 and diluted 1:1,000 in EMEM to obtain 105 CFU/ml. Equal volumes (70 μl) of bacterial suspension and diluted serum were combined and incubated in triplicate wells of a 96-well microtiter plate for 1 h at 37°C in 5% CO2. The number of viable bacteria present in each well following this incubation period was determined by standard quantitative culture. No significant decrease in the number of viable gonococci occurred in EMEM without serum during the 1-h incubation period. The degree of serum bactericidal activity was measured by comparing the number of CFU that were isolated from wells containing serum to that obtained from wells in which bacteria were incubated in EMEM alone. The experiments were repeated twice to test reproducibility, and the results were similar.
RESULTS
Experimental infection of estradiol-treated BALB/c mice.
Attempts to establish long-term infection in untreated mice (DBA, CD-1, and BALB/c) following intravaginal inoculation with N. gonorrhoeae were unsuccessful. As reported by others (7, 28, 54), gonococci were recovered for an average of 24 to 48 h after inoculation. High numbers of PMNs were seen in vaginal smears from the majority of mice within 48 h after inoculation due to either the occurrence of inflammation or the natural transition into the metestrus and/or diestrus phase of the estrus cycle. Therefore, based on the report that estradiol increases the susceptibility of mice to gonococcal genital tract infection (57), estradiol was used as described in Materials and Methods to promote prolonged infection of mice. Antibiotics were included in the infection protocol based on pilot studies which showed that simultaneous treatment with 17-β-estradiol and antibiotics resulted in a markedly longer duration of recovery of N. gonorrhoeae from mice than did treatment with either agent alone. As shown in Fig. 1, the average duration of bacterial recovery from the vaginas of estradiol-treated BALB/c mice was 4.8 days (n = 5) and 12.8 days (n = 5) following inoculation with 105 or 106 CFU, respectively, of wild-type strain FA1090. Gonococci were recovered from mice for an average of 10.8 days (n = 4) and 12.2 days (n = 6) following inoculation with 105 or 106 CFU, respectively, of strain MS11A. A mouse-passaged variant of strain MS11A was isolated following two sequential passages through mice and tested to see if it was better adapted for murine infection. The infectious dose for this variant (MS11var.72) was unexpectedly higher than that of the wild-type parent, however, with 4 × 106 CFU required for long-term recovery (≥5 days) in > 80% of mice.
FIG. 1.
Duration of recovery of N. gonorrhoeae from vaginal swabs of estradiol-treated BALB/c mice following intravaginal inoculation with wild-type strains FA1090 var. A21 and MS11A or the mouse-passaged variant MS11var.72. The bacterial doses shown were tested two or more times for each strain, and the results were similar.
Quantitative culture results suggest that bacterial replication occurred in vivo. In most cases, the number of gonococci recovered from vaginal swabs decreased a few days after inoculation, followed by a 2- to 3-log10-unit increase in recoverable gonococci (Fig. 2A). In all experiments, higher numbers of gonococci than the number that was inoculated were recovered from at least one mouse in each experimental group. Interestingly, intervals of 2 to 5 days during which only a few or no gonococci were detected occurred in some mice, followed by a dramatic increase in recovery. These intervals of reduced recovery typically occurred 5 to 10 days after inoculation. Similar quantitative culture data were obtained from experiments with strains MS11A and MS11var.72 (data not shown). There was no evidence of enhanced growth of the mouse-passaged variant occurring in vivo.
FIG. 2.
(A) Number of gonococci recovered over time from estradiol-treated BALB/c mice following inoculation with 106 CFU of strain FA1090. Values are expressed as the log10 CFU recovered in a 100-μl suspension of vaginal specimen obtained on a swab. The data for the mouse represented by open circles illustrates a period of reduced bacterial recovery, as described in the text. In this mouse, ≤10 CFU was recovered over a period of 3 days, followed by a 2- to 3-log-unit increase in recovery. (B) Percentages of vaginal PMNs for individual test mice over time. The baseline percentage (average percentage of vaginal PMNs plus one standard deviation from placebo controls [n = 5]) is shown by the dotted line. The difference between the percentage of PMNs among test mice (n = 8) and that of control mice (n = 5) was statistically significant on days 2 to 4, 6 to 8, 10, and 12 to 14 (Student t test: P ≤ 0.10 for days 2, 6, 7, 8, and 12; P ≤ 0.05 for days 3, 10, and 13; and P < 0.025 for days 4 and 14). The symbols representing each individual mouse are the same in panels A and B so that the degree of recovery of gonococci during periods of inflammation can be analyzed.
Unlike the results of Taylor-Robinson et al. (57), inflammation in response to infection was detected in the majority of mice inoculated with any of the three bacterial strains tested. The daily percentage of PMNs in vaginal smears from individual test mice inoculated with strain FA1090 compared to the baseline percentage derived from placebo-treated controls is depicted in Fig. 2B. A modest increase in the baseline percentage of PMNs occurred over time in most experiments. PMNs were rarely seen in vaginal smears from control mice during the first 5 to 7 days following inoculation with the inoculum diluent; the average percentage of PMNs then gradually increased, generally due to one or two control mice out of five having low numbers of PMNs (<45% of vaginal cells). In parallel experiments, estradiol-treated control mice that were left uninoculated showed similar results, suggesting that the appearance of PMNs was due to decreasing levels of estradiol over time rather than to the induction of low-level inflammation by ingredients in the diluent. In the representative experiment shown in Fig. 2, seven of eight (88%) estradiol-treated BALB/c mice inoculated with strain FA1090 had a higher percentage of PMNs than the baseline percentage on two or more consecutive days (Fig. 2B). Three of these mice (mice 1, 5, and 8) cleared infection concomitantly with the development of inflammation. The persistence of high numbers of gonococci in the presence of inflammation in the remaining mice is illustrated by comparing of the log10 recovery of bacteria from individual mice (Fig. 2A) with the percentage of vaginal PMNs from each corresponding mouse (Fig. 2B).
Experimental infection of SLC:ddY mice.
Based on reports that SLC:ddY mice are naturally susceptible to gonococcal infection (32, 34), a breeding colony of these mice was imported to generate female mice for experimental infection. Long-term infection could not be established in SLC:ddY mice by using the protocol of Kita et al. (32); however, estradiol treatment promoted susceptibility to infection. Gonococci were recovered from 50 to 80% of estradiol-treated SLC:ddY mice for an average of 12 days (range, 5 to 15 days) following inoculation with 107 or 108 CFU of the mouse-passaged variant MS11var.72. In contrast to infection in BALB/c mice, an influx of vaginal PMNs in SLC:ddY mice always coincided with clearance of infection. No inflammatory exudate was detected in SLC:ddY mice that were colonized for >5 days. Lower doses of MS11var.72 (105 and 106 CFU) resulted in fewer mice with inflammation, but a higher percentage of mice did not become infected. Inoculation of estradiol-treated SLC:ddY mice with 106 (n = 3) or 107 (n = 7) CFU of wild-type strain FA1090 resulted in a vigorous inflammatory response within 1 to 5 days in all mice, coinciding 100% with clearance of N. gonorrhoeae. To compensate for the ca. 40% difference in body weight between 4- to 6-week-old BALB/c and SLC:ddY mice, SLC:ddY mice were implanted with a higher-dose estradiol pellet (10 mg) and challenged with N. gonorrhoeae MS11var.72. No difference in results was observed between mice that received the 10-mg estradiol pellet and those that received the 5-mg pellet (data not shown).
Upper reproductive tract infection.
In two experiments, BALB/c mice infected with strain FA1090 were sacrificed to obtain endometrial tissue for culture. N. gonorrhoeae was recovered from the uterine horns of one of six (17%) and one of five (20%) mice, with 6 × 101 and 1.7 × 103 CFU, respectively, isolated from mucosal washings. All mice in these experiments were sacrificed at 14 days after inoculation, except for one of the two mice with positive endometrial cultures, which became moribund and was therefore sacrificed on day 11. Both of the mice that had positive endometrial cultures appeared to be hyperpyretic and demonstrated weight loss (ca. 4 g) during the first week of infection. A few gonococci were isolated from the uterine horns of one of four BALB/c mice on day 14 following inoculation with strain MS11A; no gonococci were isolated from the uterine horns of five BALB/c mice infected with strain MS11var.72. There was no evidence of ascended infection in estradiol-treated SLC:ddY mice following inoculation with strain MS11var.72 or FA1090.
Gonococcus-host cell interactions.
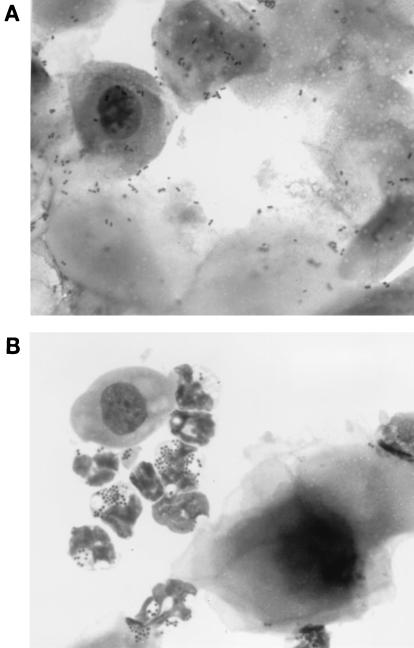
Stained vaginal smears from infected BALB/c and SLC:ddY mice revealed gram-negative diplococci associated with murine epithelial cells. High numbers of gonococci associated with squamous epithelial cells were observed during early stages of infection; adherence to columnar epithelial cells was less frequently seen (Fig. 3A). Fewer numbers of epithelial cell-associated gonococci were noted at later time points. In mice in which inflammation occurred, gram-negative diplococci associated with or within neutrophils were readily detected in vaginal smears (Fig. 3B). Greater than 99% of gonococci in the MS11A and FA1090 inocula used in these experiments were piliated as determined by the colony morphology of inoculum isolates. However, a high percentage of murine isolates exhibited a nonpiliated colony morphology within 48 h after inoculation, suggesting that gonococcal pili may not play a role in murine infection.
FIG. 3.
Vaginal smears showing N. gonorrhoeae adhering to murine epithelial cells 2 days after inoculation (A) and within murine neutrophils 12 days following inoculation (B). Smears are stained with a modified Wright stain. A Gram stain of a smear from the same mouse prepared in parallel showed gram-negative diplococci. N. gonorrhoeae (but no facultatively anaerobic commensal bacteria) was isolated from this mouse at these time points.
Opacity protein expression during murine infection.
Data from male volunteer studies suggest that the expression of gonococcal Opa proteins is selected for and/or induced in the male urethra following inoculation with a predominantly Opa-negative population of N. gonorrhoeae (27, 56). To determine if a similar phenomenon occurs during experimental murine infection, the Opa phenotypes of the inoculum and vaginal isolates from eight BALB/c mice inoculated with the Opa-negative variant A21 of FA1090 were assessed. Ninety-four percent of the inoculum isolates were of the Opa-negative phenotype; in contrast, high percentages of Opa protein-expressing gonococci were detected among vaginal isolates from four of the eight test mice. Five mice were infected for 14 days (Fig. 4A, mice 2, 3, 4, 6, and 7); greater than 50% of the vaginal isolates from three of these five mice (mice 2, 6, and 7) expressed one or more Opa proteins within 4 days after inoculation. Two mice cleared infection within 2 days; 29 and 46% of the isolates from these mice expressed Opa proteins within 24 h after inoculation (Fig. 4A, mice 1 and 8). No significant change in Opa phenotype was detected among isolates from a mouse infected for 5 days (Fig. 4A, mouse 5). Similar to previous reports (27, 56), this dramatic change in Opa phenotype did not occur when the inoculum was passaged daily on GC agar plates for 5 days. In two experiments, high percentages of Opa protein-expressing gonococci were also isolated from one of two and two of two SLC:ddY mice inoculated with the Opa-negative variant A21 (Fig. 4B). No clear association between the PMN responses and Opa phenotypes of vaginal isolates was observed for either SLC:ddY or BALB/c mice.
FIG. 4.
Percentage of vaginal isolates from estradiol-treated BALB/c (A) or SLC:ddY (B) mice that express one or more Opa proteins following inoculation with a predominantly Opa-negative population of FA1090. Percentages are based on 18 to 24 primary murine isolates for each time point and on 35 to 36 colonies isolated directly from the inoculum suspension and after in vitro passage of the inoculum for 5 days. Asterisks indicate time points for which the number of isolates was insufficient for analysis. Gonococci were recovered from BALB/c mice (A) for 2 days (mice 1 and 8), 5 days (mouse 5), and 14 days (mice 2, 3, 4, 6, and 7). Gonococci were recovered from SLC:ddY mice (B) for 4 days (mice 62, 66, and 17) or 5 days (mouse 9). Later time points are not shown for SLC:ddY mice because these mice cleared infection within 5 days after inoculation.
A single Opa antigenic variant was highly represented among vaginal isolates from individual mice in which a change in Opa phenotype occurred. However, different Opa variants predominated in different mice (Table 1). High percentages of gonococci expressing more than one Opa protein simultaneously were isolated from two of four SLC:ddY mice. A similar scenario was noted during experimental infection of male volunteers (27). This observation is interesting since isolation of gonococci expressing more than two Opa proteins is rare in vitro and expression of multiple Opa proteins is rapidly lost upon subculture (personal observation).
TABLE 1.
Opa protein phenotypes of vaginal isolates from estradiol-treated BALB/c and SLC:ddY mice following inoculation with a predominantly Opa-negative population of FA1090a
| Strain and mouse no.b | Opa protein phenotype (%) on day:
|
|||
|---|---|---|---|---|
| 1 | 4 | 5 | 8 | |
| BALB/c | ||||
| 2 | Negative (79), I (13), E/K (4), B (4) | Negative (44), I (44), B (4), A (4), D,I (4) | Negative (55), I (20), F (10), B (5), A,I (5), A (5) | |
| 6 | Negative (71), I (29) | I (100) | I (95), E/K,I (5), negative (5) | |
| 7 | Negative (42), F (33), D (17), B (8) | Negative (67), B (13), I (13), D (4), F (4) | B (67), negative (25), B,F (8) | |
| SLC:ddY | ||||
| 62 | Negative (71), B (25), I (4) | I (46), negative (42), B (8), A (4) | ||
| 9 | Negative (50), B,I (20), E/K (13), B,E/K (6), B (6), F (6) | Negative (55), I (20), C (10), C,I (10), B (5) | B,C (33), B (21), B,I (17), I (8), C,I (8), F,I (4), negative (4), B,C,E/K (4) | |
| 17 | Negative (88), B (6), F (6) | Negative (58), I (18), B (8), C (8), F (8) | ||
The inocula were 94% Opa negative, 3% OpaB, and 3% OpaA (BALB/c mice); 92% Opa negative, 4% OpaB, and 4% OpaA (SLC:ddY mouse 62); and 97% Opa negative and 3% OpaB (SLC:ddY mice 9 and 17). Other Opa variants were undoubtedly present in the inoculum suspensions but were below the limits of detection.
Sensitivity of N. gonorrhoeae to killing by mouse serum.
To test the hypothesis that Opa-negative bacteria in the urogenital tract may be selected against by complement-mediated bactericidal activity (6), the sensitivity of FA1090 variant A21 to serum from estradiol-treated SLC:ddY mice was tested. Normal human serum was used as a positive control, and strain MS11var.72 was included as a serum-sensitive control. No decrease in viable counts occurred for either strain following incubation in 0 to 50% mouse serum. In contrast, dose-dependent killing of strains FA1090 and MS11var.72 occurred following incubation with normal human serum. As expected, MS11var.72 was more sensitive to killing by human serum than was FA1090, with half-maximal survival (50%) detected following incubation of MS11var.72 and FA1090 in 2 and 25% serum, respectively. Heat treatment (56°C, 30 min) abolished the bactericidal activity of human serum against both MS11var.72 and FA1090. The resistance of N. gonorrhoeae to the bactericidal activity of murine serum has been reported by others (7, 54).
DISCUSSION
An animal model of gonococcal genital tract infection is greatly needed to facilitate the development of vaccines and other prophylactic agents in the face of the continuing gonorrhea epidemic. Such a model is also needed as an inexpensive tool for studying gonococcal pathogenesis in the context of a physiologically balanced environment in which immune defenses are intact. A female animal model is especially desirable so that adaptation of N. gonorrhoeae to the female reproductive tract can be explored in vivo. This report describes a female mouse model of long-term gonococcal genital tract infection based on a modification of the technique described by Taylor-Robinson et al. (57). N. gonorrhoeae was recovered from estradiol-treated mice for as long as 14 days, after which the experiments were terminated. Infection was limited to the lower genital tract in the majority of mice, although ascension to the uterine horns occurred in 17 to 20% of mice inoculated with strain FA1090. These statistics are reasonable in light of the fact that ascended infection in women is estimated to occur in 10 to 20% of cervical infections (24, 61). Strain FA1090 is a serum-resistant strain isolated from a patient with DGI. Future studies will focus on the capacity of serum-resistant versus serum-sensitive gonococcal strains to ascend to the upper reproductive tract and to disseminate to the bloodstream following lower genital tract infection in mice.
It is not clear why exogenously administered estradiol increases the susceptibility of mice to N. gonorrhoeae. Estrogens have an anti-inflammatory effect (33, 47) and also cause a thickening of the vaginal mucosa via stimulation of the proliferation of epithelial cells. By maintaining an estrus-like state, inhibitory factors associated with later stages or their effects are perhaps reduced. The mouse estrus cycle lasts 4 to 6 days and consists of five stages (53). Proestrus and estrus are anabolic stages during which estrogens produced by developing ovarian follicles promote active cellular growth. These stages are marked by the presence of columnar or squamous epithelial cells, respectively, in vaginal smears. In normally cycling mice, proestrus is the most hospitable phase for N. gonorrhoeae. Following ovulation, progesterone is secreted by the ruptured corpus luteum and high numbers of PMNs infiltrate into the vaginal lumen. Mice in these postovulatory stages (metestrus I and II and diestrus) are resistant to gonococcal infection, perhaps due to the abundance of phagocytes in the vaginal lumen, high numbers of commensal flora during metestrus, increased progesterone levels, and changes in mucus and cervical histology (7, 14). Interestingly, an association between culture positivity in infected women and the proliferative (high-estrogen and low-progesterone) stage of the menstrual cycle is documented in several (26, 29, 35, 41) but not all (20, 39) clinical studies; this association is consistent with the influence of reproductive hormones on the susceptibility of mice to gonococcal infection.
One complication to the model as presented is the need to suppress the overgrowth of commensal flora resulting from the massive accumulation of glycogen that occurs under the influence of estradiol. To avoid the expense of using germfree animals, an antibiotic treatment regimen which adequately suppresses inhibitory commensal flora in the majority of mice was developed. Occasional mice are colonized with inhibitory gram-negative rods or staphylococcus-like organisms, however, and it is therefore necessary to monitor commensal flora in individual mice during experiments in which susceptibility to infection is being analyzed. Otherwise, conclusions regarding the effect of inflammation, immunization, or infectious dose on the rate and/or duration of infection may be false. For example, in the work presented here, mice with inhibitory commensal flora were excluded so that the infectious doses of different gonococcal strains could be compared without differences in commensal load confounding the data.
The reliability of any nonhuman model of gonorrhea to predict events that are relevant to human infection is challenged by the fact that N. gonorrhoeae utilizes several human-specific factors during natural infection. In particular, nonhuman models may be inappropriate for the study of gonococcus-host cell interactions due to the absence of the human-specific adherence receptors. Specifically, the CD46 receptor utilized by gonococcal pili (30) is not expressed in mice (43), and no murine homologues to the carcinoembryonic antigen (CEA) family members utilized by gonococcal Opa proteins (10, 59) have been described. The availability of transgenic mice that express the CD46 (43) and CEA (19) receptors may at least partially overcome some of these host restrictions. Achievement of meaningful gonococcus-host cell interactions in any mouse background may necessitate the use of intrauterine inoculation, however, since the columnar epithelium of the murine endometrium is more relevant to human infection than is the predominantly squamous vaginal epithelium that is characteristic of mice under the influence of estradiol (14).
In light of the constant shedding of mucus from the murine vagina (52), however, it is likely that gonococcal adherence to the vaginal mucosa occurred during the prolonged infection of mice described here. Indeed, gonococci were seen closely associated with epithelial cells in stained smears from infected BALB/c mice. The gonococcal ligands responsible for these interactions are not known at this time. The receptor(s) for the hemagglutination activity associated with gonococcal pilin has not been identified (49); however, the loss of the piliation phenotype among murine isolates suggested that piliation may not play a role in experimental murine infection. Porat et al. (46) demonstrated that the terminal lacto-N-neotetraose moeity of gonococcal LOS binds to the asialoglycoprotein receptor. It is not known if similar binding occurs between this LOS species and the murine counterpart of this molecule (1). In the present work, gonococci were recovered from the mucosal surface of the murine vaginal lumen and endometrium; it is not known at this time if intracellular bacteria were also present.
The inability of gonococci to utilize nonhuman transferrin or lactoferrin as an iron source (15, 37) is another obstacle that may impede the precise reproduction of human infection in mice. The importance of the gonococcal transferrin binding protein during gonococcal urethritis was recently demonstrated in male volunteers (16). The recovery of high numbers of gonococci during experimental murine infection, however, suggests that other sources of usable iron are present in cervical mucus. Candidate reservoirs include hemin, hemoglobin, and iron bound to metabolic intermediates such as isocitrate, pyruvate, malate, and pyrophosphate (42).
The gonococcal immunoglobulin A1 (IgA1) protease is also host restricted in that it cannot cleave the murine structural class of IgA. It is hypothesized that IgA1 protease increases gonococcal survival on mucosal surfaces by inactivating gonococcus-specific IgA1 (31). The relevance of this virulence factor during infection of naive individuals is questionable, however, based on the recent report that an IgA1 protease mutant was infectious in male volunteers (12) and on the inability to detect IgA1 cleavage fragments in clinical specimens from infected patients (23). Recently Lin et al. (38) demonstrated that IgA1 protease promotes intracellular survival by cleaving the phagosome maturation protein LAMP1. The host specificity of this interesting function of IgA1 protease has not been reported. Additional roles for IgA1 protease may exist, based on the report that an IgA1 protease mutant of Streptococcus pneumoniae was attenuated in a murine septicemia model (45).
Despite the limitations described above, the use of estradiol-treated BALB/c mice to study gonococcal Opa protein expression in vivo is supported by the changes in Opa phenotype that were observed during experimental murine infection. Although the susceptibility of untreated SLC:ddY mice to gonococcal genital tract infection is questionable, it should be noted that Kita et al. (34) also described changes in Opa phenotype among isolates from infected SLC:ddY mice. The factors responsible for the apparent selection and/or induction of Opa protein expression in vivo are currently unidentified. Antigenic variation of the Opa phenotype is the result of high-frequency, reversible phase variation of individual opa genes, each encoding an antigenically distinct Opa protein (reviewed in reference 17). One might hypothesize that the change in Opa phenotype observed among urethral isolates from infected male volunteers (27, 56) and vaginal isolates from experimentally infected mice is due to selection for Opa protein expression by components of the innate defense. In light of the fact that gonococcal Opa proteins mediate adherence and/or invasion of host epithelial cells in vitro, it is also possible that those Opa variants capable of mediating adherence to and/or invasion of host cells have a functional advantage in establishing infection and therefore would be more highly represented among reisolates. The latter hypothesis is not consistent with the phenomenon observed in mice, since mice do not produce the CEA family subgroup which serves as the Opa receptors (19). Finally, it is possible that Opa-positive gonococci that enter murine epithelial cells via a non-Opa-mediated pathway may have an advantage for intracellular growth, based on the demonstration by Williams et al. (62) that Opa proteins bind pyruvate kinase from human epithelial cells and that gonococci need to utilize pyruvate and/or lactate for intracellular growth.
Not all BALB/c mice demonstrated a change in Opa phenotype during infection. Interestingly, James and Swanson reported that although a predominance of opaque gonococcal variants occurred among cervical isolates from women in the proliferative stage of the menstrual cycle, transparent variants predominated among cervical isolates isolated during the luteal phase (25, 26). This observation, together with the report that opaque variants are more sensitive to progesterone in vitro than are transparent variants (48), suggests that progesterone may select against Opa protein expression during infection of women. One might therefore hypothesize that the secretion of endogenous progesterone during experimental murine infection may also select against the expression of Opa proteins. While the mice used in the present study were under exogenous estradiol treatment, they were not ovariectomized, and therefore, progesterone secretion may have occurred during the course of the experiment. The influx of vaginal PMNs in some placebo-treated controls is consistent with this possibility, as is the absence of Opa-positive isolates in some mice following inoculation with an Opa-negative FA1090 variant. Ovariectomized mice may provide a useful background for addressing this hypothesis as well as for exploring the effects of reproductive hormones on gonococcal infection.
In summary, the change in Opa phenotype observed in both male volunteers and experimentally infected mice is consistent with the theory that Opa protein expression confers an advantage to the gonococcus in the urogenital tract. It is still not clear if the observed changes in Opa phenotype during infection reflect a selection against certain Opa phenotypes or if increased phase variation of opa genes occurs in vivo. Future studies using the murine model may elucidate the host factors and genetic mechanisms that promote gonococcal adaptation through changes in Opa protein expression.
ACKNOWLEDGMENTS
I thank Diane Ruffner and Iris Enid Valentin-Bon for technical assistance; Janne Cannon, Milan Blake, and Hank Seifert for providing antibodies or strains used in this study; David Hone for helpful reading of the manuscript; and Alison O'Brien and Sam Formal for valuable discussions.
This work was supported by USUHS Intramural Research grant RO73-FD, bridge grant GW173-GU from the Henry M. Jackson Foundation, and NIH grant RO1-AI42053-01A1.
REFERENCES
- 1.Abdullah M, Kierszenbaum A L. Identification of rat testis galactosyl receptor using antibodies to liver asialoglycoprotein receptor: purification and localization on surfaces of spermatogenic cells and sperm. J Cell Biol. 1989;108:367–375. doi: 10.1083/jcb.108.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achtman M, Neibert M, Crowe B A, Strittmatter W, Kusecek B, Weyse E, Walsh M J, Slawig B, Morelli G, Moll A, Blake M. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J Exp Med. 1988;168:507–525. doi: 10.1084/jem.168.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arko R J. Animal models for pathogenic Neisseria species. Clin Microbiol Rev. 1989;2:S56–S59. doi: 10.1128/cmr.2.suppl.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barritt D S, Schwalbe R S, Klapper D G, Cannon J G. Antigenic and structural differences among six proteins II expressed by a single strain of Neisseria gonorrhoeae. Infect Immun. 1987;55:2026–2031. doi: 10.1128/iai.55.9.2026-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black W J, Schwalbe R S, Nachamkin I, Cannon J G. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984;45:453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos M P, Hogan D, Belland R J. Selection of Opa+Neisseria gonorhoeae by limited availability of normal human serum. Infect Immun. 1997;65:645–650. doi: 10.1128/iai.65.2.645-650.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braude A I, Corbeil L B, Levine S, Ito J, McCutchan J A. Possible influence of cyclic menstrual changes on resistance to the gonococcus. In: Brooks G F, Gotschlich E C, Holmes K K, Sawyer W D, Young F E, editors. Immunobiology of Neisseria gonorrhoeae. Washington, D.C: American Society for Microbiology; 1978. pp. 328–337. [Google Scholar]
- 8.Centers for Disease Control. Ten leading nationally notifiable infectious diseases—United States, 1995. Morbid Mortal Weekly Rep. 1996;45:8834–8835. [PubMed] [Google Scholar]
- 9.Chen T, Swanson J, Wilson J, Belland R J. Heparin protects Opa+Neisseria gonorrhoeae from the bactericidal action of normal human serum. Infect Immun. 1995;63:1790–1795. doi: 10.1128/iai.63.5.1790-1795.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Gotschlich E C. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen M S, Cannon J G, Jerse A E, Charniga L M, Isbey S F, Whicker L G. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994;169:532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]
- 12.Cohen M S, Cannon J G. Human experimentation with Neisseria gonorrhoeae: progress and goals. J Infect Dis. 1999;179:S375–379. doi: 10.1086/513847. [DOI] [PubMed] [Google Scholar]
- 13.Connell T D, Shaffer D, Cannon J G. Characterization of the repertoire of hypervariable regions in the protein II (opa) gene family of Neisseria gonorrhoeae. Mol Microbiol. 1990;4:439–449. doi: 10.1111/j.1365-2958.1990.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 14.Corbeil L B, Chatterjee A, Foresman L, Westfall J A. Ultrastructure of cyclic changes in the murine uterus, cervix and vagina. Tissue Cell. 1985;17:53–68. doi: 10.1016/0040-8166(85)90015-1. [DOI] [PubMed] [Google Scholar]
- 15.Cornelissen C N, Biswas G D, Sparling P F. Expression of gonococcal transferrin-binding protein 1 causes Escherichia coli to bind human transferrin. J Bacteriol. 1993;175:2448–2450. doi: 10.1128/jb.175.8.2448-2450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 17.Dehio C, Gray-Owen S D, Meyer T F. The role of neisserial opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 18.Dillard J P, Seifert H S. A peptidoglycan hydrolase similar to bacteriophage endolysins acts as an autolysin in Neisseria gonorrhoeae. Mol Microbiol. 1997;25:893–901. doi: 10.1111/j.1365-2958.1997.mmi522.x. [DOI] [PubMed] [Google Scholar]
- 19.Eades-Perner A M, van der Putten H, Hirth A, Thompson J, Neumaier M, van Kleist S, Zimmermann W. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994;54:4169–4176. [PubMed] [Google Scholar]
- 20.Falk V, Krook G. Do results of culture for gonococci vary with sampling phase of menstrual cycle? Acta Derm Venereol. 1967;47:190–193. [PubMed] [Google Scholar]
- 21.Flemming T J, Wallsmith D E, Rosenthal R S. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect Immun. 1986;52:600–608. doi: 10.1128/iai.52.2.600-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldenberg D L, Chisholm P L, Rice P A. Experimental models of bacterial arthritis: a microbiologic and histopathologic characterization of the arthritis after the intraarticular injections of Neisseria gonorrhoeae, Staphylococcus aureus, group A streptococci, and Escherichia coli. J Rheumatol. 1983;10:5–11. [PubMed] [Google Scholar]
- 23.Hedges R R, Mayo M S, Kallman L, Mestecky J, Hook E W, Russell M W. Evaluation of immunoglobulin A1 (IgA1) protease and IgA1 protease-inhibitory activity in human female genital infection with Neisseria gonorrhoeae. Infect Immun. 1998;66:5826–5832. doi: 10.1128/iai.66.12.5826-5832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hook E W, Handsfield H H. Gonococcal infections in the adult. In: Holmes K K, Sparling P F, Mardh P A, Lemon S M, Stamm W E, Piot P, Wasserheit J N, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill Companies, Inc.; 1999. pp. 451–466. [Google Scholar]
- 25.James J F, Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978;19:332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James J F, Swanson J. Color/opacity colonial variants of Neisseria gonorrhoeae and their relationship to the menstrual cycle. In: Brooks G F, Gotschlich E C, Holmes K K, Sawyer W D, Young F E, editors. Immunobiology of Neisseria gonorrhoeae. Washington, D.C: American Society for Microbiology; 1978. pp. 338–343. [Google Scholar]
- 27.Jerse A E, Cohen M S, Drown P M, Whicker L G, Isbey S F, Seifert H S, Cannon J G. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson A P, Tuffrey M, Taylor-Robinson D. Resistance of mice to genital infection with Neisseria gonorrhoeae. J Med Microbiol. 1989;30:33–36. doi: 10.1099/00222615-30-1-33. [DOI] [PubMed] [Google Scholar]
- 29.Johnson D W, Holmes K K, Kvale P S. An evaluation of gonorrhea case findings in the chronically infected female. Am J Epidemiol. 1969;90:438–448. doi: 10.1093/oxfordjournals.aje.a121090. [DOI] [PubMed] [Google Scholar]
- 30.Kallstrom H, Liszewski M K, Atkinson J P, Jonsson A B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 31.Kilian M, Mestecky J, Russell M W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kita E, Matsuura H, Kashiba S. A mouse model for the study of gonococcal genital infection. J Infect Dis. 1981;143:67–70. doi: 10.1093/infdis/143.1.67. [DOI] [PubMed] [Google Scholar]
- 33.Kita E, Takahashi S, Yasui K, Kashiba S. Effect of estrogen (17β-estradiol) on the susceptibility of mice to disseminated gonococcal infection. Infect Immun. 1985;49:238–243. doi: 10.1128/iai.49.1.238-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kita E, Katsui N, Emoto N, M, Sawaki M, Oku D, Nishikawa F, Hamuro A, Kashiba S. Virulence of transparent and opaque colony types of Neisseria gonorrhoeae for the genital tract of mice. J Med Microbiol. 1991;34:355–362. doi: 10.1099/00222615-34-6-355. [DOI] [PubMed] [Google Scholar]
- 35.Koch M L. A study of cervical cultures taken in cases of acute gonorrhea with special reference to the phases of the menstrual cycle. Am J Obstet Gynecol. 1947;54:861–866. doi: 10.1016/s0002-9378(16)39663-6. [DOI] [PubMed] [Google Scholar]
- 36.Lambden P R, Heckels J E, Watt P J. Effect of anti-pilus antibodies on survival of gonococci within guinea pig subcutaneous chambers. Infect Immun. 1982;38:27–30. doi: 10.1128/iai.38.1.27-30.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee B C, Schryvers A B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988;2:827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin L, Ayala P, Larson J, Mulks M, Fukuda M, Carlsson S R, Enns C, So M. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol. 1997;24:1983–1094. doi: 10.1046/j.1365-2958.1997.4191776.x. [DOI] [PubMed] [Google Scholar]
- 39.Lowe T L, Krauss S J. Quantitation of Neisseria gonorrhoeae from women with gonorrhea. J Infect Dis. 1976;133:621–626. doi: 10.1093/infdis/133.6.621. [DOI] [PubMed] [Google Scholar]
- 40.McBride H M, Lamden P R, Heckels J E, Watt P J. The role of outer membrane proteins in the survival of Neisseria gonorrhoeae P9 within guinea-pig subcutanous chambers. J Gen Microbiol. 1981;126:63–67. doi: 10.1099/00221287-126-1-63. [DOI] [PubMed] [Google Scholar]
- 41.McCormack W M, Reynolds G H. Effect of menstrual cycle and method of contraception on recovery of N. gonorrhoeae. JAMA. 1982;247:1292–1294. [PubMed] [Google Scholar]
- 42.Mickelson P A, Sparling P F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz C J, Hourcade D, Atkinson J P, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowicki S, Martens M G, Nowicki B J. Gonococcal infection in a nonhuman host is determined by human complement C1q. Infect Immun. 1995;63:4790–4794. doi: 10.1128/iai.63.12.4790-4794.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, Ferrari L, Simon D. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porat N, Apicella M A, Blake M S. Neisseria gonorrhoeae utilizes and enhances the biosynthesis of the asialoglycoprotein receptor expressed on the surface of the hepatic HepG2 cell line. Infect Immun. 1995;63:1498–1506. doi: 10.1128/iai.63.4.1498-1506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pottratz S T, Bellido T, Mocharia H, Crabb D, Manolagas S C. 17 β-estradiol inhibits expression of human interleukin-6 promoter-reporter constructs by a receptor-dependent mechanism. J Clin Invest. 1994;93:944–950. doi: 10.1172/JCI117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salit I E. The differential susceptibility of gonococcal opacity variants to sex hormones. Can J Biochem. 1982;60:301–306. doi: 10.1139/m82-044. [DOI] [PubMed] [Google Scholar]
- 49.Scheuerpflug I, Thomas R, Ryll R, Pandit J, Meyer T F. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial and epithelial cells. Infect Immun. 1999;67:834–843. doi: 10.1128/iai.67.2.834-843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider H, Griffiss J M, Boslego J W, Hitchcock P J, Zahos K M, Apicella M A. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J Exp Med. 1991;174:1601–1605. doi: 10.1084/jem.174.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seifert H S, Wright C J, Jerse A E, Cohen M S, Cannon J G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest. 1994;93:2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherwood J K, Zeitlin L, Chen X, Whaley K J, Cone R A, Saltzman W M. Residence half-life of IgG administered topically to the mouse vagina. Biol Reprod. 1996;54:264–269. doi: 10.1095/biolreprod54.1.264. [DOI] [PubMed] [Google Scholar]
- 53.Snell G D. Reproduction. In: Snell G D, editor. Biology of the laboratory mouse. New York, N.Y: Dover Publications, Inc.; 1941. pp. 55–88. [Google Scholar]
- 54.Streeter P R, Corbeil L B. Gonococcal infection in endotoxin-resistant and endotoxin-susceptible mice. Infect Immun. 1981;32:105–110. doi: 10.1128/iai.32.1.105-110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey M J. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swanson J, Barrera O, Sola J, Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988;168:2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor-Robinson D, Furr P M, Hetherington C M. Neisseria gonorrhoeae colonises the genital tract of oestradiol-treated germ-free female mice. Microb Pathog. 1990;9:369–374. doi: 10.1016/0882-4010(90)90071-w. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Department of Health and Human Services. Pelvic inflammatory disease. Research directions in the 1990s. Sex Transm Dis. 1991;18:46–64. doi: 10.1097/00007435-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Virji M, Makepeace K, Ferguson D J P, Watt S M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 60.Washington E, Katz P. Cost of and payment source for pelvic inflammatory disease. JAMA. 1991;13:2565–2569. [PubMed] [Google Scholar]
- 61.Westrom L, Eschenbach D. Pelvic inflammatory disease. In: Holmes K K, Sparling P F, Mardh P A, Lemon S M, Stamm W E, Piot P, Wasserheit J N, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill Companies, Inc.; 1999. pp. 783–809. [Google Scholar]
- 62.Williams J M, Chen G C, Zhu L, Rest R F. Using the yeast two-hybrid system to identify human epithelial cell proteins that bind gonococcal Opa proteins: intracellular gonococci bind pyruvate kinase via Opa proteins and require host pyruvate for growth. Mol Microbiol. 1998;27:171–186. doi: 10.1046/j.1365-2958.1998.00670.x. [DOI] [PubMed] [Google Scholar]