Abstract
The adrenal glands are paired endocrine organs that produce steroid hormones and catecholamines required for life. Adrenocortical carcinoma (ACC) is a rare and often fatal cancer of the peripheral domain of the gland, the adrenal cortex. Recent research in adrenal development, homeostasis, and disease have refined our understanding of the cellular and molecular programs controlling cortical growth and renewal, uncovering crucial clues into how physiologic programs are hijacked in early and late stages of malignant neoplasia. Alongside these studies, genome-wide approaches to examine adrenocortical tumors have transformed our understanding of ACC biology, and revealed that ACC is composed of distinct molecular subtypes associated with favorable, intermediate, and dismal clinical outcomes. The homogeneous transcriptional and epigenetic programs prevailing in each ACC subtype suggest likely susceptibility to any of a plethora of existing and novel targeted agents, with the caveat that therapeutic response may ultimately be limited by cancer cell plasticity. Despite enormous biomedical research advances in the last decade, the only potentially curative therapy for ACC to date is primary surgical resection, and up to 75% of patients will develop metastatic disease refractory to standard-of-care adjuvant mitotane and cytotoxic chemotherapy. A comprehensive, integrated, and current bench-to-bedside understanding of our field’s investigations into adrenocortical physiology and neoplasia is crucial to developing novel clinical tools and approaches to equip the one-in-a-million patient fighting this devastating disease.
Keywords: adrenocortical carcinoma, targeted therapies, molecular biomarkers, genomics, adrenocortical development and homeostasis
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
Adrenocortical carcinoma (ACC) is a rare and aggressive cancer, with no curative medical therapies to date
Recent advances in mouse modeling of adrenocortical development, homeostasis, and disease highlight a crucial role for maintenance of physiologic paracrine (Wnt/β-catenin) and endocrine (adrenocorticotropin/protein kinase A [ACTH/PKA]) signaling throughout neoplastic evolution
Recent integrated pangenomic molecular profiling studies reveal that ACC is composed of 3 molecular subtypes, COC1, COC2, and COC3, associated with good, intermediate, and uniformly dismal prognosis, respectively
COC1 ACC possesses the highest degree of immune infiltration, and no recurrent somatic alterations except loss of imprinting of the IGF2 locus (present in 90% of ACC)
COC2-COC3 ACC possess minimal immune infiltration, with recurrent somatic alterations leading to constitutive Wnt/β-catenin signaling, increased adrenal differentiation, and cortisol production in the setting of epigenetic reprogramming
COC3 ACC are distinguished by profound disruption of epigenetic programming (genome-wide CpG island hypermethylation, CIMP-high), recurrent somatic alterations leading to constitutive cell cycle activation, and genomic instability
These studies illuminate important therapeutic targets for ACC that include adrenal differentiation, steroidogenesis, immune checkpoint activation, cell cycle and genomic instability, Wnt/β-catenin signaling, epigenetics, metabolism, and cellular plasticity
A drenocortical carcinoma (ACC) is a rare cancer with an overall dismal prognosis. Despite that approximately half of patients present with metastatic disease at diagnosis (American Joint Committee on Cancer/European Network for the Study of Adrenal Tumors stage IV), surgery remains the only therapy with the potential to cure and is limited to patients with locoregional disease (American Joint Committee on Cancer/European Network for the Study of Adrenal Tumors stage I-III). Furthermore, up to 50% of R0-resected patients recur, indicating an urgent need for improved adjuvant management (1). Limited evidence suggests that adjuvant therapy with the DDT-derived adrenolytic agent mitotane may offer a survival benefit (2). Current clinical guidelines recommend adjuvant mitotane to nearly all patients with ACC (3); however, clinical responses on this regimen are highly heterogeneous. While a subgroup of patients exhibits rapid recurrence despite standard-of-care adjuvant mitotane (4, 5), it has been recognized that patients with low-risk disease (ie, localized, with low mitotic activity) do not benefit (6). Furthermore, expert opinion suggests that platinum-based adjuvant cytotoxic chemotherapy should be offered to all high-risk patients (3); this recommendation has been recently supported by a small retrospective study (7), but definitive and robust evidence is still lacking. In addition, the definition of high risk lacks objectivity, relying on clinical judgment with nuanced interpretation of standardized prognostic markers—a decision-making strategy likely feasible only in expert centers. A phase 3 clinical trial to assess the efficacy of platinum-based therapies for patients with high mitotic activity measured by the Ki67 proliferation index is ongoing (NCT03583710). However, heterogeneous outcomes are observed in existing proliferation-based low-risk and high-risk strata, limiting the value of this strategy to individualize adjuvant therapies (8).
Standard-of-care systemic agents for metastatic ACC include mitotane either as a single agent or in combination with cytotoxic chemotherapy (3). Though mitotane remains the only agent approved by the US Food and Drug Administration (FDA) specifically for ACC, its antitumor effects as a single agent are modest (9). This partially results from its poor pharmacokinetic properties. Because it is an extremely lipophilic compound with a highly variable metabolic clearance among individuals, only a subset of patients will ever achieve therapeutic levels (14-20 mg/L, established in retrospective studies) after many months of therapy. Furthermore, its narrow therapeutic window, wide array of toxic effects, and potent induction of CYP3A4 activity and hence catabolism of other drugs (including adrenal hormone replacement) are associated with frequent interruptions in therapy (10-12). Recent work has made advances in characterizing some molecular aspects of mitotane action (13, 14); however, predicting individual responses to mitotane remains elusive, and response is likely a complex function of interindividual differences in drug metabolism and intrinsic tumor-specific vulnerabilities (12, 15, 16). A rigorous understanding the molecular basis of mitotane vulnerability will ultimately be required for optimizing clinical strategies using this agent, and for the development of alternative agents targeting these vulnerabilities. Because of its favorable effect in mitigating morbid endocrine manifestations of ACC, mitotane remains a widely prescribed and useful drug for advanced cases with anecdotal reports of complete response (17, 18). Mitotane has also been reported to have favorable interactions with cytotoxic agents via inhibition of efflux pumps (19), and combinations of mitotane and different chemotherapies have been proposed (20). In fact, a randomized phase 3 trial supports the use of combination of etoposide, doxorubicin, and cisplatin plus mitotane (EDP + M) as first-line therapy for advanced disease, albeit with minimal survival benefit (3). Second-line and salvage therapies with other agents, such as gemcitabine, capecitabine, trofosfamide, and streptozotocin have been proposed by a few studies with limited benefit (reviewed in [21]). Since the original FDA approval of mitotane in the 1960s, few novel systemic therapies for ACC have been considered for implementation. This is largely secondary to the rarity of ACC restricting our ability to enroll patients in clinical trials, limited risk stratification with widely available tools, and historic lack of knowledge on molecular mechanisms of ACC pathogenesis, all of which would seed development of rational, subtype-directed therapeutic strategies.
Recent advances in genome-wide molecular approaches, such as next-generation sequencing, single-nucleotide polymorphism [SNP] arrays, and methylation arrays, have enabled an unbiased characterization of the somatic landscape of human cancers. Furthermore, large-scale and systematic studies using these approaches, such as The Cancer Genome Atlas project (TCGA), have provided the opportunity for a high-resolution multiplatform characterization of large and multi-institutional cohorts of ACC (22-25). These initiatives seeded explosive gains in our understanding of the molecular underpinnings of several different cancers, leading to the discovery of novel recurrent somatic alterations, abnormal pathway activation, and the characterization of previously unappreciated molecular subtypes of virtually all cancers in this study. Indeed, with regard to ACC, the data generated by pangenomic, multiplatform studies (22, 23, 25) provided enormous insights into the molecular pathogenesis of ACC. However, practical translation of this knowledge into clinically meaningful concepts and tools remains challenging. In this review, we summarize these most recent advances in our understanding of the molecular pathogenesis of ACC and discuss how they expose targetable therapeutic vulnerabilities.
Overview of Physiologic Signaling Pathways Stabilized in Adrenocortical Neoplasia
Developmental and homeostatic signaling pathways, including cell cycle, Wnt/β-catenin, and protein kinase A (PKA) pathways are almost universally dysregulated in ACC through a variety of mechanisms that we will detail here. We postulate that in ACC, as recently demonstrated in other cancers (26), such discordant pathway engagement creates a plastic cell state with the capacity to traverse the full spectrum of differentiation without terminal differentiation commitment (27). This allows ACC cells to sample transcriptional programs that confer sustained proliferation potential and intrinsic therapy resistance. To place those programs in context, we will first detail the paracrine and endocrine pathways controlling development, zonation, and renewal of the adrenal cortex.
Development of the Adrenal Cortex
The adrenal cortex derives from cells of the coelomic epithelium, a monolayer of squamous cells that lines the surfaces of viscera and the internal body wall. Around E9.5 in mice (fourth to sixth gestational weeks in humans), the coelomic epithelium condenses within the intermediate mesoderm between the urogenital ridge and the dorsal mesentery to form the adrenogonadal primordium (AGP). By E10.5 in mice (eighth gestational week in humans), the AGP has matured with discrete dorsomedial and ventrolateral segments, which give rise to the adrenal and gonadal primordia, respectively. At E13.5 in mice (gestational weeks 8-9 in humans), migrating cells derived from the neural crest invade the adrenal primordium and accumulate centrally to nucleate the adrenal medulla (28, 29). Concomitantly, mesenchymal cells envelope the adrenal primordium and coalesce as a multilayered fibrous structure, the adrenal capsule (30). This process is termed encapsulation. The capsule accompanies all subsequent steps of adrenal development and persists into adulthood. In addition to serving as a physical barrier that defines the organ limits and a scaffold that maintains tissue architecture, the capsule is also required for zonation and renewal of steroidogenic cells throughout life (31-36).
At encapsulation, different histological compartments can be distinguished in the cortex: a central area of large polyhedral eosinophilic cells, and a peripheral zone of small basophilic cells, enveloped by the fibrous capsule. These regions are termed the fetal zone and definitive zone, respectively. By the end of human gestation, the fetal zone comprises approximately 80% of the adrenal mass; however, it completely regresses by apoptosis in the first few weeks of life (37-39).
Adrenal formation is dependent on the master transcription factor steroidogenesis factor 1 (SF1, encoded by NR5A1). SF1 expression can be detected early in fetal life during AGP formation, and persists throughout adult life in all steroidogenic cells of adrenal and gonadal lineages (33, 40). Genetic models of SF1 deficiency (either targeting Nr5a1 itself or alternative upstream regulators Pbx1, Wt1, and Cited2) exhibit a spectrum of phenotypes characterized by adrenal hypoplasia, underlying the critical role for Nr5a1 in adrenal organogenesis (28, 30, 39, 41-45). Intact SF1 expression is a hallmark of steroidogenic lineages throughout all stages of life; however, epigenetic mechanisms are responsible for initiation and maintenance of gene expression at different stages of murine fetal development, suggesting the engagement of alternative and context-dependent distal regulators. In mice, Nr5a1 expression is initially maintained by the fetal adrenal-specific enhancer (FAdE), which becomes inactive when the definitive cortex forms. Interestingly, lineage tracing experiments have demonstrated that the definitive cortex originates from FAdE-active cells in the fetal cortex that transiently shut down Nr5a1 expression and get incorporated into the capsule as Gli1-expressing cells (39, 46). These capsular cells ultimately reactivate Nr5a1 expression in a FAdE-independent manner, and give rise to virtually all steroidogenic cells in the definitive adrenal cortex. In the postnatal period and into adulthood, Gli1-positive/SF1-negative cells from the capsule serve as an alternative progenitor cell pool that differentiates into steroidogenic cells in response to homeostatic demands, recapitulating the cascade that originates the definitive cortex during development (32, 36). In addition to its essential role in organogenesis, differentiation, and steroidogenesis, SF1 controls an array of cellular processes, including glucose metabolism, angiogenesis, cell motility, and cell proliferation, that are critical for cell survival (47, 48).
Paracrine Control of Cell Identity in Adrenocortical Development and Homeostasis
Fine transcriptional regulation of signaling programs is a defining feature of adrenocortical homeostasis (49, 50). In addition to SF1, several other transcription factor families and coactivators play a critical role in modulating these programs. Through transduction of paracrine and endocrine signaling cues, these transcriptional modules establish the steroidogenic and differentiation states of adrenocortical cells. For example, progenitor adrenocortical cells, in addition to expressing SF1, express sonic hedgehog (SHH) (33, 51). SHH is a paracrine signaling molecule that centrifugally signals to the capsule and initiates Gli1 expression in the capsular cells that ultimately serve en masse as a signaling center to initiate centripetal Wnt signaling to the underlying cortex, thereby establishing a closed-loop SHH-Wnt relay system critical for adrenocortical homeostasis. SHH/GLI signaling is rarely targeted for somatic alteration in adrenocortical neoplasia, and the nuances of this pathway are beyond the scope of this review. However, a classic paracrine signaling pathway that is frequently targeted for activation by driver somatic alterations in adrenocortical neoplasia is the Wnt signaling pathway (22, 23, 52) (Fig. 1).
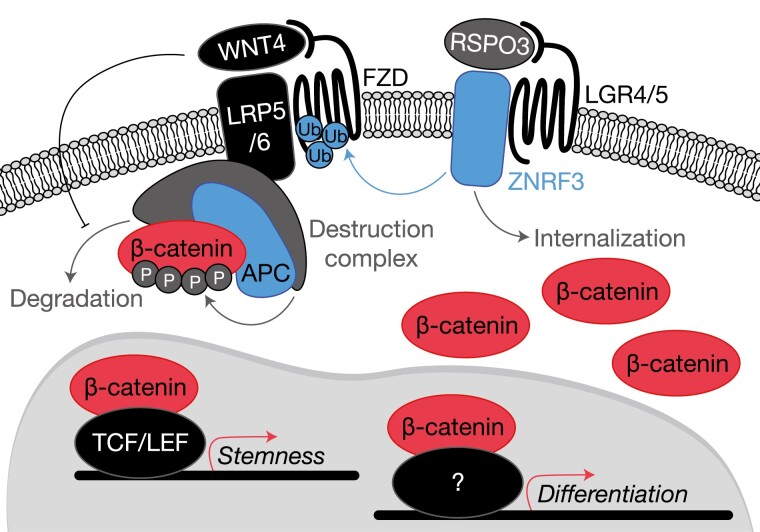
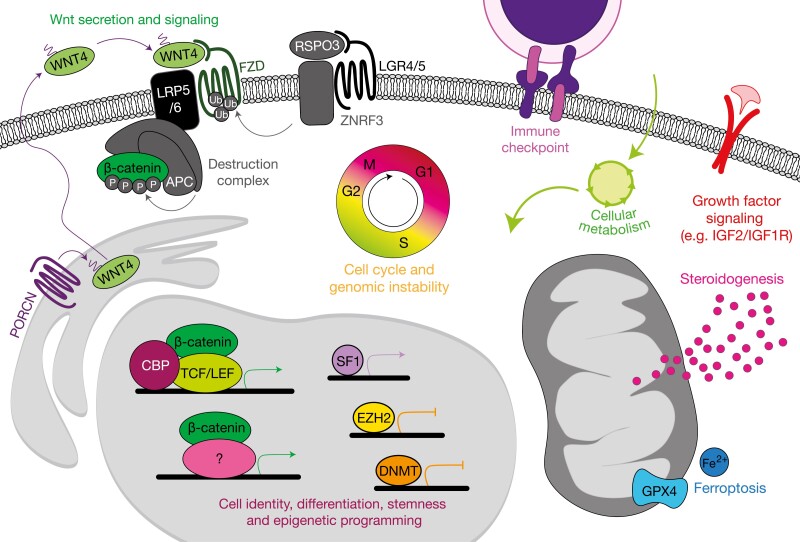
Figure 1.
Recurrent somatic alterations activate Wnt/β-catenin signaling in adrenocortical neoplasia. Benign and malignant tumors of the adrenal cortex harbor frequent somatic alterations leading to constitutive activation of the Wnt/β-catenin signaling pathway, classically culminating in high expression of a β-catenin and TCF/LEF-driven stemness program facilitating tumor growth. Activation of this pathway is regulated at several levels, primarily through availability of Wnt receptors (Frizzled receptors, FZD) and stability of β-catenin. Membrane availability of FZDs is regulated by R-spondins (in the adrenal cortex, RSPO3) and E3 ubiquitin ligases such as ZNRF3. In the absence of RSPO3, ZNRF3 ubiquitinates FZDs, targeting these receptors for internalization and degradation. When RSPO3 binds its receptors (in the adrenal cortex, these are LGR4/5), the ZNRF3/LGR/RSPO complex is internalized, permitting the activation of FZDs by Wnt ligands (in the adrenal cortex, this is WNT4). β-catenin stability is regulated by the destruction complex, a large multiprotein complex containing classical tumor suppressor APC. The destruction complex phosphorylates cytoplasmic β-catenin and targets it for degradation. When Wnt ligands such as WNT4 bind FZD receptors, the destruction complex is localized to the cell membrane and can no longer efficiently target β-catenin for degradation. Intracellular β-catenin therefore accumulates and translocates to the nucleus to drive its transcriptional programs. Importantly, in ACC, activating mutations in this pathway are associated with a higher degree of adrenal differentiation, suggesting that β-catenin may engage a herein unknown transcription factor to drive expression of a genome-wide differentiation program. Signaling components encoded by genes targeted for somatic gain of function (GOF) alterations are depicted in red (β-catenin, recurrent mutations prevent phosphorylation), and somatic loss of function (LOF) alterations are depicted in blue (APC, and ZNRF3).
Wnt signaling is crucial for embryonic development and morphogenesis, as well as for maintenance of stem/progenitor cell pools in virtually all mammalian organs. Wnt signaling through transcriptional coactivator β-catenin (the canonical Wnt pathway) is essential for adrenal formation, zonation, and renewal. Signaling is initiated by the binding of a Wnt ligand (in the adrenal cortex, this is presumed to be WNT4) to a Frizzled (Fzd) receptor. In the canonical pathway, activation of Fzd neutralizes a major negative regulator of cytoplasmic β-catenin stability (the destruction complex), enabling rapid cytoplasmic accumulation and nuclear translocation of β-catenin, where β-catenin will classically coactivate a TCF/LEF-dependent transcriptional program. Notably, cytoplasmic β-catenin stability is regulated by phosphorylation; the destruction complex resides in the cytoplasm and phosphorylates key residues on β-catenin exon 3 to target it for proteasome-mediated degradation. Active Fzd neutralizes the destruction complex by sequestering it to the cell membrane (53).
While the importance of Wnt/β-catenin signaling for adrenal development and homeostasis have been demonstrated by several groups, its precise molecular mechanisms remain incompletely understood. Studies using adrenocortical-specific Cre recombinases to activate or delete β-catenin in the mouse adrenal have demonstrated that both gain and loss of function (LOF) are associated with a spectrum of phenotypes. While LOF β-catenin models invariably result in adrenal agenesis or hypoplasia, gain-of-function models may cause both adrenal agenesis/hypoplasia and hyperplasia with zona glomerulosa (zG) differentiation and increased aldosterone production, dependent on the timing of the genetic hit and identity of adrenocortical cells affected (54-58). Similar phenotypes are observed in patients with SERKAL syndrome, an autosomal recessive disorder caused by LOF mutations in WNT4. Among several multisystemic malformations, patients with SERKAL syndrome also exhibit adrenal agenesis/dysgenesis (59). Interestingly, mice engineered to bear adrenocortical-specific Wnt4 deletion have a relatively mild phenotype characterized by zG hypoplasia and aldosterone deficiency, suggesting that other Wnt ligands may explain interspecies differences (60). Collectively, these observations suggest that Wnt/β-catenin signaling is important not only for adrenal organogenesis and homeostasis, but also for promoting functions of the differentiated adrenal cortex such as aldosterone production. These paradoxical actions of Wnt/β-catenin, supporting both stemness and differentiation, may also be mediated by interactions with distinct transcriptional regulators. In fact, it has been demonstrated that β-catenin binds SF1, suggesting that this complex might control tissue-specific functions such as aldosterone production (61-64).
Wnt/β-catenin activity in the adrenal cortex (measured by intensity of nuclear staining for β-catenin) is zonally distributed, forming a centripetal gradient where it peaks in the zG, and progressively fades into the inner zones (65). The molecular basis of this compartmentalized expression has been recently elucidated and involves both cell autonomous and nonautonomous mechanisms. The onset of Wnt/β-catenin signaling in the adrenal cortex overlaps with encapsulation and formation of the definitive cortex (28, 29, 55, 66). Indeed, as previously discussed, the entire definitive cortex is derived from capsular precursors, and capsular cells can serve as an alternative progenitor pool throughout life (32, 36, 39). Furthermore, the temporal overlap between encapsulation and the onset of Wnt signaling suggests that these 2 processes are interconnected. In fact, it has been recently demonstrated that the adrenal capsule is a source of R-spondins (RSPO), a family of secreted proteins that potentiate canonical Wnt/β-catenin signaling by interacting with members of the leucine-rich repeat containing G-protein coupled receptors (LGR) family. In the presence of RSPO, LGRs form a complex with membrane-bound E3 ubiquitin ligases ZNRF3 and RNF43 (negative Wnt pathway regulators), causing their internalization and degradation. In the absence of RSPO, ZNRF3 and RNF43 inhibit Wnt signaling by promoting internalization and proteasomal degradation of Fzd receptors (53). Vidal et al (31) have demonstrated that capsular cells expressing Gli1 produce RSPO3 in the mouse. Gli1-driven loss of RSPO3 leads to profound disruption of adrenal zonation, with adrenal hypoplasia and an absent zG, obliteration of the SHH + progenitor pool, and downregulation of Wnt/β-catenin target genes. Consistent with the role of RSPO3 in augmenting Wnt signaling, a reduction in Wnt4 expression was also observed, indicating that Wnt4 is likely a Wnt/β-catenin transcriptional target in the adrenal cortex and maintains Wnt/β-catenin signaling in an autocrine manner (65).
These observations led to important conceptual advances in the previously proposed model of the corticocapsular homeostatic unit (Fig. 2). According to this model, signaling between SHH-producing cells in the peripheral cortex (SHH+/SF1+) and SHH-responsive cells in the capsule (GLI1+/SF1–) is critical for establishing and maintaining the stem/progenitor cell niche, and the Wnt/β-catenin signaling gradient in the cortex. This is achieved by a bidirectional, interdependent paracrine loop (SHH-Wnt relay), in which cortex-derived SHH induces the expression of GLI targets in the capsule, including RSPO3, which signals back to the cortex, promoting activation of Wnt/β-catenin signaling. Consistent with this model, mice bearing deletion of either Shh or Gli1 also exhibit reduced Wnt/β-catenin signaling, adrenal hypoplasia, and disrupted zonation with reduced expression of zG markers (31, 67, 68).
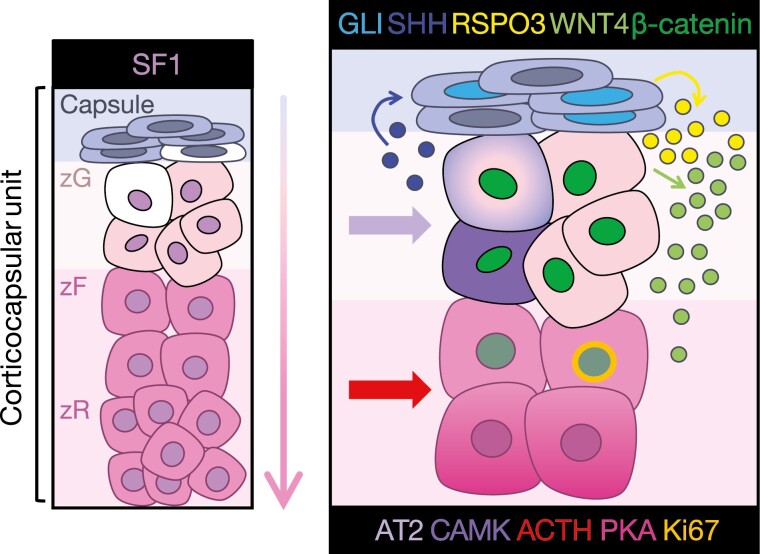
Figure 2.
Paracrine and endocrine signaling programs support homeostasis and renewal in the adrenal corticocapsular unit. Shown left, stem and progenitor cells (white) residing in the capsule or subcapsular cortex (histological zG) may be deployed for cortical renewal in response to physiologic homeostatic and endocrine demands. Differentiation is centripetal (as indicated by the arrow), and lower zR cells (humans) or lower zF cells (mice, which do not possess a zR) are terminally differentiated and undergo apoptosis at the boundary between the cortex and the medulla. Detailed in panel right, this process is regulated by interplay between capsule- and cortex-derived paracrine factors and systemic endocrine regulators, which together coordinate stem/progenitor cell maintenance, anatomic and functional zonation, lineage conversion, and steroidogenesis. Sonic hedgehog (SHH, dark blue) produced by zG cells centrifugally activates GLI family transcription factors in the capsule (cyan), which drive the expression of RSPO3 (yellow), an essential positive regulator of Wnt signaling in the cortex. Wnt-responsive cells in the cortex (possessing nuclear β-catenin, dark green) produce WNT4 (light green), further perpetuating Wnt signaling throughout the lower zG and upper zF and maintaining SHH expression throughout the zG. Endocrine signaling, through angiotensin II/calmodulin kinase (AT2/CAMK) and adrenocorticotropic hormone/protein kinase A (ACTH/PKA), establish discrete differentiation states required for zone-specific steroidogenesis. Importantly, cells at the zG-zF boundary possess mitotic activity (Ki67, gold) and represent a “transit-amplifying” population that can rapidly expand in response to ACTH. Despite responding to a zF endocrine cue, transit amplification of this population also requires intact Wnt/β-catenin signaling. This current model of adrenocortical homeostasis is supported by numerous studies, as detailed in this review.
Endocrine Control of Functional Zonation, Transdifferentiation, and Interplay With Paracrine Signaling
Endocrine factors are also major determinants of cortical function and zonation. The most important endocrine factors that regulate steroid production in the adrenal cortex are angiotensin II (ATII) and adrenocorticotropin (ACTH). These hormones are the effectors of 2 independent endocrine systems predicated on both feed-forward amplification/activation and feedback inhibition, the renin-angiotensin-aldosterone system and the hypothalamus-pituitary-adrenal axis. The renin-angiotensin-aldosterone system and hypothalamus-pituitary-adrenal axis independently regulate aldosterone and cortisol production, respectively, according to physiologic demands (Fig. 3). Though all the cells in the cortex are derived from the same progenitor pool, different cell populations are deployed to respond to ATII or ACTH with hormone production and secretion. ZG cells respond to ATII and zona fasciculata (zF) cells respond to ACTH (69-73). This is achieved through distinct differentiation states established in zG and zF cells in response to the Wnt/β-catenin signaling gradient. Indeed, murine studies have demonstrated that Wnt/β-catenin signaling promotes zG fate and restrains zF differentiation, at least partially through induction of phosphodiesterases, enzymes that convert cyclic adenosine 5′-monophosphate (cAMP) to adenosine 5′-diphosphate terminating cAMP-induced PKA activation (56, 58). As the cells migrate centripetally, progressively lower Wnt/β-catenin signaling enables maximal zF cellular response to ACTH with initiation of increased expression of the ACTH receptor (melanocortin receptor type 2 [MC2R]) and the essential melanocortin receptor 2 accessory protein (MRAP) (74). Interestingly, despite this antagonistic role, β-catenin is required for ACTH-dependent zF renewal (32).
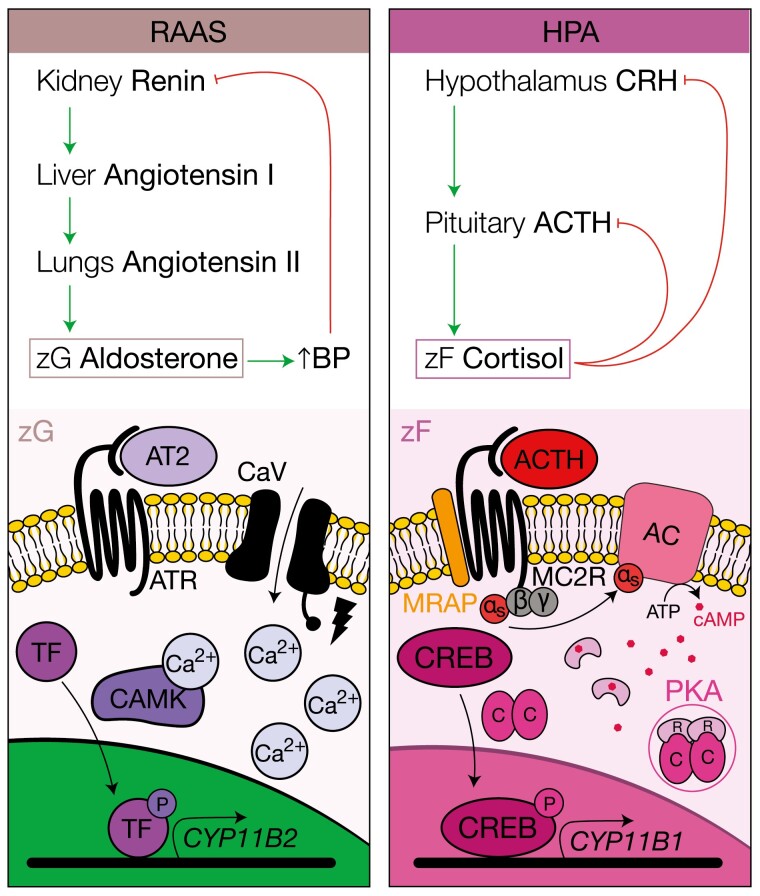
Figure 3.
Endocrine feedback loops and cellular mechanisms supporting aldosterone and cortisol production. The renin-angiotensin-aldosterone system (RAAS) and the hypothalamus-pituitary-adrenal (HPA) axis are the endocrine feedback loops that regulate aldosterone and cortisol production, respectively. These feedback loops are activated by distinct physiologic demands and target diverse cell populations in different zones of the cortex according to their differentiation state (and therefore the expression of hormone receptors). Shown left, the angiotensin II receptor (ATR) is expressed by zG cells. On angiotensin II (ATII or AT2) binding to ATR, membrane depolarization occurs, leading to opening of voltage-gated calcium channel CaV, triggering a calcium-dependent intracellular signaling cascade that activates calmodulin kinase (CAMK), and subsequently transcription factors that drive expression of critical regulators of aldosterone production such as aldosterone synthase, encoded by CYP11B2. Shown right, the adrenocorticotropin (ACTH) receptor MC2R and its accessory protein, MRAP, are expressed by zF cells. On binding of ACTH to MC2R, the Gαs subunit dissociates and activates adenylyl cyclase (AC), which triggers cellular accumulation of cyclic adenosine 5′-monophosphate (cAMP), and dissociation of the protein kinase A (PKA) tetramer with liberation of the catalytic subunits. These subunits phosphorylate and activate cAMP response element binding protein (CREB), enabling transcription of machinery required for glucocorticoid synthesis, for example CYP11B1. Not shown, this signaling program is extinguished by intracellular phosphodiesterases.
MC2R belongs to the G protein–coupled receptor family. On ACTH binding, it signals through the Gαs subunit to activate adenylate cyclase, which converts adenosine 5′-triphosphate (ATP) to cAMP to activate PKA. In its inactive form, PKA is part of a tetramer formed between two catalytic and 2 regulatory subunits. In the presence of cAMP, the tetramer dissociates, releasing the PKA catalytic subunits, which promote phosphorylation of cAMP response element-binding protein (CREB) transcription factors. pCREB activates the transcription of target genes, including immediate early response genes encoding for AP-1 components, NR5A1, and several steroidogenic enzymes including HSD3B2, CYP17A1, and CYP11B1 (75-77). In addition, PKA activation strongly represses targets of Wnt/β-catenin signaling, facilitating the zG to zF transition in cooperation with epigenetic regulators (56, 78). The contribution of PKA activation to zG-zF transdifferentiation is well demonstrated in Mc2r and Mrap knockout mouse models (79, 80). Similarly to patients with familial glucocorticoid deficiency, these mice exhibit severe corticosterone deficiency with normal aldosterone secretion and die shortly after birth (rescued by in utero corticosterone administration). Histologically, adrenals from these mice possess profoundly disrupted zonation characterized by overall cortical atrophy, thickened capsule, expanded zG, and absent zF. These abnormalities are accompanied by an increased number of cells positive for Shh, β-catenin, and Wnt4, with a profound decrease in the expression of Nr5a1 and genes coding for steroidogenic enzymes other than Cyp11b2. Accumulation of Shh-expressing cells with expansion of Wnt/β-catenin gradient is also observed after dexamethasone-induced cortical atrophy, which is rapidly restored on dexamethasone withdrawal (32). Collectively, these observations demonstrate the importance of PKA signaling in providing differentiation cues that are essential for zG-zF transition, which involves both downregulation of Wnt/β-catenin signaling at the zG-zF border and increased activation of the PKA-driven zF program.
The ability of the physiologic adrenal cortex to produce mineralocorticoids and glucocorticoids independently, according to specific demands, relies on anatomic and functional compartmentalization. The structural basis of this compartmentalization is the corticocapsular unit, established in embryogenesis shortly after encapsulation (see Fig. 2). The paracrine crosstalk between peripheral cortical progenitors (SHH+/SF1+ cells), and GLI+ capsular cells establishes a zone of high Wnt/β-catenin by releasing capsular RSPO3 into the upper cortex (31). High Wnt/β-catenin activity is essential to maintain the progenitor cell pool, to promote zG differentiation, and to avoid premature zF differentiation by antagonizing ACTH/PKA (56). In addition, several lines of evidence suggest high Wnt/β-catenin activity is required for ATII-mediated aldosterone production (31, 60). As Wnt/β-catenin signaling fades centripetally, and cortical cells acquire the ability to respond to ACTH, PKA activity promotes a dramatic transition in the cell state and establishes the zG-zF boundary. In addition to promoting zF differentiation, ACTH also induces cell proliferation in the upper zF (32, 81). Interestingly, the mitogenic response to ACTH requires Wnt/β-catenin signaling, as genetic ablation of Ctnnb1 in Wnt-responsive cells during cortical regeneration after dexamethasone-induced zF atrophy in mice significantly blunts cortical regrowth and zF differentiation (32). In the lower zF, cortical cells ultimately reach terminal differentiation and undergo apoptosis at the inner cortex (82). Cortical cells, therefore, follow a unidirectional trajectory of differentiation that is shaped both by signaling gradients, most important, Wnt/β-catenin signaling, and endocrine signaling (ATII and ACTH). Loss of these key paracrine and endocrine mediators lead to abnormal zonation, differentiation, and organ hypofunction. Interestingly, constitutive activation of paracrine and endocrine signaling also disrupts the differentiation trajectory by locking cells in specific differentiation states that accumulate and may be prone to malignant transformation (54, 58, 83-86). In fact, benign and malignant adrenocortical neoplasms are characterized by recurrent somatic events targeting these pathways. However, while recurrent driver events provide an opportunity for targeted therapies, considerable challenges for implementing molecular targeted agents in ACC remain. These will be discussed in the following sections.
Unique Clinical Features of Adrenocortical Carcinoma Allude to Mechanisms of Disease
Our current understanding of adrenocortical carcinogenesis is largely informed by the aforementioned murine studies as well as familial genetics and genome-wide investigations that we will discuss in the subsequent sections. However, some interesting and unexpected clinical features of ACC have provided important hints about disease pathogenesis and heterogeneity, especially when we consider adrenocortical tumors (ACT) as existing along a spectrum of neoplasia, in which both cell identity programs and paracrine/endocrine signaling are uncoupled.
ACT are common human neoplasms affecting approximately 5% to 10% of the population older than 60 years (87). ACT are usually found incidentally during radiological exams for unrelated complaints. Most ACT are benign adrenocortical adenomas (ACA), managed conservatively, with periodic clinical and radiologic follow-up. ACT associated with malignant radiologic features or hormone excess syndromes are managed by surgery (88). In contrast, ACC is a rare tumor, with a global incidence of 1/million to 1.5/million per year. However, while the prevalence of ACC among adrenal incidentalomas is low, approximately 10% of all ACC are diagnosed as incidentalomas (89). While ACA increase in frequency with each decade of adulthood, the incidence of ACC has a bimodal distribution peaking around age 5 years and between the fourth and fifth decades of life (1, 90). These observations suggest that molecular events supporting benign tumorigenesis in the adrenal cortex are frequent (50). While there have been anecdotal reports of collision tumors containing adenomatous and carcinomatous compartments and carcinomas arising from longstanding incidentalomas (91, 92), these epidemiological data also suggest that a benign to malignant progression in the adrenal cortex is an exquisitely rare event, perhaps unlikely to account for the vast majority of ACC (91, 93, 94). Importantly, observations of malignant adrenocortical phenomena are currently limited by the histological criteria used to diagnose ACC, which necessarily rely on several features that are achieved with sufficiently large tumor size (95, 96). The prevalence of carcinoma in situ that may progress to ACC is unknown.
The male-to-female ratio across all ACC is 1:1.5. Clinical manifestations of ACC are associated with mass effects of the primary tumor or metastasis, and steroid excess syndromes including primary aldosteronism, Cushing syndrome, and virilization/feminization. In contrast to hormonally active ACA, which will routinely secrete one class of hormones (eg, aldosterone or cortisol), ACC often produce mixed syndromes (most commonly secondary to androgen and cortisol cosecretion). While approximately 40% of ACC do not present with hormonal excess syndromes, accumulation of steroid precursors (eg, 11-deoxycortisol) can be detected in up to 90% of cases (1, 90, 97). This is also in marked contrast to silent or hormonally active ACA, which produce significantly lower levels of steroid precursors. These unique features of ACC illustrate a profound disruption of adrenocortical differentiation specifically in malignancy, characterized by discordance between steroidogenesis and endocrine- or paracrine-mediated cell identity programs. Indeed, ACC with activating mutations in the Wnt/β-catenin program (driving zG fate in physiology) are associated with cortisol rather than aldosterone production and exhibit features of zF differentiation (23).
Disease stage is the single most important clinical prognostic factor, as metastatic disease is refractory to standard therapies. For patients with localized disease, histological grade (assessed by either mitotic counts or the Ki67 score) is a commonly used tool for risk assessment and prognosis prediction. High-grade ACC, characterized by either more than 20 mitoses/50 high-power field or a Ki67 greater than 10% to 20%, bears a significantly higher risk of recurrence after an R0 surgical resection (8). In addition, the presence of hypercortisolism is also associated with an increased risk of recurrence, and a faster progression rate among advanced-disease patients (98, 99). These observations reveal the heterogeneity that exists across ACC, and suggest that certain differentiation and proliferation cancer cell states support aggressive carcinogenesis.
Although most ACC is sporadic, inherited forms associated with multiple neoplasia syndromes such as Li-Fraumeni syndrome, Beckwith-Wiedemann syndrome, Lynch syndrome, Birt-Hogg-Dube syndrome, adenomatous polyposis coli, and multiple endocrine neoplasia type I comprise 5% to 10% of the cases (100-103). The prevalence of germline TP53 mutations is particularly high among pediatric cases, ranging from 50% to 96%. In Southern and Southeastern Brazil, where the overall incidence of ACC is reported to be 10% to 15% higher than elsewhere and up to 4- to 6-fold higher in specific populations (104), more than 90% of pediatric cases and up to 20% of adult cases are associated with the p.R337H TP53 germline variant (103). Early molecular studies on sporadic ACC, based on candidate gene approaches, were informed by the aforementioned rare inherited syndromes featuring ACC. This approach led to identification of prevalent somatic events in sporadic ACC, including loss of imprinting leading to overexpression of IGF2 (> 90%), activating mutations of CTNNB1 (~15%), and inactivating mutations of tumor suppressors TP53 (~20%), APC, and Lynch syndrome–associated genes MSH2 and MSH6 (1, 103). Recent studies performed on larger multi-institutional cohorts using unbiased methods such as next-generation sequencing–based approaches and SNP arrays led to the identification of additional recurrently altered genes, including the Wnt/β-catenin regulator ZNRF3 (~20%); cell cycle regulators RB1, CDKN2A, CDK4, and CCNE1; telomere maintenance genes TERT, TERF2, ATRX, and DAXX, epigenetic regulators including MEN1 and genes encoding MLL and ATP-dependent SWI/SNF chromatin remodelers; and PKA regulator PRKAR1A (22-24). Together, disruption of homeostatic paracrine (Wnt/β-catenin), and endocrine (PKA) signaling by somatic events is observed in approximately 30% of ACC, suggesting that dysregulation of these pathways during ACC tumorigenesis is critical for sustained proliferation, recapitulating their importance in adrenal development and homeostasis.
Multiplatform Profiling of Adrenocortical Carcinoma Identifies Distinct Molecular Subtypes
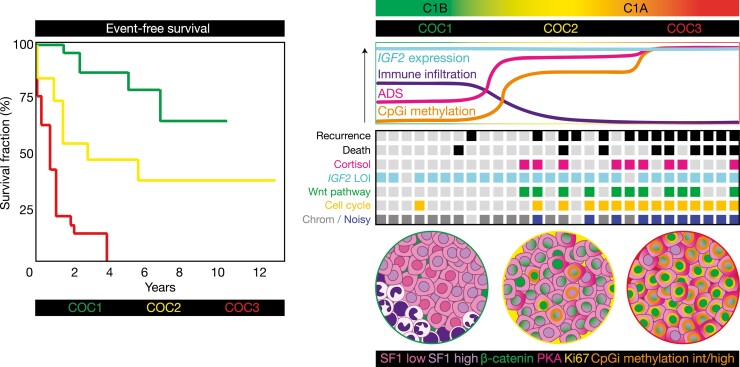
High-throughput characterization of ACC using multiomics approaches, including exome sequencing, SNV arrays, Illumina methylation arrays, RNA sequencing, and microRNA sequencing, demonstrated that ACC is composed of distinct molecular subtypes (22, 23). According to ACC-TCGA, the largest and most comprehensive of these studies, 3 molecular subtypes can be distinguished by multiomics clustering, so-called COC1, COC2, and COC3 (Fig. 4) (23). Importantly, these 3 subgroups largely explain the clinical and hormonal heterogeneity that characterizes ACC. Briefly, COC1 ACC are the least aggressive (in terms of disease stage, event-free survival, and high-grade disease), and composed mostly of non–cortisol-producing tumors (including silent tumors and androgen-secreting). COC2 ACC present with intermediate levels of aggressiveness, and a higher prevalence of cortisol-producing tumors. COC3 ACC is the most aggressive and exhibits the highest proportion of cortisol-producing tumors and high-grade disease. Broadly, COC1 and COC2-COC3 overlap with the C1B and C1A transcriptome subgroups, respectively, as first described and correlated with survival outcomes by Assie, de Reynies, Bertherat and colleagues in a series of landmark publications (22, 105). As expected (22), recurrent somatic events are also unevenly distributed among ACC-TCGA molecular subclasses. While few recurrent somatic SNVs and focal gains and losses are present in COC1, most events targeting Wnt/β-catenin and cell cycle genes are concentrated in COC2 and COC3 (the latter is particularly enriched for variants in cell cycle genes). Furthermore, while COC1-COC2 ACC have a somatic copy number alteration (SCNA) profile characterized by whole-chromosome gains and losses (an SCNA signature called “chromosomal”), COC3 ACC possess a high degree of genomic instability with numerous focal gains and losses (an SCNA signature called “noisy”).
Figure 4.
Adrenocortical carcinoma (ACC) is composed of 3 homogeneous molecular subtypes associated with distinct clinical outcomes. Multiplatform profiling in ACC-TCGA (23) revealed that ACC is composed of 3 molecular subtypes: COC1, COC2, and COC3. COC1 ACC is associated with favorable clinical outcomes (few recurrences and deaths in this group, longest event-free and overall survival), COC2 is associated with intermediate outcomes, and COC3 is associated with dismal clinical outcomes (accounting for up to 40% of all ACC but nearly 70% of recurrences and more than half of deaths) (4, 23). COC2-COC3 ACC are associated with clinically significant cortisol production. On a molecular level, virtually all ACC is characterized by loss of imprinting (LOI) leading to constitutive expression of IGF2; however, COC1-COC3 possess distinct somatic alteration profiles and differential immune infiltration, expression of adrenal differentiation score (ADS) and methylation of CpG islands (CpGi). COC1 ACC possess a higher degree of immune infiltration, lower ADS, minimal CpGi methylation, no recurrent driver somatic alterations, and a chromosomal somatic copy number alteration (SCNA) profile. COC2 ACC also possess a chromosomal SCNA profile. COC2-COC3 ACC are characterized by frequent driver somatic alterations leading to constitutive activation of the Wnt pathway. COC2-COC3 ACC also possess higher ADS (with COC3 ACC at the higher end of this spectrum), suggesting that Wnt pathway activation in these tumors facilitates steroidogenesis. COC3 ACC possess the highest degree of cell cycle activation, enriched for driver alterations promoting constitutive cell cycle activation, and possess a noisy SCNA profile. COC2 ACC possess intermediate levels of CpGi methylation (CIMP-int) while COC3 ACC possess high levels of CpGi methylation (CIMP-high), suggesting that these classes of ACC are characterized by profound disruption in epigenetic patterning. Example event-free survival curves adapted from ACC-TCGA are depicted left. Molecular features are depicted right in the curves (top panel), theoretical heat map (middle panel, columns represent patients; light gray squares indicate “null values,” eg, no recurrence, death, mutation, cortisol production or a quiet SCNA profile, while colored squares indicate the presence of the abnormality), and pseudomicroscope images depict tumor genetic, epigenetic, and cell type heterogeneity (bottom panel).
Interesting and paradoxical observations emerged from these analyses. Unlike other cancers, in which dedifferentiation is associated with aggressive disease, the most aggressive subtype of ACC, COC3, is also the most differentiated from the perspective of the adrenal differentiation score (ADS), a score composed of a combination of several genes exclusively/differentially expressed by the normal adrenal cortex (23). As previously alluded to, cortisol-producing tumors are enriched for somatic events targeting genes encoding members of the Wnt/β-catenin pathway, ZNRF3, CTNNB1, and APC. While in the physiological adrenal cortex, the highest levels of Wnt/β-catenin activation are associated with zG differentiation and aldosterone production, in ACC the highest Wnt/β-catenin activity is associated with zF differentiation and cortisol production. Although these observations seem counterintuitive, they inform us about the potential cell of origin of these particular molecular subtypes of ACC (COC2-COC3). As suggested by observations in human and mouse studies, mitotic activity in the adrenal cortex is concentrated in the upper zF (81, 82). Furthermore, ACTH-dependent adrenal regeneration after dexamethasone-induced atrophy relies on increased cell proliferation in the zG-zF border in a Wnt/β-catenin dependent-manner before zF replenishment and differentiation (32). These observations are consistent with a model in which the cell of origin of COC2-COC3 ACC (cortisol-producing; Wnt/β-catenin-active) are transit-amplifying cells from the zG-zF border. These cells physiologically exhibit intermediate levels of Wnt/β-catenin activity (65) but are already committed to zF differentiation and respond to ACTH preferentially with proliferation (Fig. 5).
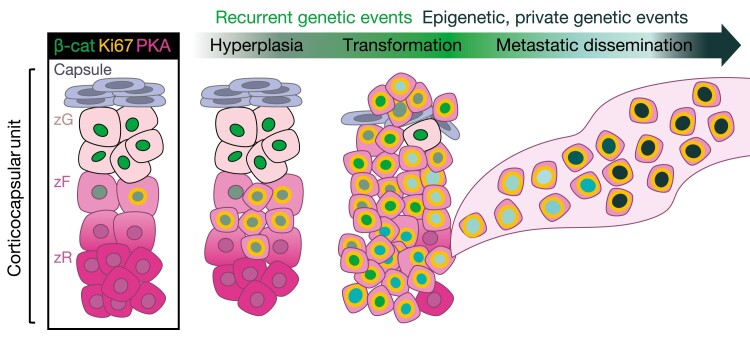
Figure 5.
Putative hyperplasia to carcinoma sequence originating from zG-zF boundary cells in COC2-COC3 ACC. COC2-COC3 adrenocortical carcinoma (ACC) are characterized by active Wnt/β-catenin signaling, high levels of adrenal differentiation (measured by ADS), and profound epigenetic rewiring (possessing intermediate and high levels of CpG island methylation genome-wide; ie, CIMP-int and CIMP-high signatures) (23). Intriguingly, despite the well-established role of β-catenin in supporting zG differentiation in physiology, COC2-COC3 tumors also frequently produce glucocorticoids (cortisol) (23). Recent mouse models of adrenocorticotropin (ACTH)-driven zF renewal (32), expanded Wnt/β-catenin signaling driven by ZNRF3 deficiency (65), sustained proliferation triggered by adrenocortical expression of the SV40 Large T antigen (106), or combined simultaneous Wnt/β-catenin and cell cycle activation (107) also demonstrate a unique interplay between Wnt/β-catenin and ACTH/PKA signaling in enabling proliferation of cells residing in the zG-zF boundary. Taken together, these studies support the existence of a small population of cells in the zG-zF boundary that are capable of rapid proliferation in response to sustained Wnt/β-catenin and/or ACTH signaling. We postulate that COC2-COC3 ACC arise from this vulnerable population through recurrent genetic events that drive hyperplasia and malignant transformation (eg, activating alterations in the Wnt pathway and/or driver alterations leading to constitutive cell cycle activation). Given the relatively homogeneous abnormal epigenetic patterns in these 2 groups of tumors, and recent studies suggesting that metastatic ACC do not acquire novel recurrent genetic events (245, 246), we speculate that tumor growth during and after transformation as well as metastatic dissemination are facilitated by epigenetic reprogramming and/or private genetic events.
Two additional genetic mouse models that spontaneously develop ACC further support this framework. The first is a model developed Batisse-Lignier et al (106), featuring adrenocortical expression of the SV40 Large T antigen (AdTag), which simultaneously inactivates pRb and p53 to induce sustained cell cycle activation. The second is a model developed by Borges and colleagues (107) possessing genetic activation of β-catenin and inactivation of p53 in all cells of the definitive adrenal cortex that have ever expressed Cyp11b2, termed BPCre. Intriguingly, both models exhibit similar tumor phenotypes with invariable progression to metastatic, glucocorticoid-producing ACC with a preceding dysplasia to carcinoma progression that starts at the zG-zF boundary. Despite genetically encoded differences in the 2 models regarding initial levels of Wnt/β-catenin activation (BPcre is characterized by intrinsic constitutive Wnt/β-catenin activation, whereas AdTag is not), both models converge to the same phenotype, with selection for cells possessing autonomous nuclear β-catenin. We hypothesize that in COC2-COC3 ACC, epigenetic or genetic hits that impair differentiation and lock cells in a Wnt/β-catenin-active/transit amplifying state are selected for and vulnerable to secondary genetic and epigenetic events that promote rapid cell growth (see Fig. 5).
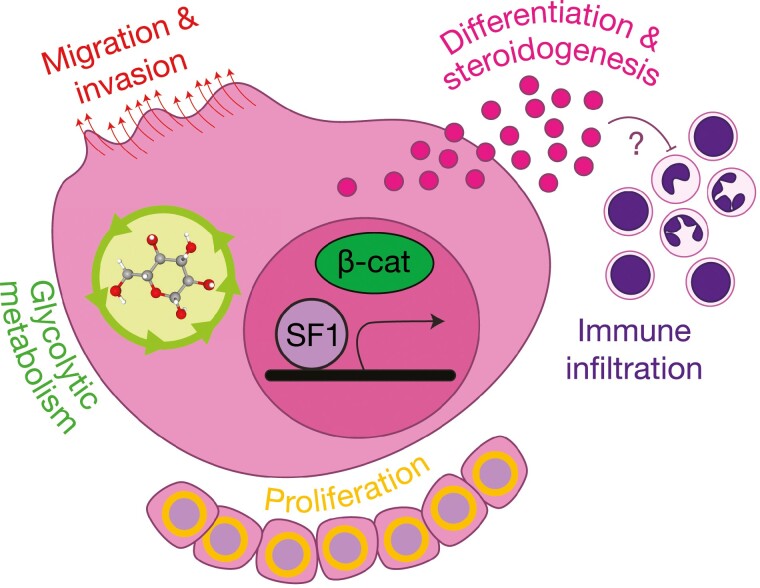
The maintenance and selection pressure for a differentiated state (suggested also by recurrent somatic alterations in PRKAR1A in ACC-TCGA) support an oncogenic role for SF1-mediated transcription in ACC tumorigenesis (Fig. 6). As previously mentioned, SF1 is known to have metabolic and proliferative effects that might be advantageous to tumor cells. In fact, a role for increased SF1 expression and copy number has been demonstrated in pediatric ACC (108, 109). In addition, enforced high expression of NR5A1 in vitro is associated with increased proliferation and invasiveness (110), as well as increased migratory capacity through regulation of cellular cytoskeleton (111). Another interesting observation that supports a tumorigenic role for SF1-mediated zF differentiation is that ACC is among the tumor types with the least immune infiltration in TCGA. Transcriptome analysis of ACC-TCGA data, and from other published microarray studies, indicates an inverse correlation between ADS (and therefore the ability to synthesize cortisol) and immune cell–associated genes, suggesting that intratumoral cortisol synthesis is a mechanism of immune exclusion in ACC (23). In fact, a negative correlation between immune infiltration and survival was later demonstrated in an independent cohort (112).
Figure 6.
Pleiotropic, oncogenic actions of steroidogenesis factor 1 (SF1) in ACC. High expression of NR5A1 (or its gene product, SF1) is retained in adrenocortical neoplasia, through recurrent genetic and/or alternative noncoding mechanisms (23, 108, 109). Studies using in vitro models bearing endogenous or enforced high SF1 expression have demonstrated that SF1 plays a critical role in cytoskeletal remodeling and cell migration/invasion, glycolytic metabolism, and proliferation (47, 110, 111). Importantly, adult adrenocortical carcinoma (ACC) with constitutive Wnt/β-catenin pathway activation possess higher expression of NR5A1 and the SF1-driven differentiation program (23). Given SF1 and β-catenin are known to cooperate to drive expression of specific gene loci (61-64), it is possible (though not yet proven) that these factors also cooperate to drive adrenal differentiation, offering a possible mechanism for glucocorticoid production in Wnt-pathway–mutated adrenocortical tumors. The inverse relationship between immune infiltration and adrenal differentiation in ACC (23) suggests that this program (alone or synergistically with the Wnt/β-catenin-driven programs) may promote immune cell exclusion. These consequences of SF1’s pleiotropic actions, facilitating tumor development through cell-autonomous and non–cell-autonomous mechanisms, likely underlie the events that facilitate selection for the SF1-driven transcriptional program throughout adrenocortical carcinogenesis.
While ACC-TCGA molecular subtypes provide powerful information on mechanisms of tumorigenesis, prognostication, and opportunities for prediction of subtype-specific response to therapies, translating these information to the clinic is challenging. We and others have proposed the use of targeted molecular biomarkers for risk stratification (4, 105, 113, 114). In fact, a combination of a DNA methylation biomarker (hypermethylation of the G0S2 locus), and the expression levels of 2 transcripts (BUB1B and PINK1) stratifies ACC into 3 groups according to recurrence risk. Importantly, G0S2 hypermethylation recapitulates a signature of genome-wide CpG island hypermethylation (CIMP-high) that is almost exclusively observed in COC3 ACC (see Fig. 4) (4). Furthermore, the rapid and homogeneous recurrence kinetics observed in CIMP-high/G0S2 methylated tumors despite adjuvant mitotane suggest intrinsic resistance to this agent, the subject of our ongoing studies.
Clinical Investigation of Targeted Molecular Agents for Adrenocortical Carcinoma
Targeted molecular agents have emerged as useful therapeutic approaches for several malignancies, including hematological and solid tumors. However, the success of these therapies relies on the presence of tumor-specific molecular vulnerabilities that can be targeted by such agents, for example, highly expressed fusion transcripts or hotspot-activating mutations of tyrosine kinase receptors, and reliable assays to detect these alterations. Many of these therapeutic agents, therefore, are used in an individualized manner as so-called precision medicine. In addition to increasing therapeutic responses, a major potential advantage of such approaches is to restrict the toxic effects associated with classic cytotoxic agents. In ACC, early molecular studies demonstrating increased expression of IGF2, epidermal growth factor receptor (EGFR), and vascular endothelial growth factor provided the rationale for clinical trials testing tyrosine kinase inhibitors (reviewed in [21]). However, overall, these agents demonstrated overall little to no benefit. The most extensively studied targeted agents for ACC are insulin-like growth factor receptor 1 (IGF1R) inhibitors. IGF1R is a tyrosine-kinase coupled receptor by which IGF2 exerts its progrowth effects (115). This receptor appeared to be the ideal molecular target in ACC, since IGF2 is overexpressed in 90% of cases, and preclinical studies using different IGF1R inhibitors demonstrated promising antitumor effects both in vitro and in vivo (116, 117). However, therapeutic responses were limited to 5% to 10% of patients in phase 1 to 3 clinical trials, failing to reach the threshold of uniform efficacy (improved overall survival compared to placebo-treated patients) (118-120).
While these clinical trials had disappointing results, further studies illuminated possible reasons for these therapeutic failures. These include inadequate patient selection (all comers), and pharmacological interactions between several of these agents and mitotane (10, 11, 121, 122), later demonstrated to be a potent inducer of CYP3A4 in the liver. Along these lines, newer studies investigating application of tyrosine kinase inhibitors including cabozantinib in patients with undetectable mitotane levels suggest potential for therapeutic response (123). Importantly, a major barrier to success in prior trials was an incomplete understanding of the landscape of somatic alterations of ACC before high-throughput molecular profiling studies. Remarkably, these high-throughput studies revealed that molecular heterogeneity defines key classes of ACC with homogeneous and distinct clinical outcomes, suggesting that “one-size-fits-all” approaches are bound to fail.
These data support a possible molecular explanation for intrinsic resistance to IGF1R inhibitors (and other targeted therapies): In addition to IGF2 overexpression, COC2-COC3 tumors exhibit strong activation of other progrowth signaling pathways, including constitutive Wnt/β-catenin signaling and cell cycle activation due to TP53/RB1 loss. In these cases, IGF1R inhibition would be compensated by these other oncogenic hits. Several lines of evidence support the idea that resistance to monotherapy-targeted agents relies on the activation of additional oncogenic signaling pathways, rendering the cells independent from the original oncogenic hit (124). In fact, an in vivo model of ACC supports the synergism between IGF2 and Wnt/β-catenin signaling for tumorigenesis (83). In other words, these tumors may exhibit prosurvival plastic responses to monotherapy with targeted agents. A recent in vitro study characterizing molecular mechanisms of acquired mitotane resistance supports this hypothesis (125). On treatment with low doses of mitotane, transcriptome analysis shows that the NCI-H295R cells progressively downregulate cholesterol metabolism/steroidogenesis genes (including SOAT1, a target of mitotane), and upregulate Wnt/β-catenin target genes (125). These changes are in parallel with downregulation of genes associated with endoplasmic reticulum (ER) stress, revealing that resistant cells have bypassed the principal mechanism of mitotane toxicity through SOAT1 (13, 126).
More recently, clinical studies using immune checkpoint inhibitors have been conducted in ACC, with heterogeneous responses (127-132). In one of the largest studies, a phase 2 clinical enrolling 39 patients to receive the programmed cell death protein 1 (PD1) inhibitor pembrolizumab, an objective response was observed in 9 patients (23%), with an additional 7 (18%) patients achieving disease stabilization. In a large phase 1 study in metastatic ACC evaluating checkpoint blockade with avelumab targeting PD-L1, nearly 50% of patients achieved disease stabilization, and 6% exhibited an objective response (132). These results clearly indicate that pembrolizumab exhibited clinically meaningful antitumoral activity in a considerable subset of patients. However, it remains unclear which patients would benefit most from pembrolizumab since response was not correlated with traditional biomarkers of response such as PD1 expression, mismatch-repair deficiency, and microsatellite instability (127). These promising results were recapitulated by another study using a combination of ipilimumab and nivolumab, anti-CTLA4 and anti-PD1 antibodies, respectively (130). Out of the 6 patients with ACC enrolled in this open-label, multicenter phase 2 trial, 2 exhibited partial response, and 2 exhibited disease stabilization, and tumors from these 4 patients uniformly exhibited MSI-H microsatellite instability. This observation is in striking contrast with the results reported by Raj et al (127), in which the MSI-H phenotype was not associated with response to pembrolizumab alone. However, 5 patients exhibited severe (grade 3/4) toxicity, including 4 cases of hepatitis requiring discontinuation of the treatment, adrenalitis, and neutropenia. Collectively, these observations indicate that immunotherapy is a promising systemic option for ACC, with a substantial proportion of patients exhibiting durable therapeutic responses—a result that has not yet been observed with any other class of conventional or targeted systemic agents. Furthermore, immunotherapy may increase antitumorigenic effects of agents targeting other pathways, such as tyrosine kinase inhibitors (133). Additional studies to identify predictive biomarkers, and a better understanding of the molecular mechanisms of response to treatment in the setting of accurate and informative preclinical models (134), are needed to escalate this therapeutic modality to its full potential.
Molecular Subtypes Expose Novel Therapeutic Vulnerabilities in Adrenocortical Carcinoma
Multiplatform studies illuminated that ACC is defined by homogeneous molecular subtypes with distinct clinical outcomes (23) amenable to identification using targeted approaches (4). Importantly, no immediately actionable novel targets, for example, recurrent fusion transcripts or readily targetable hotspot mutations, emerged from these studies. These data suggest that characterization of homogeneous molecular classes and the prominent defining features of each class might provide a rationale for patient selection to specific therapeutic agents. Subtype-defining molecular features that can potentially be used to guide future therapeutic interventions include the degree of immune cell infiltration, SF1-dependent differentiation (including steroidogenesis capacity), Wnt/β-catenin activity, constitutive activation of cell cycle genes, genomic/chromosomic instability with associated activation of DNA repair pathways, and epigenetic dysregulation.
Adrenal Differentiation
In most human cancers, including solid tumors and hematological malignancies, dedifferentiation is associated with aggressive disease. As we previously discussed, ACC is unique in that the most aggressive molecular subclass, COC3, bears the highest degree of differentiation with high expression of tissue-specific transcripts (including several SF1 and PKA targets) culminating in increased cortisol production. This suggests that SF1-mediated transcription is exploited to confer a proliferative advantage to ACC cells (see Fig. 6); however, it also exposes several vulnerabilities that can be therapeutically targeted, and is the subject of the following sections.
Steroid Production and Immune Exclusion in Adrenocortical Carcinoma
In the last decade, immunotherapy has reemerged as an exciting therapeutic avenue for solid tumors, with several landmark pancancer studies revealing that tumors with mismatch repair deficiency are exquisitely sensitive to inhibition of physiologic checkpoints that restrain autoimmunity (eg, PD1/PD-L1, CTLA4) (135, 136). A widely accepted mechanism for this sensitivity is that cancer cells upregulate immune checkpoints to evade immune detection, and mismatch repair deficiency leads to translation of mutant proteins that may serve as neoantigens with potential for immune recognition (137). These studies heralded accelerated pancancer approval of immune checkpoint blockade for mismatch repair-deficient tumors. In fact, limited evidence supports that mismatch repair-deficient ACC might indeed respond to immune checkpoint inhibition as discussed in prior sections (128, 130, 138). Contemporaneously and thereafter, a plethora of clinical studies revealed astonishing, long-term remission of previously non–mismatch repair-deficient lethal cancers like metastatic melanoma and non–small cell lung cancer, leading to clinical practice changes that have significantly prolonged overall survival for patients with advanced forms of these diseases (139, 140). In the search for additional predictors of therapeutic response, investigators have also identified clinically significant roles for measurement of immune infiltration and preexisting activation of the checkpoint (141).
As we previously discussed, ACC as a whole have a mixed response to immune checkpoint blockade. Indeed, from a molecular subtype perspective, the vast majority of ACC (COC2-COC3) are immune poor (23, 142). This is consistent with the low level of PD-L1 expression identified in another study, suggesting low activity of this checkpoint in ACC (143). Only COC1 have significant immune infiltration, with non-ACC cells accounting for up to 50% of these tumors (23). This is particularly perplexing in light of the observation that COC3 possesses the highest mutational burden across ACC-TCGA, enriched for the noisy SCNA profile. These data suggest that additional factors that define COC2-COC3 may prevent immune infiltration and therefore checkpoint activation, even in the setting of high potential for neoantigen presentation. COC2-COC3 possess frequent somatic alterations leading to constitutive activation of Wnt/β-catenin activity, known to suppress immune infiltration in other cancer types (144-146) though not clearly associated with resistance to immunotherapy in ACC (127, 130).
Importantly, COC2-COC3 also possess higher degrees of adrenal differentiation, with higher expression of steroidogenic enzymes and clinically meaningful hypercortisolism. Cortisol is a well-characterized immune suppressant—patients with Cushing syndrome present with increased risk for infections and disrupted immune cell function including glucocorticoid-induced apoptosis of lymphocytes and defects in myeloid cell migration (147, 148). These observations suggest that glucocorticoid production may act as a shield to prevent tumor immune infiltration, subverting a requirement for activation of autoimmunity checkpoints. Inhibition of steroidogenesis or glucocorticoid receptor signaling may therefore be a promising therapeutic strategy to sensitize COC2-COC3 tumors and trigger therapeutic response to immune checkpoint blockade. This area is the subject of ongoing research by our group and others (112). Indeed, combined glucocorticoid receptor antagonist relacorilant and pembrolizumab in advanced ACC is the subject of an actively recruiting clinical trial (NCT04373265). Overcoming the intrinsic barriers to immune infiltration in COC2-COC3 ACC will likely be required before evaluating efficacy of engineered cell therapies (eg, CAR T cells or other forms of engineered T cells); however, given the high mutational burden (and likely neoantigen presentation) in COC3, this represents a promising therapeutic avenue. Conversely, it remains to be seen if androgen production or COC1 status predict intrinsic susceptibility to immunotherapy.
Steroidogenesis as an Intrinsic Vulnerability
Steroid production is the principal and essential physiologic function of the adrenal gland; early mortality in mice with adrenal agenesis can be prevented with exogenous glucocorticoid supplementation (149). Steroidogenesis involves a series of enzymatic oxidative reactions that take place in the mitochondria and ER in which cholesterol is converted into the different classes of steroid hormones. Furthermore, steroidogenesis intrinsically releases a series of toxic byproducts, including reactive oxygen species (150). Therefore, steroidogenesis is an energetically expensive process requiring numerous transient and sustained adaptations to facilitate cholesterol transport, scavenging, cellular detoxification, and timely and appropriate expression of synthetic enzymes. This is evidenced by specialized cells in a variety of tissues that metabolize cholesterol and may even engage in steroidogenesis (151-153). In the adrenal cortex, expression of steroidogenic enzymes, lipid transporters, cholesterol scavengers, and other detoxification genes are thought to be directly or indirectly regulated by SF1 (47, 48, 154, 155). The machinery encoded by these genes represent a first-line defense mechanism against toxic byproducts of steroidogenesis in an adrenocortical cell.
As we previously described, ACC is characterized by mixed steroid production with the accumulation of steroid precursors even in the setting of overt hypercortisolism, sometimes also with concurrent secretion of mineralocorticoids, estrogens, and/or androgens. Patients with ACC and signs/symptoms of hormone excess often have a higher disease burden (89), suggesting that malignant steroidogenesis is intrinsically inefficient, particularly when compared to the physiologic adrenal cortex and benign tumors (which, when large, may be only < 4 cm in diameter). This cellular program therefore represents a promising therapeutic vulnerability for ACC from multiple standpoints. It offers a high therapeutic index, given the rarity of cells in the body that engage in this program; it would mitigate hormone excess-associated morbidity in ACC; and it possesses multiple avenues for therapeutic targeting.
Clinical utility of targeting steroidogenesis is widely supported by the putative mechanisms of action of mitotane. While the range of molecular targets of mitotane remains poorly characterized, mitotane-induced toxicity is preceded by mitochondria swelling and degeneration, suggesting that this organelle is a major target (156). In fact, later studies demonstrated that mitotane induces a dysfunction in mitochondria-associated membranes, causing impairment of the respiratory chain (and hence steroidogenesis) and inducing caspase 3- and 7-dependent apoptosis (157, 158). Biochemical studies have suggested that mitotane’s cytotoxic actions require an enzymatic activation step, in which the drug is converted to an unstable acyl-chloride intermediate before being metabolized to o,p’-DDA (159, 160). This unstable acyl-chloride intermediate reacts with unknown mitochondrial proteins, forming adducts that impair mitochondrial function (159, 160). Studies using an I125-labeled analogue of mitotane suggest that one such target is the p450-scc enzymes, consistent with inhibitory effects of mitotane on steroidogenesis, and indicating that CYP11A1 might be the enzyme that activates mitotane and therefore explain why adrenal cells are exquisitely sensitive to this compound (161, 162).
More recently, SOAT1, an enzyme that is essential for cholesterol esterification in the ER, was identified as a molecular target of mitotane. Inhibition of SOAT1 by genetic or pharmacological approaches leads to lipid-dependent toxicity preceded by activation of ER stress signaling (126). While efforts have been made to develop a more toxic form of mitotane (163), a complete characterization of its molecular targets as well as the mechanisms by which it induces cell death would be required to develop alternative and more specific compounds, and to illuminate additional therapeutic targets.
Recent application of this strategy is exemplified by the use of the SOAT1 inhibitor nevanimibe (ATR-101). Initially developed as cholesterol-lowering agents, this class of drugs exhibited unexpected adrenal toxicity secondary to high adrenal expression of SOAT1 (164, 165). Recently, this agent has been repurposed as an investigational compound to inhibit steroidogenesis in Cushing syndrome, congenital adrenal hyperplasia, and ACC (166, 167). Nevanimibe induces cholesterol-dependent apoptotic ACC cell death in vitro and in vivo (126, 168). More recently, a phase 1 clinical trial conducted in 63 patients with metastatic ACC has demonstrated disease stabilization in a subset of patients with few toxic effects (167).
Because steroidogenesis is a process that releases substantial amounts of reactive oxygen species, defects in clearing these toxic byproducts can induce extensive damage in steroidogenic cells. It has been recently demonstrated that the normal adrenal cortex and ACC express high levels of glutathione peroxidase 4 (GPX4), an enzyme that reduces hydroperoxides in a glutathione-dependent manner. Inhibition of GPX4 or depletion of glutathione by pharmacological agents in steroidogenic ACC cell lines potently induces ferroptosis, a nonapoptotic iron-dependent form of cell death associated with lipid peroxidation. These observations expose a potential target for future therapies in ACC (169, 170).
Another therapeutic approach that exploits the adrenal differentiation/steroidogenesis program is based on the nonbarbiturate imidazole compound metomidate. Originally designed as an anesthetic, this compound strongly binds to 11β-hydroxylase (encoded by CYP11B1 and CYP11B2) and inhibits its activity (171, 172). Because of this property, metomidate has been used as a radiotracer in positron emission tomography and single-photon emission computed tomography imaging techniques (including 11C-methomidate, 123I-methomidate, and 18F-FETO) to distinguish cortical from noncortical adrenal tumors, to identify laterality of aldosterone-producing adenomas, and to identify ACC metastasis (173-175). Hahner and colleagues (176, 177) tested the efficacy of 131I-methomidate as a therapeutic agent in a series of 11 patients with advanced ACC. One patient exhibited a partial response, with a 51% decrease in the size of target lesions, and 5 patients achieved disease stabilization (including sustained stabilization for > 24 months in some patients). Given the overall good tolerability of this treatment, these results suggest that radiopharmaceuticals are a viable option for advanced ACC and warrant further development and research.
Targeting Genomic Instability and Cell Cycle
ACC is distinguished from ACA by significant upregulation of the cell cycle, measured by mitotic counts, Ki67, and even molecular markers like the BUB1B-PINK1 score (8, 96, 105, 178). Genome-wide multiplatform studies on ACC have revealed that even within malignant lesions, cell cycle activation exists along a spectrum, with COC3 tumors possessing the highest expression of proliferation-dependent genes. COC3 ACC is characterized by enrichment for somatic events leading to constitutive cell cycle activation (23). These include amplification of genes encoding cyclins and cyclin-dependent kinases (CDKs), epigenetic silencing or deletions of genes encoding CDK inhibitors (eg, CDKN2A), and recurrent LOF alterations in genes encoding guardians of the G1/S checkpoint (TP53 and RB1). The high degree of autonomous cell cycle activation in COC3 ACC is also evidenced by significant enrichment for the noisy SCNA signature in these tumors, characterized by numerous focal gains and losses throughout the genome (23).
COC3 tumors have dismal clinical outcomes with invariable progression to metastatic disease (4, 23). In light of historical observations that a subset of patients with advanced ACC exhibit clinically meaningful responses to cytotoxic chemotherapy known to preferentially target rapidly proliferative cells (with cytoreduction culminating in partial response in some cases) (179), it is possible that patients with COC3 disease may also respond to these traditional agents (4, 180, 181). These observations of course also point to a potentially meaningful role for novel, specific small-molecule inhibitors of CDKs (eg, palbociclib) that have been associated with disease regression for patients with other solid tumors (182, 183). Preliminary in vitro studies have suggested that ACC is susceptible to palbociclib (184, 185). Given the high frequency of LOF TP53 mutations in ACC, it is unlikely that therapeutic strategies targeting intact p53 (eg, MDM2 inhibition) will be effective for COC3 tumors as a class, but these agents remain an option for patients with COC3 disease and intact p53 signaling. Other potential cell cycle–associated targets amendable for therapeutic intervention in COC3 tumors include polo-like kinase 1 (PLK1), maternal embryonic leucine zipper kinase (MELK), and aurora kinases (186-188).
Given the profound chromosomal instability that prevails in COC3 ACC, it is also possible that patients with anatomically accessible metastatic disease may be responsive to attempted cytoreduction with other strategies that induce further DNA damage, such as targeted radiation. Importantly, while COC3 tumors invariably possess high cell-cycle activation, traditional markers currently implemented in clinical practice to measure proliferation index may be insensitive to capture all tumors that reside in this class (189); using alternative surrogates to identify patients with COC3 tumors (eg, aberrant DNA methylation) can capture this class even in patients with low-grade disease (4).
Recent pancancer studies incorporating ACC-TCGA samples have also revealed that ACC is characterized by genomic instability signatures that may render them susceptible to therapies exploiting DNA damage response machinery. A subset of ACC possesses a genomic signature revealing evidence of defective homologous recombination (190). Tumors with homologous recombination deficiency, classically through inactivating mutations in genes encoding BRCA, FANC, and Rad50 family members, are exquisitely sensitive to PARP inhibitors (191, 192). ACC do not possess recurrent mutations in these genes. However, the application of this class of therapies to tumors with frequent BRCA mutations (eg, ovarian cancer) has significantly prolonged patient survival, and also revealed that genetic events leading to homologous recombination deficiency are not required for therapeutic efficacy (193, 194). Other targets that can be therapeutically exploited in this subgroup of ACC include the heat shock protein 90 (Hsp90) and the Wee1 kinase (195). These proteins are essential for an effective DNA damage response, and their inhibition in different experimental models leads to cell death by mitotic catastrophe. Hsp90 inhibition has demonstrated antitumor activity in different ACC cell lines (196). While molecular predictors of response to these agents have not been fully characterized, dysfunctional p53 has been demonstrated to increase sensitivity to Wee1 inhibitors in different cancer types, making this target particularly interesting in ACC. Moreover, these agents can be combined with radiation therapy and cytotoxic chemotherapy to overcome intrinsic resistance to any single modality (197-199). In light of new evidence that cytoreduction even by surgical resection of oligometastatic disease is associated with prolonged survival for patients with ACC (200), these strategies hold substantial promise as adjuvant or neoadjuvant approaches.
Targeting Wnt/β-Catenin Signaling
As previously discussed, one-third of ACC possess activating mutations in the Wnt/β-catenin pathway, including activating mutations in CTNNB1, and LOF alterations of Wnt/β-catenin–negative regulators APC and ZNRF3 (22-24). Somatic alterations in ZNRF3, CTNNB1, and APC are enriched in C1A/COC2-COC3 ACC, suggesting subtype specificity of targeting this pathway. While the common denominator for these alterations is the constitutive activation of Wnt/β-catenin signaling, CTNNB1- and APC-mutated tumors are ligand independent, and ZNRF3-deleted tumors require the presence of an extracellular Wnt ligand for pathway activation (201). Different strategies for targeting Wnt/β-catenin signaling have been proposed depending on the molecular defect that leads to constitutive pathway activation. These include new monoclonal antibodies or small-molecule agents to promote inhibition of acylation and secretion of Wnt ligands (eg, porcupine inhibitors), antagonism of Fzd receptors and/or Wnt ligands, degradation of β-catenin, or restriction of β-catenin’s actions as a transcriptional coactivator by inhibiting its interactions with transcription factors like TCF/LEF family members or epigenetic machinery like CBP (202, 203). Importantly, the high frequency of recurrent deletions in ZNRF3 (ligand-dependent activation) has opened new venues for targeting Wnt/β-catenin in ACC with agents that restrict ligand activity, such as porcupine inhibitors (204). However, despite the wide availability of a plethora of compounds, the clinical utility of pan-Wnt pathway inhibition has yet to be demonstrated. Wnt/β-catenin is essential for stem/progenitor cell maintenance in a variety of tissues; pathway inhibition has been associated with numerous on-target toxicities (eg, diarrhea secondary to intestinal mucosa atrophy), preventing any of these agents from advancing to phase 3 clinical trials (205, 206). Indeed, the observation that these agents have a low therapeutic index is actually not surprising, as patients with germline LOF mutations in ligand-dependent Wnt signaling components also exhibit intestinal malabsorption and several additional life-limiting abnormalities (59, 207, 208).
The high toxicity associated with different pan-Wnt pathway inhibition strategies suggest that novel approaches to target tissue-specific Wnt/β-catenin signaling components are required. Possible strategies are targeting production or signaling through tissue-specific Wnt ligands (eg, WNT4), tissue-specific Fzd receptors, or tissue-specific nuclear partners of β-catenin. More research into such components of this pathway will be required to develop novel agents with a high therapeutic index; ongoing investigations into this area are being led by our group and others. Furthermore, patients bearing tumors with Wnt pathway activation (regardless of ligand dependence of the alteration) also develop metastatic disease, necessitating systemic therapies. However, it remains to be seen if tumors with ligand-dependent alterations possess pathway activation at metastatic sites, for example, through autocrine stimulation by WNT4 or paracrine stimulation by other Wnt ligands native to the metastatic site. If metastatic seeding away from the Wnt-active adrenal cortex erases evidence of Wnt signaling in ligand-dependent tumors, it is possible that Wnt pathway activation may be required only for early stages of tumorigenesis, limiting the spectrum of ACC for which targeting Wnt signaling would be clinically meaningful.
Targeting Epigenetic Programs
Transcriptional dysregulation is a core hallmark of cancer, and ACC is no exception. Abnormal transcription is sustained by alterations in protein-coding regions, encoding transcription factors, upstream signaling components, and regulators of chromatin dynamics; and also through alterations in noncoding regions like cis-regulatory elements. The contributions of each class of alterations to transcriptional dysregulation in ACC have been discussed previously and elsewhere (142). A unique type of epigenetic dysregulation in ACC involves the disruption of imprinted genes. The first evidence of dysregulation of imprinting in ACC came from studies exploring the mechanisms of IGF2 overexpression in these tumors. Loss of imprinting within the 11p15 locus could be detected in most cases, usually associated with reduced expression of the cell-cycle regulator CDKN1C (209-211). Later studies have demonstrated abnormal activity of another imprinted region, the MEG3-DLK1 locus, characterized by hypermethylation and reduced expression of a cluster of microRNAs located within this region. This abnormal pattern is almost exclusively observed in the C1B/COC1 molecular subclass of ACC (22, 23). In ACC, dysregulation of these imprinted regions is frequently associated with whole chromosome loss-of-heterozygosity (LOH) events, indicating a selective pressure for the chromosomal SCNA signature (and perhaps explaining why hypodiploidy is so frequently observed in ACC). Furthermore, widespread whole-chromosomal LOH and hypodiploidy may confer vulnerabilities to secondary recurrent events that inactivate tumor-suppressor genes. For example, in ACC-TCGA almost all tumors that exhibit focal deletion of ZNRF3 already lost the first copy by whole-chromosome LOH events involving chr22 (23).
Abnormal DNA methylation is perhaps the next most prominent feature of epigenetic dysregulation in ACC. In particular, as previously discussed, widespread DNA hypermethylation targeting CpG islands (CIMP-high) is a feature of COC3 ACC and a marker of aggressive disease, frequently associated with constitutive cell-cycle activation, differentiation, and activation of Wnt/β-catenin signaling (4, 23). However, the role of CIMP-high in transcriptional dysregulation has not been characterized, and it is possible that this is a bystander phenomenon secondary to rapid proliferation, widespread genomic instability (212), or an abnormal metabolic state (213). DNA hypermethylation is written by the DNA methyltransferases DNMT1 and DNMT3A/B, which are also regulated in a cell cycle–dependent manner by transcription factors of the E2F family—explaining the link with a constitutively active cell cycle observed in ACC (214, 215). While DNMT inhibitors are clinically available and are standard of care for certain hematological malignancies, their role for solid tumors remains to be demonstrated (216). Our preliminary in vitro studies using zebularine, a cytidine analogue that inhibits DNMT activity, demonstrated a mild cytostatic effect in the NCI-H295R (which possesses the CIMP-high signature) (217) after a prolonged treatment, suggesting that targeting DNA hypermethylation in ACC may not be a viable therapeutic option, or the inhibitory effects on DNMTs obtained with conventional cytidine analogues are not potent enough. More recently, a new class of DNMT inhibitors that relies on allosteric inhibition has shown extremely potent inhibitory effects in other models (218) and remains to be tested in ACC. Given the strong association between CIMP-high and dismal outcomes for patients with ACC, a better understanding of the biological processes driving CIMP-high, including a full characterization of metabolic dysregulation in ACC subtypes, and other epigenetic alterations involving histone marks may reveal additional opportunities to target epigenetic dysregulation in ACC (181).
While considerable advances in therapeutic approaches targeting epigenetic dysregulation in solid tumors have been reported, it remains a challenging enterprise and an active area of research. Novel approaches targeting chromatin regulators have recently been described for several solid tumors (219, 220), but they largely rely on tumor-specific vulnerabilities and metabolic profiles. Recently, alterations in several chromatin remodelers have been described in ACC, adding to early studies that identified ACC as part of the MEN1 spectrum. In addition to recurrent mutations in MEN1 in sporadic ACC, other somatic alterations target genes encoding MLL and SWI/SNF family members (23, 221), suggesting that dysregulation of chromatin dynamics is a feature of the molecular pathogenesis of ACC. Interestingly, dysregulation of chromatin dynamics seems to converge on activation of an oncogenic SF1-driven transcriptional program, as suggested by a recent pancancer chromatin accessibility study incorporating samples from ACC-TCGA. This study revealed that ACCs possess a unique epigenetic profile, defined by accessibility and activation of the SF1-dependent transcriptional and epigenetic program characterized by increased accessibility not only in the promoter regions of target genes, but also several de novo putative distal regulatory elements. These observations suggest inhibition of pioneer factors unique to ACC and adrenal tissue may offer a high therapeutic index through targeting of a cell identity/differentiation program that is selected for in COC2-COC3 ACC (222).
Advances in the understanding of epigenetic reprogramming in cancer have illuminated several novel cancer codependencies. Given the variable activating and repressive effects of different epigenetic marks, cancers that possess decreased activation of one pathway may be vulnerable to inhibition of the other pathway. This is best exemplified by tumors possessing LOF alterations in SWI/SNF machinery, which are exquisitely sensitive to inhibition of an antagonistic repressive epigenetic complex, the Polycomb repressive complex 2 (PRC2) (223, 224). The catalytically active member of the PRC2 is a histone H3 lysine 27 methyltransferase, EZH2, that has been the subject of extensive investigation in many cancers including ACC secondary to its high cell-cycle dependence (225-227). SWI/SNF mutations are frequent in human cancers (228), and the discovery of this therapeutic vulnerability along with the simultaneous discovery of recurrent activating EZH2 mutations in lymphomas (229, 230) has spurred the rapid development of several small molecules targeting PRC2 (231), culminating in the recent FDA approval of tazemetostat for patients with tumors bearing gain-of-function alterations in EZH2. Intriguingly, the physiologic role of EZH2 is to promote stemness and pluripotency (232-234); however, in the adrenal, EZH2 facilitates zG to zF transdifferentiation and response to ACTH (78). These data suggest that targeting EZH2 may also target the differentiation program that prevails COC3 ACC, supported by our ongoing work (217).
Conclusion and Future Directions
Despite substantial advances in our understanding of the molecular pathogenesis of ACC, treatment outcomes remain dismal for the majority of patients. Therapeutic responses to conventional and targeted agents are heterogeneous, indicating that the current “one-size-fits-all” approaches are suboptimal, at best, in all settings. Furthermore, recent multiomics studies have provided important conceptual advances that can be used to guide the development of new therapeutic strategies using existing and novel agents. Clinical heterogeneity in ACC is mirrored by distinct molecular subtypes that possess unique features, including recurrent genomic alterations targeting few core signaling pathways; distinct epigenetic patterning; distinct forms of chromosomal instability; differential engagement of DNA repair programs; and transcriptional modules that capture variable degrees of adrenocortical differentiation, immune cell infiltration, mitotic activity, and Wnt/β-catenin signaling (Fig. 7). Overcoming the limitations of current therapeutic approaches necessitates accounting for molecular heterogeneity. Molecular information can lead to improved risk stratification strategies to guide adjuvant therapies, and therapeutic interventions for advanced disease. However, while several studies have recently demonstrated the clinical relevance of molecular data, translating these discoveries into clinical practice remains challenging. Minimalistic targeted approaches that capture the most clinically relevant molecular information from widely available clinical specimens such as formalin-fixed, paraffin-embedded tissue (235) and plasma (236-239) need to be further developed and validated. These putative biomarkers can be used for guiding mechanism-based therapeutic interventions to specific and well-defined subgroups both in the adjuvant setting and in late-stage disease.
Figure 7.
Schematic of cellular pathways that represent promising therapeutic targets for adrenocortical carcinoma (ACC). In COC2-COC3 ACC, Wnt signaling (which is likely at least partially autocrine via WNT4) can be targeted through agents that inhibit Wnt secretion via Porcupine (PORCN), through as yet undiscovered agents that may target tissue-specific Wnt ligands or Frizzled receptors (FZDs), or through agents that restrict the actions of β-catenin in the nucleus (inhibitors of β-catenin’s interactions with TCF/LEF, tissue-specific partners, or histone acetyltransferases such as CBP that facilitate transcriptional programming). High levels of cell cycle activation and genomic instability in COC3 tumors can targeted by traditional cytotoxic agents, targeted radiation, novel CDK inhibitors, and also through novel small molecules that take advantage of vulnerabilities that occur in cells with specific patterns of genomic instability (eg, inhibitors of PARP). We suspect that sole therapy with immune checkpoint blockade is likely to be most effective in patients with tumors that possess preexisting immune checkpoint activation (eg, COC1) and mismatch repair deficiency; however, immune checkpoint blockade may also be effective in patients with COC2-COC3 tumors treated with inhibitors of glucocorticoid secretion or action. Similarly, while inhibiting growth factor signaling has not demonstrated global efficacy as monotherapy (and is likely highly susceptible to acquired resistance), we believe that agents targeting these programs can be combined with other therapies to facilitate tumor regression. Patients with COC2-COC3 ACC or steroidogenic ACC (regardless of androgen or glucocorticoid pattern of secretion) may be susceptible to agents that lead to the cellular accumulation of toxic steroidogenesis byproducts and other reactive oxygen species. Given the physiologic importance of detoxifying steroidogenesis and other cellular process in adrenal cells, these classes of tumors may be vulnerable to agents that restrict detoxification (eg, inhibitors of glutathione peroxidase 4; GPX4) and promote cell death via iron-dependent nonapoptotic mechanisms (ferroptosis). Putative tissue-specific transcriptional programs that coordinate differentiation states favorable for cancer development and evolution (eg, driven by steroidogenesis factor 1; SF1) represent unique vulnerabilities for all classes of ACC with a potentially high therapeutic index, and are active areas of investigation. Importantly, COC2-COC3 ACC exhibit profound epigenetic rewiring that may facilitate cancer cell plasticity and can be targeted through inhibitors of epigenetic programming (eg, inhibitors of EZH2 or DNA methyltransferases [DNMT]). Given the emergent understanding of the interface between cellular metabolism and epigenetic programming, it is also promising to consider therapeutic targeting of cancer cell–specific metabolic states in combination with other strategies.
Given the rarity of ACC and therefore the challenges of rapidly accruing clinical data on a timeline at pace with scientific and mechanistic discoveries, it will be crucial to reconsider the clinical trial model. For patients with slower-growing disease (eg, COC1 and some COC2), practice-changing clinical data through traditional phase 1 to 3 clinical trials may emerge only after a decade or more of enrollment (6). For patients with rapidly growing disease (eg, COC2-COC3), this approach is viable only in the relapsed/recurrent setting and at high risk of trial failure. Given the recent advances in preclinical modeling of ACC (134, 138, 240-242), bench-to-bedside approaches hold considerable promise improving clinical trial outcomes by prioritizing therapeutic agents that have a higher potential to provide therapeutic benefit. If comprehensive care centers develop pipelines adopting these approaches in combination with rapid molecular subtyping and high-throughput screening platforms (eg, patient-derived organoids), targeted small-molecule based therapies may soon have opportunities for success in ACC.
ACC is widely known for being resistant to several forms of systemic therapy. Understanding the molecular basis of resistance is essential to develop new therapeutic strategies, even provided the existence of high-throughput screening platforms. Moreover, within a single patient with ACC, different foci of disease (eg, metastatic vs adrenal sites) may exhibit discordant responses to medical agents. These data suggest that cancer-cell heterogeneity plays an important and clinically relevant role. While genetic, genomic, and epigenetic heterogeneity has been documented among primary and metastatic ACC lesions (4, 243-245), cellular plasticity might ultimately be the culprit of therapeutic resistance (246). Plasticity can be defined as an ability to adopt different identities along the phenotypic spectra that resemble distinct developmental lineages, cell identities, and metabolic states. This is achieved by dynamic and reversible epigenetic reprogramming of different transcriptional modules. Plasticity is thought to be an adaptive response to stressors, including hypoxia, fuel deprivation, immune system activity, and systemic therapies, enabling adaptation and survival (247, 248). Examples of plasticity include epithelial-to-mesenchymal transition, a process by which cells from carcinomas assume mesenchymal characteristics rendering the migratory and invasive capacity essential for metastatic spread (249). Recently, plasticity has been documented as a mechanism of resistance to systemic targeted therapies. Prostate cancer and EGFR-mutated non–small cell lung cancer that initially respond to agents targeting the androgen receptor and EGFR, respectively, assume a neuroendocrine-like identity as therapeutic resistance emerges (250, 251, 252). This new cellular identity, characterized by the expression of several neuroendocrine markers, renders cells independent of the initial oncogenic hits, and therefore, resistant to agents targeting these programs (124, 254). Plasticity may emerge as a result of therapeutic interventions (125); however, recent studies using lineage-tracing techniques in murine models of prostate and lung cancers have demonstrated the existence of plastic cell states within a tumor even before therapeutic intervention. Changes in cell identity in these systems are associated with certain genetic hits, including RB1 and TP53 loss, and the APOBEC signature (26, 253, 254). These data support a model that certain cell types within a tissue with intrinsic lineage infidelity may be selected for in early stages of carcinogenesis.
While plasticity has been recently recognized as a major driver of therapeutic resistance, there are currently no efficacious therapeutic interventions that restrict or mitigate plasticity. Preclinical studies suggest that inhibition of epigenetic programs may restrict plasticity, and clinical trials evaluating combination therapy with agents targeting the original oncogenic hit and emerging plasticity programs are ongoing (124, 248). Little is known about cellular states that ACC can assume spontaneously or as a result of systemic therapies. A comprehensive characterization of cell heterogeneity in ACC using single-cell technologies both in human samples and mouse models may provide useful biological insights. Indeed, these studies in the physiologic adrenal gland are currently underway (255). Understanding the diversity of states a single ACC cell can adopt might be the most critical insight to enable the development of new strategies to overcome therapy resistance for patients facing this devastating disease.
Glossary
Abbreviations
- ACA
adrenocortical adenoma;
- ACC
adrenocortical carcinoma;
- ACT
adrenocortical tumor;
- ACTH
adrenocorticotropin;
- ADS
adrenal differentiation score;
- AGP
adrenogonadal primordium;
- ATII
angiotensin II;
- ATP
adenosine 5′-triphosphate;
- cAMP
cyclic adenosine 5′-monophosphate;
- CDK
cyclin-dependent kinase;
- DNMT
DNA methyltransferase;
- EGFR
epidermal growth factor receptor;
- ER
endoplasmic reticulum;
- FAdE
fetal adrenal-specific enhancer;
- FDA
US Food and Drug Administration;
- Fzd
frizzled;
- IGF1R
insulin-like growth factor receptor 1;
- LGR
leucine-rich repeat containing G-protein coupled receptor;
- LOF
loss of function;
- LOH
loss of heterozygosity;
- MC2R
melanocortin receptor type 2;
- PD1
programmed cell death protein 1;
- PKA
protein kinase A;
- PRC2
polycomb repressive complex 2;
- RSPO
R-spondins;
- SCNA
somatic copy number alteration;
- SF1
steroidogenesis factor 1;
- SHH
sonic hedgehog;
- SNP
single nucleotide polymorphism;
- SNV
single nucleotide variant;
- TCGA
The Cancer Genome Atlas project;
- zF
zona fasciculata;
- zG
zona glomerulosa
Contributor Information
Antonio Marcondes Lerario, Department of Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, Michigan 48109-2200, USA.
Dipika R Mohan, Medical Scientist Training Program, University of Michigan, Ann Arbor, Michigan 48109-2200, USA.
Gary D Hammer, Department of Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, Michigan 48109-2200, USA; Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, Michigan 48109-2200, USA; Rogel Cancer Center, University of Michigan, Ann Arbor, Michigan 48109-2200, USA; Department of Cell & Developmental Biology, University of Michigan, Ann Arbor, Michigan 48109-2200, USA.
Financial Support
This work was supported by the National Institutes of Health (grant Nos. R01 DK043140 to A.M.L. and R01 DK062027 to A.M.L. and G.D.H.), the United States Department of Defense (grant Nos. CA180750 and CA18751 A.M.L., D.R.M., and G.D.H.), the Cissell-Roell Innovation Fund (to A.M.L., D.R.M., and G.D.H.), the University of Michigan Medical Scientist Training Program (No. T32 GM7863 to D.R.M.), and the Drew O’Donoghue Fund (to D.R.M. and G.D.H.).
Disclosures
A.M.L., D.R.M., and G.D.H. are coinventors on 3 pending patent applications owned by the Regents of the University of Michigan on methods for characterizing and treating adrenocortical carcinoma. G.D.H. is founder and stock owner of Vasaragen, Inc (private).
References
- 1. Else T, Kim A, Sabolch A, et al. . Adrenocortical carcinoma. Endocr Rev. 2014;35(2):2372-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Terzolo M, Angeli A, Fassnacht M, et al. . Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372-2380. [DOI] [PubMed] [Google Scholar]
- 3. Fassnacht M, Dekkers OM, Else T, et al. . European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1-G46. [DOI] [PubMed] [Google Scholar]
- 4. Mohan DR, Lerario AM, Else T, et al. . Targeted assessment of G0S2 methylation identifies a rapidly recurrent, routinely fatal molecular subtype of adrenocortical carcinoma. Clin Cancer Res. 2019;25(11):3276-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glenn JA, Else T, Hughes DT, et al. . Longitudinal patterns of recurrence in patients with adrenocortical carcinoma. Surgery. 2019;165(1):186-195. [DOI] [PubMed] [Google Scholar]
- 6. Terzolo M, Fassnacht M, Perotti P, et al. . Results of the ADIUVO Study, the first randomized trial on adjuvant mitotane in adrenocortical carcinoma patients. J Endocr Soc. 2021;5(Suppl 1):A166-A167. [Google Scholar]
- 7. Kimpel O, Bedrose S, Megerle F, et al. . Adjuvant platinum-based chemotherapy in radically resected adrenocortical carcinoma: a cohort study. Br J Cancer. 2021;125(9):1233-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beuschlein F, Weigel J, Saeger W, et al. . Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100(3):841-849. [DOI] [PubMed] [Google Scholar]
- 9. Megerle F, Herrmann W, Schloetelburg W, et al. . German ACC Study Group. Mitotane monotherapy in patients with advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2018;103(4):1686-1695. [DOI] [PubMed] [Google Scholar]
- 10. Chortis V, Taylor AE, Schneider P, et al. . Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5α-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98(1):161-171. [DOI] [PubMed] [Google Scholar]
- 11. Kroiss M, Quinkler M, Lutz WK, Allolio B, Fassnacht M. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin Endocrinol (Oxf). 2011;75(5):585-591. [DOI] [PubMed] [Google Scholar]
- 12. Puglisi S, Calabrese A, Basile V, et al. . New perspectives for mitotane treatment of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2020;34(3):101415. [DOI] [PubMed] [Google Scholar]
- 13. Sbiera S, Leich E, Liebisch G, et al. . Mitotane inhibits sterol-O-Acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology. 2015;156(11):3895-3908. [DOI] [PubMed] [Google Scholar]
- 14. Warde KM, Schoenmakers E, Ribes Martinez E, et al. . Liver X receptor inhibition potentiates mitotane-induced adrenotoxicity in ACC. Endocr Relat Cancer. 2020;27(6):361-373. [DOI] [PubMed] [Google Scholar]
- 15. Altieri B, Sbiera S, Herterich S, et al. . Effects of germline CYP2W1*6 and CYP2B6*6 single nucleotide polymorphisms on mitotane treatment in adrenocortical carcinoma: a multicenter ENSAT study. Cancers (Basel). 2020;12(2):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Avolio A, De Francia S, Basile V, et al. . Influence of the CYP2B6 polymorphism on the pharmacokinetics of mitotane. Pharmacogenet Genomics. 2013;23(6):293-300. [DOI] [PubMed] [Google Scholar]
- 17. Reidy-Lagunes DL, Lung B, Untch BR, et al. . Complete responses to mitotane in metastatic adrenocortical carcinoma—a new look at an old drug. Oncologist. 2017;22(9):1102-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El Ghorayeb N, Rondeau G, Latour M, et al. . Rapid and complete remission of metastatic adrenocortical carcinoma persisting 10 years after treatment with mitotane monotherapy: case report and review of the literature. Medicine (Baltimore). 2016;95(13):e3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bates SE, Shieh CY, Mickley LA, et al. . Mitotane enhances cytotoxicity of chemotherapy in cell lines expressing a multidrug resistance gene (mdr-1/P-glycoprotein) which is also expressed by adrenocortical carcinomas. J Clin Endocrinol Metab. 1991;73(1):18-29. [DOI] [PubMed] [Google Scholar]
- 20. Dogliotti L, Berruti A, Pia A, Paccotti P, Alì A, Angeli A. Cytotoxic chemotherapy for adrenocortical carcinoma. Minerva Endocrinol. 1995;20(1):105-109. [PubMed] [Google Scholar]
- 21. Megerle F, Kroiss M, Hahner S, Fassnacht M. Advanced adrenocortical carcinoma—what to do when first-line therapy fails? Exp Clin Endocrinol Diabetes. 2019;127(2-03):109-116. [DOI] [PubMed] [Google Scholar]
- 22. Assié G, Letouzé E, Fassnacht M, et al. . Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46(6):607-612. [DOI] [PubMed] [Google Scholar]
- 23. Zheng S, Cherniack AD, Dewal N, et al. . Cancer Genome Atlas Research Network. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 2016;29(5):723-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Juhlin CC, Goh G, Healy JM, et al. . Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J Clin Endocrinol Metab. 2015;100(3):E493-E502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pinto EM, Chen X, Easton J, et al. . Genomic landscape of paediatric adrenocortical tumours. Nat Commun. 2015;6:6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marjanovic ND, Hofree M, Chan JE, et al. . Emergence of a high-plasticity cell state during lung cancer evolution. Cancer Cell. 2020;38(2):229-246.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453(7194):544-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134(12):2349-2358. [DOI] [PubMed] [Google Scholar]
- 29. Xing Y, Lerario AM, Rainey W, Hammer GD. Development of adrenal cortex zonation. Endocrinol Metab Clin North Am. 2015;44(2):243-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keegan CE, Hammer GD. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab. 2002;13(5):200-208. [DOI] [PubMed] [Google Scholar]
- 31. Vidal V, Sacco S, Rocha AS, et al. . The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes Dev. 2016;30(12):1389-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finco I, Lerario AM, Hammer GD. Sonic hedgehog and WNT signaling promote adrenal gland regeneration in male mice. Endocrinology. 2018;159(2):579-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106(50):21185-21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Revest JM, Spencer-Dene B, Kerr K, De Moerlooze L, Rosewell I, Dickson C. Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev Biol. 2001;231(1):47-62. [DOI] [PubMed] [Google Scholar]
- 35. Guasti L, Cavlan D, Cogger K, et al. . Dlk1 up-regulates Gli1 expression in male rat adrenal capsule cells through the activation of beta1 integrin and ERK1/2. Endocrinology. 2013;154(12):4675-4684. [DOI] [PubMed] [Google Scholar]
- 36. Grabek A, Dolfi B, Klein B, Jian-Motamedi F, Chaboissier MC, Schedl A. The adult adrenal cortex undergoes rapid tissue renewal in a sex-specific manner. Cell Stem Cell. 2019;25(2):290-296.e2. [DOI] [PubMed] [Google Scholar]
- 37. Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18(3):378-403. [DOI] [PubMed] [Google Scholar]
- 38. Narasaka T, Suzuki T, Moriya T, Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol. 2001;174(1-2):111-120. [DOI] [PubMed] [Google Scholar]
- 39. Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28(23):7030-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993;268(10):7494-7502. [PubMed] [Google Scholar]
- 41. Bamforth SD, Bragança J, Eloranta JJ, et al. . Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet. 2001;29(4):469-474. [DOI] [PubMed] [Google Scholar]
- 42. Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126(9):1845-1857. [DOI] [PubMed] [Google Scholar]
- 43. Schnabel CA, Selleri L, Cleary ML. Pbx1 is essential for adrenal development and urogenital differentiation. Genesis. 2003;37(3):123-130. [DOI] [PubMed] [Google Scholar]
- 44. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481-490. [DOI] [PubMed] [Google Scholar]
- 45. Achermann JC, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22(2):125-126. [DOI] [PubMed] [Google Scholar]
- 46. Wood MA, Acharya A, Finco I, et al. . Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140(22):4522-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baba T, Otake H, Sato T, et al. . Glycolytic genes are targets of the nuclear receptor Ad4BP/SF-1. Nat Commun. 2014;5:3634. [DOI] [PubMed] [Google Scholar]
- 48. Lalli E, Doghman M, Latre de Late P, El Wakil A, Mus-Veteau I. Beyond steroidogenesis: novel target genes for SF-1 discovered by genomics. Mol Cell Endocrinol. 2013;371(1-2):154-159. [DOI] [PubMed] [Google Scholar]
- 49. Nishimoto K, Rigsby CS, Wang T, et al. . Transcriptome analysis reveals differentially expressed transcripts in rat adrenal zona glomerulosa and zona fasciculata. Endocrinology. 2012;153(4):1755-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nishimoto K, Tomlins SA, Kuick R, et al. . Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112(33):E4591-E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laufer E, Kesper D, Vortkamp A, King P. Sonic hedgehog signaling during adrenal development. Mol Cell Endocrinol. 2012;351(1):19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tissier F, Cavard C, Groussin L, et al. . Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65(17):7622-7627. [DOI] [PubMed] [Google Scholar]
- 53. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985-999. [DOI] [PubMed] [Google Scholar]
- 54. Berthon A, Sahut-Barnola I, Lambert-Langlais S, et al. . Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19(8):1561-1576. [DOI] [PubMed] [Google Scholar]
- 55. Kim AC, Reuter AL, Zubair M, et al. . Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593-2602. [DOI] [PubMed] [Google Scholar]
- 56. Drelon C, Berthon A, Sahut-Barnola I, et al. . PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat Commun. 2016;7:12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang CC, Liu C, Yao HH. Investigating the role of adrenal cortex in organization and differentiation of the adrenal medulla in mice. Mol Cell Endocrinol. 2012;361(1-2):165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pignatti E, Leng S, Yuchi Y, et al. . Beta-catenin causes adrenal hyperplasia by blocking zonal transdifferentiation. Cell Rep. 2020;31(3):107524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mandel H, Shemer R, Borochowitz ZU, et al. . SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet. 2008;82(1):39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heikkilä M, Peltoketo H, Leppäluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143(11):4358-4365. [DOI] [PubMed] [Google Scholar]
- 61. Gummow BM, Winnay JN, Hammer GD. Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin alpha gene. J Biol Chem. 2003;278(29):26572-26579. [DOI] [PubMed] [Google Scholar]
- 62. Mizusaki H, Kawabe K, Mukai T, et al. . Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by Wnt4 in the female developing gonad. Mol Endocrinol. 2003;17(4):507-519. [DOI] [PubMed] [Google Scholar]
- 63. Hossain A, Saunders GF. Synergistic cooperation between the β-catenin signaling pathway and steroidogenic factor 1 in the activation of the mullerian inhibiting substance type II receptor. J Biol Chem. 2003;278(29):26511-26516. [DOI] [PubMed] [Google Scholar]
- 64. Kennell JA, O’Leary EE, Gummow BM, Hammer GD, MacDougald OA. T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with beta-catenin to coactivate C/EBPalpha and steroidogenic factor 1 transcription factors. Mol Cell Biol. 2003;23(15):5366-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Basham KJ, Rodriguez S, Turcu AF, et al. . A ZNRF3-dependent Wnt/β-catenin signaling gradient is required for adrenal homeostasis. Genes Dev. 2019;33(3-4):209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lerario AM, Finco I, LaPensee C, Hammer GD. Molecular mechanisms of stem/progenitor cell maintenance in the adrenal cortex. Front Endocrinol (Lausanne). 2017;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis. 2009;47(9):628-637. [DOI] [PubMed] [Google Scholar]
- 68. Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151(3):1119-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yates R, Katugampola H, Cavlan D, et al. . Adrenocortical development, maintenance, and disease. Curr Top Dev Biol. 2013;106:239-312. [DOI] [PubMed] [Google Scholar]
- 70. Chan LF, Metherell LA, Clark AJL. Effects of melanocortins on adrenal gland physiology. Eur J Pharmacol. 2011;660(1):171-180. [DOI] [PubMed] [Google Scholar]
- 71. Kramer RE, Gallant S, Brownie AC. Actions of angiotensin II on aldosterone biosynthesis in the rat adrenal cortex. Effects on cytochrome P-450 enzymes of the early and late pathway. J Biol Chem. 1980;255(8):3442-3447. [PubMed] [Google Scholar]
- 72. Fujita K, Aguilera G, Catt KJ. The role of cyclic AMP in aldosterone production by isolated zona glomerulosa cells. J Biol Chem. 1979;254(17):8567-8574. [PubMed] [Google Scholar]
- 73. Aguilera G, Capponi A, Baukal A, Fujita K, Hauger R, Catt KJ. Metabolism and biological activities of angiotensin II and des-Asp1-angiotensin II in isolated adrenal glomerulosa cells. Endocrinology. 1979;104(5):1279-1285. [DOI] [PubMed] [Google Scholar]
- 74. Chan LF, Webb TR, Chung TT, et al. . MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci U S A. 2009;106(15):6146-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baccaro RB, Mendonça PO, Torres TE, Lotfi CF. Immunohistochemical Jun/Fos protein localization and DNA synthesis in rat adrenal cortex after treatment with ACTH or FGF2. Cell Tissue Res. 2007;328(1):7-18. [DOI] [PubMed] [Google Scholar]
- 76. Rosenberg D, Groussin L, Jullian E, Perlemoine K, Bertagna X, Bertherat J. Role of the PKA-regulated transcription factor CREB in development and tumorigenesis of endocrine tissues. Ann N Y Acad Sci. 2002;968:65-74. [DOI] [PubMed] [Google Scholar]
- 77. Aesøy R, Mellgren G, Morohashi K, Lund J. Activation of cAMP-dependent protein kinase increases the protein level of steroidogenic factor-1. Endocrinology. 2002;143(1):295-303. [DOI] [PubMed] [Google Scholar]
- 78. Mathieu M, Drelon C, Rodriguez S, et al. . Steroidogenic differentiation and PKA signaling are programmed by histone methyltransferase EZH2 in the adrenal cortex. Proc Natl Acad Sci U S A. 2018;115(52):E12265-E12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chida D, Sato T, Sato Y, et al. . Characterization of mice deficient in melanocortin 2 receptor on a B6/Balbc mix background. Mol Cell Endocrinol. 2009;300(1-2):32-36. [DOI] [PubMed] [Google Scholar]
- 80. Novoselova TV, Hussain M, King PJ, et al. . MRAP deficiency impairs adrenal progenitor cell differentiation and gland zonation. FASEB J. 2018;32(11):fj201701274RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chang SP, Morrison HD, Nilsson F, Kenyon CJ, West JD, Morley SD. Cell proliferation, movement and differentiation during maintenance of the adult mouse adrenal cortex. PLoS One. 2013;8(12):e81865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sasano H, Imatani A, Shizawa S, Suzuki T, Nagura H. Cell proliferation and apoptosis in normal and pathologic human adrenal. Mod Pathol. 1995;8(1):11-17. [PubMed] [Google Scholar]
- 83. Heaton JH, Wood MA, Kim AC, et al. . Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am J Pathol. 2012;181(3): 1017-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sahut-Barnola I, de Joussineau C, Val P, et al. . Cushing’s syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet. 2010;6(6):e1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Morin E, Mete O, Wasserman JD, Joshua AM, Asa SL, Ezzat S. Carney complex with adrenal cortical carcinoma. J Clin Endocrinol Metab. 2012;97(2):E202-E206. [DOI] [PubMed] [Google Scholar]
- 86. Taylor MJ, Ullenbruch MR, Frucci EC, et al. . Chemogenetic activation of adrenocortical Gq signaling causes hyperaldosteronism and disrupts functional zonation. J Clin Invest. 2020;130(1): 83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arnaldi G, Boscaro M. Adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26(4):405-419. [DOI] [PubMed] [Google Scholar]
- 88. Fassnacht M, Arlt W, Bancos I, et al. . Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1-G34. [DOI] [PubMed] [Google Scholar]
- 89. Abiven G, Coste J, Groussin L, et al. . Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91(7):2650-2655. [DOI] [PubMed] [Google Scholar]
- 90. Wajchenberg BL, Albergaria Pereira MA, Medonca BB, et al. . Adrenocortical carcinoma: clinical and laboratory observations. Cancer. 2000;88(4):711-736. [PubMed] [Google Scholar]
- 91. Kohli HS, Manthri S, Jain S, et al. . An adrenocortical carcinoma evolving after nine years of latency from a small adrenal incidentaloma. Cureus. 2021;13(8):e16851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nogueira TM, Lirov R, Caoili EM, et al. . Radiographic characteristics of adrenal masses preceding the diagnosis of adrenocortical cancer. Horm Cancer. 2015;6(4):176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Belmihoub I, Silvera S, Sibony M, et al. . From benign adrenal incidentaloma to adrenocortical carcinoma: an exceptional random event. Eur J Endocrinol. 2017;176(6):K15-K19. [DOI] [PubMed] [Google Scholar]
- 94. Rebielak ME, Wolf MR, Jordan R, Oxenberg JC. Adrenocortical carcinoma arising from an adrenal adenoma in a young adult female. J Surg Case Rep. 2019;2019(7):rjz200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163-169. [DOI] [PubMed] [Google Scholar]
- 96. Weiss LM, Medeiros LJ, Vickery AL. Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. 1989;13(3):202-206. [DOI] [PubMed] [Google Scholar]
- 97. Arlt W, Biehl M, Taylor AE, et al. . Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96(12):3775-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Libé R, Borget I, Ronchi CL, et al. ; ENSAT Network. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol. 2015;26(10):2119-2125. [DOI] [PubMed] [Google Scholar]
- 99. Berruti A, Fassnacht M, Haak H, et al. . Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur Urol. 2014;65(4):832-838. [DOI] [PubMed] [Google Scholar]
- 100. Raymond VM, Everett JN, Furtado LV, et al. . Adrenocortical carcinoma is a Lynch syndrome-associated cancer. J Clin Oncol. 2013;31(24):3012-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Raymond VM, Long JM, Everett JN, et al. . An oncocytic adrenal tumor in a patient with Birt-Hogg-Dubé syndrome. Clin Endocrinol (Oxf). 2014;80(6):925-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Raymond VM, Else T, Everett JN, Long JM, Gruber SB, Hammer GD. Prevalence of germline TP53 mutations in a prospective series of unselected patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(1):E119-E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Mol Cell Endocrinol. 2014;386(1-2):67-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Costa TEJ, Gerber VKQ, Ibañez HC, et al. . Penetrance of the TP53 R337H mutation and pediatric adrenocortical carcinoma incidence associated with environmental influences in a 12-year observational cohort in Southern Brazil. Cancers (Basel). 2019;11(11):1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. de Reyniès A, Assié G, Rickman DS, et al. . Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27(7):1108-1115. [DOI] [PubMed] [Google Scholar]
- 106. Batisse-Lignier M, Sahut-Barnola I, Tissier F, et al. . P53/Rb inhibition induces metastatic adrenocortical carcinomas in a preclinical transgenic model. Oncogene. 2017;36(31):4445-4456. [DOI] [PubMed] [Google Scholar]
- 107. Borges KS, Pignatti E, Leng S, et al. . Wnt/β-catenin activation cooperates with loss of p53 to cause adrenocortical carcinoma in mice. Oncogene. 2020;39(30):5282-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Figueiredo BC, Cavalli LR, Pianovski MAD, et al. . Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2005;90(2):615-619. [DOI] [PubMed] [Google Scholar]
- 109. Almeida MQ, Soares IC, Ribeiro TC, et al. . Steroidogenic factor 1 overexpression and gene amplification are more frequent in adrenocortical tumors from children than from adults. J Clin Endocrinol Metab. 2010;95(3):1458-1462. [DOI] [PubMed] [Google Scholar]
- 110. Doghman M, Karpova T, Rodrigues GA, et al. . Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21(12):2968-2987. [DOI] [PubMed] [Google Scholar]
- 111. Ruggiero C, Doghman-Bouguerra M, Sbiera S, et al. . Dosage-dependent regulation of VAV2 expression by steroidogenic factor-1 drives adrenocortical carcinoma cell invasion. Sci Signal. 2017;10(469):eaal2464. [DOI] [PubMed] [Google Scholar]
- 112. Landwehr LS, Altieri B, Schreiner J, et al. . Interplay between glucocorticoids and tumor-infiltrating lymphocytes on the prognosis of adrenocortical carcinoma. J Immunother Cancer. 2020;8(1):e000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jouinot A, Assié G, Libé R, et al. . DNA methylation is an independent prognostic marker of survival in adrenocortical cancer. J Clin Endocrinol Metab. 2017;102(3):923-932. [DOI] [PubMed] [Google Scholar]
- 114. Assié G, Jouinot A, Fassnacht M, et al. . Value of molecular classification for prognostic assessment of adrenocortical carcinoma. JAMA Oncol. 2019;5(10):1440-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fürstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3(5):298-302. [DOI] [PubMed] [Google Scholar]
- 116. Almeida MQ, Fragoso MCBV, Lotfi CFP, et al. . Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab. 2008;93(9):3524-3531. [DOI] [PubMed] [Google Scholar]
- 117. Barlaskar FM, Spalding AC, Heaton JH, et al. . Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94(1):204-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Haluska P, Worden F, Olmos D, et al. . Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65(4):765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lerario AM, Worden FP, Ramm CA, et al. . The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial. Horm Cancer. 2014;5(4):232-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fassnacht M, Berruti A, Baudin E, et al. . Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426-435. [DOI] [PubMed] [Google Scholar]
- 121. van Erp NP, Guchelaar HJ, Ploeger BA, Romijn JA, den Hartigh J, Gelderblom H. Mitotane has a strong and a durable inducing effect on CYP3A4 activity. Eur J Endocrinol. 2011;164(4):621-626. [DOI] [PubMed] [Google Scholar]
- 122. Kroiss M, Quinkler M, Johanssen S, et al. . Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97(10):3495-3503. [DOI] [PubMed] [Google Scholar]
- 123. Kroiss M, Megerle F, Kurlbaum M, et al. . Objective response and prolonged disease control of advanced adrenocortical carcinoma with cabozantinib. J Clin Endocrinol Metab. 2020;105(5):1461-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Boumahdi S, de Sauvage FJ. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat Rev Drug Discov. 2020;19(1):39-56. [DOI] [PubMed] [Google Scholar]
- 125. Seidel E, Walenda G, Messerschmidt C, et al. . Generation and characterization of a mitotane-resistant adrenocortical cell line. Endocr Connect. 2020;9(2):122-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. LaPensee CR, Mann JE, Rainey WE, Crudo V, Hunt SW III, Hammer GD. ATR-101, a selective and potent inhibitor of Acyl-CoA acyltransferase 1, induces apoptosis in H295R adrenocortical cells and in the adrenal cortex of dogs. Endocrinology. 2016;157(5):1775-1788. [DOI] [PubMed] [Google Scholar]
- 127. Raj N, Zheng Y, Kelly V, et al. . PD-1 blockade in advanced adrenocortical carcinoma. J Clin Oncol. 2020;38(1):71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Head L, Kiseljak-Vassiliades K, Clark TJ, et al. . Response to immunotherapy in combination with mitotane in patients with metastatic adrenocortical cancer. J Endocr Soc. 2019;3(12):2295-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Habra MA, Stephen B, Campbell M, et al. . Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J ImmunoTher Cancer. 2019;7(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Klein O, Senko C, Carlino MS, et al. . Combination immunotherapy with ipilimumab and nivolumab in patients with advanced adrenocortical carcinoma: a subgroup analysis of CA209-538. Oncoimmunology. 2021;10(1):1908771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mota JM, Sousa LG, Braghiroli MI, et al. . Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden: two case reports. Medicine (Baltimore). 2018;97(52):e13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Le Tourneau C, Hoimes C, Zarwan C, et al. . Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J ImmunoTher Cancer. 2018;6(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bedrose S, Miller KC, Altameemi L, et al. . Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J ImmunoTher Cancer. 2020;8(2):e001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lang J, Capasso A, Jordan KR, et al. . Development of an adrenocortical cancer humanized mouse model to characterize anti-PD1 effects on tumor microenvironment. J Clin Endocrinol Metab. 2020;105(1):26-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Marabelle A, Le DT, Ascierto PA, et al. . Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Azad NS, Gray RJ, Overman MJ, et al. . Nivolumab is effective in mismatch repair-deficient noncolorectal cancers: results from arm Z1D-A subprotocol of the NCI-MATCH (EAY131) study. J Clin Oncol. 2020;38(3):214-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Le DT, Durham JN, Smith KN, et al. . Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kiseljak-Vassiliades K, Zhang Y, Bagby SM, et al. . Development of new preclinical models to advance adrenocortical carcinoma research. Endocr Relat Cancer. 2018;25(4):437-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. . Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Borghaei H, Gettinger S, Vokes EE, et al. . Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Taube JM, Klein A, Brahmer JR, et al. . Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Crona J, Beuschlein F. Adrenocortical carcinoma—towards genomics guided clinical care. Nat Rev Endocrinol. 2019;15(9):548-560. [DOI] [PubMed] [Google Scholar]
- 143. Fay AP, Signoretti S, Callea M, et al. . Programmed death ligand-1 expression in adrenocortical carcinoma: an exploratory biomarker study. J Immunother Cancer. 2015;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Trujillo JA, Luke JJ, Zha Y, et al. . Secondary resistance to immunotherapy associated with β-catenin pathway activation or PTEN loss in metastatic melanoma. J Immunother Cancer. 2019;7(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, et al. . β-Catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231-235. [DOI] [PubMed] [Google Scholar]
- 147. Savino W, Mendes-da-Cruz DA, Lepletier A, Dardenne M. Hormonal control of T-cell development in health and disease. Nat Rev Endocrinol. 2016;12(2):77-89. [DOI] [PubMed] [Google Scholar]
- 148. Ince LM, Weber J, Scheiermann C. Control of leukocyte trafficking by stress-associated hormones. Front Immunol. 2018;9:3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Majdic G, Young M, Gomez-Sanchez E, et al. . Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143(2):607-614. [DOI] [PubMed] [Google Scholar]
- 150. Prasad R, Kowalczyk JC, Meimaridou E, Storr HL, Metherell LA. Oxidative stress and adrenocortical insufficiency. J Endocrinol. 2014;221(3):R63-R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pezzi V, Mathis JM, Rainey WE, Carr BR. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol. 2003;87(2-3):181-189. [DOI] [PubMed] [Google Scholar]
- 152. Sirianni R, Seely JB, Attia G, et al. . Liver receptor homologue-1 is expressed in human steroidogenic tissues and activates transcription of genes encoding steroidogenic enzymes. J Endocrinol. 2002;174(3):R13-R17. [DOI] [PubMed] [Google Scholar]
- 153. Acharya N, Madi A, Zhang H, et al. . Endogenous glucocorticoid signaling regulates CD8+ T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity. 2020;53(3):658-671.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ferraz-de-Souza B, Hudson-Davies RE, Lin L, et al. . Sterol O-acyltransferase 1 (SOAT1, ACAT) is a novel target of steroidogenic factor-1 (SF-1, NR5A1, Ad4BP) in the human adrenal. J Clin Endocrinol Metab. 2011;96(4):E663-E668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6(8):1249-1258. [DOI] [PubMed] [Google Scholar]
- 156. Hart MM, Reagan RL, Adamson RH. The effect of isomers of DDD on the ACTH-induced steroid output, histology and ultrastructure of the dog adrenal cortex. Toxicol Appl Pharmacol. 1973;24(1):101-113. [DOI] [PubMed] [Google Scholar]
- 157. Hescot S, Slama A, Lombès A, et al. . Mitotane alters mitochondrial respiratory chain activity by inducing cytochrome c oxidase defect in human adrenocortical cells. Endocr Relat Cancer. 2013;20(3):371-381. [DOI] [PubMed] [Google Scholar]
- 158. Lehmann TP, Wrzesiński T, Jagodziński PP. The effect of mitotane on viability, steroidogenesis and gene expression in NCI-H295R adrenocortical cells. Mol Med Rep. 2013;7(3):893-900. [DOI] [PubMed] [Google Scholar]
- 159. Cai W, Counsell RE, Djanegara T, Schteingart DE, Sinsheimer JE, Wotring LL. Metabolic activation and binding of mitotane in adrenal cortex homogenates. J Pharm Sci. 1995;84(2):134-138. [DOI] [PubMed] [Google Scholar]
- 160. Martz F, Straw JA. The in vitro metabolism of 1-(o-chlorophenyl)-1-(p-chlorophenyl)-2,2-dichloroethane (o,p’-DDD) by dog adrenal mitochondria and metabolite covalent binding to mitochondrial macromolecules: a possible mechanism for the adrenocorticolytic effect. Drug Metab Dispos. 1977;5(5):482-486. [PubMed] [Google Scholar]
- 161. Cai W, Counsell RE, Schteingart DE, Sinsheimer JE, Vaz AD, Wotring LL. Adrenal proteins bound by a reactive intermediate of mitotane. Cancer Chemother Pharmacol. 1997;39(6):537-540. [DOI] [PubMed] [Google Scholar]
- 162. Waszut U, Szyszka P, Dworakowska D. Understanding mitotane mode of action. J Physiol Pharmacol. 2017;68(1):13-26. [PubMed] [Google Scholar]
- 163. Schteingart DE, Sinsheimer JE, Benitez RS, Homan DF, Johnson TD, Counsell RE. Structural requirements for mitotane activity: development of analogs for treatment of adrenal cancer. Anticancer Res. 2012;32(7):2711-2720. [PubMed] [Google Scholar]
- 164. Dominick MA, McGuire EJ, Reindel JF, Bobrowski WF, Bocan TM, Gough AW. Subacute toxicity of a novel inhibitor of acyl-CoA: cholesterol acyltransferase in beagle dogs. Fundam Appl Toxicol. 1993;20(2):217-224. [DOI] [PubMed] [Google Scholar]
- 165. Reindel JF, Dominick MA, Bocan TM, Gough AW, McGuire EJ. Toxicologic effects of a novel acyl-CoA:cholesterol acyltransferase inhibitor in cynomolgus monkeys. Toxicol Pathol. 1994;22(5):510-518. [DOI] [PubMed] [Google Scholar]
- 166. El-Maouche D, Merke DP, Vogiatzi MG, et al. . A phase 2, multicenter study of nevanimibe for the treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(8):2771-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Smith DC, Kroiss M, Kebebew E, et al. . A phase 1 study of nevanimibe HCl, a novel adrenal-specific sterol O-acyltransferase 1 (SOAT1) inhibitor, in adrenocortical carcinoma. Invest New Drugs. 2020;38(5):1421-1429. [DOI] [PubMed] [Google Scholar]
- 168. Burns VE, Kerppola TK. ATR-101 inhibits cholesterol efflux and cortisol secretion by ATP-binding cassette transporters, causing cytotoxic cholesterol accumulation in adrenocortical carcinoma cells. Br J Pharmacol. 2017;174(19):3315-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Belavgeni A, Bornstein SR, von Mässenhausen A, et al. . Exquisite sensitivity of adrenocortical carcinomas to induction of ferroptosis. Proc Natl Acad Sci U S A. 2019;116(44):22269-22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Weigand I, Schreiner J, Röhrig F, et al. . Active steroid hormone synthesis renders adrenocortical cells highly susceptible to type II ferroptosis induction. Cell Death Dis. 2020;11(3):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bergström M, Bonasera TA, Lu L, et al. . In vitro and in vivo primate evaluation of carbon-11-etomidate and carbon-11-metomidate as potential tracers for PET imaging of the adrenal cortex and its tumors. J Nucl Med. 1998;39(6):982-989. [PubMed] [Google Scholar]
- 172. Weber MM, Lang J, Abedinpour F, Zeilberger K, Adelmann B, Engelhardt D. Different inhibitory effect of etomidate and ketoconazole on the human adrenal steroid biosynthesis. Clin Investig. 1993;71(11):933-938. [DOI] [PubMed] [Google Scholar]
- 173. Hahner S, Stuermer A, Kreissl M, et al. . [123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J Clin Endocrinol Metab. 2008;93(6):2358-2365. [DOI] [PubMed] [Google Scholar]
- 174. Hahner S, Kreissl MC, Fassnacht M, et al. . Functional characterization of adrenal lesions using [123I]IMTO-SPECT/CT. J Clin Endocrinol Metab. 2013;98(4):1508-1518. [DOI] [PubMed] [Google Scholar]
- 175. Kreissl MC, Schirbel A, Fassnacht M, et al. . [¹²³I]Iodometomidate imaging in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(7):2755-2764. [DOI] [PubMed] [Google Scholar]
- 176. Hahner S, Kreissl MC, Fassnacht M, et al. . [131I]Iodometomidate for targeted radionuclide therapy of advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2012;97(3):914-922. [DOI] [PubMed] [Google Scholar]
- 177. Hahner S, Hartrampf PE, Mihatsch PW, et al. . Targeting 11-beta hydroxylase with [131I]IMAZA: a novel approach for the treatment of advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2022;107(4):e1348-e1355. [DOI] [PubMed] [Google Scholar]
- 178. Giordano TJ, Kuick R, Else T, et al. . Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15(2):668-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fassnacht M, Terzolo M, Allolio B, et al. . FIRM-ACT Study Group. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189-2197. [DOI] [PubMed] [Google Scholar]
- 180. Mohan DR, Lerario AM, Hammer GD. Therapeutic targets for adrenocortical carcinoma in the genomics era. J Endocr Soc. 2018;2(11):1259-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Mohan DR, Lerario AM, Finco I, Hammer GD. New strategies for applying targeted therapies to adrenocortical carcinoma. Curr Opin Endocr Metab Res. 2019;8:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Turner NC, Ro J, André F, et al. . PALOMA3 Study Group. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219. [DOI] [PubMed] [Google Scholar]
- 183. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417-430. [DOI] [PubMed] [Google Scholar]
- 184. Liang R, Weigand I, Lippert J, et al. . Targeted gene expression profile reveals CDK4 as therapeutic target for selected patients with adrenocortical carcinoma. Front Endocrinol (Lausanne). 2020;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Fiorentini C, Fragni M, Tiberio GAM, et al. . Palbociclib inhibits proliferation of human adrenocortical tumor cells. Endocrine. 2018;59(1):213-217. [DOI] [PubMed] [Google Scholar]
- 186. Bussey KJ, Bapat A, Linnehan C, et al. . Targeting polo-like kinase 1, a regulator of p53, in the treatment of adrenocortical carcinoma. Clin Transl Med. 2016;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kiseljak-Vassiliades K, Zhang Y, Kar A, et al. . Elucidating the role of the maternal embryonic leucine zipper kinase in adrenocortical carcinoma. Endocrinology. 2018;159(7):2532-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Borges KS, Andrade AF, Silveira VS, et al. . The aurora kinase inhibitor AMG 900 increases apoptosis and induces chemosensitivity to anticancer drugs in the NCI-H295 adrenocortical carcinoma cell line. Anticancer Drugs. 2017;28(6):634-644. [DOI] [PubMed] [Google Scholar]
- 189. Papathomas TG, Pucci E, Giordano TJ, et al. . An international Ki67 reproducibility study in adrenal cortical carcinoma. Am J Surg Pathol. 2016;40(4):569-576. [DOI] [PubMed] [Google Scholar]
- 190. Knijnenburg TA, Wang L, Zimmermann MT, et al. . Genomic and molecular landscape of DNA damage repair deficiency across The Cancer Genome Atlas. Cell Rep. 2018;23(1):239-254.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Mateo J, Carreira S, Sandhu S, et al. . DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Moore K, Colombo N, Scambia G, et al. . Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495-2505. [DOI] [PubMed] [Google Scholar]
- 193. Takaya H, Nakai H, Takamatsu S, Mandai M, Matsumura N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci Rep. 2020;10(1):2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Mirza MR, Monk BJ, Herrstedt J, et al. . ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164. [DOI] [PubMed] [Google Scholar]
- 195. Pennisi R, Ascenzi P, di Masi A. Hsp90: a new player in DNA repair? Biomolecules. 2015;5(4):2589-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Siebert C, Ciato D, Murakami M, et al. . Heat shock protein 90 as a prognostic marker and therapeutic target for adrenocortical carcinoma. Front Endocrinol (Lausanne). 2019;10:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Bridges KA, Hirai H, Buser CA, et al. . MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin Cancer Res. 2011;17(17):5638-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJF, Würdinger T. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17(13):4200-4207. [DOI] [PubMed] [Google Scholar]
- 199. Rajeshkumar NV, De Oliveira E, Ottenhof N, et al. . MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17(9):2799-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Srougi V, Bancos I, Daher M, et al. . Cytoreductive surgery of the primary tumor in metastatic adrenocortical carcinoma: impact on patients’ survival. J Clin Endocrinol Metab. 2022;107(4):964-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kleeman SO, Leedham SJ. Not all Wnt activation is equal: ligand-dependent versus ligand-independent Wnt activation in colorectal cancer. Cancers (Basel). 2020;12(11):3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol. 2017;51(5):1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Koo BK, van Es JH, van den Born M, Clevers H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc Natl Acad Sci U S A. 2015;112(24):7548-7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Chatterjee A, Paul S, Bisht B, Bhattacharya S, Sivasubramaniam S, Paul MK. Advances in targeting the WNT/β-catenin signaling pathway in cancer. Drug Discov Today. 2022;27(1):82-101. [DOI] [PubMed] [Google Scholar]
- 206. Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. O’Connell AE, Zhou F, Shah MS, et al. . Neonatal-onset chronic diarrhea caused by homozygous nonsense WNT2B mutations. Am J Hum Genet. 2018;103(1):131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Chai G, Szenker-Ravi E, Chung C, et al. . A human pleiotropic multiorgan condition caused by deficient Wnt secretion. N Engl J Med. 2021;385(14):1292-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gicquel C, Bertagna X, Schneid H, et al. . Rearrangements at the 11p15 locus and overexpression of insulin-like growth factor-II gene in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1994;78(6):1444-1453. [DOI] [PubMed] [Google Scholar]
- 210. Gicquel C, Raffin-Sanson ML, Gaston V, et al. . Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: study on a series of 82 tumors. J Clin Endocrinol Metab. 1997;82(8):2559-2565. [DOI] [PubMed] [Google Scholar]
- 211. Liu J, Kahri AI, Heikkilä P, Voutilainen R. Ribonucleic acid expression of the clustered imprinted genes, p57KIP2, insulin-like growth factor II, and H19, in adrenal tumors and cultured adrenal cells. J Clin Endocrinol Metab. 1997;82(6):1766-1771. [DOI] [PubMed] [Google Scholar]
- 212. Vaz M, Hwang SY, Kagiampakis I, et al. . Chronic cigarette smoke-induced epigenomic changes precede sensitization of bronchial epithelial cells to single-step transformation by KRAS mutations. Cancer Cell. 2017;32(3):360-376.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Venneti S, Thompson CB. Metabolic modulation of epigenetics in gliomas. Brain Pathol. 2013;23(2):217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Petryk N, Bultmann S, Bartke T, Defossez PA. Staying true to yourself: mechanisms of DNA methylation maintenance in mammals. Nucleic Acids Res. 2021;49(6):3020-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. McCabe MT, Davis JN, Day ML. Regulation of DNA methyltransferase 1 by the pRb/E2F1 pathway. Cancer Res. 2005;65(9):3624-3632. [DOI] [PubMed] [Google Scholar]
- 216. Mehdipour P, Murphy T, De Carvalho DD. The role of DNA-demethylating agents in cancer therapy. Pharmacol Ther. 2020;205:107416. [DOI] [PubMed] [Google Scholar]
- 217. Mohan DR, Finco I, LaPensee CR, et al. . SAT-LB34 repressive epigenetic programs reinforce steroidogenic differentiation and Wnt/β-catenin signaling in aggressive adrenocortical carcinoma. J Endocr Soc. 2020;4(Suppl 1):SAT-LB34. [Google Scholar]
- 218. Pappalardi MB, Keenan K, Cockerill M, et al. . Discovery of a first-in-class reversible DNMT1-selective inhibitor with improved tolerability and efficacy in acute myeloid leukemia. Nat Cancer. 2021;2(10):1002-1017. [PMC free article] [PubMed] [Google Scholar]
- 219. Chung C, Sweha SR, Pratt D, et al. . Integrated metabolic and epigenomic reprograming by H3K27M mutations in diffuse intrinsic pontine gliomas. Cancer Cell. 2020;38(3):334-349.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Sweha SR, Chung C, Natarajan SK, et al. . Epigenetically defined therapeutic targeting in H3.3G34R/V high-grade gliomas. Sci Transl Med. 2021;13(615):eabf7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Pozdeyev N, Fishbein L, Gay LM, et al. . Targeted genomic analysis of 364 adrenocortical carcinomas. Endocr Relat Cancer. 2021;28(10):671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Corces MR, Granja JM, Shams S, et al. . Cancer Genome Atlas Analysis Network. The chromatin accessibility landscape of primary human cancers. Science. 2018;362(6413):eaav1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kim KH, Kim W, Howard TP, et al. . SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med. 2015;21(12):1491-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kadoch C, Copeland RA, Keilhack H. PRC2 and SWI/SNF chromatin remodeling complexes in health and disease. Biochemistry. 2016;55(11):1600-1614. [DOI] [PubMed] [Google Scholar]
- 225. Drelon C, Berthon A, Mathieu M, et al. . EZH2 is overexpressed in adrenocortical carcinoma and is associated with disease progression. Hum Mol Genet. 2016;25(13):2789-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22(20):5323-5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Varambally S, Dhanasekaran SM, Zhou M, et al. . The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624-629. [DOI] [PubMed] [Google Scholar]
- 228. Kadoch C, Hargreaves DC, Hodges C, et al. . Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45(6):592-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Bödör C, Grossmann V, Popov N, et al. . EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood. 2013;122(18):3165-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Morin RD, Johnson NA, Severson TM, et al. . Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42(2):181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Knutson SK, Kawano S, Minoshima Y, et al. . Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther. 2014;13(4):842-854. [DOI] [PubMed] [Google Scholar]
- 232. Kamminga LM, Bystrykh LV, de Boer A, et al. . The polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107(5):2170-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Ezhkova E, Pasolli HA, Parker JS, et al. . Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136(6):1122-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21(13):4330-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Lippert J, Appenzeller S, Liang R, et al. . Targeted molecular analysis in adrenocortical carcinomas: a strategy toward improved personalized prognostication. J Clin Endocrinol Metab. 2018;103(12):4511-4523. [DOI] [PubMed] [Google Scholar]
- 236. Garinet S, Nectoux J, Neou M, et al. . Detection and monitoring of circulating tumor DNA in adrenocortical carcinoma. Endocr Relat Cancer. 2018;25(3):L13-L17. [DOI] [PubMed] [Google Scholar]
- 237. Creemers SG, Korpershoek E, Atmodimedjo PN, et al. . Identification of mutations in cell-free circulating tumor DNA in adrenocortical carcinoma: a case series. J Clin Endocrinol Metab. 2017;102(10):3611-3615. [DOI] [PubMed] [Google Scholar]
- 238. Szabó DR, Luconi M, Szabó PM, et al. . Analysis of circulating microRNAs in adrenocortical tumors. Lab Invest. 2014;94(3):331-339. [DOI] [PubMed] [Google Scholar]
- 239. Perge P, Butz H, Pezzani R, et al. . Evaluation and diagnostic potential of circulating extracellular vesicle-associated microRNAs in adrenocortical tumors. Sci Rep. 2017;7(1):5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Beuschlein F, Jakoby J, Mentz S, et al. . IGF1-R inhibition and liposomal doxorubicin: progress in preclinical evaluation for the treatment of adrenocortical carcinoma. Mol Cell Endocrinol. 2016;428:82-88. [DOI] [PubMed] [Google Scholar]
- 241. Hantel C, Shapiro I, Poli G, et al. . Targeting heterogeneity of adrenocortical carcinoma: evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget. 2016;7(48):79292-79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Pinto EM, Morton C, Rodriguez-Galindo C, et al. . Establishment and characterization of the first pediatric adrenocortical carcinoma xenograft model identifies topotecan as a potential chemotherapeutic agent. Clin Cancer Res. 2013;19(7):1740-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Jouinot A, Lippert J, Fassnacht M, et al. . Intratumor heterogeneity of prognostic DNA-based molecular markers in adrenocortical carcinoma. Endocr Connect. 2020;9(7):705-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Gara SK, Lack J, Zhang L, Harris E, Cam M, Kebebew E. Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors. Nat Commun. 2018;9(1):4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Fojo T, Huff L, Litman T, et al. . Metastatic and recurrent adrenocortical cancer is not defined by its genomic landscape. BMC Med Genomics. 2020;13(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Yuan S, Norgard RJ, Stanger BZ. Cellular plasticity in cancer. Cancer Discov. 2019;9(7):837-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Quintanal-Villalonga Á, Chan JM, Yu HA, et al. . Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 2020;17(6):360-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21-45. [DOI] [PubMed] [Google Scholar]
- 250. Sequist LV, Waltman BA, Dias-Santagata D, et al. . Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol. 2018;15(5):271-286. [DOI] [PubMed] [Google Scholar]
- 252. Davies A, Nouruzi S, Ganguli D, et al. . An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat Cell Biol. 2021;23(9):1023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lee JK, Lee J, Kim S, et al. . Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35(26):3065-3074. [DOI] [PubMed] [Google Scholar]
- 254. Aggarwal R, Huang J, Alumkal JJ, et al. . Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36(24):2492-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Lopez JP, Brivio E, Santambrogio A, et al. . Single-cell molecular profiling of all three components of the HPA axis reveals adrenal ABCB1 as a regulator of stress adaptation. Sci Adv. 2021;7(5):eabe4497. [DOI] [PMC free article] [PubMed] [Google Scholar]