Abstract
Anaplasma marginale is an ehrlichial pathogen of cattle, in the order Rickettsiales, that establishes persistent cyclic rickettsemia in the infected host. Within each rickettsemic cycle, A. marginale expressing antigenically variant major surface protein 2 (MSP2) emerge. By cloning 17 full-length msp2 transcripts expressed during cyclic rickettsemia, we determined that emergent variants have a single, central hypervariable region encoding variant B-cell epitopes. The N- and C-terminal regions are highly conserved among the expressed A. marginale variants, and similar sequences define the MSP2 homologues in the agent of human granulocytic ehrlichiosis (HGE). This is in contrast to the MSP2 homologues in ehrlichial genogroup I pathogens, Ehrlichia chaffeensis, Ehrlichia canis, and Cowdria ruminantium, that have multiple hypervariable regions. By defining the variable and conserved regions, we were able to show that the single hypervariable region of A. marginale MSP2 encodes epitopes that are immunogenic and induce variant-specific antibody responses during persistent infection. These findings demonstrate that the MSP2 structural variants that emerge during each cycle of persistent rickettsemia are true antigenic variants, consistent with MSP2 antigenic variation as a mechanism of A. marginale persistence.
Anaplasma marginale, a member of the ehrlichial genogroup II, is an intraerythrocytic pathogen that infects cattle, resulting in severe anemia, abortion, or death (11, 27). Animals that survive acute infection develop lifelong persistent infection (4, 9, 11) and serve as reservoirs for tick transmission of A. marginale (5, 22). Persistent A. marginale infection is characterized by sequential, microscopically undetectable cycles of rickettsemia that rise to levels of 107 rickettsiae/ml of blood followed by a rapid decline to <103 rickettsiae/ml of blood (4, 6, 9). This logarithmic rise in rickettsemia followed by a precipitous decrease during each persistent rickettsemic cycle is similar to acute infection where high-level rickettsemia is controlled by a primary immune response (9). We have proposed that each cycle of persistent rickettsemia reflects emergence of antigenically variant A. marginale that are subsequently controlled by variant-specific primary immune responses (6, 21). Our studies have focused on the A. marginale major surface protein 2 (MSP2), an immunodominant outer membrane protein (6, 12, 16, 17, 26). MSP2 is encoded by a large, polymorphic, multigene family that provides the genetic capacity for variation (3, 16), and we have shown that transcripts encoding unique, polymorphic MSP2 proteins are expressed in sequential rickettsemic cycles (6, 21). Whether these MSP2 structural variants are true antigenic variants and thus contain unique epitopes that induce variant-specific primary immune responses during persistent infection is unknown. If they are, specific antibody to a unique MSP2 variant should be absent at the time of when the variant first emerges but be detectable when the rickettsemic cycle terminates.
To test this hypothesis, we needed to first define the variable regions within expressed MSP2. Sequence comparison of two msp2 genes, 11.2 and DF5, revealed deletions, insertion, and substitutions resulting in polymorphism within the encoded proteins (3). In this initial comparison, the main site of variation was limited to a 37-amino-acid (aa) region (aa 234 to 271 in pCKR 11.2 msp2 [16]) in which sequence identity was 54% (3). In contrast, comparison of transcripts expressed during persistent rickettsemia, done by sequence analysis of 595-bp amplicons derived by reverse transcription-PCR (RT-PCR) (nucleotides 375 to 965 based on pCKR 11.2 msp2), showed a larger region of polymorphism encompassing aa 185 to 277 (6). Whether this is the only polymorphic region expressed in persistent rickettsemia is unknown. The MSP2 outer membrane protein homologues in Ehrlichia chaffeensis, E. canis, and Cowdria ruminantium, members of the ehrlichial genogroup I, all contain one semivariable and three hypervariable regions (14, 15, 19, 20, 23). Our previous analysis of A. marginale MSP2 variation expressed in persistent rickettsemic cycles, based on the central 595-bp amplicon, would not have detected the presence of the semivariable, the N-terminal hypervariable, or the C-terminal hypervariable region (6). Thus, in this study, we first sequenced full-length msp2 transcripts from sequential persistent rickettsemic cycles to identify possible additional regions of A. marginale MSP2 amino acid sequence hypervariability. With this information, we then tested whether antibody was generated to the hypervariable regions of the MSP2 variants that emerge during cyclic rickettsemia.
MATERIALS AND METHODS
Cloning and sequencing of full-length msp2 cDNA.
Total RNA was extracted from whole blood taken at the peak of each rickettsemic cycle and reverse transcribed by using random hexamers, as described previously (6). Primers were derived from the 5′ and 3′ ends of the open reading frames of existing full-length genomic clones, DF5 msp2 and pCKR11.2 msp2 (3, 16). The 5′ and 3′ primers for the full-length msp2 cDNA clones were ATGAGTGCTGTAAGTAATAG and CTAGAAGGCAAACCTAACAC, respectively. PCR products were ligated into pCR2.1 by using a TA cloning kit (Invitrogen). Competent Escherichia coli XL-1 Blue bacteria were transformed with the ligated vector and plated with 5 mM isopropyl-1-β-d-thiogalactopyranoside and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside for blue/white screening. Presence of msp2 inserts in plasmids from transformed colonies was confirmed by restriction digests or PCR. Plasmid DNA was extracted from each clone and sequenced in both directions.
Sequence analysis.
For alignment and presentation of amino acid sequences, the PILEUP and PRETTYBOX programs in Genetics Computer Group package, version 8.1, were used (7). For hydropathic profiles, the Kyte-Doolittle method (10) was used. GenBank accession numbers for ehrlichial MSP2 homologues used in comparisons with A. marginale MSP2 clones are as follows: HGE MSP2a, AF029322 (13); HGE MSP2b, AF029323 (13); HGE MSP2c, AF029323 (13); HGE-44 (p44hge-ijdo), AF037599 (8); HGE rP44 (p44hge-zhi), AF059181 (28); E. chaffeensis p28 U72291 (19); E. chaffeensis OMP1a-f, AF021338 (14); E. canis p30, AF078553 (15); E. canis p30-1 AF078554 (15); and E. canis p30a, AF078555 (15).
Detection of MSP2 variant-specific antibodies.
To determine if emergent MSP2 variants express unique B-cell epitopes recognized by sera obtained at the beginning and end of a rickettsemic cycle, msp2 cDNA from each variant was subcloned and expressed to generate recombinant proteins of the different MSP2 variants. Two of the variant msp2 clones obtained from animal 808 peak 2 (5-10-96 [month-day-year]), designated pk2-4 and pk2-1, were randomly selected from the five variant types expressed during this peak (6) and subcloned in frame into pET19b, and the expressed proteins were designated V4 and V1, respectively. V4 and V1 were expressed as His-tagged proteins and purified on Ni2+-charged columns under denaturing conditions as previously described (6). Serum collected from animal 808 on 4-12-96, 1 month prior to peak 2, and serum collected on 6-4-96, 1 month following peak 2, were adsorbed with two unrelated variants from animal 808, pk3-4 and pk3-6. For adsorption, pk3-4 and pk3-6 were subcloned into pET19b, expressed as His-tagged proteins, and purified as described above. Adsorption was done with 40 μg of the unrelated variants electrophoresed on a sodium dodecyl sulfate (SDS)-polyacrylamide gel, transferred to nitrocellulose, and then incubated with the pre- or post-peak 2 sera diluted 1/500 in TNT (0.01 M Tris, 0.067 M NaCl, 0.05% Tween 20 [pH 8.0]) and 3% bovine serum albumin (Sigma). In addition, 6 μg of each unrelated variant was added to the diluted sera. The adsorbed sera collected pre- and post-peak 2 were tested by Western blotting for reactivity with V4 and V1 MSP2 variants which emerged in peak 2. Briefly, purified V4 and V1 were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. Detection used a 1/500 dilution of each adsorbed serum followed by peroxidase-conjugated protein G and development by enhanced chemiluminescence, as previously described (6).
Pairwise comparison of clones from peak 1 through 3 of animals 808 and 807 demonstrated that several clones from peak 1 in animal 807 recurred in peaks 2 or 3 (6). Western blots were used to test whether variant-specific antibody was generated following the first emergence of recurrent clones and whether an anamnestic response was elicited on reemergence of the recurrent variants. The recurrent msp2 variants, pk3-5 and pk3-6, were subcloned into pET19b, and the expressed proteins were designated V5 and V6, respectively. As described above, V5 and V6 were applied to an SDS-polyacrylamide gel, electrophoresed, and transferred to a nitrocellulose membrane. The membranes were incubated with sera collected from animal 807 on 3-1-96 (pre-peak 1), 5-24-96 (post-peak 1), 7-2-96 (post-peak 2), or 8-20-96 (post-peak 3). Peaks 1 through 3 occurred on 4-19-96, 6-11-96, and 7-26-96, respectively. Sera were first adsorbed as described above, using an unrelated variant, pk2-9, to remove antibody against conserved epitopes. The adsorbed sera were diluted 1/500 in TNT, and detection was done as described above. As a negative control, sera from a seronegative, uninfected animal (96B05 5-9-96) was similarly diluted and adsorbed.
Immunoblotting was performed with monoclonal antibodies (MAbs) specific for bovine immunoglobulin G2 (IgG2) to determine whether this isotype of IgG was induced in the response to emergent variants. The variants V5 and V6 described above were applied to a SDS-polyacrylamide gel, electrophoresed, and transferred to nitrocellulose. For a positive control, 10 μg of A. marginale (Florida strain) was applied in a single lane. The membranes were blocked overnight in 10% horse serum–TNT. Variant-specific bovine sera previously adsorbed as described above were diluted 1/500 in 10% horse serum–TNT. The membranes were incubated first with pre-peak 1 serum, post-peak 1 serum, post-peak 3 serum, or, as a positive control, serum from an outer membrane protein immunized animal (96B09 6-20-96) shown to have MSP2-specific IgG2 (1). As a negative control, serum from a seronegative, uninfected animal (96B05 5-9-96) was similarly diluted and adsorbed. The membranes were incubated for 2 h at room temperature. Following three 10-min washes with TNT, the membranes were incubated for 1 h with murine anti-bovine IgG2 MAb (Serotec Ltd., Oxford, United Kingdom) diluted 1/1,000 with TNT and 2% horse serum (1). Following three 10-min washes in TNT, the membranes were incubated for 1 h at room temperature with peroxidase-conjugated, affinity-purified donkey anti-mouse IgG (heavy and light chains; Jackson Immunoresearch Laboratories, West Grove, Pa.) diluted 1/5,000 in TNT buffer containing 1% horse serum. The membranes were washed three times in TNT and developed by enhanced chemiluminescence.
RESULTS
Cloning and sequencing of full-length msp2 cDNA.
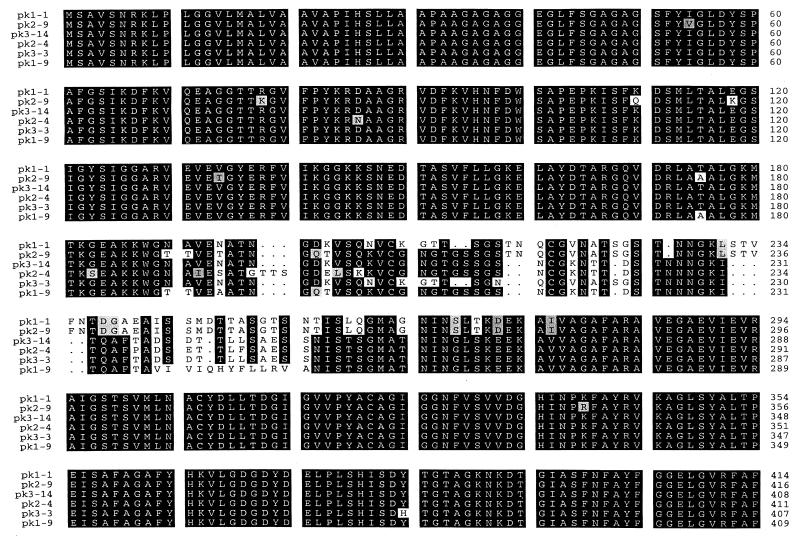
Rickettsemic cycles were previously identified in two Holstein steers, 808 and 807, persistently infected with the Florida strain of A. marginale. Three sequential cycles that occurred at 6- to 8-week intervals in each animal were identified by using msp5 competitive PCR to quantitate rickettsemia and are shown in a prior publication (6). For animal 808, major peaks, defined as ≥106 infected erythrocytes/ml of blood, occurred on 3-12-96, 5-10-96, and 7-9-96. For animal 807, rickettsemia peaked on 4-19-96, 6-11-96, and 7-26-96. To clone the full-length msp2 transcripts, primers were designed based on 5′ and 3′ sequences identically conserved in the pCKR11.2 msp2 and DF5 msp2 genes (3, 16). These sequences are also highly conserved among msp2 homologues in genogroup I and II ehrlichiae (13–15, 19, 20, 23, 25, 28). RT-PCR products were ligated into pCR2.1, individual clones were randomly selected, and the insert cDNA was sequenced. A total of 17 clones were sequenced from animal 808 peak 2 (four clones) and animal 807 peaks 1 (four clones) and 3 (nine clones). These full-length msp2 cDNA clones varied in size, ranging from 1,215 to 1,242 bp, resulting from nucleotide substitutions, insertions, and deletions in a central region spanning nucleotides 540 to 825. All full-length clones obtained from both animals have the predicted open reading frame, with variation in the number of encoded amino acids ranging from 404 to 416 (Fig. 1), compared to 410 aa for the previously described genomic clone pCKR11.2 MSP2 (16). Six representative full-length clones are shown in Fig. 1 and were obtained from peak rickettsemia of three different cycles as follows: pk1-1 and pk1-9 are from the first peak in animal 807, pk2-9 and pk2-4 are from the second peak in animal 808, and pk3-3 and 3-14 are from the third peak in animal 807.
FIG. 1.
Amino acid sequence alignment of full-length A. marginale MSP2 clones expressed during three peaks of cyclic rickettsemia. pk1, pk2, and pk3 refer to persistent rickettsemic cycles; numbers following the hyphens designates specific molecular clones. Areas of amino acid substitutions, insertions, and deletions are indicated by a white background, areas of amino acid identity have a black background, and grey shading indicates conservative amino acid substitutions.
Definition of variable and conserved regions of MSP2.
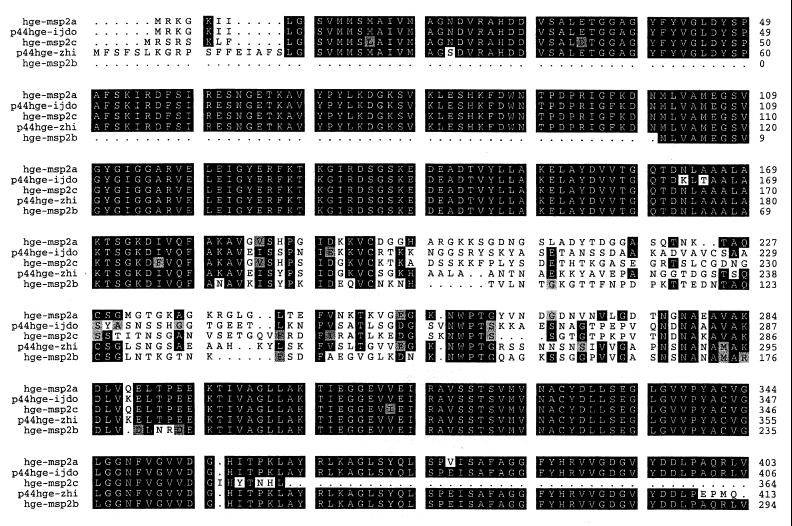
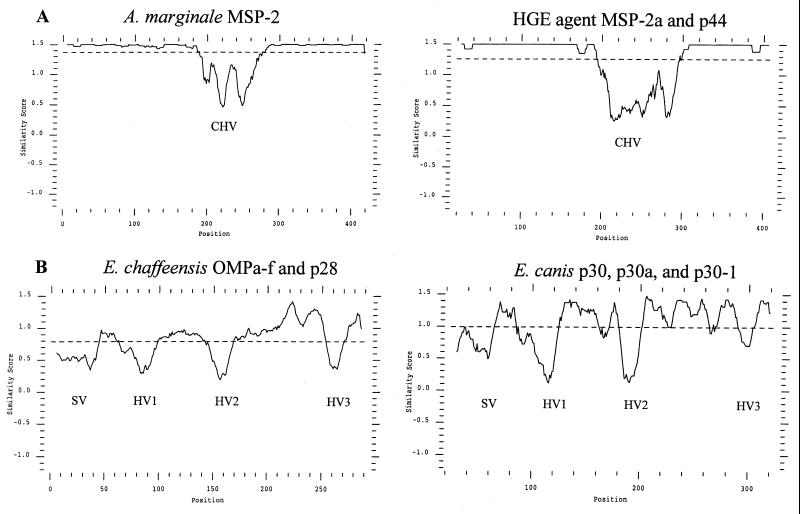
The six representative full-length A. marginale MSP2 clones shown in Fig. 1 have a single, central hypervariable region that spans aa 180 to 275 and is flanked by highly conserved N- and C-terminal regions. Examination of the additional 11 full-length expressed A. marginale MSP2 revealed that each contained only this central hypervariable region (data not shown). The MSP2 amino acid sequences were invariant in the regions corresponding to the semivariable, N-terminal hypervariable, and C-terminal hypervariable regions of E. chaffeensis (14, 19), E. canis (15), and C. ruminantium (19, 20). In contrast, the structure of A. marginale MSP2 variants expressed during persistent rickettsemia is very similar to that defined by sequencing genes encoding HGE (human granulocytic ehrlichiosis) MSP2. Comparison of the HGE MSP2 sequences derived from genes of the USG3[MSP2a,b,c], HZ (isolate 13)[p44hge-zhi], and NCH-1[p44hge-ijdo] strains also revealed a high level of conservation in the N and C termini and the same prominent central hypervariable region (Fig. 2). This single hypervariable region in HGE spans aa 184 to 287 (numbering based on the HGE-44[p44hge-ijdo] [8]). Thus, the MSP2 homologues in genogroup I and II ehrlichiae differ in the number and sites of variation. This is shown graphically by similarity plot analysis in Fig. 3.
FIG. 2.
Amino acid sequence alignment of MSP2 homologues encoded by the agent of HGE. These sequences are derived from genes of the USG3[MSP2a,b,c], HZ (isolate 13)[p44hge-zhi], and NCH-1[p44hge-ijdo] strains of HGE. GenBank accession numbers and references for initial publication of these sequences: MSP2a, AF029322 (13); MSP2b, AF029323 (13); MSP2c, AF029323 (13); p44hge-ijdo (HGE-44), AF037599 (8); p44hge-zhi (rP44), AF059181 (28). Areas of amino acid substitutions, insertions, and deletions are indicated by a white background, areas of amino acid identity have a black background, and grey shading indicates conservative amino acid substitutions.
FIG. 3.
The numbers and positions of the variable regions differ between genogroup I and II ehrlichiae. (A) Plot similarity of amino acid sequences of the genogroup II ehrlichiae based on the 17 full-length expressed A. marginale MSP2 clones and of the HGE MSP2 homologues MSP2b and p44 (p44hge-zhi and p44hge-ijdo). Similarity score is plotted as a function of amino acid position, and the central hypervariable region is designated CHV. The dashed line that transects the y axes at 1.4 (A. marginale) and 1.3 (HGE) indicates the average similarity score for all clones. (B) Plot similarity of amino acid sequences of the genogroup I ehrlichiae E. chaffeensis and E. canis. The hypervariable regions are designated HV1 to HV3, and the single semivariable region is designated SV. The average similarity score is indicated by the dashed lines at 0.58 for E. chaffeensis and 1.0 for E. canis.
The MSP2 homologues have been defined within genogroup I and II ehrlichiae based on overall nucleotide and amino acid similarity. The amino acid similarity between the MSP2 homologues of A. marginale and HGE has been reported as approximately 60% (8, 13, 28). However, this similarity is skewed by the presence of the hypervariable regions. Exclusion of the regions that are hypervariable within each species reveals a much higher similarity. The N-terminal regions (aa 132 to 220) of the 17 expressed full-length A. marginale MSP2 clones were 80 to 96% similar to those regions (aa 41 to 128) in MSP2a and -c from the HGE agent (data not shown). Likewise, comparison of the C-terminal regions of A. marginale (aa 301 to 416) with those of HGE MSP2a (aa 321 to 435), MSP2b (aa 211 to 326), and MSP2c (aa 323 to 364) revealed 85 to 87% similarity. Notably, Kyte-Doolittle analysis of both A. marginale and HGE MSP2 revealed a high probability that the central hypervariable region is surface exposed (data not shown). Within the hypervariable region of A. marginale MSP2 (aa 180 to 275), 74% of the amino acids are hydrophilic, in contrast to 45 and 30% of the amino acids in the conserved N-terminal (aa 1 to 180) and C-terminal (aa 276 to 411) regions, respectively. For HGE MSP2a, the central variable region (aa 184 to 287) has 83% hydrophilicity, compared to 52% over the N-terminal region (aa 1 to 184) and 36% over the C-terminal region (aa 287 to 430).
Variant-specific antibody responses to emergent MSP2 variants.
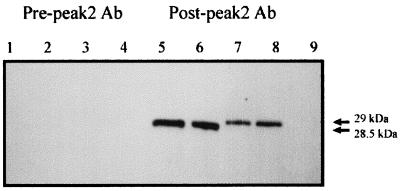
Whether B-cell epitope variation occurs during persistent A. marginale infection and whether variant-specific antibodies are induced are unknown. If expression of unique MSP2 variants within each cycle of persistent rickettsemia reflects emergence of new antigenic variants, B-cell epitopes encoded within the hypervariable region should be recognized by antibody at the end, but not the beginning, of the cycle. This was tested by using two unique, nonrecurrent MSP2 variants that emerged in peak 2 of animal 808. The two emergent variants were randomly selected from the five variant types that occurred during this peak. Sequence comparison of the emergent variants with the 30 msp2 cDNA clones obtained from animal 808 verified that these two variants did not occur in any other peaks (6). By adsorption of sera using MSP2 variants that differ in the hypervariable region, antibody to conserved epitopes can be removed to yield variant-specific sera. Sera obtained 1 month before or 1 month after peak 2 was adsorbed with unrelated variants expressed as His-tagged fusion proteins until the sera were no longer reactive. These unrelated variants, 808 pk3-4 and pk3-6, differ from the emergent variants, V1 and V4, only over the central hypervariable region, aa 180 to 275 (6). The adsorbed serum collected prior to emergence of the peak 2 variants (pre-peak 2) did not react with V1 and V4, whereas serum collected following control of the second cycle (post-peak 2) reacted specifically with V1 and V4 (Fig. 4).
FIG. 4.
Primary variant-specific antibody responses to emergent MSP2 variants V1 and V4. These variants emerged in peak 2 of animal 808. Recombinant V4 (1 μg [lanes 1 and 5] or 2 μg [lanes 2 and 6]) or V1 (1 μg [lanes 3 and 7] or 2 μg [lanes 4 and 8]) or the negative control RAP-1 (lane 9) was purified, separated by SDS-PAGE, and transferred to nitrocellulose. Membranes were reacted with either adsorbed serum obtained before (Pre-peak 2) or after (Post-peak 2) the peak in which V1 and V4 emerged. Antibody (Ab) binding was detected with peroxidase-conjugated protein G and chemiluminescence. Molecular sizes of the expressed V1 and V4 polypeptides are indicated at the right.
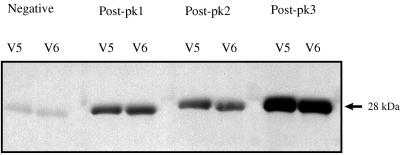
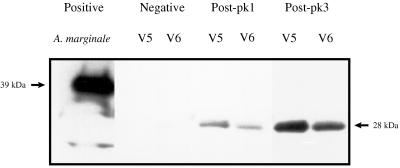
This experiment was repeated with a second set of variants, V5 and V6, derived from the cDNA clones 3-5 and 3-6 identified in peak 1 of animal 807. Variant-specific antibody to V5 and V6 was detected only in serum obtained after resolution of peak 1 (Fig. 5). Neither negative control serum (Fig. 5) nor serum obtained 1 month prior to peak 1 reacted with V5 or V6. This consistent pattern of antibody binding to each of four variants only after control of the rickettsemic cycle demonstrates that the emergent MSP2 are true antigenic variants. In addition, sera obtained following control of peak 3, the peak in which V5 and V6 recurred, had an increased level of specific antibody to the recurrent variants, consistent with an anamnestic response following recurrence (Fig. 5).
FIG. 5.
Primary and anamnestic variant-specific antibody responses to emergent and recurrent variants V5 and V6. These variants emerged in peak 1 of animal 807 and recurred in peak 3. Recombinant V5 and V6 were purified, separated, and tested by Western blotting as described for V1 and V4 in the legend to Fig. 4. Membranes were reacted with adsorbed serum following their initial emergence in peak 1 (Post-pk1), after peak 2 (Post-pk2), and after their recurrence in peak 3 (Post-pk3). Serum from an uninfected calf was used as a negative control (Negative). The molecular size of the expressed V5 and V6 polypeptides is indicated at the right.
Protective immunity against A. marginale has been postulated to require induction of opsonizing IgG2 antibody, and high IgG2 titers have been shown to be associated with clearance of A. marginale rickettsemia (1, 18). To test whether IgG2 was induced in response to the variants, the IgG2 response to V5 and V6 was determined following peaks 1 and 3 in animal 807. IgG2 was first detected following emergence of V5 and V6 in peak 1, with higher levels present following recurrence in peak 3 (Fig. 6). Specific IgG2 was not detected in serum from a seronegative animal used as a negative control. As a positive control, serum from an animal previously shown to generate a specific IgG2 response to MSP2 following immunization with A. marginale outer membrane proteins (1) bound native full-length MSP2 (39 kDa) (Fig. 6).
FIG. 6.
Primary and anamnestic variant-specific IgG2 antibody responses to emergent and recurrent variants V5 and V6. Recombinant V5 and V6 were purified, separated, and tested by Western blotting as described for Fig. 5 except that bound IgG2 was detected with a MAb specific for bovine IgG2. Membranes were reacted with adsorbed serum following their initial emergence in peak 1 (Post-pk1) and after recurrence in peak 3 (Post-pk3). Serum from an immunized calf shown to contain specific IgG2 to MSP2 (1) was reacted with native full-length MSP2 as a positive control (Positive); serum from an uninfected calf was used as a negative control (Negative). The molecular size of the native MSP2 is indicated at the left, and the size of the expressed V5 and V6 polypeptides is indicated at the right.
DISCUSSION
A. marginale MSP2 variation, defined by full-length sequences of 17 transcripts expressed during cyclic rickettsemia, occurs only in a single central region of the protein. The semivariable, N-terminal, and C-terminal variable regions defined by analysis of genes encoding MSP2 homologues in the genogroup I ehrlichiae (E. chaffeensis, E. canis, and C. ruminantium) were not detected in any of the A. marginale MSP2 variants. In contrast, these regions were all highly conserved among the expressed A. marginale MSP2 variants, as well as among the proteins encoded by the previously reported msp2 genes that are polymorphic within and between HGE strains (8, 13, 28). Our analysis excluding the central region that is hypervariable within each genogroup II species revealed a much higher similarity between A. marginale and HGE MSP2 than previously reported. For example, the full-length A. marginale MSP2 pk1-1 shares 72% similarity with HGE MSP2b, whereas pairwise comparison of the N-terminal and C-terminal regions reveals 96 and 87% similarity, respectively. Thus, the MSP2 structure, a single hypervariable region flanked by highly conserved, hydrophobic N and C termini, is a common feature of genogroup II ehrlichiae and is notably different from that of MSP2 homologues in the genogroup I pathogens (14, 15, 19, 20). This finding suggests that the mechanism and role of MSP2 variation may differ between the two genogroups of tick-transmitted ehrlichial pathogens.
MSP2 is encoded in both A. marginale and HGE by multiple, polymorphic genes that are widely distributed throughout the chromosome (16, 29). For HGE, there are an estimated 18 to 20 genes at least partially homologous to msp2 (29), and a similar number can be predicted for A. marginale (16). Transcription of different individual msp2 genes appears to be a significant source of expressed variation (3, 29). However, the detection of multiple unique transcripts in each cycle of persistent A. marginale rickettsemia (6), which occurs every 6 to 8 weeks for years (5, 6), suggests that additional mechanisms to generate variation are required. The structure of msp2 genes provides the basis for homologous recombination, and the presence of small blocks of homology within the hypervariable regions between different msp2 transcripts (Fig. 1) supports gene conversion as a second mechanism of variation. In contrast to A. marginale, very little is known about the temporal expression of HGE MSP2 variants in vivo. As shown in Fig. 2, HGE MSP2 variants are encoded by multiple unique genes within a strain and also between strains (8, 13, 28). Recently, Zhi et al. have shown that the HZ strain (isolate 13) of HGE expresses at least five transcripts of MSP2 (designated P44) in vitro and that variation occurs only in the central region (29). Interestingly, both expression of individual polymorphic genes and transcript splicing were shown to be likely sources of variation in HGE MSP2 (29). Whether these variant transcripts are expressed in the persistently infected reservoir host for HGE, as shown for A. marginale MSP2 during cyclic rickettsemia, is unknown. However, the similarities in both genetic organization and protein structure among A. marginale and HGE support a role for MSP2 variation in HGE persistence.
Whether the MSP2 structural variants expressed in each rickettsemic cycle contain epitopes that induce variant-specific primary immune responses during persistent infection has not been previously addressed. As shown in Fig. 4 and 5, the MSP2 variants that arise in each rickettsemic cycle are unrecognized by the immune system at emergence, and the cycle terminates concomitantly with a primary, variant-specific antibody response. This observation, which was shown for each of the four variants, V1, V4, V5, and V6, is the first demonstration that the expressed MSP2 variants are true antigenic variants. Clearance of A. marginale acute rickettsemia following vaccination is associated with development of antibodies against MSP2 (17, 24) and induction of MSP2-specific, gamma interferon (IFN-γ-secreting CD4+ T cells (1, 2). The proposed mechanism of clearance centers on CD4+ T-cell production of IFN-γ for coordinated activation of B cells for secretion of opsonizing IgG2 antibody and activation of macrophages for opsonization (1, 18). The induction of IgG2 in response to initial emergence of V5 and V6 (Fig. 6) indicates that this IFN-γ mediated class switching also occurs in response to MSP2 variants in persistent infection. Although MSP2-specific, memory CD4+ T cells have been demonstrated following vaccination (1, 2), whether variant-specific T cells are induced during persistent rickettsemia is unknown. However, the increased variant-specific antibody, including IgG2, to recurrent MSP2 variants, as typified by the reactivity of serum from post-peak 3 to V5 and V6, is consistent with a CD4+ T-cell-dependent anamnestic response.
In summary, we have identified the central hydrophilic region as the sole site of MSP2 structural polymorphism among variants expressed during sequential cycles of persistent A. marginale rickettsemia. This is similar to the genomic polymorphism (8, 13, 28) and in vitro expression of variant transcripts (29) shown for the HGE MSP2 homologue and suggests the presence of a common mechanism of variant generation among these genogroup II ehrlichae. Using this defined hypervariable site, we have shown that A. marginale MSP2 structural variants are true antigenic variants that emerge during persistent infection. It remains unknown whether any of the conserved regions of MSP2 can be targeted by the immune system. This will likely be a key question in determining whether effective vaccines can be generated against these antigenically variable pathogens.
ACKNOWLEDGMENTS
This work was supported by NIH grants R01 AI44005 and K08 AI01371.
We thank Donald P. Knowles, Jr., and Travis C. McGuire for helpful discussion and review of the manuscript and Jeff Abbott for assistance with the figures.
We acknowledge Beverly Hunter, Carla Robertson, Kay Morris, and Dauming Zhu for excellent technical assistance.
REFERENCES
- 1.Brown W C, Shkap V, Zhu D, McGuire T C, Tuo W, McElwain T F, Palmer G H. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect Immun. 1998;66:5406–5413. doi: 10.1128/iai.66.11.5406-5413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown W C, Zhu D, Shkap V, McGuire T C, Blouin E F, Kocan K M, Palmer G H. The repertoire of Anaplasma marginale antigens recognized by CD4+ T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect Immun. 1998;66:5414–5422. doi: 10.1128/iai.66.11.5414-5422.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eid G, French D M, Lundgren A M, Barbet A F, McElwain T F, Palmer G H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriks I S, Palmer G H, McGuire T C, Allred D R, Barbet A F. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J Clin Microbiol. 1989;27:279–284. doi: 10.1128/jcm.27.2.279-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriks I S, Stiller D, Palmer G H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol. 1993;31:2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French D M, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genetics Computer Group. Program manual for the GCG package. Madison, Wis: Genetics Computer Group; 1994. [Google Scholar]
- 8.Ijdo J W, Sun W, Zhang Y, Magnarelli L A, Fikrig E. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect Immun. 1998;66:3264–3269. doi: 10.1128/iai.66.7.3264-3269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieser S T, Eriks I S, Palmer G H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990;58:1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 11.Losos G J. Anaplasmosis. In: Losos G J, editor. Infectious tropical diseases of domestic animals. Essex, United Kingdom: Longman House; 1986. pp. 743–795. [Google Scholar]
- 12.McGuire T C, Davis W C, Brassfield A L, McElwain T F, Palmer G H. Identification of Anaplasma marginale long-term carrier cattle by detection of serum antibody to isolated MSP-3. J Clin Microbiol. 1991;29:788–793. doi: 10.1128/jcm.29.4.788-793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy C I, Storey J R, Recchia J, Doros-Richert L A, Gingrich-Baker C, Munroe K, Bakken J S, Coughlin R T, Beltz G A. Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect Immun. 1998;66:3711–3718. doi: 10.1128/iai.66.8.3711-3718.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi N, Nhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1999;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohashi N, Unver A, Zhi N, Rikihisa Y. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol. 1998;36:2671–2680. doi: 10.1128/jcm.36.9.2671-2680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer G H, Oberle S M, Barbet A F, Davis W C, Goff W L, McGuire T C. Immunization with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988;56:1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer G H, Rurangirwa F R, Kocan K M, Brown W C. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol Today. 1999;15:281–286. doi: 10.1016/s0169-4758(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 19.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 20.Reddy G R, Sulsona C R, Harrison R H, Mahan S M, Burridge M J, Barbet A F. Sequence heterogeneity of the major antigenic protein 1 genes from Cowdria ruminantium isolates from different geographical areas. Clin Diagn Lab Immunol. 1996;3:417–422. doi: 10.1128/cdli.3.4.417-422.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rurangirwa F R, Stiller D, French D M, Palmer G H. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc Natl Acad Sci USA. 1999;96:3171–3176. doi: 10.1073/pnas.96.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stich R W, Kocan K M, Palmer G H, Ewing S A, Hair J A, Barron S J. Transstadial and attempted transovarial transmission of Anaplasma marginale by Dermacentor variabilis. Am J Vet Res. 1989;50:1377–1380. [PubMed] [Google Scholar]
- 23.Sulsona C R, Mahan S M, Barbet A F. The map1 gene of Cowdria ruminantium is a member of a multigene family containing both conserved and variable genes. Biochem Biophys Res Commun. 1999;257:300–305. doi: 10.1006/bbrc.1999.0459. [DOI] [PubMed] [Google Scholar]
- 24.Tebele N, McGuire T C, Palmer G H. Induction of protective immunity using Anaplasma marginale initial body membranes. Infect Immun. 1991;59:3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Vliet A H, Jongejan F, van Kleef M, van der Zeijst B A. Molecular cloning, sequence analysis, and expression of the gene encoding the immunodominant 32-kilodalton protein of Cowdria ruminantium. Infect Immun. 1994;62:1451–1456. doi: 10.1128/iai.62.4.1451-1456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidotto M C, McGuire T C, McElwain T F, Palmer G H, Knowles D P. Intermolecular relationships of major surface proteins of Anaplasma marginale. Infect Immun. 1994;62:2940–2946. doi: 10.1128/iai.62.7.2940-2946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker D H, Dumler S J. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormser G P, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–17836. doi: 10.1074/jbc.274.25.17828. [DOI] [PubMed] [Google Scholar]