Abstract
For the development of vaccines against oral and pharyngeal pathogens invading the mucosal epithelia, both secretory and serum immunoglobulin A (IgA) and IgG antibodies and cytotoxic T lymphocytes (CTL) have been induced. We used a novel approach, targeted salivary gland (TSG) immunization, using plasmid pcDNA3/fimA, coding for Porphyromonas gingivalis fimbriae. Expression of subunit protein, fimbrillin, was observed in eukaryotic cells growing in vitro following transfection with pcDNA3/fimA. In this study, we obtained good humoral and cell-mediated immune responses in BALB/c mice by TSG administration using the above-mentioned DNA vaccine. The production of fimbria-specific IgA and IgG antibodies in saliva and serum IgG antibody was significantly stimulated by TSG immunization. Injection of DNA vaccine into salivary gland elicited high-level production of antigen-specific IgG antibody, similar to that induced following intramuscular immunization. The major IgG subclass that recognized fimbriae was IgG2a in serum from pcDNA3/fimA-immunized mice. Reverse transcription-PCR analysis of mononuclear cells from salivary glands showed that levels of Th2 cytokine-specific mRNA were higher in the immunized group than in the nonimmunized group. In addition, TSG DNA immunization resulted in the generation of antigen-specific CTL in spleen. These results indicate that TSG immunization with plasmid DNA may represent a genetic immunization strategy against infection by oral and pharyngeal pathogens that may invade local, mucosal surfaces.
Antigen-encoding plasmid DNA immunization can induce cellular and humoral immune responses against a variety of pathogens, including viruses, parasites, and bacteria (26, 28, 29, 31), and tumor cells (3, 4). In previous studies, most of the plasmid DNA was applied either intramuscularly or intradermally and should be taken up by muscle cells or keratinocytes of the injection site (24, 35). The proteins induced by plasmid DNA vaccines in the cells are similarly processed and presented by major histocompatibility complex class I and II molecules, resulting in the induction of T helper (Th) cells and plasma cells. This process mimics the natural infection by viruses (5, 25).
The mucosal immune system can be functionally divided into two sites, inductive and effector tissues. The immunoglobulin A (IgA)-inductive tissues are where mucosally applied antigens preferentially stimulate CD4+ Th subsets (Th1/Th2 cells) and IgA-committed surface IgA+ B cells. Following mucosal antigen stimulation, these activated lymphocytes leave the inductive site and home to distant mucosal effector tissues via the common mucosal immune system (or pathway) to release secretory IgA (sIgA) specific for the antigen (20). Another advantage of mucosal administration is that systemic immune responses are frequently induced (11, 27). For example, it was shown that oral immunization with a combined vaccine containing tetanus toxoid and cholera toxin induces antigen-specific serum IgG antibodies in addition to sIgA antibodies that can neutralize tetanus toxin (11). Thus, the development of an appropriate mucosal vaccine could lead to the induction of two layers of pathogen-specific immunity in both mucosal and systemic immune compartments.
It was recently shown that local administration of simian immunodeficiency virus p27 in the proximity of the internal iliac lymph nodes, termed targeted lymph node immunization, via the genitourinary-rectal tissues in rhesus macaques results in the generation of p27-specific CD4+ T cells as well as IgA and IgG responses at both mucosal and systemic sites (13, 16, 17). Mucosal immunizations using protein antigens have been reported extensively; however, few studies have used DNA immunization via mucosal or local routes (14, 30, 33). In this study, a comparison was made between targeted salivary gland (TSG) and systemic immunizations using plasmid DNA vaccines. We report here that the former approach may be useful for studying oral and pharyngeal infection and immunity.
MATERIALS AND METHODS
Animals.
Female BALB/c mice (Charles River Japan, Yokohama, Japan) were maintained in horizontal flow cabinets and provided sterile food and water ad libitum. All mice used in this study were 6 weeks of age.
Bacterial strains and growth conditions.
Escherichia coli XL1-Blue (Stratagene, La Jolla, Calif.) was cultured in Luria-Bertani medium or on Luria-Bertani agar supplemented with ampicillin (100 μg/ml). DNA manipulations were carried out according to the manufacturer's instructions.
Preparation of fimbriae and antifimbria antibody.
Fimbriae were prepared as described previously (8, 22). Fimbria-specific antiserum was obtained from rabbits immunized subcutaneously with fimbria protein.
Isolation of mononuclear cells.
A mononuclear cell suspension from spleen was prepared by gentle passage through a sterile stainless steel screen. Mononuclear cells in submandibular glands (SMG) of mice were prepared as described previously (9). Briefly, SMG were digested with collagenase type IV (Worthington Biochemical Corporation, Freehold, N.J.), followed by a discontinuous Percoll gradient consisting of 40, 55, and 75% Percoll. Almost 1 × 106 to 2 × 106 mononuclear cells per mouse with >97% viability were obtained.
Construction of DNA vaccine.
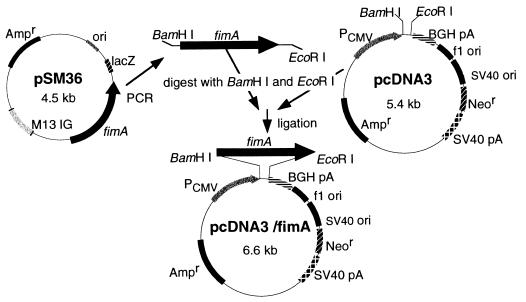
The eukaryotic expression vector pcDNA3 (Invitrogen, Groningen, The Netherlands) with the fimA gene encoding Porphyromonas gingivalis fimbrillin was constructed as shown in Fig. 1. Briefly, the fimA gene was amplified from plasmid pSM36 (8) with primers (sense, 5′-GAGCGAACCCCGCTCCCTGAATTCCGATATAGAC-3′; antisense, 5′-GGATCCTGTTGGGACTTGCTGCTCTTGCTATGACAG-3′) containing a BamHI or EcoRI site. PCR was performed with a model PCR2400 thermal cycler (Perkin-Elmer, Norwalk, Conn.) according to the manufacturer's manual. The resultant fragment was inserted into pcDNA3 predigested with BamHI or EcoRI, and the newly constructed plasmid, pcDNA3/fimA, was used as a DNA vaccine.
FIG. 1.
Construction of a DNA vaccine encoding P. gingivalis fimbriae, using pcDNA3. SV40, simian virus 40; PCMV, cytomegalovirus promoter; BGH, bovine growth hormone gene (provides polyadenylation [pA] signal); Ampr, ampicillin resistance gene; Neor, neomycin resistance gene.
Expression of recombinant fimbriae in transfected cells.
A mouse embryo cell line, NIH 3T3, was transfected with either pcDNA3/fimA or pcDNA3, using Lipofectamine reagent (Life Technologies, Rockville, Md.) according to the manufacturer's instructions. The transfected cells were harvested 48 h later. Samples were mixed with the same volume of 2× sodium dodecyl sulfate (SDS) gel-loading buffer and boiled for 5 min. SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis were performed as described previously (8), with some modifications. Briefly, the proteins were transferred electrophoretically onto a polyvinylidene difluoride membrane (Immobilon; Millipore, Bedford, Mass.), which was saturated in Block Ace (1 μg/ml; Dainippon Pharmaceutical Co., Osaka, Japan). The membrane was further incubated with rabbit antifimbria antibody (1:1,000 dilution), washed three times, and then incubated with alkaline phosphatase-conjugated swine anti-rabbit immunoglobulin serum (Dako, Glostrup, Denmark). 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium solutions (Moss Inc., Pasadena, Md.) were used to reveal positive bands.
Immunization of mice.
Five groups of BALB/c mice were used, as shown in Table 1. We performed three separate experiments.
TABLE 1.
Experimental protocol for immunization of BALB/c mice
| Group (no. in group) | Vaccine used | Days of immunizationa
|
|||
|---|---|---|---|---|---|
| 0 | 14 | 28 | 42 | ||
| I (5) | Fimbriae | s.c. | s.c. | s.c. | s.c. |
| II (18) | pcDNA3/fimA | i.m. | i.m. | i.m. | i.m. |
| III (30) | pcDNA3/fimA | TSG | TSG | TSG | TSG |
| IV (9) | pcDNA3 | TSG | TSG | TSG | TSG |
| V (5) | None | — | — | — | — |
Each vaccine (50 μg) was injected biweekly for a total of four inoculations. Blood, saliva, and fecal samples were collected biweekly through day 84. s.c., injection subcutaneously with emulsion of fimbriae and Freund's complete adjuvant; i.m., injection into the quadriceps muscles with pcDNA3/fimA, using a 27-gauge needle. TSG, injection into the SMG with pcDNA3/fimA, using a 27-gauge needle; —, no immunization.
Antigen-specific ELISA.
Samples were analyzed for fimbria-specific IgG and IgA antibodies by enzyme-linked immunosorbent assay (ELISA) as described previously (13), with some modifications. Briefly, 96-well ELISA plates (Sumitomo Bakelite, Tokyo, Japan) were coated with fimbriae (1 μg/ml) in phosphate-buffered saline and incubated at 4°C. The wells were blocked with phosphate-buffered saline containing Block Ace overnight at 4°C. Duplicate serial two-fold dilutions of samples in an appropriate range for the particular analysis were incubated on the plates overnight at 4°C. The wells were washed and incubated with goat anti-mouse IgA or IgG antibody conjugated with horseradish peroxidase (Southern Biotechnology Associates, Birmingham, Ala.) overnight at 4°C. The wells were then washed and developed with 3,3′,5,5′-tetramethylbenzidine solution (Moss Inc.). After 15 min of incubation, the enzyme reaction was stopped by adding 0.5 N HCl, and the plates were read at an optical density of 450 nm with a microplate reader (model Titertek MK11; Flow Laboratories, McLean, Va.). The endpoint titers for antigen-specific IgG and IgA were defined as the last dilution giving an optical density at 450 nm of ≧0.1.
RT-PCR for cytokine-specific mRNA.
Total RNA was extracted with TRIzol reagent (Life Technologies), and reverse transcription-PCR (RT-PCR) amplification was done according to the established protocol as previously described (13). Primers specific for murine β-actin, gamma interferon (IFN-γ), interleukin-4 (IL-4), IL-5, and IL-6 were obtained from Amersham Pharmacia Biotech (Tokyo, Japan). The primers used were as follows: sequences: β-actin, sense (5′-TGGAATCCTGTGGCATCCATGAAAC-3′) and antisense (5′-TAAAACGCAGCTCAGTAACAGTCCG-3′); IFN-γ, sense (5′-TGAACGCTACACACTGCATCTTGG-3′) and antisense (5′-CGACTCCTTTTCCGCTTCCTGAG-3′); IL-4, sense (5′-ATGGGTCTCAACCCCCAGCTAGT-3′) and antisense (5′-GCTCTTTAGGCTTTCCAGGAAGTC-3′); IL-5, sense (5′-ATGACTGTGCCTCTGTGCCTGGAGC-3′) and antisense (5′-CTGTTTTTCCTGGAGTAAACTGGGG-3′); IL-6, sense (5′-TGGAGTCACAGAAGGAGTGGCTAAG-3′) and antisense (5′-TCTGACCACAGTGAGGAATGTCCAC-3′). The reaction was carried out in a thermal cycler (model PCR2400) under optimal conditions for 33 cycles. The PCR products were electrophoresed on a 2% agarose gel and visualized by staining with ethidium bromide (0.2 μg/ml). The intensity of PCR products was quantified by using NIH Image software (version 1.59; National Institutes of Health, Bethesda, Md.). The level of cytokine-specific mRNA was normalized to the corresponding β-actin signal (37).
Splenocyte proliferation assay.
Mononuclear cells (106 cells/ml) from spleen were added to 96 wells precoated with 100 μl of fimbria solution (final concentration, 10 μg/ml) for 72 h at 37°C in a humid atmosphere of 5% CO2. During the last 16 h of incubation, 0.5 μCi of [3H]TdR incorporation was determined by a liquid scintillation counter (Aloka, Tokyo, Japan). The stimulation index (SI) was defined as the ratio of counts per minute of stimulated and medium-only cultures.
Cytotoxic T-lymphocyte (CTL) assay.
Splenocytes (106 cells/ml) from DNA-vaccinated mice (day 84) were restimulated in vitro with fimbriae (5 μg/ml) as effector cells for 4 days at 37°C (29). Target cells were prepared by adsorption of fimbriae (10 μg/ml) to mastocytoma p815 (5 × 106 cells/ml) for 1 h at 37°C (28). Cytotoxicity was determined by using CytoTox96 (Promega, Madison, Wis.) according to the manufacturer's manual. CytoTox96 quantitatively measures the lactate dehydrogenase (LDH) that is released upon cell lysis. The percentage of specific LDH release was calculated as [(experimental − effector spontaneous − target spontaneous)/(target maximum − target spontaneous)] × 100.
Statistical evaluations.
The significance of differences of the means between the groups was evaluated by a nonparametric Mann-Whitney U test. All conclusions were based on significance levels of P < 0.05.
RESULTS
Expression of the fimA gene and recombinant fimbriae in vitro.
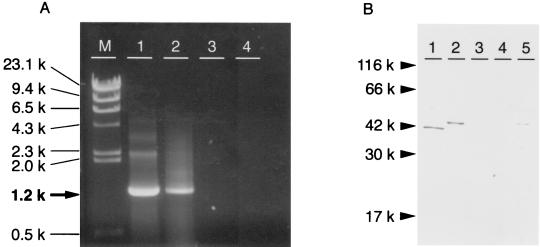
A DNA vaccine, pcDNA3/fimA, was constructed to encode fimbrillin of P. gingivalis (Fig. 1). To ensure that the DNA vaccines were intact and functional, the expression of the plasmid was analyzed in a mammalian cell line, NIH 3T3 (Fig. 2). The fimA-specific mRNA was detected in pcDNA3/fimA transfected but not pcDNA3-transfected cells (Fig. 2A). A protein of ∼43 kDa was expressed in NIH 3T3 cells transfected with pcDNA3/fimA, as detected by immunoblotting with polyclonal rabbit antifimbria serum. Since recombinant fimbriae were detected in the culture supernatant from NIH 3T3 cells transfected with pcDNA3/fimA, a portion of the protein was considered to be secreted extracellularly. On the other hand, no immunoreactive proteins were detected in cultures of pcDNA3-transfected or nontransfected NIH 3T3 cells (Fig. 2B).
FIG. 2.
Expression of the fimA gene and fimbrial protein in NIH 3T3 cells. Plasmid pcDNA3/fimA or pcDNA3 was transfected with Lipofectamine into NIH 3T3 cells. Production of specific mRNA and protein was assessed 48 h later. (A) Total RNA was purified from transfected cells and amplified by RT-PCR using fimA-specific primers. Lanes: M, λ/HindIII size marker; 1, pSM36 containing fimA (positive control); 2, pcDNA3/fimA-transfected NIH 3T3 cells; 3, pcDNA3-transfected cells; 4, NIH 3T3 cells. (B) Protein obtained from NIH 3T3 cells was subjected to SDS-PAGE and Western blotting with rabbit anti-fimbria antibody. Lanes: 1, fimbrial protein (positive control); 2, pcDNA3/fimA-transfected NIH 3T3 cells; 3, pcDNA3-transfected cells; 4, NIH 3T3 cells; 5, culture supernatant of pcDNA3/fimA-transfected NIH 3T3 cells.
Humoral immune responses to DNA vaccines.
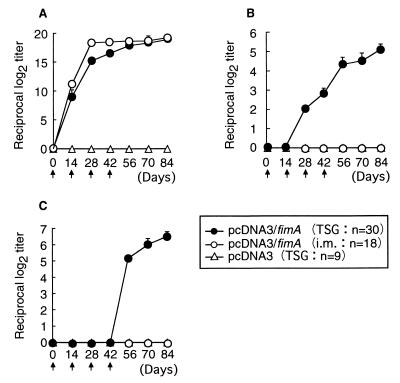
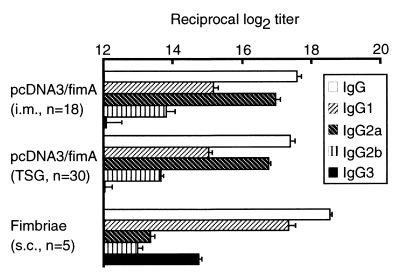
Following the first immunization, fimbria-specific IgG antibody was produced in both pcDNA3/fimA-immunized groups (Fig. 3A), while specific IgA and IgG responses were induced in mice only when TSG immunization was done with pcDNA3/fimA as an antigen (Fig. 3B and C). Two negative control groups that were vaccinated with the uninserted pcDNA3 or saline only did not exhibit any detectable immune responses to fimbriae. A specific IgG response in serum but not IgA or IgG response in saliva was elicited in the mice immunized subcutaneously with the protein (data not shown). Neither fimbria-specific IgA nor IgG antibody was detected in fecal extracts from mice administered TSG immunization with pcDNA3/fimA (<log2 of 3). Specific IgG immune responses in serum from DNA-immunized and fimbria-immunized mice showed similar patterns throughout the experiment from days 0 to 84. However, the major subclass of IgG induced by DNA immunization via either quadriceps muscles or SMG was IgG2a, whereas subcutaneous immunization with purified fimbrial protein induced mainly IgG1 production (Fig. 4).
FIG. 3.
Induction of fimbria-specific IgA and IgG responses in serum and saliva samples from DNA-immunized mice. Each vaccine was injected biweekly as shown by black arrows for a total of four inoculations. For each sample, the endpoint titer of fimbria-specific antibody was defined as described in Materials and Methods. The results are expressed as means ± standard deviations of log2 ELISA antibody titers for triplicate wells. Levels of fimbria-specific IgG antibody in serum (A) and of IgA (B) and IgG (C) in saliva samples were determined. i.m., intramuscular.
FIG. 4.
IgG subclasses against fimbriae induced by immunization with DNA vaccines or purified fimbriae. Mice were immunized by injection intramuscularly into the quadriceps muscles (i.m.) or by injection into SMG (TSG) with 50 μg of pcDNA3/fimA. Mice were also immunized subcutaneously (s.c.) with 50 μg of purified fimbriae. The endpoint titer of fimbria-specific antibody in serum (day 42) was defined as described in Materials and Methods. The results are expressed as means ± standard deviations of log2 ELISA antibody titers for triplicate wells.
Mononuclear cell proliferation and CTL induction in the spleen.
Splenocyte responses to fimbriae in mice administered plasmid DNA were measured 84 days after the first immunization. We observed fimbria-specific proliferation in splenocytes with pcDNA3/fimA but found no proliferative reactions in spleen cells from pcDNA3-immunized or nonimmunized mice (Table 2). Regardless of the immunization route, proliferation occurred in splenocytes from mice immunized with pcDNA3/fimA through quadriceps muscles and SMG glands (SI = 9.3 and 9.5, respectively).
TABLE 2.
Proliferative responses of splenocytes from mice immunized with DNA vaccinea
| Vaccines used (method) | SI (mean ± SD) |
|---|---|
| pcDNA3/fimA (i.m.) | 9.3 ± 0.34 |
| pcDNA3/fimA (TSG) | 9.5 ± 0.11 |
| pcDNA3 (TSG) | 1.4 ± 0.10 |
| None | 1.4 ± 0.05 |
Splenocytes from mice on day 84 were added to 96 wells precoated with fimbria solution for 72 h at 37°C as described in Materials and Methods. During the last 16 h of incubation, 0.5 μCi of [3H]TdR incorporation was determined. The SI was defined as the ratio of counts per minute of stimulated and medium-only cultures. i.m., intramuscular.
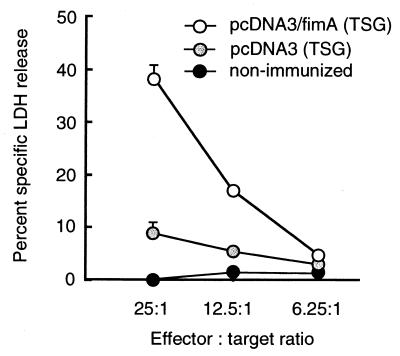
It was essential to determine if TSG immunization with pcDNA3/fimA induces not only fimbria-specific antibody but also antigen-specific CTL in mice (Fig. 5). We found that immunization of mice with pcDNA3/fimA elicited a stronger CTL response in the spleen (P < 0.01) than that with pcDNA3 (38 and 9% specific cell lysis, respectively, at an effector/target cell ratio of 25:1).
FIG. 5.
Fimbria-specific CTL in mice immunized with plasmid DNA. Five mice were chosen randomly from each immunized group (day 84), and the pooled splenocytes were restimulated in vitro with fimbriae as effector cells for 4 days at 37°C. Target cells were prepared by adsorption of fimbriae to mastocytoma p815 for 1 h at 37°C. The amount of LDH released upon cell lysis was determined by using CytoTox96. The results express representative data for three experiments.
Cytokine-specific mRNA expression of mononuclear cells in SMG.
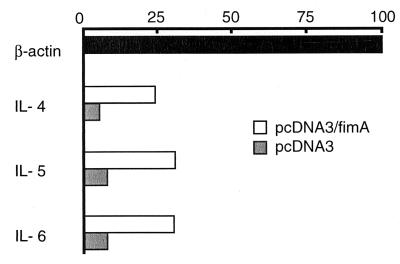
Since cytokines produced by effector cells in SMG influence antigen-specific responses, it was important to examine cytokine-specific mRNA expression of SMG mononuclear cells from mice immunized with plasmid DNA (Fig. 6). Cytokine-specific RT-PCR revealed that SMG cells from pcDNA3/fimA-immunized mice synthesized higher levels of IL-4, IL-5, and IL-6 mRNAs than the pcDNA3-immunized group. Numerous IFN-γ producing cells are present in freshly isolated lymphocytes in mouse SMG. In this experiment, high expression levels of IFN-γ-specific mRNA in SMG mononuclear cells in nonimmunized mice were seen, and no difference between pcDNA3/fimA and pcDNA3 immunized groups could be detected in terms of IFN-γ-specific mRNA expression (data not shown).
FIG. 6.
Cytokine-specific messages from mononuclear cells in the SMG detected by RT-PCR. The RNA samples were obtained from SMG mononuclear cells on day 84 and amplified by RT-PCR. The PCR products were electrophoresed and visualized by ethidium bromide staining. The intensity of PCR products was quantified with NIH Image software. The levels of cytokine mRNA expression were expressed as a relative value based on that of β-actin (=100). The results express representative data for three experiments.
DISCUSSION
Our study demonstrates that both TSG and intramuscular immunization with pcDNA3/fimA can induce humoral and cellular immune responses to P. gingivalis fimbriae. The former immunization especially results in enhanced levels of specific salivary IgA and IgG antibodies (Fig. 3). Eukaryotic expression vectors carrying a cytomegalovirus CMV promoter have been used to induce effective immune responses to antigens in vivo by intramuscular immunization (18, 26, 28, 29). Considerable quantities of recombinant fimbrillin protein were produced in E. coli when the fimA gene was introduced (8). Recombinant fimbrillin was also produced in NIH 3T3 cells transfected with pcDNA3/fimA. These results indicate that the combination of a CMV promoter and the codon usage of the fimA gene should work satisfactorily in eukaryotic cells.
Mucosal immunity is the first line of the defense system against pathogens to prevent systemic or local infection. An important factor for protective mucosal immunity is the induction of specific sIgA antibody. Intramuscular DNA immunization can induce humoral immune responses in systemic (e.g., spleen, blood, and draining lymph nodes) but not mucosal (e.g., salivary glands, genitourinary tracts, and alimentary canals) compartments. Some trials to induce mucosal immune responses have been performed by intravaginal immunization (6, 19). These studies demonstrated that local immune responses such as vaginal IgA and IgG production are induced and maintained following the immunization. Significant levels of fimbriae-specific salivary IgA and IgG as well as serum IgG were observed until at least day 138 following the first TSG immunization (result not shown). Since plasmid vectors containing reporter genes have been shown to persist in muscle for over 1 year (34), strong humoral immune responses to fimbriae in mice with pcDNA3/fimA could be expected.
Since we could demonstrate a similar pattern of serum IgG production and splenocyte proliferative response in mice that had been injected via either muscle or SMG, TSG immunization might be effective as intramuscular DNA immunization against infectious diseases. To examine whether pcDNA3/fimA immunization could induce a Th1- or Th2-type immune response, fimbria-specific serum IgG subclasses were determined (Fig. 4). Mice vaccinated with pcDNA3/fimA via both muscle and SMG predominantly showed IgG2a production, whereas immunization with purified fimbriae protein mainly induced IgG1 antibody. DNA vaccination was found to induce preferentially a Th1 type response (5). Although intramuscular or intranasal immunization with DNA encoding human immunodeficiency virus HIV antigen induces an IgG1>IgG2a-type response (23), the induction of a Th1 type response by DNA immunization may depend on the plasmid vector itself rather than the delivery route and the nature of the antigen.
Our results indicate that TSG DNA immunization elicits mucosal immunity in the oral cavity and that this administration is an effective regimen to induce immunity at this site. The present study did not show specific antibody responses in fecal samples from mice immunized with pcDNA3/fimA, although DNA immunization via oral (12) and Peyer's patch (33) routes induced specific fecal IgA production. Recently, intranasal but not intramuscular immunization with plasmid DNA-lipid complexes has been reported to elicit the production of specific IgA and IgG antibodies in rectal fluid (14). Induction of local immunity at any given site that is used for immunization with a vaccine may be advantageous, because only necessary immune responses are induced.
Under the influence of Th2-type cytokines such as IL-5, IL-6, and IL-10, antigen-activated surface IgA+ B cells develop into IgA-producing cells in effector tissues such as salivary glands and rectourinary tissues (1, 7, 36). Higher levels of Th2 cytokine (IL-4, IL-5, and IL-6) mRNA expression were detected in SMG mononuclear cells from mice TSG-immunized with pcDNA3/fimA than with pcDNA3 or saline (Fig. 6). Since IL-6 has been shown to be a key cytokine for the terminal differentiation of IgA-committed B cells to IgA plasma cells in both murine and human systems (2, 7), the increased expression of IL-6 is consistent with induction of fimbria-specific IgA-producing B-cell responses in addition to systemic IgG responses.
P. gingivalis plays an important role in the initiation and progression of periodontal disease. This bacterium adheres to and invades gingival epithelial cells (15, 32). The adherence and invasion of a fimbria-deficient mutant in human oral epithelial cells had lower efficiencies than those of the wild strain (21). Preincubation of the wild strain with antifimbria antibody resulted in a complete inhibition of the bacterial adherence to epithelial cells. The proportions of IgA, IgM, and IgG are similar between sera and gingival fluid (10). Since TSG immunization with pcDNA3/fimA elicits a fimbria-specific antibody response in systemic and mucosal sites and induces specific CTL, the humoral and cellular immune responses to fimbriae may inhibit bacterial adherence to and invasion of gingival epithelial cells.
The work presented here explores a new area in vaccine development to induce mucosal and systemic immunity. Although safety considerations may limit the use of such vaccines in humans, DNA immunization against pathogens could be applied in animals. Further studies are needed to examine immune responses when this vaccine is codelivered with adjuvants and/or cytokines to a specific mucosal site as well as systemic routes.
ACKNOWLEDGMENTS
This work was supported by grants from the Japan Society for the Promotion of Science and the Ministry of Health and Welfare.
REFERENCES
- 1.Beagley K W, Eldridge J H, Kiyono H, Everson M P, Koopman W J, Honjo T, McGhee J R. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J Immunol. 1988;141:2035–2042. [PubMed] [Google Scholar]
- 2.Beagley K W, Eldridge J H, Lee F, Kiyono H, Everson M P, Koopman W J, Hirano T, Kishimoto T, McGhee J R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA committed B cells. J Exp Med. 1989;169:2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conry R M, LoBuglio A F, Kantor J, Schlom J, Loechel F, Moore S E, Sumerel L A, Barlow D L, Abrams S, Curiel D T. Immune response to a carcinoembryonic antigen polynucleotide vaccine. Cancer Res. 1994;54:1164–1168. [PubMed] [Google Scholar]
- 4.Conry R M, LoBuglio A F, Loechel F, Moore S E, Sumerel L A, Barlow D L, Curiel D T. A carcinoembryonic antigen polynucleotide vaccine has in vitro antitumor activity. Gene Ther. 1995;2:59–65. [PubMed] [Google Scholar]
- 5.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccine. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 6.Fennelly G J, Khan S A, Abadi M A, Wild T F, Bloom B R. Mucosal DNA vaccine immunization against measles with a highly attenuated Shigella flexneri vector. J Immunol. 1999;162:1603–1610. [PubMed] [Google Scholar]
- 7.Fujihashi K, McGhee J R, Lue C, Beagley K W, Taga T, Hirano T, Kishimoto T, Mestecky J, Kiyono H. Human appendix B cells naturally express receptors for and respond to interleukin 6 with selective IgA1 and IgA2 synthesis. J Clin Investig. 1991;88:248–252. doi: 10.1172/JCI115284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara T, Morishima S, Takahashi I, Hamada S. Molecular cloning and sequencing of the fimbrillin gene of Porphyromonas gingivalis strains and characterization of recombinant proteins. Biochem Biophys Res Commun. 1993;197:241–247. doi: 10.1006/bbrc.1993.2467. [DOI] [PubMed] [Google Scholar]
- 9.Hiroi T, Fujihashi K, McGhee J R, Kiyono H. Characterization of cytokine-producing cells in mucosal effector sites: CD3+ T cells of Th1 and Th2 types in salivary gland-associated tissues. Eur J Immunol. 1994;24:2653–2658. doi: 10.1002/eji.1830241113. [DOI] [PubMed] [Google Scholar]
- 10.Holmberg K, Killander J. Quantitative determination of immunoglobulin (IgG, IgA and IgM) and identification of IgA-type in the gingival fluid. J Periodontal Res. 1971;6:1–8. doi: 10.1111/j.1600-0765.1971.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 11.Jackson R J, Fujihashi K, Xu-Amano J, Kiyono H, Elson C O, McGhee J R. Optimizing oral vaccines: induction of systemic and mucosal B cell and antibody responses to tetanus toxoid by use of cholera toxin as adjuvant. Infect Immun. 1993;61:4272–4279. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones D H, Corris S, McDonald S, Clegg J C S, Farrar G H. Poly (dl-lactide-co-glycolide)-encapsulated plasmid DNA elicits systemic and mucosal antibody responses to encoded protein after oral administration. Vaccine. 1997;15:814–817. doi: 10.1016/s0264-410x(96)00266-6. [DOI] [PubMed] [Google Scholar]
- 13.Kawabata S, Miller C J, Lehner T, Fujihashi K, Kubota M, McGhee J R, Imaoka K, Hiroi T, Kiyono H. Induction of Th2 cytokine expression for p27-specific IgA B cell responses after targeted lymph node immunization with simian immunodeficiency virus antigens in rhesus macaques. J Infect Dis. 1998;177:26–33. doi: 10.1086/513811. [DOI] [PubMed] [Google Scholar]
- 14.Klavinskis L S, Barnfield C, Gao L, Parker S. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J Immunol. 1999;15:1858–1868. [PubMed] [Google Scholar]
- 15.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehner T, Bergmeier L A, Tao L, Panagiotidi C, Klavinskis L S, Hussin L, Ward R G, Meyers N, Adams S E, Gearing A J, Brookes R. Targeted lymph node immunization with simian immunodeficiency virus p27 antigen on elicit genital, rectal, and urinary immune responses in nonhuman primates. J Immunol. 1994;153:1858–1868. [PubMed] [Google Scholar]
- 17.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Sambhara S, Li C X, Ewasyshyn M, Parrington M, Caterini J, James O, Cates G, Du R, Klein M. Protection against respiratory syncytial virus infection by DNA immunization. J Exp Med. 1998;188:681–688. doi: 10.1084/jem.188.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livingston J B, Lu S, Robinson H, Anderson D J. Immunization of female genital tract with a DNA-based vaccine. Infect Immun. 1998;66:322–329. doi: 10.1128/iai.66.1.322-329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mestecky J, McGhee J R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 21.Njoroge T, Genco R J, Sojar H T, Hamada N, Genco C A. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect Immun. 1997;65:1980–1984. doi: 10.1128/iai.65.5.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa T, Shimauchi H, Hamada S. Mucosal and systemic immune responses in BALB/c mice to Bacteroides gingivalis fimbriae administered orally. Infect Immun. 1989;57:3466–3471. doi: 10.1128/iai.57.11.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Miyazaki J, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J Immunol. 1997;159:3638–3647. [PubMed] [Google Scholar]
- 24.Raz E, Carson D A, Parker S E, Parr T B, Abai A M, Aichinger G, Gromkowski S H, Singh M, Lew D, Yankauckas M A, Baird S M, Rhodes G H. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson H L, Torres C A T. DNA vaccines. Semin Immunol. 1997;9:271–283. doi: 10.1006/smim.1997.0083. [DOI] [PubMed] [Google Scholar]
- 26.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura S, Ito Y, Asanuma H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross protection against influenza virus infection afforded by trivalent inactivated vaccine inoculation intranasally with cholera toxin as adjuvant. J Immunol. 1992;149:981–988. [PubMed] [Google Scholar]
- 28.Tascon R, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 29.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Dang K, Agadjanyan M G, Srikantan V, Li F, Ugen K E, Boyer J, Merva M, Williams W V, Weiner D B. Mucosal immunization with a DNA vaccine induces immune responses against HIV-1 at a mucosal site. Vaccine. 1997;15:821–825. doi: 10.1016/s0264-410x(96)00259-9. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Refaeli Y, Sato A I, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberg A, Belton C M, Park Y, Lamont R J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winchell J M, Routray S, Betts P W, van Kruiningen H J, Silbart L K. Mucosal and systemic antibody responses to a C4/V3 construct following DNA vaccination of rabbits via the Peyer's patch. J Infect Dis. 1998;178:850–853. doi: 10.1086/515341. [DOI] [PubMed] [Google Scholar]
- 34.Wolff J A, Ludtke J J, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 35.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 36.Xu-Amano J, Kiyono H, Jackson R J, Staats H F, Fujihashi K, Burrows P D, Elson C O, Pillai S, McGhee J R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto M, Kawabata K, Fujihashi K, McGhee J R, Van Dyke T E, Bamberg T V, Hiroi T, Kiyono H. Absence of exogeneous interleukin-4-induced apoptosis of gingival macrophages may contribute to chronic inflammation in periodontal diseases. Am J Pathol. 1996;148:331–339. [PMC free article] [PubMed] [Google Scholar]