Abstract
COVID-19 is the most devastating disease in recent times affecting most people globally. The higher rate of transmissibility and mutations of SARS-CoV-2 along with the lack of potential therapeutics has made it a global crisis. Potential molecules from natural sources could be a fruitful remedy to combat COVID-19. This systematic review highlights the detailed therapeutic implication of naturally occurring glycyrrhizin and its related derivatives against COVID-19. Glycyrrhizin has already been established for blocking different biomolecular targets related to the SARS-CoV-2 replication cycle. In this article, several experimental and theoretical evidences of glycyrrhizin and related derivatives have been discussed in detail to evaluate their potential as a promising therapeutic strategy against COVID-19. Moreover, the implication of glycyrrhizin in traditional Chinese medicines for alleviating the symptoms of COVID-19 has been reviewed. The potential role of glycyrrhizin and related compounds in affecting various stages of the SARS-CoV-2 life cycle has also been discussed in detail. Derivatization of glycyrrhizin for designing potential lead compounds along with combination therapy with other anti-SARS-CoV-2 agents followed by extensive evaluation may assist in the formulation of novel anti-coronaviral therapy for better treatment to combat COVID-19.
Keywords: Glycyrrhizin, SARS-CoV-2, Traditional Chinese medicine, Experimental and computational evidences, Molecular docking interactions
Graphical abstract
1. Introduction
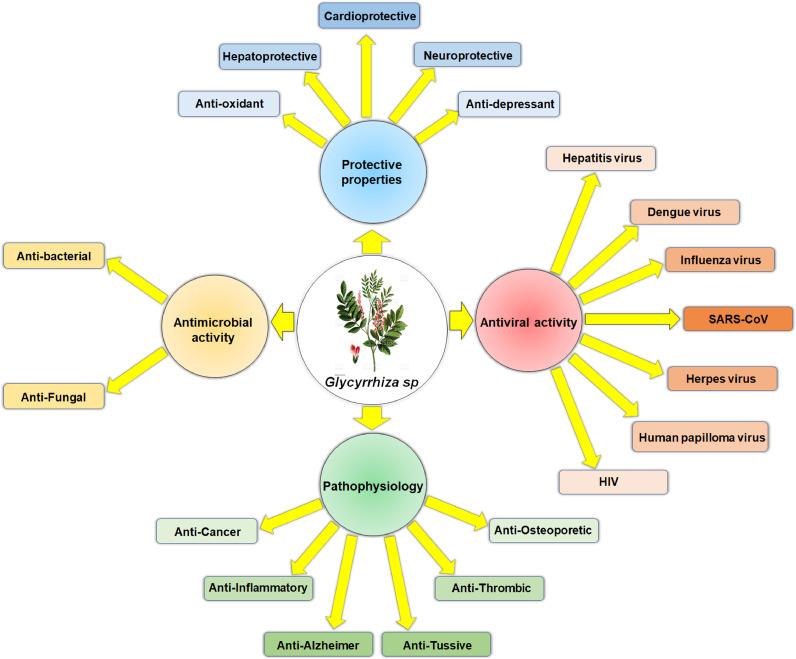
Glycyrrhizin or glycyrrhizic acid (GA) and related terpenoid derivatives (such as glycyrrhetinic acid) are found in the rhizomes of Glycyrrhiza species belonging to the family Fabaceae and possess diverse biological properties [1]. The name ‘Glycyrrhiza’ comes from two Greek words, i.e., ‘glykos’ (sweet) and ‘rhiza’ (root). Therefore, Glycyrrhiza is commonly known as licorice or sweet wood [1]. The components of licorice and different Glycyrrhiza species exert diverse potential functions as anticancer [2,3], antibacterial [4,5], antifungal [6], anti-allergic [7], asthma [8], anti-inflammatory [9], [10], [11], [12], antioxidant [13], [14], [15], hepatoprotective [16,17], cardioprotective [18], neuroprotective [19,20], anti-Alzheimer's [21], antidepressant [22], hypnotic [23,24], antiulcer [25], antitussive and expectorant [26], antithrombotic [27], anti-osteoporotic [28] activities and many more (Fig. 1 ).
Fig. 1.
The potential effect of compounds of Glycyrrhiza species against diverse disease conditions.
Apart from that, molecules obtained from Glycyrrhiza species possess a wide range of antiviral efficacies against different viruses such as hepatitis virus [29], dengue virus [30], influenza virus [31,32], HIV [33], porcine reproductive and respiratory syndrome virus [34], coxsackievirus A16 and enterovirus 71 [35], rotavirus [36,37], duck hepatitis virus [38], human respiratory syncytial virus [39], Japanese encephalitis virus [40], Epstein-Barr virus [41], human papillomavirus [42], bovine viral diarrhea virus [43], porcine epidemic diarrhea virus [44], varicella-zoster virus [45], herpes virus [46] as well as SARS-CoV [47,48] (Fig. 1).
Recently, the emergence of SARS-CoV-2 has led to a global viral outbreak, affecting around 222 countries worldwide and causing a pneumonia-like infection with symptoms such as fever, muscle pain, dry cough, and breathing shortness [49]. To date, about 584 million cases of SARS-CoV-2 infection have been reported worldwide causing a massive number of deaths more than 6.4 million [50]. In this desperate situation, it is of utmost importance to develop newer and more effective anti-coronaviral therapy that can be a salvation to humankind from this deadly COVID-19 disease. In this context, compounds from natural sources may be an effective remedy to discover newer chemical entities against SARS-CoV-2 as a valuable treatment for COVID-19 [48,51,52]. Not only that, recent studies reported that dietary supplements including vitamins and minerals from natural sources along with antiviral drugs can be effectively utilized as adjuvant therapy for COVID-19 [53]. Apart from that, several natural products including many Traditional Chinese Medicines (TCMs) can be considered safer than conventional synthetic antivirals because these are already used as dietary supplements [54]. Among these naturally occurring plant-derived compounds, glycyrrhizin and related derivatives have emerged as the effective source for developing the long-desired remedy for COVID-19 infection [55,56]. Here, in this article, the implication of glycyrrhizin for the treatment of COVID-19 has been discussed in detail in terms of several experimental pieces of evidence as well as computational and intuitive evidence that may be utilized further to develop effective therapies as armaments against COVID-19.
2. A brief overview of SARS-CoV-2 and its related coronaviruses
Coronavirus symbolizing a crown-like appearance is a diverse group of positive single-stranded RNA viruses causing infections in the respiratory and gastrointestinal tract. Coronaviruses belong to the Nidovirales order and Coronaviridae sub-family alike [49,[57], [58], [59]]. Additional codification leads to the ortho-coronaviridae sub-class that with further classification can be sub-categorized into a) alphacoronavirus, b) betacoronavirus, c) gammacoronavirus, and d) deltacoronavirus [57], [58], [59].
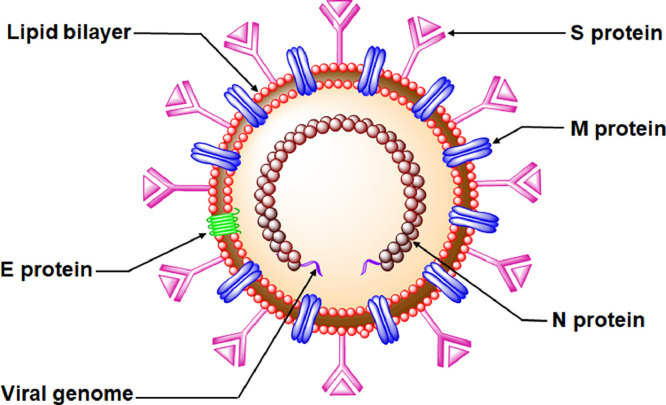
Typically, a single coronavirus virion is spherical with spike-like projections at the envelope surface made up of spike (S) proteins [60,61]. The outer shell of these viruses consists of a lipid bilayer with the envelop (E), membrane (M), and spike (S) proteins. Inside the envelope, the viral genome is found encapsulated by the nucleocapsid (N) phosphoprotein, providing an extra level of protection to the viral RNA [60], [61], [62] (Fig. 2 ).
Fig. 2.
Structural morphology of coronavirus.
The M proteins, assisting in forming the virion assembly, comprise three major domains namely the N-terminal ectodomain and C-terminal endodomain (NTD and CTD) with a conserved domain (CD), and three transmembrane domains situated between the NTD and CTD [60,61]. Integrated into the lipid bilayer, these E proteins contain a hydrophobic transmembrane domain comprising conserved cysteine and proline residues that resides between the N-terminal ectoplasmic residues and C-terminal endoplasmic residues [60,63,64]. E proteins participate in viral replication including germination, envelop formation, virion assembly, and pathogenesis [60,63,64]. The S glycoproteins are anchored into the lipid bilayer for providing the virion a crown-like appearance [60]. In both glycosylated and non-glycosylated forms, S proteins consist of two major subunits (S1 and S2). The S1 subunit aids the binding with the host cell receptors through its receptor-binding domain (RBD). However, the S2 subunit executes the S protein for anchoring to the receptor and assists the entry into the host cell by membrane fusion [60,65,66]. The N phosphoproteins reside inside the envelope and are a part of the helical nucleocapsid of coronavirus [60]. Three well-defined domains are the NTD and CTD and a linker region (LR) between these domains. Apart from encapsulation and protection of viral genetic material, these N proteins not only interact with viral membrane protein during virus assembly but also execute critical roles in viral transcription [60]. Apart from these major viral proteins, other viral accessory proteins are found in coronaviruses assisting in the replication process [60,63].
Coronaviruses contain a large 26–32 kbp 5′-3′ non-segmented positive single-stranded RNA as the genetic material. A major portion (nearly 2/3rd) of the 5′ end contains two overlapping open reading frames (ORF1a and ORF1b) which are responsible for the synthesis of polyprotein 1a and 1ab (pp1a and pp1ab) [49,60,67,68]. The ORF1a encodes 11 nonstructural proteins (nsp1-nsp11) whereas the ORF1ab encrypts 4 nsps (nsp12-nsp16) [49,60]. Besides, the coronaviruses also contain a 5′ untranslated region (UTR) as well as a 3′ UTR with a 3′ poly(A) tail [49,60,69].
2.1. SARS-CoV and MERS-CoV
SARS-CoV is responsible for the severe acute respiratory syndrome (SARS) which is a respiratory tract infection (RTI) in healthy human hosts [70], [71], [72]. Invading from China, 8000 cases along with 774 deaths were reported initially till mid-2003 [71]. The Chinese horseshoe bat was supposed to be the natural reservoir for SARS-CoV and transmittance occurred to humans via civet cats acting as intermediatory hosts [72]. The Middle East respiratory syndrome coronavirus (MERS-CoV) is another betacoronavirus responsible for RTI that emerged in the middle-east countries almost a decade after the appearance of SARS-CoV. The MERS-CoV was transmitted from the dromedary camels to human hosts. Both these viruses are found to produce common symptoms like cough, fever, chills, and headache, as well as diarrhea, nausea, and vomiting. They also exhibit upper RTI and viral shedding [72].
2.2. SARS-CoV-2
SARS-CoV-2 bears around 79% similarity with its predecessor SARS-CoV and has 96% genomic similarity with the bat coronavirus RaTG13 [[58], [59], [60],72,73]. The six ORFs of SARS-CoV-2 are arranged in a replicase (ORF1a/1b)-S-E-M-N arrangement from 5′ to 3′ while ORF1a and ORF1b are the largest [49]. Except for the S protein, both the SARS-CoV and SARS-CoV-2 share almost 90% similarity in their amino acid sequences and the majority of SARS-CoV-2 nsps sequentially resemble the SARS-CoV at least 85%. However, the S protein of SARS-CoV-2 shows around 77% similarity with SARS-CoV [49,[74], [75], [76]].
3. Replication cycle of SARS-CoV-2
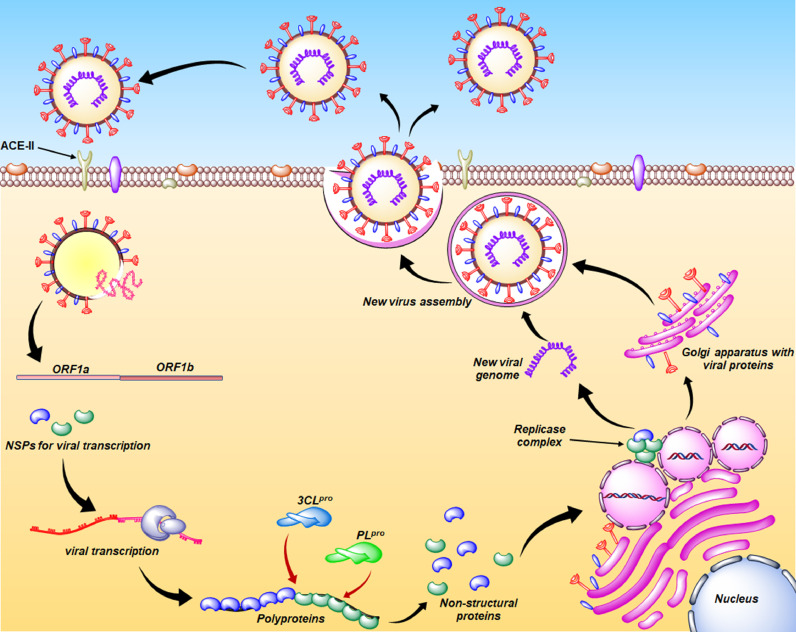
COVID-19 infection in the human host follows several steps from host cell entry to exocytosis (Fig. 3 ).
Fig. 3.
Replication cycle of SARS-CoV-2.
3.1. Host cell entry
In contact with the host cells, the angiotensin-converting enzyme 2 (ACE2) receptor of the host cell surface is targeted by the S1 subunit of the S protein. The RBD of the S1 subunit assists the binding of the S protein to ACE2 while the S2 subunit enables the viral fusion and entry into the host cell [58].
3.2. Genome expression
Upon entering the host cell, through ribosomal translation, the viral genome produces the pp1a and pp1ab from the ORF1a and ORF1b. In the post-transcriptional stage, the cysteine proteases such as the papain-like protease (PLpro) and 3-chymotrypsin-like protease (3CLpro/Mpro) reside inside the nsp3 and nsp5, respectively, and are responsible for the proteolysis of viral polyproteins [60].
3.3. RNA synthesis
These nsps are crucial for performing replication inside the host. The nsp1 targets the host cell machinery while nsp2-nsp11 may assist replication-transcription complexes (RTC). Again, nsp12-nsp16 assist in viral replication among which the RNA-dependent RNA polymerase (RdRp) with co-factor nsp7 and nsp8 executes the viral RNA synthesis process [58,[77], [78], [79], [80]]. Primarily, a negative-sense viral RNA is produced which is utilized as a template to generate new copies of positive-sense viral RNA [58]. Additionally, viral replication is known to occur in the replication organelles inside the host cells where different nsps (like nsp3, nsp4, and nsp6) detract from the host cell endo-membranes to form these replication organelles [58,[81], [82], [83]].
3.4. Accessory synthesis and assembly
The other SARS-CoV-2 ORFs proximal to the 3′ terminal encode the structural proteins (such as the S, M, E, and N) of the viral genome for such protein synthesis. Several accessory genes containing ORFs such as ORF3a, ORF6, ORF7a, ORF7b, and ORF8 along with ORF3b and ORF9b are found in the SARS-CoV-2 genome [58,84,85].
3.5. Exocytosis
Following the synthesis of structural and genomic materials, the M proteins contribute to a majority of protein-protein interactions required for the assembly, and finally, through the exocytosis involving the secretory vesicles, the assembled viruses are released from the host cells to infect a new host [86].
4. Glycyrrhizin and related derivatives
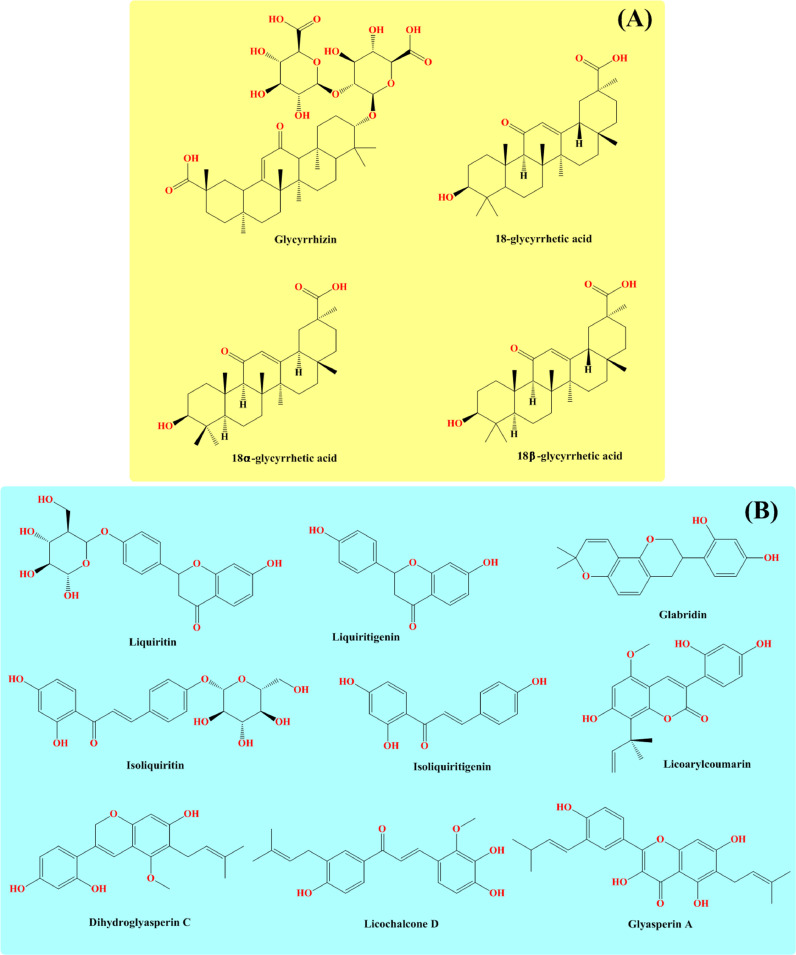
Among the 30 species of Glycyrrhiza, the root and other parts contain a variety of active phytoconstituents including glycosides, tannins, phytosterols, coumarins, various vitamins, saponins, terpenoids, and essential oils [1,[87], [88], [89], [90], [91], [92], [93]]. Among these compounds, the major important compounds are glycyrrhizin, 18α-glycyrrhetinic acid (triterpenoids); liquiritin, liquiritigenin, isoliquiritin, isoliquiritigenin (flavonoids); glabridin (isoflavone); licochalcone A (chalcone); licoarylcoumarin (coumarin) as well as essential oils [13,[94], [95], [96], [97]] (Fig. 4 ).
Fig. 4.
Compounds obtained from Glycyrrhiza species (A) Triterpenoid derivatives (B) Flavones, chalcones, and coumarins.
5. Glycyrrhizin and related terpenoids as effective anti-SARS-CoV/SARS-CoV-2 agents: Experimental evidences
Various studies were conducted earlier to examine the effect of glycyrrhizin and related derivatives on SARS-CoV [47,48]. Initially, the anti-SARS-CoV activities of such compounds in Vero cells were examined [98]. Noticeably, glycyrrhizin displayed better SARS-CoV inhibitory efficacy (EC50 = 300 mg/l) during viral adsorption and penetration compared to the other antivirals namely ribavirin, 6-azouridine, pyrazofurine, and mycophenolic acid with a greater selectivity index (CC50/EC50 > 67). Though the mechanism is unclear, it is supposed to modulate several cellular signaling processes such as modulation of protein kinase C, and casein kinase II along with various transcription factors namely activator protein 1 and NF-κB. Glycyrrhizin may act on SARS-CoV-2 through several mechanisms [99] such as:
-
(a)
by increasing nitric oxide (NO) production in macrophages.
-
(b)
by acting on several transcriptional and cell signaling pathways.
-
(c)
by altering the viral lipid-bilayer membrane.
-
(d)
by strongly binding to the ACE2 enzyme.
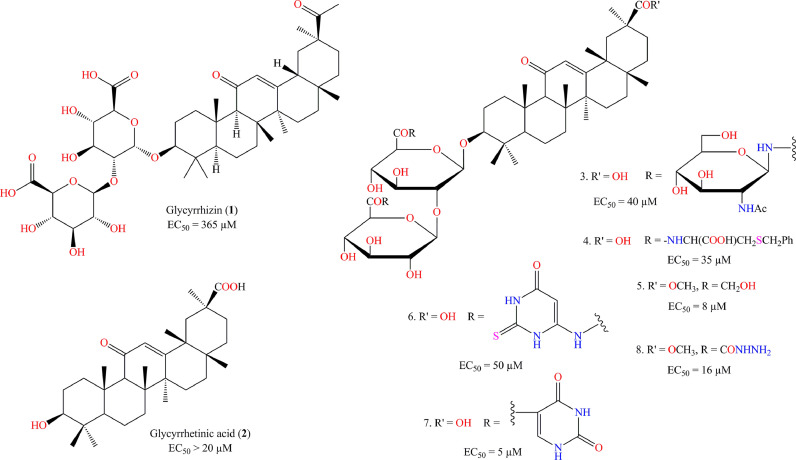
Hoever et al. [100] synthesized 15 glycyrrhizin derivatives and evaluated these against SARS-CoV. Some of these compounds were highly effective anti-SARS-CoV agents better than glycyrrhizin (1, Fig. 5 , EC50 = 365 µM; SI = CC50/EC50 > 65) and glycyrrhetinic acid (2, Fig. 5, EC50 > 20 µM). The N-acetylgycosamine derivative (3, Fig. 5) showed promising SARS-CoV inhibition (EC50 = 40 µM) with a good selectivity index (SI > 75). The 2-acetamido derivative (4, Fig. 5) showed almost similar inhibition (EC50 = 35 µM) compared to the former one but the SI is slightly reduced (SI = 41). Apart from that, the hydroxymethyl derivative (5, Fig. 5), heterocyclic amides (6, 7, Fig. 5) as well as acyl hydrazide derivative (8, Fig. 5) resulted in a potential inhibition of SARS-CoV replication in Vero cells (IC50 range 5–50 µM) along with a good SI value.
Fig. 5.
Synthesized glycyrrhizin derivatives and their activity against SARS-CoV.
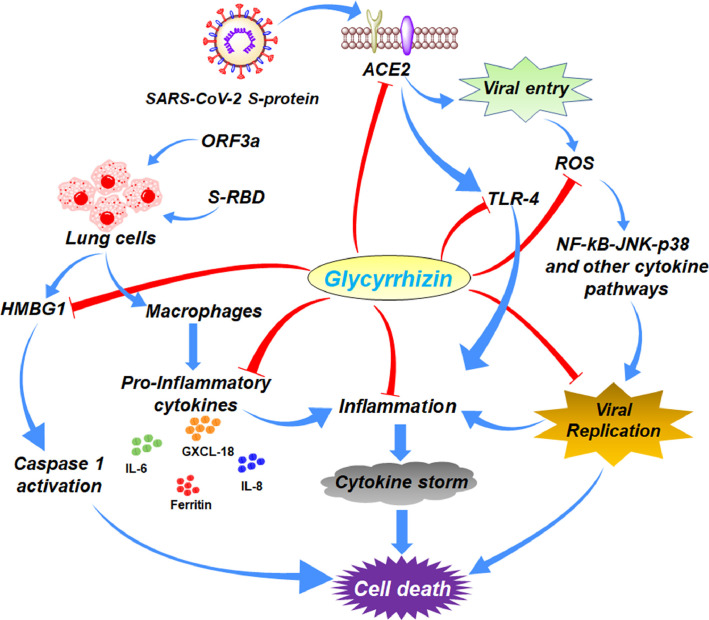
Glycyrrhizin inhibits viral adsorption as well as penetration and was found to exert good efficacy during administration before and after the viral adsorption period [101,102]. It also interferes with the viral replication as well as the cytopathogenicity of several viruses [98,101,103]. Nevertheless, it may be utilized as a cytokine-modulating agent to enhance the immune response where the cytokine storm is highly correlated with the severity of SARS-CoV-2-infected patients with clinical illness [101,104]. COVID-19 patients with cytokine storm subsequently produce acute respiratory distress syndrome (ARDS) including septic shock followed by multiple organ failure [101,105]. Glycyrrhizin by inhibiting the reactive oxygen species (ROS) can reduce the activation of several signaling factors (such as NF-κB, JNK, p38, etc.) related to the viral replication process [101,103,106] (Fig. 6 ).
Fig. 6.
Summary of the possible mode of action of glycyrrhizin against SARS-CoV-2 infection.
Nevertheless, being a thrombin inhibitor, glycyrrhizin may also be able to prevent the coagulation process related to multi-organ failure during COVID-19 infection [27,101]. Again, due to the inhibitory effect on airway mucus production through MUC5AC gene transcription, glycyrrhizin may relieve anorexia in SARS-CoV-2 infection [101,107]. Furthermore, glycyrrhizin may also be able to trigger the endogenous interferon and subsequently hinder viral replication and stimulate the immune cell population in viral replication [101,108]. It has been proposed that glycyrrhizin triggers cholesterol-mediated disorganization of lipid rafts that is crucial for SARS-CoV-2 entry into the host cells [109]. Again, glycyrrhizin binds to the high-mobility-group box1 (HMGB1) DNA binding site to reduce coronavirus-mediated inflammation and viral replication. Therefore, glycyrrhizin alone or in combination with hydroxychloroquine and tenofovir may be effective as a potential antiviral agent against SARS-CoV-2.
Glycyrrhizin may also reverse the S-RBD and the release of ORF3a-induced extracellular HMGB1 and subsequently, may abrogate caspase-1 activation and prevent lung cell death [110] (Fig. 6). Again, glycyrrhizin showed efficacy in dampening overall macrophage activation by reducing viral protein-induced expression of markers related to M1 and M2 phenotypes. Nevertheless, glycyrrhizin prevents S-RBD and ORF3a-conditioned media-induced expression of proinflammatory cytokines such as IL-6, IL-1β, IL8, and CXCL18 in macrophages (Fig. 6). Glycyrrhizin also decreases the release of ferritin (a major intracellular iron storage protein regulating various inflammatory mediators) from macrophages (Fig. 6). Treatment with glycyrrhizin resulted in significant dose-dependent inhibition of SARS-CoV-2 replication as evidenced by qRT-PCR for SARS-CoV-2 envelop (E) and nucleocapsid (N) proteins.
Furthermore, Q-PCR analysis of ACE2 mRNA in Calu-3 cells pointed out that glycyrrhizin inhibited the HMGB1-induced ACE2 mRNA expression in vitro in both dose and time-dependent manner in SARS-CoV-2 infection [111]. A number of Chinese medicines consisting of Glycyrrhiza have been tested for diagnosis and treatment and are supposed to modulate or inhibit viral invasion, adsorption, and replication process (Fig. 6) [112]. As per the historical records, research evidence, and current use of Chinese medicine for the prevention of COVID-19, Glycyrrhiza Radix et Rhizome is one of the potential and promising herbs that can be effective for the treatment of COVID-19 [113].
The guidelines for pattern identifications of herbal formulae effective against COVID-19 have recently been analyzed [114,115]. Interestingly, Glycyrrhiza Radix et Rhizome was the most frequently used herb as per the Chinese guidelines and it is effective in treating COVID-19 patients in mild, moderate, severe, and recovery stages. Further investigations revealed that Glycyrrhiza Radix et Rhizome, a component of different Chinese medicine may be able to reduce clinical manifestations (such as fever, fatigue, chills, dry cough, upper respiratory tract infection, gastrointestinal complications, myalgia, etc.) of COVID-19 infection [114,115]. The network analysis also revealed that Glycyrrhiza Radix et Rhizome should be used with Citri Reticulata Pericarpium as the medication for COVID-19. Nevertheless, Glycyrrhiza Radix et Rhizome or licorice root is one of the top five ranked Chinese herbs effective for the management of COVID-19 [116].
Sini decoction consisting of Glycyrrhiza glabra as one of the components is effective in preventing Escherichia coli-induced acute lung injury by decreasing the inflammatory mediators as well as by reducing the expression of ACE and angiotensin II receptors and thus, effective in preventing SARS-CoV-2 infection [117]. Again, glycyrrhizin in the form of diammonium glycyrrhizinate has effectively been used as enteric-coated capsules for the treatment of COVID-19 in open-label clinical trials [51]. Recently, it has been found that diammonium glycyrrhizinate in combination with vitamin C may be effective for curing patients with SARS-CoV-2 infection during self-quarantine [118]. In a randomized, controlled, open clinical trial in Hubei, China, diammonium glycyrrhizinate in combination with vitamin C was approved for the treatment of COVID-19 though it is still not clinically used [119]. Again, it has been displayed that there is a moderate to high rate of abnormal liver functions (38.1% alanine aminotransferase, 54.4% aspartate aminotransferase, 66.7% lactic dehydrogenase, 27.2% γ-glutamyl aminotransferase, 25.9% alkaline phosphatase, 27.2% superoxide dismutase) for COVID-19 patients [120]. Moreover, for 50.3% of patients, there is an abnormal increase in alanine aminotransferase and lactic dehydrogenase. Though there was no significant decrease in these two enzymes for severe COVID-19 patients, serum level of alanine aminotransferase and aspartate aminotransferase was found to be quite lower after treatment with diammonium glycyrrhizinate.
The formulation of Glycyrrhiza glabra was able to enhance the pulmonary function of asthma patients compared to prednisolone as found in clinical trials [121]. It was also proven that glycyrrhizin enhanced the survival time for influenza virus-infected mice along with inhibiting the proliferation of SARS-CoV. Apart from that, glycyrrhizin was found to inhibit influenza virus growth as well as reduced inflammatory cytokines and the cytopathic effect conferred by the respiratory syncytial virus [47,97]. Though there are some complications for hypertension and kidney disease patients, it has been assessed that glycyrrhizin alone provides relief of respiratory symptoms, and produces a soothing effect on the respiratory tract with greater clinical implications. Thus, the safety rate is presumably higher for asthmatic patients [97]. Therefore, the use of Glycyrrhiza glabra is suggested to be safe and superior compared to the reference drug molecules due to their effective clinical use as adjuvant treatment in early or mild cases of COVID-19 [97].
The aqueous licorice root extract exhibited the antiviral effect against SARS-CoV-2 at a lower concentration (2 mg/ml) in the Vero E6 cell line [122]. Interestingly, glycyrrhizin at a lower concentration (0.5 mg/ml for pre-entry condition and 1 mg/ml for post-entry condition) was found to obstruct the SARS-CoV-2 replication without any toxicity at a dose of 4 mg/ml. After 48 h of treatment, glycyrrhizin showed an EC50 of 0.44 mg/ml and significantly reduced SARS-CoV-2 RNA level in Vero E6 cells. Moreover, glycyrrhizin was found to exert 70.3% and 100% inhibition of SARS-CoV-2 3CLpro at 30 µM (0.024 mg/ml) and 2000 µM (1.6 mg/ml) respectively, to inhibit viral replication. Again, magnesium isoglycyrrhizinate which belongs to the 4th generation of glycyrrhizin preparations is capable of exerting various pharmacological activities including antiviral, anti-inflammatory, antioxidant, and immunomodulatory properties, and protects several vital organs such as the kidney, heart, and lungs [123]. Magnesium isoglycyrrhizinate may be a supportive therapy in the “Management Standard for Mild and Common Patients of COVID-19” which is issued by the National Health Commission of the People's Republic of China and the National Administration of Traditional Chinese Medicine. Therefore, magnesium isoglycyrrhizinate may play an active role clinically in combating mild to critical illness, preventing organ failure, and reducing mortality in COVID-19.
Glycyrrhizin also binds to the ACE2 enzyme and blocks the entry of SARS-CoV-2 as well as inhibits the viral replication process [124]. Again, the experimental NanoBiT-based SARS-CoV-2 S-RBD/ACE2 interaction with glycyrrhizin exhibited that it disrupted the binding interaction at a lower concentration (IC50 = 22 µM) [125]. The NanoLuc luciferase inhibition also suggested that glycyrrhizin was not a false positive hit. Therefore, it is strongly suggested that glycyrrhizin should be a promising inhibitor of SARS-CoV-2 S-RBD/ACE2 (Fig. 6). In addition, glycyrrhizin was found to be nontoxic when evaluated in mouse aorta muscle cells (MASMCs) (CC50 > 100 µM) as well as normal human lung cells 16HBE (CC50 > 100 µM). Due to exerting a potential inhibitory effect on SARS-CoV-2 S-RBD and ACE2 as well as being a nontoxic and effective inhibitor of SARS-CoV and MERS-CoV, glycyrrhizin is supposed to be a promising hit candidate with broad-spectrum anticoronaviral effects.
The extract of Glycyrrhiza Radix et Rhizome was found to inhibit phytohemagglutinin-mediated proliferation in human peripheral blood mononuclear cells (PBMCs) as well as the production of TNF-α, IL-10, and INF-γ [126]. It also significantly inhibited LPS-induced NO production in the macrophages of the mouse. Therefore, Glycyrrhiza has been suggested to produce direct action on inflammation [127]. Again, glycyrrhizin is found to modulate the signaling pathways namely protein kinase C, casein kinase II, NF-κB, and activator protein 1. Nevertheless, the aglycone metabolite of glycyrrhizin, i.e., 18β-glycyrrhetinic acid upregulates the NO synthase and subsequently, enhances the production of NO in macrophages [128,129]. Glycyrrhizin also decreases the interleukins (namely IL-4, IL-5, and IL-13), increases interferon-γ (INF-γ) levels in the bronchiolar lavage fluid as well as inhibits ovalbumin-induced eosinophilia and increases regulatory T cells in lung tissues [130]. Moreover, isoliquiritigenin and naringenin enhance Treg cell induction and promote and execute therapeutic efficacy in autoimmune and inflammatory diseases [131]. Again, glycyrrhizin, a major component of Ma Xing Shi Gan (MXSG) decoction, was found to decrease the release of IL-6 markedly from LPS-induced macrophages [132]. It was also found to inhibit Toll-like receptor (TLR)-induced IL-6 production in RAW 264.7 cells.
Glycyrrhetinic acid, a metabolite of glycyrrhizin, was found to inhibit 11β-hydroxysteroid dehydrogenase (11β-HSD) which allows cortisol to access mineralocorticoid receptor (MR) in tissues where aldosterone is found in lung, kidney, nasal route endothelial cells [133]. Inhibition of 11β-HSD increases aldosterone activation of MR through cortisol. Further, higher aldosterone level downregulates ACE2 in the kidney expressing 11β-HSD2 in the lung, epithelial cells, and other entry points for COVID-19 [133,134]. Apart from that, the serine protease TMPRSS2 expression is regulated by glycyrrhetinic acid [133,135]. Moreover, the TLR4 antagonism by glycyrrhetinic acid decreases inflammatory responses in the lung [133,136]. On the other hand, glycyrrhizin reduces TLR expression in the lung and heart and subsequently reduces cytokine release [133,137] and demonstrates a protective effect in TLR-mediated ARDS [136]. Again, glycyrrhizin reduces TLR4 expression to induce anti-inflammatory activity via a downstream regulation of ACE2 [138]. Nevertheless, glycyrrhizin exerts similar anti-inflammatory mechanisms in the central nervous system (CNS) [139,140] that may be effective in a neurological phenomenon related to COVID-19. From the analysis of the Glycyrrhiza containing Xiao Chai Hu decoction (XCHD) formulation against SARS-CoV-2, it has been summarized that although active ingredients present in the XCHD formulation were ineffective against alveolar inflammation, they can execute several actions against SARS-CoV-2 infection that includes immunomodulation, inactivation of SARS-CoV-2 invasion pathway and can inhibit cytokine storm [141].
Nevertheless, glycyrrhizin along with intravenous immunoglobulin may be effective in treating COVID-19 infection along with severe ARDS [142]. It has been examined that highly compatible glycyrrhizin nanoparticles (GANPs) inhibit the proliferation of murine coronavirus MHV-A59 [143]. Moreover, GANPs significantly decreased the MHV-A59 mRNA expression in a dose-dependent manner. Treatment with GANPs (24 mg per kg body weight) on five female BALB/c mice for seven days via i.v. route significantly reduced the inflammatory responses and decreased the ROS level. Moreover, the GANPs target these areas with severe inflammation and increased the efficacy of treatment as seen in the in vivo MHV-A59 surrogate mouse model. GANPs not only exert antiviral and anti-inflammatory effects but also relieve organ damage to confer a significant survival advantage to COVID-19-infected mice. Again, it has further been proposed that licorice root membrane may be utilized to produce a bio-based antiviral face mask to limit COVID-19 spreading [144]. Again, based on the carrier stability scoring (CSS) method, glycyrrhizin was selected as the potential compound against SARS-CoV-2 [145] and was subjected to the formulation of micelle nanoparticles. In combination with nafamostat mesylate micelle, it was observed that these two compounds yielded synergistic activity against viral infection.
6. Glycyrrhiza-containing TCMs and COVID-19
TCM is a popular and effective method of treatment that was originated and practiced in China over years [146]. It is an aggregated process of treatment that includes different techniques such as acupuncture, Chinese herbal products/medicines (CHMs), tai-chi, etc. In the current scenario, TCM has been a major part of anti-COVID-19 treatment and has been claimed to have a promising role in alleviating disease-related mortality, symptoms, and recurrence including the improvement of symptom management in patients [116,[146], [147], [148]].
The active ingredients of Glycyrrhiza were suggested to be effective for the treatment of SARS-CoV-2 [147]. In combination with naturally-occurring compounds like baicalin, hesperidin, and nicotinamide, glycyrrhizin has been used against SARS-CoV-2 for binding with ACE2 [147,148]. Glycyrrhizin can also exhibit hormone-like anti-inflammatory and antiviral effects. It inhibits SARS viral replication and can reduce late-hormone withdrawal incidence in COVID-19 patients [98,149]. Again, the LHQW formulation was found to act against COVID-19 via multiple mechanisms and suggested a good binding affinity of Glycyrrhetinic acid toward ACE2 [150].
To identify the influences of the HSBD formulation against COVID-19, Cai et al. [151] investigated 223 active ingredients including the glycyrrhetinic acid, glycyrrhiza flavanol A, the ammonium salt of glycyrrhizin, liquiritin, glabridin, and licochalcone B from the formation against 358 HSBD targets, 5555 ACE2 related targets, COVID-19 targets and 84 common targets for complex diseases that include ACE, HDAC1, ESR1, and ADRA1A. This study revealed the good binding affinity of the HSBD bioactive components to the SARS-CoV-2 3CLpro. In a systematic literature analysis of QFPD formulation, Ren et al. [152] summarized the prime targets of QFPD and their mechanisms against SARS-CoV-2. Glycyrrhizin was suggested to be a prime ingredient for blocking viral entry and inhibiting cytokine storm.
7. An overlook on Glycyrrhiza-containing TCMs in clinical studies
As the CHMs have been majorly used in the treatment of COVID-19 in China, several clinical evaluations were also performed on these Glycyrrhiza/non-Glycyrrhiza containing TCM formulations to evaluate their efficacy against SARS-CoV-2 [116,147,[151], [152], [153], [154], [155], [156]]. The TCMs/CHMs containing glycyrrhiza tested in clinical studies are summarized in Table 1 .
Table 1.
Summary of the glycyrrhiza-containing TCMs/CHMs in clinical studies.
| Sl No | Study Type | Study Size | The intervention of glycyrrhiza containing TCM/CHM | Study Design | Treatment duration | Adverse effect | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Randomized | 80 | 6 gm JHQG granules twice per day | Test: 44 Control: 36 |
7 days | No | [156] |
| 2 | – | 123 | 10 gm JHQG granules thrice per day | Test: 82 Control: 41 |
5 days | Yes | [147] |
| 3 | Retrospective | 102 | 6 gm LXQW granules thrice per day | Test: 51 Control: 51 |
7 days | No | [157] |
| 4 | Retrospective | 101 | 6 gm LXQW granules thrice per day | Test: 63 Control: 38 |
10 days | yes | [158] |
| 5 | Randomized | 283 | LXQW granules (6 gm per bag) one bag thrice per day, LXQW (6 gm per bag) thrice per day with Houzian zhengqi drop pills (2.6 gm per bag) one bag twice per day | LXQW: 94 Houzian zhengqi with LXQW: 95 Western medicine: 94 |
14 days | No | [159] |
| 6 | Randomized | 284 | LHQW capsules (1.4 gm) 4 capsules thrice per day | Test: 142 Control: 142 |
14 days | No | [160] |
| 7 | Randomized | 14 | LHQW 4.2 gm | – | 8 days | – | [161] |
| 8 | Retrospective | 70 | SFJD capsules of 6 gm thrice a day | Test: 40 Control: 30 |
10 days | No | [162] |
| 9 | – | 200 | SFDJ capsules of 6 gm thrice per day with arbidor (antiviral) tablet | Test: 100 Control: 100 |
14 days | Yes | [164] |
| 10 | Retrospective | 68 | SFJD | – | 7 days | No | [166] |
| 11 | – | 80 | MXSG | – | 5–7 days | – | [165] |
| 12 | – | 214 | QFPD | – | 3 days | – | [167] |
| 13 | – | 100 | Quinfei Touxie Fuzheng recipe as one dose per day | Test: 51 Control: 49 |
10 days | Yes | [168] |
| 14 | Retrospective | 662 | Mahuang Liu Jun Tang decoction as 200 ml oral administration twice per day | Test: 484 Control: 178 |
– | – | [169] |
| 15 | Randomized | 60 | Licorice-based formulation (760 mg thrice per day) | Test: 30 Control: 30 |
7 days | Yes | [172] |
| 16 | – | 68 | Glycyrrhiza containing CHM as 1 dose per day | Test: 53 Control: 15 |
10 days | Yes | [170] |
| 17 | – | 67 | Glycyrrhiza containing Chinese herbal medicine | Test: 49 Control: 18 |
6 days | No | [116] |
| 18 | – | 52 | Glycyrrhiza containing Chinese herbal medicine | Test: 32 Control: 18 |
10 days | Yes | [171] |
| 19 | – | 20 | Glycyrrhiza containing Chinese herbal medicine | Test: 10 Control: 10 |
7 days | No | [116] |
| 20 | – | 517 | Glycyrrhiza containing Chinese herbal medicine | Test: 249 Control: 268 |
9 days | No | [116] |
| 21 | Non-Randomized | 70 | Licorice extract (250 mg capsules containing 62.5 mg glycyrrhizin) as a dietary supplement | – | 10 days | – | [173] |
| 22 | Randomized | 120 | Glycyrrhiza Radix containing dietary supplement | – | 3–6 weeks | – | [173] |
| 23 | Non-Randomized | 2000 | Glycyrrhiza-containing combination product | – | 3 weeks | – | [173] |
| 24 | Case-Only | 150 | Licorice (6 gm) containing CHM | – | 9 months | – | [173] |
In a randomized clinical trial on a group of 80 COVID-19 patients [156], treatment with 6 gm JHQG granules twice per day for 7 days exhibited a reduced viral nucleic acid detection (56.82% and 27.78% for the treatment and control cohorts, respectively), shortening of pneumonia reduction time with higher viral clearance compared to the controlled cohort without any significant adverse reactions. In another study, additional treatment of JHQG for 5 days in 123 patients demonstrated a superior fever disappearance rate (85%) compared to the control (58%) [116,147].
A clinical study on 102 COVID-19 patients was conducted by LXQW granules where a 7-day treatment on patients showed improvements with a reduction of symptoms like fatigue, fever, and cough [116,157]. In a retrospective cohort trial, LXQW formulation while tested thrice daily on 101 COVID-19 patients, suggested a significant decrease in disease-related symptoms compared to the control sub-cohort [116,158]. Another clinical study (ChiCTR2000029601) dealt with 283 patients treated by a combination of Western medicine and LXQW granules or combined therapy of Western medicine, LXQW granules, and Huozian zhengqi dropping pills for 14 days. The results suggested the benefits of using Huozian zhengqi dropping pills and the LXQW granules besides Western medicine for reducing severe illness, vomiting, nausea, and sore limbs [159]. In an open-label randomized controlled study (ChiCTR2000029434), the anti-COVID-19 effects of the LHQW capsule were tested on 284 patients without any serious adverse effects except elevated alanine aminotransferase or aspartate aminotransferase levels. Also, when compared to the controlled group, the cohort with 14 days of LHQW treatment (4 capsules thrice per day) induced an increase in recovery rate along with improvements in symptom recovery time, lung CT results, and clinical cure rate [160]. On the other hand, during the identification of anti-COVID-19 activities of LHQW components, it was suggested [161] that glycyrrhizin can demonstrate high in vivo exposure and binding affinity for the ACE2 enzyme. In this randomized, open, single-center multi-dose study (Clinical trial registration No: 2019LS-002), 14 healthy patients were treated with a 4.2 gm dose of LHQW capsules for 8 days. From this study, it was reported that, besides other constituents of LHQW, glycyrrhizin exhibited high affinity (KD = 4.39 μM/l) toward ACE2.
The capabilities of the SFJD capsules against the SARS-CoV-2 were evaluated in a group of 70 patients for a 10-day treatment showing positive outcomes against disease-related symptoms and virus nucleic acid conversion [162]. In this retrospective study, 7 days of treatment of SFJD in 68 patients reported an excellent efficacy rate (91.2%) without any adverse effects compared to the controlled (70.6%) group [163]. In another study conducted on 200 participants, 14 days of SFJD treatment improved the clinical cure rate and lowered the chances of common patients developing severe disease compared to the controlled cohort indicating the benefits of SFJD treatment against COVID-19 [164].
The treatment of MXSG in 80 patients for 5–7 days reported 95% efficacy with a disappearance rate of 96.8%, 100%, and 81.8% for disease-related symptoms like fever, fatigue, and cough, respectively [147,165,166]. Also, the treatment of QFPD as a 3-day course in 214 patients exhibited an excellent (>90%) efficacy rate. Among these patients, the 98 patients having treatment of 9 days were reported to show a total efficacy rate of around 92% [147,167]. In another study, the evaluation of Qingfei touxie fuzheng recipe as a treatment of one dose per day was performed on a group of 100 COVID-19 patients where the efficacy of the formulation in reduction of disease-related symptoms and improvements in lung CT results were reported [116,168].
In a single-center retrospective cohort study (ChiCTR2000030719), the anti-COVID-19 effects of a Glycyrrhiza containing CHM have been tested on 662 patients with severe illness. A lesser number of disease-related deaths were reported in the TCM-treated cohort compared to the controlled/non-TCM cohort (13 vs 36 deaths). Moreover, a further multifunctional adjustment in the TCM treatment improved the mortality reduction rate compared to the controlled/non-TCM cohort [169].
In another study, the efficacy of Glycyrrhiza containing CHM was diagnosed in a group of 68 patients for 10 days. The treatment resulted in improvements in viral nucleic acid conversion, weight conversion, and lung CT results while improving WBC, CRP-H, IL-6, and LYM status compared to the controlled cohort [116,170]. In a clinical study, the effects of a Glycyrrhiza-containing CHM formulation were evaluated in 67 COVID-19 patients who exhibited improvements in mild to critical cases including fever reduction [116]. Another Glycyrrhiza-based CHM formulation while evaluated in 52 patients for 10 days, exhibited fever reduction, improvements in clinical cure rate, and a lower rate of disease severity compared to the Western medicine-treated cohort [171]. In a clinical study of 20 patients, a Glycyrrhiza containing CHM formulation demonstrated positive effects against COVID-19 by improving clinical symptom reduction and lung CT results. Again, another study for 9 days aided improvements in clinical cure rate, and symptom reduction when tested on 517 patients [116].
A randomized controlled trial (IRCT20200506047323N2) on 60 COVID-19 patients with a licorice-derived herbal formulation D-Reglis® by Irandarouk Pharmaceutical Company, Iran (760 mg 3 times per day for 1 week), reduced the clinical signs and symptoms of COVID-19 including dry cough, fever, thrombocytopenia, and lymphocytopenia [172].
Furthermore, in a non-randomized clinical trial (NCT04487964), the licorice capsules containing 25% glycyrrhizin (62.5 mg) have been evaluated as a dietary supplement against COVID-19 in a cohort of 70 participants [173]. Such supplementary treatment with glycyrrhizin decreased additional respiratory support with the rapid recovery of patients.
In a phase-I and phase-II non-randomized clinical study (NCT04846010) of 2000 patients, Glycyrrhiza Radix et Rhizome, in combination with other herbal ingredients was used as an anti-inflammatory formulation (PurWet®) to combat the COVID-19 related inflammations [173]. For the post-COVID-19 treatment (NCT04544605), licorice (6 g) was also evaluated with other herbal ingredients as Chinese medicine in an estimated group of 150 patients [173].
8. Effective binding of glycyrrhizin and related terpenoids with SARS-CoV-2 protein targets: theoretical and hypothetical evidences
Apart from experimental studies, several molecular modeling studies also pointed out the potential efficacies of glycyrrhizin and related compounds in SARS-CoV-2 inhibition. These compounds are found to bind different SARS-CoV-2 proteins as evidenced by several molecular modeling studies. Therefore, these observations further justify that glycyrrhizin and related derivatives may be potential anti-SARS-CoV-2 agents.
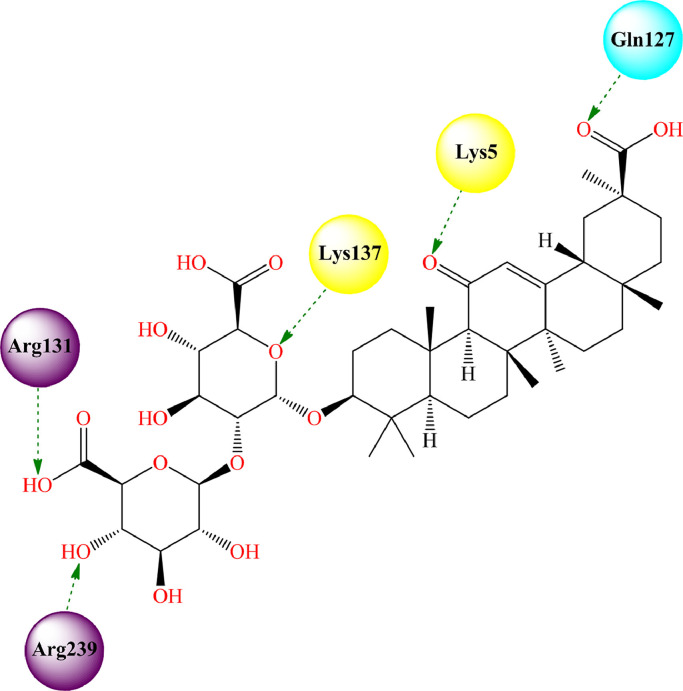
Narkhede et al. [174] showed that glycyrrhizin resulted in a strong binding affinity (-8.19 kcal/mol) with the SARS-CoV-2 Mpro (PDB: 6LU7) [175] through several hydrogen bonding interactions with active site amino acid residues namely Gln127, Lys5, Lys137, Arg131 and Tyr239 (Fig. 7 , Table 2 ).
Fig. 7.
Glycyrrhizin bound to the active site of SARS-CoV-2 Mpro (PDB: 6LU7). [Hydrogen bonding interactions are depicted in dotted green arrow].
Table 2.
Binding mode of interactions of glycyrrhizin and related derivatives with several life cycle targets of SARS-CoV-2.
| Compound | Viral Protein | PDB ID | Amino acid residues involved in hydrogen bonding interactions | Amino acid residues with other important interactions | Binding affinity/Docking Score | Ref. |
|---|---|---|---|---|---|---|
| Glycyrrhizin | 3CLpro | 6LU7 | Gln127, Lys5, Lys137, Arg131 and Tyr239 | Tyr126, Leu287 | -8.1 kcal/mol | [174] |
| Glycyrrhizin | 3CLpro | 6Y2F | Glu166, Gln189, Ser144, and Phe140 | Cys145, Met49, Met165, Pro168 | -8.21 kcal/mol | [176] |
| Glycyrrhizin | 3CLpro | 2AMD | Gln189, Met165, Asn142, Ser144, Cys145, His164, Asp187, Arg188 | – | – | [177] |
| Glycyrrhizin | 3CLpro | 6Y2E | Glu166, Pro168 | Cys145, Leu27, His41 | -8.0 kcal/mol | [178] |
| Glycyrrhizin | 3CLpro | 6LU7 | His163, Phe140, Glu166, Asp197 | His41, Gln189, Met165, Met49, His164, Asp187, Arg187, Arg188, Thr190, Ala191, Leu50, His172, Ser144, Leu141, Asn142. | -8.7 kcal/mol | [180] |
| Glycyrrhizin | RdRp | 6M71 | Arg555, Thr556, Ser682, Arg624, Ala558, Ala762, Trp800 | Trp617, Trp800, Gly616, Asp618, Lys798, Val763, Phe812, Asp452, Glu811 | -9.9 kcal/mol | [180] |
| Glycyrrhizin | PLpro | 4MM3 | Arg285, Tyr297, Thr266 | Lys298, Gly299, Pro300, His273, Cys271, Trp107, Glu251, Glu264, Met294, Thr292, Asn110 | -8.2 kcal/mol | [180] |
| Glycyrrhizin | SGp-RDB |
2GHV | Phe360, Arg426, Asn427, Trp423, Thr333, Ser363 | Tyr356, Asn357, Ser358, Phe361, Ser362, Ile489, Gln492, Asn424, Thr359, Thr425, Arg495, Ile428, Asn330, Ala332 | -9.3 kcal/mol | [180] |
| Glycyrrhizin | ACE2 | 6M17 | Arg273, His374, Tyr515, Asn394 | Phe40, His40, Glu402, Arg393, Tyr385, Glu402, Asp350, Ala348, Trp349, Asp382, His505, Phe504 |
-9.5 kcal/mol | [180] |
| Glycyrrhizin | 3CLpro | – | Gln189, His164, Glu166, His163, Ser144, Asn142, Asn119 | – | −8.8 | [182] |
| Glycyrrhizin | PLpro | – | Glu487, Ser925, Ser1560, Asp924, Arg1546, Thr1456 | – | −10.7 | [182] |
| Glycyrrhizin | ACE2 | – | Thr196, Glu208, Glu87, Asn194, Gln101, Asn397, Glu402, His401, Asp382, Ala348, Ser47, Ser43, Gly395 | – | −8.8 | [182] |
| Glycyrrhizin | RBD | – | Arg457, Asp467, Ile488, Cys480, Gly482, Ser469, Glu411, Arg454 | – | -8.6 | [182] |
| Glycyrrhizin | ACE2 | 6LZG | Lys68, Phe72, Glu75, Tyr83 | Lys68 | -10.3 (Autodock) -9.53 (DockThor) |
[183] |
| Glycyrrhizin | ACE2 | 6ACD | Leu464, Val492, Glu493 and Phe499 | Phe465, Tyr430, Phe495, Tyr498 | -7.474 kcal/mol | [184] |
| Glycyrrhizin | ACE2 | 6LZG | Asn501, Gln498, Gly496, Tyr449, Tyr453, Glu484 | Tyr489, Phe456, Leu455 | -9.5 kcal/mol | [185] |
| Glycyrrhizin | 3CLpro | 7BQY | Asp289, Asn238 | Thr199, Tyr239, Lys137, Asp197, Thr198, Glu290, Ser284, Leu287, Leu286, Leu272, Tyr237, Gly275, Leu271, Gln273, Asn274, Met276 | -8.7 kcal/mol | [186] |
| Glycyrrhizin | PLpro | 6XAA | Gln174, Asp179, Asn128 | Ala176, Asn177, Leu178, Phe173, Val202, Asp76, Asn156, Arg82, Thr74, Phe79, His175, Tyr154, His73, Phe69 | -7.9 kcal/mol | [186] |
| Glycyrrhizin | Nucleocapsid protein | 6M3M | Arg150, Tyr110, Arg89, Thr92, Arg94 | Asn49, Thr55, Ala157, Phe54, Ala51, Pro152, Thr150, Tyr112, Pro118, Ala91, Arg90 | -7.9 kcal/mol | [186] |
| Glycyrrhizin (Diammonium glycyrrhizinate) | ACE2 | 6VW1 | Tyr449,Gln493, Ser494, Gly496,Gln498, Tyr505, Lys403,Asp406,Gln409, Val417, Gly485 | Tyr453,Gly485, Phe486, Asn487, Tyr489, Tyr495, Asn501, Arg408, Ile418, Tyr453, Cys488, Glu484, Gly485, Phe486, Asn487, Cys488, Tyr489 | -11.7545 kcal/mol | [187] |
| Glycyrrhizin | RBD-ACE2 | – | Asp405, Arg403, Tyr453 | – | – | [125] |
| Glycyrrhizin | ACE2 | – | Asp405, Arg403, Tyr453 | -9 kcal/mol | [188] | |
| Glycyrrhizin | Spike glycoprotein | 6VSB | His625, Glu298, Arg319, Gln321, Val320 | Gln321, Val320, Tyr343, His625, The323, Ile624 | -9.2 kcal/mol | [189] |
| Glyasperin A | nsp15 endoribonuclease | 6W01 | Lys290, Leu346, Asn278, Lys345, Tyr343 | Tyr343, Val292, Lys345, Phe269 | -9.2 kcal/mol | [189] |
| Liquiritin | Spike glycoprotein | 6VSB | His625, Val620 Gln321, Cys291 | Gln321, Ile624, Val320 | -7.7 kcal/mol | [189] |
| Isoliquiritinapioside | Spike glycoprotein | 6VSB | His625, Val320, Gln321, Glu298 | Val620, Gln321, Phe318, Ile624, Tyr612 |
-7.4 kcal/mol | [189] |
| Glyasperin A | Spike glycoprotein | 6VSB | Val620, Val320 Glu298, Gln321 |
Val320, Val620, Val595, Gln321, Thr323, Phe318, Ile624 |
-7.9 kcal/mol | [189] |
| Glycyrrhizin | nsp15 endoribonuclease | 6W01 | His235, Ser294, Cys291, Thr341 |
Tyr343 His250, Lys290, His235, His250, Lys290 |
-8.3 kcal/mol | [189] |
| Dehydroglyasperin C | nsp15 endoribonuclease | 6W01 | His250, Ser294, Tyr343, Thr341, Val292 |
Lys345, Tyr343, Thr341, Trp333 | -8.4 kcal/mol | [189] |
| Liquiritin | nsp15 endoribonuclease | 6W01 | Lys290, Gln245, Leu246, Leu346, Asn278 |
Lys290, Tyr343, Val292 Lys290, His235 | -8.8 kcal/mol | [189] |
| Glyasperin A | nsp15 endoribonuclease | 6W01 | Lys290, Leu346, Asn278, Lys345, Tyr343 |
Tyr343, Val292, Lys345, Phe269 | -9.2 kcal/mol | [189] |
| Isoliquiritinadioside | nsp15 endoribonuclease | 6W01 | Asp240, Glu340, Ser294 | Thr341, Ser294, Val292 His235 Thr341 | -9.0 kcal/mol | [189] |
| Licochalcone D | nsp15 endoribonuclease | 6W01 | His250, Lys290, Asn278, Gly248, Leu346, Ser294, Pro344 |
Trp343, Trp333, Thr341, Lys290, Lys345 |
-8.3 kcal/mol | [189] |
| Glycyrrhizin | nsp15 endoribonuclease | 6W01 | Thr341, Asn278, Glu245, Hip250, Gly248 | Lys290 | -8.168 kcal/mol | [194] |
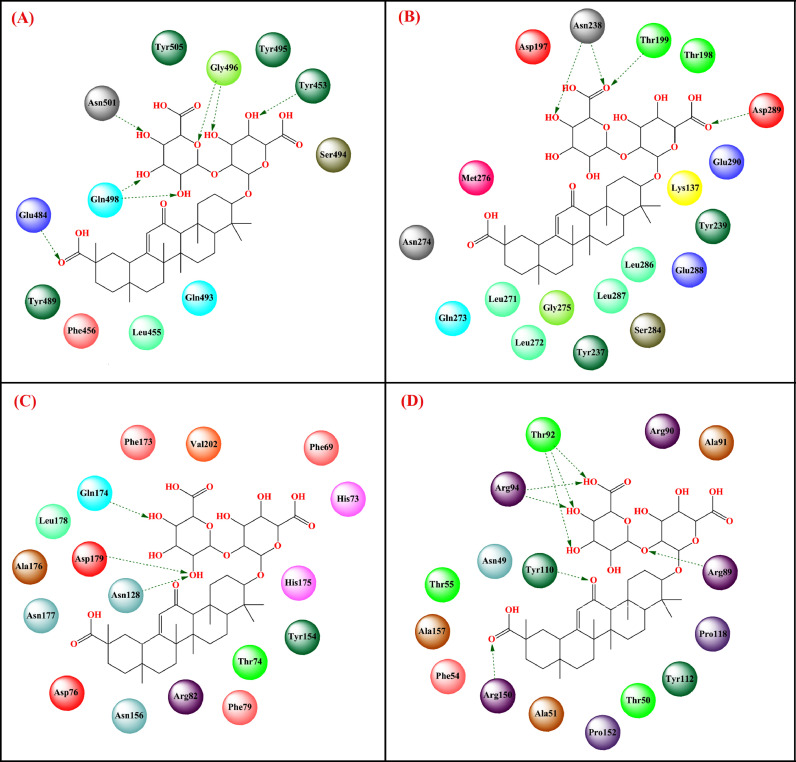
| Glycyrrhizin | nucleocapsid (N) protein | 6VYO | Lys65, Phe66, Pro67, Arg68, Gly69, Gln70, Tyr123, Gly124, Trp132, Ala134 | – | -12.61 kcal/mol | [197] |
| Glycyrrhizin | nsp1 | 2HSX | Lys47 | – | -9.24 kcal/mol | [196] |
Rai et al. [176] examined that glycyrrhizin is effectively bound to the active site of SARS-CoV-2 3CLpro (PDB: 6Y2F) [175] (binding free energy = -8.21 kcal/mol) showing potential hydrogen bonding and hydrophobic alkyl interactions with active site amino acid residues (Fig. 8 , Table 2).
Fig. 8.
Glycyrrhizin bound to the active site of SARS-CoV-2 3CLpro (PDB: 6Y2F). [Hydrogen bonding interactions are depicted in dotted green arrow].
Glycyrrhizin completely aligned into the substrate-binding cleft through a flipped C-shape form. The β-D-glucopyranosyl and α-D-glucopyranosyl moieties occupied the S4 pocket and S1 pocket, respectively. The icosahydropicene-2-carboxylic acid moiety flanked into the S2 and S3 subsites. Again, the molecular dynamics (MD) simulation study revealed that glycyrrhizin had a stable interaction with the protein as suggested by lower root mean square deviation (RMSD) and root mean square fluctuation (RMSF) values. Another study exhibited that glycyrrhizin formed 11 hydrogen bonds with the SARS-CoV-2 Mpro active site amino acid residues (namely Asn142, Ser144, Cys145, Arg188, His164, Gln189, Met165, and Asp187) [177].
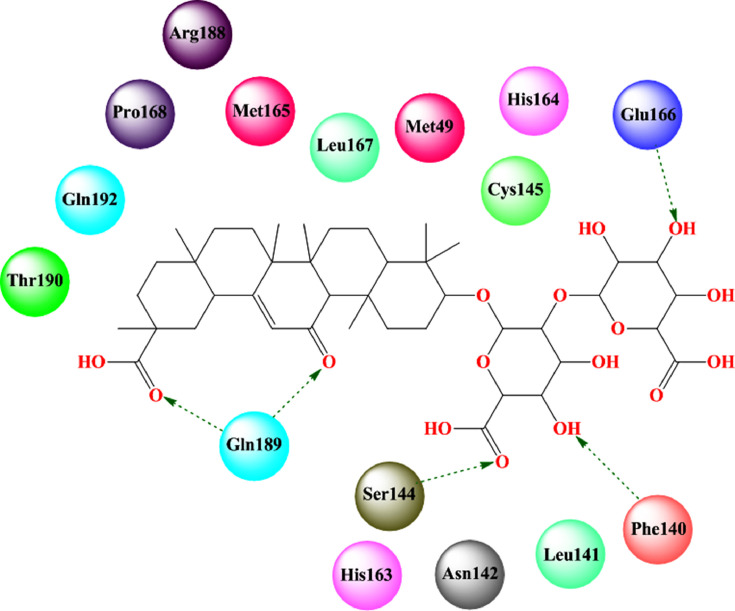
Srivastava and co-workers [178] reported the molecular docking study and ADMET analysis of some Glycyrrhiza glabra-derived bioactive compounds. Glycyrrhizin bound to the active site of SARS-CoV-2 3CLpro through hydrogen bonding interaction with Glu166, C-H bonding with Ser46, Pro168, and Asn142 along with alkyl and π-alkyl interactions with Cys145 and Pro168 (Fig. 9 , Table 2). Again, liquiritigenin also formed potential π-sulfur interaction with Met49 and Cys145; hydrogen bonding with Thr25; π-π stacking interaction with His41. Moreover, the ADMET study revealed that glycyrrhizin had a better ADMET profile than glabridin and liquiritigenin.
Fig. 9.
Glycyrrhizin bound to the active site of SARS-CoV-2 3CLpro (PDB: 6Y2E). [Hydrogen bonding interactions are depicted in dotted green arrow].
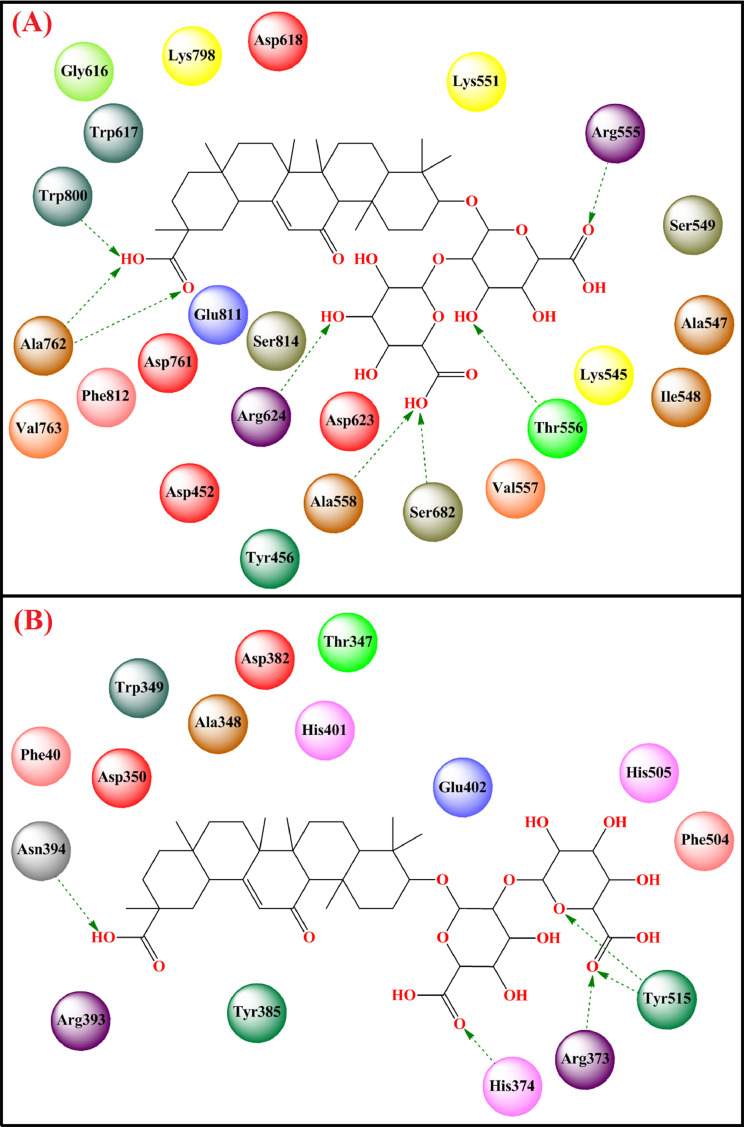
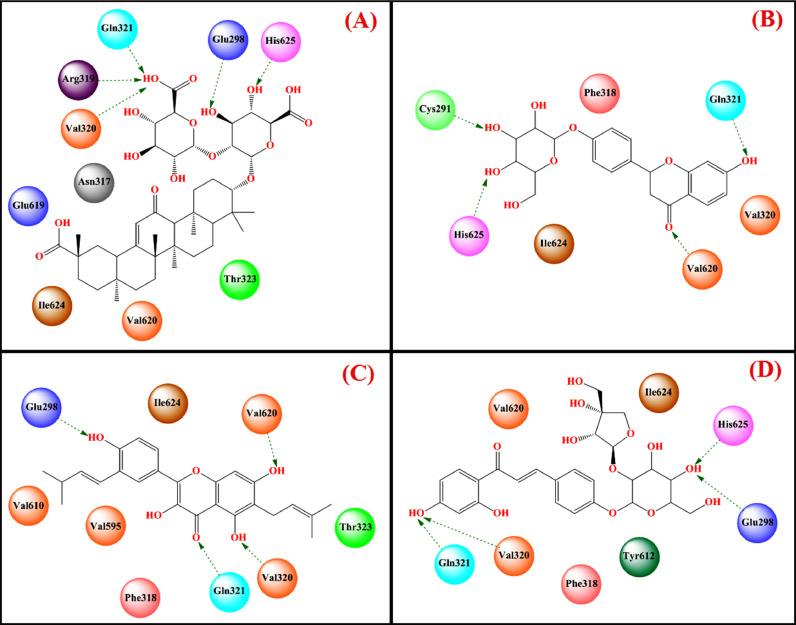
Manikyam and Joshi [179] showed that glycyrrhizin strongly bound to the SARS-CoV-2 3CLpro (Free energy of binding = -6.75 kcal/mol, Ki = 11.26 µM) and human furin protease (Free energy of binding = -5.84 kcal/mol, Ki = 52.76 µM). Again, Vardhan and Sahoo [180] showed that glycyrrhizin had an effective binding with various crucial SARS CoV-2 target proteins namely Mpro (binding energy = -8.7 kcal/mol), PLpro (binding energy = -8.2 kcal/mol), RdRp (binding energy = -9.9 kcal/mol) and ACE2 (binding energy = -9.5 kcal/mol) through several important interactions at the active site. Glycyrrhizin also strongly bound to the active site of RdRp (PDB: 6M71) [175] through several hydrogen bonding interactions, π-alkyl interactions, and van der Waals interactions with active site amino acid residues (Fig. 10 A, Table 2).
Fig. 10.
Binding mode of interaction of glycyrrhizin with SARS-CoV-2 (A) RdRp (PDB: 6M71) (B) ACE2 (PDB: 6M17). [Hydrogen bonding interactions are depicted in dotted green arrow].
While binding with the ACE2 (PDB: 6M17) [175], it formed potential hydrogen bonding along with carbon-hydrogen bonding and π-alkyl interactions with active site amino acid residues (Fig. 10B, Table 2). While binding to SARS-CoV-2 3CLpro (PDB: 6LU7) [175], glycyrrhizin formed effective hydrogen bonding with His163, Phe140, Glu166, and Asp197 along with several carbon-hydrogen bonds. Regarding binding to SARS-CoV-2 PLpro (PDB: 4MM3) [175], it formed potential hydrogen-bonding interactions with Arg285, Tyr297, and Thr266 along with carbon-hydrogen bonds, π-alkyl interactions, and van der Waals interactions with other amino acids at the active site. While binding to spike glycoprotein-RBD domain (PDB: 2GHV) [175], it showed effective hydrogen-bonding interactions with Phe360, Arg426, Asn427, Trp423, Thr333, and Ser363 as well as van der Waals interactions with active site amino acid residues.
On the other hand, glycyrrhetinic acid showed a stronger binding affinity with SARS-CoV-2 3CLpro (-8.03 kcal/mol) through hydrogen-bonding interactions with Gln189, Asn142, and Thr26 as well as hydrophobic interaction with Leu4 [181]. However, glycyrrhizin produced the strongest binding affinity with SARS-CoV-2 S protein (-19.22 kcal/mol) through several hydrophobic interactions with amino acids of chain A and chain C compared to the reference COVID-19 drug like umifenovir (-7.47 kcal/mol), chloroquine (-7.25 kcal/mol) and hydroxychloroquine (-5.30 kcal/mol) [181]. Mahdian et al. [182] performed a homology modeling study on five proteins of SARS-CoV-2 (3CLpro, PLpro, cleavage site, HR1, and RBD of spike protein) and subsequently screened 2471 FDA-approved drugs to identify 18 effective drug candidates. Glycyrrhizin was one of the best-repurposed drugs which showed good binding interactions with all these target proteins tested. Glycyrrhizin displayed the highest number of interactions with ACE2 (14 hydrogen bonds) and RBD (11 hydrogen bonds) compared to 3CLpro (7 hydrogen bonds) and PLpro (7 hydrogen bonds) (Table 2). Further study conducted by Huang et al. [112] reflected that glycyrrhizin effectively bound to the target proteins of SARS-CoV-2, i.e., 3CLpro (binding energy = -6.9 kcal/mol), PLpro (binding energy = -7.3 kcal/mol), RdRp (binding energy = -7.2 kcal/mol) and ACE2 (binding energy = -7 kcal/mol).
Ahmad et al. [183] performed a similarity search of DrugBank database molecules, and 40 molecules were screened. Glycyrrhizin was found to dock effectively into the active site of the ACE2 of SARS-CoV-2 (PDB: 6LZG) [175] showing good binding affinity with multiple hydrogen bonds and hydrophobic interactions (Fig. 11 , Table 2).
Fig. 11.
Binding mode of interaction of glycyrrhizin with ACE2 enzyme of SARS-CoV-2 (PDB: 6LZG). [Hydrogen bonding interactions are depicted in dotted green arrow].
The MD simulation revealed that the glycyrrhizin-ACE2 complex had a lower mean RMSD (1.95 Å). The MM/GBSA and MM/PBSA binding free energy calculation study suggested the energy values of -31.32 kcal/mol and -28.75 kcal/mol, respectively. Moreover, in the case of MM/GBSA analysis, it was noticed that Lys50, Phe54, Leu21, Ala47, Asn43, and Glu24 contributed significantly to the binding interaction with ACE2 and glycyrrhizin.
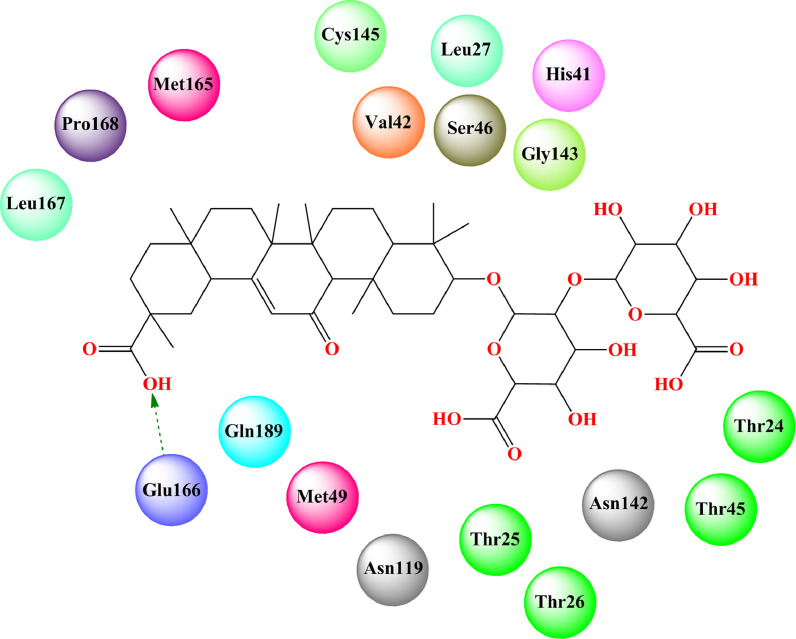
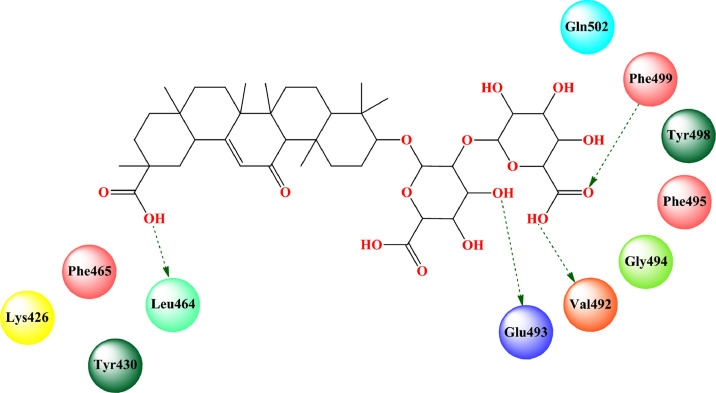
Bharath et al. [184] found that glycyrrhizin bound to the ACE2 binding site of RBD of SARS-CoV-2 spike protein (PDB: 6ACD) [175] (docking score: -7.474) through several hydrogen-bonding interactions and hydrophobic interactions (Fig. 12 , Table 2). The MD simulation study revealed that the glycyrrhizin-SARS-CoV-2-S complex became in equilibrium condition at 100 ns run. The RMSD and RMSF analysis suggested less fluctuation and stable interaction.
Fig. 12.
Binding mode of interaction of glycyrrhizin with ACE2 binding site of the RBD of SARS-CoV-2 spike protein (PDB: 6ACD). [Hydrogen bonding interactions are depicted in dotted green arrow].
Muhseen et al. [185] screened 1000 terpenoids from the NPACT database through molecular docking and found 19 hit compounds active against SARS-CoV-2 RBD bound with ACE2 (PDB: 6LZG) [175]. Glycyrrhizin (binding energy = -9.5 kcal/mol) exhibited effective hydrogen bonding interactions along with important hydrophobic interactions at the ACE2 active site (Fig. 13 , Table 2). Stable binding interactions were noticed between glycyrrhizin and SARS-CoV-2 RBD bound with ACE2 (RMSD = 0.24 nm, RMSF = 0.13 nm, SASA = 109 nm2). The ADMET study reflected that glycyrrhizin was a drug-like molecule with non-mutagenic and noncarcinogenic properties along with non-inhibitors of CYP enzymes.
Fig. 13.
Binding mode of interaction of glycyrrhizin with SARS-CoV-2 (A) RBD bound with ACE2 (PDB: 6LZG) (B) 3CLpro (PDB: 7BQY) (C) PLpro (PDB: 6XAA) and (D) Nucleocapsid (N) protein (PDB: 6M3M). [Hydrogen bonding interactions are depicted in dotted green arrow].
Muhseen et al. [186] further conducted a structure-based virtual screening study and found that glycyrrhizin effectively bound to the active site of SARS-CoV-2 3CLpro (PDB: 7BQY) [175], PLpro (PDB: 6XAA) [175] and nucleocapsid protein (PDB: 6M3M) [175] (Fig. 13, Table 2). It was noticed that glycyrrhizin while bound to SARS-CoV-2 3CLpro, formed hydrogen bond interactions with Asp289 and Asn238 along with several van der Waals interactions with active site amino acids. Similarly, in the case of binding with SARS-CoV-2 PLpro, it produced hydrogen-bonding interaction with Gln174, Asp179, and Asn128 along with several van der Waals interactions with active site amino acid residues (Fig. 13, Table 2). Moreover, in the case of binding with SARS-CoV-2 nucleocapsid protein, it formed effective hydrogen bonding interaction with Arg150, Tyr110, Arg89, Thr92, and Arg94 along with various van der Waals interactions at the active site (Fig. 13, Table 2). The MD simulation study revealed that glycyrrhizin had a mean RMSF value of 1.97Å, 2.59Å, and 1.32Å with SARS-CoV-2 3CLpro, PLpro, and N protein, respectively suggesting good intermolecular stability. Similarly, the conformational stability study revealed that glycyrrhizin had a mean RMSD value of 0.93Å, 0.96Å, and 3.48Å with SARS-CoV-2 3CLpro, PLpro, and N protein, respectively. Moreover, the binding complexes are quite stable as suggested by the radius of gyration analysis. Glycyrrhizin also showed a strong binding affinity with all these three target proteins as supported by the MM-GBSA, MM-PBSA, and WaterSwap binding free energy studies.
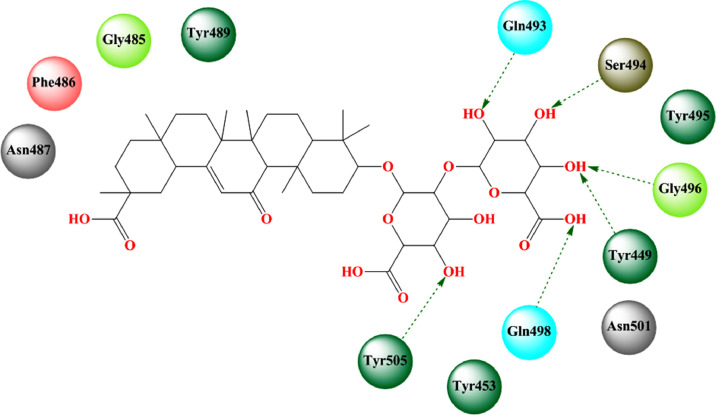
Kalhor et al. [187] tried to identify some potential inhibitors of SARS-CoV-2 from an FDA-approved drug database of 1364 compounds through the structure-based virtual screening and molecular docking approach. Diammonium glycyrrhizinate was found to produce the highest binding affinity (-11.7545 kcal/mol) while binding to the SARS-CoV-2 RBD domain complexed with ACE2 (Fig. 14 , Table 2). The glycoside portion formed potential hydrogen bonds with Gln493, Ser494, Gly496, Tyr449, Gln498, and Tyr505 along with several important hydrophobic interactions. The MD simulation study suggested that SARS-CoV-2 RBD/diammonium glycyrrhizinate complex formed a strong and stable complex (RMSD Avg. = 0.24 nm, RMSF = 0.5 nm). The toxicity risk parameters suggested that it was a nontoxic and non-mutagenic compound though it deviated from Lipinski's rule of 5.
Fig. 14.
Binding interaction of glycyrrhizin with SARS-CoV-2 RBD domain complexed with ACE2. [Hydrogen bonding interactions are depicted in dotted green arrow].
As per the frequency analysis by Yu et al. [125], Glycyrrhiza uralensis was the most common herb used in 30 TCM prescriptions. The molecular docking revealed that glycyrrhizin displayed a strong binding affinity with the SARS-CoV-2 S1 subunit (KD = 0.87 µM) positioned between the interface of RBD and ACE2 through several hydrogen-bonding interactions with Asp405, Arg403, and Tyr453. Chen et al. [188] also investigated that glycyrrhizin bound effectively to the SARS-CoV-2 ACE2 enzyme (binding energy = -9 kcal/mol) through interactions with Arg559, Gln388, Arg393, and Asp30.
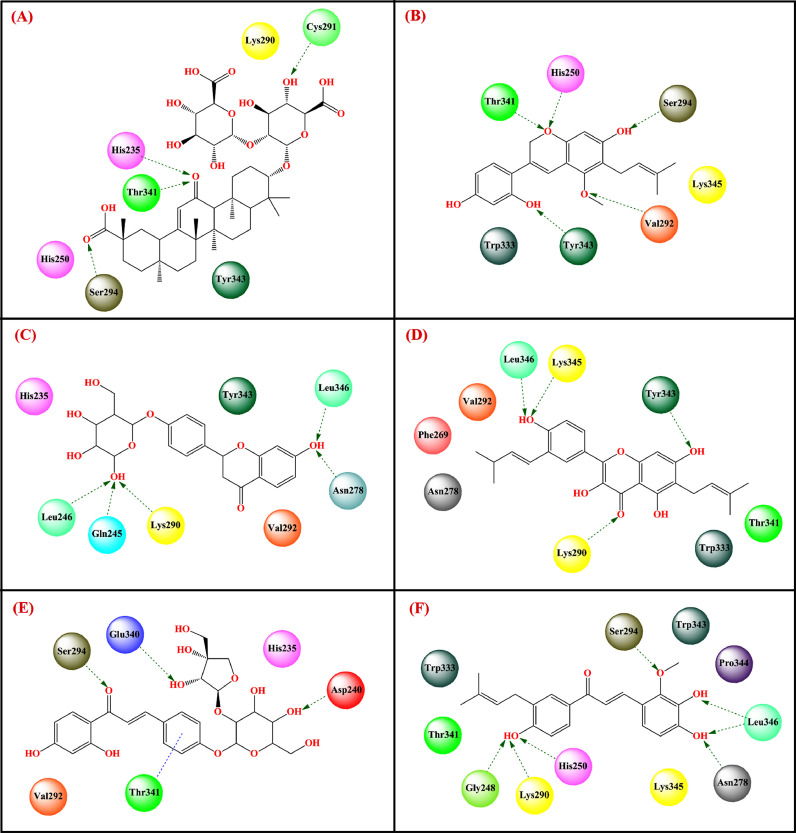
Sinha et al. [189] performed a virtual screening through the molecular docking study of 20 reported compounds obtained from Glycyrrhiza species at the active site of spike glycoprotein (PDB: 6VSB) [175] and nsp15 endoribonuclease (PDB: 6W01) [175]. Several compounds namely glycyrrhizin, dehydroglyasperin C, liquiritin, gyasperin A, isoliquiritin apioside and licochalcone D were found to be either better effective or comparable to the reference antivirals, i.e., lopinavir and ribavirin. Among these compounds glycyrrhizin (-9.2 kcal/mol) and glyasperin A (-9.2 kcal/mol) resulted in the highest binding energy with spike glycoprotein and nsp15 endoribonuclease, respectively (Figs. 15 and 16 , Table 2).
Fig. 15.
Binding interaction of (A) Glycyrrhizin (B) Liquiritin (C) Glyasperin A and (D) Isoliquiritin apioside with SARS-CoV-2 spike glycoprotein (PDB: 6VSB). [Hydrogen bonding interactions are depicted in dotted green arrow].
Fig. 16.
Binding interaction of (A) Glycyrrhizin (B) Dihydroglyasperin C (C) Liquiritin (D) Glyasperin A (E) Isoliquiritin apioside and (F) Licochalcone D with SARS-CoV-2 nsp15 endoribonuclease (PDB: 6W01). [Hydrogen bonding interactions are depicted in dotted green arrow].
Apart from that, other related compounds such as liquiritin, isoliquiritin apioside, and glyasperin A effectively bound to the active site of spike glycoprotein with binding energies of -7.7 kcal/mol, -7.4 kcal/mol, and -7.9 kcal/mol, respectively. Glycyrrhizin bound to spike glycoprotein through hydrogen bonding interaction with His625, Glu298, Arg319, Gln321, and Val320 along with several hydrophobic interactions. However, glyasperin A bound to nsp15 endoribonuclease through hydrogen-bonding interactions with Lys290, Leu346, Asn278, Lys345, and Tyr343 as well as some hydrophobic interactions. On the other hand, compounds namely dehydroglyasperin C, liquiritin, isoliquiritin apioside, and licochalcone D strongly bound to the nsp15 endoribonuclease with a binding energy score of -8.3 kcal/mol, -8.4 kcal/mol, -8.8 kcal/mol, -9.0 kcal/mol and -8.3 kcal/mol, respectively. The MD simulation study revealed that both these compounds made stable complexes with respective enzymes (for glycyrrhizin-S protein complex: RMSD Avg = 1.149 nm, RMSF Avg = 0.634 nm and Rg Avg = 4.403 nm; For glyasperin-nsp15 endoribonuclease complex: RMSD Avg = 0.231 nm, RMSF Avg = 0.148 nm and Rg Avg = 2.250 nm). Therefore, it may be postulated that glycyrrhizin may hinder the binding of virus spike protein with ACE2 enzyme at the entry-level, whereas glyasperin A by binding to nsp15 endoribonuclease may inhibit the viral replication process.
Mathew et al. [190] screened more than 100 natural compounds and found that glycyrrhizin was the best binder of the SARS-CoV-2 spike protein (docking interaction energy = -6.32 kcal/mol, binding affinity = -42.55 kcal/mol). It produced several hydrogen bonding interactions with amino acid residues such as Val382, Tyr380, Gly431, Asp428, and Asp427 (PDB: 6VSB) [175]. Recently, Vardhan and Sahoo [191] performed a computational study to know the binding mode of interaction with glycyrrhizin and SARS-CoV-2 spike glycoprotein RBD of three variants namely Omicron, Delta, and WT. Glycyrrhizin displayed a good docking score and binding interaction energies in all three types of variants. In the case of Omicron SGp-RBD, it formed conventional hydrogen bonding with Ser496 and Arg403 while forming effective van der Waals interactions with amino acids residues His505, Tyr495, Tyr453, Leu455, Tyr489, Ala484, Gly485, Cys488, and Phe456. The MD simulation study indicated that the glycyrrhizin-SGp RBD complex formed a conformational stable complex during the period of 100 ns simulation.
Kandeel and co-workers [192] screened a total of 1664 FDA-approved drugs through molecular docking analysis with SARS-CoV-2 RdRp (PDB: 7BV2) [175]. It was noticed that glycyrrhizin displayed a good docking score (-7.6 kcal/mol) with SARS-CoV-2 RdRp. Rehman et al. [193] reported that glycyrrhizin strongly bound to the SARS-CoV-2 RdRp, 3CLPro, spike protein, helicase, and E channel protein (binding energy of -10.52 kcal/mol, -9.57 kcal/mol, -9.49 kcal/mol, -11.57 kcal/mol, and -10.07 kcal/mol, respectively). Similarly, 18,β-Glycyrrhetinic acid also exhibited effective binding with SARS-CoV-2 spike protein, 3CLPro, helicase, and E channel protein (binding energy of -8.08 kcal/mol, -9.19 kcal/mol, -9.91 kcal/mol, and -9.72 kcal/mol, respectively). The glycyrrhizin-SARS-CoV-2 3CLPro complex was equilibrated at 25 ns with lower flexibility of amino acids at domain 1 and domain 2. Similarly, glycyrrhizin formed stable complexes with RdRp and spike glycoprotein.
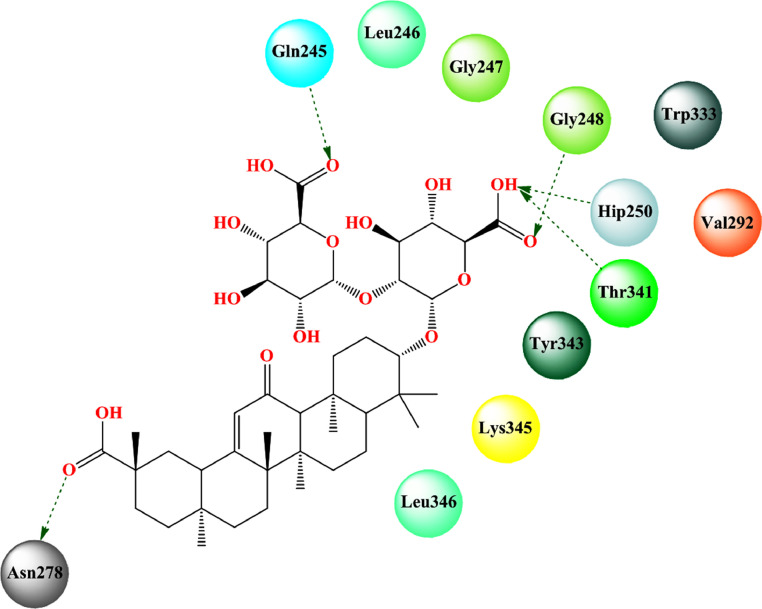
Patil et al. [194] found that glycyrrhizin strongly bound to the SARS-CoV-2 nsp15 endoribonuclease (PDB: 6OW1) [175] (docking score = -8.168, MM-PBSA binding energy = -97.599 kcal/mol). The aglycone moiety binds to the hydrophobic pocket surrounded by amino acid residues Leu346, Tyr343, Val292, and Trp333 along with some charged amino acid residues Lys345, Hip235, and polar amino acid Thr341. Apart from that, Thr341, Asn278, Glu245, and Gly248 also formed hydrogen bonding interactions (Fig. 17 , Table 2).
Fig. 17.
Binding mode of interaction of glycyrrhizin with SARS-CoV-2 nsp15 endoribonuclease (PDB: 6OW1). [Hydrogen bonding interactions are depicted in dotted green arrow].
The MD simulation study revealed that the complex with glycyrrhizin showed a low average RMSD of 0.29 nm. The protein-ligand complex also showed a lower RMSF suggesting its stable interaction with the nsp15 endoribonuclease.
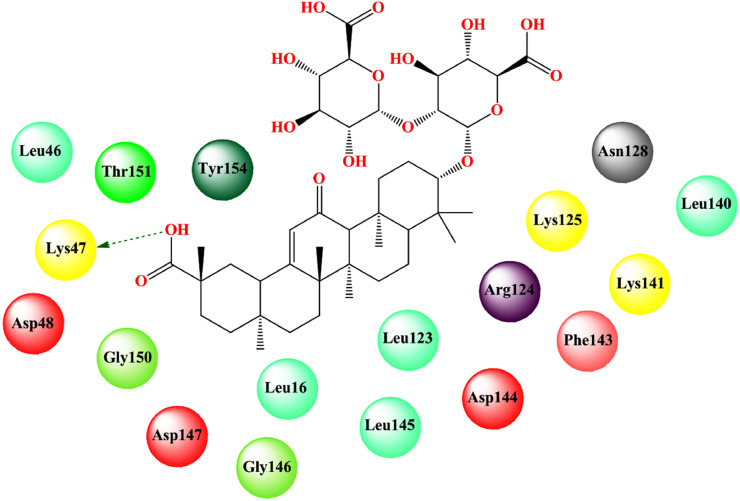
Khater et al. [195] performed a homology modeling of ExoN/nsp14 of SARS-CoV to build a 3D structure of the SARS-CoV-2 nsp14 model. The MD docking study showed that glycyrrhizin bound strongly to the ExoN active site (binding free energy = -8.6 kcal/mol). It showed hydrogen bonding interaction with catalytic amino acids Asp90, Glu92, and Glu191 as well as hydrophobic contracts with His268. Vankadari et al. [196] studied the molecular docking interaction of glycyrrhizin with the SARS-CoV-2 nsp1 protein. Glycyrrhizin strongly bound to nsp1 (binding affinity = -9.24 kcal/mol) showing hydrogen bonding interaction with Lys47 (Fig. 18 , Table 2).
Fig. 18.
Binding interaction of glycyrrhizin with SARS-CoV-2 nsp1 protein. [Hydrogen bonding interactions are depicted in dotted green arrow].
Ray et al. [197] showed that glycyrrhizin strongly bound to the SARS-CoV-2 nucleocapsid (N) protein (PDB: 6VYO) [175] (Docking energy score = -12.61 kcal/mol, Ki = 573.72 pM). It showed a large number of hydrogen-bonding interactions with amino acids Lys65, Phe66, Pro67, Arg68, Gly69, Gln70, Tyr123, Gly124, Trp132, and Ala134.
Fatima et al. [198] showed that glycyrrhizin effectively bound to the SARS-CoV-2 envelope protein (E) through hydrogen bond interactions with Arg61, Asn64, and Ile46 whereas forming effective hydrophobic interactions with amino acids Phe23, Leu28, Tyr57, Val29, Leu27 and Ala32 for disruption of helical formation and to promote disorganization of protein integrity. Interestingly, it was also observed that glycyrrhizin had a better binding affinity (-9.1 kcal/mol) compared to the currently used drugs for COVID-19 like azithromycin, hydroxychloroquine, remdesivir, dexamethasone and thymoquinone (-5.9 kcal/mol, -6.4 kcal/mol, -7.7 kcal/mol, -7.2 kcal/mol, -5.3 kcal/mol, respectively). UV-spectral studies revealed that treatment with glycyrrhizin showed a disruption of SARS-CoV-2 E protein at 280 nm. The fluorescence spectroscopy study exhibited the destabilization of the E protein α-helix structure at a dose of 50 µg/ml. Again, fluorescence quenching in glycyrrhizin resulted in an immediate major alteration in the microenvironment.
Zhao et al. [199] through a comprehensive network and pathway analysis identified that glycyrrhizin, one of the active components of TCM Qing-Fei-Pai-Du (QFPD) decoction, may act through modulating 7 biomolecular targets (such as AKT1, TNF-α, IL-6, PTGS2, HMOX1, IL-10, and TP13) in SARS-CoV-2 infection. The molecular docking study suggested that glycyrrhizin may bind to the HMOX1 protein (PDB: 1N45) (docking score = -6.923 kcal/mol), which interacts with SARS-CoV-2 protein and may regulate immunomodulation, anti-infective and anti-inflammatory effects.
Chen et al. [200] through Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis along with network analysis found that glycyrrhizin along with curcumin and vitamin C may act on a variety of targets responsible for modulating inflammatory responses. This combination may modulate immune responses by acting on NOD-like and Toll-like signaling pathways for interferon production, activation of T-cells as well as modulation of inflammatory responses by inhibiting PI3K/AKT, NF-κB, and MAPK signaling cascades. Regulation or modulation of the inflammatory pathways is crucial as these incidences are responsible for the cytokine storm during SARS-CoV-2 infection. In that condition, several pro-inflammatory cytokines (namely IL-1, IL-6, TNF-α) along with chemokines (namely CCL3, CCL5, CCL2, and CXCL10) are released. Therefore, there may be hypertension, hemorrhage, and subsequently multiorgan failure leading to death [201]. During cytokine storm by coronaviral infection, several pathways like NF-κB signaling [202,203], TNF signaling [204], PI3K/AKT signaling [205,206], and MAPK signaling pathways [205] play crucial roles in modulating inflammatory responses. Glycyrrhizin along with curcumin and vitamin C may prevent the cytokine storm by modulating several inflammatory responses related to those pathways [200]. Further, Zheng et al. [207] by KEGG pathway enrichment analysis demonstrated that glycyrrhizin was involved in the inflammatory and immune-related signaling pathways including PI3K-Akt-mTOR, IL-17, and MAPK signaling pathways. During COVID-19 infection, higher production of pro-inflammatory cytokines, as well as interplay mechanisms between coagulation cascades and inflammatory modulators, may lead to a cytokine storm followed by multiple organ failure [208]. Last but not least, from the study of Zheng et al. [207], it has been revealed that glycyrrhizin may be able to ameliorate potentially the inflammatory mechanisms related to cytokine storm during COVID-19.
9. Conclusions and future perspectives
Glycyrrhizin and related derivatives obtained from various Glycyrrhiza species have been widely used for combating multiple disease conditions including in the management of earlier SARS-CoV infections. Moreover, due to the high degree of structural and functional similarities between the earlier SARS-CoV and the current SARS-CoV-2, glycyrrhizin and related derivatives are supposed to be effective against COVID-19. Various studies that reflected the importance of glycyrrhizin in SARS-CoV and SARS-CoV-2 along with COVID-19-related clinical trials have been selected for further study. Thus, this current study involves the versatility of glycyrrhizin and related derivatives in different pathophysiological conditions, recapitulation of SARS-CoV-2 morphology while encapsulating the crucial in silico, in vitro, in vivo as well as clinical evidences of the effectivity of glycyrrhizin and related derivates in the treatment of COVID-19.
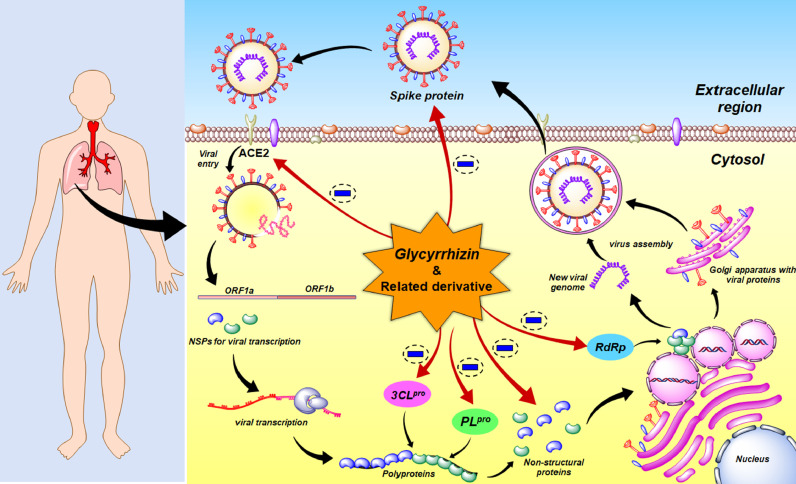
TCM and molecular modeling have been discussed to get an insight into the effective treatment of COVID-19. It was suggested that glycyrrhizin can act as a potential compound to reduce the signs and symptoms of COVID-19. Though the exact mechanism of action is still unclear, in this article, the experimental and computational evidences have been discussed in detail, reflecting the importance of glycyrrhizin and related derivatives as a promising therapeutic strategy to fight against the dreaded COVID-19 infection. From the experimental studies to date, it was observed that Glycyrrhiza, as well as Glycyrrhiza-containing formulations and TCMs, explored the implication of glycyrrhizin for treating as well as for alleviating the symptoms of COVID-19. Some of these also provided some optimistic outcomes and reduction of COVID-19 related to the clinical conditions. Nevertheless, the experimental and theoretical studies explored and claimed that glycyrrhizin can interfere with the viral life cycle at a molecular level. Also, clinical studies suggested the efficacy of Glycyrrhiza containing CHMs/TCMs as a standalone intervention, additional dietary supplement, and adjuvant therapy besides the Western/conventional treatment against COVID-19 treatment. Therefore, glycyrrhizin and related compounds may be a crucial armament to affect simultaneously in various stages of the life cycle of SARS-CoV-2 (Fig. 19 ).
Fig. 19.
Glycyrrhizin and related derivatives simultaneously block targets of SARS-CoV-2 life cycle.
Theoretical and computational evidences also supported that glycyrrhizin and related derivatives have multiple crucial functions to block several stages of the SARS-CoV-2 life cycle. However, glycyrrhizin or related derivatives that have been evaluated against SARS-CoV-2 to date have shown promising outcomes. Nevertheless, diammonium glycyrrhizinate in combination with vitamin C has been tried in clinical phases to target COVID-19. Some trials also explored that licorice extract can prevent or reduce the clinical signs and symptoms of COVID-19. Thus, careful modification of Glycyrrhiza-related derivatives to design novel inhibitors or repurposing of drugs and drug-like candidates similar to these natural products can be a promising approach for developing desirable therapeutics. In this context, it may be suggested that there is a huge scope either to derivatize such molecules to design better effective lead compounds or to formulate newer combination drugs comprising glycyrrhizin as the prime one, followed by extensive evaluation against SARS-CoV-2 to achieve a novel anti-coronaviral therapy for better treatment to combat COVID-19.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgment
The authors are thankful to the Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India, and the Department of Pharmacy, BITS-Pilani, Hyderabad, India for providing the research facilities. SB is thankful to the Swami Vivekananda Merit-cum-Means (SVMCM) scholarship, Govt. of West Bengal, India for awarding the fellowship. SKB sincerely thanks to the Indian Council of Medical Research (ICMR), New Delhi, India for awarding the senior research fellowship (SRF) [FILE NO.: 45/29/2019-PHA, dated: 21-06-2019]. NA is thankful to the authority of Jadavpur University for providing the research grant to conduct the research work. The research has been supported by the research fund provided by the Council of Scientific and Industrial Research (CSIR- 37(1722)/19/EMR-II) to Dr. Balaram Ghosh.
Data availability
No data was used for the research described in the article.
References
- 1.Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M.B.P. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother. Res. 2018;32:2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S., Shen Y., Qiu R., Chen Z., Chen Z., Chen W. 18 β-glycyrrhetinic acid exhibits potent antitumor effects against colorectal cancer via inhibition of cell proliferation and migration. Int. J. Oncol. 2017;51:615–624. doi: 10.3892/ijo.2017.4059. [DOI] [PubMed] [Google Scholar]
- 3.Nazari S., Rameshrad M., Hosseinzadeh H. Toxicological effects of Glycyrrhiza glabra (Licorice): a review. Phytother. Res. 2017;31:1635–1650. doi: 10.1002/ptr.5893. [DOI] [PubMed] [Google Scholar]
- 4.Gupta V.K., Fatima A., Faridi U., Negi A.S., Shanker K., Kumar J.K., Rahuja N., Luqman S., Sisodia B.S., Saikia D., Darokar M.P., Khanuja S.P. Antimicrobial potential of Glycyrrhiza glabra roots. J. Ethnopharmacol. 2008;116:377–380. doi: 10.1016/j.jep.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 5.He J., Chen L., Heber D., Shi W., Lu Q.Y. Antibacterial compounds from glycyrrhiza uralensis. J. Nat. Prod. 2006;69:121–124. doi: 10.1021/np058069d. [DOI] [PubMed] [Google Scholar]
- 6.Fatima A., Gupta V.K., Luqman S., Negi A.S., Kumar J.K., Shanker K., Saikia D., Srivastava S., Darokar M.P., Khanuja S.P. Antifungal activity of glycyrrhiza glabra extracts and its active constituent glabridin. Phytother. Res. 2009;23:1190–1193. doi: 10.1002/ptr.2726. [DOI] [PubMed] [Google Scholar]
- 7.Han S., Sun L., He F., Che H. Anti-allergic activity of glycyrrhizic acid on IgE-mediated allergic reaction by regulation of allergy-related immune cells. Sci. Rep. 2017;7:7222. doi: 10.1038/s41598-017-07833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W.C., Liu C.Y., Shen S.C., Chen L.C., Yeh K.W., Liu S.H., Liou C.J. Protective effects of licochalcone A improve airway hyper-responsiveness and oxidative stress in a mouse model of asthma. Cells. 2019;8:617. doi: 10.3390/cells8060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang R., Yuan B.C., Ma Y.S., Zhou S., Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017;55:5–18. doi: 10.1080/13880209.2016.1225775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah S.L., Wahid F., Khan N., Farooq U., Shah A.J., Tareen S., Ahmad F., Khan T. Inhibitory effects of Glycyrrhiza glabra and its major constituent glycyrrhizin on inflammation-associated corneal neovascularization. Evid. Based Complement. Alternat. Med. 2018;2018 doi: 10.1155/2018/8438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain H., Green I.R., Shamraiz U., Saleem M., Badshah A., Abbas G., Rehman N.U., Irshad M. Therapeutic potential of glycyrrhetinic acids: a patent review (2010-2017) Expert Opin. Ther. Pat. 2018;28:383–398. doi: 10.1080/13543776.2018.1455828. [DOI] [PubMed] [Google Scholar]
- 12.Zhai K.F., Duan H., Cui C.Y., Cao Y.Y., Si J.L., Yang H.J., Wang Y.C., Cao W.G., Gao G.Z., Wei Z.J. Liquiritin from glycyrrhiza uralensis attenuating rheumatoid arthritis via reducing inflammation, suppressing angiogenesis, and inhibiting MAPK signaling pathway. J. Agric. Food Chem. 2019;67:2856–2864. doi: 10.1021/acs.jafc.9b00185. [DOI] [PubMed] [Google Scholar]
- 13.Frattaruolo L., Carullo G., Brindisi M., Mazzotta S., Bellissimo L., Rago V., Curcio R., Dolce V., Aiello F., Cappello A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: identification of licoflavanone as a modulator of NF-κB/MAPK pathway. Antioxidants. 2019;8:186. doi: 10.3390/antiox8060186. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y.Y., Yang Y.N., Feng Z.M., Jiang J.S., Zhang P.C. Eight new triterpenoid saponins with antioxidant activity from the roots of Glycyrrhiza uralensis Fisch. Fitoterapia. 2019;133:186–192. doi: 10.1016/j.fitote.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Reigada I., Moliner C., Valero M.S., Weinkove D., Langa E., Rincón C.G. Antioxidant and antiaging effects of licorice on the Caenorhabditis elegans model. J. Med. Food. 2020;23:72–78. doi: 10.1089/jmf.2019.0081. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y.F., Wei J.H., Fang S.Q., Tang Y.P., Cheng H.B., Wang T.L., Li C.Y., Peng G.P. Hepatoprotective triterpene saponins from the roots of Glycyrrhiza inflate. Molecules. 2015;20:6273–6283. doi: 10.3390/molecules20046273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y., Kuang Y., Li K., Wang S., Ji S., Chen K., Song W., Qiao X., Ye M. Nrf2 activators from Glycyrrhiza inflata and their hepatoprotective activities against CCl4-induced liver injury in mice. Bioorg. Med. Chem. 2017;25:5522–5530. doi: 10.1016/j.bmc.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Ojha S., Golechha M., Kumari S., Bhatia J., Arya D.S. Glycyrrhiza glabra protects from myocardial ischemia-reperfusion injury by improving hemodynamic, biochemical, histopathological and ventricular function. Exp. Toxicol. Pathol. 2013;65:219–227. doi: 10.1016/j.etp.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Nakatani Y., Kobe A., Kuriya M., Hiroki Y., Yahagi T., Sakakibara I., Matsuzaki K., Amano T. Neuroprotective effect of liquiritin as an antioxidant via an increase in glucose-6-phosphate dehydrogenase expression on B65 neuroblastoma cells. Eur. J. Pharmacol. 2017;815:381–390. doi: 10.1016/j.ejphar.2017.09.040. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.E., Lim C., Lee M., Kim C.H., Kim H., Lee B., Cho S. Assessing neuroprotective effects of Glycyrrhizae Radix et Rhizoma extract using a transient middle cerebral artery occlusion mouse model. J. Vis. Exp. 2018;142:e58454. doi: 10.3791/58454. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y., Xu W., Huang Y., Zeng X. Licochalcone B, a chalcone derivative from Glycyrrhiza inflata, as a multifunctional agent for the treatment of Alzheimer's disease. Nat. Prod. Res. 2020;34:736–739. doi: 10.1080/14786419.2018.1496429. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Z., Wang W., Guo H., Zhou D. Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav. Brain Res. 2008;194:108–113. doi: 10.1016/j.bbr.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann K.M., Beltrán L., Ziemba P.M., Hatt H., Gisselmann G. Potentiating effect of glabridin from glycyrrhiza glabra on GABAA receptors. Biochem. Biophys. Rep. 2016;6:197–202. doi: 10.1016/j.bbrep.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho S., Park J.H., Pae A.N., Han D., Kim D., Cho N.C., No K.T., Yang H., Yoon M., Lee C., Shimizu M., Baek N.I. Hypnotic effects and GABAergic mechanism of licorice (Glycyrrhiza glabra) ethanol extract and its major flavonoid constituent glabrol. Bioorg. Med. Chem. 2012;20:3493–3501. doi: 10.1016/j.bmc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Fukai T., Marumo A., Kaitou K., Kanda T., Terada S., Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71:1449–1463. doi: 10.1016/s0024-3205(02)01864-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuang Y., Li B., Fan J., Qiao X., Ye M. Antitussive and expectorant activities of licorice and its major compounds. Bioorg. Med. Chem. 2018;26:278–284. doi: 10.1016/j.bmc.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Mendes-Silva W., Assafim M., Ruta B., Monteiro R.Q., Guimaraes J.A., Zingali R.B. Antithrombotic effect of Glycyrrhizin, a plant-derived thrombin inhibitor. Thromb. Res. 2003;112:93–98. doi: 10.1016/j.thromres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Carnovali M., Banfi G., Mariotti M. Liquiritigenin reduces osteoclast activity in zebrafish model of glucocorticoid-induced osteoporosis. J. Pharmacol. Sci. 2020;143:300–306. doi: 10.1016/j.jphs.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Adianti M., Aoki C., Komoto M., Deng L., Shoji I., Wahyuni T.S., Lusida M.I., Soetjipto H.F., Kawahara N., Hotta H. Anti-hepatitis C virus compounds obtained from glycyrrhiza uralensis and other glycyrrhiza species. Microbiol. Immunol. 2014;58:180–187. doi: 10.1111/1348-0421.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baltina L.A., Tasi Y.T., Huang S.H., Lai H.C., Baltina L.A., Petrova S.F., Yunusov M.S., Lin C.W. Glycyrrhizic acid derivatives as dengue virus inhibitors. Bioorg. Med. Chem. Lett. 2019;29 doi: 10.1016/j.bmcl.2019.126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolkerstorfer A., Kurz H., Bachhofner N., Szolar O.H. Glycyrrhizin inhibits influenza a virus uptake into the cell. Antiviral Res. 2009;83:171–178. doi: 10.1016/j.antiviral.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang S., Li M., Yu X., Jin H., Zhang Y., Zhang L., Zhou D., Xiao S. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019;166:328–338. doi: 10.1016/j.ejmech.2019.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., Baba M., De Clercq E., Nakashima H., Yamamoto N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV) Antiviral Res. 1988;10:289–298. doi: 10.1016/0166-3542(88)90047-2. [DOI] [PubMed] [Google Scholar]
- 34.Duan E., Wang D., Fang L., Ma J., Luo J., Chen H., Li K., Xiao S. Suppression of porcine reproductive and respiratory syndrome virus proliferation by glycyrrhizin. Antiviral Res. 2015;120:122–125. doi: 10.1016/j.antiviral.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Chen X., Wang W., Zhang Y., Yang Z., Jin Y., Ge H.M., Li E., Yang G. Glycyrrhizic acid as the antiviral component of glycyrrhiza uralensis fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease. J. Ethnopharmacol. 2013;147:114–121. doi: 10.1016/j.jep.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfajaro M.M., Kim H.J., Park J.G., Ryu E.H., Kim J.Y., Jeong Y.J., Kim D.S., Hosmillo M., Son K.Y., Lee J.H., Kwon H.J., Ryu Y.B., Park S.J., Park S.I., Lee W.S., Cho K.O. Anti-rotaviral effects of glycyrrhiza uralensis extract in piglets with rotavirus diarrhea. Virol. J. 2012;9:310. doi: 10.1186/1743-422X-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon H.J., Kim H.H., Ryu Y.B., Kim J.H., Jeong H.J., Lee S.W., Chang J.S., Cho K.O., Rho M.C., Park S.J., Lee W.S. In vitro anti-rotavirus activity of polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg. Med. Chem. 2010;18:7668–7674. doi: 10.1016/j.bmc.2010.07.073. [DOI] [PubMed] [Google Scholar]
- 38.Soufy H., Yassein S., Ahmed A.R., Khodier M.H., Kutkat M.A., Nasr S.M., Okda F.A. Antiviral and immune stimulant activities of glycyrrhizin against duck hepatitis virus. Afr. J. Tradit. Complement Altern. Med. 2012;9:389–395. doi: 10.4314/ajtcam.v9i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh C.F., Wang K.C., Chiang L.C., Shieh D.E., Yen M.H., San Chang J. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013;148:466–473. doi: 10.1016/j.jep.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badam L. In vitro antiviral activity of indigenous glycyrrhizin, licorice and glycyrrhizic acid (sigma) on Japanese encephalitis virus. J. Commun. Dis. 1997;29:91–99. [PubMed] [Google Scholar]
- 41.Lin J.C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antiviral Res. 2003;59:41–47. doi: 10.1016/s0166-3542(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 42.Farooqui A., Khan F., Khan I., Ansari I.A. Glycyrrhizin induces reactive oxygen species-dependent apoptosis and cell cycle arrest at G0/G1 in HPV18+ human cervical cancer HeLa cell line. Biomed. Pharmacother. 2018;97:752–764. doi: 10.1016/j.biopha.2017.10.147. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Wang X., Zhang K., Zhang X., Li S., Li Y., Fan W., Leng F., Yang M., Chen J. Extraction kinetics, thermodynamics, rheological properties and anti-BVDV activity of the hot water assisted extraction of Glycyrrhiza polysaccharide. Food Funct. 2020;11:4067–4080. doi: 10.1039/d0fo00608d. [DOI] [PubMed] [Google Scholar]
- 44.Huan C.C., Wang H.X., Sheng X.X., Wang R., Wang X., Mao X. Glycyrrhizin inhibits porcine epidemic diarrhea virus infection and attenuates the proinflammatory responses by inhibition of high mobility group box-1 protein. Arch. Virol. 2017;162:1467–1476. doi: 10.1007/s00705-017-3259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baba M., Shigeta S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antiviral Res. 1987;7:99–107. doi: 10.1016/0166-3542(87)90025-8. [DOI] [PubMed] [Google Scholar]
- 46.Curreli F., Friedman-Kien A.E., Flore O. Glycyrrhizic acid alters kaposi sarcoma-associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J. Clin. Invest. 2005;115:642–652. doi: 10.1172/JCI23334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiore C., Eisenhut M., Krausse R., Ragazzi E., Pellati D., Armanini D., Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytother. Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19) Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.https://www.worldometers.info/as accessed in August 2022.
- 51.Wang Z., Yang L. Chinese herbal medicine: fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 2021;270 doi: 10.1016/j.jep.2021.113869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan T., Khan M.A., Ullah N., Nadhman A. Therapeutic potential of medicinal plants against COVID-19: the role of antiviral medicinal metabolites. Biocatal. Agric. Biotechnol. 2020;31 doi: 10.1016/j.bcab.2020.101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Islam M.T., Quispe C., Martorell M., Docea A.O., Salehi B., Calina D., Reiner Ž., Sharifi-Rad J. Dietary supplements, vitamins and minerals as potential interventions against viruses: perspectives for COVID-19. Int. J. Vitam. Nutr. Res. 2022;92:49–66. doi: 10.1024/0300-9831/a000694. [DOI] [PubMed] [Google Scholar]
- 54.Ayatollahi S.A., Sharifi-Rad J., Tsouh Fokou P.V., Mahady G.B., Ansar Rasul Suleria H., Krishna Kapuganti S., Gadhave K., Giri R., Garg N., Sharma R., Ribeiro D., Rodrigues C.F., Reiner Ž., Taheri Y., Cruz-Martins N. Naturally occurring bioactives as antivirals: emphasis on coronavirus infection. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.575877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Kamel H., Grundmann O. Glycyrrhizin as a potential treatment for the novel coronavirus (COVID-19) Mini Rev. Med. Chem. 2021;21:2204–2208. doi: 10.2174/1389557521666210210160237. [DOI] [PubMed] [Google Scholar]
- 56.Gomaa A.A., Abdel-Wadood Y.A. The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomed. Plus. 2021;1 doi: 10.1016/j.phyplu.2021.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chathappady House N.N., Palissery S., Sebastian H. Corona viruses: a review on SARS, MERS and COVID-19. Microbiol. Insights. 2021;14 doi: 10.1177/11786361211002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.V'kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Artika I.M., Dewantari A.K., Wiyatno A. Molecular biology of coronaviruses: current knowledge. Heliyon. 2020;6:e04743. doi: 10.1016/j.heliyon.2020.e04743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lalchhandama K. The chronicles of coronaviruses: the bronchitis, the hepatitis and the common cold. Sci. Vis. 2020;20:43–53. [Google Scholar]
- 62.Adhikari N., Amin S.A., Jha T. In: Silico Modeling of Drugs Against Coronaviruses. Methods in Pharmacology and Toxicology. Roy K., editor. Humana; 2020. Dissecting the drug development strategies against SARS-CoV-2 through diverse computational modeling techniques; pp. 1–103. [Google Scholar]
- 63.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hulswit R.J.G, De Haan C.A.M., Bosch B.J. Coronavirus spike protein and tropism changes. Adv. Virus Res. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tortorici M.A., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D., Boons G.J., Bosch B.J., Rey F.A., de Groot R.J., Veesler D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struc. Mol. Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Hüttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu-Ozturk D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., García-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang D., Leibowitz J.L. The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res. 2015;206:120–133. doi: 10.1016/j.virusres.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alanagreh L.A., Alzoughool F., Atoum M. The human coronavirus disease COVID-19: its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens. 2020;9:331. doi: 10.3390/pathogens9050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdelrahman Z., Li M., Wang X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza a respiratory viruses. Front. Immunol. 2020;11:2309. doi: 10.3389/fimmu.2020.552909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ullrich S., Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.W., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Snijder E.J., Decroly E., Ziebuhr J. The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv. Virus Res. 2016;96:59–126. doi: 10.1016/bs.aivir.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L.W., Wang Q., Lou Z., Rao Z. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tvarogová J., Madhugiri R., Bylapudi G., Ferguson L.J., Karl N., Ziebuhr J. Identification and characterization of a human coronavirus 229E nonstructural protein 8-associated RNA 3′-terminal adenylyl transferase activity. J. Virol. 2019;93 doi: 10.1128/JVI.00291-19. e00291-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio. 2013;4 doi: 10.1128/mBio.00524-13. e00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oudshoorn D., Rijs K., Limpens R.W., Groen K., Koster A.J., Snijder E.J., Kikkert M. M. Bárcena, expression and cleavage of middle east respiratory syndrome coronavirus nsp3-4 polyprotein induce the formation of double-membrane vesicles that mimic those associated with coronaviral RNA replication. mBio. 2017;8 doi: 10.1128/mBio.01658-17. e01658-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lundin A., Dijkman R., Bergström T., Kann N., Adamiak B., Hannoun C., Kindler E., Jonsdottir H.R., Muth D., Kint J., Forlenza M. Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the middle east respiratory syndrome virus. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Konno Y., Kimura I., Uriu K., Fukushi M., Irie T., Koyanagi Y., Sauter D., Gifford R.J., Nakagawa S., Sato K. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Münch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hayashi H., Tamura S., Chiba R., Fujii I., Yoshikawa N., Fattokhov I., Saidov M. Field survey of glycyrrhiza plants in central Asia (4). characterization of G. glabra and G. bucharica collected in Tajikistan. Biol. Pharm. Bull. 2016;39:1781–1786. doi: 10.1248/bpb.b16-00251. [DOI] [PubMed] [Google Scholar]
- 88.Siracusa L., Saija A., Cristani M., Cimino F., D'Arrigo M., Trombetta D., Rao F., Ruberto G. Phytocomplexes from liquorice (Glycyrrhiza glabra L.) leaves–chemical characterization and evaluation of their antioxidant, anti-genotoxic and anti-inflammatory activity. Fitoterapia. 2011;82:546–556. doi: 10.1016/j.fitote.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Wang Q., Qian Y., Wang Q., Yang Y.F., Ji S., Song W., Qiao X., Guo D.A., Liang H., Ye M. Metabolites identification of bioactive licorice compounds in rats. J. Pharm. Biomed. Anal. 2015;115:515–522. doi: 10.1016/j.jpba.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 90.Rizzato G., Scalabrin E., Radaelli M., Capodaglio G., Piccolo O. A new exploration of licorice metabolome. Food Chem. 2017;221:959–968. doi: 10.1016/j.foodchem.2016.11.068. [DOI] [PubMed] [Google Scholar]
- 91.Yu J.Y., Ha J.Y., Kim K.M., Jung Y.S., Jung J.C., Oh S. Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules. 2015;20:13041–13054. doi: 10.3390/molecules200713041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukui H., Goto K., Tabata M. Two antimicrobial flavanones from the leaves of glycyrrhiza glabra. Chem. Pharm. Bull. 1988;36:4174–4176. doi: 10.1248/cpb.36.4174. [DOI] [PubMed] [Google Scholar]
- 93.Chouitah O., Meddah B., Aoues A., Sonnet P. Chemical composition and antimicrobial activities of the essential oil from glycyrrhiza glabra leaves. J. Essent. Oil Bear. Plants. 2011;14:284–288. [Google Scholar]
- 94.Saxena S. Glycyrrhiza glabra: medicine over the millennium. Nat. Prod. Rad. 2005;4:358–367. [Google Scholar]
- 95.Öztürk M., Altay V., Hakeem K.R., Akçiçek E. Liquorice: From Botany to Phytochemistry. Springer International Publishing; Cham: 2017. Pharmacological activities and phytochemical constituents; pp. 45–72. [Google Scholar]
- 96.El-Saber Batiha G., Magdy Beshbishy A., El-Mleeh A.M., Abdel-Daim M., Devkota H.P. Traditional Uses, Bioactive chemical constituents, and pharmacological and toxicological activities of glycyrrhiza glabra L. (Fabaceae) Biomolecules. 2020;10:352. doi: 10.3390/biom10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silveira D., Prieto-Garcia J.M., Boylan F., Estrada O., Fonseca-Bazzo Y.M., Jamal C.M., Magalhães P.O., Pereira E.O., Tomczyk M., Heinrich M. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharmacol. 2020;11:1479. doi: 10.3389/fphar.2020.581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chrzanowski J., Chrzanowska A., Graboń W. Glycyrrhizin: an old weapon against a novel coronavirus. Phytother. Res. 2021;35:629–636. doi: 10.1002/ptr.6852. [DOI] [PubMed] [Google Scholar]
- 100.Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., Doerr H.W., Cinatl J. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 101.Luo P., Liu D., Li J. Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujii T., Nakamura T., Iwamoto A. Current concepts in SARS treatment. J. Infect. Chemother. 2004;10:1–7. doi: 10.1007/s10156-003-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Michaelis M., Geiler J., Naczk P., Sithisarn P., Leutz A., Doerr H.W., Cinatl Jr J. Glycyrrhizin exerts antioxidative effects in H5N1 influenza a virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS ONE. 2011;6:e19705. doi: 10.1371/journal.pone.0019705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Yu T. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tong T., Hu H., Zhou J., Deng S., Zhang X., Tang W., Fang L., Xiao S., Liang J. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16 doi: 10.1002/smll.201906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishimoto Y., Hisatsune A., Katsuki H., Miyata T., Yokomizo K., Isohama Y. Glycyrrhizin attenuates mucus production by inhibition of MUC5AC mRNA expression in vivo and in vitro. J. Pharmacol. Sci. 2010;113:76–83. doi: 10.1254/jphs.09344fp. [DOI] [PubMed] [Google Scholar]
- 108.Wang B.X., Fish E.N. Global virus outbreaks: interferons as 1st responders. Semin. Immunol. 2019;43 doi: 10.1016/j.smim.2019.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bailly C., Vergoten G. Glycyrrhizin: an alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020;214 doi: 10.1016/j.pharmthera.2020.107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gowda P., Patrick S., Joshi S.D., Kumawat R.K., Sen E. Glycyrrhizin prevents SARS-CoV-2 S1 and ORF3A induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine. 2021;142 doi: 10.1016/j.cyto.2021.155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen R., Huang Y., Quan J., Liu J., Wang H., Billiar T.R., Lotze M.T., Zeh H.J., Kang R., Tang D. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon. 2020;6:e05672. doi: 10.1016/j.heliyon.2020.e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang F., Li Y., Leung E.L.H., Liu X., Liu K., Wang Q., Lan Y., Li X., Yu H., Cui L., Luo H. A review of therapeutic agents and Chinese herbal medicines against SARS-COV-2 (COVID-19) Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., Liu J.P. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020;26:243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luo L., Jiang J., Wang C., Fitzgerald M., Hu W., Zhou Y., Zhang H., Chen S. Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm. Sin. B. 2020;10:1192–1204. doi: 10.1016/j.apsb.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ang L., Lee H.W., Choi J.Y., Zhang J., Lee M.S. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr. Med. Res. 2020;9 doi: 10.1016/j.imr.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boozari M., Hosseinzadeh H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother. Res. 2021;35:864–876. doi: 10.1002/ptr.6873. [DOI] [PubMed] [Google Scholar]
- 118.Ding H., Deng W., Ding L., Ye X., Yin S., Huang W. Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in non-hospitalized COVID-19 patients. J. Med. Virol. 2020;92:2200–2204. doi: 10.1002/jmv.26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adhikari B., Marasini B.P., Rayamajhee B., Bhattarai B.R., Lamichhane G., Khadayat K., Adhikari A., Khanal S., Parajuli N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: a review. Phytother. Res. 2021;35:1298–1312. doi: 10.1002/ptr.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liao F.L., Peng D.H., Chen W., Hu H.N., Tang P., Liu Y.Y., Luo Y., Yao T. Evaluation of serum hepatic enzyme activities in different COVID-19 phenotypes. J. Med. Virol. 2021;93:2365–2373. doi: 10.1002/jmv.26729. [DOI] [PubMed] [Google Scholar]
- 121.Al-Jawad F.H., Al-Razzuqi R.A., Hashim H.M., Al-Bayati N.J. Glycyrrhiza glabra versus Boswellia carterii in chronic bronchial asthma: a comparative study of efficacy, Ind. J. Allergy Asthma Immunol. 2012;26:6–8. [Google Scholar]
- 122.Van de Sand L., Bormann M., Alt M., Schipper L., Heilingloh C.S., Steinmann E., Todt D., Dittmer U., Elsner C., Witzke O., Krawczyk A. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses. 2021;13:609. doi: 10.3390/v13040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tang C., Ding H., Sun Y., Han Z., Kong L. A narrative review of COVID-19: magnesium isoglycyrrhizinate as a potential adjuvant treatment. Ann. Palliat. Med. 2021;10:4777–4798. doi: 10.21037/apm-20-1971. [DOI] [PubMed] [Google Scholar]
- 124.Omrani M., Keshavarz M., Nejad Ebrahimi S., Mehrabi M., McGaw L.J., Ali Abdalla M., Mehrbod P. Potential natural products against respiratory viruses: a perspective to develop anti-COVID-19 medicines. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.586993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu S., Zhu Y., Xu J., Yao G., Zhang P., Wang M., Zhao Y., Lin G., Chen H., Chen L., Zhang J. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhong L.L.D., Lam W.C., Yang W., Chan K.W., Sze S.C.W., Miao J., Yung K.K.L., Bian Z., Wong V.T. Potential Targets for treatment of coronavirus disease 2019 (COVID-19): a review of Qing-Fei-Pai-Du-Tang and its major herbs. Am. J. Chin. Med. 2020;48:1051–1071. doi: 10.1142/S0192415X20500512. [DOI] [PubMed] [Google Scholar]
- 127.Yue G.G., Chan B.C., Kwok H.F., To M.H., Hon K.L., Fung K.P., Lau C.B., Leung P.C. Screening for anti-inflammatory and bronchorelaxant activities of 12 commonly used Chinese herbal medicines. Phytother. Res. 2012;26:915–925. doi: 10.1002/ptr.3659. [DOI] [PubMed] [Google Scholar]
- 128.Jeong H.G., Kim J.Y. Induction of inducible nitric oxide synthase expression by 18β-glycyrrhetinic acid in macrophages. FEBS Lett. 2002;513:208–212. doi: 10.1016/s0014-5793(02)02311-6. [DOI] [PubMed] [Google Scholar]
- 129.Khare P., Sahu U., Pandey S.C., Samant M. Current approaches for target-specific drug discovery using natural compounds against SARS-CoV-2 infection. Virus Res. 2020;290 doi: 10.1016/j.virusres.2020.198169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma C., Ma Z., Liao X.L., Liu J., Fu Q., Ma S. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4(+)CD25(+)Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J. Ethnopharmacol. 2013;148:755–762. doi: 10.1016/j.jep.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 131.Guo A., He D., Xu H.B., Geng C.A., Zhao J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci. Rep. 2015;5:14046. doi: 10.1038/srep14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R., Wu S., Wang J., Leung E., Chang H., Li P. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan decoction against coronavirus disease 2019 (COVID-19): in silico and experimental study. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murck H. Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19 infection? Front. Immunol. 2020;11:1239. doi: 10.3389/fimmu.2020.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fukuda S., Horimai C., Harada K., Wakamatsu T., Fukasawa H., Muto S., Itai A., Hayashi M. Aldosterone-induced kidney injury is mediated by NF-κB activation. Clin. Exp. Nephrol. 2011;15:41–49. doi: 10.1007/s10157-010-0373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun Y., Jiang M., Park P.H., Song K. Transcriptional suppression of androgen receptor by 18β-glycyrrhetinic acid in LNCaP human prostate cancer cells. Arch. Pharm. Res. 2020;43:433–448. doi: 10.1007/s12272-020-01228-z. [DOI] [PubMed] [Google Scholar]
- 136.Yu Z., Ohtaki Y., Kai K., Sasano T., Shimauchi H., Yokochi T., Takada H., Sugawara S., Kumagai K., Endo Y. Critical roles of platelets in lipopolysaccharide-induced lethality: effects of glycyrrhizin and possible strategy for acute respiratory distress syndrome. Int. Immunopharmacol. 2005;5:571–580. doi: 10.1016/j.intimp.2004.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seo E.H., Song G.Y., Kwak B.O., Oh C.S., Lee S.H., Kim S.H. Effects of glycyrrhizin on the differentiation of myeloid cells of the heart and lungs in lipopolysaccharide-induced septic mice. Shock. 2017;48:371–376. doi: 10.1097/SHK.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 138.Ingraham N.E., Lotfi-Emran S., Thielen B.K., Techar K., Morris R.S., Holtan S.G., Dudley R.A., Tignanelli C.J. Immunomodulation in COVID-19. Lancet Resp. Med. 2020;8:544–546. doi: 10.1016/S2213-2600(20)30226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kong Z.H., Chen X., Hua H.P., Liang L., Liu L.J. The oral pretreatment of glycyrrhizin prevents surgery-induced cognitive impairment in aged mice by reducing neuroinflammation and Alzheimer's-related pathology via HMGB1 inhibition. J. Mol. Neurosci. 2017;63:385–395. doi: 10.1007/s12031-017-0989-7. [DOI] [PubMed] [Google Scholar]
- 140.Lai S., Wu G., Jiang Z. Glycyrrhizin treatment facilitates extinction of conditioned fear responses after a single prolonged stress exposure in rats. Cell Physiol. Biochem. 2018;45:2529–2539. doi: 10.1159/000488271. [DOI] [PubMed] [Google Scholar]
- 141.Sun K.B., Zhang X.Y., Liu J., Sun R. Network pharmacological analysis and mechanism prediction of Xiaochaihu Decoction in treatment of COVID-19 with syndrome of pathogenic heat lingering in lung and obstructive cardinalate. Chin. Trad. Herb. Drugs. 2020;51:1750–1760. [Google Scholar]
- 142.Jitsuiki K., Katayama I., Iida T., Nagatomo S., Yanagawa Y. Successful treatment of elderly male with COVID-19 infection with severe acute respiratory distress syndrome using multimodal therapy, including immune modulation therapy. Cureus. 2020;12:12402. doi: 10.7759/cureus.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao Z., Xiao Y., Xu L., Liu Y., Jiang G., Wang W., Li B., Zhu T., Tan Q., Tang L., Zhou H. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl. Mater. Interfaces. 2021;13:20995–21006. doi: 10.1021/acsami.1c02755. [DOI] [PubMed] [Google Scholar]
- 144.Chowdhury M.A., Shuvho M.B.A., Shahid M.A., Haque A.M., Kashem M.A., Lam S.S., Ong H.C., Uddin M.A., Mofijur M. Prospect of biobased antiviral face mask to limit the coronavirus outbreak. Environ. Res. 2021;192 doi: 10.1016/j.envres.2020.110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cho T., Han H.S., Jeong J., Park E.M., Shim K.S. A novel computational approach for the discovery of drug delivery system candidates for COVID-19. Int. J. Mol Sci. 2021;22:2815. doi: 10.3390/ijms22062815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li X., Qiu Q., Li M., Lin H., Cao S., Wang Q., Chen Z., Jiang W., Zhang W., Huang Y., Luo H. Chemical composition and pharmacological mechanism of ephedra-glycyrrhiza drug pair against coronavirus disease 2019 (COVID-19) Aging. 2021;13:4811. doi: 10.18632/aging.202622. (Albany N.Y.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dai Y.J., Wan S.Y., Gong S.S., Liu J.C., Li F., Kou J.P. Recent advances of traditional chinese medicine on the prevention and treatment of COVID-19, Chin. J. Nat. Med. 2020;18:881–889. doi: 10.1016/S1875-5364(20)60031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peng X.J., Yang X.J., Xu G., Chen Y.B., Yang C.H., Gong W.L. Investigating clinical efficacy and mechanism of qingfei paidu decoction for treatment of COVID-19 based on integrative pharmacology. Chin. J. Exp. Tradit. Med. Form. 2020;26:6–13. [Google Scholar]
- 149.Yang L., Liu J.Q., Bian Y. Feasibility analysis of compounds glycyrrhizin in the treatment of COVID-19. West China J. Pharm. Sci. 2020;35:342–346. [Google Scholar]
- 150.Ling X.Y., Tao J.L., Sun X., Yuan B. Exploring material basis and mechanism of Lianhua Qingwen prescription against coronavirus based on network pharmacology. Chin. Trad. Herb. Drugs. 2020;7:1723–1730. [Google Scholar]
- 151.Cai Y., Zeng M., Chen Y.Z. The pharmacological mechanism of huashi baidu formula for the treatment of COVID-19 by combined network pharmacology and molecular docking. Ann. Palliat. Med. 2021;10:3864–3895. doi: 10.21037/apm-20-1759. [DOI] [PubMed] [Google Scholar]
- 152.Ren W., Ma Y., Wang R., Liang P., Sun Q., Pu Q., Dong L., Mazhar M., Luo G., Yang S. Research advance on Qingfei Paidu decoction in prescription principle, mechanism analysis and clinical application. Front. Pharmacol. 2021;11 doi: 10.3389/fphar.2020.589714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.An X., Zhang Y., Duan L., Jin D., Zhao S., Zhou R., Duan Y., Lian F., Tong X. The direct evidence and mechanism of traditional chinese medicine treatment of COVID-19. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li L.C., Zhang Z.H., Zhou W.C., Chen J., Jin H.Q., Fang H.M., Chen Q., Jin Y.C., Qu J., Kan K.D. Lianhua qingwen prescription for coronavirus disease 2019 (COVID-19) treatment: advances and prospects. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin H., Wang X., Liu M., Huang M., Shen Z., Feng J., Yang H., Li Z., Gao J., Ye X. Exploring the treatment of COVID-19 with Yinqiao powder based on network pharmacology. Phytother. Res. 2021;35:2651–2664. doi: 10.1002/ptr.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Z., Li X., Gou C., Li L., Luo X., Zhang C., Zhang Y., Zhang J., Jin A., Li H., Zeng Y. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. J. Tradit. Chin. Med. 2020;40:467–472. doi: 10.19852/j.cnki.jtcm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 157.Cheng D.Z., Wang W.J., Li Y., Wu X.D., Zhou B., Song Q.Y. Analysis of curative effect of 51 patients with novel coronavirus pneumonia treated with chinese medicine lianhua qingwen: A multicentre retrospective study. Tianjin Trad. Chin. Med. 2020;37:509–516. Y. [Google Scholar]
- 158.Lv R., Wang W., Li X. Clinical observation on 63 cases of suspected new coronavirus pneumonia treated by lianhuaqingwen. J. Trad. Chin. Med. 2020;61:655–659. [Google Scholar]
- 159.Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., Peng M., Zhang Y., Yan D., Lang S., Zhang Q., Fan A., Ke J., Li X., Liu B., Jiang M., Liu Q., Zhu J., Yang L., Zhu Z., Zeng K., Li C., Zheng Y., Wu H., Lin J., Lian F., Li X., Tong X. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomed. 2021;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen X., Wu Y., Chen C., Gu Y., Zhu C., Wang S., Chen J., Zhang L., Lv L., Zhang G., Yuan Y., Chai Y., Zhu M., Wu C. Identifying potential anti-COVID-19 pharmacological components of traditional chinese medicine lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm. Sin. B. 2021;11:222–236. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qu X.K., Hao S.L., Ma J.H., Wei G.Y., Song K.Y., Tang C., Gao Y.F., Liang S.Q., Du W.J. Observation on clinical effect of Shufeng Jiedu capsule combined with arbidol hydrochloride capsule in treatment of COVID-19. Chin. Trad. Herbal Drugs. 2020;5:1167–1170. [Google Scholar]
- 163.Cheng L., Liu F., Wu J.H. Clinical efficacy of shufeng jiedu capsule combined with western medicine in treatment of common COVID-19 patients by retrospective analysis. Chin. J. Exp. Tradit. Med. Formulae. 2020;26:14–20. [Google Scholar]
- 164.Xiao Q., Jiang Y.J., Wu S.S., Wang Y., An J., Xu W.P., Wu J.J. Analysis of the value of Chinese medicine Shufeng Jiedu capsule combined with abidor in the treatment of mild new coronavirus pneumonia. J. Emergency Trad. Chin. Med. 2020;29:756–758. [Google Scholar]
- 165.Gong X.Y., Wei D.R., Gong X., Xiong Y., Wang T., Mou F.Z. Analysis on TCM clinical characteristics and syndromes of 80 patients with novel coronavirus pneumonia. Chin. J. Inf. TCM. 2020;27:1–6. [Google Scholar]
- 166.Qu Y.F., Fang W., Jin Y.Z., Qin C., Niu X.C., Zhang N. Forty cases of common COVID-19 treated with modified Ephedra and Apricot Kernel and Gypsum and Licorice Decoction combined with western medicine routine treatment. Tradit. Chin. Med. 2020;40:666–669. [Google Scholar]
- 167.Wang R.Q., Yang S.J., Xie C.G., Shen Q., Li M., Lei X., Li J., Huang M. Clinical observation of Qingfeipaidu Decoction in the treatment of COVID-19. Pharmacol. Clin. Chin. Mater. Med. 2020;36:13–18. [Google Scholar]
- 168.Ding X.J., Zhang Y., He D.C., Zhang M.Y., Tan Y.J., Yu A., Yu Q., Wu W., Yang W., Huang H., Liu L. Clinical effect and mechanism of qingfei touxie fuzheng recipe in the treatment of novel coronavirus pneumonia. Herald Med. 2020;39:640–644. [Google Scholar]
- 169.Chen G., Su W., Yang J., Luo D., Xia P., Jia W., Li X., Wang C., Lang S., Meng Q., Zhang Y., Ke Y., Fan A., Yang S., Zheng Y., Fan X., Qiao J., Lian F., Wei L., Tong X. Chinese herbal medicine reduced mortality in patients with severe and critical coronavirus disease 2019: a retrospective cohort study. Front. Med. 2020;14:752–759. doi: 10.1007/s11684-020-0813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang H., Zhao Y., Zuo X.H., Jin J.S., Guo Y. Treatment of COVID-19 by pneumonia No. 1 prescription and pneumonia No. 2 prescription. Acta Chin. Med. 2020:1–11. [Google Scholar]
- 171.Xia W.G., An C.Q., Zheng C.J., Zhang J.X., Huang M., Wang Y. Clinical observation on 34 patients with novel coronavirus pneumonia (COVID-19) treated with intergrated traditional Chinese and western medicine. J. Tradit. Chin. Med. 2020;61:375–382. [Google Scholar]
- 172.Safa O., Hassani-Azad M., Farashahinejad M., Davoodian P., Dadvand H., Hassanipour S., Fathalipour M. Effects of Ginger on clinical manifestations and paraclinical features of patients with Severe Acute Respiratory Syndrome due to COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:841. doi: 10.1186/s13063-020-04765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.https://clinicaltrials.gov/as accessed by April 2022.
- 174.Narkhede R.R., Pise A.V., Cheke R.S., Shinde S.D. Recognition of natural products as potential inhibitors of COVID-19 main protease (Mpro): in-silico evidences. Nat. Prod. Bioprospect. 2020;10:297–306. doi: 10.1007/s13659-020-00253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.https://www.rcsb.org/as accessed by May 2022.
- 176.Rai H., Barik A., Singh Y.P., Suresh A., Singh L., Singh G., Nayak U.Y., Dubey V.K., Modi G. Molecular docking, binding mode analysis, molecular dynamics, and prediction of ADMET/toxicity properties of selective potential antiviral agents against SARS-CoV-2 main protease: an effort toward drug repurposing to combat COVID-19. Mol. Divers. 2021;25:1905–1927. doi: 10.1007/s11030-021-10188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li Y., Chu F., Li P., Johnson N., Li T., Wang Y., An R., Wu D., Chen J., Su Z., Gu X. Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J. Ethnopharmacol. 2021;271 doi: 10.1016/j.jep.2021.113854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Srivastava V., Yadav A., Sarkar P. Molecular docking and ADMET study of bioactive compounds of glycyrrhiza glabra against main protease of SARS-CoV2, Mater. Today Proc. 2022;49:2999–3007. doi: 10.1016/j.matpr.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Manikyam H.K., Joshi S.K. Whole genome analysis and targeted drug discovery using computational methods and high throughput screening tools for emerged novel coronavirus (2019-nCoV) J. Pharm. Drug Res. 2020;3:341. [PMC free article] [PubMed] [Google Scholar]
- 180.Vardhan S., Sahoo S.K. In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19. Comput. Biol. Med. 2020;124 doi: 10.1016/j.compbiomed.2020.103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zígolo M.A., Goytia M.R., Poma H.R., Rajal V.B., Irazusta V.P. Virtual screening of plant-derived compounds against SARS-CoV-2 viral proteins using computational tools. Sci.Tot. Env. 2021;781 doi: 10.1016/j.scitotenv.2021.146400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mahdian S., Ebrahim-Habibi A., Zarrabi M. Drug repurposing using computational methods to identify therapeutic options for COVID-19. J. Diabetes Metab. Disord. 2020;19:691–699. doi: 10.1007/s40200-020-00546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ahmad S., Waheed Y., Abro A., Abbasi S.W., Ismail S. Molecular screening of glycyrrhizin-based inhibitors against ACE2 host receptor of SARS-CoV-2. J. Mol. Model. 2021;27:206. doi: 10.1007/s00894-021-04816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bharath B.R., Damle H., Ganju S., Damle L. In silico screening of known small molecules to bind ACE2 specific RBD on spike glycoprotein of SARS-CoV-2 for repurposing against COVID-19. F1000Research. 2020;9:663. doi: 10.12688/f1000research.24143.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Muhseen Z.T., Hameed A.R., Al-Hasani H.M., ul Qamar M.T., Li G. Promising terpenes as SARS-CoV-2 spike receptor-binding domain (RBD) attachment inhibitors to the human ACE2 receptor: Integrated computational approach. J. Mol. Liq. 2020;320 doi: 10.1016/j.molliq.2020.114493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Muhseen Z.T., Hameed A.R., Al-Hasani H.M., Ahmad S., Li G. Computational determination of potential multiprotein targeting natural compounds for rational drug design against SARS-COV-2. Molecules. 2021;26:674. doi: 10.3390/molecules26030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kalhor H., Sadeghi S., Abolhasani H., Kalhor R., Rahimi H. Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches. J. Biomol. Struct. Dyn. 2020;40:1299–1315. doi: 10.1080/07391102.2020.1824816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen H., Du Q. Preprints; 2020. Potential Natural Compounds for Preventing SARS-CoV-2 (2019-nCoV) Infection. 2020010358. [DOI] [Google Scholar]
- 189.Sinha S.K., Prasad S.K., Islam M.A., Gurav S.S., Patil R.B., AlFaris N.A., Aldayel T.S., AlKehayez N.M., Wabaidur S.M., Shakya A. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: a pharmacoinformatics study. J. Biomol. Struct. Dyn. 2021;39:4686–4700. doi: 10.1080/07391102.2020.1779132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mathew S.M., Benslimane F., Althani A.A., Yassine H.M. Identification of potential natural inhibitors of the receptor-binding domain of the SARS-CoV-2 spike protein using a computational docking approach. Qatar Med. J. 2021;2021:12. doi: 10.5339/qmj.2021.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vardhan S., Sahoo S.K. Computational studies on the interaction of SARS-CoV-2 Omicron SGp RBD with human receptor ACE2, limonin and glycyrrhizic acid. Comput. Biol. Med. 2022;144 doi: 10.1016/j.compbiomed.2022.105367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kandeel M., Kitade Y., Almubarak A. Repurposing FDA-approved phytomedicines, natural products, antivirals and cell protectives against SARS-CoV-2 (COVID-19) RNA-dependent RNA polymerase. PeerJ. 2020;8:e10480. doi: 10.7717/peerj.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rehman M.F., Akhter S., Batool A.I., Selamoglu Z., Sevindik M., Eman R., Mustaqeem M., Akram M.S., Kanwal F., Lu C., Aslam M. Effectiveness of Natural Antioxidants against SARS-CoV-2? Insights from the in-silico world. Antibiotics. 2021;10:1011. doi: 10.3390/antibiotics10081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Patil R., Chikhale R., Khanal P., Gurav N., Ayyanar M., Sinha S., Prasad S., Dey Y.N., Wanjari M., Gurav S.S. Computational and network pharmacology analysis of bioflavonoids as possible natural antiviral compounds in COVID-19. Inform. Med. Unlocked. 2021;22 doi: 10.1016/j.imu.2020.100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Khater S., Kumar P., Dasgupta N., Das G., Ray S., Prakash A. Combining SARS-CoV-2 proofreading exonuclease and RNA-dependent RNA polymerase inhibitors as a strategy to combat COVID-19: a high-throughput in silico screening. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.647693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vankadari N., Jeyasankar N.N., Lopes W.J. Structure of the SARS-CoV-2 Nsp1/5′-untranslated region complex and implications for potential therapeutic targets, a vaccine, and virulence. J. Phys. Chem. Lett. 2020;11:9659–9668. doi: 10.1021/acs.jpclett.0c02818. [DOI] [PubMed] [Google Scholar]
- 197.Ray M., Sarkar S., Rath S.N. Druggability for COVID-19: in silico discovery of potential drug compounds against nucleocapsid (N) protein of SARS-CoV-2. Genomics Inform. 2020;18:e43. doi: 10.5808/GI.2020.18.4.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fatima S.W., Alam S., Khare S.K. Molecular and structural insights of β-boswellic acid and glycyrrhizic acid as potent SARS-CoV-2 Envelope protein inhibitors. Phytomed. Plus. 2022;2 doi: 10.1016/j.phyplu.2022.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F., Tu D., Ge G., Zheng Y., Shi T., Xu X. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., DuJ J. A novel combination of vitamin c, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: a perspective from system biology analysis. Nutrients. 2020;12:1193. doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lau S.K., Lau C.C., Chan K.H., Li C.P., Chen H., Jin D.Y., Chan J.F., Woo P.C., Yuen K.Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J. Gen. Virol. 2013;94:2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 202.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C., Castaño-Rodriguez C., Perlman S., Enjuanes L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Peteranderl C., Herold S. The impact of the interferon/TNF-related apoptosis-inducing ligand signaling axis on disease progression in respiratory viral infection and beyond. Front. Immunol. 2017;8:313. doi: 10.3389/fimmu.2017.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kindrachuk J., Ork B., Mazur S., Holbrook M.R., Frieman M.B., Traynor D., Johnson R.F., Dyall J., Kuhn J.H., Olinger G.G., Hensley L.E. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for middle east respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agent. Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mizutani T. Signal transduction in SARS-CoV-infected cells. Ann. N. Y. Acad. Sci. 2007;1102:86–95. doi: 10.1196/annals.1408.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zheng W., Huang X., Lai Y., Liu X., Jiang Y., Zhan S. Glycyrrhizic acid for COVID-19: findings of targeting pivotal inflammatory pathways triggered by SARS-CoV-2. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.631206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.