Abstract
Softening dry food with water is believed to be more beneficial to the intestinal health and nutrients absorption of dogs by some owners, but there appears to be little scientific basis for this belief. Thus, this study aimed to compare feeding dry food (DF) and water-softened dry food (SDF) on stress response, intestinal microbiome, and metabolic profile in dogs. Twenty healthy 5-month-old beagle dogs were selected and divided into two groups according to their gender and body weight using a completely randomized block design. Both groups were fed the same basal diet, with one group fed DF and the other fed SDF. The trial lasted for 21 days. The apparent total tract digestibility (ATTD) of nutrients, inflammatory cytokines, stress hormones, heat shock protein-70 (HSP-70), fecal microbiota, short-chain fatty acids (SCFAs), branch-chain fatty acids (BCFAs), and metabolomics were measured. Results showed that there was no significant difference in body weight, ATTD, and SCFAs between the DF and SDF groups (p > 0.05), whereas feeding with SDF caused a significant increase in serum cortisol level (p < 0.05) and tended to have higher interleukin-2 (p = 0.062) and HSP-70 (p = 0.097) levels. Fecal 16S rRNA gene sequencing found that the SDF group had higher alpha diversity indices (p < 0.05). Furthermore, the SDF group had higher levels of Streptococcus, Enterococcus, and Escherichia_Shigella, and lower levels of Faecalibacterium (p < 0.05). Serum and fecal metabolomics further showed that feeding with SDF significantly influenced the purine metabolism, riboflavin metabolism, and arginine and proline metabolism (p < 0.05). Overall, feeding with SDF caused higher cortisol level and generated effects of higher intestinal microbial diversity in dogs, but it caused an increase in some pathogenic bacteria, which may result in intestinal microbiome disturbance and metabolic disorder in dogs. In conclusion, feeding with SDF did not provide digestive benefits but caused some stress and posed a potential threat to the intestinal health of dogs. Thus, SDF is not recommended in the feeding of dogs.
Keywords: water-softened dry food, pet food, beagle dog, inflammatory response, fecal microbiota, metabolomics
1. Introduction
Dogs serve as great companions, possibly due to their remarkable social cognitive abilities [1]. Owning a dog can provide emotional benefits and improve one’s physical and mental health [2,3,4]. Dog owners care deeply for their pets and treat them as family members, which is evidenced by the increased pet-related expenditure on things such as pet food, veterinary service, training, and pet-sitting [5]. Therefore, maintaining health and expanding the lifespan of dogs could be of significant interest to owners.
The gut is now considered the largest endocrine organ in mammals, and intestinal health can be a top priority in pet health. Gastrointestinal (GI) health not only refers to the aspects of digestion and absorption of nutrients, but also includes the intestinal microbiota, fermentation of product, and metabolite composition [6]. The gut microbiota is a complex ecosystem that has an impact on many areas of the host’s health, including physiology, behavior, and fitness [7]. GI bacteria can influence host physiology and metabolism through direct contact, and also indirectly by way of microbiota-derived metabolites [8,9]. For instance, the GI microbiota aids food digestion and the production of metabolites, including vitamins and short-chain fatty acids (SCFAs) [10]. When there is an imbalance in the gut microbiota, it either helps or hinders intestinal immunity [11]. On the other hand, gut microbiota and metabolites can be affected by different diets in healthy dogs and cats [12,13,14,15]. Therefore, the composition of the microbiota community and fecal metabolites are critical measures for better evaluation and understanding of the gastrointestinal health of dogs.
In the case of young animals, some pet food companies suggest that puffed foods should be softened before use in feeding. However, these statements lack rigorous experiment-based evidence. There has been some research in recent years on the effects of various diets, including fresh and raw meat diets on the apparent total tract digestibility (ATTD), microbiota, and metabolome of the canine gastrointestinal tract [16,17,18]. However, as we know, no study has been carried out on the effect of water-softened dry food (SDF) on dog’s health. Thus, the objective of this study was to evaluate whether feeding with SDF is beneficial to dogs. The effects of a three-week consumption of water-SDF on healthy dogs were examined in the current study by determining the ATTD of macronutrients, immune response, stress hormones, heat shock protein-70 (HSP-70), and fecal characteristics, microbiota, and metabolites.
2. Materials and Methods
2.1. Animals and Housing
Protocols for all experiments were approved by the Experimental Animal Ethics Committee of South China Agricultural University (protocol code 2021e028). This study included twenty beagle dogs, and their information was shown in Table 1. Subjects were all housed individually kennels measured 1.2 × 1.0 × 1.1 m in an enclosed room with constant temperature (24 ± 1 °C), relative humidity (70 ± 5%) and light control conditions (12 h dark–light cycle, light: 06:00–18:00) in the building of Experimental Animal Centre of South China Agricultural University. All dogs can interact with other dogs and staff daily and were provided opportunities to go out and play at least once a week.
Table 1.
Detailed information of dogs in this study.
| Group 1 | N (Male: Female) | Age (M) | Weight (kg) |
|---|---|---|---|
| DF | 10 (4:6) | 5.38 ± 0.35 | 7.33 ± 0.12 |
| SDF | 10 (4:6) | 5.62 ± 0.29 | 7.25 ± 0.18 |
1 DF: dry food, SDF: water-softened dry food.
2.2. Diets and Feeding
The basal diet used in this experiment was normal commercial dog food (Ramical, Guangzhou, China). The experimental dogs were fed 200 g every day (twice—at 09:00 and 17:00) to meet their energy requirements, which is calculated approximately according to the standard of the National Research Council (NRC, 2000). During the experiment, dogs had free access to clean water at any time through the water nipple. Table 2 showed the nutrient composition of the diet used in this experiment.
Table 2.
The chemical composition of the basal diet.
| Items 1 | Basal diet 2 |
|---|---|
| DM (%) | 91.19 |
| OM (% DM) | 91.57 |
| CP (% DM) | 27.69 |
| AHF (% DM) | 11.21 |
| TDF (% DM) | 3.63 |
1 DM: dry matter; OM: organic matter; CP: crude protein; AHF: acid-hydrolyzed fat; TDF: total dietary fiber. 2 Extruded diet: corn flour, flour, sweet potato, chicken powder, meat and bone meal, soybean meal, beef meal, calcium hydrophosphate, calcium chloride, lysine, methionine, vitamin A, vitamin D, vitamin E, copper sulfate, ferrous sulfate, zinc sulfate, and manganese sulfate.
2.3. Experimental Design
In this study, a total of 20 beagle dogs were chosen. All animals were physically examined at the vet clinic before the start of the trial; vaccination and deworming were completed two months before the experiment. Following an acclimation period of one month when all dogs were fed the basal diet, they were divided into two groups according to their gender and body weight (BW) using a completely randomized block design. One group continually fed the same DF as in the acclimation period (DF, n = 10, male:female = 4:6), and the other group fed SDF was made by the mixture of 1.5 mL purified water with 1 g of dry food (SDF, n = 10, male:female = 4:6). The water used in this test was filtered through a filter to ensure quality. Additionally, the water/dry food ratio was determined to ensure the pet food was neither too tough nor too soft. The whole trial lasted for 21 days. BW and body condition score (BCS) were recorded every ten days, and fecal score (FS) was recorded every day and evaluated as described previously [19]. Fresh fecal samples and blood samples were collected on the last day of the experiment period, and the total fecal collection was performed in the last 3 days.
2.4. Chemical Analysis and Digestibility Measurements
Digestibility was measured by the total fecal collection method. A mass of 200 g dry food of basal diet was collected every 10 days, and all feces was collected and 10% HCl was added to the nitrogen fixation for the last three consecutive days in this study. All fecal samples were oven-dried for 48 h (60 °C), then diet and dry fecal samples were ground in a grinder with a 1 mm screen for further chemical analysis. Dry matter (DM) and organic matter (OM) of the diet and feces were determined according to Association of Official Analytical Chemists (AOAC) (2000; method 950.46 and 942.05). A fatty analyzer (FT640, Grand Analytical Instrument Co., Ltd., Guangzhou, China) was used to determine the acid-hydrolyzed fat level (AHF) of the diet and feces (AOAC, 2000; method 954.01). An automatic fiber analyzer (FIBRETHERM FT12, C. Gerhardt GmbH & Co. KG, Königswinter, Germany) was used to analyze the total dietary fiber (TDF) content of the diet and feces (AOAC, 2000; method 962.09). Crude protein (CP) was determined using AOAC with the semi-automatic Kjeldahl apparatus (VAPODEST 200, C. Gerhardt GmbH & Co. KG, Germany). Besides, ATTD values were calculated using the equation as follows: [nutrient intake (g/d, DM basis) − fecal output (g/d, DM basis)]/nutrient intake (g/d, DM basis) × 100.
2.5. Blood Sample Collection and Analysis
On day 21, after overnight fasting, 5 mL of blood was taken via forelimb venipuncture. The samples were then clotted for 30 min and centrifugated for 15 min to separate the supernatant for further analyses. Commercial canine enzyme-linked immunosorbent assay (ELISA) kits (MEIMIAN, Jiangsu Meimian Industrial Co., Ltd., Yancheng, China) were used to measure the serum cortisol (COR), adrenocorticotropin hormone (ACTH), glucocorticoids (GC), HSP-70, immunoglobulin G (IgG), tumor necrosis factor-alpha (TNF-α), interferon-γ (INF-γ), interleukin-6 (IL-6), interleukin-4 (IL-4), and interleukin-2 (IL-2).
2.6. Microbial Analyses
2.6.1. DNA Extraction, Amplification, and Sequencing
Total genomic DNA was isolated from fresh fecal samples using the cetyltrimethylammonium bromide method. DNA concentration and purity were monitored on a 1% agarose gel. The DNA was diluted to 1 ng/μL with sterile water based on the concentration. The 16S rRNA genes for 16S V3-V4 were amplified using primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGGTATCTAAT-3′) with barcodes. For all PCR reactions, a volume of 15 µL Phusion High Fidelity PCR Master Mix (New England Biolabs) was used, along with 2 µM of forward and reverse primers and nearly 10 ng of template DNA. The thermal cycling process was set as follows: initial denaturation for 1 min (98 °C), denaturation for 10 s (98 °C), annealing for 30 s (50 °C), and extension twice for 30 s and 5 min, respectively (72 °C). The PCR products were added with the same volume of (1X) loading buffer (including SYB Green) in an equal density ratio which was subsequently analyzed by electrophoretic manipulation on a 2% agarose gel. Using the Qiagen Gel Extraction Kit, the PCR product combination was then purified (Qiagen, Hilden, Germany). The TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA) was used to build sequencing libraries, and index codes were then added. A Qubit@ 2.0 fluorometer (Thermo Scientific, Waltham, MA, USA) and an Agilent Bioanalyzer 2100 system were used to evaluate the library’s quality. Finally, the Illumina NovaSeq technology was used to generate final paired-end reads of 250 bp.
2.6.2. Bioinformatics Analysis
Pairs of reads were combined using FLASH (V1.2.7, http://ccb.jhu.edu/software/FLASH/, accessed on 10 June 2022) [20], and the spliced sequence was referred to as the original label. The raw labels were quality filtered according to the quality control process of QIIME (V1.9.1, http://qiime.org/scripts/split_libraries_fastq.html, accessed on 10 June 2022) [21], resulting in high-quality clean labels [22]. Chimeric sequences were detected by comparing the tags with a reference database (Silva database, https://www.arb-silva.de/, accessed on 10 June 2022) using the UCHIME algorithm (UCHIME http://www.drive5.com/usearch/manual/uchime_algo.html, accessed on 10 June 2022) [23]. Then, we removed the chimeric sequences [24] and finally obtained the valid tags.
We used Uparse software (Uparse v7.0.1001, http://drive5.com/uparse/, accessed on 10 June 2022) (Edgar, 2013) to perform sequence analysis. Same OTU were defined as those sequences with ≥97% similarity. For each representative sequence, the classification information was annotated according to the Mothur algorithm and using the Silva database (http://www.arb-silva.de/, accessed on 10 June 2022) [25]. We used MUSCLE software Multiple to performing sequence comparisons (version 3.8.31, http://www.drive5.com/muscle/, accessed on 10 June 2022) [26] to investigate the phylogenetic relationships of different OTUs. Abundance information of OTUs was normalized using sequence number criteria corresponding to the least sequenced sample for further analyses of both alpha-diversity and beta-diversity. Alpha-diversity was measured by six indices, including Observed_species, Chao1, Shannon, Simpson, Ace, and Goods_coverage. All these indices in our samples were calculated using QIIME (version 1.7.0) and displayed using R software (version 2.15.3).
2.7. Fecal Short-Chain Fatty Acids and Branch-Chain Fatty Acids Analyses
The gas chromatograph (Shimadzu, Kyoto, Japan) coupled to a flame ionization detector and chromatographic separation capillary column (DB-FFAP, 30 m × 0.25 mm × 0.25 μm) was used in this study to analyze the concentrations of fecal SCFAs (acetic acid, butyric acid, and propionic acid) and branch-chain fatty acids (BCFAs) (isovaleric acid, isobutyric acid, and valeric acid). The instrument parameters and sample processing procedures of extraction of fecal volatiles referred to our previous study [27].
2.8. Untargeted Fecal and Serum Metabolomics Analysis
The sample processing procedures of extraction of fecal and serum metabolites referred to our previous study [27]. UPLC-Orbitrap-MS/MS analysis method was used as in a previous study [28], only with slight alterations. Thermo Fisher Scientific’s compound Discover 2.1 data analysis tool was used to scan the mzCloud and mzVault libraries for metabolites. Performing multivariate analysis, MetaboAnalyst 5.0 (https://www.metaboanalyst.ca, accessed on 10 June 2022) was used. In this study, principal component analysis (PCA) and pathway enrichment analyses were performed on MetaboAnalyst 5.0.
2.9. Statistical Analysis
SPSS 26.0 was used for statistical analysis, and GraphPad Prism 8.0.3 software was used for graphical presentation. Student’s t tests were employed to compare two groups. The mean ± standard error (SE) was used to express all data. Significance was set at p < 0.05, a trend was defined by 0.05 < p < 0.10. Selecting the metabolites whose adjusted p value < 0.05 (calculated by Student’s t test) and the variable importance in the projection (VIP) >2 to obtain the differential metabolites.
3. Results
3.1. Effects of SDF on Body Weight, Body Condition Score, and Fecal Score
As shown in Table 3, there was no difference in BW or BCS between the DF and SDF groups throughout the trial period (p > 0.05). The total fecal score (TFS) of the SDF group was markedly higher than that of the DF group (p < 0.05). The total soft feces ratio (TFSR) of the DF and SDF groups were both 0.48%. The total diarrhea ratio (TDR) of the SDF group was 0.95%, which was 2.38% higher than TDR of the DF group (3.33%).
Table 3.
Body weight, body condition score, and fecal score in dogs fed DF and SDF on days 11 and 21.
| Item 1 | DF 2 | SDF 3 | p Value |
|---|---|---|---|
| BW (kg) | 8.01 ± 0.14 | 7.87 ± 0.18 | 0.540 |
| BCS | 5.05 ± 0.17 | 4.65 ± 0.11 | 0.660 |
| TFS | 2.55 ± 0.14 | 2.62 ± 0.25 | 0.028 |
| TSFR (%) | 0.48 | 0.48 | - |
| TDR (%) | 0.95 | 3.33 | - |
1 BW: body weight; BCS: body condition score; all the test dogs were weighed and BCS were recorded with a nine-point scale on days 1, 11, and 21 before morning feeding. TFS: total fecal score. All samples were scored in 0.5 units increments using the following scale: 1 = hard, dry pellets, small hard masses; 2 = hard, stool, remains firm and soft; 3 = soft, formed, and moist stool, retains shape; 4 = soft, unformed stool, assumes shape of container; 5 = watery, liquid that can be poured; in which, 1 ≤ FS < 2 constipation, 2 ≤ FS ≤ 3 normal, 3 < FS < 4 soft stool, 4 ≤ FS ≤ 5 diarrhea. TSFR: total soft feces ratio. Ratio of the number of soft feces to the total number of fecal scores. TDR: total diarrhea ratio. Ratio of the number of diarrhea to the total number of fecal scores. 2 DF: dry food. 3 SDF: water-softened dry food.
3.2. Effects of Soften Dry Food on Apparent Total Tract Macronutrient Digestibility
As shown in Table 4, fecal output, ATTD of DM, OM, CP, AHF, and TDF all showed no significant difference between the two groups (p > 0.05).
Table 4.
Food intake, fecal characteristics, and apparent total tract macronutrient digestibility in dogs fed DF and SDF.
| Item | DF 1 | SDF 2 | p Value |
|---|---|---|---|
| Food intake 3, g/d (DM basis) | 184.00 | 184.00 | - |
| Fecal output, g/d (as is) | 91.07 ± 4.78 | 89.00 ± 1.81 | 0.693 |
| Fecal output, g/d (DM basis) | 24.90 ± 1.27 | 23.82 ± 1.73 | 0.628 |
| Digestibility, % | |||
| Dry matter | 88.55 ± 0.01 | 89.04 ± 0.01 | 0.628 |
| %DM basis | |||
| Organic matter | 81.28 ± 0.26 | 82.39 ± 0.10 | 0.693 |
| Crude protein | 83.88 ± 0.01 | 85.52 ± 0.01 | 0.301 |
| Acid hydrolyzation fat | 94.83 ± 0.01 | 95.84 ± 0.01 | 0.104 |
| Total dietary fiber | 70.73 ± 0.02 | 66.82 ± 0.01 | 0.202 |
1 DF: dry food. 2 SDF: water-softened dry food. 3 All dogs were fed a restricted diet during the trial and all dogs had no leftovers at each meal.
3.3. Effects of SDF on Serum Hormones, Heat Shock Protein-70, IgG, and Inflammatory Factors
As shown in Table 5, the SDF group tended to have higher IL-2 (p = 0.062) and HSP-70 (p = 0.097) levels than the DF group. The SDF group also had a higher level of COR compared with the DF group (p < 0.05). Besides, there was no difference shown in IgG, IFN-γ, IL-6, IL-4, TNF-α, GC, and ACTH of the two groups (p > 0.05).
Table 5.
Serum IgG, inflammatory factors, hormones, and HSP-70 in dogs fed DF and SDF on day 21.
| Item 1 | DF 2 | SDF 3 | p Value |
|---|---|---|---|
| IgG | 258.88 ± 16.04 | 227.38 ± 14.90 | 0.167 |
| IFN-γ | 42.10 ± 1.39 | 45.23 ± 1.23 | 0.108 |
| IL-6 | 541.77 ± 20.15 | 567.00 ± 21.69 | 0.405 |
| IL-4 | 163.72 ± 14.20 | 146.66 ± 9.65 | 0.334 |
| IL-2 | 254.86 ± 11.54 | 287.23 ± 11.42 | 0.062 |
| TNF-α | 77.08 ± 4.15 | 77.12 ± 4.69 | 0.995 |
| GC | 321.91 ± 13.13 | 318.80 ± 9.23 | 0.887 |
| ACTH | 67.23 ± 3.31 | 68.10 ± 2.45 | 0.836 |
| COR | 394.24 ± 13.13 | 438.50 ± 9.23 | 0.014 |
| HSP-70 | 334.50 ± 18.05 | 376.07 ± 15.42 | 0.097 |
1 IgG = immunoglobulin G; IFN-γ = interleukin-γ; IL-6 = interleukin-6; IL-4 = interleukin-4; IL-2 = interleukin-2; ACTH = adreno-cortico-tropic-hormone; TNF-α = tumor necrosis factor; GC = glucocorticoid; COR = cortisol; HSP-70 = heat stress protein 70. 2 DF: dry food. 3 SDF: water-softened dry food.
3.4. Effects of SDF on Fecal Microbiota
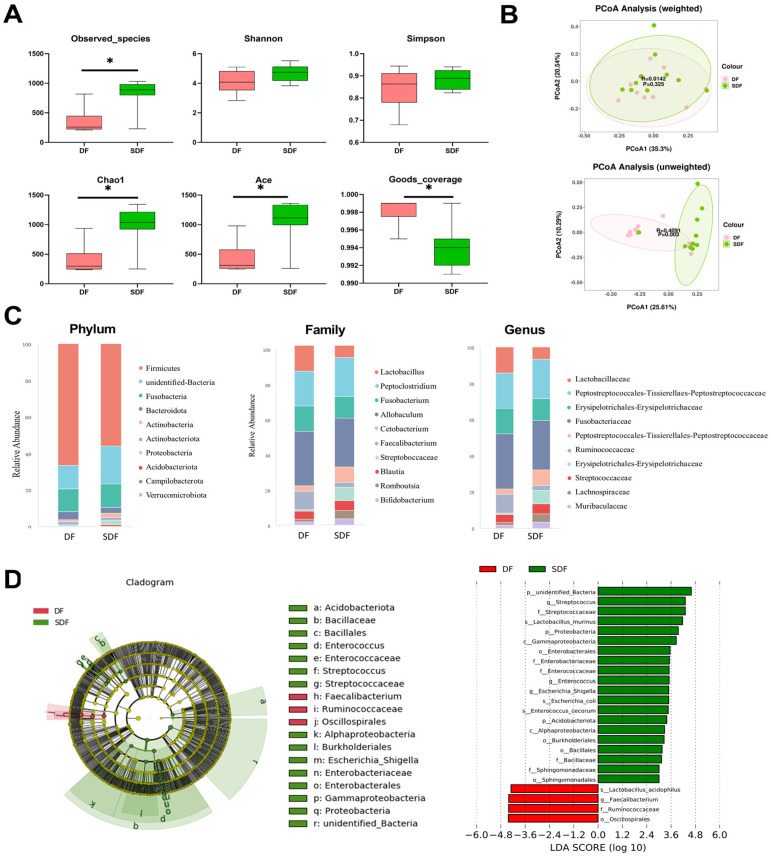
As shown in Figure 1A, alpha diversity analyses of all samples were performed. Chao1 and Ace represent species richness, while the Shannon and Simpson indices are indicative of a diverse microbial population. We can see that the fecal microbial communities of the SDF group had more Observed_species, higher Ace and Chao1 indices levels than that of the DF group, whereas the fecal microbial of the SDF group had lower Goods_coverage over that of the DF group (p < 0.05). Besides, fecal samples were evaluated for beta diversity to analyze the structural difference of microbial community (Figure 1B). There was no separation between the DF and SDF groups in PCoA of weighted UniFrac distances (p > 0.05). However, PCoA of unweighted UniFrac distances revealed distinct separation between two groups (p < 0.05), which showed that SDF could influence the gut microbiota composition and structure in dogs.
Figure 1.
Alpha diversity indices (Observed_species, Shannon, Simpson, Chao1, Ace and Goods_coverage) of fecal microbial communities of the DF and SDF groups on day 21 (A). The symbol (*) indicates statistically significant differences between two groups. Principal Coordinate Analysis (PCoA) based on weighted and unweighted UniFrac distances of fecal microbial communities of the DF and SDF groups on day 21 (B). Predominant fecal microbial communities and different bacteria at the phylum, family, and genus levels of the DF and SDF groups on day 21 (C). LEfSe analysis identified gut bacterial biomarkers of the DF and SDF groups on day 21 (D). The histogram of the LDA scores presents species (biomarker) whose abundance showed significant differences between different groups. The LDA score represents the effect size. In the cladogram, circles radiating from the inner side to outer side represent taxonomic level from phylum to genus (species). Each circle’s diameter is proportional to the taxon’s relative abundance. Yellow nodes refer to the bacteria that contributed a lot in the DF group and blue nodes refer to the bacteria dominant in the SDF group. DF = dry food; SDF = softened dry food.
The top 6 abundant phyla included Firmicutes, Fusobacteria, Bacteroidetes, Actinobacteria, Actinobacteriota, and Proteobacteria (Figure 1C). Additionally, the relative abundances at the family level indicated that the top 8 abundant families included Lactobacillaceae, Peptostreptococcaceae, Erysipelotrichaceae, Fusobacteriaceae, Ruminococcaceae, Streptococcaceae, Lachnospiraceae, and Muribaculaceae (Figure 1C). Finally, the top 10 abundant genera were Lactobacillus, Peptoclostridium, Fusobacterium, Allobaculum, Cetobacterium, Faecalibacterium, Streptoboccaceae, Blautia, Romboutsia, and Bifidobacterium (Figure 1C).
To select the differential bacteria, differential abundant taxa were confirmed by LEfSe analysis (LDA > 3.0). Proteobacteria and Acidobacteriota were enriched in the SDF group at the phylum level. At the family level, Proteobacteria, Enterobacteriaceae, Enterococcaceae, and Sphingomonadaies were enriched in the SDF group, and Ruminococcaceae was enriched in the DF group. Furthermore, at a genus level, Streptococcus, Enterococcus, and Escherichia_Shigella were enriched in the SDF group, and Faecalibacterium was enriched in the DF group (Figure 1D).
3.5. Effects of SDF on Fecal Short-Chain Fatty Acids and Branch-Chain Fatty Acids
As shown in Table 6, no significant difference occurred in the concentrations of fecal total SCFAs, acetic acid, propionic acid, butyric acid, total BCFAs, valeric acid, isovaleric acid, and isobutyric acid (p > 0.05) between the DF and SDF groups.
Table 6.
Fecal SCFAs and BCFAs concentrations in dogs fed DF and SDF.
| Item (µg/g) | DF 1 | SDF 2 | p Value |
|---|---|---|---|
| Acetic acid | 1476.31 ± 35.88 | 1479.08 ± 54.89 | 0.968 |
| Propionic acid | 1079.94 ± 23.41 | 1063.59 ± 43.24 | 0.751 |
| Butyric acid | 442.42 ± 19.53 | 443.47 ± 13.93 | 0.891 |
| Total SCFAs 3 | 2998.66 ± 72.02 | 2986.13 ± 103.37 | 0.924 |
| Isobutyric acid | 92.21 ± 7.12 | 91.06 ± 9.95 | 0.955 |
| Isovaleric acid | 142.52 ± 12.76 | 141.41 ± 14.25 | 0.550 |
| Valeric acid | 19.70 ± 3.26 | 17.35 ± 2.20 | 0.891 |
| Total BCFAs 4 | 254.43 ± 20.20 | 249.82 ± 25.68 | 0.922 |
1 DF: dry food. 2 SDF: softened dry food. 3 Total SCFAs: total short-chain fatty acids = acetic acid + propionic acid + butyric acid. 4 Total BCFAs: total branch-chain fatty acids = isobutyric acid + isovaleric acid + valeric acid.
3.6. Effects of SDF on Fecal Metabolome
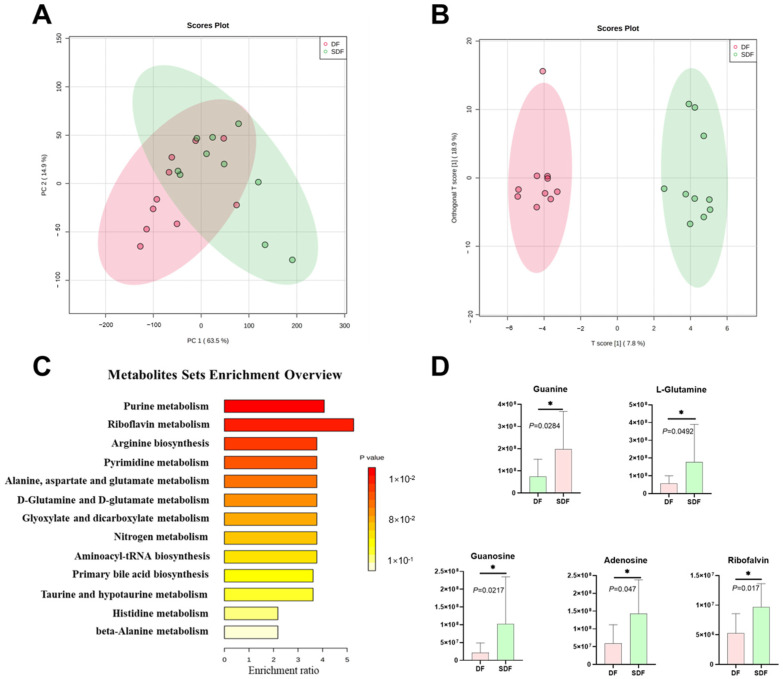
To investigate the metabolic regulation in the gut of SDF, the fecal metabolites were analyzed using UPLC-Orbitrap-MS/MS. Metabolomics analysis from all fecal samples identified 249 metabolites. PCA of the fecal metabolomics shows that SDF influenced the fecal metabolites in the gut of dogs (Figure 2A). Furthermore, the Orthogonal Partial Least-Squares-Discriminant Analysis (ortho PLS-DA) for fecal metabolomics in two groups shows significant separation (Figure 2B). This result confirms the effects of SDF on fecal metabolites.
Figure 2.
Principal components analysis (PCA) for fecal metabolomics data of dogs fed DF and SDF on day 21 (A). Orthogonal Partial Least-Squares-Discriminant Analysis (orthoPLS-DA) for fecal metabolomics data of dogs fed DF and SDF on day 21 (B). Enrichment bar plot based on the fecal differential metabolites of dogs fed DF and SDF on day 21 (C). Bar plot based on the fecal differential metabolites which were enriched in the differential metabolite pathways between two groups (D). The symbol (*) indicates the difference between the dry food (DF) and softened dry food (SDF) groups.
Twenty-four fecal metabolites were significantly different between the DF and SDF groups (Table 7). Dogs fed SDF had higher contents of isoquinoline, N-Acetylmuramic acid, riboflavin, guanosine, glutamylglutamine, tigilc acid, 5-Aminopentanoic acid, taurine, L-Glutamine, guanine and deoxyadenosine, and lower contents of 16-Hydroxy hexadecenoic acid, stearoylethanolamide, tryptophanol, N, N-Dimethylsphingosine, 4-Dodecylbenzenesulfonic acid Na salt, oleamide, anandamide, sebacic aced, 2-Piperidinone, carnosine, palmitic amide, adenosine, and fluoxymesteron. The enrichment analysis platform of Metaboanalyst 5.0 was adopted to explore the differential metabolic pathways (Figure 2C). Among these, purine metabolism (nucleotide metabolism) and riboflavin metabolism (metabolism of cofactors and vitamins) were significantly influenced. The specific fecal metabolites enriched in the differential pathways were showed in the bar plot (Figure 2D).
Table 7.
Fecal differential metabolites were identified according to the standard of VIP > 1.5 and p < 0.05 between the DF and SDF groups.
| Metabolites | p Value | VIP 1 | KEGG 2 | Trend (SDF 3 vs. DF 4) |
|---|---|---|---|---|
| 16-Hydroxy hexadecenoic acid | 0.000 | 2.03 | - | Down |
| Stearoylethanolamide | 0.000 | 1.96 | - | Down |
| Isoquinoline | 0.002 | 2.01 | C06323 | Up |
| Tryptophanol | 0.002 | 2.26 | C00955 | Down |
| N, N-Dimethylsphingosine | 0.002 | 2.17 | C13914 | Down |
| N-Acetylmuramic acid | 0.004 | 1.58 | C02713 | Up |
| Riboflavin | 0.004 | 2.28 | C00255 | Up |
| 4-Dodecylbenzenesulfonic acid Na salt | 0.004 | 2.24 | - | Down |
| Oleamide | 0.005 | 2.03 | C19670 | Down |
| Anandamide | 0.005 | 1.63 | C11695 | Down |
| Guanosine | 0.006 | 1.98 | C00387 | Up |
| Sebacic acid | 0.006 | 2.15 | C08277 | Down |
| Glutamylglutamine | 0.012 | 1.92 | - | Up |
| 2-Piperidinone | 0.020 | 2.12 | - | Down |
| Carnosine | 0.027 | 2.08 | C00386 | Down |
| Tiglic acid | 0.027 | 1.73 | C08279 | Up |
| Palmitic amide | 0.023 | 1.82 | - | Down |
| 5-Aminopentanoic acid | 0.028 | 1.72 | C00431 | Up |
| Taurine | 0.029 | 1.61 | C00245 | Up |
| Adenosine | 0.034 | 1.51 | C00212 | Down |
| L-Glutamine | 0.035 | 1.56 | C00064 | Up |
| Fluoxymesterone | 0.036 | 1.57 | - | Down |
| Guanine | 0.039 | 1.89 | C00242 | Up |
| Deoxyadenosine | 0.040 | 1.51 | C00559 | Up |
1 VIP: variable importance in the projection. 2 KEGG: Kyoto Encyclopedia of Genes and Genomes. 3 SDF: water-softened dry food. 4 DF: dry food.
3.7. Effects of SDF on Serum Metabolome
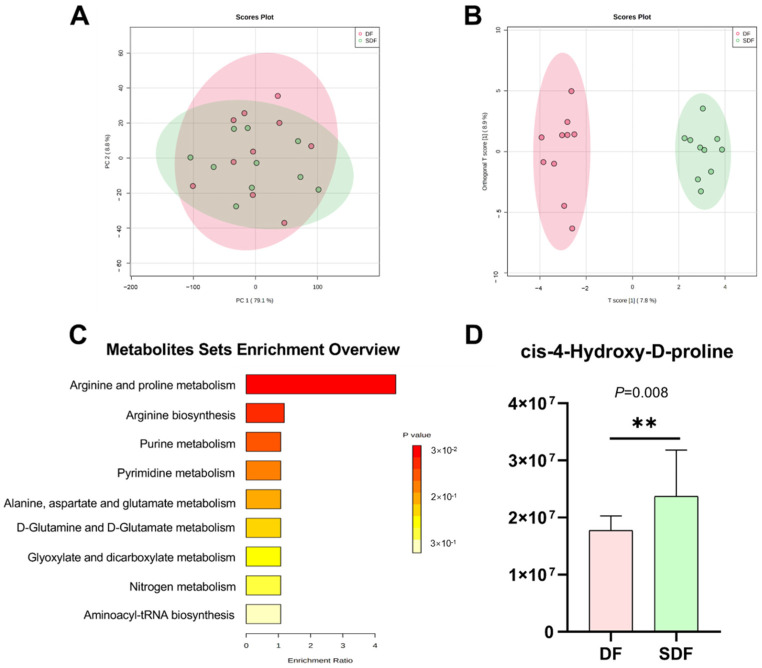
To further investigate the effects of an SDF diet on metabolic regulation in dogs, we used UPLC-Orbitrap-MS/MS to analyze the serum metabolites. Metabolomics analysis from all serum samples identified 126 metabolites. PCA of the serum metabolomics showed no significant separation between two groups (Figure 3A). However, the ortho PLS-DA for serum metabolomics in the two groups shows significant separation (Figure 3B). This result shows that the SDF influenced the serum metabolites in dogs.
Figure 3.
Principal components analysis (PCA) for serum metabolomics data of dogs fed dry food (DF) and softened dry food (SDF) on day 21 (A). Orthogonal Partial Least-Squares-Discriminant Analysis (orthoPLS-DA) for serum metabolomics data of dogs fed dry food (DF) and softened dry food (SDF) on day 21 (B). Enrichment bar plot based on the serum differential metabolites of dogs fed dry food (DF) and softened dry food (SDF) on day 21 (C). Bar plot based on the serum differential metabolites which were enriched in the differential metabolite pathways between two groups (D). The symbol (**) indicates the difference between the dry food (DF) and softened dry food (SDF) groups.
We selected the differential metabolites with VIP > 1.5 and p < 0.05, and 13 serum metabolites were significantly different between the DF and SDF groups (Table 8). Dogs fed SDF had higher contents of 3-Hydroxycapric acid, 3-indolebutyric acid, cis-4-hydroxy-D-proline, isoquinoline, alpha-hydroxyisobutyric acid, and L-glutamine, lower contents of N-acetylornithine, fluoren-9-one, 3-cresotinic acid, 3,4-dihydroxyhydrocinnamic acid, N-acetyl-L-phenylalanine, and indole-3-propionic acid. The enrichment analysis platform of Metaboanalyst 5.0 was adopted to explore the differential metabolic pathways (Figure 3C). Among these, arginine and proline metabolism were significantly influenced. Furthermore, the specific serum metabolites enriched in the differential pathways were showed in the bar plot (Figure 3D).
Table 8.
The serum differential metabolites were identified according to the standard of VIP > 1.5 and p < 0.05 between the DF and SDF groups.
| Metabolites | p Value | VIP 1 | KEGG 2 | Trend (SDF 3 vs. DF 4) |
|---|---|---|---|---|
| 3-Hydroxycapric acid | 0.001 | 2.49 | - | Up |
| N-Acetylornithine | 0.004 | 2.13 | C00437 | Down |
| 3-Indolebutyric acid | 0.006 | 2.16 | C11284 | Up |
| cis-4-Hydroxy-D-proline | 0.008 | 2.02 | C03440 | Up |
| 3-Hydroxybutyric acid | 0.011 | 1.94 | C01089 | Up |
| Isoquinoline | 0.011 | 2.05 | C06323 | Up |
| Fluoren-9-one | 0.012 | 1.87 | C06712 | Down |
| 3-Cresotinic acid | 0.022 | 1.89 | C14088 | Down |
| 3,4-Dihydroxyhydrocinnamic acid | 0.024 | 1.84 | C10447 | Down |
| N-Acetyl-L-phenylalanine | 0.026 | 1.85 | C03519 | Down |
| Alpha-Hydroxyisobutyric acid | 0.027 | 1.90 | - | Up |
| L-Glutamine | 0.030 | 1.76 | C00064 | Up |
| Indole-3-propionic acid | 0.042 | 1.83 | - | Down |
1 VIP: variable importance in the projection. 2 KEGG: Kyoto Encyclopedia of Genes and Genomes. 3 SDF: water-softened dry food. 4 DF: dry food.
3.8. Correlation between Differential Metabolites and Fecal Bacteria at the Genus Level
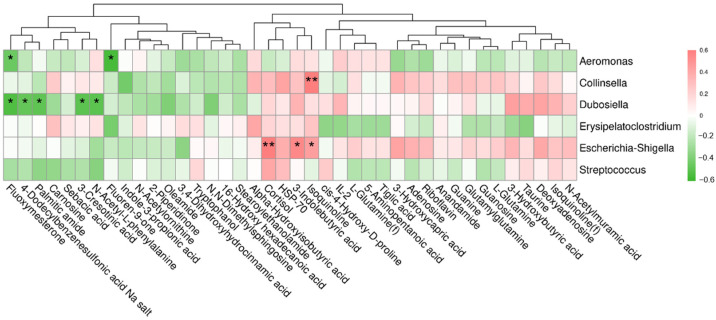
Correlation between differential metabolites (fecal and serum) and fecal bacteria (at the genus level) is displayed in the heatmap (Figure 4). As we can see that Aeromounas were negatively associated with fluoxymesterone and fluoren-9-one, Dubosiella were negatively associated with fluxoymesterone, 4-Dodecylbenzenesulfonic acid Na salt, palmitic amide, 3-cresotinic acid and N-acetyo-L-phenylalanine. Besides, Collinsella had a positive association with isoquinoline, and Escherichia-Shigella had a positive association with serum cortisol, 3-indolebutyric acid, and isoquinoline.
Figure 4.
Correlation heatmap between differential metabolites and differential genera in the dogs on day 21. The symbol (*) indicates a significant correlation between serum metabolites and fecal bacteria (* p < 0.05, ** p < 0.01). Pink color indicates a positive correlation, and green color indicates a negative correlation.
4. Discussion
With scientific and healthy concepts being introduced more in pet feeding strategies, pet owners are paying extra attention to the diet quality and intestinal health of their pets. Although some pet food companies recommend that feeding pets with SDF is better for their health, there is no rigorous experiment-based evidence to suggest this. Therefore, we investigated the effects of SDF on ATTD, immune response, stress indicators, and fecal characteristics, microbiota, and metabolites in dogs.
Nutrient digestibility is an important measurement for evaluating the quality of a diet for dogs [29]. Many factors, including dietary ingredients, processing mode, and animal’s physiology state can affect the nutrient digestibility, while fecal output may also be influenced by food intake, the chemical composition of the diet, physiological state, and nutrient digestibility of animals [30]. Higher nutrient digestibility usually results in lower fecal output. This can correspond to the results of the current study. Under the premise of daily intake of the same amounts of foods, there were no significant differences in fecal output and ATTD. Additionally, SDF had no effect on nutrient digestibility in dogs. In addition, nutrient digestibility, fiber content, DM intake, and fat tolerance can affect the fecal quality [31,32]. Even though the basal diet was the same for both groups in the present study, SDF resulted in softer stools and a higher diarrhea ratio than DF. This may be caused by the disturbance of the intestinal bacteria.
In this study, serum COR of the SDF group was significantly higher than that of the DF group on day 21. An increase in COR is commonly observed in stress response where internal and external stimulus activates the hypothalamic-pituitary-adrenal axis and triggers a cascade of hormonal release [33,34]. Besides, we found that serum COR level was positively associated with Escherichia-Shigella, indicating that the increase in Escherichia-Shigella may cause stress to dogs fed with SDF, thereby inducing an increase in th serum COR level. Additionally, the SDF group tended to have higher serum IL-2 and HSP-70 levels than the DF group. IL-2 is the major regulatory hormone of the immune system [35]. Heat shock proteins are induced by a wide variety of stressors and have broad cytoprotective functions; in particular, HSP-70 plays a vital role in cellular protection [36,37,38]. This may suggest that feeding with SDF may exert stress and have a tendency to cause inflammation in dogs.
In addition to the signs of gastrointestinal health (e.g., fecal state, diarrhea ratio, and volume) that are noticeable to pet owners, the abundance and activity of gut microbiota are critical to the long-term health [39,40,41]. Different diets can regulate the gut microbiota [12]. In our study, the results of alpha diversity in fecal microbial communities revealed significant differences between the two groups on day 21. Changes in the microbial communities occurred in the SDF group, which had more Observed_species, higher Ace and Chao1 indices, and lower Goods_coverage than the DF group. Moreover, a significant separation occurred between two groups in PCoA analysis based on unweighted UniFrac distances. In short, feeding with SDF could increase the species richness, the diversity of the gut microbiota and change the intestinal microbiome structure in dogs. The most abundant phyla found in the two groups were Firmicutes, Fusobacteria, Bacteroidetes, Actinobacteria, and Proteobacteria, which are similar to the previous studies [16,17,42]. The results of LEfSe showed that feeding dogs with SDF significantly changed with phyla Proteobacteria and Acidobacteriota, families Streptococcaceae, Enterococcaceae, Bacillaceae, Sphingomonadaceae, and Ruminococcaceae. Besides, genus Streptococcus, Collinsella, Dubosiella, Escherichia_Shigella, and Enterococcus were proved to be significantly enriched in the SDF group. Among these bacteria, Streptococcus, Escherichia_Shigella, and Enterococcus are known as pathogen bacterial associated with idiopathic small intestinal irritable bowel disease [40,43], and a previous study showed that Escherichia_Shigella had a strong negative correlation with Faecalibacterium [44], indicating that SDF caused the growth of some pathogenic bacteria. Similar to this study, our study results also showed that Escherichia_Shigella was enriched in the SDF group and Faecalibacterium was enriched in the DF group. Collinsella, the major taxon of the Coriobacteriaceae family, has a close correlation to insulin circulation [45]. Dubosiella has been recently identified as a novel member of the family Erysipeotrichaceae which thrives in such conditions, and a study showed it was enriched in the gut of obese LKO mice fed a high-fat diet [46,47]. The exact functions of Dubosiella are not clear, but a study showed the abundances of Dubosiella increased in DSS-induced mice [48]. Thus, we can speculate that the increase in fecal Collinsella and Dubosiella in the SDF group may result in some potential inflammation in the present study. In conclusion, changes in bacterial phylogeny observed are not always indicative of functional changes. However, according to the results of previous studies, most of the increased bacteria genera in the SDF group are potential pathogenic bacteria from previous study; that is, SDF may lead to an increase to the intestinal pathogens. Moisture content is an important factor in the growth of bacteria in pet food, and SDF may increase the risk.
An abundance of gut microbial-derived metabolites exists in the digestive tract [49]. In this study, there was no difference in fecal SCFAs and BCFAs between the SDF and DF groups. However, fecal metabolites derived from microbiome activity are usually secreted into the intestine and transferred to the circulatory system through the intestinal barrier, serving as very important regulators of host metabolism [9,50]. Metabolomics helps to identify the key metabolites in different diets. Our study used UPLC-Orbitrap-MS/MS to analyze and investigate the changes in fecal and serum metabolites in dogs with 21 days of SDF feeding.
The results of fecal metabolites analyses showed that SDF significantly influenced the purine metabolism and riboflavin metabolism in dogs, and that there were five differential metabolites (guanine, L-glutamine, guanosine, adenosine, and ribofalvin) enriched in these two differential pathways. A study indicated that the release of guanine increases following hypoxia/hypoglycemia and permits astrocytes to exert neurotrophic effects [51]. Guanosine may have a function in stress response, biofilm development, and cellular damage protection [52,53,54]. A study reported that L-glutamine is an abundant nitrogen source in host serum and cells and serves as an environmental indicator and inducer of virulent gene expression [55]. Adenosine is an inhibitory modulator of neuronal activity, with potent inhibitory effects on synaptic activity. In a study, extracellular adenosine levels increase dramatically in the brain via hydrolysis of adenosine triphosphate during conditions of high energy demand [56]. Another study demonstrated that adenosine interferes with the production and release of various inflammatory mediators, and it activates cellular antioxidant defense systems, thus providing protective effects at multiple levels in the pathogenesis of ischemia and reperfusion [57]. Except for the above metabolites, some other differential metabolites are also worth noting. Taurine has been linked to antioxidant functions and defense against oxygen free radicals [58], as well as apoptosis, inflammation, cell death, and increasing NO production in endothelial cells [59,60]. Taurine can alleviate oxidative stress either directly by converting superoxides to taurine chloramine [61], or indirectly through a variety of processes, including the renin-angiotensin system [62]. In conclusion, the higher contents of guanosine and taurine in the SDF group also seem to indicate oxidative stress. In both in vitro and in vivo studies, oleamide has been shown to have anti-inflammatory and anti-allergenic properties [63,64,65]. Conversely, a study showed that oleamide and palmitic amide may significantly and positively correlate with activities circulating inflammatory markers, including TNF-α, hypersensitive-c-reactive-protein, and Lipoprotein-associated phospholipaseA2 activities [66]. While in this study, the lower contents of oleamide and palmitic amide may increase inflammation in dogs fed with SDF.
Further serum metabolites analyses showed that SDF significantly influenced the arginine and proline metabolism, and that cis-4-Hydroxy-D-proline was the differential metabolite in the differential pathway. However, the function and mechanism of cis-4-Hydroxy-D-proline in the serum are still unknown. Besides, high levels of 3-indolebutyric acid (IBA) and isoquinoline were observed in the SDF group. IBA, the naturally occurring metabolite of tryptophan, a study shows that it was the metabolic product of Clostridia species [67]. In another study, IBA was proven to co-occur with the incidence of inflammatory bowel syndromes in schizophrenic patients [68], and a potential mechanism for IBA to control inflammation is competitive inhibition of phospholipase A2 [69]. Isoquinoline was a type of cardiac glycosides and potent apoptosis inducers. Its derivatives are widely presented in many plants and foodstuffs, and readily cross the blood–brain barrier. In addition, isoquinoline derivatives are oxidized by monoamine oxidases to produce isoquinolinium cations with the concomitant generation of reactive oxygen species [70]. According to correlation analysis, we found that 3-indolebutyric acid and isoquinoline were positively associated with Escherichia-Shigella. Therefore, we suggest that 3-indolebutyric acid and isoquinoline could serve as potential biomarkers of the SDF stress response. However, the relationship between intestinal microbiome and metabolites needs further investigation.
These results could adequately explain the result of the increased diarrhea ratio and FS in dogs, and the increase in the pathogens caused some stress to dogs, thereby leading to the elevated serum IL-2, COR, and HSP-70. Based on the changes of specific bacteria and different metabolites, we speculated that bacteria may breed in the process of water-SDF, which leads to the increase in TFS and TDR, as well as abnormal fecal shape. However, although we observed that the contents of some bacteria and metabolites were changed and had some correlation, the exact biological functions and the underlying mechanisms in dogs still need further exploration.
5. Conclusions
We found that feeding with SDF did not influence the ATTD, fecal SCFAs and BCFAs in dogs. However, this feeding method increased the diarrhea ratio, serum stress indicators levels, and risk of inflammation. Although feeding with SDF increased the diversity of the intestinal microbiotas in dogs, it caused a significant increase in some pathogenic bacteria. Meanwhile, fecal metabolomics further revealed that feeding with SDF can induce metabolic disorders in dogs. In conclusion, feeding with SDF did not provide digestive benefits, but posed some stress and a potential threat to the intestinal health of dogs. Thus, SDF is not recommended in dogs.
Author Contributions
Conceptualization, L.Z. (Limeng Zhang) and K.Y.; methodology, L.Z. (Limeng Zhang); software, Z.X. and C.W.; validation, B.D., J.D. and L.Z. (Lingna Zhang); formal analysis, L.Z. (Limeng Zhang); data curation, L.Z. (Limeng Zhang); writing—original draft preparation, L.Z. (Limeng Zhang); writing—review and editing, K.Y.; visualization, S.J.; supervision, B.D. and J.H.; project administration, J.D., L.Z. (Lingna Zhang) and B.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Protocols for all experiments were approved by the Experimental Animal Ethics Committee of South China Agricultural University (protocol code 2021e028).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/sra/PRJNA898496 (accessed on 5 November 2022).
Conflicts of Interest
L.Z. is employed by Guangzhou Qingke Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed a s a potential conflict of interest.
Funding Statement
This research was funded by National Key R&D Program of China (Grant No. 2021YFD1300400), National Natural Science Foundation of China (Grant Nos. 31790411, 32002186), Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010322), Guangzhou Basic and Applied Basic Research Foundation (Grant No. 202102020850), and Start-up Research Project of Maoming Laboratory (Grant No. 2021TDQD002). The study meets with the approval of the university’s review board.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Udell M.A., Brubaker L. Are dogs social generalists? Canine social cognition, attachment, and the dog-human bond. Curr. Dir. Psychol. Sci. 2016;25:327–333. doi: 10.1177/0963721416662647. [DOI] [Google Scholar]
- 2.Christian H.E., Westgarth C., Bauman A., Richards E.A., Rhodes R.E., Evenson K.R., Mayer J.A., Thorpe R.J. Dog ownership and physical activity: A review of the evidence. J. Phy. Act. Health. 2013;10:750–759. doi: 10.1123/jpah.10.5.750. [DOI] [PubMed] [Google Scholar]
- 3.Islam A., Towell T. Cat and dog companionship and well-being: A systematic review. Int. J. Appl. Psychol. 2013;3:149–155. [Google Scholar]
- 4.Carr E.C., Norris J.M., Alix Hayden K., Pater R., Wallace J.E. A scoping review of the health and social benefits of dog ownership for people who have chronic pain. Anthrozoös. 2020;33:207–224. doi: 10.1080/08927936.2020.1719761. [DOI] [Google Scholar]
- 5.Dotson M.J., Hyatt E.M. Understanding dog–human companionship. J. Bus. Res. 2008;61:457–466. doi: 10.1016/j.jbusres.2007.07.019. [DOI] [Google Scholar]
- 6.Beloshapka A.N., Wolff A.K., Swanson K.S. Effects of feeding polydextrose on faecal characteristics, microbiota and fermentative end products in healthy adult dogs. Br. J. Nutr. 2012;108:638–644. doi: 10.1017/S0007114511005927. [DOI] [PubMed] [Google Scholar]
- 7.Bordenstein S.R., Theis K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015;13:e1002226. doi: 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suchodolski J.S. Companion animals symposium: Microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 2011;89:1520–1530. doi: 10.2527/jas.2010-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canfora E.E., Meex R.C., Venema K., Blaak E.E. Gut microbial metabolites in obesity, nafld and t2dm. Nat. Rev. Endocrinol. 2019;15:261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 10.D’Argenio V., Salvatore F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta. 2015;451:97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Pereira A.M., Clemente A. Dogs’ microbiome from tip to toe. Top. Companion Anim. Med. 2021;45:100584. doi: 10.1016/j.tcam.2021.100584. [DOI] [PubMed] [Google Scholar]
- 12.Handl S., Dowd S.E., Garcia-Mazcorro J.F., Steiner J.M., Suchodolski J.S. Massive parallel 16s rrna gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 2011;76:301–310. doi: 10.1111/j.1574-6941.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 13.Swanson K.S., Dowd S.E., Suchodolski J.S., Middelbos I.S., Vester B.M., Barry K.A., Nelson K.E., Torralba M., Henrissat B., Coutinho P.M., et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011;5:639–649. doi: 10.1038/ismej.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Mazcorro J.F., Dowd S.E., Poulsen J., Steiner J.M., Suchodolski J.S. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. Microbiol. Open. 2012;1:340–347. doi: 10.1002/mbo3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hand D., Wallis C., Colyer A., Penn C.W. Pyrosequencing the canine faecal microbiota: Breadth and depth of biodiversity. PLoS ONE. 2013;8:e53115. doi: 10.1371/journal.pone.0053115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beloshapka A.N., Dowd S.E., Suchodolski J.S., Steiner J.M., Duclos L., Swanson K.S. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol. Ecol. 2013;84:532–541. doi: 10.1111/1574-6941.12081. [DOI] [PubMed] [Google Scholar]
- 17.Sandri M., Dal Monego S., Conte G., Sgorlon S., Stefanon B. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 2016;13:65. doi: 10.1186/s12917-017-0981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanprasertsuk J., Perry L.A.M., Tate D.E., Honaker R.W., Shmalberg J. Apparent total tract nutrient digestibility and metabolizable energy estimation in commercial fresh and extruded dry kibble dog foods. Transl. Anim. Sci. 2021;5:txabo71. doi: 10.1093/tas/txab071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Middelbos I.S., Fastinger N.D., Fahey G.C. Evaluation of fermentable oligosaccharides in diets fed to dogs in comparison to fiber standards. J. Anim. Sci. 2007;85:3033–3044. doi: 10.2527/jas.2007-0080. [DOI] [PubMed] [Google Scholar]
- 20.Magoc T., Salzberg S.L. Flash: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas B.J., Gevers D., Earl A.M., Feldgarden M., Ward D.V., Giannoukos G., Ciulla D., Tabbaa D., Highlander S.K., Sodergren E. Chimeric 16s rrna sequence formation and detection in sanger and 454-pyrosequenced pcr amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The silva ribosomal rna gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang K., Deng X., Jian S., Zhang M., Wen C., Xin Z., Zhang L., Tong A., Ye S., Deng B. Gallic acid alleviates gut dysfunction and boosts immune and antioxidant activity in puppies under environmental stress based on microbiome-metabolomics analysis. Front. Immunol. 2022;5848:813890. doi: 10.3389/fimmu.2021.813890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuli R., Azhati J., Cai J.X., Kadeer A., Zhang B., Mohemaiti P. Metagenomics and faecal metabolomics integrative analysis towards the impaired glucose regulation and type 2 diabetes in uyghur-related omics. J. Diabetes Res. 2019;2019:2893041. doi: 10.1155/2019/2893041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furniss G. The influence of nutrition on puppy and kitten growth and development. Ir. Vet. J. 2008;61:191–194. [Google Scholar]
- 30.Do S., Phungviwatnikul T., de Godoy M.R.C., Swanson K.S. Nutrient digestibility and fecal characteristics, microbiota, and metabolites in dogs fed human-grade foods. J. Anim. Sci. 2021;99:skab028. doi: 10.1093/jas/skab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brito C.B.M.D., Lima D.C.D., Souza C.M.M., Vasconcellos R.S., Oliveira S.G.D., Félix A.P. Evaluation of dried apple pomace on digestibility and palatability of diets for cats. Braz. J. Anim. Sci. 2020;49:1. doi: 10.37496/rbz4920190219. [DOI] [Google Scholar]
- 32.Wakshlag J.J., Simpson K.W., Struble A.M., Dowd S.E. Negative fecal characteristics are associated with ph and fecal flora alterations during dietary change in dogs. Int. J. Appl. Res. Vet. M. 2011;9:278–283. [Google Scholar]
- 33.McEwen B.S. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S., Janicki-Deverts D., Miller G.E. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 35.Helfand S.C., Modiano J.F., Nowell P.C. Immunophysiological studies of interleukin-2 and canine lymphocytes. Vet. Immunol. Immunopathol. 1992;33:1–16. doi: 10.1016/0165-2427(92)90030-T. [DOI] [PubMed] [Google Scholar]
- 36.Hammarqvist F., Wernerman J., von der Decken A., Vinnars E. Alanyl-glutamine counteracts the depletion of free glutamine and the postoperative decline in protein synthesis in skeletal muscle. Ann. Sur. 1990;212:637. doi: 10.1097/00000658-199011000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karner J., Roth E. Alanyl-glutamine infusions to patients with acute pancreatitis. Clin. Nurt. 1990;9:43–44. doi: 10.1016/0261-5614(90)90079-8. [DOI] [PubMed] [Google Scholar]
- 38.Weiss Y.G., Maloyan A., Tazelaar J., Raj N., Deutschman C.S. Adenoviral transfer of hsp-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J. Clin. Invest. 2002;110:801–806. doi: 10.1172/JCI0215888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inness V., McCartney A., Khoo C., Gross K., Gibson G. Molecular characterisation of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence in situ hybridisation with special reference to desulfovibrio spp. J. Anim. Physiol. Anim. Nutr. 2007;91:48–53. doi: 10.1111/j.1439-0396.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 40.Janeczko S., Atwater D., Bogel E., Greiter-Wilke A., Gerold A., Baumgart M., Bender H., McDonough P., McDonough S., Goldstein R. The relationship of mucosal bacteria to duodenal histopathology, cytokine mrna, and clinical disease activity in cats with inflammatory bowel disease. Vet. Microbiol. 2008;128:178–193. doi: 10.1016/j.vetmic.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Clavel T., Desmarchelier C., Haller D., Gerard P., Rohn S., Lepage P., Daniel H. Intestinal microbiota in metabolic diseases from bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes. 2014;5:544–551. doi: 10.4161/gmic.29331. [DOI] [PubMed] [Google Scholar]
- 42.Kim J., An J.U., Kim W., Lee S., Cho S. Differences in the gut microbiota of dogs (canis lupus familiaris) fed a natural diet or a commercial feed revealed by the illumina miseq platform. Gut Pathog. 2017;9:68. doi: 10.1186/s13099-017-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xenoulis P.G., Palculict B., Allenspach K., Steiner J.M., Van House A.M., Suchodolski J.S. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol. Ecol. 2008;66:579–589. doi: 10.1111/j.1574-6941.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 44.Thorkildsen L.T., Nwosu F.C., Avershina E., Ricanek P., Perminow G., Brackmann S., Vatn M.H., Rudi K. Dominant fecal microbiota in newly diagnosed untreated inflammatory bowel disease patients. Gastroenterol. Res. Pract. 2013;2013:636785. doi: 10.1155/2013/636785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Arango L.F., Barrett H.L., Wilkinson S.A., Callaway L.K., Mcintyre H.D., Morrison M., Nitert M.D. Low dietary fiber intake increases collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes. 2018;9:189–201. doi: 10.1080/19490976.2017.1406584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H., DiBaise J.K., Zuccolo A., Kudrna D., Braidotti M., Yu Y., Parameswaran P., Crowell M.D., Wing R., Rittmann B.E. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox L.M., Sohn J., Tyrrell K.L., Citron D.M., Lawson P.A., Patel N.B., Iizumi T., Perez-Perez G.I., Goldstein E.J., Blaser M.J. Description of two novel members of the family erysipelotrichaceae: Ileibacterium valens gen. Nov., sp. Nov. And dubosiella newyorkensis, gen. Nov., sp. Nov., from the murine intestine, and emendation to the description of faecalibacterium rodentium. Int. J. Syst. Evol. Microbiol. 2017;67:1247. doi: 10.1099/ijsem.0.001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheng K., Zhang G., Sun M., He S., Kong X., Wang J., Zhu F., Zha X., Wang Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020;11:7817–7829. doi: 10.1039/D0FO01418D. [DOI] [PubMed] [Google Scholar]
- 49.Chamorro S., Romero C., Brenes A., Sanchez-Patan F., Bartolome B., Viveros A., Arija I. Impact of a sustained consumption of grape extract on digestion, gut microbial metabolism and intestinal barrier in broiler chickens. Food Funct. 2019;10:1444–1454. doi: 10.1039/C8FO02465K. [DOI] [PubMed] [Google Scholar]
- 50.Vitek L., Haluzik M. The role of bile acids in metabolic regulation. J. Endocrinol. 2016;228:R85–R96. doi: 10.1530/JOE-15-0469. [DOI] [PubMed] [Google Scholar]
- 51.Ciccarelli R., Di Iorio P., Giuliani P., D’Alimonte I., Ballerini P., Caciagli F., Rathbone M.P. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25:93–98. doi: 10.1002/(SICI)1098-1136(19990101)25:1<93::AID-GLIA9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 52.Cornforth D.M., Foster K.R. Competition sensing: The social side of bacterial stress responses. Nat. Rev. Microbiol. 2013;11:285–293. doi: 10.1038/nrmicro2977. [DOI] [PubMed] [Google Scholar]
- 53.Rowlett V.W., Mallampalli V.K.P.S., Karlstaedt A., Dowhan W., Taegtmeyer H., Margolin W., Vitrac H. Impact of membrane phospholipid alterations in escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017;199:e00849-16. doi: 10.1128/JB.00849-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bange G., Bedrunka P. Physiology of guanosine-based second messenger signaling in bacillus subtilis. Biol. Chem. 2020;401:1307–1322. doi: 10.1515/hsz-2020-0241. [DOI] [PubMed] [Google Scholar]
- 55.Haber A., Friedman S., Lobel L., Burg-Golani T., Sigal N., Rose J., Livnat-Levanon N., Lewinson O., Herskovits A.A. L-glutamine induces expression of listeria monocytogenes virulence genes. PLoS Pathog. 2017;13:e1006161. doi: 10.1371/journal.ppat.1006161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ådén U., O’Connor W.T., Berman R.F. Changes in purine levels and adenosine receptors in kindled seizures in the rat. Neuroreport. 2004;15:1585–1589. doi: 10.1097/01.wnr.0000133227.94662.c9. [DOI] [PubMed] [Google Scholar]
- 57.Bouma M.G., van den Wildenberg F.A.J.M., Buurman W.A. The anti-inflammatory potential of adenosine in ischemia-reperfusion injury: Established and putative beneficial actions of a retaliatory metabolite. Shock. 1997;8:313–320. doi: 10.1097/00024382-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Giriş M., Depboylu B., Doğru-Abbasoğlu S., Erbil Y., Olgaç V., Alış H., Aykaç-Toker G., Uysal M. Effect of taurine on oxidative stress and apoptosis-related protein expression in trinitrobenzene sulphonic acid-induced colitis. Clin. Exp. Immunol. 2008;152:102–110. doi: 10.1111/j.1365-2249.2008.03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franconi F., Loizzo A., Ghirlanda G., Seghieri G. Taurine supplementation and diabetes mellitus. Curr. Opin. Clin. Nutr. 2006;9:32–36. doi: 10.1097/01.mco.0000196141.65362.46. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira M.W.S., Minotto J.B., de Oliveira M.R., Zanotto A., Behr G.A., Rocha R.F., Moreira J.C.F., Klamt F. Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol. Rep. 2010;62:185–193. doi: 10.1016/S1734-1140(10)70256-5. [DOI] [PubMed] [Google Scholar]
- 61.Kim C., Cha Y.-N. Production of reactive oxygen and nitrogen species in phagocytes is regulated by taurine chloramine. Taurine. 2009;7:463–472. doi: 10.1007/978-0-387-75681-3_48. [DOI] [PubMed] [Google Scholar]
- 62.Hu J., Xu X., Yang J., Wu G., Sun C., Lv Q. Antihypertensive effect of taurine in rat. Taurine. 2009;7:75–84. doi: 10.1007/978-0-387-75681-3_8. [DOI] [PubMed] [Google Scholar]
- 63.Sudhahar V., Shaw S., Imig J.D. Mechanisms involved in oleamide-induced vasorelaxation in rat mesenteric resistance arteries. Eur. J. Pharmacol. 2009;607:143–150. doi: 10.1016/j.ejphar.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moon S.-M., Lee S.A., Hong J.H., Kim J.-S., Kim D.K., Kim C.S. Oleamide suppresses inflammatory responses in lps-induced raw264. 7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018;56:179–185. doi: 10.1016/j.intimp.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 65.Kita M., Ano Y., Inoue A., Aoki J. Identification of p2y receptors involved in oleamide-suppressing inflammatory responses in murine microglia and human dendritic cells. Sci. Rep. 2019;9:3135. doi: 10.1038/s41598-019-40008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M., Kim M., Han J.Y., Lee S.-H., Jee S.H., Lee J.H. The metabolites in peripheral blood mononuclear cells showed greater differences between patients with impaired fasting glucose or type 2 diabetes and healthy controls than those in plasma. Diabetes Vasc. Dis. Res. 2017;14:130–138. doi: 10.1177/1479164116678157. [DOI] [PubMed] [Google Scholar]
- 67.Lombard G.L., Dowell V., Jr. Comparison of three reagents for detecting indole production by anaerobic bacteria in microtest systems. J. Clin. Mic. 1983;18:609–613. doi: 10.1128/jcm.18.3.609-613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai H., Li H., Yan X., Sun B., Zhang Q., Yan M., Zhang W.-Y., Jiang P., Zhu R.-H., Liu Y.-P. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J. Proteomoe Res. 2012;11:4338–4350. doi: 10.1021/pr300459d. [DOI] [PubMed] [Google Scholar]
- 69.Dileep K.V., Remya C., Tintu I., Haridas M., Sadasivan C. Interactions of selected indole derivatives with phospholipase a2: In silico and in vitro analysis. J. Mol. Model. 2013;19:1811–1817. doi: 10.1007/s00894-012-1741-4. [DOI] [PubMed] [Google Scholar]
- 70.McNaught K.S.P., Carrupt P.-A., Altomare C., Cellamare S., Carotti A., Testa B., Jenner P., Marsden C.D. Isoquinoline derivatives as endogenous neurotoxins in the aetiology of parkinson’s disease. Biochem. Pharmacol. 1998;56:921–933. doi: 10.1016/S0006-2952(98)00142-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/sra/PRJNA898496 (accessed on 5 November 2022).