Abstract
HVAC systems have a significant impact on the indoor environment, and microbial contamination in HVAC systems has a significant effect on the indoor air quality. In this study, to gain a better understanding of the microbial contamination inside ACs, we used NGS to analyze the 16S rRNA gene of bacteria adhering to AC filters, cooling coils, fans, and air outlet surfaces. The five phyla in terms of the highest relative abundance were Proteobacteria, Firmicutes, Actinobacteria, Cyanobacteria, and Bacteroidetes. The surface of an AC filter provides a history of indoor airborne bacterial contamination, and of the 10 bacterial genera we detected with the highest abundance (in the following order: Pseudomonas > Staphylococcus > Paracoccus > Corynebacterium > Acinetobacter > Streptococcus > Methylobacterium > Enhydrobacter > Sphingomonas > Actinotignum) on the filter surface, the top 6 genera were Gram-negative bacteria. Furthermore, the seventh-most abundant genus adhering to the filter surface (Methylobacterium) was the second-most abundant genus on the cooling coil and fan, and the ninth-most abundant genus on the air filter (Sphingomonas) was the third-most abundant genus on the cooling coil. Various factors impact the bacterial flora inside AC units, including the location of the house, AC unit usage, and occupant activity.
Keywords: residential building, air conditioner, air filter, cooling coil, fan, air outlet, bacteria, next-generation sequencing, 16S rRNA gene
1. Introduction
In developed countries, people spend around 90% of their time indoors [1]. The indoor environment plays host to a variety of microbes, such as bacteria, fungi, and viruses [2,3], and includes a wide variety of bacterial sources [4], such as the outside air [5], occupants [5,6,7,8,9,10,11], pets [12,13], houseplants [14,15], and air-conditioning (AC) systems [7,16,17,18,19,20,21,22,23,24]. The heating, ventilation, and air-conditioning (HVAC) systems have a significant impact on the indoor environment, and microbial contamination in HVAC systems has a significant effect on indoor air quality.
Air conditioning is common in developed countries. In Japan, AC units are installed in more than 90% of the homes [25], and microbial contamination inside AC systems is an important factor in people’s health. Banaszak et al. [26], Baur et al. [27], and Acierno et al. [28] have reported that thermophilic actinomycetes growing inside humidifiers and AC units cause hypersensitivity pneumonia. There are also reports that endotoxins, a component of the cell wall in Gram-negative bacteria, may be associated with allergic sensitization in humans [29,30] and exposure to airborne endotoxins is associated with workplace-related illnesses [31,32,33].
When an AC unit operates in cooling mode, the cooling coil inside the AC unit works to cool and dehumidify the air in the room. To achieve this, condensation forms on the cooling coil and a high-humidity environment is created inside the AC unit. Given a high-humidity environment, bacteria will proliferate on a wide variety of component surfaces. Dannemiller et al. used glass chambers to investigate the effect of relative humidity on microbial growth in floor dust and observed fungal growth after 1 week at 90% relative humidity and bacterial growth after 1 week at 100% relative humidity [34]. Hyvärinen et al. also investigated microbial growth in actual indoor environments on seven different moisture-damaged building materials (wood, paper, non-wooden building boards, ceramic products, mineral-based insulation materials, paints and glues, and plastics) and observed bacterial growth on all materials but particularly pronounced growth on insulation materials [35]. Not only is bacterial proliferation on cooling coils an important factor in the health of occupants, but bacteria can also form biofilms [18,19,22,36] that reduce the heat-exchange efficiency of the equipment.
Various published reports provide details of the phyla, genera, and species of bacteria that have been detected to date in HVAC systems. Streptophyta is the predominant phylum found adhering to HVAC system filters, a phenomenon reportedly affected by both indoor and outdoor air [7]. Using culture-based analysis, Hugenholtz et al. detected Acinetobacter, Arthrobacter, Bacillus, Corynebacterium, Pseudomonas, and Staphylococcus on air handling system coils [19]. Using next-generation sequencing (NGS), Bakker et al. identified Methylobacteriaceae, Propionibacterium, Acetobacteraceae, Sphingomonas, Pseudanabaenaceae, Streptophyta, Acinetobacter, Bacillales, Hymenobacter, and Corynebacterium as the predominant bacteria on AC unit coil surfaces [21]. Using NGS, Hatayama et al. identified the genus Methylobacterium and the family Sphingomonadaceae as the predominant bacteria on the evaporator of split-type air conditioners in Japanese homes [20].
Most of the studies published to date have focused on bacteria attached to the air filters and cooling coils of AC systems in commercial buildings. Although Hatayama et al. did publish a report on the bacteria found on coil surfaces in residential AC units [20], almost no other reports that focus on residential homes examine in detail the ductless mini-split-type AC units widely used in Japanese homes. Looking at the AC unit in terms of the airflow, the air filter, the coil, the fan, and the air outlet surface, each feature has different temperature and relative humidity conditions. Hence, each presents an environment suited to the proliferation of different types of bacteria. Understanding the bacterial flora on each of these features will help to determine the effects of the bacteria in air conditioners on indoor environments. Therefore, we examined the bacterial flora attached to filters, coils, fans, and air outlet surfaces of room air conditioners in 17 homes in Kanagawa prefecture, Japan, and investigated the characteristic features of the bacterial flora on each of these components.
2. Materials and Methods
2.1. Measured Locations
Measurements were performed in the living rooms of 17 homes in Kanagawa prefecture, Japan, between 3 July and 26 September 2021 (during summer). Table 1 provides an overview of the homes where measurements were taken, AC unit usage, and the number of occupants.
Table 1.
Information on the measured residential buildings and room air conditioners.
| ID | Building Age | Type of Residental Building | AC Set Point | Frequency of Use | Hours of Opretion |
Years of Use | Number of Residents |
|
|---|---|---|---|---|---|---|---|---|
| (Years) | (Floors) | (°C) | (Day/Week) | (h/day) | (Years) | |||
| 004 | 13 | Detached house | 1 | 29 | 7 | 12 | 13 | 2 |
| 005 | 16 | Detached house | 1 | 28 | 7 | 24 | 16 | 2 |
| 006 | 19 | Detached house | 1 | 26 | 7 | 17 | 6 | 5 |
| 018 | 42 | Comdminium | 2 | 26 | 4.5 | 12 | 10 | 2 |
| 045 | 51 | Detached house | 1 | 28 | 1 | 2 | 5 | 2 |
| 048 | 31 | Comdminium | 1 | 27 | 7 | 8 | 6 | 2 |
| 049 | 44 | Detached house | 1 | 26 | 7 | 9 | 10 | 2 |
| 050 | 45 | Detached house | 1 | 26 | 7 | 15 | 8 | 2 |
| 060 | 11 | Detached house | 1 | 23~25 | 7 | 12 | 1.6 | 2 |
| 062 | 24 | Detached house | 1 | 27 | 7 | 14 | 13 | 2 |
| 065 | 49 | Detached house | 1 | 26 | 7 | 10 | 4 | 2 |
| 072 | 16 | Detached house | 1 | 27 | 7 | 16 | 2 | 3 |
| 083 | 28 | Detached house | 2 | 28 | 7 | 4 | 8 | 4 |
| 087 | 23 | Comdminium | 13 | 28 | 7 | 24 | 12 | 2 |
| 113 | 41 | Detached house | 1 | 28 | 7 | 5 | 13 | 2 |
| 116 | 27 | Comdminium | 3 | 26 | 7 | 24 | 10 | 2 |
| 118 | 7 | Detached house | 1 | 26 | 5 | 10 | 7 | 2 |
2.2. Temperature and Relative Humidity
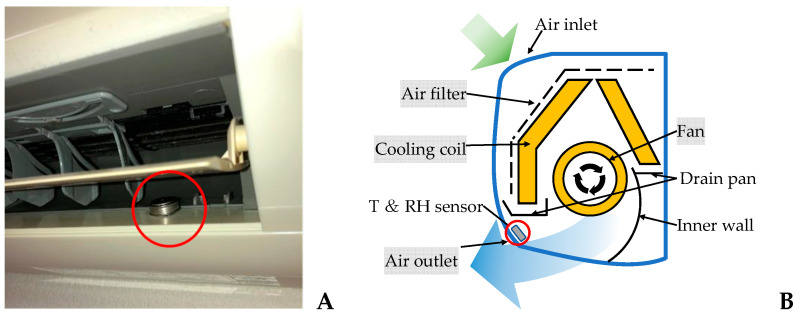
To understand the temperature (T) and relative humidity (RT) conditions inside an AC unit operating in cooling mode, temperature and relative humidity were measured continuously at 30-min intervals by a compact temperature/humidity sensor (Hygrochron, KN Lab Series). To represent the conditions at the air inlet of the AC unit, measurements were taken in indoor air. To represent the conditions at the cooling coil in the AC unit and the air downstream from the cooling coil, measurements were taken at the air outlet of the AC unit (Figure 1). To investigate the bacterial flora adhered to each component in the room AC unit, the bacteria were analyzed in order of airflow, i.e., bacteria adhered to the air filter, bacteria adhered to the cooling coil, bacteria adhered to the fan, and bacteria adhered to the air outlet surfaces. A sterile cotton swab was used to wipe bacteria from a 25 cm2 surface area on each component. The cotton swabs were then stored individually in a container (ST-25, ELMEX) containing 10 mL of sterile phosphate-buffered solution and taken to be processed in a laboratory. Samples were collected from two sites of each component, for a total sampled surface area of 50 cm2.
Figure 1.
Location of sensors for measuring T and RH at air the conditioner air outlet (A), configuration of the ductless mini- split-type AC unit (B).
2.3. DNA Extraction, Amplification, and Sequencing
2.3.1. DNA Extraction
After samples of adhered bacteria were collected as described above, the cotton swabs were processed with a stomacher (MiniMix 100 P CC Interscience), 3 mL of DNase-free water was combined with 2 mL of the sample solution, and the DNA was extracted with a Stomacher Biomaster device. The processed sample was then removed from the stomacher bag and placed in a 1.5 mL test tube and centrifuged (KUBO-TA5911) at 4 °C and 3000 rpm for 30 min to extract the bacteria. DNA was purified using a NucleoSpin® Tissue kit (740952, MACHEREY-NAGEL) and by mixing the process liquid in a vortex mixer, heating it, combining it with ethanol, performing centrifugal separation, and carrying out other process steps.
2.3.2. DNA Amplification and Sequencing
For each sample, the variable region 4 (V4) of the bacterial 16S ribosomal RNA (rRNA) gene was amplified by polymerase chain reaction (PCR) using the primer “ACACTCTTTCCCTACACGACGCTCTTCCGATCT-GTGCCAGCMGCCGCGGTAA (1st_515F)” [37], and “GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-GACTACHVGGGTWTCTAAT (1st_806R)”; “AATGATACGGCGACCACCGAGATCTACACxxxxxxxxACACTCTTTCCCTACACGACGC (2nd forward primer)”, and “CAAGCAGAAGACGGCATACGAGATxxxxxxxxGTGACTGGAGTTCAGACGTGTG (2nd reverse primer)”. DNA amplification and 16S rRNA gene analysis performed on Illumina NGS, the collected DNA was outsourced to a commercial laboratory.
2.3.3. DNA Sequencing and Analysis
DNA quality was verified using an Agilent 2200 TapeStation, and all samples containing nucleic acid concentrations of the quality and quantity required for analysis were analyzed. The generated sequence libraries were combined, and the re-amplified PCR products were purified with AMPure XP beads (bead volume ratio 1:1) to improve the quality of the sequence libraries. Data were analyzed using QIIME (Ver.1.9.0, Silva 132 Database).
3. Results
3.1. Temperature and Humidity
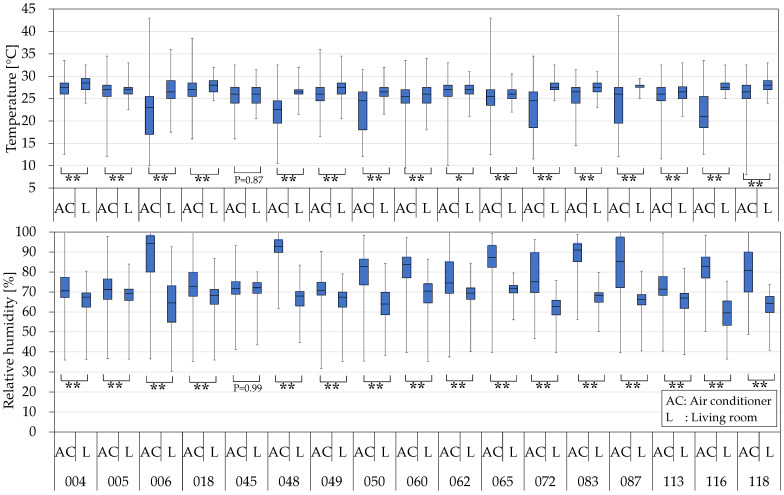
During the cooling operation, the temperature at the air outlet of an air conditioner (AC) is lower than the living room temperature (L), and the relative humidity is higher. Statistical analysis using SPSS Statistics 29 revealed significant differences between the indoor and air outlet temperatures and relative humidity, except for house 045. (Figure 2). House 045 had the lowest air conditioning usage frequency (Table 1).
Figure 2.
The quartile values of air temperature and relative humidity of living room and air conditioner air Outlet. Statistical significance was evaluated by Mann−Whitney U test. * p < 0.01, and ** p < 0.001.
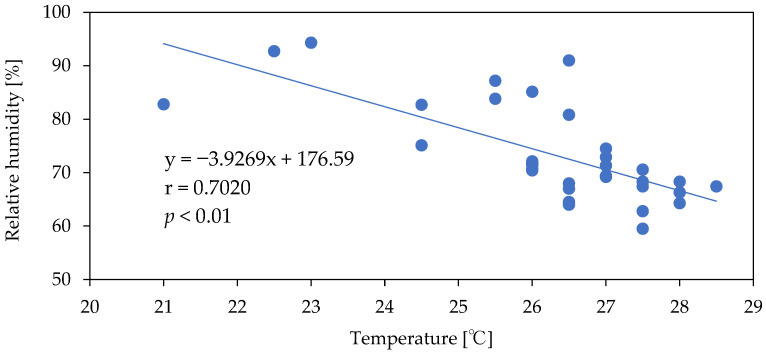
The temperature and relative humidity measurements show a large degree of variation because they were collected both while the AC unit was operating in cooling mode and while the AC unit was not in operation (Figure 2). Overall, the relative humidity tends to increase when the outlet temperature is low (Figure 3). In addition, because the temperature/humidity sensor at the air outlet is exposed to indoor air, when the AC unit is not in operation, the sensor is affected by the temperature and relative humidity in the room. This explains why house 045, which had the lowest frequency of AC unit usage (Table 1), produced almost identical 75th percentile, median, and 25th percentile results for the living room temperature measurements and the AC unit air-outlet-temperature measurements. The maximum temperature of the AC unit was higher than that of the living room due to the warm air produced when the AC unit operates in drying mode. In drying mode, a four-way valve in the AC unit refrigerant flowline temporarily reverses the flow of the refrigerant, the evaporator in the AC unit acts as a condenser, and the AC unit coils are dried with warm air. The median relative humidity for all AC units was above 70%, confirming the presence of a favorable environment for microbe growth. In addition, the frequency of appearance of relative humidity values of 90% or higher in homes was 60% in house 006, 74% in house 048, and 58% in house 083, confirming fairly high humidity levels.
Figure 3.
Relationship between median temperature and median relative humidity.
3.2. Taxonomic Analysis
3.2.1. Taxonomic Identification
Organisms are currently divided into three domains: Eukarya (eukaryotes), Bacteria (eubacteria), and Archaea (archaea). Organisms in each of these domains are further divided by phylum, class, order, family, genus, and species. To allow comparison with previous reports, this study mainly discusses bacteria in terms of phyla and genera.
3.2.2. Phylum
In total, 39 bacterial phyla were detected. The five phyla with the highest mean relative abundance among all samples were Proteobacteria (52.21 ± 24.71%), Firmicutes (14.19 ± 12.33%), Actinobacteria (8.30 ± 6.97%), Cyanobacteria (2.15 ± 6.37%), and Bacteroidetes (1.50 ± 1.40%) (Figure S1).
3.2.3. Genus
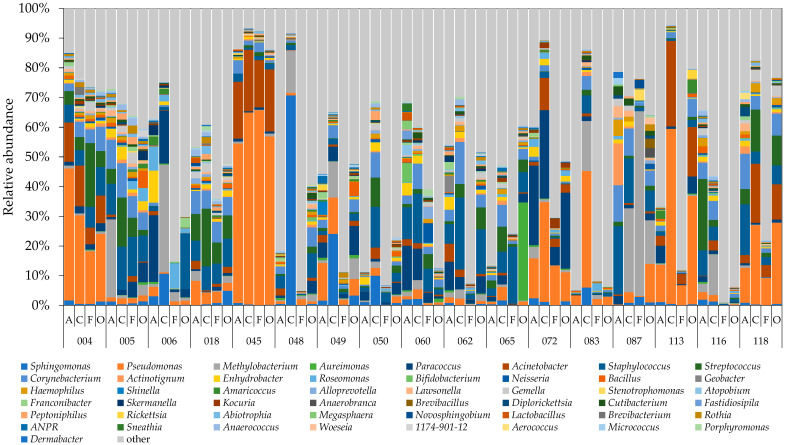
The 10 highest-ranked bacterial genera were Sphingomonas (Proteobacteria phylum, Gram-negative), Pseudomonas (Proteobacteria phylum, Gram-negative), Methylobacterium (Proteobacteria phylum, Gram-negative), Aureimonas (Proteobacteria phylum, Gram-negative), Paracoccus (Proteobacteria phylum, Gram-negative), Acinetobacter (Proteobacteria phylum, Gram-negative), Staphylococcus, (Firmicutes phylum, Gram-positive), Streptococcus (Firmicutes phylum, Gram-positive), Corynebacterium (Actinobacteria phylum, Gram-positive), and Actinotignum (Actinomycetota phylum, Gram-positive) (Figure 4). The S. paucimobilis species, of the genus Sphingomonas, is reportedly associated with pseudobacteraemia, and cases of severe and invasive infections, such as bacterial arthritis and osteomyelitis, are also not uncommon [38]. The genera Pseudomonas, Methylobacterium, and Acinetobacter are also known to include species that cause opportunistic infections.
Figure 4.
Relative abundance of 2% or higher of bacterial genera for all samples from the 17 houses. A, C, F, and O corresponded to air filter, cooling coil, fan, and air outlet. Genus ANPR: Allorhizobium-Neorhizobium -Pararhizobium-Rhizobium.
The top 10 relative abundance of the mean of each genus were Pseudomonas, Staphylococcus, Methylobacterium, Paracoccus, Acinetobacter, Corynebacterium, Streptococcus, Sphingomonas, Enhydrobacter, and Roseomonas—of which 7 were Gram-negative (Figure S2). Furthermore, the median result for the five genera Pseudomonas, Methylobacterium, Corynebacterium, Streptococcus, and Sphingomonas was higher at the cooling coil than at other locations, showing that many bacteria grow predominantly on the coil surface, which is a high-humidity environment. Using culture-based analysis, Hugenholtz et al. detected Acinetobacter, Corynebacterium, Pseudomonas, and Staphylococcus on the coil of an air handling system [19]. Using NGS, Bakker et al. detected predominantly Methylobacteriaceae, Acetobacteraceae, Sphingomonas, Pseudanabaenaceae, Streptophyta, Acinetobacter, and Corynebacterium on the surface of an AC unit coil [22].
3.2.4. Change in Ranking of Bacteria Adhering to Various Parts of Air Conditioners
The ten bacterial genera detected with the highest relative abundance on the air filter surface across all 17 residences were analyzed to understand the changes in the relative abundance of bacterial genera in each part of the air conditioner downstream from the air filter. Pseudomonas was the most abundant genus at all locations, showing that the bacteria belonging to the genus Pseudomonas grow not only in the general environment but also inside AC units. The nine most abundant genera on the air filter matched the nine most abundant genera on the cooling coil and also the seven most abundant genera on the fan and the air outlet, indicating that these bacteria pass through the air filter to adhere to and grow on the cooling coil, fan, and air outlet surface (Table 2). This result is probably due to the generally less-than-excellent collection efficiency of air filters found in room AC units.
Table 2.
Change in the ranking of the relative abundances of the top 10 air-filter-adherent bacteria.
| Air Filter | Cooling Coil | Fan | Air Outlet | ||||
|---|---|---|---|---|---|---|---|
| Pseudomonas | 1 | → | 1 | → | 1 | → | 1 |
| Staphylococcus | 2 | ⤵ | 4 | ⤴ | 3 | ⤴ | 2 |
| Paracoccus | 3 | ⤵ | 8 | ⤴ | 7 | ⤴ | 3 |
| Corynebacterium | 4 | ⤵ | 6 | → | 6 | ⤴ | 5 |
| Acinetobacter | 5 | → | 5 | → | 5 | ⤴ | 4 |
| Streptococcus | 6 | ⤵ | 7 | ⤴ | 4 | ⤵ | 7 |
| Methylobacterium | 7 | ⤴ | 2 | → | 2 | ⤵ | 9 |
| Enhydrobacter | 8 | ⤵ | 9 | ⤵ | 16 | ⤴ | 12 |
| Sphingomonas | 9 | ⤴ | 3 | ⤵ | 17 | ⤵ | 11 |
| Actinotignum | 10 | ⤵ | 29 | ⤴ | 25 | ⤴ | 24 |
3.2.5. Diversity of Bacterial Community
The Shannon index was used to assess alpha diversity. The Shannon index is higher when the number of bacterial species is high and each species is equally present. The order of the highest mean value and the standard deviation of the Shannon index was air outlet (4.858 ± 1.369), air filter (4.725 ± 1.100), cooling coil (4.556 ± 1.077), and fan (4.127 ± 0.816). The bacterial flora on the air outlets was affected both by the indoor environment (when the AC was not in operation) and the AC interior (when the AC was in operation), and the bacterial flora on the air filters was affected by the indoor environment, so the Shannon index was higher. In the air conditioner, the Shannon index was higher on the surface of the condensation coil than on the surface of the fan.
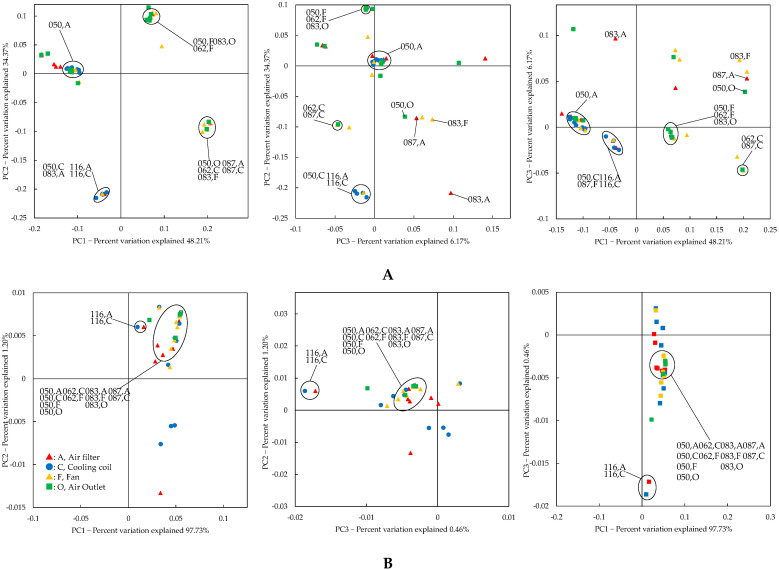
Beta diversity is shown by principal coordinate analysis (PCoA) using the unweighted and weighted UniFrac distance [39]. The air filter, the coil, the fan, and the air outlet were plotted close together in house 050; the air filter and the coil were plotted close together in houses 087 and 166; the coil and the fan were plotted close together in house 062; and the air filter, the fan, and the air outlet were plotted close together in house 083 (Figure 5B). AC parts plotted close to each other have similar bacterial flora. However, the flora of the air filter in house 049 and the fan in house 087 was significantly different from that in the other houses: the largest relative abundance of adherent bacteria in the air filter of house 049 was 12.70%, for Pseudomonas, and the largest relative abundance of adherent bacteria in the fan in house 087 was 62.20%, for Methylobacterium (Figure S3).
Figure 5.
Principal coordinate analysis (PCoA) of partial expansion of the unweighted UniFrac distance (A) and weighted UniFrac distance (B). A, C, F, and O corresponded to air filter, cooling coil, fan, and air outlet.
4. Discussion
Bacterial contamination in air conditioning units affects the health of occupants. The establishment of bacterial flora in air conditioners is influenced by the location of the residence, occupant activity, frequency of air conditioner use, and air conditioner maintenance. Although there have been reports of studies on some parts of air conditioners (filters and coils), there have been almost no reports on the bacterial flora in different components of air conditioners. Therefore, to better understand the bacterial flora inside room AC units, this study sampled bacteria adhering to the air filter, cooling coil, fan, and air outlet of AC units and analyzed the 16S rRNA gene using NGS-based amplicon sequencing.
The five top-ranked phyla (Proteobacteria, Firmicutes, Actinobacteria, Cyanobacteria, and Bacteroidetes) were detected in high relative abundance on the filters, coils, fans, and air outlets of all AC units. A study of house dust by Täubel et al. detected Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes at 1% or greater abundance by sequencing. A study by Dannemiller et al. [34] of bacterial flora in dust collected from the carpets or floors of residential homes detected similarly large amounts of bacteria belonging to the phyla Firmicutes and Actinobacteria. These findings suggest that these bacterial phyla favor growing in the home environment and appear to pass through the air filter to affect the interior of the AC unit.
We also investigated the ten highest-ranked bacterial genera adhering to air filters and their subsequent alterations in downstream coils, fans, and air outlets to better understand the characteristics and differences of the bacterial flora in each component of the air conditioner and the differences among them. Because some of the airborne bacteria present at the AC unit air inlet are normally removed by the air filter, if no bacterial proliferation occurs inside the AC unit, the surface of the air filter will contain the largest number of bacteria. Put differently, if the relative abundance of a given bacterium is higher downstream of the air filter (on the coil, etc.), that bacterium probably proliferated at the downstream location. Conversely, because the air outlet of the AC unit is exposed to the air in the room, the indoor environment has a significant impact on the air outlet flora when the AC unit is not in operation. Of the top 10 genera of bacteria detected on the filter surface, 6 genera were Gram-negative (Pseudomonas, Paracoccus, Acinetobacter, Methylobacterium, Enhydrobacter, and Sphingomonas) and 4 genera were Gram-positive (Staphylococcus, Corynebacterium, Streptococcus, and Actinotignum). As mentioned above, Gram-negative bacteria have endotoxin in their cell walls, which may lead to allergic sensitization in humans and to workplace-related illnesses. Gram-positive bacteria, however, are known for their cell-wall-associated proteins that play key roles in both colonization and pathogenesis [40]. When an AC unit operates in cooling mode, condensation on the surface of the cooling coil creates a high-humidity environment that favors bacterial proliferation. The seventh-ranked genus on the air filter (Methylobacterium) became the second-ranked genus on the cooling coil and the fan, and the ninth-ranked genus on the air filter (Sphingomonas) became the third-ranked genus on the cooling coil. Given that Methylobacterium bacteria are also detected in bathrooms [41], this genus seems to proliferate readily in high-humidity environments. Bacteria of the genus Methylobacterium are also known to form biofilms. The bacterial genera detected in abundance on the cooling coil surface in this study have also been detected in previous studies. Bacteria belonging to the genera Methylobacteriaceae and Sphingomonas were detected in abundance on AC unit cooling coils by Bakker et al. [22], and bacteria of the genus Methylobacterium were detected in abundance on the surface of the heat exchanger (coil) of AC units in four Japanese homes by Hatayama et al. [20]. In the same report, Hatayama et al. also detected an abundance of bacteria of the family Sphingomonadaceae, genus Sphingomonas. Thus, we can infer that bacteria of the genera Methylobacterium and Sphingomonas grow readily inside AC units. In this study, bacteria of the genera Staphylococcus, Paracoccus, and Streptococcus were detected with a higher relative abundance on the fan than on the cooling coil. Furthermore, because the AC unit air outlet is affected by the indoor environment when not in operation, the same five bacterial genera were the most abundant on the air filter and on the air outlet. Nevertheless, we did observe some difference in the relative abundance based on location, with the genus Gemella ranked in the top 10 on the cooling coil and the fan and the genera Roseomonas and Neisseria ranked in the top 10 on the fan, but none of these 3 genera ranked among the top 10 on the air filter. The genus Gemella is a Gram-positive bacterium that is ubiquitous on the mucous membranes of the human oral cavity, the upper respiratory tract, and the gastro-intestinal tract. Bacteria of the genus Neisseria are Gram-negative and, except for N. gonorrhoeae and N. meningitidis, which are pathogenic, they are endemic in the oral cavity. Bacteria of the genus Roseomon are Gram-negative and usually found in the environment.
The PCoA analysis revealed that five residences (050, 062, 083, 087, and 116) had remarkably comparable flora inside the air conditioner, confirming that the same bacterial genera on the surface of the air filter also influence the bacterial contamination inside the air conditioner. However, in many other homes in our study, the bacterial flora inside the air conditioners was not so similar. In other words, the bacterial flora was changing in ACs. Various factors, including the location of the house, ventilation conditions (that is, affected by both indoor and outdoor air [7]), AC unit operating conditions, and occupant activity, make it difficult to quantitatively evaluate the effect of each of these factors on bacterial flora inside the AC unit.
5. Conclusions
The AC unit air filter surface provides a history of airborne bacteria in the indoor environment. In our study, of the top 10 genera of bacteria detected on the filter surface, 6 genera were Gram-negative (Pseudomonas, Paracoccus, Acinetobacter, Methylobacterium, Enhydrobacter, and Sphingomonas) and 4 were Gram-positive (Staphylococcus, Corynebacterium, Streptococcus, and Actinotignum). Since bacteria on the surface of the air filter affect the bacterial contamination inside the air conditioner, it is important to clean air filters before and during the air conditioning season from the standpoint of occupants’ health. In addition, periodic cleaning of the air conditioner’s internal components is important. However, in many homes in our study, the bacterial flora inside the air conditioners was not so similar. In other words, the bacterial flora was changing. The presence of various impacting factors, including the location of the house (that is, influence from outside air), ventilation conditions, AC unit operating conditions, and occupant activity, makes it difficult to quantitatively evaluate the effect of each of these factors on bacterial flora inside the AC unit. A more detailed investigation of this topic is required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10112246/s1, Figure S1: Relative abundance of bacterial phyla for all samples from the 17 houses. A, C, F, and O corresponded to air filter, cooling coil, fan, and air outlet; Figure S2: Relative abundances (quartile value) of the main genus. A, C, F, and O corresponded to air filter, cooling coil, fan, and air outlet; Figure S3: Principal coordinate analysis (PCoA) of the unweighted UniFrac distance (A) and weighted UniFrac distance (B).
Author Contributions
Conceptualization: K.A., U.Y. and K.W.; methodology: K.W. and U.Y.; investigation: K.H., F.O. and Y.S.; writing—original draft preparation: K.W. and U.Y.; writing—review and editing: U.Y., K.A., Y.S., K.H., F.O. and K.W.; visualization: K.W.; supervision: U.Y.; project administration: K.A., Y.S. and U.Y.; funding acquisition: K.A. and U.Y. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was supported in part by Research Grants on Allergic Disease and Immunology (21ek0410055, 22ek0410097) from the Japan Agency for Medical Research and Development, and the Japan Society for the Promotion of Science (JSPS) (17H06216).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klepeis N.E., Nelson W.C., Ott W.R., Robinson T.A.M., Switzer P., Behar J.V., Hern S.C., Engelmann W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Env. Epid. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 2.Adams R.I., Bhangar S., Dannemiller K.C., Eisen J.A., Fierer N., Gilbert J.A., Green J.L., Miller S.L., Siegel J.A., Stephens B., et al. Ten questions concerning the microbiomes of buildings. Build. Environ. 2016;109:224–234. doi: 10.1016/j.buildenv.2016.09.001. [DOI] [Google Scholar]
- 3.Gilbert J.A., Stephens B. Microbiology of the built environment. Nat. Rev. Microbiol. 2018;16:661–670. doi: 10.1038/s41579-018-0065-5. [DOI] [PubMed] [Google Scholar]
- 4.Prussin A.J., Marr L.C. Sources of airborne microorganisms in the built environment. Microbiome. 2015;3:78. doi: 10.1186/s40168-015-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams R.I., Bateman A.C., Bik H.M., Meadow J.F. Microbiota of the indoor environment: A meta-analysis. Microbiome. 2015;3:1–18. doi: 10.1186/s40168-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoisington A., Maestre J.P., Kinney K.A., Siegel J.A. Characterizing the bacterial communities in retail stores in the United States. Indoor Air. 2016;26:857–868. doi: 10.1111/ina.12273. [DOI] [PubMed] [Google Scholar]
- 7.Hospodsky D., Qian J., Nazaroff W.W., Yamamoto N., Bibby K., Rismani-Yazdi H., Peccia J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE. 2012;7:e34867. doi: 10.1371/journal.pone.0034867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian J., Hospodsky D., Yamamoto N., Nazaroff W.W., Peccia J. Size-resolved emission rates of airborne bacteria and fungi in an occupied classroom. Indoor Air. 2012;22:339–351. doi: 10.1111/j.1600-0668.2012.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Täubel M., Rintala H., Pitkäranta M., Paulin L., Laitinen S., Pekkanen J., Hyvärinen A., Nevalainen A. The occupant as a source of house dust bacteria. J. Allergy Clin. Immunol. 2009;124:834–840. doi: 10.1016/j.jaci.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 10.Leung M.H.Y., Lee P.K.H. The roles of the outdoors and occupants in contributing to a potential pan-microbiome of the built environment: A review. Microbiome. 2016;4:21. doi: 10.1186/s40168-016-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanagi U., Kato S., Nagano H., Ito K., Yamanaka T., Momoi Y., Kobayashi H., Hayama H. Dispersion characteristics of oral microbial communities in a built environment. Jpn. Archit. Rev. 2022;5:225–232. doi: 10.1002/2475-8876.12261. [DOI] [Google Scholar]
- 12.Barberán A., Dunn R.R., Reich B.J., Pacifici K., Laber E.B., Menninger H.L., Morton J.M., Henley J.B., Leff J.W., Miller S.L., et al. The ecology of microscopic life in household dust. Proc. R. Soc. B. 2015;282:20151139. doi: 10.1098/rspb.2015.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ownby D.R., Johnson C.C., Peterson E.L. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. J. Am. Med. Assoc. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 14.Berg G., Mahnert A., Moissl-Eichinger C. Beneficial effects of plant-associated microbes on indoor microbiomes and human health? Front. Microbiol. 2014;5:15. doi: 10.3389/fmicb.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burge H.A., Solomon W.R., Muilenberg M.L. Evaluation of indoor plantings as allergen exposure sources. J. Allergy Clin. Immunol. 1982;70:101–108. doi: 10.1016/0091-6749(82)90236-6. [DOI] [PubMed] [Google Scholar]
- 16.Li A., Xiong J., Yao L., Gou L., Zhang W. Determination of dust and microorganism accumulation in different designs of AHU system in Shaanxi History Museum. Build. Environ. 2016;104:232–242. doi: 10.1016/j.buildenv.2016.05.014. [DOI] [Google Scholar]
- 17.Ager B.P., Tickner J.A. The control of microbiological hazards associated with air-conditioning and ventilation systems. Ann. Occup. Hyg. 1983;27:341–358. doi: 10.1093/annhyg/27.4.341. [DOI] [PubMed] [Google Scholar]
- 18.Batterman S.A., Burge H. HVAC systems as emission sources affecting indoor air quality: A critical review. HVAC R Res. 1995;1:61–78. doi: 10.1080/10789669.1995.10391309. [DOI] [Google Scholar]
- 19.Hugenholtz P., Fuerst J.A. Heterotrophic bacteria in an air handling system. Appl. Environ. Microbiol. 1992;58:3914–3920. doi: 10.1128/aem.58.12.3914-3920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatayama K., Oikawa Y., Ito H. Bacterial community structures in air conditioners installed in Japanese residential buildings. Antonie Leeuwenhoek. 2018;11:45–53. doi: 10.1007/s10482-017-0925-4. [DOI] [PubMed] [Google Scholar]
- 21.Bakker A., Siegel J.A., Mendell M.J., Peccia J. Building and environmental factors that influence bacterial and fungal loading on air conditioning cooling coils. Indoor Air. 2018;28:689–696. doi: 10.1111/ina.12474. [DOI] [PubMed] [Google Scholar]
- 22.Bakker A., Siegel J.A., Mendell M.J., Prussin A.J., Marr L.C., Peccia J. Bacterial and fungal ecology on air conditioning cooling coils is influenced by climate and building factors. Indoor Air. 2019;30:326–334. doi: 10.1111/ina.12632. [DOI] [PubMed] [Google Scholar]
- 23.Simmons R.B., Rose L.J., Crow S.A., Ahearn D.G. The Occurrence and Persistence of Mixed Biofilms in Automobile Air Conditioning Systems. Curr. Microbiol. 1999;39:141–145. doi: 10.1007/s002849900435. [DOI] [PubMed] [Google Scholar]
- 24.Diekmann N., Burghartz M., Remus L., Kaufholz A.L., Nawrath T., Rohde M., Schulz S., Roselius L., Schaper J., Mamber O., et al. Microbial communities related to volatile organic compound emission in automobile air conditioning units. Appl. Microbiol. Biotechnol. 2013;97:8777–8793. doi: 10.1007/s00253-012-4564-4. [DOI] [PubMed] [Google Scholar]
- 25.Japanese Agency for Natural Resources and Energy. [(accessed on 7 October 2022)]. Available online: https://www.meti.go.jp/shingikai/enecho/shoene_shinene/sho_energy/air_denki/pdf/002_04_00.pdf. (In Japanese)
- 26.Banaszak E.F., Thiede W.H., Fink J.N. Hypersensitivity pneumonitis due to contamination of an air conditioner. N. Engl. J. Med. 1970;283:271–276. doi: 10.1056/NEJM197008062830601. [DOI] [PubMed] [Google Scholar]
- 27.Baur X., Behr J., Dewair M., Ehret W., Fruhmann G., Vogelmeier C., Weiss W., Zinkernagel V. Humidifier Lung and Humidifier Fever. Lung. 1988;166:113–124. doi: 10.1007/BF02714035. [DOI] [PubMed] [Google Scholar]
- 28.Acierno L.J., Lytle J.S., Sweeney M.J. Acute Hypersensitivity Pneumonitis Related to Forced Air Systems—A Review of Selected Literature And a Commentary on Recognition and Prevention. J. Environ. Health. 1985;48:138–141. [Google Scholar]
- 29.Braun-Fahrlander C., Riedler J., Herz U., Eder W.T., Waser M., Grize L., Maish A., Carr D., Gerlach F., Bufe A., et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Eng. J. Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 30.Williams L.K., Ownby D.R., Maliarik M.J., Johnson C.C. The role of endotoxin and its receptors in allergic disease. Ann. Allergery Asthma Immunol. 2005;94:323–332. doi: 10.1016/S1081-1206(10)60983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flaherty D.K., Deck F.H., Cooper J., Bishop K., Winzenburger P.A., Smith L.R., Bynum L., Witmer W.B. Bacterial endotoxin isolated from a water spray air humidification system as a putative agent of occupation-rated lung disease. Infect. Immunol. 1984;43:206–212. doi: 10.1128/iai.43.1.206-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heederik D., Brouwer R., Biersteker K., Boleij J.S. Relationship of airborne endotoxin and bacterial levels in pig farms with the lung fuction and respiratory symptoms of farmers. Int. Arch. Occup. Environ. Health. 1991;62:595–601. doi: 10.1007/BF00381114. [DOI] [PubMed] [Google Scholar]
- 33.Milton D.K., Wypij D., Walters M., Hammond S.K., Evans J. Endotoxin exposure-response in a fiberglass manufacturing plant. Am. J. Indust. Med. 1996;29:3–13. doi: 10.1002/(SICI)1097-0274(199601)29:1<3::AID-AJIM2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 34.Dannemiller K.C., Weschler C.J., Peccia J. Fungal and bacterial growth in floor dust at elevated relative humidity levels. Indoor Air. 2017;27:354–363. doi: 10.1111/ina.12313. [DOI] [PubMed] [Google Scholar]
- 35.Hyvärinen A., Meklin T., Vepsäläinen A., Nevalainen A. Fungi and actinobacteria in moisture-damaged building materials— concentrations and diversity. Int. Biodeterior. Biodegradation. 2002;49:27–37. doi: 10.1016/S0964-8305(01)00103-2. [DOI] [Google Scholar]
- 36.Schmidt M.G., Attaway H.H., Terzieva S., Marshall A., Steed L.L., Salzberg D., Hamoodi H.A., Khan J.A., Feigley C.E., Michels H.T. Characterization and Control of the Microbial Community Affiliated with Copper or Aluminum Heat Exchangers of HVAC Systems. Curr. Microbiol. 2012;65:141–149. doi: 10.1007/s00284-012-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone G.A., Turnbaugh P.J., Fierer N., Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA. 2011;108((Suppl. S1)):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan M.P., Adley C.C. Sphingomonas paucimobilis: A persistent Gram negative nosocomial infectious organism. J. Hosp. Infect. 2010;75:153–157. doi: 10.1016/j.jhin.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Lozupone C.A., Hamady M., Kelley S.T., Knight R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007;73:1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickering A.C., Fitzgerald J.R. The Role of Gram-Positive Surface Proteins in Bacterial Niche- and Host-Specialization. Front. Microbiol. 2020;11:1–9. doi: 10.3389/fmicb.2020.594737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano T., Miyahata Y., Yokohata R., Hanai J., Matsuo S., Hiratsuka E., Okano T., Kubota H. Analyses and Regulation of Biofilms in Actual Environments. [(accessed on 1 October 2022)];J. Environ. Biotecthnol. 2015 14:125–129. Available online: https://www.jseb.jp/wordpress/wp-content/uploads/14-02-125.pdf. (In Japanese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.