Summary
Background
Spontaneous regression of advanced solid tumors is infrequent but may occur. Quantifying response rates from placebo in cancer drug trials may provide important information for physicians, patients, and regulators. We aimed to provide a pooled placebo response rate from drug trials in advanced solid tumors.
Methods
We pooled the overall response rate (ORR), complete response rate (CR) and partial response rates (PR) in the placebo arm of placebo-controlled randomized controlled trials (RCTs) of cancer drugs for advanced solid tumors published during 2015–2021 using random-effects model.
Findings
45 phase 3 RCTs including 5684 patients on placebo met our inclusion criteria and formed the study cohort. The pooled overall ORR, CR and PR rates in the placebo arm were 1% (95% CI, 0%–2%), 0% (95% CI, 0%–0%), and 1% (95% CI, 0%–2%) respectively. Higher placebo responses were observed in prostate cancer and sarcoma trials.
Interpretation
Overall, 1% patients with advanced solid tumors can expect to achieve some response even in absence of treatment. However, complete regression without treatment is extremely rare, almost zero percent. This information will be helpful to patients in their decisions, as well as regulators in evaluating cancer drugs’ efficacy based on response rates alone.
Funding
None.
Keywords: Objective response rates, Placebo, RCT
Research in context.
Evidence before this study
Some patients with cancer believe that their cancer may undergo spontaneous regression without cancer treatment. Regulators also offer drug approvals based on response rates alone. The chances of spontaneous regression of cancer may be studied by assessing the response rates of placebo monotherapy arm in randomized trials. Two past such studies have put this around 2%, the latest of which included studies up until 2014.
Added value of this study
Our meta-analysis of RCTs from 2015 to 2021 finds the overall placebo response rate in patients with cancer at 1%, almost all of which were partial responses. This placebo response rates differed by tumor types.
Implications of all available evidence
There is a less than 2% chance, at best, of achieving any spontaneous response and almost zero chance of spontaneous complete remission in absence of cancer treatment. Regulators, however, should consider that there are some chances of partial responses even from placebo alone when making approval decisions based on response rates alone. Complete response rate is a better marker of drug activity than partial response rates.
Introduction
An increasing number of new cancer drugs are approved on the basis of overall response rates (ORR) from single-arm trials. ORR is defined as the percentage of patients who achieve a response, which can either be complete response (complete disappearance of lesions) or partial response (reduction in the sum of maximal tumor diameters by at least 30% or more).1 Between 2017 and 2021, 36% (58/161) of cancer drugs approved by the U.S. Food and Drug Administration (FDA) were based on ORR from single-arm trials.2
However, ORR is a poor marker of drug efficacy as it doesn't correlate with improvement in survival.3 Despite this poor correlation with survival, one reason for the frequent use of ORR as an endpoint in cancer drug trials is the hypothesis that a malignant tumor would not shrink spontaneously, if not for the drug. However, some studies in the past have shown that solid tumors may also occasionally regress even without treatment, either spontaneously or due to placebo effect. A 2003 meta-analysis showed a response rate of 2% among patients with advanced solid tumors randomized to receive placebo or best supportive care in randomized controlled trials (RCTs).4 This finding was subsequently corroborated in a 2016 study that revealed a similar 2% objective response rates among patients with advanced solid tumors randomized to receive no active treatment in RCTs of cancer drugs.5
Such spontaneous regressions or responses are intriguing from both scientific and regulatory standpoints. Therefore, we conducted an updated analysis to discover the ORR, complete response rates (CR), and partial response rates (PR) from placebo in the RCTs conducted in the modern era in which treatment landscapes have changed and 40% Americans believe that cancer can be cured solely through alternative therapies.6
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline.7 This is a meta-analysis of trial-level data from the published literature, and individual patient level data were not collected. Therefore, in absence of individual patient level data, ethical approval was not deemed necessary.
Study selection
We conducted a systematic search of PubMed to identify all RCTs of anti-cancer drugs in patients with advanced solid tumors conducted between January 1, 2015 and December 31, 2021 and published in English. The most recent study of placebo response rates in advanced solid tumors had included studies until 2014, so we decided to study the RCTs conducted after 2014, i.e. 2015–2021. Trials that met the following criteria were included: (1) assessed advanced adult solid tumours only; (2) not a (neo) adjuvant therapy; (3) did not study local therapies such as surgery or radiation, cell-based therapies, or supportive care; (4) had a sample size greater than 20 participants in each arm; (5) randomly allocated patients to either treatment or placebo arms; (6) the placebo arm was a monotherapy or was used in combination with the best supportive care (i.e. placebo was not a part of combination therapy such as chemotherapy plus placebo or immunotherapy plus placebo); (7) was not a duplicate trial or subgroup analysis; (8) measured objective response rates as outlined by the Response Evaluation Criteria in Solid Tumours (RECIST). Both phase 2 and phase 3 RCTs in either first-line or later-lines of therapy would be included, if eligible. Non-randomized trials, trials in pediatric population, trials with placebo in combination with an active treatment, and trials without response rate data were excluded. In addition, RCTs of maintenance therapies where the trial tests a maintenance therapy versus placebo after the completion of initial therapy were also excluded because any responses observed in the placebo arm could have been from the initial treatment with active anticancer agent.
Data extraction
After title and abstract screening independently by the two authors (AS and BG), the full texts of potentially relevant studies were downloaded, and relevant data were independently extracted from published reports by the two authors. This was also subsequently verified separately by the other two authors (IS and MB) and any discrepancy was resolved by mutual consensus.
We collected key trial characteristics: study name, year of publication, treatment setting (lines of treatment, use of maintenance therapy etc.), sample size, the number of patients randomized into each trial arm, and the rates of ORR, CR, and PR for each arm. We used the number of response evaluable patients for assessing response rates. Both the rates and the 95% confidence intervals were recorded.
Endpoints
The primary endpoint of our study was to estimate a summary response rate of placebo in cancer clinical trials. The secondary endpoints included estimation of complete and partial response rates for placebos, and subgroup analyses by tumor type and line of therapy (first-line versus later-line).
Statistical analysis
The statistical analyses for this study were performed using R statistical software, version 4.1.2. Proportions of patients in each trial were used to derive the ORR, CR, and PR rates.
The meta-analysis was performed using a random-effects model based on the inverse-variance approach to account for clinical heterogeneity. The Freedman-Tukey double arcsine transformation was used, removing the need to apply a correction for the studies which reported zero patient values for either ORR, CR, or PR.8 The Clopper-Pearson method was used to determine confidence intervals for each study.9 Statistical heterogeneity among the included studies was assessed using the DerSimonian and Laird estimate for tau-squared (τ2), which quantifies the variance of the effect size of the data using the Cochrane's Q statistic.10 Confidence intervals for tau (τ) and τ2 were derived using the Jackson method.11 Statistical heterogeneity was quantified using the I-squared (I2) statistic and a value above 75% was deemed to be considerable heterogeneity.12
Forest plots were generated for ORR, CR and PR to assess the placebo effect among patients with cancer. Additional subgroup analyses was performed to examine whether the placebo effect differed based on cancer type (prostate, colorectal, non-small cell lung cancer (NSCLC), and other) or based on lines of therapy (first-line versus later-lines).
Role of funding
No funding support was received for this study.
Results
Of the 963 studies initially identified, 45 RCTs involving 5684 patients in the placebo arm met our inclusion criteria and were included in this study (Fig. 1 and Supplementary Appendix). General characteristics of the included studies are summarized in Table 1. The commonest cancer type was hepatocellular cancer (20%) and the commonest treatment type studied was targeted therapy (80%). Only 6 trials (12%) were conducted in treatment naïve patients, the rest were trials conducted in second or subsequent lines of therapy. The number of patients included in the placebo arm were 5684 but the number of patients evaluable for ORR, CR and PR were 4760, 3808, and 3808 respectively. The denominators for ORR, CR, and PR differ due to (un)availability of data.
Fig. 1.
Flow-diagram of included studies.
Table 1.
Characteristics of clinical trials included in the study.
| Characteristics | Number (percentage) |
|---|---|
| Total number of trials | 45 |
| Total number of patients in the placebo arm | 5684 |
| Total number of patients evaluable for ORR in placebo arm | 4760 |
| Cancer types | |
| Lung | 5 (11%) |
| Colorectal | 7 (16%) |
| Prostate | 3 (7%) |
| Hepatocellular | 9 (20%) |
| Gastro-esophageal | 5 (11%) |
| Sarcoma | 4 (9%) |
| Others | 12 (27%) |
| Treatment type | |
| Immunotherapy | 4 (9%) |
| Targeted therapy | 36 (80%) |
| Hormone therapy | 4 (9%) |
| Chemotherapy | 1 (2%) |
| Previous treatment | |
| None (treatment naïve or first-line) | 6 (12%) |
| Yes (second and subsequent lines) | 39 (87%) |
Pooled ORR
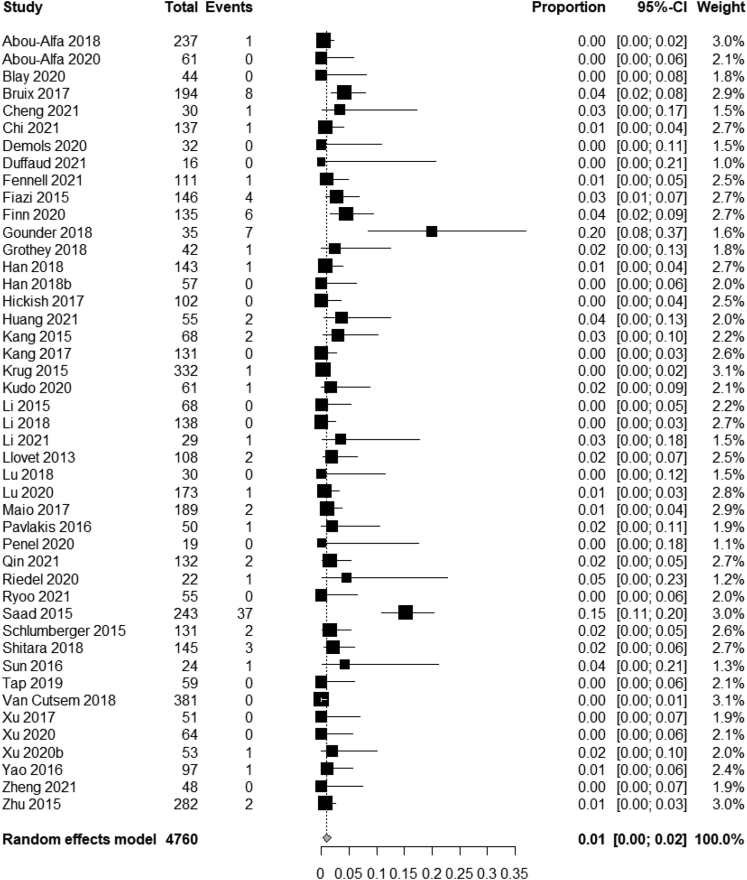
Of the total 4760 evaluable patients for ORR in the placebo arm of RCTs, 94 achieved a response for an overall response rate of 1.97%. By using random-effects model, the pooled ORR for placebo was 1% (95% CI, 0%–2%) (Fig. 2),
Fig. 2.
Pooled analysis of ORR in the placebo arm across the trials of adult solid tumors.
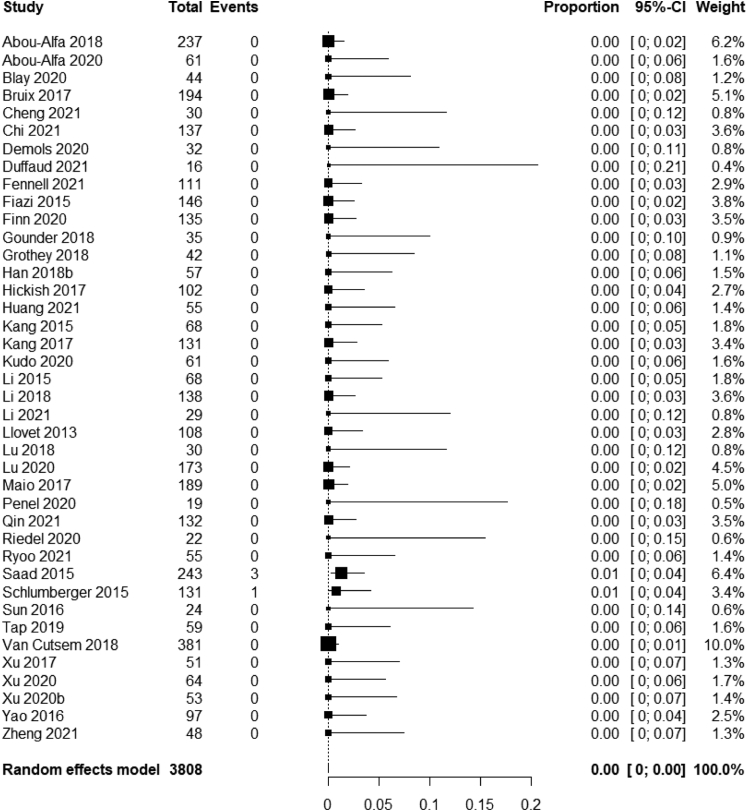
Some studies didn't provide ORR data differentiated by CR and PR rates. Of the 3808 patients where this was evaluable, only 4 (0.1%) achieved a complete response. The pooled CR rate was 0% (95% CI, 0%–0%) (Fig. 3).
Fig. 3.
Meta-analysis of complete response rates of placebo across adult solid tumors.
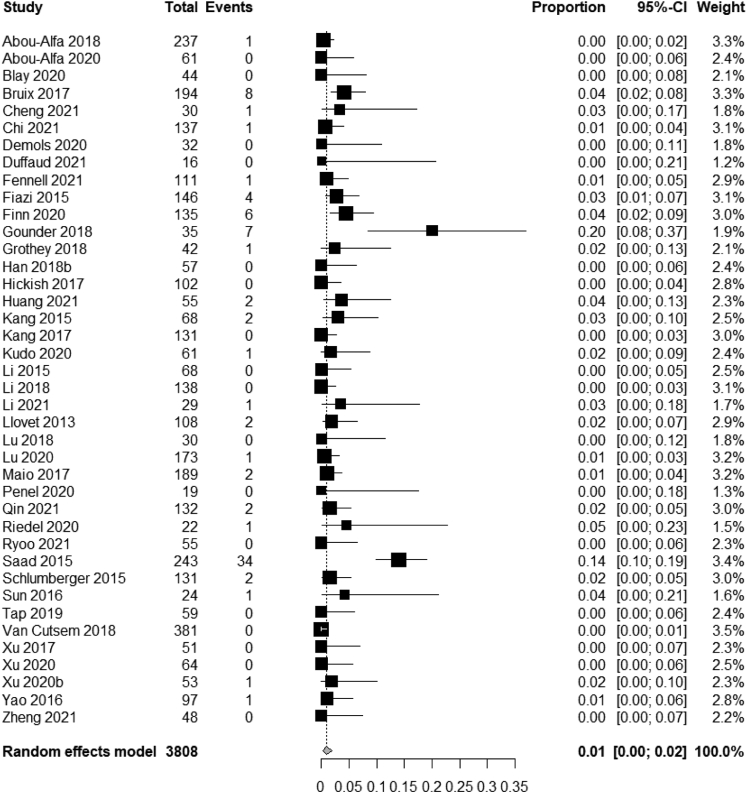
Partial response was observed in 2.18% of patients receiving placebo (83/3808). The pooled partial response rate across the trials was 1% (95% CI, 0%–2%) (Fig. 4).
Fig. 4.
Meta-analysis of partial response rates of placebo across adult solid tumors.
Subgroup analyses
There was substantial heterogeneity in placebo ORR across the trials (I2 = 72%, Q = 157, p < 0.0001) and therefore, subgroup analyses based on tumor type and treatment-line were performed (Supplementary Appendix). Subgroup analyses based on tumor types revealed important differences in placebo response rates based on tissue of origin (7% in prostate cancer, 4% in sarcoma, 2% in hepatocellular cancer vs 1% in gastroesophageal and 0% in colorectal, lung and other tumor types; between-groups I2 = 72%, p < 0.01). However, subgroup analysis based on treatment-line did not reveal significant differences between first-line treatment trials or treatment-naïve trials (4%) versus second or subsequent line treatments (1%) (Between-group I2 = 72%, p = 0.12). However, this lack of significance could be due to fewer number of trials in the treatment-naïve subgroup.
Discussion
In this study, we found that the ORR from placebo in RCTs of cancer drugs for adult solid tumors was 1%, almost all of which was partial responses. This information will be useful for multiple stakeholders, including patients, physicians, and regulators as discussed below.
Patients may wish to know the chance of spontaneous regression of their malignant tumors without treatment. Some other patients also forego medicinal treatment in favour of alternative modalities.13 The best estimate for the chances of spontaneous regression can be inferred through the placebo response rates in randomized trials since placebo, by definition, are inert substances that do not have any anti-tumor effects of their own. Our study highlights that expectation of complete regression of tumors in absence of treatment is extremely rare as only four of 3808 patients in our study achieved CR with placebo alone, for a pooled CR rate of 0%. However, our study also shows that in 1% of patients, there may be partial response even without any active cancer treatment. This may explain the occasional anecdotes about some patients achieving a response without any cancer treatment or with the use of an alternative unproven therapy.
In subgroup analysis, we found a higher response in prostate cancer and sarcoma of 7% and 4% respectively. A higher response rate in prostate cancer may be explained by some therapeutic effect of prednisone that patients with prostate cancer are usually on, even on placebo arm14 or the effects of the use of ongoing androgen blockade such as due to GnRH analogue outside of the trial. However, a 4% response rate in sarcoma patients is intriguing, and lacks clear explanation. We did not perform subgroup analysis based on treatment type because the differences in the type of therapy in the experimental arm will not have any bearings on the responses from the placebo arm.
Our overall response rate across tumor types of 1% is somewhat similar to previously reported placebo response rates in adult solid tumors4,5 although the time frame of our study was different. The 2003 report by Chvetzoff and Tannock reported a placebo ORR of 2.7% in 375 patients; however, they did not conduct a pooled analysis accounting for the heterogeneity.4 We also report a 1.97% ORR using absolute numbers, similar to the methods used by Chvetzoff and Tannock. The 2015 report by Ghatalia et al. estimated placebo ORR using random effects pooled analysis and reported it to be 1.95%; their estimate may have been slightly inflated since they did not exclude maintenance therapy trials.5 Similar to our results, they also do not show any significant differences in response rates between first-line and subsequent-line trials.
We believe that our estimate of 1% ORR to be the closest to the truth answer for placebo response rate or chances of spontaneous regression. It is reassuring to see that all published reports of placebo response rates so far put this number at less than 2%, which is also the upper bound of our 95% confidence interval. Thus, we can safely communicate with our patients that the chances of response without treatment is probably 1%, and not more than 2% even in the most optimistic scenario. Furthermore, the similarity in placebo ORR in these three reports conducted in different time frames solidifies this metric because there is no reason for placebo responses to change with time.
A previous study has shown that patients who receive complementary medicine are more likely to refuse conventional cancer therapy and were at a higher risk of death.15 A national survey from the American Society of Clinical Oncology also revealed that four in ten Americans believed that cancer could be completely cured with alternative treatments alone.6 Combined with our study showing almost zero chance of complete regression and very small chance of any response in absence of cancer treatment, we believe these results will help persuade several patients to not forego cancer treatment in hope of achieving spontaneous regression via other means.
Previous studies have shown that the response rates of cancer drugs in single-arm trials are higher than in randomized trials.16,17 Furthermore, placebo effects aren't captured in single-arm trials. Therefore, regulators should be very cautious in offering drug approval based on response rates from single arm trials. Indeed, one therapy trial in our study showed a response rate of up to 20% with placebo alone.18 It is noteworthy that several FDA drug approvals are based on response rates lower than this (12% ORR in Checkmate 032,19 17% in Keynote 224,20 15% in EZH-20221). Thus, our data suggest that for drug approval, it is important to evaluate response rates in the context of a RCT. Additionally, our study suggests that complete regressions are almost zero in absence of therapy; so regulators may wish to focus more on complete response rates rather than overall response rates in evaluating the preliminary activity of a cancer drug.
Only 6 RCTs in our sample were conducted in treatment naïve patients. One could reasonably argue, therefore, that some responses observed in the placebo arm of second or subsequent line trials maybe a carryover effect from the treatments offered before the patients were enrolled onto this trial. This may be true in a few selected patients whose previous treatment was stopped due to toxicities. However, in most cases, the previous treatment will have been stopped due to disease progression and therefore, one would expect continued disease progression in absence of any treatment in the placebo arm. In any case, the ORR was instead higher in first-line trials in our analysis than in subsequent line trials.
Possible publication bias and heterogeneity of included studies are potential limitations of our study. Publication bias against negative studies could impact our results via exclusion of studies with greater placebo response rates. However, there is consistency in the reported overall placebo response rates between our study and the two previous studies conducted across different time frames.4,5 We also included studies published only in English language. Although most placebo-controlled RCTs are published in journals that publish at least the abstract in English language, there is a possibility that some placebo-controlled RCTs may have been missed with the use of English language as an inclusion criterion. However, there are no reasons to believe that placebo-controlled RCTs published in non-English language would be systematically different from those published in English language. In addition, some partial responses may also be attributed to variability in response assessment based on imaging criteria. There is also no granular data available on imaging modalities such as CT-based versus MRI-based versus PET-scan-based response assessment for patients in placebo-arm, although they wouldn't be systematically different than those for patients in the intervention arm. Furthermore, the years selected in this study may not be representative of all the studies; however there is no reason to believe that placebo response rates should differ with time. The similarity of our findings with previous studies affirms the absence of chronological impact on placebo response rates. Finally, not all tumor types have been represented in this study reflecting the advance in treatment and unsuitability of placebo monotherapy arm in some tumor types.
In conclusion, while approximately 1% of patients with advanced solid tumours will experience partial response from placebo alone, complete regression is extremely rare in absence of treatment. Patients should not expect complete regression of cancers without treatment. Regulators should not rely on response rate of cancer drugs from single arm trials for drug approval because some partial responses are possible even from placebo.
Contributors
AS- Data collection, Data curation, Investigation, Writing-original draft; IS and MB- Data collection, Data curation, Methodology, Writing-review and editing; CB- Writing-review and editing; BG- Conceptualization, Data Curation, Supervision, Writing- original draft, Writing-review and editing.
Data sharing statement
The data underlying this article are available upon reasonable request to the corresponding author.
Declaration of interests
Dr. Gyawali declares receiving consulting fees from Vivio Health unrelated to the manuscript. Other authors have no conflicts of interest to disclose.
Acknowledgments
Dr. Gyawali has received salary support from Ontario Institute for Cancer Research, funded by the government of Ontario. The views expressed in the publication are the views of the authors and do not necessarily reflect those of the Government of Ontario.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101753.
Appendix A. Supplementary data
References
- 1.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Cherny N.I. An appraisal of FDA approvals for adult solid tumours in 2017–2021: has the eagle landed? Nat Rev Clin Oncol. 2022;19:486–492. doi: 10.1038/s41571-022-00636-y. [DOI] [PubMed] [Google Scholar]
- 3.Cooper K., Tappenden P., Cantrell A., Ennis K. A systematic review of meta-analyses assessing the validity of tumour response endpoints as surrogates for progression-free or overall survival in cancer. Br J Cancer. 2020;123(11):1686–1696. doi: 10.1038/s41416-020-01050-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chvetzoff Gl, Tannock I.F. Placebo effects in oncology. J Natl Cancer Inst. 2003;95(1):19–29. doi: 10.1093/jnci/95.1.19. [DOI] [PubMed] [Google Scholar]
- 5.Ghatalia P., Morgan C.J., Sonpavde G. Meta-analysis of regression of advanced solid tumors in patients receiving placebo or no anti-cancer therapy in prospective trials. Crit Rev Oncol Hematol. 2016;98:122–136. doi: 10.1016/j.critrevonc.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 6.ASCO National survey reveals surprising number of Americans believe alternative therapies can cure cancer. 2018. https://www.asco.org/about-asco/press-center/news-releases/national-survey-reveals-surprising-number-americans-believe Accessed at.
- 7.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3(3) doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clopper C.J., Pearson E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 10.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.rdrr.io. metaprop:Meta-analysis of single proportions. 2022. https://rdrr.io/cran/meta/man/metaprop.html
- 12.Ryan R. Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Group reviews: planning the analysis at protocol stage. 2022. https://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/heterogeneity_subgroup_analyses_revising_december_1st_2016.pdf
- 13.Molassiotis A., Fernández-Ortega P., Pud D., et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16(4):655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 14.Teply B.A., Luber B., Denmeade S.R., Antonarakis E.S. The influence of prednisone on the efficacy of docetaxel in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2016;19(1):72–78. doi: 10.1038/pcan.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson S.B., Park H.S., Gross C.P., Yu J.B. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;4(10):1375–1381. doi: 10.1001/jamaoncol.2018.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gyawali B., D'Andrea E., Franklin J.M., Kesselheim A.S. Response rates and durations of response for biomarker-based cancer drugs in nonrandomized versus randomized trials. J Natl Compr Canc Netw. 2020;18(1):36–43. doi: 10.6004/jnccn.2019.7345. [DOI] [PubMed] [Google Scholar]
- 17.Zia M., Siu L., Pond G. Comparison of outcomes of phase II studies and subsequent randomized control studies using identical chemotherapeutic regimens. J Clin Oncol. 2005;23:6982–6991. doi: 10.1200/jco.2005.06.679. [DOI] [PubMed] [Google Scholar]
- 18.Gounder M.M., Mahoney M.R., Van Tine B.A., et al. Sorafenib for advanced and refractory desmoid tumors. N Engl J Med. 2018;379(25):2417–2428. doi: 10.1056/NEJMoa1805052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attard G., Borre M., Gurney H., et al. Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol. 2018;36(25):2639–2646. doi: 10.1200/jco.2018.77.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalaf D.J., Annala M., Taavitsainen S., et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20(12):1730–1739. doi: 10.1016/s1470-2045(19)30688-6. [DOI] [PubMed] [Google Scholar]
- 21.Gyawali B., Bouche G., Crisp N., André N. Challenges and opportunities for cancer clinical trials in low- and middle-income countries. Nat Cancer. 2020;1(2):142–145. doi: 10.1038/s43018-020-0030-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.