Abstract
Introduction
Polymer materials used in medical devices and treatments invariably encounter cellular networks. For the device to succeed in tissue engineering applications, the polymer must promote cellular interactions through adhesion and proliferation. To predict how a polymer will behave in vitro, these material–cell interactions need to be well understood.
Methods
To study polymer structure-property relationships, microparticles of four chemically distinct tyrosol-derived poly(ester-arylate) polymers and a commercially available poly(lactic acid-co-glycolic acid) (PLGA) copolymer were prepared and their interactions with cells investigated. Cell loading concentration was optimized and cell adhesion and proliferation evaluated. Particles were also tested for their ability to adsorb bone morphogenetic protein-2 (BMP-2) and differentiate a myoblast cell line towards an osteoblast lineage through BMP-2 loading and release.
Results
While cell adhesion was observed on all particles after 24 h of incubation, the highest degree of cell adhesion occurred on polymers with smaller crystallites. At longer incubation times, cells proliferated on all particle formulations, regardless of the differences in polymer properties. High BMP-2 loading was achieved for all particle formulations and all formulations showed a burst release. Even with the burst release, cells cultured on all formulations showed an upregulation in alkaline phosphatase (ALP) activity, a measure of osteoblast differentiation.
Conclusions
As with cell adhesion, the polymer with the smaller crystallite showed the most ALP activity. We suggest that smaller crystallites serve as a proxy for topographical roughness to elicit the observed responses from cells. Furthermore, we have drawn a correlation between the polymer crystallite with the hydration potential using surface analysis techniques.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12195-022-00729-9.
Keywords: Cell–material interactions, Tissue regeneration, Bone-morphogenetic protein-2, Crystallite size, Hydration potential
Introduction
The use of polymeric biomaterials in tissue engineering applications has been the focus of regenerative medicine for the past few decades. Designing a polymer that is both noncytotoxic and stimulates the appropriate biological response is important to both reduce rejection and to create a surface that allows cells to both adhere and proliferate. Understanding the material–cell interactions that are affected by the polymer’s chemical, mechanical, and morphological properties is important to succeed in tissue regeneration. When a cell first comes in contact with a biomaterial with properties favorable for attachment, the cell will bind to the surface either passively, allowing for easy removal if disturbed, or actively, through focal adhesions to the adsorbed serum proteins and deposited extracellular matrix (ECM).5 The physiochemical interactions between the polymer surface, adsorbed proteins, and the cell dictate if and how strongly a cell will adhere.16 It has previously been shown that a polymer that is too hydrophilic will inhibit cell adhesion due to the hydration layer that prevents protein adsorption; however, if a polymer is too hydrophobic, it will denature the ECM proteins during adsorption and reduce cell binding.4,35 Polymer stiffness, surface roughness, thermal transitions, and crystallinity also influence cell adhesion, with values close to that of natural ECM facilitating cell adhesion.15,27,34 Methods to modify polymer surfaces with anti-fouling coatings to change the hydrophobicity, patterning to alter the topography, and functionalizing the surface with proteins to attract cells have been investigated.1,24,32 After a cell has adhered to the polymer surface, the cells must begin to proliferate for the device to be useful for promoting tissue regeneration. Cell proliferation, like cell adhesion, are affected by polymer properties.37
Novel polymeric materials are continuously being designed for tissue regenerative applications; however, developing a relationship between a polymer’s chemical and physical properties and cellular response is key to predicting how the polymer will perform in vivo. Here, we explore the effect of polymer crystallinity and glass transition temperature (Tg), which control polymer rigidity and chain flexibility, on cell adhesion and proliferation.
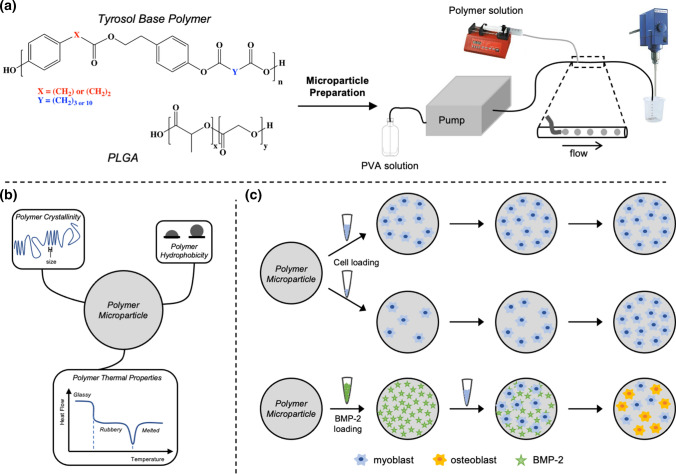
For tissue regenerative applications, polymeric 3D scaffolds that release proteins and drugs are often used to fill defect sites.26,29 Polymer properties again control protein adsorption and release rates.8,9 The same balance between a polymer's crystalline properties that determines polymer hydration and therefore cell adhesion will also dictate growth factor loading and stability in a polymer scaffold. To evaluate the impact of polymer properties on cellular interactions and protein adsorption, microparticles were prepared out of four tyrosol-derived poly(ester-arylate) polymers that have different physical and thermal properties (Fig. 1). These tyrosol-derived polymers have shown promise in microparticle drug delivery systems due to their biocompatibility, biodegradability, and ability to delivery drugs out to several months.18,20 Additionally, poly(lactic-co-glycolic acid) (PLGA) was used as a control polymer as it is commonly used in tissue regeneration applications.21,25 Furthermore, these 3D microparticle-based scaffolds were studied for their use in bone regeneration applications, testing bone morphogenetic protein-2 (BMP-2) loading and release and their ability to promote osteoblast differentiation in vitro.
Figure 1.
Experimental design schematic. (a) Polymer selection of a series of tyrosol-derived poly(ester-arylate)s with differring physical and thermal properties. A continuous flow method was used to prepare microparticles ranging from 40 to 200 µm in diameter. (b) Polymer properties investigated for their influence on cellular interactions. (c) Polymer particles were studied for their cell adhesion, proliferation, and ability to release BMP-2 to promote osteogenic differentiation.
Materials and Methods
Chemicals and Materials
PLGA-Resomer® 506 (50:50 ratio of lactic acid:glycolic acid) was purchased from Boehringer Ingelheim. Tyrosol-derived polymers were synthesized as previously described.7 All chemicals were purchased from Sigma Aldrich unless otherwise noted. Dulbecco’s phosphate-buffered saline (DPBS) and water for injection (WFI) were purchased from Fisher Scientific. All solvents used were purchased through VWR and Sigma-Aldrich.
Microparticle Preparation
Microparticles were prepared as previously described.19 Briefly, an oil/water (o/w) coaxial flow system fitted with a syringe pump to add the polymer solution to the aqueous carrier solution (2.5% w/v polyvinyl alcohol (PVA) in DI water) was used (Fig. 1a) The polymer solution was prepared by dissolving polymer in dichloromethane (DCM) at 9% w/v. Prepared particles were collected in a beaker with PVA solution and gently agitated with overhead stirring. After 30 min, the PVA solution was removed, particles washed with DI water, sieved to a range of 40–200 µm using cell strainers (PluriSelect), then lyophilized. Particles were sterilized by soaking particles in isopropyl alcohol (IPA) for 30 min, then dried overnight on a filter with vacuum to remove residual IPA.
Polymer and Microparticle Characterization
All polymers and prepared microparticles were characterized by gel permeation chromatography (GPC) and dynamic scanning calorimetry (DSC). The air-water contact angle (AWCA) was measured using polymer spin coated films and water miscibility and logP values were calculated using a polymer degree of polymerization (DP) of one. Microparticles were analyzed using X-ray diffraction (XRD), measured using optical images, and surface texture analyzed using scanning electron microscopy (SEM). Methods for all analysis and calculations are described in a previous publication.18
Cell Culture
Human dermal fibroblasts (HDF, Life Technologies) and C2C12 cells (ATCC) were maintained in complete Dulbecco’s Modified Essential Medium [DMEM (Life Technologies), supplemented with 10% fetal bovine serum (FBS, Gibco) and 35 µg/mL gentamicin (Sigma Aldrich)]. W-20-12 (ATCC) cells were maintained in basal medium according to ASTM Standard (F2131-02). Briefly, 13.3 g of DMEM with 4500 mg/L glucose, 4 mM l-glutamine, and 2.226 g sodium bicarbonate were dissolved in 800 mL purified water. HCl (0.2 N) was added to adjust the pH to 7.3 ± 0.1, and the final volume brought to 1 L. The basal medium was sterile filtered using a 0.2 µm polyethersulfone (PES) filter and 10% heat-inactivated FBS, 8 mM l-glutamine, and 50 µg/mL gentamicin added. Cells were passaged before reaching confluence and medium changed every 3–4 days. Cells were used with a passage number between 4 and 12. All cells were cultured at 37 °C, 5% CO2, and >95% humidity.
Cytocompatibility Study
To evaluate particle cytocompatibility, extracts from prepared particles were incubated with HDFs, and their effect on cell survival was evaluated. To obtain the extract, 10 mg of particles were weighed into a non-treated 48-well plate and incubated in 300 µL complete DMEM (supplemented with 10% FBS and 35 µg/mL gentamicin) for 24 h at 37 °C. Complete DMEM with supplements was used as a negative control, and each polymer was run in triplicate. After incubation, the plates were centrifuged to gravimetrically separate the particles, and the media was removed. The extracted media represents any leachable present either from the microparticle preparation process or polymer degradation; however, the polymer concentration in the media is expected to be low due to the insignificant degradation of the polymers in the first 24 h.7,17 HDF cells were seeded into a tissue culture treated 96-well plate at a density of 64,000 cells/cm2. After 24 h of culture, the media was removed from the cells and replaced with 100 µL of undiluted particle extract media. After 24 h of incubation, media was removed, cells were washed two times with DPBS, then frozen in 80 µL lysis buffer (Cell Signaling Technologies) at − 80 °C. Plates underwent two thaw-freeze cycles prior to analysis. Recovered DNA was quantified using Picogreen assay (ThermoFisher) according to manufacturer’s protocols described in the SI.
Cell Adhesion and Proliferation Studies
Particles (10 mg) were weighed into well-plates and hydrated in DPBS for 12 h. W-20-17 or C2C12 cells were added at either 5000, 15,000, and 50,000 cells/mg particles concentrations. For initial cell adhesion studies particles were weighed into a 24-well plate and W-20-17 cells were stained using CellTracker™ Deep Red dye (ThermoFisher) according to the manufacturer’s protocol and cell adhesion was monitored after 30 min, 2 h, and 3.5 h using fluorescent microscopy at an excitation-emission wavelength of 630–660 nm. Particles were transferred to a round bottom 96-well plate and incubated at 37 °C. For the remaining cell adhesion and proliferation studies, particles were weighed directly into a round bottom 96-well plate. AlamarBlue® (Serotec) assays were performed to monitor cell metabolic activity and a Picogreen assay was performed for cell quantification according to manufacturer’s protocols described in the SI. Samples were imaged, stained, and fixed according to the methods described in the SI. A complete protocol for each experiment is described in the SI.
BMP-2 Loading and Release Studies
Lyophilized BMP-2 (Peprotech, E.coli-derived) was reconstituted according to manufacturer’s instructions and stored at − 80 °C until use. 50 µL of BMP-2 was added at either 3.14 µg/mL or 0.314 µg/mL in WFI to particles (10 mg) in a non-treated round bottom 96-well plate. An aliquot of starting BMP-2 solution was frozen for downstream quantification. Each concentration was tested in triplicate and one sample without BMP-2 was used as a control. The well plate was placed into the incubator at 37 °C for 12 h then BMP-2 solution removed, particles washed three times with 100 µL DPBS to remove free BMP-2, 200 µL complete DMEM added, and the well plate replaced in the incubator. At pre-determined timepoints, 150 µL of release media was removed from each sample and replaced with fresh complete DMEM, removed media was stored at − 80 °C. ALP activity was quantified by ELISA (Peprotech) according to manufacturer’s protocols described in the SI.
Cell Differentiation using C2C12 Cells
Polymer particles were weighed (10 mg) into a non-treated round bottom 96-well plate and 50 µL of BMP-2 solution added at 3.14 µg/mL in WFI. Each polymer was tested in triplicate both with and without BMP-2. The well plate was placed into the incubator at 37 °C for 12 h. The solution was removed, particles washed to remove free BMP-2. C2C12 cells were cultured with particles at a concentration 5000 cells/mg particles in a final volume of 100 µL for 24 h with gentle agitation every hour for the first 4 h. After 24 h, the media was removed, and particles were washed two times with fresh complete DMEM (100 µL) to remove unadhered cells. Fresh DMEM was added, and culture continued for 7 days. Cell metabolic activity was quantified by AlamarBlue® assay, which was performed after 3 and 7 days of incubation. After 7 days of incubation, particles were washed 3 times with 50 µL DPBS, then frozen in 80 µL WFI at − 80 °C. Plates and the aliquot of the initial cell concentration underwent two thaw-freeze cycles, then brought to room temperature before analysis. Recovered DNA was quantified using a Picogreen assay. ALP activity was quantified using a fluorometric ALP activity assay (Biovision) according to manufacturer’s protocols described in the SI.
Cell Staining and Imaging
Cells were fixed by incubating samples in 500 µL fixating solution: 4% w/v glutaraldehyde, 8.6% w/v sucrose, 50% v/v cacodylate buffer (0.2 M). Samples sat at room temperature for 2 h with gentle agitation every 30 min. Media was removed, 500 µL fresh fixing solution was added, and samples placed at 4 °C overnight. Mouse monoclonal antibody specific to SC35 (Abcam, ab11826), goat anti-mouse IgG1 conjugated to Alexa Fluor 488 (Life Technologies), Alexa Fluor 488 phalloidin (Life Technologies), and Hoechst (Sigma) were used for cell staining experiments. Samples were stained for fibronectin or for actin and nuclei as described in the SI. After staining, media was removed, and samples embedded in optimal cutting temperature (O.C.T., Sakura Finetek). Particles were cross-sectioned to 5 µm thickness using a Cryostat Leica 1850 (Leica, Buffalo Grove, IL) and sectioned samples mounted on charged microscope slides (VWR, 1358A). Samples were imaged using a Zeiss Fluorescent Microscope.
Statistical Analysis
One-way analysis of variance (ANOVA) and a Tukey multiple comparison post-test were used with significance set at p < 0.05. Data are expressed as mean ± standard deviation (SD). All statistical analyses were carried out using Origin 18.0 software.
Results and Discussion
Polymer Selection and Microparticle Preparation
Four tyrosol-derived poly(ester-arylate)s and PLGA were selected to study cell and protein interactions due to their differences in physical and thermal properties. Polymer abbreviations, physical (molecular weight number (Mn), air-water contact angle (AWCA), polymer–water miscibility (χpolymer–water), logP, crystallinity), and thermal properties [Tg, melting temperature (Tm), and crystallization temperature (Tc)] are shown in Table 1. Polymer chemical structures are shown in Fig. 1a. The tyrosol base polymer consists of a tyrosol-derived monomer and a diacid, where X is the number of carbons in the tyrosol monomer (one or two), and Y is the number of carbons in the diacid monomer (3–10). When X is one carbon, the monomer is denoted as 4-hydroxyphenethyl 2-(4-hydroxyphenyl)acetate (HTy), and 4-hydroxyphenethyl 3-(4-hydroxyphenyl)propanoate (DTy) denotes the monomer containing two carbons. The measured pKa of both the HTy and DTy monomers at saturation are > 7. The published pKa of glutaric acid (3 carbons) is 3.76 and dodecanedioic acid (10 carbons) is 4.65.10,11 In comparison, PLGA degrades into lactic and glycolic acid which have a pKa of 3.86 and 3.83, respectively.22,23 At their solubility limit, the monomers comprising the tyrosol-derived polymers will produce a less acidic local environment compared to those of PLGA, reducing the potential for acid-induced inflammation as a side effect.38 Microparticles were prepared out of all five polymers using the o/w continuous flow method shown in Fig. 1a. The small size of these microparticles (40-200 µm, Fig. S1) allows them to be used as an injectable drug delivery system or implanted during an operation.28 The polymers were all of high Mn, ranging from 77 to 101 kDa. Degradation will be slow for all polymers at these high molecular weights, with PLGA expected to degrade to ~ 50% of its starting Mn in ~ 25 days, whereas the tyrosol polymers are expected to degrade to ~50% Mn retention in 80, 35, 170, 150 days for pHTy3, pDTy3, pHTy10, and pDTy10, respectively.7,17 While particle preparation did not change the Mn of the particles, sterilization in IPA reduced the particles’ Mn by ~ 20 to 40% (Table S1). However, it has previously been shown that at higher polymer molecular weights such as those used in this study, differences in drug release are minimal, and therefore it is expected that BMP-2 loading and release will not be impacted by the differences in the starting Mn of the polymer particles.36
Table 1.
Polymer abbreviations, physical, and thermal properties of poly(ester-arylate)s and PLGA control.
| Polymer | Abbreviation | Mn (kDa)a | AWCA (deg.)b | χsubstrate-waterc | logPd | Crystallinity (%)e | Crystallite size (Å)e | Tg (°C)f | Tm (°C)f | Tc (°C)f |
|---|---|---|---|---|---|---|---|---|---|---|
| Poly(HTy glutarate) ester | pHTy3 | 98 | 73.5 (0.8) | 4.22 | 3.63 | 37 | 93 | 31 | 137 (118) | 86 |
| Poly(DTy glutarate) ester | pDTy3 | 101 | 71.0 (0.8) | 4.37 | 4.08 | 39 | 96 | 29 | 157 (134) | 118 |
| Poly(HTy dodecanedioate) ester | pHTy10 | 92 | 76.2 (0.5) | 5.02 | 6.75 | 43 | 117 | 6 | 88 (99) | 66 |
| Poly(DTy dodecanedioate) ester | pDTy10 | 77 | 77.2 (0.8) | 5.10 | 7.19 | 40 | 103 | 6 | 153 | 131 |
| Poly(lactic-co-glycolic acid) | PLGA | 91 | 65.5 (0.8) | 3.71 | 0.80 | 0 | – | 48 | – | – |
aValues were measured on CHCl3 GPC relative to polystyrene standards
bAWCA values were measured in triplicate on polymer spin coated coverslips, standard deviation (SD) is shown in parenthesis
cHoftyzer-van-Krevelen (HVK) group contribution theory was used to calculate polymer-water miscibility
dMarvinView software was used to calculate polymer logP values where only a single monomer repeat unit was considered
eXRD was used to determine polymer crystalline properties on prepared microparticles. PLGA was completely amorphous and therefore does not have a crystallite size
fThermal properties were measured using DSC. Tg and Tm values were from the second heat and Tm values in parenthesis represent a second, minor melting temperature of the polymer. PLGA was completely amorphous and therefore does not have a Tm or Tc
Increasing the diacid chain length of the tyrosol polymers from 3 to 10 increased polymer hydrophobicity, and the HTy polymers had a lower calculated χpolymer–water, and logP than their corresponding DTy polymers. Of the polymers, PLGA is the only amorphous polymer exhibiting only a Tg and no Tm. The tyrosol polymers are all semi-crystalline and all had Tm’s above 37 °C, which ensures that the polymers will not melt during incubation at 37 °C. The Tg and Tm of the dry particles and particles post-sterilization remained unchanged. At 37 °C, the tyrosol-derived polymeric particles, which have a Tg below 37 °C, have more chain mobility compared to PLGA, which has a Tg of 48 °C. Polymer crystallinity and crystallite size was evaluated using microparticles incubated in DI water for 24 h at 37 °C (Table 1). These conditioned particles mimic the surface state of the particle during cell culture. XRD results show that the pHTy3 and pDTy3 polymers had a main polymer peak with a shoulder whereas the pHTy10 and pDTy10 polymers were composed of two and three main peaks, respectively (Fig. 2a). The crystallinity of the tyrosol polymers was between 37 and 43%, whereas PLGA was amorphous. In the tyrosol-derived polymers, large crystallites were observed, ranging from 93 to 117 Å. The details of these calculations are described in the SI. We have previously shown that for this tyrosol-derived polymer library, increasing the crystallinity and crystallite size increases the rate of drug release.18 Therefore, faster protein release is expected for the tyrosol-10 polymers (pHTy10 and pDTy10) than for pHTy3. The surface morphology of the particles was examined using SEM (Figs. 2c–2g). The particles varied in surface porosity and smoothness with pHTy3 showing the most surface porosity, especially when compared to PLGA which appeared completely smooth with limited porosity.
Figure 2.
(a) Polymer XRD spectra of conditioned microparticles. (b) Cytocompatibility study of microparticles where cell viability of microparticles was evaluated with HDF cells using a Picogreen assay. Samples were run in triplicate and normalized to the control (incubated cell media without the presence of particles). Error bars represent the SD between the three samples. Representative SEM images of (c) pHTy3; (d) pDTy3; (e) pHTy10; (f) pDTy10; (g) PLGA particles at × 2000 magnification.
Cytocompatibility of Polymeric Microparticles
The percent viability of HDF cells after exposure to the polymeric microspheres was analyzed using a Picogreen assay. The results shown in Fig. 2b indicate that cell viability is not affected by the addition of microparticles into the media. All particles showed a HDF cell viability of 100% ± 7 compared to the control after 24 h of incubation. According to ISO 10993-5, 2009 standards, the prepared microparticles were nontoxic because cell viability remained > 80%.14
Optimization of Cell Attachment and Proliferation on Microparticles
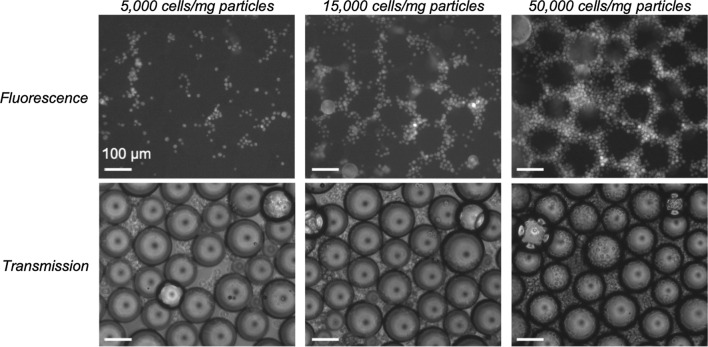
PLGA particles were used to optimize the cell density required to study both cell adhesion and proliferation. It has been shown that the W-20-17 mouse bone marrow stromal cells and C2C12 mouse myoblast cells line responds to BMP-2 induction in a dose-dependent manner through increased ALP activity.6 The BMP-2 detection range for these cells is 10–100 ng/mL and 200–2000 ng/mL, respectively.12 As the seeding density to reach confluence is similar for both cell lines, W-20-17 cells were used for initial optimization studies when no BMP-2 was used; however, C2C12 cells were later selected to ensure that we can test the upper end of BMP-2 release while ensuring a dose response curve. First, cell attachment was visualized by staining the W-20-17 cells using a fluorescent dye to monitor cell localization in the microsphere environment. Cells were added to PLGA particles at three different cell densities (5000, 15,000, and 50,000 cells/mg particles) and cell attachment was tracked after 30 min, 2 h, and 3.5 h of incubation. After 30 min, cells were clearly attaching to the particles at all cell densities, with a higher cell attachment observed at 50,000 cells/mg particles (Fig. 3). Images of the highest cell density after 2 and 3.5 h of incubation, showed that the cells continued to adhere to the particles with time (Fig. S2).
Figure 3.
CellTracker studies to optimize the cell loading density for cell adhesion to PLGA particles. Three cell densities (5000, 15,000, and 50,000 cells/mg particles) were imaged after 30 min of incubation with the particles. Images were taken at × 10 magnification.
After 4 h of incubation, the particles were transferred from the 24-well flat bottom plate to a round bottom 96-well plate and metabolic activity monitored after 1, 4, and 8 days of incubation. At the lowest cell density, cells proliferated 300% after 4 days and 1200% after 8 days of incubation (Fig. 4). At the highest cell density, there was no increase in cell metabolic activity after one day of incubation, indicating that the cells had reached a high enough density such that their metabolic activity remained constant. At 15,000 cells/mg particles some growth was still observed, but it was significantly slower than for the lower cell density, suggesting that the available surface area had been fully saturated. Therefore, for studying the effect of polymer properties on cell growth, the lower cell seeding density will be used. However, when testing cell adhesion, 15,000 cells/mg particles will be used to ensure enough cells are introduced to the particles to see a difference in initial cell adhesion.
Figure 4.

AlamarBlue® assay analysis of cell proliferation on PLGA particles at three different W-20-17 cell densities (5000, 15,000, and 50,000 cells/mg particles). Results were normalized to the 1-day time point. Samples were run in triplicate and error bars represent the SD between the three samples removed at every time point.
To confirm that a cell matrix is forming around the particles, after 8 days of incubation, cells were fixed, permeabilized, and stained for fibronectin and actin, then embedded and sectioned. Fluorescence microscopy images show that there are cell matrix proteins spanning the gap between the particles for the lower 5000 and 15,000 cells/mg particles densities (Fig. 5), and this expression is less pronounced in particles seeded with the higher cell seeding density. This is consistent with the AlamarBlue® results, where the lower cell densities had available particle surface area for the cells to grow, thereby creating a cell matrix around the particles during proliferation. The highest cell density did not have a lot of cell matrix present, indicating that the cells either did not grow during the 8 days of incubation, or the high initial cell density promoted the formation of cell-cell attachment over cell-material attachment, and these cell clusters were washed away during sample processing. The particles cultured with 15,000 cells/mg particles appeared the most clumped before embedding, suggesting that this cell density is the optimal loading to achieve a high initial cell adhesion while still providing surface area for expansion.
Figure 5.
PLGA particles exposed to different W-20-17 cell densities for 8 days were stained for fibronectin and actin to visualize the cell matrix and cell wall, respectively. Images were taken at × 10 magnification.
Growing cells in a round bottom 96-well plate proved successful in keeping the particles close together, thereby maximizing the contact between the cells and the particles while reducing cell contact with the well plate. Two different C2C12 cell densities (5000 and 15,000 cells/mg particles) were tested using PLGA particles in round bottom 96-well plates. After 3, 6, and 10 days of incubation, cell metabolic activity was used to monitor cell growth. While the higher seeding density resulted in a greater absolute measurement of metabolic activity, at the 3-day timepoint, the relative change in metabolic activity over the 10-day incubation period was insignificant, suggesting little opportunity for cell growth under these conditions. Initial cell metabolic activity was reduced under the lower seeding density condition, but the cells proliferated quickly after six and ten days of incubation. (Fig. 6a). This was confirmed from the Picogreen assay that was run on the starting cell concentration and of the particles after 10 days of incubation as shown in Fig. 6b.
Figure 6.
C2C12 cell proliferation on PLGA particles tested at 5000 and 15,000 cells/mg particles densities. (a) AlamarBlue® assay monitoring cell viability after 3, 6, and 10 days where results are either normalized to the 3-day time point (orange) or plotted as the RFU (blue). (b) Picogreen analysis of starting cells and cell content after 10 days of incubation. Samples were run in triplicate and error bars represent the SD between the three samples removed at every time point.
Cell Adhesion and Proliferation on Microparticles
To determine how polymer properties affect both short- and long-term exposure to cells, a 24 h cell adhesion study was performed using all five polymer particle formulations (Fig. 7a). Cell attachment was less than 100% for all particle formulations, indicating that the differences in cell adhesion are due to differences in the polymer properties and not surface area. The shorter diacid chain tyrosol polymer particles (pHTy3 and pDTy3) had the highest cell attachment compared to the longer diacid chain polymers (pHTy10 and pDTy10) and PLGA. For a constant diacid chain length, the HTy polymers resulted in greater cell attachment than their DTy counterparts. In the absence of any other characteristic differences, the differences in cell adhesion between the polymers can be attributed to the surface morphology and crystallite size of the polymers where the tyrosol-3 polymers (pHTy3 and pDTy3) have a smaller crystallite size (~ 95 Å) compared to the tyrosol-10 polymers (~ 110 Å).
Figure 7.
(a) Picogreen analysis of cell adhesion was tested using 15,000 cells/mg particles seeding density with a 24 h incubation period. (b) AlamarBlue® assay of cell proliferation tested using 5,000 cells/mg particles seeding density analyzing the samples after 3, 7, 10, and 14 days of incubation and normalized to the 3-day time point. (c) AlamarBlue® assay of cell proliferation tested using 5000 cells/mg particles seeding density after 3 days of incubation and (d) after 14 days of incubation. Samples were run in triplicate and error bars represent the SD between the three samples removed at every time point. Statistical analysis of (c) is in respect to the pHTy3 particles.
It has previously been reported that the crystallite size impacts the macroscale roughness of the polymer surface.2 The larger the crystallite size, the larger the macroscale pits on the surface.33 Surface topography is known to influence cell response, adhesion, proliferation, and differentiation.30,31 Comparison of the particles surface morphology using SEM images (Figs. 2c–2g) showed that visually, pHTy3 has the most surface porosity. This polymer also promoted the most cell adhesion after 24 h which is consistent with previously reported results that show that microscale surface roughness promotes cell adhesion.3 At the nanometer length scale, a smaller crystallite size resulted in more cell adhesion due to less water absorbed in the pits on the surface of the particles. A correlation between the measured polymer hydrophilicity and polymer hydration, which is correlated to the crystallite size, was not observed. This suggests that different methods for determining polymer hydrophilicity other than contact angle measurements are needed. Contact angle measurements are often performed on spin coated films which changes the crystalline nature of the polymer; however, often the fabricated device is a 3D scaffold which has a different hydrophilicity than the spin coated films. Therefore, by using surface area analysis to measure crystallite size, a polymers hydration potential can be determined. Interestingly, the amorphous nature of PLGA, resulted in similar cell adhesion as the tyrosol-10 polymers. This can be attributed to a more amorphous polymer being more prone to water hydration, again creating a competitive environment for cell adhesion.
When particles were exposed to a lower cell concentration (5000 cells/mg particles) for 3 days, only pHTy3 showed a significantly higher metabolic activity than the other polymers (Fig. 7c). PLGA particles resulted in the fastest initial increase in metabolic activity; however, the growth rate at later timepoints was reduced as the surface area became saturated with cells. On the contrary, cells cultured on pHTy10 particles showed a delayed proliferation, with the growth rate increasing at later timepoints (Fig. 7b). The delayed cell growth on pHTy10 particles is hypothesized to be a result of the lower initial cell metabolic activity observed after 3 days of incubation. Interestingly, after 14 days, the particles with the largest crystallite size resulted in the most total cell growth, consistent with the higher metabolic activity observed at the end of the study (Fig. 7d). This suggests that the larger pits create a competitive environment for cells to initially adhere to, but after adhesion, the cells split and grow on all surfaces.
Cell adhesion and proliferation experiments were performed on the series of five polymer particles using C2C12 cells at 15,000 cells/mg particles and an incubation time of 8 days. Cells were stained for fibronectin, actin, and nuclei. Samples showed good cell attachment and growth for all polymers and a cell matrix formed around the particles, creating particle-cell clumps (Fig. S3). pDTy10 particles images showed minimal actin staining; however, the fibronectin and Hoechst staining suggests that the cells were disturbed and washed off during the fibronectin staining procedure resulting in minimal fluorescence. No difference between the amount of cell matrix, actin, and nuclei present on the particles was observed.
BMP-2 Loading and Release from Polymeric Microparticles
A BMP-2 concentration of 3.14 µg/mL was used to load all five polymer particles. BMP-2 loading and release from the particles was quantified by ELISA. The resulting loading efficiency of all formulations was high, ~ 14.4 ng BMP-2/mg particles (92% encapsulation efficiency), and there were no significant differences observed between the different polymers indicating that the polymer properties did not impact protein adsorption (Table S2). BMP-2 release was monitored out to 14 days. All formulations underwent a high burst release followed by a slow sustained release out to 14 days (Fig. 8). Incubation of particles in complete DMEM without the presence of BMP-2 was used as a control. Results showed a negligible amount of BMP-2 present after 14 days of incubation (Fig. S4). For sustained release applications, the protein would need to be loaded during particle preparation so that that diffusion controls release, not the rate of protein desorption.
Figure 8.
BMP-2 release profile from all polymer particle formulations. The insert shows BMP-2 release at early time points. Samples were run in triplicate and error bars represent the SD between the three samples.
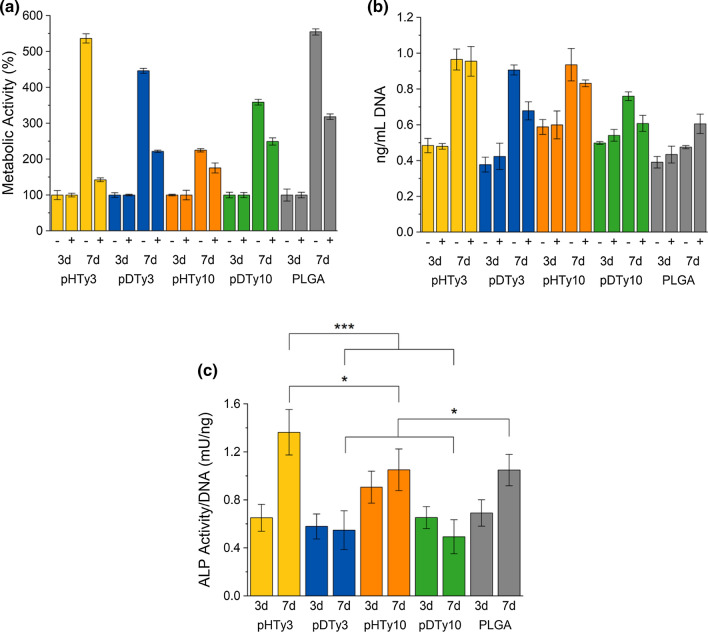
Cell Differentiation Towards Osteoblasts
Particles were evaluated for their effectiveness in bone regeneration by comparing BMP-2 loaded particles (3.14 µg/mL) to those without BMP-2. Both sets of particles were incubated with C2C12 cells (5000 cells/mg particles) and ALP activity monitored after 3 and 7 days of culture. Incubation was stopped at 7 days because BMP-2 release studies showed minimal release after this time. Cell metabolic activity increased from 3 to 7 days, and in the presence of BMP-2, cells proliferated less, which is typical for differentiating cells (Fig. 9a).13 Trends were consistent with initial proliferation studies where the smaller crystallite size resulted in the most cell proliferation, Fig. 9b. Finally, ALP activity was quantified and the results normalized to cell DNA content (Fig. 9c). Particles that were incubated without BMP-2 induced no cellular ALP activity above the background noise. After 3 days, ALP activity was similar in cells cultured on all formulations; however, after 7 days, pHTy3 particles loaded with BMP-2 induced the highest ALP activity. The differences in ALP activity, a marker of osteogenesis, can be attributed to the higher initial cell adhesion for pHTy3 particles. A polymer that successfully supports osteogenic differentiation will both promote cell attachment, proliferation, and differentiation to the highest degree, and of the polymers tested, pHTy3 is superior to its other tyrosol-based analogues.
Figure 9.
Cell differentiation studies testing all polymeric particles for osteoblast differentiation. (a) AlamarBlue® assay comparing particles with (+) and without (−) BMP-2 exposure after 3 and 7 days of incubation at 37 °C. Results are normalized to the 3-day time points. (b) Picogreen analysis quantifying DNA content with (+) and without (−) BMP-2 exposure after 3 and 7 days of incubation at 37 °C. (c) ALP activity normalized to the Picogreen results for particles exposed to BMP-2 solution and incubated for 3 and 7 days at 37 °C. Samples were run in triplicate and error bars represent the SD between the three samples removed at every time point.
Conclusions
A series of tyrosol-derived polymers and commercially available PLGA microparticles were evaluated for their cellular interactions, adhesion, proliferation, and ability to differentiate a myoblast cell line towards an osteoblast lineage through BMP-2 release. Particles with smaller crystallite size (pHTy3) promoted cell adhesion and proliferation; this is due to smaller macroscale pits. However, eventually regardless of the crystallite size, cell proliferation occurred on all polymers until all available surface area was saturated. BMP-2 was successfully adsorbed onto all particle formations. The burst release observed indicates that for sustained release, the BMP-2 should be encapsulated during particle preparation as opposed to after fabrication. Even with the burst release, all polymers induced cellular ALP activity after 3 and 7 days of incubation indicating that the C2C12 myoblasts had begun to differentiate towards osteoblasts and that the amount of BMP-2 release was above the minimal required to induce ALP activity. As with cell adhesion and proliferation, pHTy3 particles had the most ALP activity when normalized to the concentration of cells present. This work demonstrates the importance of considering polymer properties when designing new polymers for tissue regeneration. Using a subset of a larger polymer library we have demonstrated that crystallinity plays a crucial role in determining the ability of a polymer to support cell attachment, proliferation, and differentiation. Furthermore, we have drawn a correlation between polymer crystallite size with the hydration potential, which offers a better predictor for cellular interactions. Using traditional contact angle measurements, a complete understanding of a polymers ability to interact with cells is not well understood; however, by using in depth surface analysis such as XRD, the crystallite texture of the devices surface can be well understood, giving insight into how these polymers will behave in vivo.
Associated Content
Supporting Information
The supporting information is available free of charge and includes bioactivity assay protocols, cell staining and imaging protocols, and cell adhesion and proliferation protocols, comparison of Mn, Tg, and Tm after particle sterilization, particle size analysis, fluorescent images of particles stained for fibronectin, actin, and nuclei, BMP-2 loading encapsulation efficiency, BMP-2 release from particles incubated without the presence of BMP-2, and ALP activity for particles incubated without the presence of BMP-2.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank Jarrod Cohen for providing the polymers to complete these studies.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Funding
The authors acknowledge NIH NIGMS R35GM138296, the Rutgers University’s TechAdvance program, and Lubrizol Life Science for their help in funding this project.
Conflict of interest
Catherine E. Miles, Stephanie L. Fung, and N. Sanjeeva, Murthy have received research funding from Rutgers University’s TechAdvance program and Lubrizol Life Science, but do not have a conflict of interest with these organizations. Adam J. Gormley has received research grant number R35GM138296 from NIH but does not have a conflict of interest with this organization.
Ethical Approval
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Abbreviations
- BMP-2
Bone morphogenetic protein-2
- ALP
Alkaline phosphatase
- PLGA
Poly(lactide-co-glycolide)
- HTy
4-Hydroxyphenethyl 2-(4-hydroxyphenyl)acetate
- DTy
4-Hydroxyphenethyl 3-(4-hydroxyphenyl)propanoate
- GPC
Gel permeation chromatography
- Mn
Molecular weight number
- DSC
Differential scanning calorimetry
- Tg
Glass transition temperature
- Tm
Melting temperature
- AWCA
Air–water contact angle
- XRD
X-ray diffraction
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amani H, Arzaghi H, Bayandori M, Dezfuli AS, Pazoki-Toroudi H, Shafiee A, Moradi L. Controlling cell behavior through the design of biomaterial surfaces: a focus on surface modification techniques. Adv. Mater. Interfaces. 2019;6:1900572. doi: 10.1002/admi.201900572. [DOI] [Google Scholar]
- 2.Amorin LHC, da Silva Martins L, Urbano A. Commitment between roughness and crystallite size in the vanadium oxide thin film opto-electrochemical properties. Mat. Res. 2018;22:e20180245. doi: 10.1590/1980-5373-mr-2018-0245. [DOI] [Google Scholar]
- 3.Andrukhov O, Huber R, Shi B, Berner S, Rausch-Fan X, Moritz A, Spencer ND, Schedle A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016;32:1374–1384. doi: 10.1016/j.dental.2016.08.217. [DOI] [PubMed] [Google Scholar]
- 4.Cai S, Wu C, Yang W, Liang W, Yu H, Liu L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020;9:971–989. doi: 10.1515/ntrev-2020-0076. [DOI] [Google Scholar]
- 5.Chen L, Yan C, Zheng Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today. 2018;21:38–59. doi: 10.1016/j.mattod.2017.07.002. [DOI] [Google Scholar]
- 6.Chiu Y-C, Fong EL, Grindel BJ, Kasper FK, Harrington DA, Farach-Carson MC. Sustained delivery of recombinant human bone morphogenetic protein-2 from perlecan domain I - functionalized electrospun poly(ε-caprolactone) scaffolds for bone regeneration. J. Exp. Orthop. 2016;3:25–25. doi: 10.1186/s40634-016-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J, Shultz RB, Mullaghy A, Gwin C, Kohn J. Bioresorbable tyrosol-derived poly(ester-arylate)s with tunable properties. J. Polym. Sci. 2021;59:860–869. doi: 10.1002/pol.20210047. [DOI] [Google Scholar]
- 8.Cordeiro AL, Rückel M, Bartels F, Maitz MF, Renner LD, Werner C. Protein adsorption dynamics to polymer surfaces revisited—a multisystems approach. Biointerphases. 2019;14:051005. doi: 10.1116/1.5121249. [DOI] [PubMed] [Google Scholar]
- 9.Dashtimoghadam E, Fahimipour F, Tongas N, Tayebi L. Microfluidic fabrication of microcarriers with sequential delivery of VEGF and BMP-2 for bone regeneration. Sci. Rep. 2020;10:11764. doi: 10.1038/s41598-020-68221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Database, H. M. “HMBD metabocard for HMDB0000661, glutaric acid.” Human Metabolome Database 2021, hmdb.ca/metabolites/HMDB0000661.
- 11.Database, H. M. “HMBD metabocard for HMDB0000623, dodecanedioic acid.” Human Metabolome Database 2021, hmdb.ca/metabolites/HMDB0000623.
- 12.Fung SL, Wu X, Maceren JP, Mao Y, Kohn J. In vitro evaluation of recombinant bone morphogenetic protein-2 bioactivity for regenerative medicine. Tissue Eng. Part C. 2019;25:553–559. doi: 10.1089/ten.tec.2019.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Infante A, Rodríguez CI. Osteogenesis and aging: lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018;9:244. doi: 10.1186/s13287-018-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ISO/EN 10993-5. Biological evaluation of medical devices—Part 5. Tests for cytotoxicity. In vitro methods: 8.2 tests on extract. 2009, nhiso.com/wp-content/uploads/2018/05/ISO-10993-5-2009.
- 15.Jubeli E, Khzam A, Yagoubi N. Cells integration onto scaffolds prepared from polyester based polymers—importance of polymer thermal properties in addition to hydrophilicity. Int. J. Polym. Mater. Polym. Biomater. 2019;68:1068–1077. doi: 10.1080/00914037.2018.1525549. [DOI] [Google Scholar]
- 16.Kikuchi A, Okano T. Nanostructured designs of biomedical materials: applications of cell sheet engineering to functional regenerative tissues and organs. J. Controlled Release. 2005;101:69–84. doi: 10.1016/j.jconrel.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miles CE, Bernstein AD, OsbornPopp TM, Murthy SN, Nieuwkoop AJ, Gormley AJ. Control of drug release from microparticles by tuning their crystalline textures: a structure–activity study. ACS Appl. Polym. Mater. 2021;3(12):6548–6561. doi: 10.1021/acsapm.1c01254. [DOI] [Google Scholar]
- 19.Miles CE, Gwin C, Zubris KAV, Gormley AJ, Kohn J. Tyrosol derived poly(ester-arylate)s for sustained drug delivery from microparticles. ACS Biomater. Sci. Eng. 2021;7:2580–2591. doi: 10.1021/acsbiomaterials.1c00448. [DOI] [PubMed] [Google Scholar]
- 20.Miles CE, Lima MRN, Buevich F, Gwin C, Murthy SN, Kohn J. Comprehensive hydrolytic degradation study of a new poly(ester-amide) used for total meniscus replacement. Polym. Degrad. Stab. 2021;190:109617. doi: 10.1016/j.polymdegradstab.2021.109617. [DOI] [Google Scholar]
- 21.Motamedian SR, Hosseinpour S, Ahsaie MG, Khojasteh A. Smart scaffolds in bone tissue engineering: a systematic review of literature. World J. Stem Cells. 2015;7:657–668. doi: 10.4252/wjsc.v7.i3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCBI “PubChem compound summary for CID 757, glycolic acid.” National Center for Biotechnology Information, 2021, pubchem.ncbi.nlm.nih.gov/compound/Glycolic-acid.
- 23.NCBI “PubChem compound summary for CID 612, lactic acid.” National Center for Biotechnology Information, 2021, pubchem.ncbi.nlm.nih.gov/compound/Lactic-acid.
- 24.Neděla O, Slepička P, Švorčík V. Surface modification of polymer substrates for biomedical applications. Materials. 2017;10:1115. doi: 10.3390/ma10101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortega-Oller I, Padial-Molina M, Galindo-Moreno P, O'Valle F, Jódar-Reyes AB, Peula-García JM. Bone regeneration from PLGA micro-nanoparticles. BioMed Res. Int. 2015;2015:415289. doi: 10.1155/2015/415289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasadh S, Wong RCW. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018;15:48–55. doi: 10.1016/S1348-8643(18)30005-3. [DOI] [Google Scholar]
- 27.Romanazzo S, Forte G, Ebara M, Uto K, Pagliari S, Aoyagi T, Traversa E, Taniguchi A. Substrate stiffness affects skeletal myoblast differentiation in vitro. Sci. Technol. Adv. Mater. 2012;13:064211–064211. doi: 10.1088/1468-6996/13/6/064211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarmadi M, Behrens AM, McHugh KJ, Contreras HTM, Tochka ZL, Lu X, Langer R, Jaklenec A. Modeling, design, and machine learning-based framework for optimal injectability of microparticle-based drug formulations. Sci. Adv. 2020;6:eabb6594. doi: 10.1126/sciadv.abb6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stępień K, Miles C, McClain A, Wiśniewska E, Sobolewski P, Kohn J, Puskas J, Wagner HD, El Fray M. Biocopolyesters of poly(butylene succinate) containing long-chain biobased glycol synthesized with heterogeneous titanium dioxide catalyst. ACS Sustain. Chem. Eng. 2019;7:10623–10632. doi: 10.1021/acssuschemeng.9b01191. [DOI] [Google Scholar]
- 30.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 31.Vega SL, Arvind V, Mishra P, Kohn J, Sanjeeva Murthy N, Moghe PV. Substrate micropatterns produced by polymer demixing regulate focal adhesions, actin anisotropy, and lineage differentiation of stem cells. Acta Biomater. 2018;76:21–28. doi: 10.1016/j.actbio.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Víšová I, Smolková B, Uzhytchak M, Vrabcová M, Chafai DE, Houska M, Pastucha M, Skládal P, Farka Z, Dejneka A, Vaisocherová-Lísalová H. Functionalizable antifouling coatings as tunable platforms for the stress-driven manipulation of living cell machinery. Biomolecules. 2020;10:1146. doi: 10.3390/biom10081146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vyner MC, Amsden BG. Polymer chain flexibility-induced differences in fetuin a adsorption and its implications on cell attachment and proliferation. Acta Biomater. 2016;31:89–98. doi: 10.1016/j.actbio.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Kempen DHR, Yaszemski MJ, Lu L. The roles of matrix polymer crystallinity and hydroxyapatite nanoparticles in modulating material properties of photo-crosslinked composites and bone marrow stromal cell responses. Biomaterials. 2009;30:3359–3370. doi: 10.1016/j.biomaterials.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winblade ND, Nikolic ID, Hoffman AS, Hubbell JA. Blocking adhesion to cell and tissue surfaces by the chemisorption of a poly-l-lysine-graft-(poly(ethylene glycol); phenylboronic acid) copolymer. Biomacromolecules. 2000;1:523–533. doi: 10.1021/bm000040v. [DOI] [PubMed] [Google Scholar]
- 36.Wu XS. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers: part III. Drug delivery application. Artif. Cells. 2004;32:575–591. doi: 10.1081/BIO-200039635. [DOI] [PubMed] [Google Scholar]
- 37.Zhang N, Kohn DH. Using polymeric materials to control stem cell behavior for tissue regeneration. Birth Defects Res. Part C. 2012;96:63–81. doi: 10.1002/bdrc.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. J. Controlled Release. 2007;122:338–344. doi: 10.1016/j.jconrel.2007.05.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.