Abstract
Background
Older people with dementia (PWD) in nursing homes (NHs) tend to have decreased cognitive function, which may cause behavioral and psychological symptoms of dementia (BPSDs) and hinder activities of daily living (ADLs). Therefore, taking measures against the cognitive decline of PWD in NH and, in turn, the decline of BPSDs and ADLs is crucial. The purpose of this study was to test whether a multimodal non-pharmacological intervention (MNPI) is effective in maintaining and improving global cognitive function, BPSDs, and ADLs in PWD in NHs.
Methods
An intervention study using a single-case AB design was conducted in three subjects in NHs. During the non-intervention phase, participants underwent follow-up assessments, and during the intervention phase, they participated in an MNPI. The ABC Dementia Scale (which concurrently assesses ADLs [“A”], BPSDs [“B”], and cognitive function [“C”]) was used for the assessment.
Results
One of the three patients showed improvement in dementia severity, global cognitive function, ADLs, and BPSDs. However, the other two participants showed no improvement following the MNPI, although the possibility of a maintenance effect remained.
Conclusion
Although there is room for improvement of the MNPI, it may be effective in maintaining and improving cognitive function, ADLs, and BPSD, in PWD in NHs.
Trial registration
The University Hospital Medical Information Network Clinical Trials Registry (http://www.umin.ac.jp/, No. UMIN000045858, registration date: November 1, 2021).
Keywords: Multimodal, Non-pharmacological interventions, Cognitive function, Nursing home, Dementia
Introduction
Cognitive function has been reported to be more prone to decline in older people with dementia (PWD) in nursing homes (NHs) than in older people living in the community [1–3]. Behavioral and psychological symptoms of dementia (BPSDs) have a variety of contributing factors (e.g., neurobiologically related disease factors, acute medical illness, unmet needs, pre-existing personality and psychiatric illness factors, caregiver factors, and environmental factors), and cognitive decline, a core symptom, has also been shown to be an important contributing factor [4–7]. The occurrence of BPSDs follows a vicious cycle in which cognitive decline accelerates, resulting in impaired activities of daily living (ADLs) and increased mortality [8–13]. In addition, even PWD who have the same cognitive dysfunctions may differ in the degree of specific cognitive dysfunctions, such as memory and orientation; accordingly, BPSDs and ADLs may also differ between individuals. Such BPSD and ADL disorders may lead to greater burdens on long-term care and medical and long-term care costs [14, 15]. Therefore, developing measures to maintain and improve the cognitive function of PWD in NHs is crucial.
Several interventions for cognitive decline and other dementia-related problems in PWD in NHs have been reported to be as effective as pharmacological interventions. However, pharmacological interventions do not provide adequate improvement of symptoms and are also associated with adverse effects, such as nausea and vomiting, diarrhea, weight loss, leg cramps, and increased mortality [16–20]. Therefore, non-pharmacological interventions (NPIs), which are considered as effective as pharmacological interventions, have become the first-line treatment option, although further development is required [2, 21].
In PWD, NPIs alone, such as reminiscence, music, and cognitive training, have been reported to improve cognitive function [22–24]. However, the effectiveness of a single NPI is limited; moreover, it is not effective in improving global cognitive function. Therefore, multimodal non-pharmacological interventions (MNPIs), which combine several NPIs to improve and maintain global cognitive function [25, 26], have recently received attention. A previous study reported that an MNPI that comprises exercise, cognitive training, and ADL training in PWD in NHs is effective in maintaining and improving cognitive functions, such as global cognitive function, executive function, attention, and memory [25]. However, few reports have focused on the effectiveness of MNPI in maintaining and improving the cognitive function of PWD in NHs, and the effectiveness of MNPI has not yet been fully verified. Therefore, we proposed a new MNPI based on previous research. Our newly designed MNPI consisted of a single 30-minute session that combined interventions related to exercise, cognitive function, and ADLs that had high clinical utility without the need for special equipment. Our aim was to determine the effectiveness of the proposed MNPI to enable its implementation as a valuable and clinically useful intervention that considers the burdens placed on PWD in NHs.
In this study, we aimed to test whether our MNPI, which we developed according to previous research, is effective in maintaining and improving global cognitive function in PWD in NHs, using a single-case experimental design (SCED). We also examined the effects of the MNPI on dementia severity, ADLs, and BPSDs.
Methods
This study was conducted in accordance with the Single-Case Reporting Guideline in Behavioral Interventions (SCRIBE) 2016 Checklist [27].
Design
Case series are suitable for assessing the feasibility of new interventions [28]. The MNPI in this study was a newly developed intervention based on previous research. Therefore, we decided to use a non-randomized single-case AB design, with phase A as the no-intervention phase and phase B as the intervention period, to evaluate the feasibility as well as the effect of the MNPI.
This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000045858; public title: A preliminary study of the effects of multimodal non-pharmacological interventions for PWD in NHs; registration date: November 1, 2021).
Setting and study population
The participants were three PWD from two cooperating NH facilities. Inclusion criteria were individuals who (1) were aged 65 years or older; (2) had been admitted for at least 3 months; (3) had a Mini Mental State Examination-Japanese (MMSE-J) [29, 30] score of 23 or less with mild to moderate dementia according to the ABC Dementia Scale (ABC-DS) (which concurrently assesses ADLs [“A”], BPSDs [“B”], and cognitive function [“C”]) [31–38]; (4) were able to communicate and perform the tests and tasks; (5) provided consent or their family (or guardian) provided consent; and (6) had a diagnosis of Alzheimer’s disease or were suspected by a physician as having Alzheimer’s disease if the diagnosis was dementia. Exclusion criteria were individuals (1) with severe behavioral disorders or medical requirements; (2) with severe visual or hearing impairments; (3) who expressed their refusal to participate in the research; and (4) with cerebrovascular dementia, frontotemporal dementia, dementia with Lewy bodies, or other secondary dementias without a diagnosis of Alzheimer’s disease.
Data collection
Basic characteristics of age, sex, marital status (married, widowed, divorced, or single), educational attainment (elementary school, junior high school, high school, or university), medication status, diagnosis of dementia, mode of transportation, and Clinical Dementia Rating (CDR) scores were collected for all participants from the medical records of the institutions. The Japanese version of the Neurobehavioral Cognitive Status Examination Five (COGNISTAT Five) was used to evaluate participants’ memory (short memory and recall), orientation, and construction ability. The COGNISTAT Five is a shortened version of the COGNISTAT [39, 40] that consists of four subsets: memory (word recall), score range 0–7; orientation, score range 0–12; construction, score range 0–6; and memory (delayed recall), score range 0–12 (higher scores indicate more severe impairment for word recall only). The validity and reliability of the Japanese version have been confirmed previously [41, 42].
Multimodal non-pharmacological intervention
Previous research has suggested that MNPIs that combine exercise, cognitive tasks, and ADL training three times per week for 30 min per session for at least 8 weeks are recommended for PWD in NHs [25]. In addition, exercise and cognitive training are easy to introduce to PWD, even those with severe cognitive dysfunction; thus, structuring MNPIs around exercise and cognitive training may be useful in clinical practice [43]. According to the results of these previous studies, we proposed a specific and realistic MNPI based on the experiences of five occupational therapists (OTs) from a cooperating NH facility. In many of the previous studies, the duration of interventions ranged from 45 to 120 min per session, and the frequency was four or more times per week, which often presented difficulties in terms of time and labor to incorporate MNPIs into usual care practices. Therefore, we developed a highly effective MNPI that is short, simple, requires no special qualifications, and has the minimum time, frequency, and duration recommended in a previous systematic review [25].
The MNPI consisted of light-impact exercises, such as gymnastics and stretching, cognitive tasks, such as calculations and puzzles that could be performed in a short time, and ADL training, which included eating with chopsticks and spoons and changing clothes. In addition, tasks related to orientation, such as identifying the date, time, and place, were incorporated into the beginning and end of the session. Each intervention within the MNPI took approximately 10 min to perform, and the entire session was designed to be completed in 30–40 min (Table 1). The MNPI was usually administered as an individual intervention between 5:00 PM and 6:00 PM, which avoided the usual care time, and facility users were relatively free (the MNPI was administered individually to minimize group situations to prevent COVID-19 infection). The MNPI was implemented by the staff (OTs, physical therapists, and speech therapists) at each NH. The intervention period, phase B, lasted 8 weeks, and a total of 24 MNPI sessions were administered to each participant. The details of the MNPI were shared with the representative OTs at each site prior to the start of the study via materials and online meetings to ensure uniformity.
Table 1.
Contents of the multimodal non-pharmacological intervention
| Time frame | Component | Examples of the content of the intervention |
|---|---|---|
| 10–15 min | Reality orientation and exercises |
Reality orientation: confirmation of the date, place, and weather Exercises: gymnastics, stretching, and strength training |
| 10 min | Cognitive activation | Calculations, card games, finger gymnastics, and puzzles |
| 10–15 min | ADL training and reality orientation |
Manipulation of jackets and trousers (simulated), use of chopsticks and spoons to transfer beans to a bowl, and brushing hair and teeth (simulated) Reality orientation: confirmation of the date, place, and weather, and reviewing the implemented program |
ADL, activities of daily living
Outcome measure
Assessments of dementia severity, global cognitive function, ADLs, and BPSDs
We used the ABC-DS to assess dementia severity. The ABC-DS is a dementia assessment scale that was developed in Japan that evaluates patients by asking their caregivers who have knowledge of the patient’s condition [31–38]. Questions included, “How well does the patient change their clothes?” and “How well can the patient remember the location of a familiar item?” Each of the 13 items is scored from 1 to 9 points. The maximum total score is 117 points and represents the severity of dementia. In addition, the maximum total score of the six items on ADLs is 54 points and represents the individual’s ability to carry out ADLs. The maximum total score for the three BPSD items is 27 points and represents the severity of BPSDs. The maximum total score for the four cognitive function questions is 36 points and represents global cognitive function. Higher total and individual item scores indicate milder dementia. The validity and reliability of the test have been confirmed previously. Furthermore, because the assessment is conducted by interviewing the caregiver, it places little burden on the patient. Another advantage of the ABC-DS is that the assessment takes only 10 min. Because the ABC-DS is not affected by learning effects, it was administered approximately once a week (18 times in total) from the time of participant recruitment to the end of the follow-up survey. The ABC-DS was administered by the staff (OTs, physical therapists, and speech therapists) at each NH.
Blinding
This study used an open-label, single-case design to prevent new coronavirus infections. Wherever possible, different people carried out the evaluation and interventions.
Statistical analysis
There were 18 observation points of the ABC-DS (total score and scores for each item) time-series data for each participant, and therefore we applied the Bayesian unknown change-point (BUCP) model [44, 45] to analyze the effect of the MNPI on each participant. This analysis is applicable if there are at least three observation points in each phase, and more stable estimates are possible if there are more than eight observations in each phase [44]. The observed outcome variables (the total score and item scores of the ABC-DS) were assumed continuous and normally distributed. The analysis yielded the parameters β11, β21, σ, and ρ. β11 represents phase A, β21 represents phase B, σ represents the standard deviation (SD), and ρ represents the autocorrelation. For parameter estimation, Bayesian estimation and the Markov chain Monte Carlo method (MCMC) was used, and MCMC sampling was set to 120,000 times (chain = 4, burn-in = 5000). The change point (CP) detects where the change in the relationship between the explanatory variable and the objective variable occurs at a continuous time point. The effect size (es) in the two phases was calculated as the standardized mean difference of the intercept estimates
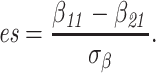 |
The 95% Bayesian confidence interval (CI) of the posterior distribution of the standardized mean difference determined the 95% limits of the credible value of the effect size under this distribution. Results were considered significant if the 95% Bayesian CIs of each estimated posterior distribution for phases A and B did not overlap, and the 95% Bayesian CI of the posterior distribution of the effect size was considered significant if it did not include zero [45].
For convergence determination, MCMC was considered to have converged to a steady state when the potential scale reduction factor (PSRF) was < 1.05 [46]. When the posterior distribution converged to a stationary state, the analysis was judged to have been performed appropriately with the assumed probability distribution (i.e., normal distribution).
The statistical software R (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria) with the runjags (version 2.2.0–2) and rjags (version 4–10) packages were used for all statistical analyses.
Results
The estimation result using the BUCP model had a PSRF of < 1.05 for all posterior distributions, and MCMC was regarded as having converged to a steady state. Participants 1 and 2 completed all 24 MNPI sessions. Participant 3 completed 23 sessions because of the potential influence of the new coronavirus infection. None of the participants experienced any adverse events during the study period.
Demographic characteristics
Participants were three PWD (participants 1–3: women, aged 92, 87, and 85 years, respectively) selected from two NHs who received the MNPI between November 2021 and May 2022. Participants 1 and 3 had a diagnosis of Alzheimer’s disease, and participant 2 had a diagnosis of dementia. All participants were taking anti-dementia medications. MMSE-J scores of the three patients were 21, 16, and 9, respectively, and the CDR scale score was 2 for all three participants. All patients had moderate dementia severity based on the initial ABC-DS (Table 2).
Table 2.
Characteristics of the study participants
| Participant 1 | Participant 2 | Participant 3 | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, year | 92 | 87 | 85 |
| Sex | Female | Female | Female |
| Education | Unknown | High school | Junior high school |
| Diagnosis of dementia | Alzheimer’s disease | Dementia | Alzheimer’s disease |
| Length of stay, months | 15 | 16 | 21 |
| Marital status | Widowed | Widowed | Widowed |
| Antidementia drug | Yes | Yes | Yes |
| Locomotion | walking frame | walking frame | cane |
| Initial assessment | |||
| MMSE-J | 21 | 16 | 9 |
| CDR | 2 | 2 | 2 |
| COGNISTAT Five | |||
| Short term memory | 3 | 7 | 2 |
| Orientation | 3 | 6 | 4 |
| Construction | 3 | 0 | 0 |
| Recall | 0 | 0 | 5 |
| ABC-DS | |||
| Dementia severity (total score) | 78 | 71 | 81 |
| ADLs | 39 | 40 | 41 |
| BPSDs | 26 | 24 | 24 |
| Cognitive function | 13 | 7 | 16 |
MMSE-J, Mini Mental State Examination Japanese; CDR, Clinical Dementia Rating; COGNISTAT Five, The Japanese version of the Neurobehavioral Cognitive Status Examination Five; ABC-DS, ABC Dementia Scale; ADLs, activities of daily living; BPSDs, behavioral and psychological symptoms of dementia
Results of the estimates for ABC-DS using the BUCP model
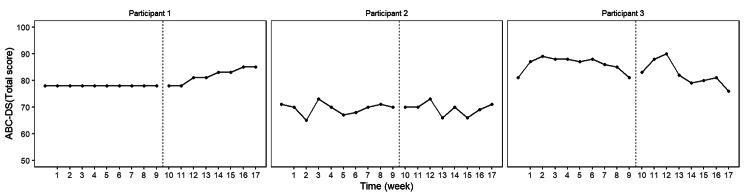
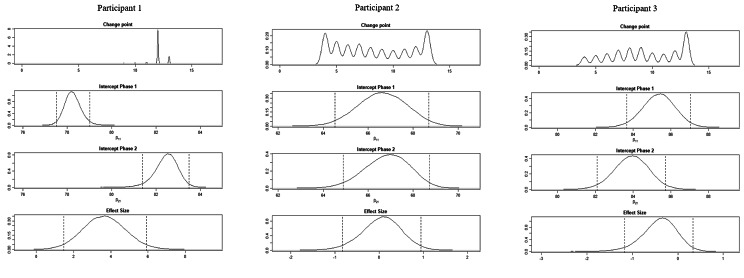
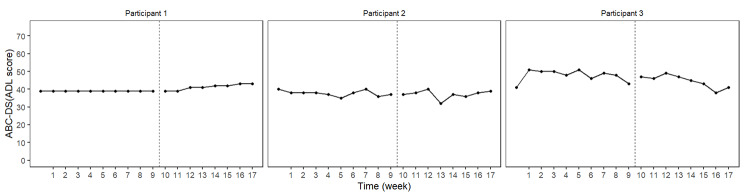
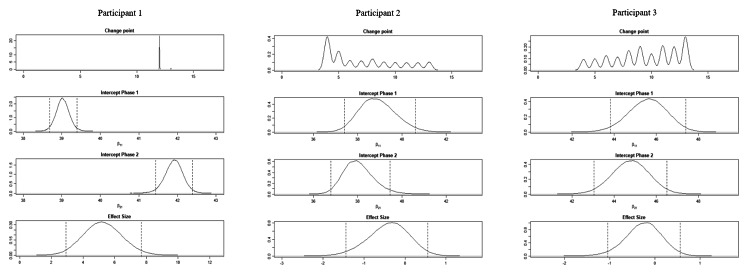
The BUCP model results for the ABC-DS in participant 1 showed that the mean (standard deviation [SD]) CP was 12.14 (0.45) weeks for total score (severity), 12.03 (0.17) weeks for ADLs, 12.93 (0.43) weeks for BPSDs, and 12.04 (0.23) weeks for cognitive function. The estimated posterior distributions (95% Bayesian CIs) for the scores of phases A (β11) and B (β21) did not overlap for any of the ABC-DS items. For the 95% Bayesian CIs of the effect size, none of the items contained zero, and the effect of the intervention was considered statistically significant (Table 3; Figs. 1, 2, 3, 4, 5, 6, 7 and 8).
Table 3.
Estimated result for each ABC-DS item score using the BUCP model
| Total score (severity) | ADLs | BPSDs | Cognitive function | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% Bayesian CI | Mean (SD) | 95% Bayesian CI | Mean (SD) | 95% Bayesian CI | Mean (SD) | 95% Bayesian CI | |||||||||||||
| Participant 1 | CP | 12.14 | (0.45) | 12.03 | (0.17) | 12.93 | (0.43) | 12.04 | (0.23) | |||||||||||
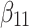
|
78.25 | (0.38) | [77.52, 79.00] | 39.03 | (0.18) | [38.68, 39.39] | 25.99 | (0.08) | [25.84, 26.14] | 13.01 | (0.10) | [12.81, 13.21] | ||||||||
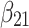
|
82.46 | (0.53) | [81.38, 83.49] | 41.91 | (0.24) | [41.44, 42.38] | 26.77 | (0.12) | [26.52, 27.02] | 14.35 | (0.14) | [14.08, 14.63] | ||||||||
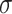
|
1.24 | (0.31) | [0.75, 1.84] | 0.57 | (0.12) | [0.37, 0.55] | 0.26 | (0.06) | [0.16, 0.37] | 0.33 | (0.07) | [0.21, 0.47] | ||||||||
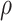
|
−0.001 | (0.02) | [− 0.04, 0.03] | 0.00 | (0.02) | [− 0.03, 0.03] | 0.00 | (0.01) | [− 0.02, 0.02] | 0.00 | (0.03) | [− 0.05, 0.06] | ||||||||
| es | 3.65 | (1.13) | [1.47, 5.91] | 5.25 | (1.22) | [2.93, 7.67] | 3.17 | (0.87) | [1.46, 4.89] | 4.20 | (0.97) | [2.28, 6.09] | ||||||||
| Participant 2 | CP | 8.36 | (3.20) | 7.16 | (2.99) | 8.40 | (2.93) | 8.62 | (2.56) | |||||||||||
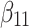
|
66.63 | (1.08) | [64.52, 68.70] | 38.91 | (0.83) | [37.40, 40.61] | 24.69 | (0.60) | [23.48, 25.89] | 7.48 | (0.55) | [6.43, 8.61] | ||||||||
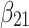
|
66.84 | (0.99) | [64.88, 68.73] | 38.04 | (0.69) | [36.80, 39.47] | 24.68 | (0.54) | [23.62, 25.77] | 8.13 | (0.54) | [7.16, 9.26] | ||||||||
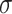
|
3.53 | (0.68) | [2.36, 4.89] | 2.35 | (0.52) | [1.47, 3.41] | 1.84 | (0.36) | [1.21, 2.55] | 1.39 | (0.33) | [0.84, 2.04] | ||||||||
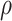
|
0.06 | (0.05) | [− 0.05, 0.17] | −0.02 | (0.06) | [− 0.14, 0.11] | 0.01 | (0.08) | [− 0.15, 0.16] | 0.02 | (0.18) | [− 0.33, 0.39] | ||||||||
| es | 0.06 | (0.45) | [− 0.83, 0.95] | −0.40 | (0.51) | [− 1.44, 0.56] | 0.00 | (0.45) | [− 0.88, 0.90] | 0.51 | (0.56) | [− 0.56, 1.64] | ||||||||
| Participant 3 | CP | 9.33 | (2.87) | 9.43 | (2.79) | 8.75 | (3.08) | 7.04 | (2.99) | |||||||||||
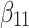
|
85.37 | (0.86) | [83.65, 87.04] | 45.59 | (0.92) | [43.82, 47.39] | 25.71 | (0.70) | [24.26, 26.97] | 13.95 | (0.91) | [12.23, 15.72] | ||||||||
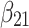
|
83.92 | (0.93) | [82.09, 85.73] | 44.78 | (0.89) | [43.05, 46.51] | 25.48 | (0.62) | [24.20, 26.61] | 13.15 | (0.79) | [11.74, 14.76] | ||||||||
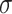
|
3.85 | (0.58) | [2.90, 4.99] | 3.48 | (0.59) | [2.43, 4.69] | 1.96 | (0.39) | [1.29, 2.76] | 2.98 | (0.62) | [1.89, 4.27] | ||||||||
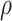
|
0.04 | (0.05) | [− 0.06, 0.14] | 0.18 | (0.09) | [0.01, 0.36] | −0.02 | (0.09) | [− 0.19, 0.15] | −0.20 | (0.17) | [− 0.53, 0.16] | ||||||||
| es | −0.40 | (0.38) | [− 1.17, 0.33] | −0.25 | (0.41) | [− 1.05, 0.56] | −0.13 | (0.53) | [− 1.14, 0.92] | −0.30 | (0.47) | [− 1.23, 0.60] | ||||||||
ADL, activities of daily living; BPSDs, behavioral and psychological symptoms of dementia: SD: standard deviation; CI, confidence interval; CP, change point; es, effect size
Fig. 1.
ABC Dementia Scale (ABC-DS) total score plot of each participant
Fig. 2.
Plot of the estimates of the ABC Dementia Scale (ABC-DS) total score using the Bayesian unknown change-point (BUCP) model of each participant. The BUCP results of the participants are shown from the top in order of change point, phase A (intercept phase 1) posterior distribution, phase B posterior distribution (intercept phase 2), and effect size posterior distribution. The vertical dotted lines on either side of the posterior distributions are 95% Bayesian confidence intervals
Fig. 3.
ABC Dementia Scale (ABC-DS) activities of daily living (ADL) score plot of each participant
Fig. 4.
Plot of the estimates for the ABC Dementia Scale (ABC-DS) activities of daily living score using the Bayesian unknown change-point (BUCP) model of each participant. The BUCP results of the participants are shown from the top in order of change point, phase A (intercept phase 1) posterior distribution, phase B posterior distribution (intercept phase 2), and effect size posterior distribution. The vertical dotted lines on either side of the posterior distributions are 95% Bayesian confidence intervals
Fig. 5.
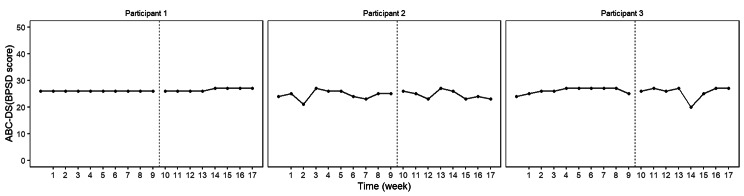
ABC Dementia Scale (ABC-DS) Bayesian unknown change-point (BUCP) score plot of each participant
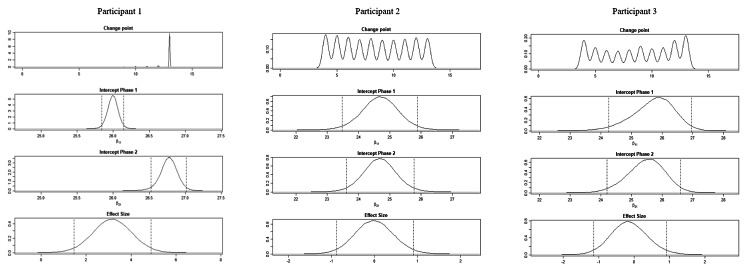
Fig. 6.
Plot of estimates of the ABC Dementia Scale (ABC-DS) Bayesian unknown change-point (BUCP) score using the BUCP model of each participant. The BUCP results of the participants are shown from the top in order of change point, phase A (intercept phase 1) posterior distribution, phase B posterior distribution (intercept phase 2), and effect size posterior distribution. The vertical dotted lines on either side of the posterior distributions are 95% Bayesian confidence intervals
Fig. 7.
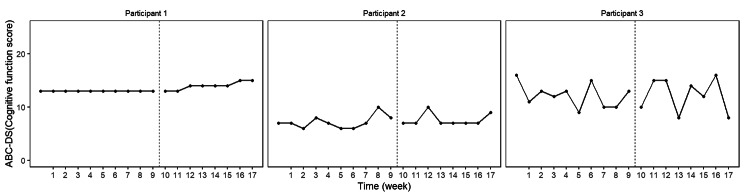
ABC Dementia Scale (ABC-DS) cognitive function score plot of each participant
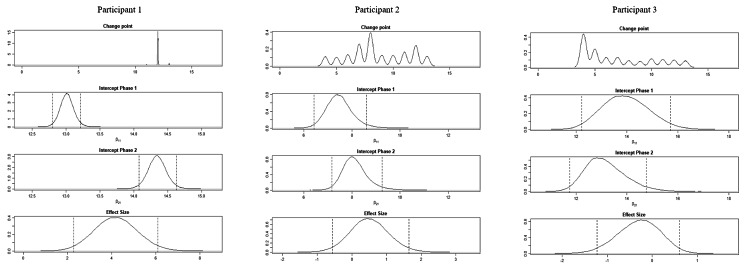
Fig. 8.
Plot of the estimates of the ABC Dementia Scale (ABC-DS) cognitive function score using the Bayesian unknown change-point (BUCP) model of each participant. The BUCP results of the participants are shown from the top in order of change point, phase A (intercept phase 1) posterior distribution, phase B posterior distribution (intercept phase 2), and effect size posterior distribution. The vertical dotted lines on either side of the posterior distributions are 95% Bayesian confidence intervals
The BUCP model results for the ABC-DS in participant 2 showed that the mean (SD) CP was 8.36 (3.20) weeks for total score (severity), 7.16 (2.99) weeks for ADLs, 8.40 (2.93) weeks for BPSDs, and 8.62 (2.56) weeks for cognitive function. The estimated posterior distributions (95% Bayesian CIs) for the scores of phases A (β11) and B (β21) overlapped for all ABC-DS items, which indicated that there was no difference in the distribution of phases A and B in participant 2. The 95% Bayesian CIs of the effect size included zero for all items, and the effect of the intervention was considered non-significant (Table 3; Figs. 1, 2, 3, 4, 5, 6, 7 and 8).
The BUCP model results for the ABC-DS in participant 3 showed that the mean (SD) CP was 9.33 (2.87) weeks for total score (severity), 9.43 (2.79) weeks for ADLs, 8.75 (3.08) weeks for BPSDs, and 7.04 (2.99) weeks for cognitive function. The estimated posterior distributions (95% Bayesian CIs) for the scores of phases A (β11) and phase B (β21) overlapped for all ABC-DS items, which indicated that there was no difference in the distribution of phases A and B in participant 3. The 95% Bayesian CIs of the effect size included zero for all items, and the effect of the intervention was determined to be non-significant (Table 3; Figs. 1, 2, 3, 4, 5, 6, 7 and 8).
Discussion
The purpose of this study was to evaluate the effect of our proposed MNPI on the cognitive function of PWD in NH using a SCED. In recent years, several studies using a SCED have proposed analyses using statistical methods in addition to visual inspection, attracting increased attention [45]. Because the ABC-DS is not impacted by learning effects, the frequency of assessment required for use of the BUCP model can be achieved even over a short period. Moreover, the Bayesian framework allows “acceptance” of the null or alternative hypothesis. Thus, unlike the classical framework, the possibility of no effect of this intervention can be deduced, even if the result is non-significant [45]. Therefore, the analysis using the BUCP model was suitable for this study.
Effect of the MNPI on participant 1
The MNPI was effective in improving the ABC-DS scores (dementia severity, ADLs, BPSDs, and cognitive function) in participant 1. CP was detected approximately 2 weeks after the start of the intervention (12 weeks after the start of the study), which suggested that the effect emerged gradually from this period. Participant 1 had been in the NH for 15 months, and her condition was stable with no major changes in medication status, including during the study period. Therefore, the MNPI likely improved the score of each ABC-DS item. The results of participant 1 supported our hypothesis that the MNPI would be effective in improving global cognitive function, ADLs, and BPSDs in PWD in NHs.
Effect of the MNPI on participant 2
The model BUCP model results for the ABC-DS in participant 2 showed that none of the items improved. Although the expected value of the CP was generally estimated at the intervention time point (i.e., 7–8 weeks after the start of the study), there were no obvious changes in any of the items, as shown in Figs. 1, 2, 3, 4, 5, 6, 7 and 8. The results of the MNPI for participant 2 indicated that the intervention did not improve dementia severity, ADLs, BPSDs, or cognitive function.
Effect of the MNPI on participant 3
The results of the BUCP model for the ABC-DS in participant 3 showed that none of the items improved. Although the expected value of the CP was generally estimated at the intervention time point (i.e., 7–9 weeks after the start of the study), we detected no obvious changes in any of the items, as shown in Figs. 1, 2, 3, 4, 5, 6, 7 and 8. The results of the MNPI for participant 3 indicated that the intervention did not improve dementia severity, ADLs, BPSDs, or cognitive function.
The effectiveness of the MNPI
In this study, all participants continued to engage in recreational activities and exercises as a daily routine, and no additional interventions or major care policy changes were introduced other than the MNPI. Therefore, the results were likely attributed to the MNPI. The effectiveness of the MNPI was confirmed in participant 1. However, the validity of our MNPI was not confirmed in participant 2 or 3. In contrast to participant 1, the ABC-DS phase A of participants 2 and 3 varied each week. It is also possible that factors that were not controlled for in this study, such as differences in the care policies of the target NHs at the time of the evaluation (participant 1 was from a different NH), may have affected the results. In addition, because participants 2 and 3 had a more unstable dementia status than participant 1, it may have been useful to consider tasks and BPSDs according to dementia status when administering the MNPI. Incorporating these into future MNPIs may enable greater efficacy. However, it is also possible that in PWD in NHs, cognitive function declines over a short period, such as that of the current study [1–3]. Therefore, the condition of participants 2 and 3 may have been maintained by the MNPI.
The results of our study suggest that our MNPI works to maintain or improve the severity of dementia, global cognitive function, ADLs, and BPSD in PWD in NHs. However, only one of three participants showed an improvement in their ABC-DS score, which suggested that the time, frequency, and duration of our MNPI design were inadequate. A review of the intervention period, time per session, and frequency of implementation should be considered. Consideration should also be given to the intervention provider adjusting the difficulty of the intervention according to the core symptoms, BPSD, or ADL impairments of the participant. The effectiveness of the MNPI for improving dementia severity, global cognitive function, ADLs, and BPSD in PWDs in NHs then requires examination in a larger number of participants. In the future, the problems of our MNPI identified in the present study should be addressed to enable a comparison between the AB and AA conditions (i.e., subjects participate in the non-intervention phase only) or a two-group design study to examine the maintenance and improvement effects of the MNPI.
The fact that the MNPI implemented in this study did not result in a worsening of ABC-DS score or other adverse events indicates that the MNPI can be safely implemented in NHs and is a useful intervention. Our MNPI was implemented using a minimal intervention design because our priority was to ensure clinical practicality in NHs. However, the time, frequency, and duration of the MNPI may need refinement to optimize the improvement of dementia symptoms. Nevertheless, we believe that our findings are valuable for clinical practitioners in NHs because our MNPI is a feasible intervention that can be implemented in NHs that has a small chance of slowing the progression of dementia symptoms.
Limitations
Proxy-rated instruments, such as the ABC-DS, have lower reliability in measuring cognitive function than performance tests, such as the MMSE-J. Therefore, it is necessary to validate our findings using performance tests in the future while taking learning effects into consideration. Cognitive function in PWD in NHs may decline, even 8 weeks after admission [3]. Moreover, it is thought that the longer the length of stay, the more likely that decline will occur. In our AB design, the non-intervention phase was 9 weeks, and it is uncertain whether cognitive function would have declined if no intervention was provided after the intervention period of 10 weeks. Furthermore, we did not include a control group. Therefore, the maintenance effects and delays in the decline of cognitive function could not be determined. In addition, the possibility that the improvement effect in participant 1 was due to natural improvement cannot be ruled out completely. Nevertheless, our results provide a basis to further develop our MNPI and examine its effectiveness using a study design that offers a higher level of evidence.
Conclusion
Our MNPI may be effective in maintaining and improving cognitive function, ADLs, and BPSD in PWD in NHs. However, refinement of the frequency, duration, and intervention period is required. Moreover, consideration of the intervention provider adjusting the difficulty of the intervention according to the observed impairment of the patient may be necessary.
Acknowledgements
The authors would like to thank Geriatric Health Service Facility Shousidou and Geriatric Health Service Facility Otowanomori for their support of the study. The authors would also like to thank all the rehabilitation, nursing, care, and administrative staff, the study participants who provided data, and participants’ family members or legal representatives who provided consent to participate. We thank Sarina Iwabuchi, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
List of abbreviations
- PWD
older people with dementia.
- NH
nursing home.
- BPSD
behavioral and psychological symptoms of dementia.
- ADL
activities of daily living.
- NPI
non-pharmacological intervention.
- MNPI
multimodal non-pharmacological intervention.
- SCED
single-case experimental design.
- SCRIBE
Single-Case Reporting Guideline in Behavioural Intervention.
- MMSE-J
Mini Mental State Examination-Japanese.
- ABC-DS
ABC Dementia Scale.
- CDR
Clinical Dementia Rating.
- COGNISTAT Five
Japanese version of the Neurobehavioral Cognitive Status Examination Five.
- OT
occupational therapist.
- BUCP
Bayesian unknown change point.
- MCMC
Markov chain Monte Carlo method.
- CP
change point.
- CI
confidence interval.
- PSRF
potential scale reduction factor.
- SD
standard deviation.
Author contributions
Data curation: Kyosuke Yorozuya and Hideaki Hanaoka. Formal analysis: Kyosuke Yorozuya, Yuta Kubo, and Hideaki Hanaoka. Investigation: Kyosuke Yorozuya and Yoshihiro Asaoka. Methodology: Kyosuke Yorozuya, Yoshihito Tsubouchi, Yuta Kubo, Hiroyuki Hayashi, Takashi Fujita, and Hideaki Hanaoka. Project administration: Kyosuke Yorozuya and Hideaki Hanaoka. Supervision: Hiroyuki Hayashi, Takashi Fujita, and Hideaki Hanaoka. Validation: Hideaki Hanaoka. Writing – original draft: Kyosuke Yorozuya. Writing – review and editing: Yoshihito Tsubouchi, Yuta Kubo, Yoshihiro Asaoka, Hiroyuki Hayashi, Takashi Fujita, and Hideaki Hanaoka. All authors read and approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI [Grant Number JP 21K21126] and Seijoh University.
Data Availability
Because of privacy and ethical concerns, the datasets generated and analyzed during the current study cannot be made publicly available.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Seijoh University (approval number: 2021C0005). The participants and their caregivers provided informed consent before participating in the study.
Consent for publication
Written informed consent was obtained from the participants for publication of this study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson RS, McCann JJ, Li Y, Aggarwal NT, Gilley DW, Evans DA. Nursing home placement, day care use, and cognitive decline in Alzheimer’s disease. Am J Psychiatry. 2007;164(6):910–5. doi: 10.1176/ajp.2007.164.6.910. [DOI] [PubMed] [Google Scholar]
- 2.Graessel E, Stemmer R, Eichenseer B, Pickel S, Donath C, Kornhuber J, et al. Non-pharmacological, multicomponent group therapy in patients with degenerative dementia: a 12-month randomized, controlled trial. BMC Med. 2011;9(129):1–11. doi: 10.1186/1741-7015-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luca RD, Bramanti A, Cola MCD, Leonardi S, Torrisi M, Aragona B, et al. Cognitive training for patients with dementia living in a Sicilian nursing home: a novel web-based approach. Neurol Sci. 2016;37(10):1685–91. doi: 10.1007/s10072-016-2659-x. [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532–9. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3:73. doi: 10.3389/fneur.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubo Y, Hayashi H, Kozawa S, Okada S. Relevant factors of depression in dementia modifiable by non-pharmacotherapy: a systematic review. Psychogeriatrics. 2019;19(2):181–91. doi: 10.1111/psyg.12371. [DOI] [PubMed] [Google Scholar]
- 8.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–83. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 9.Aalten P, de Vugt ME, Jaspers N, Jolles J, Verhey FRJ. The course of neuropsychiatric symptoms in dementia. Part I: findings from the two-year longitudinal Maasbed study. Int J Geriatr Psychiatry. 2005;20(6):523–30. doi: 10.1002/gps.1316. [DOI] [PubMed] [Google Scholar]
- 10.Kales HC, Kim HM, Zivin K, Valenstein M, Seyfried LS, Chiang C, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71–9. doi: 10.1176/appi.ajp.2011.11030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luppa M, Luck T, Weyerer S, König HH, Brähler E, Riedel-Heller SG. Prediction of institutionalization in the elderly. A systematic review. Age Ageing. 2010;39(1):31–8. doi: 10.1093/ageing/afp202. [DOI] [PubMed] [Google Scholar]
- 12.Chen RC, Liu CL, Lin MH, Peng LN, Chen LY, Liu LK, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440–6. doi: 10.1111/ggi.12126. [DOI] [PubMed] [Google Scholar]
- 13.Prizer LP, Zimmerman S. Progressive Support for Activities of Daily Living for Persons Living With Dementia. Gerontologist. 2018;58(Suppl 1):74–87. doi: 10.1093/geront/gnx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borsje P, Lucassen PLBJ, Bor H, Wetzels RB, Pot AM, Koopmans RTCM. The course of neuropsychiatric symptoms in patients with dementia in primary care. Fam Pract. 2019;36(4):437–44. doi: 10.1093/fampra/cmy117. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization: Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 2 Sept 2021.
- 16.Loy C, Schneider L. Galantamine for Alzheimer’s disease and mild cognitive impairment (Review). Cochrane Database Syst Rev 2006 Jan; (1): CD001747. doi:10.1002/14651858.CD001747. [DOI] [PMC free article] [PubMed]
- 17.Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med. 2007;4(11):1818–28. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. JABFM. 2012;25:350–66. doi: 10.3122/jabfm.2012.03.100183. [DOI] [PubMed] [Google Scholar]
- 19.Cooper C, Li R, Livingston G. A systematic review of treatments for Mild Cognitive Impairment. Br J Psychiatry. 2013;203(3):255–64. doi: 10.1192/bjp.bp.113.127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 21.Kurz AF, Leucht S, Lautenschlager NT. The clinical significance of cognition-focused interventions for cognitively impaired older adults: a systematic review of randomized controlled trials. Int Psychogeriatr. 2011;23(9):1364–75. doi: 10.1017/S1041610211001001. [DOI] [PubMed] [Google Scholar]
- 22.Woods B, Spector AE, Jones CA, Orrell M, Davies SP. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2): doi:10.1002/14651858. [DOI] [PubMed]
- 23.Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161–78. doi: 10.1159/000316119. [DOI] [PubMed] [Google Scholar]
- 24.Vasionyte I, Madison G. Musical intervention for patients with dementia: a meta-analysis. JCN. 2013;22(9–10):1203–16. doi: 10.1111/jocn.12166. [DOI] [PubMed] [Google Scholar]
- 25.Yorozuya K, Kubo Y, Tomiyama N, Yamane S, Hanaoka H. A systematic review of multimodal non-pharmacological interventions for cognitive function in older people with dementia in nursing homes. Dement Geriatr Cogn Disord. 2019;48(1–2):1–16. doi: 10.1159/000503445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akram A, Nicosia F, Lee J, Lee M, Martin L, Martinez S, et al. Implementation of an integrative movement program for residents with dementia in a VA nursing home. BMC Geriatr. 2021;21(1):607. doi: 10.1186/s12877-021-02494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tate RL, Perdices M, Rosenkoetter U, Shadish W, Vohra S, Barlow DH, et al. The Single-Case Reporting Guideline In BEhavioural Interventions (SCRIBE) 2016 Statement. Neuropsychol Rehabil. 2017;27(1):1–15. doi: 10.1080/09602011.2016.1190533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanstrup M, Kontio E, Geranmayeh A, Lauri KO, Moulds ML, Holmes EM. A single case series using visuospatial task interference to reduce the number of visual intrusive memories of trauma with refugees. Clin Psychol Psychother. 2021;28(1):109–23. doi: 10.1002/cpp.2489. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Sugishita M. Mini Mental State Examination-Japanese (MMSE-J) Tokyo: Nihon Bunka Kagakusha Co., Ltd.; 2019. [Google Scholar]
- 31.Kikuchi T, Mori T, Wada-Isoe K, Umeda-Kameyama Y, Kagimura T, Kojima S, et al. Novel dementia scale for Alzheimer’s disease. J Alzheimers Dis Parkinsonism. 2018;8(2):2–7. doi: 10.4172/2161-0460.1000429. [DOI] [Google Scholar]
- 32.Mori T, Kikuchi T, Umeda-Kameyama Y, Wada-Isoe K, Kojima S, Kagimura T, et al. ABC Dementia Scale: a quick assessment tool for determining Alzheimer’s disease severity. Dement Geriatr Cogn Dis Extra. 2018;8(1):85–97. doi: 10.1159/000486956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuchi T, Wada-Isoe K, Mori T, Nakamura Y, Umeda-Kameyama U, Akishita M. Concurrent validity of EQ-5D-5L by caregiver proxy rating with the ABC Dementia Scale for Alzheimer patients. J Brain Res. 2018;2(1):105. [Google Scholar]
- 34.Umeda-Kameyama Y, Mori T, Wada-Isoe K, Kikuchi T, Kojima S, Kagimura T, et al. Development of a novel convenient Alzheimer’s disease assessment scale, the ABC Dementia Scale, using item response theory. Geriatr Gerontol Int. 2019;19(1):18–23. doi: 10.1111/ggi.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada-Isoe K, Kikuchi T, Umeda-Kameyama Y, Mori T, Kudoh C, Ueda T, et al. ABC Dementia Scale can evaluate the changes of symptoms caused by alternation or addition of anti-Alzheimer’s disease drugs. Japanese J Geriatric Psychiatry. 2019;30(1):73–83. [Google Scholar]
- 36.Wada-Isoe K, Kikuchi T, Umeda-Kameyama Y, Mori T, Akishita M, Nakamura Y. Global clinical dementia rating score of 0.5 may not be an accurate criterion to identify individuals with mild cognitive impairment. J Alzheimers Dis Rep. 2019;3(1):233–9. doi: 10.3233/ADR-190126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada-Isoe K, Kikuchi T, Umeda-Kameyama Y, Mori T, Akishita M, Nakamura Y. ABC Dementia Scale classifies Alzheimer’s disease patients into subgroups characterized by activities of daily living, behavioral and psychological symptoms of dementia, and cognitive function. J Alzheimers Dis. 2020;73(1):383–92. doi: 10.3233/JAD-190767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada-Isoe K, Kikuchi T, Umeda-Kameyama Y, Mori T, Akishita M, Nakamura Y. Validation of the neuropsychiatric inventory based on item response theory. J Alzheimers Dis Rep. 2020;4(1):151–9. doi: 10.3233/ADR-200172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiederman MW, Morgan CD. The Neurobehavioral Cognitive Status Exam (NCSE) with geriatric inpatients. Clin Gerontologist. 1995;15(4):35–47. doi: 10.1300/J018V15N04_04. [DOI] [Google Scholar]
- 40.Matsuda O, Nakatani M. Manual for Japanese version of the Neurobehavioral Cognitive Status Examination (COGNISTAT) Tokyo: World Planning; 2004. [Google Scholar]
- 41.Arai H, Takayama T, Takayama Y. The manual for Japanese version of Cognistat Five® 2015. Tokyo: World Planning; 2019. [Google Scholar]
- 42.Takayama T, Takayama Y, Shibata N, Nakata T, Toda A, Ohno K, et al. A study of the reliability, validity and availability of the Japanese version of Cognistat Five. Japanese J Geriatric Psychiatry. 2019;30:785–93. [Google Scholar]
- 43.Yorozuya K, Yamane S, Nobuhisa M, Owaki H, Suzuki T, Okahara H, et al. Bayesian analysis of the association between effective strategies of multimodal nonpharmacological intervention and characteristics of cognitive function in nursing home residents with cognitive impairment: a cross-sectional study. Medicine. 2020;99(37):e22154. doi: 10.1097/MD.0000000000022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Natesan P, Hedges LV. Bayesian unknown change-point models to investigate immediacy in single case designs. Psychol Methods. 2017;22(4):743–59. doi: 10.1037/met0000134. [DOI] [PubMed] [Google Scholar]
- 45.Batley PN, Nandakumar R, Palka JM, Shrestha P. Comparing the Bayesian unknown change-point model and simulation modeling analysis to analyze single case experimental designs. Front Psychol. 2021;11:617047. doi: 10.3389/fpsyg.2020.617047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gelman A. Bayesian data analysis. 3. Oxford: Chapman and Hall/CRC; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because of privacy and ethical concerns, the datasets generated and analyzed during the current study cannot be made publicly available.