Abstract
The visual system uses ON and OFF pathways to signal luminance increments and decrements. Increasing evidence suggests that ON and OFF pathways have different signaling properties and serve specialized visual functions. However, it is still unclear the contribution of ON and OFF pathways to visual behavior. Therefore, we examined the effects on optomotor response and the retinal dopamine system in nob mice with ON pathway dysfunction and Vsx1−/− mice with partial OFF pathway dysfunction. Spatial frequency and contrast sensitivity thresholds were determined, and values were compared to age-matched wild-type controls. Retinas were collected immediately after visual testing to measure levels of dopamine and its metabolite, DOPAC. At 4 weeks of age, we found that nob mice had significantly reduced spatial frequency (19%) and contrast sensitivity (60%) thresholds compared to wild-type mice. Vsx1−/− mice also exhibited reductions in optomotor responses (3% in spatial frequency; 18% in contrast sensitivity) at 4 weeks, although these changes were significantly smaller than those found in nob mice. Furthermore, nob mice had significantly lower DOPAC levels (53%) and dopamine turnover (41%) compared to controls while Vsx1−/− mice displayed a transient increase in DOPAC levels at 4 weeks of age (55%). Our results show that dysfunction of ON pathways leads to reductions in contrast sensitivity, spatial frequency threshold, and retinal dopamine and DOPAC levels whereas partial loss of the OFF pathway has minimal effect. We conclude that ON pathways play a critical role in visual reflexes and retinal dopamine signaling, highlighting a potential association for future investigations.
Keywords: nob, Vsx1 −/− , dopamine, DOPAC, optomotor response (OMR)
Visual information is processed by ON and OFF pathways that detect luminance increments and decrements. ON and OFF pathways are differentiated at the first retinal synapse between the photoreceptors and bipolar cells and are maintained through the visual cortex (Kuffler, 1953). It has become increasingly clear that ON and OFF pathways differ in more than contrast polarity detection. Rather, ON and OFF pathway signaling differences begin in the retina (Chichilnisky and Kalmar, 2002; Zaghoul et al., 2003), are amplified in the visual cortex (Kremkow et al., 2014), and are important to visual function and behavior. For example, the ON pathway is associated with the accessory optic system (AOS) that controls image stabilization and stimulus tracking reflexes in many species including humans (Emran et al., 2007; Joesch et al., 2010; Sun et al., 2015; Winkelman et al., 2019).
The contribution of ON and OFF pathways to visual behavior can be probed by studying cases where either pathway is dysfunctional. Patients with congenital stationary night blindness (CSNB) resulting from a mutation in the Nyx gene leading to ON bipolar cell dysfunction, have night blindness, myopia, nystagmus, and strabismus (MacDonald et al., 2008). The same mutation is shared by ‘nob’ mice (Gregg et al., 2003; Pardue et al., 1998), which lack the ON-driven b-wave component of the electroretinogram (ERG) composed of rod and ON cone bipolar cell signals (Gregg et al., 2007; Morgans et al., 2006; Quigley et al., 1996). Nob mice have reduced ability to maintain eye tracking after an initial smooth movement (Winkelman et al., 2019) and reduced avoidance detection (Gregg et al., 2003). Deficits in visual reflexes have also been reported in other mouse models of genetic ON pathway disruption when measuring eye tracking (optokinetic responses) (Iwakabe et al., 1997; Sugita et al., 2013) or head tracking (optomotor response, OMR) (Orhan et al., 2021; Pinto et al., 2007). Moreover, mice with dysfunctional mGluR6 receptors found on ON bipolar cells have pronounced deficits in cortical orientation selectivity, contrast sensitivity, and absent ON signals in the superior colliculus and primary visual cortex (Sarnaik et al., 2014).
While less pronounced, mutations in the predominantly OFF pathway-expressing Vsx1 gene are associated with keratoconus, altered corneal shape, and development of myopia (Bisceglia et al., 2005). Vsx1 encodes for a transcription factor expressed in mammalian cone bipolar cells, which primarily impacts terminal differentiation in over half of OFF cone bipolar cells (types 1, 2, and 3a) but also affects type 7 ON cone bipolar cells (Chow et al., 2004; Shi et al., 2012; Shi et al., 2011). Vsx1−/− mice have decreased OFF ganglion cell responses, with minimal reductions in ON responses (Chow et al., 2004; Shi et al., 2011). Therefore, Vsx1−/− mice represent a partial, but still substantial model of primarily OFF pathway dysfunction. However, it remains unclear the contribution of ON and OFF pathways to the OMR which combines motion detection, acuity, and contrast sensitivity in a natural reflex in unrestrained mice (Kretschmer et al., 2017).
In addition to understanding the contribution of ON and OFF pathways to visual behavior, the retinal mechanisms associated with these behaviors need to be elucidated. One potential candidate for modulating visual reflex pathways is dopamine. Retinal dopamine is a prominent neuromodulator for visual function and eye growth (Jackson et al., 2012; Witkovsky, 2004; Zhou et al., 2017) and its release is driven by luminance increments and ON pathways (Contini et al., 2010; Dumitrescu et al., 2009; Qiao et al., 2016). Therefore, in this study, we investigated the contribution of ON and OFF pathways to visual reflex behavior by measuring spatial frequency thresholds and contrast sensitivity using OMR assessment in mice with ON and OFF pathway dysfunction. Additionally, we measured levels of retinal dopamine and its primary metabolite, L-3,4-dihydroxyphenylalanine (DOPAC) in the same animals to examine any potential links between dopamine signaling and changes in visual reflexes. We hypothesized that ON pathway dysfunction (nob mice) would result in greater reductions in spatial frequency and contrast sensitivity thresholds that would be accompanied by reduced retinal dopamine and DOPAC levels in comparison to partial OFF pathway dysfunction (Vsx1−/− mice).
To assess the contribution of the ON pathway to visual acuity and contrast sensitivity, nob mice with defective Nyx gene (Nyxnob/nob or Nyxy/nob mice), referred collectively in this paper as nob mice) (Gregg et al., 2007) were compared to their background strain C57BL/6J wild-type mice (Nyxwt/wt). To examine the role of OFF pathways in visual function, Vsx1−/− mice were compared to their 129S1/Sv wild-type background strain (Vsx1+/+). Visual function was evaluated in two cohorts at either 4 or 8 weeks post-natal. All mice were housed under 12:12 light-dark cycle with food and water ad libitum. All procedures were approved by the Atlanta VA Institutional Animal Care and Use Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
The OMR was measured using an optomotor assessment system (OptoMotry system, Cerebral Mechanics, Lethbridge, Canada), which has been described previously (Prusky et al., 2004). In brief, the mouse was placed on a platform surrounded by four gamma-corrected computer monitors that displayed a vertical drifting sine-wave grating (at 100% contrast, white bar: 374.8 cd/m2, black bar: 15.08 cd/m2) rotating at 12 deg/s. The mouse was un-restrained and was observed in real time via video camera placed over the platform. The mouse’s response to stimuli was assessed by looking for head turns in the direction of the drifting grating that was presented continuously for ~10 minutes. The drifting gratings rotated clockwise and counterclockwise, randomly, between trials to selectively test each eye (Douglas et al., 2005). To measure visual acuity, gratings were presented with spatial frequencies at 100% contrast that increased in a staircase paradigm starting at 0.042 cyc/deg. The spatial frequency threshold was measured as the highest spatial frequency that could produce an OMR. To assess contrast sensitivity, gratings were evaluated at the spatial frequency that produced the highest contrast sensitivity threshold (i.e. peak contrast sensitivity). Gratings were initially presented at 100% contrast, which was gradually reduced by half in a staircase paradigm (e.g. 100%, 75%, 50%, 25%, 12.5%, etc.) until no OMR was detected (i.e. a positive head-turn response). The contrast sensitivity threshold was then calculated as the reciprocal of the Michelson contrast from the screen luminance ([maximum + minimum]/[maximum − minimum]) (Prusky et al., 2006). Both the spatial frequency and contrast sensitivity thresholds were determined after three positive responses and calculated as the average of the reported values for both eyes.
After OMR assessment, animals were sacrificed between 4 and 6 hours after light-cycle onset under typical indoor lighting conditions. Retinas were collected from enucleated eyes, stored at −80°C, and then analyzed with high-performance liquid chromatography (HPLC) to measure levels of retinal dopamine and DOPAC. Dopamine and DOPAC levels were normalized to total retinal protein content as determined by the method of Lowry et al.,1951 (Lowry et al., 1951). The DOPAC:dopamine ratio was calculated as an indirect assessment of dopamine turnover. All OMR and dopamine, DOPAC, and DOPAC:dopamine values were compared separately between each mutant strain to its respective wild-type control. When directly compared, the two mutant strains were normalized to their respective wild-type strains to address differences between C57BL6/J and 129S1/Sv animals. Two-way repeated-measures mixed-effects analysis, or ANOVA, with Holm-Sidak multiple comparisons post hoc tests (GraphPad Prism 9.1, San Diego, CA) was used for statistical comparisons. Data was collected and analyzed by experimenters blinded to animals’ genotypes. In some instances, measurements of individual mice were not performed across all testing parameters so the sample sizes in the figures reflect the range of data points for each group. All reported statistics were performed with these tests, unless otherwise noted. All data are expressed as mean ± standard error of mean (SEM).
We found that both mutant strains showed significantly reduced spatial frequency thresholds, with nob mice more severely affected than Vsx1−/− mice. Compared to wild-type mice, the average threshold of nob mice was reduced by 19% at 4 weeks (0.31 ± 0.01 vs. 0.39 ± 0.003 cyc/deg, p<0.001) and by 9% at 8 weeks post-natal (0.37 ± 0.003 vs. 0.41 ± 0.002 cyc/deg, p=0.001; main effect of genotype, F(1,33) = 66.52, p<0.001; Figure 1A). The spatial frequency threshold increased with age (main effect of age, F(1,33) = 36.48, p<0.001), but there was a greater age-related increase in spatial frequency threshold for nob than Nyxwt/wt mice (age by genotype interaction, F(1,33) = 7.682, p=0.009). In contrast, Vsx1−/− mice had a modest but significant 3% reduction in spatial frequency threshold at 4 weeks (0.44 ± 0.003 vs. 0.43 ± 0.01 cyc/deg, p=0.03) and 2% at 8 weeks post-natal (0.44 ± 0.002 vs. 0.45 ± 0.004 cyc/deg, p=0.04; main effect of genotype, F(1,34) = 10.90, p=0.002; Figure 1B). Both Vsx1−/− and Vsx1+/+ mice also showed higher spatial frequency threshold with age (main effect of age, F(1,34) = 4.572, p=0.04; age by genotype interaction, F(1,34) = 0.07489, p=0.79). When normalized to wild-type values, the magnitude of spatial frequency threshold reduction was significantly greater in nob than Vsx1−/− mice at both 4 (0.81 ± 0.03 vs. 0.97 ± 0.011 normalized cyc/deg, p<0.001) and 8 weeks post-natal (0.91 ± 0.01 vs. 0.98 ± 0.01 normalized cyc/deg, p=0.02; main effect of genotype, F(1,32)=38.07, p<0.001; age by genotype interaction, F(1,32)=7.016, p=0.01; Figure 1C).
Figure 1.
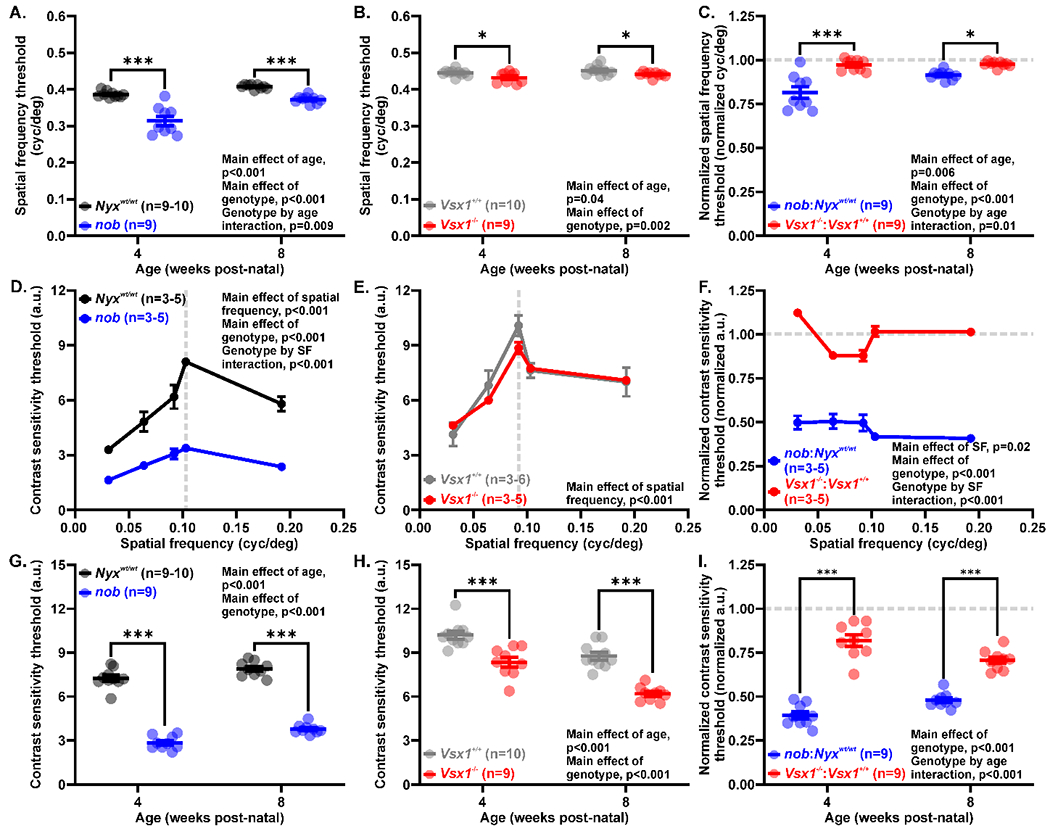
Greater reduction in spatial frequency and contrast sensitivity thresholds in nob than Vsx1−/− mice. (A-C) Comparison of spatial frequency thresholds of nob and Vsx1−/− mice against their wild-type controls. Both (A) nob and (B) Vsx1−/− mice had significant reductions in spatial frequency at 4 and 8 weeks post-natal. Relative to their background strains, nob mice had a larger relative decrease in normalized spatial frequency threshold compared to Vsx1−/− mice (C). (D-E) Contrast sensitivity measured at increasing spatial frequency gratings for (D) nob (blue) and Nyxwt/wt mice (black) as well as (E) Vsx1−/− (red) and Vsx1+/+ mice (gray). Contrast sensitivity in nob, but not Vsx1−/− mice was significantly reduced across all spatial frequencies when compared to wild-type animals. (F) Contrast sensitivity normalized to the wild-type background strain across spatial frequencies. nob mice had greater reduction in relative contrast sensitivity across all tested spatial frequencies than Vsx1−/− mice. (G-I) Contrast sensitivity measured at the peak spatial frequency for (G) nob and (H) Vsx1−/− mice at 4 and 8 weeks post-natal. Both nob and Vsx1−/− mice had significantly reduced contrast sensitivity compared to their age-matched controls. nob mice had a greater relative decrease in normalized contrast sensitivity than Vsx1−/− mice (I). Data are mean ± SEM and significance is *p<0.05, **p<0.01, and ***p<0.001. Comparisons were performed with repeated measures (RM) two-way mixed-effect analysis (MEA) (A-C) or two-way ANOVA (D-I) both with Holm-Sidak multiple comparisons tests.
To determine the contrast sensitivity curves for each mouse strain, a subset of mice between post-natal day 28 and 35 were assessed for their peak contrast sensitivity over a range of spatial frequencies (0.031 to 0.192 cyc/deg). In both Nyxwt/wt and mutant nob mice, the contrast sensitivity threshold peaked at 0.103 cyc/deg (Figure 1D). As we hypothesized, nob mice exhibited reduced contrast sensitivity thresholds at all tested spatial frequencies (peak contrast sensitivity: 3.38 ± 0.12 vs. 8.09 ± 0.15 a.u., p<0.001; spatial frequency by genotype interaction, F(4,20)=10.71, p<0.001; Figure 1D). In contrast, Vsx1−/− mice did not differ from Vsx1+/+ mice in either their peak spatial frequency threshold (0.092 cyc/deg) or contrast sensitivity curve (peak contrast sensitivity: 8.84 ± 0.32 vs. 10.07 ± 0.55 a.u., p=0.38; main effect of genotype, F(1,9) = 0.5832, p=0.46; Figure 1E). Furthermore, when normalizing contrast sensitivity of mutant animals to their wild-type averages at each spatial frequency, nob mice had significantly reduced contrast sensitivity compared to Vsx1−/− mice (main effect of genotype, F(1,8)= 689.1, p<0.001; Figure 1F).
After the spatial frequency that elicited the maximal contrast sensitivity was determined for each mouse mutant (0.103 cyc/deg for nob and 0.092 cyc/deg for Vsx1−/− mice), we measured how ON and OFF pathway dysfunction affected peak contrast sensitivity across development. Compared to age-matched Nyxwt/wt mice, peak contrast sensitivity of nob mice was reduced by 60% at 4 weeks (2.84 ± 0.15 vs. 7.25 ± 0.20 a.u., p<0.001) and 52% at 8 weeks post-natal (3.78 ± 0.11 vs. 7.89 ± 0.17 a.u., p<0.001; main effect of genotype, F(1, 33) = 667.3, p<0.001; Figure 1G). Peak contrast sensitivity increased across age similarly for both nob and Nyxwt/wt mice (main effect of age, F(1,33) = 22.65, p<0.001). Likewise, Vsx1−/− mice exhibited a 18% reduction at 4 weeks (8.34 ± 0.34 vs. 10.19 ± 0.26 a.u., p<0.001) and 29% reduction at 8 weeks post-natal (6.18 ± 0.16 vs. 8.76 ± 0.26 a.u., p<0.001; main effect of genotype, F(1,34) = 69.96, p<0.001; Figure 1H) in their peak contrast sensitivity compared to their wild-type counterparts. In contrast to the nob and Nyxwt/wt mice, the peak contrast sensitivity for Vsx1+/+ and Vsx1−/− mice decreased with age (main effect of age, F(1,34) = 46.06, p<0.001). As expected, when comparing the normalized peak contrast sensitivity deficits between nob and Vsx1−/− mice, nob mice demonstrated more significant reductions at both 4 and 8 weeks post-natal (4 weeks: 0.39 ± 0.02 vs. 0.82 ± 0.03 normalized a.u., p<0.001; 8 weeks: 0.39 ± 0.02 vs. 0.71 ± 0.02 normalized a.u., p<0.001; Figure 1I).
Despite significant changes in visual function, retinal dopamine levels were not significantly altered when comparing each mutant strain to their wild-type controls (main effect of genotype: nob, F(1,33)=3.961, p=0.05, Figure 2A; Vsx1−/−, F(1,34)=3.499, p=0.07, Figure 2B). Interestingly however, at 4 weeks post-natal, there was on average a 10% reduction in retinal dopamine in nob mice and a 10% increase in retinal dopamine in Vsx1−/− mice. When retinal dopamine levels for each mutant strain were normalized to their wild-type strains, we found that Vsx1−/− mice had significantly higher dopamine levels than nob mice (0.926 ± 0.043 vs. 1.039 ± 0.084 normalized pg/mg retinal protein, p=0.20; main effect of genotype, F(1,32) = 15.94, p<0.001; age by genotype interaction, F(1,32) = 4.544, p=0.04; Figure 2C). Additionally, both strains showed a significant reduction with age (main effect of age: nob, F(1,33) = 38.21, p<0.001; Vsx1−/−, F(1,34) = 6.233, p=0.02; Figure 2A–B).
Figure 2.
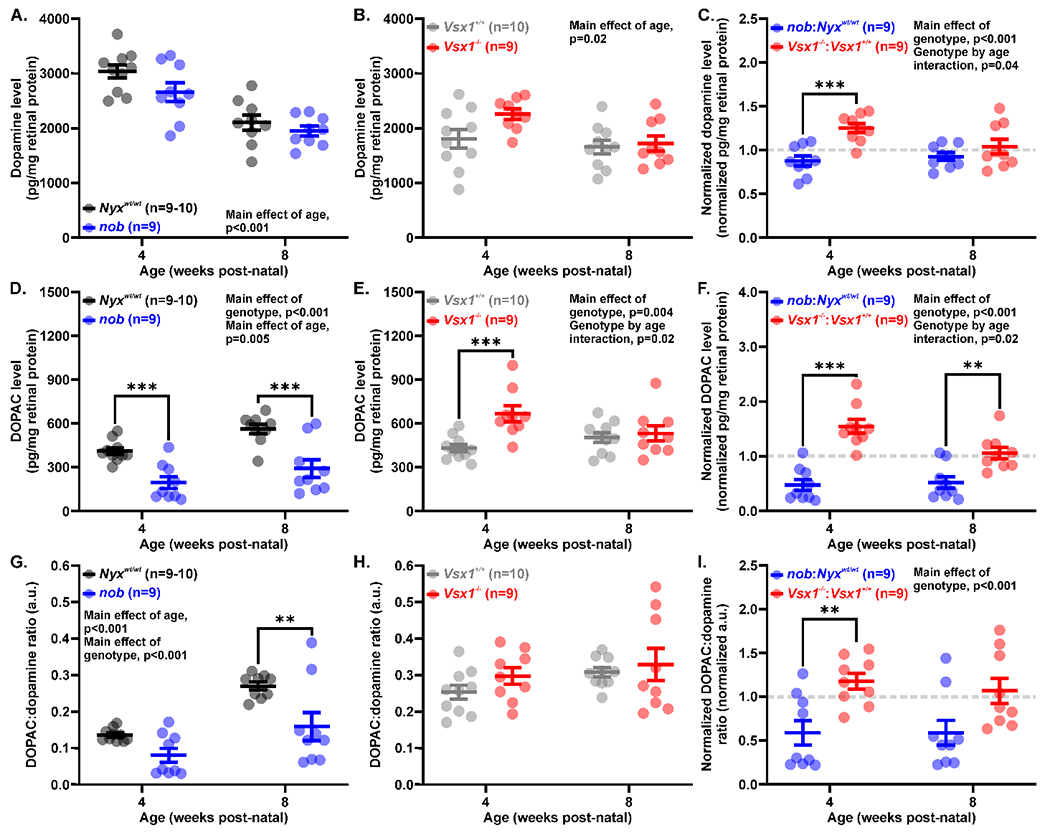
Greater modulation in the retinal dopaminergic system in nob than Vsx1−/− mice. (A-B) Retinal dopamine levels measured with HPLC in (A) nob (blue) and Nyxwt/wt mice (black) as well as (B) Vsx1−/− (red) and Vsx1+/+ mice (gray). Retinal dopamine levels were unchanged in both genotypes, but both Nyxwt/wt and nob mice had reduced dopamine with age. (C) Dopamine levels normalized to wild-type average dopamine levels at each age. nob mice had significantly less retinal dopamine at 4 weeks post-natal than Vsx1−/− mice. (D-E) Retinal DOPAC levels measured in (D) nob and (E) Vsx1−/− mice as well as their wild-type counterparts. nob mice had reduced DOPAC levels at both ages, while Vsx1−/− mice had increased DOPAC at 4 but not 8 weeks post-natal. (F) Normalized DOPAC levels at 4 and 8 weeks post-natal. nob mice had reduced DOPAC levels compared to Vsx1−/− mice. (G-H) Ratio of DOPAC to dopamine levels, an indirect measurement of dopamine turnover, in (G) nob and (H) Vsx1−/− mice. nob mice had reduced dopamine turnover but Vsx1−/− mice did not. (I) Normalized DOPAC:dopamine ratio. Dopamine turnover was significantly more affected in nob than Vsx1−/− mice. Data are mean ± SEM and significance is *p<0.05, **p<0.01, and ***p<0.001. Comparisons were performed with two-way ANOVA (A-I) with Holm-Sidak multiple comparisons tests.
In contrast to dopamine levels, mice with ON and OFF pathway dysfunction exhibited significantly altered retinal DOPAC levels. Specifically, nob mice had significantly reduced DOPAC levels at both 4 and 8 weeks post-natal (main effect of genotype, F(1,33) = 35.59, p<0.001; main effect of age, F(1,33)=9.170, p=0.005; Figure 2D). However, Vsx1−/− mice had an increase in retinal DOPAC at 4 weeks (665 ± 54.7 vs. 430 ± 25.8 pg/mg retinal protein, p<0.001) that was not present at 8 weeks post-natal (532 ± 52.2 vs. 503 ± 34.1 pg/mg retinal protein, p=0.63; main effect of genotype, F(1,34) = 9.748, p=0.004; age by genotype interaction, F(1,34) = 5.919, p=0.02; Figure 2E). This opposite effect of ON and OFF pathway dysfunction on retinal DOPAC levels can be further demonstrated when DOPAC levels are normalized to their wild-type strains and compared. At 4 weeks post-natal, nob mice had a 53% reduction while Vsx1−/− mice had a 55% increase in retinal DOPAC level (0.475 ± 0.101 vs. 1.546 ± 0.127 normalized pg/mg retinal protein, p<0.001). At 8 weeks post-natal, nob mice had a similar change in DOPAC but Vsx1−/− mice were similar to wild-type controls (0.518 ± 0.106 vs. 1.058 ± 0.104 normalized pg/mg retinal protein, p=0.001; main effect of genotype, F(1,32) = 53.69, p<0.001; age by genotype interaction, F(1,32) = 5.834, p=0.02; Figure 2F).
Dopamine turnover, calculated as the ratio of DOPAC to dopamine levels, was significantly reduced in nob mice (main effect of genotype, F(1,33) = 14.65, p<0.001). Both nob and Nyxwt/wt mice experienced an age-dependent increase in dopamine turnover from 4 to 8 weeks post-natal (main effect of age, F(1,33) = 24.07, p<0.001; Figure 2G). Vsx1−/− and Vsx1+/+ mice showed no change in dopamine turnover with genotype (p=0.22) or age (p=0.11; Figure 2H). When normalized to wild-type strains, nob mice across both timepoints had a ~40% reduction in dopamine turnover, which was significantly reduced compared to Vsx1−/− mice which showed no change (main effect of genotype, F(1,32) = 16.52, p<0.001; Figure 2I).
In this study we found that asymmetries between the ON and OFF pathways persist in functional visual behavior as measured by differences in OMR thresholds between nob and Vsx1−/− strains that have dysfunctional ON and OFF pathways, respectively. Consistent with our hypothesis, we found that nob mice had significantly greater reductions in spatial acuity and more difficulty perceiving low contrast stimuli when compared to Vsx1−/− mice. The differences in visual tracking reflexes in mice with ON or partial OFF pathway deficits could be related to alterations in retinal dopaminergic system as nob mouse retinas also contained lower levels of dopamine and DOPAC compared to Vsx1−/− mice.
The primary pathway for the OMR involves retinal signaling and the AOS. Visual signals route through ON direction-selective retinal ganglion cells (ON DS RGCs) to the nuclei of optic tract (NOT) and dorsal terminal nucleus (DTN) (Dhande et al., 2013; Oyster et al., 1972). Despite the significant reductions in OMR that we discovered in nob mice, these mice can still see, as can patients with CSNB (Bijveld et al., 2013; Dryja et al., 2005). Our results are also consistent with previous findings measuring optokinetic responses in nob mice (Winkelman et al., 2019) and in both optokinetic and optomotor measurements in other ON-pathway mutant mice (Iwakabe et al., 1997; Orhan et al., 2021; Pinto et al., 2007; Sugita et al., 2013). However, the OMR can also be driven by ON-OFF direction-selective retinal ganglion cells (ON-OFF DS RGCs) (Dunn and Wong, 2014). ON-OFF DS RGCs are tuned to respond optimally to higher stimulus speeds (~25 deg/s) whereas ON DS RGCs are tuned to slower speeds (~1-2 deg/s) (Sun et al., 2006; Weng et al., 2005). The balance between DS RGC inputs to the OMR is thought to shift with stimulus speed, and our testing speed of 12 deg/s, the optimal speed for the OMR in wild-type mice (Prusky et al., 2004; Umino et al., 2008), likely elicits mixed contribution from both cell types (Kretschmer et al., 2017). The large effect on the OMR in nob mice using our slower stimulus speed also aligns with the ON pathway preferred cortical stimulus of slow motion (Joesch et al., 2010; Luo-Li et al., 2018; Mazade et al., 2019). Importantly, projections from visual cortex to the AOS modulate the optokinetic reflex in mice (Liu et al., 2016) and nearly half of the optokinetic nystagmus cells in the rat NOT-DTN receive direct input from the visual cortex (Schmidt et al., 1993). Therefore, changes in retinal output to higher visual centers in nob mice are likely similar to other mouse models of ON pathway dysfunction (Sarnaik et al., 2014), which could amplify the deficits seen in nob mice OMRs.
Additionally, we found slight but significant OMR reductions in Vsx1−/− mice. Due to the partial and non-specific pathway loss in Vsx1−/− mice, our results could be explained by the partial loss of OFF pathway input to ON-OFF DS RGCs or by the loss of type 7 ON bipolar cell input to ON or ON-OFF DS RGCs (Shi et al., 2011). However, it is important to note that the Vsx1 gene affects 60-70% of cone bipolar cells, and more than half of all OFF bipolar cell subtypes (Chow et al., 2004; Shi et al., 2012; Shi et al., 2011). Vsx1−/− mice have significantly reduced, but still present, OFF ganglion cell responses with more sustained ON ganglion cell responses (Chow et al., 2004; Shi et al., 2011) as well as reduced ERG b-wave amplitudes (Ohtoshi et al., 2004). Despite such compromised electrophysiologic OFF ganglion cell responses in Vsx1−/− mice, we only saw a modest reduction in contrast sensitivity and almost no change in spatial frequency thresholds. One interpretation of our result is that remaining OFF pathway input to ON-OFF direction-selective cells is sufficient to drive an OMR, and thus inputs from the dysfunctional OFF pathways are sufficient but not necessary for normal OMR. An alternative interpretation is that the loss of type 7 ON bipolar cells led to the deficits in the OMR. However, these cells only comprise a small portion of all ON bipolar inputs to ON DS RGCs (Matsumoto et al., 2019) and this loss was not sufficient to elicit the magnitude of deficits seen in nob mice. Teasing these mechanisms apart would be an interesting future study. Overall, we conclude that ON more than OFF pathways are vital for visual reflexes due to their connections to the AOS.
In addition to deficits in spatial acuity and contrast sensitivity, we found reduced levels of DOPAC in nob but not Vsx1−/− mice, replicating our earlier work (Chakraborty et al., 2014; Pardue et al., 2008). Previous studies have suggested associations between the OMR and dopamine signaling in wild-type (Jackson et al., 2012) and diabetic rodents (Aung et al., 2014). Dopamine signaling is driven by ON pathways and modulates visual receptive fields which could have pronounced consequences for visual tracking (Contini et al., 2010; Witkovsky, 2004). In this study, we found that genetic disruption of the ON pathway has pronounced effects on both the OMR and dopamine signaling, whereas partial disurption of the OFF pathway does not. If dopamine signaling is disrupted in nob mice, then it could be one mechanism to partly explain the reductions in contrast sensitivity and visual acuity that we observed. For example, low levels of dopamine signaling could result in (1) reduced D1 receptor activation causing lower spatial frequency thresholds (e.g. through increased horizontal cell coupling), and (2) decreased D4 receptor activation leading to lower contrast sensitivity (e.g. through reduced photoreceptor coupling) (Jackson et al., 2012). Interestingly, Vsx1−/− mice were found to have normal or higher than normal retinal dopamine and DOPAC levels, which could attenuate any effect on contrast sensitivity and spatial frequency thresholds. However, we have not demonstrated or assessed causation between dopamine signaling and OMR in either of these mutant strains, and further work using genetic models and pharmacologic treatment paradigms is crucial to understand the interplay between ON and OFF pathways, retinal dopamine, and visual reflexes.
Overall, we demonstrate that the ON pathway is necessary for normal visual reflex behavior, whereas mice with partial dysfunction of some OFF pathways still had near-normal visual reflexes. Furthermore, while we find a reduction in retinal dopamine signaling only in nob mice, the link between visual reflexes and dopamine in nob and Vsx1−/− mice still needs to be tested directly to determine a causal link. Our findings support the importance of the ON pathway for non-image forming visual functions. Nob mice, as well as mice with null mutation in the metabotropic glutamate receptor 6 required for ON pathway activation, exhibit increased myopia susceptibility after form-deprivation (Chakraborty et al., 2015; Pardue et al., 2008) and it is becoming increasingly clear that the ON pathway plays a direct role in visual function, eye growth, and refractive development. Therefore, it is important to fully characterize mutant mouse models that have disrupted visual pathways which may provide potential targets for visual therapies.
Highlights.
Optomotor responses are more affected in nob than Vsx1−/− mice
Contrast sensitivity is affected more by pathway dysfunction than spatial frequency
Retinal dopamine signaling is reduced in nob more than Vsx1−/− mice
Partial OFF dysfunction has minimal effects on optomotor responses
ON pathways are necessary for visual reflex behavior and retinal dopamine signaling
Funding:
This project was supported by the National Institutes of Health [NIH R01 EY016435 (MTP), NIH R01 EY004864 and NIH R01 EY027711 (PMI), NIH P30 EY006360], Department of Veterans Affairs [Rehabilitation R&D Service Research Career Scientist Award IK6 RX003134 (MTP)], and Research to Prevent Blindness [Departmental Award]. The funding organizations had no role in the design or conducting of this research.
Footnotes
Disclosures: MH Aung, None; K Hogan, None; RE Mazade, None; H Park, None; CS Sidhu, None; PM Iuvone, None; MT Pardue, None
References
- Aung MH, Park H, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, Iuvone PM, Pardue MT, 2014. Doapmine Deficiency Contributes to Early Visual Dysfunction in a Rodent Model of Type 1 Diabetes. J Neurosci 34, 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijveld MM, van Genderen MM, Hoeben FP, Katzin AA, van Nispen RM, Riemslag FC, Kappers AM, 2013. Assessment of night vision problems in patients with congenital stationary night blindness. PLoS One 8, e62927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisceglia L, Ciaschetti M, De Bonis P, Campo PAP, Pizzicoli C, Scala C, Grifa M, Ciavarella P, Noci ND, Vaira F, Macaluso C, Zelante L, 2005. VSX1 Mutational Analysis in a Series of Italian Patients Affected by Keratoconus: Detection of a Novel Mutation. Investigative Ophthalmology & Visual Science 46, 39–45. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Park HN, Hanif AM, Sidhu CS, Iuvone PM, Pardue MT, 2015. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res 137, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky EJ, Kalmar RS, 2002. Functional Asymmetries in ON and OFF Ganglion Cells of Primate Retina. J Neurosci 22, 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Volgyi B, Szilard RK, Ng D, McKerlie C, Bloomfield SA, Birch DG, McInnes RR, 2004. Control of late off-center cone bipolar cell differentiation and late signaling by the homeobox gene Vsx1. Proc Natl Acad Sci 101, 1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini M, Lin B, Kobayashi K, Okano H, Masland RH, Raviola E, 2010. Synaptic input of ON-bipolar cells onto the dopaminergic neurons of the mouse retina. The Journal of comparative neurology 518, 2035–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Estevez ME, Quattrochi LE, El-Danaf RN, Nguyen PL, Berson DM, Huberman AD, 2013. Genetic dissection of retinal input to brainstem nuclei controlling image stabilization. J Neurosci 33, 17797–17813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT, 2005. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci 22, 677–684. [DOI] [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Berson EL, Fishman GA, Sandberg MA, Alexander KR, Derlacki DJ, Rajagopalan AS, 2005. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci U S A 102, 4884–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, Berson DM, 2009. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. The Journal of comparative neurology 517, 226–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Wong ROL, 2014. Wiring patterns in the mouse retina: collecting evidence across the connectome, physiology and light microscopy. J Physiol 592, 4809–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Rihel J, Adolph AR, Wong KY, Kraves S, Dowling JE, 2007. OFF ganglion cells cannot drive the optokinetic reflex in zebrafish. Proc Natl Acad Sci U S A 104, 19126–19131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, Vessey KA, McCall MA, 2007. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol 98, 3023–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Mukhopadhyay S, Candille SI, Ball SL, Pardue MT, McCall MA, Peachey NS, 2003. Identification of the gene and the mutation responsible for the mouse nob phenotype. Invest Ophthalmol Vis Sci 44, 378–384. [DOI] [PubMed] [Google Scholar]
- Iwakabe H, Katsuura G, Ishibashi C, Nakanishi S, 1997. Impairment of pupillary responses and optokinetic nystagmus in the mGluR6-deficient mouse. Neuropharmacology 36, 135–143. [DOI] [PubMed] [Google Scholar]
- Jackson CR, Ruan G, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG, 2012. Retinal Dopamine Mediates Multiple Dimensions of Light-Adapted Vision. J Neurosci 32, 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesch M, Schnell B, Raghu SV, Reiff DF, Borst A, 2010. ON and OFF pathways in Drosophila motion vision. Nature 468, 300–304. [DOI] [PubMed] [Google Scholar]
- Kremkow J, Jin J, Komban SJ, Wang Y, Lashgari R, Li X, Jensen M, Zaidi Q, Alonso J, 2014. Neuronal nonlinearity explains greater visual spatial resolution for darks than lights. Proc Natl Acad Sci 111, 3170–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer F, Tarqiq M, Chatila W, Wu B, Badea TC, 2017. Comparison of optomotor and optokinetic reflexes in mice. J Neurophysiol 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, 1953. Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16, 37–68. [DOI] [PubMed] [Google Scholar]
- Liu BH, Huberman AD, Scanziani M, 2016. Cortico-fugal output from visual cortex promotes plasticity of innate motor behaviour. Nature 538, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ, 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193, 265–275. [PubMed] [Google Scholar]
- Luo-Li G, Mazade R, Zaidi Q, Alonso JM, Freeman AW, 2018. Motion changes response balance between ON and OFF visual pathways. Commun Biol 1, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald IM, Hoang S, Tuupanen S, 2008. Congential Stationary Night Blindness, GeneReviews. University of Washington, Seattle, WA. [PubMed] [Google Scholar]
- Matsumoto A, Briggman KL, Yonehara K, 2019. Spatiotemporally Asymmetric Excitation Supports Mammalian Retinal Motion Sensitivity. Curr Biol 29, 3277–3288 e3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazade R, Jin J, Pons C, Alonso J, 2019. Functional Specialization of ON and OFF Cortical Pathways for Global-Slow and Local-Fast Vision. Cell Rep 27, 2881–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Ren G, Akileswaran L, 2006. Localization of nyctalopin in the mammalian retina. Eur J Neurosci 24, 1664–1674. [DOI] [PubMed] [Google Scholar]
- Ohtoshi A, Wang SW, Maeda H, Saszik SM, Frishman LJ, Klein WH, Behringer RR, 2004. Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr Biol 14, 530–536. [DOI] [PubMed] [Google Scholar]
- Orhan E, Neuille M, de Sousa Dias M, Pugliese T, Michiels C, Condroyer C, Antonio A, Sahel JA, Audo I, Zeitz C, 2021. A New Mouse Model for Complete Congenital Stationary Night Blindness Due to Gpr179 Deficiency. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyster CW, Takahashi E, Collewijn H, 1972. Direction-selective retinal ganglion cells and control of optokinetic nystagmus in the rabbit. Vision Res 12, 183–193. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, WIlliams RW, Pozdeyev K, Iuvone PM, 2008. High susceptibility to experimental myopia in a mouse model with a retinal ON pathway defect. Invest Ophthalmol Vis Sci 49, 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS, 1998. A Naturally Occurring Mouse Model of X-Linked Congenital Stationary Night Blindness. Invest Ophthalmol Vis Sci 39, 2443–2449d. [PubMed] [Google Scholar]
- Pinto LH, Vitaterna MH, Shimomura K, Siepka SM, Balannik V, McDearmon EL, Omura C, Lumayag S, Invergo BM, Glawe B, Cantrell DR, Inayat S, Olvera MA, Vessey KA, McCall MA, Maddox D, Morgans CW, Young B, Pletcher MT, Mullins RF, Troy JB, Takahashi JS, 2007. Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis Neurosci 24, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM, 2004. Rapid Quantification of Adult and Developing Mouse Spatial Vision Using a Virtual Optomotor System. Invest Ophthalmol Vis Sci 45, 4611–4616. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Douglas RM, 2006. Enhancement of vision by monocular deprivation in adult mice. J Neurosci 26, 11554–11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao SN, Zhang Z, Ribelayga CP, Zhong YM, Zhang DQ, 2016. Multiple cone pathways are involved in photic regulation of retinal dopamine. Sci Rep 6, 28916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M, Roy M, Barsoum-Homsy M, Chevrette L, Jacob J, Milot J, 1996. On- and off-responses in the photopic electroretinogram in complete-type congenital stationary night blindness. Doc Ophthalmol 92, 159–165. [DOI] [PubMed] [Google Scholar]
- Sarnaik R, Chen H, Liu X, Cang J, 2014. Genetic disruption of the On visual pathway affects cortical orientation selectivity and contrast sensitivity in mice. J Neurophysiol 111, 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Zhang H-Y, Hoffman K-P, 1993. OKN-Related Neurons in the Rat Nucleus of the Optic Tract and Dorsal Terminal Nucleus of the Accessory Optic System Receive a Direct Cortical Input. The Journal of comparative neurology 330, 147–157. [DOI] [PubMed] [Google Scholar]
- Shi Z, Jervis D, Nickerson PE, Chow RL, 2012. Requirement for the paired-like homeodomain transcription factor VSX1 in type 3a mouse retinal bipolar cell terminal differentiation. The Journal of comparative neurology 520, 117–129. [DOI] [PubMed] [Google Scholar]
- Shi Z, Trenholm S, Zhu M, Buddingh S, Star EN, Awatramani GB, Chow RL, 2011. Vsx1 Regulates Terminal Differentiation of Type 7 ON Bipolar Cells. J Neurosci 37, 13118–13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y, Miura K, Araki F, Furukawa T, Kawano K, 2013. Contributions of retinal direction-selective ganglion cells to optokinetic responses in mice. Eur J Neurosci 38, 2823–2831. [DOI] [PubMed] [Google Scholar]
- Sun LO, Brady CM, Cahill H, Al-Khindi T, Sakuta H, Dhande OS, Noda M, Huberman AD, Nathans J, Kolodkin AL, 2015. Functional assembly of accessory optic system circuitry critical for compensatory eye movements. Neuron 86, 971–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Deng Q, Levick WR, He S, 2006. ON direction-selective ganglion cells in the the mouse retina. J Physiol 576, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umino Y, Solessio E, Barlow RB, 2008. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J Neurosci 28, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S, Sun W, He S, 2005. Identification of ON-OFF direction-selective ganglion cells in the mouse retina. J Physiol 562, 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman BHJ, Howlett HC, Hölzel M, Joling C, Fransen KH, Pangeni G, Kamermans S, Sakuta H, Noda M, Simonsz HJ, McCall MA, De Zeeuw CI, Kamermans M, 2019. Nystagmus in patients with congenital stationary night blindness (CSNB) originates from synchronously firing retinal ganglion cells. PLoS Biol 17, e3000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, 2004. Dopamine and retinal function. Doc Ophthalmol 108, 17–40. [DOI] [PubMed] [Google Scholar]
- Zaghoul KA, Boahen K, Demb JB, 2003. Different Circuits for ON and OFF Retinal Ganglion Cells Cause Different Contrast Sensitivities. J Neurosci 23, 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Pardue MT, Iuvone PM, Qu J, 2017. Dopamine signaling and myopia development: What are the key challenges. Prog Retin Eye Res 61, 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


