Abstract
Fibrolamellar hepatocellular carcinoma (FLC) is a rare primary liver cancer that affects primarily adolescents and young adults. It is associated with a poor overall prognosis. There is a need to better define risk factors, but small sample size has limited such studies. An FLC patient registry now provides data sufficient for statistically robust inferences. We leveraged a unique patient community–based FLC registry to analyze the prognostic impact of demographic and clinical characteristics evident at diagnosis. Variables were analyzed using Cox proportional hazards regression to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). In multivariable models of 149 patients (88 females and 61 males), female gender was associated with statistically significant improved survival with HR of 0.52 (95% CI 0.29–0.93). Factors evident at diagnosis that are associated with worse survival included the presence of 10 or more tumors within the liver (HR 7.1; 95% CI 2.4–21.04), and metastases at diagnosis (HR 2.17; 95% CI 1.19–3.94). Positive lymph nodes at diagnosis, despite being found significantly associated with worse survival in a univariate analysis, did not remain significant when adjusted for covariates in a multivariable analysis. We found no statistically significant effect of age at diagnosis nor tumor size at diagnosis on survival. Female gender may confer a favorable prognosis in FLC. Established high‐risk prognostic factors that we confirmed in this Registry included the diagnostic presence of numerous intrahepatic tumors, and metastases. This is the first study derived from a FLC patient community–based registry, and highlights how registries of rare tumors can empower patients to meaningfully advance clinical and scientific discoveries.
INTRODUCTION
Fibrolamellar hepatocellular carcinoma (FLC) is a liver cancer affecting children and young adults. The reported overall 5‐year survival rates are 33.6%–42.6%.[ 1 , 2 ] Surgery remains the cornerstone of therapy, and there is no approved systemic therapy. FLC is classified as a subtype of noncirrhotic hepatocellular carcinoma (HCC)[ 3 ] and is treated similarly, although FLC has clinical, pathological, and molecular features distinct from HCC.
The transcriptome, proteome,[ 4 ] and the preclinical drug response profile of FLC[ 5 ] are consistent across patients and distinct from HCC. On a molecular level, all patients with FLC have a dysregulation of protein kinase A. A somatic deletion produces a gene fusion of DNAJB1, a heatshock protein cofactor, to PRKACA, the catalytic subunit α of protein kinase A (PKA).[ 6 ] For liver tumors with hepatocellular differentiation, this fusion is pathognomonic for FLC.[ 7 ] Expression of this gene fusion in mouse liver is sufficient to produce tumors that recapitulate the histology and molecular changes of human FLC.[ 8 , 9 ] In a few cases the PKA pathway can be dysregulated by another mechanism: 3 patients have mutations in a regulatory subunit of PKA.[ 10 ]
There is no consensus as to the prognostic factors associated with patient survival.[ 2 , 11 , 12 , 13 ] Risk‐stratification models for patients with FLC are modeled after those for HCC.[ 14 ] Given that FLC is distinct from HCC, we hypothesized that defining prognostically relevant risk factors at diagnosis will improve risk stratification.
The fibrolamellar patient community has been collecting clinical data in a patient‐run registry (http://fibroregistry.org). The data are provided through self‐reported answers to surveys by patients or family members and, when available, validated by cross‐referencing hospital medical records. Using these data, we tested for associations between demographic and clinical factors and overall survival.
METHODS
Data source
The Fibrolamellar Registry is an international, community‐based, non‐profit launched in October 2016. The Registry is approved by the Genetic Alliance Institutional Review Board and belongs to the REDCap Consortium. The data are de‐identified of specific patient factors and are in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
The Fibrolamellar Registry enrolls patients with a clinical diagnosis of FLC. Participants first answer the main survey containing demographic questions (e.g., gender, race/ethnicity, date of birth) as well as clinical questions (e.g., medical history before diagnosis, age at diagnosis, symptoms of the disease, treatments, complications). Then, subgroups of patients may answer additional surveys depending on treatments they had received (i.e., surgery, immunotherapy, chemotherapy) or cases of complications (i.e., hyperammonemia, a rare but potentially lethal consequence of FLC).
The data in the registry were provided by the patients themselves (77, 51.6% of entries), by family members of living patients (34, 22.8%), and by family members of deceased patients (38, 25.5%). For 23% of the patients, the registry has received medical records and a clinical oncology nurse who, when applicable, checked the patient responses against the medical records.
Study design
In this retrospective cohort study, we included patients with a diagnosis of FLC who joined the Fibrolamellar Registry from its initiation in October 2016 to January 2021. Survival status was followed through direct contact with members and their family and, if they could not be ascertained, patient data were excluded from analysis.
The demographic and clinical characteristics at diagnosis of these patients were analyzed for their frequencies and for their association with survival in unadjusted univariate analysis. Variables found significant were included in a multivariable analysis. The variables measured were gender, race/ethnicity, and, at the time of diagnosis, the patient's age, tumor size, lymph node involvement, and metastasis status. A separate analysis evaluated the association between treatment modalities (surgery only, surgical and systemic therapy, systemic therapy only, and no therapy) and survival. Survival was defined as the time from diagnosis with FLC until death or the end of the follow‐up period (April 2021).
Statistical analysis
Kaplan–Meier survival plots[ 15 ] were created for each group. The log‐rank test[ 16 ] was used to assess statistical differences in survival among categories. Variables significantly associated with survival in the univariable analysis were included in a multivariable Cox proportional hazards model[ 17 ] to assess hazard ratios (HRs) for risk of death at any time among categories. p‐Values < 0.05 were considered statistically significant. Because data were not provided fully for all of the variables examined, we analyzed the data in a complete case approach.
Tools
The survival analysis and the Kaplan‐Meier and Cox proportional hazard plots were generated using R Software v4.0.3 and Rstudio v1.4.1103, with packages survival v3.2.11 (https://CRAN.R‐project.org/package=survival) and survminer v0.4.9 (https://CRAN.R‐project.org/package=survminer). Some plots were created using Microsoft Excel v16.4.
RESULTS
Study population
As of January 2021, the Fibrolamellar Registry included data from 171 patients. We analyzed 149 records with complete survival data. Demographic characteristics, clinical presentations, and treatments are summarized in Table 1.
TABLE 1.
Demographic and clinical characteristics of patients with FLC
| Characteristics | Total (n = 149) |
|---|---|
| Age at diagnosis, n (%) | |
| ≤10 years | 4 (2.7%) |
| 10–20 years | 52 (34.9%) |
| 20–30 years | 68 (45.6%) |
| 30–40 years | 17 (11.4%) |
| 40–50 years | 5 (3.4%) |
| 50–60 years | 2 (1.3%) |
| >60 years | 1 (0.7%) |
| Median (years) | 22.4 |
| Gender, n (%) | |
| Male | 61 (40.9%) |
| Female | 88 (59.1%) |
| Race/ethnicity, n (%) | |
| Non‐Hispanic White | 133 (89.3%) |
| Non‐Hispanic Black | 1 (0.7%) |
| Hispanic | 8 (5.4%) |
| Asian | 4 (2.7%) |
| Mixed | 3 (2%) |
| Number of tumors within the liver, n = 133 | |
| 1 | 113 (76.8%) |
| 2–5 | 15 (10.1%) |
| 6–9 | 0 |
| ≥10 | 5 (3.4%) |
| Tumor size at diagnosis, n = 129 | |
| 0–5 | 12 (9.3%) |
| 6–10 | 56 (43.4%) |
| 11–15 | 39 (30.2%) |
| 16–20 | 12 (9.3%) |
| ≥21 | 10 (7.8%) |
| Median | 10 cm |
| Lymph node involvement, n = 146 | 48 (32.9%) |
| Metastases at diagnosis, n = 146 | 41 (28.1%) |
| Treatment modality, n = 143 | |
| Surgery alone | 54 (37.8%) |
| Surgery & systemic therapy | 67 (46.9%) |
| Systemic therapy alone | 16 (11.2%) |
| Neither surgery or systemic therapy | 6 (4.2%) |
Abbreviation: FLC, fibrolamellar hepatocellular carcinoma.
Most patients were female (59%). The median age at diagnosis was 22.4 years, with 37.6% of the patients diagnosed under the age of 20. Most participants (133 of 149) were non‐Hispanic White. Due to the limited numbers in race/ethnicity categories, we did not perform survival analysis by that variable.
The median primary tumor size at diagnosis was 10 cm (interquartile 9–14 cm). There were positive lymph nodes and metastases at diagnosis in 32.9% and 28.1% of patients (n = 146), respectively. Sites outside of the liver were in order of prevalence: lungs, gallbladder, stomach, diaphragm, portal vein, peritoneum, inferior vena cava, small intestine, abdominal cavity, adrenal glands, brain, chest cavity, colon, omentum, ovaries, retroperitoneum, and spine (most received surgery [84.6%] and 58% reported receiving any systemic therapy [n = 143]).
Most patients in the registry (57%, n = 154) were seen for diagnosis and/or treatment, at two or more different facilities. For diagnosis alone, 44% visited two or more facilities for a second opinion on their diagnosis.
Overall survival
As of April 2021, 65 (43%) patients had died. The median survival was 8.2 years, and the 5‐year survival rate was 62.2% (54.4–71.2) (Figure 1). The median follow‐up time for living patients was 6 years. The latest death was 13.7 years after diagnosis, with no deaths for the 19 patients who survived longer. The longest time from diagnosis to our follow‐up was 35.1 years.
FIGURE 1.
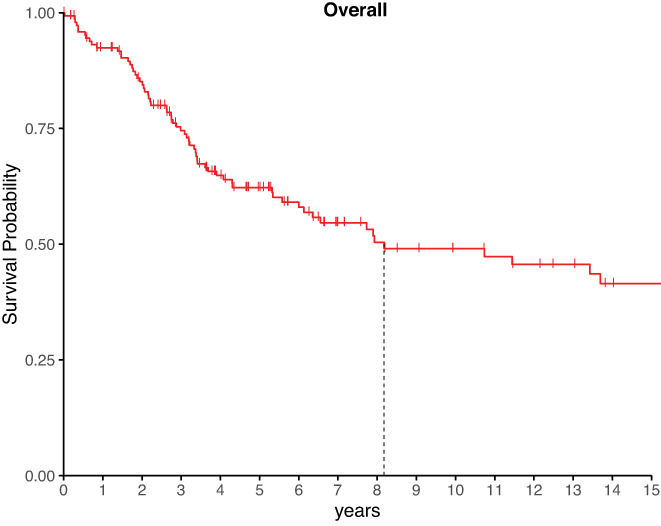
Kaplan–Meier curve of survival of the participants. Verticle notches mark the most recent follow‐up for patients who are censored from the plot for subsequent times. A vertical line marks when survival dropped below 50%.
Univariate survival analysis
The univariate analysis of factors at diagnosis is found in Table 2. The log‐rank test found no significant survival difference between those diagnosed older or younger than 22 years old (p = 0.43), the rounded median age of patient diagnosis (Figure 2A).
TABLE 2.
Univariable analysis for patients with FLC included in this study
| Variables | No. of patients | Five‐year survival (95% CI) | p (log‐rank) |
|---|---|---|---|
| Age | 0.43 | ||
| <22 years | 71 | 57.1% (45.9%–71.0%) | |
| ≥22 years | 78 | 66.8% (56.5%–79.0%) | |
| Gender | 0.018 | ||
| Male | 61 | 50.7% (38.6%–66.5%) | |
| Female | 88 | 69.9% (60.4%–80.8%) | |
| Number of tumors inside the liver | <0.01 | ||
| 1 | 114 | 68.7% (60.1%–78.6%) | |
| 2–5 | 15 | 54.5% (33.2%–89.5%) | |
| 6–9 | 0 | N/A | |
| ≥10 | 5 | 0 | |
| Tumor size | 0.44 | ||
| <10 cm | 38 | 68.7% (54.2%–87.0%) | |
| ≥10 cm | 91 | 58.9% (48.9%–70.9%) | |
| Lymph node involvement | <0.01 | ||
| Negative | 98 | 67.7% (58.6%–78.2%) | |
| Positive | 48 | 49.9% (36.2%–68.7%) | |
| Metastases | <0.01 | ||
| Negative | 105 | 69.6% (60.7%–79.7%) | |
| Positive | 41 | 44.3% (30.8%–63.7%) | |
| Treatment modality | <0.0001 | ||
| Surgery only | 54 | 87.3% (78.2%–97.4%) | |
| Surgery & systemic therapy | 67 | 54.3% (43.0%–68.5%) | |
| Systemic therapy only | 16 | 25.4% (10.2%–62.9%) | |
| Neither surgery nor systemic therapy | 6 | N/A |
Abbreviations: CI, confidence interval; N/A, not applicable.
FIGURE 2.
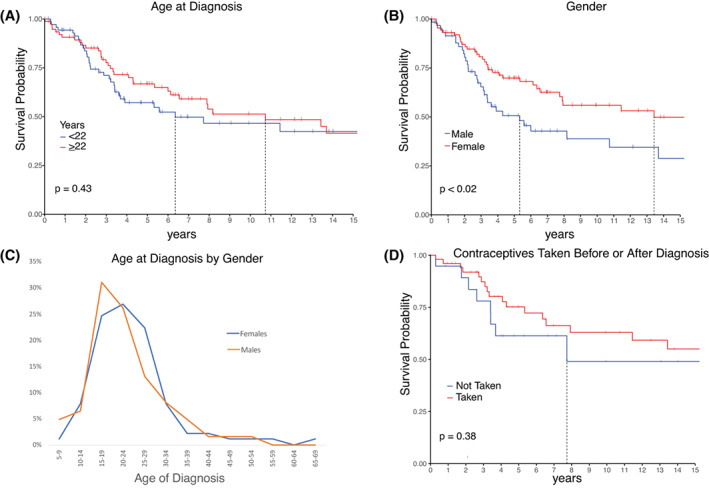
(A) Kaplan–Meier curve of survival by age at diagnosis. (B) Kaplan–Meier curve of survival by gender. (C) Comparison of ages at diagnosis according to gender. (D) Kaplan–Meier curve of survival by use of contraceptives either before or after diagnosis. Vertical notches mark the most recent follow‐up for patients who are censored from the plot for subsequent times. A vertical line marks when survival dropped below 50%.
Female patients had a better prognosis than males (log‐rank test p = 0.018; Figure 2B). The 5‐year survival for females was 69.9% (60.4–80.8) and 50.7% (38.6–66.5) for males. Median overall survival for males was 5.33 years, and 13.43 years for females. There was no statistical (two‐sample t test p = 0.39) gender‐based difference in the age (Figure 2C) nor the stage at diagnosis (25 of 32 females and 17 of 25 males had stage IV).
Because of the marked difference in survival between males and females, we assessed for association between survival and contraceptive use before or after diagnosis. Among the 71 female patients who gave information, no significant difference in survival was found among those who had (n = 52) and those who had not (n = 19) been taking contraceptives. Those who had been taking contraceptives had a 5‐year survival of 75.2% (63.4–89.2), and those who had not, had a 5‐year survival of 61.3% (42.5–88.5) (log‐rank test p = 0.38; Figure 2D).
A greater number of tumors within the liver was associated with poorer survival (Figure 3A). Patients had 5‐year survival of 68.7% (60.1–78.6) with a single tumor, 54.5% (33.2–89.5) with two to five tumors, while no patients with ≥10 tumors survived 5 years. The populations with one or ≥10 tumors were distinct in their survival (log‐rank test p < 0.003). No patients reported six to nine tumors.
FIGURE 3.
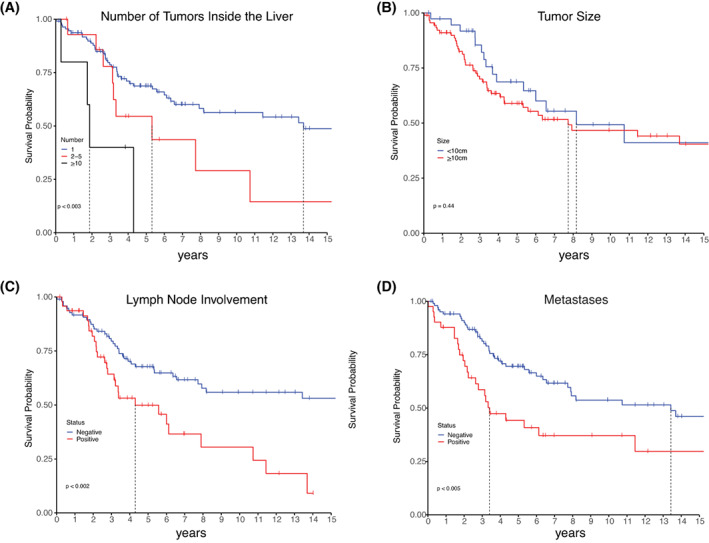
Kaplan‐Meier curves of survival by (A) Number of tumors in the liver; (B) Tumor size; (C) Involvement of lymph nodes; (D) Presence of metastases. All values were determined at time of diagnosis. Vertical notches mark the most recent follow‐up for patients who are censored from the plot for subsequent times. A vertical line marks when survival dropped below 50%.
Survival was assessed by size of the tumor at diagnosis (Figure 3B). No significant difference in survival was found between patients with tumors greater than or less than the median size, 10 cm (log‐rank test p = 0.44). Further testing did not find significance at any size cutoff. The Cox hazard test using size as a continuous variable had an HR of 1 (95% CI 0.98–1.1), indicating no association. No association was seen between tumor size and intrahepatic spread, nodal metastases, and metastatic disease.
The detection of metastatic disease at diagnosis was associated with poorer prognosis. Patients who had a positive lymph node at diagnosis had a 5‐year survival of 49.9% (36.2–68.7) compared with 67.7% (58.6–78.2) survival for those who did not report positive lymph nodes (log‐rank test p < 0.002; Figure 3C). Patients with metastases had a 5‐year survival of 44.3% (30.8–63.7) versus 69.9% (60.7–79.7) for patients with no metastases (log‐rank test p < 0.005; Figure 3D).
Treatment modalities
Additional analysis examined the treatment modalities patients received (Figure 4A, Table 2). Patients who underwent surgical treatment alone, without additional systemic therapy, had a 5‐year survival of 87.3% (78.2–97.4). Those who had surgery with systemic therapy had a 5‐year survival of 54.3% (43–68.5). Those with systemic therapy alone had a 5‐year survival of 25.4% (10.2–62.9), and those who were treated with neither surgery nor systemic therapy did not survive until the 5‐year mark. Patients treated with surgery alone had a better overall survival than patients with systemic therapy, or systemic therapy alone or no treatment (log‐rank test p < 0.0001).
FIGURE 4.
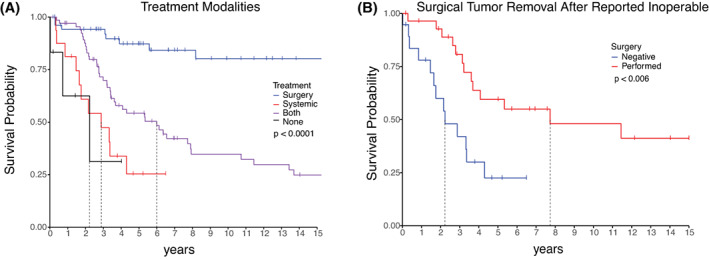
(A) Kaplan–Meier curve of survival by treatment modalities. (B) Kaplan–Meier curve of survival of patients told that their tumor cannot be resected, classified by whether their tumor was eventually surgically resected or not. Vertical notches mark the most recent follow‐up for patients who are censored from the plot for subsequent times. A vertical line marks when survival dropped below 50%.
Of the 48 patients who were diagnosed as having nonoperable tumors, those who subsequently had surgical resection had a 5‐year survival of 59.5% (42.9–82.6), and those who did not had a 5‐year survival of 22.5% (8.9–56.6) (log‐rank test p < 0.006; Figure 4B).
Nine patients underwent liver transplant. Three patients are still alive, 2 of whom had tumors only in the liver and 1 had a single metastasis that was surgically removed. Six patients had a mean survival of 3.7 years, 5 of whom had metastases and were at Stage 3 or 4, and one patient who had at least 10 tumors in the liver, but no detectable metastases.
Multivariable survival analysis
Significant diagnostic variables in the univariate analysis were tested in a Cox proportional hazards regression analysis. Most variables remained significant, even when adjusted for the covariates (Table 3). The HR is the risk of death at any time point during follow‐up for one group relative to another. Relative to a control group, an HR > 1 is worse, HR < 1 is better, and HR = 1 indicates no difference in outcome. Female gender had a HR = 0.52 (0.29–0.93) relative to males. Ten or more tumors in the liver had a HR = 7.1 (2.4–21.04). Positive metastases had a HR = 2.17 (1.19–3.94). Positive lymph nodes did not remain significant with a HR = 1.75 (0.97–3.18).
TABLE 3.
Multivariable analysis of hazard ratio of risk of death at any time adjusting for variables found to be significant in the univariate analysis
| Variables | No. of patients | HR (95% CI) | p |
|---|---|---|---|
| Gender | |||
| Male | 61 | Reference | |
| Female | 88 | 0.52 (0.29–0.93) | 0.027 |
| Number of tumors inside the liver | |||
| 1 | 113 | Reference | |
| 2–5 | 15 | 1.21 (0.56–2.6) | 0.632 |
| 6–9 | 0 | N/A | |
| ≥10 | 5 | 7.1 (2.4–21.04) | <0.001 |
| Lymph node involvement | |||
| Negative | 98 | Reference | |
| Positive | 48 | 1.75 (0.97–3.18) | 0.064 |
| Metastases | |||
| Negative | 105 | Reference | |
| Positive | 41 | 2.17 (1.19–3.94) | 0.011 |
Abbreviation: HR, hazard ratio.
DISCUSSION
We queried a patient community–driven rare tumor registry to assess the association of factors with survival in patients with FLC. A notable observation is the survival advantage of females over males, even when adjusting for covariables. Most participants in the Fibrolamellar Registry are females, matching earlier reports,[ 18 , 19 ] which is different from HCC with a 4–8‐times higher incidence in males.[ 20 ] Interestingly, in hepatocellular adenoma, there is also a predominance of female patients with a much lower rate of malignant transition compared with males.[ 21 ]
One possible explanation for the better survival of females is “lead time bias”; female patients may come to medical attention earlier than males.[ 22 ] Diagnosis at an earlier stage could lead to longer survival. However, this is unlikely, as there was no difference in the age nor stage at diagnosis based on gender.
A second possible explanation stems from the observation that females have two X‐chromosomes and there is not always a full inactivation of one X‐chromosome, which increases expression of some genes, including a few involved in the immune response.[ 23 ] This increased expression has been implicated in the higher rate of autoimmune disorders in women.[ 24 ] This increased gene dose of immune function transcripts may be partially protective against fibrolamellar carcinoma. There are also some tumor suppressors on the X‐chromosome. Women would require two hits to lose these tumor‐suppressor genes, and men, with only one copy, would be more vulnerable to their loss.[ 25 ]
Another possibility is that sex hormones may affect tumor progression. Estrogen may be protective against liver carcinogenesis, as it represses HCC growth in mice.[ 26 ] Also, women with HCC have a significantly longer survival, independent of age, race/ethnicity, stage of disease, or treatment modalities.[ 27 ] The advantage was more pronounced among ages 18–64, and it was hypothesized that the advantage was associated with estrogen. Conversely, androgens may have an oncogenic effect on liver cells. Transforming growth factor beta 1 (TGF‐β1) expression was activated by androgen and androgen receptor in isolated Huh7 cells,[ 28 ] and expression of TGF‐β1 increases during the progression of HCC.[ 29 ] The higher estrogen/androgen ratio for women could be protective, but our analysis did not find an association between contraceptive use and survival. We should note that patients did not verify what kind of oral contraceptives they had taken, and at least some could have potentially used progestin‐only pills.
A second notable result is the lack of association between primary tumor size and survival. Surprisingly, survival was the same for patients with tumors larger or smaller than 10 cm, our median tumor size. We tested other size cutoffs, none of which showed association with survival. A different study[ 12 ] also did not find an independent association for size (±10 cm). Similarly, there was no correlation between size and the presence of metastases. Some had very large tumors without metastases, and others had very small tumors with metastases.
Thus, there may be two variants of FLC: one that is more aggressive in its metastatic potential, which has metastasized by the time the primary tumor has been detected; and another that is more quiescient, and continues to grow with a low probability of metastases. In some cancers, “synchronous metastases” can be seeded from an early carcinoma cell, years before diagnosis, and develop into colorectal, lung, or breast cancers.[ 30 ] If that process occurs in FLC, patient prognosis may depend less on tumor size and more on metastatic status. It is possible that something in the genomic background of the patient modulates the metastatic potential of the tumor.[ 31 ]
Accordingly, we found that a worse prognosis was associated with the presence of metastases or with mulitple intrahepatic tumors. While some liver tumors, such as HCC, are usually the consequence of widespread underlying liver damage, FLC is the consequence of a very rare somatic mutation. Thus, multiple intrahepatic tumors may represent local metastasis, consistent with the worse outcome with metastases.
Our 32.9% of positive lymph nodes is lower than the 70% in earlier studies.[ 11 ] Despite positive lymph node being associated with worse prognosis in the univariate analysis, it was not significant when adjusting for covariates (p = 0.064). The effect of lymph nodes on survival may be co‐dependent on another factor such as positive metastasis; or the low rate of lymph nodes and small sample of patients led to inadequate statistical power. Patients did not identify in the registry whether the positive lymph nodes were regional or distant, whereas previous publications addressed positive lymph nodes and distant organ metastases together, as they are the components of stage IV disease according to the American Joint Committee on Cancer staging manual.[ 12 , 16 , 19 ] Elucidation of the association of positive regional versus distant lymph nodes with prognosis would benefit from further studies.
We did not find significant association between survival and age of diagnosis. Another study found worse survival for patients over the age of 40,[ 12 ] and another found worse survival for patients over the age of 58.[ 11 ] We found no significant difference in survival at age cutoffs of either 40 years or 58 years. Patients in this study were diagnosed at an earlier median age than those other studies.[ 11 , 12 ] Until all patients receive a molecular analysis, it is unresolved whether FLC is a different disease in younger patients than older patients, or whether the older patients included in previous studies were misdiagnosed. FLC is believed to grow slowly in most patients with fairly innocuous symptoms that are overlooked.[ 32 ] Thus, there has been no way to determine how long the tumors were growing before diagnosis. This complicates evaluating the utility of early diagnosis. Therefore, even if a correlation is not seen between the age of diagnosis and survival, individual patients may still benefit from a diagnosis at an earlier stage.
All systemic treatment options, when compared with surgery alone, were found to have significantly poorer prognosis. This finding is not surprising, as patients who were treated with surgery alone predominantly included those with lower‐stage disease. For patients who were told their tumor cannot be resected, there was better survival for those who eventually underwent resection (Figure 4B). This suggests the importance of resecting all radiographic evidence of disease.
This analysis of a patient community–based registry of FLC aimed to assess the prognostic factors associated with survival. There are advantages of such a registry. The patient community running the registry has ongoing direct communication with its members, facilitating updates on clinical status. This is especially important for FLC, because, for diagnosis, 44% went for a second opinion, and most (57%) were seen for diagnosis and/or treatment at two or more facilities. This is higher than the 16% of new oncology patients who went for a second opinion.[ 33 ] Because the Fibrolamellar Registry follows patients as they switch institutions or, in some cases, even countries, it has the potential of allowing more complete data collection, especially of patient outcomes. A second advantage is that those involved in managing the registry remain in touch with the patients with updates and advocate for participants to have their clinicians re‐evaluate their samples to ensure the accuracy of the original diagnosis of FLC. Historically, the diagnosis of FLC was based on morphology plus immunostains, but now the diagnosis is based on morphology, immunostains, and molecular testing. Although FLC has distinctive histologic features, about 9% of the cases reported as FLC were actually HCC,[ 32 ] and about the same percentage of HCC cases were FLC.[ 4 ] It has been suggested that other databases may include patients incorrectly classified as FLC[ 32 , 34 ]. Thus, morphological classification as well as confirmatory genetic testing has been recommended to diagnose FLC.[ 30 ] This potential problem may also exist in the registry's data. However, because most of its patients have been diagnosed since the discovery of the chimeric gene, it may be that there are fewer misdiagnoses in the registry,[ 6 , 34 ] and indeed we know of 29 participants (19%) whose diagnosis has been verified with molecular testing. Furthermore, this dynamic interaction with the patients allowed the registry management to go back and remove from the records patients who had been misdiagnosed as having FLC. Five such patients have been removed since the beginning of the registry.
As a consequence of these patient record updates, we may be looking at a different population of patients than other studies. This may impact our observations on median age of diagnosis and survival time. The median age at diagnosis in the Fibrolamellar Registry (22 years old) is younger than in earlier publications (27–39 years old).[ 12 , 18 , 19 ] Because HCC occurs more frequently with increasing age, the erroneous inclusion of patients with HCC would raise the derived median age of diagnosis, as well as affect other variables. The overall median survival of the Fibrolamellar Registry participants was 98 months, longer than reported previously (57.2–80.4 months).[ 13 , 35 , 36 ] This was surprising for a few reasons. First, while the registry is actively pursuing data from patients diagnosed years ago, most of those have been actively recruited in the past few years with a medium follow‐up of 6.3 years. We were concerned that this might bias the survey toward a shorter survival time. Instead, our survival is longer. Second, one study that reported a shorter survival included only patients who had surgery.[ 13 ] Yet, our results indicate that those undergoing surgery have longer survival. The reported shorter survival time was associated with patients over 58 years old with worse survival in univariate or multivariable analysis. The Fibrolamellar Registry has only 2 patients over age 58, which might account for the longer overall survival in our study. This absence of older patients is the consequence of some patients who were initially diagnosed with FLC aged over 40, who were later removed when it was discovered they had HCC.
There are some potential limitations in working from a patient registry. First, the data are self‐reported by individuals, many of whom do not have medical training. To address this potential issue, the registry has been collecting medical records to supplement specific targeted studies in collaboration with researchers. An oncology nurse has been scanning those records for additional information as well as comparing the self‐reporting with the health records for discrepancies. Second, it is possible that the registry is missing a population of patients who had a short survival after diagnosis. These patients may not have survived long enough after diagnosis to get involved in the patient registry, and the family members of a deceased patient may not be as motivated to upload the information. Another possible limitation is the population of participants who participate. Many do so after hearing about it from other patients in forums, social media groups, or a web search. The registry may bias toward patients who use these online resources, which could skew the representation to particular countries, economic strata, gender, or age groups. Another potential limitation is rarity of the disease. With time, as the patient‐run medical registry gathers more data and reaches more countries, we will re‐examine whether there are any shifted outcomes in the analyses. As is typical for such studies, we have some censored observations in our data set for when we lost patient contact. Because we try to keep in touch with the patients and family members, we believe that the survival probabilities apply to both censored and uncensored observations. We will continue to update the analyses across time and as more patients are entered into the database. Determination of the demographic and clinical predictors of outcome for patients with FLC may help guide risk stratification for patients with this rare disease.
CONCLUSIONS
In this patient registry–based study on FLC, a patient's gender was found to be a prognostic factor, with females having a better outcome than males. Age at diagnosis and tumor size at diagnosis were not found to have significant effects on survival. Ten or more tumors inside the liver, as well as metastases at diagnosis, were associated with poorer prognosis.
AUTHOR CONTRIBUTIONS
Study concept: Amichai Berkovitz, Rachael D. Migler, Erin Marcotte, Sanford M. Simon. Data curation: Rachael D. Migler, Sanford M. Simon. Formal analysis: Amichai Berkovitz, Rachael D. Migler, Adam Qureshi, Carly Rosemore, Michael S. Torbenson, RV, Erin Marcotte, Sanford M. Simon. Methodology: Amichai Berkovitz, Adam Qureshi, RV, Erin Marcotte, Sanford M. Simon. Investigation: Amichai Berkovitz, Rachael D. Migler, Adam Qureshi, Carly Rosemore, Michael S. Torbenson, Roger Vaughan, Erin Marcotte, Sanford M. Simon. Manuscript draft: Amichai Berkovitz and Sanford M. Simon. Manuscript review and editing: Amichai Berkovitz, Rachael D. Migler, Adam Qureshi, Carly Rosemore, Michael S. Torbenson, Roger Vaughan, Erin Marcotte, Sanford M. Simon. Funding obtainment: Sanford M. Simon, Rachael D. Migler.
FUNDING INFORMATION
Supported by the Rally Foundation and the Richard Lounsbery Foundation; National Institutes of Health (NIH)/National Cancer Institute (P50CA210964 and 1U54CA243126); NIH Clinical and Translational Science Award through the Rockefeller University (UL1TR001866); and the Center for Basic and Translational Research on Disorders of the Digestive System, through the generosity of the Leona M. and Harry B. Helmsley Charitable Trust.
CONFLICT OF INTEREST
Nothing to report.
ACKNOWLEDGMENT
The authors thank Gail Trecosta, Julie Pickard, Lisa Walters, Carol Hendrix, Elana P. Simon, D. Love, Michelle Desmond, and Siobhan Lett of the FibroRegistry for their assistance. Most importantly, they want to thank all of the patients and their caregivers in the fibrolamellar community.
Berkovitz A, Migler RD, Qureshi A, Rosemore C, Torbenson MS, Vaughan R, et al. Clinical and demographic predictors of survival for fibrolamellar carcinoma patients—A patient community, registry‐based study. Hepatol Commun. 2022;6:3539–3549. 10.1002/hep4.2105
REFERENCES
- 1. Eggert T, McGlynn KA, Duffy A, Manns MP, Greten TF, Altekruse SF. Fibrolamellar hepatocellular carcinoma in the USA, 2000‐2010: a detailed report on frequency, treatment and outcome based on the surveillance, epidemiology, and end results database. United European Gastroenterol J. 2013;1:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darcy DG, Malek MM, Kobos R, Klimstra DS, DeMatteo R, La Quaglia MP. Prognostic factors in fibrolamellar hepatocellular carcinoma in young people. J Pediatr Surg. 2015;50:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fritz AG. International classification of diseases for oncology: ICD‐O. Geneva: World Health Organization; 2013. viii, 242. [Google Scholar]
- 4. Simon EP, Freije CA, Farber BA, Lalazar G, Darcy DG, Honeyman JN, et al. Transcriptomic characterization of fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2015;112:E5916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lalazar G, Requena D, Ramos‐Espiritu L, Ng D, Bhola PD, de Jong YP, et al. Identification of novel therapeutic targets for fibrolamellar carcinoma using patient‐derived xenografts and direct‐from‐patient screening. Cancer Discov. 2021;11:2544–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Honeyman JN, Simon EP, Robine N, Chiaroni‐Clarke R, Darcy DG, Lim II, et al. Detection of a recurrent DNAJB1‐PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham RP, Jin L, Knutson DL, Kloft‐Nelson SM, Greipp PT, Waldburger N, et al. DNAJB1‐PRKACA is specific for fibrolamellar carcinoma. Modern Pathol. 2015;28:822–9. [DOI] [PubMed] [Google Scholar]
- 8. Kastenhuber ER, Lalazar G, Houlihan SL, Tschaharganeh DF, Baslan T, Chen CC, et al. DNAJB1‐PRKACA fusion kinase interacts with beta‐catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2017;114:13076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engelholm LH, Riaz A, Serra D, Dagnaes‐Hansen F, Johansen JV, Santoni‐Rugiu E, et al. CRISPR/Cas9 engineering of adult mouse liver demonstrates that the Dnajb1‐Prkaca gene fusion is sufficient to induce tumors resembling fibrolamellar hepatocellular carcinoma. Gastroenterology. 2017;153:1662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graham RP, Lackner C, Terracciano L, Gonzalez‐Cantu Y, Maleszewski JJ, Greipp PT, et al. Fibrolamellar carcinoma in the carney complex: PRKAR1A loss instead of the classic DNAJB1‐PRKACA fusion. Hepatology. 2018;68:1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamashita S, Vauthey JN, Kaseb AO, Aloia TA, Conrad C, Hassan MM, et al. Prognosis of fibrolamellar carcinoma compared to non‐cirrhotic conventional hepatocellular carcinoma. J Gastrointest Surg. 2016;20:1725–31. [DOI] [PubMed] [Google Scholar]
- 12. Assi HA, Mukherjee S, Machiorlatti M, Vesely S, Pareek V, Hatoum H. Predictors of outcome in patients with fibrolamellar carcinoma: analysis of the National Cancer Database. Anticancer Res. 2020;40:847–55. [DOI] [PubMed] [Google Scholar]
- 13. Mayo SC, Mavros MN, Nathan H, Cosgrove D, Herman JM, Kamel I, et al. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg. 2014;218:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 16. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 17. Cox DR. Regression models and life‐tables. J R Stat Soc B Methodol. 1972;34:187–220. [Google Scholar]
- 18. El‐Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population‐based study. Hepatology. 2004;39:798–803. [DOI] [PubMed] [Google Scholar]
- 19. Stipa F, Yoon SS, Liau KH, Fong Y, Jarnagin WR, D'Angelica M, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106:1331–8. [DOI] [PubMed] [Google Scholar]
- 20. Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol. 2006;23:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152:880–94.e6. [DOI] [PubMed] [Google Scholar]
- 22. Viera AJ, Thorpe JM, Garrett JM. Effects of sex, age, and visits on receipt of preventive healthcare services: a secondary analysis of national data. BMC Health Serv Res. 2006;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Youness A, Miquel CH, Guery JC. Escape from X chromosome inactivation and the female predominance in autoimmune diseases. Int J Mol Sci. 2021;22:1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunford A, Weinstock DM, Savova V, Schumacher SE, Cleary JP, Yoda A, et al. Tumor‐suppressor genes that escape from X‐inactivation contribute to cancer sex bias. Nat Genet. 2017;49:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88‐dependent IL‐6 production. Science. 2007;317:121–4. [DOI] [PubMed] [Google Scholar]
- 27. Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a surveillance, epidemiology, and end results analysis. Cancer. 2014;120:3707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon G, Kim JY, Choi YK, Won YS, Lim IK. Direct activation of TGF‐beta1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma cells and its tumor in nude mice. J Cell Biochem. 2006;97:393–411. [DOI] [PubMed] [Google Scholar]
- 29. Matsuzaki K, Date M, Furukawa F, Tahashi Y, Matsushita M, Sakitani K, et al. Autocrine stimulatory mechanism by transforming growth factor beta in human hepatocellular carcinoma. Cancer Res. 2000;60:1394–402. [PubMed] [Google Scholar]
- 30. Hu Z, Li Z, Ma Z, Curtis C. Multi‐cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat Genet. 2020;52:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ostendorf BN, Bilanovic J, Adaku N, Tafreshian KN, Tavora B, Vaughan RD, et al. Common germline variants of the human APOE gene modulate melanoma progression and survival. Nature Medicine. 2020;26:1048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lalazar G, Simon SM. Fibrolamellar carcinoma: recent advances and unresolved questions on the molecular mechanisms. Semin Liver Dis. 2018;38:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olver I, Carey M, Bryant J, Boyes A, Evans T, Sanson‐Fisher R. Second opinions in medical oncology. BMC Palliat Care. 2020;19:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malouf G, Falissard B, Azoulay D, Callea F, Ferrell LD, Goodman ZD, et al. Is histological diagnosis of primary liver carcinomas with fibrous stroma reproducible among experts? J Clin Pathol. 2009;62:519–24. [DOI] [PubMed] [Google Scholar]
- 35. Ang CS, Kelley RK, Choti MA, Cosgrove DP, Chou JF, Klimstra D, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res. 2013;6:3–9. [PMC free article] [PubMed] [Google Scholar]
- 36. Kaseb AO, Shama M, Sahin IH, Nooka A, Hassabo HM, Vauthey J‐N, et al. Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology. 2013;85:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]


