Abstract
Arylamine N-acetyltransferase 2 (NAT2) is well-known for its role in phase II metabolism of xenobiotics and drugs. More recently, genome wide association studies and murine models implicated NAT2 in regulation of insulin sensitivity and plasma lipid levels. However, the mechanism remains unknown. Transcript levels of human NAT2 varied dynamically in HepG2 (hepatocellular) cells, depending on the nutrient status of the culture media. Culturing the cells in the presence of glucose induced NAT2 mRNA expression as well as its N-acetyltransferase activity significantly. In addition, insulin or acetate treatment also significantly induced NAT2 mRNA. We examined and compared the glucose- and acetate-dependent changes in NAT2 expression to those of genes involved in glucose and lipid metabolism, including FABP1, CPT1A, ACACA, SCD, CD36, FASN, ACLY, G6PC, and PCK1. Genes that are involved in fatty acid transport and lipogenesis, such as FABP1 and CD36, shared a similar pattern of expression with NAT2. In silico analysis of genes co-expressed with NAT2 revealed an enrichment of biological processes involved in lipid and cholesterol biosynthesis and transport. Among these, A1CF (APOBEC1 complementation factor) showed the highest correlation with NAT2 in terms of its expression in normal human tissues. The current study shows, for the first time, that human NAT2 is transcriptionally regulated by glucose and insulin in liver cancer cell lines and that the gene expression pattern of NAT2 is similar to that of genes involved in lipid metabolism and transport.
Keywords: N-acetyltransferase 2, transcriptional regulation, glucose, insulin
Arylamine N-acetyltransferase 2 (NAT2) and its isozyme, arylamine N-acetyltransferase 1 (NAT1), play key roles in the detoxification of carcinogenic arylamines and xenobiotics by catalyzing acetyl-coenzyme A (CoA)-dependent biotransformation of arylamines to N-arylacetamides (Sim et al., 2014). NAT2 expresses a well-defined genetic polymorphism in humans that translates into differential xenobiotic metabolism (Fathzadeh et al., 2017). Based on the combination of alleles, individuals can be categorized into 3 distinct acetylator phenotypes (ie, rapid, intermediate and slow acetylators) and exhibit clinically meaningful differences in ability to metabolize NAT2 substrates, including drugs (eg, isoniazid) and carcinogens (eg, 4-aminobiphenyl) (Hein, 2002; Sim et al., 2014).
More recently, a novel and unexpected role of NAT2 has been reported. Genome-wide association studies (GWAS) have identified links between polymorphisms of human NAT2 gene and insulin resistance, high serum triglyceride, and coronary artery disease as well as high fasting plasma glucose level (Fathzadeh et al., 2017; Knowles et al., 2015). Analysis of Nat1 knockout (KO) mice revealed that NAT1 enzyme deficiency leads to development of insulin resistance and metabolic defects at the systemic level, further supporting the previously unrecognized role of human homologue NAT2 in regulating metabolism and insulin sensitivity (Camporez et al., 2017; Chennamsetty et al., 2016). Moreover, the follow-up studies have attributed insulin resistance in Nat1 KO mice to mitochondrial dysfunction (Camporez et al., 2017; Chennamsetty et al., 2016). However, it is currently unknown how human NAT2 influences cellular metabolism and insulin sensitivity, or if NAT2 expression or activity is reciprocally regulated by the energy or metabolic state.
Endogenous NAT2 expression is limited to the liver and small and large intestines (Sim et al., 2014), suggesting that NAT2 plays organ-specific roles. For this reason, we have been working with human hepatocellular carcinoma cell lines, including HepG2, to study the role of human NAT2 in the liver, where its expression is the highest. In our preliminary experiments, we cultured HepG2 cells in culture media containing different nutrients (eg, glucose, pyruvate, and l-glutamine, etc.) and discovered that NAT2 mRNA level fluctuated dynamically in HepG2 cells depending on the availability of certain nutrients, suggesting that NAT2 expression is differentially regulated by energy or metabolic state of the cells. To our knowledge, no previous studies have reported such finding. In the present study, we report the effects of glucose and insulin on NAT2 expression in HepG2 cells. In addition, the glucose-dependent expression pattern of NAT2 was compared with those of genes involved in glucose and lipid metabolism. Finally, to better understand the transcriptional regulation and novel cellular roles of NAT2, we performed an in silico analysis of genes co-expressed with NAT2 in human tissues.
MATERIALS AND METHODS
Cell culture
HepG2 and Hep3B cells were obtained from ATCC and cultured in DMEM (Cytiva) containing d-glucose (5.5 mM), sodium pyruvate (1 mM), l-glutamine (4 mM), fetal bovine serum (FBS; 10%), and penicillin-streptomycin (1%) at 37°C with 5% CO2 in humidified air.
Reagents and treatments
d-glucose, sodium pyruvate, l-glutamine, dexamethasone, sodium acetate, and insulin (bovine pancreas; 10 mg/ml solution) were purchased from Sigma-Aldrich. Cell membrane-permeable, 8-bromo-cAMP (sodium salt) was purchased from Tocris. The final treatment concentrations of individual reagents used in the study were the following: d-glucose (5 mM), pyruvate (1 mM), l-glutamine (4 mM), 8-bromo-cAMP (500 µM), dexamethasone (1 µM), acetate (5 mM), insulin (100 nM), and FBS (10%). For treatments, cells were plated on either 12-well or 24-well plates the day before the treatment. For the treatment with selected nutrients (eg, d-glucose), the nutrients (either individually or in combination) were added to a control DMEM (“Control”) which was free of d-glucose, pyruvate, l-glutamine, FBS, and phenol red (Cytiva), and the cells were treated for 24 h. For one of the experiments, HepG2 cells were treated in the absence or presence of insulin (100 nM final) for 6 h in regular, serum-free DMEM which contained 5.5 mM d-glucose, 1 mM sodium pyruvate, and 4 mM l-glutamine. Bovine serum albumin (BSA, heat shock fraction, protease-free, fatty acid-free, and essentially globulin-free, pH 7, ≥98%) and sodium palmitate were purchased from Sigma-Aldrich. BSA (10%)-Palmitic acid (5 mM) (BSA-PA) complex stock solution was prepared as described previously (Cousin et al., 2001). Fatty acid-free BSA solution (10%) was used for vehicle control. For the palmitate treatment, HepG2 cells were incubated with BSA (0.5%)-PA (0.25 mM) or 0.5% BSA alone in Control DMEM for 24 h.
RNA isolation and RT-qPCR
Cells were approximately 50%–70% confluent at the time of harvest. For isolation of total RNA, E.Z.N.A. Total RNA Kit I (Omega Bio-Tek) was used according to the manufacturer’s instructions with an added, in-column DNase digestion step. RNA concentration was measured based on absorbance at 260 nm using NanoDrop spectrophotometer (Thermo Scientific). For cDNA synthesis, 500 ng total RNA was processed in a 10-µl reaction using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s instructions. For qPCR, 1 µl of cDNA, the gene-specific primer set, and iTaq Universal SYBR Green Supermix (Bio-Rad) were used in a 20-µl reaction. For PCR amplification and quantification of SYBR Green signal, StepOne Real-Time PCR System (Applied Biosystems) was used. Samples underwent 40 cycles of 2-step PCR (denaturation at 96°C [15 s] followed by annealing and extension at 60°C [1 min]). For calculation of relative levels of the gene of interest, delta-delta Ct method (a.k.a., the 2–ΔΔCt method) was used using 18S rRNA as an internal control. The sequences of the PCR primers used in the current study are available in Table 1.
Table 1.
List of qPCR Primer Sequences Used in the Study
| Gene (Human) | Description | Sequence | |
|---|---|---|---|
| 18S rRNA | Internal control | Forward | GGAAGGGCACCACCAGGAGT |
| Reverse | TGCAGCCCCGGACATCTAAG | ||
| ACACA | Acetyl-CoA carboxylase alpha | Forward | CTGGCTGTCTTACATGCCCA |
| Reverse | TAGCCTGGCTCTACCAACCA | ||
| ACLY | ATP citrate lyase | Forward | GGTGCTCCGGATTTTGC |
| Reverse | ACATGGCTGCAGAGAGACCT | ||
| ACOX1 | Acyl-CoA oxidase 1 | Forward | GTAGCAGTCTGGCCAACCAT |
| Reverse | GCTCCCCTGAAGGAAATCCC | ||
| ACSS1 | Acyl-CoA synthetase short chain family member 1 | Forward | ACCAAGATCGCCAAATATGC |
| Reverse | TGCTTGTCCTTGCACTTCTG | ||
| ACSS2 | Acyl-CoA synthetase short chain family member 2 | Forward | GGATTCCAGCTGCAGTCTTC |
| Reverse | CATGCCACCACAAGTCAATC | ||
| ACTB | Actin beta; β-actin | Forward | GCAGTCGGTTGGAGCGAGCA |
| Reverse | ATCACCTCCCCTGTGTGGACTTGG | ||
| CD36 | Fatty acid translocase (FAT) | Forward | CAGCCTCATTTCCACCTTTTGT |
| Reverse | GCAAAGGCCTTGGATGGAAGA | ||
| CPT1A | Carnitine palmitoyltransferase 1A; CPT1 | Forward | GATGAGTCGTGCCACCAAGA |
| Reverse | GCTCTTGCTGCCTGAATGTG | ||
| FABP1 | Fatty acid binding protein 1 | Forward | GGGAAGGGAGCCCCCTATAA |
| Reverse | TGGATCACTTTGGACCCAGC | ||
| FASN | Fatty acid synthase (FAS) | Forward | CACACACGATGGACCCTCAG |
| Reverse | GAAGAAGGAGAGCCGGTTGG | ||
| NR1H4 | Nuclear receptor subfamily 1 group H member 4; Farnesoid X-activated receptor (FXR) | Forward | GACCTCGACAACAAAGTCATGC |
| Reverse | ATAGCTTCAACCGCAGACCC | ||
| NR1I2 | Nuclear receptor subfamily 1 group I member 2; pregnane X receptor (PXR) | Forward | ATGGGCCATCTGGGGTCTAT |
| Reverse | ATGATGTGGCCGGAATGAGG | ||
| G6PC | Glucose-6-phosphatase catalytic subunit 1 | Forward | ACGAATCTACCTTGCTGCTCA |
| Reverse | AAAATCCGATGGCGAAGCTG | ||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Forward | GGTGAAGCAGGCGTCGGAGG |
| Reverse | GAGGGCAATGCCAGCCCCAG | ||
| NAT1 | N-Acetyltransferase 1 | Forward | CACTGTTTGGTGGGCTTCAC |
| Reverse | GCACAAGCTTTCTCTGCAAGG | ||
| NAT2 | N-Acetyltransferase 2 | Forward | TGGACCAAATCAGGAGAGAGC |
| Reverse | GCCCACCAAACAGTAAACCC | ||
| PCK1 | Phosphoenolpyruvate carboxykinase 1; PEPCK-C | Forward | TGATGAGCCGCTAGCTTCAG |
| Reverse | GCCTTTATGTTCTGCAGCCG | ||
| PPARGC1A | PPARG coactivator 1 alpha; PGC1α | Forward | CACGGACAGAACTGAGGGAC |
| Reverse | TTCGTTTGACCTGCGCAAAG | ||
| SCD | Stearoyl-CoA desaturase; SCD1 | Forward | GCAGCCGAGCTTTGTAAGAG |
| Reverse | GTTCTACACCTGGCTTTGGG |
Note: The table shows the complete list of all RT-qPCR primers used in the current study. All primer sets target corresponding genes in human. Primers were synthesized at Integrated DNA Technologies.
Western blot
The cells were lysed in Laemmli buffer (50 mM Tris-Cl [pH 6.8], 2% sodium dodecyl sulfate [SDS; w/v], 0.1% bromophenol blue, 10% [v/v] glycerol) and boiled for 10 min. Protein concentrations were determined using Pierce BCA Assay kit (Thermo Scientific) per manufacturer’s instructions. β-mercaptoethanol was then added to each sample and boiled for additional 5 min. Fifty micrograms of protein per sample was loaded and separated on a 4%–12% gradient Bis-Tris Plus polyacrylamide gel (Invitrogen). The gel was transferred to a PVDF membrane and blocked in 5% (w/v) skim milk in tris-buffered saline containing 0.1% Tween 20 (TBST) for 30 min. Membranes were incubated with primary antibody at 4°C overnight. Membranes were washed with TBST and then were incubated for 1 h at room temperature with HRP-conjugated secondary antibodies (1:5000 in TBST). Membranes were washed and the protein-antibody complex was detected using Clarity MAX ECL substrate (BioRad). Monoclonal rabbit anti-NAT2 antibody (ab194114) was purchased from Abcam and used at 1:1000 dilution. The antibody against GAPDH was purchased from Cell Signaling Technology and used at 1:5000 dilution.
In vitro NAT2 assay
The N-acetylated product of the NAT2-selective substrate sulfamethazine (SMZ) was quantified by separation of substrate and acetylated product by reverse phase high-performance liquid chromatography (HPLC) as described (Hein et al., 2006). Briefly, treated HepG2 cells were lysed in a lysis buffer containing 20 mM sodium phosphate pH 7.4, 1 mM EDTA, 0.2% Triton X-100, 1 mM DTT, 100 µM PMSF, 1 μg/ml aprotinin, and 2 µM pepstatin A (MilliporeSigma). Cell lysate was centrifuged at 15 000 × g for 20 min in the cold room, and the resulting supernatant was used for the assay. NAT2 enzymatic assay reactions containing 50 µl of cell lysate, 300 µM SMZ, and acetyl-CoA (1 mM), were incubated at 37°C for 10 min. Reactions were terminated by the addition of 10 µl of 1 M acetic acid, then proteins were precipitated by centrifugation (15 000 × g for 10 min) and supernatant was injected onto a C-18 (250 mm × 4 mm; 5 µm) reverse phase column using an Agilent 1260 Infinity II system (Agilent Technologies). Separation of N-acetyl-SMZ was achieved using a gradient of 100:0 of 55 mM sodium phosphate pH 4.0: Methanol to 0:100 55 mM sodium phosphate: Methanol over 20 min. Absorbance was recorded at 260 nm. In vitro NAT2 activity was normalized to total protein (and to incubation time) and expressed as N-acetylated-SMZ nmol/min/mg protein. Each value was then normalized to the control (no glucose) group and expressed as a relative level.
NAT2 genotyping of HepG2
Genomic DNA was isolated from pelleted HepG2 cells by using the QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer’s instructions. NAT2 genotype and deduced phenotype were determined as described previously (Doll and Hein, 2001). The analysis showed that HepG2 has NAT2*6A and NAT2*14C alleles, and thus is a slow acetylator.
Data analysis and presentation
Graphical presentation and statistical analysis of the data was performed with Prism (GraphPad). One-way ANOVA with Bonferroni post hoc test was used for multiple group comparisons. For studies involving comparison of 2 experimental groups, unpaired t test was used. Inter-group differences with p < .05 were considered statistically significant.
In silico analysis of genes co-expressed with NAT2
The following 4 different, online analytical engines were used to identify genes whose expression patterns were similar to that of human NAT2 (ie, co-expressed genes): GEPIA (http://gepia.cancer-pku.cn/; last accessed August 2022) (Tang et al., 2017), COXPRESdb (https://coxpresdb.jp/; last accessed August 2022) (Obayashi et al., 2019), SEEK (https://seek.princeton.edu/seek/; last accessed August 2022) (Zhu et al., 2015), and Archs4 (https://maayanlab.cloud/archs4; last accessed August 2022) (Lachmann et al., 2018). The list of top 50 genes identified by each engine is available in Table 2. Based on these lists, we compiled a combined gene list by identifying genes that appear more than once in the lists, which resulted in a total of 40 genes (see Table 3). The list of co-expressed genes were then analyzed for enrichment of biological processes they are associated with using GOnet (https://tools.dice-database.org/GOnet/; last accessed August 2022) (Pomaznoy et al., 2018) and PANTHER (http://www.pantherdb.org/; last accessed August 2022) (Mi et al., 2013). The top 50 Gene Ontology (GO) terms/biological processes significantly enriched in the list (q < 0.001) are shown in Table 4. For analyzing and graphing correlations between NAT2 and individual gene expression (shown in Figure 5) in multiple human tissues, the graphing and statistical tools available at GEPIA (http://gepia.cancer-pku.cn/; last accessed August 2022) (Tang et al., 2017) were employed using Genotype-Tissue Expression (GETx) database on normal human tissues.
Table 2.
List of Top 50 Genes Co-Expressed With Human NAT2
| Sources |
||||
|---|---|---|---|---|
| Rank | Archs4 | COXPRESdb | SEEK | GEPIA |
| 1 | FLJ22763 | MOGAT2 | A1CF | CYP2C8 |
| 2 | OTC | C3orf85 | TM4SF5 | NR1I2 |
| 3 | TMEM82 | IYD | SLC39A5 | MOGAT2 |
| 4 | FABP1 | OTC | LEAP2 | KDM8 |
| 5 | MOGAT2 | SLC17A4 | SLC10A1 | C8A |
| 6 | CLRN3 | CYP2C9 | OTC | SLC25A47 |
| 7 | PLA2G12B | ADGRG7 | FABP1 | AFM |
| 8 | MTND4P20 | FABP1 | NR1I2 | PLGLA |
| 9 | TM4SF5 | CLRN3 | MTTP | LINC01093 |
| 10 | GUCA2B | AGXT2 | APOA5 | RDH16 |
| 11 | CYP2C19 | NR1I2 | SLC17A4 | MAT1A |
| 12 | ADGRG7 | NAT1 | HPX | F9 |
| 13 | ABCG8 | ALDOB | AFM | C3P1 |
| 14 | HMGCS2 | GLOD5 | CYP4F2 | CYP2C9 |
| 15 | NR1H4 | APOB | RDH16 | GDF2 |
| 16 | SLC17A4 | CYP2C19 | CYP3A4 | ADH4 |
| 17 | APOC3 | A1CF | ADH4 | SMUG1P1 |
| 18 | CYP2C18 | HSD3B2 | PRAP1 | APOF |
| 19 | ALDOB | HMGCS2 | C8A | SAA4 |
| 20 | GBA3 | TAT | NPC1L1 | ABCG5 |
| 21 | APOA4 | CDH17 | CYP3A43 | GYS2 |
| 22 | UGT2A3 | GC | ABCG8 | CYP4A22 |
| 23 | PRAP1 | LOC285733 | APOC3 | RP11-622A1.2 |
| 24 | NR1I2 | NR1H4 | SLC30A10 | PLGLB2 |
| 25 | SLC2A2 | MALRD1 | GLYCTK | HP |
| 26 | UGT1A4 | IGSF5 | KHK | CYP8B1 |
| 27 | TM6SF2 | SI | CYP2B6 | TTC36 |
| 28 | LRRC31 | FMO4 | CYP2C9 | PLG |
| 29 | SLC28A1 | ZG16 | APOB | PROZ |
| 30 | SLC39A5 | GBA3 | SLC2A2 | APOA5 |
| 31 | CCL15-CCL14 | PPP1R1C | ADH6 | F11 |
| 32 | CYP3A4 | ANKS4B | GBA3 | CYP4F2 |
| 33 | ACE2 | GLYATL1 | CPN2 | AGXT |
| 34 | AGXT2 | ABCC6P1 | AGXT | C9 |
| 35 | ADH1C | LECT2 | CYP2C19 | HAO1 |
| 36 | RP11-400G3.5 | ZNF503-AS1 | C8G | HPX |
| 37 | APCS | KNG1 | AKR1C4 | C6 |
| 38 | F11 | CFHR5 | ABCB11 | UROC1 |
| 39 | CFHR2 | SLC26A3 | HAO1 | MBL2 |
| 40 | ADH6 | LOC100509856 | ALDOB | GCKR |
| 41 | GSTA2 | CYP2C18 | SERPINA4 | KLKB1 |
| 42 | OIT3 | SLC2A2 | GYS2 | RP11-290F5.1 |
| 43 | F9 | LRRC31 | NR1I3 | SERPINA10 |
| 44 | GOLT1A | MS4A12 | HRG | RP11-116D2.1 |
| 45 | GSTA1 | SULT1B1 | PLA2G12B | C8B |
| 46 | RNF186 | MEP1B | HNF4A | SLC22A1 |
| 47 | RP4-608O15.3 | F9 | MOGAT3 | ADH6 |
| 48 | UGT2B10 | UGT2B15 | SULT2A1 | ACOT12 |
| 49 | CFHR5 | SLC15A1 | OSTALPHA | ITIH4 |
| 50 | AFM | BCO1 | CYP2C18 | ORM2 |
Note: Four separate analytical engines (ie, Archs4, COXPRESdb, SEEK, and GEPIA) were used to identify top 50 genes that share a similar expression pattern (ie, co-expressed) with human NAT2. Only the gene symbols are shown here. The genes that appear more than once in the lists are shown in Table 3.
Table 3.
List of Genes Co-Expressed With Human NAT2 (in Alphabetical Order)
| Gene ID | Gene Name | Protein Class/Type | No. Hits (out of 4)a | |
|---|---|---|---|---|
| 1 | A1CF | APOBEC1 complementation factor | RNA metabolism | 2 |
| 2 | ABCG8 | ATP-binding cassette sub-family G member 8 | ATP-binding cassette (ABC) transporter | 2 |
| 3 | ADGRG7 | Adhesion G-protein coupled receptor G7; ADGRG7 | G-protein coupled receptor | 2 |
| 4 | ADH4 | All-trans-retinol dehydrogenase [NAD(+)] ADH4 | Dehydrogenase | 2 |
| 5 | ADH6 | Alcohol dehydrogenase 6 | Dehydrogenase | 3 |
| 6 | AFM | Afamin | Transfer/carrier protein | 3 |
| 7 | AGXT | Alanine-glyoxylate and serine-pyruvate aminotransferase | Transaminase | 2 |
| 8 | AGXT2 | Alanine-glyoxylate aminotransferase 2 | Transaminase | 2 |
| 9 | ALDOB | Fructose-bisphosphate aldolase B | Aldolase | 3 |
| 10 | APOA5 | Apolipoprotein A-V | Apolipoprotein | 2 |
| 11 | APOB | Apolipoprotein B-100 | Apolipoprotein | 2 |
| 12 | APOC3 | Apolipoprotein C-III | Apolipoprotein | 2 |
| 13 | C8A | Complement component C8 alpha chain | Complement component | 2 |
| 14 | CFHR5 | Complement factor H-related protein 5 | Complement component | 2 |
| 15 | CLRN3 | Clarin-3; transmembrane Protein 12; TMEM12; USH3AL1 | – | 2 |
| 16 | CYP2C18 | Cytochrome P450 2C18 | Oxygenase | 3 |
| 17 | CYP2C19 | Cytochrome P450 2C19 | Oxygenase | 3 |
| 18 | CYP2C9 | Cytochrome P450 2C9 | Oxygenase | 3 |
| 19 | CYP3A4 | Cytochrome P450 3A4 | Oxygenase | 2 |
| 20 | CYP4F2 | Cytochrome P450 4F2 | Oxygenase | 2 |
| 21 | F11 | Coagulation factor XI | Serine protease | 2 |
| 22 | F9 | Coagulation factor IX | Serine protease | 3 |
| 23 | FABP1 | Fatty acid-binding protein, liver | Transfer/carrier protein | 3 |
| 24 | FLJ22763 | Chromosome 3 open reading frame 85 (c3orf85) | – | 2 |
| 25 | GBA3 | Cytosolic beta-glucosidase | Glycosidase | 3 |
| 26 | GYS2 | Glycogen [starch] synthase, liver | – | 2 |
| 27 | HAO1 | Hydroxyacid oxidase 1 | Oxidoreductase | 2 |
| 28 | HMGCS2 | Hydroxymethylglutaryl-CoA synthase, mitochondrial | HMG-CoA synthase; ketogenesis | 2 |
| 29 | HPX | Hemopexin; HPX | Metalloprotease | 2 |
| 30 | LRRC31 | Leucine-rich repeat-containing protein 31 | – | 2 |
| 31 | MOGAT2 | 2-acylglycerol O-acyltransferase 2 | Acyltransferase | 3 |
| 32 | NR1H4 | Bile acid receptor; FXR | C4 zinc finger nuclear receptor | 2 |
| 33 | NR1I2 | Nuclear receptor subfamily 1 group I member 2; PXR | C4 zinc finger nuclear receptor | 4 |
| 34 | OTC | Ornithine transcarbamylase, mitochondrial | Transferase | 3 |
| 35 | PLA2G12B | Group XIIB secretory phospholipase A2-like protein | Phospholipase | 2 |
| 36 | PRAP1 | Proline-rich acidic protein 1 | – | 2 |
| 37 | SLC17A4 | Probable small intestine urate exporter | Secondary carrier transporter | 3 |
| 38 | SLC2A2 | Solute carrier family 2, facilitated glucose transporter member 2; GLUT2 | Secondary carrier transporter | 3 |
| 39 | SLC39A5 | Zinc transporter ZIP5 | Secondary carrier transporter | 2 |
| 40 | TM4SF5 | Transmembrane 4 L6 family member 5 | – | 2 |
Note: These genes were identified to be co-expressed with NAT2 by more than one of 4 analytical engines (ie, Archs4, COXPRESdb, SEEK, and GEPIA). The original lists of co-expressed genes are shown in Table 2.
Number of times the gene appeared in 4 separate analyses of co-expressed genes.
Table 4.
Gene Ontology Biological Processes Enriched Among Genes Co-Expressed With NAT2 (Selected)
| Category | Rank | GO Term ID | GO Term | p Value | P_FDR_adja | No. Genes | Genes |
|---|---|---|---|---|---|---|---|
| Xenobiotic metabolism | 5 | GO : 0017144 | Drug metabolic process | 1.00E-10 | 2.47E-07 | 12 | ADH4|ADH6|AGXT|AGXT2|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|HAO1|HMGCS2|NR1I2 |
| 31 | GO : 0042737 | Drug catabolic process | 3.74E-07 | 1.33E-04 | 6 | CYP2C18|CYP2C19|CYP2C9|CYP3A4|HAO1|NR1I2 | |
| 6 | GO : 0042738 | Exogenous drug catabolic process | 3.10E-09 | 5.77E-06 | 5 | CYP2C18|CYP2C19|CYP2C9|CYP3A4|NR1I2 | |
| GO : 0097267 | Omega-hydroxylase P450 pathway | 8.43E-07 | 2.51E-04 | 3 | CYP2C19|CYP2C9|CYP4F2 | ||
| 17 | GO : 0019373 | Epoxygenase P450 pathway | 7.54E-08 | 4.89E-05 | 4 | CYP2C18|CYP2C19|CYP2C9|CYP4F2 | |
| Lipid; fatty acid; cholesterol |
21 | GO : 0033344 | Cholesterol efflux | 1.11E-07 | 5.84E-05 | 4 | ABCG8|APOA5|APOB|APOC3 |
| 50 | GO : 0030301 | Cholesterol transport | 3.80E-06 | 8.38E-04 | 4 | ABCG8|APOA5|APOB|APOC3 | |
| 42 | GO : 0006869 | Lipid transport | 1.64E-06 | 4.29E-04 | 7 | ABCG8|APOA5|APOB|APOC3|FABP1|NR1H4|PLA2G12B | |
| 44 | GO : 0010876 | Lipid localization | 2.63E-06 | 6.58E-04 | 7 | ABCG8|APOA5|APOB|APOC3|FABP1|NR1H4|PLA2G12B | |
| 10 | GO : 0042632 | Cholesterol homeostasis | 9.80E-09 | 1.06E-05 | 6 | ABCG8|APOA5|APOB|APOC3|NR1H4|PLA2G12B | |
| 26 | GO : 0055088 | Lipid homeostasis | 2.15E-07 | 8.95E-05 | 6 | ABCG8|APOA5|APOB|APOC3|NR1H4|PLA2G12B | |
| 3 | GO : 0006629 | Lipid metabolic process | 0.00E + 00 | 1.13E-08 | 18 | ADH4|ADH6|APOA5|APOB|APOC3|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|FABP1|GBA3|HAO1|HMGCS2|MOGAT2|NR1H4|NR1I2|PLA2G12B | |
| 4 | GO : 0044255 | Cellular lipid metabolic process | 0.00E + 00 | 2.73E-08 | 16 | ADH4|ADH6|APOA5|APOB|APOC3|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|FABP1|GBA3|HAO1|HMGCS2|MOGAT2|PLA2G12B | |
| 43 | GO : 0034370 | Triglyceride-rich lipoprotein particle remodeling | 2.54E-06 | 6.52E-04 | 3 | APOA5|APOB|APOC3 | |
| 8 | GO : 0016042 | Lipid catabolic process | 4.60E-09 | 6.39E-06 | 9 | APOA5|APOB|APOC3|CYP3A4|CYP4F2|FABP1|GBA3|HAO1|PLA2G12B | |
| 18 | GO : 0044242 | Cellular lipid catabolic process | 8.62E-08 | 5.03E-05 | 7 | APOA5|APOB|APOC3|CYP4F2|FABP1|GBA3|HAO1 | |
| 27 | GO : 0019433 | Triglyceride catabolic process | 2.19E-07 | 8.95E-05 | 4 | APOA5|APOB|APOC3|FABP1 | |
| 35 | GO : 0046464 | Acylglycerol catabolic process | 6.47E-07 | 1.98E-04 | 4 | APOA5|APOB|APOC3|FABP1 | |
| 47 | GO : 0046503 | Glycerolipid catabolic process | 3.53E-06 | 8.27E-04 | 4 | APOA5|APOB|APOC3|FABP1 | |
| 29 | GO : 0006641 | Triglyceride metabolic process | 3.34E-07 | 1.24E-04 | 5 | APOA5|APOB|APOC3|FABP1|MOGAT2 | |
| 39 | GO : 0006639 | Acylglycerol metabolic process | 1.36E-06 | 3.84E-04 | 5 | APOA5|APOB|APOC3|FABP1|MOGAT2 | |
| 34 | GO : 0070328 | Triglyceride homeostasis | 6.47E-07 | 1.98E-04 | 4 | APOA5|APOC3|NR1H4|PLA2G12B | |
| 36 | GO : 0055090 | Acylglycerol homeostasis | 6.47E-07 | 1.98E-04 | 4 | APOA5|APOC3|NR1H4|PLA2G12B | |
| 46 | GO : 0001676 | Long-chain fatty acid metabolic process | 3.13E-06 | 7.49E-04 | 5 | CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2 | |
| 33 | GO : 0046461 | Neutral lipid catabolic process | 6.47E-07 | 1.98E-04 | 4 | APOA5|APOB|APOC3|FABP1 | |
| 41 | GO : 0006638 | Neutral lipid metabolic process | 1.43E-06 | 3.84E-04 | 5 | APOA5|APOB|APOC3|FABP1|MOGAT2 | |
| Sterols; Steroids | 11 | GO : 0055092 | Sterol homeostasis | 1.06E-08 | 1.06E-05 | 6 | ABCG8|APOA5|APOB|APOC3|NR1H4|PLA2G12B |
| 14 | GO : 0008202 | Steroid metabolic process | 3.17E-08 | 2.50E-05 | 8 | APOA5|APOB|CYP2C19|CYP2C9|CYP3A4|HMGCS2|NR1H4|NR1I2 | |
| 1 | GO : 0006721 | Terpenoid metabolic process | 0.00E + 00 | 1.13E-08 | 9 | ADH4|ADH6|APOB|APOC3|CYP2C18|CYP2C19|CYP2C9|CYP3A4|HMGCS2 | |
| 2 | GO : 0006720 | Isoprenoid metabolic process | 0.00E + 00 | 1.84E-08 | 9 | ADH4|ADH6|APOB|APOC3|CYP2C18|CYP2C19|CYP2C9|CYP3A4|HMGCS2 | |
| 15 | GO : 0001523 | Retinoid metabolic process | 4.89E-08 | 3.59E-05 | 6 | ADH4|ADH6|APOB|APOC3|CYP2C18|CYP3A4 | |
| 16 | GO : 0016101 | Diterpenoid metabolic process | 6.87E-08 | 4.73E-05 | 6 | ADH4|ADH6|APOB|APOC3|CYP2C18|CYP3A4 | |
| 48 | GO : 0034754 | Cellular hormone metabolic process | 3.69E-06 | 8.38E-04 | 5 | ADH4|ADH6|CYP2C18|CYP2C9|CYP3A4 | |
| 24 | GO : 0016098 | Monoterpenoid metabolic process | 1.41E-07 | 6.49E-05 | 3 | CYP2C19|CYP2C9|CYP3A4 | |
| Other | 22 | GO : 0065008 | Regulation of biological quality | 1.32E-07 | 6.49E-05 | 23 | A1CF|ABCG8|ADH4|ADH6|AFM|AGXT|AGXT2|APOA5|APOB|APOC3|CYP2C18|CYP2C9|CYP3A4|CYP4F2|F11|F9|GBA3|HPX|NR1H4|OTC|PLA2G12B|SLC2A2|SLC39A5 |
| 25 | GO : 0015711 | Organic anion transport | 1.88E-07 | 8.30E-05 | 9 | ABCG8|AGXT|APOA5|APOC3|FABP1|NR1H4|PLA2G12B|SLC17A4|SLC2A2 | |
| 40 | GO : 0006820 | Anion transport | 1.42E-06 | 3.84E-04 | 9 | ABCG8|AGXT|APOA5|APOC3|FABP1|NR1H4|PLA2G12B|SLC17A4|SLC2A2 | |
| 32 | GO : 0050892 | Intestinal absorption | 4.46E-07 | 1.54E-04 | 4 | ABCG8|FABP1|MOGAT2|SLC2A2 | |
| 9 | GO : 0044281 | Small-molecule metabolic process | 7.90E-09 | 9.68E-06 | 17 | ADH4|ADH6|AGXT|AGXT2|ALDOB|APOA5|APOB|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|HAO1|HMGCS2|MOGAT2|NR1H4|OTC | |
| 12 | GO : 1901615 | Organic hydroxy compound metabolic process | 1.16E-08 | 1.07E-05 | 10 | ADH4|ADH6|APOA5|APOB|CYP3A4|CYP4F2|HAO1|HMGCS2|MOGAT2|NR1H4 | |
| 23 | GO : 0006066 | Alcohol metabolic process | 1.38E-07 | 6.49E-05 | 8 | ADH4|ADH6|APOA5|APOB|CYP3A4|HAO1|HMGCS2|MOGAT2 | |
| 38 | GO : 0046185 | Aldehyde catabolic process | 1.16E-06 | 3.36E-04 | 3 | ADH4|AGXT|AGXT2 | |
| 49 | GO : 0006081 | Cellular aldehyde metabolic process | 3.80E-06 | 8.38E-04 | 4 | ADH4|AGXT|AGXT2|ALDOB | |
| 13 | GO : 0009056 | Catabolic process | 1.28E-08 | 1.09E-05 | 18 | ADH4|AGXT|AGXT2|ALDOB|APOA5|APOB|APOC3|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|FABP1|GBA3|HAO1|NR1I2|OTC|PLA2G12B | |
| 45 | GO : 1901575 | Organic substance catabolic process | 3.11E-06 | 7.49E-04 | 14 | ADH4|AGXT|AGXT2|ALDOB|APOA5|APOB|APOC3|CYP3A4|CYP4F2|FABP1|GBA3|HAO1|OTC|PLA2G12B | |
| 19 | GO : 0044248 | Cellular catabolic process | 8.67E-08 | 5.03E-05 | 16 | ADH4|AGXT|AGXT2|APOA5|APOB|APOC3|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|FABP1|GBA3|HAO1|NR1I2|OTC | |
| 7 | GO : 0032787 | Monocarboxylic acid metabolic process | 3.70E-09 | 5.83E-06 | 11 | ADH6|AGXT|AGXT2|ALDOB|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|HAO1|NR1H4 | |
| 20 | GO : 0019752 | Carboxylic acid metabolic process | 9.66E-08 | 5.32E-05 | 12 | ADH6|AGXT|AGXT2|ALDOB|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|HAO1|NR1H4|OTC | |
| 28 | GO : 0043436 | Oxoacid metabolic process | 2.70E-07 | 1.06E-04 | 12 | ADH6|AGXT|AGXT2|ALDOB|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|HAO1|NR1H4|OTC | |
| 30 | GO : 0006082 | Organic acid metabolic process | 3.38E-07 | 1.24E-04 | 12 | ADH6|AGXT|AGXT2|ALDOB|CYP2C18|CYP2C19|CYP2C9|CYP3A4|CYP4F2|HAO1|NR1H4|OTC |
Note: The list of genes that are co-expressed with NAT2 (listed in Table 2) were analyzed using GOnet (Pomaznoy et al. 2018) to identify Gene Ontology biological processes (GO terms and IDs) that are enriched by the group. Some GO terms were categorized according to common biological processes they belong to (eg, Xenobiotic Metabolism). Among the co-expressed genes in Table 2, the genes that contribute to each GO term are shown under the column, “Genes.” Only top 50 GO biological processes are shown here.
False discovery rate-adjusted p value (or q value).
Figure 5.
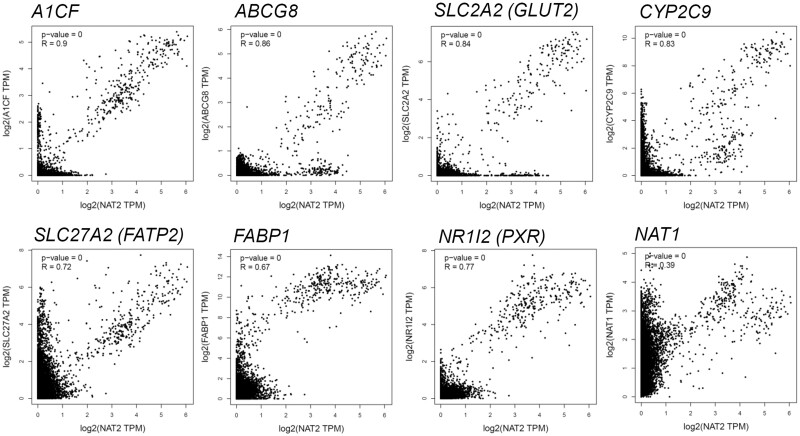
Representative genes which are co-expressed with NAT2 among nondiseased human tissues. The dot plots show the mRNA levels (expressed in log2 of transcripts per kilobase million [TPM]) of NAT2 (x-axis) and the corresponding mRNA level of the gene of interest (eg, A1CF) (y-axis) in the same individual tissue samples. R, Pearson correlation coefficient. The analysis was done using tools available at GEPIA (Tang et al., 2017).
RESULTS
Culture Condition-Dependent Changes in NAT2 mRNA in Liver Cancer Cell Lines
While culturing HepG2 cells, we observed that NAT2 mRNA levels fluctuated substantially depending on the culture conditions. Because no previous reports have described such phenomenon, we investigated this further. Starting with DMEM media devoid of glucose, pyruvate, l-glutamine, and serum (“Control”), we cultured HepG2 cells in media which contained cell culture components/nutrients either individually or in various combinations for 24 h and measured NAT2 mRNA via reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR). Compared with Complete Media (containing 5.5 mM d-glucose, 1 mM pyruvate, 4 mM l-glutamine, and 10% FBS), there was a significant and marked reduction in NAT2 mRNA level in cells cultured in Control DMEM (Figure 1A). Addition of d-glucose (5 mM) or serum (10% FBS) alone was able to recover the NAT2 mRNA level (13.5-fold increase by glucose and 18.2-fold increase by FBS). Although pyruvate (1 mM) or l-glutamine (4 mM) alone was also able to moderately increase the NAT2 mRNA level (2.1- and 6.4-fold, respectively), the induction was not found to be statistically significant (Figure 1A). Combining glucose with pyruvate or combining glucose with pyruvate and l-glutamine did not significantly increase the NAT2 mRNA further, compared with the glucose treatment alone (Figure 1A). However, the NAT2 mRNA level in Complete Media (23.9-fold increase) was significantly higher than that in glucose treatment alone (13.5-fold increase), suggesting a moderately additive effect of serum and other components on NAT2 expression (Figure 1A). Taken together, these results suggest that NAT2 expression is influenced by the presence of glucose and serum in HepG2 cells.
Figure 1.
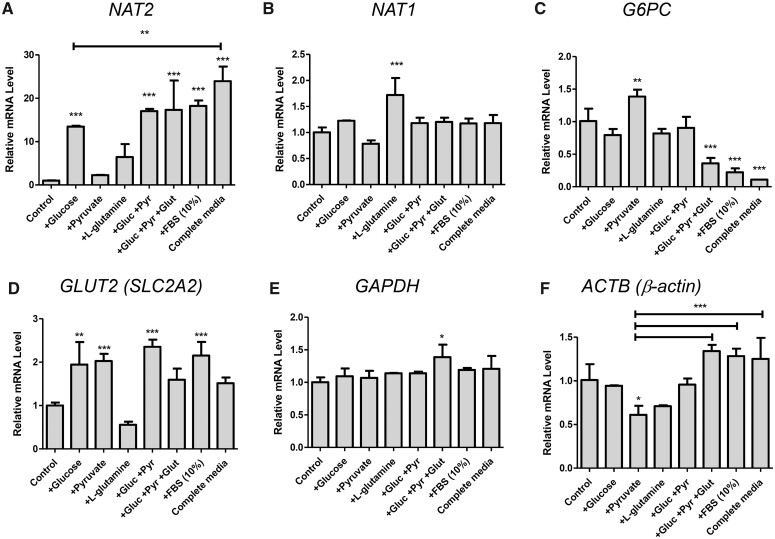
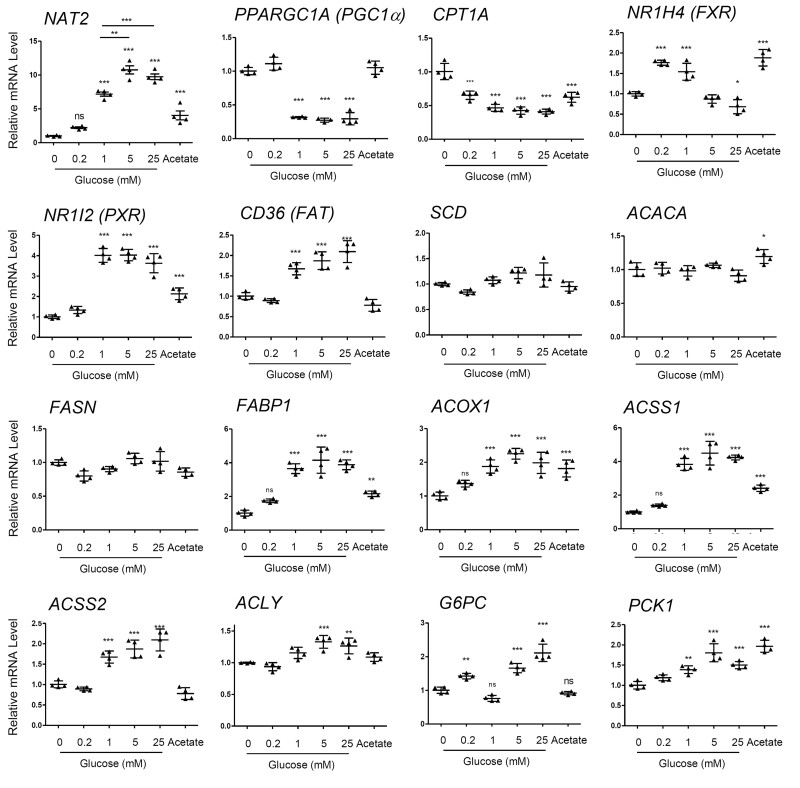
Effect of different culture components on the mRNA levels of NAT2 and other genes assayed for comparison. HepG2 cells were cultured in the presence of the indicated components/nutrients (either individually or in combinations) for 24 h. DMEM free of d-glucose, pyruvate, l-glutamine, and FBS (Control) served as a treatment control. Gluc, d-glucose (5.5 mM). Pyr, pyruvate (1 mM). Glut, l-glutamine (4 mM). “Complete Media” contained all components listed here, including FBS (10%). A–F, The relative mRNA level of the indicated gene was measured by RT-qPCR using 18S ribosomal RNA as an internal control. Bar graphs represent mean ± SD (standard deviation) (n = 4 per group). *p < .05; **p < .01; ***p < .001. The asterisks located above the bars are for the comparison with the control DMEM treatment group (ie, Control), unless otherwise specified. The asterisks above the bracketed lines are for the comparison between 2 treatment groups.
We also examined the mRNA levels of its closely related isozyme, NAT1, and additional genes in the same set of samples (Figure 1). The NAT1 transcript did not share the same culture condition-dependent gene expression pattern with NAT2 and remained relatively steady. For instance, the presence or absence of glucose or serum did not affect the NAT1 transcript level (Figure 1B). Interestingly, there was a modest, yet significant, increase (1.7-fold) in NAT1 mRNA when cells were cultured in l-glutamine alone. However, such effect on NAT1 expression was gone when l-glutamine was combined with other components, such as glucose and pyruvate (Figure 1B). For comparison, we also analyzed the same set of samples for G6PC (glucose-6-phosphatase catalytic subunit 1) mRNA, which is involved in gluconeogenesis and is one of the insulin receptor target genes (Barthel and Schmoll, 2003). Its expression pattern largely contrasted with that of NAT2. Although there was a significant, yet marginal, increase (1.4-fold) in G6PC mRNA in pyruvate-treated cells, glucose failed to induce its level (Figure 1C). Compared with Control DMEM, G6PC transcript was significantly and markedly downregulated when the cells were cultured in complete media (0.11-fold) as well as in media containing serum (0.22-fold) or the combination of components (0.36-fold) (Figure 1C). We additionally examined the expression of GLUT2 (SLC2A2), the major glucose transporter in hepatocytes. Its expression was significantly induced (approximately 2-fold) by glucose, pyruvate, and FBS (Figure 1D). Such induction of GLUT2 was not observed when the cells were treated with l-glutamine alone, and, interestingly, addition of l-glutamine prevented the gene induction by other components (ie, glucose, pyruvate, or FBS). Transcript levels of commonly used housekeeping genes, GAPDH and ACTB (β-actin), remained largely unchanged between culture conditions tested (when normalized to 18S rRNA), although ACTB (β-actin) expression was more variable than GAPDH, questioning its validity as an internal control (Figs. 1E and 1F). Taken together, these results indicated that the culture condition-dependent expression of NAT2 is not a global but a gene-specific phenomenon.
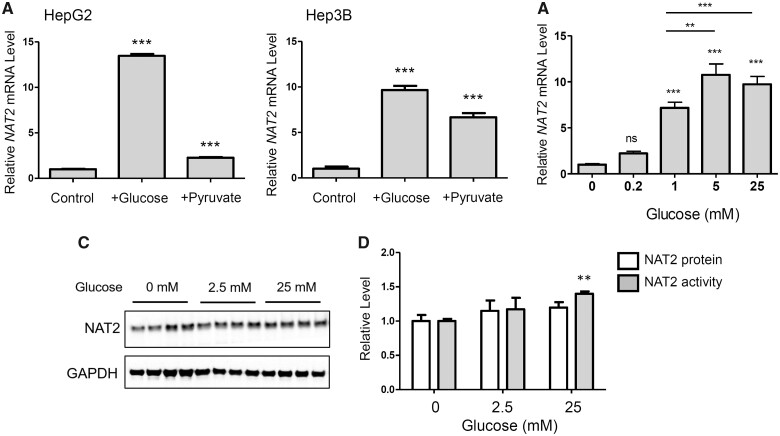
We also tested an additional liver cancer cell line, Hep3B, to see if glucose or pyruvate produced similar effects on NAT2 expression in multiple cell lines. In Hep3B cells, glucose (5 mM) alone significantly increased the relative NAT2 mRNA level (9.7-fold) (Figure 2A). Interestingly, treatment with pyruvate (1 mM) alone also significantly induced NAT2 mRNA (6.7-fold) in Hep3B cells (Figure 2A). The effects of pyruvate on NAT2 mRNA was not as robust with HepG2 cells (2.3-fold increase) in the same experiment (Figure 2A). These results indicate that induction of NAT2 mRNA by glucose is not a cell line-specific effect.
Figure 2.
Induction of NAT2 mRNA and NAT2 N-acetyltransferase activity by glucose in liver cancer cell lines. HepG2 or Hep3B cells were cultured in the presence of the indicated components/nutrients for 24 h. DMEM free of d-glucose, pyruvate, l-glutamine, and FBS served as a treatment control (Control). A, HepG2 (left panel) and Hep3B (right panel) were incubated with 5 mM d-glucose (+Glucose) or 1 mM sodium pyruvate (+Pyruvate) in Control DMEM. The relative NAT2 mRNA level was measured by RT-qPCR using 18S ribosomal RNA as an internal control. B, Concentration-dependent increases in NAT2 mRNA in HepG2 cells by glucose. HepG2 cells was incubated in the indicated concentration of d-glucose in Control DMEM for 24 h. The relative NAT2 mRNA level was measured by RT-qPCR. C, HepG2 cells were incubated in the indicated concentrations of d-glucose (in Control DMEM) for 24 h and analyzed for NAT2 protein level using Western blot (left panel). NAT2 band intensity was quantified and normalized to that of GAPDH and expressed as a relative level (panel D). D, relative level of NAT2 protein and enzymatic activity. HepG2 cells were incubated in the indicated concentrations of d-glucose (in Control DMEM) for 24 h, lysed, and measured for NAT2 protein (white bars) by Western blot or NAT2 activity (gray bars) using sulfamethazine (SMZ) as its substrate. The level of N-acetylated-SMZ (Ac-SMZ) was measured using HPLC and normalized to the time of incubation (min) and total protein amount (mg protein). The protein and activity measurements were expressed as relative values to the untreated control. According to 2-way ANOVA analysis, glucose concentration significantly contributed to variation (p = .0005). Bar graphs represent mean ± SD (n = 4 per group). *, p < .05; **, p < .01; ***, p < .001. ns, not statistically significant. The asterisks located above the bars are for the comparison with the control DMEM treatment group (ie, Control), unless otherwise specified. The asterisks above the bracketed lines are for the comparison between 2 treatment groups.
Concentration-Dependent Induction of NAT2 mRNA by Glucose
To investigate if induction of NAT2 mRNA by glucose was concentration-dependent, we exposed HepG2 cells to different concentrations of glucose (0, 0.2, 1, 5, and 25 mM) in Control DMEM for 24 h. Between 0 and 5 mM glucose, the relative level of NAT2 mRNA increased in a concentration-dependent manner (Figure 2B). However, the mRNA level peaked at 5 mM glucose, and did not increase further at 25 mM treatment (Figure 2B).
Changes in NAT2 Protein Level and N-Acetyltransferase Activity by Glucose
HepG2 cells were incubated in Control DMEM containing 0, 2.5, or 25 mM of d-glucose for 24 h, and the treated cells were analyzed by Western blot using an antibody against human NAT2 (Figure 2C). Following the glucose treatment, NAT2 activity was measured in vitro in the presence of a NAT2-selective substrate, SMZ and acetyl-CoA. There was a significant increase (1.4-fold) in in vitro NAT2 activity following treatment with 25 mM glucose, compared with the no-glucose treatment group (Figure 2D), and there was significant variation in both protein and activity with respect to concentration (p = .005). Glucose treatment marginally increased NAT2 protein to an extent that did not differ significantly from its increase in NAT2 catalytic activity.
Induction of NAT2 by Insulin
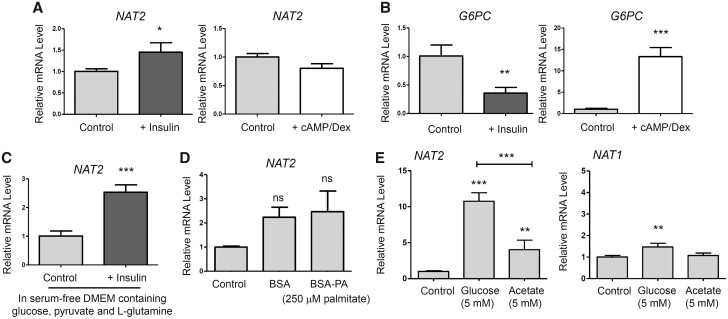
Previous studies on mouse NAT1 showed that it plays an important role in maintaining insulin sensitivity in mice (Camporez et al., 2017; Chennamsetty et al., 2016; Knowles et al., 2015). We questioned if NAT2 mRNA expression was controlled by insulin or a fasting condition in HepG2 cells. Insulin (100 nM) treatment for 24 h significantly induced NAT2 mRNA in HepG2 cells (Figure 3A). The level of induction (1.4-fold), however, was not as robust as that observed with glucose treatments (13.5-fold; see Figure 1A). We also subjected the cells to treatment with 8-bromo-cAMP and dexamethasone (cAMP/Dex), which mimics the effect of glucagon and “starvation” condition in vitro. cAMP/Dex did not alter or further decrease the NAT2 mRNA level (Figure 3A). G6PC served as the treatment control. As expected, insulin treatment significantly reduced G6PC mRNA (0.36-fold), whereas cAMP/Dex treatment significantly induced its expression (13.3-fold) (Figure 3B). Next, we examined if insulin can induce NAT2 expression even in the presence of normal, serum-free, culture media containing glucose, pyruvate, and l-glutamine. Insulin treatment (100 nM for 6 h) induced NAT2 mRNA expression (approximately 2.5-fold) even in the presence of glucose (Figure 3C). This finding indicates that insulin induces NAT2 expression in HepG2 cells and that NAT2 may be a novel insulin receptor target gene.
Figure 3.
Effect of insulin, cAMP/Dex, palmitate, or acetate on mRNA levels of NAT2, G6PC, and NAT1. A and B, HepG2 cells were cultured in the presence of insulin (100 nM) (+ Insulin) or 8-br-cAMP (500 µM) + dexamethasone (1 µM) (+ cAMP/Dex). DMEM free of d-glucose, pyruvate, l-glutamine, and FBS served as a treatment control (Control). C, HepG2 cells were cultured in serum-free DMEM containing d-glucose, pyruvate, and l-glutamine, plus insulin (100 nM) (+ Insulin) for 6 h. D, HepG2 cells were cultured in Control DMEM containing 0.5% BSA or 0.5% BSA-250 µM palmitate (BSA-PA) for 24 h. E, HepG2 cells were cultured in Control DMEM containing 5 mM d-glucose or 5 mM acetate for 24 h. Following the treatments, the relative mRNA level of the indicated gene (NAT2, G6PC, or NAT1) was measured by RT-qPCR using 18S ribosomal RNA as an internal control. Bar graphs represent mean ± SD (n = 4 per group). *, p < .05; **, p < .01; ***, p < .001. ns, not statistically significant. The asterisks located above the bars are for the comparison with the control DMEM treatment group (ie, Control), unless otherwise specified. The asterisks above the bracketed lines are for the comparison between 2 treatment groups.
We next questioned if NAT2 expression is influenced by free fatty acids, an alternative energy source. HepG2 cells were treated with BSA (control) or BSA-conjugated palmitic acid (BSA-PA; 250 µM) in Control DMEM for 24 h. In contrast to the glucose treatment, BSA-PA treatment did not modulate the NAT2 mRNA level (Figure 3D).
Induction of NAT2 Expression by Acetate
Our group has demonstrated previously that NAT1 hydrolyzes acetyl-CoA in the presence of folate (Stepp et al., 2015), and CRISPR/Cas9-mediated deletion of NAT1 in MDA-MB-231 breast cancer cells leads to a significant increase in cellular acetyl-CoA levels (Stepp et al., 2019). These findings suggest that NAT1 regulates cellular acetyl-CoA level. Based on the link between NAT1 and acetyl-CoA, we questioned if NAT2 expression can be regulated reciprocally by the cellular acetyl-CoA level in HepG2 cells. Acetyl-CoA can be generated from acetate in various types of cancers (Mashimo et al., 2014), which is catalyzed by acetyl-CoA synthetase 1 and 2 (ACSS1 and ACSS2) in mammals (Fujino et al., 2001; Luong et al., 2000). To test this, we treated HepG2 cells with 5 mM glucose or 5 mM acetate in Control DMEM for 24 h and measured the changes in the NAT2 mRNA level. The acetate concentration (5 mM) used in the study was based on previous studies (Gao et al., 2016). Acetate treatment led to a significant increase in NAT2 mRNA (4.0-fold) (Figure 3E), although the level of induction was not as high as the one in the glucose (5 mM) treatment group (10.7-fold) (Figure 3E). In contrast, NAT1 transcript was not induced by acetate (Figure 3E).
Genes Involved in Glucose and Lipid Metabolism and Transport
To explore the mechanism of glucose-mediated transcriptional regulation of NAT2, we sought to compare the pattern of NAT2 mRNA expression to the expression of genes involved in glucose and lipid metabolism and transport. Gene polymorphisms in human NAT2 have been previously linked to insulin resistance and differential plasma lipid levels (Hoffmann et al., 2018; Knowles et al., 2015; Willer et al., 2013), which raises the possibility that NAT2 expression and/or activity may be regulated by energy or metabolic state of the cell. To explore this possibility, we screened a set of genes implicated in cellular metabolism to see if their gene expression patterns resembled that of NAT2 in HepG2 cells. For this, we characterized glucose- and acetate-dependent changes in the transcript levels of multiple genes.
PPARGC1A and CPT1A
PGC1α (a.k.a., PPARGC1A) regulates the expression of key mitochondrial genes and is an important regulator of mitochondrial biogenesis, and CPT1A (carnitine palmitoyl transferase 1A; CPT1) catalyzes an essential step for the mitochondrial uptake of long-chain fatty acids and their subsequent β-oxidation. Glucose-dependent changes in the expression of PGC1α and CPT1A contrasted sharply with that of NAT2. PGC1α and CPT1A were significantly downregulated in the presence of glucose (Figure 4). This likely reflects the switch to glycolysis (vs oxidative phosphorylation) and a reduction in utilization of β-oxidation of fatty acids upon introduction of glucose in the culture media, and suggests that the cellular roles of NAT2 are different from the ones played by PGC1α and CPT1A.
Figure 4.
Glucose and acetate-dependent expression patterns of genes involved in glucose and lipid metabolism. HepG2 cells were cultured in the indicated concentration of d-glucose or 5 mM acetate (Acetate) for 24 h. The relative mRNA level of the indicated gene (eg, NAT2) was measured by RT-qPCR using 18S ribosomal RNA as an internal control. The horizontal lines represent mean ± SD (n = 4 per group). *, p < .05; **, p < .01; ***, p < .001. ns, not statistically significant. The asterisks or any marks located above the lines are for the comparison with the control DMEM treatment group (ie, Control).
FXR and PXR
Although NAT1 is ubiquitously expressed in various organs and tissues, expression of NAT2 is limited to specific organs, including liver and intestines (Sim et al., 2014), suggesting NAT2 expression is likely regulated by tissue-specific transcription factors. We examined the glucose-dependent mRNA expression patterns of 2 transcription factors that are known to mediate tissue-specific (ie, liver and intestines) gene expression, FXR (farnesoid X receptor; NR1H4), and PXR (pregnane X receptor; NR1I2). FXR, which is a regulator of bile acid synthesis and also regulates lipid and glucose homeostasis (Preidis et al., 2017), exhibited an interesting biphasic pattern where its expression was upregulated at low concentrations of glucose (0.2 and 1 mM) but downregulated at higher concentrations (5 and 25 mM) (Figure 4). PXR is known to regulate genes involved in xenobiotic metabolism and secretion, but also has been implicated in energy metabolism (Wada et al., 2009). There was a concentration-dependent increase in PXR mRNA by glucose, the pattern of which was similar to that of NAT2 (Figure 4).
Genes in fatty acid metabolism and transport
Gene polymorphisms in human NAT2 have been previously linked to differential plasma lipid levels (Hoffmann et al., 2018; Willer et al., 2013), which led to the question if NAT2 expression and/or activity is co-regulated along with genes involved in fatty acid metabolism and transport. We observed glucose- and acetate-dependent changes in the transcript levels of multiple genes involved in this category. These included CD36 (fatty acid transporter; FAT), SCD (acyl-CoA desaturase), ACACA (acetyl-CoA carboxylase alpha; ACC), FASN (fatty acid synthase; FAS), FABP1 (fatty acid binding protein 1), and ACOX1 (acyl-CoA oxidase 1). The transcript levels of SCD, ACACA, and FASN, which are involved in fatty acid synthesis, did not change dynamically in response to glucose or acetate treatment in HepG2 cells (Figure 4). Among the genes tested, FABP1 and CD36 (FAT), which are involved in fatty acid transport, showed glucose concentration-dependent induction, and their expression patterns largely coincided with that of NAT2 (Figure 4). ACOX1 which mediates the first step in β-oxidation of fatty acids also showed a similar pattern of expression in response to glucose and acetate (Figure 4).
Genes in acetyl-CoA synthesis and gluconeogenesis
Our analysis included genes involved in acetyl-CoA synthesis. ACLY (ATP Citrate Lyase) catalyzes the cleavage of citrate into oxaloacetate and acetyl-CoA, and ACSS1 and ACSS2 are known to generate acetyl-CoA from free acetate (Fujino et al., 2001; Luong et al., 2000). The glucose and acetate-dependent pattern of the genes encoding them was similar to that of NAT2 (Figure 4). G6PC and PCK1 represented gluconeogenic genes. G6PC induction by glucose was biphasic in which there was a significant induction at 0.2, 5.0, or 25 mM glucose but not at 1 mM, and acetate failed to induce G6PC (Figure 4). In contrast, PCK1 showed concentration-dependent increases in mRNA by glucose and also was induced by acetate alone. Interestingly, the level of PCK1 induction by acetate was comparable with that by 25 mM glucose. Hence, the expression patterns of G6PC and PCK1 were not only dissimilar to each other’s but also to that of NAT2.
Genes co-expressed with NAT2: In silico analysis
In order to better understand the transcriptional regulation of NAT2 and its energy metabolism-related roles (eg, in insulin sensitivity), we analyzed genes whose expression patterns in various human tissues resembled that of NAT2 (ie, “co-expressed” genes), using publicly available gene expression databases and analytical tools available at GEPIA (Tang et al., 2017), COXPRESdb (Obayashi et al., 2019), SEEK (Zhu et al., 2015), and Archs4 (Lachmann et al., 2018). The list of co-expressed genes differed depending on the algorithm (Table 2). We compared the lists of the top 50 co-expressed genes from all 4 engines and selected the ones that appeared more than once. This retrieved a total of 40 genes (Table 3). The only gene that appeared on all 4 lists was NR1I2 which encodes pregnane X receptor (PXR). There were 13 genes that appeared on 3 out of 4 lists, and they included FABP1, MOGAT2 (monoacylglycerol O-acyltransferase 2), and GLUT2 (SLC2A2) (Table 3).
The list of co-expressed genes was then analyzed for biological pathways (Gene Ontology [GO] terms) that are significantly enriched, using GONet (Pomaznoy et al., 2018). Genes encoding xenobiotic metabolizing enzymes, such as ADH4, ADH6, CYP2C18, CYP2C19, CYP2C9, CYP3A4, CYP4F2, HAO1, and NR1I2 (PXR) contributed to the enrichment of biological processes related to xenobiotic or drug metabolism (Tables 3 and 4). The GO terms in this category included “drug metabolic process” (GO: 0017144), “epoxygenase P450 pathway” (GO: 0019373), and “xenobiotic metabolic process” (GO: 0006805), and “cellular response to xenobiotic stimulus” (GO: 0071466) (Table 4). Similarly, among the co-expressed genes which were not included in the final list, there were also multiple genes encoding phase II metabolic enzymes including sulfotransferases (SULT1B1 and SULT2A1) and UDP-glucuronosyltransferases (UGT1A4, UGT2A3, UGT2B10, and UGT2B15) (Table 2), suggesting that NAT2 expression is co-regulated along with other genes involved in xenobiotic, phase II metabolism.
Interestingly, GO terms that are related to cholesterol, triglyceride, lipid, and lipoprotein synthesis and transport were overwhelmingly enriched among the selected 40 genes (Table 4). These included “cholesterol homeostasis” (GO: 0042632), “lipid homeostasis” (GO: 0055088), “triglyceride homeostasis” (GO: 0070328), “regulation of cholesterol transport” (GO: 0032374), “plasma lipoprotein particle assembly” (GO: 0034377), and “plasma lipoprotein particle remodeling” (GO: 0034369) (Table 4). Among the co-expressed genes, APOA5, APOB, APOC2, APOC3, ABCG8, ANGPTL3, FABP1, MOGAT2, and PLA2G12B contributed to the enrichment of these biological processes (Tables 3 and 4).
Next, we examined the correlation between the transcript levels of NAT2 and those of individual genes from the list using the GTEx (Genotype-Tissue Expression) dataset (non-diseased human tissues) and graphing and statistical tools available at GEPIA (Tang et al., 2017). Among the genes whose expression highly correlated with that of NAT2 (Pearson correlation coefficient, R > 0.8), A1CF (APOBEC1 complementation factor) showed the highest correlation with NAT2 with a R value of 0.9 (Figure 5). Others included ABCG8 (ATP-binding cassette sub-family G member 8) (R = 0.86), GLUT2 (glucose transporter 2; SLC2A2) (R = 0.84), CYP2C9 (R = 0.83), SLC27A2 (a.k.a., FATP2, fatty acid transport protein 2) (R = 0.72) and FABP1 (R = 0.67), (Figure 5). ABCG8 is involved in transport of cholesterol and sterols (Kerr et al., 2021). CYP2C9 is a member of the cytochrome P450 superfamily of enzymes and the most abundantly expressed human CYP2C isoform in the liver (Miners and Birkett, 1998). SLC27A2 (FATP2) activates long-chain fatty acids as a very long-chain acyl-CoA synthetase and also transports long-chain fatty acids as a fatty acid transporter (Qiu et al., 2020). Expression of NR1I2 (PXR), which was the only gene that was identified to be co-expressed with NAT2 by all 4 engines (Table 2), also correlated with that of NAT2 (R = 0.77). In contrast, NAT1, whose expression is normally found in multiple tissues and organs, did not correlate well with NAT2 (R = 0.39) whose expression is largely restricted to liver and intestines (Figure 5).
DISCUSSION
The current study shows, for the first time, that human NAT2 expression at the transcript level and its enzymatic activity is regulated by glucose in hepatocellular carcinoma cell lines. It has not been determined, however, if NAT2 is transcriptionally regulated similarly in normal human hepatocytes or liver. It is possible that liver cancer cells (eg, HepG2) are more sensitive to changes in the glucose level due to their dependency on glycolysis for energy production (ie, Warburg effect). We also speculate that it is not glucose per se, but rather its metabolism or metabolites that are responsible for the changes in the NAT2 expression. This is supported by the current finding that reduced NAT2 expression in glucose-depleted media is recovered, in part, by pyruvate, a metabolite in the glycolytic pathway, (see Figure 2A) or acetate supplementation (see Figure 3E). Notably, pyruvate can be decarboxylated and converted to acetyl-CoA by the mitochondrial pyruvate dehydrogenase complex, and free acetate can be converted to acetyl-CoA by acyl-CoA short-chain synthetases, ACSS1 and ACSS2 (Moffett et al., 2020). It would require additional studies to determine if glucose induces NAT2 expression in normal human hepatocytes and if such transcriptional regulation is mediated by its downstream metabolites, such as acetyl-CoA.
Glucose plays a central role in metabolism, serving as the preferred source of energy and carbon. Glucose (and its metabolites) induces changes in metabolism by affecting gene expression in multiple tissues and cell types, including pancreatic β islet cells, adipose tissue, and liver. One mechanism by which glucose influences the expression of metabolic genes is through carbohydrate response element binding protein (ChREBP; MLXIPL), an important regulator of an intracellular carbohydrate-sensing mechanism (Ortega-Prieto and Postic, 2019). ChREBP expression is present in multiple organs in the gut and adipose tissues, but most abundant in active sites of de novo lipogenesis including liver and adipose tissues (Iizuka et al., 2004). In response to glucose and its metabolites (eg, glucose-6-phosphate), ChREBP undergoes post-translational modifications that modulate its subcellular localization, protein stability and/or its transcriptional activity (Ortega-Prieto and Postic, 2019). Once activated, it binds to a cis-acting regulatory element called “carbohydrate response element” and transactivates the target genes. Its target genes encode key enzymes of de novo lipogenesis, such as fatty acid synthase (FAS; FASN), acetyl-CoA carboxylase (ACC; ACACA) and stearoyl-CoA desaturase (SCD) (Kawaguchi et al., 2001). In the liver, it is also a key regulator of glycolysis and VLDL secretion by modulating expression of l-pyruvate kinase (PKLR), a rate-limiting enzyme in glycolysis, and microsomal triglyceride transfer protein (MTTP), respectively (Kawaguchi et al., 2001). In the intestine, ChREBP stimulates expression of sucrase-isomaltase (SI), GLUT5 (SLC2A5), GLUT2 (SLC2A2) and ketohexokinase (KHK), which improves sucrose tolerance and fructose absorption (Kato et al., 2018). Thus, one of the possible mechanisms by which glucose regulates NAT2 expression in HepG2 cells is via ChREBP. However, it should be noted that in the current study, glucose did not affect or only had marginal effects on mRNA expression of known ChREBP target genes, such as FASN, ACACA, and SCD (see Figure 4), suggesting the experimental conditions used in the study might have been insufficient to activate ChREBP in HepG2 cells.
Another mechanism by which glucose influences gene expression and cellular metabolism is through insulin. Elevated blood glucose induces secretion of insulin which suppresses gluconeogenesis and promotes lipogenesis in the liver. Genes that are involved in lipogenesis are induced by insulin in hepatocytes, and this is mediated through the transcription factor, sterol regulatory element-binding protein 1 (SREBF1) (Horton et al., 2002). Activation of SREBF1 promotes fatty acid and cholesterol biosynthesis, for its target genes include the rate-limiting lipogenic and cholesterol biosynthetic genes, such as fatty acid synthase (FASN; FAS), acetyl-CoA carboxylase (ACC1; ACACA), HMG-CoA reductase, and the LDL receptor (Osborne and Espenshade, 2009). Another major effect of insulin on liver is inhibition of hepatic gluconeogenesis (ie, de novo synthesis of glucose), and it does so by suppressing expression of genes involved in this process, such as G6PC (Streeper et al., 1997) and PCK1 (phosphoenolpyruvate carboxykinase 1; PEPCK) (Granner et al., 1983). Conversely, these gluconeogenic genes are induced by glucagon and glucocorticoids during fasting and stress, respectively (Lamers et al., 1982; Schmoll et al., 1999). In accordance to this, we observed that (overnight) insulin treatment significantly reduced the G6PC mRNA level in HepG2 cells, whereas treatment with cAMP/Dex (which is commonly used to mimic the effects of glucagon and glucocorticoids) markedly upregulated it (see Figure 3B). In contrast to G6PC, insulin induced a marginal, yet significant, increase in NAT2 mRNA while cAMP/Dex did not alter NAT2 expression (see Figure 3A). Moreover, NAT2 transcript was significantly induced following only a 6-h treatment with insulin in the presence of glucose (5 mM) (see Figure 3C), indicating that NAT2 may be a novel insulin receptor target gene in hepatocytes. A recent study reported that reduction in mouse NAT1 level contributes to cerebral endothelial necroptosis and Aβ accumulation in Alzheimer’s disease (Zou et al., 2020). The authors showed that insulin induces mouse Nat1 expression in a concentration-dependent manner in bEnd.3, a mouse endothelioma cell line (Zou et al., 2020). In addition, cerebro-microvessels isolated from endothelial cell-specific insulin receptor KO mice express reduced levels of both mRNA and protein of mouse Nat1, suggesting that insulin regulates expression of mouse Nat1 in endothelial cells in vivo (Zou et al., 2020). These previous and current findings collectively suggest that expression of human NAT2 (and mouse Nat1) is regulated by insulin in multiple cell types, including hepatocytes and endothelial cells.
Although the biological significance of the current findings would require additional studies, human NAT2 may be involved in cellular and physiological processes brought by elevated glucose level and insulin actions. Based on previous studies performed in murine Nat1 KO mice (Camporez et al., 2017; Chennamsetty et al., 2016; Knowles et al., 2015), such regulation of NAT2 expression by insulin appears to be important in maintaining insulin sensitivity. One possibility is that human NAT2 may be involved in lipid and/or cholesterol biosynthesis and transport. First, its expression is induced by insulin and glucose along with other lipogenic genes (see discussions above). Second, a group of genes that are co-expressed with human NAT2 (eg, APOA5, APOB, APOC2, APOC3, ABCG8, ANGPTL3, FABP1, MOGAT2, and PLA2G12B) (see Tables 3 and 4) is involved in lipid and cholesterol biosynthesis and transport. For example, MOGAT2 (2-acylglycerol O-acyltransferase 2; MGAT2) catalyzes the synthesis of diacylglycerol from 2-monoacylglycerol and fatty acyl-CoA as a part of the triglyceride biosynthetic process. It also plays a role in uptake of dietary fat by the small intestine (Cao et al., 2004). As a result, the enrichment analysis revealed that the GO terms that describe cholesterol, triglyceride, lipid, and lipoprotein synthesis and transport are overwhelmingly enriched among co-expressed genes (see Table 4).
Third, the novel link between human NAT2 and lipid and cholesterol metabolism is also supported by multiple GWAS. In particular, one of NAT2 single-nucleotide polymorphisms (SNPs), rs1495741, has been linked to differential plasma lipid and (LDL and total) cholesterol, as well as apolipoprotein B levels by independent GWAS (Hoffmann et al., 2018; Richardson et al., 2020; Ripatti et al., 2020; Willer et al., 2013). SNP rs1495741 is located approximately 14 kb downstream of human NAT2 coding region and thus represents a non-coding, inter-genic SNP. We have previously reported that rs1495741 (a.k.a., “tag SNP”) genotype is a good predictor of the NAT2 acetylator phenotype (García-Closas et al., 2011). In that study, the N-acetyltransferase activity of NAT2 was measured in human hepatocytes from 154 individuals and compared between genotypes at rs1495741 (ie, GG, GA, vs AA). Hepatocytes from individuals that carry the A allele exhibit significantly lower NAT2 activity compared with those with the G allele, and thus GG, GA, and AA alleles of rs1495741 predicts rapid, intermediate, and slow acetylator phenotypes, respectively (García-Closas et al., 2011). The aforementioned GWAS investigating the genetic variants associated with differential serum lipid levels reported that the G (ie, rapid) allele of rs1495741 is associated with increased serum triglyceride and LDL and total cholesterol levels (Hoffmann et al., 2018; Ripatti et al., 2020). Collectively, these findings suggest that individuals carrying rapid alleles of NAT2 (eg, G allele at rs1495741) are likely to produce higher serum lipid levels and imply that higher NAT2 activity may contribute to the development of dyslipidemia. Although there are differences between human and rat NAT2, nevertheless, rapid acetylator rats congenic for Nat2 exhibit greater dyslipidemia than slow acetylator rats (Hong et al., 2020). The GWAS and rat study findings support a hypothesis that human NAT2 is involved in metabolism and transport of lipid and cholesterol. Although the mechanism is unknown, the inter-genic NAT2 SNP (ie, rs1495741) may represent an important cis-acting element (eg, enhancer) that regulates NAT2 expression and NAT2 activity is a novel determinant of the serum lipid levels.
According to the analysis of the GTEx dataset (see Figure 5), the gene that shows the highest correlation with NAT2 in terms of its transcript level in various human tissues is A1CF (APOBEC1 complementation factor). Of note, genetic polymorphisms in A1CF also have been implicated in differential serum triglyceride and cholesterol levels by GWAS (Liu et al., 2017; Sinnott-Armstrong et al., 2021). A1CF is expressed only in liver, intestine, and kidney, with the highest levels in the liver, and its hepatic expression in humans is decreased in obese subjects compared with that in lean donors (Nikolaou et al., 2019). A1CF was originally identified as an AU-rich RNA-binding protein that regulates RNA editing activity of the cytidine deaminase APOBEC1 and posttranscriptional editing of ApoB mRNA (Mehta et al., 2000). However, studies in A1cf KO mice revealed that A1CF is dispensable for hepatic ApoB mRNA editing and expression (Snyder et al., 2017). For this reason, the cellular functions of A1CF had remained elusive until recently. In a recent study (Nikolaou et al., 2019), investigators reported that A1CF functions as a hepatocyte-specific regulator of alternative splicing. The authors showed that it regulates alternative splicing of specific genes that are involved in fructose, lipid, and glycerophospholipid metabolism, such as ketohexokinase (Khk) and glycerol kinase (Gk) genes (Nikolaou et al., 2019). Interestingly, liver-specific A1cf KO mice showed a slight increase in serum triglyceride under fed state and improvement in glucose homeostasis (Nikolaou et al., 2019), suggesting A1CF is involved in regulation of lipid and glucose homeostasis via controlling liver-specific alternative splicing of its target genes. In another recent paper, Blanc et al. studied transgenic mice that overexpress A1cf under the liver-specific ApoE enhancer (Blanc et al., 2021). The authors observed that hepatic overexpression of A1cf resulted in spontaneous hepatic steatosis in young mice, and this was concomitant with increases in expression of genes involved in fatty acid uptake and lipogenesis, including Cidea, Mogat1, Mogat2, and Cd36 (Blanc et al., 2021). Because the expression patterns of NAT2 and A1CF are highly correlative, it would be of interest to examine the role of NAT2 in the context of regulation of lipid metabolism by A1CF.
The glucose treatment significantly increased NAT2 catalytic activity. It marginally increased NAT2 protein to an extent that did not differ significantly from its increase in NAT2 catalytic activity (see Figs. 2C and 2D), suggesting that the increase in catalytic activity is secondary to the increase in protein. This contrasted with the robust increase in NAT2 mRNA by glucose. Previous studies have reported similar cases in which changes in NAT2 mRNA did not correlate with changes NAT2 protein/activity. A transcriptomics study by Carlisle et al. (2021) reported that one of the transcripts that are highly and significantly upregulated in NAT1 KO MDA-MB-231 cells (compared with the parental cells) is NAT2, and yet, the authors were not able to detect NAT2 enzyme activity in NAT1 KO cells. In a study by Salazar-González et al. (2020), an in-cell Western technique was used to measure NAT2 protein levels in cryopreserved human hepatocytes with distinctive acetylator genotypes (ie, rapid, intermediate, and slow acetylators) and found that NAT2 protein level correlated well with the acetylator genotype. The NAT2 mRNA level, however, did not correlate with either NAT2 protein level or the acetylator genotype, indicating that NAT2 mRNA level is a poor predictor of the NAT2 protein or activity levels in human hepatocytes. An additional factor that needs to be considered is the acetylator genotype of HepG2 cells used in the current study. Our analysis showed that HepG2 has NAT2*6A and NAT2*14C alleles, and thus is a slow acetylator. Given that HepG2 carries 2 slow acetylator alleles, it is not surprising that the NAT2 transcriptional upregulation conferred by glucose did not directly translate into a similar increase in protein and/or enzymatic activity. Future investigations are necessary to understand the actual phenotypic effects of this association. The underlying mechanism for such disconnects between NAT2 mRNA and protein levels observed in the previous and present studies is unknown. It is possible that NAT2 is regulated at the post-transcriptional level, for recent studies have identified microRNAs that target NAT2 (Yang et al., 2019).
In summary, expression of human NAT2 was upregulated by glucose and insulin in liver cancer cell lines, indicating that NAT2 expression can change dynamically depending on the energy and metabolic status of the cells. The pattern of culture condition-dependent NAT2 expression as well as its expression in human tissues suggests a novel role of NAT2 in lipid and cholesterol metabolism and transport. We only examined transcriptional regulation of NAT2 in cancer cells of hepatocyte origin. It is currently unknown if the same mechanism of regulation is present in normal human hepatocytes or other normal human cells. Additional studies are required to (1) validate the current findings in normal human hepatocytes; (2) explore possible mechanisms of transcriptional regulation of NAT2 by glucose and insulin; and (3) test if NAT2 is involved in lipid and cholesterol metabolism.
FUNDING
National Institutes of Health (NIEHS [National Institute of Environmental Health Sciences] T32-ES011564, NIEHS P30-ES030283, and NIGMS [National Institute of General Medical Sciences] P20-GM113226).
AUTHORSHIP CONTRIBUTIONS
K.U.H.: Conceptualization, project administration, investigation, formal analysis, validation, visualization, writing—original draft preparation. R.A.S.-G.: Investigation, formal analysis, visualization, writing—review and editing. K.M.W.: Investigation, formal analysis, visualization, writing—review and editing. D.W.H.: Conceptualization, supervision, project administration, funding acquisition, writing—review and editing.
DECLARATION OF CONFLICTING INTERESTS
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Contributor Information
Kyung U Hong, Department of Pharmacology & Toxicology and Brown Cancer Center, University of Louisville School of Medicine, Louisville, Kentucky 40202, USA.
Raúl A Salazar-González, Department of Pharmacology & Toxicology and Brown Cancer Center, University of Louisville School of Medicine, Louisville, Kentucky 40202, USA.
Kennedy M Walls, Department of Pharmacology & Toxicology and Brown Cancer Center, University of Louisville School of Medicine, Louisville, Kentucky 40202, USA.
David W Hein, Department of Pharmacology & Toxicology and Brown Cancer Center, University of Louisville School of Medicine, Louisville, Kentucky 40202, USA.
REFERENCES
- Barthel A., Schmoll D. (2003). Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 285, E685–E692. [DOI] [PubMed] [Google Scholar]
- Blanc V., Riordan J. D., Soleymanjahi S., Nadeau J. H., Ilk N., Xie Y., Molitor E. A., Madison B. B., Brunt E. M., Mills J. C., et al. (2021). Apobec1 complementation factor overexpression promotes hepatic steatosis, fibrosis, and hepatocellular cancer. J. Clin. Invest. 131, 138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camporez J. P., Wang Y., Faarkrog K., Chukijrungroat N., Petersen K. F., Shulman G. I. (2017). Mechanism by which arylamine N-acetyltransferase 1 ablation causes insulin resistance in mice. Proc. Natl. Acad. Sci. U.S.A. 114, E11285–E11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Hawkins E., Brozinick J., Liu X., Zhang H., Burn P., Shi Y. (2004). A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J. Biol. Chem. 279, 18878–18886. [DOI] [PubMed] [Google Scholar]
- Carlisle S. M., Trainor P. J., Doll M. A., Hein D. W. (2021). Human arylamine N-acetyltransferase 1 (NAT1) knockout in MDA-MB-231 breast cancer cell lines leads to transcription of NAT2. Front. Pharmacol. 12, 803254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennamsetty I., Coronado M., Contrepois K., Keller M. P., Carcamo-Orive I., Sandin J., Fajardo G., Whittle A. J., Fathzadeh M., Snyder M., et al. (2016). Nat1 deficiency is associated with mitochondrial dysfunction and exercise intolerance in mice. Cell Rep. 17, 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin S. P., Hügl S. R., Wrede C. E., Kajio H., Myers M. G., Rhodes C. J. (2001). Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology 142, 229–240. [DOI] [PubMed] [Google Scholar]
- Doll M. A., Hein D. W. (2001). Comprehensive human NAT2 genotype method using single nucleotide polymorphism-specific polymerase chain reaction primers and fluorogenic probes. Anal. Biochem. 288, 106–108. [DOI] [PubMed] [Google Scholar]
- Fathzadeh M., Hein D. W., Knowles J. W. (2017). The human arylamine N-acetyltransferase type 2 gene: Genomics and cardiometabolic risk. In: Arylamine N-Acetyltransferases in Health and Disease, pp. 43–67. World Scientific. Available at: https://www.worldscientific.com/doi/10.1142/9789813232013_0002. Accessed 2022 May 18. [Google Scholar]
- Fujino T., Kondo J., Ishikawa M., Morikawa K., Yamamoto T. T. (2001). Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 276, 11420–11426. [DOI] [PubMed] [Google Scholar]
- Gao X., Lin S.-H., Ren F., Li J.-T., Chen J.-J., Yao C.-B., Yang H.-B., Jiang S.-X., Yan G.-Q., Wang D., et al. (2016). Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 7, 11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Closas M., Hein D. W., Silverman D., Malats N., Yeager M., Jacobs K., Doll M. A., Figueroa J. D., Baris D., Schwenn M., et al. (2011). A single nucleotide polymorphism tags variation in the arylamine N-acetyltransferase 2 phenotype in populations of European background. Pharmacogenet Genomics 21, 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granner D., Andreone T., Sasaki K., Beale E. (1983). Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature 305, 549–551. [DOI] [PubMed] [Google Scholar]
- Hein D. W. (2002). Molecular genetics and function of NAT1 and NAT2: Role in aromatic amine metabolism and carcinogenesis. Mutat. Res. 506–507, 65–77. [DOI] [PubMed] [Google Scholar]
- Hein D. W., Doll M. A., Nerland D. E., Fretland A. J. (2006). Tissue distribution of N-acetyltransferase 1 and 2 catalyzing the N-acetylation of 4-aminobiphenyl and O-acetylation of N-hydroxy-4-aminobiphenyl in the congenic rapid and slow acetylator Syrian hamster. Mol. Carcinog. 45, 230–238. [DOI] [PubMed] [Google Scholar]
- Hoffmann T. J., Theusch E., Haldar T., Ranatunga D. K., Jorgenson E., Medina M. W., Kvale M. N., Kwok P.-Y., Schaefer C., Krauss R. M., et al. (2018). A large electronic-health-record-based genome-wide study of serum lipids. Nat. Genet. 50, 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K. U., Doll M. A., Lykoudi A., Salazar-González R. A., Habil M. R., Walls K. M., Bakr A. F., Ghare S. S., Barve S. S., Arteel G. E., et al. (2020). Acetylator genotype-dependent dyslipidemia in rats congenic for N-acetyltransferase 2. Toxicol. Rep. 7, 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. D., Goldstein J. L., Brown M. S. (2002). SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K., Bruick R. K., Liang G., Horton J. D., Uyeda K. (2004). Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. U.S.A. 101, 7281–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Iizuka K., Takao K., Horikawa Y., Kitamura T., Takeda J. (2018). ChREBP-knockout mice show sucrose intolerance and fructose malabsorption. Nutrients 10, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T., Takenoshita M., Kabashima T., Uyeda K. (2001). Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc. Natl. Acad. Sci. U.S.A. 98, 13710–13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. D., Hutchison E., Gerard L., Aleidi S. M., Gelissen I. C. (2021). Mammalian ABCG-transporters, sterols and lipids: To bind perchance to transport? Biochim. Biophys. Acta. Mol. Cell Biol. Lipids. 1866, 158860. [DOI] [PubMed] [Google Scholar]
- Knowles J. W., Xie W., Zhang Z., Chennamsetty I., Chennemsetty I., Assimes T. L., Paananen J., Hansson O., Pankow J., Goodarzi M. O., et al. ; SAPPHIRe (Stanford Asian and Pacific Program for Hypertension and Insulin Resistance) Study. (2015). Identification and validation of N-acetyltransferase 2 as an insulin sensitivity gene. J. Clin. Invest. 125, 1739–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann A., Torre D., Keenan A. B., Jagodnik K. M., Lee H. J., Wang L., Silverstein M. C., Ma’ayan A. (2018). Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 9, 1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. (1982). cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc. Natl. Acad. Sci. U.S.A. 79, 5137–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. J., Peloso G. M., Yu H., Butterworth A. S., Wang X., Mahajan A., Saleheen D., Emdin C., Alam D., Alves A. C., et al. ; VA Million Veteran Program. (2017). Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 49, 1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong A., Hannah V. C., Brown M. S., Goldstein J. L. (2000). Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 275, 26458–26466. [DOI] [PubMed] [Google Scholar]
- Mashimo T., Pichumani K., Vemireddy V., Hatanpaa K. J., Singh D. K., Sirasanagandla S., Nannepaga S., Piccirillo S. G., Kovacs Z., Foong C., et al. (2014). Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Kinter M. T., Sherman N. E., Driscoll D. M. (2000). Molecular cloning of apobec-1 complementation factor, a novel RNA-binding protein involved in the editing of apolipoprotein B mRNA. Mol. Cell. Biol. 20, 1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Casagrande J. T., Thomas P. D. (2013). Large-scale gene function analysis with PANTHER classification system. Nat. Protoc. 8, 1551–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners J. O., Birkett D. J. (1998). Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol. 45, 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett J. R., Puthillathu N., Vengilote R., Jaworski D. M., Namboodiri A. M. (2020). Acetate revisited: A key biomolecule at the nexus of metabolism, epigenetics and oncogenesis—Part 1: Acetyl-CoA, acetogenesis and Acyl-CoA short-chain synthetases. Front. Physiol. 11, 580167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou K. C., Vatandaslar H., Meyer C., Schmid M. W., Tuschl T., Stoffel M. (2019). The RNA-binding protein A1CF regulates hepatic fructose and glycerol metabolism via alternative RNA splicing. Cell Rep. 29, 283–300.e8. [DOI] [PubMed] [Google Scholar]
- Obayashi T., Kagaya Y., Aoki Y., Tadaka S., Kinoshita K. (2019). COXPRESdb v7: A gene coexpression database for 11 animal species supported by 23 coexpression platforms for technical evaluation and evolutionary inference. Nucleic Acids Res. 47, D55–D62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Prieto P., Postic C. (2019). Carbohydrate sensing through the transcription factor ChREBP. Front. Genet. 10, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T. F., Espenshade P. J. (2009). Evolutionary conservation and adaptation in the mechanism that regulates SREBP action: What a long, strange tRIP it’s been. Genes Dev. 23, 2578–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomaznoy M., Ha B., Peters B. (2018). GOnet: A tool for interactive gene ontology analysis. BMC Bioinform. 19, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preidis G. A., Kim K. H., Moore D. D. (2017). Nutrient-sensing nuclear receptors PPARα and FXR control liver energy balance. J. Clin. Invest. 127, 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P., Wang H., Zhang M., Meng Z., Peng R., Zhao Q., Liu J. (2020). FATP2-targeted therapies—A role beyond fatty liver disease. Pharmacol. Res. 161, 105228. [DOI] [PubMed] [Google Scholar]
- Richardson T. G., Sanderson E., Palmer T. M., Ala-Korpela M., Ference B. A., Davey Smith G., Holmes M. V. (2020). Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 17, e1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripatti P., Rämö J. T., Mars N. J., Fu Y., Lin J., Söderlund S., Benner C., Surakka I., Kiiskinen T., Havulinna A. S., FinnGen, et al. (2020). Polygenic hyperlipidemias and coronary artery disease risk. Circ. Genom. Precis. Med. 13, e002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-González R. A., Doll M. A., Hein D. W. (2020). Human arylamine N-acetyltransferase 2 genotype-dependent protein expression in cryopreserved human hepatocytes. Sci. Rep. 10, 7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll D., Wasner C., Hinds C. J., Allan B. B., Walther R., Burchell A. (1999). Identification of a cAMP response element within the glucose- 6-phosphatase hydrolytic subunit gene promoter which is involved in the transcriptional regulation by cAMP and glucocorticoids in H4IIE hepatoma cells. Biochem. J. 338, 457–463. [PMC free article] [PubMed] [Google Scholar]
- Sim E., Abuhammad A., Ryan A. (2014). Arylamine N-acetyltransferases: From drug metabolism and pharmacogenetics to drug discovery. Br. J. Pharmacol. 171, 2705–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott-Armstrong N., Tanigawa Y., Amar D., Mars N., Benner C., Aguirre M., Venkataraman G. R., Wainberg M., Ollila H. M., Kiiskinen T., et al. ; FinnGen. (2021). Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 53, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. M., McCarty C., Mehalow A., Svenson K. L., Murray S. A., Korstanje R., Braun R. E. (2017). APOBEC1 complementation factor (A1CF) is dispensable for C-to-U RNA editing in vivo. RNA 23, 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp M. W., Mamaliga G., Doll M. A., States J. C., Hein D. W. (2015). Folate-dependent hydrolysis of acetyl-coenzyme A by recombinant human and rodent arylamine N-acetyltransferases. Biochem. Biophys. Rep. 3, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp M. W., Salazar-González R. A., Hong K. U., Doll M. A., Hein D. W. (2019). N-acetyltransferase 1 knockout elevates acetyl coenzyme A levels and reduces anchorage-independent growth in human breast cancer cell lines. J. Oncol. 2019, 3860426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeper R. S., Svitek C. A., Chapman S., Greenbaum L. E., Taub R., O'Brien R. M. (1997). A multicomponent insulin response sequence mediates a strong repression of mouse glucose-6-phosphatase gene transcription by insulin. J. Biol. Chem. 272, 11698–11701. [DOI] [PubMed] [Google Scholar]
- Tang Z., Chenwei L., Kang B., Gao G., Cheng L., Zhang Z. (2017). GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., Gao J., Xie W. (2009). PXR and CAR in energy metabolism. Trends Endocrinol. Metab. 20, 273–279. [DOI] [PubMed] [Google Scholar]
- Willer C. J., Schmidt E. M., Sengupta S., Peloso G. M., Gustafsson S., Kanoni S., Ganna A., Chen J., Buchkovich M. L., Mora S. (2013). Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-L., Zheng X.-L., Ye K., Sun Y.-N., Lu Y.-F., Ge H., Liu H. (2019). Effects of microRNA-217 on proliferation, apoptosis, and autophagy of hepatocytes in rat models of CCL4-induced liver injury by targeting NAT2. J. Cell. Physiol. 234, 3410–3424. [DOI] [PubMed] [Google Scholar]
- Zhu Q., Wong A. K., Krishnan A., Aure M. R., Tadych A., Zhang R., Corney D. C., Greene C. S., Bongo L. A., Kristensen V. N., et al. (2015). Targeted exploration and analysis of large cross-platform human transcriptomic compendia. Nat. Methods. 12, 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C., Mifflin L., Hu Z., Zhang T., Shan B., Wang H., Xing X., Zhu H., Adiconis X., Levin J. Z., et al. (2020). Reduction of mNAT1/hNAT2 contributes to cerebral endothelial necroptosis and Aβ accumulation in Alzheimer’s disease. Cell Rep. 33, 108447. [DOI] [PubMed] [Google Scholar]