Abstract
Background and objective
The operative priority in the setting of traumatic cervical spinal cord injury (SCI) is to decompress the injured spinal cord and stabilize the vertebral column. Currently, there is a relative paucity of evidence regarding associations of patient and surgical factors with in-hospital mortality following traumatic SCI. In light of this, the aim of this study was to investigate the correlation of injury, patient, and surgical factors with in-hospital morbidity and mortality.
Methods
The study was designed as a retrospective cohort study. The electronic medical records (EMR) at a single tertiary centre in Australia were retrospectively reviewed over a five-year period (2016-2021). All adults who were admitted to undergo emergency surgery for cervical SCI were identified and reviewed for patient factors (age, sex, comorbidities), injury factors [injury severity score (ISS), American Spinal Cord Injury Association (ASIA) classification], and surgical factors (anterior/posterior/360 instrumentation, greater than five levels instrumented, operative time). Factors were correlated to in-hospital complications (infection, pressure injury, ventilator dependency, venous thromboembolism, stroke) and in-hospital mortality by using univariate analysis and multivariable logistic regression models.
Results
A total of 92 patients were identified from the EMR. The median patient age was 54.5 years [interquartile range (IQR): 2.5]; 77 (82.2%) of the participants were male. The median ASIA classification was C4 ASIA C. In-hospital mortality following surgery was 6.5% (n=6). Of these patients, the primary cause of death was respiratory failure in 83.3% (n=5). In-hospital mortality was associated with anticoagulation (p=0.01), coronary disease (p=0.012), complete injury (p=0.011), and ventilator dependency (p<0.001). Postoperative pneumonia was associated with complete injury (p=0.009) and polytrauma (p=0.002). Ventilator dependency was associated with complete injuries (p<0.001) and polytrauma (p<0.001). A logistic regression analysis found complete neurological injury to be significant in predicting in-hospital mortality [odds ratio (OR): 184.53, 95% confidence interval (CI): 2.41-14106.65, p=0.018, R2=0.58].
Conclusion
To improve surgical outcomes in patients with traumatic cervical SCI, a concerted effort must be made to prevent postoperative complications. Cardiovascular comorbidities present significant risk factors for patients. Patient age appears to insignificantly influence postoperative complication rates; however, this finding may have been influenced by selection bias. Postoperative respiratory complications, especially in patients with complete neurological deficits, can be particularly devastating.
Keywords: australia, inhospital mortality, early postoperative complication, logistic regression model, cervical spinal cord injury, spinal cord injury
Introduction
Acute cervical spinal cord injury (SCI) is a devastating injury that requires highly complex and sub-specialized multidisciplinary care. The estimated incidence of traumatic SCI in Australia is 21.0-32.3 per million population [1]. The rates are highest in young adult males, with the majority of injuries caused by accidental trauma [2,3]. The effects of surgery are both immediate and long-lasting. Decompression of the spinal cord may prevent the neurotoxic biochemical cascade of secondary injury mechanisms, while stabilization prevents the morbidity associated with chronic deformity [4-7].
Postoperative complications following surgery for cervical SCI can contribute to early mortality, affect neurological recovery and functional outcomes, and result in high healthcare costs [8]. Respiratory complication following cervical SCI is the main cause of in-hospital mortality, responsible for 42-87% of deaths [9-11]. Specialized respiratory care for cervical SCI patients can improve outcomes [12]. Increased risk of mortality has been associated with the development of pulmonary embolism (PE), which is most likely to occur in the immediate post-injury period [13]. Pressure ulcers (PU) are the most common complication of SCI and a major cause of chronic re-hospitalisation [14-16]. Currently, the levels of evidence about risk factors for the development of PU in the acute period remain insufficient [16].
Patient factors including age and comorbidities can influence the risk profile of SCI patients [9,10,17-19]. Complications in elderly patients are more likely to be cardiorespiratory in origin [myocardial infarction, deep vein thrombosis (DVT), PE] whereas infections (pneumonia, respiratory and urinary infection) are more frequent in younger groups of patients [10,17]. Studies have demonstrated a strong association between complete SCI and increased in-hospital mortality [9,11,19-21]. In-hospital mortality for patients aged over 65 years with complete cervical SCI is five times higher than those with incomplete injuries [19]. The severity of polytrauma has also been significantly associated with postoperative in-hospital mortality among SCI patients [18,21].
Surgical factors also influence complication rates. Existing evidence supports improved neurological recovery among cervical SCI patients undergoing early surgery [22], as well as lower in-hospital mortality [9]. A growing body of evidence supports early surgery as a means to reduce the time spent in intensive care and the overall hospital length of stay [23]. Medical complications such as PE may also be reduced by the early mobilization achieved by surgery [23]. Longer surgical duration is associated with increased postoperative complications [24]; however, anterior and posterior approaches may be equivalent in terms of outcomes in the long term [25]. Posterior approaches may result in greater blood loss, longer operative times, and longer inpatient admission duration [26].
There is a paucity of evidence regarding the association of these factors with in-hospital mortality and postoperative complications from an Australian cohort. Hence, this study aimed to investigate these associations by using a predictive model.
Materials and methods
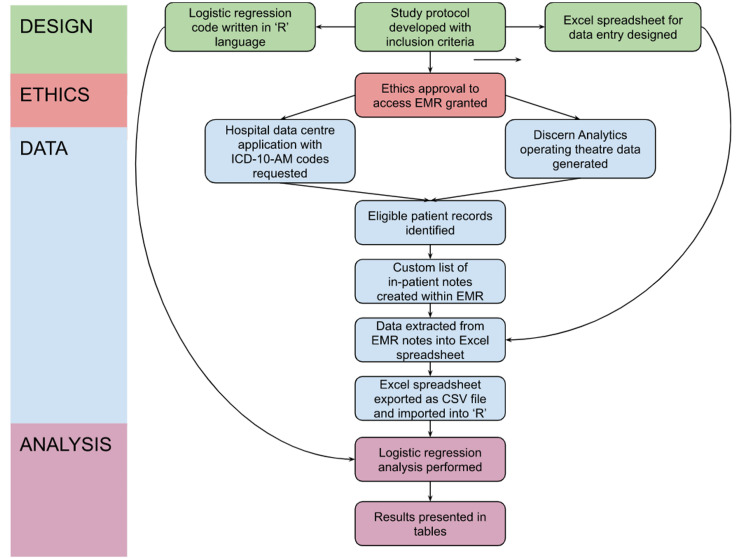
An overview of the study procedure is outlined in Figure 1.
Figure 1. Flowchart depicting the study procedure.
EMR: electronic medical records; ICD-10-AM: the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification
Ethical approval
The authority to access patient data through electronic medical records (EMR) was granted by the Northern Sydney Local Health District (NSLHD) Hospital Research Ethics Committee (HREC).
Study population
The Royal North Shore Hospital (RNSH) Spinal Cord Injury Service is one of two hospitals in the state of New South Wales that provides acute surgical and rehabilitation services for acute traumatic SCI patients. The RNSH caters to roughly half the population of NSW, which has a total population of 8.1 million people [27]. Adult patients over 16 years of age who sustained acute, traumatic cervical SCI from June 1, 2016, to June 1, 2021, and underwent operative management at RNSH under the orthopaedics team were eligible for inclusion in the study. The inclusion and exclusion criteria are outlined in Table 1.
Table 1. Patient inclusion and exclusion criteria.
RNSH: The Royal North Shore Hospital; SCI: spinal cord injury
| Inclusion criteria | Exclusion criteria |
| Greater than 16 years of age | Subacute presentation (>48 hours after injury) |
| Acute traumatic SCI (injury within 48 hours before presentation) | Cervical SCI of aetiology other than trauma (i.e., malignancy, infection, ischemia, complications of medical or surgical intervention, periprosthetic fracture, or hardware failure) |
| Cervical decompression +/- fusion +/- fixation +/- bone grafting performed | Patients managed nonoperatively |
| Admission to RNSH under the orthopaedics team | Patients who died before surgery |
| Patients managed with halo external fixation |
Data collection
The hospital coding centre was approached with a request for data on admissions during the study period with unique billing codes. These codes were chosen from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification (ICD-10-AM) [28] and are listed in Table 2.
Table 2. ICD-10-AM codes provided by the hospital data centre for patient search.
ICD-10-AM: the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification
| ICD-10-AM code | Code description |
| S14.0 | Concussion and oedema of cervical spinal cord |
| S14.10 | Unspecified injury of cervical spinal cord |
| S14.11 | Complete lesion of cervical spinal cord |
| S14.12 | Central cord syndrome (incomplete cord injury) of the cervical spinal cord |
| S14.13 | Other incomplete cord syndrome of cervical spinal cord |
| S14.70 | Function spinal cord injury, cervical level unspecified |
| S14.71 | Function spinal cord injury, C1 |
| S14.72 | Function spinal cord injury, C2 |
| S14.73 | Function spinal cord injury, C3 |
| S14.74 | Function spinal cord injury, C4 |
| S14.75 | Function spinal cord injury, C5 |
| S14.76 | Function spinal cord injury, C6 |
| S14.77 | Function spinal cord injury, C7 |
| S14.78 | Function spinal cord injury, C8 |
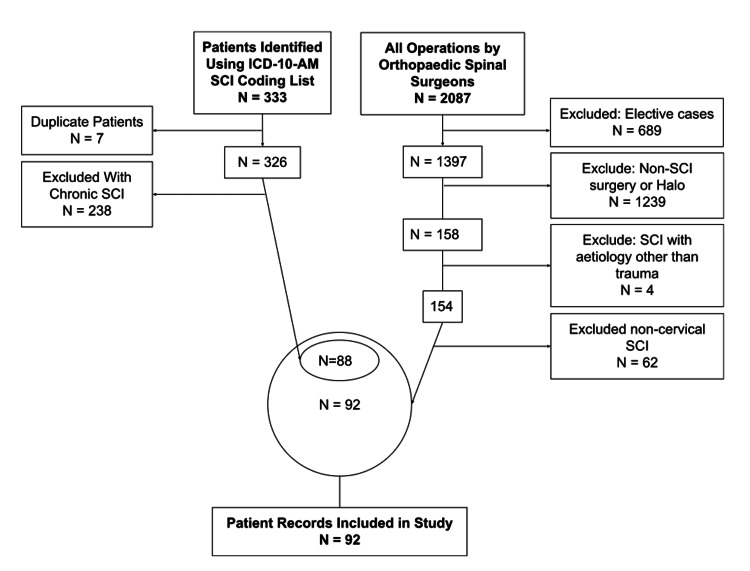
This process identified 88 patients who fulfilled the inclusion criteria (Figure 2). Additionally, a manual search of all emergency operations documented in the operating theatre EMR was performed using the data collection tool, Discern Analytics 2.0 (DA2) within Surginet (Oracle Cerner, 2016, Austin, TX), which yielded an additional four patients missed by hospital coding. The triage note, admission notes, operation reports, and medical imaging were reviewed and relevant data were extracted to a pre-designed Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA).
Figure 2. Flow diagram of sample selection.
ICD-10-AM: the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification; SCI: spinal cord injury
Patient age, sex, and comorbidities including smoking, hypertension, diabetes, anticoagulation, chronic alcohol use, and coronary disease, and the American Society of Anesthesiologists (ASA) classification and American Spinal Cord Injury Association (ASIA) classification were recorded. The Injury Severity Score (ISS) was calculated manually based on the admission notes and imaging reports. Surgical factors including time to surgery, operative time, number of levels instrumented, and surgical approach were extracted from the EMR and validated against the intraoperative imaging to ensure accuracy. Postoperative adverse outcomes recorded included in-hospital mortality, any type of cerebrovascular accident (CVA), venous thromboembolic disease (DVT/PE), pressure injury, respiratory infection, urinary infection, and ventilator dependency.
Data analysis
All data analysis was performed using “R” (Ver 4.2.2 The Free Software Foundation Inc. Boston, MA). The Shapiro-Wilk test for normality was used, demonstrating that the data was not normally distributed. Continuous variables were expressed in terms of the median and interquartile range (IQR). Categorical variables were expressed as a percentage of the total sample number. Continuous variables were changed to categorical variables for the purpose of univariate analysis using the Chi-squared test.
Statistical model
A univariable selection method of logistic regression modeling was used. Models were constructed using all independent variables from the univariate analysis with p<0.20 [29,30]. Collinearity was assessed using the variance inflation factor (VIF), with VIF >10 used to define collinear variables. This threshold was chosen as it has been used in similar studies [9,31]. If collinearity was detected, the offending variable was removed from the analysis. This process was used to separately create regression models for each dependent variable. Independent variables were equally weighted within each model. The dataset was randomly split in a ratio of 70:30 for the purposes of creating training and testing subsets. These subsets were used to calculate the area under the curve (AUC) and McFadden’s R2 for each model.
Results
Study population
A total of 92 patients met the inclusion criteria. The median patient age was 54.5 years (IQR: 32.5); 77 (82.2%) of the patients were male. The median ASA score was 3 (IQR: 2), with comorbidities including smoking (n=16, 17.2%), hypertension (n=26, 28.0%), diabetes (n=12, 13.0%), anticoagulation (n=4 4.3%), chronic alcoholism (n=6, 6.5%), and coronary disease (n=10 10.7%). The median ISS was 20 (IQR: 9). The median cervical level of injury was C4, and the median ASIA classification was ASIA C. The median number of levels instrumented was 3 (IQR: 3) with greater than 5 levels instrumented in 15 (16.3%) cases. The median surgical time was 171 minutes (IQR: 130).
Postoperative complications included CVA (n=1, 1.1%), DVT/PE (n=33, 35.5%), PU (n=5, 5.4%), respiratory infection (n=28, 30.1%), urinary tract infection (n=25, 26.9%), and ventilatory dependence (n=17, 18.3%). In-hospital mortality following surgery was 6.5% (n=6). Of these patients, the primary cause of death was respiratory failure in 83.3% (n=5).
The basic characteristics of the sample are presented in Table 3.
Table 3. Characteristics of the study participants (n=92).
ASA: American Society of Anesthesiologists; ASIA: American Spinal Cord Injury Association; CVA: cerebrovascular accident; DVT/PE: deep vein thrombosis and/or pulmonary embolism; HTN: hypertension; IQR: interquartile range; ISS: Injury Severity Score; SD: standard deviation
| Characteristics | ||
| Demographics | ||
| Number of patients | 92 | |
| Age, years, median (IQR) | 54.5 (32.5) | |
| Males, n (%) | 77 (82.8) | |
| Injury factors | ||
| ISS, median (IQR) | 20.0 (9.0) | |
| Cervical level of injury, median (SD) | 4.0 (1.0) | |
| Cervical 1-4 (high tetraplegia), n (%) | 58 (63.0) | |
| Cervical 5-8 (low tetraplegia), n (%) | 41 (37.0) | |
| ASIA classification, median | C | |
| Surgery factors | ||
| Number of levels instrumented, median (SD) | 3.0 (3.0) | |
| Greater than 5 levels instrumented, n (%) | 15 (16.3) | |
| Surgery time, minutes, median (IQR) | 171.0 (130.0) | |
| Patient factors | ||
| ASA classification, median (IQR) | 3 (2) | |
| Smoking, n (%) | 16 (17.2) | |
| HTN, n (%) | 26 (28.0) | |
| Diabetes, n (%) | 12 (13.0) | |
| Anticoagulation, n (%) | 4 (4.3) | |
| Chronic alcoholism, n (%) | 6 (6.5) | |
| Coronary disease, n (%) | 10 (10.7) | |
| Complications | ||
| CVA, n (%) | 1 (1.1) | |
| DVT/PE, n (%) | 33 (35.5) | |
| Pressure ulcers, n (%) | 5 (5.4) | |
| Respiratory infection, n (%) | 28 (30.1) | |
| Urinary tract infection, n (%) | 25 (26.9) | |
| Ventilator dependency, n (%) | 17 (18.3) | |
| Postoperative in-hospital death, n (%) | 6 (6.5) | |
Univariate analysis
In-hospital mortality was associated with age >65 years (Χ2=2.6, p=0.107), hypertension (Χ2=2.9, 0.091), anticoagulation (Χ2=6.6, p=0.01), chronic alcoholism (Χ2=3.6, p=0.058), coronary disease (Χ2=6.2, p=0.012), complete injury (Χ2=6.4, p=0.011), polytrauma (Χ2=2.7, p=0.099), greater than 5 levels of instrumentation (Χ2=3.02, p=0.0819), anterior instrumentation (Χ2=1.9, p=0.174), in-hospital thromboembolic disease (Χ2=2.1, p=0.145), and ventilator dependency (Χ2=13.6, p<0.001). Postoperative CVA was associated with chronic alcoholism (Χ2=3.1, p=0.076). Postoperative DVT/PE was associated with polytrauma (ISS >15) (Χ2=2.0, p=0.156). Postoperative pneumonia was associated with male gender (Χ2=3.5, p=0.06), age >65 years (Χ2=2.6, p=0.064), hypertension (Χ2=2.9, 0.085), complete injury (Χ2=2.6, 0.009), and polytrauma (Χ2=2.6, 0.002). Urinary tract infection was associated with high-level C-spine injury (C1-4) (Χ2=2.6, p=0.113). Ventilator dependence was associated with complete injuries (Χ2=19.6, p<0.001), and polytrauma (Χ2=12.1, p<0.001). These results are presented in Tables 4-10.
Table 4. Univariate analysis for dependent complication variable: in-hospital mortality.
Degrees of freedom=1; sample size=92
P-value <0.2 was the threshold for inclusion in multivariable logistic regression analysis
DVT/PE: deep vein thrombosis and/or pulmonary embolism; ISS: Injury Severity Score; UTI: urinary tract infection
| Χ2 statistic | P-value | |
| Patient factors | ||
| Male gender | 0.3 | 0.585 |
| Age >65 years | 2.6 | 0.107 |
| Age >80 years | 0.5 | 0.469 |
| Smoking | 0.3 | 0.611 |
| hypertension | 2.9 | 0.091 |
| Diabetes | 0.8 | 0.368 |
| Anticoagulation | 6.6 | 0.01 |
| Alcoholism | 3.6 | 0.058 |
| Coronary disease | 6.2 | 0.012 |
| Injury factors | ||
| Complete injury | 6.4 | 0.011 |
| High level (C1-C4) | 0 | 1 |
| Polytrauma (ISS >15) | 2.7 | 0.099 |
| Surgery factors | ||
| Surgical time >120 minutes | 0.01 | 0.901 |
| Greater than 5 levels instrumented | 3.02 | 0.0819 |
| Anterior instrumentation | 1.9 | 0.174 |
| Posterior instrumentation | 0.6 | 0.413 |
| Anterior and posterior instrumentation | 0 | 1 |
| Complications | ||
| In-hospital stroke | 0 | 1 |
| In-hospital DVT/PE | 2.1 | 0.145 |
| In-hospital pressure injury | 0 | 1 |
| In-hospital respiratory infection | 0.4 | 0.536 |
| In-hospital UTI | 1.2 | 0.283 |
| Ventilator dependency | 13.6 | <0.001 |
Table 10. Univariate analysis for dependent complication variable: ventilator dependency.
Degrees of freedom=1; sample size=92
P-value <0.2 was the threshold for inclusion in multivariable logistic regression analysis
ISS: Injury Severity Score
| Χ2 statistic | P-value | |
| Patient factors | ||
| Male gender | 0 | 0.843 |
| Age >65 years | 0.8 | 0.379 |
| Age >80 years | 0 | 1 |
| Smoking | 0.1 | 0.746 |
| Hypertension | 0 | 0.855 |
| Diabetes | 0 | 0.821 |
| Anticoagulation | 0 | 1 |
| Alcoholism | 0 | 1 |
| Coronary disease | 0 | 1 |
| Injury factors | ||
| Complete injury | 19.6 | <0.001 |
| High level (C1-C4) | 0.2 | 0.663 |
| Polytrauma (ISS >15) | 12.1 | <0.001 |
| Surgical factors | ||
| Surgical time >120 minutes | 0.005 | 0.942 |
| Greater than 5 levels instrumented | 0.3 | 0.596 |
| Anterior instrumentation | 0 | 0.896 |
| Posterior instrumentation | 0 | 0.873 |
| Anterior and posterior instrumentation | 0 | 0.906 |
Table 5. Univariate analysis for dependent complication variable: CVA.
Degrees of freedom=1; sample size=92
P-value <0.2 was the threshold for inclusion in multivariable logistic regression analysis
CVA: cerebrovascular accident; ISS: Injury Severity Score
| Χ2 statistic | P-value | |
| Patient factors | ||
| Male gender | 0.8 | 0.359 |
| Age >65 years | 0.2 | 0.648 |
| Age >80 years | 0 | 1 |
| Smoking | 0 | 1 |
| Hypertension | 0.2 | 0.627 |
| Diabetes | 1.2 | 0.269 |
| Anticoagulation | 0 | 1 |
| Alcoholism | 3.1 | 0.076 |
| Coronary disease | 0 | 1 |
| Injury factors | ||
| Complete injury | 0 | 1 |
| High level (C1-C4) | 0 | 1 |
| Polytrauma (ISS >15) | 0.04 | 0.841 |
| Surgery factors | ||
| Surgical time >120 minutes | 0.2 | 0.605 |
| Greater than 5 levels instrumented | 0 | 1 |
| Anterior instrumentation | 0 | 1 |
| Posterior instrumentation | 0.02 | 0.877 |
| Anterior and posterior instrumentation | 0 | 1 |
Table 6. Univariate analysis for dependent complication variable: DVT/PE.
Degrees of freedom=1; sample size=92
P-value <0.2 was the threshold for inclusion in multivariable logistic regression analysis
DVT/PE: deep vein thrombosis and/or pulmonary embolism; ISS: Injury Severity Score
| Χ2 statistic | P-value | |
| Patient factors | ||
| Male gender | 0 | 1 |
| Age >65 years | 0.2 | 0.697 |
| Age >80 years | 0 | 1 |
| Smoking | 1.6 | 0.199 |
| Hypertension | 0 | 0.933 |
| Diabetes | 0.3 | 0.603 |
| Anticoagulation | 0.9 | 0.319 |
| Alcoholism | 0.1 | 0.759 |
| Coronary disease | 0.4 | 0.523 |
| Injury factors | ||
| Complete injury | 0.8 | 0.386 |
| High level (C1-C4) | 0.6 | 0.445 |
| Polytrauma (ISS >15) | 9 | 0.002 |
| Surgery factors | ||
| Surgical time >120 minutes | 0.5 | 0.473 |
| Greater than 5 levels instrumented | 0.4 | 0.51 |
| Anterior instrumentation | 1.4 | 0.228 |
| Posterior Instrumentation | 5.8 | 0.015 |
| Anterior and posterior Instrumentation | 1.1 | 0.283 |
Table 7. Univariate analysis for dependent complication variable: pressure injury.
Degrees of freedom=1; sample size=92
P-value <0.2 was the threshold for inclusion in multivariable logistic regression analysis
ISS: Injury Severity Score
| Χ2 statistic | P-value | |
| Patient factors | ||
| Male gender | 0.2 | 0.694 |
| Age >65 years | 0 | 1 |
| Age >80 years | 0 | 1 |
| Smoking | 0.2 | 0.653 |
| Hypertension | 0 | 1 |
| Diabetes | 0.04 | 0.835 |
| Anticoagulation | 0 | 1 |
| Alcoholism | 0.1 | 0.746 |
| Coronary disease | 0 | 1 |
| Injury factors | ||
| Complete injury | 0 | 0.973 |
| High level (C1-C4) | 0 | 1 |
| Polytrauma (ISS >15) | 2 | 0.156 |
| Surgical factors | ||
| Surgical time >120 minutes | 0 | 1 |
| Greater than 5 levels instrumented | 0 | 1 |
| Anterior instrumentation | 0 | 1 |
| Posterior instrumentation | 0.1 | 0.723 |
| Anterior and posterior instrumentation | 0.3 | 0.579 |
Table 8. Univariate analysis for dependent complication variable: respiratory infection.
Degrees of freedom=1; sample size=92
P-value <0.2 was the threshold for inclusion in multivariable logistic regression analysis
ASIA: American Spinal Cord Injury Association; ISS: Injury Severity Score
| Χ2 statistic | P-value | |
| Patient factors | ||
| Male gender | 3.5 | 0.06 |
| Age >65 years | 3.4 | 0.064 |
| Age >80 years | 0 | 1 |
| Smoking | 0.9 | 0.329 |
| Hypertension | 2.9 | 0.085 |
| Diabetes | 0.6 | 0.438 |
| Anticoagulation | 0.6 | 0.425 |
| Alcoholism | 0.1 | 0.764 |
| Coronary disease | 0.2 | 0.692 |
| Injury factors | ||
| Complete injury (ASIA A) | 6.9 | 0.009 |
| High level (C1-C4) | 0.3 | 0.589 |
| Polytrauma (ISS >15) | 9.7 | 0.002 |
| Surgical factors | ||
| Surgical time >120 minutes | 1.2 | 0.282 |
| Greater than 5 levels instrumented | 1.4 | 0.235 |
| Anterior instrumentation | 0 | 1 |
| Posterior Instrumentation | 0 | 1 |
| Anterior and posterior instrumentation | 0.3 | 0.559 |
Table 9. Univariate analysis for dependent complication variable: UTI.
Degrees of freedom=1; sample size=92
P-value <0.2 was the threshold for inclusion in multivariable logistic regression analysis
ISS: Injury Severity Score; UTI: urinary tract infection
| Χ2 statistic | P-value | |
| Patient factors | ||
| Male gender | 0.1 | 0.788 |
| Age >65 years | 0.4 | 0.549 |
| Age >80 years | 0.2 | 0.677 |
| Smoking | 0 | 1 |
| Hypertension | 0.1 | 0.821 |
| Diabetes | 0.3 | 0.596 |
| Anticoagulation | 0.2 | 0.635 |
| Alcoholism | 0 | 0.901 |
| Coronary disease | 0.8 | 0.359 |
| Injury factors | ||
| Complete injury | 0.2 | 0.666 |
| High level (C1-C4) | 2.5 | 0.113 |
| Polytrauma (ISS >15) | 1.4 | 0.222 |
| Surgery factors | ||
| Surgical time >120 minutes | 0.5 | 0.495 |
| Greater than 5 levels instrumented | 0.8 | 0.366 |
| Anterior instrumentation | 0.9 | 0.34 |
| Posterior instrumentation | 0.2 | 0.669 |
| Anterior and posterior instrumentation | 0.1 | 0.719 |
Multivariable analysis
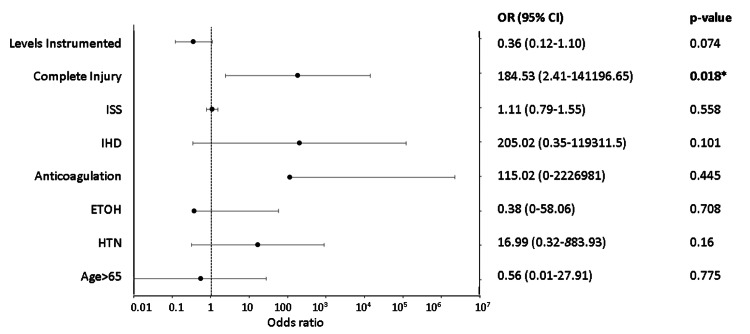
The independent variables used to create a regression model for in-hospital mortality were as follows: age >65 years, hypertension, alcoholism, anticoagulation, coronary disease, complete injury, ventilator dependency, and PE/DVT. The process of collinearity assessment and model selection is outlined in Table 11. The results of the chosen regression model for in-hospital mortality are depicted in Figure 3. In this model, complete injury was significantly associated with in-hospital mortality [odds ratio (OR): 184.53, 95% confidence interval (CI): 2.41-14106.65, p=0.018]. The AUC for this model was 0.9615 and R2=0.58.
Table 11. Collinearity analysis for dependent variable: in-hospital mortality.
⧫Model chosen for multivariable analysis
Variance inflation factors (VIF) for parameters of each model. Model variables were chosen from univariate analysis where p<0.2. VIF cut-off threshold was 10
ASIA: American Spinal Cord Injury Association classification; DVT/PE: deep vein thrombosis and/or pulmonary embolism; ETOH: chronic alcoholism; HTN: hypertension; IHD: ischemic heart disease; ISS: Injury Severity Score
| Age >65 years | HTN | ETOH | Anticoagulation | IHD | ISS | ASIA | Anterior | Levels instrumented | Ventilator dependency | DVT/PE | |
| Age >65 years + HTN | 1.4 | 1.4 | |||||||||
| Age >65 years + HTN + ETOH | 1.4 | 1.4 | 1.1 | ||||||||
| Age >65 years + HTN + ETOH + IHD | 1.6 | 1.4 | 1.2 | 1.3 | |||||||
| Age >65 years + HTN + ETOH + IHD + anticoagulation | 1.9 | 1.6 | 1.2 | 1.2 | 1.3 | ||||||
| Age >65 years + HTN + ETOH + IHD + anticoagulation + ISS | 1.9 | 1.7 | 1.2 | 1.3 | 1.4 | 1.1 | |||||
| Age >65 years + HTN + ETOH + IHD + anticoagulation + ISS + ASIA | 2.5 | 2 | 1.5 | 1 | 2.6 | 1.4 | 2.4 | ||||
| Age >65 years + HTN + ETOH + IHD + anticoagulation + ISS + ASIA + anterior | 2.1 | 2.3 | 1.8 | 1 | 1535.2 | 1.6 | 1.5 | 1535.2 | |||
| Age >65 years + HTN + ETOH + IHD + anticoagulation + ISS + ASIA + levels instrumented⧫ | 2.4 | 2.5 | 2.2 | 1.1 | 5.7 | 1.6 | 3.9 | - | 2.8 | ||
| Age >65 years + HTN + ETOH + IHD + anticoagulation + ISS + ASIA + levels instrumented + ventilator dependency | 39.9 | 2.7 | 3.1 | 1.1 | 33.1 | 1.9 | 3.2 | - | 1 | 4.2 | |
| Age >65 years + HTN + ETOH + IHD + anticoagulation + ISS + ASIA + levels instrumented + DVT/PE | 72.4 | 13.8 | 1.4 | 1 | 69.2 | 1.01 | 26.7 | - | 1 | - | 4.3 |
Figure 3. Plot of logistic regression model for in-hospital mortality.
*Significant at p<0.05
Formula: in-hospital mortality ~age >65 + HTN + ETOH + anticoagulation + IHD + complete injury + levels Instrumented
CI: confidence interval; ETOH: chronic alcoholism; HTN: hypertension; IHD: ischemic heart disease; ISS: Injury Severity Score; OR: odds ratio
Discussion
Significant results
Our strongest finding pertaining to in-hospital mortality was its association with anticoagulation, coronary disease, complete injury, and ventilator dependency. The logistic regression model found complete injury to be significantly associated with postoperative in-hospital mortality. Studies have demonstrated a significant association between a high number of medical comorbidities and in-hospital mortality following SCI [10,19,32]. However, there is a paucity of evidence as to which specific chronic comorbidities contribute the most to postoperative complications in these patients. We have shown that in-hospital mortality risk after surgery for cervical SCI may be more strongly associated with cardiovascular comorbidities than diabetes and chronic alcohol abuse. We found that postoperative pneumonia and ventilator dependency was significantly associated with complete injury and polytrauma, as has been shown previously [33]. These findings highlight the vigilance required to prevent deterioration from respiratory decompensation in the ASIA A cohort of patients. Evidence supports providing specialized respiratory care for cervical SCI patients as a critical component of early management to avoid adverse outcomes [34].
Comparison of our findings with the literature
Table 12 provides a summary of previous studies investigating the risk factors for in-hospital mortality and complications among SCI patients using predictive models. The study population’s mean age of 51 years and its male predominance are consistent with similar studies [10,34-37]. The average level of injury was C4-5, which aligns with other studies [38-40]. However, the sample size of 92 was smaller when compared to larger studies in the literature with over 1000 patients [9,11,17,18,21]. Beck et al. investigated 706 patients with SCI in an Australian population and observed a median age of 50 years and an in-patient mortality of 15%, which is higher than our rate of 6.5%. However, their study was not limited to cervical SCI, and the inclusion criteria limited patients to those suffering from major trauma (ISS >12, ICU admission), which may account for their higher rate of in-hospital death. The majority of deaths in our study were secondary to respiratory complications, which is consistent with the literature [9-11]. Several studies have demonstrated minor differences in early complication rates between patients managed conservatively and those treated surgically [18,33], which may allow us to draw comparisons between our study cohort and nonoperatively managed patients.
Table 12. Summary of articles that investigate in-hospital mortality and complications with statistical relationships to various risk factors for SCI patients.
ASIA: American Spinal Cord Injury Association; GCS: Glasgow Coma Scale; ISS: Injury Severity Score; SCI: spinal cord injury; TBI: traumatic brain injury
| Author, year, country | Sample | In-hospital mortality | The primary cause of death | Analysis technique: factors associated with in-hospital mortality |
| Shao et al. [9], 2011, China | N=1163, cervical SCI | 9.4% (n=109) | Respiratory failure, 87.2% (n=95) | Logistic regression: -age -ASIA A -time to surgery -malnutrition -tracheotomy |
| Selassie et al. [17], 2012, USA | N=3389, all SCI | 8.0% (n=272) | - | Cox proportional hazard regression model: -age -level of injury -2 or more comorbidities -concomitant injuries -pulmonary embolism |
| Durga et al. [33], 2010, India | N=101, postoperative, cervical SCI | 17.8% | - | Logistic regression: -ASIA -haemodynamic instability -pulmonary infection |
| Chhabra et al. [10], 2019, India | N=758, all SCI | 10% (n=79) | Respiratory complications including pulmonary embolism and pneumonia (42%) | Cox proportional hazard regression: -age -low tetraplegia -ventilator use -associated injuries -cardiovascular complication |
| Inglis et al. [19], 2020, Canada | N=826, postoperative all SCI, age over 65 years | 9.5% (n=78) | - | Logistical regression: -age -ASIA A -Charlson Comorbidity Index -ventilation dependence |
| Gong et al. [11], 2022, China | N=3176, all SCI | 0.7% (n=23) | Respiratory complication, 73.9% (n=17) | Logistic regression: -cervical level -abdominal visceral injury -ASIA classification -surgery |
| Blex et al. [32], 2022, Germany | N=321, all SCI | 6.2% (n=20) | Multiorgan failure, 40.0% (n=8) | Condition inference tree analysis: -age -Charlson Comorbidity Index -kidney or liver impairment |
| Shibahashi et al. [21], 2019, Japan | N=8069, all SCI | 5.6% | - | Logistic regression: -age -male -GCS -hypotension on arrival -bradycardia on arrival -severe head injury -ISS -ASIA classification |
| Varma et al. [18], 2010, USA | N=1995, all SCI | 12.6% (n=251) | - | Logistic regression: -age -male -ISS >15 -TBI -1 or more comorbidities -ASIA classification |
| Jackson et al. [20], 2005, USA | N=458, postoperative, cervical SCI | 3.9% | - | -Age >65 years -ASIA A |
| Beck et al. [41], 2019, Australia | N=706, all SCI | 15% (n=104) | - | Χ2 test: -age |
Beck et al. showed that Australia is witnessing an increase in the number of older adults with traumatic SCI [41], which is consistent with other studies [36,42]. These findings exceed the rate of geriatric population growth and reflect an increasing rate of trauma from low falls in this age group [43,44]. Most studies modeling risk factors for complications following SCI report increasing age as significantly associated with in-hospital mortality [9,17-20,32,41]. Inglis et al. specifically investigated traumatic SCI in a geriatric population (n=1340) and determined that the odds of dying 50 days post-surgery are six times higher for patients aged ≥77 years versus those aged 65-76 years [19]. Our study did not find a strong association between age and postoperative complications. Some studies in the literature also demonstrate an insignificant relationship between age and postoperative mortality following cervical SCI surgery [33]. Wilson et al. investigated a group of geriatric (age >65 years) cervical SCI patients managed surgically [45]. Mortality at 90 days was significantly associated with complete injury; however, there was no significant association between mortality and age subgroups. A possible explanation for this inconsistency could be selection bias, as older patients are more likely to be conservatively managed without surgery, which avoided their inclusion in the study.
Strengths, limitations, and future research
The main strength of this study lies in the reliability of the dataset, which was rigorously extracted using multiple ICD codes and validated using multiple sources [46]. Evidence suggests significant inaccuracies inherent in hospital coding data [47-50] and underreporting of adult variants of SCI without radiological abnormality (SCIWORA) [51,52]. The data collection methodology employed in this study used multiple validation points between coding, EMR documentation, and medical imaging in an attempt to reduce information bias.
The relatively small sample size of this project likely contributed to statistical errors. A power analysis during the study planning phase would have been useful [53], as it is generally accepted that a larger sample size can improve the validity of a model by reducing the confounding errors caused by multicollinearity [54]. A larger sample size could be derived from local and state-wide trauma registries. A more detailed dataset (including time of death) would allow for Kaplan-Meier survival curves to be drawn and a Cox proportional hazard model to be constructed, as performed in similar studies [10,17]. Logistic regression models may be less precise than a Cox proportional hazard model and may have difficulty in separating the effects of inter-related, time-dependent covariates [55,56]. This model could then be validated on an external, compatible dataset to improve external validity, as has been performed in similar studies [33,57].
This study was limited to a single centre and did not include patients admitted under the neurosurgical team. Additionally, the study design was retrospective and conducted by a single author, reducing overall reliability and allowing for potential bias. As with most retrospective, non-randomised surgical studies, selection bias towards healthier, operative candidates is an inherent factor [58]. The scope of this study did not include nonoperative patients, a group more likely to be palliated secondary to old age and comorbidities, precluding surgical management. Additionally, the scope of this study was limited to patients admitted under orthopaedics. The neurosurgical team admits approximately 30% of acute SCI patients at RNSH and there is evidence to suggest that there is a lack of consensus between neurosurgeons and orthopaedic surgeons on the value of surgical intervention after SCI [59], which may have influenced the results. The grouping of venous thromboembolic disease into PE + DVT may have confounded the association of PE with mortality risk, which has been established in the literature [17].
The future of this type of research will involve the development of machine-learning algorithms to better understand non-linear associations between variables and possibly model mortality risk with greater precision. One recent study has suggested the superiority of a machine-learning-derived tool, the SCI Risk Score (SCIRS), which can predict in-hospital and one-year mortality using the following variables: age, neurological level and completeness of injury, fracture classification, and Abbreviated Injury Scale scores. This tool has been validated in a separate cohort and demonstrated superior AUC and sensitivity than ISS [60].
Conclusions
To improve surgical outcomes for patients with traumatic cervical SCI, a concerted effort must be made to prevent postoperative complications. This study investigated the patient, injury, and surgical factors contributing to in-hospital mortality and postoperative complications following surgery for traumatic SCI. Cardiovascular comorbidities including anticoagulation and coronary disease appear to present significant patient risk factors contributing to in-hospital mortality. A logistic regression analysis found complete neurological injury to be significant in predicting in-hospital mortality. Patient age appears to insignificantly influence postoperative complication rates; however, this finding may have been influenced by selection bias. Postoperative respiratory complications, especially in the setting of complete injury and ventilator dependency, are particularly devastating. The findings of this study endorse the existing literature advocating for vigilant respiratory management in these fragile patients.
Acknowledgments
I would like to thank The Royal North Shore Hospital, Sydney, NSW: Department of Orthopaedics and Trauma Surgery and NSRHS Data and Analytics Unit for their assistance with data collection for this project.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Northern Sydney Local Health District (NSLHD) Human Research Ethics Committee (HREC) issued approval N/A
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Estimating the incidence and prevalence of traumatic spinal cord injury in Australia. New PW, Baxter D, Farry A, Noonan VK. Arch Phys Med Rehabil. 2015;96:76–83. doi: 10.1016/j.apmr.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Incidence and patterns of spinal cord injury in Australia. O'Connor P. Accid Anal Prev. 2002;34:405–415. doi: 10.1016/s0001-4575(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 3.Spinal cord injury facts and figures at a glance. National Spinal Cord Injury Statistical Center. J Spinal Cord Med. 2013;36:1–2. doi: 10.1179/1079026813Z.000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Central nervous system injury and nicotinamide adenine dinucleotide phosphate oxidase: oxidative stress and therapeutic targets. von Leden RE, Yauger YJ, Khayrullina G, Byrnes KR. J Neurotrauma. 2017;34:755–764. doi: 10.1089/neu.2016.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Oyinbo CA. https://pubmed.ncbi.nlm.nih.gov/21731081/ Acta Neurobiol Exp (Wars) 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 7.Acute spinal cord injury, part I: pathophysiologic mechanisms. Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Clin Neuropharmacol. 2001;24:254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Cervical spine injuries with acute traumatic spinal cord injury: spinal surgery adverse events and their association with neurological and functional outcome. Liebscher T, Ludwig J, Lübstorf T, et al. Spine (Phila Pa 1976) 2022;47:0–26. doi: 10.1097/BRS.0000000000004124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Factors associated with early mortality after cervical spinal cord injury. Shao J, Zhu W, Chen X, et al. J Spinal Cord Med. 2011;34:555–562. doi: 10.1179/2045772311Y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.In-hospital mortality in people with complete acute traumatic spinal cord injury at a tertiary care center in India-a retrospective analysis. Chhabra HS, Sharawat R, Vishwakarma G. Spinal Cord. 2022;60:210–215. doi: 10.1038/s41393-021-00657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A new scale for predicting the risk of in-hospital mortality in patients with traumatic spinal cord injury. Gong Y, Du J, Hao D, et al. Front Neurol. 2022;13:894273. doi: 10.3389/fneur.2022.894273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A systematic review of intensive cardiopulmonary management after spinal cord injury. Casha S, Christie S. J Neurotrauma. 2011;28:1479–1495. doi: 10.1089/neu.2009.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Incidence of pulmonary embolism after the first 3 months of spinal cord injury. Alabed S, de Heredia LL, Naidoo A, Belci M, Hughes RJ, Meagher TM. Spinal Cord. 2015;53:835–837. doi: 10.1038/sc.2015.105. [DOI] [PubMed] [Google Scholar]
- 14.Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Arch Phys Med Rehabil. 2004;85:1757–1763. doi: 10.1016/j.apmr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Secondary conditions following spinal cord injury in a population-based sample. Johnson RL, Gerhart KA, McCray J, Menconi JC, Whiteneck GG. Spinal Cord. 1998;36:45–50. doi: 10.1038/sj.sc.3100494. [DOI] [PubMed] [Google Scholar]
- 16.Pressure ulcer risk factors in persons with SCI: Part I: acute and rehabilitation stages. Gélis A, Dupeyron A, Legros P, Benaïm C, Pelissier J, Fattal C. Spinal Cord. 2009;47:99–107. doi: 10.1038/sc.2008.107. [DOI] [PubMed] [Google Scholar]
- 17.Determinants of in-hospital death after acute spinal cord injury: a population-based study. Selassie AW, Varma A, Saunders LL, Welldaregay W. Spinal Cord. 2013;51:48–54. doi: 10.1038/sc.2012.88. [DOI] [PubMed] [Google Scholar]
- 18.Predictors of early mortality after traumatic spinal cord injury: a population-based study. Varma A, Hill EG, Nicholas J, Selassie A. Spine (Phila Pa 1976) 2010;35:778–783. doi: 10.1097/BRS.0b013e3181ba1359. [DOI] [PubMed] [Google Scholar]
- 19.In-hospital mortality for the elderly with acute traumatic spinal cord injury. Inglis T, Banaszek D, Rivers CS, et al. J Neurotrauma. 2020;37:2332–2342. doi: 10.1089/neu.2019.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cervical spine injuries in the elderly: acute postoperative mortality. Jackson AP, Haak MH, Khan N, Meyer PR. Spine (Phila Pa 1976) 2005;30:1524–1527. doi: 10.1097/01.brs.0000167822.75063.8c. [DOI] [PubMed] [Google Scholar]
- 21.Epidemiological state, predictors of early mortality, and predictive models for traumatic spinal cord injury: a multicenter nationwide cohort study. Shibahashi K, Nishida M, Okura Y, Hamabe Y. Spine (Phila Pa 1976) 2019;44:479–487. doi: 10.1097/BRS.0000000000002871. [DOI] [PubMed] [Google Scholar]
- 22.The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Badhiwala JH, Wilson JR, Witiw CD, et al. Lancet Neurol. 2021;20:117–126. doi: 10.1016/S1474-4422(20)30406-3. [DOI] [PubMed] [Google Scholar]
- 23.Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. Papadopoulos SM, Selden NR, Quint DJ, Patel N, Gillespie B, Grube S. J Trauma. 2002;52:323–332. doi: 10.1097/00005373-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Anesthesia duration as an independent risk factor for early postoperative complications in adults undergoing elective ACDF. Phan K, Kim JS, Kim JH, Somani S, Di'Capua J, Dowdell JE, Cho SK. Global Spine J. 2017;7:727–734. doi: 10.1177/2192568217701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comparison of anterior and posterior approaches in cervical spinal cord injuries. Brodke DS, Anderson PA, Newell DW, Grady MS, Chapman JR. J Spinal Disord Tech. 2003;16:229–235. doi: 10.1097/00024720-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Comparison of anterior and posterior approaches for treatment of traumatic cervical dislocation combined with spinal cord injury: minimum 10-year follow-up. Ren C, Qin R, Wang P, Wang P. Sci Rep. 2020;10:10346. doi: 10.1038/s41598-020-67265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Australian Bureau of Statistics: Snapshot of New South Wales. Australian Bureau of Statistics: Canberra, ABS. [ Nov; 2022 ]. 2022. https://www.abs.gov.au/articles/snapshot-nsw-2021 https://www.abs.gov.au/articles/snapshot-nsw-2021
- 28.Australian Institute of Health and Welfare. Australian Institute of Health and Welfare: Australian Family of Health and Related Classifications - ICD-10-AM. 2022. [ Nov; 2022 ]. 2022. https://www.aihw.gov.au/suicide-self-harm-monitoring/data/technical-notes/codes-and-classifications p. 0.https://www.aihw.gov.au/suicide-self-harm-monitoring/data/technical-notes/codes-and-classifications
- 29.The impact of confounder selection criteria on effect estimation. Mickey RM, Greenland S. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 30.Variable selection strategies and its importance in clinical prediction modelling. Chowdhury MZ, Turin TC. Fam Med Community Health. 2020;8:0. doi: 10.1136/fmch-2019-000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Multicollinearity and misleading statistical results. Kim JH. Korean J Anesthesiol. 2019;72:558–569. doi: 10.4097/kja.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baseline predictors of in-hospital mortality after acute traumatic spinal cord injury: data from a level I trauma center. Blex C, Kreutzträger M, Ludwig J, et al. Sci Rep. 2022;12:11420. doi: 10.1038/s41598-022-15469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Development and validation of predictors of respiratory insufficiency and mortality scores: simple bedside additive scores for prediction of ventilation and in-hospital mortality in acute cervical spine injury. Durga P, Sahu BP, Mantha S, Ramachandran G. Anesth Analg. 2010;110:134–140. doi: 10.1213/ANE.0b013e3181c293a9. [DOI] [PubMed] [Google Scholar]
- 34.Mortality and longevity after a spinal cord injury: systematic review and meta-analysis. Chamberlain JD, Meier S, Mader L, von Groote PM, Brinkhof MW. Neuroepidemiology. 2015;44:182–198. doi: 10.1159/000382079. [DOI] [PubMed] [Google Scholar]
- 35.Risk factors for mortality after spinal cord injury in the USA. Cao Y, Krause JS, DiPiro N. Spinal Cord. 2013;51:413–418. doi: 10.1038/sc.2013.2. [DOI] [PubMed] [Google Scholar]
- 36.Recent trends in mortality and causes of death among persons with spinal cord injury. DeVivo MJ, Krause JS, Lammertse DP. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- 37.Stability of predictors of mortality after spinal cord injury. Krause JS, Saunders LL, Zhai Y. Spinal Cord. 2012;50:281–284. doi: 10.1038/sc.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervical injuries in Sweden, a national survey of patient data from 1987 to 1999. Brolin K, von Holst H. Inj Control Saf Promot. 2002;9:40–52. doi: 10.1076/icsp.9.1.40.3318. [DOI] [PubMed] [Google Scholar]
- 39.Epidemiology of traumatic spinal cord injury in Canada. Pickett GE, Campos-Benitez M, Keller JL, Duggal N. Spine (Phila Pa 1976) 2006;31:799–805. doi: 10.1097/01.brs.0000207258.80129.03. [DOI] [PubMed] [Google Scholar]
- 40.Spinal cord injuries in Ilorin, Nigeria. Solagberu BA. West Afr J Med. 2002;21:230–232. doi: 10.4314/wajm.v21i3.28037. [DOI] [PubMed] [Google Scholar]
- 41.Traumatic spinal cord injury in Victoria, 2007-2016. Beck B, Cameron PA, Braaf S, et al. Med J Aust. 2019;210:360–366. doi: 10.5694/mja2.50143. [DOI] [PubMed] [Google Scholar]
- 42.Incidence of traumatic spinal cord injuries in Finland over a 30-year period. Ahoniemi E, Alaranta H, Hokkinen EM, Valtonen K, Kautiainen H. Spinal Cord. 2008;46:781–784. doi: 10.1038/sc.2008.53. [DOI] [PubMed] [Google Scholar]
- 43.The changing face of major trauma in the UK. Kehoe A, Smith JE, Edwards A, Yates D, Lecky F. Emerg Med J. 2015;32:911–915. doi: 10.1136/emermed-2015-205265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Major trauma in older persons. Beck B, Cameron P, Lowthian J, Fitzgerald M, Judson R, Gabbe BJ. BJS Open. 2018;2:310–318. doi: 10.1002/bjs5.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morbidity and mortality of traumatic cervical spinal cord injuries in a geriatric cohort. Wilson KV, McDonnell JM, O'Malley S, et al. Ir J Med Sci. 2022;3:1–7. doi: 10.1007/s11845-022-03169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diagnostic coding accuracy for traumatic spinal cord injuries. Hagen EM, Rekand T, Gilhus NE, Gronning M. Spinal Cord. 2009;47:367–371. doi: 10.1038/sc.2008.118. [DOI] [PubMed] [Google Scholar]
- 47.Clinical coders' perspectives on pressure injury coding in acute care services in Victoria, Australia. Weller CD, Turnour L, Connelly E, Banaszak-Holl J, Team V. Front Public Health. 2022;10:893482. doi: 10.3389/fpubh.2022.893482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical documentation requirements for the accurate coding of hospital-acquired urinary tract infections in Australia. Liu S, Kim D, Penfold S, Doric A. Aust Health Rev. 2022;2:1–5. doi: 10.1071/AH22155. [DOI] [PubMed] [Google Scholar]
- 49.Accuracy of ICD-9-CM codes in hospital morbidity data, Victoria: implications for public health research. MacIntyre CR, Ackland MJ, Chandraraj EJ, Pilla JE. Aust N Z J Public Health. 1997;21:477–482. doi: 10.1111/j.1467-842x.1997.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 50.Accuracy of injury coding in Victorian hospital morbidity data. MacIntyre CR, Ackland MJ, Chandraraj EJ. https://pubmed.ncbi.nlm.nih.gov/9489199/ Aust N Z J Public Health. 1997;21:779–783. [PubMed] [Google Scholar]
- 51.Spinal cord injury without radiologic abnormalities in adults: a systematic review. Boese CK, Lechler P. J Trauma Acute Care Surg. 2013;75:320–330. doi: 10.1097/TA.0b013e31829243c9. [DOI] [PubMed] [Google Scholar]
- 52.The adult spinal cord injury without radiographic abnormalities syndrome: magnetic resonance imaging and clinical findings in adults with spinal cord injuries having normal radiographs and computed tomography studies. Kasimatis GB, Panagiotopoulos E, Megas P, Matzaroglou C, Gliatis J, Tyllianakis M, Lambiris E. J Trauma. 2008;65:86–93. doi: 10.1097/TA.0b013e318157495a. [DOI] [PubMed] [Google Scholar]
- 53.The relationship between statistical power and predictor distribution in multilevel logistic regression: a simulation-based approach. Olvera Astivia OL, Gadermann A, Guhn M. BMC Med Res Methodol. 2019;19:97. doi: 10.1186/s12874-019-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Multicollinearity in regression analyses conducted in epidemiologic studies. Vatcheva KP, Lee M, McCormick JB, Rahbar MH. Epidemiology (Sunnyvale) 2016;6:3–7. doi: 10.4172/2161-1165.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evaluation of Cox's model and logistic regression for matched case-control data with time-dependent covariates: a simulation study. Leffondré K, Abrahamowicz M, Siemiatycki J. Stat Med. 2003;22:3781–3794. doi: 10.1002/sim.1674. [DOI] [PubMed] [Google Scholar]
- 56.Empirical comparisons of proportional hazards, poisson, and logistic regression modeling of occupational cohort data. Callas PW, Pastides H, Hosmer DW. Am J Ind Med. 1998;33:33–47. doi: 10.1002/(sici)1097-0274(199801)33:1<33::aid-ajim5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 57.External validation of a Cox prognostic model: principles and methods. Royston P, Altman DG. BMC Med Res Methodol. 2013;13:33. doi: 10.1186/1471-2288-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bias in surgical research. Paradis C. Ann Surg. 2008;248:180–188. doi: 10.1097/SLA.0b013e318176bf4b. [DOI] [PubMed] [Google Scholar]
- 59.Variations in practice patterns among neurosurgeons and orthopaedic surgeons in the management of spinal disorders. Hussain M, Nasir S, Moed A, Murtaza G. Asian Spine J. 2011;5:208–212. doi: 10.4184/asj.2011.5.4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Development of a machine learning algorithm for predicting in-hospital and 1-year mortality after traumatic spinal cord injury. Fallah N, Noonan VK, Waheed Z, et al. Spine J. 2022;22:329–336. doi: 10.1016/j.spinee.2021.08.003. [DOI] [PubMed] [Google Scholar]