Abstract
Cryptococcosis is an hematogenously disseminated meningoencephalitis during which the relationship between the disease severity and the immune response remains unclear. We thus analyzed, by enzyme-linked immunosorbent assay, proinflammatory (tumor necrosis factor alpha [TNF-α] and interleukin-6 [IL-6]) and anti-inflammatory (IL-10) cytokine levels in plasma at the time of diagnosis in 51 AIDS patients with culture-proven cryptococcosis. We used a murine model to determine the correlation between cytokine levels and fungal burden in blood and tissues and the kinetics of the immune response and of the formation of cerebral lesions. In AIDS patients, plasma TNF-α and IL-10, but not IL-6, levels were significantly higher in the case of fungemia or disseminated infection than in their absence, whereas the presence of meningitis had no influence on these levels. In mice, none of these cytokines were detected within the first day after inoculation. Later on, TNF-α and IL-10, but not IL-6, levels in plasma correlated significantly with the fungal burden in the blood and spleen but not the brain. In the brain, cytokine levels were low compared to those in other compartments, and tissue lesions and a degree of infection similar to those observed in humans were seen, further suggesting the relevance of this experimental model. Thus, AIDS patients with cryptococcosis produce an immune response that reflects the dissemination but not the meningeal involvement. This murine model of disseminated cryptococcosis can be used to investigate the pathophysiology of cryptococcosis and new therapeutic approaches.
Cryptococcus neoformans is an encapsulated yeast responsible for severe meningitis and disseminated infections, including fungemia, mostly in patients with AIDS (14). In this group, 10 to 25% of patients die during initial antifungal therapy (32). Thus, improving the prognosis of this life-threatening opportunistic mycosis may require new therapeutic approaches such as immunointervention. However, immunotherapeutic trials cannot be designed without a comprehensive understanding of the immune response to C. neoformans in humans. Although there is a multitude of nonspecific effector cells capable of killing or inhibiting C. neoformans, cell-mediated immunity is a confirmed key host defense mechanism against C. neoformans (6, 35). Studies with mice have documented the roles of several cytokines, such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and interleukin 12 (IL-12), in the host defense against C. neoformans through their exogenous administration (22, 23, 25), the administration of specific anticytokine antibodies (1, 18, 21) or the use of cytokine-deficient mice generated by gene disruption (11, 36, 41). In addition, several recent in vitro studies focused on the production of various cytokines by human neutrophils and mononuclear cells incubated with C. neoformans. However, the use of different experimental conditions prevents any definitive conclusion on the role of these cytokines and their relationship during cryptococcosis (7, 28, 37, 38). Nevertheless, some of these studies clearly demonstrated a dose-dependent induction of cytokine secretion by human cells after stimulation with the cryptococcal glucuronoxylomannan (GXM) or with intact cells (13, 37, 38). Whether these experimental data will reflect the immune activation induced by C. neoformans in humans remains to be determined.
Thus, the main purpose of the present study was to investigate the cytokine response to C. neoformans infection in AIDS patients and to assess whether there was a relationship between cytokine profiles in plasma and the initial severity of the disease as evaluated by the presence of fungemia, meningeal involvement, or dissemination. Because precise quantification of tissue infection in humans is precluded, a murine model was needed to assess the influence of fungal load on cytokine responses in the target compartments. Furthermore, the model was also mandatory to evaluate the kinetics of the immune response to C. neoformans infection. Since the human disease is usually a disseminated meningoencephalitis, we chose a route of inoculation that leads to progressive disseminated infection in outbred mice and assessed the clinical relevance of this model. We did so by comparing fungal loads and histopathologies of brain tissues obtained from a rapidly fatal case of AIDS-associated disseminated cryptococcosis and from mice sacrificed at various times after inoculation.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998 [30a].)
MATERIALS AND METHODS
Human study.
The human study was done in accordance with a prospective protocol approved by the Ethical Committee of the Groupe Hospitalier Necker Enfants-Malades, Paris, France (DGS 970089, French Ministry of Health). Patients were enrolled anonymously, and all samples were assayed blindly. Plasma samples obtained within 2 days after the diagnosis of cryptococcosis from 51 AIDS patients with culture-confirmed cryptococcosis were studied. All of the patients had at least a culture of blood, cerebrospinal fluid, and urine before receiving antifungal therapy, and their median CD4 cell count was 28/mm3. Patients were considered to have disseminated infection if C. neoformans was cultured from at least two sites. Plasma samples were kept frozen at −80°C until assayed. Upon thawing, the samples were used for the measurement of TNF-α and IL-10 within the same day and then aliquoted and stored at −80°C prior to the measurement of IL-6. TNF-α, IL-6, and IL-10 concentrations were determined by an enzyme-linked immunosorbent assay (R & D Systems, Abingdon, United Kingdom) by comparison with standard curves. All samples were tested individually. According to the manufacturer, the minimum detectable levels of TNF-α, IL-6, and IL-10 in plasma were 4.4, 0.7, and 2.0 pg/ml, respectively.
Experimental studies. (i) Infecting organism.
The isolate of C. neoformans (NIH 52D) was subcultured in yeast nitrogen base broth supplemented with 2% glucose (Difco Laboratories, Detroit, Mich.) for 18 h on a rotary shaker at 30°C. The inoculum was prepared in sterile saline, as reported before (15), and 200 μl was injected into the lateral tail vein of each mouse.
(ii) Experimental infections.
Outbred male OF1 mice (Ico: OF1 [I.O.P.S. Caw]; mean body weight, 22 g) (Iffa Credo, l'Arbresle, France) were used. Five to eight mice per cage were housed in our animal facilities and received food and water ad libitum. Animal experimentation guidelines were respected in these studies.
The cytokine responses and the fungal burdens in blood and target organs in groups of five mice were studied as a function of the inoculum size (2 × 104, 2 × 105, or 2 × 106 per mouse) or the time of sacrifice (day 1, 3, 6, 8 or 10). The experiments were repeated twice or thrice (CFU counts and cytokine production on days 1 and 6 to 8). In this case, results from one representative experiment are shown.
(iii) Blood and tissue cultures.
From animals that had been euthanized, approximately 1 ml of blood was obtained by cardiac puncture. The plasma samples were individually aliquoted and frozen at −80°C. Fungemia was assessed by culturing buffy coats as previously described (31). The lung, spleen, and brain were aseptically removed, weighed, and ground in 1,000 μl of sterile phosphate-buffered saline containing 3% bovine serum albumin (Miles Laboratories, Spokane, Wash.). Tenfold dilutions of the homogenates were plated (100 μl) in duplicate on Sabouraud-chloramphenicol agar-coated petri dishes and incubated at 28°C for 48 h. Results are expressed as log10 CFU per gram of organ. When appropriate, the remaining homogenates were then centrifuged (14,000 rpm, 10 min), and the supernatants were immediately frozen at −80°C for subsequent determination of cytokine levels (see below).
(iv) Murine cytokine immunoassays.
Cytokine concentrations in plasma and supernatants of ground organs were assessed by enzyme-linked immunosorbent assay (R & D Systems) and determined by comparison with standard curves. Preliminary results showed that the curves obtained with the internal standard diluted in the provided buffer and with phosphate-buffered saline–3% bovine serum albumin were superposable (data not shown). All samples were tested individually. Cytokine concentrations in organs were expressed in picograms per gram by using the following formula: concentration in the supernatant in picograms per milliliter/organ weight in grams. According to the manufacturer, the minimum detectable levels of TNF-α, IL-6, IFN-γ, and IL-10 in plasma were 5, 3, 2, and 4 pg/ml, respectively. The corresponding lowest possible detection thresholds, expressed in picograms per gram of organ for the mice used in these experiments, were calculated based on the heaviest organs excised and were, respectively, 10, 6, 4, and 8 pg/g. Cytokine concentrations in all samples from naive mice were undetectable.
(v) Comparative histopathological study.
The brain recovered during the autopsy of an AIDS patient who died 2 days after the diagnosis of disseminated cryptococcosis was studied, as were those from mice inoculated with this patient's strain or with NIH 52D (2 × 105 cryptococci/mouse, as described above) and sacrificed (groups of three mice) at days 1, 3, 8, and 15 postinoculation. The experiment using NIH 52D was repeated twice. After removal, all of the specimens were fixed in 10% formalin solution, paraffin embedded, and sectioned coronally at 4-μm thickness. Sections were stained with hematoxylin-eosin, periodic acid-Schiff stain, blue-alcian, and Gomori-Grocott. All of the slides were analyzed blindly by one of the authors (F.G.). Upon removal, a sample from the brain of the patient and a sample from each mouse were immediately processed for CFU enumeration as described above.
(vi) Statistical methods.
Statistical analyses were performed with Statview 4.5 (Abacus Concepts, Inc., Berkeley, Calif.). CFU and cytokine levels were compared by using the Mann-Whitney U test or Kruskal-Wallis test, depending on the number of groups. The Spearman test was used to establish correlations between cytokine levels and CFU or between levels of two cytokines after pooling results (for mice used in at least three independent experiments starting on day 6 and sacrificed 6 to 10 days after infection with 2 × 106 cryptococci per mouse, n = 15 to 27). A correlation was taken into account as of rs ≥ 0.80. Significance was defined as P ≤ 0.05.
RESULTS
Histopathological study.
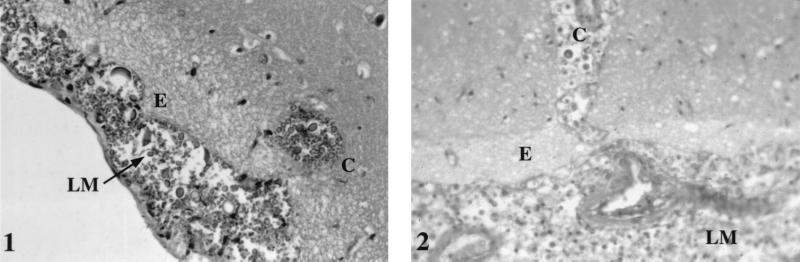
The microscopic examination of the patient's brain showed occasional inflammatory cells and numerous cryptococci in the leptomeninges, extending into the brain parenchyma along the perivascular spaces, where they formed cysts. In mice, lesions appeared 3 days after inoculation, but significant changes were not obvious until day 8 after infection with both the patient's strain and NIH 52D. At that time, lesions were similar to those observed in the patient's brain (Fig. 1), and enumeration of CFU per gram of organ found a similar degree of infection (the log10 CFU per gram of brain was 6.5 for the patient, 7.4 ± 0.2 for the mice inoculated with the corresponding strain, and 6.8 ± 0.3 for mice inoculated with NIH 52D). As cerebral lesions and fungal loads in the mouse brains at 8 days after inoculation were similar to those observed in the AIDS patient who died shortly after the diagnosis of cryptococcosis, we considered day 8 to be relevant for the study of the immune response in mice.
FIG. 1.
Comparative histopathological analysis of brain sections of mice sacrificed 8 days after inoculation with 2 × 105 C. neoformans organisms/mouse (panel 1) and of an AIDS patient who died of disseminated cryptococcosis (panel 2). Magnification, ×1,000. The strain inoculated into mice was cultured from the patient. LM, leptomeninges; E, edema; C, cyst.
Cytokine patterns in the plasma of AIDS patients with cryptococcosis.
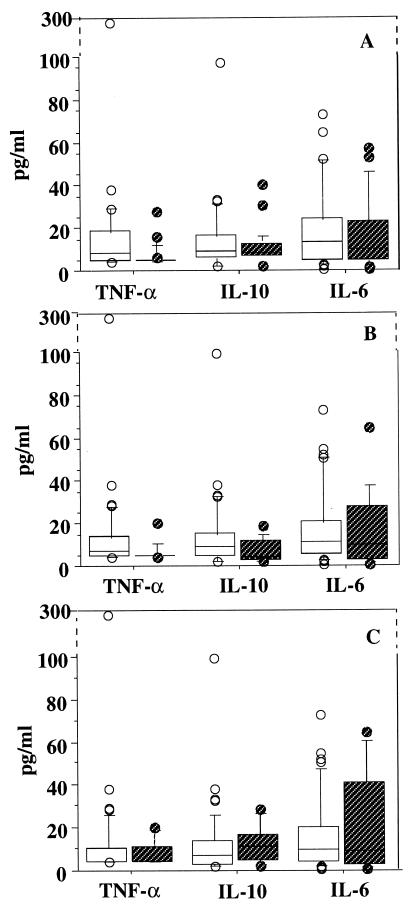
Since quantitative cultures for the detection of C. neoformans in blood and tissues are not routinely performed for humans, results are interpreted here in the light of positive or negative cultures. Median plasma TNF-α and IL-10, but not IL-6, levels were significantly higher in AIDS patients with cryptococcemia than in patients with negative blood culture and were significantly higher in patients with disseminated cryptococcosis than in patients with a single site infected (Fig. 2). Levels of these three cytokines in plasma were similar whether the patients had culture-confirmed meningitis or no meningeal involvement.
FIG. 2.
Distribution of TNF-α, IL-10, and IL-6 baseline levels in plasma of AIDS patients with cryptococcosis according to the presence (□) or absence ( ) of fungemia (A) (n = 27 and 24, respectively), dissemination (B) (n = 36 and 15, respectively), and culture-confirmed meningitis (C) (n = 44 and 7, respectively). Lines through boxes show medians, with other quartiles at either end. Bars show the 10th and 90th centiles. Dots represent individual values above the 90th centile or below the 10th centile. Significant differences between patients with or without fungemia and with or without dissemination were found for TNF-α (P = 0.001 and P = 0.01, respectively) and IL-10 (P = 0.002 and P = 0.04, respectively) in plasma.
Course of infection in OF1 mice.
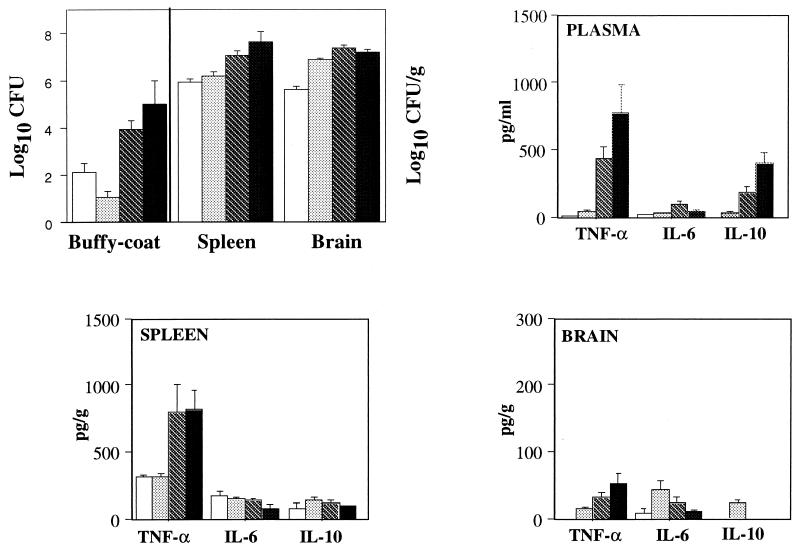
As previously determined with this model, survival and early CFU counts in blood and tissues depended on the inoculum size (31). At the early phase of infection (day 6 or earlier), for a given inoculum the groups were very homogeneous regarding fungal burdens in all organs and in blood (≤10% variation). Afterwards, a plateau (median log10 CFU, 7.01; range, 6.47 to 7.71) was reached in the brain whatever the size of the inoculum, whereas the fungal burden in the other compartments (spleen, lungs, and blood) varied more from mouse to mouse (31). A representative course of infection in mice inoculated with 2 × 106/mouse is shown in Fig. 3.
FIG. 3.
Fungal burden and TNF-α, IL-6, and IL-10 levels in plasma and in spleen and brain homogenates in OF1 mice. Mice were sacrificed on days 3 (□), 6 (░⃞), 8 (▧), and 10 (■) after inoculation with 2 × 106 C. neoformans organisms. Results are expressed as means ± standard errors of the means (n = 5 in each group).
Impact of fungal load on cytokine expression in mice.
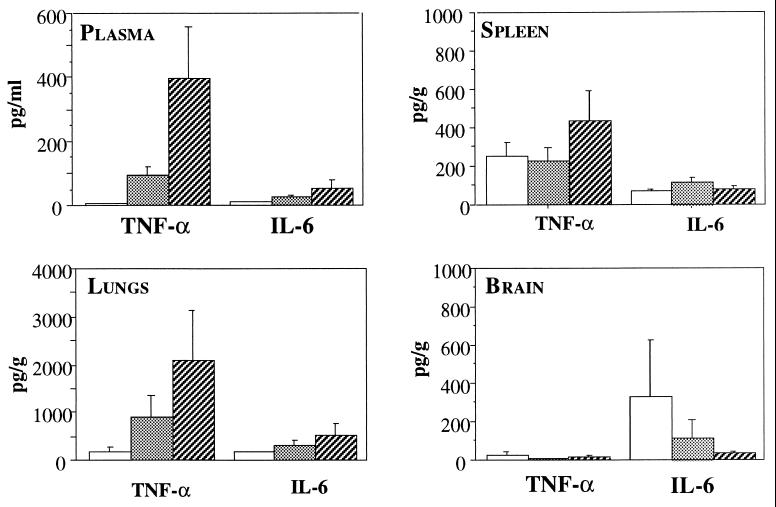
On day 1, despite fungemia, none of the mice (except one infected with the highest inoculum) had detectable plasma TNF-α (15 pg/ml) and IL-6 (40 pg/ml), and none had detectable IL-10 levels. It was verified in other experiments that no TNF-α or IL-6 was produced even earlier (1.5 and 5 h) after intravenous inoculation (data not shown). Plasma TNF-α and IL-10 levels increased significantly in parallel during the course of the infection (P < 0.004), while those of IL-6 did not (Fig. 3). The influence of fungal load was also seen on day 8, when plasma TNF-α levels differed significantly as a function of the inoculum size (P < 0.005) but those of IL-6 did not (Fig. 4). Significant correlations were established between plasma TNF-α and IL-10 levels (rs = 0.947).
FIG. 4.
TNF-α and IL-6 levels in plasma and in spleen, lung, and brain homogenates 8 days after inoculation of OF1 mice with 2 × 104 (□), 2 × 105 (░⃞), or 2 × 106 ( ) C. neoformans organisms/mouse. Results are expressed as means ± standard errors of the means (n = 5 in each group).
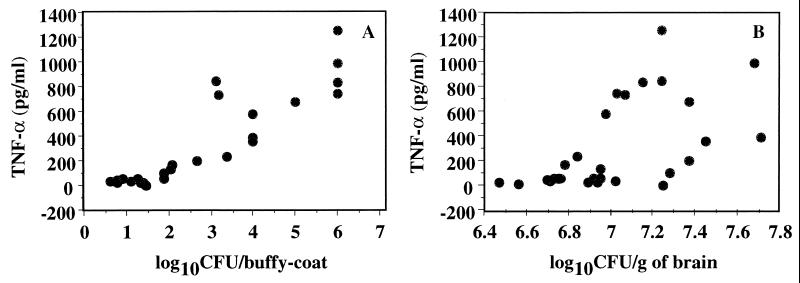
Significant correlations were also established between plasma TNF-α levels and spleen CFU (rs = 0.890) and fungemia (rs = 0.853), but not brain CFU (rs = 0.608) (Fig. 5), and similarly between plasma IL-10 levels and spleen CFU (rs = 0.860) and fungemia (rs = 0.819), but not brain CFU (rs = 0.574).
FIG. 5.
Correlations between TNF-α in plasma plotted versus fungal burden in buffy coat (A) or brain (B) in OF1 mice. Mice were sacrificed between days 6 and 10 after inoculation with 2 × 106 C. neoformans organisms. Each dot represents an individual sample (n = 27).
Locally in infected tissues, the size of the inoculum had no significant influence on TNF-α and IL-6 concentrations in the spleens and lungs on day 1 (data not shown) and day 8 (Fig. 4). Spleen TNF-α levels varied significantly over time (Fig. 3) (P < 0.001), while IL-6 and IL-10 concentrations remained stable. In brains, despite the local infection and regardless of the inoculum tested, no TNF-α and IL-6 were detected on day 1. Thereafter, brain TNF-α levels increased significantly over time (P < 0.02), while IL-6 concentrations peaked on day 6 after infection and decreased thereafter (P < 0.03), and IL-10 levels in most of samples remained below the detection threshold (Fig. 3). Overall, no correlations were found between local cytokine levels and the concomitant fungal burdens in the organs studied.
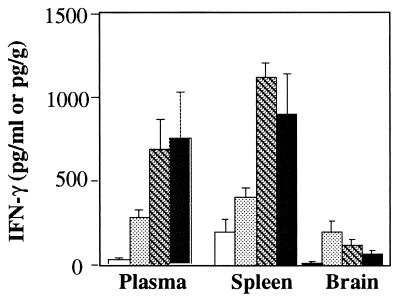
As IFN-γ is known to enhance the in vitro TNF-α production induced by C. neoformans (28), we wondered if the lack of TNF-α production early after inoculation was related to the absence of IFN-γ production. Plasma IFN-γ was detected as early as day 1 (median, 8 pg/ml; range, 5 to 12 pg/ml) and increased over time. Significant correlations were established between plasma TNF-α and IFN-γ (rs = 0.871). Spleen IFN-γ levels varied significantly over time (Fig. 3) (P < 0.001), while brain IFN-γ concentrations peaked on day 6 after infection and decreased thereafter (P < 0.03) (Fig. 6).
FIG. 6.
IFN-γ levels in plasma and in spleen and brain homogenates of OF1 mice. Mice were sacrificed on days 3 (□), 6 (░⃞), 8 ( ), and 10 (■) after inoculation with 2 × 106 C. neoformans organisms. Results are expressed as means ± standard errors of the means (n = 5 in each group).
DISCUSSION
We demonstrated in the present study the clinical relevance of an experimental model of disseminated cryptococcosis obtained after intravenous inoculation in outbred mice. Indeed, we were able to show that histopathological lesions and fungal loads in the brain were similar late in the course of experimental infection to what was observed in an AIDS patient who died shortly after the diagnosis of disseminated cryptococcosis and to the brain lesions reported by Lee et al. for an autopsy series of 13 human immunodeficiency virus-infected patients with cryptococcal meningitis (27). We also observed similar cytokine profiles in immunocompetent mice and in AIDS patients and an influence of fungal burden on the expression of some cytokines in both settings. For AIDS patients, we found evidence that fungemia and dissemination of C. neoformans infection influenced the production of TNF-α, as measured in the blood compartment. To better study the correlation between the cytokine response and the fungal load, we used quantitative cultures of yeasts in the blood and in target tissues of infected mice. We demonstrated a clear correlation between plasma TNF-α levels and fungal loads in blood and spleen, independently of the duration of infection in mice. Overall, our data show that the proinflammatory cytokine TNF-α is a marker of fungal load during disseminated cryptococcosis in humans and mice. Several in vitro studies have already shown that capsule components or cryptococcal cells themselves are able to stimulate TNF-α production by various types of cells (8, 10, 13, 20, 28, 37, 39), and some of these papers have pointed out a dose-dependent secretion of TNF-α by cells that had been stimulated with GXM (37) or intact cryptococci (13).
Our failure to demonstrate any correlation between plasma IL-6 levels and fungal loads in both mice and humans differs from the data reported for bacterial sepsis, where higher plasma IL-6 levels are found for nonsurviving individuals (17, 34). The lack of correlation between plasma IL-6 and TNF-α levels, however, is in agreement with the TNF-α-independent IL-6 production previously demonstrated during experimental bacterial sepsis (2, 17) and for human monocytes stimulated with C. neoformans components (12). Very little is known about the parameters influencing IL-6 synthesis after exposure to C. neoformans or its components. However, it was found that complement was required to trigger IL-6 secretion by human monocytes (12) and to transcribe IL-6 mRNA in rat alveolar macrophages (30). Another group has demonstrated that the magnitude of IL-6 release by human neutrophils reflected capsule thickness (37). Thus, the differences that we observed between plasma IL-6 and TNF-α profiles and fungal loads and their independent evolution in the host suggest the systematic study of the various parameters in vitro, as done by Retini et al. (37).
Another fact to be noted is the delayed and low expression of proinflammatory cytokines in the plasma of infected animals. Indeed, although fungemia was documented within the first 24 h, no proinflammatory cytokine response was observed in the plasma until day 3 after inoculation. These results contrast markedly with those observed after intravenous injection of bacterial lipopolysaccharide (LPS), when TNF-α and IL-6 peaked at high levels 1.5 h after inoculation and then declined (2, 9), but they agree with in vitro data showing that C. neoformans induction of TNF-α synthesis by human monocytes occurred late (≥18 h) compared to LPS-induced production (≥3 h) (28, 39).
We wondered whether the lack of an early inflammatory response in the plasma after C. neoformans inoculation reflected imbalances in the cytokine network which justifies our kinetic study of the expression of IFN-γ and IL-10. The first explanation would be a GXM-induced down regulation of TNF-α secretion, like that reported for human monocytes (39). This possibility seems unlikely, as the cryptococcal antigen concentration is low early after inoculation (4). Second, IL-10 could have down regulated TNF-α in vivo, as observed in vitro with human monocytes or peripheral blood mononuclear cells in response to LPS or C. neoformans (29, 38, 40). However, we were unable to detect IL-10 in the plasma early during the course of the experimental infection, and its concentrations in plasma became correlated significantly with TNF-α levels later on. In addition, the higher IL-10 levels found in AIDS patients with cryptococcemia compared to those with negative blood cultures and the correlation between the plasma IL-10 level and fungal load found in mice are in agreement with the dose-dependent induction of IL-10 secretion by human monocytes after stimulation with GXM (38). Since IFN-γ is known to enhance the in vitro TNF-α production by C. neoformans-activated macrophages (28), we verified in the mouse model that the delayed secretion of TNF-α was not due to an absence of IFN-γ stimulation.
Thus, our results with mice and humans disagree with the classical concept of Th1-Th2 balance (33), since plasma TNF-α, IFN-γ, and IL-10 concentrations rose in concert. Interestingly, when measuring cytokines produced in vitro by pulmonary T cells from mice infected intratracheally with C. neoformans, Huffnagle observed that both Th1- and Th2-type cytokines were secreted (19). Furthermore, using the same pulmonary model of cryptococcosis, Kawakami et al. found that IL-12 administration increased pulmonary IFN-γ and IL-10 levels (24).
The lack of early production of proinflammatory cytokines could prevent activation of defense mechanisms against C. neoformans and result in progressive disease. Indeed, TNF-α is necessary for the induction of the protective immune response against C. neoformans, as shown by the exogenous administration of TNF-α (23) or anti-TNF-α serum (21). Using the same model of disseminated infection after intravenous inoculation of strain NIH 52D, we were also able to show that mice deficient in both TNF-α and lymphotoxin-α genes were more susceptible to disseminated C. neoformans infection than their wild counterparts (36), thus confirming the importance of TNF-α and lymphotoxin-α in the cytokine network involved in the host defense against C. neoformans.
Keeping in mind that the brain is the target organ during cryptococcosis (14), one of the most important observations is the compartmentalization of cytokine production, as shown by differences between the brain and the other compartments. This was evidenced by identical cytokine levels in the plasma samples from AIDS patients with meningeal involvement and from those without such involvement and by the absence of a correlation between cytokine levels in plasma and the severity of cerebral infection in mice. In addition, despite the use of various inoculum sizes and study up to premortem stages with CFU counts as high as 7 log10 CFU/g of brain, all brain cytokine levels except those of IFN-γ remained low compared to those measured in the other compartments. Interestingly, after inoculating C. neoformans intracisternally, Blasi et al. observed IL-6, but not TNF-α, gene expression in the mouse brain (5). This group also showed that the detection of TNF-α, IL-6, and IFN-γ transcripts in the brain was delayed (3). The low expression of proinflammatory cytokines in the brain is in accordance with the occasional inflammatory reaction seen in pathological sections in AIDS patients and even in immunocompetent mice and might contribute to explain why the infection evolves in the brain independently of the other compartments (31). The reason for this compartmentalization could be the rare direct contacts occurring between the particulate antigens, mostly within the Virchow-Robin spaces, and the local effector cells (16) such as microglial macrophages and astrocytes (5, 26), compared to the ubiquitous contacts that can take place in other organs. A study of proinflammatory and anti-inflammatory cytokine levels in the cerebrospinal fluid of a large group of AIDS patients with cryptococcosis to confirm the in vivo contribution of these cytokines is under way.
We think that our murine model appropriately mimics the human infection with the fungemia-meningitis sequence and can be useful for further investigation of the pathophysiology of cryptococcosis, the unique behavior of the brain, and new therapeutic approaches. Other in vivo studies are needed to better explain the mutual influence of C. neoformans and human immunodeficiency virus on cytokine production.
ACKNOWLEDGMENTS
Olivier Lortholary is the recipient of fellowships from the Assistance-Publique-Hôpitaux de Paris and Sidaction and of an ASM travel grant for this work. This work was supported in part by a grant from the Pasteur Institute (Contrat Interne de Recherche Clinique to Françoise Dromer).
We thank the members of the French Cryptococcosis Study Group for enrolling patients in the clinical study and collecting biological samples. We thank Marlène Nicolas for her help in animal studies, Karine Sitbon and Amaury de Gouvello for monitoring the clinical study, and Janet Jacobson for reviewing the English text.
REFERENCES
- 1.Aguirre K, Havell E A, Gibson G W, Johnson L J. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformansin the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiot F, Fitting C, Tracey K J, Cavaillon J M, Dautry F. Lipopolysaccharide-induced cytokine cascade and lethality in LTα/TNFα-deficient mice. Mol Med. 1997;3:864–875. [PMC free article] [PubMed] [Google Scholar]
- 3.Barluzzi R, Mazzolla R, Brozzetti A, Puliti M, Mariucci G, Mosci P, Bistoni F, Blasi E. A low virulent strain of Candida albicansenhances brain anticryptococcal defenses: characterization of the local immune reaction by RT-PCR and histochemical analysis. J Neuroimmunol. 1997;79:37–48. doi: 10.1016/s0165-5728(97)00105-7. [DOI] [PubMed] [Google Scholar]
- 4.Bennett J E, Hasenclever H F. Cryptococcus neoformanspolysaccharide: studies of serologic properties and role in infection. J Immunol. 1965;94:916–920. [PubMed] [Google Scholar]
- 5.Blasi E, Barluzzi R, Mazzolla R, Pitzurra L, Puliti M, Saleppico S, Bistoni F. Biomolecular events involved in anticryptococcal resistance in the brain. Infect Immun. 1995;63:1218–1222. doi: 10.1128/iai.63.4.1218-1222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C: American Society for Microbiology; 1998. [Google Scholar]
- 7.Chaka W, Verheul A F M, Hoepelman A I M. Influence of different conditions on kinetics of tumor necrosis factor alpha release by peripheral blood mononuclear cells after stimulation with Cryptococcus neoformans: a possible explanation for different results. Clin Diagn Lab Med. 1997;4:792–794. doi: 10.1128/cdli.4.6.792-794.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaka W, Verheul A F M, Vaishnav V V, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hopelman I M. Cryptococcus neoformansand cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect Immun. 1997;65:272–278. doi: 10.1128/iai.65.1.272-278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chensue S W, Terebuh P D, Remick D G, Scales W E, Kunkel S L. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991;138:395–402. [PMC free article] [PubMed] [Google Scholar]
- 10.Cross C E, Bancroft G J. Ingestion of acapsular Cryptococcus neoformansoccurs via mannose and β-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect Immun. 1995;63:2604–2611. doi: 10.1128/iai.63.7.2604-2611.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delfino D, Cianci L, Lupis E, Celeste A, Petrelli M L, Curro F, Cusumano V, Teti G. Interleukin-6 production by human monocytes stimulated with Cryptococcus neoformanscomponents. Infect Immun. 1997;65:2454–2456. doi: 10.1128/iai.65.6.2454-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delfino D, Cianci L, Migliardo M, Mancuso G, Cusumano V, Corradini C, Teti G. Tumor necrosis factor-inducing activities of Cryptococcus neoformanscomponents. Infect Immun. 1996;64:5199–5204. doi: 10.1128/iai.64.12.5199-5204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond R D. Cryptococcus neoformans. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2331–2340. [Google Scholar]
- 15.Dromer F, Charreire J. Improved amphotericin B (AMB) activity by a monoclonal anti-Cryptococcus neoformansantibody. J Infect Dis. 1991;163:1114–1120. doi: 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- 16.Fabry Z, Raine C S, Hart M N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1994;15:218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 17.Havell E A, Sehgal P B. Tumor necrosis factor-independent IL-6 production during murine listeriosis. J Immunol. 1991;146:756–761. [PubMed] [Google Scholar]
- 18.Hoag K A, Lipscomb M F, Izzo A A, Street N E. Il-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 19.Huffnagle G B. Role of cytokines in T-cell immunity to a pulmonary Cryptococcus neoformansinfection. Biol Signals. 1996;5:215–222. doi: 10.1159/000109193. [DOI] [PubMed] [Google Scholar]
- 20.Huffnagle G B, Chen G H, Curtis J L, McDonald R A, Strieter R M, Toews G B. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- 21.Huffnagle G B, Toews G B, Burdick M D, Boyd M B, McAllister K S, McDonald R A, Kunkel S L, Strieter R M. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 22.Joly V, Saint-Julien L, Carbon C, Yeni P. In vivo activity of interferon-gamma in combination with amphotericin B in the treatment of experimental cryptococcosis. J Infect Dis. 1994;170:1331–1334. doi: 10.1093/infdis/170.5.1331. [DOI] [PubMed] [Google Scholar]
- 23.Kawakami K, Qifeng X, Tohyama M, Qureshi M H, Saito A. Contribution of tumour necrosis factor-alpha (TNF-α) in host defence mechanism against Cryptococcus neoformans. Clin Exp Immunol. 1996;106:468–474. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakami K, Tohyama M, Qifeng X, Saito A. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect Immun. 1997;65:1307–1312. doi: 10.1128/iai.65.4.1307-1312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami K, Tohyama M, Sie Q, Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin Exp Immunol. 1996;104:208–214. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S C, Dickson D W, Brosnan C F, Casadevall A. Human astrocytes inhibit Cryptococcus neoformansgrowth by a nitric oxide-mediated mechanism. J Exp Med. 1994;180:365–369. doi: 10.1084/jem.180.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S C, Dickson D W, Casadevall A. Pathology of cryptococcal meningoencephalitis. Analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;27:839–847. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- 28.Levitz S M, Tabuni A, Kornfeld H, Reardon C C, Golenbock D T. Production of tumor necrosis factor alpha in human leukocytes stimulated by Cryptococcus neoformans. Infect Immun. 1994;62:1975–1981. doi: 10.1128/iai.62.5.1975-1981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitz S M, Tabuni A, Nong S, Golenbock D T. Effects of interleukin-10 on human peripheral blood mononuclear cell responses to Cryptococcus neoformans, Candida albicans, and lipopolysaccharide. Infect Immun. 1996;64:945–951. doi: 10.1128/iai.64.3.945-951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R K, Mitchell T G. Induction of interleukin-6 mRNA in rat alveolar macrophages by in vitro exposure to both Cryptococcus neoformans and anti-C. neoformansantiserum. J Med Vet Mycol. 1997;35:327–334. [PubMed] [Google Scholar]
- 30a.Lortholary O, Improvisi L, Rayhane N, Nichols M, Fitting C, DuPont B, Cavaillon J M, Dromer F. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, abstr. J-055. Washington, D.C: American Society for Microbiology; 1998. [Google Scholar]
- 31.Lortholary O, Improvisi L, Nicolas M, Provost F, Dupont B, Dromer F. Fungemia during murine cryptococcosis sheds some light on pathophysiology. Med Mycol. 1999;37:169–174. [PubMed] [Google Scholar]
- 32.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 34.Munoz C, Misset B, Fitting C, Blériot J P, Carlet J, Cavaillon J M. Dissociation between plasma and monocyte-associated cytokines during sepsis. Eur J Immunol. 1991;21:2177–2184. doi: 10.1002/eji.1830210928. [DOI] [PubMed] [Google Scholar]
- 35.Murphy J W. Immunoregulation in cryptococcosis. In: Kurstak E, editor. Immunology of fungal diseases. New York, N.Y: Marcel Dekker, Inc.; 1989. pp. 319–345. [Google Scholar]
- 36.Rayhane, N., O. Lortholary, C. Fitting, J. Callebert, M. Huerre, F. Dromer, and J. M. Cavaillon. Enhanced sensitivity of tumor necrosis factor/lymphotoxin-α deficient mice to Cryptococcus neoformans infection despite increased levels of nitrite/nitrate, gamma-interferon and interleukin 12. J. Infect. Dis., in press. [DOI] [PubMed]
- 37.Retini C, Vecchiarelli A, Monari C, Tascini C, Bistoni F, Kozel T R. Capsular polysaccharide of Cryptococcus neoformansinduces proinflammatory cytokine release by human neutrophils. Infect Immun. 1996;64:2897–2903. doi: 10.1128/iai.64.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformansinduces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994;153:811–816. [PubMed] [Google Scholar]
- 41.Yuan R, Casadevall A, Oh J, Scharff M D. T cells cooperate with passive antibody to modify Cryptococcus neoformansinfection in mice. Proc Natl Acad Sci USA. 1997;94:2483–2488. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]