Abstract
Background:
Bipolar disorder (BD) presents with high obesity and type 2 diabetes (T2D) and pathophysiological and phenomenological abnormalities shared with cardiometabolic disorders. Genomic studies may help define if they share genetic liability. This selective review of BD with obesity and T2D will focus on genomic studies, stress their current limitations and guide future steps in developing the field.
Methods:
We searched electronic databases (PubMed, Scopus) until December 2021 to identify genome-wide association studies, polygenic risk score analyses, and functional genomics of BD accounting for body mass index (BMI), obesity, or T2D.
Results:
The first genome-wide association studies (GWAS) of BD accounting for obesity found a promising genome-wide association in an intronic gene variant of TCF7L2 that was further replicated. Polygenic risk scores of obesity and T2D have also been associated with BD, yet, no genetic correlations have been demonstrated. Finally, human-induced stem cell studies of the intronic variant in TCF7L2 show a potential biological impact of the products of this genetic variant in BD risk.
Limitations:
The narrative nature of this review.
Conclusions:
Findings from BD GWAS accounting for obesity and their functional testing, have prompted potential biological insights. Yet, BD, obesity, and T2D display high phenotypic, genetic, and population-related heterogeneity, limiting our ability to detect genetic associations. Further studies should refine cardiometabolic phenotypes, test gene-environmental interactions and add population diversity.
Keywords: Bipolar disorder, Obesity, Diabetes type 2, Genome-wide association study, Genomics, Polygenic risk score
1. Introduction
Globally, the standardized mortality ratio for individuals with bipolar disorder (BD) is two-times the rate for the general population caused mainly by cardiovascular and neurovascular events (Hayes et al., 2015). For example, a real-world analysis including BD patients (N = 124,803) revealed that 60.5 % had at least one cardiometabolic comorbidity, while 33.4 % had two or more (Correll et al., 2017). More recently, a large case-controlled study of BD participants (N = 661) and age-sex-matched controls (N = 706) identified a significant association between BD and obesity [odds ratio (CI) 1.62 (1.22–2.15)], elevated systolic blood pressure (SBP) [2.18 (1.55–3.06)] and elevated triglycerides [1.58(1.13–2.2)] (Cuellar-Barboza et al., 2021), adjusting for smoking and cardiometabolic medications. All of these cardiometabolic markers can drive increased risk for cardiovascular and stroke mortality (Foroughi et al., 2022; Prieto et al., 2016).
Obesity is a chronic and progressive disease that has doubled in global prevalence in the last four decades (Bray et al., 2017). Currently prevalence estimates for 2030 predicted to surpass 50 % of the world’s adult population (Global Burden of Disease, 2017; Kelly et al., 2008). Clinically, obesity is defined by a body mass index (BMI) >30 kg/m2. While BMI is an estimate of adiposity based upon weight and height, it has been argued that abdominal (or visceral or central) obesity (defined as a waist circumference >88 cm in women and >102 cm in men), should be included when stratifying obesity-related health risk, given the failure of BMI to fully capture cardiometabolic risk (Ross et al., 2020). Despite obesity’s high general population’s prevalence, substantial evidence indicates that rates of obesity (OR 1.65, 95 % [CI] 1.45–1.89) (Goldstein et al., 2011) and central obesity are even higher among BD individuals than non-BD controls (Cardenas et al., 2008; Fagiolini et al., 2002; Grothe et al., 2014; Holgerson et al., 2021; Y.K. Liu et al., 2021; McElroy et al., 2004; McElroy et al., 2016b), with more than half of the BD population having abdominal obesity (prevalence of 51.1 %; 95 % CI, 45.0–57.3 %) (Y.K. Liu et al., 2021). Conversely, when obese versus non-obese individuals are compared, lifetime prevalence rates of BD are significantly higher among individuals with obesity (OR 1.47; 95 % [CI] 1.12–1.93) (Simon et al., 2006). Factors associated with obesity among individuals with BD include atypical depressive symptoms (i.e., increased appetite, weight gain, hypersomnia, psychomotor inhibition), binge eating behavior and co-occurring binge eating disorder and bulimia nervosa, delayed circadian phase with a preference for later timing of sleep and daily activities (also called evening chronotype), poor dietary quality, and the weight-gaining side effects of psychotropics (especially antipsychotics but also lithium, valproate, and antidepressants) (Calkin et al., 2013; Gardea-Resendez et al., 2022; Cuellar-Barboza et al., 2019; Mason et al., 2020; McElroy et al., 2013; McElroy et al., 2016a; Melo et al., 2017; Miola et al., 2022; Nestsiarovich et al., 2020; Romo-Nava et al., 2020; Sen et al., 2021).
Of the cardiometabolic complications of obesity evaluated among BD individuals, diabetes type 2 (T2D) is perhaps the best studied. Similar to obesity, the prevalence of T2D in BD is higher than in the general population (RR 1.98; 95 % [CI] 1.6–2.4) (Lindekilde et al., 2021; Vancampfort et al., 2015), with a pooled prevalence of T2D in BD being 9.6 % (95 % CI, 7.3–12.2 %) (Y.K. Liu et al., 2021). Although specific moderators behind the BD-T2D have been studied to less extent, similarly to those associated with obesity, atypical depressive symptoms, disturbances in circadian rhythms, increased rates of psychological childhood trauma/maltreatment, immune system activation, unhealthy lifestyle habits including alcohol and substance abuse, smoking, poor dietary patterns, and sedentary life have been reported (Aas et al., 2020; Huang et al., 2015; Huffhines et al., 2016; Kemp et al., 2010; McIntyre et al., 2005; Solmi et al., 2021).
While multifactorial in etiology, BD, obesity, and T2D, share some common biological and behavioral mechanisms which includes: a) insulin resistance (Charles et al., 2016; Cuperfain et al., 2020), b) inflammation (Goldstein et al., 2009; SayuriYamagata et al., 2017), c) lifestyle (Elmslie et al., 2001; Morriss and Mohammed, 2005), d) environmental influences (Łojko et al., 2019). However, a comprehensive biological model of BD with cardiometabolic abnormalities is lacking for appropriate interventions. Genomic studies represent an important opportunity to model this complex phenomenon by determining if BD patients with/without obesity and/or T2D have a distinct genetic underpinning, investigating shared common risk variants, and exploring the functional impact of such findings. For example, a recent genome-wide association studies (GWAS) for BD risk accounting for the effect of BMI identified a genome-wide significant SNP signal mapping to an intronic region of the TCF7L2 gene, a well-established T2D risk gene (D. Liu et al., 2021). Here, we aim to produce the first narrative review of the genomic contributions to BD with obesity and T2D and discuss future steps to construct a comprehensive model of BD with cardiometabolic abnormalities of translational impact.
2. Methods
For this narrative review of genomics of obesity and T2D in BD, we completed electronic databases (PubMed, Scopus) search up to December 17, 2021 to identify genomic studies of obesity or T2D in BD. The search terms used were: ((“bipolar disorder”[MeSH] OR “bipolar”) AND (“body mass index” [MeSH] OR “BMI” OR “obesity”[MeSH] OR “obese” OR “diabetes type 2” OR “diabetes mellitus, type 2”[MeSH] “T2DM” OR “T2D” OR “insulin resistance”[MeSH] OR “IR”) AND (“genetic association studies”[MeSH] OR “polymorphism, genetic”[MeSH] OR “SNP” OR “SNPs” OR “VNTR” OR “genome-wide association study”[MeSH] OR “genomics”[MeSH] OR “polygenic risk score” OR “PRS” OR “mendelian randomization” OR “mendelian randomization analysis”[MeSH] OR “genetic expression” OR “functional genomics” OR “pluripotent stem cells”[MeSH]).
Studies were selected based on subject matter expertise in bipolar disorder (AM, ABCB) and diabetes/obesity (EDF). The authors selected studies based on their relevance to the topic. For the genomic section, we focused on GWAS, gene-level association studies, polygenic risk scores (PRS), Mendelian Randomization and functional genetics of BD with obesity or T2D, and all the studies with these characteristics were included.
3. Pathophysiology
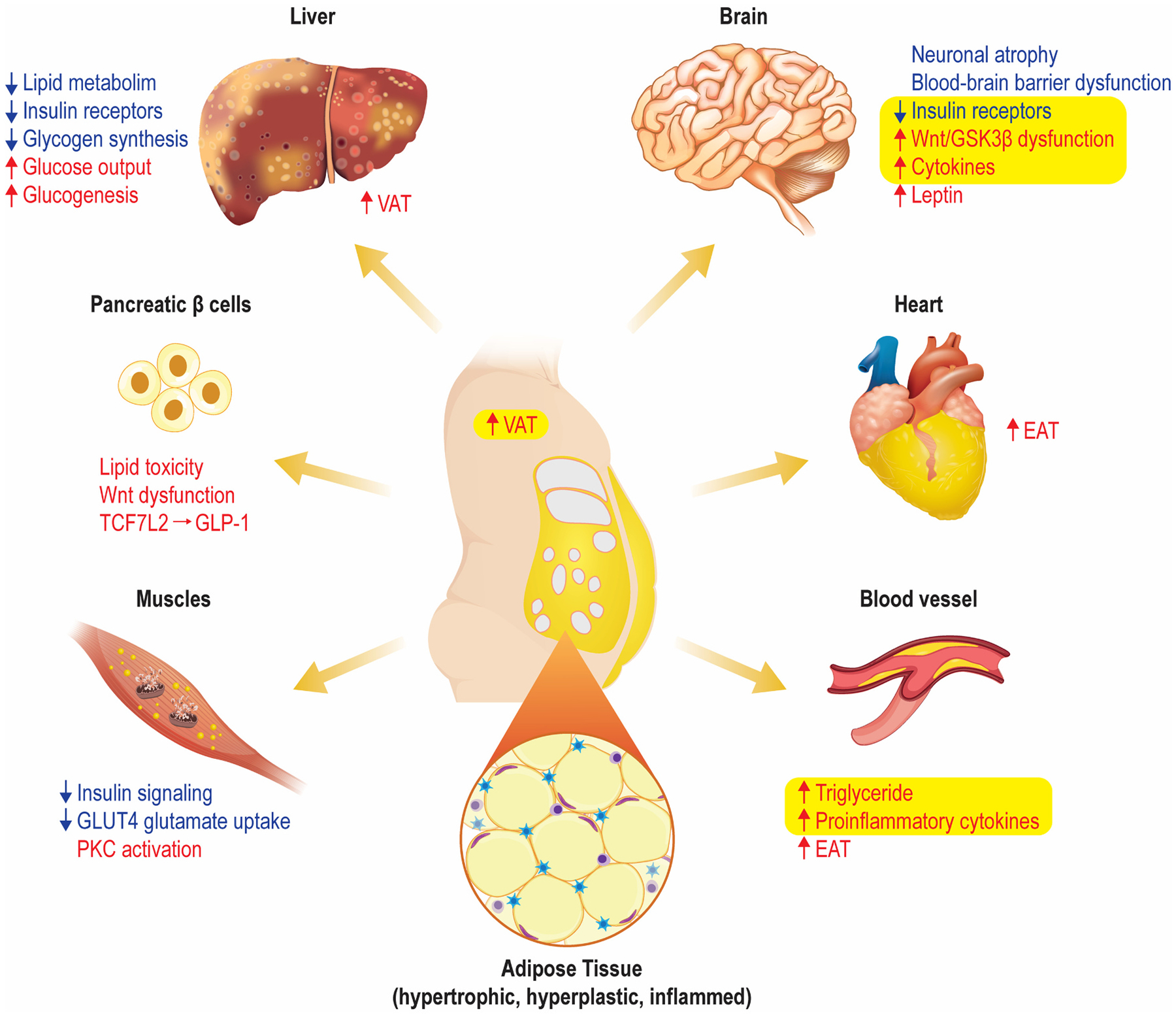
Obesity and T2D can independently or additively contribute to cardiometabolic disease (Grundy, 2016; Mechanick et al., 2020), and share a complex interplay (Kahn et al., 2006). Obesity is considered the strongest risk factor for T2D and is associated with metabolic abnormalities resulting in insulin resistance. Insulin resistance typically precedes the development of T2D (Bellou et al., 2018; DeFronzo and Tripathy, 2009; Galicia-Garcia et al., 2020; Groop, 1999). However, the exact mechanisms by which obesity induces insulin resistance and T2D are still poorly understood. Increasing evidence shows that the variabilities of both fat composition and adipose tissue distribution contribute to an increased risk of developing insulin resistance (Liu et al., 2018; Shulman, 2014). Specifically, an increase in visceral adipose tissue (VAT) is closely related to an increased incidence of insulin resistance (Liu et al., 2018), T2D and a higher risk of CVD (González et al., 2017; Lalia et al., 2016). With increased VAT, hypertrophied adipocytes are infiltrated by macrophage, in turn, leading to increased production of inflammatory cytokines (TNFα, IL-6, and IL-1b). This further impairs insulin signaling as well as lipolysis and endothelial function (Devaraj et al., 2004; Wilcox, 2005). In parallel, there is a decreased production of protective adipokines, such as adiponectin, an insulin sensitizer (Kadowaki et al., 2006; Neeland et al., 2019), and fatty acid esters of hydroxy fatty acids (FAHFAs) (Smith and Kahn, 2016). With a reduction in adiponectin, insulin-sensitive cells lose the ability to respond to insulin (i.e., they become insulin resistant) with secondary changes in pancreatic β-cell function. Of note, systemic and brain inflammation has been associated with BD pathophysiology (Goldstein et al., 2009; Hajek et al., 2014; Sayana et al., 2017), which positions the mechanisms of inflammation as an underlying biological underpinning common to BD, obesity, and T2D. However, this mechanism is not unique to these three diseases and multiple additional contributing factors likely contribute to the ratio of pro-inflammatory versus protective cytokine responses (i.e., genetic, stress, lifestyle, etc.). Further studies are needed to better elucidate the directionality of these associations (Wilcox, 2005).
In the early stages of insulin resistance, over secretion of insulin occurs, but normoglycemia is maintained. However, over time the ability to increase insulin secretion is exhausted, leading to the development of hyperglycemia and T2D (Czech, 2017; Kahn, 2003; Reaven, 1988). Genetic risk likely contributes to this physiological mechanism as T2D has a highly polygenic architecture with previous GWAS leading to the discovery of >100 associated genomic regions or loci (Langenberg and Lotta, 2018; McCarthy, 2010; Morris et al., 2012). Defects in pancreatic β-cell function also play a key role in developing T2D which is partially genetically determined (Galicia-Garcia et al., 2020; Halban et al., 2014; Langenberg and Lotta, 2018).
In the pancreas, Wnt signaling has been shown to control islet β cell proliferation (Rulifson et al., 2007). In particular, TCF7L2, an important downstream target of the canonical Wnt signaling pathway, is an important regulator of β-cell function and survival in human pancreatic islets (Shu et al., 2008). Wnt signaling has been implicated in glucose homeostasis through regulation of pro-glucagon gene expression, which encodes glucagon-like peptide 1 (GLP-1) in the brain and intestinal cells (Shao et al., 2013; Yi et al., 2005). Variants of the TCF7L2 gene confer an increased risk of developing T2D by altering levels of the insulinotropic hormone GLP-1, whose expression in enteroendocrine cells is transcriptionally regulated by TCF7L2 (Grant et al., 2006). Moreover, the Wnt signaling pathway regulates crucial processes in the development of mammalian nervous system, including patterning, cell fate specification, proliferation and neuronal morphology (Muneer, 2017; Valvezan and Klein, 2012). This pathway and one of its key enzymes, glycogen synthase kinase 3β, may regulate synaptic plasticity, cell survival, and circadian rhythms which have been implicated in the pathophysiology and treatment of BD (Gould and Manji, 2002; Madison et al., 2015). Proposed multisystemic alterations and their interactions in obesity, T2D and BD are shown in Fig. 1.
Fig. 1.
T2D pathophysiology and its interplay with obesity and BD.
Defects at the early steps of insulin signaling such as INSR, IRS1, PI3K, and Akt activity (Caro et al., 1987; Cusi et al., 2000; Griffin et al., 2000), impair insulin ability to stimulate GLUT4 translocation and subsequent glycogen synthesis (Petersen and Shulman, 2018). Unlike starvation, compensatory hyperinsulinemia promotes insulin mitogenic effects (Wilcox, 2005). Furthermore, the liver increases free fatty acid delivery which results in rising circulating lipids which will accumulate in the liver and further compromise triglyceride content and VLDL secretion (Krauss and Siri, 2004). Expanded visceral adipose tissue (VAT) becomes inflamed leading to increased production of pro-inflammatory cytokines, leptin, RBP4 and PAI-I that impair further insulin signaling (Devaraj et al., 2004; Wilcox, 2005) in part related to the increased presence of pro-inflammatory macrophages, and cause systemic and brain inflammation (Goldstein et al., 2009; Sayana et al., 2017). Wnt signaling pathway regulates the development of mammalian nervous system (Muneer, 2017; Valvezan and Klein, 2012), as well as glucose homeostasis through the regulation of pro-glucagon gene expression, which encodes glucagon-like peptide 1 (GLP-1) in intestinal cells (Yi et al., 2005).
4. Genomics
4.1. Heritability
BD, obesity, and T2D are each complex and heritable traits fitting a polygenetic risk model (Goes, 2016; Kim et al., 2014; Prasad and Groop, 2015; Willyard, 2014). BD has an estimated heritability of 60–85 % (Lichtenstein et al., 2009; McGuffin et al., 2003) with recent estimates of single nucleotide polymorphism (SNP)-based heritability of about 18–20 % (Mullins et al., 2021; Stahl et al., 2019). For obesity, heritability estimates vary depending on the applied definition (Yang et al., 2007) and ethnicity (Salinas et al., 2016). It is estimated that anywhere between 40 % and 80 % of body size variation is due to genetic factors (Barsh et al., 2000); BMI heritability estimates range between 40 % and 50 % (McQueen et al., 2003) or greater in different populations (Stunkard et al., 1986). The heritability of other obesity measurements such as waist circumference (WC) and fat percentage also showed great variability. T2D heritability also shows high variation, from 25 % to 80 % (Medici et al., 1999; Meigs et al., 2000; Poulsen et al., 1999).
Most genetic variants that contribute to these phenotypes have not been identified, in part due to the aforementioned polygenic risk model. However, complex traits such as BD, obesity, and T2D also show high phenotypic, genetic, and population-related heterogeneity, limiting the ability to detect genetic associations (Hodgson et al., 2017). One strategy to reduce phenotypic heterogeneity is to consider sub-phenotypes that are expected to be more genetically homogeneous (Saunders et al., 2008). Also, many genes have shared effects with complex diseases (Pickrell et al., 2016; Sivakumaran et al., 2011) which may be the case for BD, obesity, or T2D. For thorough reviews on the genetics of BD, obesity, and T2D (which are beyond the scope of this review), please refer to Goes (2016), Willyard (2014), and Prasad and Groop (2015).
4.2. Genetic association studies
Over a decade ago, genome-wide association studies (GWAS) initiated a new era of genetic research (WTCCC 2007). While a recent GWAS completed by the Psychiatric Genomics Consortium (PGC) Bipolar Disorder Work Group identified 64 loci associated with BD risk (Mullins et al., 2021), BMI was not routinely collected and a number of other ascertainment challenges for data harmonization are challenging to explore sub-phenotypes of bipolar disorder with obesity and or T2D. In our work that investigated the genetic association in BD with the consideration of obesity, we identified a significant association between an intronic SNP located in TCF7L2, rs12772424, and BD when the SNPBD interaction was included in the model (Cuellar-Barboza et al., 2016; Winham et al., 2014). We found that as BMI increased, so did the risk of BD in the minor allele carriers of rs12772424. These results were later replicated (Cuellar-Barboza et al., 2016). Additional TCF7L2 SNPs showed significant BMI-BD interaction at the gene level and in several haplotype analyses (Cuellar-Barboza et al., 2016). Importantly, variants in TCF7L2 have been widely demonstrated as risk SNPs for T2D (Grant et al., 2006). Similar to our BD studies, obesity also showed to be interacting with TCF7L2 rs7903146 C > T to confer an increase in T2D risk (Corella et al., 2016). TCF7L2 is an effector of the Wnt canonical signaling pathway (Saito-Diaz et al., 2013), which plays a critical role in metabolism and energy utilization (Benzler et al., 2013), neurobiological functions such as synaptic development, neurogenesis, neuronal migration and differentiation (Kim and Snider, 2011), as well as lithium drug mechanism of action (Klein and Melton, 1996), and has been proposed, through pluripotent stem cell modeling, as a model for the neurodevelopment and neuroplasticity of BD (Madison et al., 2015).
TCF7L2 rs12772424 identified in BD and obesity was independent of the TCF7L2 rs7903146 which was associated with T2D risk. These two SNPs are located approximately 122.2 kilo bases (kb) away from each other and are not in linkage disequilibrium in European (r2 = 0.0013, D′ = 0.0596) or other ancestral groups, indicating that TCF7L2 might have differing functions in T2D and in BD with increased BMI. Indeed, our subsequent studies of TCF7L2 function in human central nervous system (CNS) cell models revealed a unique CNS-based mechanism underlying BD-BMI genetic risk that involved a novel TCF7L2 non-coding transcript (D. Liu et al., 2021).
Since TCF7L2 is well recognized as a T2D risk gene, its function in T2D and glucose metabolism had been studied extensively in peripheral tissues in animal models with results that suggests it has a complex molecular role (Nobrega, 2013). Specifically, TCF7L2 dysfunction in hepatic (Boj et al., 2012; Ip et al., 2015; Oh et al., 2012) and pancreatic (da Silva Xavier et al., 2017; Mitchell et al., 2015) tissue, as well as involvement in the gut-brain axis (Shao et al., 2013), have all been thought to contribute to T2D risk. Despite mounting evidence suggesting that TCF7L2 may link psychiatric disorders and metabolic dysregulation, its function in the CNS was not well studied until recent efforts mentioned in the next section.
Potential genetic factors shared between BD and obesity have been most comprehensively investigated by Bahrami et al. (2020) using summary statistics from the PGC BD GWAS (N = 20,352 cases and N = 31,358 controls) and the BMI GWAS from the UK Biobank and GIANT data (n = 795,640) (Bahrami et al., 2020). The authors identified 679 loci associated with BMI conditionally on BD, and 52 BD associated variants conditionally on BMI. Additionally, 17 variants were of shared risk between BD and BMI (one was novel for BMI while nine were novel for BD). The functional analysis of these SNPs confirmed associations with molecular adhesion and neurite growth pathways (Bahrami et al., 2020). Among the overlapping BD and BMI SNPs, some had the same direction of effects on the two phenotypes while others had opposite direction of effect, and overall, no genome-wide genetic correlation was found between BD and BMI. Nonetheless, further evidence of genetic overlap was recently published by Rødevand et al. (2021) also using data from the PGC BD GWAS and the UK Biobank and GIANT data. They found extensive polygenic overlap between BD and BMI (81.5 % of BD variants influence BMI), including 69 overlapping loci between the two disorders. As in the previous study, no genetic correlations were established. This lack of genome-wide correlation underscores the complexity of the genetic relationship between BMI and BD. For instance, gene*environment interactions may play an important role, in which loci that typically have “neutral” effects on weight and metabolic regulation could become pathogenic genetic variants in the context of medication intake, sedentarism, or high calorie-intake diets (Prasad and Groop, 2015). Also, weight increments and metabolic alterations could affect normal brain processes via genetic factors. For example, in rodent models, obesity impairs brain glucose homeostasis by altering the genetic expression of Wnt-signaling ligands and targets (Benzler et al., 2013).
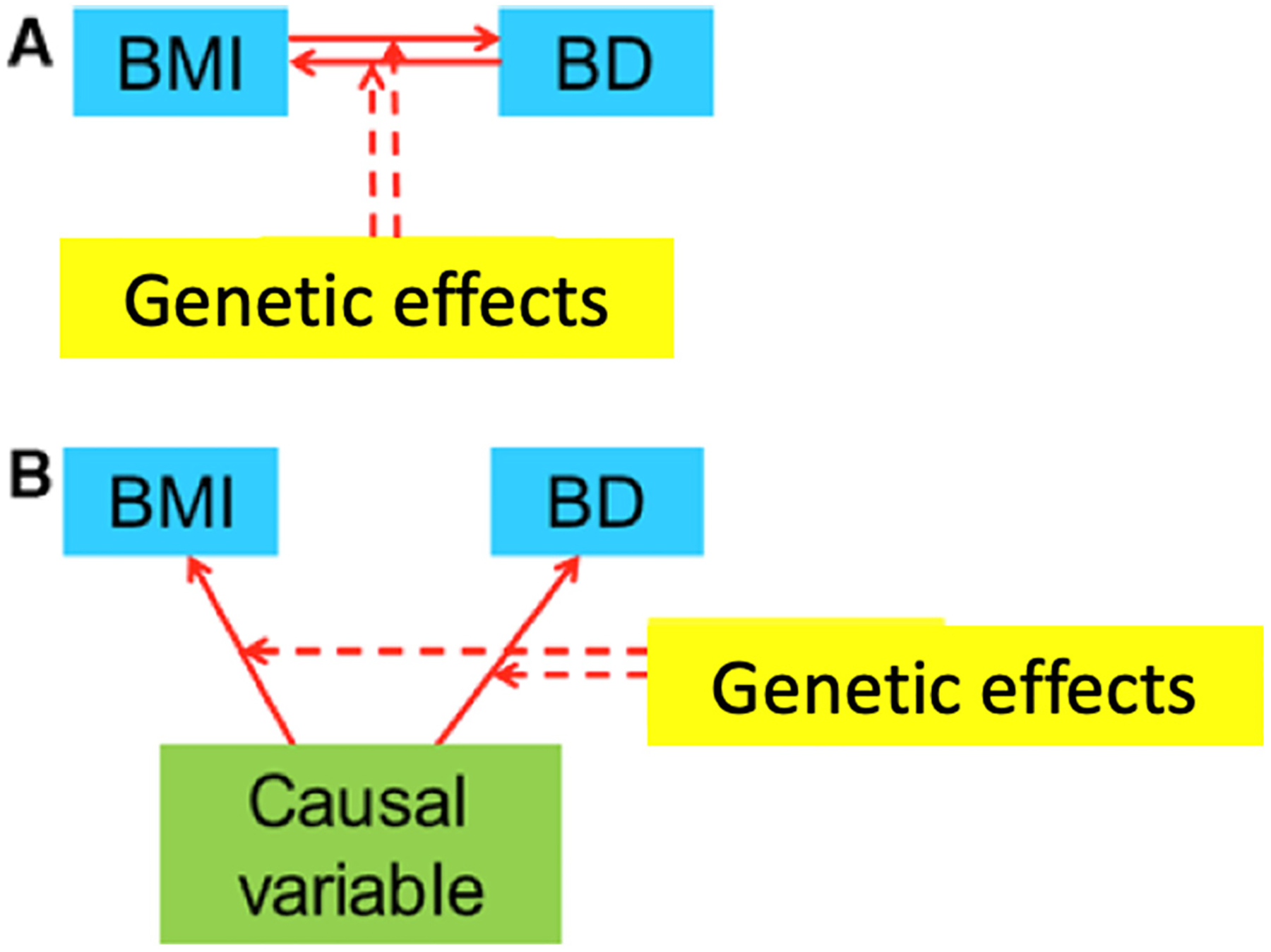
A meta-GWAS by Amare et al. (2017) attempted to find shared variants between “mood disorders”, including BD, and several common metabolic traits, including T2D, IR, lipid markers, and BMI. They found, associations between neuroplasticity and apoptosis-related SNPs common to obesity, lipid markers, and mood disorders. Also, IR, T2D, and mood disorders shared associations with TCF7L2 variants, among other genes (Amare et al., 2017). In addition, pathway analysis showed enrichment for monoaminergic, circadian rhythm, and leptin signaling, among others (Amare et al., 2017). This study provided interesting hypothesis-generating results, but its design precluded the determination of the direction of associations or the underlying mechanisms (Fig. 2).
Fig. 2.
Possible models of obesity/T2D-gene interaction.
Similarly, Pisanu et al. (2019) sought to find gene-based associations shared between BD and BMI or T2D. Fifty-two genes were significantly associated with both BD and BMI, twelve genes were significantly associated with BD and T2D, and three were associated with all three conditions (Pisanu et al., 2019). Pathway analysis showed a potential biological relationship among these conditions since enrichment was found in the protein networks established from the gene products shared by these disorders (Pisanu et al., 2019). Moreover, BD and BMI showed a larger number of gene-based associations and significant functional enrichment, than expected by chance; most notably in two Hedgehog signaling pathways, which are critically involved in cell survival and differentiation (Carballo et al., 2018).
4.3. Polygenic risk score analysis
Polygenic risk scores (PRS) for a given trait account for a combination of genetic risk variants to estimate the individual cumulative risk for the trait (Janssens, 2019). Using UK Biobank including N = 4155 participants with “possible bipolar disorder”, Fürtjes et al. (2021) tested the association between this BD phenotype and the PRSs for several cardiometabolic traits. PRS for obesity, waist to hip ratio (WHR), WHR adjusted for BMI, triglycerides, T2D, and coronary artery disease showed positive associations with this BD definition. Directions of associations were consistent when a more restricted BD phenotype definition was used (N = 920), but were not statistically significant (Fürtjes et al., 2021). As in the findings of Bahrami et al., genetic correlations between BD and these cardiometabolic traits were not statistically significant. The authors hypothesized that their PRS analysis captured individual-level genotype data, while genetic correlations relied on summary statistics, therefore deeming the former more powerful to capture genetic overlap.
4.4. Functional genomic studies
4.4.1. Other genomic studies
Functional studies are essential to determine tissue specificity, biological effects, and the pathophysiological role of genetic variants associated with BD in interaction with obesity and T2D. For instance, our findings of an association between TCF7L2 and BD could be, in fact, due to BD-dependent effects on obesity, rather than direct effects on BD (e.g., the SNP may impact the risk of obesity in patients with BD but not in controls). Our initial studies found marginal evidence that the identified variants were also associated with TCF7L2 expression in the brain (Cuellar-Barboza et al., 2016). Recently, a study by Liu and colleagues used induced pluripotent stem cells (iPSC) to explore the functional implications of the SNP that we had found to be associated with obesity and BD, rs12772424 (D. Liu et al., 2021). Those studies began with a review of human brain single-nucleus and single-cell RNA-seq data to determine that TCF7L2 was highly expressed in astrocytes. Further examination of the genomic region showed that the rs12772424 variant generated a half palindrome glucocorticoid response element (GRE), raising the possibility that TCF7L2 transcription might be regulated by glucocorticoid signaling in a SNP-dependent manner. After treatment of iPSC-derived astrocytes with dexamethasone, a potent synthetic glucocorticoid, expression levels of two TCF7L2 long non-coding transcripts (lncRNA-TCF7L2) were significantly repressed. One of those lncRNA-TCF7L2 transcripts, “T-3”, was predominantly expressed in the human brain but not in peripheral tissues such as the liver or pancreas. Finally, RNA-seq after knock-down of the lncRNA-TCF7L2 was performed. The lncRNA-TCF7L2 “T-3” was shown to influence expression in astrocytes of the parent gene as well as other genes involved in energy metabolism, including insulin signaling. Since insulin signaling in astrocytes is known to co-regulate CNS glucose sensing and systemic glucose metabolism (García-Cáceres et al., 2019, 2016), the identified glucocorticoid-responsive lncRNA-TCF7L2 T-3, might influence systemic glucose metabolism through the regulation of insulin signaling pathway genes in astrocytes, a mechanism that could contribute to the comorbidity of BD and obesity. In summary, the functional genomic study of our original GWAS signal resulted in the discovery of novel biological mechanisms in the brain that may contribute to the BD-obesity comorbidity risk.
Bioinformatic genomic tools will also be crucial to implement genomic markers, for instance, to define which BD patients are at risk of developing obesity and/or T2D and vice versa. Thus far, such models have had mixed findings. For example, using machine learning, Harrison et al. aimed to determine the best model to predict changes in BMI over one year of treatment in 284 BD subjects recruited for the IMPACT clinical trial (Harrison et al., 2017). Their model including genotyping and gene-expression data was less effective than the clinical model, which may have been in part due to the smaller sample size in the former and the use of peripheral gene-expression data, which may be less specific. In contrast, in a naturalistic one-year weight gain follow-up study of similar size, a predictive model incorporating candidate gene data improved the prediction over a model using only clinical data (Vandenberghe et al., 2016). Thus, larger sample sizes and multiple omic data from multiple tissues will likely be necessary to design better prediction tools.
5. Discussion
5.1. Summary
Compared with the general population, patients with BD are more frequently obese (Amare et al., 2017) and more likely to have T2D (Calkin et al., 2015; Gomes et al., 2013; Salvi et al., 2020).
Genetic association studies initially showed that BMI may moderate the association between BD risk and variants in TCF7L2 (Cuellar-Barboza et al., 2016; Winham et al., 2014). TCF7L2 is the effector of the Wnt signaling pathway, and is widely involved in obesity (Cauchi et al., 2008), T2D (Chen et al., 2021), and mood disorders (Madison et al., 2015) pathogenesis. Thus, TCF7L2 is a compelling molecular target for cardiometabolic (Chen and Wang, 2018) and mood disorders treatment (Muneer, 2017). These findings are particularly promising because SNPs in TCF7L2 are prominent risk factors for T2D and have potential common ground with BD, as shown by a meta-GWAS (Amare et al., 2017). Moreover, recent iPSC studies support a CNS-based mechanism behind the previously observed genetic BD-BMI interaction (D. Liu et al., 2021). However, further functional studies that test tissue specificity, different cardiometabolic phenotypes and pharmacological effects are needed.
Studies have found common genetic variants of risk for BD and BMI with functional enrichment of molecular adhesion and neurite growth pathways. Similarly, gene-level associations found common genetic risk between BD and BMI, and BD and T2D, but only BD and BMI showed pathway enrichment suggesting common biology (Pisanu et al., 2019). Finally, while BD and BMI were found to not be genetically correlated (Bahrami et al., 2020), studies have found that BMI and T2D PRS seem to be associated with a broad BD phenotype from the UK Biobank (Fürtjes et al., 2021).
5.2. Limitations and future perspectives
Previous investigations in the field suffer from limitations that should be considered when designing upcoming efforts:
Most studies exploring the relationship between BD and obesity used BMI to estimate the degree of obesity instead of other parameters that may better define this condition, including WC and central and peripheral fat mass. For instance, the largest BD GWAS to date found a genetic correlation between BD and waist-to-hip ratio (WHR rg = 0.07, p = .006; extreme WHR rg = 0.175, p = .0034) that should be further explored.
Methodologies for assessing obesity (i.e., BMI or WC) or T2D (i.e., fasting glucose, HbA1c, etc.) are highly heterogeneous across recruitment centers. Similarly, other cardiometabolic factors have not been captured in detail in many large BD repositories.
Environmental factors are heterogeneously assessed too (or not assessed at all) in current repositories. Consequently, G*E interactions have not been thoroughly tested in this field. Therefore, exploring lifestyle, diet, and especially medications in future studies will be crucial.
Well-powered genetic studies of the relationship between BMI or T2D and BD, testing overall genetic correlation or pleiotropic effects, have relied on BMI or T2D GWAS in the general population. However, there may be genetic determinants of BMI or T2D that are specific to populations of patients with BD (e.g., under the G*E hypothesis described above). Large studies of the genetics of BMI or T2D in patients with BD would be needed to identify such genetic effects.
Healthy obesity (obesity alone with no medical complication) has not been differentiated from unhealthy obesity in the reviewed studies, nor their different illness course. However, exploring the progression and interconnectedness of obesity and T2D (and other cardiometabolic factors) in BD will be important in future steps.
Psychiatric comorbidities and sub-phenotypes that are potential modifiers of obesity and T2D, such as binge eating behavior and chronotypes, have not been explored to date.
White European is the predominant ancestry included in the revised studies, with considerably smaller samples of African American and Eastern Asian participants. The scarce population diversity, in turn, hinders the generalizability of these genetic findings.
Finally, we want to underscore that investigating the genomics behind the BD obesity and T2D phenomenon (Mullins et al., 2021) merits a multidisciplinary approach. Geneticists, psychiatrists, and endocrinologists will need to discuss the genetic, clinical, and environmental factors impacting these complex disorders. Finally, pharmacogenetic trials will be vital in developing individualized interventions for people dealing with these conditions.
6. Conclusions
Genetic correlations between BD and obesity or T2D have not been established to date. Yet, markedly clinical correlations between BD, obesity, and T2D; and promising genetic signals of seemingly CNS functional implications warrant future studies in this field. We point at many limitations of prior studies that will impact this domain when addressed, including a lack of consistent phenotyping and the scarce investigation of genetic by environmental interactions.
Acknowledgements
This work was supported by the generous benefactors: the Huang Family.
Conflict of interest
NA Nuñez is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008685.
Francisco Romo-Nava MD, PhD, receives grant support from the National Institute of Mental Health K23 Award (K23MH120503) and from a 2017 NARSAD Young Investigator Award from the Brain and Behavior Research Foundation; has a U.S. Patent and Trademark Office patent # 10,857,356; and has received non-financial research support from Soterix Medical.
Mark Frye M.D. Grant Support; Assurex Health, Mayo Foundation and Intellectual property licensed to Chymia LLC. with rights to receive future royalties.
Other co-authors have no conflict of interest to declare.
Footnotes
CRediT authorship contribution statement
Alessandro Miola, Alfredo B. Cuellar-Barboza, Eleanna De Filippis designed the study, managed the literature searches, and wrote the first draft of the manuscript.
Marin Veldic, Stacey J. Winham, Susan L. McElroy, Joanna M. Biernacka, Mark A. Frye designed the study, and wrote the first draft of the manuscript.
Ada Man-Choi Ho, Mariana Mendoza, Francisco Romo-Nava, Nicolas A. Nunez, Manuel Gardea Resendez, Miguel L. Prieto managed the literature searches and wrote the draft of the manuscript.
All authors contributed to and have approved the final manuscript.
References
- Aas M, Bellivier F, Bettella F, Henry C, Gard S, Kahn J-P, Lagerberg TV, Aminoff SR, Melle I, Leboyer M, Jamain S, Andreassen OA, Etain B, 2020. Childhood maltreatment and polygenic risk in bipolar disorders. Bipolar Disord. 22, 174–181. 10.1111/bdi.12851. [DOI] [PubMed] [Google Scholar]
- Amare AT, Schubert KO, Klingler-Hoffmann M, Cohen-Woods S, Baune BT, 2017. The genetic overlap between mood disorders and cardiometabolic diseases: a systematic review of genome wide and candidate gene studies. Transl. Psychiatry 7, e1007. 10.1038/tp.2016.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami S, Steen NE, Shadrin A, O’Connell K, Frei O, Bettella F, Wirgenes KV, Krull F, Fan CC, Dale AM, Smeland OB, Djurovic S, Andreassen OA, 2020. Shared genetic loci between body mass index and major psychiatric disorders: a genome-wide association study. JAMA Psychiatry 77, 503–512. 10.1001/jamapsychiatry.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh GS, Farooqi IS, O’Rahilly S, 2000. Genetics of body-weight regulation. Nature 404, 644–651. 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- Bellou V, Belbasis L, Tzoulaki I, Evangelou E, 2018. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PloS One 13, e0194127. 10.1371/journal.pone.0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzler J, Andrews ZB, Pracht C, Stöhr S, Shepherd PR, Grattan DR, Tups A, 2013. Hypothalamic WNT signalling is impaired during obesity and reinstated by leptin treatment in male mice. Endocrinology 154, 4737–4745. 10.1210/en.2013-1746. [DOI] [PubMed] [Google Scholar]
- Boj SF, van Es JH, Huch M, Li VSW, José A, Hatzis P, Mokry M, Haegebarth A, van den Born M, Chambon P, Voshol P, Dor Y, Cuppen E, Fillat C, Clevers H, 2012. Diabetes risk gene and wnt effector Tcf7l2/TCF4 controls hepatic response to perinatal and adult metabolic demand. Cell 151, 1595–1607. 10.1016/j.cell.2012.10.053. [DOI] [PubMed] [Google Scholar]
- Bray GA, Kim KK, Wilding JPH, 2017. Obesity: a chronic relapsing progressive disease process. A position statement of the world obesity federation. Obes. Rev 18, 715–723. 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- Calkin CV, Gardner DM, Ransom T, Alda M, 2013. The relationship between bipolar disorder and type 2 diabetes: more than just co-morbid disorders. Ann. Med 45, 171–181. 10.3109/07853890.2012.687835. [DOI] [PubMed] [Google Scholar]
- Calkin CV, Ruzickova M, Uher R, Hajek T, Slaney CM, Garnham JS, O’Donovan MC, Alda M, 2015. Insulin resistance and outcome in bipolar disorder. Br. J. Psychiatry 10.1192/bjp.bp.114.152850. [DOI] [PubMed] [Google Scholar]
- Carballo GB, Honorato JR, de Lopes GPF, de S.E. Spohr TCL, 2018. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. CCS 16, 11. 10.1186/s12964-018-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas J, Frye MA, Marusak SL, Levander EM, Chirichigno JW, Lewis S, Nakelsky S, Hwang S, Mintz J, Altshuler LL, 2008. Modal subcomponents of metabolic syndrome in patients with bipolar disorder. J. Affect. Disord 106, 91–97. 10.1016/j.jad.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Caro JF, Sinha MK, Raju SM, Ittoop O, Pories WJ, Flickinger EG, Meelheim D, Dohm GL, 1987. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J. Clin. Invest 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchi S, Choquet H, Gutiérrez-Aguilar R, Capel F, Grau K, Proença C, Dina C, Duval A, Balkau B, Marre M, Potoczna N, Langin D, Horber F, Sørensen TIA, Charpentier G, Meyre D, Froguel P, 2008. Effects of TCF7L2 polymorphisms on obesity in european populations. Obes. Silver Spring Md 16, 476–482. 10.1038/oby.2007.77. [DOI] [PubMed] [Google Scholar]
- Charles EF, Lambert CG, Kerner B, 2016. Bipolar disorder and diabetes mellitus: evidence for disease-modifying effects and treatment implications. Int. J. Bipolar Disord 4, 13. 10.1186/s40345-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ning C, Mu J, Li D, Ma Y, Meng X, 2021. Role of wnt signaling pathways in type 2 diabetes mellitus. Mol. Cell. Biochem 476, 2219–2232. 10.1007/s11010-021-04086-5. [DOI] [PubMed] [Google Scholar]
- Chen N, Wang J, 2018. Wnt/β-catenin signaling and obesity. Front. Physiol 9, 792. 10.3389/fphys.2018.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corella D, Coltell O, Sorlí JV, Estruch R, Quiles L, Martínez-González MÁ, Salas-Salvadó J, Castañer O, Arós F, Ortega-Calvo M, Serra-Majem L, Gómez-Gracia E, Portolés O, Fiol M, Díez Espino J, Basora J, Fitó M, Ros E, J. M, 2016. Polymorphism of the transcription factor 7-like 2 gene (TCF7L2) interacts with obesity on Type-2 diabetes in the PREDIMED study emphasizing the heterogeneity of genetic variants in Type-2 diabetes risk prediction: time for obesity-specific genetic risk scores. Nutrients 8. 10.3390/nu8120793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Ng-Mak DS, Stafkey-Mailey D, Farrelly E, Rajagopalan K, Loebel A, 2017. Cardiometabolic comorbidities, readmission, and costs in schizophrenia and bipolar disorder: a real-world analysis. Ann. Gen. Psychiatry 16, 9. 10.1186/s12991-017-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Barboza AB, Cabello-Arreola A, Winham SJ, Colby C, Romo-Nava F, Nunez NA, Morgan RJ, Gupta R, Bublitz JT, Prieto ML, De Filippis EA, Lopez-Jimenez F, McElroy SL, Biernacka JM, Frye MA, Veldic M, 2021. Body mass index and blood pressure in bipolar patients: target cardiometabolic markers for clinical practice. J. Affect. Disord 282, 637–643. 10.1016/j.jad.2020.12.121. [DOI] [PubMed] [Google Scholar]
- Cuellar-Barboza AB, Winham SJ, Biernacka JM, Frye MA, McElroy SL, 2019. Clinical phenotype and genetic risk factors for bipolar disorder with binge eating: an update. Expert. Rev. Neurother 19, 867–879. 10.1080/14737175.2019.1638764. [DOI] [PubMed] [Google Scholar]
- Cuellar-Barboza AB, Winham SJ, McElroy SL, Geske JR, Jenkins GD, Colby CL, Prieto ML, Ryu E, Cunningham JM, Frye MA, Biernacka JM, 2016. Accumulating evidence for a role of TCF7L2 variants in bipolar disorder with elevated body mass index. Bipolar Disord. 18, 124–135. 10.1111/bdi.12368. [DOI] [PubMed] [Google Scholar]
- Cuperfain AB, Kennedy JL, Gonçalves VF, 2020. Overlapping mechanisms linking insulin resistance with cognition and neuroprogression in bipolar disorder. Neurosci. Biobehav. Rev 111, 125–134. 10.1016/j.neubiorev.2020.01.022. [DOI] [PubMed] [Google Scholar]
- Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ, 2000. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech MP, 2017. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med 23, 804–814. 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Xavier G, Mondragon A, Mourougavelou V, Cruciani-Guglielmacci C, Denom J, Herrera PL, Magnan C, Rutter GA, 2017. Pancreatic alpha cell-selective deletion of Tcf7l2 impairs glucagon secretion and counter-regulatory responses to hypoglycaemia in mice. Diabetologia 60, 1043–1050. 10.1007/s00125-017-4242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D, 2009. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, S157–S163. 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Rosenson RS, Jialal I, 2004. Metabolic syndrome: an appraisal of the pro-inflammatory and procoagulant status. Endocrinol. Metab. Clin. N. Am 10.1016/j.ecl.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Elmslie JL, Mann JI, Silverstone JT, Williams SM, Romans SE, 2001. Determinants of overweight and obesity in patients with bipolar disorder. J. Clin. Psychiatry 62, 486–491. 10.4088/jcp.v62n0614 quiz 492–493. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Frank E, Houck PR, Mallinger AG, Swartz HA, Buysse DJ, Ombao H, Kupfer DJ, 2002. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J. Clin. Psychiatry 63, 528–533. 10.4088/jcp.v63n0611. [DOI] [PubMed] [Google Scholar]
- Foroughi M, Medina Inojosa JR, Lopez-Jimenez F, Saeidifard F, Suarez L, Stokin GB, Prieto ML, Rocca WA, Frye MA, Morgan RJ, 2022. Association of bipolar disorder with major adverse cardiovascular events: a population-based historical cohort study. Psychosom. Med 84, 97–103. 10.1097/PSY.0000000000001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürtjes AE, Coleman JRI, Tyrrell J, Lewis CM, Hagenaars SP, 2021. Associations and limited shared genetic aetiology between bipolar disorder and cardiometabolic traits in the UK biobank. Psychol. Med 1–10 10.1017/S0033291721000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C, 2020. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci 21, 6275. 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cáceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi C-X, Chowen JA, Verkhratsky A, Araque A, Bechmann I, Tschöp MH, 2019. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci 22, 7–14. 10.1038/s41593-018-0286-y. [DOI] [PubMed] [Google Scholar]
- García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi C-X, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Alemán I, Kahn CR, Götz M, Horvath TL, Tschöp MH, 2016. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166, 867–880. 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardea-Resendez M, Winham SJ, Romo-Nava F, Cuellar-Barboza A, Clark MM, Andreazza AC, Cabello-Arreola A, Veldic M, Bond DJ, Singh B, Prieto ML, Nunez NA, Betcher H, Moore KM, Blom T, Colby C, Pendegraft RS, Kelpin SS, Ozerdem A, Miola A, De Filippis E, Biernacka JM, McElroy SL, Frye MA, 2022. Quantification of diet quality utilizing the rapid eating assessment for participants-shortened version in bipolar disorder: implications for prospective depression and cardiometabolic studies. J. Affect. Disord 310, 150–155. 10.1016/j.jad.2022.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease, 2017. Global Burden of Disease Study 2015 (GBD 2015) Obesity and Overweight Prevalence 1980–2015 | GHDx [WWW Document]. Inst. Health Metr. Eval. IHME [Google Scholar]
- Goes FS, 2016. Genetics of bipolar disorder: recent update and future directions. Psychiatr. Clin. North Am 39, 139–155. 10.1016/j.psc.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS, 2009. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J. Clin. Psychiatry 70, 1078–1090. 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Liu S-M, Zivkovic N, Schaffer A, Chien L-C, Blanco C, 2011. The burden of obesity among adults with bipolar disorder in the United States. Bipolar Disord. 13, 387–395. 10.1111/j.1399-5618.2011.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FA, Almeida KM, Magalhães PV, Caetano SC, Kauer-Sant’Anna M, Lafer B, Kapczinski F, 2013. Cardiovascular risk factors in outpatients with bipolar disorder: a report from the Brazilian research network in bipolar disorder. Rev. Bras. Psiquiatr. Sao Paulo Braz 1999 (35), 126–130. 10.1590/1516-4446-2011-0768. [DOI] [PubMed] [Google Scholar]
- González N, Moreno-Villegas Z, González-Bris A, Egido J, Lorenzo, 2017. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc. Diabetol 10.1186/s12933-017-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Manji HK, 2002. The wnt signaling pathway in bipolar disorder. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 8, 497–511. 10.1177/107385802237176. [DOI] [PubMed] [Google Scholar]
- Grant SFA, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K, 2006. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet 38, 320–323. 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI, 2000. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C θ and alterations in the insulin signaling cascade. Diabetes. 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- Groop LC, 1999. Insulin resistance: the fundamental trigger of type 2 diabetes. Diabetes Obes. Metab 1 (Suppl. 1), S1–S7. 10.1046/j.1463-1326.1999.0010s1001.x. [DOI] [PubMed] [Google Scholar]
- Grothe KB, Mundi MS, Himes SM, Sarr MG, Clark MM, Geske JR, Kalsy SA, Frye MA, 2014. Bipolar disorder symptoms in patients seeking bariatric surgery. Obes. Surg 24, 1909–1914. 10.1007/s11695-014-1262-6. [DOI] [PubMed] [Google Scholar]
- Grundy SM, 2016. Metabolic syndrome update. Trends Cardiovasc. Med 26, 364–373. 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Hajek T, Calkin C, Blagdon R, Slaney C, Uher R, Alda M, 2014. Insulin resistance, diabetes mellitus, and brain structure in bipolar disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 39, 2910–2918. 10.1038/npp.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC, 2014. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J. Clin. Endocrinol. Metab 99, 1983–1992. 10.1210/jc.2014-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RNS, Gaughran F, Murray RM, Lee SH, Cano JP, Dempster D, Curtis CJ, Dima D, Patel H, de Jong S, Breen G, 2017. Development of multivariable models to predict change in body mass index within a clinical trial population of psychotic individuals. Sci. Rep 7, 14738. 10.1038/s41598-017-15137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JF, Miles J, Walters K, King M, Osborn DPJ, 2015. A systematic review and meta-analysis of premature mortality in bipolar affective disorder. Acta Psychiatr. Scand 131, 417–425. 10.1111/acps.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson K, McGuffin P, Lewis CM, 2017. Advancing psychiatric genetics through dissecting heterogeneity. Hum. Mol. Genet 26, R160–R165. 10.1093/hmg/ddx241. [DOI] [PubMed] [Google Scholar]
- Holgerson AA, Clark MM, Frye MA, Kellogg TA, Mundi MS, Veldic M, Grothe K, 2021. Symptoms of bipolar disorder are associated with lower bariatric surgery completion rates and higher food addiction. Eat. Behav 40, 101462 10.1016/j.eatbeh.2020.101462. [DOI] [PubMed] [Google Scholar]
- Huang H, Yan P, Shan Z, Chen S, Li M, Luo C, Gao H, Hao L, Liu L, 2015. Adverse childhood experiences and risk of type 2 diabetes: a systematic review and meta-analysis. Metabolism 64, 1408–1418. 10.1016/j.metabol.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Huffhines L, Noser A, Patton SR, 2016. The link between adverse childhood experiences and diabetes. Curr. Diab. Rep 16, 54. 10.1007/s11892-016-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip W, Shao W, Song Z, Chen Z, Wheeler MB, Jin T, 2015. Liver-specific expression of dominant-negative transcription factor 7-like 2 causes progressive impairment in glucose homeostasis. Diabetes 64, 1923–1932. 10.2337/db14-1329. [DOI] [PubMed] [Google Scholar]
- Janssens ACJW, 2019. Validity of polygenic risk scores: are we measuring what we think we are? Hum. Mol. Genet 28, R143–R150. 10.1093/hmg/ddz205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K, 2006. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, 2003. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM, 2006. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846. 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kelly T, Yang W, Chen C-S, Reynolds K, He J, 2008. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes 2005 (32), 1431–1437. 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- Kemp DE, Gao K, Chan PK, Ganocy SJ, Findling RL, Calabrese JR, 2010. Medical comorbidity in bipolar disorder: relationship between illnesses of the endocrine/metabolic system and treatment outcome. Bipolar Disord. 12, 404–413. 10.1111/j.1399-5618.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W-Y, Snider WD, 2011. Functions of GSK-3 signaling in development of the nervous system. Front. Mol. Neurosci 4, 44. 10.3389/fnmol.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Xia K, Tao R, Giusti-Rodriguez P, Vladimirov V, van den Oord E, Sullivan PF, 2014. A meta-analysis of gene expression quantitative trait loci in brain. Transl. Psychiatry 4, e459. 10.1038/tp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA, 1996. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U. S. A 93, 8455–8459. 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss RM, Siri PW, 2004. Metabolic abnormalities: triglyceride and low-density lipoprotein. Endocrinol. Metab. Clin. N. Am 10.1016/j.ecl.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Lalia AZ, Dasari S, Johnson ML, Robinson MM, Konopka AR, DIstelmaier K, Port JD, Glavin MT, Esponda RR, Nair KS, Lanza IR, 2016. Predictors of whole-body insulin sensitivity across ages and adiposity in adult humans. J. Clin. Endocrinol. Metab 10.1210/jc.2015-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg C, Lotta LA, 2018. Genomic insights into the causes of type 2 diabetes. Lancet Lond. Engl 391, 2463–2474. 10.1016/S0140-6736(18)31132-2. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM, 2009. Common genetic determinants of schizophrenia and bipolar disorder in swedish families: a population-based study. Lancet Lond. Engl 373, 234–239. 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindekilde N, Scheuer SH, Rutters F, Knudsen L, Lasgaard M, Rubin KH, Henriksen JE, Kivimäki M, Andersen GS, Pouwer F, 2021. Prevalence of type 2 diabetes in psychiatric disorders: an umbrella review with meta-analysis of 245 observational studies from 32 systematic reviews. Diabetologia. 10.1007/s00125-021-05609-x. [DOI] [PubMed] [Google Scholar]
- Liu D, Nguyen TTL, Gao H, Huang H, Kim DC, Sharp B, Ye Z, Lee J-H, Coombes BJ, Ordog T, Wang L, Biernacka JM, Frye MA, Weinshilboum RM, 2021. TCF7L2 lncRNA: a link between bipolar disorder and body mass index through glucocorticoid signaling. Mol. Psychiatry 1–11. 10.1038/s41380-021-01274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Feng J, Zhang G, Yuan X, Li F, Yang T, Hao S, Huang D, Hsue C, Lou Q, 2018. Visceral adipose tissue is more strongly associated with insulin resistance than subcutaneous adipose tissue in chinese subjects with pre-diabetes. Curr. Med. Res. Opin 10.1080/03007995.2017.1364226. [DOI] [PubMed] [Google Scholar]
- Liu YK, Ling S, Lui LMW, Ceban F, Vinberg M, Kessing LV, Ho RC, Rhee TG, Gill H, Cao B, Mansur RB, Lee Y, Rosenblat J, Teopiz KM, McIntyre RS, 2021. Prevalence of type 2 diabetes mellitus, impaired fasting glucose, general obesity, and abdominal obesity in patients with bipolar disorder: a systematic review and meta-analysis. J. Affect. Disord 300, 449–461. 10.1016/j.jad.2021.12.110. [DOI] [PubMed] [Google Scholar]
- Łojko D, Stelmach-Mardas M, Suwalska A, 2019. Diet quality and eating patterns in euthymic bipolar patients. Eur. Rev. Med. Pharmacol. Sci 23, 1221–1238. 10.26355/eurrev_201902_17016. [DOI] [PubMed] [Google Scholar]
- Madison JM, Zhou F, Nigam A, Hussain A, Barker DD, Nehme R, van der Ven K, Hsu J, Wolf P, Fleishman M, O’Dushlaine C, Rose S, Chambert K, Lau FH, Ahfeldt T, Rueckert EH, Sheridan SD, Fass DM, Nemesh J, Mullen TE, Daheron L, McCarroll S, Sklar P, Perlis RH, Haggarty SJ, 2015. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol. Psychiatry 20, 703–717. 10.1038/mp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason IC, Qian J, Adler GK, Scheer FAJL, 2020. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia 63, 462–472. 10.1007/s00125-019-05059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MI, 2010. Genomics, type 2 diabetes, and obesity. N. Engl. J. Med 363, 2339–2350. 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Crow S, Biernacka JM, Winham S, Geske J, Cuellar Barboza AB, Prieto ML, Chauhan M, Seymour LR, Mori N, Frye MA, 2013. Clinical phenotype of bipolar disorder with comorbid binge eating disorder. J. Affect. Disord 150, 981–986. 10.1016/j.jad.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy Susan L., Crow S, Blom TJ, Biernacka JM, Winham SJ, Geske J, Cuellar-Barboza AB, Bobo WV, Prieto ML, Veldic M, Mori N, Seymour LR, Bond DJ, Frye MA, 2016. Prevalence and correlates of DSM-5 eating disorders in patients with bipolar disorder. J. Affect. Disord 191, 216–221. 10.1016/j.jad.2015.11.010. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kemp DE, Friedman ES, Reilly-Harrington NA, Sylvia LG, Calabrese JR, Rabideau DJ, Ketter TA, Thase ME, Singh V, Tohen M, Bowden CL, Bernstein EE, Brody BD, Deckersbach T, Kocsis JH, Kinrys G, Bobo WV, Kamali M, McInnis MG, Leon AC, Faraone S, Nierenberg AA, Shelton RC, 2016. Obesity, but not metabolic syndrome, negatively affects outcome in bipolar disorder. Acta Psychiatr. Scand 133, 144–153. 10.1111/acps.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB, 2004. Are mood disorders and obesity Related? A review for the mental health professional. J. Clin. Psychiatry 65, 634–651. 10.4088/JCP.v65n0507. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A, 2003. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch. Gen. Psychiatry 60, 497–502. 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Konarski JZ, Misener VL, Kennedy SH, 2005. Bipolar disorder and diabetes mellitus: epidemiology, etiology, and treatment implications. Ann. Clin. Psychiatry off. J. Am. Acad. Clin. Psychiatr 17, 83–93. 10.1080/10401230590932380. [DOI] [PubMed] [Google Scholar]
- McQueen MB, Bertram L, Rimm EB, Blacker D, Santangelo SL, 2003. A QTL genome scan of the metabolic syndrome and its component traits. BMC Genet. 4 (Suppl. 1), S96. 10.1186/1471-2156-4-S1-S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanick JI, Farkouh ME, Newman JD, Garvey WT, 2020. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers. J. Am. Coll. Cardiol 75, 525–538. 10.1016/j.jacc.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici F, Hawa M, Ianari A, Pyke DA, Leslie RD, 1999. Concordance rate for type II diabetes mellitus in monozygotic twins: actuarial analysis. Diabetologia 42, 146–150. 10.1007/s001250051132. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Cupples LA, Wilson PW, 2000. Parental transmission of type 2 diabetes: the Framingham offspring study. Diabetes 49, 2201–2207. 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- Melo MCA, Abreu RLC, Linhares Neto VB, de Bruin PFC, de Bruin VMS, 2017. Chronotype and circadian rhythm in bipolar disorder: a systematic review. Sleep Med. Rev 34, 46–58. 10.1016/j.smrv.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Miola A, Pinna M, Manchia M, Tondo L, Baldessarini RJ, 2022. Overweight in mood disorders: effects on morbidity and treatment response. J. Affect. Disord 297, 169–175. 10.1016/j.jad.2021.10.032. [DOI] [PubMed] [Google Scholar]
- Mitchell RK, Mondragon A, Chen L, Mcginty JA, French PM, Ferrer J, Thorens B, Hodson DJ, Rutter GA, Da Silva Xavier G, 2015. Selective disruption of Tcf7l2 in the pancreatic β cell impairs secretory function and lowers β cell mass. Hum. Mol. Genet 24, 1390–1399. 10.1093/hmg/ddu553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Müller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJF, Vedantam S, Chen H, Florez JC, Fox C, Liu C-T, Rybin D, Couper DJ, Kao WHL, Li M, Cornelis MC, Kraft P, Sun Q, van Dam RM, Stringham HM, Chines PS, Fischer K, Fontanillas P, Holmen OL, Hunt SE, Jackson AU, Kong A, Lawrence R, Meyer J, Perry JRB, Platou CGP, Potter S, Rehnberg E, Robertson N, Sivapalaratnam S, Stančáková A, Stirrups K, Thorleifsson G, Tikkanen E, Wood AR, Almgren P, Atalay M, Benediktsson R, Bonnycastle LL, Burtt N, Carey J, Charpentier G, Crenshaw AT, Doney ASF, Dorkhan M, Edkins S, Emilsson V, Eury E, Forsen T, Gertow K, Gigante B, Grant GB, Groves CJ, Guiducci C, Herder C, Hreidarsson AB, Hui J, James A, Jonsson A, Rathmann W, Klopp N, Kravic J, Krjutškov K, Langford C, Leander K, Lindholm E, Lobbens S, Männistö S, Mirza G, Mühleisen TW, Musk B, Parkin M, Rallidis L, Saramies J, Sennblad B, Shah S, Sigurðsson G, Silveira A, Steinbach G, Thorand B, Trakalo J, Veglia F, Wennauer R, Winckler W, Zabaneh D, Campbell H, van Duijn C, Uitterlinden AG, Hofman A, Sijbrands E, Abecasis GR, Owen KR, Zeggini E, Trip MD, Forouhi NG, Syvänen A-C, Eriksson JG, Peltonen L, Nöthen MM, Balkau B, Palmer CNA, Lyssenko V, Tuomi T, Isomaa B, Hunter DJ, Qi L, Consortium, Wellcome Trust Case Control, Meta-Analyses of Glucose Insulin-related traits Consortium (MAGIC) Investigators, Genetic Investigation of ANthropometric Traits (GIANT) Consortium, Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium, South Asian Type 2 Diabetes (SAT2D) Consortium, Shuldiner AR, Roden M, Barroso I, Wilsgaard T, Beilby J, Hovingh K, Price JF, Wilson JF, Rauramaa R, Lakka TA, Lind L, Dedoussis G, Njølstad I, Pedersen NL, Khaw K-T, Wareham NJ, Keinanen-Kiukaanniemi SM, Saaristo TE, Korpi-Hyövälti E, Saltevo J, Laakso M, Kuusisto J, Metspalu A, Collins FS, Mohlke KL, Bergman RN, Tuomilehto J, Boehm BO, Gieger C, Hveem K, Cauchi S, Froguel P, Baldassarre D, Tremoli E, Humphries SE, Saleheen D, Danesh J, Ingelsson E, Ripatti S, Salomaa V, Erbel R, Jöckel K-H, Moebus S, Peters A, Illig T, de Faire U, Hamsten A, Morris AD, Donnelly PJ, Frayling TM, Hattersley AT, Boerwinkle E, Melander O, Kathiresan S, Nilsson PM, Deloukas P, Thorsteinsdottir U, Groop LC, Stefansson K, Hu F, Pankow JS, Dupuis J, Meigs JB, Altshuler D, Boehnke M, McCarthy MI, DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, 2012. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet 44, 981–990. 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss R, Mohammed FA, 2005. Metabolism, lifestyle and bipolar affective disorder. J. Psychopharmacol. Oxf. Engl 19, 94–101. 10.1177/0269881105058678. [DOI] [PubMed] [Google Scholar]
- Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, Als TD, Bigdeli TB, Børte S, Bryois J, Charney AW, Drange OK, Gandal MJ, Hagenaars SP, Ikeda M, Kamitaki N, Kim M, Krebs K, Panagiotaropoulou G, Schilder BM, Sloofman LG, Steinberg S, Trubetskoy V, Winsvold BS, Won H-H, Abramova L, Adorjan K, Agerbo E, Al Eissa M, Albani D, Alliey-Rodriguez N, Anjorin A, Antilla V, Antoniou A, Awasthi S, Baek JH, Bækvad-Hansen M, Bass N, Bauer M, Beins EC, Bergen SE, Birner A, Bøcker Pedersen C, Bøen E, Boks MP, Bosch R, Brum M, Brumpton BM, Brunkhorst-Kanaan N, Budde M, Bybjerg-Grauholm J, Byerley W, Cairns M, Casas M, Cervantes P, Clarke T-K, Cruceanu C, Cuellar-Barboza A, Cunningham J, Curtis D, Czerski PM, Dale AM, Dalkner N, David FS, Degenhardt F, Djurovic S, Dobbyn AL, Douzenis A, Elvsåshagen T, Escott-Price V, Ferrier IN, Fiorentino A, Foroud TM, Forty L, Frank J, Frei O, Freimer NB, Friśen L, Gade K, Garnham J, Gelernter J, Giørtz Pedersen M, Gizer IR, Gordon SD, Gordon-Smith K, Greenwood TA, Grove J, Guzman-Parra J, Ha K, Haraldsson M, Hautzinger M, Heilbronner U, Hellgren D, Herms S, Hoffmann P, Holmans PA, Huckins L, Jamain S, Johnson JS, Kalman JL, Kamatani Y, Kennedy JL, Kittel-Schneider S, Knowles JA, Kogevinas M, Koromina M, Kranz TM, Kranzler HR, Kubo M, Kupka R, Kushner SA, Lavebratt C, Lawrence J, Leber M, Lee H-J, Lee PH, Levy SE, Lewis C, Liao C, Lucae S, Lundberg M, MacIntyre DJ, Magnusson SH, Maier W, Maihofer A, Malaspina D, Maratou E, Martinsson L, Mattheisen M, McCarroll SA, McGregor NW, McGuffin P, McKay JD, Medeiros H, Medland SE, Millischer V, Montgomery GW, Moran JL, Morris DW, Mühleisen TW, O’Brien N, O’Donovan C, Olde Loohuis LM, Oruc L, Papiol S, Pardiñas AF, Perry A, Pfennig A, Porichi E, Potash JB, Quested D, Raj T, Rapaport MH, DePaulo JR, Regeer EJ, Rice JP, Rivas F, Rivera M, Roth J, Roussos P, Ruderfer DM, Sánchez-Mora C, Schulte EC, Senner F, Sharp S, Shilling PD, Sigurdsson E, Sirignano L, Slaney C, Smeland OB, Smith DJ, Sobell JL, Søholm Hansen C, Soler Artigas M, Spijker AT, Stein DJ, Strauss JS, Świątkowska B, Terao C, Thorgeirsson TE, Toma C, Tooney P, Tsermpini E-E, Vawter MP, Vedder H, Walters JTR, Witt SH, Xi S, Xu W, Yang JMK, Young AH, Young H, Zandi PP, Zhou H, Zillich L, All-In Psychiatry HUNT, Adolfsson R, Agartz I, Alda M, Alfredsson L, Babadjanova G, Backlund L, Baune BT, Bellivier F, Bengesser S, Berrettini WH, Blackwood DHR, Boehnke M, Børglum AD, Breen G, Carr VJ, Catts S, Corvin A, Craddock N, Dannlowski U, Dikeos D, Esko T, Etain B, Ferentinos P, Frye M, Fullerton JM, Gawlik M, Gershon ES, Goes FS, Green MJ, Grigoroiu-Serbanescu M, Hauser J, Henskens F, Hillert J, Hong KS, Hougaard DM, Hultman CM, Hveem K, Iwata N, Jablensky AV, Jones I, Jones LA, Kahn RS, Kelsoe JR, Kirov G, Landén M, Leboyer M, Lewis CM, Li QS, Lissowska J, Lochner C, Loughland C, Martin NG, Mathews CA, Mayoral F, McElroy SL, McIntosh AM, McMahon FJ, Melle I, Michie P, Milani L, Mitchell PB, Morken G, Mors O, Mortensen PB, Mowry B, Müller-Myhsok B, Myers RM, Neale BM, Nievergelt CM, Nordentoft M, Nöthen MM, O’Donovan MC, Oedegaard KJ, Olsson T, Owen MJ, Paciga SA, Pantelis C, Pato C, Pato MT, Patrinos GP, Perlis RH, Posthuma D, Ramos-Quiroga JA, Reif A, Reininghaus EZ, Ribaśes M, Rietschel M, Ripke S, Rouleau GA, Saito T, Schall U, Schalling M, Schofield PR, Schulze TG, Scott LJ, Scott RJ, Serretti A, Shannon Weickert C, Smoller JW, Stefansson H, Stefansson K, Stordal E, Streit F, Sullivan PF, Turecki G, Vaaler AE, Vieta E, Vincent JB, Waldman ID, Weickert TW, Werge T, Wray NR, Zwart J-A, Biernacka JM, Nurnberger JI, Cichon S, Edenberg HJ, Stahl EA, McQuillin A, Di Florio A, Ophoff RA, Andreassen OA, 2021. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet 10.1038/s41588-021-00857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneer A, 2017. Wnt and GSK3 signaling pathways in bipolar disorder: clinical and therapeutic implications. Clin. PsychopharmacolNeurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol 15, 100–114. 10.9758/cpn.2017.15.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart JC, Eckel RH, 2019. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- Nestsiarovich A, Kerner B, Mazurie AJ, Cannon DC, Hurwitz NG, Zhu Y, Nelson SJ, Oprea TI, Crisanti AS, Tohen M, Perkins DJ, Lambert CG, 2020. Diabetes mellitus risk for 102 drugs and drug combinations used in patients with bipolar disorder. Psychoneuroendocrinology 112, 104511. 10.1016/j.psyneuen.2019.104511. [DOI] [PubMed] [Google Scholar]
- Nobrega MA, 2013. TCF7L2 and glucose metabolism: time to look beyond the pancreas. Diabetes 62, 706–708. 10.2337/db12-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K-J, Park J, Kim SS, Oh H, Choi CS, Koo S-H, 2012. TCF7L2 modulates glucose homeostasis by regulating CREB- and FoxO1-dependent transcriptional pathway in the liver. PLoS Genet. 8, e1002986 10.1371/journal.pgen.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, Shulman GI, 2018. Mechanisms of insulin action and insulin resistance. Physiol. Rev 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell JK, Berisa T, Liu JZ, Śegurel L, Tung JY, Hinds DA, 2016. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet 48, 709–717. 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanu C, Williams MJ, Ciuculete DM, Olivo G, Del Zompo M, Squassina A, Schiöth HB, 2019. Evidence that genes involved in hedgehog signaling are associated with both bipolar disorder and high BMI. Transl. Psychiatry 9, 315. 10.1038/s41398-019-0652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H, 1999. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance–a population-based twin study. Diabetologia 42, 139–145. 10.1007/s001250051131. [DOI] [PubMed] [Google Scholar]
- Prasad RB, Groop L, 2015. Genetics of type 2 diabetes-pitfalls and possibilities. Genes 6, 87–123. 10.3390/genes6010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto ML, Schenck LA, Kruse JL, Klaas JP, Chamberlain AM, Bobo WV, Bellivier F, Leboyer M, Roger VL, Brown RD, Rocca WA, Frye MA, 2016. Long-term risk of myocardial infarction and stroke in bipolar I disorder: a population-based cohort study. J. Affect. Disord 194, 120–127. 10.1016/j.jad.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM, 1988. Role of insulin resistance in human disease. Diabetes. 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Rødevand L, Bahrami S, Frei O, Chu Y, Shadrin A, O’Connell KS, Smeland OB, Elvsåshagen T, Hindley GFL, Djurovic S, Dale AM, Lagerberg TV, Steen NE, Andreassen OA, 2021. Extensive bidirectional genetic overlap between bipolar disorder and cardiovascular disease phenotypes. Transl. Psychiatry 11, 407. 10.1038/s41398-021-01527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo-Nava F, Blom TJ, Cuellar-Barboza AB, Winham SJ, Colby CL, Nunez NA, Biernacka JM, Frye MA, McElroy SL, 2020. Evening chronotype as a discrete clinical subphenotype in bipolar disorder. J. Affect. Disord 266, 556–562. 10.1016/j.jad.2020.01.151. [DOI] [PubMed] [Google Scholar]
- Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin BA, Zambon A, Barter P, Fruchart JC, Eckel RH, Matsuzawa Y, Despŕes J-P, 2020. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat. Rev. Endocrinol 16, 177–189. 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK, 2007. Wnt signaling regulates pancreatic β cell proliferation. Proc. Natl. Acad. Sci. U. S. A 104, 6247–6252. 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito-Diaz K, Chen TW, Wang X, Thorne CA, Wallace HA, Page-McCaw A, Lee E, 2013. The way wnt works: components and mechanism. Growth Factors Chur Switz. 31, 1–31. 10.3109/08977194.2012.752737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas YD, Wang L, DeWan AT, 2016. Multiethnic genome-wide association study identifies ethnic-specific associations with body mass index in hispanics and african americans. BMC Genet. 17, 78. 10.1186/s12863-016-0387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi V, Di Salvo G, Kořcáková J, Torriero S, Aragno E, Kolenič M, Ungrmanová M, Maina G, Mencacci C, Hajek T, 2020. Insulin resistance is associated with verbal memory impairment in bipolar disorders. J. Affect. Disord 266, 610–614. 10.1016/j.jad.2020.01.145. [DOI] [PubMed] [Google Scholar]
- Saunders EH, Scott LJ, McInnis MG, Burmeister M, 2008. Familiality and diagnostic patterns of subphenotypes in the National Institutes of mental health bipolar sample. Am. J. Med. Genet. Part B neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet 147B, 18–26. 10.1002/ajmg.b.30558. [DOI] [PubMed] [Google Scholar]
- Sayana P, Colpo GD, Simões LR, Giridharan VV, Teixeira AL, Quevedo J, Barichello T, 2017. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J. Psychiatr. Res 92, 160–182. 10.1016/j.jpsychires.2017.03.018. [DOI] [PubMed] [Google Scholar]
- SayuriYamagata A, Brietzke E, Rosenblat JD, Kakar R, McIntyre RS, 2017. Medical comorbidity in bipolar disorder: the link with metabolic-inflammatory systems. J. Affect. Disord 211, 99–106. 10.1016/j.jad.2016.12.059. [DOI] [PubMed] [Google Scholar]
- Sen ZD, Danyeli LV, Woelfer M, Lamers F, Wagner G, Sobanski T, Walter M, 2021. Linking atypical depression and insulin resistance-related disorders via low-grade chronic inflammation: integrating the phenotypic, molecular and neuroanatomical dimensions. Brain Behav. Immun 93, 335–352. 10.1016/j.bbi.2020.12.020. [DOI] [PubMed] [Google Scholar]
- Shao W, Wang D, Chiang Y-T, Ip W, Zhu L, Xu F, Columbus J, Belsham DD, Irwin DM, Zhang H, Wen X, Wang Q, Jin T, 2013. The wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes 62, 789–800. 10.2337/db12-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K, 2008. Transcription factor 7-like 2 regulates β-cell survival and function in human pancreatic islets. Diabetes 57, 645–653. 10.2337/db07-0847. [DOI] [PubMed] [Google Scholar]
- Shulman GI, 2014. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC, 2006. Association between obesity and psychiatric disorders in the US adult population. Arch. Gen. Psychiatry 63, 824–830. 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumaran S, Agakov F, Theodoratou E, Prendergast JG, Zgaga L, Manolio T, Rudan I, McKeigue P, Wilson JF, Campbell H, 2011. Abundant pleiotropy in human complex diseases and traits. Am. J. Hum. Genet 89, 607–618. 10.1016/j.ajhg.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U, Kahn BB, 2016. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med 280, 465–475. 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M, Suresh Sharma M, Osimo EF, Fornaro M, Bortolato B, Croatto G, Miola A, Vieta E, Pariante CM, Smith L, Fusar-Poli P, Shin JI, Berk M, Carvalho AF, 2021. Peripheral levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and interleukin-1β across the mood spectrum in bipolar disorder: a meta-analysis of mean differences and variability. Brain Behav. Immun 97, 193–203. 10.1016/j.bbi.2021.07.014. [DOI] [PubMed] [Google Scholar]
- Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, Mattheisen M, Wang Y, Coleman JRI, Gaspar HA, de Leeuw CA, Steinberg S, Pavlides JMW, Trzaskowski M, Byrne EM, Pers TH, Holmans PA, Richards AL, Abbott L, Agerbo E, Akil H, Albani D, Alliey-Rodriguez N, Als TD, Anjorin A, Antilla V, Awasthi S, Badner JA, Bækvad-Hansen M, Barchas JD, Bass N, Bauer M, Belliveau R, Bergen SE, Pedersen CB, Bøen E, Boks MP, Boocock J, Budde M, Bunney W, Burmeister M, Bybjerg-Grauholm J, Byerley W, Casas M, Cerrato F, Cervantes P, Chambert K, Charney AW, Chen D, Churchhouse C, Clarke T-K, Coryell W, Craig DW, Cruceanu C, Curtis D, Czerski PM, Dale AM, de Jong S, Degenhardt F, Del-Favero J, DePaulo JR, Djurovic S, Dobbyn AL, Dumont A, Elvsåshagen T, Escott-Price V, Fan CC, Fischer SB, Flickinger M, Foroud TM, Forty L, Frank J, Fraser C, Freimer NB, Friśen L, Gade K, Gage D, Garnham J, Giambartolomei C, Pedersen MG, Goldstein J, Gordon SD, Gordon-Smith K, Green EK, Green MJ, Greenwood TA, Grove J, Guan W, Guzman-Parra J, Hamshere ML, Hautzinger M, Heilbronner U, Herms S, Hipolito M, Hoffmann P, Holland D, Huckins L, Jamain S, Johnson JS, Juŕeus A, Kandaswamy R, Karlsson R, Kennedy JL, Kittel-Schneider S, Knowles JA, Kogevinas M, Koller AC, Kupka R, Lavebratt C, Lawrence J, Lawson WB, Leber M, Lee PH, Levy SE, Li JZ, Liu C, Lucae S, Maaser A, MacIntyre DJ, Mahon PB, Maier W, Martinsson L, McCarroll S, McGuffin P, McInnis MG, McKay JD, Medeiros H, Medland SE, Meng F, Milani L, Montgomery GW, Morris DW, Mühleisen TW, Mullins N, Nguyen H, Nievergelt CM, Adolfsson AN, Nwulia EA, O’Donovan C, Loohuis LMO, Ori APS, Oruc L, Ösby U, Perlis RH, Perry A, Pfennig A, Potash JB, Purcell SM, Regeer EJ, Reif A, Reinbold CS, Rice JP, Rivas F, Rivera M, Roussos P, Ruderfer DM, Ryu E, Sánchez-Mora C, Schatzberg AF, Scheftner WA, Schork NJ, Shannon Weickert C, Shehktman T, Shilling PD, Sigurdsson E, Slaney C, Smeland OB, Sobell JL, Søholm Hansen C, Spijker AT, St Clair D, Steffens M, Strauss JS, Streit F, Strohmaier J, Szelinger S, Thompson RC, Thorgeirsson TE, Treutlein J, Vedder H, Wang W, Watson SJ, Weickert TW, Witt SH, Xi S, Xu W, Young AH, Zandi P, Zhang P, Zöllner S, eQTLGen Consortium, Consortium, B.I.O.S., Adolfsson R, Agartz I, Alda M, Backlund L, Baune BT, Bellivier F, Berrettini WH, Biernacka JM, Blackwood DHR, Boehnke M, Børglum AD, Corvin A, Craddock N, Daly MJ, Dannlowski U, Esko T, Etain B, Frye M, Fullerton JM, Gershon ES, Gill M, Goes F, Grigoroiu-Serbanescu M, Hauser J, Hougaard DM, Hultman CM, Jones I, Jones LA, Kahn RS, Kirov G, Landén M, Leboyer M, Lewis CM, Li QS, Lissowska J, Martin NG, Mayoral F, McElroy SL, McIntosh AM, McMahon FJ, Melle I, Metspalu A, Mitchell PB, Morken G, Mors O, Mortensen PB, Müller-Myhsok B, Myers RM, Neale BM, Nimgaonkar V, Nordentoft M, Nöthen MM, O’Donovan MC, Oedegaard KJ, Owen MJ, Paciga SA, Pato C, Pato MT, Posthuma D, Ramos-Quiroga JA, Ribaśes M, Rietschel M, Rouleau GA, Schalling M, Schofield PR, Schulze TG, Serretti A, Smoller JW, Stefansson H, Stefansson K, Stordal E, Sullivan PF, Turecki G, Vaaler AE, Vieta E, Vincent JB, Werge T, Nurnberger JI, Wray NR, Di Florio A, Edenberg HJ, Cichon S, Ophoff RA, Scott LJ, Andreassen OA, Kelsoe J, Sklar P, Bipolar Disorder Working Group of the Psychiatric Genomics Consortium, 2019. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet 51, 793–803. 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Sørensen TI, Hanis C, Teasdale TW, Chakraborty R, Schull WJ, Schulsinger F, 1986. An adoption study of human obesity. N. Engl. J. Med 314, 193–198. 10.1056/NEJM198601233140401. [DOI] [PubMed] [Google Scholar]
- Valvezan AJ, Klein PS, 2012. GSK-3 and wnt signaling in neurogenesis and bipolar disorder. Front. Mol. Neurosci 5, 1. 10.3389/fnmol.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Mitchell AJ, De Hert M, Sienaert P, Probst M, Buys R, Stubbs B, 2015. Prevalence and predictors of type 2 diabetes mellitus in people with bipolar disorder: a systematic review and meta-analysis. J. Clin. Psychiatry 76, 1490–1499. 10.4088/JCP.14r09635. [DOI] [PubMed] [Google Scholar]
- Vandenberghe F, Saigí-Morgui N, Delacrétaz A, Quteineh L, Crettol S, Ansermot N, Gholam-Rezaee M, von Gunten A, Conus P, Eap CB, 2016. Prediction of early weight gain during psychotropic treatment using a combinatorial model with clinical and genetic markers. Pharmacogenet. Genomics 26, 547–557. 10.1097/FPC.0000000000000249. [DOI] [PubMed] [Google Scholar]
- Wilcox G, 2005. Insulin and insulin resistance. Clin. Biochem. Rev 26 (2), 19–39. [PMC free article] [PubMed] [Google Scholar]
- Willyard C, 2014. Heritability: the family roots of obesity. Nature 508, S58–S60. 10.1038/508S58a. [DOI] [PubMed] [Google Scholar]
- Winham SJ, Cuellar-Barboza AB, Oliveros A, McElroy SL, Crow S, Colby C, Choi D-S, Chauhan M, Frye M, Biernacka JM, 2014. Genome-wide association study of bipolar disorder accounting for effect of body mass index identifies a new risk allele in TCF7L2. Mol. Psychiatry 19, 1010–1016. 10.1038/mp.2013.159. [DOI] [PubMed] [Google Scholar]
- Yang W, Kelly T, He J, 2007. Genetic epidemiology of obesity. Epidemiol. Rev 29, 49–61. 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- Yi F, Brubaker PL, Jin T, 2005. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J. Biol. Chem 280, 1457–1464. 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]


