Abstract
Background:
Prior studies have consistently demonstrated that blacks have an approximate two-fold higher incidence of sudden cardiac death (SCD) than whites; however, these analyses have lacked individual level sociodemographic, medical comorbidity, and behavioral health data.
Objectives:
To evaluate whether racial differences in SCD incidence are attributable to differences in the prevalence of risk factors or rather to underlying susceptibility to fatal arrhythmias.
Methods:
The Reasons for Geographic and Racial Differences in Stroke study is a prospective, population-based cohort of adults from across the US. Associations between race and SCD defined per NHLBI criteria were assessed.
Results:
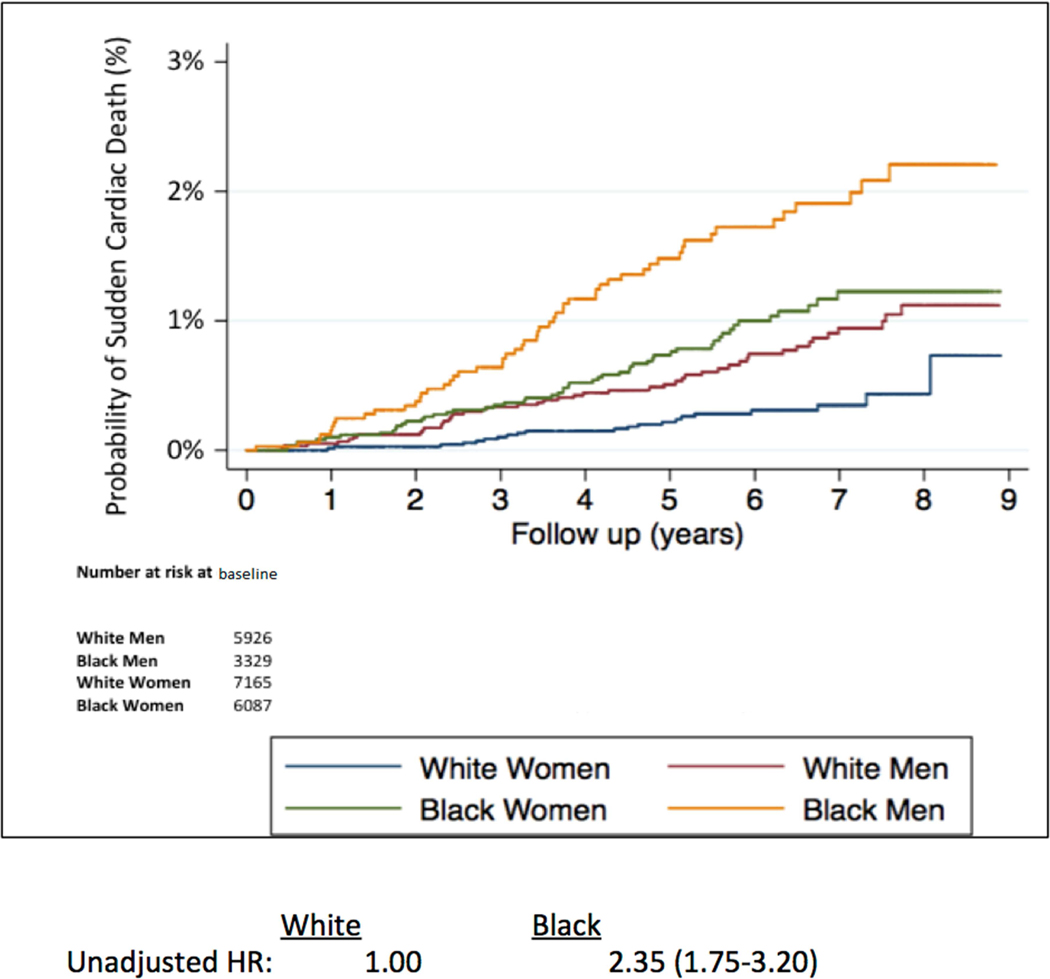
Among 22,507 participants (9,416 blacks and 13,091 whites) without a history of clinical cardiovascular disease (CVD), there were 174 SCD events (67 whites and 107 blacks) over a median follow-up of 6.1 [interquartile range 4.6–7.3] years. The age-adjusted SCD incidence rate (per 1,000 person years) was higher in blacks (1.8; 95% CI, 1.4–2.2) compared to whites (0.7; 95% CI 0.6–0.9) with an unadjusted HR of 2.35; 95% CI 1.74, 3.20. The association of black race with SCD risk remained significant after adjustment for sociodemographics, comorbidities, behavioral measures of health, intervening cardiovascular events and competing risks of non-SCD mortality (HR 1.97; 95% CI, 1.39–2.77).
Conclusions:
In a large biracial population of adults without a history of cardiovascular disease, SCD rates were significantly higher in blacks as compared to whites. These racial differences were not fully explained by demographics, adverse socioeconomic measures, cardiovascular risk factors, and behavioral measures of health.
Keywords: race, population science, epidemiology, sudden cardiac death, risk factor, risk stratification
CONDENSED ABSTRACT:
Our objective was to evaluate whether racial differences in SCD incidence are attributable to differences in the prevalence of risk factors or rather to underlying susceptibility to fatal arrhythmias among 22,507 participants without a history of clinical cardiovascular disease enrolled in the Reasons for Geographic and Racial Differences in Stroke study, a prospective, population-based cohort of adults from across the US. Among individuals without a history of cardiovascular disease, SCD rates were significantly higher in blacks as compared to whites. These racial differences were not fully explained by demographics, adverse socioeconomic measures, cardiovascular risk factors, and behavioral measures of health.
Introduction
Multiple studies have demonstrated that rates of fatal coronary heart disease, which are comprised of inpatient and outpatient deaths attributable to myocardial infarction, are higher in blacks compared with whites (1–3). Surveillance studies from the past 2–3 decades have reported similar racial differences in the incidence of sudden cardiac death (SCD) (4–7). Recently, the Oregon Sudden Unexpected Death Study (SUDS) estimated that the age-adjusted annual incidence of SCD in blacks was more than twice the incidence observed in whites (6), and blacks with SCD were >6 years younger than white patients. Possible explanations raised for these observed racial differences include the higher burden of adverse socioeconomic and/or environmental factors, cardiovascular disease (CVD) risk factors, and behavioral measures (8). However, a rigorous, prospective population-based analysis controlling for these factors has not been performed.
The pathology underlying SCD may also differ in blacks as compared to whites. In autopsy series, coronary artery disease accounts for a lower percentage of sudden unexpected cardiovascular deaths in blacks versus whites (47% versus 67%) (9) despite the known higher burden of hypertension (10), diabetes (11,12), and chronic kidney disease (13) in blacks. Further, among SCDs with coronary disease documented at autopsy, plaque rupture was more commonly found in whites as compared to blacks (9). Alternatively, left ventricular hypertrophy is more commonly found at autopsy in black SCD victims (6,9). Therefore, risk factors for SCD may differ between blacks and whites, and traditional coronary disease risk factors may have less of an impact in blacks.
To improve our understanding of racial differences in SCD, we compared the incidence and relative hazards of SCD in black and white participants, who did not have a history of clinical cardiovascular disease at the time of enrollment in a large, contemporary, nationwide population-based study. In staged, multivariable models, we evaluated whether racial differences in SCD risk were attributable to sociodemographic measures, cardiovascular risk factors, behavioral health metrics, intervening non-fatal cardiac events, and competing risks of non-SCD.
Methods
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study
The REGARDS Study is a population-based study of 30,239 participants (12,534 blacks and 17,705 whites) that was originally established to understand the racial disparities in the incidence of stroke among U.S. adults aged 45 years and older (14). The investigators recruited potential participants >45 years from communities spread across the lower 48 states of the United States. The study randomly enrolled participants and then oversampled often underrepresented groups to arrive at a roughly equal representation of whites and blacks, and men and women. As such, in the final cohort, 58% of participants were women and 42% of participants were black race, which was within the original designed proportions. In addition, there was oversampling from the stroke belt and buckle (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana), which represent geographic regions in the southeastern United States with higher stroke mortality rates than the rest of the country (15). Trained personnel conducted computer-assisted telephone interviews to obtain information including participants’ sociodemographics, cardiovascular health profile, previous medical interventions, health behaviors (smoking, exercise, alcohol use), and measures of general health. Biometric data (blood pressure, electrocardiography, anthropometrics, fasting blood and urine samples) were collected by a health professional during an in-home visit. The REGARDS study protocol was approved by the institutional review boards at the participating centers, and all participants provided written informed consent.
Study Population for Analysis
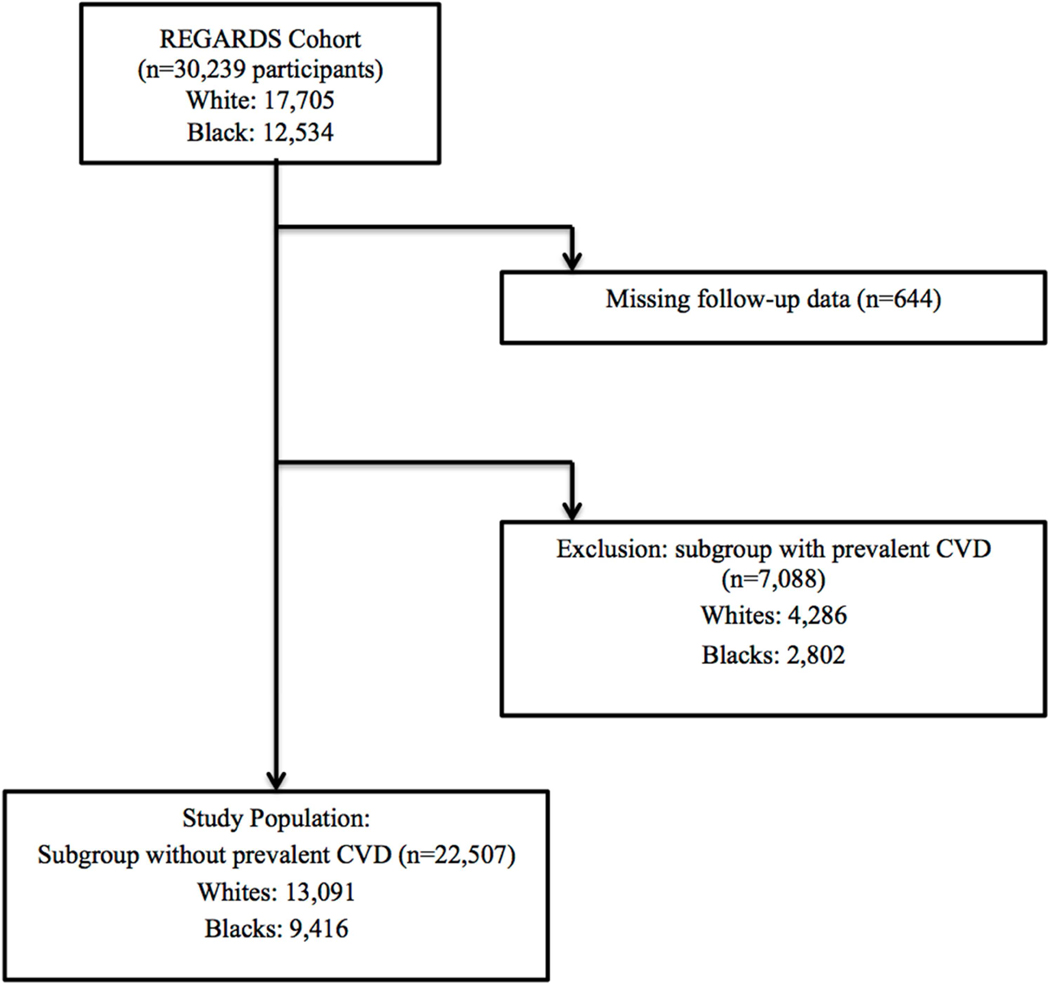
We limited our study population to the 22,507 participants (9,416 blacks and 13,091 whites) without prevalent CVD at baseline. A similar percentage of blacks and whites from the original cohort had prevalent CVD (22% blacks, 24% whites) and were excluded from the primary analysis (Figure 1). Prevalent CVD was defined as a self-reported history of myocardial infarction (MI), coronary revascularization procedure, stroke, or heart failure. Patients with ECG evidence of MI were also considered to have prevalent CVD and were excluded.
Figure 1: Study Overview.
REGARDS indicates Reasons for Geographic and Racial Differences in Stroke study; CVD, cardiovascular disease.
Demographics and Baseline Risk Factor Assessment
Detailed descriptions of the baseline assessment of clinical covariates have been provided in prior publications from the REGARDS study (16–18). Demographic variables including age, sex, race, and socioeconomic measures (income and education) were self-reported. Based on previous studies, annual income was dichotomized at $35,000, and education was categorized as greater than versus high-school grade or less (19–21). The presence of health insurance was collected from the baseline interview. Diabetes was defined as a fasting glucose of ≥126 mg/dL, a nonfasting glucose of ≥200 mg/dL, or self-reported use of medications for glycemic control. Atrial fibrillation was identified in study participants at baseline by the scheduled ECG and also from self-reported history of a physician diagnosis during the computer-assisted telephone interview surveys. Prevalent coronary heart disease (CHD) was defined as a self-reported myocardial infarction (MI), ECG evidence of prior MI or a self-report of coronary artery bypass, angioplasty, or stent. Prevalent stroke was defined as a self-reported episode that had been diagnosed by a physician. Height and weight were measured on all participants during the in-home visit, and body mass index (BMI) was calculated. Blood pressure was obtained using an aneroid sphygmomanometer after a seated rest of 5 minutes. Two measures were obtained following a standardized protocol and averaged. The use of antihypertensive medications was defined by self-report. Total cholesterol and high-density lipoprotein (HDL) cholesterol were measured.
Behavioral Health Measures
Health behaviors were assessed at the baseline interview and included cigarette smoking (current, past or never), physical activity (never or at least once a week), and alcohol use (current, past or never). Psychosocial factors included measurements of depressive symptoms and perceived stress. Depressive symptoms were assessed with the 4-item Center for Epidemiologic Studies Depression (CES-D) questionnaire (8,22). Consistent with previous research (8,23–26), we classified participants as having elevated depressive symptoms if they had a CES-D score ≥ 4 (22). Perceived stress was measured with a well-validated 4-item version of the Perceived Stress Scale (27). Participants with a total score ≥ 5 are classified as having elevated levels of perceived stress.
Outcome Definition
Our definition for SCD was consistent with that proposed by the NHLBI-sponsored, expert panel: (a) unexpected death without an obvious extracardiac cause occurring with a rapid witnessed collapse; (b) for unwitnessed events, SCD is defined as an event that occurs within one hour after symptom onset or an unexpected death without obvious extracardiac cause that occurred within the previous 24 hours (20,28). Outcomes were ascertained through regular 6 month telephone contact with participants, and all out-of-hospital cardiovascular deaths underwent further evaluation as a possible SCD event. These events could have occurred in the emergency room, but may not have been the consequence of acute trauma, intoxication, or the culmination of a terminal illness (cancer or end stage lung disease).
Details of the adjudication protocol
As part of the adjudication process, our working group generated a SCD supplemental questionnaire that has been incorporated into the standard, exit interview from all out-of-hospital deaths. The SCD supplemental questionnaire consists of a distinct series of questions for witnessed versus unwitnessed events and was designed to collect specific information on the circumstances and timing of all death events. We also abstracted information from emergency medical service reports and emergency room accounts. All data are recorded on a SCD data form that is subsequently reviewed for the adjudication of SCD. Expert adjudicators (CA, TB. RD, MS) independently assessed cause of death, including whether the death was SCD with kappa >0.80. Disagreements were adjudicated by committee. End of follow-up for this analysis was December 31, 2011.
Statistical Analysis
For our primary analysis, we first computed incidence rates per 1,000 person-years for SCD in blacks and whites without prevalent CVD using methods for censored data. Additional stratification by sex was pre-specified. After confirming the proportionality of hazards, we constructed Cox proportional hazards regression models to estimate associations between race and time to SCD. For each participant, the time at risk was defined from the baseline exam until the date of SCD, other death, censorship due to loss to follow-up, or December 31, 2011— whichever came first. We adjusted for confounding variables in a staged regression approach and constructed models based upon biological plausibility. Models were first adjusted for age, sex, region, education, income, and health insurance status (Model 1). Then adjustment was made for standard coronary heart disease risk factors and preventive therapies including systolic and diastolic blood pressure, total cholesterol, HDL, diabetes, BMI, statins and use of antihypertensive therapies (Model 2). Model 3 further adjusted for other SCD risk factors that differed by race including estimated glomerular filtration rate, the urinary albumin to creatinine ratio, left ventricular hypertrophy by electrocardiogram, and atrial fibrillation. Model 4 further adjusted for behavioral health measures including smoking, alcohol use, physical activity, and psychosocial stress. Model 5 further adjusted for intervening MI and/or heart failure hospitalizations as time dependent covariates. Missing covariates were imputed using chained equations with 5 data sets as in prior REGARDS papers (29).
We repeated the above analysis using competing risk methods proposed by Fine and Gray to ensure that the risk for SCD was not biased by the higher risk of non-SCD deaths in blacks compared with whites (30). These models distinguish between participants who are alive and those who have experienced competing causes of clinical endpoints. In the competing risk method, participants who die of a non-sudden cause are considered to have had alternative events when calculating the event-free survival, and are distinguished from persons with uninformative censoring.
In pre-specified secondary analyses, given known age and sex differences in SCD incidence (28,31,32), we explored effect modification by sex and age through the use of stratified analyses and interaction terms in multivariable-adjusted Cox models using a post-estimation Wald test to obtain an omnibus P value for interaction. All analyses were conducted using SAS software version 9.4 (SAS Institute), STATA version 12 (STATA Inc), and R 3.0.1 (R Foundation for Statistical Computing).
Results
In the REGARDS study, a total of 388 SCDs were confirmed over a median follow-up of 6.1 [interquartile range 4.6–7.3] years. Black participants who experienced SCD were less likely to have CVD at baseline than white participants (56% blacks, 34% whites) despite both groups having a similar overall prevalence of CVD at the baseline visit (23% blacks, 25% whites). In our primary analysis of the 22,507 REGARDS participants (9,416 blacks and 13,091 whites) without a history of CVD upon enrollment into the study, there were 107 and 67 SCD events in blacks and whites, respectively. The absolute incidence of SCD in the population was 0.18% per year in blacks and 0.07% per year in whites.
Compared to white participants, blacks in our analysis were more likely to be women, less likely to graduate high school, more likely to have a lower income, and less likely to have health insurance (Table 1). Blacks were also more likely to smoke, not participate in exercise, and to have depressive symptoms and perceived stress. Blacks were also more likely to have a history of diabetes mellitus and LVH. No significant differences were observed in the ECG measures for heart rate, QRS duration, and the corrected QT interval. Blacks were more likely to have an eGFR >90 ml/min/1.72m2; however, they were also more likely to have albuminuria >30mg/g. Finally, blacks were more likely to be on an antihypertensive medication.
Table 1.
Baseline characteristics of REGARDS participants free of cardiovascular diseas
| Characteristics | Black (n=9,416) | White (n=13,091) |
|---|---|---|
| Age (years)±SD | 63±9 | 64±9 |
| Women n(%) | 6,087 (65) | 7,165 (55) |
| Education <high school n(%) | 1,656 (18) | 771 (6) |
| Income <$35,000 n(%) | 4,783 (51) | 4,098 (31) |
| Region | ||
| Belt* n(%) | 3,178 (34) | 4,618 (35) |
| Buckle† n(%) | 1,714 (18) | 3,016 (23) |
| Non-Belt n(%) | 4,524 (48) | 5,457 (42) |
| Insurance Status | ||
| Yes | 8,398 (89%) | 12,445 (95%) |
| No | 1,006 (11%) | 636 (5%) |
| BMI kg/m2±SD | 31±7 | 28±6 |
| Systolic blood pressure ±SD mmHg | 130±17 | 125±15 |
| Behavioral Health Measures | ||
| Current smoking n(%) | 1,553 (17) | 1,553(12) |
| Never exercises n(%) | 3,296 (36) | 3,923 (30) |
| Alcohol Use n(%) | ||
| Current | 4,125 (44) | 7,959 (61) |
| Never | 3,287 (35) | 3,497 (27) |
| Past | 2,004 (21) | 1,635 (13) |
| Elevated Depressive Symptoms | 1,165 (12) | 1,067 (8) |
| Elevated Stress | 3,056 (33) | 3,172 (24) |
| Comorbidities | ||
| Diabetes n(%) | 2,442 (27) | 1,528 (12) |
| Atrial fibrillation n(%) | 507 (6) | 757 (6) |
| Left ventricular hypertrophy n(%) | 1,251 (14) | 718(6) |
| EKG Variables | ||
| Heart rate ±SD, bpm | 68±12 | 66±11 |
| QRS interval ±SD, ms | 86±13 | 88±14 |
| Corrected QT interval ±SD, ms | 406±22 | 406±22 |
| Laboratory Parameters | ||
| Estimated Glomerular filtration rate | ||
| ≥90 n(%) | 4,999 (56) | 5,590 (44) |
| 60–89 n(%) | 3,083 (35) | 6,091 (48) |
| 45–59 n(%) | 519 (6) | 740 (6) |
| <45 n(%) | 320 (4) | 270 (2) |
| Albumin to Creatinine Ratio | ||
| <15 n(%) | 6,501 (73) | 10,084 (80) |
| 15–30 n(%) | 951 (11) | 1,293 (10) |
| >30 n(%) | 1,470 (17) | 1,214 (10) |
| Total cholesterol mg/dL ±SD | 195±40 | 196±38 |
| HDL mg/dL ±SD | 54±16 | 52±17 |
| Medications | ||
| Statins n(%) | 2,250 (24) | 3,325 (26) |
| Antihypertensives n(%) | 6,012 (64) | 5,856 (45) |
The stroke belt represents regions of the southeastern United States and have a higher incidence of stroke and stroke-associated mortality than other parts of the country. This region is represented by Georgia, Alabama, Mississippi, Tennessee, Arkansas, Louisiana and regions of North Carolina and South Carolina.
The stroke buckle, which are regions of the US with stroke-associated mortality rates that are higher than the stroke belt, are represented by the coastal plain regions of North Carolina, South Carolina, and Georgia.
In unadjusted models, blacks had a significantly greater SCD risk than whites (HR 2.35; 95% CI, 1.74–3.20; p <0.001). In the primary models evaluating race and SCD, adjustment for age, sex, region, education, income, and health insurance status did not materially alter the higher risk of SCD in blacks compared with whites (Table 2). Further, in the staged multivariable analysis, the adjustment for standard cardiovascular risk factors and preventive therapies resulted in the largest attenuation of the elevated risk of SCD. However, after controlling for all other potential confounders including behavioral measures of health, intervening cardiovascular events and competing risks of non-SCD, blacks continued to have a highly significant near two-fold higher risk of SCD than whites (HR 1.97; 95% CI, 1.39–2.77; p <0.001).
Table 2.
Association of Race with Sudden Cardiac Death among Participants without Baseline Cardiovascular Disease
| Participants (n=22,507) | White (n=13,091) |
Black (9,416) |
p-value |
|---|---|---|---|
| Sudden Cardiac Death Events, n | 67 | 107 | |
| Incidence Rate* (per 1,000 py) | 0.7 (0.6, 0.9) | 1.8 (1.4, 2.2) | |
| Unadjusted; HR (95% CI) | 1.00 | 2.35 (1.74, 3.20) | <0.001 |
| Model 1† | 1.00 | 2.35 (1.70, 3.24) | <0.001 |
| Model 2‡ | 1.00 | 1.89 (1.35, 2.66) | <0.001 |
| Model 3§ | 1.00 | 1.98 (1.41, 2.80) | <0.001 |
| Model 4‖ | 1.00 | 1.88 (1.33, 2.67) | <0.001 |
| Model 5# | 1.00 | 1.97 (1.39, 2.77) | <0.001 |
Age-adjusted
Model 1 adjusts for age, sex, region, education, income, and health insurance
Model 2 adjusts for Model 1 plus standard coronary heart disease risk factors and preventive therapies including systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, diabetes, body mass index, statins, and use of antihypertensive medication
Model 3 adjusts for Model 2 plus ACR, eGFR, LVH, and atrial fibrillation
Model 4 adjusts for Model 3 plus behavioral factors including smoking, alcohol use, physical activity, perceived stress and elevated depressive symptoms
Model 5 adjusts for Model 4 plus intervening non-fatal myocardial infarction and/or heart failure hospitalization as time-variant covariates and the competing risks of non-SCD
Sex- and Age-stratified Analyses
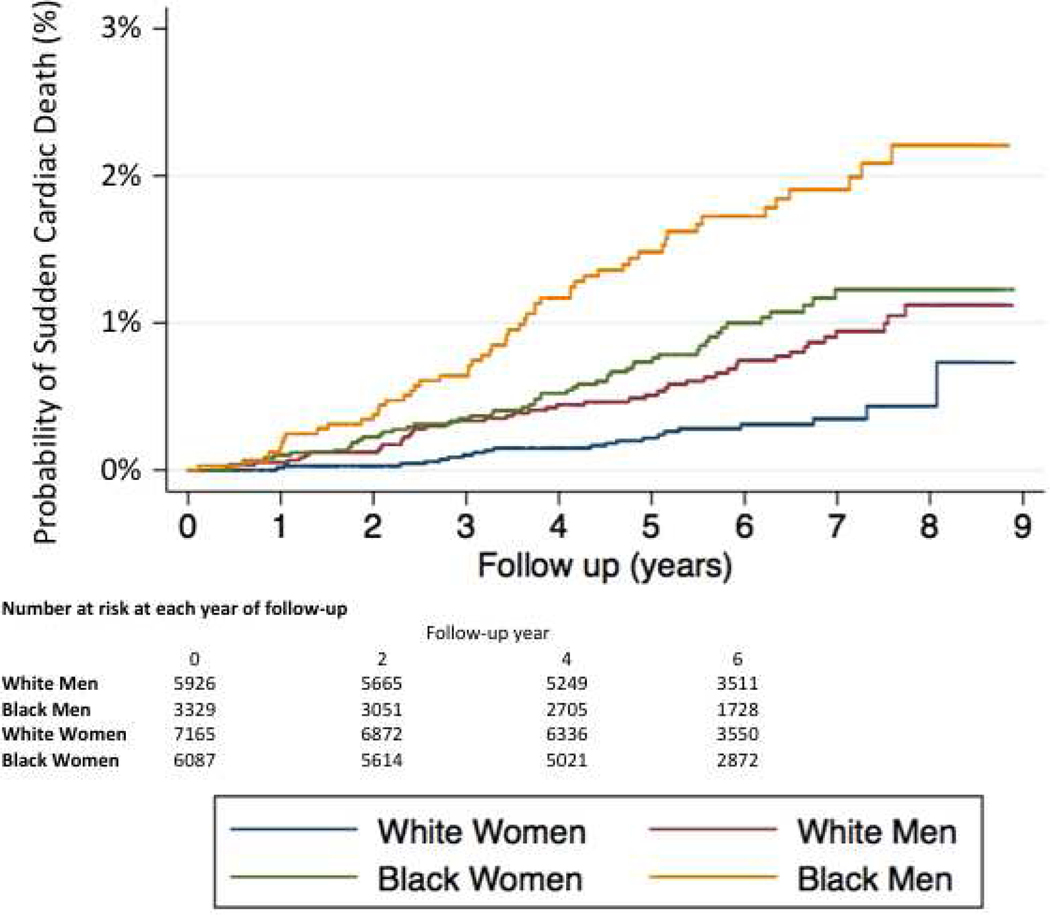
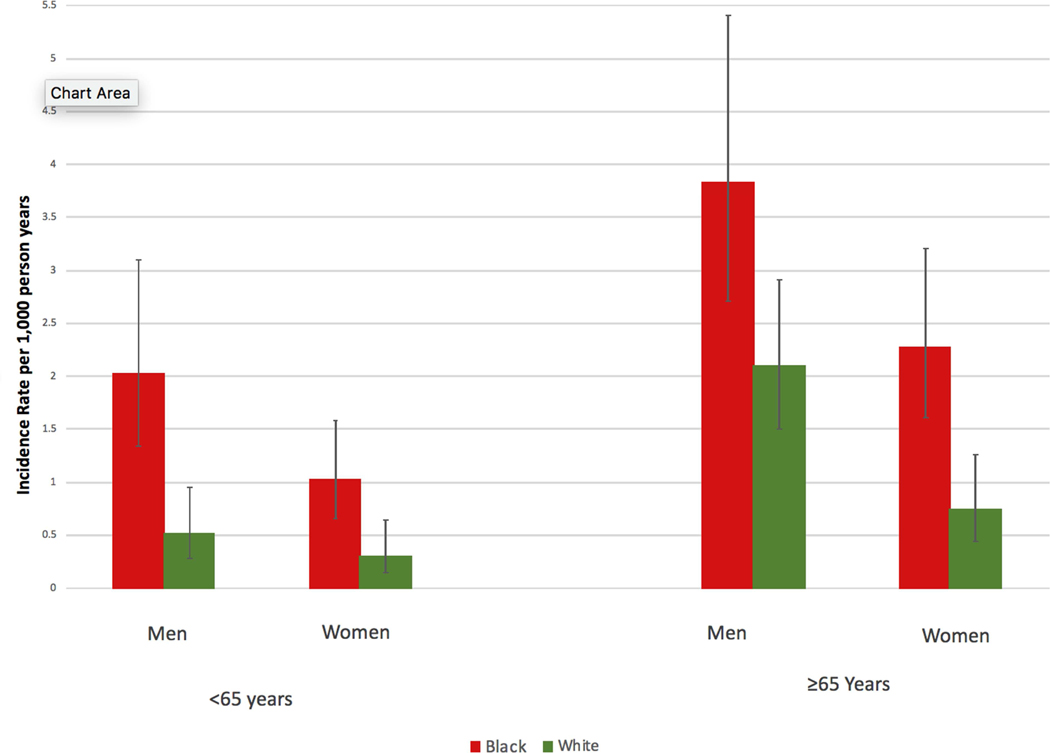
Comparisons in baseline characteristics between black and white men and black and white women in our primary study population are provided in Online Table 1. Most racial differences observed in the overall population were also observed within sex strata; however, racial differences in BMI appeared greater in women than men. Black men had the highest incidence of SCD followed by black women, white men, and white women (Figure 2), and these racial differences remained when the population was further stratified at age 65 (Figure 3). In unadjusted analyses, black race was associated with a higher SCD risk in women (HR 3.08; 95% CI, 1.86–5.11; p <0.001) and men (HR 2.26; 95% CI, 1.52–3.34; p <0.001). Socioeconomic inequalities among men and women attenuated this risk slightly. Similar to the total population, adjustment for standard cardiovascular risk factors resulted in the greatest attenuation in these relative risks, and black race remained significantly associated with SCD risk in the fully adjusted model for both men (HR 1.69; 95% CI, 1.08–2.63; p = 0.003) and women (HR 2.39, 95% CI, 1.36–4.19; p =0.021) (Table 3). Neither the race x sex nor the race x age interactions were significant.
Figure 2: Sudden Cardiac Death Incidence According to Race and Sex.
The probabilities of sudden cardiac death for white women (blue), white men (red), black women (green), and black men (orange) are depicted.
Figure 3: Rates of Sudden Cardiac Death According to Race, Sex and Age.
The unadjusted incident rates of sudden cardiac death in black men, white men, black women, and white women are stratified by age 65 years.
Table 3.
Association of Race and Sex with Sudden Cardiac Death among Participants without Baseline Cardiovascular Disease
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Participants (n=22,507) | White (n=5,926) |
Black (3,329) |
p-value | White (n=7,165) |
Black (6,087) |
p-value |
| Sudden Cardiac Death Events, n | 46 | 54 | 21 | 53 | ||
| Incidence Rate* (per 1,000 py) | 1.0 (0.8, 1.4) | 2.5 (1.9, 3.2) | 0.4 (0.3, 0.7) | 1.4 (1.0, 18) | ||
| Unadjusted; HR (95% CI) | 1.00 | 2.26 (1.52, 3.34) | <0.001 | 1.00 | 3.08 (1.86, 5.11) | <0.001 |
| Model 1† | 1.00 | 2.00 (1.32, 3.04) | 0.001 | 1.00 | 3.00 (1.77, 5.07) | <0.001 |
| Model 2‡ | 1.00 | 1.62 (1.04, 2.50) | 0.032 | 1.00 | 2.27 (1.30, 3.94) | 0.004 |
| Model 3§ | 1.00 | 1.72 (1.10, 1.67) | 0.017 | 1.00 | 2.39 (1.36, 4.20) | 0.002 |
| Model 4‖ | 1.00 | 1.63 (1.04, 2.55) | 0.033 | 1.00 | 2.28 (1.30, 4.02) | 0.004 |
| Model 5# | 1.00 | 1.69 (1.08, 2.63) | 0.003 | 1.00 | 2.39 (1.36, 4.19) | 0.021 |
Age-adjusted
Model 1 adjusts for age, region, education, income, and health insurance
Model 2 adjusts for Model 1 plus standard coronary heart disease risk factors and preventive therapies including systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, diabetes, body mass index, statins, and use of antihypertensive medication
Model 3 adjusts for Model 2 plus ACR, eGFR, LVH, and atrial fibrillation
Model 4 adjusts for Model 3 plus behavioral factors including smoking, alcohol use, physical activity, perceived stress and elevated depressive symptoms
Model 5 adjusts for Model 4 plus intervening non-fatal myocardial infarction and/or heart failure hospitalization as time-variant covariates and the competing risks of non-SCD
Discussion
In this contemporary, U.S. population-based analysis of individuals without a history of cardiovascular disease, blacks had a near 2-fold higher risk of SCD that remained significant after adjusting for sociodemographics, cardiovascular risk factors and comorbidities, behavioral measures of health, intervening cardiovascular events, and competing risks (Central Illustration). In the staged multivariable modeling, the higher prevalence of CVD risk factors in blacks appears to explain part, but not all, of the racial differences in SCD incidence. Similar racial differences were observed in men and women; and in those either younger than or 65 years and older. While more comprehensive public health efforts toward risk factor modification in black populations will be a critical step to reduce the higher burden of SCD in blacks compared with whites, our data suggest that it may not eliminate the racial disparity. Therefore, additional research will be needed to understand the factors that underlie these persistent racial differences in SCD risk in order to prevent the excess of SCD in blacks.
Central Illustration: Racial Differences in Sudden Cardiac Death Risk among Individuals without a History of Cardiovascular Disease.
HR indicates hazards ratio; CVD, cardiovascular disease; MI, myocardial infarction; and HF, heart failure.
SCD rates in blacks and whites without baseline CVD in the REGARDS cohort were similar to those reported in a similar population within the Atherosclerosis Risk in Communities (ARIC) Study, another US-population based study that initiated enrollment 16 years prior to REGARDS (33). These consistent SCD rates suggest that declines in SCD may not be occurring at the same rate as contemporaneous reductions in MI and fatal CHD (2,34,35). Although CHD underlies a substantial proportion of SCD, a recent autopsy study involving a multiethnic population suggests that a sizeable proportion of SCD cases do not have underlying CHD at autopsy (36). In addition, other non-atherosclerotic pathologies such as alterations in cardiac hypertrophy, conduction, and repolarization have been documented to contribute to SCD risk (33,36,37). In black SCD cases, CHD is even less likely to be documented at autopsy (5,9,36) and other cardiac pathologies such as cardiac hypertrophy, or other non-cardiac causes are more commonly found (9,36). These racial differences in the underlying pathophysiology of SCD may explain, in part, the racial differences that persisted in our analyses of SCD risk after controlling for sociodemographic and cardiovascular risk factors. In a multi-cohort study, which included participants from ARIC and REGARDS, the elevated fatal CHD risk observed in blacks versus whites was completely attenuated and no longer significant after controlling for the same sociodemographic and cardiovascular risk factors (38). These divergent findings suggest that cardiovascular risk factor modification may have a lesser impact on the non-atherosclerotic pathologies and mechanisms underlying SCD in blacks.
In additional analysis of all REGARDS participants, we found that blacks are more likely to present with SCD as their initial manifestation of CVD. Our study’s prospective design and comprehensive assessment of baseline characteristics complement prior surveillance studies and suggest that modification of traditional risk factors may have a limited impact on racial disparities in SCD risk. Further, the higher burden of poor health behaviors including smoking, alcohol use, low physical activity and psychosocial distress in blacks compared with whites did not appear to impact directly SCD risk. As a result, public health initiatives that include greater awareness about SCD and potential warning symptoms in minority populations may be important interventions to reduce the burden of racial disparities in SCD.
Several limitations of our study should be considered. Our analysis did not assess direct measures of cardiac remodeling such as left ventricular function, and it is potentially possible that blacks may be more likely to have undiagnosed LV dysfunction. Although a depressed left ventricular ejection fraction (LVEF) is a strong risk factor for SCD, multiple studies have demonstrated that the majority of SCD in the community occur in individuals with normal LVEF (39). Given the design of this national cohort where in-home visits were performed, it was not feasible to obtain measures of LVEF using echocardiograms in this population. In addition, we were limited in quantifying the extent to which the racial differences present in our analysis were attributable to disparities in care. All participants were members of the REGARDS study; however, differences in the quality of or access to healthcare may have impacted the timely diagnosis of cardiovascular conditions. Further, all demographic, risk factor, and comorbidity characteristics were assessed at a single time point. As such, we cannot examine whether treatment of these conditions affects racial differences. Further, autopsies were not systematically performed in the REGARDS study and were only available on a small minority of deaths. We also acknowledge that prior CVD status was self-reported and racial differences in under-reporting or under-detection may exist. Finally, as with any observational study, we cannot rule out the possibility that residual confounding by imperfectly measured or unmeasured confounders could account for part of the association observed. For example, finer control for education and income and/or a variety of unmeasured factors might explain part of the associations between black race and SCD.
Our study is one of the first to systematically compare the risk of SCD in blacks and whites across the general population after accounting for sociodemographic, behavioral, and cardiovascular risk factors. Among those without clinical cardiovascular disease, blacks are more likely than whites to present with SCD, and the elevation in risk does not appear to be explained by known risk factors. Future research is needed to understand the mechanisms underlying these racial differences in SCD incidence. In the interim, the current findings underscore the importance of community-based interventions to increase awareness about SCD, warning symptoms, and improve resuscitation rates from cardiac arrest.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
In a population-based study, rates of sudden cardiac death were higher in blacks than whites. These racial differences were not explained by demographics, socioeconomic measures, cardiovascular risk factors, or behavior.
Translational Outlook:
Further studies are needed to clarify the mechanisms underlying racial disparity in arrhythmia risk and develop effective strategies for prevention.
Acknowledgements:
The authors thank the other investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org. We would also like to thank Ms. Gabriela Daszweska-Smith for her technical assistance in preparing this manuscript.
Funding Information:
This research project is supported by a cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. Additional support was provided by grants R01-HL080477 and K24-HL111154 from the National Heart, Lung, and Blood Institute. Representatives of the National Institutes of Health have been involved in the review of the manuscript but were not directly involved in the collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. This project was also supported, in part, by the Winkelman Family Fund in Cardiovascular Innovation.
ABBREVIATIONS
- SCD
sudden cardiac death
- CHD
coronary heart disease
- MI
myocardial infarction
- BMI
body mass index
- CVD
cardiovascular disease
Footnotes
Disclosures: No relationships with industry
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012;308:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation 2012;125:1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. [DOI] [PubMed] [Google Scholar]
- 4.Becker LB, Han BH, Meyer PM et al. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med 1993;329:600–6. [DOI] [PubMed] [Google Scholar]
- 5.Steinhaus DA, Vittinghoff E, Moffatt E, Hart AP, Ursell P, Tseng ZH. Characteristics of sudden arrhythmic death in a diverse, urban community. Am Heart J 2012;163:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinier K, Nichols GA, Huertas-Vazquez A, et al. Distinctive Clinical Profile of Blacks Versus Whites Presenting With Sudden Cardiac Arrest. Circulation 2015;132:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowie MR, Fahrenbruch CE, Cobb LA, Hallstrom AP. Out-of-hospital cardiac arrest: racial differences in outcome in Seattle. Am J Public Health 1993;83:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumner JA, Khodneva Y, Muntner P, et al. Effects of Concurrent Depressive Symptoms and Perceived Stress on Cardiovascular Risk in Low- and High-Income Participants: Findings From the Reasons for Geographical and Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke AP, Farb A, Pestaner J, et al. Traditional risk factors and the incidence of sudden coronary death with and without coronary thrombosis in blacks. Circulation 2002;105:419–24. [DOI] [PubMed] [Google Scholar]
- 10.Henderson SO, Haiman CA, Wilkens LR, Kolonel LN, Wan P, Pike MC. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS One 2007;2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA 2000;283:2253–9. [DOI] [PubMed] [Google Scholar]
- 12.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care 2009;32:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int 2005;68:914–24. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke study: objectives and design. Neuroepidemiology 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 15.Borhani NO. Changes and Geographic Distribution of Mortality from Cerebrovascular Disease. Am J Public Health Nations Health 1965;55:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redmond N, Richman J, Gamboa CM, et al. Perceived stress is associated with incident coronary heart disease and all-cause mortality in low- but not high-income participants in the Reasons for Geographic And Racial Differences in Stroke study. J Am Heart Assoc 2013;2:e000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pullicino PM, McClure LA, Wadley VG, et al. Blood pressure and stroke in heart failure in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Stroke 2009;40:3706–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard VJ, Woolson RF, Egan BM, et al. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens 2010;4:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA 2011;305:1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deo R, Khodneva YA, Shlipak MG, et al. Albuminuria, kidney function, and sudden cardiac death: Findings from The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Heart Rhythm 2017;14:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez OM, Khodneva YA, Muntner P, et al. Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA 2013;310:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melchior LAHG, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas 1993;53:1117–25. [Google Scholar]
- 23.Cummings DM, Kirian K, Howard G, et al. Consequences of Comorbidity of Elevated Stress and/or Depressive Symptoms and Incident Cardiovascular Outcomes in Diabetes: Results From the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Diabetes Care 2016;39:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcantara C, Muntner P, Edmondson D, et al. Perfect storm: concurrent stress and depressive symptoms increase risk of myocardial infarction or death. Circ Cardiovasc Qual Outcomes 2015;8:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye S, Muntner P, Shimbo D, et al. Behavioral mechanisms, elevated depressive symptoms, and the risk for myocardial infarction or death in individuals with coronary heart disease: the REGARDS (Reason for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol 2013;61:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sims M, Redmond N, Khodneva Y, Durant RW, Halanych J, Safford MM. Depressive symptoms are associated with incident coronary heart disease or revascularization among blacks but not among whites in the Reasons for Geographical and Racial Differences in Stroke study. Ann Epidemiol 2015;25:426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen SWG. Perceived stress in a probability sample of the United States. Newbury Park, CA: Sage, 1988. [Google Scholar]
- 28.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 2010;122:2335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc 1996;91:473–89. [Google Scholar]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 31.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation 2012;125:620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert CM, McGovern BA, Newell JB, Ruskin JN. Sex differences in cardiac arrest survivors. Circulation 1996;93:1170–6. [DOI] [PubMed] [Google Scholar]
- 33.Deo R, Norby FL, Katz R, et al. Development and Validation of a Sudden Cardiac Death Prediction Model for the General Population. Circulation 2016;134:806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics−−2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurt RD, Weston SA, Ebbert JO, et al. Myocardial infarction and sudden cardiac death in Olmsted County, Minnesota, before and after smoke-free workplace laws. Arch Intern Med 2012;172:1635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseng ZH, Olgin JE, Vittinghoff E, et al. Prospective Countywide Surveillance and Autopsy Characterization of Sudden Cardiac Death: POST SCD Study. Circulation 2018;137:2689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waks JW, Sitlani CM, Soliman EZ, et al. Global Electric Heterogeneity Risk Score for Prediction of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation 2016;133:2222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colantonio LD, Gamboa CM, Richman JS, et al. Black-White Differences in Incident Fatal, Nonfatal, and Total Coronary Heart Disease. Circulation 2017;136:152–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narayanan K, Reinier K, Uy-Evanado A, et al. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation 2013;128:1733–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.