Abstract
Our laboratory has reported on a biphasic pattern of nuclear factor κB (NF-κB) activation in cultured human umbilical vein endothelial cells during infection with Rickettsia rickettsii, an obligate, intracellular bacterium, and the etiologic agent of Rocky Mountain spotted fever. Transcriptional activation of the tissue factor (TF) gene during this infection has been shown to involve NF-κB. To further understand the signal transduction events underlying these phenomena, we studied the role of protein kinase C (PKC), a ubiquitous family of phospholipid-dependent enzymes implicated in the regulation of a variety of cell signaling pathways. Two inhibitors of PKC, namely, bisindolylmaleimide I hydrochloride (BM-1) and calphostin C, which exhibit different inhibitory properties towards various isozymes of PKC, were used. Infection of cells with R. rickettsii in the presence of BM-1 (50 nM) did not significantly affect NF-κB, whereas calphostin C (2.5 μM) completely blocked the early phase of NF-κB activation. The late, sustained phase also was not affected by treatment with BM-1. Downregulation of phorbol ester-sensitive PKCs by long-term treatment with phorbol 12-myristate 13-acetate (PMA) did not inhibit NF-κB activation. Likewise, this downregulation had no effect on induction of TF activity. The activity of TF was, however, sensitive to BM-1 and calphostin C, whereas expression of TF mRNA was inhibited only by calphostin C. Overall, these results suggest the lack of involvement of classical PKC pathways in R. rickettsii-induced NF-κB activation but the possible involvement of a non-PMA-responsive PKC isoform in the posttranscriptional control of TF expression.
Infection of cultured vascular endothelial cells (EC) with Rickettsia rickettsii, an obligate, intracellular, and gram-negative bacterium, results in the expression of a proinflammatory and procoagulant cellular phenotype characterized by the induction of tissue factor (TF; factor III, thromboplastin) (33, 39), E-selectin (32), interleukin-1α (IL-1α) (34), and plasminogen activator inhibitor-1 (8, 27). Since the endothelial cell is the primary target of infection, these alterations likely contribute significantly to the vascular pathology associated with the human rickettsial disease, Rocky Mountain spotted fever.
The transcription factor, nuclear factor κB (NF-κB), controls the expression of many genes involved in responses to injurious or inflammatory stimuli, including TF, E-selectin, and interleukins (20, 22, 29, 42) and appears to participate as a major regulator of the host cell response to rickettsial infection. Indeed, NF-κB activation mediates R. rickettsii-induced expression of prothrombotic TF (28), a cell surface receptor and essential cofactor for coagulation factor VII, which is an important initiator of intravascular coagulation (1). Its activation is also required to inhibit host cell apoptosis during infection (4), probably by inducing expression of survival factors. NF-κB is a ubiquitous family of transcription factors which resides in the cell cytoplasm bound to a member of the family of structurally related inhibitor proteins known as IκB. Upon appropriate stimulation, IκB is phosphorylated on specific serine residues which targets it for degradation by the proteasome (31), thus exposing nuclear translocation sequences and DNA-binding domains. R. rickettsii infection of endothelial cells results in a biphasic pattern of NF-κB activation, with an early transient phase evident at about 3 h, with a decline back to basal levels by 14 h, followed by a second, sustained phase appearing at 18 to 24 h (35). Aside from the fact that activation requires intracellular uptake of viable organisms (35), little is known about signaling pathways involved in R. rickettsii-induced activation of NF-κB.
Although activation of NF-κB is a common response of the host cell to a variety of infectious agents, including Mycobacterium tuberculosis (40), Shigella flexneri (9), Listeria monocytogenes (13, 14), Salmonella typhimurium (15), parasitic protozoans such as Theileria parva (18), and many viruses (31), little is known about the intracellular signaling pathways involved. S. typhimurium infection of cultured intestinal epithelial cells was recently reported to trigger activation of mitogen-activated protein (MAP) kinases, ERK, JNK, and p38, and such activation could be linked to the resulting nuclear responses including interleukin-8 (IL-8) expression (15). L. monocytogenes invasion also activates several host cell MAP kinases (37), but involvement of these kinases in NF-κB activation has not been explored. Yersinia pseudotuberculosis, on the other hand, inhibits NF-κB activation in its host by a mechanism involving the YopJ protein (26).
Several studies have suggested a role for protein kinase C (PKC), a family of structurally related, phospholipid-dependent, serine-threonine kinases (17), in the activation of NF-κB by infectious agents and other stimuli. The persistent but not the initial activation of NF-κB by respiratory syncytial virus involves signaling through PKC (3). Lysophosphatidylcholine, an atherogenic phospholipid, induces biphasic activation of NF-κB, and its effect is partly mediated through a PKC-dependent pathway (36). Furthermore, activated PKC itself is sufficient to induce NF-κB activation in vitro (30). Exploring the potential involvement of PKC in R. rickettsii-induced NF-κB activation is a logical first step in elucidation of signal transduction pathways. In the present study, we have utilized pharmacologic inhibitors of PKC as well as phorbol ester-induced downregulation of this enzyme to investigate whether R. rickettsii-induced activation of NF-κB and TF expression in the host endothelial cell involve PKC.
(A portion of this work was presented at the American Society for Microbiology conference entitled “A Cell Biology Approach to Microbial Pathogenesis” held in Portland, Oreg., 1999.)
MATERIALS AND METHODS
Reagents.
Bisindolylmaleimide I hydrochloride (BM-1; GF 109203X; Gö 6850), phorbol 12-myristate 13-acetate (PMA), calphostin C, H7 dihydrochloride, and staurosporine were obtained from Sigma Chemical Co. (St. Louis, Mo.). Stock concentrations of BM-1 and calphostin C were prepared in dimethyl sulfoxide, and PMA was dissolved in ethanol. TRI Reagent was purchased from Molecular Research Center, Inc., Cincinnati, Ohio. [γ-32P]ATP (3,000 Ci/mmol) was obtained from Dupont NEN (Boston, Mass.).
Cell culture.
Human umbilical vein EC were isolated as described previously (10) and cultured in McCoy's 5a medium (Flow Laboratories, McLean, Va.) supplemented with 20% fetal bovine serum, EC growth supplement (50 μg/ml; Collaborative Research Inc., Bedford, Mass.), heparin (100 μg/ml; Sigma), and insulin (25 μg/ml; Sigma). All experiments used cells at passage 2, which were plated so as to achieve confluence after 4 to 5 days in culture.
Infection with R. rickettsii and treatment with inhibitors.
Near-confluent cell cultures were infected with R. rickettsii as previously described (35). The Sheila Smith strain of R. rickettsii was used as a plaque-purified seed stock (1 × 107 to 5 × 107 PFU/cm2) prepared in Vero cells (African green monkey kidney; American Type Culture Collection, Rockville, Md.). EC were infected with approximately 6 × 104 PFU/cm2 of cell culture area, and infection was monitored on cells cultured in parallel on Thermanox plastic coverslips (33). To study the effects of treatments, cells were incubated with the desired concentrations of the inhibitor in complete culture medium for 1 h at 37°C prior to and during infection with R. rickettsii.
Preparation of nuclear extracts and gel shift analysis.
After infection and/or inhibitor treatment, EC nuclei were isolated, and nuclear proteins were extracted as previously described (35). Approximately 2 × 106 EC were used per experimental condition. The protein concentration in the nuclear extracts was measured by using the Bradford reagent (Bio-Rad, Hercules, Calif.) and typically ranged between 0.2 and 0.5 mg/ml. HeLa cell nuclear extracts containing activated NF-κB (Promega Corporation, Madison, Wis.) were used as controls for all gel shift experiments. Gel shift analyses were performed with the Promega Gel Shift Assay System according to the manufacturer's specifications by using 2 μg of nuclear protein for each gel shift reaction. A double-stranded oligonucleotide containing consensus NF-κB binding sequence (5′-AGT TGA GGG TTT CCC AGG C-3′) was end labeled with [γ-32P]ATP by using T4 polynucleotide kinase, as instructed by the manufacturer (Promega). Competition studies were performed by adding a 10-fold molar excess of unlabeled oligonucleotide to the reaction mixture prior to the addition of radiolabeled probe. Reaction mixtures were analyzed on 4% nondenaturing polyacrylamide gels in 0.5× TBE (89 mM Tris-HCl, pH 8.0; 89 mM boric acid; 2 mM EDTA) as the running buffer. Electrophoresis was performed at 100 V for 2 to 3 h, followed by drying of the gel at 80°C under vacuum and visualization of DNA-protein complexes by autoradiography for 12 to 18 h.
Semiquantitative RT-PCR.
Total RNA was isolated from EC cultured in 25-cm2 flasks by using Tri Reagent (Molecular Research Center, Inc., Cincinnati, Ohio) according to the manufacturer's protocol. Total RNA (0.5 μg) was then reverse-transcribed by using Superscript RNase H reverse transcriptase (RT; Bethesda Research Laboratories, Gaithersburg, Md.) and amplified by using standard PCR protocols in a Perkin-Elmer Cetus Thermal Cycler. The cycles comprised an initial incubation at 95°C for 105 s followed by cycling at 95°C for 30 s, 65°C for 30 s, and 72°C for 60 s, with a final incubation at 72°C for 7 min. The nucleotide sequences of the primers used were as follows: TF forward primer, 5′-ACT CCC CAG AGT TCA CAC CTT ACC-3′; TF reverse primer, 5′-TGA CCA CAA ATG CCA CAG CTC C-3′ (398-bp product); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward primer, 5′-CCA CCC ATG GCA AAT TCC ATG GCA-3′; GAPDH reverse primer, 5′-TCT AGA CGG CAG GTC AGG TCC ACC-3′ (588-bp product). The amplification products, after completion of 25, 30, 35, and 40 cycles respectively, were separated by electrophoresis on a 1.5% agarose gel, visualized by ethidium bromide staining, and analyzed by comparison with a 1-kb DNA ladder (Gibco BRL).
Measurement of TF activity.
EC cultured in 12-well plates were washed twice with Tris-buffered saline (TBS; 0.05 M Tris, 0.1 M NaCl, pH 7.5) and lysed in 0.16 ml of TBS containing 10 mg of bovine serum albumin per ml. After three freeze-thaw cycles, the TF activity in cell lysates was assayed by a two-stage clotting assay (33). Results were quantitated based on a standard curve generated by using purified human brain TF reconstituted into phospholipid vesicles (kindly provided by Yale Nemerson), as previously described (2, 25).
RESULTS
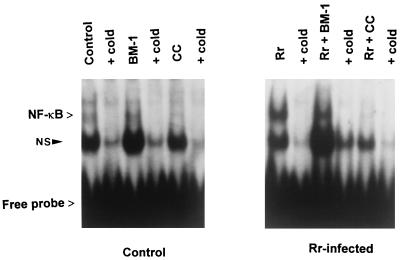
To explore the involvement of PKC during the early phase of NF-κB activation during R. rickettsii infection, cultured endothelial cells were infected for 3 h in the presence or absence of a potent and highly specific inhibitor of PKC, BM-1 (Ki = 10 nM) or calphostin C (50% inhibitory concentration = 50 nM). BM-1 (50 nM) was used to selectively inhibit the calcium-dependent, phorbol ester-sensitive classical PKCs (cPKCs; α, β, and γ isozymes) (23, 41). Calphostin C interacts with the common regulatory domain in all isozymes of PKC (19) and inhibits the calcium-independent, phorbol ester-insensitive, atypical PKCs (aPKCs, ζ and λ) as well (12). A third class of PKC isozymes, the novel PKCs (nPKCs, δ and ɛ) do not require calcium but are phorbol ester responsive. Nuclear extracts were prepared, and activated NF-κB was measured by gel shift assay with a 32P-labeled oligonucleotide probe. Very low levels of activated NF-κB were present in the nuclear extracts from uninfected endothelial cells. Treatment with the inhibitors BM-1 and calphostin C alone did not alter this basal level of activation. As reported previously (35), a 3-h infection with R. rickettsii led to a dramatic increase in the intensity of the gel-shifted complexes, indicating activation of NF-κB. This activation was not affected by BM-1 but was completely blocked by calphostin C (Fig. 1). Immunofluorescence staining for R. rickettsii, performed in parallel by using cells cultured on coverslips, indicated that these treatments had no effect on the level of endothelial cell infection (not shown). Treatment of cells with the PKC inhibitors, H7 or staurosporine, dramatically enhanced basal levels of NF-κB activation (results not shown) and thus could not be used to assess involvement of PKC in R. rickettsii-induced responses.
FIG. 1.
Effect of PKC inhibitors on R. rickettsii-induced NF-κB activation. Cultured endothelial cells were incubated with BM-1 or calphostin C (CC) for 1 h prior to and during a 3-h infection with R. rickettsii. Nuclear extracts were prepared, and activation of NF-κB was measured by gel shift analysis by using a 32P-labeled oligonucleotide containing a consensus NF-κB binding sequence. A 10-fold molar excess of unlabeled oligonucleotide was added (+ cold) to demonstrate specificity of complex formation. Autoradiographic exposures were typically for 18 to 24 h. The positions of gel-shifted NF-κB complexes and free probe are indicated. NS represents a nonspecific and noninducible complex.
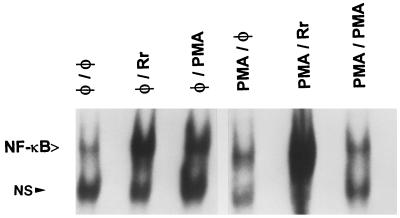
Endothelial cells were preincubated with PMA (100 ng/ml) for 48 h to downregulate and deplete the cells of PMA-sensitive PKCs (11, 16). Cells were subsequently infected with R. rickettsii (3 h) or challenged with PMA (20 ng/ml). As shown in Fig. 2, in the absence of PMA downregulation, both R. rickettsii infection (lane 2) and PMA stimulation (lane 3) resulted in similar levels of NF-κB activation. PMA-pretreated endothelial cells, as expected, were refractory to subsequent PMA stimulation (lane 6). PMA pretreatment did not inhibit and actually appeared to potentiate the activation response to R. rickettsii infection (lane 5). The extent of endothelial cell infection was not affected by pretreatment of cells with PMA (not shown). These results, in conjunction with the inhibitor studies described above, suggest that R. rickettsii-induced NF-κB activation occurred independently of the involvement of PMA-sensitive PKCs, which include the classical and novel PKC isoforms. Furthermore, since BM-1 treatment had no effect on the level of activation seen during the late phase of R. rickettsii-induced NF-κB activation (not shown), it is likely that classical PKCs are similarly not involved in this phase as well. It was not possible to assess the effects of calphostin C on the late phase of activation, since its complete inhibitory effect on the early phase led to rapid host cell loss by apoptosis.
FIG. 2.
Effect of downregulation of phorbol ester-sensitive PKCs on R. rickettsii-induced NF-κB activation. Activated NF-κB was assayed by gel shift analysis of nuclear extracts derived from the following EC samples: untreated, uninfected EC (φ/φ); EC infected with R. rickettsii in the absence of PMA pretreatment (φ/Rr); EC challenged with PMA (20 ng/ml) in the absence of PMA pretreatment (φ/PMA); EC pretreated with PMA (100 ng/ml) with no subsequent challenge or infection (PMA/φ); EC pretreated for 48 h with PMA (100 ng/ml), followed by R. rickettsii-infection (3 h) (PMA/Rr); and EC pretreated for 48 h with PMA (100 ng/ml), followed by PMA (20 ng/ml) challenge for 30 min (PMA/PMA).
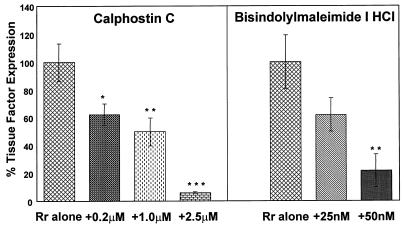
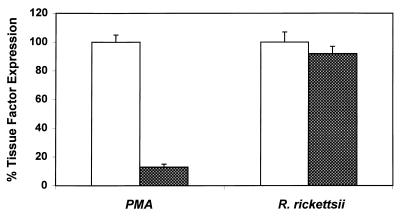
The effects of BM-1, calphostin C, and PMA downregulation of PKC on R. rickettsii-induced expression of TF procoagulant activity were then studied. As reported previously, infection of endothelial cells for 8 h resulted in enhanced expression of TF activity (28). Untreated endothelial cells expressed very low basal levels of TF activity. As expected, based on its ability to block R. rickettsii-induced NF-κB activation, calphostin C abrogated R. rickettsii-induced TF activity in a dose-dependent manner. Surprisingly, treatment of cells with BM-1 (25 and 50 nM), which did not inhibit R. rickettsii-induced NF-κB activation, also resulted in substantial, dose-dependent inhibition of R. rickettsii-induced TF activity (Fig. 3). Downregulation of the phorbol ester-responsive PKCs by prolonged PMA treatment, however, had no effect on R. rickettsii-induced TF activity, whereas it completely blocked the response to a second challenge with PMA (Fig. 4).
FIG. 3.
Effect of PKC inhibitors on R. rickettsii-induced TF activity. The effects of increasing concentrations of calphostin C and BM-1 on R. rickettsii-induced expression of TF procoagulant activity are shown. Untreated endothelial cells expressed low levels of TF activity, whereas a 10- to 20-fold increase in expression occurred after 8 h of infection with R. rickettsii. Within each experiment, the percent TF activity was calculated, with the amount present in R. rickettsii-infected EC (8 h) in the absence of inhibitor assigned a value of 100%. Results shown represent the mean ± the standard error of the mean (SEM) from three separate experiments performed in duplicate. ∗, P < 0.05, ∗∗, P < 0.02; ∗∗∗, P < 0.002. P values were calculated in relation to results with no inhibitor present during infection.
FIG. 4.
Effect of downregulation of PMA-sensitive PKCs on R. rickettsii-induced TF activity. EC were treated with PMA (100 ng/ml) for 48 h and infected with R. rickettsii for 8 h or else rechallenged with PMA (20 ng/ml). PMA and R. rickettsii-induced TF activities in untreated EC were assigned a value of 100% (open bars), and changes are expressed relative to this value (shaded bars). The values shown are the mean ± the SEM (n ≥ 9).
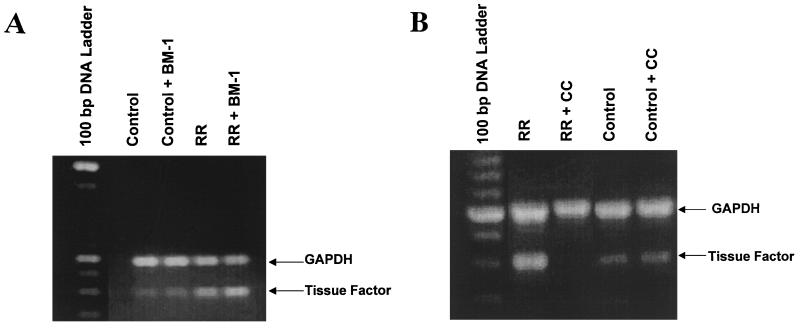
To determine whether BM-1 blocked the induced expression of TF activity by preventing transcriptional activation of the TF gene, steady-state levels of TF mRNA were measured by semiquantitative RT-PCR. Total RNA was isolated from endothelial cells infected for 4 h and from uninfected cells (control) in the presence or absence of BM-1 (50 nM). As previously reported, R. rickettsii infection caused an increase in TF mRNA levels (28). BM-1 treatment resulted in no apparent decrease in R. rickettsii-induced levels of TF mRNA (Fig. 5A). Calphostin C, however, completely blocked TF mRNA expression (Fig. 5B), a result consistent with its inhibitory effect on R. rickettsii-induced NF-κB activation. Neither R. rickettsii infection nor the presence of either inhibitor affected steady-state levels of the housekeeping mRNA species, GAPDH.
FIG. 5.
(A) Effect of BM-1 on R. rickettsii-induced expression of TF mRNA. Levels of TF mRNA (398-bp product) and GAPDH mRNA (588-bp product) were analyzed in total RNA samples prepared from uninfected EC (Control), uninfected EC in the presence of BM-1 (Control + BM-1), R. rickettsii-infected EC (RR), and infected EC in the presence of BM-1 (RR + BM-1) by RT-PCR analysis with specific primer pairs. The amplification products generated after 30 amplification cycles are shown. (B) Effect of calphostin C (CC) on R. rickettsii-induced expression of TF mRNA. Levels of TF mRNA and GAPDH mRNA were analyzed in total RNA preparations from R. rickettsii-infected EC (RR), infected EC in the presence of CC (RR + CC), uninfected EC (Control), and uninfected EC in the presence of CC (Control + CC) by RT-PCR analysis with the primer sequences described in Materials and Methods.
DISCUSSION
Studies were undertaken to explore the potential involvement of PKC in R. rickettsii-induced activation of NF-κB and TF expression in endothelial cells. Two inhibitors of PKC, BM-1 and calphostin C, each of which displays unique structural characteristics, inhibitory properties, and mode of action, were employed. BM-1 inhibits PKC by competing for the ATP binding site and, at the concentrations used in this study, exhibits high selectivity for classical isoforms of PKC (Ki = 10 nM). It can inhibit other isozymes, including atypical PKCs, but only at much higher concentrations; it has little effect on cyclic AMP-dependent protein kinase, myosin light-chain kinase, or phosphorylase kinase and is inactive against tyrosine kinases (41). BM-1 had no effect on the degree of NF-κB activation induced by infection, either at the peak of the early phase (3 h) (Fig. 1) or during the late, sustained phase of activation (18 h) (results not shown). Calphostin C, which inhibits PKC by competing at the binding site of diacylglycerol and phorbol esters, is known to inhibit atypical PKCs (12) and completely blocked R. rickettsii-induced activation of NF-κB (Fig. 1). Consistently, prolonged treatment with the phorbol ester, PMA, which downregulates and depletes cells of classical and novel PKC isoforms, but not atypical PKCs (11, 16), did not diminish NF-κB activation induced by R. rickettsii infection (Fig. 2).
Taken together, these results argue strongly against the involvement of classical (α, β, and γ), as well as novel (δ and ɛ), PKC isoforms in R. rickettsii-induced NF-κB activation. Involvement of an atypical PKC such as PKCζ remains a likely possibility since this diacylglycerol-insensitive and calcium-independent isozyme of PKC is not activated or downregulated by exposure to PMA (5) and is not inhibited by BM-1 at the concentrations used and yet is blocked by calphostin C. PKCζ is thought to be required for the activation of NF-κB in mammalian cells (21), possibly through the activation of an IκB kinase leading to the phosphorylation and inactivation of IκBα (7). Further, overexpression of PKCζ is sufficient to activate NF-κB, and transfection of NIH 3T3 fibroblasts with a kinase-defective dominant-negative mutant of PKCζ dramatically blocks κB-dependent transactivation (6).
Expression of the procoagulant protein, TF, is an important consequence of NF-κB activation, since its expression could contribute to the inflammatory and thrombotic consequences of disease. NF-κB controls expression of many genes involved in rapid responses of endothelial cells to injury or inflammatory signals, including E-selectin, interleukin-1 (IL-1), and tissue factor. We have previously demonstrated that R. rickettsii infection induces NF-κB-dependent TF expression (28). An active infection is necessary for turning on the signaling machinery leading to these phenomena since treatment of EC with cytochalasin B, which causes inhibition of rickettsial entry into the host cell without eliminating adherence of the organisms to the surface by disrupting cell's actin cytoskeleton, inhibited activation of NF-κB by about 70% and eliminated the TF response (33, 35). Similarly, rendering R. rickettsii noninfective by tetracycline treatment or UV exposure also eliminated TF induction (33). Rickettsial lipopolysaccharide (LPS) also appears not to function in the signaling events resulting in transcriptional activation, since adsorption of suspensions of intact, viable R. rickettsii on polymyxin B had no significant effect on TF expression (33).
The role of PKC in the induced expression of endothelial cell TF has been investigated in response to several stimuli, with no clear consensus on its involvement. The PKC inhibitors, H7 and sphingosine, blocked expression of TF activity in response to tumor necrosis factor alpha (TNF-α), IL-1, LPS, and stimulation with allogenic T-cell subpopulations. PMA downregulation of PKC, however, did not block expression of TF activity in response to LPS and IL-1 (24). In the present study, TF activity during R. rickettsii infection was completely unaffected by PMA-induced downregulation (Fig. 4). Further analysis of R. rickettsii-induced TF mRNA and functional activity in the presence of the PKC inhibitors BM-1 and calphostin C revealed that PKC may be involved as an important mediator in the expression of TF at the levels of mRNA as well as the protein.
While our studies suggest that R. rickettsii-induced activation of NF-κB likely involves an atypical PKC, it appears that the expression of TF activity on the cell surface may be regulated at the posttranscriptional level. Although expression of TF activity during R. rickettsii infection was insensitive to PMA-induced downregulation of PKC (Fig. 4), it was inhibited by BM-1 in a concentration-dependent manner (Fig. 3). It was interesting, however, that BM-1 had no significant inhibitory effect on R. rickettsii-induced expression of TF mRNA (Fig. 5A), indicating that its effect is not exerted at the level of gene expression. It is not yet clear whether BM-1-mediated inhibition of TF activity is specific to or independent of its PKC inhibitory activity. In contrast, calphostin C, which has been shown to inhibit TF mRNA expression in response to IL-1α and TNF-α (38), had an almost complete inhibitory effect on the mRNA and activity levels of TF. It was recently reported that NF-κB-induced expression of IκBα after stimulation with IL-1α, but not TNF-α, involves PKC-dependent, posttranscriptional regulation of the IκBα transcript. Specifically, inhibition of PKCα resulted in nuclear retention of this mRNA species (12). R. rickettsii-induced expression of TF appears to be at least partially regulated by a similar mechanism, perhaps involving one or more isozymes of PKC. It is important to note that the inhibitor studies described here do not reveal the entire spectrum of activation of various PKC isoforms during infection. Such studies may reveal that more than one isozyme of PKC is activated, perhaps at various times throughout the course of infection, but particular isozymes are involved in the various steps leading to the altered host cell phenotype.
NF-κB activation likely constitutes a pivotal event in R. rickettsii-induced activation of the host endothelial cell. The data presented clearly suggest that NF-κB activation occurs independently of classical PKCs but likely involves a phorbol ester-insensitive, atypical isoform. Since PKC isotypes are unique with respect to their primary structure, expression patterns, and responsiveness to extracellular ligands and might have separate and unique functions in the cell (17), the resultant change in cell phenotype may be influenced by other mechanisms involving PKC isozymes that are exerting a regulatory influence at the posttranscriptional level.
ACKNOWLEDGMENTS
We thank Li Hua Rong for assistance with cell culture, Norma B. Lerner for help with the RT-PCR, David J. Silverman and Lisa Domotor for providing R. rickettsii, and Catherine Farrell for help with the bibliography.
This work was supported in part by grants AI 40689, AI 17416, and HL 30616 from the National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Bach R. Initiation of coagulation by tissue factor. Crit Rev Biochem. 1988;23:339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 2.Bach R, Gentry R, Nemerson Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: induction of cooperativity by phosphatidylserine. Biochemistry. 1986;25:4007–4020. doi: 10.1021/bi00362a005. [DOI] [PubMed] [Google Scholar]
- 3.Bitko V, Barik S. Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IκBβ, and sequestration of protein phosphatase 2A by the viral phosphoprotein. J Virol. 1998;72:5610–5618. doi: 10.1128/jvi.72.7.5610-5618.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clifton D R, Goss R A, Sahni S K, vanAntwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsiiinfection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crespo P, Mischak H, Gutkind J S. Overexpression of mammalian protein kinase C-zeta does not affect the growth characteristics of NIH 3T3 cells. Biochem Biophys Res Commun. 1995;213:266–272. doi: 10.1006/bbrc.1995.2125. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Meco M T, Berra E, Municio M M, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera M T, Alcamí J, Payá C V, Arenzana-Seisdedos F, Virelizier J-L, Moscat J. A dominant negative protein kinase C ζ subspecies blocks NF-κB activation. Mol Cell Biol. 1993;13:4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Meco M T, Dominguez I, Sanz L, Dent P, Lozano J, Municio M M, Berra E, Hay R T, Sturgill T W, Moscat J. ζPKC induces phosphorylation and inactivation of IκB-α in vitro. EMBO J. 1994;13:2842–2848. doi: 10.1002/j.1460-2075.1994.tb06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drancourt M, Alessi M-C, Levy P-Y, Juhan-Vague I, Raoult D. Selection of tissue-type plasminogen activator and plasminogen activator inhibitor by Rickettsia conorii- and Rickettsia rickettsii-infected cultured endothelial cells. Infect Immun. 1990;58:2459–2463. doi: 10.1128/iai.58.8.2459-2463.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyer R B, Collaco C R, Niesel D W, Herzog N K. Shigella flexneriinvasion of HeLa cells induces NF-κB DNA-binding activity. Infect Immun. 1993;61:4427–4433. doi: 10.1128/iai.61.10.4427-4433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimbrone M A, Jr, Cotran R S, Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974;60:673–680. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godson C, Weiss B A, Insel P A. Differential activation of protein kinase C α is associated with arachidonate release in Madin-Darby canine kidney cells. J Biol Chem. 1990;265:8369–8372. [PubMed] [Google Scholar]
- 12.Han Y, Meng T, Murray N R, Fields A P, Brasier A R. Interleukin-1-induced nuclear factor-κB-IκBα autoregulatory feedback loop in hepatocytes. J Biol Chem. 1999;274:939–947. doi: 10.1074/jbc.274.2.939. [DOI] [PubMed] [Google Scholar]
- 13.Hauf N, Goebel W, Serfling E, Kuhn M. Listeria monocytogenes infection enhances transcription factor NF-κB in P388D1macrophage-like cells. Infect Immun. 1994;62:2740–2747. doi: 10.1128/iai.62.7.2740-2747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauf N, Goebel W, Fiedler F, Sokolovic Z, Kuhn M. Listeria monocytogenes infection of P388D1macrophages results in a biphasic NF-κB (RelA/p50) activation induced by lipoteichoic acid and bacterial phospholipases and mediated by IκBα and IκBβ degradation. Proc Natl Acad Sci USA. 1997;94:9394–9399. doi: 10.1073/pnas.94.17.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobbie S, Chen L M, Davis R J, Galán J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimuriumin cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 16.Huang F L, Yoshida Y, Cunha-Melo J R, Beaven M A, Huang K-P. Differential down-regulation of protein kinase C isozymes. J Biol Chem. 1989;264:4238–4243. [PubMed] [Google Scholar]
- 17.Hug H, Sarre T F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov V, Stein B, Baumann I, Dobbelaere D A E, Herrlich P, Williams R O. Infection with the intracellular protozoan parasite Theileria parvainduces constitutively high levels of NF-κB in bovine T lymphocytes. Mol Cell Biol. 1989;9:4677–4686. doi: 10.1128/mcb.9.11.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 20.Kunsch C, Rosen C A. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano J, Berra E, Municio M M, Diaz-Meco M T, Dominguez I, Sanz L, Moscat J. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 22.Mackman N, Brand K, Edgington T S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THO-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991;174:1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martiny-Baron G, Kazanietz M G, Mischak H, Blumberg P M, Kochs G, Hug H, Marmé D, Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 24.Pettersen K S, Wiiger M T, Narahara N, Andoh K, Gaudernack G, Prydz H. Induction of tissue factor synthesis in human umbilical vein endothelial cells involves protein kinase C. Thromb Haemostasis. 1992;67:473–477. [PubMed] [Google Scholar]
- 25.Pitlick F A, Nemerson Y. Purification and characterization of tissue factor apoprotein. Methods Enzymol. 1976;45:37–42. doi: 10.1016/s0076-6879(76)45007-3. [DOI] [PubMed] [Google Scholar]
- 26.Schesser K, Spiik A-K, Dukuzumuremyi J-M, Neurath M F, Pettersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 27.Shi R-J, Simpson-Haidaris P J, Marder V J, Silverman D J, Sporn L A. Increased expression of plasminogen activator inhibitor-1 in R. rickettsii-infected endothelial cells. Thromb Haemostasis. 1996;75:600–606. [PubMed] [Google Scholar]
- 28.Shi R-J, Simpson-Haidaris P J, Lerner N B, Marder V J, Silverman D J, Sporn L A. Transcriptional regulation of endothelial cell tissue factor expression during Rickettsia rickettsiiinfection: involvement of the transcription factor NF-κB. Infect Immun. 1998;66:1070–1075. doi: 10.1128/iai.66.3.1070-1075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu H, Mitomo K, Watanabe T, Koamoto S, Yamamoto K. Involvement of the NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990;10:561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirakawa F, Mizel S B. In vitro activation and nuclear translocation of NF-κB catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989;9:2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 32.Sporn L A, Lawrence S O, Silverman D J, Marder V J. E-selectin-dependent neutrophil adhesion to Rickettsia rickettsii-infected endothelial cells. Blood. 1993;81:2406–2412. [PubMed] [Google Scholar]
- 33.Sporn L A, Simpson-Haidaris P J, Shi R-J, Nemerson Y, Silverman D J, Marder V J. Rickettsia rickettsiiinfection of cultured human endothelial cells induces tissue factor expression. Blood. 1994;83:1527–1534. [PubMed] [Google Scholar]
- 34.Sporn L A, Marder V J. Interleukin-1α production during Rickettsia rickettsii-infection of cultured endothelial cells: potential role in autocrine cell stimulation. Infect Immun. 1996;64:1609–1613. doi: 10.1128/iai.64.5.1609-1613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sporn L A, Sahni S K, Lerner N B, Marder V J, Silverman D J, Turpin L C, Schwab A L. Rickettsia rickettsiiinfection of cultured human endothelial cells induces NF-κB activation. Infect Immun. 1997;65:2786–2791. doi: 10.1128/iai.65.7.2786-2791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugiyama S, Kugiyama K, Ogata N, Doi H, Ota Y, Ohgushi M, Matsumura T, Oka H, Yasue H. Biphasic regulation of transcription factor nuclear factor-κB activity in human endothelial cells by lysophosphatidylcholine through protein kinase C-mediated pathway. Arterioscler Thromb Vasc Biol. 1998;18:568–576. doi: 10.1161/01.atv.18.4.568. [DOI] [PubMed] [Google Scholar]
- 37.Tang P, Sutherland C L, Gold M R, Finlay B B. Listeria monocytogenesinvasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect Immun. 1998;66:1106–1112. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terry C M, Callahan K S. Protein kinase C regulates cytokine-induced tissue factor transcription and procoagulant activity in human endothelial cells. J Lab Clin Med. 1996;127:81–93. doi: 10.1016/s0022-2143(96)90169-9. [DOI] [PubMed] [Google Scholar]
- 39.Teysseire N, Arnoux D, George F, Sampol J, Raoult D. von Willebrand factor release, thrombomodulin activity and tissue factor expression in Rickettsia conorii-infected endothelial cells. Infect Immun. 1992;60:4388–4393. doi: 10.1128/iai.60.10.4388-4393.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toossi Z, Hamilton B D, Phillips M H, Averill L E, Ellner J J, Salvekar A. Regulation of nuclear factor-κB and its inhibitor IκB-α/MAD-3 in monocytes by Mycobacterium tuberculosisand during human tuberculosis. J Immunol. 1997;159:4109–4116. [PubMed] [Google Scholar]
- 41.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 42.Whelan J, Ghersa P, Hooft van Huijsduijnen T, Gray J, Chandra G, Talabot F, DeLamarter J F. An NF kappa B-like factor is essential but not sufficient for cytokine induction of endothelial leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Nucleic Acids Res. 1991;19:2645–2653. doi: 10.1093/nar/19.10.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]